Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
|
|
|
- Esther Park
- 5 years ago
- Views:
Transcription
1 Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline Authors C. Spada 1, C. Hassan 1, J. P. Galmiche 2, H. Neuhaus 3, J. M. Dumonceau 4, S. Adler 5, O. Epstein 6,G.Gay 7, M. Pennazio 8, D. K. Rex 9, R. Benamouzig 10, R. de Franchis 11, M. Delvaux 7, J. Devière 12, R. Eliakim 13, C. Fraser 14, F. Hagenmuller 15, J. M. Herrerias 16, M. Keuchel 17, F. Macrae 18, M. Munoz-Navas 19, T. Ponchon 20, E. Quintero 21, M. E. Riccioni 1, E. Rondonotti 22,R.Marmo 23, J. J. Sung 24, H. Tajiri 25, E. Toth 26, K. Triantafyllou 27, A. Van Gossum 12, G. Costamagna 1 Institutions Institutions are listed at the end of article. Bibliography DOI /s Published online: 2012 Endoscopy Georg Thieme Verlag KG Stuttgart New York ISSN X Corresponding author C. Spada, MD Digestive Endoscopy Unit Università Cattolica del Sacro Cuore Largo A. Gemelli Roma Italy Fax: cristianospada@gmail.com 1. Introduction Colorectal cancer (CRC) represents a major cause of morbidity and mortality in Western countries [1]. Although CRC prevention, based on the identification and removal of precancerous adenomatous polyps as well as on downstaging of CRC that has already developed, has been shown to be highly effective in high quality randomized and observational studies [2 4], uptake in CRC screening programs is still disappointingly low, especially when compared with the high rate of participation in breast, cervical, and prostate screening programs [5, 6]. Colon capsule endoscopy (CCE; Given Imaging Ltd, Yoqneam, Israel) is a new endoscopic technique which might have the potential for improving uptake of CRC screening [7 20]. It represents a minimally invasive and painless imaging system that allows exploration of the colon without the need for sedation and gas insufflation. Recently, a second-generation CCE device has been released that provides a higher number of images per second and a larger viewing angle [18 20]. Although CCE technology is now available on the market in many countries, its clinical indications as well as CCE reporting and post-cce work-up have not yet been standardized. PillCam colon capsule endoscopy (CCE) is an innovative noninvasive, and painless ingestible capsule technique that allows exploration of the colon without the need for sedation and gas insufflation. Although it is already available in European and other countries, the clinical indications for CCE as well as the reporting and work-up of detected findings have not yet been standardized. The aim of this evidence-based and consensusbased guideline, commissioned by the European Society of Gastrointestinal Endoscopy (ESGE) is to furnish healthcare providers with a comprehensive framework for potential implementation of this technique in a clinical setting. 2. Methods The European Society of Gastrointestinal Endoscopy (ESGE) commissioned this Guideline, which was then endorsed by its Governing Board. The guideline development process was similar to that used in creating other ESGE guidelines [21]: it included meetings, telephone conferences, and electronic media-based discussions among subgroups and members of the entire committee during October 2010 and March Subgroups were formed, each being charged with a series of clearly defined key questions (see Appendix e1, available online). The committee chair (J.P.G.) worked with subgroup leaders (S.A., O.E., G.G., M.P.) to identify pertinent search terms that always included, as a minimum, colon capsule endoscopy as well as words pertinent to specific key questions. Searches were performed on Medline (via Pubmed), the Cochrane Library, Embase, and the internet. Articles were first selected by title; their relevance was then confirmed by review of the corresponding manuscript, and publications with content that was considered irrelevant were excluded. Additional articles, including abstracts presented at international conferences, were identified by manually searching the reference lists of retrieved papers. A central repository of selected literature was made available to all members of the guideline development group. Evidence tables were generated for each key question, summarizing the level of evidence of the available studies. The number of articles selected for each task force is indicated in the Evidence
2 Table1 Definitions of categories for evidence levels and recommendation grades used in this Guideline [21]. Levels of evidence 1 ++ High quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias 1 + Well conducted meta-analyses, systematic reviews, or RCTs with a low risk of bias 1 Meta-analyses, systematic reviews, or RCTs with a high risk of bias 2 ++ High quality systematic reviews of case control or cohort studies High quality case control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal 2 + Well conducted case control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal 2 Case control or cohort studies with a high risk of confounding or bias and a significant risk that the relationship is not causal 3 Nonanalytic studies, e. g. case reports, case series 4 Expert opinion Grades of recommendation A At least one meta-analysis, systematic review, or RCT rated as 1 ++, and directly applicable to the target population; or A body of evidence consisting principally of studies rated as 1+, directly applicable to the target population, and demonstrating overall consistency of results B A body of evidence including studies rated as 2++, directly applicable to the target population, and demonstrating overall consistency of results; or Extrapolated evidence from studies rated as 1++or 1+ C A body of evidence including studies rated as 1 or 2+, directly applicable to the target population and demonstrating overall consistency of results; or Extrapolated evidence from studies rated as 2++ D Evidencelevel2 or 3 or 4; or Extrapolated evidence from studies rated as 2+ RCT, randomized controlled trial. Note: The grade of recommendation relates to the strength of the evidence on which the recommendation is based. It does not reflect the clinical importance of the recommendation. table (see " Appendix e1 available online). For important outcomes, articles were individually assessed by using the Method for Evaluating Research and Guideline Evidence checklists as amended by the Scottish Intercollegiate Guidelines Network (SIGN) [22]. Evidence levels and recommendation grades used in this guideline were those recommended by the amended SIGN system ( " Table 1) [22]. Subgroups agreed by online communication on draft proposals that were presented to the entire group for general discussion during a meeting held in February 2011 (Tarquinia, Italy). During the meeting and following discussion, competing proposals for wording of recommendations or assigning strength of evidence were resolved by formal voting. Consensus was defined as agreement on a statement by 66 % of participants; this was then termed a consensus statement, and numbered for convenience in the document. Where the guideline development group was unable to achieve a 66 % agreement on a specific recommendation, the difference of opinion was formally recorded and the reasons for dissent were noted. The results of that discussion were incorporated into the subsequent guideline version and again discussed using . Searches were re-run in March Studies that were published after this date were not considered for inclusion. In March 2011, the final draft was sent to all individual ESGE members. After incorporation of comments made by the individual ESGE members, the manuscript was then sent to the Editorial Board of the journal Endoscopy for critique and international peer review. It underwent international peer review and the final version was approved by all members of the guideline development group. Evidence statements and recommendations are stated in italics, key evidence statements and recommendations are in bold. This Guideline was issued in 2011 and will be considered for review in 2014, or sooner if new evidence becomes available. Any updates to the guideline in the interim period will be noted on the ESGE website: 3. Summary of statements and recommendations Indications and contraindications for colon capsule endoscopy (CCE) CCE is feasible and safe and appears to be accurate when used in average-risk individuals (Evidence level 2 + +, Recommendation grade C). There is a lack of specific studies based in the setting of screening. CCE screening may be cost-effective if it increases screening uptake compared with colonoscopy (Evidence level 4, Recommendation Patients at high risk for CRC, because of alarm symptoms or signs, or a family or personal history of CRC, are at increased risk of advanced colorectal neoplasia and cancer. These patients should be referred to colonoscopy. However, in patients for whom colonoscopy is inappropriate or not possible, the use of CCE could be discussed with the patient CCE is a feasible and safe tool for visualization of the colonic mucosa in patients with incomplete colonoscopy and without stenosis (Evidence level 3, Recommendation Randomized studies comparing CCE with radiological imaging or conventional endoscopic modalities are needed to confirm the efficacy of CCE in this setting and to better define the patients for whom CCE is most suitable To date, there are insufficient data to support the use of CCE in the diagnostic work-up or in the surveillance of patients with suspected or known inflammatory bowel disease (IBD) (Evidence level 4, Recommendation On the basis of preliminary data, CCE may be useful to monitor inflammation in ulcerative colitis, which may help to guide therapy (Evidence level 4, Recommendation Contraindications for CCE are similar to those for small-bowel capsule endoscopy. The use of sodium phosphate as a booster should be avoided in patients at increased risk of sodium phosphate toxicity. Other kinds of booster preparations are under investigation and may be considered in patients at increased risk of sodium phosphate toxicity The risk of capsule retention with CCE is very low. In the case of capsule retention either in the small or the large bowel, endoscopy or surgery can be considered on the basis of the clinical background (EL, 3 Recommendation Bowel preparation for CCE Patients must follow a liquid diet the day before the procedure, whilst the role of low-residue diet is yet to be clarified (Evidence level 4, Recommendation A total of 4 L of polyethylene glycol should be administered before the CCE procedure. A split-dosage regimen with intake the day before and on the day of the examination seems advisable to increase the tolerability and the efficacy of the preparation (Evidence level 4, Recommendation
3 Table2 Diagnostic accuracy of colon capsule endoscopy (CCE) for significant findings (polyps 6mm in size, or 3 polyps). Author, year Patients with significant findings, n (%) Sensitivity Specificity PPV NPV Eliakim, 2006 [7] 1 16 (19) 50 % 82 % 40 % 88 % Schoofs, 2006 [8] 1 13 (36) 77 % 70 % 59 % 84 % Van Gossum, 2009 [9] 1 87 (27) 64 % 84 % 60 % 86 % Gay, 2010 [11] 1 67 (53) 76 % 76 % 78 % 74 % Sacher-Huvelin, 2010 [12] (21) 39 % 88 % 47 % 85 % Pilz, 2010 [13] 1 6(10) 50% 75% 19% 93% Spada, 2011 [14] 1 13 (33) 62 % 85 % 67 % 82 % Spada, 2011 [15] 1 7 (15) 100% 95 % 78 % 100 % Eliakim, 2009 [18] 2 18 (19) 89 % 76 % 46 % 97 % Spada, 2011 [20] 2 45 (41) 84 % 64 % 62 % 85 % All studies 384 (20) 63 % 83 % 57 % 86 % CCE-1 studies 321 (19) 58 % 85 % 57 % 86 % CCE-2 studies 63 (30) 86 % 71 % 56 % 92 % PPV, positive predictive value; NPV; negative predictive value. 1 First-generation colon capsule endoscopy (CCE-1) studies. 2 Second-generation (CCE-2) studies. Booster preparations need to be given to improve capsule egestion rates and to complete visualization of the colonic mucosa (Evidence level 4, Recommendation In patients without contraindications to sodium phosphate, boosters based on sodium phosphate should be used (Evidence level 1+, Recommendation grade B). Lowdose sodium phosphate boosts have been shown to achieve an adequate CCE egestion rate and should be preferred over higher sodium phosphate doses During the CCE procedure, the use of prokinetics is recommended when the capsule stays in the stomach longer than 1 hour (Evidence level 4, Recommendation Written and oral information about CCE bowel preparation should be delivered by healthcare professionals (physician or nurse) involved in gastroenterology and with experience regarding the technique Reporting and work-up of CCE results The CCE report should provide information on the quality of preparation, technical details of the examination, completeness of the procedure, and on the significant findings (polyps/masses 6mm or 3 polyps, irrespective of size) in a standardized fashion (Evidence level 4, Recommendation In particular, size, morphology, and location should be separately described for each polyp. Extracolonic findings should also be reported when clinically meaningful Patients found to have a polyp 6mm at CCE, as well as those with 3 polyps irrespective of size, should be sent for post-cce colonoscopy for polypectomy Patients without significant findings at CCE should repeat CCE or a different screening test after 5 years, unless bowel preparation at CCE was inadequate If overall bowel preparation was inadequate or CCE was incomplete, repetition of CCE or another colon imaging study should be considered 4. Indications and contraindications for colon capsule endoscopy (CCE) CCE is feasible and safe and appears to be accurate when used in average-risk individuals (Evidence level 2 + +, Recommendation grade C). There is a lack of specific studies based in the setting of screening. CCE screening may be cost-effective if it increases screening uptake compared with colonoscopy (Evidence level 4, Recommendation In the validation of noninvasive colorectal imaging tests for CRC screening and diagnosis, detection of advanced neoplasia has been regarded as a reliable intermediate end point. For instance, computed tomography (CT) colonography and immunochemical fecal tests have been extensively validated using this end point [23, 24]. The validation process generally consists in assessing the accuracy of noninvasive tests for advanced neoplasia, using colonoscopy as an independent gold standard [25]. Because noninvasive colorectal imaging tests cannot provide a histological diagnosis, morphological criteria (i. e. polyp/mass 6mm in size, or 3 polyps) are accepted as surrogate markers of advanced neoplasia (high grade dysplasia, presence of villous component, or malignancy) [7, 8, 23]. Matching algorithms, based on size matching between the noninvasive test and the reference standard, have usually been incorporated in these studies to assure a reliable assessment of the new tests [7, 8,23]. Of note, the accuracy values shown in these studies are independent from the prevalence of the disease. This means that values assessed in a population enriched with regard to advanced neoplasia remain accurate in settings where different prevalences may be expected, such as in asymptomatic average-risk populations. The average sensitivity of the first generation of CCE (CCE-1) devices for significant findings ( 6mm size, or 3 polyps irrespective of size) was 58% [7 17], substantially improving to 86 % with the second generation CCE (CCE-2) devices ( " Table 2) [18 20]. These rates are higher than the 50% cutoff for sensitivity that has been adopted by the American Cancer Society to define a test acceptable for screening purposes [25], and are comparable or superior to those of other noninvasive screening tests [26]. It should be emphasized that the ultimate aim of CRC screening is not to detect all the prevalent CRC (i.e. 100 % sensitivity), but to
4 reduce the prevalence significantly, also taking into account other variables, such as uptake, safety, costs, and complications. The apparently low specificity shown by CCE (see " Table 2; including CCE-2) has been shown to be mainly related to size mismatching between CCE and colonoscopy, resulting in an overestimation of size by CCE for diminutive lesions [18 20]. Although diminutive polyps do not represent a primary target for noninvasive imaging tests (see the work-up section below), it cannot be excluded at this stage that their detection may nevertheless be associated with CRC prevention, so that this low specificity should not be considered to be a significant obstacle to CCE implementation in a screening setting [20]. CCE has consistently been shown to be a very safe procedure: no major complication has been reported in over 1500 procedures, of which around 40 % were in asymptomatic individuals [7 20]. CCE also appears to be a feasible procedure, with a very low rate of technical failures (i.e. 3 %) and a high capsule excretion rate of about 90 % [7 20]. On the other hand, no data are available on the possible uptake of CCE in a screening context. A previous cost effectiveness analysis has compared first-generation CCE with colonoscopy in a screening setting [27]. Although CCE was not a cost-effective alternative when equal uptake was assumed, it became an efficient option when it was assumed that uptake of CCE would be higher than that of colonoscopy for CRC screening, a premise that has not been demonstrated yet. Patients with non-alarm symptoms do not appear to be at increased risk of colorectal neoplasia [28]. For this reason, noninvasive tests may be proposed in this setting as an alternative to colonoscopy [29]. Among noninvasive tests, however, imaging tests might be preferred over non-imaging ones (i. e. fecal tests), because of the ability, that may be regarded as clinically useful, to detect non-neoplastic conditions (e. g. vascular malformations). Among noninvasive imaging tests, CCE may be applicable in the context of screening because of the abovementioned considerations of feasibility, safety and accuracy. Although CCE has been shown to detect diverticular disease as well as inflammatory changes of the bowel mucosa [7 20], no study has specifically addressed its performance in detecting non-neoplastic alterations. Patients at high risk for CRC, because of alarm symptoms or signs, or a family or personal history of CRC, are at increased risk of advanced colorectal neoplasia and cancer. These patients should be referred for colonoscopy. However, in patients for whom colonoscopy is inappropriate or not possible, the use of CCE could be discussed with the patient Patients with alarm symptoms (rectal bleeding, anemia, weight loss, intestinal subocclusion) are at increased risk of colorectal neoplasia. In particular, these patients are at a 5 10-fold increased risk of malignancy [30]. Patients with a positive fecal blood test are also at increased risk for CRC and advanced neoplasia [24 26]. In this setting, a test with a very high sensitivity is desirable and, therefore, colonoscopy or, alternatively, computed tomography (CT)-colonography or barium enema should be considered as the primary options [31]. CCE sensitivity for cancer is still unclear. As shown in " Table 3, the sensitivity of first-generation CCE (CCE-1) for CRC detection was suboptimal compared with that of colonoscopy [7 17]. Although CCE-2 would seem to be more sensitive (i. e. 4 cancers detected out of 4 true-positive cases, thus sensitivity 100 %) [18, 20] than CCE-1 for malignancy detection, data from a larger number of patients are needed before recommending the use of CCE-2 in this setting. Moreover, Table3 True-positive and false-negative diagnoses of histologically verified colorectal cancer reported with colon capsule endoscopy (CCE). Author, year Patients with cancer, n First-generation CCE studies Eliakim, 2006 [7] Schoofs, 2006 [8] Van Gossum, 2009 [9] Sieg, 2009 [10] Gay, 2010 [11] Sacher-Huvelin, 2010 [12] Pilz, 2010 [13] Spada, 2011 [14] Spada, 2011 [15] Second-generation CCE studies Eliakim, 2009 [18] Spada, 2011 [20] Truepositive Falsenegative subocclusive symptoms represent a contraindication to CCE because of the high risk of capsule retention. CCE is a feasible and safe tool for visualization of the colonic mucosa in patients with incomplete colonoscopy and without stenosis (Evidence level 3, Recommendation Randomized studies comparing CCE with radiological imaging or conventional endoscopic modalities are needed to confirm the efficacy of CCE in this setting and to better define the patients for whom CCE is most suitable The cecal intubation rate in colonoscopy has been reported to range widely from 60 % to over 90 % [32, 33]. Because of the risk of missed neoplasia in the nonvisualized colon, further tests may be advisable depending on patients risk factors (i. e. left-sided polyps, family history, clinical indication). Such tests usually consist of radiological imaging (CT-colonography or barium enema) and/or colonoscopy using different endoscopes (pediatric or variable-stiffness colonoscopes, balloon-assisted enteroscopes) or with anaesthetist assistance [34]. The most frequent causes of incomplete colonoscopy include left-sided angulations caused by diverticular disease or post-surgical adhesions, extensive looping, or stenosing colorectal cancer [35]. In preliminary studies, CCE-1 has been shown to be feasible in this setting, although results for complete visualization of the colorectal mucosa have been conflicting, varying between 50% and 89 % in small numbers of patients [36 38]. To date, there are insufficient data to support the use of CCE in the diagnostic work-up or in the surveillance of patients with suspected or known inflammatory bowel disease (IBD) (Evidence level 4, Recommendation On the basis of preliminary data, CCE may be useful to monitor inflammation in ulcerative colitis, which may help to guide therapy (Evidence level 4, Recommendation Inflammatory bowel diseases (IBDs) frequently involve the colorectal mucosa, with lesions reported in this location in % of patients with Crohn s disease and virtually always in those with ulcerative colitis [39]. Endoscopy is a suitable test for IBD diagnosis, because of its high sensitivity for the detection of mucosal lesions and its ability to sample the digestive mucosa. However, biopsy is usually required to improve test specificity, because of the possibility of false-positive results [40]. For this reason, colonoscopy is primarily recommended in this setting [41]. It is also recognized that treatment of ulcerative colitis should be
5 tailored to the severity of colonic inflammation and that longlasting ulcerative colitis is associated with an increased risk of colorectal dysplasia and cancer [42, 43]. Small-bowel capsule endoscopy provides a very high diagnostic yield for small-bowel mucosal lesions and its use is recommended in specific scenarios of IBD [40]. Similarly, CCE could be used to identify mucosal changes in the colorectal mucosa. To date, the role of CCE in IBD has been evaluated in only one series [44]. Specifically, CCE has been compared with colonoscopy with the aim of evaluating its accuracy in monitoring colonic inflammation in patients with suspected or known ulcerative colitis. In this preliminary experience, CCE yielded encouraging results for detecting active ulcerative colitis (i. e., sensitivity 77 %, specificity 78 %) and substantial agreement with colonoscopy [44]. Contraindications for CCE are similar to those for small-bowel capsule endoscopy. The use of sodium phosphate as a booster should be avoided in patients at increased risk of sodium phosphate toxicity. Other kinds of booster preparations are under investigation and may be considered in patients at increased risk of sodium phosphate toxicity CCE entails a similar technology to small-bowel capsule endoscopy, so that contraindications to small-bowel capsule endoscopy apply to CCE. The most frequent contraindications to capsule endoscopy are: dysphagia or swallowing disorder, prior major abdominal surgery of the gastrointestinal tract, known or suspected bowel obstruction, presence of a cardiac pacemaker or other implanted electromedical devices, and pregnancy [45]. In contrast to small-bowel capsule endoscopy, no data on safety of CCE in pediatric patients are available. In addition to contraindications inherent to the capsule device, further contraindications can exist because the CCE procedure may imply the administration of sodium phosphate, although at lower doses than those usually used for bowel preparation for colonoscopy [18, 20]. However, to be prudent, all the contraindications to use of sodium phosphate should be extended to CCE. In particular, sodium phosphate use should be avoided in elderly persons as well as in patients with hypovolemia, baseline kidney disease, bowel obstruction, or active colitis, and also in those who are using medications that affect renal perfusion or function [46]. In patients with contraindications to sodium phosphate, alternative booster agents (see section on bowel preparation) may be considered. The risk of capsule retention with CCE is very low. In the case of capsule retention either in the small or the large bowel, endoscopy or surgery can be considered on the basis of the clinical background (Evidence level 3, Recommendation Capsule retention is a major complication of small-bowel capsule endoscopy, with an overall incidence of 1 2 %, although reported rates widely vary between 0 % and 21 % depending on the indication for the examination [45]. Available studies with CCE, despite the larger capsule size, have not reported any capsule retention or sticking either in the small bowel or in the colon [7 20]. Of note, a case of CCE where a stuck colonic capsule was passed through a malignant colonic stricture and removed with the aid of flexible colonoscopy has recently been reported [47]. Removal of colonic capsule retained in the small bowel may require surgery, although removal by device-assisted enteroscopy may be an option [45]. Patients symptoms and the possible pathology identified by CCE must also be taken into account in choosing the most appropriate treatment for capsule retention. Albeit infrequently, mild nausea, abdominal pain, and vomiting may occur during CCE and are attributed to colon preparation [7 18,20]. Antiemetic drugs have been empirically used to manage these situations. 5. Bowel preparation for CCE Patients must follow a liquid diet the day before the procedure, whilst the role of low-residue diet is yet to be clarified (Evidence level 4, Recommendation For conventional colonoscopy, a low-residue diet is often recommended during the 3 to 5 days before the bowel cleansing protocol itself. The aim is to decrease the amount of solid stool in the colon and thus reinforce the lavage effect of polyethylene glycol (PEG) solutions. This recommendation is reinforced when lowvolume cleansing protocols are proposed. However, very few studies have adequately addressed the efficacy of this low-residue diet before colonoscopy. On the other hand, when CCE is considered, recent experience seems to suggest that a clear liquid diet on the day before the procedure might improve the quality of bowel cleansing and consequently the diagnostic yield of CCE [9, 11, 12, 18, 20]. Therefore, diet recommendations (i. e., liquid diet the day before, low residue diet 3 5 days before CCE) have generally been adopted in the studies published so far. However, the prescription of diet restrictions over several days before the CCE may impair patient compliance with the cleansing protocol. A total of 4L of PEG should be administered before the CCE procedure. A split-dosage regimen with intake the day before and on the day of the examination seems advisable to increase the tolerability and the efficacy of the preparation (Evidence level 4, Recommendation Booster preparations need to be given to improve capsule egestion rates and to complete visualization of the colonic mucosa (Evidence level 4, Recommendation In patients without contraindications to sodium phosphate, boosters based on sodium phosphate should be used (Evidence level 1+, Recommendation grade B). Lowdose sodium phosphate boosts have been shown to achieve an adequate CCE egestion rate and should be preferred over higher sodium phosphate doses During CCE, as the capsule is not equipped to insufflate the colon, aspirate liquids, wash the mucosal surface, and move actively along the gut, the cleansing protocol cannot be restricted to the time before the procedure but must be continued intraprocedurally [7 15, 18,20]. The cleansing protocol for CCE aims at: (i) adequately cleansing the colonic mucosa, (ii) filling the colonic lumen with clear liquids to improve mucosal visualization and to decrease the number of air bubbles, and (iii) facilitating capsule progression so that it reaches the anal verge before the end of the battery life. Unpublished preliminary trials showed that a standard colonoscopy preparation could not achieve these tasks. Three trials demonstrated the efficacy of a protocol combining PEG and boosts with sodium phosphate to provide adequate bowel cleansing and high rates of complete colon examination [9, 10,12]. Such a combined protocol has been applied in most subsequent studies although modifications in the timing and doses of the components have been reported. Starting from the experience with bowel cleansing for colonoscopy, lavage solutions with large volumes (3 4L) of PEG solutions have been used in most studies with CCE [7 15, 18, 20]. Very little experience with lower doses of PEG or substitutes such as sodium phosphate or magnesium citrate is currently available [48]. Studies demonstrating the equivalence of lower-volume cleansing and PEG pro-
6 Rating Description Table4 Cleansing level scale and bubbles effect scale [51]. Cleansing level scale Poor Inadequate Large amount of fecal residue precludes a complete examination Inadequate but examination completed Enough feces or turbid fluid to prevent a reliable examination Adequate Small amount of feces or turbid fluid not interfering with examination Adequate No more than small bits of adherent feces Bubbles that interfere with the examination More than 10 % of surface area obscured by bubbles No bubbles or bubbles that do not interfere with the examination Less than 10 % of surface area obscured by bubbles Fair Good Excellent Bubbles effect scale Significant Insignificant tocols for conventional colonoscopies cannot therefore be extended to CCE [49]. Two main split regimens of PEG have been proposed: 3+1 (i. e., 3 L of PEG the day before and 1 L on the day of CCE) [7 9, 11,14], or 2+2 (i.e., 2 L of PEG the day before and 2L on the day of CCE) [15, 18,20]. Although no comparative study is available, the 2 +2 regimen seems to be more acceptable to patients and equally effective in terms of colon cleanliness. To meet the specific goals of bowel cleansing for CCE discussed above, sodium phosphate boosts have been added to the usual PEG and diet recommendations used for conventional colonoscopy. The role of the boosts administered during capsule progression is not limited to increasing and/or maintaining colonic cleanliness but also includes a propulsive effect by means of a volume effect allowing the capsule to move in a watery environment. The propulsive effect of the sodium phosphate boosts results in an effective transit of the CCE device through the small and large bowels with a higher rate of capsule expulsion within the limited operating time of the capsule battery [8 15, 18,20]. Magnesium sulfate has not been tested in this indication. However, a recent randomized study compared boosting with sodium phosphate to boosting with small volumes of PEG and concluded that the rate of capsule expulsion was higher with sodium phosphate [14]. The cumulative dose of sodium phosphate booster adopted initially in CCE studies was 75 ml [8, 9]. In order to reduce the risk of side effects from sodium phosphate administration, a lower dose (45 or 55 ml, in total) has been used in the most recent studies on CCE-2, with no apparent decrease in capsule egestion rate [18, 20]. Potential adverse events caused by bowel preparation for CCE mainly appear to be related to sodium phosphate administration. Although no serious sodium phosphate-related adverse event has been reported up to now, after more than 1000 CCE examinations [7 18,20], use of sodium phosphate should be avoided in patients at higher risk of sodium phosphate toxicity (i. e. elderly persons, patients with hypovolemia, baseline kidney disease, bowel obstruction, or active colitis, patients on medications affecting renal perfusion or with function kidney failure, or having concomitant treatment with angiotensin-converting enzyme (ACE)-inhibitors, etc.) [46]. During the CCE procedure, the use of prokinetics is recommended when the capsule stays in the stomach longer than 1 hour (Evidence level 4, Recommendation Prokinetics have been added to the protocol of bowel preparation for CCE mainly to stimulate the progression of the capsule in the upper gut, especially the stomach. In initial studies, tegaserod [7] and domperidone [8] were used. Domperidone was also used in multicenter trials [9, 11, 12]. No comparative study has so far been reported that assessed the usefulness of one dose of a prokinetic administered 15 minutes before capsule ingestion. With CCE-2, the data recorder allows real-time viewing of endoscopic images provided by the capsule; administration of prokinetics has thus been limited to cases where the capsule had not entered the small bowel within 1 hour after ingestion [18, 20]. Written and oral information about CCE bowel preparation should be delivered by healthcare professionals (physician or nurse) involved in gastroenterology and with experience regarding the technique It is essential to strongly emphasize to the patient the need for good bowel preparation, since the quality of bowel preparation has been found to relate to the accuracy of the CCE procedure [9]. Experience with conventional colonoscopy shows that detailed information must be given to the patient before the procedure. The instructing person may be the endoscopist himself or herself, the referring physician, or an experienced and endoscopy-trained nurse [50]. Beyond the issues of quality and necessity of information with regard to bowel preparation, monitoring or coaching the patient during the cleansing protocol might be helpful to improve bowel cleansing. 6. Reporting and work-up of CCE results The CCE report should provide information on the quality of preparation, technical details of the examination, completeness of the procedure, and on the significant findings (polyps/masses 6mm, or 3 polyps irrespective of size) in a standardized fashion (Evidence level 4, Recommendation In particular, size, morphology, and location should be separately described for each polyp. Extracolonic findings should also be reported when clinically meaningful (Evidence level 4, Recommendation Patients found to have a polyp 6mm at CCE, as well as those with 3 polyps irrespective of size, should be sent for post-cce colonoscopy for polypectomy Patients without significant findings at CCE should repeat CCE or a different screening test after 5 years, unless bowel preparation at CCE was inadequate Quality of bowel preparation has been related to CCE accuracy for relevant lesions [9]. Mucosal visualization by CCE may be hampered by feces, turbid fluids, or bubbles. Scoring on all these items has recently been included in a grading system that allowed evaluation of colon cleansing for CCE with good interobserver agreement ( " Table 4) [51]. A CCE examination may be incomplete, mainly because a slow colonic transit that may mean that the battery is exhausted while the CCE device is still in the colon. Therefore, some colorectal
7 segments (usually the left colon) may not be visualized by CCE, potentially leading to false-negative results. Most colonic polyps discovered at screening are diminutive, with negligible risk of harboring advanced features (high grade dysplasia, villous component, or malignancy) [52 55]. Moreover, 40 % of diminutive colonic polyps are hyperplastic rather than adenomatous [56]. Diminutive lesions identified by a noninvasive test may also be missed by the post-test colonoscopy, because of the imperfect sensitivity of the latter for diminutive lesions [57, 58]. By extrapolating data from CT colonography studies that modelled the impact of colonoscopy or continued surveillance for diminutive polyps discovered at CT-colonography, it can be concluded that referral for removal of diminutive lesions found at CCE might carry an unjustified burden of costs and complications relative to a minimal gain in clinical efficacy [59, 60]. Moreover, studies on second-generation CCE only provide accuracy data relating to lesions 6 mm in size, its specificity for diminutive lesions being largely unknown [18, 20]. Thus, the only exception regarding post-cce referral for diminutive polyps is the simultaneous presence of at least 3 of these lesions. Polyp multiplicity has appeared as a strong predictive factor of subsequent advanced neoplasia development in post-polypectomy follow-up studies [61]. Most advanced neoplasias have been shown to be restricted to the relatively small proportion of patients with polyps 6 mm in size [55]. Consequently, post-cce colonoscopy referral of such patients may be expected to lead to a substantial reduction of the prevalence of advanced neoplasia in patients initially evaluated with CCE. Although it has been shown that patients with polyps of size 6 9 mm may be safely followed up for a relatively short period of time [62 64], there is no evidence that CCE repetition after 2 3 years may lead to re-identification of the previously unremoved polyp. The efficacy of a noninvasive test such as CCE ultimately depends on the identification and removal/biopsy sampling at post-cce colonoscopy of the lesions found at CCE. For this reason, it is critically important that the size, morphology, and location of lesions should be accurately described, to facilitate the post-cce identification at colonoscopy. The Paris classification may be extended to CCE to standardize polyp reporting [65]. Similarly to small-bowel capsule endoscopy, CCE may detect clinically important extracolonic findings, such as preneoplastic (i.e. Barrett esophagus), ulcerative, or malignant processes of the upper gastrointestinal tract or the small bowel. Although the impact of CCE diagnosis on the course of these extracolonic diseases has not been addressed, appropriate patient care requires that the presence of clinically important abnormalities should be identified and effectively communicated. The long-term efficacy of CCE with regard to CRC prevention is unknown at present. A 5-year interval before repeating a new screening test has been recommended for other noninvasive imaging procedures, such as flexible sigmoidoscopy, CT colonography, or barium enema [25]. Considering that the sensitivity of CCE appears to be comparable to that of these tests, the 5- year interval may be extended to CCE by analogy. If overall bowel preparation was deemed inadequate or CCE was incomplete, repetition of an imaging study should be considered [Evidence level 4, Recommendation grade D]. There is an association between the cleanliness level and the accuracy of CCE for detecting polyps [9]. False-negative CCE findings have also been related to incomplete visualization of the colorectal mucosa because of CCE battery exhaustion [16]. Although possible predictors of inadequate bowel preparation at CCE have not yet been analyzed, the effectiveness of bowel preparation has been shown at colonoscopy to depend on a number of factors, the most important of which is compliance [66 68]. Other independent risk factors for incomplete colon preparation include a history of constipation, inpatient status, use of antidepressants, and male gender [68]. In the case of inadequate colon cleansing at CCE or of incomplete visualization of the colorectal mucosa, it may not be possible to exclude even large lesions in the poorly visualized or unvisualized areas. For this reason, based on clinical background, repetition of CCE or a different imaging test may be considered. For a follow-on procedure, whichever one is recommended, the potential reasons for inadequate CCE preparation should be addressed. Poor compliance might be addressed by better education and supervision, and by changing the type and/or intensity of bowel preparation. Use of this Guideline The aim of this Guideline is to provide caregivers with a comprehensive framework on how to implement and practice CCE in a clinical setting. ESGE Guidelines represent a consensus of best practice based on the available evidence at the time of preparation. They may not apply in all situations and should be interpreted in the light of specific clinical situations and resource availability. Further controlled clinical studies may be needed to clarify aspects of these statements, and revision may be necessary as new data appear. Clinical consideration may justify a course of action at variance to these recommendations. ESGE Guidelines are intended to be an educational device to provide information that may assist endoscopists in providing care to patients. They are not rules and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. Competing interests: Given Imaging funded the consensus meeting in Tarquinia. C. Spada, C. Hassan, J. P. Galmiche, S. Adler, M. Pennazio, D. K. Rex, R. de Franchis, and E. Toth are consultants for Given Imaging. M. Keuchel, F. Macrae, F. Hagenmueller, T. Ponchon, J. J. Sung, and G. Costamagna have received research support and are consultants for Given Imaging. R. Benamouzig and J. Devière received research support from Given Imaging. H. Neuhaus, J. M. Dumonceau, O. Epstein, G. Gay, M. Delvaux, R. Eliakim, C. Fraser, J. M. Herrerias, M. Munoz-Navas, E. Quintero, M. E. Riccioni, E. Rondonotti, R. Marmo, H. Tajiri, K. Triantafyllou, and A. Van Gossum declare no conflict of interest to disclose. Institutions 1 Digestive Endoscopy Unit, Catholic University, Rome, Italy 2 Department of Gastroenterology and Hepatology, Nantes University, Nantes, France 3 Department of Gastroenterology, Evangelisches Krankenhaus, Düsseldorf, Germany 4 Service of Gastroenterology and Hepatology, Geneva University Hospitals, Geneva, Switzerland 5 Department of Gastroenterology, Bikur Holim Hospital; Jerusalem, Israel 6 Department of Gastroenterology, Royal Free and University College Medical School, London, UK 7 Department of Hepato-Gastroenterology, CHU Strasbourg, Strasbourg, France 8 Division of Gastroenterology 2, San Giovanni Battista University Teaching Hospital, Turin, Italy 9 Department of Gastroenterology, Indiana University Hospital, Indianapolis, USA 10 Department of Gastroenterology, Avicenne Hospital, University of Paris, Bobigny, France
8 11 Gastroenterology Unit, Luigi Sacco University Hospital, Milan, Italy 12 Department of Gastroenterology, Erasme Hospital, Université Libre de Bruxelles, Brussels, Belgium 13 Chaim Sheba Medical Center, Tel-Aviv, Israel 14 Department of Gastroenterology, St Mark's Hospital, London, UK 15 Department of Medicine I, Altona General Hospital, Hamburg, Germany 16 Gastroenterology Service, Virgen Macarena University Hospital, Seville, Spain 17 Clinic for Internal Medicine, Bethesda Krankenhaus Bergedorf, Hamburg, Germany 18 Department of Gastroenterology and Clinical Nutrition Service, Royal Melbourne Hospital, Melbourne, Australia 19 Digestive Endoscopy Unit, Clinica Universitaria de Navarra, Pamplona, Spain 20 Department of Gastroenterology, Hôpital Edouard Herriot, Lyon, France 21 Department of Gastroenterology, Hospital Universitario de Canarias, Tenerife, Spain 22 Gastroenterology Unit, Ospedale Valduce, Como, Italy 23 Division of Gastroenterology, Curto Hospital, Polla, Italy 24 Department of Medicine and Therapeutics, Division of Gastroenterology, Prince of Wales Hospital, Shatin, N.T., Hong Kong 25 Division of Gastroenterology and Hepatology, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan 26 Endoscopy Unit, Skane University Hospital, Lund University, Malmö, Sweden 27 Hepatogastroenterology Unit, 2nd Department of Internal Medicine- Propaedeutic, Attikon University General Hospital, Athens University, Athens, Greece References 1 Ferlay J, Autier P, Boniol M et al. Estimates of the cancer incidence and mortality in Europe in Ann Oncol 2007; 18: Atkin WS, Edwards R, Kralj-Hans I et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010; 375: Winawer SJ, Zauber AG, Ho MN. The National Polyp Study Workgroup. et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993; 329: Citarda F, Tomaselli G, Capocaccia R et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48: Segnan N, Senore C, Andreoni B et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: Lisi D, Hassan C, Crespi M et al. Participation to colorectal cancer screening with FOBT and colonoscopy: An Italian, multicentre, randomized population study. Dig Liver Dis 2010; 42: Eliakim R, Fireman Z, Gralnek IM et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy 2006; 38: Schoofs N, Devière J, Van Gossum A et al. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy 2006; 38: Van Gossum A, Navas MM, Fernandez-Urien I et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009; 361: Sieg A, Friedrich K, Sieg U et al. Is PillCam COLON capsule endoscopy ready for colorectal cancer screening? A prospective feasibility study in a community gastroenterology practice Am J Gastroenterol 2009; 104: Gay G, Delvaux M, Frederic M et al. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy?: results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening Am J Gastroenterol 2010; 105: Sacher-Huvelin S, Coron E, Gaudric M et al. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther 2010; 32: Pilz JB, Portmann S, Peter S et al. Colon capsule endoscopy compared to conventional colonoscopy under routine screening conditions. BMC Gastroenterol 2010; 10: Spada C, Riccioni ME, Hassan C et al. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol 2011; 45: Spada C, Hassan C, Ingrosso M et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: a pilot study. Dig Liver Dis 2011; 43: Spada C, Hassan C, Marmo R et al. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol 2010; 8: Rokkas T, Papaxoinis K, Triantafyllou K et al. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc 2010; 71: Eliakim R, Yassin K, Niv Y et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009; 41: Spada C, Hassan C, Riccioni ME et al. False positive at colon capsule endoscopy or false negative at conventional colonoscopy? Endoscopy 2010; 42: Spada C, Hassan C, Munoz-Navas M et al. Second-generation colon capsule compared with colonoscopy. Gastrointest Endosc 2011; 74: Dumonceau JM, Andriulli A, Deviere J et al. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ercp pancreatitis. Endoscopy 2010; 42: Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001; 323: Johnson CD, Chen MH, Toledano AY et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008; 359: Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med 2009; 150: Levin B, Lieberman DA, McFarland B et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134: Lieberman DA. Clinical practice. Screening for colorectal cancer. N Engl J Med 2009; 361: Hassan C, Zullo A, WinnSet al. Cost effectiveness of capsule endoscopy in screening for colorectal cancer. Endoscopy 2008; 40: Rex DK, Mark D, Clarke B et al. Flexible sigmoidoscopy plus air-contrast barium enema versus colonoscopy for evaluation of symptomatic patients without evidence of bleeding. Gastrointest Endosc 1995; 42: Pickhardt PJ, Choi JR, Hwang I et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349: Goulston KJ, Cook I, Dent OF. How important is rectal bleeding in the diagnosis of bowel cancer and polyps? Lancet 1986; 2: American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52: Bowles CJ, Leicester R, Romaya C et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004; 53: Lieberman DA, Weiss DG, Bond JH. Veterans Affairs Cooperative Study Group 380. et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med 2000; 343: Rex DK. Achieving cecal intubation in the very difficult colon. Gastrointest Endosc 2008; 67: Hanson ME, Pickhardt PJ, Kim DH et al. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol 2007; 189: Spada C, Riccioni ME, Petruzziello L et al. The new PillCam Colon capsule: difficult colonoscopy? No longer a problem? Gastrointest Endosc 2008; 68: Triantafyllou K, Tsibouris P, Kalantzis C et al. PillCam Colon capsule endoscopy does not always complement incomplete colonoscopy. Gastrointest Endosc 2009; 69: Triantafyllou K, Viazis N, Tsibouris P et al. Colon capsule endoscopy complements incomplete colonoscopy in clinical practice. Endoscopy 2010; 42 : 01A27 39 Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361: Bourreille A, Ignjatovic A, Aabakken L et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy 2009; 41: Strange EF, Travis SPL, Vermeire S et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohn s Colitis 2008; 2: 1 23
Interview with Prof. Guido Costamagna
 Interview with Prof. Guido Costamagna Extraxts of his curriculum vitae: Full Professor of Surgery, Università Cattolica del Sacro Cuore, Rome, Italy Director, Digestive Endoscopy Unit, Policlinico A. Gemelli,
Interview with Prof. Guido Costamagna Extraxts of his curriculum vitae: Full Professor of Surgery, Università Cattolica del Sacro Cuore, Rome, Italy Director, Digestive Endoscopy Unit, Policlinico A. Gemelli,
Colon Cancer Detection by Rendezvous Colonoscopy : Successful Removal of Stuck Colon Capsule by Conventional Colonoscopy
 19 Colon Cancer Detection by Rendezvous Colonoscopy : Successful Removal of Stuck Colon Capsule by Conventional Colonoscopy István Rácz Márta Jánoki Hussam Saleh Department of Gastroenterology, Petz Aladár
19 Colon Cancer Detection by Rendezvous Colonoscopy : Successful Removal of Stuck Colon Capsule by Conventional Colonoscopy István Rácz Márta Jánoki Hussam Saleh Department of Gastroenterology, Petz Aladár
Colon Capsule Endoscopy: Where Are We and Where Are We Going
 REVIEW Clin Endosc 2016;49:449-453 http://dx.doi.org/10.5946/ce.2016.095 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Colon Capsule Endoscopy: Where Are We and Where Are We Going Yoo Min Han
REVIEW Clin Endosc 2016;49:449-453 http://dx.doi.org/10.5946/ce.2016.095 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Colon Capsule Endoscopy: Where Are We and Where Are We Going Yoo Min Han
Second-generation colon capsule endoscopy compared with colonoscopy
 Second-generation colon capsule endoscopy compared with colonoscopy Cristiano Spada, MD, Cesare Hassan, MD, PhD, Miguel Munoz-Navas, MD, PhD, Horst Neuhaus, MD, Jacques Deviere, MD, PhD, Paul Fockens,
Second-generation colon capsule endoscopy compared with colonoscopy Cristiano Spada, MD, Cesare Hassan, MD, PhD, Miguel Munoz-Navas, MD, PhD, Horst Neuhaus, MD, Jacques Deviere, MD, PhD, Paul Fockens,
ORIGINAL ARTICLE: Clinical Endoscopy
 ORIGINAL ARTICLE: Clinical Endoscopy A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps Theodore Rokkas, MD, PhD, Konstantinos Papaxoinis, MD, Konstantinos Triantafyllou,
ORIGINAL ARTICLE: Clinical Endoscopy A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps Theodore Rokkas, MD, PhD, Konstantinos Papaxoinis, MD, Konstantinos Triantafyllou,
Cecal intubation is a recognized measure of colonoscopy
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2013;11:534 540 Effects of Colon Capsule Endoscopy on Medical Decision Making in Patients With Incomplete Colonoscopies ONOFRE ALARCÓN FERNÁNDEZ,* LAURA RAMOS,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2013;11:534 540 Effects of Colon Capsule Endoscopy on Medical Decision Making in Patients With Incomplete Colonoscopies ONOFRE ALARCÓN FERNÁNDEZ,* LAURA RAMOS,*
Colorectal cancer screening A puzzle of tests and strategies
 Colorectal cancer screening A puzzle of tests and strategies A. Van Gossum, MD, PhD Head of the Clinic of Intestinal Diseases and Nutritional Support Department of Gastroenterology Hôpital Erasme ULB -
Colorectal cancer screening A puzzle of tests and strategies A. Van Gossum, MD, PhD Head of the Clinic of Intestinal Diseases and Nutritional Support Department of Gastroenterology Hôpital Erasme ULB -
PillCam Colon Capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy
 1130-0108/2011/103/2/69-75 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS Copyright 2011 ARÁN EDICIONES, S. L. REV ESP ENFERM DIG (Madrid) Vol. 103. N. 2, pp. 69-75, 2011 ORIGINAL PAPERS PillCam Colon Capsule
1130-0108/2011/103/2/69-75 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS Copyright 2011 ARÁN EDICIONES, S. L. REV ESP ENFERM DIG (Madrid) Vol. 103. N. 2, pp. 69-75, 2011 ORIGINAL PAPERS PillCam Colon Capsule
Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology
 Original article 473 Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology Authors Grainne Holleran 1, 2, Ronan Leen 1,2, Colm
Original article 473 Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology Authors Grainne Holleran 1, 2, Ronan Leen 1,2, Colm
Early detection and screening for colorectal neoplasia
 Early detection and screening for colorectal neoplasia Robert S. Bresalier Department of Gastroenterology, Hepatology and Nutrition. The University of Texas. MD Anderson Cancer Center. Houston, Texas U.S.A.
Early detection and screening for colorectal neoplasia Robert S. Bresalier Department of Gastroenterology, Hepatology and Nutrition. The University of Texas. MD Anderson Cancer Center. Houston, Texas U.S.A.
Protocol. Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus, and Colon
 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders (60133) Medical Benefit Effective Date: 01/01/14 Next Review Date: 09/14 Preauthorization Yes Review Dates: 02/07, 03/08, 11/08, 09/09,
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders (60133) Medical Benefit Effective Date: 01/01/14 Next Review Date: 09/14 Preauthorization Yes Review Dates: 02/07, 03/08, 11/08, 09/09,
Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer
 The new england journal of medicine original article Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer André Van Gossum, M.D., Miguel Munoz-Navas, M.D., Iñaqui Fernandez-Urien,
The new england journal of medicine original article Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer André Van Gossum, M.D., Miguel Munoz-Navas, M.D., Iñaqui Fernandez-Urien,
Capsule endoscopy screening. Carlo SENORE
 Capsule endoscopy screening Carlo SENORE Possible conflicts of interest Medtronics provides CCE-2 devices to conduct a multicenter independent, non profit study, aimed to assess CCE-2 diagnostic accuracy
Capsule endoscopy screening Carlo SENORE Possible conflicts of interest Medtronics provides CCE-2 devices to conduct a multicenter independent, non profit study, aimed to assess CCE-2 diagnostic accuracy
Citation for published version (APA): Wijkerslooth de Weerdesteyn, T. R. (2013). Population screening for colorectal cancer by colonoscopy
 UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
Advice Statement. Advice Statement November Advice for NHSScotland. Why is SHTG looking at this topic?
 Advice Statement 014-18 November 2018 Advice Statement Colon capsule endoscopy (CCE-2) for the detection of colorectal polyps and cancer in adults with signs or symptoms of colorectal cancer or at increased
Advice Statement 014-18 November 2018 Advice Statement Colon capsule endoscopy (CCE-2) for the detection of colorectal polyps and cancer in adults with signs or symptoms of colorectal cancer or at increased
French National Colon Capsule Endoscopy
 French National Colon Capsule Endoscopy Observatory (ONECC) Evaluation and first lessons Jean-Christophe Saurin On behalf of the comité scientifique de l Oservatoire national de l endoscopie par capsule
French National Colon Capsule Endoscopy Observatory (ONECC) Evaluation and first lessons Jean-Christophe Saurin On behalf of the comité scientifique de l Oservatoire national de l endoscopie par capsule
/2014/106/5/ Revista Española de Enfermedades Digestivas Vol. 106, N.º 5, pp , 2014 ORIGINAL PAPERS
 1130-0108/2014/106/5/312-317 Revista Española de Enfermedades Digestivas Copyright 2014 Arán Ediciones, S. L. Rev Esp Enferm Dig (Madrid Vol. 106, N.º 5, pp. 312-317, 2014 ORIGINAL PAPERS Preparations
1130-0108/2014/106/5/312-317 Revista Española de Enfermedades Digestivas Copyright 2014 Arán Ediciones, S. L. Rev Esp Enferm Dig (Madrid Vol. 106, N.º 5, pp. 312-317, 2014 ORIGINAL PAPERS Preparations
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon. Original Policy Date
 MP 6.01.23 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Medical Policy Section Radiology Issue 12:2013 Original Policy Date 12:2013 Last Review
MP 6.01.23 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Medical Policy Section Radiology Issue 12:2013 Original Policy Date 12:2013 Last Review
Accepted Manuscript. En bloc resection for mm polyps to reduce post-colonoscopy cancer and surveillance. C. Hassan, M. Rutter, A.
 Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Colorectal Cancer Screening: A Clinical Update
 11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
효과적인대장정결법 김태준 삼성서울병원소화기내과
 효과적인대장정결법 김태준 삼성서울병원소화기내과 부적절한장정결 Efficacy blurring - 긴검사시간 - 낮은맹장도달율 & 선종발견율 Risk intensification - 시술관련합병증증가 Waste of cost - 검사반복 - 내시경의사의 workload 증가 Patient dissatisfaction 장정결에따른선종발견율차이 Multi-centers
효과적인대장정결법 김태준 삼성서울병원소화기내과 부적절한장정결 Efficacy blurring - 긴검사시간 - 낮은맹장도달율 & 선종발견율 Risk intensification - 시술관련합병증증가 Waste of cost - 검사반복 - 내시경의사의 workload 증가 Patient dissatisfaction 장정결에따른선종발견율차이 Multi-centers
Same-day colon capsule endoscopy is a viable means to assess unexplored colonic segments after incomplete colonoscopy in selected patients
 Original Article Same-day colon capsule endoscopy is a viable means to assess unexplored colonic segments after incomplete colonoscopy in selected patients United European Gastroenterology Journal 2018,
Original Article Same-day colon capsule endoscopy is a viable means to assess unexplored colonic segments after incomplete colonoscopy in selected patients United European Gastroenterology Journal 2018,
Virtual Colonoscopy/CT Colonography
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
Japan Journal of Medicine 2018; 1(4): doi: /jjm
 Jpn J Med 2018,1:4 191 Review Colon capsule endoscopy for colorectal cancer screening in patients with the positive fecal occult blood test (FOBT) in Japan Konosuke Nakaji * Endoscopy Center, Aishinkai
Jpn J Med 2018,1:4 191 Review Colon capsule endoscopy for colorectal cancer screening in patients with the positive fecal occult blood test (FOBT) in Japan Konosuke Nakaji * Endoscopy Center, Aishinkai
Colorectal Cancer Screening
 Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
LIST OF ABBREVIATIONS
 Gastroenter oenterology 2005 Royal College of Physicians of Edinburgh Screening and surveillance for upper and lower gastrointestinal cancer JN Plevris Consultant Gastroenterologist and Honorary Senior
Gastroenter oenterology 2005 Royal College of Physicians of Edinburgh Screening and surveillance for upper and lower gastrointestinal cancer JN Plevris Consultant Gastroenterologist and Honorary Senior
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
The Usefulness of Capsule Endoscopy
 The Usefulness of Capsule Endoscopy David J. Hass, MD, FACG Associate Clinical Professor of Medicine Yale University School of Medicine Gastroenterology Center of Connecticut INDICATIONS FOR USE PillCam
The Usefulness of Capsule Endoscopy David J. Hass, MD, FACG Associate Clinical Professor of Medicine Yale University School of Medicine Gastroenterology Center of Connecticut INDICATIONS FOR USE PillCam
CRC Risk Factors. U.S. Adherence Rates Cancer Screening. Genetic Model of Colorectal Cancer. Epidemiology and Clinical Consequences of CRC
 10:45 11:45 am Guide to Colorectal Cancer Screening SPEAKER Howard Manten M.D. Presenter Disclosure Information The following relationships exist related to this presentation: Howard Manten MD: No financial
10:45 11:45 am Guide to Colorectal Cancer Screening SPEAKER Howard Manten M.D. Presenter Disclosure Information The following relationships exist related to this presentation: Howard Manten MD: No financial
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Review Article Colon Capsule Endoscopy: Review and Perspectives
 Gastroenterology Research and Practice Volume 2016, Article ID 9643162, 6 pages http://dx.doi.org/10.1155/2016/9643162 Review Article Colon Capsule Endoscopy: Review and Perspectives David Friedel, Rani
Gastroenterology Research and Practice Volume 2016, Article ID 9643162, 6 pages http://dx.doi.org/10.1155/2016/9643162 Review Article Colon Capsule Endoscopy: Review and Perspectives David Friedel, Rani
Summary. Cezary ŁozińskiABDF, Witold KyclerABCDEF. Rep Pract Oncol Radiother, 2007; 12(4):
 Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Therapeutic impact of colon capsule endoscopy with PillCam COLON 2 after incomplete standard colonoscopy: a Spanish multicenter study
 1130-0108/2017/109/5/322-327 Revista Española de Enfermedades Digestivas Copyright 2017. SEPD y ARÁN EDICIONES, S.L. Rev Esp Enferm Dig 2017, Vol. 109, N.º 5, pp. 322-327 ORIGINAL PAPERS Therapeutic impact
1130-0108/2017/109/5/322-327 Revista Española de Enfermedades Digestivas Copyright 2017. SEPD y ARÁN EDICIONES, S.L. Rev Esp Enferm Dig 2017, Vol. 109, N.º 5, pp. 322-327 ORIGINAL PAPERS Therapeutic impact
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Therapeutic Advances in Gastroenterology. Original Research
 586355TAG0010.1177/1756283X15586355Therapeutic Advances in GastroenterologyP.K. Kashyap and R. Peled research-article2015 Therapeutic Advances in Gastroenterology Original Research Polyethylene glycol
586355TAG0010.1177/1756283X15586355Therapeutic Advances in GastroenterologyP.K. Kashyap and R. Peled research-article2015 Therapeutic Advances in Gastroenterology Original Research Polyethylene glycol
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus, and Colon
 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus, and Colon Policy Number: 6.01.33 Last Review: 4/2014 Origination: 4/2003 Next Review: 4/2015 Policy Blue
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus, and Colon Policy Number: 6.01.33 Last Review: 4/2014 Origination: 4/2003 Next Review: 4/2015 Policy Blue
Quality Measures In Colonoscopy: Why Should I Care?
 Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
Capsule Endoscopy: Is it Really Helpful in the Diagnosis of Small Bowel Diseases? Kashif Malik, Muhammad Joher Amin, Syed Waqar Hassan Shah
 Original Article Capsule Endoscopy: Is it Really Helpful in the Diagnosis of Small Bowel Diseases? Kashif Malik, Muhammad Joher Amin, Syed Waqar Hassan Shah ABSTRACT Objective: To determine the diagnostic
Original Article Capsule Endoscopy: Is it Really Helpful in the Diagnosis of Small Bowel Diseases? Kashif Malik, Muhammad Joher Amin, Syed Waqar Hassan Shah ABSTRACT Objective: To determine the diagnostic
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING
 CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls. Disclosures: None. CRC: still a major public health problem
 Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls Disclosures: None Jonathan P. Terdiman, M.D. Professor of Clinical Medicine University of California, San Francisco CRC: still a major public
Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls Disclosures: None Jonathan P. Terdiman, M.D. Professor of Clinical Medicine University of California, San Francisco CRC: still a major public
C olorectal adenomas are reputed to be precancerous
 568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University WCC, Melbourne
 Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
Colorectal Cancer Screening and Surveillance
 1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
Digestive Health Southwest Endoscopy 2016 Quality Report
 Digestive Health 2016 Quality Report Our 2016 our quality and value management program focused on one primary area of interest: Performing high quality colonoscopy High quality Colonoscopy We selected
Digestive Health 2016 Quality Report Our 2016 our quality and value management program focused on one primary area of interest: Performing high quality colonoscopy High quality Colonoscopy We selected
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer
 Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Title Description Type / Priority
 Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Colorectal Neoplasia. Dr. Smita Devani MBChB, MRCP. Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi
 Colorectal Neoplasia Dr. Smita Devani MBChB, MRCP Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi Case History BT, 69yr male Caucasian History of rectal bleeding No change
Colorectal Neoplasia Dr. Smita Devani MBChB, MRCP Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi Case History BT, 69yr male Caucasian History of rectal bleeding No change
Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005
 Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005 David Lieberman MD Chief, Division of Gastroenterology Oregon Health and Science University Portland VAMC Portland, Oregon
Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005 David Lieberman MD Chief, Division of Gastroenterology Oregon Health and Science University Portland VAMC Portland, Oregon
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
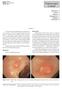 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications
 Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications This document contains a listing of the clinical quality measures which the New Hampshire
Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications This document contains a listing of the clinical quality measures which the New Hampshire
How to characterize dysplastic lesions in IBD?
 How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North
 Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North Kargar Avenue 14666 Tehran, Iran. Tel: +98-21-82415415 Fax:
Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North Kargar Avenue 14666 Tehran, Iran. Tel: +98-21-82415415 Fax:
Description. Section: Radiology Effective Date: October 15, 2014 Subsection: Radiology Original Policy Date: December 7, 2011 Subject:
 Last Review Status/Date: September 2014 Page: 1 of 13 Description Computed tomography (CT) colonography, also known as virtual colonoscopy, is an imaging technique of the colon. CT colonography has been
Last Review Status/Date: September 2014 Page: 1 of 13 Description Computed tomography (CT) colonography, also known as virtual colonoscopy, is an imaging technique of the colon. CT colonography has been
IEHP UM Subcommittee Approved Authorization Guidelines Colorectal Cancer Screening with Cologuard TM for Medicare Beneficiaries
 for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
1101 First Colonial Road, Suite 300, Virginia Beach, VA Phone (757) Fax (757)
 1101 First Colonial Road, Suite 300, Virginia Beach, VA 23454 www.vbgastro.com Phone (757) 481-4817 Fax (757) 481-7138 1150 Glen Mitchell Drive, Suite 208 Virginia Beach, VA 23456 www.vbgastro.com Phone
1101 First Colonial Road, Suite 300, Virginia Beach, VA 23454 www.vbgastro.com Phone (757) 481-4817 Fax (757) 481-7138 1150 Glen Mitchell Drive, Suite 208 Virginia Beach, VA 23456 www.vbgastro.com Phone
GIQIC18 Appropriate follow-up interval of not less than 5 years for colonoscopies with findings of 1-2 tubular adenomas < 10 mm
 GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it
 Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
COLON CANCER SCREENING: AN UPDATE
 Overview COLON CANCER SCREENING: AN UPDATE Siddharth Verma, DO, JD Rutgers New Jersey Medical School Background Screening Updates in Specific Populations African Americans CRC in the younger age USPSTF
Overview COLON CANCER SCREENING: AN UPDATE Siddharth Verma, DO, JD Rutgers New Jersey Medical School Background Screening Updates in Specific Populations African Americans CRC in the younger age USPSTF
EXPERT WORKING GROUP Surveillance after neoplasia removal. Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum
 EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
What Questions Should You Ask?
 ? Your Doctor Has Ordered a Colonoscopy. What Questions Should You sk? From the merican College of Gastroenterology www.acg.gi.org Normal colon Is the doctor performing your colonoscopy a Gastroenterologist?
? Your Doctor Has Ordered a Colonoscopy. What Questions Should You sk? From the merican College of Gastroenterology www.acg.gi.org Normal colon Is the doctor performing your colonoscopy a Gastroenterologist?
Sequential screening in the early diagnosis of colorectal cancer in the community
 Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
ACG Clinical Guideline: Colorectal Cancer Screening
 ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an open-label, randomized controlled trial
 Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
Colorectal Cancer Screening What are my options?
 069-Colorectal cancer (Rosen) 1/23/04 12:59 PM Page 69 What are my options? Wayne Rosen, MD, FRCSC As presented at the 37th Annual Mackid Symposium: Cancer Care in the Community (May 22, 2003) There are
069-Colorectal cancer (Rosen) 1/23/04 12:59 PM Page 69 What are my options? Wayne Rosen, MD, FRCSC As presented at the 37th Annual Mackid Symposium: Cancer Care in the Community (May 22, 2003) There are
Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema
 Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
1. Introduction. Catalonia, Spain. Correspondence should be addressed to Begoña González-Suarez;
 Hindawi Gastroenterology Research and Practice Volume 2017, Article ID 1507914, 7 pages https://doi.org/10.1155/2017/1507914 Research Article Accuracy of Colon Capsule Endoscopy in Detecting Colorectal
Hindawi Gastroenterology Research and Practice Volume 2017, Article ID 1507914, 7 pages https://doi.org/10.1155/2017/1507914 Research Article Accuracy of Colon Capsule Endoscopy in Detecting Colorectal
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Capsule Endoscopy. Medical Coverage Policy. Related Coverage Resources. Table of Contents. Coverage Policy. Omnibus Codes
 Medical Coverage Policy Effective Date...12/15/2017 Next Review Date...12/15/2018 Coverage Policy Number... 0008 Capsule Endoscopy Table of Contents Coverage Policy... 1 Overview... 2 General Background...
Medical Coverage Policy Effective Date...12/15/2017 Next Review Date...12/15/2018 Coverage Policy Number... 0008 Capsule Endoscopy Table of Contents Coverage Policy... 1 Overview... 2 General Background...
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Updates in Colorectal Cancer Screening & Prevention
 Updates in Colorectal Cancer Screening & Prevention Swati G. Patel, MD MS Assistant Professor of Medicine Division of Gastroenterology & Hepatology Gastrointestinal Cancer Risk and Prevention Clinic University
Updates in Colorectal Cancer Screening & Prevention Swati G. Patel, MD MS Assistant Professor of Medicine Division of Gastroenterology & Hepatology Gastrointestinal Cancer Risk and Prevention Clinic University
COMPUTED TOMOGRAPHIC COLONOGRAPHY
 COMPUTED TOMOGRAPHIC COLONOGRAPHY Protocol: GAS021 Effective Date: November 1, 2017 Table of Contents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 2 DESCRIPTION OF
COMPUTED TOMOGRAPHIC COLONOGRAPHY Protocol: GAS021 Effective Date: November 1, 2017 Table of Contents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 2 DESCRIPTION OF
Policy Specific Section: March 1, 2005 January 30, 2015
 Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Policy #: 017 Latest Review Date: August 2013
 Name of Policy: Wireless Capsule Endoscopy (Given Video Capsule) Policy #: 017 Latest Review Date: August 2013 Category: Radiology Policy Grade: B Background/Definitions: As a general rule, benefits are
Name of Policy: Wireless Capsule Endoscopy (Given Video Capsule) Policy #: 017 Latest Review Date: August 2013 Category: Radiology Policy Grade: B Background/Definitions: As a general rule, benefits are
A Trip Through the GI Tract: Common GI Diseases and Complaints. Jennifer Curtis, MD
 A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
Colorectal Cancer Screening and Risk Assessment Workflow. Documentation Guide for Health Center NextGen Users
 Colorectal Cancer Screening and Risk Assessment Workflow Documentation Guide for Health Center NextGen Users Colorectal Cancer Screening and Risk Assessment Workflow and Documentation Guide for Health
Colorectal Cancer Screening and Risk Assessment Workflow Documentation Guide for Health Center NextGen Users Colorectal Cancer Screening and Risk Assessment Workflow and Documentation Guide for Health
Improving Access to Endoscopy at Safety-Net Hospitals. Lukejohn W. Day MD Assistant Professor of Medicine
 Improving Access to Endoscopy at Safety-Net Hospitals Lukejohn W. Day MD Assistant Professor of Medicine Goals Background Improving Access to Endoscopic Care Electronic referral: ereferral Direct Access
Improving Access to Endoscopy at Safety-Net Hospitals Lukejohn W. Day MD Assistant Professor of Medicine Goals Background Improving Access to Endoscopic Care Electronic referral: ereferral Direct Access
Improving you ADR. Robert Enns Colonoscopy Education Day October 2018
 Improving you ADR Robert Enns Colonoscopy Education Day October 2018 ADR Applying to CSP Assume 50% ADR in FIT positive patients Out of 40 patients only 20 will have polyps Out of 20 likely 15 will be
Improving you ADR Robert Enns Colonoscopy Education Day October 2018 ADR Applying to CSP Assume 50% ADR in FIT positive patients Out of 40 patients only 20 will have polyps Out of 20 likely 15 will be
2. Describe pros/cons of screening interventions (including colonoscopy, CT colography, fecal tests)
 Learning Objectives 1. Review principles of colon adenoma/cancer biology that permit successful prevention regimes 2. Describe pros/cons of screening interventions (including colonoscopy, CT colography,
Learning Objectives 1. Review principles of colon adenoma/cancer biology that permit successful prevention regimes 2. Describe pros/cons of screening interventions (including colonoscopy, CT colography,
GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY
 Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Page 1. Is the Risk This High? Dysplasia in the IBD Patient. Dysplasia in the Non IBD Patient. Increased Risk of CRC in Ulcerative Colitis
 Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
The Prevalence Rate and Anatomic Location of Colorectal Adenoma and Cancer Detected by Colonoscopy in Average-Risk Individuals Aged Years
 American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00430.x Published by Blackwell Publishing The Prevalence Rate and Anatomic Location
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00430.x Published by Blackwell Publishing The Prevalence Rate and Anatomic Location
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1258 ISSN 1175 8716 A survey of colonoscopy capacity in New Zealand s public hospitals Andrew Yeoman, Susan Parry Abstract Aims Population screening for colorectal
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1258 ISSN 1175 8716 A survey of colonoscopy capacity in New Zealand s public hospitals Andrew Yeoman, Susan Parry Abstract Aims Population screening for colorectal
Quality in Endoscopy: Can We Do Better?
 Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
Colon capsule endoscopy: What we know and what we would like to know
 Submit a Manuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx DOI: 10.3748/wjg.v20.i45.16948 World J Gastroenterol 2014 December 7; 20(45): 16948-16955 ISSN 1007-9327
Submit a Manuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx DOI: 10.3748/wjg.v20.i45.16948 World J Gastroenterol 2014 December 7; 20(45): 16948-16955 ISSN 1007-9327
Accepted Manuscript. Hyperosmotic low-volume bowel preparations: Is NER1006 safe? Douglas K. Rex, MD
 Accepted Manuscript Hyperosmotic low-volume bowel preparations: Is NER1006 safe? Douglas K. Rex, MD PII: S0016-5107(18)33271-1 DOI: https://doi.org/10.1016/j.gie.2018.11.009 Reference: YMGE 11335 To appear
Accepted Manuscript Hyperosmotic low-volume bowel preparations: Is NER1006 safe? Douglas K. Rex, MD PII: S0016-5107(18)33271-1 DOI: https://doi.org/10.1016/j.gie.2018.11.009 Reference: YMGE 11335 To appear
Wireless Capsule Endoscopy to Diagnose Disorders of the Small Bowel, Esophagus, and Colon
 Wireless Capsule Endoscopy to Diagnose Disorders of the Small Bowel, Esophagus, and Colon Policy Number: 6.01.33 Last Review: 4/2018 Origination: 4/2003 Next Review: 4/2019 Policy Blue Cross and Blue Shield
Wireless Capsule Endoscopy to Diagnose Disorders of the Small Bowel, Esophagus, and Colon Policy Number: 6.01.33 Last Review: 4/2018 Origination: 4/2003 Next Review: 4/2019 Policy Blue Cross and Blue Shield
COMPUTED TOMOGRAPHIC COLONOGRAPHY
 MEDICAL POLICY COMPUTED TOMOGRAPHIC COLONOGRAPHY Policy Number: 2013T0320M Effective Date: November 1, 2013 Table of Contents COVERAGE RATIONALE... BACKGROUND... CLINICAL EVIDENCE... U.S. FOOD AND DRUG
MEDICAL POLICY COMPUTED TOMOGRAPHIC COLONOGRAPHY Policy Number: 2013T0320M Effective Date: November 1, 2013 Table of Contents COVERAGE RATIONALE... BACKGROUND... CLINICAL EVIDENCE... U.S. FOOD AND DRUG
Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps
 Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps Full guideline Draft for consultation, May 00 0 This guideline
Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps Full guideline Draft for consultation, May 00 0 This guideline
Structured Follow-Up after Colorectal Cancer Resection: Overrated. R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007
 Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Colonic lesions in patients undergoing small bowel capsule endoscopy: incidence, diagnostic and therapeutic impact
 1130-0108/2017/109/7/498-502 Revista Española de Enfermedades Digestivas Copyright 2017. SEPD y ARÁN EDICIONES, S.L. Rev Esp Enferm Dig 2017, Vol. 109, N.º 7, pp. 498-502 ORIGINAL PAPERS Colonic in patients
1130-0108/2017/109/7/498-502 Revista Española de Enfermedades Digestivas Copyright 2017. SEPD y ARÁN EDICIONES, S.L. Rev Esp Enferm Dig 2017, Vol. 109, N.º 7, pp. 498-502 ORIGINAL PAPERS Colonic in patients
COLON CAPSULE ENDOSCOPY VERSUS COLONOSCOPY IN PATIENTS AT AVERAGE OR INCREASED RISK OF COLORECTAL CANCER
 COLON CAPSULE ENDOSCOPY VERSUS COLONOSCOPY IN PATIENTS AT AVERAGE OR INCREASED RISK OF COLORECTAL CANCER Sylvie Sacher-Huvelin, Emmanuel Coron, Marianne Gaudric, Lucie Planche, Robert Benamouzig, Vincent
COLON CAPSULE ENDOSCOPY VERSUS COLONOSCOPY IN PATIENTS AT AVERAGE OR INCREASED RISK OF COLORECTAL CANCER Sylvie Sacher-Huvelin, Emmanuel Coron, Marianne Gaudric, Lucie Planche, Robert Benamouzig, Vincent
