Pravit Asawanonda, MD, DSc, and Yaowalak Nateetongrungsak, MD Bangkok, Thailand
|
|
|
- Alan Banks
- 6 years ago
- Views:
Transcription
1 Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: A randomized, placebo-controlled study Pravit Asawanonda, MD, DSc, and Yaowalak Nateetongrungsak, MD Bangkok, Thailand Background: Combining phototherapy with topical and oral agents allows clinicians to treat recalcitrant psoriasis with reduced number of treatments and cumulative UV exposures. Objective: This study was designed to determine the number of treatments necessary to clear plaque-type psoriasis when narrowband (NB) UVB is administered with methotrexate (MTX) or placebo in a randomized, controlled fashion. Methods: MTX (15 mg/wk) or placebo was administered 3 weeks before standard NB UVB phototherapy was started. Treatments with the oral agent and phototherapy were continued until Psoriasis Area and Severity Index scores were reduced to less than 10% of the original scores or 24 weeks. Follow-up was performed until lesional scores returned to 50% of the original ones. Results: A total of 24 patients were enrolled and 19 patients completed the study. Kaplan-Meier analysis revealed that the median time to clear psoriasis in the MTX/NB UVB group was 4 weeks, which was significantly less than that for the placebo/nb UVB group. Limitations: Our sample size was relatively small (24 patients) with 5 dropouts. In addition, the study was conducted in skin types III to IV, Asian patients. Follow-up was limited to 4 to 6 months after completion of phototherapy. Conclusion: MTX pretreatment allows physicians to clear psoriasis in fewer phototherapy sessions than when phototherapy is administered alone. ( J Am Acad Dermatol 2006;54: ) Treatment for psoriasis is not totally harmless. The use of methotrexate (MTX) is notoriously associated with liver toxicity especially when long-term treatment is used. Retinoids are teratogenic and cause a plethora of adverse effects, although mostly pharmacologic and dose-related. Abbreviations used: DLQI: Dermatology Life Quality Index MED: minimal erythema dose MTX: methotrexate NB: narrowband PASI: Psoriasis Area and Severity Index PUVA: psoraleneuva From the Division of Dermatology, Department of Medicine, Chulalongkorn University. Funding sources: None. Conflicts of interest: None identified. Accepted for publication January 6, Reprint requests: Pravit Asawanonda, MD, DSc, Division of Dermatology, Department of Medicine, King Chulalongkorn Memorial Hospital, Rama 4 Rd, Bangkok Thailand. pravit@adsl.loxinfo.com /$32.00 ª 2006 by the American Academy of Dermatology, Inc. doi: /j.jaad Cyclosporin A is nephrotoxic. UV therapies can cause skin to age prematurely and, more significantly, skin cancers. Because most of the adverse effects from the above-mentioned modalities occur after prolonged treatments, rotational, 1 sequential, 2 and combination therapies are often recommended. Combining two or more modalities often allows treating physicians to clear psoriasis faster, thereby exposing patients to fewer doses of each treatment. 1013
2 1014 Asawanonda and Nateetongrungsak JAM ACAD DERMATOL JUNE 2006 Table I. Baseline characteristics of study patients Characteristics MTX/NB UVB (n = 11) Placebo/NB UVB (n = 13) P value Age, y Range Mean 6 SD * Sex Male y Female 5 4 Skin type III y IV 8 9 Mean duration of disease (mo) 6 SD * PASI score at baseline Range Mean 6 SD * PASI score after 3 wk of MTX or placebo Range Mean 6 SD * PASI score at the end of NB UVB treatment Range Mean 6 SD * Mean DLQI score 6 SD * DLQI, Dermatology Life Quality Index; MTX, methotrexate; NB, narrowband; PASI, Psoriasis Area and Severity Index. *Student t test. y Chi-square test. In the developing countries, retinoids, cyclosporine, let alone biologics, are often times prohibitively expensive. MTX is, thus, the mainstay when costs are taken into consideration. Phototherapy is a relatively safe and very effective treatment modality. However, several weeks are often required to achieve satisfactory clearing of lesions. Our study was designed to evaluate the efficacy of MTX plus narrowband (NB) UVB phototherapy, the combination that has never been tested to clear plaque-type psoriasis. METHODS The study protocol was approved by the our ethics committee. Informed consent was obtained from each participant. Patients with plaque-type psoriasis, with at least 20% of body surface area involvement, whose disease activity had been stable in the 3 months before entering the study were eligible. Patients had to have discontinued systemic treatments, including psoraleneuva (PUVA), for the past 8 weeks, UVB phototherapy for 4 weeks, and all topical treatments for 2 weeks before entering study. Patients with known history of MTX intolerance, photosensitivity, immunosuppression, and alcohol abuse, and those who were pregnant or lactating were excluded. Patients were randomized by way of randomization cards to receive either MTX or placebo, which were identical in appearance. MTX (15 mg) or placebo was given in 3 divided weekly doses for 3 weeks before starting NB UVB phototherapy. The oral medications were given on weekends and continued until clearance or end of 24-week study period. At the end of the 3-week run-in period and before starting phototherapy, minimal erythema dose (MED) was determined in all patients. The fluences used at MED testing were 0.28, 0.40, 0.56, 0.80, and 1.12 J/cm 2. Total body irradiation was administered thrice weekly using stand-up cabinets (Daavlin Spectra 311, Bryan, Ohio; TL W lamps, Philips, Eindhoven, The Netherlands). Before each phototherapy session, liberal use of mineral oil was encouraged. Starting UVB dose and dose escalations were standard as used in many treatment centers, ie, initial UVB dose was 70% of the MED then increased by 20% if no reactions developed from previous treatment, 10% if minimal reactions occurred, and 0% if erythema lasting longer than 24 hours developed. If no erythema developed from MED determination, UVB fluence was started at 0.70 J/cm 2 (rounding down from 0.78 J/cm 2, which was 70% of the highest fluence given at testing) for safety reasons. Dose increments continued until lesion clearance, which was defined as 90% reduction in Psoriasis Area and Severity Index (PASI) scores. At clearance or 24 weeks, all forms of therapy were discontinued without tapering or maintenance.
3 JAM ACAD DERMATOL VOLUME 54, NUMBER 6 Asawanonda and Nateetongrungsak 1015 Table II. Time to achieve Psoriasis Area and Severity Index 90 and time to relapse after clearance Patient no. Time to clearance, wk No. of NB UVB treatments Cumulative UVB fluence, J/cm 2 Time to relapse, wk MTX/NB UVB Not relapsed at 16 wk Not relapsed at 16 wk Not relapsed at 24 wk Not relapsed at 16 wk Not relapsed at 16 wk Not relapsed at 16 wk 11 Lost to follow-up 0 0 Lost to follow-up Placebo/NB UVB 12 Not cleared at 24 wk Not cleared at 24 wk Not cleared at 24 wk Not cleared at 24 wk Not relapsed at 24 wk Not relapsed at 4 wk 19 Erythroderma N/A 20 Lost to follow-up Lost to follow-up 21 Lost to follow-up Lost to follow-up 22 Lost to follow-up Lost to follow-up MTX, Methotrexate; NB, narrowband. PASI scores were given by an investigator blinded to the treatment assignment (P. A.) at baseline, after the 3-week pretreatment period, and every 2 weeks thereafter. Follow-up was done every 4 weeks after clearance or end of 24-week phototherapy period until relapse, which was defined as PASI scores returning to 50% of original scores or 6 months. Dermatology Life Quality Index (DLQI) questionnaire, which was translated to Thai with the permission of Dr Andrew Finlay and validated in more than 200 patients with variable dermatologic conditions, was used to evaluate the quality of life of patients every week until clearance. Complete blood cell counts and blood chemistry were performed every 4 weeks while patients were taking oral treatment. Statistical analysis Data from all randomized patients were included on an intent-to-treat basis. We used the t test, where equal variance was demonstrated, and the chi-square test in the initial exploration of the data. Otherwise, equivalent nonparametric statistics (Wilcoxon rank sum test) was used. Time-to-event analyses were performed for clearing and relapse of lesions by using Kaplan-Meier method to account for truncated observations. We analyzed differences between event curves by means of the log rank test. RESULTS Twenty-four patients were enrolled in the study. Of these, 11 were randomized to receive MTX plus NB UVB and 13 were in the placebo/nb UVB group. Table I illustrates the demographic data of the participants. Age, sex, skin types, baseline PASI scores, and baseline DLQI scores did not differ significantly between patients in the two treatment groups (Table I). In the MTX-NB UVB group the mean MED was 0.96 J/cm whereas that of the placebo-nb UVB group was 0.80 J/cm In all, 9 patients in the MTX-NB UVB group and 6 patients in the placebo-nb UVB group had MED beyond 1.12 J/cm 2. In our experience, the starting fluence larger than 0.70 J/cm 2 occasionally resulted in unexpected phototoxicity, even when MED testing yielded negative results; thus, in these patients, the starting NB UVB fluence was 0.70 J/cm 2 for safety reasons. Of 11 patients in the MTX/NB UVB group, 10 completed the study whereas 3 of 13 patients in the placebo/nb UVB dropped out. All 4 dropouts were a result of conflicts in work schedule. One patient
4 1016 Asawanonda and Nateetongrungsak JAM ACAD DERMATOL JUNE 2006 Fig 1. Kaplan-Meier analysis of time to clearance. MTX, Methotrexate; NB, narrowband. Fig 2. Kaplan-Meier analysis of time to relapse. MTX, Methotrexate; NB, narrowband. (No. 19) had erythroderma early in the course of phototherapy and had to discontinue the treatment. Nineteen patients, 10 male and 9 female, completed the study (Table II). Both pretreatments with MTX and placebo resulted in reduction of PASI scores. As expected, the score reduction was greater in the MTX group. The median difference in score reduction in the MTX/NB UVB versus placebo group was 5.6 (1.1, 9.7) (P =.013, Wilcoxon rank sum test). Of 11 patients who received MTX/NB UVB, 10 achieved clearance, again defined as PASI 90, whereas only 5 of 13 patients in the placebo-nb UVB did (P =.01). At the end of the treatment period, in the MTX-NB UVB group the median PASI score was In the placebo-nb UVB group the median PASI was 3.15 (P \.001, Wilcoxon rank sum test). The median time to clear in the MTX/NB UVB group was 4 weeks whereas that in the placebo/nb UVB group could not be determined because at the end of the 24-week study period, more than half of the patients were still not cleared or lost to follow-up (Fig 1). The difference in median time to clear in the two groups was statistically significant (P\.0001, log rank test). The mean cumulative MTX dose received was The mean cumulative UVB dose received in those who cleared in the MTX/NB UVB group was J/cm 2 ( J/cm 2 ) whereas in the placebo/nb UVB group this dose was J/cm 2 ( J/cm 2 )(Table II). The difference in cumulative UVB dose received in the two groups was statistically significant (P =.0026, Student t test). The median time to relapse in the placebo/nb UVB group was 4 weeks whereas that of the MTX/NB UVB group could not be evaluated because more than half of the patients still maintained their PASI scores below 50% of the original ones (Fig 2). The difference was marginally nonsignificant (P =.078, log rank test). The results obtained from DLQI assessment revealed that both groups had improvement in their quality of life with mean score reduction of in the MTX/NB UVB group and in the placebo/nb UVB group. This difference in score reduction was, however, not statistically significant (P =.74, Student t test). The number of adverse events in both groups did not vary with 4 episodes of mild erythema, two in each treatment group, and generalized hyperpigmentation in all patients. Lesional postinflammatory hyperpigmentation occurred in 5 and 7 patients in the MTX/NB UVB and placebo/nb UVB groups, respectively. Erythroderma developed in one patient in the placebo/nb UVB group. Treatments were otherwise well tolerated by all patients. Complete blood cell counts and blood chemistry findings were all within normal limits in all patients tested. DISCUSSION In modern phototherapy, UV is almost always administered with at least one other agent, topical or systemic, to minimize the dose of light necessary to clear lesions. Among the systemic agents used, retinoids are favored by many authors. 1,3-5 Retinoids have been shown to enhance the therapeutic
5 JAM ACAD DERMATOL VOLUME 54, NUMBER 6 Asawanonda and Nateetongrungsak 1017 response of broadband UVB, 6,7 NB UVB, and PUVA 8 treatments. This is reflected in the fewer numbers of treatments and cumulative UV doses required to clear lesions than when UV or the retinoid is used alone. 6,8-13 Despite the obvious efficacy, retinoids are prohibitively expensive in some developing countries, including Thailand where MTX is more widely used. The use of MTX in combination with phototherapy is not as popular as retinoids because of theoretic concern of increased carcinogenic risks. 14 This combination, although first reported in 1982, 15 is not mentioned even in some extensive reviews. 5 In their study, Paul et al 15 reported that all 26 patients were cleared after a mean of 12 broadband UVB treatments and 7 weeks of MTX intake. The mean cumulative MTX dose to achieve clearance was 112 mg (range mg). The authors mentioned that with historic controls, the number of phototherapy sessions and the dose of UVB used in the combination study was less than half when broadband UVB alone was used. The use of MTX plus PUVA was also reported in by the same group of authors. They demonstrated that with the combination, the mean number of treatments required to clear psoriasis in 93% of the patients was 9.3 and the mean treatment time was 5.7 weeks. Mean cumulative MTX dose was 93.0 mg ( mg). Again, when compared with prior treatments in the same patients, the number of treatments and treatment time and the cumulative UVA dose were less than half. To our knowledge this is the first randomized, placebo-controlled study of the combined use of MTX and NB UVB phototherapy. We have demonstrated that the combination of a 3-week course of MTX followed by NB UVB clears more patients with psoriasis (ie, 90.9% vs 38.5%), and in significantly fewer treatments than when NB UVB is used alone. Our results compare favorably with those obtained from similar studies. 15,16 The fact that NB UVB alone cleared only 38.5% of our patients warrants discussion as this clearance rate may not represent typical results normally obtained from this modality. Our explanations are 2-fold. First, our institution is a referral center where patients with psoriasis seen are relatively more recalcitrant to treatment. Secondly, PASI 90 represents an end point where lesions are almost totally cleared, thus, much more difficult to achieve than when less stringent end points are used. Most of our patients were of skin phototypes III and IV for which higher fluences are often necessary to clear the disease. Again, we were able to demonstrate that cumulative UVB doses can be significantly reduced with MTX administration. In addition, the cumulative MTX doses received were very low. With such low doses, MTX can be given for years before the total cumulative doses are high enough to pose any risks for hepatotoxicity. MTX, NB UVB, 17 and PUVA 18 are known to cause apoptosis in the infiltrating lymphocytes in psoriatic lesions. It is, thus, not surprising that the combination would be synergistic resulting in faster clearing of lesions. Moreover, MTX has been reported to result in reduction in the scaliness and thickness of the lesions, thereby explaining the improved skin optics when UVB phototherapy is given. 15,16 Our patients in both treatment groups reported significantly better quality of life compared with pretreatment baseline data as evident from the DLQI assessments. Unfortunately, our sample size was too small to demonstrate significant differences in DLQI scores between the two treatment groups despite the much better results with MTX-NB UVB treatment. The duration of remission obtained in our study is comparable with results obtained from other studies whereby patients maintained their remission for 3 to 6 months after NB UVB phototherapy with or without systemic therapeutic agents. 19 Short-term adverse effects associated with this treatment combination are not different from when either treatment is used alone. The MED obtained when patient is taking MTX is not at all different from unprimed skin. Erythroderma developed in one patient in the placebo/nb UVB group. The MED detected in this particular patient was more than 1.12 J/cm 2 and, thus, the initial UVB fluence administered was 0.70 J/cm 2. The erythema happened after the first phototherapy session, which might have resulted from too large incremental doses when MED was performed. The erythema recall phenomenon, although reported, 20 does not seem to occur so commonly as feared. 15 Subacute phototoxicity, reported when MTX was used in combination with PUVA, 16 was not seen in our study. In summary, we propose that the combination of MTX and NB UVB phototherapy is a useful and relatively safe treatment for plaque-type psoriasis. This combination could be especially useful in developing countries. The authors wish to thank Dr Andrew Finlay for his kind permission to use the DLQI questionnaire and Dr Chaichana Nimnuan for his assistance in statistical analysis. REFERENCES 1. Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol 2004;50:
6 1018 Asawanonda and Nateetongrungsak JAM ACAD DERMATOL JUNE Koo J. Systemic sequential therapy of psoriasis: a new paradigm for improved therapeutic results. J Am Acad Dermatol 1999;41(Suppl):S Lebwohl M, Drake L, Menter A, Koo J, Gottlieb AB, Zanolli M, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol 2001;45: Zanolli M. Phototherapy arsenal in the treatment of psoriasis. Dermatol Clin 2004;22: Honigsmann H. Phototherapy for psoriasis. Clin Exp Dermatol 2001;26: Iest J, Boer J. Combined treatment of psoriasis with acitretin and UVB phototherapy compared with acitretin alone and UVB alone. Br J Dermatol 1989;120: Lowe NJ, Prystowsky JH, Bourget T, Edelstein J, Nychay S, Armstrong R. Acitretin plus UVB therapy for psoriasis: comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol 1991;24: Saurat JH, Geiger JM, Amblard P, Beani JC, Boulanger A, Claudy A, et al. Randomized double-blind multicenter study comparing acitretin-puva, etretinate-puva and placebo-puva in the treatment of severe psoriasis. Dermatologica 1988; 177: Green C, Lakshmipathi T, Johnson BE, Ferguson J. A comparison of the efficacy and relapse rates of narrowband UVB (TL-01) monotherapy vs etretinate (re-tl-01) vs etretinate- PUVA (re-puva) in the treatment of psoriasis patients. Br J Dermatol 1992;127: Spuls PI, Rozenblit M, Lebwohl M. Retrospective study of the efficacy of narrowband UVB and acitretin. J Dermatol Treat 2003;14: Ruzicka T, Sommerburg C, Braun-Falco O, Koster W, Lengen W, Lensing W, et al. Efficiency of acitretin in combination with UV-B in the treatment of severe psoriasis. Arch Dermatol 1990;126: Tanew A, Guggenbichler A, Honigsmann H, Geiger JM, Fritsch P. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol 1991;25: Muchenberger S, Schopf E, Simon JC. The combination of oral acitretin and bath PUVA for the treatment of severe psoriasis. Br J Dermatol 1997;137: MacKie RM, Fitzsimons MB. Risk of carcinogenicity in patients with psoriasis treated with methotrexate or PUVA singly or in combination. J Am Acad Dermatol 1983;9: Paul BS, Momtaz K, Stern RS, Arndt KA, Parrish JA. Combined methotrexate-ultraviolet B therapy in the treatment of psoriasis. J Am Acad Dermatol 1982;7: Morison WL, Momtaz K, Parrish JA, Fitzpatrick TB. Combined methotrexate-puva therapy in the treatment of psoriasis. J Am Acad Dermatol 1982;6: Ozawa M, Ferenczi K, Kikuchi T, Cardinale I, Austin LM, Coven TR, et al. 312-Nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med 1999;189: Coven TR, Walters IB, Cardinale I, Krueger JG. PUVA-induced lymphocyte apoptosis: mechanism of action in psoriasis. Photodermatol Photoimmunol Photomed 1999;15: Koo J, Lebwohl M. Duration of remission of psoriasis therapies. J Am Acad Dermatol 1999;41: Korossy KS, Hood AF. Methotrexate reactivation of sunburn reaction. Arch Dermatol 1981;117:310-1.
A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients
 Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis
 Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Combination Nonbiologic Therapy in Psoriasis. Sushil Tahiliani, MBBS, MD
 Combination Nonbiologic Therapy in Psoriasis Sushil Tahiliani, MBBS, MD Agenda Rationale Preferred and less preferred combination Morphology-specific preferred combinations Doses used in combinations Potential
Combination Nonbiologic Therapy in Psoriasis Sushil Tahiliani, MBBS, MD Agenda Rationale Preferred and less preferred combination Morphology-specific preferred combinations Doses used in combinations Potential
Comparison of PUVA and UVB therapy in moderate plaque psoriasis. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman*
 Comparison of PUVA and UVB therapy in moderate plaque psoriasis Arfan ul Bari et al. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman* Department of Dermatology, PAF Hospital, Sargodha. * Department of
Comparison of PUVA and UVB therapy in moderate plaque psoriasis Arfan ul Bari et al. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman* Department of Dermatology, PAF Hospital, Sargodha. * Department of
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service:
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis
 Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
STUDY. The Development of Practice Guidelines for the Treatment of Severe Plaque Form Psoriasis
 STUDY The Development of Practice Guidelines for the Treatment of Severe Plaque Form Psoriasis Phyllis I. Spuls, MD; Patrick M. M. Bossuyt, PhD; Jannes J. E. van Everdingen, MD, PhD; Leonard Witkamp, MD,
STUDY The Development of Practice Guidelines for the Treatment of Severe Plaque Form Psoriasis Phyllis I. Spuls, MD; Patrick M. M. Bossuyt, PhD; Jannes J. E. van Everdingen, MD, PhD; Leonard Witkamp, MD,
Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris
 IJMAMR 5 (2017) 1-6 ISSN 2053-1834 Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris Tran Hau Khang* and Le Huu Doanh National Hospital of Dermatology and Venereology,
IJMAMR 5 (2017) 1-6 ISSN 2053-1834 Comparison of the efficacy of PUVA versus BBUVB in the treatment of psoriasis vulgaris Tran Hau Khang* and Le Huu Doanh National Hospital of Dermatology and Venereology,
Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial
 Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
PUVA: Shall we still use it for psoriasis in 2019?
 PUVA: Shall we still use it for psoriasis in 2019? Ben Stoff MD, MA Associate Professor Emory Department of Dermatology Phototherapy: F003 March 1, 2019 DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY
PUVA: Shall we still use it for psoriasis in 2019? Ben Stoff MD, MA Associate Professor Emory Department of Dermatology Phototherapy: F003 March 1, 2019 DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY
Summary. DOI /j x
 PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C
 A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
STUDY. A Randomized Comparison of Methods of Selecting Narrowband UV-B Starting Dose to Treat Chronic Psoriasis
 ONLINE FIRST STUDY A Randomized Comparison of Methods of Selecting Narrowband UV-B Starting Dose to Treat Chronic Psoriasis Robert S. Dawe, MBChB, MD, FRCP; Heather M. Cameron, MBChB, MRCGP; Susan Yule,
ONLINE FIRST STUDY A Randomized Comparison of Methods of Selecting Narrowband UV-B Starting Dose to Treat Chronic Psoriasis Robert S. Dawe, MBChB, MD, FRCP; Heather M. Cameron, MBChB, MRCGP; Susan Yule,
Introduction Psoriasis is a common, chronic, disfiguring, inflammatory and proliferative condition of the skin and has remitting and relapsing course.
 Methotrexate plus narrow band ultraviolet B (NBUVB) versus methotrexate alone in the treatment of moderate to severe plaque psoriasis: A randomized clinical trial Khadka DK 1, Agrawal S 2, Dhali TK 3 1
Methotrexate plus narrow band ultraviolet B (NBUVB) versus methotrexate alone in the treatment of moderate to severe plaque psoriasis: A randomized clinical trial Khadka DK 1, Agrawal S 2, Dhali TK 3 1
STUDY. Efficacy of Acitretin and Commercial Tanning Bed Therapy for Psoriasis
 STUDY Efficacy of Acitretin and Commercial Tanning Bed Therapy for Psoriasis Christopher S. Carlin, MD; Kristina P. Callis, MD; Gerald G. Krueger, MD Objective: To assess the efficacy of acitretin and
STUDY Efficacy of Acitretin and Commercial Tanning Bed Therapy for Psoriasis Christopher S. Carlin, MD; Kristina P. Callis, MD; Gerald G. Krueger, MD Objective: To assess the efficacy of acitretin and
SYSTEMIC THERAPY OF MODERATE AND SEVERE PSORIASIS WITH METHOTREXATE
 Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 SYSTEMIC THERAPY OF MODERATE AND SEVERE PSORIASIS WITH METHOTREXATE M. FRÎNCU 1 A. OANŢĂ 1 Abstract:
Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 SYSTEMIC THERAPY OF MODERATE AND SEVERE PSORIASIS WITH METHOTREXATE M. FRÎNCU 1 A. OANŢĂ 1 Abstract:
Light Therapy for Psoriasis Protocol Medical Benefit Effective Date Next Review Date Preauthorization Review Dates Preauthorization is required.
 Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
BJD. Summary. British Journal of Dermatology THERAPEUTICS
 THERAPEUTICS BJD British Journal of Dermatology Efficacy of psoralen plus ultraviolet A therapy vs. biologics in moderate to severe chronic plaque psoriasis: retrospective data analysis of a patient registry
THERAPEUTICS BJD British Journal of Dermatology Efficacy of psoralen plus ultraviolet A therapy vs. biologics in moderate to severe chronic plaque psoriasis: retrospective data analysis of a patient registry
EFFECTIVENESS AND SAFETY OF NARROW BAND ULTRAVIOLET B THERAPY IN CHRONIC PLAQUE PSORIASIS
 ORIGINAL ARTICLE EFFECTIVENESS AND SAFETY OF NARROW BAND ULTRAVIOLET B THERAPY IN CHRONIC PLAQUE PSORIASIS 1 4 Mohammad Majid Paracha, Irfanullah, Zafar Ali, Said Amin ABSTRACT Objectives: To determine
ORIGINAL ARTICLE EFFECTIVENESS AND SAFETY OF NARROW BAND ULTRAVIOLET B THERAPY IN CHRONIC PLAQUE PSORIASIS 1 4 Mohammad Majid Paracha, Irfanullah, Zafar Ali, Said Amin ABSTRACT Objectives: To determine
chemotherapeutic agents in
 Use of biologics and chemotherapeutic agents in cutaneous emergencies: Focus on lifethreatening forms of psoriasis Alice Bendix Gottlieb MD, PhD Professor of Dermatology New York Medical College Metropolitan
Use of biologics and chemotherapeutic agents in cutaneous emergencies: Focus on lifethreatening forms of psoriasis Alice Bendix Gottlieb MD, PhD Professor of Dermatology New York Medical College Metropolitan
A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital
 Original article A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital 1Y Roja Ramani, 2 Benu Panigrahy, 3 Sailenkumar Mishra, 4 BTPS Singh
Original article A study of treatment modalities in psoriasis in dermatology outpatient department of a tertiary care teaching hospital 1Y Roja Ramani, 2 Benu Panigrahy, 3 Sailenkumar Mishra, 4 BTPS Singh
Light Therapy for Psoriasis. Description
 Subject: Light Therapy for Psoriasis Page: 1 of 11 Last Review Status/Date: June 2015 Light Therapy for Psoriasis Description Light therapy for psoriasis includes both targeted phototherapy and photochemotherapy
Subject: Light Therapy for Psoriasis Page: 1 of 11 Last Review Status/Date: June 2015 Light Therapy for Psoriasis Description Light therapy for psoriasis includes both targeted phototherapy and photochemotherapy
Vitiligo is an acquired cutaneous disorder of
 Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo Lubomira Scherschun, MD, Jane J. Kim, MD, and Henry W. Lim, MD Detroit, Michigan Background: The treatment of vitiligo remains
Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo Lubomira Scherschun, MD, Jane J. Kim, MD, and Henry W. Lim, MD Detroit, Michigan Background: The treatment of vitiligo remains
Atopic dermatitis (AD) is a common chronic skin. Phototherapy in the management of atopic dermatitis: a systematic review.
 Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Narrow band UVB (311 nm), psoralen UVB (311 nm) and PUVA therapy in the treatment of early-stage mycosis fungoides: a right left comparative study
 Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
Narrow-band UVB PHOTOTHERAPY for Skin Diseases
 Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Original Policy Date
 MP 2.01.58 Light Therapy for Vitiligo Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical Policy
MP 2.01.58 Light Therapy for Vitiligo Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical Policy
Phototherapy for Psoriasis. Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA
 Phototherapy for Psoriasis Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA Disclosure Investigator: Clinuvel Estée Lauder Ferndale Incyte
Phototherapy for Psoriasis Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA Disclosure Investigator: Clinuvel Estée Lauder Ferndale Incyte
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Follow this and additional works at: Part of the Skin and Connective Tissue Diseases Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
TRANSPARENCY COMMITTEE OPINION. 26 April 2006
 TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17
 Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Ixekizumab (Taltz) Reference Number: ERX.SPA.122 Effective Date:
 Clinical Policy: (Taltz) Reference Number: ERX.SPA.122 Effective Date: 10.01.16 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Taltz) Reference Number: ERX.SPA.122 Effective Date: 10.01.16 Last Review Date: 11.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Use of light for the treatment of skin diseases
 An Update on At-Home UVB Phototherapy At-home options increase accessibility to phototherapy, which is effective and generally safe for psoriasis management. By Joseph Bikowski, MD Use of light for the
An Update on At-Home UVB Phototherapy At-home options increase accessibility to phototherapy, which is effective and generally safe for psoriasis management. By Joseph Bikowski, MD Use of light for the
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
The effects of oral cyclosporine in plaque-type psoriasis: the experience of Andreas Sygros Hospital
 The effects of oral cyclosporine in plaque-type psoriasis: the experience of Andreas Sygros Hospital RESEARCH ARTICLE Antoniou C, Stratigos A, Stefanaki C, Stavropoulos P, Potouridou I, Katsambas AD &
The effects of oral cyclosporine in plaque-type psoriasis: the experience of Andreas Sygros Hospital RESEARCH ARTICLE Antoniou C, Stratigos A, Stefanaki C, Stavropoulos P, Potouridou I, Katsambas AD &
THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS
 Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS Mădălina FRÎNCU 1 Abstract: Biological
Bulletin of the Transilvania University of Braşov Series VI: Medical Sciences Vol. 5 (54) No. 2-2012 THE THERAPY OF THE REBEL SEVERE PSORIAZIS WITH BIOLOGICAL PREPARATS Mădălina FRÎNCU 1 Abstract: Biological
Medical Policy. MP Light Therapy for Psoriasis
 Medical Policy MP 2.01.47 BCBSA Ref. Policy: 2.01.47 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.44 Dermatologic Applications of Photodynamic Therapy 2.01.86
Medical Policy MP 2.01.47 BCBSA Ref. Policy: 2.01.47 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.44 Dermatologic Applications of Photodynamic Therapy 2.01.86
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
COST-EFFECTIVENESS OF TREATMENT FOR MODERATE-TO-SEVERE PSORIASIS
 COST-EFFECTIVENESS OF TREATMENT FOR MODERATE-TO-SEVERE PSORIASIS Cheryl S. Hankin PhD 1 ; Steven R. Feldman, MD, PhD 2 ; Daniel Pearce, MD 2 1 BioMedEcon, San Jose, CA, USA; 2 Wake Forest University School
COST-EFFECTIVENESS OF TREATMENT FOR MODERATE-TO-SEVERE PSORIASIS Cheryl S. Hankin PhD 1 ; Steven R. Feldman, MD, PhD 2 ; Daniel Pearce, MD 2 1 BioMedEcon, San Jose, CA, USA; 2 Wake Forest University School
Update on systemic therapies and emerging treatments How do I choose a systemic agent?
 Update on systemic therapies and emerging treatments How do I choose a systemic agent? Amy S. Paller, M.D. Walter J. Hamlin Professor and Chair of Dermatology Professor of Pediatrics Northwestern University
Update on systemic therapies and emerging treatments How do I choose a systemic agent? Amy S. Paller, M.D. Walter J. Hamlin Professor and Chair of Dermatology Professor of Pediatrics Northwestern University
Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan-
 Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan- Chih-Sheng Lai Po-Han Huang Ji-Chen Ho Despite the recent advances in treatment for psoriasis, it is still
Treatment for Psoriasis with Modified Goeckerman Regimen -One Year Experience in Southern Taiwan- Chih-Sheng Lai Po-Han Huang Ji-Chen Ho Despite the recent advances in treatment for psoriasis, it is still
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
Therapeutic Management of Early Cutaneous Mycosis Fungoides
 Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
During the last 20 years, the number of topical
 THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
Laser, Light Therapy, and Cryotherapy for Acne Vulgaris Non-Pharmacologic Treatment of Rosacea
 2.01.47 Light Therapy for Psoriasis Section 2.0 Medicine Subsection Effective Date October 31, 2014 Original Policy Date June 13, 2001 Next Review Date October 2015 Description Plaque psoriasis, also called
2.01.47 Light Therapy for Psoriasis Section 2.0 Medicine Subsection Effective Date October 31, 2014 Original Policy Date June 13, 2001 Next Review Date October 2015 Description Plaque psoriasis, also called
Psoriasis: Therapeutic goals
 Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Clinical Policy: Apremilast (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17
 Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Brodalumab (Siliq) Reference Number: CP.PHAR.375 Effective Date: Last Review Date: 05.18
 Clinical Policy: (Siliq) Reference Number: CP.PHAR.375 Effective Date: 06.01.18 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Siliq) Reference Number: CP.PHAR.375 Effective Date: 06.01.18 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Ixekizumab (Taltz) Reference Number: CP.PHAR.257 Effective Date: Last Review Date: 05.18
 Clinical Policy: (Taltz) Reference Number: CP.PHAR.257 Effective Date: 08.01.16 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Taltz) Reference Number: CP.PHAR.257 Effective Date: 08.01.16 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Original Article. A Study of Histopathological Changes Seen in Chronic Plaque Psoriasis, Before and After Treatment with Narrow Band Ultraviolet B
 Original Article A Study of Histopathological Changes Seen in Chronic Plaque Psoriasis, Before and After Treatment with Narrow Band Ultraviolet B Amoolya Bhat 1 *, Subramanya H 2, PS Murthy 3 and Santosh
Original Article A Study of Histopathological Changes Seen in Chronic Plaque Psoriasis, Before and After Treatment with Narrow Band Ultraviolet B Amoolya Bhat 1 *, Subramanya H 2, PS Murthy 3 and Santosh
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 13 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
Methotrexate versus Cyclosporine in Moderateto-Severe Chronic Plaque Psoriasis
 The new england journal of medicine original article Methotrexate versus Cyclosporine in Moderateto-Severe Chronic Plaque Psoriasis Vera M.R. Heydendael, M.D., Phyllis I. Spuls, M.D., Ph.D., Brent C. Opmeer,
The new england journal of medicine original article Methotrexate versus Cyclosporine in Moderateto-Severe Chronic Plaque Psoriasis Vera M.R. Heydendael, M.D., Phyllis I. Spuls, M.D., Ph.D., Brent C. Opmeer,
USTEKINUMAB Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA GUIDELINES FOR USE
 Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
5 European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris
 87 5 European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris Supported by the EDF/EADV/IPC Pathirana, D.; Ormerod, A. D.; Saiag, P.; Smith, C.; Spuls, P. I.; Nast, A.; Barker, J.; Bos, J.
87 5 European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris Supported by the EDF/EADV/IPC Pathirana, D.; Ormerod, A. D.; Saiag, P.; Smith, C.; Spuls, P. I.; Nast, A.; Barker, J.; Bos, J.
Predicting the Response to Phototherapy for Psoriasis Patients
 A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
UVB phototherapy and skin cancer risk: a review of the literature
 Oxford, IJD International 0011-9059 Blackwell 45 UK Publishing Journal Ltd. Ltd, of Dermatology 2003 Review Lee, Koo, phototherapy and Berger and skin cancer risk UVB phototherapy and skin cancer risk:
Oxford, IJD International 0011-9059 Blackwell 45 UK Publishing Journal Ltd. Ltd, of Dermatology 2003 Review Lee, Koo, phototherapy and Berger and skin cancer risk UVB phototherapy and skin cancer risk:
A.HANNUKSELA-SVAHN, B.SIGURGEIRSSON,* E.PUKKALA,² B.LINDELOÈ F,³ B.BERNE, M.HANNUKSELA, K.POIKOLAINEN AND J.KARVONEN
 British Journal of Dermatology 1999; 141: 497±501. Trioxsalen bath PUVA did not increase the risk of squamous cell skin carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and
British Journal of Dermatology 1999; 141: 497±501. Trioxsalen bath PUVA did not increase the risk of squamous cell skin carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and
Table 2.1. Cohort studies of treatment with methoxsalen plus UV radiation and cutaneous and extracutaneous cancers
 skin Forman et al. (1989) The PUVA-48 Cooperative Study (multicentre ) Retrospective cohort of 551 psoriatic patients of both sexes treated with PUVA since 1975 in seven medical centres; cancer incidence
skin Forman et al. (1989) The PUVA-48 Cooperative Study (multicentre ) Retrospective cohort of 551 psoriatic patients of both sexes treated with PUVA since 1975 in seven medical centres; cancer incidence
Scientific Report On PhotoTherapy
 Scientific Report On PhotoTherapy What exactly is psoriasis? Psoriasis is an immune-mediated disease. This means that your immune system causes your skin cells to reproduce in 4 days instead of 30 days,
Scientific Report On PhotoTherapy What exactly is psoriasis? Psoriasis is an immune-mediated disease. This means that your immune system causes your skin cells to reproduce in 4 days instead of 30 days,
PUVATHERAPY tor PSORIASIS AND OTHER SKIN DISEASES
 PUVATHERAPY tor PSORIASIS AND OTHER SKIN DISEASES AN INITIAL REPORT by HILARY A. LAVERY and D. BURROWS Department of Dermatology, Royal Victoria Hospital, Belfast SUMMARY Fifty-two patients with severe
PUVATHERAPY tor PSORIASIS AND OTHER SKIN DISEASES AN INITIAL REPORT by HILARY A. LAVERY and D. BURROWS Department of Dermatology, Royal Victoria Hospital, Belfast SUMMARY Fifty-two patients with severe
The Natural History of Psoriasis and Treatment Goals
 The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S
 Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S ABSTRACT Background Department of Dermatology Dhulikhel Hospital, Kathmandu University
Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S ABSTRACT Background Department of Dermatology Dhulikhel Hospital, Kathmandu University
Pediatric Use: Safety and effectiveness of Ustekinumab (STELARA ) in pediatric patients have not been evaluated.
 Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Clinical Policy: Apremilast (Otezla) Reference Number: CP.PHAR.245 Effective Date: Last Review Date: Line of Business: HIM, Medicaid
 Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08.16 Last Review Date: 11.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08.16 Last Review Date: 11.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Cosentyx, Cosentyx Sensoready) Reference Number: HIM.PA.SP29 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See
Clinical Policy: (Cosentyx, Cosentyx Sensoready) Reference Number: HIM.PA.SP29 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK)
 Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK) TREATMENT OF SKIN CONDITIONS BY UVB PHOTHERAPY Ultraviolet radiation from artificial light sources (UV therapy) has been used by Dermatologists for almost 100 years
Dr Ravi C. Ratnavel DM (Oxon) FRCP (UK) TREATMENT OF SKIN CONDITIONS BY UVB PHOTHERAPY Ultraviolet radiation from artificial light sources (UV therapy) has been used by Dermatologists for almost 100 years
Bilaga 1.till rapport. Bilaga 1 Tabell över inkluderade studier/ Appendix 1 Description of included studies
 Bilaga 1.till rapport Ljusbehandling och systemisk behandling av psoriasis, rapport nr 278 (2018) Bilaga 1 Tabell över inkluderade studier/ Appendix 1 Description of included studies Description of included
Bilaga 1.till rapport Ljusbehandling och systemisk behandling av psoriasis, rapport nr 278 (2018) Bilaga 1 Tabell över inkluderade studier/ Appendix 1 Description of included studies Description of included
Review Article. Narrow band UVB phototherapy in dermatology
 Review Article Narrow band UVB phototherapy in dermatology Sunil Dogra, Amrinder Jit Kanwar Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education & Research,
Review Article Narrow band UVB phototherapy in dermatology Sunil Dogra, Amrinder Jit Kanwar Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education & Research,
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441
 Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
SCIENTIFIC PAPER ABSTRACT
 SCIENTIFIC PAPER ABSTRACT Vitiligo Treatment with Monochromatic Excimer Light and Tacrolimus: Results of an Open Randomized Controlled Study Nistico` S., Chiricozzi A., M.D., Rosita Saraceno R., Schipani
SCIENTIFIC PAPER ABSTRACT Vitiligo Treatment with Monochromatic Excimer Light and Tacrolimus: Results of an Open Randomized Controlled Study Nistico` S., Chiricozzi A., M.D., Rosita Saraceno R., Schipani
Clinical Policy: Ixekizumab (Taltz) Reference Number: CP.PHAR.257 Effective Date: Last Review Date: 11.18
 Clinical Policy: (Taltz) Reference Number: CP.PHAR.257 Effective Date: 08.01.16 Last Review Date: 11.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Taltz) Reference Number: CP.PHAR.257 Effective Date: 08.01.16 Last Review Date: 11.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Psoriasis is a chronic, inflammatory, T-cell mediated
 Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis Seyamak Saleky, MD; Işıl Bulur, MD; Zeynep Nurhan Saraçoğlu, MD PRACTICE POINTS The 25-hydroxyvitamin
Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis Seyamak Saleky, MD; Işıl Bulur, MD; Zeynep Nurhan Saraçoğlu, MD PRACTICE POINTS The 25-hydroxyvitamin
An otherwise healthy 12-year-old
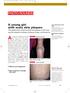 1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese
 Hong Kong J. Dermatol. Venereol. (2011) 19, 59-64 Original Article Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese Ustekinumab SKF Loo, KH Lau, KM Ho Introduction:
Hong Kong J. Dermatol. Venereol. (2011) 19, 59-64 Original Article Ustekinumab for severe treatment-resistant psoriasis: a 24-week pilot study in Hong Kong Chinese Ustekinumab SKF Loo, KH Lau, KM Ho Introduction:
NIH Public Access Author Manuscript J Am Acad Dermatol. Author manuscript; available in PMC 2013 May 14.
 NIH Public Access Author Manuscript Published in final edited form as: J Am Acad Dermatol. 2011 October ; 65(4): 733 738. doi:10.1016/j.jaad.2010.08.006. Narrowband ultraviolet B phototherapy for the treatment
NIH Public Access Author Manuscript Published in final edited form as: J Am Acad Dermatol. 2011 October ; 65(4): 733 738. doi:10.1016/j.jaad.2010.08.006. Narrowband ultraviolet B phototherapy for the treatment
Clinical Policy: Secukinumab (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17
 Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Elements of Successful PBS Applications. Barbara Radulski RN. Copyright
 Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Corporate Medical Policy
 Corporate Medical Policy Ultraviolet Light Therapy in the Home Setting(UVB) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultraviolet_light_therapy_in_the_home 3/1996 11/2017 11/2018
Corporate Medical Policy Ultraviolet Light Therapy in the Home Setting(UVB) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultraviolet_light_therapy_in_the_home 3/1996 11/2017 11/2018
PSORIASIS IS A COMMON
 STUDY Clinical Severity of Psoriasis in Last Years of PUVA Study Tamar Nijsten, MD, PhD; Caspar W. N. Looman, PhD; Robert S. Stern, MD Objective: To assess the severity of psoriasis over time. Design:
STUDY Clinical Severity of Psoriasis in Last Years of PUVA Study Tamar Nijsten, MD, PhD; Caspar W. N. Looman, PhD; Robert S. Stern, MD Objective: To assess the severity of psoriasis over time. Design:
Corporate Medical Policy
 Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Clinical Policy: Ustekinumab (Stelara) Reference Number: CP.PHAR.264
 Clinical Policy: (Stelara) Reference Number: CP.PHAR.264 Effective Date: 08/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Stelara) Reference Number: CP.PHAR.264 Effective Date: 08/16 Last Review Date: 05/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Ustekinumab (Stelara) Reference Number: ERX.SPA.01 Effective Date:
 Clinical Policy: (Stelara) Reference Number: ERX.SPA.01 Effective Date: 04.01.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Stelara) Reference Number: ERX.SPA.01 Effective Date: 04.01.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
National Managed Clinical Network For Phototherapy DOSIMETRY PROTOCOLS
 National Managed Clinical Network For Phototherapy DOSIMETRY PROTOCOLS Photonet Dosimetry Protocols Revised March 2013 Review Date March 2015 1 MANAGED CLINICAL NETWORK SCOTLAND Photonet CONTENT DOSIMETRY
National Managed Clinical Network For Phototherapy DOSIMETRY PROTOCOLS Photonet Dosimetry Protocols Revised March 2013 Review Date March 2015 1 MANAGED CLINICAL NETWORK SCOTLAND Photonet CONTENT DOSIMETRY
The Treatment Toolbox for Severe Pediatric Psoriasis
 The Treatment Toolbox for Severe Pediatric Psoriasis Dr. Kim A. Papp, MD, PhD, FRCPC, FAAD K Papp Clinical Research and Probity Medical Research Objectives: Treating severe pediatric psoriasis 1. Challenges
The Treatment Toolbox for Severe Pediatric Psoriasis Dr. Kim A. Papp, MD, PhD, FRCPC, FAAD K Papp Clinical Research and Probity Medical Research Objectives: Treating severe pediatric psoriasis 1. Challenges
Clinical Policy: Secukinumab (Cosentyx) Reference Number: ERX.SPA.165 Effective Date:
 Clinical Policy: (Cosentyx) Reference Number: ERX.SPA.165 Effective Date: 10.01.16 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Cosentyx) Reference Number: ERX.SPA.165 Effective Date: 10.01.16 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study
 American Journal of Therapeutics 13, 121 126 (2006) Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study Steve Bernstein, Howard Donsky,*
American Journal of Therapeutics 13, 121 126 (2006) Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study Steve Bernstein, Howard Donsky,*
The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit
 ORIGINAL ARTICLE The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit Vaani Valerie Visuvanathan, AdvMDerm 1, Min Moon Tang, AdvMDerm 2, Li Lian Tan,
ORIGINAL ARTICLE The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit Vaani Valerie Visuvanathan, AdvMDerm 1, Min Moon Tang, AdvMDerm 2, Li Lian Tan,
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/04/900. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
CHAPTER 3. Diagnostic phototesting in polymorphous. Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations
 CHAPTER 3 Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations Diagnostic phototesting in polymorphous light eruption: Ines Schornagel, Edward the optimal Knol, Huib
CHAPTER 3 Diagnostic phototesting in polymorphous light eruption: the optimal number of irradiations Diagnostic phototesting in polymorphous light eruption: Ines Schornagel, Edward the optimal Knol, Huib
Citation for published version (APA): Coevorden, A. M. V. (2005). Hand eczema: clinical efficacy of interventions, and burden of disease s.n.
 University of Groningen Hand eczema Coevorden, Anthony Marco van IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Hand eczema Coevorden, Anthony Marco van IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
ADALIMUMAB Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Clinical Policy: Secukinumab (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: Last Review Date: Line of Business: HIM, Medicaid
 Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08.16 Last Review Date: 11.18 Line of Business: HIM, Medicaid Coding Implications Revision Log See Important Reminder at the end
Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08.16 Last Review Date: 11.18 Line of Business: HIM, Medicaid Coding Implications Revision Log See Important Reminder at the end
Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions
 Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review
Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review
