Clinical commissioning policy: Hyperbaric oxygen therapy for malignant otitis externa (all ages) For implementation from 1 April 2019
|
|
|
- Kristina Hortense Fleming
- 5 years ago
- Views:
Transcription
1 Clinical commissioning policy: Hyperbaric oxygen therapy for malignant otitis externa (all ages) NHS England Reference: 1745P
2 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Commissioning Strategy Finance Publications Gateway Reference: 763 Document Purpose Document Name Author Publication Date Target Audience Policy Clinical commissioning policy: Hyperbaric oxygen therapy for malignant otitis externa (all ages) Specialised Commissioning Team 2 July 218 CCG Clinical Leaders, Care Trust CEs, Foundation Trust CEs, Medical Directors, Directors of PH, Directors of Nursing, NHS England Regional Directors, NHS England Directors of Commissioning Operations, Directors of Finance, NHS Trust CEs Additional Circulation List #VALUE! Description Not for routine commissioning Cross Reference Superseded Docs (if applicable) Action Required Timing / Deadlines (if applicable) Contact Details for further information By January 19 england.specialisedcommissioning@nhs.net Document Status This is a controlled document. Whilst this document may be printed, the electronic version posted on the intranet is the controlled copy. Any printed copies of this document are not controlled. As a controlled document, this document should not be saved onto local or network drives but should always be accessed from the intranet. 2
3 Standard Operating Procedure: Clinical Commissioning Policy: Hyperbaric oxygen therapy for malignant otitis externa (all ages) First published: July 218 Prepared by NHS England Specialised Services Clinical Reference Group for Hyperbaric oxygen therapy Published by NHS England, in electronic format only. 3
4 Contents 1 Introduction Definitions Aims and objectives Epidemiology and needs assessment Evidence base Documents which have informed this policy Date of review References
5 Policy statement NHS England will not routinely commission hyperbaric oxygen therapy for malignant otitis externa (all ages) in accordance with the criteria outlined in this document. In creating this policy NHS England has reviewed this clinical condition and the options for its treatment. It has considered the place of this treatment in current clinical practice, whether scientific research has shown the treatment to be of benefit to patients, (including how any benefit is balanced against possible risks) and whether its use represents the best use of NHS resources. This policy document outlines the arrangements for funding of this treatment for the population in England. Equality statement Promoting equality and addressing health inequalities are at the heart of NHS England s values. Throughout the development of the policies and processes cited in this document, we have: given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 21) and those who do not share it; and given regard to the need to reduce inequalities between patients in access to, and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities. Plain language summary About malignant otitis externa Malignant otitis externa is a disorder that involves infection of and damage to the bones of the ear canal and at the base of the skull. Malignant otitis externa is caused 5
6 by the spread of an outer ear infection (otitis externa) also called swimmer's ear. It is not common (NHS Choices, 215). About current treatments The goal of treatment is to cure the infection. The treatment is taking medicines that fight infections (antibiotics) for a long period of time. The medicines may be given through a vein (intravenously), or by mouth. In some cases, surgery might be needed to remove dead or damaged tissue in the skull. About the new treatment Hyperbaric oxygen therapy (HBOT) has been suggested as an adjuvant treatment (another treatment used together with the primary treatment) of malignant otitis externa. HBOT is delivered by giving a patient oxygen to breathe while in a pressurised chamber so that a higher level of oxygen can be dissolved in the patient s blood plasma. Inhaling oxygen at increased pressure is intended to improve oxygen supply to the infected tissue. During HBOT, the patient is in a pressure chamber, usually for 6 to 12 minutes at least once daily. On average, patients require between 15 and 3 treatments. What we have decided NHS England has carefully reviewed the evidence to treat malignant otitis externa with hyperbaric oxygen therapy. We have concluded that there is not enough evidence to consider making the treatment available at this time. 6
7 1 Introduction About Malignant Otitis Externa Malignant Otitis Externa (MOE) is an uncommon condition mainly found in the elderly or in diabetics (Phillips and Jones 213). It is an aggressive infection involving the external ear canal and the surrounding skull base, mainly the temporal bone. It was first reported in the literature by Toulmouche in Signs and symptoms of malignant otitis externa can include: o severe ear pain and headaches o exposed bone visible in the ear canal o facial nerve palsy where the face droops on the side of the affected ear (NHS Choices, 215). Occasionally, MOE can also be complicated by infections of the parts of the body near to the ear. This can cause parotitis, mastoiditis, jugular vein thrombosis, meningitis and death (Glamarellou 1992). Pseudomonas aeruginosa is the infective organism most commonly isolated from the aural drainage in more than 9% of MOE cases. Diagnosis is based upon clinical, microbiological and radiological findings (Ali et al 21). Current treatment The mainstay of treatment for MOE has been prolonged antibiotic therapy, stringent diabetes control, the repeated removal of dead tissue by surgical management (Glamarellou 1992, Illing and Olaleye 211, Phillips and Jones 213). Proposed intervention HBOT is the breathing of 1% oxygen at pressures above one atmosphere absolute (ATA) (~1 kilopascals [kpa]). HBOT involves placing the patient in a compression chamber, increasing the environmental pressure within the chamber and administering 1% oxygen through a face mask or a transparent hood. In this way, it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. Typically, treatments involve pressurisation to between 23 to 34 kilopascals (kpa) [two and three atmospheres absolute (ATA)] for periods between 7
8 6 and 12 minutes once or twice daily. A typical course might involve 15 to 3 such treatments (Phillips and Jones 213). Hyperbaric oxygen has been proposed as an adjuvant (additional) therapy for MOE and has been included in treatment pathways in places where a therapeutic pressure chamber is available (Shupak et al 1989). It is postulated that HBOT works by increasing oxygen supply to hypovascular tissues. This leads to efficient leukocyte (white blood cell) function which is essential for soft tissue and bone healing as well as infection resolution (Phillips and Jones 213). There are no national evidence-based policies or guidance for the management of patients with MOE. 2 Definitions Avascular: characterized by or associated with a lack of blood vessels. Case reports: An uncontrolled observational study involving an intervention and an outcome in a single patient. Case series: Reports of several patients with a given condition, usually covering the course of the condition and the response to treatment. There is no comparison (control) group of patients. Computerised tomography (CT): a scan that uses X-rays and a computer to create detailed images of the inside of the body. Granulation tissue: new connective tissue and microscopic blood vessels that form on the exposed surfaces of a wound during the healing process. Hypovascular: characterized by or associated with fewer blood vessels than normal. Immunocompromised: inability to respond normally to an infection due to an impaired or weakened immune system. Jugular vein thrombosis: an extremely rare vascular disease. Leukaemia: a cancer which starts in blood-forming tissue, usually the bone marrow. Lymphoma: is an uncommon cancer that develops in the lymphatic system, which is a network of vessels and glands spread throughout the body. 8
9 Mastoiditis: result of an infection that extends to the air cells of the skull behind the ear. Specifically, it is an inflammation of the mucosal lining of the mastoid antrum and mastoid air cell system inside the mastoid process. The mastoid process is the portion of the temporal bone of the skull that is behind the ear which contains open, air-containing spaces. Meningitis: an infection of the protective membranes that surround the brain and spinal cord (meninges). Neoplasia: the presence or formation of new, abnormal growth of tissue. Post-transplantation immunosuppression: drugs or medicines that lower the body's ability to reject a transplanted organ. Parotitis: an inflammation of one or both parotid glands, the major salivary glands located on either side of the face in humans. The parotid gland is the salivary gland most commonly affected by inflammation. Pulmonary: relating to the lungs. Retrospective study: A research study that focuses on the past and present. The study examines past exposure to suspected risk factors for the disease or condition. Splenectomy: is a surgical procedure to remove the spleen Systematic review: A review that summarises the evidence on a clearly formulated review question according to a predefined protocol, using systematic and explicit methods to identify, select and appraise relevant studies, and to extract, analyse, collate and report their findings. 3 Aims and objectives This policy aims to consider the evidence underpinning the use of HBOT for malignant otitis externa. The objectives are to consider whether: the evidence base supports HBOT as a routine adjuvant treatment in the management of malignant otitis externa the evidence base identifies the place of HBOT in the care pathway for the management of malignant otitis externa. there is a subgroup of patients who are most likely to benefit 9
10 the evidence identifies a treatment schedule that is most effective in achieving best outcomes HBOT is cost effective as an adjuvant treatment for malignant otitis externa. 4 Epidemiology and needs assessment MOE is an uncommon condition mainly found in the elderly or in diabetics (Phillips and Jones 213). It is generally suspected in diabetic patients with pseudomonal otitis externa, especially when pain is a prominent feature. Recent reviews have quoted the prevalence of diabetes in MOE cases as 9% to 1% (Berenholz, Katzenell and Harell 22, Mani et al 27). Other immunocompromised states also represent risk factors for MOE development (with or without diabetes) (Shpitzer et al 1993), including human immunodeficiency virus (HIV) infection, acquired immunodeficiency syndrome (AIDS), neoplasia, leukaemia, lymphoma, splenectomy and post-transplantation immunosuppression (Hollis and Evans 211). MOE is more common in males than females (Phillips and Jones 213). The mortality of MOE has been reported to be as high as one third, but when cranial nerves are affected it may be as high as 8% (Phillips and Jones 213). Chawdhary, Liow & Whiteside (215) undertook an analysis of Hospital Episode Statistics in the UK and found a six-fold increase in the number of cases from 1999 (n=67) to 213 (n=421). 5 Evidence base Summary of evidence NHS England commissioned a review of the published evidence on the use of HBOT treatment for malignant otitis externa. To aid in the search for clinically relevant literature, experts in the field of HBOT guided the development of a Population, Intervention, Comparison, Outcome (PICO) framework. Key findings were: Clinical effectiveness The literature search for this rapid evidence review found one systematic review (Phillips and Jones 213), one retrospective controlled study (Sabra et al 215) 1
11 and one case series (Saxby et al 21) which assess the use of HBOT in the management of MOE. A variety of outcomes were reported including pain, ear discharge, complications of HBOT and mortality. Sabra et al (215) carried out a retrospective controlled study to assess the usefulness of HBOT as an adjunctive treatment in diabetic patients with MOE. 28 patients received ciprofloxacin (antibiotics) only while 15 patients were treated with ciprofloxacin and HBOT. HBOT was administered at 2.5 ATA (~253 kpa) for 9 minutes per session every other day for two months. All the patients were evaluated clinically (in terms of ear discharge, granulations, and pain severity) and radiologically by a temporal bone computed tomography scan. The patients were followed up for at least two months. The authors reported the following in patients treated with ciprofloxacin plus HBOT versus those treated with ciprofloxacin only: an improvement in pain in 86.7% vs. 32.1% of patients at one month after 15 sessions (no p-value was reported); freedom from pain in 46.7% vs. % of patients at one month and 93.3% vs. at 28.5% two months (p<.1 for both follow up periods); the absence of purulent ear discharge in 8% vs. % 1 (p<.1) at one month and 93.3% vs. 28.5% (p<.1) at two months (Sabra et al 215). The systematic review did not identify any RCTs; it described four case reports and five case series which included a total of 73 patients (range was not reported). The authors reported that the individual papers described the use of HBOT as adjuvant therapy with antibiotics in the majority of cases. Most regimens used 2 to 4 doses of hyperbaric oxygen treatment. Each treatment was of 9 minutes duration at 2.5 ATA (~253 kpa). However due to the poor quality of studies, lack of randomisation or other controls, they were unable to assess statistically the effectiveness of treatment with HBOT compared with other treatments (Phillips and Jones 213). The retrospective case series of patients with a diagnosis of MOE, referred to a hyperbaric unit for treatment over a period of six years, conducted by Saxby et al included 17 patients. All the patients received HBOT once daily on Monday to Friday on a standard 9-minutes schedule at 243 kpa (2.4 ATA).They reported 1 Not reported to one decimal place therefore inconsistent with other results 11
12 that 12 patients (7%) were disease-free at follow up, so were considered cured of their disease; this included four patients who had died of other causes but were symptom-free at the time of death. Three patients (18%) died from MOE, one after a recurrence of their disease. Two further patients (12%) had recurrent disease, both successfully treated with a second cycle of HBOT and antibiotics (Saxby et al 21). Safety The study carried out by Sabra et al did not report any complications associated with HBOT. The case reports and case series described in the Cochrane review did not report any complications or adverse events related to hyperbaric oxygen treatment (Phillips and Jones 213). However, the retrospective review conducted by Saxby et al reported that five out of 17 patients (29%) had complications attributable to HBOT; acute pulmonary oedema (n = 2), seizure (n = 1), tympanic membrane perforation (n = 1) and claustrophobia (n = 1). Cost-effectiveness No studies on cost-effectiveness were identified. Conclusion The published literature on the use of HBOT in MOE is limited to very small unrandomised studies; it is therefore not robust enough to make blanket recommendations. The evidence for the clinical effectiveness of HBOT as adjunctive treatment in the management of MOE is limited to small case series and retrospective studies. Although the reported results suggest that this intervention may be effective, the studies have a number of limitations. For this reason the results may not be reliable or generalisable. In summary, while HBOT may be useful as an adjunct in the management of MOE, there is insufficient evidence to make clear recommendations particularly in comparison to alternative treatments. The low prevalence of MOE is likely to make 12
13 randomised control trials of HBOT in this indication very difficult to complete. However study designs that encompass a more systematic approach to the diagnosis, standard care, outcome measures and place of HBOT in the care pathway would help to reduce the uncertainty. 6 Documents which have informed this policy This document replaces the present NHS England Clinical Commissioning Policy (213): Hyperbaric Oxygen Therapy: NHSCB/D11/P/a: 7 Date of review This document will be reviewed when information is received which indicates that the policy requires revision. 13
14 References Ali, T., Meade, K., Anari, S., ElBadawey, M. and Zammit-Maempel, I. (21). Malignant otitis externa: case series. The Journal of Laryngology & Otology, 124(8), pp Berenholz, L., Katzenell, U. and Harell, M. (22). Evolving Resistant Pseudomonas to Ciprofloxacin in Malignant Otitis Externa. The Laryngoscope, 112(9), pp Chandler, J. (1968). Malignant external otitis. The Laryngoscope, 78(8), pp Glamarellou, H. (1992). Malignant otitis externa: the therapeutic evolution of a lethal infection. Journal of Antimicrobial Chemotherapy, 3(6), pp Hollis, S. and Evans, K. (211). Management of malignant (necrotising) otitis externa. The Journal of Laryngology & Otology, 125(12), pp Illing, E and Olaleye, O. (211). Malignant otitis externa: a review of aetiology, presentation, investigations and current management strategies. WebmedCentral Otorhinolaringology 211; 2(3):WMC1725 doi: /journal.wmc Mani, N., Sudhoff, H., Rajagopal, S., Moffat, D. and Axon, P. (27). Cranial Nerve Involvement in Malignant External Otitis: Implications for Clinical Outcome. The Laryngoscope, 117(5), pp NHS Choices, 215. Otitis externa Complications. [online] Available at: [Accessed 16 Oct. 217]. Phillips JS and Jones SEM. (213). Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database of Systematic Reviews Issue 5. Art. No.: CD
15 Sabra, R., Taha, M., Elsamny, T. and Khafagy, A. (215). Value of hyperbaric oxygen therapy in the management of malignant otitis externa patients. The Egyptian Journal of Otolaryngology, 31(3), p.143. Saxby A, Barakate M, Kertesz T, James J, Bennett M. (21). Malignant otitis externa: experience with hyperbaric oxygen therapy. Diving Hyperb Med 4 pp Shpitzer, T., Levy, R., Stern, Y., Segal, K., Cohen, O. and Feinmesser, R. (1993). Malignant External Otitis in Nondiabetic Patients. Annals of Otology, Rhinology & Laryngology, 12(11), pp Shupak, A., Greenberg, E., Hardoff, R., Gordon, C., Melamed, Y. and Meyer, W. (1989). Hyperbaric Oxygenation for Necrotizing (Malignant) Otitis Externa. Archives of Otolaryngology - Head and Neck Surgery, 115(12), pp Sobie, S., Brodsky, L. and Stanievich, J. (1987). Necrotizing External Otitis in Children. The Laryngoscope, 97(5), pp
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages)
 Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults
 Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages)
 Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England: 16029/P NHS England INFORMATION READER BOX Directorate Medical Operations and
Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England: 16029/P NHS England INFORMATION READER BOX Directorate Medical Operations and
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae
 Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Reference: NHS England: 16022/P
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages)
 Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages)
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages)
 Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.14 Shingles immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations
NHS public health functions agreement 2016-17 Service specification No.14 Shingles immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations
Clinical Commissioning Policy: Hyperbaric oxygen therapy for diabetic lower limb ulceration (diabetic foot ulcer) (all ages)
 Clinical Commissioning Policy: Hyperbaric oxygen therapy for diabetic lower limb ulceration (diabetic foot ulcer) (all ages) NHS England Reference: 1712P 1 NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Hyperbaric oxygen therapy for diabetic lower limb ulceration (diabetic foot ulcer) (all ages) NHS England Reference: 1712P 1 NHS England INFORMATION READER BOX Directorate
Editorial Manager(tm) for European Archives of Oto-Rhino-Laryngology and Head & Neck Manuscript Draft
 Editorial Manager(tm) for European Archives of Oto-Rhino-Laryngology and Head & Neck Manuscript Draft Manuscript Number: EAORL-D-07-00196 Title: Usefulness of CT scans in malignant external otitis: Effective
Editorial Manager(tm) for European Archives of Oto-Rhino-Laryngology and Head & Neck Manuscript Draft Manuscript Number: EAORL-D-07-00196 Title: Usefulness of CT scans in malignant external otitis: Effective
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome. September 2013 Reference: E03/PS(HSS)/a.
 Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy Proposition: Hyperbaric Oxygen Therapy for necrotising soft tissue infections
 Clinical Commissioning Policy Proposition: Hyperbaric Oxygen Therapy for necrotising soft tissue infections Reference: NHS England 1776 Prepared by NHS England Specialised Services Clinical Reference Group
Clinical Commissioning Policy Proposition: Hyperbaric Oxygen Therapy for necrotising soft tissue infections Reference: NHS England 1776 Prepared by NHS England Specialised Services Clinical Reference Group
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.7 Hib/MenC vaccination programme NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information
NHS public health functions agreement 2016-17 Service specification No.7 Hib/MenC vaccination programme NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Insert heading depending line on length; please delete delete. length; please delete other cover options once
 Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease Reference: NHS England: 16032/P 2 NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease Reference: NHS England: 16032/P 2 NHS England INFORMATION READER BOX Directorate
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.4 Immunisation against diphtheria, tetanus, poliomyelitis, pertussis and Hib programme Classification: official NHS England INFORMATION
NHS public health functions agreement 2016-17 Service specification No.4 Immunisation against diphtheria, tetanus, poliomyelitis, pertussis and Hib programme Classification: official NHS England INFORMATION
Reference: NHS England 1602
 Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult)
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Human coagulation factor X for hereditary factor X deficiency (all ages)
 Clinical Commissioning Policy: Human coagulation factor X for hereditary factor X deficiency (all ages) NHS England Reference: 170105P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Human coagulation factor X for hereditary factor X deficiency (all ages) NHS England Reference: 170105P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
How not to miss malignant otitis externa: The secrets of radiological diagnosis
 How not to miss malignant otitis externa: The secrets of radiological diagnosis Poster No.: C-1788 Congress: ECR 2010 Type: Educational Exhibit Topic: Head and Neck Authors: A. Romsauerova, J. Brunton;
How not to miss malignant otitis externa: The secrets of radiological diagnosis Poster No.: C-1788 Congress: ECR 2010 Type: Educational Exhibit Topic: Head and Neck Authors: A. Romsauerova, J. Brunton;
The Role of Hyperbaric Oxygen Therapy in Necrotizing Otitis Externa: 8 Case Reports Review
 ISSN: 2250-0359 The Role of Hyperbaric Oxygen Therapy in Necrotizing Otitis Externa: 8 Case Reports Review Borges-Costa Joana 1*, Abreu Pereira Diogo 1, Duarte Delfim 1, Viana Miguel 1, Fernandes Tiago
ISSN: 2250-0359 The Role of Hyperbaric Oxygen Therapy in Necrotizing Otitis Externa: 8 Case Reports Review Borges-Costa Joana 1*, Abreu Pereira Diogo 1, Duarte Delfim 1, Viana Miguel 1, Fernandes Tiago
Malignant Otitis Externa: A Review of Aetiology, Presentation, Investigations and Current Management Strategies
 Article ID: WMC001725 ISSN 2046-1690 Malignant Otitis Externa: A Review of Aetiology, Presentation, Investigations and Current Management Strategies Author(s):Ms. Elizabeth Illing, Mr. Oladejo Olaleye
Article ID: WMC001725 ISSN 2046-1690 Malignant Otitis Externa: A Review of Aetiology, Presentation, Investigations and Current Management Strategies Author(s):Ms. Elizabeth Illing, Mr. Oladejo Olaleye
NHS England. Evidence review: Hyperbaric oxygen therapy for necrotising soft tissue infections
 NHS England Evidence review: Hyperbaric oxygen therapy for necrotising soft tissue infections NHS England Evidence review: Hyperbaric oxygen therapy for necrotising soft tissue infections First published:
NHS England Evidence review: Hyperbaric oxygen therapy for necrotising soft tissue infections NHS England Evidence review: Hyperbaric oxygen therapy for necrotising soft tissue infections First published:
POLICY FOR TREATMENT OF UPPER RESPIRATORY TRACT INFECTIONS
 POLICY F TREATMENT OF UPPER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: July 2013 Approved by: The Drugs & Therapeutics Committee Date: April 2016 Implementation
POLICY F TREATMENT OF UPPER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: July 2013 Approved by: The Drugs & Therapeutics Committee Date: April 2016 Implementation
Management of Malignant Otitis Externa
 Bahrain Medical Bulletin, Vol.25, No. 4, December 2003 Management of Malignant Otitis Externa Ashoor Abdul Aziz, FacArzt* AbuBakr M.Yasser, FRCS Dublin** Objective: The aim of this study is to present
Bahrain Medical Bulletin, Vol.25, No. 4, December 2003 Management of Malignant Otitis Externa Ashoor Abdul Aziz, FacArzt* AbuBakr M.Yasser, FRCS Dublin** Objective: The aim of this study is to present
Hyperbaric oxygen therapy Villanueva E, Johnston R, Clavisi O, Burrows E, Bernath V, Rajendran M, Wasiak J, Fennessy P, Anderson J, Harris A, Yong K
 Hyperbaric oxygen therapy Villanueva E, Johnston R, Clavisi O, Burrows E, Bernath V, Rajendran M, Wasiak J, Fennessy P, Anderson J, Harris A, Yong K Authors' objectives To assess the effectiveness of hyperbaric
Hyperbaric oxygen therapy Villanueva E, Johnston R, Clavisi O, Burrows E, Bernath V, Rajendran M, Wasiak J, Fennessy P, Anderson J, Harris A, Yong K Authors' objectives To assess the effectiveness of hyperbaric
Clinical Commissioning Policy: Bortezomib for relapsed/refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bortezomib for relapsed/refractory mantle cell lymphoma (all ages) NHS England Reference: 1735P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Bortezomib for relapsed/refractory mantle cell lymphoma (all ages) NHS England Reference: 1735P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
ACUTE PAEDIATRIC EAR PRESENTATIONS PROF IAIN BRUCE PAEDIATRIC OTOLARYNGOLOGIST & ADULT OTOLOGIST
 www.manchesterchildrensent.com ACUTE PAEDIATRIC EAR PRESENTATIONS PROF IAIN BRUCE PAEDIATRIC OTOLARYNGOLOGIST & ADULT OTOLOGIST A CHILD WITH EARACHE UNCOMPLICATED AOM ACUTE OTITIS MEDIA Acute otitis media
www.manchesterchildrensent.com ACUTE PAEDIATRIC EAR PRESENTATIONS PROF IAIN BRUCE PAEDIATRIC OTOLARYNGOLOGIST & ADULT OTOLOGIST A CHILD WITH EARACHE UNCOMPLICATED AOM ACUTE OTITIS MEDIA Acute otitis media
GROMMET INSERTION RECURRENT ACUTE OTITIS MEDIA (WITHOUT EFFUSION) SECONDARY CARE PRIOR APPROVAL POLICY
 Version: 1718.v1 Ratified by: SCCG COG Date Ratified: 05 April 2017 Name of Originator/Author: Name of Responsible Committee/Individual: IFR SCCG CCPF/ IFR Date issued: 18 April 2017 Review date: Target
Version: 1718.v1 Ratified by: SCCG COG Date Ratified: 05 April 2017 Name of Originator/Author: Name of Responsible Committee/Individual: IFR SCCG CCPF/ IFR Date issued: 18 April 2017 Review date: Target
Malignant otitis externa: An assessment of emerging pathogens and the prognostic factors
 Vol. 9(7), pp. 86-91, July 2017 DOI: 10.5897/IJMMS2017.1305 Article Number: B9FAA0D64801 ISSN 2006-9723 Copyright 2017 Author(s) retain the copyright of this article http://www.academicjournals.org/ijmms
Vol. 9(7), pp. 86-91, July 2017 DOI: 10.5897/IJMMS2017.1305 Article Number: B9FAA0D64801 ISSN 2006-9723 Copyright 2017 Author(s) retain the copyright of this article http://www.academicjournals.org/ijmms
Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages)
 Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages) NHS England Reference: 170065P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages) NHS England Reference: 170065P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information
NHS public health functions agreement Service specification No.14 Shingles (herpes zoster) immunisation programme
 NHS public health functions agreement 2018-19 Service specification No.14 Shingles (herpes zoster) immunisation programme 1 NHS public health functions agreement 2018-19 Service specification No.14 Shingles
NHS public health functions agreement 2018-19 Service specification No.14 Shingles (herpes zoster) immunisation programme 1 NHS public health functions agreement 2018-19 Service specification No.14 Shingles
ACUTE MASTOIDITIS IN CHILDREN: SUSCEPTIBILITY FACTORS AND MANAGEMENT
 & ACUTE MASTOIDITIS IN CHILDREN: SUSCEPTIBILITY FACTORS AND MANAGEMENT Slobodan Spremo*, Biljana Udovčić Clinic for Otorhinolaryngology, Clinic Center in Banja Luka, Zdrave Korde 1, 78000 Banja Luka, Bosnia
& ACUTE MASTOIDITIS IN CHILDREN: SUSCEPTIBILITY FACTORS AND MANAGEMENT Slobodan Spremo*, Biljana Udovčić Clinic for Otorhinolaryngology, Clinic Center in Banja Luka, Zdrave Korde 1, 78000 Banja Luka, Bosnia
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care
 CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care Technical guidance NHS England INFORMATION READER BOX Directorate
CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care Technical guidance NHS England INFORMATION READER BOX Directorate
Brain Tumors. What is a brain tumor?
 Scan for mobile link. Brain Tumors A brain tumor is a collection of abnormal cells that grows in or around the brain. It poses a risk to the healthy brain by either invading or destroying normal brain
Scan for mobile link. Brain Tumors A brain tumor is a collection of abnormal cells that grows in or around the brain. It poses a risk to the healthy brain by either invading or destroying normal brain
Clinical commissioning policy statement: Arsenic trioxide for the treatment of high risk acute promyelocytic leukaemia (all ages)
 Clinical commissioning policy statement: Arsenic trioxide for the treatment of high risk acute promyelocytic leukaemia (all ages) NHS England Reference: 170072P 1 Contents 1 Plain language summary... 3
Clinical commissioning policy statement: Arsenic trioxide for the treatment of high risk acute promyelocytic leukaemia (all ages) NHS England Reference: 170072P 1 Contents 1 Plain language summary... 3
POLICY FOR TREATMENT OF UPPER RESPIRATORY TRACT INFECTIONS
 POLICY F TREATMENT OF UPPER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: Date: July 2018 The Drugs & Therapeutics Committee Implementation
POLICY F TREATMENT OF UPPER RESPIRATY TRACT INFECTIONS Written by: Dr M Milupi, Consultant Microbiologist Date: June 2018 Approved by: Date: July 2018 The Drugs & Therapeutics Committee Implementation
Clinical Commissioning Policy: Bendamustine with rituximab for first line treatment of mantle cell lymphoma (all ages)
 Clinical Commissioning Policy: Bendamustine with rituximab for first line treatment of mantle cell lymphoma (all ages) NHS England Reference: 17088P NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for first line treatment of mantle cell lymphoma (all ages) NHS England Reference: 17088P NHS England INFORMATION READER BOX Directorate Medical
DRAFT FOR CONSULTATION. Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain
 Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages and over, with Publications Gateway
Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages and over, with Publications Gateway
Aligning the Publication of Performance Data Statistics Consultation
 Aligning the Publication of Performance Data Statistics Consultation NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information Nursing Trans. & Corp. Ops.
Aligning the Publication of Performance Data Statistics Consultation NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information Nursing Trans. & Corp. Ops.
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency
 Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Case Report Synchronous Malignant Otitis Externa and Squamous Cell Carcinoma of the External Auditory Canal
 Case Reports in Otolaryngology Volume 2013, Article ID 837169, 4 pages http://dx.doi.org/10.1155/2013/837169 Case Report Synchronous Malignant Otitis Externa and Squamous Cell Carcinoma of the External
Case Reports in Otolaryngology Volume 2013, Article ID 837169, 4 pages http://dx.doi.org/10.1155/2013/837169 Case Report Synchronous Malignant Otitis Externa and Squamous Cell Carcinoma of the External
Research Article Temporal Bone Osteomyelitis: The Relationship with Malignant Otitis Externa, the Diagnostic Dilemma, and Changing Trends
 e Scientific World Journal, Article ID 591714, 10 pages http://dx.doi.org/10.1155/2014/591714 Research Article Temporal Bone Osteomyelitis: The Relationship with Malignant Otitis Externa, the Diagnostic
e Scientific World Journal, Article ID 591714, 10 pages http://dx.doi.org/10.1155/2014/591714 Research Article Temporal Bone Osteomyelitis: The Relationship with Malignant Otitis Externa, the Diagnostic
If you have any further questions, please speak to a doctor or nurse caring for you.
 Surgical Removal of a Paraganglioma of the Temporal Bone This leaflet explains more about surgery for the removal of a paraganglioma of the temporal bone, including the benefits, risks and any alternatives
Surgical Removal of a Paraganglioma of the Temporal Bone This leaflet explains more about surgery for the removal of a paraganglioma of the temporal bone, including the benefits, risks and any alternatives
QUESTIONS What are the effects of empirical treatments for otitis externa?... 4
 Search date October 213 Daniel Hajioff and Samuel Mackeith ABSTRACT INTRODUCTION: is thought to affect 1% of people at some stage, and can present in acute, chronic, or necrotising forms. may be associated
Search date October 213 Daniel Hajioff and Samuel Mackeith ABSTRACT INTRODUCTION: is thought to affect 1% of people at some stage, and can present in acute, chronic, or necrotising forms. may be associated
NHS public health functions agreement Service specification No.1 Neonatal hepatitis B immunisation programme
 NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Clinical Commissioning Policy: Continuous aztreonam lysine for cystic fibrosis (all ages)
 Clinical Commissioning Policy: Continuous aztreonam lysine for cystic fibrosis (all ages) Reference: NHS England: 16001/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Continuous aztreonam lysine for cystic fibrosis (all ages) Reference: NHS England: 16001/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Informed Consent for Liver Transplant Patients
 Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages)
 Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages) Reference: NHS England 1607 1 First published: TBC Prepared by NHS England
Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages) Reference: NHS England 1607 1 First published: TBC Prepared by NHS England
Management of Common ENT Cases MS ANN O CONNOR MD FRCS (ORL-HNS) BEACON HOSPITAL 21 ST APRIL 2018
 Management of Common ENT Cases MS ANN O CONNOR MD FRCS (ORL-HNS) BEACON HOSPITAL 21 ST APRIL 2018 Introduction GP referrals to ENT services Highest of all specialities Number of patients waiting an OPD
Management of Common ENT Cases MS ANN O CONNOR MD FRCS (ORL-HNS) BEACON HOSPITAL 21 ST APRIL 2018 Introduction GP referrals to ENT services Highest of all specialities Number of patients waiting an OPD
OFFICIAL. Document Status. NHS England INFORMATION READER BOX
 NHS England INFORMATION READER BOX OFFICIAL Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Strategy & Innovation Finance Publications Gateway Reference:
NHS England INFORMATION READER BOX OFFICIAL Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Strategy & Innovation Finance Publications Gateway Reference:
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
SYLLABUS FOR M.S. DEGREE COURSE IN E.N.T.
 SYLLABUS FOR M.S. DEGREE COURSE IN E.N.T. First year POST GRADUATE M.S. DEGREE COURSE IN E.N.T. TRAINING PROGRAMME E.N.T. 5 months General Surgery 1 month General Medicine Ward 1 month Anaesthesia 1 month
SYLLABUS FOR M.S. DEGREE COURSE IN E.N.T. First year POST GRADUATE M.S. DEGREE COURSE IN E.N.T. TRAINING PROGRAMME E.N.T. 5 months General Surgery 1 month General Medicine Ward 1 month Anaesthesia 1 month
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate
 Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate Reference: NHS England B01X09 First published: March 2016 Prepared by NHS England Specialised Services Clinical
Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate Reference: NHS England B01X09 First published: March 2016 Prepared by NHS England Specialised Services Clinical
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV infection (all ages) Reference: NHS England F03/P/b
 Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV infection (all ages) Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV infection (all ages) Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Skull Base Tumour Service. The Multi-Disciplinary Team (MDT) Explained. Jan 2018 v1
 Skull Base Tumour Service The Multi-Disciplinary Team (MDT) Explained Jan 2018 v1 Skull base tumours grow in the bones of the skull that form the bottom of the head and the body ridge between the nose
Skull Base Tumour Service The Multi-Disciplinary Team (MDT) Explained Jan 2018 v1 Skull base tumours grow in the bones of the skull that form the bottom of the head and the body ridge between the nose
NHS public health functions agreement Service specification No.5 Rotavirus immunisation programme
 NHS public health functions agreement 2018-19 Service specification No.5 Rotavirus immunisation programme Classification: official 1 NHS public health functions agreement 2018-19 Service specification
NHS public health functions agreement 2018-19 Service specification No.5 Rotavirus immunisation programme Classification: official 1 NHS public health functions agreement 2018-19 Service specification
CAT 13. Coping with cancer. The charity dedicated to helping sick, injured and homeless pets since 1897.
 CAT 13 Coping with cancer The charity dedicated to helping sick, injured and homeless pets since 1897. Coping with cancer As with people, cats commonly get cancer, especially as they get older although
CAT 13 Coping with cancer The charity dedicated to helping sick, injured and homeless pets since 1897. Coping with cancer As with people, cats commonly get cancer, especially as they get older although
Mastoidectomy or combined approach tympanoplasty
 PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a cholesteatoma? This is a cyst of skin cells behind your eardrum. As it gets
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a cholesteatoma? This is a cyst of skin cells behind your eardrum. As it gets
Blood Cancer: Chronic Myelogenous Leukaemia
 Published on: 30 May 2017 Blood Cancer: Chronic Myelogenous Leukaemia What Is Cancer? The body is made up of cells that grow and die in a controlled way. Sometimes, cells keep dividing and growing without
Published on: 30 May 2017 Blood Cancer: Chronic Myelogenous Leukaemia What Is Cancer? The body is made up of cells that grow and die in a controlled way. Sometimes, cells keep dividing and growing without
Radiotherapy in feline and canine head and neck cancer
 Bettina Kandel Like surgery radiotherapy is usually a localized type of treatment. Today it is more readily available for the treatment of cancer in companion animals and many clients are well informed
Bettina Kandel Like surgery radiotherapy is usually a localized type of treatment. Today it is more readily available for the treatment of cancer in companion animals and many clients are well informed
What is head and neck cancer? How is head and neck cancer diagnosed and evaluated? How is head and neck cancer treated?
 Scan for mobile link. Head and Neck Cancer Head and neck cancer is a group of cancers that start in the oral cavity, larynx, pharynx, salivary glands, nasal cavity or paranasal sinuses. They usually begin
Scan for mobile link. Head and Neck Cancer Head and neck cancer is a group of cancers that start in the oral cavity, larynx, pharynx, salivary glands, nasal cavity or paranasal sinuses. They usually begin
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis. Reference: NHS England: /PS
 Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Paediatric ENT problems
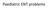 Paediatric ENT problems Ears Otitis media Otitis media with effusion FBs Otitis externa Ruptured TM Nose FBs Allergic rhinitis Septal perforation expistaxis Throat FB Croup Stidor Tonsillitis Paediatric
Paediatric ENT problems Ears Otitis media Otitis media with effusion FBs Otitis externa Ruptured TM Nose FBs Allergic rhinitis Septal perforation expistaxis Throat FB Croup Stidor Tonsillitis Paediatric
Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults)
 Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults) Reference: NHS England: 16050/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults) Reference: NHS England: 16050/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Royal Victoria Hospital Montreal General Hospital Jewish General Hospital. Department of Otolaryngology Head and Neck Surgery
 Royal Victoria Hospital Montreal General Hospital Jewish General Hospital Department of Otolaryngology Head and Neck Surgery A. GENERAL COMPETENCIES ( )denotes optional competencies At the completion of
Royal Victoria Hospital Montreal General Hospital Jewish General Hospital Department of Otolaryngology Head and Neck Surgery A. GENERAL COMPETENCIES ( )denotes optional competencies At the completion of
Specialised Services Policy Position PP104
 Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Retroperineal Lymph Node Dissection (RPLND)
 Acute Services Division Information for patients about Retroperineal Lymph Node Dissection (RPLND) Introduction This booklet gives you information about surgery to remove the residual lymph nodes at the
Acute Services Division Information for patients about Retroperineal Lymph Node Dissection (RPLND) Introduction This booklet gives you information about surgery to remove the residual lymph nodes at the
Gangrene. Introduction Gangrene is the death of tissues in your body. It happens when a part of your body loses its blood supply.
 Gangrene Introduction Gangrene is the death of tissues in your body. It happens when a part of your body loses its blood supply. Gangrene can happen on the surface of the body, such as on the skin. It
Gangrene Introduction Gangrene is the death of tissues in your body. It happens when a part of your body loses its blood supply. Gangrene can happen on the surface of the body, such as on the skin. It
Evidence Review: Title. Month/ Year. Evidence Summary: Hyperbaric Oxygen Therapy
 Evidence Review: Title Month/ Year Evidence Summary: Hyperbaric Oxygen Therapy May 2015 1 Standard Operating Procedure: NHS England Evidence Summary: Hyperbaric Oxygen Therapy First published: Updated:
Evidence Review: Title Month/ Year Evidence Summary: Hyperbaric Oxygen Therapy May 2015 1 Standard Operating Procedure: NHS England Evidence Summary: Hyperbaric Oxygen Therapy First published: Updated:
Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages)
 Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England E03X05/01 Information Reader Box (IRB) to be inserted on inside
Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England E03X05/01 Information Reader Box (IRB) to be inserted on inside
NHS England. Evidence review: Hyperbaric oxygen therapy for diabetic lower limb ulceration refractory to best standard care
 NHS England Evidence review: Hyperbaric oxygen therapy for diabetic lower limb ulceration refractory to best standard care NHS England Evidence review: Hyperbaric oxygen therapy for diabetic lower limb
NHS England Evidence review: Hyperbaric oxygen therapy for diabetic lower limb ulceration refractory to best standard care NHS England Evidence review: Hyperbaric oxygen therapy for diabetic lower limb
THE MANAGEMENT of COMPLICATED OTITIS MEDIA. IFOS, Lima, 2018
 THE MANAGEMENT of COMPLICATED OTITIS MEDIA IFOS, Lima, 2018 VINCENT C COUSINS ENT-Otoneurology Unit, The Alfred Hospital & Department of Surgery. Monash University MELBOURNE, AUSTRALIA Otologic Complications
THE MANAGEMENT of COMPLICATED OTITIS MEDIA IFOS, Lima, 2018 VINCENT C COUSINS ENT-Otoneurology Unit, The Alfred Hospital & Department of Surgery. Monash University MELBOURNE, AUSTRALIA Otologic Complications
diagnosis Temporal bone fractures: a clinical
 Archives of Emergency Medicine, 1988, 5, 146-150 Temporal bone fractures: a clinical diagnosis J. WALDRON & S. E. J. HURLEY Department of Ear Nose and Throat Surgery, St Mary's Hospital, London, England
Archives of Emergency Medicine, 1988, 5, 146-150 Temporal bone fractures: a clinical diagnosis J. WALDRON & S. E. J. HURLEY Department of Ear Nose and Throat Surgery, St Mary's Hospital, London, England
The AAO- HNS s position statement on Point- of- Care Imaging in Otolaryngology states that the AAO- HNS,
 AAO- HNS Statement on Diagnostic Imaging Reimbursement for Otolaryngologist Head and Neck Surgeons (September 2014) The American Academy of Otolaryngology Head and Neck Surgery (AAO- HNS), with approximately
AAO- HNS Statement on Diagnostic Imaging Reimbursement for Otolaryngologist Head and Neck Surgeons (September 2014) The American Academy of Otolaryngology Head and Neck Surgery (AAO- HNS), with approximately
All about the adult cystic fibrosis service
 All about the adult cystic fibrosis service All about the adult cystic fibrosis service Lay Introduction to Cystic Fibrosis Service Specification Since April 2013, NHS England has taken on direct responsibility
All about the adult cystic fibrosis service All about the adult cystic fibrosis service Lay Introduction to Cystic Fibrosis Service Specification Since April 2013, NHS England has taken on direct responsibility
Clinical Commissioning Policy: Sapropterin for Phenylketonuria (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Sapropterin for Phenylketonuria (all ages) NHS England Reference: 1713P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning
Clinical Commissioning Policy: Sapropterin for Phenylketonuria (all ages) NHS England Reference: 1713P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning
London Strategic Clinical Networks. My AKI. Guidance for patients with, or recovering from, acute kidney injury
 London Strategic Clinical Networks My AKI Guidance for patients with, or recovering from, acute kidney injury Supporting the delivery of equitable, high quality AKI care through collaboration www.londonaki.net
London Strategic Clinical Networks My AKI Guidance for patients with, or recovering from, acute kidney injury Supporting the delivery of equitable, high quality AKI care through collaboration www.londonaki.net
Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis
 Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis Reference: NHS England: 16025/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised
Clinical Commissioning Policy: Gastroelectrical stimulation for gastroparesis Reference: NHS England: 16025/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised
Kingdom of Bahrain Arabian Gulf University College of Medicine and Medical Sciences Year 6 ENT SMC Otitis Media (Dr.
 Kingdom of Bahrain Arabian Gulf University College of Medicine and Medical Sciences Year 6 ENT SMC Otitis Media (Dr. Jalal Almarzooq) - Anatomy of the ear: The ear is divided into 3 parts: External ear.
Kingdom of Bahrain Arabian Gulf University College of Medicine and Medical Sciences Year 6 ENT SMC Otitis Media (Dr. Jalal Almarzooq) - Anatomy of the ear: The ear is divided into 3 parts: External ear.
Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults)
 Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults) Reference: NHS England 1621 First published: Month Year Prepared
Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults) Reference: NHS England 1621 First published: Month Year Prepared
Clinical commissioning policy: Bendamustine with rituximab for first line treatment of advanced indolent non-hodgkin s lymphoma (all ages)
 Clinical commissioning policy: Bendamustine with rituximab for first line treatment of advanced indolent non-hodgkin s lymphoma (all ages) NHS England Reference: 170055P 1 NHS England INFORMATION READER
Clinical commissioning policy: Bendamustine with rituximab for first line treatment of advanced indolent non-hodgkin s lymphoma (all ages) NHS England Reference: 170055P 1 NHS England INFORMATION READER
Head and neck cancer - patient information guide
 Head and neck cancer - patient information guide The development of reconstructive surgical techniques in the last 20 years has led to major advances in the treatment of patients with head and neck cancer.
Head and neck cancer - patient information guide The development of reconstructive surgical techniques in the last 20 years has led to major advances in the treatment of patients with head and neck cancer.
Radiotherapy for lymphoma
 Radiotherapy for lymphoma The name of your consultant is: The radiographer who explained the treatment to you is: You can contact us on: What is radiotherapy? Radiotherapy treats cancer by using high energy
Radiotherapy for lymphoma The name of your consultant is: The radiographer who explained the treatment to you is: You can contact us on: What is radiotherapy? Radiotherapy treats cancer by using high energy
Case Report Squamous Cell Carcinoma of the External Auditory Canal: ACaseReport
 Case Reports in Otolaryngology Volume 2011, Article ID 615210, 4 pages doi:10.1155/2011/615210 Case Report Squamous Cell Carcinoma of the External Auditory Canal: ACaseReport Harry Boamah, 1 Glenn Knight,
Case Reports in Otolaryngology Volume 2011, Article ID 615210, 4 pages doi:10.1155/2011/615210 Case Report Squamous Cell Carcinoma of the External Auditory Canal: ACaseReport Harry Boamah, 1 Glenn Knight,
Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults)
 Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults) NHS England Reference: 170083P 1 Contents 1. Plain Language Summary... 3 2.
Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults) NHS England Reference: 170083P 1 Contents 1. Plain Language Summary... 3 2.
Squamous cell carcinoma
 Squamous cell carcinoma Skin Oncology Team Patient Information Leaflet Introduction This leaflet has been written to help you understand more about squamous cell carcinomas of the skin. It tells you what
Squamous cell carcinoma Skin Oncology Team Patient Information Leaflet Introduction This leaflet has been written to help you understand more about squamous cell carcinomas of the skin. It tells you what
Stereotactic ablative body radiotherapy to the lung Information for patients
 Oxford University Hospitals NHS Trust Stereotactic ablative body radiotherapy to the lung Information for patients page 2 Introduction This leaflet aims to provide information for patients and relatives
Oxford University Hospitals NHS Trust Stereotactic ablative body radiotherapy to the lung Information for patients page 2 Introduction This leaflet aims to provide information for patients and relatives
KTE-C19 for relapsed or refractory mantle cell lymphoma
 NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
Klinikleitung: Dr. Kessler Dr. Kosfeld Dr. Tassani-Prell Dr. Bessmann. Radiotherapy in feline and canine head and neck cancer.
 Radiotherapy in feline and canine head and neck cancer Bettina Kandel Like surgery radiotherapy is usually a localized type of treatment. Today it is more readily available for the treatment of cancer
Radiotherapy in feline and canine head and neck cancer Bettina Kandel Like surgery radiotherapy is usually a localized type of treatment. Today it is more readily available for the treatment of cancer
Bowel Cancer Information Leaflet THE DIGESTIVE SYSTEM
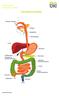 THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
