Virtual Mentor American Medical Association Journal of Ethics January 2007, Volume 9, Number 1:
|
|
|
- Roland Beasley
- 5 years ago
- Views:
Transcription
1 Virtual Mentor American Medical Association Journal of Ethics January 2007, Volume 9, Number 1: Policy forum Setting fair prices for life-saving drugs by Bruce A. Chabner, MD, and Thomas G. Roberts Jr., MD, MSocSci Cancer drugs are big business. Worldwide sales are projected to reach $25 billion in 2006 and to increase to almost $50 billion by 2010 [1]. This represents a startling growth in a segment of the drug industry once shunned by major pharmaceutical manufacturers as too high-risk and unprofitable. While a few drug companies, notably Bristol-Myers Squibb (BMS) and Pharmatalia, made significant profits on cancer drugs between 1970 and 1990 when the first effective combination therapies came into common practice, the turning point in this industrial segment occurred in 1992 with the approval of Bristol-Myers Squibb s paclitaxel, which became a multibillion-dollar-per-year product by To understand our current concerns with cancer drug costs and their potential effect on medical care financing and access, one needs to be familiar with the paclitaxel experience. The story of paclitaxel s discovery and commercial development reflects both the lack of interest that industry had in cancer drugs at that time and the sudden emergence of drug cost as a social justice issue. In 1964 Monroe Wall and associates, working at the Research Triangle Institute under a National Cancer Institute (NCI) contract, isolated the active compound in paclitaxel from the bark of the common yew tree [2]. Its tortuous development, complicated by difficulties in material procurement, compound purification and formulation, delayed its entry into clinical trials until 1983, and its efficacy in treating ovarian cancer was not demonstrated until 1987 [3]. Because of the need to procure large amounts of plant material for its isolation and the tendency of the necessary solvent (Cremophor EL) to cause hypersensitivity reactions, there was little commercial interest in the compound. When NCI announced an open competition for clinical development of the compound in 1990, only four companies responded. Two of the applicants were small firms, unprepared for the task of drug production and clinical development. A third was a foreign company that already held rights to a competitor compound in the same class. BMS was the only major U.S. company to apply for the development rights and won the contract in Shortly thereafter, the drug s effectiveness against breast cancer became apparent, and it emerged as a blockbuster. Congressional involvement Pricing of the drug immediately became a concern for the U.S. Congress [4]. 38 Virtual Mentor, January 2007 Vol 9
2 Because it had been discovered and developed under government contracts with the NCI, members of Congress scrutinized the price set by BMS. The NCI had urged BMS to set a price consistent with that of competitive compounds in the field of ovarian cancer. NCI directors believed that the production costs, limited range of uses and novelty of the compound justified a cost per treatment of approximately $2,000, and the BMS price was consistent with this target price. NCI also wanted to assure that patients could get the drug regardless of their insurance status, a concern to which BMS responded by setting up a program of free distribution to indigent patients. But members of Congress castigated BMS at open hearings, pointing out the government s key role in the drug s discovery and deploring the profit BMS was making. An unstated, but recurring theme in these hearings was the plight of cancer patients who had no choice but to pay or seek insurer reimbursement for this uniquely effective medication. Even though the drug was shown to confer a survival advantage in multiple forms of cancer, most notably breast and ovarian, some countries, including those in the United Kingdom, refused to grant approval based on a cost-benefit analysis. Parenthetically, the U.K. National Health Service continues to deny use of other expensive new drugs, such as bortezomib, on the same basis. During this tumultuous period of paclitaxel marketing, when the U.S. Congress and the public first directly confronted the pharmaceutical industry over the cost of lifesaving cancer drugs, it became apparent that the public had no other option but to pay the price. Federally mandated price controls were openly discussed at Senate hearings, but rejected as impractical (what is a fair price?) and potentially fatal to the rapidly growing biotechnology industry. No one neither physicians nor the patients in need could place an appropriate dollar value on the worth of one year of human life [5]. Challenges for pricing drugs in the 21st century The paclitaxel experience set the stage for the dilemma of cancer drug pricing that is now playing out on a much larger scale. Since the mid-1990s the biotechnology industry has made major contributions to cancer treatment with targeted therapies agents that have specific molecular targets and which, alone, are not toxic to cells at standard doses. (Traditional chemotherapy agents are cytotoxins that cause cell death at standard doses.) Targeted therapies include monoclonal antibodies such as bevacizumab (Avastin) and trastuzumab (Herceptin) from Genentech; selective small molecule tyrosine kinase inhibitors such as erlotinib (Tarceva) from OSI pharmaceuticals/genentech Inc.; and multi-targeted kinase inhibitors such as sorafenib (Nexavar; Onyx Pharmaceuticals/Bayer AG) and sunitinib (Sutent; Pfizer Inc.). The antibodies have achieved annual sales in excess of $1 billion each, and their potential for further expansion seems unlimited (figure 1). Virtual Mentor, January 2006 Vol 9 39
3 $60,000 $50,000 Annual Sales $40,000 $30,000 $20, $10,000 $0 Chemotherapeutics Targeted Therapies Total Market Figure 1. Estimated Worldwide Market for Oncology Drugs by Class (in millions) in 2005 and 2010 (Data are from S.G. Cowen and Company; Reference 1). The cost of these medications ranges from approximately $3,000 per treatment cycle for the small molecules to $7,000-$10,000 per treatment cycle for the antibodies [6]. One antibody in particular, cetuximab (Erbitux; Imclone Systems Inc/BMS), an EGFR (epidermal growth factor receptor) inhibitor indicated for treatment of colorectal and head and neck cancer, has attracted significant publicity because of its high cost and low response rates. While each of the companies has established mechanisms that provide assistance for uninsured or indigent patients, the high cost of these new medications has attracted considerable attention in the medical and lay press [7]. How can it be justified? The risk-reward ratio for companies engaged in cancer drug discovery and development remains unfavorable. The cost begins with a major investment in basic research, often heavily supplemented by NCI and other grants, followed by extensive preclinical evaluations and clinical trials involving hundreds of patients and many years of effort. If one takes into account the expenses associated with failed drugs, the industry spends approximately $1 billion for each compound that reaches the market [8]. Obviously, for the individual successful compound and its company, the cost is significantly less, but there is no doubt that this is not an industry for the fainthearted or for those with shallow pockets. No more than 7 percent of cancer compounds that enter clinical trials end up reaching the market. While several hundred biotech companies are now engaged in cancer drug discovery and development, the number of new drugs approved each year remains in the single digits, and most companies ultimately fail to earn a profit. Certain classes of 40 Virtual Mentor, January 2007 Vol 9
4 promising compounds, such as vaccines and cell cycle inhibitors, in which multiple companies have invested hugely, have not yet produced a single approved drug. Recent public and congressional concerns about the need for post-marketing surveillance to ensure safety would further increase the cost of drug development. Pretrial systems for predicting clinical success, based on mouse models of human disease or human tumor cell lines, have largely failed. When breakthrough drugs such as imatinib (Gleevec; Novartis AG) for chronic myelogenous leukemia do succeed, their period of uniqueness is often brief, as competitors quickly produce new and perhaps better drugs, such as dasatinib (Sprycel; BMS), for the same target illness [9]. And, finally, the period of patent protection, typically 20 years from the time of patent filing, is too brief, considering the time 10 years on average spent in development and the fact that the pharmaceutical industry must reinvest up to 30 percent of its profits in new drug research. Forces that may reduce the cost of cancer drugs While talk of price controls continues in Congress, other factors are likely to mitigate pricing. The first is competition. A new antibody, panitumumab (Vectibix; Amgen Inc.), an effective EGFR inhibitor, has entered the market in competition with cetuximab (Erbitux) and costs less. A number of small molecules are in the late stage of development and are being groomed to compete with the most expensive drugs, namely the monoclonal antibodies. There are differences between the antibodies and their small molecule competitors (e.g., site of action, target access, pharmacokinetics, etc.), so a lot of comparative development remains to be done. Nonetheless, the small compounds, traditionally priced below the antibodies, will, if approved, most likely drive down the cost of cancer care. Orally administered small molecules have the additional attractive feature of not requiring a hospital visit and thus obviate the cost of intravenous infusion, a major economic benefit for the health care system. As prescription drugs, however, they are not always covered completely by the insurance of those who need them; that is, they can fall into Medicare s proverbial donut hole of noncoverage. A second force for reducing the cost of care will be improvements in patient selection for therapy. With few exceptions, cancer drugs are presently used in settings in which only a fraction of patients will benefit. Cetuximab, which is among the most costly antibodies, produces responses in percent of patients with chemotherapy-resistant colorectal cancer. It may benefit a larger subset of patients when given in combination with irinotecan, but there is no test currently available to identify the responsive subset of patients. Through the use of molecular diagnostics (biomarkers), it may be possible to improve the response rates and eliminate the needless expense of shotgun therapy. Examples of successful patient selection include the use of imatinib in chronic myelogenous leukemia (CML), in which all patients have tumors with translocations involving the same gene. Further, CML patients who develop resistance to imatinib harbor further mutations, most of which are sensitive to dasatinib [9]. Molecular Virtual Mentor, January 2006 Vol 9 41
5 analysis clearly has a role in creating a treatment plan for these resistant patients. The experience with EGFR inhibitors such as erlotinib and gefitinib has yielded a strong correlation between receptor mutations and responsiveness, a relationship that could lead to up-front or adjuvant use of these drugs in selected patients [10]. The NCI and Food and Drug Administration (FDA) have jointly endorsed the development of biomarkers for drug selection in cancer and have outlined an ambitious research effort. At the same time, however, the FDA is tightening its oversight of home brew diagnostic tests, asking for stronger prospective validation of assays. In the past, reimbursement for these tests was determined by the regional Medicare carrier and by individual insurers. Depending on the standard to which these tests will be held, the development of molecular diagnostics for cancer could encounter significant regulatory delays in the future. Finally, the government possesses a strong weapon in bargaining for lower prices of cancer drugs. It is a major purchaser of pharmaceuticals through the Veterans Administration system and sets reimbursement rates for medical procedures and services in the Medicare program. Congress is threatening to become more involved in issues of drug pricing, using its bully pulpit congressional hearings to expose excessive profits. Through legislative action, it could ask for a cost-benefit analysis as part of Medicare reimbursement policy and could extend patent life as a reward for corporate programs that expand free access to drugs, ensuring that all patients will benefit from the federally funded research that underlies virtually all of these new discoveries. In conclusion, the high cost of cancer drugs tests corporate responsiveness to public needs, while challenging scientific innovation and the potential of the competitive marketplace to control prices. The federal government will surely exercise indirect influence over pricing through its ability to expose the issue in congressional hearings and through the multiple points of intersection of the executive branch and industry. Meanwhile scientific progress is driving the cost of cancer care ever upward. This progress is saving lives, but there have to be limits. At this point, no one has a clear idea what these limits might be. References 1. S.G. Cowen and Company. Pharmaceutical Therapeutic Categories Outlook: Comprehensive Study. New York, NY: Cowen and Company; 2006: Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93: Rowinsky EK, Donehower RC. Paclitaxel (Taxol). N Engl J Med. 1995;332: Goodman J, Walsh V. The Story of Taxol: Nature and Politics in the Pursuit of an Anti-Cancer Drug. Cambridge, UK: Cambridge University Press; Virtual Mentor, January 2007 Vol 9
6 5. Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11: Schrag D. The price tag on progress chemotherapy for colorectal cancer. N Engl J Med. 2004;351: Berenson A. A cancer drug shows promise, at a price that many can t pay. New York Times. February 15, 2006:A1, C2. Available at: 15drug.html?ex= &en=bc6aaaf25acffa44&ei=5088. Accessed December 12, Brock DW. How much is more life worth? Hastings Cent Rep. 2006;36: Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P. Dasatinib. Nat Rev Drug Discov. 2006;5: Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350: Bruce A. Chabner, MD, is clinical director for the Massachusetts General Hospital Cancer Center and a professor of medicine at Harvard Medical School. His main fields of research focus on the biochemistry and pharmacology of folate antagonists, experimental therapeutics and clinical trial design. He serves as the associate director of clinical science at Dana Farber/Harvard Cancer Center and has held additional appointments, including the position of director of the Division of Cancer Treatment at the National Cancer Institute from 1982 to Thomas G. Roberts Jr., MD, MSocSci, is a portfolio manager and senior health care analyst at Noonday Asset Management L.P. in Charlotte, N.C. Dr. Roberts also holds appointments at the Massachusetts General Hospital Institute of Technology Assessment and the Massachusetts Institute of Technology Program on the Pharmaceutical Industry. His research interests include optimizing the process of cancer drug development, government approval and reimbursement policy, and pharmacoeconomic modeling. Virtual Mentor welcomes your response to recently published articles and commentaries. Send your correspondence to the Virtual Mentor address: virtualmentor@ama-assn.org. The viewpoints expressed on this site are those of the authors and do not necessarily reflect the views and policies of the AMA. Copyright 2006 American Medical Association. All rights reserved. Virtual Mentor, January 2006 Vol 9 43
Chronic Myeloid Leukemia (CML)
 Chronic Myeloid Leukemia (CML) Japan Drug Forecast and Market Analysis to 2022 GDHC1088CFR / Published April 2013 Executive Summary Sales for CML in Japan The Japan CML therapeutics market in the 7MM is
Chronic Myeloid Leukemia (CML) Japan Drug Forecast and Market Analysis to 2022 GDHC1088CFR / Published April 2013 Executive Summary Sales for CML in Japan The Japan CML therapeutics market in the 7MM is
Targeted Medicine and Molecular Therapeutics. Angus McIntyre, M.D. Medical Oncologist, Addison Gilbert Hospital and Beverly Hospital October 6, 2009
 Targeted Medicine and Molecular Therapeutics Angus McIntyre, M.D. Medical Oncologist, Addison Gilbert Hospital and Beverly Hospital October 6, 2009 Approaches to Cancer Prevention Screening and early diagnosis
Targeted Medicine and Molecular Therapeutics Angus McIntyre, M.D. Medical Oncologist, Addison Gilbert Hospital and Beverly Hospital October 6, 2009 Approaches to Cancer Prevention Screening and early diagnosis
Synribo (Chronic Myeloid Leukemia)
 Synribo (Chronic Myeloid Leukemia) Forecast and Market Analysis to 2022 GDHC1141DFR / Published May 2013 Executive Summary Synribo (omacetaxine mepesuccinate): Key Metrics in CML Markets 2012 Synribo Sales
Synribo (Chronic Myeloid Leukemia) Forecast and Market Analysis to 2022 GDHC1141DFR / Published May 2013 Executive Summary Synribo (omacetaxine mepesuccinate): Key Metrics in CML Markets 2012 Synribo Sales
Oncology Market Forecast To 2013
 Oncology Market Forecast To 2013 How do you uncover the threats and opportunities that your oncology drug will face this year, next year and each year after that? Our new report answers your questions
Oncology Market Forecast To 2013 How do you uncover the threats and opportunities that your oncology drug will face this year, next year and each year after that? Our new report answers your questions
Oncology Market Opportunities, Strategies, and Forecasts, 2006 to Oncology. Picture by Susie Eustis MOUNTAINS OF OPPORTUNITY
 Oncology Market Opportunities, Strategies, and Forecasts, 2006 to 2012 Oncology Picture by Susie Eustis MOUNTAINS OF OPPORTUNITY WinterGreen Research, Inc. Lexington, Massachusetts www.wintergreenresearch.com
Oncology Market Opportunities, Strategies, and Forecasts, 2006 to 2012 Oncology Picture by Susie Eustis MOUNTAINS OF OPPORTUNITY WinterGreen Research, Inc. Lexington, Massachusetts www.wintergreenresearch.com
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Krysta Pellegrino (650) Investor Contact: Sue Morris (650) Advocacy Contact: Kristin Reed (650)
 NEWS RELEASE Media Contact: Krysta Pellegrino (650) 225-8226 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Kristin Reed (650) 467-9831 FDA APPROVES AVASTIN IN COMBINATION WITH CHEMOTHERAPY
NEWS RELEASE Media Contact: Krysta Pellegrino (650) 225-8226 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Kristin Reed (650) 467-9831 FDA APPROVES AVASTIN IN COMBINATION WITH CHEMOTHERAPY
Introduction to Targeted Therapy
 Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF HER2-POSITIVE NODE-POSITIVE BREAST CANCER
 NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE
 37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
08:FORECAST. The View From the Office: How Community Oncologists See 2008 By Peter Carlin and Don Stark of Market Strategies International
 08:FORECAST A Survey of Oncologists Provides Insight Into the Coming Year The View From the Office: How Community Oncologists See 2008 By Peter Carlin and Don Stark of Market Strategies International While
08:FORECAST A Survey of Oncologists Provides Insight Into the Coming Year The View From the Office: How Community Oncologists See 2008 By Peter Carlin and Don Stark of Market Strategies International While
ISPOR 4 th Asia Pacific Conference IP2 Gilberto de Lima Lopes
 Health Economic Considerations for Personalized Medicine in Asia: Using Genomic Profiling to Guide Treatment Decisions in Early Breast Cancer Gilberto de Lima Lopes, Jr., M.D., M.B.A Assistant Director
Health Economic Considerations for Personalized Medicine in Asia: Using Genomic Profiling to Guide Treatment Decisions in Early Breast Cancer Gilberto de Lima Lopes, Jr., M.D., M.B.A Assistant Director
Like others here today, we are very conflicted on the FDA s proposal on behind-thecounter medications, but thank you for raising the issue.
 Statement of Consumers Union William Vaughan, Senior Policy Analyst before the US Food and Drug Administration Public Meeting on Behind the Counter Availability of Drugs November 14, 2007 Consumers Union
Statement of Consumers Union William Vaughan, Senior Policy Analyst before the US Food and Drug Administration Public Meeting on Behind the Counter Availability of Drugs November 14, 2007 Consumers Union
Cell, Volume 141. Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger
 Cell, Volume 141 Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger Figure S1. RTK Mutations in Diseases Locations of gain-of-function (green arrows)
Cell, Volume 141 Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger Figure S1. RTK Mutations in Diseases Locations of gain-of-function (green arrows)
REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics of Cyramza for colorectal cancer (CRC) in the eight major pharmaceutical
REFERENCE CODE GDHC476DFR PUBLICAT ION DATE NOVEMBER 2014 CYRAMZA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics of Cyramza for colorectal cancer (CRC) in the eight major pharmaceutical
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Executive Summary. Reproduction prohibited v
 Kinases are a large family of proteins that have now become firmly established as a major class of drug targets for the pharmaceutical industry. The sequencing of the Human Genome has led to the identification
Kinases are a large family of proteins that have now become firmly established as a major class of drug targets for the pharmaceutical industry. The sequencing of the Human Genome has led to the identification
HALOZYME REPORTS SECOND QUARTER 2018 RESULTS
 Contacts: Robert H. Uhl Managing Director Westwicke Partners, LLC 858-356-5932 robert.uhl@westwicke.com Laurie Stelzer 858-704-8222 ir@halozyme.com FOR IMMEDIATE RELEASE HALOZYME REPORTS SECOND QUARTER
Contacts: Robert H. Uhl Managing Director Westwicke Partners, LLC 858-356-5932 robert.uhl@westwicke.com Laurie Stelzer 858-704-8222 ir@halozyme.com FOR IMMEDIATE RELEASE HALOZYME REPORTS SECOND QUARTER
Access to cancer drugs: The role for a stakeholder alliance?
 Access to cancer drugs: The role for a stakeholder alliance? John Zalcberg Co-Chair, Cancer Drugs Alliance (CDA) Disclosures Research support, travel support and honoraria from a wide variety of pharmaceutical
Access to cancer drugs: The role for a stakeholder alliance? John Zalcberg Co-Chair, Cancer Drugs Alliance (CDA) Disclosures Research support, travel support and honoraria from a wide variety of pharmaceutical
Erbitux. Erbitux (cetuximab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
United States House of Representatives. I am the Executive Director of the North America office
 Rachel M. Cohen, Executive Director Drugs for Neglected Diseases initiative, North America Written Testimony, Outside Witness Hearing Subcommittee on State and Foreign Operations, Committee on Appropriations
Rachel M. Cohen, Executive Director Drugs for Neglected Diseases initiative, North America Written Testimony, Outside Witness Hearing Subcommittee on State and Foreign Operations, Committee on Appropriations
REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Stivarga for colorectal cancer (CRC) in the eight major
REFERENCE CODE GDHC472DFR PUBLICAT ION DATE NOVEM BER 2014 STIVARGA (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Stivarga for colorectal cancer (CRC) in the eight major
DS-8201 Strategic Collaboration
 DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
New Developments in Cancer Treatment. Dulcinea Quintana, MD
 New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
Industry Company Products Pipeline Risks/Catalysts Valuation Q&A. Agenda
 Industry Company Products Pipeline Risks/Catalysts Valuation Q&A Agenda 1 Pharmaceutical/Biotech Pharmaceutical companies Simple molecules made by chemical process taken orally Biotech companies Complicated
Industry Company Products Pipeline Risks/Catalysts Valuation Q&A Agenda 1 Pharmaceutical/Biotech Pharmaceutical companies Simple molecules made by chemical process taken orally Biotech companies Complicated
Walgreens (WAG) Analyst: Juan Fabres Fall 2014
 Recommendation: Buy Target Price August 31, 2016: $77.57 1. Reasons for the Recommendation With the acquisition of Alliance Boots in Europe, Walgreens will be the first US pharmacy to operate retail stores
Recommendation: Buy Target Price August 31, 2016: $77.57 1. Reasons for the Recommendation With the acquisition of Alliance Boots in Europe, Walgreens will be the first US pharmacy to operate retail stores
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS
 MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
REFERENCE CODE GDHC207DFR PUBLICAT ION DATE JUNE 2013 GILOTRIF (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC207DFR PUBLICAT ION DATE JUNE 2013 GILOTRIF (NON-SMALL CELL LUNG CANCER) GILOTRIF (NON-SMALL CELL LUNG CANCER) - Executive Summary Gilotrif: Key Metrics in NSCLC Markets 2022 Gilotrif
REFERENCE CODE GDHC207DFR PUBLICAT ION DATE JUNE 2013 GILOTRIF (NON-SMALL CELL LUNG CANCER) GILOTRIF (NON-SMALL CELL LUNG CANCER) - Executive Summary Gilotrif: Key Metrics in NSCLC Markets 2022 Gilotrif
Vectibix. Vectibix (panitumumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.85 Subject: Vectibix Page: 1 of 5 Last Review Date: December 2, 2016 Vectibix Description Vectibix
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.85 Subject: Vectibix Page: 1 of 5 Last Review Date: December 2, 2016 Vectibix Description Vectibix
REFERENCE CODE GDHC198DFR PUBLICAT ION DATE JUNE 2013 TARCEVA (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC198DFR PUBLICAT ION DATE JUNE 2013 TARCEVA (NON-SMALL CELL LUNG CANCER) Executive Summary Tarceva (erlotinib): Key Metrics in NSCLC Markets 2012 Tarceva Sales US 5EU Japan China India
REFERENCE CODE GDHC198DFR PUBLICAT ION DATE JUNE 2013 TARCEVA (NON-SMALL CELL LUNG CANCER) Executive Summary Tarceva (erlotinib): Key Metrics in NSCLC Markets 2012 Tarceva Sales US 5EU Japan China India
GASTRIC CANCER TESTING, TREATMENT AND PREVENTION: TECHNOLOGIES AND GLOBAL MARKETS. HLC174A October Usha Nagavarapu Project Analyst
 GASTRIC CANCER TESTING, TREATMENT AND PREVENTION: TECHNOLOGIES AND GLOBAL MARKETS HLC174A October 2015 Usha Nagavarapu Project Analyst ISBN: 1-62296-167-6 BCC Research 49 Walnut Park, Building 2 Wellesley,
GASTRIC CANCER TESTING, TREATMENT AND PREVENTION: TECHNOLOGIES AND GLOBAL MARKETS HLC174A October 2015 Usha Nagavarapu Project Analyst ISBN: 1-62296-167-6 BCC Research 49 Walnut Park, Building 2 Wellesley,
Novogen Limited. An emerging oncology drug developer with two clinical-stage programs NRT NVGN. April 2017
 Novogen Limited An emerging oncology drug developer with two clinical-stage programs NRT April 2017 NVGN Forward-Looking Statements This presentation contains forward-looking statements within the meaning
Novogen Limited An emerging oncology drug developer with two clinical-stage programs NRT April 2017 NVGN Forward-Looking Statements This presentation contains forward-looking statements within the meaning
Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies selectively targeting genetic drivers of cancer
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
Corporate Presentation. August 2016
 v Corporate Presentation August 2016 Safe harbor statement Certain statements made in this presentation contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933,
v Corporate Presentation August 2016 Safe harbor statement Certain statements made in this presentation contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933,
Personalized medecine Biomarker
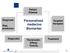 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
REFERENCE CODE GDHC471DFR PUBLICAT ION DATE NOVEM BER 2014 VECTIBIX (COLORECTAL CANCER) FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC471DFR PUBLICAT ION DATE NOVEM BER 2014 VECTIBIX (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Vectibix for colorectal cancer (CRC) in the eight major
REFERENCE CODE GDHC471DFR PUBLICAT ION DATE NOVEM BER 2014 VECTIBIX (COLORECTAL CANCER) Executive Summary Table below presents the key metrics for Vectibix for colorectal cancer (CRC) in the eight major
New Developments in Cancer Treatment. Ian Rabinowitz MD
 New Developments in Cancer Treatment Ian Rabinowitz MD Treatment Outline Angiogenesis inhibition Targeted therapy Immunotherapy Personalization of therapy Genomics and cancer Stem cells and cancer Angiogenesis
New Developments in Cancer Treatment Ian Rabinowitz MD Treatment Outline Angiogenesis inhibition Targeted therapy Immunotherapy Personalization of therapy Genomics and cancer Stem cells and cancer Angiogenesis
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
NATIVA GROUP. Inspired by Innovation and Technology
 NATIVA GROUP Inspired by Innovation and Technology INNOVATION & TECHNOLOGY IS OUR PASSION Nativa is a leading group of pharmaceutical companies in Russia. It develops and produces worldclass complex medicines
NATIVA GROUP Inspired by Innovation and Technology INNOVATION & TECHNOLOGY IS OUR PASSION Nativa is a leading group of pharmaceutical companies in Russia. It develops and produces worldclass complex medicines
1.5. Research Areas Treatment Selection
 1.5. Research Areas Cancer biomarker research embraces many areas of study, including tumorigenesis, metastasis, clinical trial and surrogate endpoints, cell isolation, target identification, drug resistance,
1.5. Research Areas Cancer biomarker research embraces many areas of study, including tumorigenesis, metastasis, clinical trial and surrogate endpoints, cell isolation, target identification, drug resistance,
Recent Surge in FDA Approvals Shakes up the Oncology Promotional Landscape. Perjeta. Stivarga. Marquibo. Bosulif. Xtandi. Krypolis.
 Krypolis Bosulif Inlyta Erivedge Xtandi Stivarga Alimta Gleevec Marquibo Perjeta Synribo Zaltrap Recent Surge in FDA Approvals Shakes up the Oncology Promotional Landscape By Bill Bowman, JD What a year
Krypolis Bosulif Inlyta Erivedge Xtandi Stivarga Alimta Gleevec Marquibo Perjeta Synribo Zaltrap Recent Surge in FDA Approvals Shakes up the Oncology Promotional Landscape By Bill Bowman, JD What a year
Health Systems Adoption of Personalized Medicine: Promise and Obstacles. Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA
 Health Systems Adoption of Personalized Medicine: Promise and Obstacles Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA Cancer Pharmaceutical Pricing Bach P. NEJM 2009;360:626 Opportunities
Health Systems Adoption of Personalized Medicine: Promise and Obstacles Scott Ramsey Fred Hutchinson Cancer Research Center Seattle, WA Cancer Pharmaceutical Pricing Bach P. NEJM 2009;360:626 Opportunities
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. What to expect during treatment with Vectibix. Important Safety Information.
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
Targeted Therapy Vijay Narang
 25 Volume 1, Issue 1, January 2013, Online: Targeted Therapy Vijay Narang ABSTRACT This is a review on targeted therapy that blocks the growth and spread of cancer by interfering with specific molecules
25 Volume 1, Issue 1, January 2013, Online: Targeted Therapy Vijay Narang ABSTRACT This is a review on targeted therapy that blocks the growth and spread of cancer by interfering with specific molecules
REPORT OF THE COUNCIL ON MEDICAL SERVICE
 REPORT OF THE COUNCIL ON MEDICAL SERVICE CMS Report -I- Subject: Presented by: Referred to: Hearing Aid Coverage (Resolutions -I- and -I-) Robert E. Hertzka, MD, Chair Reference Committee J (Jeffrey P.
REPORT OF THE COUNCIL ON MEDICAL SERVICE CMS Report -I- Subject: Presented by: Referred to: Hearing Aid Coverage (Resolutions -I- and -I-) Robert E. Hertzka, MD, Chair Reference Committee J (Jeffrey P.
Name of firm/applicant
 Recommendation:- The 18 th Subject Expert Committee (Oncology & Hematology) deliberated the proposals on 04.07.2014 and recommended the following:- Agenda no. Drug name Name of firm/applicant Recommendations
Recommendation:- The 18 th Subject Expert Committee (Oncology & Hematology) deliberated the proposals on 04.07.2014 and recommended the following:- Agenda no. Drug name Name of firm/applicant Recommendations
Regorafenib from Bayer Submitted to Health Authorities Seeking Approval in Second-Line Treatment of Liver Cancer
 News Release Not intended for U.S. and UK Media Bayer AG Communications, Government Relations & Corporate Brand 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com Regorafenib from Bayer Submitted
News Release Not intended for U.S. and UK Media Bayer AG Communications, Government Relations & Corporate Brand 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com Regorafenib from Bayer Submitted
61 Recommendations for better use of medications
 Chapter 61 Recommendations for better use of medications As stated elsewhere in this book, we are fortunate to have a number of effective medications for treatment of many medical conditions. The drug
Chapter 61 Recommendations for better use of medications As stated elsewhere in this book, we are fortunate to have a number of effective medications for treatment of many medical conditions. The drug
Nektar Investor & Analyst Call. Nektar & z Bristol-Myers Squibb Collaboration. February 14, 2018
 Nektar Investor & Analyst Call Nektar & z Bristol-Myers Squibb Collaboration February 14, 2018 This presentation includes forward-looking statements regarding Nektar s proprietary drug candidates, the
Nektar Investor & Analyst Call Nektar & z Bristol-Myers Squibb Collaboration February 14, 2018 This presentation includes forward-looking statements regarding Nektar s proprietary drug candidates, the
REFERENCE CODE GDHC199DFR PUBLICAT ION DATE JUNE 2013 XALKORI (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC199DFR PUBLICAT ION DATE JUNE 2013 XALKORI (NON-SMALL CELL LUNG CANCER) Executive Summary Xalkori (crizotinib): Key Metrics in NSCLC Markets 2012 Xalkori Sales US 5EU Japan China India
REFERENCE CODE GDHC199DFR PUBLICAT ION DATE JUNE 2013 XALKORI (NON-SMALL CELL LUNG CANCER) Executive Summary Xalkori (crizotinib): Key Metrics in NSCLC Markets 2012 Xalkori Sales US 5EU Japan China India
Pharma 2020: Which Path will you take? Automation in Pharma Congress, Leiden 8 October 2009 Loic Kubitza & Arwin van der Linden
 Pharma 2020: Which Path will you take? Automation in Pharma Congress, Leiden 8 October 2009 Loic Kubitza & Arwin van der Linden Agenda Trends and implications Potential business models Personalized medicine
Pharma 2020: Which Path will you take? Automation in Pharma Congress, Leiden 8 October 2009 Loic Kubitza & Arwin van der Linden Agenda Trends and implications Potential business models Personalized medicine
REFERENCE CODE GDHC196DFR PUBLICAT ION DATE JUNE 2013 ABRAXANE (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC196DFR PUBLICAT ION DATE JUNE 2013 ABRAXANE (NON-SMALL CELL LUNG CANCER) Executive Summary Abraxane (nab-paclitaxel): Key Metrics in NSCLC Markets 2012 Abraxane Sales US 5EU $98.34m
REFERENCE CODE GDHC196DFR PUBLICAT ION DATE JUNE 2013 ABRAXANE (NON-SMALL CELL LUNG CANCER) Executive Summary Abraxane (nab-paclitaxel): Key Metrics in NSCLC Markets 2012 Abraxane Sales US 5EU $98.34m
What are Clinical Trials
 What are Clinical Trials Clinical Trials are systematic studies designed to discover and verify the effects and/or adverse reactions of drugs (pharmacodynamics) and the absorption, distribution, metabolism
What are Clinical Trials Clinical Trials are systematic studies designed to discover and verify the effects and/or adverse reactions of drugs (pharmacodynamics) and the absorption, distribution, metabolism
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Stivarga) Reference Number: CP.PHAR.107 Effective Date: 12.01.12 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of
Clinical Policy: (Stivarga) Reference Number: CP.PHAR.107 Effective Date: 12.01.12 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of
TREAT-NMD and the Role of the Industry in Orphan Diseases. Dr. Stefanie Possekel Santhera Pharmaceuticals
 TREAT-NMD and the Role of the Industry in Orphan Diseases Dr. Stefanie Possekel Santhera Pharmaceuticals TREAT-NMD EU-funded infrastructure to accelerate therapy development in neuromuscular diseases Clinical
TREAT-NMD and the Role of the Industry in Orphan Diseases Dr. Stefanie Possekel Santhera Pharmaceuticals TREAT-NMD EU-funded infrastructure to accelerate therapy development in neuromuscular diseases Clinical
Arizona s Oral Anticancer Treatment Access Law: What Clinicians Need to Know
 Outdated coverage policies in Arizona USED TO limit cancer patients access to lifesaving drugs! Traditionally, IV chemotherapy treatments are covered under a health plan s medical benefit where the patient
Outdated coverage policies in Arizona USED TO limit cancer patients access to lifesaving drugs! Traditionally, IV chemotherapy treatments are covered under a health plan s medical benefit where the patient
Breast Cancer: Who Gets It? Who Survives? The Latest Information
 Breast Cancer: Who Gets It? Who Survives? The Latest Information James J. Stark, MD, FACP Medical Director, Cancer Program and Director of Palliative Care Maryview Medical Center Professor of Medicine
Breast Cancer: Who Gets It? Who Survives? The Latest Information James J. Stark, MD, FACP Medical Director, Cancer Program and Director of Palliative Care Maryview Medical Center Professor of Medicine
New Oncology Drugs: Nadeem Ikhlaque, M.D Subtitle Would Go Here
 New Oncology Drugs: A PowerPoint Brief Primer Cover Title Nadeem Ikhlaque, M.D 05.19.2017 Subtitle Would Go Here Learning Objectives List novel chemotherapies and the indications of these newer agents
New Oncology Drugs: A PowerPoint Brief Primer Cover Title Nadeem Ikhlaque, M.D 05.19.2017 Subtitle Would Go Here Learning Objectives List novel chemotherapies and the indications of these newer agents
Emerging Issues in Food and Drug Law: Implementation of FDASIA Innovative Drug Approvals: Breakthrough Therapies Arnold & Porter LLP, Washington DC
 Emerging Issues in Food and Drug Law: Implementation of FDASIA Innovative Drug Approvals: Breakthrough Therapies Arnold & Porter LLP, Washington DC Ryan M. Hohman, JD, MPA Managing Director, Friends of
Emerging Issues in Food and Drug Law: Implementation of FDASIA Innovative Drug Approvals: Breakthrough Therapies Arnold & Porter LLP, Washington DC Ryan M. Hohman, JD, MPA Managing Director, Friends of
GAO BRAND-NAME PRESCRIPTION DRUG PRICING
 GAO United States Government Accountability Office Report to Congressional Requesters December 2009 BRAND-NAME PRESCRIPTION DRUG PRICING Lack of Therapeutically Equivalent Drugs and Limited Competition
GAO United States Government Accountability Office Report to Congressional Requesters December 2009 BRAND-NAME PRESCRIPTION DRUG PRICING Lack of Therapeutically Equivalent Drugs and Limited Competition
Diagnostics for the early detection and prevention of colon cancer. Fourth-Quarter 2014 Earnings Call February 24, 2015
 Diagnostics for the early detection and prevention of colon cancer Fourth-Quarter 2014 Earnings Call February 24, 2015 Safe Harbor Statement Certain statements made in this presentation contain forward-looking
Diagnostics for the early detection and prevention of colon cancer Fourth-Quarter 2014 Earnings Call February 24, 2015 Safe Harbor Statement Certain statements made in this presentation contain forward-looking
Sustain and Seize Cancer Research Opportunities
 One Voice Against Cancer (OVAC) appreciates the opportunity to submit written comments for the record regarding funding for cancer programs for research, prevention, detection, and treatment as well as
One Voice Against Cancer (OVAC) appreciates the opportunity to submit written comments for the record regarding funding for cancer programs for research, prevention, detection, and treatment as well as
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK
 HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
Health Industry Forum October 2, 2007 Scott Howell, MD Senior Director, Channel and Contracting Strategy Genentech
 1 Cost Effectiveness & Pricing/Reimbursement A Biotech Case Study Health Industry Forum October 2, 2007 Scott Howell, MD Senior Director, Channel and Contracting Strategy Genentech Disclaimer 2 The views
1 Cost Effectiveness & Pricing/Reimbursement A Biotech Case Study Health Industry Forum October 2, 2007 Scott Howell, MD Senior Director, Channel and Contracting Strategy Genentech Disclaimer 2 The views
Development trends for new cancer therapeutics and vaccines
 REVIEWS Drug Discovery Today Volume 13, Numbers 1/2 January 2008 Development trends for new cancer therapeutics and vaccines Janice M. Reichert and Julia B. Wenger Tufts Center for the Study of Drug Development,
REVIEWS Drug Discovery Today Volume 13, Numbers 1/2 January 2008 Development trends for new cancer therapeutics and vaccines Janice M. Reichert and Julia B. Wenger Tufts Center for the Study of Drug Development,
Introducing NCCN Academy for Excellence & Leadership in Oncology April Program Overview
 Introducing NCCN Academy for Excellence & Leadership in Oncology April 2009 Program Overview In 2009, NCCN will launch the NCCN Academy for Excellence & Leadership in Oncology, a series of oncology certificate
Introducing NCCN Academy for Excellence & Leadership in Oncology April 2009 Program Overview In 2009, NCCN will launch the NCCN Academy for Excellence & Leadership in Oncology, a series of oncology certificate
REFERENCE CODE GDHC200DFR PUBLICAT ION DATE JUNE 2013 AVASTIN (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC200DFR PUBLICAT ION DATE JUNE 2013 AVASTIN (NON-SMALL CELL LUNG CANCER) AVASTIN (NON-SMALL CELL LUNG CANCER) - Executive Summary Avastin (bevacizumab): Key Metrics in NSCLC Markets 2012
REFERENCE CODE GDHC200DFR PUBLICAT ION DATE JUNE 2013 AVASTIN (NON-SMALL CELL LUNG CANCER) AVASTIN (NON-SMALL CELL LUNG CANCER) - Executive Summary Avastin (bevacizumab): Key Metrics in NSCLC Markets 2012
Lung Cancer Screening
 Lung Cancer Screening v Lung Cancer: America s leading cancer killer Annual US cancer mortality 158,040 221,000 new diagnoses in US 4,100 14,180 27,540 Lung 40,560 40,730 49,700 Cervix Ovary Prostate Pancreas
Lung Cancer Screening v Lung Cancer: America s leading cancer killer Annual US cancer mortality 158,040 221,000 new diagnoses in US 4,100 14,180 27,540 Lung 40,560 40,730 49,700 Cervix Ovary Prostate Pancreas
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist
 Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
Do Firms Underinvest In Long-Term Research? Evidence From Cancer Clinical Trials
 SIEPR policy brief Stanford University May 2014 Stanford Institute for Economic Policy Research on the web: http://siepr.stanford.edu Do Firms Underinvest In Long-Term Research? Evidence From Cancer Clinical
SIEPR policy brief Stanford University May 2014 Stanford Institute for Economic Policy Research on the web: http://siepr.stanford.edu Do Firms Underinvest In Long-Term Research? Evidence From Cancer Clinical
Oncology Therapeutics Market in India to 2018
 Oncology Therapeutics Market in India to 2018 Introduction of Market-Based Pricing to Fuel Price Wars and Intense Competition Among Domestic and Multinational Players GBI Research Report Guidance GBI Research
Oncology Therapeutics Market in India to 2018 Introduction of Market-Based Pricing to Fuel Price Wars and Intense Competition Among Domestic and Multinational Players GBI Research Report Guidance GBI Research
Health Care Reform Update and Advocacy Priorities
 Health Care Reform Update and Advocacy Priorities Robert Greenwald Clinical Professor of Law Director, Center for Health Law and Policy Innovation of Harvard Law School October 2012 PRESENTATION OUTLINE
Health Care Reform Update and Advocacy Priorities Robert Greenwald Clinical Professor of Law Director, Center for Health Law and Policy Innovation of Harvard Law School October 2012 PRESENTATION OUTLINE
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results
 Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
European Experience and Perspective on Assessing Value for Oncology Products. Michael Drummond Centre for Health Economics, University of York
 European Experience and Perspective on Assessing Value for Oncology Products Michael Drummond Centre for Health Economics, University of York Outline of Presentation The European landscape on access to
European Experience and Perspective on Assessing Value for Oncology Products Michael Drummond Centre for Health Economics, University of York Outline of Presentation The European landscape on access to
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS
 NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
CANCER HAS BEEN AROUND
 For decades, drug companies have been spending billions of dollars and countless hours on researching and developing treatments and cures for cancer. The reason is easy to understand. Cancer is the second-leading
For decades, drug companies have been spending billions of dollars and countless hours on researching and developing treatments and cures for cancer. The reason is easy to understand. Cancer is the second-leading
Hosts. New Treatments for Cancer
 Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education
 Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education Aims of this session Understand how genomic information will change your clinical practice Remind
Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education Aims of this session Understand how genomic information will change your clinical practice Remind
Crisis Investing: Profits From a Diabetes Drug About to Get Approved Crisis Investing: Profits From a Diabetes Drug About to Get Approved
 Crisis Investing: Profits From a Diabetes Drug About to Get Approved A Special Research Report from Bret Jensen's Biotech Gems Lexicon Pharmaceuticals (Nasdaq: LXRX): tackling one of America s biggest
Crisis Investing: Profits From a Diabetes Drug About to Get Approved A Special Research Report from Bret Jensen's Biotech Gems Lexicon Pharmaceuticals (Nasdaq: LXRX): tackling one of America s biggest
Corporate Presentation Fourth Quarter 2017
 Corporate Presentation Fourth Quarter 2017 November 2017 1 Safe harbor statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
Corporate Presentation Fourth Quarter 2017 November 2017 1 Safe harbor statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
Stratified medicine in practice: Review of predictive biomarkers in European Medicines Agency (EMA) indications
 Stratified medicine in practice: Review of predictive biomarkers in European Medicines Agency (EMA) indications Kinga Malottki, Mousumi Biswas, Jon Deeks, Richard Riley, Charles Craddock, Lucinda Billingham
Stratified medicine in practice: Review of predictive biomarkers in European Medicines Agency (EMA) indications Kinga Malottki, Mousumi Biswas, Jon Deeks, Richard Riley, Charles Craddock, Lucinda Billingham
Documenting the journey
 Documenting the journey A lung cancр patiнt s journal LUNG CANCER INITIATIVE OF NORTH CAROLINA There is no medicine like,פho no incentive so great, and no tonic so powerful as expectation of something
Documenting the journey A lung cancр patiнt s journal LUNG CANCER INITIATIVE OF NORTH CAROLINA There is no medicine like,פho no incentive so great, and no tonic so powerful as expectation of something
CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer
 November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
Restoration. JDRF is turning Type One into Type None. attack so the newly restored beta cell function is protected.
 Restoration JDRF is turning Type One into Type None Type 1 diabetes (T1D) is an extraordinarily complex disease. Finding the cure a short-term clinical intervention, with minimal side effects, after which
Restoration JDRF is turning Type One into Type None Type 1 diabetes (T1D) is an extraordinarily complex disease. Finding the cure a short-term clinical intervention, with minimal side effects, after which
Global Pulse Oximetry Project
 3.3 Introduction of new health technologies: lessons learned Over the past twenty years there have been a number of comprehensive public health projects which illustrate important lessons regarding the
3.3 Introduction of new health technologies: lessons learned Over the past twenty years there have been a number of comprehensive public health projects which illustrate important lessons regarding the
Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical
 For immediate release November 25, 2004 Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical Sumitomo Chemical Co., Ltd. announced today that its subsidiary, Sumitomo
For immediate release November 25, 2004 Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical Sumitomo Chemical Co., Ltd. announced today that its subsidiary, Sumitomo
You Get What You Pay For The Unintended Consequences of Buy and Bill in Oncology
 You Get What You Pay For The Unintended Consequences of Buy and Bill in Oncology Jeffrey Peppercorn, MD, MPH Associate Professor of Medicine Director, Cancer Survivorship Center Duke Cancer Institute Duke
You Get What You Pay For The Unintended Consequences of Buy and Bill in Oncology Jeffrey Peppercorn, MD, MPH Associate Professor of Medicine Director, Cancer Survivorship Center Duke Cancer Institute Duke
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper)
 A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. Important Safety Information. Indication and Limitation of Use. Your Doctor Discussion Guide
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
5 $3 billion per disease
 $3 billion per disease Chapter at a glance Our aim is to set a market size large enough to attract serious commercial investment from several pharmaceutical companies that see technological opportunites,
$3 billion per disease Chapter at a glance Our aim is to set a market size large enough to attract serious commercial investment from several pharmaceutical companies that see technological opportunites,
Phoenix Molecular Designs
 Phoenix Molecular Designs DESIGNING PRECISE THERAPEUTICS TO REVOLUTIONIZE TREATMENT Our Team Dr. Sandra Dunn, Ph.D. Dr. Anna Stratford, Ph.D. Dr. Aarthi Jayanthan, Ph.D. Dr. Zaihui Zhang CEO, CSO UBC Professor
Phoenix Molecular Designs DESIGNING PRECISE THERAPEUTICS TO REVOLUTIONIZE TREATMENT Our Team Dr. Sandra Dunn, Ph.D. Dr. Anna Stratford, Ph.D. Dr. Aarthi Jayanthan, Ph.D. Dr. Zaihui Zhang CEO, CSO UBC Professor
scr.zacks.com 10 S. Riverside Plaza, Suite 1600, Chicago, IL (MBVX - Nasdaq) OUTLOOK ZACKS ESTIMATES
 Small-Cap Research August 26, 2016 David Bautz, PhD 312-265-9471 dbautz@zacks.com scr.zacks.com 10 S. Riverside Plaza, Suite 1600, Chicago, IL 60606 MabVax Therapeutics Holdings, Inc. MBVX: Uplist to Nasdaq
Small-Cap Research August 26, 2016 David Bautz, PhD 312-265-9471 dbautz@zacks.com scr.zacks.com 10 S. Riverside Plaza, Suite 1600, Chicago, IL 60606 MabVax Therapeutics Holdings, Inc. MBVX: Uplist to Nasdaq
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C Form 6-K
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month
A company developing innovative, high-impact drugs for cancer
 A company developing innovative, high-impact drugs for cancer Investor Briefing March 2019 ASX: KZA NASDAQ : KZIA Twitter: @KaziaTx Forward-Looking Statements This presentation contains forward-looking
A company developing innovative, high-impact drugs for cancer Investor Briefing March 2019 ASX: KZA NASDAQ : KZIA Twitter: @KaziaTx Forward-Looking Statements This presentation contains forward-looking
Imaging Cancer Treatment Complications in the Chest
 Imaging Cancer Treatment Complications in the Chest Michelle S. Ginsberg, MD Objectives Imaging Cancer Treatment Complications in the Chest To understand the mechanisms of action of different classes of
Imaging Cancer Treatment Complications in the Chest Michelle S. Ginsberg, MD Objectives Imaging Cancer Treatment Complications in the Chest To understand the mechanisms of action of different classes of
Innovative Finance: the power of innovation to save lives
 Innovative Finance: the power of innovation to save lives GAVI s mission is to save children s lives and protect people s health by increasing access to immunisation in poor countries. To that end, the
Innovative Finance: the power of innovation to save lives GAVI s mission is to save children s lives and protect people s health by increasing access to immunisation in poor countries. To that end, the
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Stivarga) Reference Number: CP.CPA.157 Effective Date: 11.16.17 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Stivarga) Reference Number: CP.CPA.157 Effective Date: 11.16.17 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Positioned for Growth
 Positioned for Growth Annual Shareholders Meeting July 21, 2016 Paris Panayiotopoulos President and Chief Executive Officer David Sachs Non small cell lung cancer ARIAD clinical trial patient This presentation
Positioned for Growth Annual Shareholders Meeting July 21, 2016 Paris Panayiotopoulos President and Chief Executive Officer David Sachs Non small cell lung cancer ARIAD clinical trial patient This presentation
