Personalisierte Medizin in der Praxis. Dr. Bernhard Kirschbaum DVFA Life Science Conference Frankfurt, 17. Juni 2009
|
|
|
- Dorthy Robertson
- 5 years ago
- Views:
Transcription
1 Personalisierte Medizin in der Praxis Dr. Bernhard Kirschbaum DVFA Life Science Conference Frankfurt, 17. Juni 2009
2 Merck Serono The largest division of the Merck Group Merck Group Revenues FY2008: 7,558m Pharmaceuticals Revenues FY2008: 5,429m Business sectors Chemicals D i v i s i o n s Merck Serono 4,987m Consumer Health Care 442m Liquid Crystals Performance & Life Science Chemicals Cross-divisional functions / Central functions 2
3 Merck Serono Key Facts & Figures Established: January 5, 2007 Business: Innovative small molecules & biopharmaceuticals Employees: >17,500 President: Elmar Schnee Headquarters: Geneva, Switzerland Key growth drivers: 3
4 Therapeutic Area Focus: From Research and Development to Market Research Development Market Oncology Oncology Autoimmune & Inflammatory Diseases (AIID) New Specialist Therapies Osteoarthritis*, Rheumatology*, etc. Neurodegenerative diseases Neurodegenerative diseases Multiple Sclerosis, Parkinson s* Fertility Fertility Endocrinology Endocrinology CM Care & local products * Currently in Development 4
5 Stratified Medicine: Definition Stratified Medicine is about adapting the treatment (molecule, dose, schedule, ) ) according to the patient s s characteristics for better efficacy and less adverse events. Personalized Medicine versus Stratified Medicine Individual patients, e.g. cancer vaccine made from the patient s tumor Patient sub-populations 5
6 Stratified Medicine: Basic Principle 6
7 How Can Stratified Medicine Help the Patients, Clinicians and Healthcare Systems? Patients: More effective & predictive treatment options Clinicians: Therapy adjustment based on disease and patient characteristics Tool to optimize dosing Healthcare Systems: Better return on healthcare expenditure More innovation through products with higher medical value using combination of Diagnostics (Dx) and Pharma (Rx) 7
8 Stratified Medicine as a Means to Contain Health Care Expenditures Senator Obama introduces Genomics and Personalized Medicine Act (2006) President Obama s economical stimulus bill allocates US$1.1bn for comparative effectiveness research (2009) Source: Early identification of non responders has double value for the authorities increasing pressure is expected: Increased patient benefit Heath care cost containment 8
9 Stratified Medicine and Pharma Business New Opportunities / Added Complexity Improved benefit / risk ratio Faster, more directed development Smaller target market Opens doors to new indications + Business Outcome - Higher R&D costs Higher & faster market penetration Better price and reimbursement Complication through Dx testing In practice 9
10 Merck Serono Pipeline as per April 27, 2009 Phase Phase I I Phase Phase II II Phase Phase III III in Registration Aurora Kinase Inhibitor AS Solid tumors and hematological malignancies NHS-IL2-LT Solid tumors DI17E6 Solid tumors Eg 5 inhibitor Solid tumors and hematological malignancies Survivac Cancer Vaccine Solid tumors MEK Inhibitor Solid tumors IMO-2055, TLR9 immunomodulator, Solid tumors Sonepcizumab (ASONEP TM ) Solid tumors Fibroblast Growth Factor 18 Osteoarthritis Atacicept Multiple Sclerosis Erbitux (cetuximab) Breast cancer Tucotuzumab celmoleukin (EMD /huKS-IL2), Small cell lung cancer (SCLC) EMD (hu14.18-il2), immunocytokine Pediatric neuroblastoma EMD (hu14.18-il2), immunocytokine Melanoma Cilengitide SCCHN Cilengitide NSCLC Adecatumumab (MT201) Colorectal Cancer Atacicept Rheumatoid Arthritis Hyperglycosylated FSH Infertility (ART) Rebif New Formulation in CIS (REFLEX) Cladribine tablets Relapsing forms of MS Cladribine tablets in CIS Safinamide Early stage Parkinson s Safinamide Mid-to-late stage Parkinson s Erbitux (cetuximab) Adj Colon Cancer Erbitux (cetuximab) Gastric Cancer Cilengitide Glioblastoma Stimuvax NSCLC Atacicept Lupus Tesamorelin HIV patients with lipodystrophy (US only) Rebif New Formulation Relapsing forms of MS FDA: Submission filed Erbitux (cetuximab) NSCLC 1 st line therapy EMEA: Submission filed Oncology Neurodegenerative Diseases Autoimmune & Inflammatory Diseases Fertility Endocrinology ARX 201 Growth hormone deficiencies 10
11 Merck Serono Pipeline as per April 27, 2009 Phase Phase I I Phase Phase II II Phase Phase III III in Registration Aurora Kinase Inhibitor AS Solid tumors and hematological malignancies NHS-IL2-LT Solid tumors DI17E6 Solid tumors Eg 5 inhibitor Solid tumors and hematological malignancies Survivac Cancer Vaccine Solid tumors MEK Inhibitor Solid tumors IMO-2055, TLR9 immunomodulator, Solid tumors Sonepcizumab (ASONEP TM ) Solid tumors Fibroblast Growth Factor 18 Osteoarthritis Atacicept Multiple Sclerosis Erbitux (cetuximab) Breast cancer Tucotuzumab celmoleukin (EMD /huKS-IL2), Small cell lung cancer (SCLC) EMD (hu14.18-il2), immunocytokine Pediatric neuroblastoma EMD (hu14.18-il2), immunocytokine Melanoma Cilengitide SCCHN Cilengitide NSCLC Adecatumumab (MT201) Colorectal Cancer Atacicept Rheumatoid Arthritis Hyperglycosylated FSH Infertility (ART) v Rebif New Formulation in CIS (REFLEX) Cladribine tablets Relapsing forms of MS Cladribine tablets in CIS Safinamide Early stage Parkinson s Safinamide Mid-to-late stage Parkinson s Erbitux (cetuximab) Adj Colon Cancer Erbitux (cetuximab) Gastric Cancer Cilengitide Glioblastoma Stimuvax NSCLC Atacicept Lupus Tesamorelin HIV patients with lipodystrophy (US only) Rebif New Formulation Relapsing forms of MS FDA: Submission filed Erbitux (cetuximab) NSCLC 1 st line therapy EMEA: Submission filed Oncology Neurodegenerative Diseases Autoimmune & Inflammatory Diseases Fertility Endocrinology Biomarker and or stratification activities ARX 201 Growth hormone deficiencies Stratification is an important part of the Merck Serono strategy. Over 60% of our portfolio include Biomarker programs (predictive, mechanistic, surrogates). 11
12 Stratified Medicine: A Reality at Merck Serono KRAS stratification for Erbitux was key to therapeutic expansion. Two stratified Phase III programs ongoing in Oncology Cilengitide and Stimuvax Implementation of stratified medicine approach across the whole development portfolio Mandatory search for stratification biomarkers in our discovery process. Creation of a specialized function dedicated to stratified medicine. 12
13 KRAS as a Predictive Marker for Erbitux Efficacy in Metastatic Colorectal Cancer (mcrc) Chemotherapy (FOLFIRI) +/- Cetuximab in first line treatment of mcrc Progression-free survival estimate Cetuximab + FOLFIRI 65% of patients KRAS wild-type KRAS mutant 35% of patients FOLFIRI 65% of patients KRAS wild-type KRAS mutant 35% of patients Months Months KRAS patient status influences the efficacy of Erbitux 13
14 Improving tumor responses in mcrc: impact of tailored therapy and patient selection Personalized / tailored therapy a new era in mcrc ITT, intent-to-treat population; wt, wild-type; LLD, liver-limited disease 1. Folprecht et al. ESMO 2008; 2. Van Cutsem et al. ESMO 2008; 3. Bokemeyer et al. ASCO 2008; 4. Van Cutsem et al. ASCO 2008; 5. Saltz et al. WCGIC
15 Benefit-effort ratio Identified 60% pts (KRAS wt) treated with tailored therapy 100% pts treated with non-tailored therapy Target specific patient group (KRAS wt) No indicators to select patients Maximize success in clinical studies Good clinical benefit in stratified patients Avoid unnecessary adverse effects Efficient from health economics perspective Variable success in clinical studies Variable clinical benefit in allcomers Risk of unnecessary adverse effects Inefficient from health economics perspective 15
16 Extension of mcrc Indication to 1 st Line with KRAS Stratification Treatment line 3 rd line 10,200 patients 2 nd line 32,200 patients 1 st line 74,200 patients KRAS mutant 35% KRAS mutant 3,570 patients KRAS mutant patients KRAS mutant 25,970 patients KRAS wild-type 65% KRAS wild-type 6,630 patients KRAS wild-type 20,930 patients KRAS wild-type 48,230 patients Treatment duration 16 weeks 20 weeks 32 weeks Treating 2 nd and 3 rd line, both KRAS wild-type and mutant: Market = 807,200 weeks treatment Treating 1 st line, KRAS wild-type only: Market = 1,543,360 weeks treatment Bigger market enabled by stratification Higher penetration due to improved efficacy *Data for biggest 5 countries in Europe, Source: OncFoundation2007 projections for
17 1 June 2009: Breakthrough for Erbitux in the UK NICE recommendation thanks to KRAS 85 pieces of coverage for NICE (11 in major newspapers/newswires) 17
18 Cilengitide in Glioblastoma Glioblastoma is a rare tumor, difficult to treat. The methylation of the MGMT* gene promoter is a potential stratification marker. Increased likelihood to succeed in Phase III using MGMT stratification. Stratified medicine helps to meet unmet medical needs and to access additional indications *O-Methylguanine-DNA Methyltransferase 18
19 Consequences of Stratified Medicine Implementation on Technology Management New capabilities New complexities Drug Development Study Conduct & BM Logistics Biomarker Technologies Clinical Study Design 19
20 Implementation of Stratified Medicine Has a Strong Impact on Drug Development Process Early integration in discovery and development process is important Good early PK/PD research is essential Competitive advantage to learn the lessons early Analytical validation of diagnostic kit Label consideration based on marker status Clinical validation of diagnostic kit Clinical utility assessment for stratification Design clinical trials Address regulatory requests Ensure proper logstics Label consideration based on trial Identification of stratification Validation of marker assay Discovery Preclinical Development Phase I Phase II Phase III Regulatory Approval Launch 20
21 Requirements for Stratification Kits in the Pharma Business Conditions for good stratification kits from the Rx standpoints: Technical reliability: Selective Sensitive Reproducible Validated in clinical setting Short turn-around Market access: Properly distributed Technically user-friendly Financially affordable Alignment of Rx and Dx is important 21
22 Are the Current Rx/Dx Business Models Challenged in the Context of Stratified Medicine? Rx reimbursement: Value-based system Price is set according to the medical value Innovative drugs increasing the response rate - bringing real added benefit to the patients - will enjoy higher reimbursement Dx reimbursement: Cost-based system Price is set according to the product cost Innovative genotyping kit increasing the response rate will be reimbursed at the level of other genotyping methods Will Dx benefit increasingly from value-based recognition? 22
23 Dx and Rx Interactions Two extreme models: Invasive model Dx develops stratification markers for marketed drugs without Rx partnership Strong impact on Rx business since drug price is unlikely to be renegotiable Kit development risk shouldered by Dx Risk of double marginalization Dx/Rx misalignment might be detrimental for patients benefit Collaborative model Dx co-develops stratification markers with Rx Higher Dx price negotiable at launch Shared development risk, mainly covered by Rx Profit sharing & optimization Aligned commercialization efforts - Best medical benefit for patients Stratified Medicine might result in a new Dx/Rx symbiosis. 23
24 From Complexity to Oversimplification & Back to Complexity Genomics Era Post-Genomics Era 24
25 Contact information Further Contact Merck KGaA Investor Relations Frankfurter Str Darmstadt Germany Fax: Thank you for your kind attention! Dr. Thomas Kornek Claudia Nickolaus Alessandra Heinz Assistant Investor Relations
Merck Serono Expands Cilengitide Development Program
 Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Opportunities and Challenges in the Development of Companion Diagnostics
 Opportunities and Challenges in the Development of Companion Diagnostics E. Patrick Groody, Ph.D. Divisional Vice President Research and Development Abbott Molecular Agenda Value of Personalized Medicine
Opportunities and Challenges in the Development of Companion Diagnostics E. Patrick Groody, Ph.D. Divisional Vice President Research and Development Abbott Molecular Agenda Value of Personalized Medicine
Merck. Berenberg Pennyhill Conference Eva Schäfer-Jansen. Merck Investor Relations
 Merck Berenberg Pennyhill Conference 2012 Eva Schäfer-Jansen Merck Investor Relations December 7, 2012 Disclaimer Remarks All comparative figures relate to the corresponding last year s period. Important
Merck Berenberg Pennyhill Conference 2012 Eva Schäfer-Jansen Merck Investor Relations December 7, 2012 Disclaimer Remarks All comparative figures relate to the corresponding last year s period. Important
Transforming Drug Development at Biopharmaceuticals
 Transforming Drug Development at Biopharmaceuticals Annalisa Jenkins Global Head of Drug Development & Medical Darmstadt, May 15, 2012 Agenda 1. Framework to deliver productivity improvements and value
Transforming Drug Development at Biopharmaceuticals Annalisa Jenkins Global Head of Drug Development & Medical Darmstadt, May 15, 2012 Agenda 1. Framework to deliver productivity improvements and value
Pursuing Novel Pathways in Immunology: Merck KGaA, Darmstadt, Germany Presents New Clinical Data at 2017 ACR/ARHP Annual Meeting
 News Release Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products:
News Release Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products:
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO
 9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
News Release. Safety and Efficacy of Erbitux Confirmed for Combination Trials. Merck Serono. September 25, 2007
 September 25, 2007 Abstracts: 3000, 3001, 3003, 3025, 3055, 3040, 3017, 5501, 0522 Location: 14 th European Congress of Clinical Oncology (ECCO) 2007, Barcelona, Spain Safety and Efficacy of Erbitux Confirmed
September 25, 2007 Abstracts: 3000, 3001, 3003, 3025, 3055, 3040, 3017, 5501, 0522 Location: 14 th European Congress of Clinical Oncology (ECCO) 2007, Barcelona, Spain Safety and Efficacy of Erbitux Confirmed
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS
 MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
European Commission Grants Approval for Mavenclad (Cladribine Tablets)
 Your Contact Friederike Segeberg +49 6151 72-6328 Investor Relations +49 6151 72-3321 August 25, 2017 European Commission Grants Approval for Mavenclad (Cladribine Tablets) First oral short-course treatment
Your Contact Friederike Segeberg +49 6151 72-6328 Investor Relations +49 6151 72-3321 August 25, 2017 European Commission Grants Approval for Mavenclad (Cladribine Tablets) First oral short-course treatment
ASCO Abstract # Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072
 Your Contact Gangolf Schrimpf +49 6151 72-9591 ASCO Abstract Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072 May 18, 2016
Your Contact Gangolf Schrimpf +49 6151 72-9591 ASCO Abstract Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072 May 18, 2016
Global Strategic Partners Merck and Pfizer Finalize Agreement to Co-Promote XALKORI (crizotinib)
 Merck Media: Markus Talanow +49 6151 72 7144 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 News Release April 7, 2015 Not
Merck Media: Markus Talanow +49 6151 72 7144 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 News Release April 7, 2015 Not
Patient Leader Education Summit. Precision Medicine: Today and Tomorrow March 31, 2017
 Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Genomic Health. Kim Popovits, Chairman, CEO and President
 Genomic Health Kim Popovits, Chairman, CEO and President Safe Harbor Statement Various remarks that we make in this presentation that are not historical, including those about our future financial and
Genomic Health Kim Popovits, Chairman, CEO and President Safe Harbor Statement Various remarks that we make in this presentation that are not historical, including those about our future financial and
Pursuing Novel Pathways in Immunology: Merck Presents New Clinical Data at 2017 ACR/ARHP Annual Meeting
 Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products: 25,
Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products: 25,
Only In Few Cases Have Subgroups Been Defined in Advance With Formal Analysis
 Backups Session 4 Only In Few Cases Have Subgroups Been Defined in Advance With Formal Analysis Imatinib and Kit + GIST (prospective, preapproval) Dasatinib and PH + ALL (prospective, preapproval) Maraviroc
Backups Session 4 Only In Few Cases Have Subgroups Been Defined in Advance With Formal Analysis Imatinib and Kit + GIST (prospective, preapproval) Dasatinib and PH + ALL (prospective, preapproval) Maraviroc
Jefferies 2016 Healthcare Conference. Reid Huber, PhD Chief Scientific Officer
 Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
News Release. Merck and Pfizer Advance Clinical Development Program with Two Additional Phase III Trials of Avelumab.
 Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your contact. Merck and Pfizer to Present Data at ASCO for Avelumab, an Investigational Anti-PD-L1 Antibody
 Your contact Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 News Release Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 May
Your contact Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 News Release Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 May
MOLOGEN AG Jefferies 2014 London Healthcare Conference
 Jefferies 2014 London Healthcare Conference Dr. Matthias Schroff Chief Executive Officer London 20 November 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring
Jefferies 2014 London Healthcare Conference Dr. Matthias Schroff Chief Executive Officer London 20 November 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring
Personalized medecine Biomarker
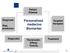 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Myriad Genetics Fiscal First-Quarter 2017 Earnings Call 11/01/2016
 Myriad Genetics Fiscal First-Quarter 2017 Earnings Call 11/01/2016 1 Forward Looking Statements Forward Looking Statements Some of the information presented here today may contain projections or other
Myriad Genetics Fiscal First-Quarter 2017 Earnings Call 11/01/2016 1 Forward Looking Statements Forward Looking Statements Some of the information presented here today may contain projections or other
Personalised Medicine
 Personalised Medicine Panacea or Pandora s box? Dr Tom Lillie Oncology Therapeutic Area Head Amgen October 2012 Amgen is the world s leading biotechnology company employs more than 17,000 staff in 39 countries
Personalised Medicine Panacea or Pandora s box? Dr Tom Lillie Oncology Therapeutic Area Head Amgen October 2012 Amgen is the world s leading biotechnology company employs more than 17,000 staff in 39 countries
News Release. March 29, 2019
 Your Contact Alice McGrail +1 781 738 8791 Investor Relations +49 6151 72 3321 March 29, 2019 FDA Approves MAVENCLAD (Cladribine) Tablets as First and Only Short-Course Oral Treatment for Relapsing-Remitting
Your Contact Alice McGrail +1 781 738 8791 Investor Relations +49 6151 72 3321 March 29, 2019 FDA Approves MAVENCLAD (Cladribine) Tablets as First and Only Short-Course Oral Treatment for Relapsing-Remitting
DS-8201 Strategic Collaboration
 DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
Your Contacts. EMA ODD is an important regulatory milestone for avelumab in metastatic Merkel cell carcinoma (MCC)
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Lisa O Neill +44 1737 331536 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Lisa O Neill +44 1737 331536 Investor
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Innovation and value creation. Severin Schwan, CEO Roche Group. Zurich, January 2015
 Innovation and value creation Severin Schwan, CEO Roche Group Zurich, January 2015 This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words
Innovation and value creation Severin Schwan, CEO Roche Group Zurich, January 2015 This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words
Bank of America Merrill Lynch 2016 Health Care Conference
 Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Systems Thinking in Personalized Medicine
 Systems Thinking in Personalized Medicine Devon C. Campbell Head, Engineering and Systems Novartis Molecular Diagnostics MIT SDM Systems Thinking Conference October 25, 2011 Agenda Personalized medicine
Systems Thinking in Personalized Medicine Devon C. Campbell Head, Engineering and Systems Novartis Molecular Diagnostics MIT SDM Systems Thinking Conference October 25, 2011 Agenda Personalized medicine
ONCOLOGY: WHEN EXPERTISE, EXPERIENCE AND DATA MATTER. KANTAR HEALTH ONCOLOGY SOLUTIONS: FOCUSED I DEDICATED I HERITAGE
 CATALYSTS DRIVING SUCCESSFUL DECISIONS IN LIFE SCIENCES ONCOLOGY: WHEN EXPERTISE, EXPERIENCE AND DATA MATTER. KANTAR HEALTH ONCOLOGY SOLUTIONS: FOCUSED I DEDICATED I HERITAGE AT KANTAR HEALTH, ONCOLOGY
CATALYSTS DRIVING SUCCESSFUL DECISIONS IN LIFE SCIENCES ONCOLOGY: WHEN EXPERTISE, EXPERIENCE AND DATA MATTER. KANTAR HEALTH ONCOLOGY SOLUTIONS: FOCUSED I DEDICATED I HERITAGE AT KANTAR HEALTH, ONCOLOGY
Case 2:09-cv FSH-PS Document 1-3 Filed 08/24/2009 Page 1 of 5. Exhibit B
 Case 2:09-cv-04329-FSH-PS Document 1-3 Filed 08/24/2009 Page 1 of 5 Exhibit B Case 2:09-cv-04329-FSH-PS Document 1-3 Filed 08/24/2009 Page 2 of 5 TO OUR FELLOW SHAREHOLDERS: In 2001 we made significant
Case 2:09-cv-04329-FSH-PS Document 1-3 Filed 08/24/2009 Page 1 of 5 Exhibit B Case 2:09-cv-04329-FSH-PS Document 1-3 Filed 08/24/2009 Page 2 of 5 TO OUR FELLOW SHAREHOLDERS: In 2001 we made significant
News Release. December 9, Not intended for UK-based media
 Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72-9591 Investor Relations: +49 6151 72-3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72-9591 Investor Relations: +49 6151 72-3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Vectibix. Vectibix (panitumumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.85 Subject: Vectibix Page: 1 of 5 Last Review Date: December 2, 2016 Vectibix Description Vectibix
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.85 Subject: Vectibix Page: 1 of 5 Last Review Date: December 2, 2016 Vectibix Description Vectibix
Developing & Commercializing Targeted Small Molecule Drugs in Cancer
 Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics of Cyramza for colorectal cancer (CRC) in the eight major pharmaceutical
REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics of Cyramza for colorectal cancer (CRC) in the eight major pharmaceutical
Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer
 Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer Initiation of expanded Phase II trials in NSCLC triggers US$10 million milestone from AstraZeneca to Chi-Med New study
Savolitinib Global Phase II Trial Initiated in EGFR Mutant Non-Small Cell Lung Cancer Initiation of expanded Phase II trials in NSCLC triggers US$10 million milestone from AstraZeneca to Chi-Med New study
Merck KGaA, Darmstadt, Germany, and Pfizer to Present Avelumab Data in Seven Different Cancers at ASCO Annual Meeting
 News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor Relations:
News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor Relations:
Panitumumab: The KRAS Story. Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd
 Panitumumab: The KRAS Story Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd Clinical Background: panitumumab in mcrc Panitumumab is a fully human IgG2 monoclonal antibody directed
Panitumumab: The KRAS Story Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd Clinical Background: panitumumab in mcrc Panitumumab is a fully human IgG2 monoclonal antibody directed
Accelerate Your Research with Conversant Bio
 Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES
 CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES Fortunato Ciardiello ESMO Past-President 2018-2019 Dipartimento di Medicina di Precisione Università degli
CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES Fortunato Ciardiello ESMO Past-President 2018-2019 Dipartimento di Medicina di Precisione Università degli
Personalised Healthcare. will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D
 Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Idenix Pharmaceuticals Building a Leading Antiviral Franchise. Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston
 Idenix Pharmaceuticals Building a Leading Antiviral Franchise Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston Safe Harbor This presentation includes forward-looking statements
Idenix Pharmaceuticals Building a Leading Antiviral Franchise Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston Safe Harbor This presentation includes forward-looking statements
JP Morgan Healthcare Conference
 JP Morgan Healthcare Conference Lamberto Andreotti Chief Executive Officer January 14, 2014 Forward-Looking Information During this meeting, we will make statements about the Company s future plans and
JP Morgan Healthcare Conference Lamberto Andreotti Chief Executive Officer January 14, 2014 Forward-Looking Information During this meeting, we will make statements about the Company s future plans and
Roche at a Glance An Introduction to our Company. February 2013
 Roche at a Glance An Introduction to our Company February 2013 Basic facts at a glance Founded 1896 in Basel, Switzerland Founding families still hold majority stake Employing 80,000 people Currently active
Roche at a Glance An Introduction to our Company February 2013 Basic facts at a glance Founded 1896 in Basel, Switzerland Founding families still hold majority stake Employing 80,000 people Currently active
Cancer Immunotherapy Survey
 CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
Presentation to AGM 9 November Deborah Rathjen CEO & Managing Director
 Presentation to AGM 9 November 2011 Deborah Rathjen CEO & Managing Director Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward looking" statements within the
Presentation to AGM 9 November 2011 Deborah Rathjen CEO & Managing Director Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward looking" statements within the
MOLOGEN AG. Company Presentation October 2015
 Company Presentation October 2015 Agenda Business Overview Market MGN1703 Cancer Immunotherapy MGN1601 Therapeutic Vaccination against Cancer EnanDIM New Generation of Immunomodulators Key Financials and
Company Presentation October 2015 Agenda Business Overview Market MGN1703 Cancer Immunotherapy MGN1601 Therapeutic Vaccination against Cancer EnanDIM New Generation of Immunomodulators Key Financials and
Myriad Genetics Corporate Presentation 6/4/13
 Myriad Genetics Corporate Presentation 6/4/13 Forward Looking Statement Some of the information presented here today may contain projections or other forward-looking statements regarding future events
Myriad Genetics Corporate Presentation 6/4/13 Forward Looking Statement Some of the information presented here today may contain projections or other forward-looking statements regarding future events
Personalized Medicine Disruptive Technology? David Logan Senior Vice President, Commercial Genomic Health Inc
 1 Personalized Medicine Disruptive Technology? David Logan Senior Vice President, Commercial Genomic Health Inc Safe Harbor Statement This presentation contains forward-looking statements within the meaning
1 Personalized Medicine Disruptive Technology? David Logan Senior Vice President, Commercial Genomic Health Inc Safe Harbor Statement This presentation contains forward-looking statements within the meaning
Credit Suisse 27 th Annual Healthcare Conference
 CHANGING THE COURSE OF HUMAN HEALTH THROUGH BOLD PURSUITS IN SCIENCE Credit Suisse 27 th Annual Healthcare Conference November 14, 2018 Forward-Looking Statements and Adjusted Financial Information This
CHANGING THE COURSE OF HUMAN HEALTH THROUGH BOLD PURSUITS IN SCIENCE Credit Suisse 27 th Annual Healthcare Conference November 14, 2018 Forward-Looking Statements and Adjusted Financial Information This
Merck KGaA, Darmstadt, Germany: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers
 News Release Your Contact Martina Brunner +49 6151 724 3959 Not intended for UK-based media Embargoed until 00:05 CEST, August 31, 2017 ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab:
News Release Your Contact Martina Brunner +49 6151 724 3959 Not intended for UK-based media Embargoed until 00:05 CEST, August 31, 2017 ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab:
Dawson James Conference
 Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE
 37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
Disruptive innovation in molecular diagnostics. Hilde Windels CEO Biocartis 25 March 2017
 Disruptive innovation in molecular diagnostics Hilde Windels CEO Biocartis 25 March 2017 1 One of the key innovations in healthcare in the last decade PERSONALISED MEDICINE or HIGH PRECISION MEDICINE From
Disruptive innovation in molecular diagnostics Hilde Windels CEO Biocartis 25 March 2017 1 One of the key innovations in healthcare in the last decade PERSONALISED MEDICINE or HIGH PRECISION MEDICINE From
MOLOGEN AG. Pioneering Immune Therapy. Annual Results Analysts Call March 25, 2014
 Pioneering Immune Therapy Annual Results 2013 Analysts Call March 25, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
Pioneering Immune Therapy Annual Results 2013 Analysts Call March 25, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
36th Annual J.P. Morgan Healthcare Conference. Kevin Conroy, Chairman and CEO January 9, 2018
 36th Annual J.P. Morgan Healthcare Conference Kevin Conroy, Chairman and CEO January 9, 2018 1 Safe harbor statement This presentation contains forward-looking statements within the meaning of Section
36th Annual J.P. Morgan Healthcare Conference Kevin Conroy, Chairman and CEO January 9, 2018 1 Safe harbor statement This presentation contains forward-looking statements within the meaning of Section
MOLOGEN AG. Pioneering Immune Therapy. Q1 Results 2014 Conference Call. Dr. Matthias Schroff, CEO
 Pioneering Immune Therapy Q1 Results 2014 Conference Call Dr. Matthias Schroff, CEO Berlin, 14 May 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to
Pioneering Immune Therapy Q1 Results 2014 Conference Call Dr. Matthias Schroff, CEO Berlin, 14 May 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies selectively targeting genetic drivers of cancer
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
JY Douillard MD, PhD Professor of Medical Oncology
 ESMO Preceptorship Colorectal Cancer Colorectal ESMO Cancer Preceptorship Vienna 26-27 Program October 2015 Prague May 22-23rd 2014 Review of the ESMO Consensus Conference on metastatic colo-rectal cancer
ESMO Preceptorship Colorectal Cancer Colorectal ESMO Cancer Preceptorship Vienna 26-27 Program October 2015 Prague May 22-23rd 2014 Review of the ESMO Consensus Conference on metastatic colo-rectal cancer
REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Stivarga for colorectal cancer (CRC) in the eight major
REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Stivarga for colorectal cancer (CRC) in the eight major
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
Changes from Third Quarter Flash Report for the Fiscal Year ending March 2015 announced on February 3, 2015 *1: Marketing authorization of Onoact 150
 Annual Flash Report (unaudited) Fiscal Year ended March 31, 2015 Supplemental Information Status of Development Pipeline as of May 12, 2015 I. Main Pipelines Other than ONO-4538 ⅰ. Developments Status
Annual Flash Report (unaudited) Fiscal Year ended March 31, 2015 Supplemental Information Status of Development Pipeline as of May 12, 2015 I. Main Pipelines Other than ONO-4538 ⅰ. Developments Status
Oncology Pipeline Analytics
 Oncology Pipeline Analytics Trials, Drug Classes, Indications, Correlations and Strategies Report Brochure O n c o l o g y P i p e l i n e A n a l y t i c s Greystone Research Associates is pleased to
Oncology Pipeline Analytics Trials, Drug Classes, Indications, Correlations and Strategies Report Brochure O n c o l o g y P i p e l i n e A n a l y t i c s Greystone Research Associates is pleased to
The summit and its purpose
 Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
Merck Announces FDA Approval of KEYTRUDA. Provided to Investors as a Reference
 Merck Announces FDA Approval of KEYTRUDA Provided to Investors as a Reference Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions
Merck Announces FDA Approval of KEYTRUDA Provided to Investors as a Reference Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions
LEVERAGING FDA S ACCELERATED PATHWAYS FOR MARKET ADVANTAGE
 WHITE PAPER LEVERAGING FDA S ACCELERATED PATHWAYS FOR MARKET ADVANTAGE Over the past 15 years, the FDA and Congress developed several accelerated pathways to provide incentives for manufacturers to develop
WHITE PAPER LEVERAGING FDA S ACCELERATED PATHWAYS FOR MARKET ADVANTAGE Over the past 15 years, the FDA and Congress developed several accelerated pathways to provide incentives for manufacturers to develop
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies. Eric H. Rubin, MD Merck Research Laboratories
 Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
National Bank 8th Annual Quebec Conference TSX: IMV. May 30, IMV Inc. All rights reserved.
 National Bank 8th Annual Quebec Conference TSX: IMV May 30, 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV Inc.
National Bank 8th Annual Quebec Conference TSX: IMV May 30, 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV Inc.
Company presentation. Claudio Bordignon, Chairman and CEO. Jefferies Global Healthcare Conference New York, 7 June 2012
 Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Myriad Genetics Corporate Presentation 06/13/2018
 Myriad Genetics Corporate Presentation 06/13/2018 Copyright 2016 2018 Myriad Genetics, Inc., all rights reserved. www.myriad.com. 1 Forward Looking Statements Some of the information presented here today
Myriad Genetics Corporate Presentation 06/13/2018 Copyright 2016 2018 Myriad Genetics, Inc., all rights reserved. www.myriad.com. 1 Forward Looking Statements Some of the information presented here today
JY Douillard MD, PhD Professor of Medical Oncology
 Colorectal Cancer ESMO Preceptorship Program Prague May 22-23rd 2014 Review of the ESMO Consensus Conference on metastatic colo-rectal cancer Basic strategy and groups (RASwt/mut, BRAF mut) JY Douillard
Colorectal Cancer ESMO Preceptorship Program Prague May 22-23rd 2014 Review of the ESMO Consensus Conference on metastatic colo-rectal cancer Basic strategy and groups (RASwt/mut, BRAF mut) JY Douillard
Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017
 Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017 Supplemental nation Status of Development Pipeline as of May 8, 2017. Main Status of Development Pipelines (Oncology) 1. Development Status
Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017 Supplemental nation Status of Development Pipeline as of May 8, 2017. Main Status of Development Pipelines (Oncology) 1. Development Status
Merck ASCO 2015 Investor Briefing
 Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
News Release. Merck: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers. August 31, 2017
 Your Contact Martina Brunner +49 6151 724 3959 August 31, 2017 Not intended for U.K. or U.S. based media ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab: 1227P, 913P, 1377TiP, 882P, 856P;
Your Contact Martina Brunner +49 6151 724 3959 August 31, 2017 Not intended for U.K. or U.S. based media ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab: 1227P, 913P, 1377TiP, 882P, 856P;
Roche Diagnostics. Daniel O Day COO Roche Diagnostics. Vontobel Summer Conference June 22, 2012
 This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words such as believes, expects, anticipates, projects, intends, should, seeks, estimates,
This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words such as believes, expects, anticipates, projects, intends, should, seeks, estimates,
Health Systems Adoption of Personalized Medicine: Promise and Obstacles. Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA
 Health Systems Adoption of Personalized Medicine: Promise and Obstacles Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA Cancer Pharmaceutical Pricing Bach P. NEJM 2009;360:626 Opportunities
Health Systems Adoption of Personalized Medicine: Promise and Obstacles Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA Cancer Pharmaceutical Pricing Bach P. NEJM 2009;360:626 Opportunities
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
KRAS G13D mutation testing and anti-egfr therapy
 KRAS G13D mutation testing and anti-egfr therapy KRAS G13D mutation and anti-egfr therapy Current data do not support a need to specifically identify this mutation for assessing anti-egfr eligibility in
KRAS G13D mutation testing and anti-egfr therapy KRAS G13D mutation and anti-egfr therapy Current data do not support a need to specifically identify this mutation for assessing anti-egfr eligibility in
MERCK KGAA, DARMSTADT, GERMANY
 MERCK KGAA, DARMSTADT, GERMANY 37TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE Belén Garijo, CEO Healthcare Udit Batra, CEO Life Science San Francisco January 7, 2019 Disclaimer Publication of Merck KGaA,
MERCK KGAA, DARMSTADT, GERMANY 37TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE Belén Garijo, CEO Healthcare Udit Batra, CEO Life Science San Francisco January 7, 2019 Disclaimer Publication of Merck KGaA,
International Partnership for Microbicides. Microbicides: New HIV Protection for Women Global Diseases: Voices from the Vanguard
 International Partnership for Microbicides Microbicides: New HIV Protection for Women Global Diseases: Voices from the Vanguard Dr. Zeda Rosenberg February 20, 2007 The Face of HIV Globally Increasingly
International Partnership for Microbicides Microbicides: New HIV Protection for Women Global Diseases: Voices from the Vanguard Dr. Zeda Rosenberg February 20, 2007 The Face of HIV Globally Increasingly
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Roche Diagnostics Daniel O Day COO Roche Diagnostics. Société Générale - The Premium Review Conference, Paris December 2, 2011
 This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words such as believes, expects, anticipates, projects, intends, should, seeks, estimates,
This presentation contains certain forward-looking statements. These forward-looking statements may be identified by words such as believes, expects, anticipates, projects, intends, should, seeks, estimates,
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Summary of Strategic Competitive Analysis and Publication Planning
 Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Presentation of ALK London, 24 May 2011 UBS GLOBAL SPECIALTY PHARMACEUTICALS CONFERENCE : LONDON : 24 MAY 2011
 Presentation of ALK London, 24 May 2011 1 ALK pharma specialist - treatment, prevention and diagnosis of allergies World leader in allergy immunotherapy ~ 33% global market share Expanding Base Business
Presentation of ALK London, 24 May 2011 1 ALK pharma specialist - treatment, prevention and diagnosis of allergies World leader in allergy immunotherapy ~ 33% global market share Expanding Base Business
State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan
 State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan Consultant GI Medical Oncologist National Cancer Centre Singapore Clinician Scientist, Genome Institute of Singapore OS (%) Overall survival
State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan Consultant GI Medical Oncologist National Cancer Centre Singapore Clinician Scientist, Genome Institute of Singapore OS (%) Overall survival
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Citi Global Healthcare Conference
 Citi Global Healthcare Conference Francis Cuss, MB BChir, FRCP Chief Scientific Officer February 24, 2014 1 Forward-Looking Information During this meeting, we will make statements about the Company s
Citi Global Healthcare Conference Francis Cuss, MB BChir, FRCP Chief Scientific Officer February 24, 2014 1 Forward-Looking Information During this meeting, we will make statements about the Company s
Nexavar in Combination with Chemotherapy Demonstrates 74 Percent Improvement in Progression-Free Survival
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Phase II Study in Advanced Breast Cancer: Nexavar in Combination with Chemotherapy Demonstrates 74 Percent Improvement
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Phase II Study in Advanced Breast Cancer: Nexavar in Combination with Chemotherapy Demonstrates 74 Percent Improvement
Rexahn Pharmaceuticals Overview
 Rexahn Pharmaceuticals Overview May 2018 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn s actual results may differ
Rexahn Pharmaceuticals Overview May 2018 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn s actual results may differ
Merrill Lynch Pharmaceutical, Biotechnology & Medical Device Conference New York, February 4, 2004
 Merrill Lynch Pharmaceutical, Biotechnology & Medical Device Conference New York, February 4, 2004 Disclaimer 2 This presentation contains forward-looking statements, i.e., current estimates or expectations
Merrill Lynch Pharmaceutical, Biotechnology & Medical Device Conference New York, February 4, 2004 Disclaimer 2 This presentation contains forward-looking statements, i.e., current estimates or expectations
Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges
 Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges Frédérique Nowak - 21 october 2015 "Putting Science into Standards event:
Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges Frédérique Nowak - 21 october 2015 "Putting Science into Standards event:
Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib in Patients with Germline BRCA-Positive Advanced Breast Cancer
 For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
