Brain and CNS Cancer Pathway Board Annual Report 2014/15
|
|
|
- Daniel Marshall Robbins
- 5 years ago
- Views:
Transcription
1 Brain and CNS Cancer Pathway Board Annual Report 2014/15 Pathway Clinical Director: Dr Catherine McBain Pathway Manager: James Leighton Version 1.4
2 Executive summary Brain and CNS cancer surgery is based at Salford Royal Foundation Trust with robust and strong links with the oncology service at the Christie NHS Foundation Trust. There are four associated MDTs (3 distinct neuro-oncology sub-specialities at SRFT, 1 supportive care at Christie), all of whom are represented on the pathway board. There are clear and well established procedures and guidelines for referral into the service and this is further supported by an electronic referral process. The Christie at Salford radiotherapy centre with the use of stereotactic radio surgery is now well developed and established. The activity volumes for this service are in appendix 1. The board is able to confirm that the service underpinning this pathway is stable, well run and mature with a strong ethos of team working across all the professions. It complies with the 2006 NICE Improving Outcomes Guidance for brain and spinal tumours. In the course of the first year of Manchester Cancer, the board reflected on how it was functioning within the confines of the prescribed structure. It felt that because of the unique nature of the service and the disease, having a pathway board based solely on organisational representation (with a majority of board members not directly involved in treating brain tumour patients) meant that the board did not benefit from the range of available expertise and experience required. To address this and increase the amount of relevant clinical input, the board decided to restructure itself. As a consequence the Pathway Board is now supported by two sub-groups that drive the agenda forward. The first sub-group is the Strategic Management Group, (SMG) which comprises clinicians directly involved with the delivery of the service. It is clinically focussed, with a responsibility to manage the clinical outcome and research objectives. The patient experience and standardisation of service objectives are managed by the Patient Experience Group (PEG). There is a broader and developing membership to this group and it is where the patient representation is expected to sit. The Pathway Board (PB) remains in place and is the body responsible for the overall governance of the pathway and delivery of the Manchester Cancer objectives. It is drawn from referring Trusts, representatives of the 4 brain and CNS MDTs, the 2 subgroups and allied health professionals. This is where GP representation is expected to sit. There are 8 meetings per year (3 SMG, 3 PEG, 2 PB). Increasing the contribution of the staff involved in managing the pathway, within a network structure, was the biggest challenge the board faced in its first year. However, the revised structure is becoming increasingly established, is demonstrating improved engagement with staff of all disciplines and backgrounds, and is beginning to deliver on the Board s objectives. 2
3 In the 2014/15 annual report it set 5 objectives, 4 of which were delivered.in full, one delivered in part. These were Introduction of 5ALA-guided resection into routine practice for suitable patients with high grade gliomas Maintenance of IOG compliance of MDTs Introduction of patient-held records Optimisation of data collection to generate outcome measures The one objective which remains outstanding is the introduction of MGMT testing for high grade gliomas. Progress towards this has been made, and it is now technically and logistically feasible; the only remaining barrier to realising it is funding agreement The Board wishes to prominently highlight to readers that this test is now routine in every other major centre in the UK, and in many smaller units. It is vital in selecting patients, particularly those over 70, for chemotherapy and is a critical prognostic marker in younger patients with grade 4 gliomas. The situation of being unable to deliver this testing, which is central to the optimisation of patient care, is becoming increasingly indefensible. The cost is 150 per sample for 150 patients per year. Because of the nature of brain cancer, there is little scope for developing early detection and prevention strategies. In addition, evidence that early detection improves outcomes is lacking, although earlier detection does reduce acute presentation. However, the board will look to support primary care colleagues in symptom recognition and earlier referral for investigation through its education programme. The board has successfully supported two bids to the Macmillan Cancer Living With and Beyond Cancer fund. Both of these bids will monitor the patient and carer experience along the pathway following treatment and, by better understanding the needs of patients and carers during this phase of the disease, aim to improve experience and survivorship. One bid focuses on patients with primary brain tumours, the other on patients with secondary (metastatic) brain tumours (further summary information is set out in Appendix 2). The work of these two projects will be within this year s action plan. The pathway board and its subgroups will also review all of the guidelines as part of what will be the on-going quality assurance of the pathway. This will be a key function of the pathway board and one that it looks forward to undertaking. Over the course of the forthcoming year the board has set the following objectives 1. Continue to pursue, possibly via a business case, the routine introduction of MGMT testing in Manchester 2. Fully revise and extend clinical guidelines to include standardised imaging follow-up policies 3. Introduce banking of brain tumour tissue 4. Successfully deliver both Macmillan funded projects supporting patients in living with and beyond their disease 3
4 5. The board will work with primary care commissioners and secondary care providers to develop a response to the recommendations for Brain & CNS cancer made in the NICE guidance Suspected cancer: recognition and referral. 4
5 1 Introduction the Pathway Board and its vision This is the annual report of the Manchester Cancer Brain and CNS cancer Pathway Board for 2014/15. This annual report is designed to: Provide a summary of the work programme, outcomes and progress of the Board alongside the minutes of its meetings, its action plan and its scorecard, it is the key document for the Board. Provide an overview to the hospital trust CEOs and other interested parties about the current situation across Manchester Cancer in this particular cancer area Meet the requirements of the National Cancer Peer Review Programme Be openly published on the external facing website. This annual report outlines how the Pathway Board has contributed in 2014/15 to the achievement of Manchester Cancer s four overarching objectives: Improving outcomes, with a focus on survival Improving patient experience Increasing research and clinical innovation Delivering compliant and high quality services 1.1. Vision The board has this year benefitted from increasing the amount of relevant clinical expertise made available to the decision making process. It has also created two more appropriate forums where the objectives of Manchester Cancer can be better managed, within the governance structure of the Pathway Board. Because of the nature of the disease there is little opportunity for early detection or preventative actions that can be taken. Therefore the focus of the board will be on supporting innovation, quality assurance of the pathway and enhancing the experience of those living with and beyond their cancer. As an example of this the board will work with primary care commissioners and secondary care providers to develop a response to the recommendations for Brain & CNS cancer made in the NICE guidance Suspected cancer: recognition and referral. The board will also deepen its knowledge base and understanding of the whole pathway and put in place actions were the patient outcomes, survival rates and experience can be improved and enhanced. 5
6 1.2. Membership The Pathway board membership is as follows - Brain & CNS Trust Nominee Profession/ specialty Christie Dr Catherine Chair McBain Bolton Dr Arun Kallat Cons Elderly Care Physician Christie Julie Emerson Neuro-oncology AHP Sara Robson Neuro-oncology AHP Elizabeth Molloy Neuro-oncology Clinical Nurse Specialist Dr Anna Tran Consultant Oncologist CMFT Prof Peter Selby Consultant East Cheshire Dr Moe Sein Consultant Pennine TBC SRFT Miss K Karabatsou Cons Neurosurgeon/Lead for Neuro-oncology Mr S Rutherford Dr T Kearney Cons Neurosurgeon/Lead for Skull Base Cons Endocrinologist/Lead for Pituitary MDT Cundliffe Sarah Neuro/Oncology Clinical Nurse Specialist Alison Gilston- Neuro/Oncology Specialist Nurse Hope Andrea Wadeson Base of Skull Clinical Nurse Specialist Stockport Dr K Dizayee Consultant Physician Tameside Dr Chris Douglass Consultant Neurologist UHSM Dr Samantha Kay Palliative Care Consultant Dr Sophie Harrison Palliative Care Consultant WWL Dr A Ismail Consultant Radiologist Salford CCG Dr Steven Elliot GP representative 1.3. Meetings The pathway board met three times in 2014 and has met within the new structure once in Both the strategic management group (SMG) and the patient experience groups (PEG) have met twice since their constitution in January The board has asked that the SMG and the PEG meet three times per year. The Pathway Board will then meet twice a year to co-ordinate the reporting of the board objectives to Manchester Cancer. 6
7 Below are the dates of the pathway board meetings and the links to the board minutes; 25 th April st July rd October st April VERSION.pdf The board attendances for these meetings are contained within appendix 3. The board has in 2015 welcomed GP representation from Salford CCG and has identified potential patient/carer representatives. Their inclusion will be managed by the Macmillan patient involvement team on behalf of the board. There remains no representation from Pennine Acute NHS Hospitals Trust. This is a persistent problem and was raised in the 2014/15 report. There have been discussions with the lead cancer manager at Pennine and these discussions have been on-going into the new structure. 7
8 2. Summary of delivery against 2014/15 plan No Objective Alignment with Provider Board objectives 1 Optimise data collection to generate Objective no 1 outcome measures Tasks By Status Green = achieved Amber = partially achieved Red = not achieved 2 Introduction of 5ALA-guided resection into routine practice of suitable patients with high grade gliomas Objective no 1 3 Maintain IOG compliance of MDTs Objective no 4 4 Introduction of MGMT testing for high grade gliomas Objective no 1 5 Introduction of patient-held records Objective no 3 Organisational and logistic aspects completed; only remaining barrier is funding.
9 3. Improving outcomes, with a focus on survival 3.1. Information Nationally Brain tumours represent 1% of all cancers diagnosed yet 3% of all deaths. Of these deaths 71% will be under the age of 75 years, compared to 47% for all cancers. Further, 58% of adults diagnosed with bran cancer die within a year compared to 5% for breast cancer and 35% for leukaemia and 7% for prostate cancer. There is considerable regional variation in incidence, ranging from 108 per million in London to 139 per million in the North-west of England. This makes the North-west the 4 th highest region with 9 more per million than the national average. Within Greater Manchester, clinical outcomes measures are collated from two main sources: 1) Information on surgical outcomes, including operated numbers, post-op complication rates and 30-day mortality and readmission rates are supplied to and collated by Dr Foster and the Society of British Neurosurgeons. This data is now available for all UK centres, although it has not yet been fully released into the public domain 2) Information for patients referred onwards to The Christie for radiotherapy and/or chemotherapy is collated via the Christie Clinical Outcomes Unit. The first report will be published on the Christie website and further interrogation of this data will be undertaken. This data can be directly compared to national outcomes via publications from the NCIN. Some example comparators are given below. This objective from the 2013/14 was successfully delivered and the Brain & CNS board is one of the first boards to produce this level of outcome data. This aspect of the Pathway Board s work will be expanded in the coming year. The outcome report can be found on the following link There are presently no national clinical lines of enquiry in the brain tumour population Progress As outlined above, the work undertaken this year to develop the infrastructure to support and develop routine data collection is now beginning to bear the expected results Challenges The main remaining gaps are that full long-term survival data is not yet available on patients not referred to Christie i.e. those with low-grade or benign tumours who do not require non-surgical treatment. However, the 5-year survival of this group is likely to be 100% (if their disease progressed, they would be referred to Christie and therefore captured). In due course, this information will be 9
10 accessible via the NCIN, but due to the indolent or benign nature of these tumours, the Board does not deem this to be a priority area. The second gap in recording outcomes is the survival in those patients of very poor prognosis and performance status whose case is discussed at the Neuro-oncology MDT but who are referred directly to community palliative care. Many palliative patients, who opt for only symptom control (without active treatment), are seen in the neuro-oncology clinics and their data is captured. The missing data is from the subgroup of patient not well enough to come to clinic and generally very near the end of their lives. Work is on-going to capture survival data in these patients as well via the NCIN and possibly The Christie COU. Improving survival in malignant brain tumours is a major international challenge; there is no quick-fix. There is still a dearth of active treatment options and of high-quality clinical trials. The Board have made progress in this area by significantly improving the quality of surgery via the routine introduction of post-operative imaging, leading to re-resection of some sub-total resections, and with the use of 5-ALA guided resections. We plan to improve our outcomes further by ensuring that management of older patients is optimised and MGMT testing is vital for this. The support we need is funding agreement to introduce routine testing. 10
11 4. Improving patient experience 4.1. Information In the 2014 national patient experience survey only 42 responses were collected therefore it is difficult to draw any conclusions that would allow targeted actions to be planned. The Brain & CNS cancer treatments are almost all delivered at two organisations, Salford Royal and the Christie. The Christie NHSFT conducts a 200 patient survey every month which includes all patients from all modalities and specialties. A patient satisfaction survey to assess the entire Pituitary patient journey was devised and distributed during This will be repeated by the Endocrinology Specialist Nurses in 2015 for any patients who have undergone Pituitary surgery and the results of this survey will be utilised when reassessing the Pituitary service as a whole. The endocrine service have also asked several patients to give their perspective through patients stories and have used these as learning tools within the MDT. Several patients have personal web pages where they have documented their medical progress, from the diagnosis of their tumour through to the treatment and subsequent on-going care. These are widely available and reflect well upon the services provided by the MDT. The Salford Skull Base service developed and distributed a disease specific patient questionnaire in order to provide detailed and constructive feedback that can be used to shape the service provided to patients. 100 questionnaires were distributed to a random selection of patients who had been diagnosed with a skull base tumour with a response rate of 61%. The Neuro-oncology specialist nurses have devised, and are in the process of distributing, a patient satisfaction questionnaire for Neuro-oncology patients; findings will be discussed at the Patient Experience Group and any necessary actions agreed and monitored. These results will be presented in next year s annual report. The SRS service regularly monitors patient satisfaction. Findings of the last study in revealed high levels of satisfaction with no major causes for concern Progress Therefore the Board are confident, in the absence of the National patient experience survey that the service continues to draw feedback from their patients. This underlines the commitment of the Board and services to improve the patient experience and collect local data as well. These local surveys will be undertaken within 2015 and reported to the board as soon as the results are available. 11
12 4.3. Challenges The Board feel confident that patient feedback will continue to support service delivery. They feel that by the nature of being a two centre service and the experience of the MDT staff in undertaking such surveys that this challenge will continue to be met. 12
13 5. Increasing research and innovative practice 5.1. Information Over 2014/15 the number of Brain & CNS cancer patients recruited into trials when is as follows Internal Christie Number Trial name (sponsor) Phase Tumour type Date opened Recruitment End Target recruitment Number recruited 10_Dog11_13 BR14/ (EORTC) III Non-co-deleted Anaplastic Glioma Jul-10 Apr _Dog11_18 OPARATIC (Cancer Research UK) I Relapsed Glioblastoma Nov-11 New cohort open _Dog11_20 NBT (ICR) Observational Glioma Dec-12 Feb _Dog11_23 SATIVEX (GW Pharma Ltd) Ib - II Recurrent Glioblastoma Jan-14 Sep _Dog11_21 MO28347 (Roche Pharma Ltd) IIIb Glioblastoma Jan-14 Aug _Dog11_22 TAVAREC (EORTC) II Recurrent grade II and grade III noncodeleted gliomas Jan-14 Apr _Dog11_27 EORTC III Recurrent GBM Aug-14 October _Dog11_26 Checkmate 143 III Recurrent glioblastoma multiforme October 2014 April _Dog11_24 HCQ III GBM in patients > 70 or PS > 2 October Table 1 - Clinical trials portfolio There are also a number of NCRN Portfolio trials that are either in set-up or under discussion, these are 13
14 Internal Christie Number 14_Dog11_29 12_Dog11_20 Trial name (sponsor) HIPPO (Cancer Research UK) CAM BMT ROAM (EORTC) VIMPAT (UCB Pharma) Trial title Phase Tumour type A randomised phase II trial of Hippocampal Sparing versus Conventional Whole Brain Radiotherapy after surgical resection or radiosurgery in favourable prognosis patients with 1-4 brain metastases Cambridge Brain Mets Trial 1: A proof of principle phase 1b/randomised phase II study of afatinib penetration into cerebral metastases for patients undergoing neurosurgical resection, both with and without prior, lowdose, targeted radiotherapy Randomized controlled trial of the addition of radiotherapy following resection of grade 2 meningioma A randomised controlled trial to assess the efficacy and toxicity of the use of prophylactic lacosimide in patients with newly diagnosed high grade glioma Table 2 NCRN Portfolio trials that are either in set-up or under discussion II Ib/II III III Brain metastases treated with surgery or SRS Brain metastases Atypical (grade 2) meningioma, post-op Newlydiagnosed grade 3 and 4 gliomas Date opened June 2015 Target recruitment 14 July 2015 Approx. 4 Nov 2015 Approx 6 TBC Approx 10 Lastly there are two trials not in the NCN portfolio but for which NCRN portfolio adoption has been applied for - 14_Dog11_28 Trial name sponsor TSPO Glioma imaging study Trial title Phase IMP Tumour type Investigation of the utility of the novel PET tracer TSPO in the identification of brain metastases Translocator Protein Expression in Transformin g Gliomas 1 None imaging study Imaging study Brain metastases patients prior to SRS patients with low grade glioma (with concerns for higher grade transformation ) Table 3 Other Clinical Trials non-ncn portfolio / portfolio adoption applied for Date opened Target recruitment Number recruited Mar Jan The Brain & CNS trials portfolio is nationally highly competitive, with great effort made by Salford and the Christie to open all possible studies. Many trials of novel agents e.g. Checkmate-143, Sativex, TAMIGA are open at only a small number of centres nationally and the Christie is consistently within this subgroup. Important achievements this year are the opening of the Checkmate 143 Nivolumab study, where The Christie is the UK lead site (Dr McBain is UK Chief Investigator) and the advancement of the HIPPO study, an NCRN-adopted multi-centre RCT written by and initiated by which Dr Whitfield (member of the Brain & CNS SMG), who is the chief investigator. 14
15 Both Dr McBain and Dr Whitfield sit on the NCRN Brain Clinical Subgroup making them well-placed to pro-actively manage Manchester s trials portfolio. Dr McBain also sits on the CSG Novel Agents Subcommittee. The service is highly committed to CNS research and all potentially eligible patients are offered study entry Progress Recruitment to both the NIHR portfolio of trials and non NIHR trials in 2014/15 are reported above Challenges The four MDTs are very active in clinical research at a local level and regularly present and publish research. Some studies require very challenging streamlining of patient pathways to meet tight study timelines, and the entire MDT functions cohesively to deliver this. Where recruitment targets have not been met, there have been clear reasons e.g. trial closed early (EORTC 26101) or cohort slots less available than anticipated (OPARATIC), or recruitment difficulties which have proved common to all investigating sites (TAVAREC). There are no national trials specific to skull base surgery that the MDT is able to recruit to. However the skull base MDT members do play a prominent role in the British Skull Base Society (including Professor King as past president and Mr Rutherford as council member) and thereby continue to act as a major influence in any future national research initiatives. Recruitment relies not just on offering and conducting trials, but on having trials to offer. The MDTs and the board will do all they can to engage with Sponsors to ensure that all possible industry-sponsored and NCRN portfolio studies are available to Manchester and Salford patients, and that all patients are considered for trial entry. 15
16 Delivering complaint and high quality services 5.4. Information All primary brain and spinal tumour cases across what was recognised as the Manchester Cancer footprint are referred to the Neuro oncology MDT, many as acute referrals initially referred to the neuro-surgical on-call service. Patients with cerebral metastases (and other indicated pathologies) are also referred if they are for consideration of neurosurgical intervention or for Stereotactic Radiosurgery (SRS). On average the agenda for the MDT now routinely comprises between forty-five and sixty-five patients per week and the time required for MDT has increased over the past 5 years from 3 hours to 5 hours. The MDT is still attempting to deliver this in one session, but this requires review. The Base of Skull MDT includes neuro-surgeons, ENT and ophthalmic surgeons who work together to provide multi-disciplinary management of patients with tumours arising at the base of the skull. It receives referrals from the local neuroscience unit (30 Neurologists and 19 Neurosurgeons based at SRFT), from a variety of clinicians and specialties across the Greater Manchester region, as well as from a wider referral base across the UK and from abroad based on their longstanding reputation. Because of the nature of the disease skull base patients are seldom discharged, which leaves an ever increasing group of patients requiring on-going multidisciplinary management. The agenda now routinely comprises up to 120 cases per fortnight. The Pituitary Tumour MDT was established pre-2002 to facilitate discussion of pituitary cases across the region and although the vast majority of cases have benign pathology, the service falls within the Brain and CNS IOG. The Pituitary surgical service in Manchester is one of the busiest in the country serving a population of approximately 3.5 million adults and 5 million children. Pituitary surgery is undertaken at both Salford Royal Hospital (adults) and Royal Manchester Children s hospitals (RMCH). As a consequence over the last few years the numbers of cases discussed has increased and the proceedings of the MDT have been formalised. The Supportive Care Cancer Network MDT was established in January 2012 when it became necessary to separate the NS (Neuro science) MDT and the Cancer Network MDT due to time restraints at the combined meeting. It is a multi- professional group serving the population of 3.2 million across what was recognised as the Greater Manchester and Cheshire Cancer Network footprint and is now well established. The MDT is made up of core members who are all involved in the management of patients with primary brain and central nervous system primary tumour including primary cerebral lymphoma, skull base and pituitary tumours. 16
17 5.5. Progress The board has had a successful year and has achieved most of its objectives from the 2013/14 report. In terms of service development it has started to use a patient held record and is now starting to interrogate service led outcome data. The guidelines and service manuals will be revised this year. Until this is complete the existing pathway and supporting documents are now located on the Manchester Cancer website and can be found on the links below. Guidelines Management guidance for acute trusts Referral pathways - At this point in time the board has no plans for educational events as it is waiting for the cancer education strategy to be developed by Manchester Cancer. Once this strategy has been agreed the board will support and contribute to all Brain and CNS cancer education as required Challenges One of the main challenges to the service is that the referrals to the MDT continue to increase. Please see table 1 below. This puts a strain on the MDT members in delivering the workload within the time resource available. The MDT and service leads will explore if a second MDT meeting needs to be set up and will test what are the costs and benefits of delivering this. Table 4 Number of MDT discussions per year Another significant challenge is the deployment of MGMT testing. This test is vital in selecting patients, particularly those over 70, for chemotherapy and is a critical prognostic marker in younger patients with grade 4 gliomas. Currently this is not provided within Greater Manchester which puts the conurbation at variance with accepted practice nationally. The Board wishes to prominently highlight that this test is now routine in every other major centre in the UK, and in many smaller units. Progress towards this objective has been made, and it is now technically and logistically feasible. The only remaining barrier to realising it is the funding agreement. The cost is 150 per sample for 150 patients per year and being unable to deliver this testing is becoming increasingly indefensible. 17
18 The board hopes to work with the relevant stakeholders to address this anomaly and provide parity for our patients with the rest of the country. The board are also mindful that there is a national review of stereotactic radiosurgery (SRS) provision currently being undertaken. The board awaits the output from this review and it will respond accordingly. It intends to work with providers, commissioners and user groups to minimise the impact and ensure that a high quality service remains in place. As previously outlined the Board has restructured and has started to manage the Brain & CNS cancer agenda in a more inclusive and integrated fashion. Therefore in its first year the board has resolved its first challenge and will now benefit from this work. The board remain confident that with the wider participation and streamlined delegation of responsibilities it will be in a position to better overcome any difficulties it might face over the coming months. 18
19 6. Objectives for 2015/16 The board have set five objectives for this year and these are 1. Continue to pursue, possibly via a business case, the routine introduction of MGMT testing in Manchester 2. Fully revise and extend clinical guidelines to include standardised imaging follow-up policies 3. Introduce banking of brain tumour tissue 4. Manage the successful delivery of the two Macmillan funded Living With and Beyond projects 5. Construct a response to the recommendations for Brain & CNS cancer made in the NICE guidance Suspected cancer: recognition and referral. The work of the board will not be limited to just these objectives. As the year unfolds new challenges and opportunities will be identified, for example the national stereotactic radiosurgery review. The board feel that as a high quality, dedicated, functioning group they are adaptable and capable of accepting and addressing all possibilities to deliver the objectives of Manchester Cancer. 19
20 Appendix 1 Stereotactic Radiotherapy Service activity YEAR TOTAL PATIENTS ISOCENTRES METASTASES ACOUSTICS MENINGIOMAS 2011/ / / / Number of patients treated by SRS 2011/ /15 20
21 7. Appendix 2 Summary of the Macmillan living with and beyond cancer projects Patients with brain and CNS tumours are a highly diverse group of people with varied diagnoses, prognoses, symptoms, ages and cultures. Treatment regimens are therefore also very varied and can range from active surveillance via scans to surgery, radiotherapy and chemotherapy interventions. Due to the nature of this group of tumours, the needs of our patients differ depending on the location, grade and histology of the tumour. Frequently-encountered issues for patients living with and beyond treatment for brain tumours include speech and communication difficulties, mobility problems, short-term memory loss and cognitive dysfunction, personality change and epilepsy, in addition to the other difficulties common to the other cancer types. The pathway board was successful in a bid to the Macmillan Living With and Beyond Cancer fund for two projects. These projects to address the identified unmet needs of two patient groups in living with and beyond their treatment: i. Patients with primary brain tumours who have completed active treatment but who continue to have disabilities and survivorship challenges. ii. Patients with brain metastases from any primary site who have been successfully treated with neurosurgery or stereotactic radiosurgery (SRS) The first project is to introduce a living with and beyond programme for patients with primary Brain and CNS tumours, and provide effective and successful health and well-being events in the future. There is presently no survivorship programme for this patient group. It is felt that to be able to do this most effectively and with the best possible outcomes, we first need to identify a true picture of our patients needs and determine the optimal method of assessing, recording and addressing those needs. While valuable, the current range of health needs assessment tools do not provide enough detail on these specific neurological symptom domains to allow a proper evaluation of the needs of our patients and how we might address those via health and well-being events. This project will address this and will identify and use the most appropriate tool. The second project is to use the funding to improve the care and experience of patients living with and beyond treatment for brain metastases from cancer of any primary site, particularly those patients who have been successfully treated with neurosurgery, radiotherapy or stereotactic radiosurgery (SRS), in any location in community, primary, secondary or tertiary care settings. Brain metastases occur in 20-30% of all cancer patients, and are particularly prevalent in patients with primary breast, lung and renal cancers and melanoma. Historically, brain metastases developed late in the course of the disease and were a terminal or near-terminal event. However, improvements in systemic treatments e.g. chemotherapy and novel agents, and more pro-active treatment of brain metastases with surgery or radiosurgery mean that more patients are living 21
22 longer following treatment. This is a comparatively new and growing patient group and the board is keen to work towards improving their care and survivorship experience. This project aims to facilitate the sharing of specialist neuro-oncology expertise in the management of areas such as epilepsy, steroids, neuro-cognitive dysfunction and optimisation of functioning in patients with residual neurological deficits, providing an educational resource and ensuring that all patients have access to the highest standards of specialist advice and care. By improving understanding of the options for treating, managing or optimising function in the presence of neurological disability, referrals are more likely to be better directed and more targeted, leading to improved patient experience and improved utilisation of existing resources e.g. community rehab teams. It would also ensure patients have access to specialist services e.g. specialist optometry clinics and epilepsy support services that more general cancer care professionals may be unaware of. The funding will be used to support the recruitment of dedicated project management to take these bids to a successful conclusion. They will be completed within the timescales agreed with the funding and administrative bodies. 22
23 8. Appendix 3 Meeting attendance NAME ROLE TRUST 06/05/ /07/2014 0/10/ /04/2015 Arun Kallat Cons Elderly Bolton Care Physician Apologies Apologies Apologies Apologies Dr catherine McBain Consultant Julie Emerson SALT Christie Sara Robson OT Apologies Elizabeth Molloy CNS Dr A Tran Consultant Peter Selby Consultant CMFT Aplogies Apologies Apologies Dr Moe Sein Consultant East Cheshire TBC Pennine K Karabatsou Cons Neurosurgeon/ Lead for Neurooncology Apologies S Rutherford Cons Neurosurgeon/ Lead for Skull Base SRFT Apologies Apologies T Kearney Cons Endocrinologist/ Lead for Pituitary MDT Apologies Apologies Apologies Apologies Andrea Wadeson CNS Sarah Cundliffe CNS Apologies Alison Gilston-Hope CNS Dr K Dizayee Consultant Stockport Physician Apologies Apologies Apologies Apologies Dr C Douglass Consultant Tameside Neurologist Apologies Apologies Palliative Care Dr Samantha Kay Consultant Apologies UHSM Palliative Care Dr Sophie Harrison Consultant Apologies Dr A Ismail Consultant WWL Radiologist Apologies Apologies Apologies Apologies Dr Steve Elliot GP Salford CCG Apologies Apologies 23
24 9. Appendix 4 - Pathway Board Annual Plan 2015/16 Brain &CNS Pathway Board Annual Plan Pathway Clinical Director: Dr Catherine McBain Pathway Board Members: Pathway Manager: Date agreed by Pathway Board: James Leighton To be ratified at next pathway board Date agreed by Medical Director: Review date: 22 nd September 2015 Summary of objectives No Objective Alignment with Provider Board objectives 1 To develop the routine introduction of MGMT testing in Manchester Objective no 1 clinical outcomes & survival 2 Fully revise and extend the clinical guidelines to include standardised imaging follow-up policies Objective no 1 clinical outcomes & survival 3 Introduce banking of brain tumour tissue Objective no 2 - Research 4 Manage the successful delivery of both Macmillan funded Living With and Beyond projects Objectives no 1 & 3 clinical outcomes & survival plus patient experience 5 The board will work with primary care commissioners and secondary care providers to develop a response to the recommendations for Brain & CNS cancer made in the NICE guidance Suspected cancer: recognition and referral. Objectives no 1 & 4 clinical outcomes & survival plus standardisation of the service 24
25 Objective 1: Objective: To develop the routine introduction of MGMT testing in Manchester Rationale: MGMT status is a vital marker of prognosis and chances of response to chemotherapy which we have not presently been able to offer. However, clinical trial evidence, particularly in the elderly, has emphasised that care not guided by this marker may be sub-standard. Introduction of this measure will help to improve outcomes and patient experience and ensure highest quality care. By (date): 31/3/15 Board measure(s): Risks to success: Support required: Testing of >90% of glioblastomas for MGMT methylation. > 90% of all tumour reports issued in line with minimum dataset requirements Improved patient satisfaction Improved 1 year survival rates Neuro-Pathology consultant staff time Funding Recognition of the vital nature of excellence in neuropathology with appropriate additional support of necessary Possible, funding for MGMT test Work programme Action Resp. By (date) Develop a business case to support the introduction of this test Service Nov 15 team Gain approval from relevant trust service team Dec 15 Full implementation, including compliance with specimen turnaround time, in > 90% patients 31/3/16
26 Objective 2: Objective: Fully revise and extend the clinical guidelines to include standardised imaging follow-up policies Rationale: The guidelines of the pathway are now due for revision and need to reflect any changes in practice, service provision and processes. In addition, growing pressure on radiology departments makes a formal review of follow-up imaging policies indicated and timely. By (date): November 15 Board measure(s): Risks to success: Support required: Revised guidelines and management policy including new standardised imaging follow-up policies Time and other commitments of involved personnel Resources None identified Work programme Action Resp. By (date) Revision of guidelines and management policy PD Oct 15 Table at SMG meeting for approval PM Nov 15 Table for adoption at Pathway Board meting PM Jan 16 26
27 Objective 3: Objective: Rationale: Introduce banking of brain tumour tissue BRAIN UK is an MRC-funded initiative supported by the British Neuropathological Society which is cataloguing the diagnostic tissue holdings of UK neuropathology centres and making these extensive archives available to the research community for high quality neurological research. To date there is access to over 60,000 cases. These valuable tissue resources are derived from post mortem examinations and contain neurological conditions from a range of conditions including cancer as well as many normal cases which are useful as controls. By (date): January 2016 Board measure(s): Risks to success: Support required: To direct and support the clinical teams in retrieving pathological material to contribute to the Brain bank The research ethical approval process and patient approval The commitment of clinical teams Possible additional resource required by Department of Pathology Support at executive level for organisational change process Work programme Action Resp. By (date) SMG approval and commitment CMcB Nov 15 Identify potential resource implications SMG Nov 15 Service teams to agree process for retrieval, despatch and SRFT Nov 15 recording of sample Agree a process for patient consent SRFT Nov 15 27
28 Objective 4: Objective: Rationale: Manage the successful delivery of both Macmillan funded Living With and Beyond projects To successfully deliver the Macmillan funded Living With and Beyond projects so that the proposed outcomes are fully realised. By (date): March 2016 Board measure(s): Risks to success: Support required: Agreed health needs assessment tool Learning tool available for clinical staff caring for patients with Brain Mets Failure to recruit project management support Time and other commitments of involved personnel Resources Support at executive level for organisational change process Work programme Action Resp. By (date) Recruitment of project management resource JE / SR Aug 15 Commencement of Project Project Sep 15 Man Report to Pathway board PD/PM Mar 16 28
29 Objective 5: Objective: The board will work with primary care commissioners and secondary care providers to develop a response to the recommendations for Brain & CNS cancer made in the NICE guidance Suspected cancer: recognition and referral. Rationale: This guidance has asked for direct access to MR scanning for primary care; the availability of direct access to this diagnostic tool to GPs is currently unknown. Equally the implication of this for providers also needs to be better understood. By (date): Dec 2015 Board measure(s): Risks to success: Support required: Audit of direct access MR scanning Protocol written to support delivery of the guidance Time and other commitments of involved personnel Resources - available scanning and reporting capability Mitigation: Aim for an efficient, unified, sustainable approach. Support at executive level for organisational change process Increased support for neuro-oncology MDT which is likely to experience an increase in referrals of patients with incidental findings. Work programme Action Resp. By (date) Audit the scanning departments on primary care access PM Q2 Liaise with commissioners to understand possible volumes PD Q2 Review at board Board Nov 15 Develop protocol to ensure correct patients access scanning Board Jan 16 29
Children s Cancer Pathway Board Annual Report 2014/15. Pathway Clinical Director: Bernadette Brennan Pathway Manager: Melissa Wright
 Children s Cancer Pathway Board Annual Report 2014/15 Pathway Clinical Director: Bernadette Brennan Pathway Manager: Melissa Wright Executive summary Cancer in children and young people is rare with only
Children s Cancer Pathway Board Annual Report 2014/15 Pathway Clinical Director: Bernadette Brennan Pathway Manager: Melissa Wright Executive summary Cancer in children and young people is rare with only
Activity Report April 2012 to March 2013
 North, South East and West of Scotland Cancer Networks Brain/Central Nervous System Tumours National Managed Clinical Network Activity Report April 2012 to March 2013 Professor Roy Rampling Emeritus Professor
North, South East and West of Scotland Cancer Networks Brain/Central Nervous System Tumours National Managed Clinical Network Activity Report April 2012 to March 2013 Professor Roy Rampling Emeritus Professor
Trust Leads Board Terms of Reference
 Manchester Cancer Trust Leads Board Terms of Reference These terms of reference were agreed on 28 th July 2014 by the Manchester Cancer Trust Leads Board. The terms of reference will be subject to future
Manchester Cancer Trust Leads Board Terms of Reference These terms of reference were agreed on 28 th July 2014 by the Manchester Cancer Trust Leads Board. The terms of reference will be subject to future
Brain & CNS Pathway Board Annual Report 2013/14
 Manchester Cancer Brain & CNS Pathway Board Annual Report 2013/14 Pathway Clinical Director: Dr Catherine McBain Pathway Manager: James Leighton Version 1.4-1 - 1.Executive summary Brain and CNS cancer
Manchester Cancer Brain & CNS Pathway Board Annual Report 2013/14 Pathway Clinical Director: Dr Catherine McBain Pathway Manager: James Leighton Version 1.4-1 - 1.Executive summary Brain and CNS cancer
National Cancer Peer Review Programme
 National Cancer Peer Review Programme Julia Hill Acting Deputy National Co-ordinator What is Cancer Peer Review? A quality assurance process for cancer services. An integral part of Improving Outcomes
National Cancer Peer Review Programme Julia Hill Acting Deputy National Co-ordinator What is Cancer Peer Review? A quality assurance process for cancer services. An integral part of Improving Outcomes
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM)
 INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MVCN EAST AND NORTH HERTFORDSHIRE Mount Vernon Cancer Centre Cancer Network MDT (11-2K-1) - 2011/12 Date Self Assessment Completed
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MVCN EAST AND NORTH HERTFORDSHIRE Mount Vernon Cancer Centre Cancer Network MDT (11-2K-1) - 2011/12 Date Self Assessment Completed
Gynaecology Cancer Pathway Board Annual Report 2015/16
 Gynaecology Cancer Pathway Board Annual Report 2015/16 Pathway Clinical Director: Dr Lisa Barraclough Pathway Manager: James Leighton Version 2.0 Executive summary The Gynaecology pathway board is now
Gynaecology Cancer Pathway Board Annual Report 2015/16 Pathway Clinical Director: Dr Lisa Barraclough Pathway Manager: James Leighton Version 2.0 Executive summary The Gynaecology pathway board is now
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM)
 INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
NATIONAL MANAGED CLINICAL NETWORK FOR ADULT NEURO-ONCOLOGY ANNUAL REPORT 2010/11
 NATIONAL MANAGED CLINICAL NETWORK FOR ADULT NEURO-ONCOLOGY ANNUAL REPORT 2/11 Hosted by West of Scotland Cancer Network (WoSCAN) North, South East and West of Scotland Cancer Networks Contents Contents...ii
NATIONAL MANAGED CLINICAL NETWORK FOR ADULT NEURO-ONCOLOGY ANNUAL REPORT 2/11 Hosted by West of Scotland Cancer Network (WoSCAN) North, South East and West of Scotland Cancer Networks Contents Contents...ii
Work Programme/Service Delivery Plan 2010/2013
 Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
Activity Report March 2013 February 2014
 West of Scotland Cancer Network Skin Cancer Managed Clinical Network Activity Report March 2013 February 2014 Dr Girish Gupta Consultant Dermatologist MCN Clinical Lead Tom Kane MCN Manager West of Scotland
West of Scotland Cancer Network Skin Cancer Managed Clinical Network Activity Report March 2013 February 2014 Dr Girish Gupta Consultant Dermatologist MCN Clinical Lead Tom Kane MCN Manager West of Scotland
Building on Success. Driving improvements in clinical outcomes through a Greater Manchester Cancer Alliance. May 2015
 Building on Success Driving improvements in clinical outcomes through a Greater Manchester Cancer Alliance May 2015 Introduction Cancer care in Greater Manchester has seen significant improvements in recent
Building on Success Driving improvements in clinical outcomes through a Greater Manchester Cancer Alliance May 2015 Introduction Cancer care in Greater Manchester has seen significant improvements in recent
Brighton and Sussex University Hospitals NHS Trust Board of Directors. Mark Smith Chief Operating Officer
 Meeting: Brighton and Sussex University Hospitals NHS Trust Board of Directors Date: 24 th August 2015 Board Sponsor: Paper Author: Subject: Mark Smith Chief Operating Officer Clinical Director and Directorate
Meeting: Brighton and Sussex University Hospitals NHS Trust Board of Directors Date: 24 th August 2015 Board Sponsor: Paper Author: Subject: Mark Smith Chief Operating Officer Clinical Director and Directorate
Greater Manchester Cancer Colorectal Pathway Board CNS Group
 Minutes Thursday 16 th March 2017, 2.00pm 4.00pm Pinewood Education Centre, Stepping Hill Hospital Greater Manchester Cancer CNS Group Attendance Sajal Rai Ian Buchanan Sarah Wemyss Julie Williams Claire
Minutes Thursday 16 th March 2017, 2.00pm 4.00pm Pinewood Education Centre, Stepping Hill Hospital Greater Manchester Cancer CNS Group Attendance Sajal Rai Ian Buchanan Sarah Wemyss Julie Williams Claire
Integrated Cancer Services Action Plan. Colchester Hospital University NHS Foundation Trust 31 March 2014
 Integrated Cancer Services Action Plan Colchester Hospital University NHS Foundation Trust 31 March KEY Implemented, clearly evidenced and externally approved On Track to deliver Some issues narrative
Integrated Cancer Services Action Plan Colchester Hospital University NHS Foundation Trust 31 March KEY Implemented, clearly evidenced and externally approved On Track to deliver Some issues narrative
The Greater Manchester Stroke Operational Delivery Network
 The Dr Jane Molloy Clinical Lead What is the GMSODN? Established in July 2015 Only Stroke ODN in the country Non-statutory body constituted from all public sector stroke provider organisations across Greater
The Dr Jane Molloy Clinical Lead What is the GMSODN? Established in July 2015 Only Stroke ODN in the country Non-statutory body constituted from all public sector stroke provider organisations across Greater
Activity Report July 2012 June 2013
 Urological Cancers Managed Clinical Network Activity Report July 2012 June 2013 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Urological Cancers Managed Clinical Network Activity Report July 2012 June 2013 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
MCIP Recruitment Pack
 MCIP Recruitment Pack Page 1 of 13 Welcome Thank you for the interest you have shown in the MCIP Programme. An exciting partnership has been established to redesign cancer care in Manchester. Funded by
MCIP Recruitment Pack Page 1 of 13 Welcome Thank you for the interest you have shown in the MCIP Programme. An exciting partnership has been established to redesign cancer care in Manchester. Funded by
Richard Watson, Chief Transformation Officer. Dr P Holloway, GP Clinical Lead for Cancer Lisa Parrish, Senior Transformation Lead
 GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
Activity Report April 2014 March 2015
 North, South East and West of Scotland Cancer Networks Brain/Central Nervous System Tumours National Managed Clinical Network Activity Report April 2014 March 2015 Dr Avinash Kanodia Consultant Radiologist
North, South East and West of Scotland Cancer Networks Brain/Central Nervous System Tumours National Managed Clinical Network Activity Report April 2014 March 2015 Dr Avinash Kanodia Consultant Radiologist
Improving services for upper GI (OG) cancer Application template (Version 2)
 Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
Breast Cancer Pathway Board Minutes of meeting
 Breast Cancer Pathway Board Minutes of meeting Monday 9 th March 2015, 2pm 5 pm Boardroom 1, New Alderley House, Victoria Road, East Cheshire NHS Foundation Trust Attendance Mohammed Absar Chandeena Roshanlall
Breast Cancer Pathway Board Minutes of meeting Monday 9 th March 2015, 2pm 5 pm Boardroom 1, New Alderley House, Victoria Road, East Cheshire NHS Foundation Trust Attendance Mohammed Absar Chandeena Roshanlall
Haemato-oncology Clinical Forum. 20 th June 2013
 Haemato-oncology Clinical Forum 20 th June 2013 Welcome Dr Majid Kazmi, LCA Haemato-oncology Pathway Group Chair Purpose of today Provide an update on progress of the LCA to date Identify priorities for
Haemato-oncology Clinical Forum 20 th June 2013 Welcome Dr Majid Kazmi, LCA Haemato-oncology Pathway Group Chair Purpose of today Provide an update on progress of the LCA to date Identify priorities for
Living with and Beyond Pathway Board Minutes of Meeting
 Living with and Beyond Pathway Board Minutes of Meeting 20 th January 2015 Location: Lecture Theatre 1, Department 17, The Christie NHS Foundation Trust Time: 3 to 5pm Attendance Wendy Makin Kathy Pantelides
Living with and Beyond Pathway Board Minutes of Meeting 20 th January 2015 Location: Lecture Theatre 1, Department 17, The Christie NHS Foundation Trust Time: 3 to 5pm Attendance Wendy Makin Kathy Pantelides
Breast Cancer Pathway Board Minutes of meeting. Wednesday 14 th January 2015, 2pm 5 pm Lecture Theatre, The Nightingale Centre, UHSM
 Breast Cancer Pathway Board Minutes of meeting Wednesday 14 th January 2015, 2pm 5 pm Lecture Theatre, The Nightingale Centre, UHSM Attendance Jane Ooi Chandeena Roshanlall Vanessa Pope Mohammed Absar
Breast Cancer Pathway Board Minutes of meeting Wednesday 14 th January 2015, 2pm 5 pm Lecture Theatre, The Nightingale Centre, UHSM Attendance Jane Ooi Chandeena Roshanlall Vanessa Pope Mohammed Absar
Primary brain tumours and cerebral metastases workshop
 Primary brain tumours and cerebral workshop 22.4.16 Summary of workshop group discussions on the content of the scope Scope section Title: Primary brain tumours and cerebral Who the guideline is for People
Primary brain tumours and cerebral workshop 22.4.16 Summary of workshop group discussions on the content of the scope Scope section Title: Primary brain tumours and cerebral Who the guideline is for People
Proposed Neurosciences National Structure. Accountability Framework Neurological Alliance (Feedback Loop) National Advisory Group (Neurosciences)
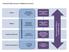 Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
BRAIN & CNS PATHWAY BOARD CONSTITUTION JULY 2014
 BRAIN & CNS PATHWAY BOARD CONSTITUTION JULY 2014 Date for Review: July 2015 Contents Measure No Measure Title Page No 14-1C-101k Network Configuration of the Brain & CNS MDTs 5 14-1C-102k Configuration
BRAIN & CNS PATHWAY BOARD CONSTITUTION JULY 2014 Date for Review: July 2015 Contents Measure No Measure Title Page No 14-1C-101k Network Configuration of the Brain & CNS MDTs 5 14-1C-102k Configuration
Colorectal Cancer Pathway Clinical Subgroup Tuesday 4 th September pm 4 pm CTCCU Seminar Room UHSM
 Manchester Cancer Colorectal Cancer Pathway Clinical Subgroup Tuesday 4 th September2014 2 pm 4 pm CTCCU Seminar Room UHSM Attendance Sarah Duff Michael Braun David Bisset Mohammed Sadat Deborah Hitchen
Manchester Cancer Colorectal Cancer Pathway Clinical Subgroup Tuesday 4 th September2014 2 pm 4 pm CTCCU Seminar Room UHSM Attendance Sarah Duff Michael Braun David Bisset Mohammed Sadat Deborah Hitchen
South Norfolk CCG Dementia Strategy and Action Plan Dr Tony Palframan, SNCCG Governing Body Member
 Agenda item: 9.4 Subject: Presented by: Submitted to: South Norfolk CCG Dementia Strategy and Action Plan Dr Tony Palframan, SNCCG Governing Body Member Governing Body Date: 28 th July Purpose of paper:
Agenda item: 9.4 Subject: Presented by: Submitted to: South Norfolk CCG Dementia Strategy and Action Plan Dr Tony Palframan, SNCCG Governing Body Member Governing Body Date: 28 th July Purpose of paper:
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SAFE PAEDIATRIC NEUROSURGERY A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS
 SAFE PAEDIATRIC NEUROSURGERY 2001 A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS SAFE PAEDIATRIC NEUROSURGERY 2001 INTRODUCTION In 1997 the SBNS agreed to the setting up of a task force to
SAFE PAEDIATRIC NEUROSURGERY 2001 A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS SAFE PAEDIATRIC NEUROSURGERY 2001 INTRODUCTION In 1997 the SBNS agreed to the setting up of a task force to
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
Haematological Oncology Pathway Board Annual Report 2013/14
 Haematological Oncology Pathway Board Annual Report 2013/14 Pathway Clinical Director: Mike Dennis Pathway Manager: Melissa Wright Executive summary The establishment of the Haematological Oncology Manchester
Haematological Oncology Pathway Board Annual Report 2013/14 Pathway Clinical Director: Mike Dennis Pathway Manager: Melissa Wright Executive summary The establishment of the Haematological Oncology Manchester
Greater Manchester Stroke Operational Delivery Network Supporting Stroke Care in Greater Manchester
 Supporting Stroke Care in Greater Manchester Dr Jane Molloy ODN Clinical Lead Who are we? Set up in July 2015 A partnership of NHS stroke-care providers across Greater Manchester and Eastern Cheshire Provider-funded
Supporting Stroke Care in Greater Manchester Dr Jane Molloy ODN Clinical Lead Who are we? Set up in July 2015 A partnership of NHS stroke-care providers across Greater Manchester and Eastern Cheshire Provider-funded
Oesophago-gastric Cancer Pathway Board Annual Report 2015/16
 Oesophago-gastric Cancer Pathway Board Annual Report 2015/16 Pathway Clinical Director: Mr Jonathan Vickers Pathway Manager: James Leighton Version 3.0 Executive summary The Oesophago-gastric (OG) pathway
Oesophago-gastric Cancer Pathway Board Annual Report 2015/16 Pathway Clinical Director: Mr Jonathan Vickers Pathway Manager: James Leighton Version 3.0 Executive summary The Oesophago-gastric (OG) pathway
HPB PATHWAY BOARD MEETING. Meeting of the HPB Pathway Board, 4 th September 2015 Bolton Royal Hospital. MacMillan / Manchester Cancer
 Manchester Cancer HPB PATHWAY BOARD MEETING Meeting of the HPB Pathway Board, 4 th September 2015 Bolton Royal Hospital IN ATTENDANCE Derek O Reilly Rebecca Price Mahesh Bhalme Michelle Storey Vicki Stevenson-Hornby
Manchester Cancer HPB PATHWAY BOARD MEETING Meeting of the HPB Pathway Board, 4 th September 2015 Bolton Royal Hospital IN ATTENDANCE Derek O Reilly Rebecca Price Mahesh Bhalme Michelle Storey Vicki Stevenson-Hornby
Head and Neck Pathway Board 13 th January 2016 Minutes of Meeting
 Head and Neck Pathway Board 13 th January 2016 Minutes of Meeting Humphrey booth Lecture theatre 2, Mayo building SRFT Time: 2-4pm Attendance Miss Susi Penney David Makin David Thomson Mazhar Iqbal Kate
Head and Neck Pathway Board 13 th January 2016 Minutes of Meeting Humphrey booth Lecture theatre 2, Mayo building SRFT Time: 2-4pm Attendance Miss Susi Penney David Makin David Thomson Mazhar Iqbal Kate
Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester
 Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester Greater Manchester Cancer Clinical Director: Mr Mohammed Absar Pathway Manager: Rebecca Price Pathway approval: 24
Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester Greater Manchester Cancer Clinical Director: Mr Mohammed Absar Pathway Manager: Rebecca Price Pathway approval: 24
Colorectal Subgroup Minutes Tuesday 22 nd September 2015, 2pm 4pm CTCCU Seminar Room, UHSM
 Colorectal Subgroup Minutes Tuesday 22 nd September 2015, 2pm 4pm CTCCU Seminar Room, UHSM ATTENDANCE Sarah Duff Tom Pharaoh Mike Braun Edwin Clark Dave Smith Vanessa Denvir Hannah Leaton Malcolm Wilson
Colorectal Subgroup Minutes Tuesday 22 nd September 2015, 2pm 4pm CTCCU Seminar Room, UHSM ATTENDANCE Sarah Duff Tom Pharaoh Mike Braun Edwin Clark Dave Smith Vanessa Denvir Hannah Leaton Malcolm Wilson
PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE
 PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: Dr V. Misra Version: Accountable Committee: V3 MSCC Network
PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: Dr V. Misra Version: Accountable Committee: V3 MSCC Network
Activity Report April 2012 March 2013
 Colorectal Cancer Managed Clinical Network Activity Report April 2012 March 2013 Paul Horgan Professor of Surgery MCN Clinical Lead Kevin Campbell Network Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Colorectal Cancer Managed Clinical Network Activity Report April 2012 March 2013 Paul Horgan Professor of Surgery MCN Clinical Lead Kevin Campbell Network Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
A Framework for Optimal Cancer Care Pathways in Practice
 A to Guide Care Cancer Care A for Care in Practice SUPPORTING CONTINUOUS IMPROVEMENT IN CANCER CARE Developed by the National Cancer Expert Reference Group to support the early adoption of the A to Guide
A to Guide Care Cancer Care A for Care in Practice SUPPORTING CONTINUOUS IMPROVEMENT IN CANCER CARE Developed by the National Cancer Expert Reference Group to support the early adoption of the A to Guide
Implementing NICE clinical guidelines on Parkinson s disease
 ORIGINAL PAPERS Clinical Medicine 2009, Vol 9, No 5: 436 40 Implementing NICE clinical guidelines on Parkinson s disease Beverly A Ryton and B Jane Liddle ABSTRACT Implementing national guidance such as
ORIGINAL PAPERS Clinical Medicine 2009, Vol 9, No 5: 436 40 Implementing NICE clinical guidelines on Parkinson s disease Beverly A Ryton and B Jane Liddle ABSTRACT Implementing national guidance such as
Brain tumours and NHS England
 Brain tumours and NHS England Andrew Brodbelt Consultant Neurosurgeon and Division Clinical Director The Walton Centre NHS Foundation Trust, Liverpool NCRAS CNS tumours workshop April 12 th 2016. National
Brain tumours and NHS England Andrew Brodbelt Consultant Neurosurgeon and Division Clinical Director The Walton Centre NHS Foundation Trust, Liverpool NCRAS CNS tumours workshop April 12 th 2016. National
Activity Report March 2012 February 2013
 Lung Cancer Managed Clinical Network Activity Report March 2012 February 2013 John McPhelim Lead Lung Cancer Nurse MCN Clinical Lead Kevin Campbell Network Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Lung Cancer Managed Clinical Network Activity Report March 2012 February 2013 John McPhelim Lead Lung Cancer Nurse MCN Clinical Lead Kevin Campbell Network Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
PEER REVIEW VISIT REPORT (MULTI-DISCIPLINARY TEAM)
 PEER REVIEW VISIT REPORT (MULTI-DISCIPLINARY TEAM) Network Organisation (Trust) Team DCN POOLE Poole THYROID ONLY MDT (11-2I-2) - 2011/12 Peer Review Visit Date 18th August 2011 Compliance THYROID ONLY
PEER REVIEW VISIT REPORT (MULTI-DISCIPLINARY TEAM) Network Organisation (Trust) Team DCN POOLE Poole THYROID ONLY MDT (11-2I-2) - 2011/12 Peer Review Visit Date 18th August 2011 Compliance THYROID ONLY
Activity Report July 2014 June 2015
 Urological Cancers Managed Clinical Network Activity Report July 2014 June 2015 Mr Gren Oades Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION 5
Urological Cancers Managed Clinical Network Activity Report July 2014 June 2015 Mr Gren Oades Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION 5
GOVERNING BODY REPORT
 GOVERNING BODY REPORT DATE OF MEETING: 20th September 2012 TITLE OF REPORT: KEY MESSAGES: NHS West Cheshire Clinical Commissioning Group has identified heart disease as one of its six strategic clinical
GOVERNING BODY REPORT DATE OF MEETING: 20th September 2012 TITLE OF REPORT: KEY MESSAGES: NHS West Cheshire Clinical Commissioning Group has identified heart disease as one of its six strategic clinical
Activity Report April 2013 March 2014
 North, South East and West of Scotland Cancer Networks HepatoPancreatoBiliary Cancers National Managed Clinical Network Activity Report April 2013 March 2014 Mr Colin McKay Consultant Surgeon NMCN Clinical
North, South East and West of Scotland Cancer Networks HepatoPancreatoBiliary Cancers National Managed Clinical Network Activity Report April 2013 March 2014 Mr Colin McKay Consultant Surgeon NMCN Clinical
Activity Report April 2013 March 2014
 North, South East and West of Scotland Cancer Networks Sarcoma National Managed Clinical Network Activity Report April 2013 March 2014 Dr Jeff White Consultant Oncologist NMCN Clinical Lead Lindsay Campbell
North, South East and West of Scotland Cancer Networks Sarcoma National Managed Clinical Network Activity Report April 2013 March 2014 Dr Jeff White Consultant Oncologist NMCN Clinical Lead Lindsay Campbell
CANCER IN SCOTLAND: ACTION FOR CHANGE The structure, functions and working relationships of Regional Cancer Advisory Groups
 CANCER IN SCOTLAND: ACTION FOR CHANGE The structure, functions and working relationships of Regional Cancer Advisory Groups Introduction/Background 1. Our National Health: A Plan for action, a plan for
CANCER IN SCOTLAND: ACTION FOR CHANGE The structure, functions and working relationships of Regional Cancer Advisory Groups Introduction/Background 1. Our National Health: A Plan for action, a plan for
Activity Report April 2012 March 2013
 North, South East and West of Scotland Cancer Networks HepatoPancreatoBiliary Cancers National Managed Clinical Network Activity Report April 2012 March 2013 Mr Colin McKay Consultant Surgeon NMCN Clinical
North, South East and West of Scotland Cancer Networks HepatoPancreatoBiliary Cancers National Managed Clinical Network Activity Report April 2012 March 2013 Mr Colin McKay Consultant Surgeon NMCN Clinical
Greater Manchester Cancer Services
 HPB PATHWAY BOARD MEETING MINUTES DATE: 14/04/2014 Greater Manchester Cancer Services part of Manchester Cancer In Attendance: Mr. Derek O Neill Ms. Caroline McCall Prof. Juan Valle Dr. Konrad Koss Ms.
HPB PATHWAY BOARD MEETING MINUTES DATE: 14/04/2014 Greater Manchester Cancer Services part of Manchester Cancer In Attendance: Mr. Derek O Neill Ms. Caroline McCall Prof. Juan Valle Dr. Konrad Koss Ms.
Project Initiation Document:
 Project Initiation Document: Lancashire Support Services for Children, Young People, Families and Carers Affected by Autistic Spectrum Disorder (ASD) and Diagnosis 1. Background The Children and Young
Project Initiation Document: Lancashire Support Services for Children, Young People, Families and Carers Affected by Autistic Spectrum Disorder (ASD) and Diagnosis 1. Background The Children and Young
PRIMARY CARE CO-COMMISSIONING COMMITTEE 8 SEPTEMBER 2015
 Part 1 Part 2 PRIMARY CARE CO-COMMISSIONING COMMITTEE 8 SEPTEMBER 2015 Title of Report Trafford Palliative care Quality Premium Scheme 2015/16 Purpose of the Report The purpose of the report is to detail
Part 1 Part 2 PRIMARY CARE CO-COMMISSIONING COMMITTEE 8 SEPTEMBER 2015 Title of Report Trafford Palliative care Quality Premium Scheme 2015/16 Purpose of the Report The purpose of the report is to detail
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)
 B3d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification B3d No.
B3d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification B3d No.
National Optimal Lung Cancer Pathways. Dr Sadia Anwar Nottingham University Hospitals NHS Trust Clinical Lead for Lung Cancer
 National Optimal Lung Cancer Pathways Dr Sadia Anwar ttingham University Hospitals NHS Trust Clinical Lead for Lung Cancer Overview How NOLCP evolved How it relates to national guidance Pathways Implementation
National Optimal Lung Cancer Pathways Dr Sadia Anwar ttingham University Hospitals NHS Trust Clinical Lead for Lung Cancer Overview How NOLCP evolved How it relates to national guidance Pathways Implementation
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician NECN NORTH TEES AND HARTLEPOOL North Tees And Hartlepool Lung MDT (11-2C-1) - 2011/12 Dr D N Leitch Compliance Self
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician NECN NORTH TEES AND HARTLEPOOL North Tees And Hartlepool Lung MDT (11-2C-1) - 2011/12 Dr D N Leitch Compliance Self
Test and Learn Community Frailty Service for frail housebound patients and those living in care homes in South Gloucestershire
 Test and Learn Community Frailty Service for frail housebound patients and those living in care homes in South Gloucestershire Introduction This document introduces South Gloucestershire Clinical Commissioning
Test and Learn Community Frailty Service for frail housebound patients and those living in care homes in South Gloucestershire Introduction This document introduces South Gloucestershire Clinical Commissioning
Safeguarding Business Plan
 Safeguarding Business Plan 2015-2018 Contents 1. Introduction 2. The Care Act 3. Organisational Development 4. Vision, Values and Strategic Objectives 5. Financial Plan 6. Appendix A Action Plan 7. Appendix
Safeguarding Business Plan 2015-2018 Contents 1. Introduction 2. The Care Act 3. Organisational Development 4. Vision, Values and Strategic Objectives 5. Financial Plan 6. Appendix A Action Plan 7. Appendix
Head and Neck Cancer surgeon level data - first report. Queen Victoria Hospital NHS Foundation Trust
 Head and Neck Cancer surgeon level data - first report Queen Victoria Hospital NHS Foundation Trust 1 of 8 Foreword This report for the first time presents data for individual surgeons relating to head
Head and Neck Cancer surgeon level data - first report Queen Victoria Hospital NHS Foundation Trust 1 of 8 Foreword This report for the first time presents data for individual surgeons relating to head
Centralising to a 7 day stroke stroke service in Greater Manchester - lessons learnt
 Centralising to a 7 day stroke stroke service in Greater Manchester - lessons learnt Sarah Rickard, Network Manager @GMStrokeODN www.gmsodn.org.uk 2010 2011 2014 2015 2016 2017 Partial centralisation of
Centralising to a 7 day stroke stroke service in Greater Manchester - lessons learnt Sarah Rickard, Network Manager @GMStrokeODN www.gmsodn.org.uk 2010 2011 2014 2015 2016 2017 Partial centralisation of
Supra Network Sarcoma Advisory Group (SAG) Annual Report
 London and South East Sarcoma Network Supra Network Sarcoma Advisory Group (SAG) Annual Report 2011-2012 Hosted by Date: December 2012 Version: 2 Review Date: September 2013 London Cancer Integrated Cancer
London and South East Sarcoma Network Supra Network Sarcoma Advisory Group (SAG) Annual Report 2011-2012 Hosted by Date: December 2012 Version: 2 Review Date: September 2013 London Cancer Integrated Cancer
Colorectal Pathway Meeting minutes Wednesday 16 th July 2014, 2 pm 4 pm Nightingale Lecture Theatre, UHSM
 Colorectal Pathway Meeting minutes Wednesday 16 th July 2014, 2 pm 4 pm Nightingale Lecture Theatre, UHSM Attendance: Sarah Duff Edwin Clark Sophie Harrison Caroline Whitaker Sarah Taylor Debbie West Mark
Colorectal Pathway Meeting minutes Wednesday 16 th July 2014, 2 pm 4 pm Nightingale Lecture Theatre, UHSM Attendance: Sarah Duff Edwin Clark Sophie Harrison Caroline Whitaker Sarah Taylor Debbie West Mark
Upper GI Cancer Network Site-Specific Group. Work Programme/Service Delivery Plan 2011/14
 Upper GI Cancer Network Site-Specific Group Work Programme/Service Delivery Plan 2011/14 1 UPPER GI NSSG Work Programme/Service Delivery Plan AGREEMENT COVER SHEET 2011/14 This Work Programme has been
Upper GI Cancer Network Site-Specific Group Work Programme/Service Delivery Plan 2011/14 1 UPPER GI NSSG Work Programme/Service Delivery Plan AGREEMENT COVER SHEET 2011/14 This Work Programme has been
CIG SCP Health Board/Velindre Implementation Plan (Aneurin Bevan University Health Board)
 CIG SCP Health Board/Velindre Implementation Plan (Aneurin Bevan University Health Board) 1 Governance, Leadership and Planning a) Describe your SCP Programme Board (or equivalent), its membership and
CIG SCP Health Board/Velindre Implementation Plan (Aneurin Bevan University Health Board) 1 Governance, Leadership and Planning a) Describe your SCP Programme Board (or equivalent), its membership and
Cancer Improvement Plan Update. September 2014
 Cancer Improvement Plan Update September 2014 1 Contents Page 1. Introduction 3 2. Key Achievements 4-5 3. Update on Independent Review Recommendations 6-13 4. Update on IST Recommendations 14-15 5. Update
Cancer Improvement Plan Update September 2014 1 Contents Page 1. Introduction 3 2. Key Achievements 4-5 3. Update on Independent Review Recommendations 6-13 4. Update on IST Recommendations 14-15 5. Update
The Ayrshire Hospice
 Strategy 2010-2015 Welcome... The Ayrshire Hospice : Strategy 2010-2015 Index 05 06 08 09 10 12 15 17 19 Foreword Our vision and purpose Our guiding principles Our achievements 1989-2010 Our priorities
Strategy 2010-2015 Welcome... The Ayrshire Hospice : Strategy 2010-2015 Index 05 06 08 09 10 12 15 17 19 Foreword Our vision and purpose Our guiding principles Our achievements 1989-2010 Our priorities
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Head and Neck Pathway Board 10 th March 2016 Minutes of Meeting
 Head and Neck Pathway Board 10 th March 2016 Minutes of Meeting Peter Mount Meeting Room, Peter Mount Building, CMFT Time: 2-4pm Attendance Miss Susi Penney David Makin Mark Price Lucie Francis Hannah
Head and Neck Pathway Board 10 th March 2016 Minutes of Meeting Peter Mount Meeting Room, Peter Mount Building, CMFT Time: 2-4pm Attendance Miss Susi Penney David Makin Mark Price Lucie Francis Hannah
Volunteering in NHSScotland Developing and Sustaining Volunteering in NHSScotland
 NG11-07 ing in NHSScotland Developing and Sustaining ing in NHSScotland Outcomes The National Group for ing in NHS Scotland agreed the outcomes below which formed the basis of the programme to develop
NG11-07 ing in NHSScotland Developing and Sustaining ing in NHSScotland Outcomes The National Group for ing in NHS Scotland agreed the outcomes below which formed the basis of the programme to develop
ROLE SPECIFICATION FOR MACMILLAN GPs
 ROLE SPECIFICATION FOR MACMILLAN GPs November 2010 History of Macmillan GPs Macmillan Cancer Support has funded GP positions from the early 1990 s, following the success of our investment in supporting
ROLE SPECIFICATION FOR MACMILLAN GPs November 2010 History of Macmillan GPs Macmillan Cancer Support has funded GP positions from the early 1990 s, following the success of our investment in supporting
Colorectal Pathway Meeting minutes Wednesday 11 th March 2015, 2 pm 4 pm Nightingale Lecture Theatre, UHSM
 Colorectal Pathway Meeting minutes Wednesday 11 th March 2015, 2 pm 4 pm Nightingale Lecture Theatre, UHSM Attendance: Sarah Duff Clinical Director and Consultant Colorectal Surgeon, UHSM Mohammed Sadat
Colorectal Pathway Meeting minutes Wednesday 11 th March 2015, 2 pm 4 pm Nightingale Lecture Theatre, UHSM Attendance: Sarah Duff Clinical Director and Consultant Colorectal Surgeon, UHSM Mohammed Sadat
Manchester Cancer Provider Board Second annual report 2014/15
 Manchester Cancer Provider Board Second annual report 2014/15 Web manchestercancer.org Twitter @GM_Cancer Email info@manchestercancer.org Phone 0161 918 2087 Contents 1 A welcome from the Independent Chair
Manchester Cancer Provider Board Second annual report 2014/15 Web manchestercancer.org Twitter @GM_Cancer Email info@manchestercancer.org Phone 0161 918 2087 Contents 1 A welcome from the Independent Chair
South East Coast Operational Delivery Network. Critical Care Rehabilitation
 South East Coast Operational Delivery Networks Hosted by Medway Foundation Trust South East Coast Operational Delivery Network Background Critical Care Rehabilitation The optimisation of recovery from
South East Coast Operational Delivery Networks Hosted by Medway Foundation Trust South East Coast Operational Delivery Network Background Critical Care Rehabilitation The optimisation of recovery from
A06/S(HSS)b Ex-vivo partial nephrectomy service (Adult)
 A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
MS Priority Setting Partnership. PROTOCOL August 2012
 MS Priority Setting Partnership PROTOCOL August 2012 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the MS Priority Setting Partnership (PSP) and the basic roles
MS Priority Setting Partnership PROTOCOL August 2012 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the MS Priority Setting Partnership (PSP) and the basic roles
Appendix 1. Cognitive Impairment and Dementia Service Elm Lodge 4a Marley Close Greenford Middlesex UB6 9UG
 Appendix 1 Mr Dwight McKenzie Scrutiny Review Officer Legal and Democratic Services Ealing Council Perceval House 14 16 Uxbridge Road Ealing London W5 2HL Cognitive Impairment and Dementia Service Elm
Appendix 1 Mr Dwight McKenzie Scrutiny Review Officer Legal and Democratic Services Ealing Council Perceval House 14 16 Uxbridge Road Ealing London W5 2HL Cognitive Impairment and Dementia Service Elm
Improving Outcomes for People with Sarcoma
 NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
National Standards for Sarcoma Services
 National Standards for Sarcoma Services 2009 TABLE OF CONTENTS 1. INTRODUCTION TO THE NATIONAL CANCER STANDARDS...2 2. METHODOLOGY...4 3. FORMAT...4 4. INTRODUCTION TO SARCOMA...5 STANDARDS TOPIC: ORGANISATION...10
National Standards for Sarcoma Services 2009 TABLE OF CONTENTS 1. INTRODUCTION TO THE NATIONAL CANCER STANDARDS...2 2. METHODOLOGY...4 3. FORMAT...4 4. INTRODUCTION TO SARCOMA...5 STANDARDS TOPIC: ORGANISATION...10
The NHS Cancer Plan: A Progress Report
 DEPARTMENT OF HEALTH The NHS Cancer Plan: A Progress Report LONDON: The Stationery Office 9.25 Ordered by the House of Commons to be printed on 7 March 2005 REPORT BY THE COMPTROLLER AND AUDITOR GENERAL
DEPARTMENT OF HEALTH The NHS Cancer Plan: A Progress Report LONDON: The Stationery Office 9.25 Ordered by the House of Commons to be printed on 7 March 2005 REPORT BY THE COMPTROLLER AND AUDITOR GENERAL
National Standards for Sarcoma Services 2009
 National Standards for Sarcoma Services 2009 Crown copyright July 2010 F1101011 Contents 1. Introduction to the National Cancer Standards 2. Methodology 3. Format 4. Introduction to Sarcoma Standards Topic:
National Standards for Sarcoma Services 2009 Crown copyright July 2010 F1101011 Contents 1. Introduction to the National Cancer Standards 2. Methodology 3. Format 4. Introduction to Sarcoma Standards Topic:
Evaluation of the Health and Social Care Professionals Programme Interim report. Prostate Cancer UK
 Evaluation of the Health and Social Care Professionals Programme Interim report Prostate Cancer UK July 2014 Contents Executive summary... 2 Summary of the research... 2 Main findings... 2 Lessons learned...
Evaluation of the Health and Social Care Professionals Programme Interim report Prostate Cancer UK July 2014 Contents Executive summary... 2 Summary of the research... 2 Main findings... 2 Lessons learned...
People living well with Dementia in the East Midlands: Improving the Quality of Care in Acute Hospitals
 PROJECT INITIATION DOCUMENT We re in it together People living well with Dementia in the East Midlands: Improving the Quality of Care in Acute Hospitals Version: 1.1 Date: February 2011 Authors: Jillian
PROJECT INITIATION DOCUMENT We re in it together People living well with Dementia in the East Midlands: Improving the Quality of Care in Acute Hospitals Version: 1.1 Date: February 2011 Authors: Jillian
Consumer Participation Strategy
 Consumer Participation Strategy Plan Implementation Period 2011-2013 Date: 24 December 2010 Developed by: NEMICS Directorate in consultation with Acknowledgements and thank you to: s, Dr Ian Roos (Cancer
Consumer Participation Strategy Plan Implementation Period 2011-2013 Date: 24 December 2010 Developed by: NEMICS Directorate in consultation with Acknowledgements and thank you to: s, Dr Ian Roos (Cancer
Urology Pathway Board Meeting Minutes of the meeting held on March 15 th 2017
 Greater Manchester Cancer Urology Pathway Board Urology Pathway Board Meeting Minutes of the meeting held on March 15 th 2017 Members in attendance Satish Maddineni (Chair) Pathway Director Steve Elliot
Greater Manchester Cancer Urology Pathway Board Urology Pathway Board Meeting Minutes of the meeting held on March 15 th 2017 Members in attendance Satish Maddineni (Chair) Pathway Director Steve Elliot
Head and Neck Cancer MCN Work Plan 2017/18
 Head and Neck Cancer MCN Work Plan /18 Objective Deliverables / Outcomes Lead 1. Manage the development/review of Head and Neck Cancer Management Guidelines and Clinical Guidance Documents. 1.1 Identify
Head and Neck Cancer MCN Work Plan /18 Objective Deliverables / Outcomes Lead 1. Manage the development/review of Head and Neck Cancer Management Guidelines and Clinical Guidance Documents. 1.1 Identify
Brain and CNS tumours Presentation pathway
 Brain and CNS tumours Presentation pathway Ref: AngCN-SSG-BC16 Page 1 of 8 1 Background and Scope This presentation pathway deals with the pathway of referral from all aspects of primary care to hospitals
Brain and CNS tumours Presentation pathway Ref: AngCN-SSG-BC16 Page 1 of 8 1 Background and Scope This presentation pathway deals with the pathway of referral from all aspects of primary care to hospitals
Patient and Carer Network. Work Plan
 Patient and Carer Network Work Plan 2016 2020 Introduction from our chair When it was established over a decade ago, the RCP s Patient and Carer Network (PCN) led the way in mapping and articulating the
Patient and Carer Network Work Plan 2016 2020 Introduction from our chair When it was established over a decade ago, the RCP s Patient and Carer Network (PCN) led the way in mapping and articulating the
Item No: 6. Meeting Date: Tuesday 12 th December Glasgow City Integration Joint Board Performance Scrutiny Committee
 Item No: 6 Meeting Date: Tuesday 12 th December 2017 Glasgow City Integration Joint Board Performance Scrutiny Committee Report By: Susanne Millar, Chief Officer, Strategy & Operations / Chief Social Work
Item No: 6 Meeting Date: Tuesday 12 th December 2017 Glasgow City Integration Joint Board Performance Scrutiny Committee Report By: Susanne Millar, Chief Officer, Strategy & Operations / Chief Social Work
Cancer Transformation Programme
 Cancer Transformation Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION November 2016 1 Introduction and Contents The Planning Guidance for 2017-2019
Cancer Transformation Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION November 2016 1 Introduction and Contents The Planning Guidance for 2017-2019
Activity Report July 2014 June 2015
 West of Scotland Cancer Network Gynaecological Cancer Managed Clinical Network Activity Report July 2014 June 2015 Nadeem Siddiqui Consultant Gynaecological Oncologist MCN Clinical Lead Kevin Campbell
West of Scotland Cancer Network Gynaecological Cancer Managed Clinical Network Activity Report July 2014 June 2015 Nadeem Siddiqui Consultant Gynaecological Oncologist MCN Clinical Lead Kevin Campbell
Acute Oncology & Chemotherapy Clinical Network Group (CNG)
 Acute Oncology & Chemotherapy Clinical Network Group (CNG) Work Programme 2014-2015 Version 1.0 This Work Programme has been agreed by: Title Name Date Agreed AO & Chemotherapy CNG Chair Ernie Marshall
Acute Oncology & Chemotherapy Clinical Network Group (CNG) Work Programme 2014-2015 Version 1.0 This Work Programme has been agreed by: Title Name Date Agreed AO & Chemotherapy CNG Chair Ernie Marshall
Diabetes in Pregnancy Network: Scoping survey March 2013
 Diabetes in Pregnancy Network: Scoping survey March 2013 Diabetes in Pregnancy Network Scoping Survey Aim To inform the development of a National Diabetes in Pregnancy Network Objectives To identify the
Diabetes in Pregnancy Network: Scoping survey March 2013 Diabetes in Pregnancy Network Scoping Survey Aim To inform the development of a National Diabetes in Pregnancy Network Objectives To identify the
HPB PATHWAY BOARD MEETING. Meeting of the HPB Pathway Board, 6 th May 2015 Macclesfield General Hospital
 Manchester Cancer HPB PATHWAY BOARD MEETING Meeting of the HPB Pathway Board, 6 th May 2015 Macclesfield General Hospital IN ATTENDANCE Derek O Reilly Amanda Corfield- Halliwell Mahesh Bhalme Juan Valle
Manchester Cancer HPB PATHWAY BOARD MEETING Meeting of the HPB Pathway Board, 6 th May 2015 Macclesfield General Hospital IN ATTENDANCE Derek O Reilly Amanda Corfield- Halliwell Mahesh Bhalme Juan Valle
2.2 The primary roles and responsibilities of the Committee are to:
 Edinburgh Child Protection Constitution 1. Introduction 1.1 This document sets out the governance arrangements established to promote the delivery of integrated, high quality child protection services
Edinburgh Child Protection Constitution 1. Introduction 1.1 This document sets out the governance arrangements established to promote the delivery of integrated, high quality child protection services
2. The role of CCG lay members and non-executive directors
 CCG Lay Members, Non-Executive Directors and STP Governance and Engagement 1. Introduction Report from network events organised by NHS England and NHS Clinical Commissioners in February 2017 This briefing
CCG Lay Members, Non-Executive Directors and STP Governance and Engagement 1. Introduction Report from network events organised by NHS England and NHS Clinical Commissioners in February 2017 This briefing
