Received for publication October 19, 2005; revised March 8, 2006; accepted March 8, 2006
|
|
|
- Roderick Brooks
- 5 years ago
- Views:
Transcription
1 American Journal of Obstetrics and Gynecology (2006) 195, Effects of Tibolone and Continuous Combined Conjugated Equine Estrogen/Medroxyprogesterone Acetate on the Endometrium and Vaginal Bleeding: Results of the OPAL Study Robert D. Langer, MD, MPH, a, * Britt Marie Landgren, MD, b Janice Rymer, MD, c Frans A. Helmond, PhD, d for the OPAL Investigators Gesinger Health System, a Danville, PA; Karolinska Hospital and Institutet, b Stockholm, Sweden; Guy s, King s, and St Thomas School of Medicine, King s College, c London, UK; Organon International, d Roseland, NJ Received for publication October 19, 2005; revised March 8, 2006; accepted March 8, 2006 KEY WORDS Endometrial safety Hormone therapy OPAL Tibolone Vaginal bleeding Objectives: The primary objective of the Osteoporosis Prevention and Arterial effects of tibo- Lone study was to compare the effect of tibolone and placebo on the progression of the common carotid artery intima-medial thickness; the common carotid artery intima-medial thickness and bone data will be presented elsewhere. A secondary objective was to assess the effects of tibolone (2.5 mg), continuous combined conjugated equine estrogen/medroxyprogesterone acetate [0.625/ 2.5 mg], and placebo on the endometrium and vaginal bleeding; these results are the subject of this report. Study design: This 3-year, three-arm, international, randomized, double-blind, parallel group, placebo-controlled clinical trial enrolled 866 postmenopausal women (aged years). The endometrium was assessed by annual transvaginal ultrasound scans and end-of-study biopsies (United States/United Kingdom centers only). Vaginal bleeding was recorded in daily diaries. Results: Endometrial thickness measured by transvaginal ultrasound scan increased slightly during the first year with tibolone and conjugated equine estrogen/medroxyprogesterone acetate, without any further progression. After 3 years, there were no significant differences between the tibolone, conjugated equine estrogen/medroxyprogesterone acetate, and placebo groups in the incidence of proliferation (1.4%, 4.8%, and 0%, respectively), endometrial hyperplasia (0% in all groups), or cancer (1, 0, and 1 case, respectively). During the first 3 months, bleeding/ spotting rates were greater with conjugated equine estrogen/medroxyprogesterone acetate (48%) than with tibolone (18%; P!.001) or placebo (3%; P!.001). During 3 years of treatment, the incidence of bleeding/spotting was 66%, 48%, and 23% for conjugated equine estrogen/medroxyprogesterone acetate, tibolone, and placebo, respectively. The mean number of bleeding/spotting days was greater in the conjugated equine estrogen/medroxyprogesterone acetate than the tibolone Funded by NV Organon, Oss, The Netherlands. * Reprint requests: Robert D. Langer, MD, MPH, Center for Health Research & Rural Advocacy, Geisinger Health System, 100 N Academy Avenue, Danville, PA rdlanger@geisinger.edu /$ - see front matter Ó 2006 Mosby, Inc. All rights reserved. doi: /j.ajog
2 Langer et al 1321 or placebo groups (61, 28, and 7 days, respectively; P =.023 vs tibolone; P!.0001 vs placebo). The mean number of bleeding/spotting episodes was also greater in the conjugated equine estrogen/medroxyprogesterone acetate group (13 episodes) compared with the tibolone group (six episodes; P!.001) and placebo group (four episodes; P!.001). Vaginal bleeding was more commonly reported as an adverse event with conjugated equine estrogen/medroxyprogesterone acetate than tibolone (26.4% vs 10.8%, P!.0001) and as the reason for premature discontinuation (9% vs 2%, P =.001). Conclusion: Compared with conjugated equine estrogen/medroxyprogesterone acetate, tibolone has a better tolerability profile with respect to vaginal bleeding but with a similar endometrial safety. These results reinforce the endometrial safety profile of tibolone. Ó 2006 Mosby, Inc. All rights reserved. Conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) is one of the most commonly used estrogen-progestogen therapy (EPT) combinations and its endometrial safety has been confirmed in largescale studies. 1-3 However, it has been associated with frequent bleeding and spotting episodes, especially in the first 6 months of treatment. 4 Tibolone is a selective tissue estrogenic activity regulator 5 that is effective in the prevention of osteoporosis and treatment of climacteric symptoms and does not stimulate endometrial and breast tissue. 6 In the endometrium, tibolone locally reduces estrogenic activity via two different routes. Tibolone decreases the activity of the enzyme sulfatase and increases the activity of the enzyme sulfotransferase. These two enzymes are involved in the activation and deactivation, respectively, of estrogenic compounds, resulting in a shift toward deactivation. The other route is the metabolization of the two estrogenic hydroxymetabolites of tibolone back into tibolone, which can be further metabolized locally into the D 4 -isomer, which has progestogenic but no estrogenic properties. 7-9 Tibolone is associated with a low incidence of endometrial hyperplasia and vaginal bleeding and does not require the addition of a progestogen The Osteoporosis Prevention and Arterial effects of tibolone (OPAL) 17 study was conducted to determine the effects of tibolone, continuous combined CEE/ MPA, and placebo on bone mineral density and carotid intima-media thickness (CIMT) in postmenopausal women. This double-blind placebo-controlled trial also captured unique long-term (3 years) comparative data on endometrial safety and vaginal bleeding for tibolone and. Methods This three-arm, multicenter, randomized, double-blind, placebo-controlled study was conducted in six centers in the United States (US) and five centers in Europe. Healthy postmenopausal women (aged years and with a body mass index of O19 and %32 kg/m 2 ) who had been amenorrheic for R1 year were enrolled. If the date of final menstruation was unclear, the woman was to have used hormone therapy (HT) for O2 years and be O53 years old or fulfill the US Food and Drug Administration (FDA) criteria for menopause (serum estradiol %20 pg/ml [or 73 pmol/l] and follicle-stimulating hormone R40 miu/ml). Per study protocol, an intact uterus was required for enrollment in the US centers, whereas in European centers, women with or without a uterus were eligible. A washout period of 8 weeks was required for oral estrogens with or without progestogens, androgens, or selective estrogen receptor modulators (SERMs), 4 weeks for transdermal or local sex steroids, and 20 weeks for injections of MPAcontaining contraceptives. Women were excluded if they had an abnormal cervical Pap smear result, double-layer endometrial thickness of O5 mm as measured by transvaginal ultrasound scan (TVUS), endometrial hyperplasia, unexplained vaginal bleeding that required follow-up, uncontrolled hypertension, current or recent alcohol and/or drug abuse, Type I diabetes mellitus, low total fasting cholesterol, recent history of myocardial infarction, heart failure requiring pharmacologic treatment, current or previous stroke, thrombophlebitis, thromboembolic disorder, gallbladder disease or malignancy (except nonmelanoma skin cancer), suspected breast malignancy, relevant abnormal electrocardiogram (ECG) or laboratory values, serious decompensated renal or liver disease, a carotid ultrasound alert, carotid arteries that were difficult to image using the study protocol, any condition that could alter the pharmacokinetics of the investigational drugs, or hypersensitivity to tibolone or. Current or recent prolonged use of systemic corticosteroids or hepatic microsomal enzymeinducing anticonvulsant medication (or other drugs known to alter the pharmacokinetics of steroids), investigational drug use within the last 60 days, a requirement for medication to treat or prevent dyslipidemia and/or osteoporosis, or a requirement for sex steroids, androgens, or SERMs were also reasons for exclusion. All women provided written informed consent according to the regulations at each participating institution. The study was conducted according to Good Clinical Practice, in full compliance with the Declaration of Helsinki including revisions, and the protocol was
3 1322 Langer et al Table I Baseline characteristics Tibolone (n = 286) (n = 284) (n = 287) Mean (G SD) age (years) 58.9 G G G 6.6 Mean (G SD) BMI (kg/m 2 ) 25.2 G G G 2.9 Mean (G SD) weight (kg) 67.3 G G G 9.1 Mean (G SD) height (cm) G G G 5.9 Race Caucasian 275 (96.1%) 275 (96.8%) 274 (95.4%) Asian 4 (1.3%) 5 (1.7%) 7 (2.4%) Other 7 (2.4%) 4 (1.4%) 6 (2.0%) Mean (G SD) time since 10.8 G G G 7.8 menopause (years) Intact uterus 228 (79.7%) 236 (83.0%) 243 (84.6%) Previous HT use 133 (46.5%) 142 (50.0%) 131 (45.6%) BMI, Body mass index. Table II Endometrial thickness (mm) assessed by TVUS at baseline and annually during treatment with tibolone,, or placebo (mean G SD) Tibolone Baseline 2.3 G 1.1 (n = 216) 2.4 G 1.1 (n = 219) 2.4 G 1.1 (n = 230) Week G 2.6 (n = 187) 3.1 G 1.8 (n = 197) 2.4 G 1.3 (n = 200) Week G 2.3 (n = 167) 3.2 G 1.6 (n = 177) 2.6 G 1.2 (n = 178) Week G 2.0 (n = 152) 3.0 G 1.3 (n = 164) 2.6 G 1.1 (n = 172) End point 3.6 G 2.0 (n = 191) 3.2 G 1.7 (n = 200) 2.6 G 1.3 (n = 211) approved by the institutional review boards of the participating clinics. Medication The women were randomized in a 1:1:1 ratio to receive oral tibolone 2.5 mg, continuous combined 0.625/2.5 mg, or placebo for 3 years (39 cycles of 28 days). The investigational products were blinded using a doubledummy technique. All subjects also received oral calcium supplements (500 mg/day). Concomitant medication (other than that specifically prohibited by the protocol) was permitted at the discretion of the investigator. Assessments Screening evaluations included a medical and gynecologic history, physical and gynecologic examination (including pelvic examination, cervical Pap smear, and TVUS), vital signs, ECG, mammography, pretreatment signs and symptoms and concurrent medications, a carotid ultrasound, dual energy x-ray absorptiometry measurements of bone mineral density, blood lipid levels, routine chemistry, hematology, and urinalysis. Eligible subjects were assigned code numbers in the order of their randomization into the study. Smoking and alcohol consumption and any symptoms and concomitant medications were recorded. The subjects also completed a quality-of-life questionnaire. Follow-up visits were performed at 3 months after randomization and every 6 months thereafter. At all centers, the endometrium was assessed by TVUS after 52, 104, and 156 weeks of treatment (or at the final visit if withdrawn prematurely). At baseline and throughout most of the study, a double-wall endometrial thickness of %5 mm was interpreted as supporting a diagnosis of no endometrial cancer. At the end of the study, to be more conservative, this was changed to %4 mm. Any relevant comments pertaining to the scan were recorded and an appropriate follow-up was conducted in the case of clinically significant findings. In the US/United Kingdom (UK) centers only (7 of 11 centers with 46.9% of women enrolled), an endometrial biopsy was performed at screening and the end of the study using Pipelle endometrial sampler or an alternative acceptable method. Additional biopsies were performed during the study as clinically indicated (eg, undiagnosed abnormal vaginal bleeding, endometrial double-wall thickness of O5 mm). Subjects with endometrial hyperplasia or cancer were not eligible to be enrolled in the study. All biopsy specimens were shipped in a vial containing 10% buffered formalin to a central cytology/pathology laboratory for processing and analysis. Biopsies were evaluated in accordance with the FDA 18 and European Agency for the Evaluation of Medicinal Products 19 guidelines. The results of the histologic examination were classified as follows 20 :
4 Langer et al 1323 Table III Summary of the endometrial biopsy findings at baseline and end point during 3 years of treatment with tibolone,, or placebo Number of women with Tibolone P value* Baseline biopsies Evaluable 86 (75.4%) 92 (77.3%) 89 (75.4%) Nonevaluable 28 (24.6%) 27 (22.7%) 29 (24.6%) 3-year biopsies Evaluable 47 (77.0%) 52 (71.2%) 41 (60.3%) Nonevaluable 14 (23.0%) 21 (28.8%) 27 (39.7%) End point biopsies Evaluable 57 (80.3%) 65 (75.6%) 49 (63.6%) Nonevaluable 14 (19.7%) 21 (24.4%) 28 (36.3%) Proliferation at baseline 0 (0.0%) 5 (4.8%) 1 (0.8%) y.2128 z.0661 x Proliferation at end point 1 (1.4%) 7 (8.1%) 0 (0.0%).4797 y.0146 z.0733 x Hyperplasia at end point NA Endometrial cancer k y at end point * P-values based on Fisher s exact test. y Tibolone vs placebo. z vs placebo. x Tibolone vs. k Non-US/UK subject: no endometrial biopsy performed (cancer diagnosis following surgery) z.4522 x Table IV Incidence of vaginal bleeding/spotting during treatment with tibolone,, or placebo (n; %) P value* Tibolone (n = 222) (n = 232) (n = 235) Tibolone vs Tibolone vs vs Bleeding/Spotting 107 (48.2%) 153 (65.9%) 53 (22.6%)!0.001!0.001!0.001 Spotting only 59 (26.6%) 76 (32.8%) 42 (17.9%) !0.01 Bleeding only 48 (21.6%) 77 (33.2%) 11 (4.7%) 0.007!0.001!0.001 CMH, Cochran-Mantel Haenzel. * P value based on CMH test adjusted for centers. 0: No tissue obtained 1: Tissue insufficient for diagnosis 2: Atrophic 3: Inactive 4: Proliferative 5: Secretory 6: Menstrual type 7: Simple hyperplasia 8: Complex hyperplasia 9: Atypical hyperplasia 10: Carcinoma (type to be specified) Biopsies were considered normal if they were classified as atrophic or inactive. If there was no or insufficient tissue available for diagnosis but the TVUS was %4 mm, the biopsy was also considered normal. Any subjects who were found to have endometrial hyperplasia during the study were managed by their physicians according to the current local standard of care. In the event that progestogen therapy was prescribed, the subject stopped investigational treatment in order to avoid inappropriate concomitant use of two progestational drugs. All other subjects continued in the study. For all nonhysterectomized study participants, vaginal bleeding was recorded in daily diaries and collected at each clinic visit (baseline and weeks 12, 28, 52, 76, 104, 128, and 156). The following definitions were used for diary recordings: Bleeding: any vaginal flow requiring more than one sanitary napkin or tampon per day
5 1324 Langer et al Endometrium The mean endometrial double-wall thickness (as assessed by TVUS) was similar in all three groups at baseline ( mm). During the first year of treatment, there was a small increase in endometrial thickness in the tibolone and the groups. After 2 and 3 years of treatment, the thickness was very similar to that observed after the first year of treatment and no further progression was observed (Table II). Biopsy results Figure Percentage of women with vaginal bleeding/spotting episodes by 12-week intervals (three treatment cycles). Spotting: any vaginal flow requiring not more than one sanitary napkin or tampon per day Details of all other assessments are provided elsewhere. 17 Statistical analyses Tabulations and summary statistics were performed for TVUS and endometrial biopsy findings. For the endometrial biopsies, the number of subjects was tabulated in terms of the histologic categories of normal (atrophic, inactive, proliferative, secretory, or menstrual), hyperplasia (simple, complex, or atypical), or cancer. In cases where the endometrial biopsy material was insufficient for diagnosis, the TVUS finding of an endometrium %4 mm resulted in inclusion in the normal category. The proportion of women reporting any bleeding or spotting was compared between the tibolone and placebo groups and between the and placebo groups using the Cochran-Mantel-Haenszel test adjusted for centers. An episode was defined as one or more consecutive days during which bleeding or spotting was recorded in the diary bounded by bleeding/ spotting-free days. Results A total of 866 women were randomized, of whom 857 received at least one dose of study medication (286 in the tibolone group, 284 in the group, and 287 in the placebo group). There were no statistically significant differences between the groups with regard to baseline characteristics (Table I). The percentage of women who completed the study was similar in each group (69% tibolone, 72%, and 70% placebo). A summary of biopsy findings is shown in Table III. The number of subjects with baseline biopsies was 351 (114 tibolone, 119, and 118 placebo). The number of end point biopsies was 234 (71, 86, and 77, respectively). The percentage of subjects with evaluable baseline or end point biopsies was similar among groups, with the possible exception of the lower percentage of evaluable end point biopsies in the placebo group. No women developed hyperplasia. Two women were diagnosed with endometrial cancer during the study. One case was a participant in the placebo group who did not have a biopsy performed (non-us/uk subject) but who was diagnosed following surgery after approximately 6 months in the study. The other case was a participant in the tibolone group who was diagnosed by endometrial biopsy 3 months after the end of the 3-year study. In that case, TVUS revealed endometrial thickness of 3 mm at baseline and 8 mm at month 39. Baseline and last visit endometrial specimens were unobtainable due to tissue being insufficient for diagnosis, but a subsequent attempt, after the study was complete, yielded adequate tissue. Vaginal bleeding The group had a higher incidence (66%, P!.001 vs both tibolone and placebo) of bleeding/ spotting episodes than either the tibolone group (48%) or the placebo group (23%) (Table IV). The higher incidence of bleeding/spotting in the group was particularly notable in the first 12 weeks, when 47.8% of women reported bleeding/spotting compared with 17.6% (P!.001) in the tibolone group and 3.4% (P!.001) in the placebo group (Figure, first 12 week interval). After 3 years of treatment, the incidence of bleeding/spotting episodes was reported in 22.3% of women given (P! vs both tibolone and placebo), 7.5% of those given tibolone and 2.8% given placebo (Figure, last 12 week interval). As shown in Table V, the mean number of bleeding/spotting days and bleeding/spotting episodes was also greater in the group than in the tibolone and placebo groups (bleeding/spotting days: 61, 28 and 7, respectively, P =.023 vs tibolone and P!.0001 vs placebo;
6 Langer et al 1325 Table V Summary of bleeding/spotting parameters in women reporting bleeding/spotting during treatment with tibolone,, or placebo (All-Subjects-Treated Group) P value* Tibolone (n = 107) (n = 153) (n = 53) Tibolone vs Tibolone vs vs Number of bleeding/spotting days Mean G SD 28.0 G G G !0.0001!.0001 Median Range Number of bleeding/spotting episodes Mean G SD 5.8 G G G 7.2! !.0001 Median Range Duration of bleeding/spotting episodes (days) Mean G SD 4.7 G G G !0.0001!.0001 Median Range * P values based on Wilcoxon test. bleeding/spotting episodes: 13, 6, and 4, respectively, P!.001 vs tibolone and P!.0001 vs placebo). The mean duration of bleeding/spotting episodes was similar in the and tibolone groups and longer than in the placebo group (Table V). Vaginal bleeding was reported as an adverse event by more women in the group (n = 75; 26.4%, P!.0001 vs both tibolone and placebo) than in the tibolone (n = 31; 10.8%) and placebo (n = 9; 3.1%) groups. The percentage of women who discontinued the study due to vaginal bleeding was 9% (n = 25, P =.001 vs tibolone) in the group compared with 2% (n = 7) in the tibolone group and!1% (n = 1) in the placebo group. Most of these discontinuations occurred during the first 3 months of treatment (14/23 [61%]), although some occurred as late as the second or third year (4/23 [17%]). Comment The TVUS and biopsy findings of the OPAL study confirm and extend previous clinical trial results with respect to the endometrial safety of tibolone and its favorable vaginal bleeding profile when compared with continuous combined preparations. 13,15 The incidence of endometrial proliferation in the OPAL study was low in all three treatment groups and none of the women in any of the treatment groups developed endometrial hyperplasia during the 3-year study. Two cases of endometrial cancer were found, one in the placebo group and one in the tibolone group. The biopsy results should be interpreted with caution because only about half of the total study population had biopsies performed, as was prespecified in the protocol. Previous clinical trials have also reported a preponderance of atrophic endometrial histology in postmenopausal women treated with tibolone, and no increased risk of hyperplasia or cancer ,16 For example, in a 2-year, open-label study conducted in 150 women, the endometrium remained atrophic in 92% of subjects taking tibolone, whereas 6.5% showed a change to a weakly proliferative pattern; the endometrial pattern was classified as simple hyperplasia in one woman. 10 Similar results were seen in a study of 168 women treated with tibolone for between 1.5 months and 6 years, with 90% showing no histologic change and the remainder having only mild proliferative changes. 11 In a 5-year observational study with standard and low-dose EPT, tibolone, and raloxifene mean endometrial thickness was not significantly affected by any of the treatment regimens studied. 21 A 2-year prevention of osteoporosis dose-finding study of postmenopausal women demonstrated that the incidence of hyperplasia was similar in tibolonetreated subjects with a uterus (3 of 600) compared with placebo-treated subjects (1 of 146). Three cases of endometrial cancer were observed in that study, but in each case, preexisting carcinoma was later detected when the initial biopsy samples were more extensively examined. 16 The present clinical trial results do not support recent findings in two UK observational studies. A casecontrol study showed an increased risk of endometrial cancer when compared with users of sequential HT (RR = 1.58; 95% CI: ). However, the authors did not present any data on tibolone-only users and reported that their data were fragile and biased and uncontrolled confounding could not be excluded. 22
7 1326 Langer et al The UK Million Women Study (MWS) recently reported follow-up data on 716,738 postmenopausal women 23 who used daily combined estrogen-progestin (22%), sequential estrogen-progestin (45%), estrogen only (4%), and tibolone (9%). During the follow-up period of an average 3.4 years, there were 1320 cases of invasive endometrial cancer. The risk of endometrial cancer was reduced with the use of continuous combined estrogen-progestin (RR = 0.71; 95% CI: ) and increased with estrogen-only (RR = 1.45; 95% CI: ) and tibolone (RR = 1.79; 95% CI: ). In the 5.6-year Women s Health Initiative (WHI) trial of estrogen plus progestin versus placebo, the risk of endometrial cancer was also reduced with estrogen-progestin preparations (RR = 0.81; 95% CI: ). 24 The WHI trial did not include tibolone as one of the comparators. It has been suggested that the tibolone results in observational trials may reflect preferential prescribing. Data from the UK MediPlus Primary Care database indicate that clinicians often prescribed tibolone for women at increased risks for breast and endometrial cancer. 25 Women who were prescribed tibolone in the UK had chronic breast disease, a personal history of breast cancer, previous dysfunctional uterine bleeding, hypertension, and previous uterine operations more often than users of other forms of postmenopausal HT. Most importantly, more women who were prescribed tibolone had a history of (long-term) treatment with unopposed estrogen. These data argue that preferential prescribing of tibolone for women at higher risk of endometrial cancer could explain the apparent increased risk reported in the UK MWS cohort. It is important to qualify the MWS as an observational study. Evidence from clinical trials of tibolone, including the present study, do not support an increase in hyperplasia, which is believed to be a precursor to endometrial carcinoma, nor do they provide support for an excess of carcinoma itself. Double-layer endometrial thickness measured by TVUS in the OPAL study showed a similar increase during the first year of treatment in both the tibolone and the groups. No increase was seen thereafter in either group. The degree of increase was small, with the mean thickness for both groups remaining!4 mm. A previous double-blind, randomized comparison of tibolone with continuous combined 17b-estradiol (2 mg) plus norethisterone acetate (NETA; 1 mg) in 100 postmenopausal women also revealed no differences in endometrial thickness between the groups at any assessment during the 1-year study. 15 However, both oral and transdermal 17b-estradiol sequentially combined with dydrogesterone caused a significant increase in endometrial thickness after 2 years in a randomized study involving 85 women, whereas there were no differences between tibolone and a control group. 26 The long-term effects of tibolone on the endometrium have been compared with those in an age-matched control group in an open-label study that recruited 110 recently postmenopausal women. 27 After 10 years, 57 subjects remained in the study and there was no significant difference between the mean endometrial thickness in the tibolone group (2.2 mm), as compared with the untreated control group (1.8 mm; P =.33). Although the OPAL study revealed no differences between tibolone and continuous combined with respect to endometrial safety, the incidence of vaginal bleeding/spotting episodes, as well as the mean number of bleeding/spotting days and bleeding/spotting episodes, was clearly higher with. This effect was particularly noticeable during the first 3 months of treatment, a period during which women often decide whether or not to continue with HT. In addition, more women given reported vaginal bleeding as an adverse event (26.4% vs 10.8%) and stated it as the reason for premature discontinuation (9% vs 2%). This bleeding profile is consistent with the results from previous studies comparing tibolone with other HT regimens, including continuous combined. In a 1-year, double-blind study involving 501 postmenopausal women, the vaginal bleeding rate was significantly (P!.01) lower with tibolone than with CEE/ MPA during weeks 1 to 12 (23% vs 41%) and weeks 13 to 24 (15% vs 27%). 13 Similar findings were seen in another smaller (n = 68), 1-year, double-blind study, in which significantly fewer women had at least 1 day of bleeding/spotting with tibolone than with CEE/ MPA (6% vs 33%; P!.05). 28 Among comparisons with other HT regimens, two large double-blind studies have shown that tibolone results in a significantly (P!.01) lower incidence of bleeding/spotting episodes than continuous combined 17b-estradiol plus NETA, as well as fewer subject discontinuations due to vaginal bleeding. 13,15 In conclusion, the OPAL study confirmed that tibolone has a favorable bleeding profile compared with, while offering similar endometrial safety. References 1. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women s Health Initiative randomized controlled trial. JAMA 2002;288: Pickar JH, Yeh I, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 2001;76: The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996;275:370-5.
8 Langer et al Lindenfeld EA, Langer RD. Bleeding patterns of the hormone replacement therapies in the postmenopausal Estrogen and Progestin Interventions Trail. Obstet Gynecol 2002;100: Kloosterboer HJ. Tissue-selectivity: the mechanism of action of tibolone. Maturitas 2004;48(Suppl 1):S Modelska K, Cummings S. Tibolone for postmenopausal women: systematic review of randomized trials. J Clin Endocrinol Metab 2002;87: Gooyer de ME, Deckers GH, Schoonen WGEJ, Verheul HAM, Kloosterboer HJ. Receptor profiling and endocrine interactions of tibolone. Steroids 2003;68: Markiewicz L, Gurpride E. In vitro evaluation of estrogenic, estrogen antagonistic and progestogenic effects of a steroidal drug (Org OD14) and its metabolites on human endometrium. J Steroid Biochem 1990;35: Gooyer de ME, Overklift Vaupel Kleyn GT, Smits KC, Ederveen AGH, Verheul HAM, Kloosterboer HJ. Tibolone: a compound with tissue specific inhibitory effects on sulfatase. Mol Cell Endocrinol 2001;183: Vo lker W, Coelingh Bennink HJT, Helmond FA. Effects of tibolone on the endometrium. Climacteric 2001;4: Genazzani AR, Benedek-Jaszmann LJ, Hart DM, Andolsek L, Kicovic PM, Tax L. Org OD 14 and the endometrium. Maturitas 1991;13: Wender MC, Edelweiss MI, Campos LS, de Castro JA, Spritzer PM. Endometrial assessment in women using tibolone or placebo: 1-year randomized trial and 2-year observational study. Menopause 2004;11: Hammar M, Christau S, Nathorst-Böo s J, Rud T, Garre K. A double-blind randomised trial comparing the effects of tibolone and continuous combined hormone replacement therapy in postmenopausal women with menopausal symptoms. Br J Obstet Gynaecol 1998;105: Huber J, Palacios S, Berglund L, Ha nggi W, Sathanandan H, Christau S, et al. The effect of tibolone compared with conjugated equine oestrogens continuously combined with medroxyprogesterone acetate on bleeding rates, quality of life and tolerability in postmenopausal women. Br J Obstet Gynaecol 2002;109: Do ren M, Ru big A, Coelingh Bennink HJT, Holzgreve W. Impact on uterine bleeding and endometrial thickness: tibolone compared with continuous combined estradiol and norethisterone acetate replacement therapy. Menopause 1999;6: Gallagher JC, Baylink DJ, Freeman R, McClung M. Prevention of bone loss with tibolone in postmenopausal women: results of the two randomized, double-blind, placebo-controlled, dose-finding studies. J Clin Endocrinol Metab 2001;365: Bots ML, Evans GW, Riley W, Meijer R, McBride KH, Paskett ED, et al. The Osteoporosis Prevention and Arterial effects of tibolone (OPAL) study: design and baseline characteristics. Cont Clin Trials 2003;24: FDA. Guidance for Clinical Evaluation of Combination Estrogen/progestin-Containing Drug Products Used for Hormone Replacement Therapy of Postmenopausal Women. Rockville (MD): FDA Center for Drug Evaluation and Research; EMEA. Points to Consider on Hormone Replacement Therapy. London (UK): Human Medicines Evaluation Unit; Kurman JR. Blaustein s Pathology of the Female Genital Tract. 4th ed. New York: Springer-Verlag Inc.; 1994: Christodoulakos GE, Botsis DS, Lambrinoudaki IV, Papagianni VD, Panoulis CP, Creatsa MG, et al. A 5-year study on the effect of hormone therapy, tibolone and raloxifene on vaginal bleeding and endometrial thickness. Maturitas 2006;53: de Vries CS, Bromley SE, Thomas H, Farmer RDT. Tibolone and endometrial cancer: a cohort and nested case-control study in the UK. Drug Safety 2005;28: Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005;365: Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. JAMA 2003; 290: Velthuis-te Wierik EJM, Hendricks PT, Boerstoel-Streefland M. Clinical background of women prescribed tibolone or combined estrogen C progestagen therapies: A UK MediPlus Ò study. Climacteric 2004;7: Haenggi W, Bersinger N, Altermatt HJ, Birkhäuser MH. Comparison of transvaginal ultrasonography and endometrial biopsy in endometrial surveillance in postmenopausal hormone replacement therapy users. Maturitas 1997;27: Bruce D, Robinson J, Rymer J. Long-term effects of tibolone on the endometrium as assessed by bleeding episodes, transvaginal scan and endometrial biopsy. Climacteric 2004;7: Chapman MG. The effect of tibolone compared with on bleeding rates and tolerability. Climacteric 2002;5(Suppl 1):214-5.
NEW SELECTIVE TISSUE ESTROGENIC ACTIVITY REGULATOR (STEAR) IN MENOPAUSAL THERAPY IN TAIWAN
 ORIGINAL ARTICLE Tibolone Compliance and Efficacy in Women Living in Taiwan NEW SELECTIVE TISSUE ESTROGENIC ACTIVITY REGULATOR (STEAR) IN MENOPAUSAL THERAPY IN TAIWAN Kuan-Chong Chao*, Peng-Hui Wang, Ming-Shyen
ORIGINAL ARTICLE Tibolone Compliance and Efficacy in Women Living in Taiwan NEW SELECTIVE TISSUE ESTROGENIC ACTIVITY REGULATOR (STEAR) IN MENOPAUSAL THERAPY IN TAIWAN Kuan-Chong Chao*, Peng-Hui Wang, Ming-Shyen
Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals
 Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals Literature Review (January 2009) Hormone Therapy for Women Women's Health
Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals Literature Review (January 2009) Hormone Therapy for Women Women's Health
Postmenopausal hormone therapy and cancer risk
 International Congress Series 1279 (2005) 133 140 www.ics-elsevier.com Postmenopausal hormone therapy and cancer risk P. Kenemans*, R.A. Verstraeten, R.H.M. Verheijen Department of Obstetrics and Gynaecology,
International Congress Series 1279 (2005) 133 140 www.ics-elsevier.com Postmenopausal hormone therapy and cancer risk P. Kenemans*, R.A. Verstraeten, R.H.M. Verheijen Department of Obstetrics and Gynaecology,
HT: Where do we stand after WHI?
 HT: Where do we stand after WHI? Hormone therapy and cardiovascular disease risk Experimental and clinical evidence indicate that hormone therapy (HT) reduces the risk of cardiovascular disease (CVD) Women
HT: Where do we stand after WHI? Hormone therapy and cardiovascular disease risk Experimental and clinical evidence indicate that hormone therapy (HT) reduces the risk of cardiovascular disease (CVD) Women
WHI Estrogen--Progestin vs. Placebo (Women with intact uterus)
 HORMONE REPLACEMENT THERAPY In the historical period it was commonly held that estrogen had two principal benefits to postmenopausal women: 1) To alleviate the constitutional symptoms related to the climacteric
HORMONE REPLACEMENT THERAPY In the historical period it was commonly held that estrogen had two principal benefits to postmenopausal women: 1) To alleviate the constitutional symptoms related to the climacteric
HRT and bone health. Management of osteoporosis and controversial issues. Delfin A. Tan, MD
 Strong Bone Asia V. Osteoporosis in ASEAN (+), Danang, Vietnam, 3 August 2013 Management of osteoporosis and controversial issues HRT and bone health Delfin A. Tan, MD Section of Reproductive Endocrinology
Strong Bone Asia V. Osteoporosis in ASEAN (+), Danang, Vietnam, 3 August 2013 Management of osteoporosis and controversial issues HRT and bone health Delfin A. Tan, MD Section of Reproductive Endocrinology
Effects of TX-001HR on Uterine Bleeding Rates in Menopausal Women with Vasomotor Symptoms
 Effects of TX-001HR on Uterine Bleeding Rates in Menopausal Women with Vasomotor Symptoms Photo (compulsory) Steven R Goldstein, MD 1 ; Ginger D Constantine, MD 2 ; David F Archer, MD 3 ; James H Pickar,
Effects of TX-001HR on Uterine Bleeding Rates in Menopausal Women with Vasomotor Symptoms Photo (compulsory) Steven R Goldstein, MD 1 ; Ginger D Constantine, MD 2 ; David F Archer, MD 3 ; James H Pickar,
LET S START WITH (AND REMEMBER WE ARE TALKING ABOUT LOCAL E2) THERAPUTIC AGENTS: ARE THEY SAFE? Systemic HT/ET. Ospemifene. Local Estrogen Therapy
 THERAPUTIC AGENTS: ARE THEY SAFE? Steven R. Goldstein, M.D. Professor of Obstetrics & Gynecology New York University School of Medicine Director of Gynecologic Ultrasound Co-Director of Bone Densitometry
THERAPUTIC AGENTS: ARE THEY SAFE? Steven R. Goldstein, M.D. Professor of Obstetrics & Gynecology New York University School of Medicine Director of Gynecologic Ultrasound Co-Director of Bone Densitometry
Menopause and HRT. John Smiddy and Alistair Ledsam
 Menopause and HRT John Smiddy and Alistair Ledsam Menopause The cessation of menstruation Diagnosed retrospectively after 1 year of amenorrhoea Average age 51 in the UK Normal physiology - Menstruation
Menopause and HRT John Smiddy and Alistair Ledsam Menopause The cessation of menstruation Diagnosed retrospectively after 1 year of amenorrhoea Average age 51 in the UK Normal physiology - Menstruation
Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia
 University of Groningen Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Long-term effects of tibolone on mammographic density. Key Words: Tibolone, mammographic density, long-term use
 FERTILITY AND STERILITY VOL. 82, NO. 5, NOVEMBER 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. MENOPAUSE Long-term effects
FERTILITY AND STERILITY VOL. 82, NO. 5, NOVEMBER 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. MENOPAUSE Long-term effects
Tibolone: Clinical recommendations and practical guidelines A report of the International Tibolone Consensus Group
 Maturitas 51 (2005) 21 28 Tibolone: Clinical recommendations and practical guidelines A report of the International Tibolone Consensus Group P. Kenemans a,, L. Speroff b for the International Tibolone
Maturitas 51 (2005) 21 28 Tibolone: Clinical recommendations and practical guidelines A report of the International Tibolone Consensus Group P. Kenemans a,, L. Speroff b for the International Tibolone
Postmenopausal hormone therapy - cardiac disease risks and benefits
 Postmenopausal hormone therapy - cardiac disease risks and benefits Tomi S. Mikkola, MD Helsinki University Central Hospital Department of Obstetrics and Gynecology Helsinki, Finland Disclosures Speaker/consulting
Postmenopausal hormone therapy - cardiac disease risks and benefits Tomi S. Mikkola, MD Helsinki University Central Hospital Department of Obstetrics and Gynecology Helsinki, Finland Disclosures Speaker/consulting
AusPharm CE Hormone therapy 23/09/10. Hormone therapy
 Hormone therapy Learning objectives: Assess options to address quality of life and health concerns of menopausal women Outline indications for hormone therapy Counsel women on the risks and benefits of
Hormone therapy Learning objectives: Assess options to address quality of life and health concerns of menopausal women Outline indications for hormone therapy Counsel women on the risks and benefits of
HORMONE THERAPY A BALANCED VIEW?? Prof Greta Dreyer
 HORMONE THERAPY A BALANCED VIEW?? Prof Greta Dreyer -- PART 1 -- Definitions HRT hormone replacement therapy HT genome therapy ERT estrogen replacement therapy ET estrogen EPT estrogen progesterone therapy
HORMONE THERAPY A BALANCED VIEW?? Prof Greta Dreyer -- PART 1 -- Definitions HRT hormone replacement therapy HT genome therapy ERT estrogen replacement therapy ET estrogen EPT estrogen progesterone therapy
Menopause and Cancer risk; What to do overcome the risks? Fatih DURMUŞOĞLU,M.D
 Menopause and Cancer risk; What to do overcome the risks? Fatih DURMUŞOĞLU,M.D Menopause and Cancer How does menopause affect a woman s cancer risk? Ø Menopause does not cause cancer.but risk of developing
Menopause and Cancer risk; What to do overcome the risks? Fatih DURMUŞOĞLU,M.D Menopause and Cancer How does menopause affect a woman s cancer risk? Ø Menopause does not cause cancer.but risk of developing
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH. Ross L. Prentice. Fred Hutchinson Cancer Research Center
 COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
Learning Objectives. Peri menopause. Menopause Overview. Recommendation grading categories
 Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
UPDATE: Women s Health Issues
 UPDATE: Women s Health Issues Renee B. Alexis, MD, MBA, MPH, FACOG Associate Professor Department of OBGYN Kiran C. Patel College of Osteopathic Medicine Disclosure of Conflicts of Interest I have no financial
UPDATE: Women s Health Issues Renee B. Alexis, MD, MBA, MPH, FACOG Associate Professor Department of OBGYN Kiran C. Patel College of Osteopathic Medicine Disclosure of Conflicts of Interest I have no financial
Virtual Mentor Ethics Journal of the American Medical Association November 2005, Volume 7, Number 11
 Virtual Mentor Ethics Journal of the American Medical Association November 2005, Volume 7, Number 11 Clinical Pearl Post Women's Health Initiative Menopausal Women and Hormone Therapy by JoAnn V. Pinkerton,
Virtual Mentor Ethics Journal of the American Medical Association November 2005, Volume 7, Number 11 Clinical Pearl Post Women's Health Initiative Menopausal Women and Hormone Therapy by JoAnn V. Pinkerton,
Disclosures. REPLENISH Trial: Objective and Design. Background Use of compounded bioidentical hormone therapy (CBHT) has become highly prevalent
 17β Estradiol/Progesterone in a Single Oral Softgel Capsule (TX 001HR) Significantly Reduced Moderate to Severe Vasomotor Symptoms without Endometrial Hyperplasia Disclosures Research support: Actavis,
17β Estradiol/Progesterone in a Single Oral Softgel Capsule (TX 001HR) Significantly Reduced Moderate to Severe Vasomotor Symptoms without Endometrial Hyperplasia Disclosures Research support: Actavis,
Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial
 DOI: 10.1111/1471-0528.12598 www.bjog.org Epidemiology Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial
DOI: 10.1111/1471-0528.12598 www.bjog.org Epidemiology Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial
SERMS, Hormone Therapy and Calcitonin
 SERMS, Hormone Therapy and Calcitonin Tiffany Kim, MD Clinical Fellow VA Advanced Women s Health UCSF Endocrinology and Metabolism I have nothing to disclose Thanks to Clifford Rosen and Steven Cummings
SERMS, Hormone Therapy and Calcitonin Tiffany Kim, MD Clinical Fellow VA Advanced Women s Health UCSF Endocrinology and Metabolism I have nothing to disclose Thanks to Clifford Rosen and Steven Cummings
Columbia University Medical Center, New York, NY 2. Clinical Research Center, Eastern Virginia Medical School, Norfolk, VA 3
 17β-Estradiol/Progesterone in a Single Oral Softgel Capsule (TX-001HR) Significantly Reduced Moderate-to-Severe Vasomotor Symptoms without Endometrial Hyperplasia Rogerio A Lobo, MD 1 ; David F Archer,
17β-Estradiol/Progesterone in a Single Oral Softgel Capsule (TX-001HR) Significantly Reduced Moderate-to-Severe Vasomotor Symptoms without Endometrial Hyperplasia Rogerio A Lobo, MD 1 ; David F Archer,
HKCOG Guidelines. Guidelines for the Administration of Hormone Replacement Therapy. Number 2 Revised November BENEFITS OF HRT
 HKCOG Guidelines Guidelines for the Administration of Hormone Replacement Therapy Number 2 Revised November 2006 Published by The Hong Kong College of Obstetricians and Gynaecologists A Foundation College
HKCOG Guidelines Guidelines for the Administration of Hormone Replacement Therapy Number 2 Revised November 2006 Published by The Hong Kong College of Obstetricians and Gynaecologists A Foundation College
MENOPAUSAL HORMONE THERAPY 2016
 MENOPAUSAL HORMONE THERAPY 2016 Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA NICE provides the National Health Service advice on effective, good value healthcare.
MENOPAUSAL HORMONE THERAPY 2016 Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA NICE provides the National Health Service advice on effective, good value healthcare.
WEIGHING UP THE RISKS OF HRT. Department of Endocrinology Chris Hani Baragwanath Academic Hospital
 WEIGHING UP THE RISKS OF HRT V. Nicolaou Department of Endocrinology Chris Hani Baragwanath Academic Hospital Background Issues surrounding post menopausal hormonal therapy (PMHT) are complex given: Increased
WEIGHING UP THE RISKS OF HRT V. Nicolaou Department of Endocrinology Chris Hani Baragwanath Academic Hospital Background Issues surrounding post menopausal hormonal therapy (PMHT) are complex given: Increased
The preferred treatment for osteoporosis
 Alternate Options to Hormone Replacement Therapy for Osteoporosis James R. Shoemaker, DO Andrea B. Klemes, DO This presentation, developed from a symposium lecture at the 40th Annual Convention of the
Alternate Options to Hormone Replacement Therapy for Osteoporosis James R. Shoemaker, DO Andrea B. Klemes, DO This presentation, developed from a symposium lecture at the 40th Annual Convention of the
Menopausal hormone therapy currently has no evidence-based role for
 IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
A cost-utility analysis of low-dose hormone replacement therapy in postmenopausal women with an intact uterus Swift J A, Conway P, Purdie D W
 A cost-utility analysis of low-dose hormone replacement therapy in postmenopausal women with an intact uterus Swift J A, Conway P, Purdie D W Record Status This is a critical abstract of an economic evaluation
A cost-utility analysis of low-dose hormone replacement therapy in postmenopausal women with an intact uterus Swift J A, Conway P, Purdie D W Record Status This is a critical abstract of an economic evaluation
Postmenopausal hormones and coronary artery disease: potential benefits and risks
 CLIMACTERIC 2007;10(Suppl 2):21 26 Postmenopausal hormones and coronary artery disease: potential benefits and risks R. A. Department of Obstetrics and Gynecology, Columbia University, New York, New York,
CLIMACTERIC 2007;10(Suppl 2):21 26 Postmenopausal hormones and coronary artery disease: potential benefits and risks R. A. Department of Obstetrics and Gynecology, Columbia University, New York, New York,
MENOPAUSE. I have no disclosures 10/11/18 OBJECTIVES WHAT S NEW? WHAT S SAFE?
 MENOPAUSE WHAT S NEW? WHAT S SAFE? I have no disclosures Sara Whetstone, MD, MHS OBJECTIVES To describe risks of HT by age and menopause onset To recommend specific HT regimen for women who undergo early
MENOPAUSE WHAT S NEW? WHAT S SAFE? I have no disclosures Sara Whetstone, MD, MHS OBJECTIVES To describe risks of HT by age and menopause onset To recommend specific HT regimen for women who undergo early
HKCOG Guidelines. Guidelines for the Administration of Hormone Replacement Therapy. Number 2 revised January 2003
 HKCOG Guidelines Guidelines for the Administration of Hormone Replacement Therapy Number 2 revised January 2003 published by The Hong Kong College of Obstetricians and Gynaecologists A Foundation College
HKCOG Guidelines Guidelines for the Administration of Hormone Replacement Therapy Number 2 revised January 2003 published by The Hong Kong College of Obstetricians and Gynaecologists A Foundation College
Women s Health: Managing Menopause. Jane S. Sillman, MD Assistant Professor of Medicine Harvard Medical School
 Women s Health: Managing Menopause Jane S. Sillman, MD Assistant Professor of Medicine Harvard Medical School Disclosures I have no conflicts of interest. Learning Objectives 1. Apply strategies to help
Women s Health: Managing Menopause Jane S. Sillman, MD Assistant Professor of Medicine Harvard Medical School Disclosures I have no conflicts of interest. Learning Objectives 1. Apply strategies to help
Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy
 MENOPAUSE Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy James H. Pickar, M.D., a I-Tien Yeh, M.D., b Gloria Bachmann, M.D.,
MENOPAUSE Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy James H. Pickar, M.D., a I-Tien Yeh, M.D., b Gloria Bachmann, M.D.,
Practical recommendations for hormone replacement therapy in the peri- and postmenopause
 CLIMACTERIC 2004;7:in press Practical recommendations for hormone replacement therapy in the peri- and postmenopause Recommendations from an Expert Workshop, February 2004 Henry Burger, Australia; David
CLIMACTERIC 2004;7:in press Practical recommendations for hormone replacement therapy in the peri- and postmenopause Recommendations from an Expert Workshop, February 2004 Henry Burger, Australia; David
North American Menopause Society (NAMS)
 North American Menopause Society (NAMS) 2012 Hormone Therapy Position Statement Cynthia B. Evans, MD Assistant Professor-Clinical Department of Obstetrics and Gynecology The Ohio State University College
North American Menopause Society (NAMS) 2012 Hormone Therapy Position Statement Cynthia B. Evans, MD Assistant Professor-Clinical Department of Obstetrics and Gynecology The Ohio State University College
Potential dangers of hormone replacement therapy in women at high risk
 ESC meeting, Stockholm, August 30, 16.30-18.00, 2010 Potential dangers of hormone replacement therapy in women at high risk Karin Schenck-Gustafsson MD, PhD, FESC Professor, Chief consultant Department
ESC meeting, Stockholm, August 30, 16.30-18.00, 2010 Potential dangers of hormone replacement therapy in women at high risk Karin Schenck-Gustafsson MD, PhD, FESC Professor, Chief consultant Department
Research. Breast cancer represents a major
 Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
Kathryn M. Rexrode, MD, MPH. Assistant Professor. Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School
 Update: Hormones and Cardiovascular Disease in Women Kathryn M. Rexrode, MD, MPH Assistant Professor Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School Overview Review
Update: Hormones and Cardiovascular Disease in Women Kathryn M. Rexrode, MD, MPH Assistant Professor Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School Overview Review
Menopause management NICE Implementation
 Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Effects of tibolone and continuous combined hormone therapy on mammographic breast density and breast histochemical markers in postmenopausal women
 FERTILITY AND STERILITY VOL. 81, NO. 3, MARCH 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. Effects of tibolone and continuous
FERTILITY AND STERILITY VOL. 81, NO. 3, MARCH 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. Effects of tibolone and continuous
5. Summary of Data Reported and Evaluation
 326 5. Summary of Data Reported and Evaluation 5.1 Exposure data Combined estrogen progestogen menopausal therapy involves the co-administration of an estrogen and a progestogen to peri- or postmenopausal
326 5. Summary of Data Reported and Evaluation 5.1 Exposure data Combined estrogen progestogen menopausal therapy involves the co-administration of an estrogen and a progestogen to peri- or postmenopausal
The 6 th Scientific Meeting of the Asia Pacific Menopause Federation
 Predicting the menopause The menopause marks the end of ovarian follicular activity and is said to have occurred after 12 months amenorrhoea. The average age of the menopause is between 45 and 60 years
Predicting the menopause The menopause marks the end of ovarian follicular activity and is said to have occurred after 12 months amenorrhoea. The average age of the menopause is between 45 and 60 years
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Estrogen and progestogen therapy in postmenopausal women
 Estrogen and progestogen therapy in postmenopausal women The Practice Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Hormone
Estrogen and progestogen therapy in postmenopausal women The Practice Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Hormone
Haemostasis, thrombosis risk and hormone replacement therapy
 Haemostasis, thrombosis risk and hormone replacement therapy Serge Motte Brussels 13.05.17 - MY TALK TODAY The coagulation cascade and its regulation Effects of hormone replacement therapy on haemostasis
Haemostasis, thrombosis risk and hormone replacement therapy Serge Motte Brussels 13.05.17 - MY TALK TODAY The coagulation cascade and its regulation Effects of hormone replacement therapy on haemostasis
Management of Perimenopausal symptoms
 Management of Perimenopausal symptoms Serge Rozenberg CHU St Pierre Université libre de Bruxelles Belgium serge_rozenberg@stpierre-bru.be serge.rozenberg@skynet.be Conflict of interest & Disclosure Conflicts
Management of Perimenopausal symptoms Serge Rozenberg CHU St Pierre Université libre de Bruxelles Belgium serge_rozenberg@stpierre-bru.be serge.rozenberg@skynet.be Conflict of interest & Disclosure Conflicts
bleeding Studies naar de diagnostiek van endom triumcarcinoom bij vrouwen met postm nopauzaal bloedverlies. Studies on the
 Studies on the diagnosis of endometria cancer in women with postmenopausal bleeding. Studies naar de diagnostiek va endometriumcarcinoom bij vrouwen m postmenopauzaal bloedverlies. Studies on the diagnosis
Studies on the diagnosis of endometria cancer in women with postmenopausal bleeding. Studies naar de diagnostiek va endometriumcarcinoom bij vrouwen m postmenopauzaal bloedverlies. Studies on the diagnosis
Natural Hormones Replacement An Evidence and Practice Based Approach
 Natural Hormones Replacement An Evidence and Practice Based Approach Andres Ruiz, PharmD, MSc, FACA President/Partner Stonegate Pharmacy PRESENTED BY THE AMERICAN COLLEGE OF APOTHECARIES 2830 SUMMER OAKS
Natural Hormones Replacement An Evidence and Practice Based Approach Andres Ruiz, PharmD, MSc, FACA President/Partner Stonegate Pharmacy PRESENTED BY THE AMERICAN COLLEGE OF APOTHECARIES 2830 SUMMER OAKS
Hormone therapy for menopausal vasomotor symptoms
 Hormone therapy for menopausal vasomotor symptoms Given our available (better) options for treating hot flashes, can we reduce our use of medroxyprogesterone acetate? OBG Manag. 2014;26(7):10,13 15. Robert
Hormone therapy for menopausal vasomotor symptoms Given our available (better) options for treating hot flashes, can we reduce our use of medroxyprogesterone acetate? OBG Manag. 2014;26(7):10,13 15. Robert
Difference between vagifem and yuvafem
 Difference between vagifem and yuvafem Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be considered to reduce the risk of endometrial cancer. Estrogen-alone
Difference between vagifem and yuvafem Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be considered to reduce the risk of endometrial cancer. Estrogen-alone
HRT in Perimenopausal Women. Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College
 HRT in Perimenopausal Women Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College 1 This is the Change But the CHANGE is not a disease 2 Introduction With a marked increase in longevity, women now
HRT in Perimenopausal Women Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College 1 This is the Change But the CHANGE is not a disease 2 Introduction With a marked increase in longevity, women now
Preventing Breast Cancer in HT users by Manuel Neves-e-Castro Portuguese Menopause Society September 2004
 Preventing Breast Cancer in HT users by Manuel Neves-e-Castro Portuguese Menopause Society September 2004 I am also PRO!... because HT does not increase breast cancer, and overall, its benefits out weight
Preventing Breast Cancer in HT users by Manuel Neves-e-Castro Portuguese Menopause Society September 2004 I am also PRO!... because HT does not increase breast cancer, and overall, its benefits out weight
2017 Position Statement of Hormone Therapy of NAMS: overview SHELAGH LARSON, MS, RNC WHNP, NCMP ACCLAIM, JPS HEALTH NETWORK
 2017 Position Statement of Hormone Therapy of NAMS: overview SHELAGH LARSON, MS, RNC WHNP, NCMP ACCLAIM, JPS HEALTH NETWORK WHI the only large, long-term RCT of HT in women aged 50 to 79 years, Drug trail
2017 Position Statement of Hormone Therapy of NAMS: overview SHELAGH LARSON, MS, RNC WHNP, NCMP ACCLAIM, JPS HEALTH NETWORK WHI the only large, long-term RCT of HT in women aged 50 to 79 years, Drug trail
Endometrial Cancer Biopsy of the endometrium Evaluation of women of all ages
 Endometrial Cancer Biopsy of the endometrium Evaluation of women of all ages Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Health System Ann Arbor, Michigan Cancer of the
Endometrial Cancer Biopsy of the endometrium Evaluation of women of all ages Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Health System Ann Arbor, Michigan Cancer of the
Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene)
 EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
Supplementary Online Content
 Supplementary Online Content Gartlehner G, Patel SV, Feltner C, et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: Evidence Report and Systematic Review for
Supplementary Online Content Gartlehner G, Patel SV, Feltner C, et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: Evidence Report and Systematic Review for
Outline. Estrogens and SERMS The forgotten few! How Does Estrogen Work in Bone? Its Complex!!! 6/14/2013
 Outline Estrogens and SERMS The forgotten few! Clifford J Rosen MD rosenc@mmc.org Physiology of Estrogen and estrogen receptors Actions of estrogen on bone BMD, fracture, other off target effects Cohort
Outline Estrogens and SERMS The forgotten few! Clifford J Rosen MD rosenc@mmc.org Physiology of Estrogen and estrogen receptors Actions of estrogen on bone BMD, fracture, other off target effects Cohort
Estetrol, the Next Generation of Hormone Therapy: Results of a Phase 2b Dose-finding Study in Postmenopausal Women (E4 Relief)
 Estetrol, the Next Generation of Hormone Therapy: Results of a Phase 2b Dose-finding Study in Postmenopausal Women (E4 Relief) Prof Wulf H Utian Case Western Reserve University School of Medicine, Cleveland,
Estetrol, the Next Generation of Hormone Therapy: Results of a Phase 2b Dose-finding Study in Postmenopausal Women (E4 Relief) Prof Wulf H Utian Case Western Reserve University School of Medicine, Cleveland,
Effective Health Care Program
 Comparative Effectiveness Review Number 17 Effective Health Care Program Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women Executive Summary Background Breast cancer
Comparative Effectiveness Review Number 17 Effective Health Care Program Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women Executive Summary Background Breast cancer
Low & Ultra Low Dose HRT The Cardiovascular Impact
 Low & Ultra Low Dose HRT The Cardiovascular Impact Wyeth Symposium, Turin 29 th Sept 2007 Nick Panay Consultant Gynaecologist Queen Charlotte s & Chelsea and Chelsea & Westminster Hospitals Honorary Senior
Low & Ultra Low Dose HRT The Cardiovascular Impact Wyeth Symposium, Turin 29 th Sept 2007 Nick Panay Consultant Gynaecologist Queen Charlotte s & Chelsea and Chelsea & Westminster Hospitals Honorary Senior
RALOXIFENE Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA Is the request for the prevention (risk reduction) of breast cancer?
 Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA 16917 GUIDELINES FOR USE 1. Is the request for the prevention (risk reduction) of breast cancer? If yes, continue to #2. If no, approve by HICL
Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA 16917 GUIDELINES FOR USE 1. Is the request for the prevention (risk reduction) of breast cancer? If yes, continue to #2. If no, approve by HICL
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
Menopausal Symptoms. Hormone Therapy Products Available in Canada for the Treatment of. Physician Desk Reference - 3rd Edition
 Hormone Therapy Products Available in Canada for the Treatment of Menopausal Symptoms Physician Desk Reference - 3rd Edition A clinical resource provided to you by: The Society of Obstetricians and Gynaecologists
Hormone Therapy Products Available in Canada for the Treatment of Menopausal Symptoms Physician Desk Reference - 3rd Edition A clinical resource provided to you by: The Society of Obstetricians and Gynaecologists
4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms
 550 4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms 4.1 Absorption, distribution, metabolism and excretion 4.1.1 Humans The pharmacokinetics of the newer progestogens, desogestrel,
550 4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms 4.1 Absorption, distribution, metabolism and excretion 4.1.1 Humans The pharmacokinetics of the newer progestogens, desogestrel,
WHI, HERS y otros estudios: Su significado en la clinica diária. Manuel Neves-e-Castro
 WHI, HERS y otros estudios: Su significado en la clinica diária III Congreso Ecuatoriano de Climaterio Menopausia y Osteoporosis por Manuel Neves-e-Castro (Lisboa-Portugal) Julho, 2003 Machala The published
WHI, HERS y otros estudios: Su significado en la clinica diária III Congreso Ecuatoriano de Climaterio Menopausia y Osteoporosis por Manuel Neves-e-Castro (Lisboa-Portugal) Julho, 2003 Machala The published
Something has changed? The literature from 2008 to present?
 Something has changed? The literature from 2008 to present? Elina Hemminki National Institute for Health and Welfare, Helsinki, Finland Rome Oct 7, 2011: Post-menopausal hormone therapy and women's information
Something has changed? The literature from 2008 to present? Elina Hemminki National Institute for Health and Welfare, Helsinki, Finland Rome Oct 7, 2011: Post-menopausal hormone therapy and women's information
Hormones friend or foe? Undertreatment and quality of life. No conflicts of interest to declare
 Hormones friend or foe? Undertreatment and quality of life Anette Tønnes Pedersen MD, Ph.D. Consultant, Associate professor Dept. Of Gynecology / Fertility Clinic Rigshospitalet No conflicts of interest
Hormones friend or foe? Undertreatment and quality of life Anette Tønnes Pedersen MD, Ph.D. Consultant, Associate professor Dept. Of Gynecology / Fertility Clinic Rigshospitalet No conflicts of interest
25 mg oestradiol implants--the dosage of first choice for subcutaneous oestrogen replacement therapy?
 Research Subcutaneous estrogen replacement therapy. Jones SC. Journal of Reproductive Medicine March, 2004; 49(3):139-142. Department of Obstetrics and Gynecology, Keesler Medical Center, Keesler Air Force
Research Subcutaneous estrogen replacement therapy. Jones SC. Journal of Reproductive Medicine March, 2004; 49(3):139-142. Department of Obstetrics and Gynecology, Keesler Medical Center, Keesler Air Force
Appendix: Reference Table of HT Brand Names
 Appendix: Reference Table of HT Brand Names This is a full reference table in alphabetical order, of Brand Name drugs used in HT. It is the basis for prescription advice throughout this handbook. Drug
Appendix: Reference Table of HT Brand Names This is a full reference table in alphabetical order, of Brand Name drugs used in HT. It is the basis for prescription advice throughout this handbook. Drug
CLINICIAN INTERVIEW CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN
 CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN Nanette K. Wenger, MD, is a recognized authority on women and coronary heart disease. She chaired the US National Heart, Lung, and Blood Institute conference
CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN Nanette K. Wenger, MD, is a recognized authority on women and coronary heart disease. She chaired the US National Heart, Lung, and Blood Institute conference
The Practice Committee of the American Society for Reproductive Medicine,
 FERTILITY AND STERILITY VOL. 81, NO. 1, JANUARY 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. PRACTICE COMMITTEE Estrogen
FERTILITY AND STERILITY VOL. 81, NO. 1, JANUARY 2004 Copyright 2004 American Society for Reproductive Medicine Published by Elsevier Inc. Printed on acid-free paper in U.S.A. PRACTICE COMMITTEE Estrogen
PERIMENOPAUSE. Objectives. Disclosure. The Perimenopause Perimenopause Menopause. Definitions of Menopausal Transition: STRAW.
 PERIMENOPAUSE Patricia J. Sulak, MD Founder, Living WELL Aware LLC Author, Should I Fire My Doctor? Author, Living WELL Aware: Eleven Essential Elements to Health and Happiness Endowed Professor Texas
PERIMENOPAUSE Patricia J. Sulak, MD Founder, Living WELL Aware LLC Author, Should I Fire My Doctor? Author, Living WELL Aware: Eleven Essential Elements to Health and Happiness Endowed Professor Texas
Department of Obstetrics and Gynecology, Osaka Medical College, Takatsuki-city, Osaka , Japan. Pituitary gonadotropin, Clinical managament
 Original Article Adequate Reduction Degree of Pituitary Gonadotropin Level in the Clinical Management of Short-Term Hormone Replacement Therapy of Women with Menopausal Symptoms Department of Obstetrics
Original Article Adequate Reduction Degree of Pituitary Gonadotropin Level in the Clinical Management of Short-Term Hormone Replacement Therapy of Women with Menopausal Symptoms Department of Obstetrics
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
James H. Liu, M.D. Arthur H. Bill Professor Chair of Reproductive Biology Dept of Obstetrics and Gynecology
 Disclosure Estrogen Therapy After Postmenopausal Hysterectomy: Issues, Challenges, Risks/Benefits James H. Liu, M.D. Arthur H. Bill Professor Chair of Reproductive Biology Dept of Obstetrics and Gynecology
Disclosure Estrogen Therapy After Postmenopausal Hysterectomy: Issues, Challenges, Risks/Benefits James H. Liu, M.D. Arthur H. Bill Professor Chair of Reproductive Biology Dept of Obstetrics and Gynecology
Post-menopausal hormone replacement therapy. Evan Klass, MD May 17, 2018
 Post-menopausal hormone replacement therapy Evan Klass, MD May 17, 2018 Are we really still talking about this? Are we really still talking about this? 1960-1975- estrogen prescriptions doubled. Pharma
Post-menopausal hormone replacement therapy Evan Klass, MD May 17, 2018 Are we really still talking about this? Are we really still talking about this? 1960-1975- estrogen prescriptions doubled. Pharma
A Practitioner s Toolkit for the Management of the Menopause
 Medicine, Nursing and Health Sciences A Practitioner s Toolkit for the Management of the Menopause Developed by the Women s Health Research Program School of Public Health and Preventive Medicine Monash
Medicine, Nursing and Health Sciences A Practitioner s Toolkit for the Management of the Menopause Developed by the Women s Health Research Program School of Public Health and Preventive Medicine Monash
Safety of tibolone in the treatment of vasomotor symptoms in breast cancer patients Design and baseline data 'LIBERATE' trial
 Safety of tibolone in the treatment of vasomotor symptoms in breast cancer patients Design and baseline data 'LIBERATE' trial E. Kubista a, P. Kenemans b, J.M. Foidart c, C.H. Yip d, B. von Schoultz e,
Safety of tibolone in the treatment of vasomotor symptoms in breast cancer patients Design and baseline data 'LIBERATE' trial E. Kubista a, P. Kenemans b, J.M. Foidart c, C.H. Yip d, B. von Schoultz e,
The Tissue-Selective Estrogen Complex (TSEC): A Promising New Therapy for Menopausal Symptoms and Postmenopausal Osteoporosis
 Curr Obstet Gynecol Rep (2012) 1:50 58 DOI 10.1007/s13669-012-0007-6 MANAGEMENT OF MENOPAUSE (K-E HUANG, SECTION EDITOR) The Tissue-Selective Estrogen Complex (TSEC): A Promising New Therapy for Menopausal
Curr Obstet Gynecol Rep (2012) 1:50 58 DOI 10.1007/s13669-012-0007-6 MANAGEMENT OF MENOPAUSE (K-E HUANG, SECTION EDITOR) The Tissue-Selective Estrogen Complex (TSEC): A Promising New Therapy for Menopausal
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
5. Summary of Data Reported and Evaluation
 168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
Evidence Synthesis Number 93
 Evidence Synthesis Number 93 Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: Systematic Review to Update the 2002 and 2005 U.S. Preventive Services Task Force Recommendations
Evidence Synthesis Number 93 Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: Systematic Review to Update the 2002 and 2005 U.S. Preventive Services Task Force Recommendations
the cumulative rates of persistence with estrogen replacement therapy, CEE/MPA and tibolone;
 Economic impact of tibolone compared with continuous-combined hormone replacement therapy in the management of climacteric symptoms in postmenopausal women Diaby V, Perreault S, Lachaine J Record Status
Economic impact of tibolone compared with continuous-combined hormone replacement therapy in the management of climacteric symptoms in postmenopausal women Diaby V, Perreault S, Lachaine J Record Status
THE WOMEN S HEALTH INITIAtive
 ORIGINAL CONTRIBUTION JAMA-EXPRESS Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women Principal Results From the Women s Health Initiative Randomized Controlled Trial Writing
ORIGINAL CONTRIBUTION JAMA-EXPRESS Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women Principal Results From the Women s Health Initiative Randomized Controlled Trial Writing
9/27/2017. Disclosure. Selecting Progestogens: Breast, Cardiovascular, and Cognitive Outcomes. James H Liu, MD. Overview
 Disclosure Selecting Progestogens: Breast, Cardiovascular, and Cognitive utcomes James H Liu, MD Arthur H Bill Professor and Chair Departments of Reproductive Biology and bstetrics and Gynecology UH Cleveland
Disclosure Selecting Progestogens: Breast, Cardiovascular, and Cognitive utcomes James H Liu, MD Arthur H Bill Professor and Chair Departments of Reproductive Biology and bstetrics and Gynecology UH Cleveland
Lessons from the WHI HT Trials: Evolving Data that Changed Clinical Practice
 Lessons from the WHI HT Trials: Evolving Data that Changed Clinical Practice JoAnn E. Manson, MD, DrPH, FACP Chief, Division of Preventive Medicine Interim Executive Director, Connors Center Brigham and
Lessons from the WHI HT Trials: Evolving Data that Changed Clinical Practice JoAnn E. Manson, MD, DrPH, FACP Chief, Division of Preventive Medicine Interim Executive Director, Connors Center Brigham and
Estrogens and progestogens
 Estrogens and progestogens Estradiol and Progesterone hormones produced by the gonads are necessary for: conception embryonic maturation development of primary and secondary sexual characteristics at puberty.
Estrogens and progestogens Estradiol and Progesterone hormones produced by the gonads are necessary for: conception embryonic maturation development of primary and secondary sexual characteristics at puberty.
Controversies in Primary Care Pros and Cons of HRT on patients with CHD
 Controversies in Primary Care Pros and Cons of HRT on patients with CHD Claire Bellone MSc Clinical Nurse Specialist Menopause Nottingham Declaration Honorariums & Sponsorship from Bayer, Novonortis, Wyeth
Controversies in Primary Care Pros and Cons of HRT on patients with CHD Claire Bellone MSc Clinical Nurse Specialist Menopause Nottingham Declaration Honorariums & Sponsorship from Bayer, Novonortis, Wyeth
OB/GYN Update: Menopausal Management What Does The Evidence Show? Rebecca Levy-Gantt D.O. PremierObGyn Napa Inc.
 OB/GYN Update: Menopausal Management What Does The Evidence Show? Rebecca Levy-Gantt D.O. PremierObGyn Napa Inc. Napa, California IMPORTANT SAFETY INFORMATION ABOUT EVAMIST: WARNING: ENDOMETRIAL CANCER,
OB/GYN Update: Menopausal Management What Does The Evidence Show? Rebecca Levy-Gantt D.O. PremierObGyn Napa Inc. Napa, California IMPORTANT SAFETY INFORMATION ABOUT EVAMIST: WARNING: ENDOMETRIAL CANCER,
Imvexxy (estradiol) NEW PRODUCT SLIDESHOW
 Imvexxy (estradiol) NEW PRODUCT SLIDESHOW Introduction Brand name: Imvexxy Generic name: Estradiol Pharmacological class: Estrogen Strength and Formulation: 4mcg, 10mcg; vaginal inserts Manufacturer: TherapeuticsMD,
Imvexxy (estradiol) NEW PRODUCT SLIDESHOW Introduction Brand name: Imvexxy Generic name: Estradiol Pharmacological class: Estrogen Strength and Formulation: 4mcg, 10mcg; vaginal inserts Manufacturer: TherapeuticsMD,
The impact of micronized progesterone on the endometrium: a systematic review
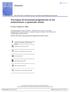 Climacteric ISSN: 1369-7137 (Print) 1473-0804 (Online) Journal homepage: http://www.tandfonline.com/loi/icmt20 The impact of micronized progesterone on the endometrium: a systematic review P. Stute, J.
Climacteric ISSN: 1369-7137 (Print) 1473-0804 (Online) Journal homepage: http://www.tandfonline.com/loi/icmt20 The impact of micronized progesterone on the endometrium: a systematic review P. Stute, J.
Sexual dysfunction: Is it all about hormones?
 Sexual dysfunction: Is it all about hormones? Angelica Lindén Hirschberg, MD, PhD, Professor Department of Women s and Children s Health, Karolinska Institutet and Karolinska University Hospital, Stockholm,
Sexual dysfunction: Is it all about hormones? Angelica Lindén Hirschberg, MD, PhD, Professor Department of Women s and Children s Health, Karolinska Institutet and Karolinska University Hospital, Stockholm,
Abnormal Uterine Bleeding. Richard Dover Specialist gynaecologist
 Abnormal Uterine Bleeding Richard Dover Specialist gynaecologist A pragmatic guide. Wide topic range What s not coming up Precocious puberty Menorrhagia well maybe just a little Topics Adolescents IMB
Abnormal Uterine Bleeding Richard Dover Specialist gynaecologist A pragmatic guide. Wide topic range What s not coming up Precocious puberty Menorrhagia well maybe just a little Topics Adolescents IMB
Menopausal hormone therapy includes various forms, Review
 Review Annals of Internal Medicine Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations Heidi
Review Annals of Internal Medicine Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations Heidi
10/07/18. Conflict of interest statement
 Care: principles, best practices in Europe and how reproductive/sexual health care providers might contribute Petra De Sutter University Hospital Gent Conflict of interest statement My department occasionally
Care: principles, best practices in Europe and how reproductive/sexual health care providers might contribute Petra De Sutter University Hospital Gent Conflict of interest statement My department occasionally
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS
 CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia
 University of Groningen Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Effects of hormone treatment on sexual functioning in postmenopausal women Nijland, Esmé Aurelia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
