Tamoxifen for the Prevention of Breast Cancer: Late Results of the Italian Randomized Tamoxifen Prevention Trial Among Women With Hysterectomy
|
|
|
- Adam Newman
- 5 years ago
- Views:
Transcription
1 ARTICLE Tamoxifen for the Prevention of Breast Cancer: Late Results of the Italian Randomized Tamoxifen Prevention Trial Among Women With Hysterectomy Umberto Veronesi, Patrick Maisonneuve, Nicole Rotmensz, Bernardo Bonanni, Peter Boyle, Giuseppe Viale, Alberto Costa, Virgilio Sacchini, Roberto Travaglini, Giuseppe D Aiuto, Pasquale Oliviero, Francesco Lovison, Giacomo Gucciardo, Marco Rosselli del Turco, Maria Grazia Muraca, Maria Antonietta Pizzichetta, Serafino Conforti, Andrea Decensi For the Italian Tamoxifen Study Group Background Methods Initial findings of the Italian Randomized Tamoxifen Prevention Trial found no reduction in risk of breast cancer with tamoxifen use, whereas the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial showed that tamoxifen treatment reduces risk of estrogen receptor positive breast cancer. Here we present an extended follow-up of the Italian trial. From October 1, 1992, to December 31, 1997, 5408 otherwise healthy women who had undergone hysterectomy were randomly assigned in a double-blind manner to tamoxifen (20 mg daily) or placebo for 5 years. Rates of breast cancer and other events in the two groups were compared by the use of risk ratios (RRs) and 95% confidence intervals (CIs). Results After 11 years of follow-up, 136 women (74 placebo, 62 tamoxifen) developed breast cancer (RR = 0.84, 95% CI = 0.60 to 1.17; annual rates were 2.48 and 2.07 per 1000 women-years, respectively). The rates of breast cancer in the two study groups were similar among women who had had bilateral oophorectomy and among women at low risk for hormone receptor positive (HR+) disease but were much lower in the tamoxifen group among women at high risk (placebo, 6.26 per 1000 women-years, tamoxifen, 1.50 per 1000 women-years; RR = 0.24, 95% CI = 0.10 to 0.59). During the treatment period, women in the tamoxifen group reported more hot flashes (RR = 1.78, 95% CI = 1.57 to 2.00), vaginal discharge (RR = 3.44, 95% CI = 2.90 to 4.09), and urinary disturbances (RR = 1.52, 95% CI = 1.23 to 1.89) but fewer headaches (RR = 0.68, 95% CI = 0.50 to 0.94) than women in the placebo group. Hypertriglyceridemia (RR = 4.33, 95% CI = 1.96 to 9.53), thromboembolic events (RR = 1.63, 95% CI = 1.02 to 2.62), and cardiac arrhythmia or atrial fibrillation (RR = 1.73, 95% CI = 1.01 to 2.98) were also more frequent in the tamoxifen group than in the placebo group. Conclusions Appropriate selection of women at high risk for HR+ disease may improve the risk benefit ratio of tamoxifen intervention. J Natl Cancer Inst 2007;99: The benefit of tamoxifen in reducing the risk of contralateral breast cancer among women with early-stage breast cancer provided the rationale for large-scale chemoprevention trials. The disclosure of a clear-cut risk reduction of breast cancer attained by tamoxifen in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (NSABP P-1) ( 1 ) prompted the publication of initial findings from several other ongoing tamoxifen trials, which had found no statistically significant reduction of breast cancers in women receiving tamoxifen ( 2, 3 ). Because the trials had similar designs (double-blind randomized studies of 20 mg tamoxifen versus placebo daily for 5 years), it was Affiliations of authors: Scientific Directorate (UV), Division of Epidemiology and Biostatistics (PM, NR), Division of Cancer Prevention and Genetics (BB, AD), Division of Pathology (GV), and Division of Senology (VS), European Institute of Oncology, Milan, Italy; School of Medicine, University of Milan, Milan, Italy (GV) ; International Agency for Research on Cancer, Lyon, France (PB); Fondazione Maugeri, Pavia, Italy (AC); Memorial Sloan-Kettering Cancer Center, New York, NY (VS); Comitato Prevenzione Tumori al seno, Milan, Italy (RT); Department of Senology, Istituto per lo Studio e la Cura dei Tumori Fondazione Pascale, Naples, Italy (GDA, PO); Lega Italiana per la lotta contro i tumori, Vicenza, Italy (FL); Ospedale San Camillo-Forlanini, Rome, Italy (GG); General Surgery Division, Centro per lo Studio e la Prevenzione Oncologica, Florence, Italy (MRDT, MGM); Centro Regionale di Riferimento Oncologico, Aviano, Italy (MAP); Ospedale di Cosenza, Cosenza, Italy (SC); Ospedali Galliera, Genova, Italy (AD). Correspondence to: Umberto Veronesi, MD, Istituto Europeo di Oncologia, Via G. Ripamonti 435, Milano, Italy ( umberto.veronesi@ieo.it ). See Appendix for a list of physicians and scientists in the Italian Tamoxifen Study Group and their affiliations. See Notes following References. DOI: /jnci/djk154 The Author Published by Oxford University Press. All rights reserved. For Permissions, please journals.permissions@oxfordjournals.org. jnci.oxfordjournals.org JNCI Articles 727
2 CONTEXT AND CAVEATS Prior knowledge The initial results from the Italian Tamoxifen Trial showed no reduction in risk of breast cancer with tamoxifen treatment compared with placebo, which was in contrast to the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. Study design Randomized double-blind phase III trial of tamoxifen treatment among healthy women who had undergone hysterectomy. Data were also analyzed based on the woman s baseline risk, low or high, for hormone receptor positive (HR+) breast cancer. Contributions Rates of breast cancer were similar in both groups among women at low risk but were lower in the tamoxifen group than in the placebo group among women at high risk. More women in the tamoxifen group than in the placebo group reported side effects, including hot flashes and cardiovascular events. Implications Women who are at high risk for breast cancer and not for cardiovascular disease may benefit from tamoxifen treatment as a prevention strategy for HR+ breast cancer. Limitations Most women were not at high risk for breast disease. postulated that differences in the study populations could be responsible for the discrepancy in the results ( 4 ). The NSABP P-1 trial included only women who were at increased risk of breast cancer, whereas the Italian trial ( 3 ) included women who had undergone hysterectomy, of whom nearly 50% had also undergone oophorectomy. The NSABP P-1 trial was stopped early due to the large and statistically significant reduction in the incidence of invasive breast cancer in the women assigned to tamoxifen, and women in the placebo group were given the opportunity either to receive a 5-year course of tamoxifen or to be randomly assigned to the Study of Tamoxifen and Raloxifene (STAR) trial ( 5 ), whereas the Italian trial ( 3 ) continued in accordance with the original protocol. Women continued treatment, blindly, for a total of 5 years and are still being followed for major events, enabling the study of the long-term efficacy of tamoxifen. Here we present the updated results of the Italian Randomized Tamoxifen Prevention Trial after 11 years of follow-up, focusing on the occurrence of breast cancer (the primary study endpoint). We also describe the variety of symptoms and adverse events reported during treatment, including cancers other than the breast and all-cause mortality (the secondary study endpoints). Patients and Methods Accrual, Drug Administration, and Follow-up The Italian Randomized Tamoxifen Prevention Trial ( 3 ) included healthy women aged years at average risk for breast cancer who had had a total hysterectomy to avoid the risk of endometrial cancer associated with tamoxifen use ( 6 ). All patients provided written informed consent, and the trial received authorization number 800.C.35/ from the Italian National Ministry of Health. The study design and preliminary results on breast cancer risk and other study outcomes have been published elsewhere ( 3, 7 10 ). In brief, the study started on October 1, 1992, and recruitment ended by December 31, Women were randomly assigned in a double-blind manner to receive tamoxifen (20 mg daily) or placebo for 5 years. The initial sample size calculation assumed a reduction of one-third in breast cancer risk as shown by the data on the incidence of new contralateral breast cancer in patients treated with tamoxifen as an adjuvant. A reevaluation of the study power based on the initial results of the NSABP P-1 trial ( 1 ) and on the observed incidence rate of breast cancer in women assigned to placebo in our trial (2.5% per women-year) ( 3 ) demonstrated that the 5408 women enrolled in the trial confer a 90% power to detect a 49% reduction of the incidence of invasive breast cancer 10 years after randomization and a 95% power to detect a 68% reduction of ER+ breast cancers at =.05. Treatment allocation used a randomized permuted block design, with stratification by institution. The allocation process was centralized at the Data Center at the European Institute of Oncology. Randomization was by telephone call (occasionally fax) to the center. Women with severe concurrent disease were excluded from random assignment. Use of estrogen replacement therapy was allowed at random assignment and/or during the trial. During the treatment period (first 5 years), women had a physical examination every 6 months and blood testing (including white blood cell and platelet counts and measures of high-density lipoproteins, low-density lipoproteins, and total cholesterol and of alanine and aspartate aminotransferase) and mammography every 12 months. After completion of treatment, or in case of dropout, women were followed on an annual basis. Information about major endpoints, such as death, serious adverse events, or cancer diagnosis, was collected continuously and submitted to the data center at the European Institute of Oncology. Breast Cancer Risk Assessment Although women were not selected for the study according to their risk of developing breast cancer, the majority (n = 2876, 53%) of participants had had both ovaries removed during hysterectomy and thus were at low risk for the disease. Because previous reports ( 1, 4 ) had demonstrated the efficacy of tamoxifen on endocrine-responsive tumors among women at high risk for breast cancer, we decided to perform an exploratory analysis by stratifying women according to their risk of developing such a tumor. We defined a group of 702 (13%) women as having high risk of hormone receptor positive (HR+) breast cancer based on their baseline characteristics. This group included women who were taller than 160 cm (the median height of the group), had at least one intact ovary, were younger than age 14 years at menarche (the upper age tertile of the group), and had no full-term pregnancy before age 24 years (the median age at first pregnancy of the group) ( 8 ). The remaining 1830 (34%) women with at least one intact ovary were classified as the low-risk group. The 2876 (53%) women who had had a bilateral oophorectomy were analyzed separately. 728 Articles JNCI Vol. 99, Issue 9 May 2, 2007
3 Immunohistochemistry Immunohistochemical investigations were performed on formalinfixed, paraffin-embedded tissues as previously described ( 11 ). Immunoreactivity for estrogen and progesterone receptors was scored according to the percentage of neoplastic cells, with sections with 10% or more immunoreactive cells identifying HR+ tumors ( 12 ). For Ki-67 labeling index, as assessed with the MIB1 mouse monoclonal antibody (1:200 dilution, Dako, Glostrup, Denmark), we used the median value (15%) of the absolute percentage of the neoplastic cells showing nuclear immunoreactivity as a cutoff for positivity ( 13 ). For HER2/neu, which was evaluated using a 1 : 100 dilution of a polyclonal antiserum (Dako, Glostrup, Denmark), overexpression (3+) was defined as the presence of moderate to strong staining of the entire membrane in >10% of the tumor cells ( 13 ). Statistical Methods The data included in this manuscript are based on information received and processed by the Italian Tamoxifen Study data center as of December 31, All analyses were performed on an intention-to-treat basis. Incidence rates for the study endpoints were calculated for each group by dividing the number of observed events by the number of event-specific person-years of follow-up. For symptoms and adverse events, which were recorded at the patient s follow-up visits while on treatment, person-years were calculated from the date of random assignment to the date of termination of the treatment, the date of development of the specific event, or the date of dropout from treatment, which ever came first. For other cancers and for deaths, person-years were calculated from the date of random assignment to the date of last follow-up (on an annual basis after termination of the 5 years of treatment) or to the date of the specific event. For breast cancer, person-years were calculated from the date of random assignment to December 31, 2005, regardless of the follow-up status of the participant. Event rates in the two groups were compared by using risk ratios (RRs) and 95% confidence intervals (CIs). Confidence intervals were determined assuming that the events followed a Poisson distribution. Because healthy, mostly middle-aged women were enrolled in the trial, mortality did not constitute a major competing risk of failure. Therefore, the cumulative incidence of the various types of events were determined using Kaplan Meier methods ( 11 ). The two treatment groups were compared by using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals were obtained using Cox proportional regression model after adjustment for patient s age (in 5-year age groups) and participating center. Before presenting results from the log-rank tests or from the Cox models, we tested the proportional hazards assumption by introducing a constructed time-dependent variable and testing for its statistical significance. All P values were two-sided. All analyses were performed with SAS software (v8, SAS, Cary, NC). Results Table 1. Women included in the analyses of the Italian Randomized Tamoxifen Prevention Trial and number followed Accrual and follow-up status Placebo Tamoxifen Total Accrual, n Women randomly assigned Early withdrawals Ineligible For major changes in protocol For major adverse events Voluntary withdrawals Lost to follow-up Died Completed 5 years of treatment Average duration of treatment, mo * Average follow-up time until last contact, mo Average follow-up time until December 31, 2005, mo Total person-years of follow-up Accumulated during treatment * Accumulated until last follow-up Accumulated until the end of the study * Used for the evaluation of the rates of intercurrent events. Used for the evaluation of the rate of cancers other than of the breast. Used for the evaluation of the rate of breast cancer. Participant Characteristics A total of 5408 women were randomly assigned to placebo (n = 2708) or to tamoxifen (n = 2700) ( Table 1 ). Of these, 2119 (39.2%) interrupted treatment before completion (1407 voluntarily, 394 for major events, such as cardiovascular, cerebrovascular or thromboembolic events, cancer or hepatic disease, 99 for major changes in protocol [previously eligible women who had incomplete hysterectomy or endometriosis which rendered the women ineligible], 56 for ineligibility [incomplete hysterectomy after protocol modification or major medical conditions not reported at the baseline visit], 154 for loss to follow-up, and 9 for death), whereas 3289 women (60.8%) completed the 5-year treatment period. On average, women underwent treatment for 4.0 years and were followed for 9.1 years, and 11.2 years elapsed from random assignment to December 31, No differences were observed between the two study groups in any of the baseline characteristics ( Table 2 ). Breast Cancer Through 11 years of follow-up, 74 women in the placebo group and 62 women in the tamoxifen group developed breast cancer ( P =.30, Fig. 1 ). The corresponding annual rates of breast cancer in the two groups were 2.48 and 2.07 per 1000 women-years, respectively ( Table 3 ). Six (8.1%) women in the placebo group and nine (14.5%) in the tamoxifen group had noninvasive tumors. Interestingly, among women taking estrogen replacement therapy at baseline, statistically significantly fewer women in the tamoxifen group than in the placebo group developed breast cancer (9 versus 21, HR = 0.43, 95% CI = 0.20 to 0.95). We further studied the effect of tamoxifen treatment on the incidence of breast cancer according to known prognostic and predictive factors (histology [invasive, noninvasive, or unknown], tumor grade [G1, G2, G3, or unknown] and size [<1, 1 2, or >2 cm or unknown], pn [negative or positive], focality [unifocal or multifocal], multicentricity [yes or no], hormone receptor status [ER negative, positive, or unknown; PgR negative, positive, or unknown], vascular invasion [absent, present, unknown], Ki-67 expression [<15%, 15%, or unknown], and HER2/Neu expression jnci.oxfordjournals.org JNCI Articles 729
4 Table 2. Participant characteristics at time of random assignment [0 2+, 3+, or unknown]. Overall, we observed a statistically significant reduction of progesterone receptor positive (PgR+) tumors among women who were taking tamoxifen (n = 27) compared with those on placebo (n = 44) (RR = 0.61, 95% CI = 0.38 to 0.99; Supplementary Table 1, available online). Tamoxifen treatment was not associated with any other difference in tumor characteristics. When analyses were restricted to subsets of women defined according to their baseline characteristics, we observed a strong reduction of breast cancer in women with at least one intact ovary and at high risk of developing HR+ tumors. In this high-risk group (702 women taller than 160 cm, with at least one intact ovary, who were <14 years of age at menarche, and who had no full-term pregnancy before age 24 years), the rate of breast cancer was 6.26 per 1000 women in the placebo group and 1.50 per 1000 womenyears in the tamoxifen group, yielding a reduction of breast cancer of 4.76 per 1000 women-years (RR = 0.24, 95% CI = 0.10 to 0.59) ( Table 3 ). In contrast, women who had undergone bilateral oophorectomy (RR = 0.86, 95% CI = 0.50 to 1.47) or women with at least one intact ovary but at low risk of HR+ tumors (RR = 1.46, 95% CI = 0.84 to 2.53) had no reduction in breast cancer risk with tamoxifen treatment ( Table 3, Fig. 2 ). In this last group of women at low risk of HR+ tumors, tamoxifen treatment was associated with a non statistically significant excess of breast cancer ( Table 3 ) that was more pronounced for ER (tamoxifen versus placebo, nine versus five; RR = 1.72, 95% CI = 0.58 to 5.13) and PgR Placebo Tamoxifen Characteristic No. % No. % All women Age at entry, y Type of hysterectomy Hysterectomy alone Hysterectomy + unilateral oophorectomy Hysterectomy + bilateral oophorectomy Hysterectomy + oophorectomy (NOS) Age at hysterectomy, y Unknown Class of risk Without ovaries Low risk * High risk * No. of first-degree relatives with breast cancer Hormone replacement therapy Neither at baseline nor during intervention At baseline During intervention only * The high-risk group includes women taller than 160 cm, with at least one intact ovary, who were younger than age 14 years at menarche, and who had no full-term pregnancy before age 24 years. The low-risk group includes the remaining women with at least one intact ovary. NOS = not otherwise specified. (tamoxifen versus placebo, 15 versus 8, RR = 1.79, 95% CI = 0.76 to 4.23) tumors than for ER+ and PgR+ tumors. Similarly, among women with a positive family history (one or more first-degree relatives with breast cancer), more breast cancers were observed in the tamoxifen group than in the placebo group, although the excess was not statistically significant ( Table 3 ). Among women in Fig. 1. Cumulative incidence of breast cancer among women in the Italian Randomized Tamoxifen Prevention Trial. Hazard ratio (HR) (and 95% confidence interval) was calculated from a Cox regression model adjusted for age and center. 730 Articles JNCI Vol. 99, Issue 9 May 2, 2007
5 Table 3. Numbers and rates of breast cancer in the placebo and tamoxifen groups by selected participant characteristics No. of events the high-risk group, the breast cancer risk reduction was similar during treatment (HR = 0.18, 95% CI = 0.04 to 0.85) and after treatment (HR = 0.20, 95% CI = 0.06 to 0.69) ( Fig. 3 ). Thus, there was evidence of benefit even after treatment stopped. During follow-up of women who developed breast cancer, one woman in the tamoxifen group and two women in the placebo group had a loco-regional relapse. Three women in the tamoxifen group and two in the placebo group developed distant metastases. Two women in each group died from breast cancer. Rate per 1000 women-years Participant characteristic Placebo Tamoxifen Placebo Tamoxifen Difference * RR (95% CI) All women (0.60 to 1.17) Age at study entry, y (0.52 to 1.71) (0.38 to 1.24) (0.46 to 1.99) (0.32 to 2.16) Type of hysterectomy Hysterectomy alone (0.56 to 1.71) Hysterectomy + unilateral oophorectomy (0.30 to 1.20) Hysterectomy + bilateral oophorectomy (0.43 to 1.31) Hysterectomy + oophorectomy (NOS) NE Age at hysterectomy, y (0.43 to 2.31) (0.40 to 1.48) (0.43 to 1.28) (0.48 to 2.24) No. of first-degree relatives with breast cancer (0.50 to 1.06) (0.65 to 3.15) Hormonal replacement therapy Never (0.67 to 1.50) At baseline (0.20 to 0.95) During intervention only (0.30 to 2.92) Class of risk Without ovaries (0.50 to 1.47) Low risk (0.84 to 2.53) High risk (0.10 to 0.59) * Rate in the placebo group minus rate in the tamoxifen group. Risk ratio (RR) for women in the tamoxifen group relative to women in the placebo group. CI = confidence interval. NOS = not otherwise specified; NE = not able to estimate. The high-risk group includes women taller than 160 cm, with at least one intact ovary, who had menarche at younger than age 14 years, and who had no full-term pregnancy before age 24 years; the low-risk group includes the remaining women, with at least one intact ovary. Adverse Events Because adverse events that occurred after termination of the intervention (or after dropout from the study) were not systematically reported to the data center, and because these late events would have less likely been attributable to the active treatment than those occurring during the active phase of the trial, we restricted the analysis to events that occurred during intervention, while women were still actively being followed. We measured the cumulative incidence of each event that occurred during the 5-year intervention period in the two study groups (Supplementary Figs. 1 3, available online). A statistically significant excess of vasomotor symptoms were observed in women in the tamoxifen group compared with the placebo group ( Table 4, Fig. 4 ). Hot flashes were the most common: among women who were free of symptoms at entry, 21.2% of the women in the placebo group reported hot flashes for the first time during treatment versus 31.3% of women in the tamoxifen arm (RR = 1.78, 95% CI = 1.57 to 2.00). Similarly, vaginal discharge was more frequent in women in the tamoxifen group than in those in the placebo group (RR = 3.44, 95% CI = 2.90 to 4.09), whereas vaginal dryness was not associated with tamoxifen treatment (RR = 1.14, 95% CI = 0.97 to 1.34). Women in the tamoxifen group complained more frequently of urinary disturbances (cystitis or incontinence) (RR = 1.52, 95% CI = 1.23 to 1.89) than women in the placebo group but less often of headache (RR = 0.68, 95% CI = 0.50 to 0.94) ( Table 4 ). All other adverse events (nausea, vomiting, diarrhea, dermatologic alterations, ophthalmologic disease, gastric complaints, weight gain, fluid retention, anxiety, and sexual disorders) were reported equally in the two groups during the active intervention period ( Table 4 ; Supplementary Table 2, available online). As previously reported, tamoxifen treatment was associated with an excess of venous thromboembolic events ( Table 4 ). Of the total of 72 women who developed thromboembolic events (51 superficial phlebitis, 17 deep venous thrombosis, 2 pulmonary embolism, including one who also had superficial phlebitis, one visceral venous thrombosis, and two retinal venous thrombosis) during the 5-year intervention period, 28 were on placebo and jnci.oxfordjournals.org JNCI Articles 731
6 Fig. 2. Cumulative incidence of breast cancer in the Italian Randomized Tamoxifen Prevention Trial according to baseline risk profile. Hazard ratios (HRs) (and 95% confidence intervals) were calculated from Cox regression models adjusted for age and center A ) in women without ovaries, B ) in women with at least one intact ovary, C ) in women at low risk with at least one intact ovary, and D ) in women at high risk with at least one intact ovary. The high-risk group includes women taller than 160 cm, with at least one intact ovary, who were younger than age 14 years at menarche, and who had no full-term pregnancy before age 24 years; the low-risk group includes the remaining women with at least one intact ovary. 44 on tamoxifen (RR = 1.63, 95% CI = 1.02 to 2.62; P =.04). Superficial phlebitis of the legs accounted for all of the excess in the tamoxifen group. No fatal thromboembolic event occurred in either group during the intervention period. One woman who had a superficial phlebitis at the beginning of the study developed a fatal pulmonary embolism 5.5 years after the end of treatment. The annual rate of thromboembolic events in the tamoxifen group was modest, however (4.45 per 1000 women-years), and the excess was restricted to women with conventional risk factors for atherosclerosis ( 10 ). Rates of cardiovascular events, angina, and de novo hypertension were similar in the two groups, whereas cardiac arrhythmia and atrial fibrillation were more often diagnosed in women who received tamoxifen (RR = 1.73, 95% CI = 1.01 to 2.98, Table 4 ). More women in the tamoxifen group (six strokes, six transient ischemic attacks) than in the placebo (two strokes, five transient Fig. 3. Cumulative incidence of breast cancer in the highrisk group during and after treatment. Hazard ratios (HRs) (and 95% confidence intervals) were calculated from Cox regression models adjusted for age and center. The high-risk group includes women taller than 160 cm, with at least one intact ovary, who were younger than age 14 years at menarche, and who had no full-term pregnancy before age 24 years; the low-risk group includes the remaining women with at least one intact ovary. 732 Articles JNCI Vol. 99, Issue 9 May 2, 2007
7 Table 4. Numbers and incidence rates of selected adverse events in the placebo and tamoxifen groups during treatment No. of events (%) Rate per 1000 women-years Events Placebo Tamoxifen Placebo Tamoxifen Difference * RR (95% CI) Hot flashes (1.57 to 2.00) Vaginal dryness (0.97 to 1.34) Vaginal discharges (2.90 to 4.09) Urinary disturbances (1.23 to 1.89) Headache (0.50 to 0.94) Cardiac arrhythmias/atrial fibrillation (1.01 to 2.98) Cerebrovascular events (0.70 to 4.52) Thromboembolic events (1.02 to 2.62) * Rate in the placebo group minus rate in the tamoxifen group. Risk ratio (RR) for women in the tamoxifen group relative to women in the placebo group. CI = confidence interval. Among women who were free of symptoms at baseline. ischemic attacks) developed a cerebrovascular event during intervention, although the difference was not statistically significant (RR = 1.78, 95% CI = 0.70 to 4.52) ( Table 4 ; Supplementary Table 3, available online). Unlike other adverse events, hypertriglyceridemia was not assessed specifically at each follow-up visit, and the analysis of this outcome relies on self-reports from patients and their physicians and subsequent confirmation from laboratory findings. A total of 37 women in the study had hypertriglyceridemia noted 8 in the placebo group and 29 in the tamoxifen group ( P <.001) (Supplementary Fig. 2, available online). Because this information likely underestimates the true occurrence of hypertriglyceridemia in women in the study, the event rates for each group were not calculated. Cancers Other Than Breast Cancer A total of 91 cancers other than breast cancer developed among women who received placebo and 106 developed among those who received tamoxifen (Supplementary Table 4 and Supplementary Fig. 4, available online). No statistically significant differences by site were observed. Cancer of the colon or rectum was the most common form of cancer (n = 39), followed by nonmelanoma skin cancer (n = 23); cancers of the lung, trachea, and bronchus (n = 20); Fig. 4. Cumulative incidence of selected adverse events during treatment. The number of women on treatment in the tamoxifen and in the placebo groups were 2700 and 2708 at baseline, 2222 and 2317 after 1 year, 2031 and 2150 after 2 years, 1879 and 1940 after 3 years, 1730 and 1797 after 4 years, and 1263 and 1352 after 5 years, respectively. Hazard ratios (HRs) (and 95% confidence intervals) were calculated from Cox regression models adjusted for age and center. jnci.oxfordjournals.org JNCI Articles 733
8 Table 5. Deaths in the placebo and tamoxifen groups Cause Placebo Tamoxifen Cancer Esophagus 1 0 Colon rectum 6 5 Stomach 4 1 Hepatobiliary 1 1 Pancreas 0 4 Lung 4 5 Breast 2 2 Melanoma 2 0 Ovary 1 0 Kidney 1 0 Brain 1 2 Lymphatic and hematopoietic 0 2 Unknown primary 2 0 Cardiovascular and cerebrovascular 5 3 disease Myocardial infarction 2 1 Pulmonary embolism 0 1 Ictus 1 0 Aneurism rupture 2 1 Other Cirrhosis 1 0 Peritonitis 0 1 Pulmonitis 0 1 Multiple sclerosis 0 1 During sleep 1 0 Suicide 1 0 Unknown 5 8 Total no. of deaths Mortality rate per 1000 women-years RR (95% CI) * 0.95 (0.60 to 1.49) * Risk ratio (RR) for women in the tamoxifen group relative to women in the placebo group. CI = confidence interval. cancer of the thyroid or adrenal glands (n = 20); and lymphatic or hematopoietic malignancies (n = 20). Cause of Death A total of 74 women (placebo, 38; tamoxifen, 36) died after the initiation of the trial. Rates of death from all causes (RR = 0.95, 95% CI = 0.60 to 1.49) or from any specific cause were similar in the two groups ( Table 5 ; Supplementary Fig. 4, available online). For 13 women, the cause of death remained unknown (the notification of death was obtained either from relatives or from municipal death registry). Cancer was the most common cause of death, with 25 women dying from malignancy in the placebo group versus 22 in the tamoxifen group. Colorectal cancer was the most common cause of cancer death (n = 11), followed by lung cancer (n = 9). Only four women, two in the placebo group and two in the tamoxifen group, died of breast cancer. Discussion The results of the Italian Randomized Tamoxifen Prevention Trial are in agreement with the preliminary reports showing overall no statistically significant reduction of breast cancer in otherwise healthy women who had undergone hysterectomy ( 3, 7, 8 ). However, this updated report now shows a statistically significant reduction of HR+ breast tumors among women in the tamoxifen group relative to the placebo group; subset analyses also substantiated a strong risk reduction among women at high risk of developing HR+ breast tumors. Preliminary and updated results of the NSABP P-1 trial ( 1, 14 ) and the STAR trial ( 5 ) reported undisputable results on the preventive effect of tamoxifen in women who were at increased risk of breast cancer, as calculated according to a modification of the Gail model ( 15, 16 ), but whether these results could be extrapolated to all women would have remained unknown without the findings of other large-scale trials. Differences in study populations among the various chemoprevention trials using tamoxifen may partly explain the different results and may also help to identify women who are most likely to benefit from treatment ( 4, 17 ). To date, there is a good evidence that tamoxifen reduces the incidence of HR+ tumors, particularly those expressing estrogen receptors. In our study, the risk reduction was stronger for tumors expressing both progesterone and estrogen receptors (ER+ and PgR+) than for tumors that were ER+ and PgR. We also found a non statistically significant increase in the number of HR tumors in the tamoxifen group of similar magnitude to that reported in the NSABP P-1 trial. Of interest, most of the HR tumors in the tamoxifen group were diagnosed among women who were in the low-risk group. More important, no excess of breast cancers defined as intermediate or high risk on the basis of their clinicopathologic characteristics were seen in the tamoxifen group. To assess whether tamoxifen treatment may be more effective in specific subgroups, we performed 20 subset analyses according to age at entry, type of hysterectomy, age at hysterectomy, family history, hormone replacement therapy use, and class of risk. None of these stratifications were proposed before the initiation of the study, but the two that led to statistically significant results have been published in previous reports [stratification by hormone replacement therapy use ( 3 ) and by class of risk ( 8 )]. Thus, we were able to assess, this time prospectively, whether the risk reduction of breast cancer was still evident after the publication of the initial reports. We previously reported ( 8 ) a strong protective effect of tamoxifen among women at high risk of HR+ tumors in particular, based on three patients in the tamoxifen group versus 15 in the placebo (HR = 0.18, 95% CI = 0.05 to 0.62). Since that report, we have observed nine new cases of breast cancer in the placebo group but only three in the tamoxifen group, thus substantiating the value of this selection criterion. Among women who were assigned to the placebo group, the annual rate of breast cancer in the high-risk group (6.26 per 1000 women-years) was similar to that reported in the NSABP P-1 trial (6.29 per 1000 women-years), but the risk reduction was even stronger in our trial (4.76 per 1000 women-years versus 2.70 per 1000 women-years in NSABP P-1), although the difference between trials was of borderline statistical significance ( P =.06). This potentially stronger risk reduction suggests that the criteria we used may further define which women would be most likely to benefit from tamoxifen treatment relative to the factors included in the Gail model, which identifies women at high risk of de - veloping breast cancer in general regardless of their endocrine responsiveness. Our long-term data confirm our previous findings ( 7 ) of a 50% 60%, statistically significant reduction of breast cancers with 734 Articles JNCI Vol. 99, Issue 9 May 2, 2007
9 tamoxifen treatment relative to placebo in women on estrogen replacement therapy at random assignment. Although general use of hormone replacement therapy has been slowed after the results of the Women s Health Initiative trial ( 18 ), a substantial group of younger women will still benefit from use of hormone replacement therapy for treatment of climacteric syndrome ( 19 ). However, the risk of breast cancer associated with hormone replacement therapy is a main barrier for many women, so much so that there is interest in adding agents like tamoxifen to possibly lower this risk. However, the use of tamoxifen as a breast cancer preventive agent is limited by the risk of endometrial cancer and venous thromboembolism, two events that might be minimized by concomitant use of hormone replacement therapy. A phase 3 trial, the HRT opposed to low-dose tamoxifen study ( 20 ) of tamoxifen at 5 mg/day versus placebo, is ongoing in women on hormone replacement therapy to assess the efficacy of this combined approach in an attempt to maintain the benefits while reducing the risks of both agents. We found no effect of tamoxifen treatment among women who had had both of their ovaries removed, regardless of their class of risk. Oophorectomy alone has been recognized as a valid breast cancer risk reduction option for women of all risk levels ( 21 ). In our study, the annual rate of breast cancer in women who had undergone oophorectomy in the placebo group was even lower (1.81 per 1000 women-years) than in women classified as being at low risk but with at least one preserved ovary (2.09 per women-years). Interestingly, Gronwald et al. ( 22 ) studied the association of tamoxifen with contralateral breast cancer in women with BRCA1- or BRCA2-associated breast cancer. Tamoxifen treatment was associated with a reduction of contralateral breast cancer in that study but, as in our study, the reduction was not seen among women who had undergone oophorectomy. In the NSABP P-1 trial, 37% of the women had had a hysterectomy before entry. It is unknown whether tamoxifen was less effective among women who had undergone oophorectomy and who were at increased risk in that trial. Our data cast doubt about the benefit of tamoxifen in women who had undergone oophorectomy and were at average risk of breast cancer. A comparison of prognostic and predictive factors for breast cancer showed little difference between the groups in our study, although patients in the tamoxifen group had less advanced disease (more tumors < 5 mm, fewer patients with positive lymph nodes, fewer with multifocality), but less endocrine responsive tumors (fewer ER+ and PR+ tumors, the latter statistically significantly so), than those in the placebo group. Nonetheless, the number of breast cancer related events was similar in the two groups. Notably, cancer was the most common cause of death (47 out of 72 deaths), colorectal cancer being the most frequent (n = 11). However, the mortality rate from breast cancer was extremely low compared with that reported in the general population and in the other breast cancer chemoprevention trials ( 4, 14 ). Whether this low rate was the result of strict surveillance or appropriate treatments will be the subject of a separate investigation. As in the NSABP P-1 trial ( 1 ), hot flashes and vaginal discharge represented the most frequent adverse events of tamoxifen in our study, but the excess was limited to the first 12 months of intervention ( Fig. 4 ). After this period, the occurrence of symptoms was similar in the two groups, suggesting a genetic susceptibility profile in a subset of women. Among other genes, the cytochrome P450 gene CYP2D6, which is implicated in the metabolic activation of tamoxifen to endoxifen, has recently been associated with the occurrence of vasomotor symptoms in women with breast cancer who were treated with tamoxifen ( 23 ). In that study, women with the CYP2D6*4/*4 genotype had a higher risk of disease relapse and a lower incidence of hot flashes than women with the wild-type gene or the CYP2D6*4/wt genotype. The potentially important role of CYP2D6 in the metabolic activation of tamoxifen, which was confirmed in a subsequent report ( 24 ), suggests that women with the CYP2D6*4/*4 genotype might be less likely to benefit from tamoxifen as a chemopreventive agent than women with the wild-type gene or the CYP2D6*4/wt genotype. In our study, women in the tamoxifen group complained more often about urinary disturbances (mostly incontinence) than women in the placebo group. Although little has been published on the effect of tamoxifen on bladder control, recent results of the STAR trial indicated an increased occurrence of urinary incontinence in the tamoxifen group relative to the raloxifene group ( 25 ). Although there was no placebo group in that study, raloxifene has been shown to have no effect on bladder control and represents a suitable control group ( 26 ). In our study, women who were allocated to tamoxifen treatment reported statistically significantly fewer headaches than women assigned to placebo. Although this association was not reported in the other chemoprevention trials, early studies of tamoxifen ( 27, 28 ) demonstrated its potential effect on the relief of severe menstrual migraine. Substantial epidemiologic, pathophysiologic, and clinical evidence suggests a link between estrogen and progesterone and migraine headache ( 29, 30 ). Recently, Martin and Behbehani ( 31, 32 ) reviewed the association between ovarian hormones and migraine headache and noted that estrogen and progesterone treatment can prevent or promote migraine headache under different circumstances, depending on their absolute serum levels, constancy of exposure, and types of estrogen/progesterone derivatives. Indeed, hormonal treatments, including oral contraceptives, estrogen replacement therapy, antiestrogen agents (tamoxifen), and gonadotropin-releasing hormone agonists followed by estrogen add-back therapy, have been proposed for the prevention of menstrual migraines as an alternative to nonsteroidal antiinflammatory drugs and ergotamine derivatives ( 33 ). We previously provided an extensive description of the thromboembolic events in our trial and tried to identify women who were most likely to develop these events ( 10 ). Our data indicated that, although tamoxifen slightly increased the risk of venous thromboembolic events in our population, the excess was restricted to superficial thrombophlebitis, which is not a serious adverse event per se, during the first 18 months of treatment. The development of thromboembolic events after 18 months of treatment was similar in the two groups. The risk of venous thromboembolic events associated with tamoxifen was particularly elevated in women at high risk for coronary heart disease. In a meta-analysis of the preliminary results of the four major primary prevention trials of tamoxifen involving a total of women ( 4 ), the use of tamoxifen was associated with 118 serious venous thromboembolic events versus 62 in the placebo groups (RR = 1.9, 95% CI = 1.4 to jnci.oxfordjournals.org JNCI Articles 735
10 2.6), including six versus two patients with fatal pulmonary emboli. The risk of superficial thrombophlebitis was doubled with tamoxifen relative to placebo (68 versus 30 events). A complete assessment of the baseline risk of venous thromboembolic events should be an important component of counseling women on the use of tamoxifen, particularly in the prevention setting. Atrial fibrillation ( 34 ), the most common type of arrhythmia in adults, is a major risk factor for stroke but has not so far been associated with tamoxifen treatment of women with breast cancer and its use in the prevention of breast cancer. Although women with major cardiac disorders and those taking anticoagulant therapy were excluded from the trial, a small proportion of participants developed atrial fibrillation during the trial, with a statistically significant excess in women receiving tamoxifen. Similarly, cerebrovascular events were more frequent among women in the tamoxifen group than in the placebo group and, as in the NSABP P-1 trial ( 14 ), the difference was more pronounced for strokes than for transient ischemic attacks. Two of the women in the tamoxifen group who had signs of atrial fibrillation continued the intervention and developed a nonfatal cerebrovascular event (one stroke and one transient ischemic attack). These and other results suggest that tamoxifen may be responsible for the development of cerebrovascular events, specifically strokes, which could be mediated by the appearance of cardiac arrhythmias. Tamoxifen withdrawal may be recommended in these women. The current analysis confirmed our preliminary finding of an excess of hypertriglyceridemia in women receiving tamoxifen compared with placebo ( 3 ). Although hypertriglyceridemia is rare, yet likely to be underestimated by the lack of a systematic search during the study, the association we observed is supported by several reports ( ). Recently, Liu and Yang ( 38 ) showed that, in breast cancer patients, reducing tamoxifen dose is associated with a decrease in the marked hypertriglyceridemia that occurs in some patients during tamoxifen treatment. Overall mortality was similar in the two treatment groups, in agreement with the results from the NSABP P-1 trial; however, the annual death rates in the Italian Randomized Tamoxifen Prevention Trial were much lower than those in the NSABP P-1 trial (1.54 per 1000 women-years versus 2.80 per 1000 womenyears in the placebo group), suggesting that these populations have markedly different risk factors and lifestyles. This difference in mortality rates could also be largely attributed to the higher proportion of elder women in the NSABP P-1 trial than in the Italian trial. The proportion of women aged 60 years and older at the time of randomization was 30.0% and 11.5% in the NSABP P-1 and the Italian trials, respectively. One potential limitation of the study is that the major findings derive from subset analyses that were not planned in the initial study protocol. Therefore, it is possible that some of our findings might be due to chance. However both the statistically significant reduction of PgR+ breast cancers and the overall statistically significant reduction of breast cancer in women at high risk of the disease have been previously observed in other chemoprevention trials (1,2,4). We also observed a statistically significant reduction of breast cancer in the subset of women on estrogen replacement therapy; however, this finding was already present in our previous report ( 3 ), which was based on a limited number of events. Thus, although we cannot completely exclude the role of chance, our findings are compatible with prior reports. In summary, this update of the Italian Randomized Tamoxifen Prevention Trial confirms that tamoxifen reduces the incidence of breast cancer in a subset of women who are at high risk of de - v eloping hormone-related tumors, including women on estrogen replacement therapy. Tamoxifen did not further reduce the risk of breast cancer in women who had had their ovaries removed but might in fact favor the development of HR tumors in women at low risk of breast cancer. Tamoxifen decreased incidence of headache but slightly increased the risk of developing menopausal symptoms, hypertriglyceridemia, superficial thromboembolic events, arrhythmias, and cerebrovascular disease. Therefore, a complete assessment of baseline cardiovascular risk should become an important component of counseling women on the use of tamoxifen, particularly in the prevention setting. References (1) Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998 ; 90 : (2) Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 1998 ; 352 : (3) Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 1998 ; 352 : (4) Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003 ; 361 : (5) Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006 ; 295 : (6) Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1989 ; 1 : (7) Veronesi U, Maisonneuve P, Sacchini V, Rotmensz N, Boyle P. Tamoxifen for breast cancer among hysterectomised women. Lancet 2002 ; 359 : (8) Veronesi U, Maisonneuve P, Rotmensz N, Costa A, Sacchini V, Travaglini R, et al. Italian randomized trial among women with hysterectomy: tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst 2003 ; 95 : (9) Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 2005 ; 330 : 932. (10) Decensi A, Maisonneuve P, Rotmensz N, Bettega D, Costa A, Sacchini V, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation 2005 ; 111 : (11) Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958 ; 53 : (12) Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, et al.; International Breast Cancer Study Group. Re- evaluating adjuvant breast cancer trials: assessing hormone receptor status by im - munohistochemical versus extraction assays. J Natl Cancer Inst 2006 ; 98 : (13) Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not 736 Articles JNCI Vol. 99, Issue 9 May 2, 2007
Disclosure Information Relationships Relevant to this Session
 Disclosure Information Relationships Relevant to this Session DeCensi, Andrea No relevant relationships to disclose. Please note, all disclosures are reported as submitted to ASCO, and are always available
Disclosure Information Relationships Relevant to this Session DeCensi, Andrea No relevant relationships to disclose. Please note, all disclosures are reported as submitted to ASCO, and are always available
Chemo-endocrine prevention of breast cancer
 Chemo-endocrine prevention of breast cancer Andrea DeCensi, MD Division of Medical Oncology Ospedali Galliera, Genova; Division of Cancer Prevention and Genetics, European Institute of Oncology, Milano;
Chemo-endocrine prevention of breast cancer Andrea DeCensi, MD Division of Medical Oncology Ospedali Galliera, Genova; Division of Cancer Prevention and Genetics, European Institute of Oncology, Milano;
Review Future possibilities in the prevention of breast cancer Breast cancer prevention trials Jack Cuzick
 Review Future possibilities in the prevention of breast cancer Breast cancer prevention trials Jack Cuzick Imperial Cancer Research Fund, London, UK Received: 17 December 1999 Accepted: 30 March 2000 Published:
Review Future possibilities in the prevention of breast cancer Breast cancer prevention trials Jack Cuzick Imperial Cancer Research Fund, London, UK Received: 17 December 1999 Accepted: 30 March 2000 Published:
Tamoxifen for prevention of breast cancer: extended longterm follow-up of the IBIS-I breast cancer prevention trial
 for prevention of breast cancer: extended longterm follow-up of the IBIS-I breast cancer prevention trial Jack Cuzick, Ivana Sestak, Simon Cawthorn, Hisham Hamed, Kaija Holli, Anthony Howell, John F Forbes,
for prevention of breast cancer: extended longterm follow-up of the IBIS-I breast cancer prevention trial Jack Cuzick, Ivana Sestak, Simon Cawthorn, Hisham Hamed, Kaija Holli, Anthony Howell, John F Forbes,
Research. Breast cancer represents a major
 Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
POSITION PAPER FOR HEALTH CARE PROVIDERS Use of Pharmacologic Intervention for Breast Cancer Risk Reduction
 P.O. Box 30195 Lansing, MI 48909 Phone: 877-588-6224 FAX: 517-335-9397 www.michigancancer.org Introduction POSITION PAPER FOR HEALTH CARE PROVIDERS Use of Pharmacologic Intervention for Breast Cancer Risk
P.O. Box 30195 Lansing, MI 48909 Phone: 877-588-6224 FAX: 517-335-9397 www.michigancancer.org Introduction POSITION PAPER FOR HEALTH CARE PROVIDERS Use of Pharmacologic Intervention for Breast Cancer Risk
William J. Gradishar MD
 Northwestern University Feinberg School of Medicine Adjuvant Endocrine Therapy For Postmenopausal Women SOBO 2013 William J. Gradishar MD Betsy Bramsen Professor of Breast Oncology Director, Maggie Daley
Northwestern University Feinberg School of Medicine Adjuvant Endocrine Therapy For Postmenopausal Women SOBO 2013 William J. Gradishar MD Betsy Bramsen Professor of Breast Oncology Director, Maggie Daley
BRIEF COMMUNICATIONS. Italian Randomized Trial Among Women With Hysterectomy: Tamoxifen and Hormone-Dependent Breast Cancer in High-Risk Women
 BRIEF COMMUNICATIONS Italian Randomized Trial Among Women With Hysterectomy: Tamoxifen and Hormone-Dependent Breast Cancer in High-Risk Women Umberto Veronesi, Patrick Maisonneuve, Nicole Rotmensz, Alberto
BRIEF COMMUNICATIONS Italian Randomized Trial Among Women With Hysterectomy: Tamoxifen and Hormone-Dependent Breast Cancer in High-Risk Women Umberto Veronesi, Patrick Maisonneuve, Nicole Rotmensz, Alberto
Breast Cancer Prevention
 Breast Cancer Prevention TREVOR J. POWLES Royal Marsden NHS Trust, and Institute of Cancer Research, London, United Kingdom Key Words. Breast cancer Chemoprevention Tamoxifen Raloxifene ABSTRACT Epidemiological,
Breast Cancer Prevention TREVOR J. POWLES Royal Marsden NHS Trust, and Institute of Cancer Research, London, United Kingdom Key Words. Breast cancer Chemoprevention Tamoxifen Raloxifene ABSTRACT Epidemiological,
Effective Health Care Program
 Comparative Effectiveness Review Number 17 Effective Health Care Program Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women Executive Summary Background Breast cancer
Comparative Effectiveness Review Number 17 Effective Health Care Program Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women Executive Summary Background Breast cancer
Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer
 Global Breast Cancer Conference 2017 21 st Apr, 2017@Chezu Island Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer Shinji Ohno, M.D., Ph.D., F.A.C.S. Breast
Global Breast Cancer Conference 2017 21 st Apr, 2017@Chezu Island Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer Shinji Ohno, M.D., Ph.D., F.A.C.S. Breast
Breast Cancer Prevention Studies. Key Points. Breast cancer prevention studies are clinical trials (research studies conducted with
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Breast Cancer Prevention
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Breast Cancer Prevention
Vascular Medicine. Effect of Tamoxifen on Venous Thromboembolic Events in a Breast Cancer Prevention Trial
 Vascular Medicine Effect of Tamoxifen on Venous Thromboembolic Events in a Breast Cancer Prevention Trial Andrea Decensi, MD; Patrick Maisonneuve, PhD; Nicole Rotmensz, PhD; Donato Bettega, MD; Alberto
Vascular Medicine Effect of Tamoxifen on Venous Thromboembolic Events in a Breast Cancer Prevention Trial Andrea Decensi, MD; Patrick Maisonneuve, PhD; Nicole Rotmensz, PhD; Donato Bettega, MD; Alberto
RALOXIFENE Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA Is the request for the prevention (risk reduction) of breast cancer?
 Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA 16917 GUIDELINES FOR USE 1. Is the request for the prevention (risk reduction) of breast cancer? If yes, continue to #2. If no, approve by HICL
Generic Brand HICL GCN Exception/Other RALOXIFENE EVISTA 16917 GUIDELINES FOR USE 1. Is the request for the prevention (risk reduction) of breast cancer? If yes, continue to #2. If no, approve by HICL
J Clin Oncol 29: by American Society of Clinical Oncology INTRODUCTION
 VOLUME 29 NUMBER 17 JUNE 10 2011 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Benefit/Risk Assessment for Breast Cancer Chemoprevention With Raloxifene or Tamoxifen for Women Age 50 Years or
VOLUME 29 NUMBER 17 JUNE 10 2011 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Benefit/Risk Assessment for Breast Cancer Chemoprevention With Raloxifene or Tamoxifen for Women Age 50 Years or
Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data
 Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data Jack Cuzick, Ivana Sestak, Bernardo Bonanni, Joseph P Costantino, Steve Cummings,
Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data Jack Cuzick, Ivana Sestak, Bernardo Bonanni, Joseph P Costantino, Steve Cummings,
Chemoprevention of Breast Cancer
 Chemoprevention of Breast Cancer Recommendations and Rationale U.S. Preventive Services Task Force This statement summarizes the current U.S. Preventive Services Task Force (USPSTF) recommendations for
Chemoprevention of Breast Cancer Recommendations and Rationale U.S. Preventive Services Task Force This statement summarizes the current U.S. Preventive Services Task Force (USPSTF) recommendations for
HORMONE REPLACEMENT THERAPY
 TRIALS OF HR RUTH (Barrett- Connor et al 29 ) JULY 2006 (Country) mean ± sd, range International trial 67.5 an Placebo component in 67.5 ± 6.7 women with Raloxifene or multiple 67.5 ± 6.6 risk factors
TRIALS OF HR RUTH (Barrett- Connor et al 29 ) JULY 2006 (Country) mean ± sd, range International trial 67.5 an Placebo component in 67.5 ± 6.7 women with Raloxifene or multiple 67.5 ± 6.6 risk factors
The NSABP Study of Tamoxifen and Raloxifene (STAR) trial
 For reprint orders, please contact reprints@expert-reviews.com The NSABP Study of Tamoxifen and Raloxifene (STAR) trial Expert Rev. Anticancer Ther. 9(1), 51 60 (2009) Victor G Vogel University of Pittsburgh
For reprint orders, please contact reprints@expert-reviews.com The NSABP Study of Tamoxifen and Raloxifene (STAR) trial Expert Rev. Anticancer Ther. 9(1), 51 60 (2009) Victor G Vogel University of Pittsburgh
The Study of Tamoxifen and Raloxifene (STAR): Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s The Study of Tamoxifen
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s The Study of Tamoxifen
Breast Cancer Risk Assessment and Prevention
 Breast Cancer Risk Assessment and Prevention Katherine B. Lee, MD, FACP October 4, 2017 STATISTICS More than 252,000 cases of breast cancer will be diagnosed this year alone. About 40,000 women will die
Breast Cancer Risk Assessment and Prevention Katherine B. Lee, MD, FACP October 4, 2017 STATISTICS More than 252,000 cases of breast cancer will be diagnosed this year alone. About 40,000 women will die
Breast Cancer Prevention for the Population at Large
 Breast Cancer Prevention for the Population at Large Jack Cuzick Centre for Cancer Prevention Wolfson Institute of Preventive Medicine St Bartholomew s Medical School Queen Mary University of London London,
Breast Cancer Prevention for the Population at Large Jack Cuzick Centre for Cancer Prevention Wolfson Institute of Preventive Medicine St Bartholomew s Medical School Queen Mary University of London London,
Should premenopausal HR+ve breast cancer receive LHRH?
 Should premenopausal HR+ve breast cancer receive LHRH? Hesham Elghazaly, MD Prof. Clinical Oncology, Ain Shams University President of the BGICS Should premenopausal HR+ve breast cancer receive LHRH? NO?
Should premenopausal HR+ve breast cancer receive LHRH? Hesham Elghazaly, MD Prof. Clinical Oncology, Ain Shams University President of the BGICS Should premenopausal HR+ve breast cancer receive LHRH? NO?
Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene)
 EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
Learning Objectives. Peri menopause. Menopause Overview. Recommendation grading categories
 Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers
 日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
BSO, HRT, and ERT. No relevant financial disclosures
 BSO, HRT, and ERT Jubilee Brown, MD Professor & Associate Director, Gynecologic Oncology Levine Cancer Institute at the Carolinas HealthCare System Charlotte, North Carolina No relevant financial disclosures
BSO, HRT, and ERT Jubilee Brown, MD Professor & Associate Director, Gynecologic Oncology Levine Cancer Institute at the Carolinas HealthCare System Charlotte, North Carolina No relevant financial disclosures
THE SAFETY CHECK LIST BEFORE STARTING HT
 THE SAFETY CHECK LIST BEFORE STARTING HT This safety checklist was designed by the Endocrine Society in 2015 to minimize the chance of giving hormone therapy to women who may be negatively affected by
THE SAFETY CHECK LIST BEFORE STARTING HT This safety checklist was designed by the Endocrine Society in 2015 to minimize the chance of giving hormone therapy to women who may be negatively affected by
Figure 1: PALLAS Study Schema. Endocrine adjuvant therapy may have started before randomization and be ongoing at that time.
 Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
Extended Adjuvant Endocrine Therapy
 Extended Adjuvant Endocrine Therapy After all, 5 years Tamoxifen works.. For women with ER+ primary breast cancer, previous studies have shown that treatment with tamoxifen for 5 years has a carry-over
Extended Adjuvant Endocrine Therapy After all, 5 years Tamoxifen works.. For women with ER+ primary breast cancer, previous studies have shown that treatment with tamoxifen for 5 years has a carry-over
FAQ-Protocol 3. BRCA mutation carrier guidelines Frequently asked questions
 ULast updated: 09/02/2015 Protocol 3 BRCA mutation carrier guidelines Frequently asked questions UQ: How accurate are the remaining lifetime and 5 year breast cancer risks in the table? These figures are
ULast updated: 09/02/2015 Protocol 3 BRCA mutation carrier guidelines Frequently asked questions UQ: How accurate are the remaining lifetime and 5 year breast cancer risks in the table? These figures are
Extended Hormonal Therapy
 Extended Hormonal Therapy Dr. Caroline Lohrisch, Medical Oncologist, BC Cancer Agency Vancouver Centre November 1, 2014 www.fpon.ca Optimal Endocrine Therapy for Women with Hormone Receptor Positive Early
Extended Hormonal Therapy Dr. Caroline Lohrisch, Medical Oncologist, BC Cancer Agency Vancouver Centre November 1, 2014 www.fpon.ca Optimal Endocrine Therapy for Women with Hormone Receptor Positive Early
Οutcomes for patients who are diagnosed with breast and endometrial cancer
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2013 Οutcomes for patients who are diagnosed with breast and endometrial cancer Tonya M. Martin-Dunlap Washington
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2013 Οutcomes for patients who are diagnosed with breast and endometrial cancer Tonya M. Martin-Dunlap Washington
Debate Axillary dissection - con. Prof. Dr. Rodica Anghel Institute of Oncology Bucharest
 Debate Axillary dissection - con Prof. Dr. Rodica Anghel Institute of Oncology Bucharest Summer School of Oncology, third edition Updated Oncology 2015: State of the Art News & Challenging Topics Bucharest,
Debate Axillary dissection - con Prof. Dr. Rodica Anghel Institute of Oncology Bucharest Summer School of Oncology, third edition Updated Oncology 2015: State of the Art News & Challenging Topics Bucharest,
The New England Journal of Medicine
 The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 O CTOBER 17, 22 NUMBER 16 TWENTY-YEAR FOLLOW-UP OF A RANDOMIZED STUDY COMPARING BREAST-CONSERVING SURGERY
The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 O CTOBER 17, 22 NUMBER 16 TWENTY-YEAR FOLLOW-UP OF A RANDOMIZED STUDY COMPARING BREAST-CONSERVING SURGERY
Oncotype DX testing in node-positive disease
 Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
So, Who are the appropriate individuals that should consider genetic counseling and genetic testing?
 Hello, I m Banu Arun, Professor of Breast Medical Oncology and Co-Director of Clinical Cancer Genetics at the University of Texas MD Anderson Cancer Center. Today I will be discussing with you Hereditary
Hello, I m Banu Arun, Professor of Breast Medical Oncology and Co-Director of Clinical Cancer Genetics at the University of Texas MD Anderson Cancer Center. Today I will be discussing with you Hereditary
Clinical Trial Results Database Page 1
 Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
Estimation of Tamoxifen s Efficacy for Preventing the Formation and Growth of Breast Tumors
 Estimation of Tamoxifen s Efficacy for Preventing the Formation and Growth of Breast Tumors Michael D. Radmacher, Richard Simon Background: Several randomized clinical trials have tested the hypothesis
Estimation of Tamoxifen s Efficacy for Preventing the Formation and Growth of Breast Tumors Michael D. Radmacher, Richard Simon Background: Several randomized clinical trials have tested the hypothesis
BREAST CANCER. Dawn Hershman, MD MS. Medicine and Epidemiology Co-Director, Breast Program HICCC Columbia University Medical Center.
 BREAST CANCER Dawn Hershman, MD MS Florence Irving Assistant Professor of Medicine and Epidemiology Co-Director, Breast Program HICCC Columbia University Medical Center Background Breast cancer is the
BREAST CANCER Dawn Hershman, MD MS Florence Irving Assistant Professor of Medicine and Epidemiology Co-Director, Breast Program HICCC Columbia University Medical Center Background Breast cancer is the
Cancer Genetics Unit Patient Information
 Chemoprevention for women at an increased risk of familial breast cancer Cancer Genetics Unit Patient Information What is chemoprevention? Chemoprevention describes drugs that are used to reduce the risk
Chemoprevention for women at an increased risk of familial breast cancer Cancer Genetics Unit Patient Information What is chemoprevention? Chemoprevention describes drugs that are used to reduce the risk
Patient Education. Breast Cancer Prevention. Cancer Center
 Patient Education Breast cancer affects one in nine women in the US by the time they reach their 80 s. It is the result of several mutations or alterations in the genes found in the DNA of normal breast
Patient Education Breast cancer affects one in nine women in the US by the time they reach their 80 s. It is the result of several mutations or alterations in the genes found in the DNA of normal breast
Delayed adjuvant tamoxifen: Ten-year results of a collaborative randomized controlled trial in early breast cancer (TAM-02 trial)
 Annals of Oncology 11: 515-519, 2000. 2000 Kluwer Academic Publishers. Printed in the Netherlands. Original article Delayed adjuvant tamoxifen: Ten-year results of a collaborative randomized controlled
Annals of Oncology 11: 515-519, 2000. 2000 Kluwer Academic Publishers. Printed in the Netherlands. Original article Delayed adjuvant tamoxifen: Ten-year results of a collaborative randomized controlled
Overdiagnosis in. breast cancers 12. chemoprevention trials. V. Sopik msc* and S.A. Narod md*
 Curr Oncol, Vol. 22, pp. e6-10; doi: http://dx.doi.org/10.3747/co.22.2191 OVERDIAGNOSIS IN BREAST CANCER CHEMOPREVENTION TRIALS C O M M E N T A R Y Overdiagnosis in breast cancer chemoprevention trials
Curr Oncol, Vol. 22, pp. e6-10; doi: http://dx.doi.org/10.3747/co.22.2191 OVERDIAGNOSIS IN BREAST CANCER CHEMOPREVENTION TRIALS C O M M E N T A R Y Overdiagnosis in breast cancer chemoprevention trials
38 years old, premenopausal, had L+snbx. Pathology: IDC Gr.II T-1.9cm N+2/4sn ER+100%st, PR+60%st, Her2-neg, KI %
 38 years old, premenopausal, had L+snbx Pathology: IDC Gr.II T-1.9cm N+2/4sn ER+100%st, PR+60%st, Her2-neg, KI67 5-10% Question: What will you do now? 1. Give adjuvant chemotherapy 2. Send for Oncotype
38 years old, premenopausal, had L+snbx Pathology: IDC Gr.II T-1.9cm N+2/4sn ER+100%st, PR+60%st, Her2-neg, KI67 5-10% Question: What will you do now? 1. Give adjuvant chemotherapy 2. Send for Oncotype
Breast cancer is the most frequently
 Charmaine Kim-Sing, MBChB, FRCPC, Lorna Weir, MD, FRCPC, and Urve Kuusk, MD, FRCPC Breast cancer risk management for moderaterisk and high-risk women An accurate assessment of breast cancer risk can help
Charmaine Kim-Sing, MBChB, FRCPC, Lorna Weir, MD, FRCPC, and Urve Kuusk, MD, FRCPC Breast cancer risk management for moderaterisk and high-risk women An accurate assessment of breast cancer risk can help
Assessment of Risk Recurrence: Adjuvant Online, OncotypeDx & Mammaprint
 Assessment of Risk Recurrence: Adjuvant Online, OncotypeDx & Mammaprint William J. Gradishar, MD Professor of Medicine Robert H. Lurie Comprehensive Cancer Center of Northwestern University Classical
Assessment of Risk Recurrence: Adjuvant Online, OncotypeDx & Mammaprint William J. Gradishar, MD Professor of Medicine Robert H. Lurie Comprehensive Cancer Center of Northwestern University Classical
What is new in HR+ Breast Cancer? Olivia Pagani Breast Unit and Institute of oncology of Southern Switzerland
 What is new in HR+ Breast Cancer? Olivia Pagani Breast Unit and Institute of oncology of Southern Switzerland Outline Early breast cancer Advanced breast cancer Open questions Outline Early breast cancer
What is new in HR+ Breast Cancer? Olivia Pagani Breast Unit and Institute of oncology of Southern Switzerland Outline Early breast cancer Advanced breast cancer Open questions Outline Early breast cancer
Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals
 Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals Literature Review (January 2009) Hormone Therapy for Women Women's Health
Hormones and Healthy Bones Joint Project of National Osteoporosis Foundation and Association of Reproductive Health Professionals Literature Review (January 2009) Hormone Therapy for Women Women's Health
Menopausal hormone therapy currently has no evidence-based role for
 IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
Figure 1: Consort diagram; the status of participants in the study
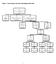 Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Repeating Conservative Surgery after Ipsilateral Breast Tumor Reappearance: Criteria for Selecting the Best Candidates
 Ann Surg Oncol (2012) 19:3771 3776 DOI 10.1245/s10434-012-2404-5 ORIGINAL ARTICLE BREAST ONCOLOGY Repeating Conservative Surgery after Ipsilateral Breast Tumor Reappearance: Criteria for Selecting the
Ann Surg Oncol (2012) 19:3771 3776 DOI 10.1245/s10434-012-2404-5 ORIGINAL ARTICLE BREAST ONCOLOGY Repeating Conservative Surgery after Ipsilateral Breast Tumor Reappearance: Criteria for Selecting the
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Hormone therapy in Breast Cancer patients with comorbidities
 Hormone therapy in Breast Cancer patients with comorbidities Diana Crivellari Centro di Riferimento Oncologico Aviano- ITALY Madrid November 9th, 2007 Main issues Comorbidities in elderly women Hormonal
Hormone therapy in Breast Cancer patients with comorbidities Diana Crivellari Centro di Riferimento Oncologico Aviano- ITALY Madrid November 9th, 2007 Main issues Comorbidities in elderly women Hormonal
Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes
 Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes G. Cancello, P. Maisonneuve, N. Rotmensz, G. Viale, M. G. Mastropasqua, G.
Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes G. Cancello, P. Maisonneuve, N. Rotmensz, G. Viale, M. G. Mastropasqua, G.
All medications are a double-edged sword with risks
 Menopause: The Journal of The North American Menopause Society Vol. 14, No. 5, pp. 1/14 DOI: 10.1097/gme.0b013e31802e8508 * 2007 by The North American Menopause Society REVIEW ARTICLE Postmenopausal hormone
Menopause: The Journal of The North American Menopause Society Vol. 14, No. 5, pp. 1/14 DOI: 10.1097/gme.0b013e31802e8508 * 2007 by The North American Menopause Society REVIEW ARTICLE Postmenopausal hormone
Issues in Cancer Survivorship. Larissa A. Korde, MD, MPH June 26, 2010
 Issues in Cancer Survivorship Larissa A. Korde, MD, MPH June 26, 2010 Estimated US Cancer Cases in Women: 2006-2008 CA Cancer J Clin 2006; 56:106-130; CA Cancer J Clin 2008;58:71 96. Relative Survival*
Issues in Cancer Survivorship Larissa A. Korde, MD, MPH June 26, 2010 Estimated US Cancer Cases in Women: 2006-2008 CA Cancer J Clin 2006; 56:106-130; CA Cancer J Clin 2008;58:71 96. Relative Survival*
BREAST CANCER RISK REDUCTION
 BREAST CANCER RISK REDUCTION Clinical Practice Guideline Update Introduction ASCO published its first breast cancer risk reduction (BCRR) guideline in 1999 ASCO Guidelines are updated at intervals by an
BREAST CANCER RISK REDUCTION Clinical Practice Guideline Update Introduction ASCO published its first breast cancer risk reduction (BCRR) guideline in 1999 ASCO Guidelines are updated at intervals by an
Study Of Letrozole Extension. Coordinating Group IBCSG IBCSG BIG 1-07
 tudy Of Letrozole Extension Coordinating Group IBCSG IBCSG 35-07 BIG 1-07 A phase III trial evaluating the role of continuous letrozole versus intermittent letrozole following 4 to 6 years of prior adjuvant
tudy Of Letrozole Extension Coordinating Group IBCSG IBCSG 35-07 BIG 1-07 A phase III trial evaluating the role of continuous letrozole versus intermittent letrozole following 4 to 6 years of prior adjuvant
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY
 Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY Methodology The test is based on the reported 50-gene classifier algorithm originally named PAM50 and is performed on the ncounter Dx Analysis System
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY Methodology The test is based on the reported 50-gene classifier algorithm originally named PAM50 and is performed on the ncounter Dx Analysis System
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY
 Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY GENE EXPRESSION PROFILING WITH PROSIGNA What is Prosigna? Prosigna Breast Cancer Prognostic Gene Signature Assay is an FDA-approved assay which provides
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY GENE EXPRESSION PROFILING WITH PROSIGNA What is Prosigna? Prosigna Breast Cancer Prognostic Gene Signature Assay is an FDA-approved assay which provides
R. A. Nout Æ W. E. Fiets Æ H. Struikmans Æ F. R. Rosendaal Æ H. Putter Æ J. W. R. Nortier
 Breast Cancer Res Treat (2008) 109:567 572 DOI 10.1007/s10549-007-9681-x EPIDEMIOLOGY The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated
Breast Cancer Res Treat (2008) 109:567 572 DOI 10.1007/s10549-007-9681-x EPIDEMIOLOGY The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated
HORMONAL THERAPY IN ADJUVANT CARE
 ADVANCES IN ENDOCRINE THERAPY FOR BREAST CANCER* Matthew J. Ellis, MD, PhD ABSTRACT Endocrine therapy is used frequently in breast cancer management, particularly in the setting of adjuvant care, but outstanding
ADVANCES IN ENDOCRINE THERAPY FOR BREAST CANCER* Matthew J. Ellis, MD, PhD ABSTRACT Endocrine therapy is used frequently in breast cancer management, particularly in the setting of adjuvant care, but outstanding
Increased Risk of Breast Cancer: Screening and Prevention. Elizabeth Pritchard, MD 4/5/2017
 Increased Risk of Breast Cancer: Screening and Prevention Elizabeth Pritchard, MD 4/5/2017 No disclosures Defining Risk Risk Factors Modifiable Lifestyle obesity physical activity alcohol consumption breast
Increased Risk of Breast Cancer: Screening and Prevention Elizabeth Pritchard, MD 4/5/2017 No disclosures Defining Risk Risk Factors Modifiable Lifestyle obesity physical activity alcohol consumption breast
Scottish Medicines Consortium
 Scottish Medicines Consortium anastrozole 1mg tablets (Arimidex ) No. (198/05) AstraZeneca UK Ltd New indication: for adjuvant treatment of postmenopausal women with hormone receptorpositive early invasive
Scottish Medicines Consortium anastrozole 1mg tablets (Arimidex ) No. (198/05) AstraZeneca UK Ltd New indication: for adjuvant treatment of postmenopausal women with hormone receptorpositive early invasive
Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer
 Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Supplemental Appendix. 1. Protocol Definition of Sustained Virologic Response. A patient has a sustained virologic response if:
 Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Hormonal therapies for the adjuvant treatment of early oestrogenreceptor-positive
 Hormonal therapies for the adjuvant treatment of early oestrogenreceptor-positive breast cancer Issued: November 2006 guidance.nice.org.uk/ta112 NICE 2006 Contents 1 Guidance... 3 2 Clinical need and practice...
Hormonal therapies for the adjuvant treatment of early oestrogenreceptor-positive breast cancer Issued: November 2006 guidance.nice.org.uk/ta112 NICE 2006 Contents 1 Guidance... 3 2 Clinical need and practice...
Trial: Take-Home Message: Executive Summary: Guidelines:
 Trial: Davies C, et al. "Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial".
Trial: Davies C, et al. "Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial".
Radiation and DCIS. The 16 th Annual Conference on A Multidisciplinary Approach to Comprehensive Breast Care and Imaging
 Radiation and DCIS The 16 th Annual Conference on A Multidisciplinary Approach to Comprehensive Breast Care and Imaging Einsley-Marie Janowski, MD, PhD Assistant Professor Department of Radiation Oncology
Radiation and DCIS The 16 th Annual Conference on A Multidisciplinary Approach to Comprehensive Breast Care and Imaging Einsley-Marie Janowski, MD, PhD Assistant Professor Department of Radiation Oncology
Clinicopathological Factors Affecting Distant Metastasis Following Loco-Regional Recurrence of breast cancer. Cheol Min Kang 2018/04/05
 Abstract No.: ABS-0075 Clinicopathological Factors Affecting Distant Metastasis Following Loco-Regional Recurrence of breast cancer 2018/04/05 Cheol Min Kang Department of surgery, University of Ulsan
Abstract No.: ABS-0075 Clinicopathological Factors Affecting Distant Metastasis Following Loco-Regional Recurrence of breast cancer 2018/04/05 Cheol Min Kang Department of surgery, University of Ulsan
Immunohistochemical phenotype of breast cancer during 25- year follow-up of the Royal Marsden Tamoxifen Prevention Trial
 Immunohistochemical phenotype of breast cancer during 25- year follow-up of the Royal Marsden Tamoxifen Prevention Trial Simone I Detre 1, Susan Ashley 2, Kabir Mohammed 2, Ian E Smith 3, Trevor J Powles
Immunohistochemical phenotype of breast cancer during 25- year follow-up of the Royal Marsden Tamoxifen Prevention Trial Simone I Detre 1, Susan Ashley 2, Kabir Mohammed 2, Ian E Smith 3, Trevor J Powles
CHL 5225 H Advanced Statistical Methods for Clinical Trials. CHL 5225 H The Language of Clinical Trials
 CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Commentary Has tamoxifen had its day? Michael Baum
 Commentary Has tamoxifen had its day? Michael Baum Department of Surgery, University College London, UK Correspondence: Michael Baum, Consulting Rooms, The Portland Hospital, 212-214 Great Portland St,
Commentary Has tamoxifen had its day? Michael Baum Department of Surgery, University College London, UK Correspondence: Michael Baum, Consulting Rooms, The Portland Hospital, 212-214 Great Portland St,
ORMONOTERAPIA ADIUVANTE: QUALE LA DURATA OTTIMALE? MARIANTONIETTA COLOZZA
 ORMONOTERAPIA ADIUVANTE: QUALE LA DURATA OTTIMALE? MARIANTONIETTA COLOZZA THE NATURAL HISTORY OF HORMONE RECEPTOR- POSITIVE BREAST CANCER IS VERY LONG Recurrence hazard rate 0.3 0.2 0.1 0 ER+ (n=2,257)
ORMONOTERAPIA ADIUVANTE: QUALE LA DURATA OTTIMALE? MARIANTONIETTA COLOZZA THE NATURAL HISTORY OF HORMONE RECEPTOR- POSITIVE BREAST CANCER IS VERY LONG Recurrence hazard rate 0.3 0.2 0.1 0 ER+ (n=2,257)
Endocrine Therapy in Premenopausal Breast Cancer. Joyce O Shaughnessy, MD Baylor Sammons Cancer Center Texas Oncology, PA US Oncology
 Endocrine Therapy in Premenopausal Breast Cancer Joyce O Shaughnessy, MD Baylor Sammons Cancer Center Texas Oncology, PA US Oncology Ovarian Ablation or Suppression vs. Not in ER + or ER UK Breast Cancer
Endocrine Therapy in Premenopausal Breast Cancer Joyce O Shaughnessy, MD Baylor Sammons Cancer Center Texas Oncology, PA US Oncology Ovarian Ablation or Suppression vs. Not in ER + or ER UK Breast Cancer
Giuseppe Viale for the BIG 1 98 Collaborative and International Breast Cancer Study Groups
 Central Review of ER, PgR and HER2 in BIG 1 98 Evaluating Letrozole vs. Letrozole Tamoxifen vs. Tamoxifen Letrozole as Adjuvant Endocrine Therapy for Postmenopausal Women with Hormone Receptor Positive
Central Review of ER, PgR and HER2 in BIG 1 98 Evaluating Letrozole vs. Letrozole Tamoxifen vs. Tamoxifen Letrozole as Adjuvant Endocrine Therapy for Postmenopausal Women with Hormone Receptor Positive
Use of Ovarian Suppression and Ablation in Breast Cancer Treatment
 Use of Ovarian Suppression and Ablation in Breast Cancer Treatment Dr Marina Parton Consultant Medical Oncologist Royal Marsden and Kingston Hospitals Overview Breast cancer phenotypes Use of ovarian manipulation
Use of Ovarian Suppression and Ablation in Breast Cancer Treatment Dr Marina Parton Consultant Medical Oncologist Royal Marsden and Kingston Hospitals Overview Breast cancer phenotypes Use of ovarian manipulation
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Menopause management NICE Implementation
 Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Contents. Version 1.0: 01/02/2010 Protocol# ISRCTN Page 1 of 7
 Contents 1. INTRODUCTION... 2 2. STUDY SYNOPSIS... 2 3. STUDY OBJECTIVES... 2 3.1. Primary Objective... 2 3.2. Secondary Objectives... 2 3.3. Assessment of Objectives... 3 3.4. Change the Primary Objective
Contents 1. INTRODUCTION... 2 2. STUDY SYNOPSIS... 2 3. STUDY OBJECTIVES... 2 3.1. Primary Objective... 2 3.2. Secondary Objectives... 2 3.3. Assessment of Objectives... 3 3.4. Change the Primary Objective
 Mdi Medical Management of Breast Cancer Morbidity and Mortality Aug 13, 2009 Irina Kovatch, PGY3 Introduction Metastatic disease is the principal cause of death from breast cancer Metastatic events often
Mdi Medical Management of Breast Cancer Morbidity and Mortality Aug 13, 2009 Irina Kovatch, PGY3 Introduction Metastatic disease is the principal cause of death from breast cancer Metastatic events often
Kathryn M. Rexrode, MD, MPH. Assistant Professor. Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School
 Update: Hormones and Cardiovascular Disease in Women Kathryn M. Rexrode, MD, MPH Assistant Professor Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School Overview Review
Update: Hormones and Cardiovascular Disease in Women Kathryn M. Rexrode, MD, MPH Assistant Professor Division of Preventive Medicine Brigham and Women s s Hospital Harvard Medical School Overview Review
SOFTly: The Long Natural History of [Trials for] [premenopausal] ER+ Breast Cancer
![SOFTly: The Long Natural History of [Trials for] [premenopausal] ER+ Breast Cancer SOFTly: The Long Natural History of [Trials for] [premenopausal] ER+ Breast Cancer](/thumbs/95/125806264.jpg) SOFTly: The Long Natural History of [Trials for] [premenopausal] ER+ Breast Cancer Charles Moertel Lecture May 12, 2017 Gini Fleming Charles Moertel Founder of NCCTG Dedication to high quality clinical
SOFTly: The Long Natural History of [Trials for] [premenopausal] ER+ Breast Cancer Charles Moertel Lecture May 12, 2017 Gini Fleming Charles Moertel Founder of NCCTG Dedication to high quality clinical
Copyright, 1995, by the Massachusetts Medical Society
 Copyright, 99, by the Massachusetts Medical Society Volume 332 APRIL 6, 99 Number ADJUVANT CYCLOPHOSPHAMIDE, METHOTREXATE, AND FLUOROURACIL IN NODE- POSITIVE BREAST CANCER The Results of Years of Follow-up
Copyright, 99, by the Massachusetts Medical Society Volume 332 APRIL 6, 99 Number ADJUVANT CYCLOPHOSPHAMIDE, METHOTREXATE, AND FLUOROURACIL IN NODE- POSITIVE BREAST CANCER The Results of Years of Follow-up
Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers
 Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers Ross Prentice Fred Hutchinson Cancer Research Center and University of Washington AICR, November 5, 2009 Outline
Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers Ross Prentice Fred Hutchinson Cancer Research Center and University of Washington AICR, November 5, 2009 Outline
Hormone replacement therapy and breast density after surgical menopause
 Hormone replacement therapy and breast density after surgical menopause Freya Schnabel*; Sarah Pivo; Esther Dubrovsky; Jennifer Chun; Shira Schwartz; Amber Guth; Deborah Axelrod Department of Surgery,
Hormone replacement therapy and breast density after surgical menopause Freya Schnabel*; Sarah Pivo; Esther Dubrovsky; Jennifer Chun; Shira Schwartz; Amber Guth; Deborah Axelrod Department of Surgery,
Prophylactic Mastectomy State of the Art
 Memorial Sloan-Kettering Cancer Center 1275 York Avenue, New York, NY 10065 6 th Brazilian Breast Cancer Conference Sao Paulo, Brazil 9 March 2012 Prophylactic Mastectomy State of the Art Monica Morrow
Memorial Sloan-Kettering Cancer Center 1275 York Avenue, New York, NY 10065 6 th Brazilian Breast Cancer Conference Sao Paulo, Brazil 9 March 2012 Prophylactic Mastectomy State of the Art Monica Morrow
ATAC Trial. 10 year median follow-up data. Approval Code: AZT-ARIM-10005
 ATAC Trial 10 year median follow-up data Approval Code: AZT-ARIM-10005 Background FDA post-approval commitment analysis to update DFS, TTR, OS and Safety Prof. Jack Cuzick on behalf of ATAC/LATTE Trialists
ATAC Trial 10 year median follow-up data Approval Code: AZT-ARIM-10005 Background FDA post-approval commitment analysis to update DFS, TTR, OS and Safety Prof. Jack Cuzick on behalf of ATAC/LATTE Trialists
Tamoxifen For Breast Cancer Chemoprevention: Low Uptake by High-Risk Women After Evaluation of a Breast Lump
 Tamoxifen For Breast Cancer Chemoprevention: Low Uptake by High-Risk Women After Evaluation of a Breast Lump Rebecca Taylor, MD, MSc 1 Kenneth Taguchi, MD, MDCM 2 1 Division of General Surgery, University
Tamoxifen For Breast Cancer Chemoprevention: Low Uptake by High-Risk Women After Evaluation of a Breast Lump Rebecca Taylor, MD, MSc 1 Kenneth Taguchi, MD, MDCM 2 1 Division of General Surgery, University
La salute dell osso nelle pazienti in trattamento adiuvante. Airoldi Mario - S.C. Oncologia Medica 2 Città della Salute e della Scienza di Torino
 La salute dell osso nelle pazienti in trattamento adiuvante Airoldi Mario - S.C. Oncologia Medica 2 Città della Salute e della Scienza di Torino BONE STRENGTH OSTEOPOROSIS SKELETAL DISORDER COMPROMISING
La salute dell osso nelle pazienti in trattamento adiuvante Airoldi Mario - S.C. Oncologia Medica 2 Città della Salute e della Scienza di Torino BONE STRENGTH OSTEOPOROSIS SKELETAL DISORDER COMPROMISING
CLINICIAN INTERVIEW CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN
 CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN Nanette K. Wenger, MD, is a recognized authority on women and coronary heart disease. She chaired the US National Heart, Lung, and Blood Institute conference
CARDIOVASCULAR DISEASE IN POSTMENOPAUSAL WOMEN Nanette K. Wenger, MD, is a recognized authority on women and coronary heart disease. She chaired the US National Heart, Lung, and Blood Institute conference
TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer
 TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer Marta Bonotto Department of Oncology University Hospital of Udine TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive
TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer Marta Bonotto Department of Oncology University Hospital of Udine TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive
PROFESSIONAL INFORMATION BROCHURE
 64207-00 Rev 05/02 PROFESSIONAL INFORMATION BROCHURE TM WARNING - For Women with Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer: Serious and life-threatening events associated
64207-00 Rev 05/02 PROFESSIONAL INFORMATION BROCHURE TM WARNING - For Women with Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer: Serious and life-threatening events associated
Update on New Perspectives in Endocrine-Sensitive Breast Cancer. James R. Waisman, MD
 Update on New Perspectives in Endocrine-Sensitive Breast Cancer James R. Waisman, MD Nothing to disclose DISCLOSURE TAILORx Oncotype Recurrence Score TAILORx Study Design Sparano, J Clin Oncol 2008;26:721-728
Update on New Perspectives in Endocrine-Sensitive Breast Cancer James R. Waisman, MD Nothing to disclose DISCLOSURE TAILORx Oncotype Recurrence Score TAILORx Study Design Sparano, J Clin Oncol 2008;26:721-728
Study Period: 27 March 2008 (first subject enrolled) to 05 May 2010 (data cutoff date for primary analysis)
 Date: 20 July 2011 Page 2 of 3375 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C US Name of Finished Product: Not applicable Name of ctive Ingredient: Ganitumab (MG 479) Title of Study: n International,
Date: 20 July 2011 Page 2 of 3375 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C US Name of Finished Product: Not applicable Name of ctive Ingredient: Ganitumab (MG 479) Title of Study: n International,
University Gynecologic Oncology Associates
 University Gynecologic Oncology Associates Medical History Form Date: Name: Date of Birth: / / GYNE HISTORY Age of first period? If you no longer have periods, at what age did they stop? Are you pregnant
University Gynecologic Oncology Associates Medical History Form Date: Name: Date of Birth: / / GYNE HISTORY Age of first period? If you no longer have periods, at what age did they stop? Are you pregnant
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
