MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS. MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS
|
|
|
- Jeffery Barker
- 5 years ago
- Views:
Transcription
1 ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL MIU 1 INJ KEY ONCOLOGICS Alemtuzumab (dosc new ATC L04AA34) Alemtuzumab (dosc new ATC L04AA34) MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS Anagrelide AGRYLIN mg 100 ORAL TEMA MEDICAL Anastrozole STRADEXA mg 30 ORAL EUROLAB Anastrozole TEVA ANASTROZOLE mg 30 ORAL CIPLA-MEDPRO PHARMACEUTICALS Asparaginase Laspar IU 1 INJ FRESENIUS KABI Azacitidine VIDAZA mg 1 INJ KEY ONCOLOGICS Bendamustine RIBOMUSTIN mg 1 INJ ASTELLAS PHARMA Bendamustine RIBOMUSTIN mg 5 INJ ASTELLAS PHARMA Bendamustine RIBOMUSTIN mg 5 INJ ASTELLAS PHARMA Bendamustine RIBOMUSTIN mg 1 INJ ASTELLAS PHARMA Bevacizumab AVASTIN mg 1 INJ ROCHE PRODUCTS Bevacizumab AVASTIN mg 1 INJ ROCHE PRODUCTS Bicalutamide CALOXA mg 30 ORAL EUROLAB Bicalutamide BICALOX mg 30 ORAL PHARMAPLAN Bicalutamide TEVA BICALUTAMIDE mg 30 ORAL CIPLA-MEDPRO PHARMACEUTICALS Bicalutamide ACCORD BICALUTAMIDE mg 30 ORAL ACCORD HEALTHCARE Bicalutamide SPEC-BICALUTAMIDE mg 30 ORAL SPECPHARM (PTY) LTD Bleomycin Bleolem IU 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Bortezomib VELCADE mg 1 INJ JANSSEN PHARMACEUTICALS Buserelin SUPREFACT 3 MONTH DEPOT mg 1 IMP SANOFI AVENTIS Busulfan BUSULFEX AMP mg 8 INJ TEMA MEDICAL Busulfan MYLERAN mg 100 ORAL ASPEN PHARMACARE
2 Capecitabine XELODA mg 60 ORAL ROCHE PRODUCTS Capecitabine XELODA mg 120 ORAL ROCHE PRODUCTS Carboplatin Accord Carboplatin mg 1 INJ ACCORD HEALTHCARE Carboplatin Carbosin mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Carboplatin ASPEN CARBOPLATIN mg 1 INJ ASPEN PHARMACARE Carboplatin Accord Carboplatin mg 1 INJ ACCORD HEALTHCARE Carboplatin Carbosin mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Carboplatin ASPEN CARBOPLATIN mg 1 INJ ASPEN PHARMACARE Carboplatin Carbosin mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Cetuximab ERBITUX mg 1 INJ MERCK Cetuximab ERBITUX VIAL mg 1 INJ MERCK Chlorambucil LEUKERAN mg 25 ORAL ASPEN PHARMACARE Cisplatin CISACOR mg 1 INJ ACCORD HEALTHCARE Cisplatin P&U CISPLATIN mg 1 INJ PFIZER LABORATORIES Cisplatin CISACOR mg 1 INJ ACCORD HEALTHCARE Cisplatin P&U CISPLATIN mg 1 INJ PFIZER LABORATORIES Cladribine LITAK mg 1 INJ KEY ONCOLOGICS Cyclophosphamide ENDOXAN mg 1 INJ SANOFI AVENTIS Cyclophosphamide ENDOXAN mg 1 INJ SANOFI AVENTIS Cyclophosphamide ENDOXAN mg 50 ORAL SANOFI AVENTIS Cyproterone ANDROCUR DEPOT mg 3 INJ BAYER CONSUMER CARE Cyproterone CIPLA-CYPROTERONE ACETATE mg 20 ORAL CIPLA-MEDPRO PHARMACEUTICALS Cytarabine P & U CYTARABINE mg 5 INJ PFIZER LABORATORIES Cytarabine Cytarabine Fauld mg 5 INJ PHARMAPLAN Cytarabine LARACIT mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Dacarbazine DACIN mg 1 INJ KEY ONCOLOGICS Dactinomycin COSMEGEN mg 1 INJ KEY ONCOLOGICS Danazol DANOGEN mg 60 ORAL CIPLA-MEDPRO PHARMACEUTICALS
3 Danazol LADAZOL mg 60 ORAL ADCOCK GENERICS Dasatinib SPRYCEL mg 60 ORAL BM SQUIBB Dasatinib SPRYCEL mg 60 ORAL BM SQUIBB Dasatinib SPRYCEL mg 60 ORAL BM SQUIBB Dasatinib SPRYCEL mg 30 ORAL BM SQUIBB Daunorubicin DAUNOBLASTIN mg 1 INJ PFIZER LABORATORIES Docetaxel ERIOX mg 1 INJ EUROLAB Docetaxel ERIOX mg 1 INJ EUROLAB Doxorubicin RUBEXET mg 1 INJ EUROLAB Doxorubicin DOXORUBICIN ACCORD mg 1 INJ ACCORD HEALTHCARE Doxorubicin DOXORUBICIN RTU mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Doxorubicin Cipla-Doxorubicin mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Doxorubicin RUBEXET mg 1 INJ EUROLAB Doxorubicin DOXORUBICIN RTU mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Doxorubicin DOXORUBICIN ACCORD mg 1 INJ ACCORD HEALTHCARE Doxorubicin Cipla-Doxorubicin mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Doxorubicin RUBEXET mg 1 INJ EUROLAB Epirubicin EPICORD mg 1 INJ EUROLAB Epirubicin Accord Epirubicin mg 1 INJ ACCORD HEALTHCARE Epirubicin ASPEN EPIRUBICIN mg 1 INJ ASPEN PHARMACARE Epirubicin EPICORD mg 1 INJ EUROLAB Epirubicin Accord Epirubicin mg 1 INJ ACCORD HEALTHCARE Epirubicin EPIRUBICIN-LEMERY mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Epirubicin ASPEN EPIRUBICIN mg 1 INJ ASPEN PHARMACARE Epirubicin ASPEN EPIRUBICIN mg 1 INJ ASPEN PHARMACARE Erlotinib TARCEVA mg 30 ORAL ROCHE PRODUCTS Erlotinib TARCEVA mg 30 ORAL ROCHE PRODUCTS Erlotinib TARCEVA mg 30 ORAL ROCHE PRODUCTS
4 Etoposide ETOPOSIDE-HEXAL mg 1 INJ SANDOZ Etoposide EPOSIN mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Etoposide ASPEN ETOPOSIDE mg 1 INJ ASPEN PHARMACARE Etoposide ACTITOP 50mg CAP ICON mg 20 ORAL Ando Pharma Etoposide ACTITOP 100mg CAP ICON mg 10 ORAL Ando Pharma Everolimus AFINITOR mg 30 ORAL NOVARTIS Everolimus AFINITOR mg 30 ORAL NOVARTIS Exemestane ASPEN EXEMESTANE mg 30 ORAL ASPEN PHARMACARE Exemestane EQUISIN mg 30 ORAL EQUITY PHARMACEUTICALS Fludarabine TEVA FLUDARABINE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Fludarabine FLUDARA ORAL mg 20 ORAL GENZYME BIOPHARMACEUTICALS Fluorouracil FLORACOR mg 1 INJ ACCORD HEALTHCARE Fluorouracil Fluracedyl mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Fluorouracil FLURACEDYL mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Fluorouracil Fluracedyl mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Flutamide SPEC-FLUTAMIDE mg 100 ORAL SPECPHARM (PTY) LTD Flutamide FLUTAHEXAL (105) mg 105 ORAL SANDOZ Fulvestrant FASLODEX mg 2 INJ ASTRAZENECA Gemcitabine CYTIGEM mg 1 INJ EUROLAB Gemcitabine MYLAN GEMCITABINE mg 1 INJ MYLAN Gemcitabine ACCORD GEMCITABINE mg 1 INJ ACCORD HEALTHCARE Gemcitabine ONCOGEM mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Gemcitabine CYTIGEM mg 1 INJ EUROLAB Gemcitabine MYLAN GEMCITABINE mg 1 INJ MYLAN Gemcitabine ACCORD GEMCITABINE mg 1 INJ ACCORD HEALTHCARE Gemcitabine ONCOGEM mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Goserelin ZOLADEX DEPOT mg 1 INJ ASTRAZENECA Goserelin ZOLADEX DEPOT mg 1 INJ ASTRAZENECA
5 Hydroxyurea HYDREA mg 100 ORAL BM SQUIBB Idarubicin ZAVEDOS mg 1 INJ PFIZER LABORATORIES Idarubicin ZAVEDOS mg 1 INJ PFIZER LABORATORIES Idarubicin ZAVEDOS mg 1 ORAL PFIZER LABORATORIES Ifosfamide HOLOXAN mg 1 INJ SANOFI AVENTIS Ifosfamide HOLOXAN mg 1 INJ SANOFI AVENTIS Ifosfamide HOLOXAN mg 1 INJ SANOFI AVENTIS Imatinib SUNMATIN mg 60 ORAL PHARMAPLAN Imatinib IMAVEC mg 60 ORAL CIPLA-MEDPRO PHARMACEUTICALS Imatinib VATIVIO mg 120 ORAL NOVARTIS Imatinib SUNMATIN mg 30 ORAL PHARMAPLAN Interferon alfa-2a ROFERON-A PREFILL MIU 1 INJ ROCHE PRODUCTS Interferon alfa-2a ROFERON-A PREFILL MIU 1 INJ ROCHE PRODUCTS Interferon alfa-2a ROFERON-A PREFILL MIU 1 INJ ROCHE PRODUCTS Interferon alfa-2a ROFERON-A PREFILL MIU 1 INJ ROCHE PRODUCTS Interferon alfa-2a INTRON A MIU 1 INJ MSD Interferon alfa-2b Intron A Redipen MIU 1 INJ MSD Interferon alfa-2b INTRON A HSA-FREE SOL MIU 1 INJ USE MSD SCHERING MERGED MSD Interferon alfa-2b Intron A HSA-Free Redipen MD MIU 1 INJ MSD Interferon alfa-2b Intron A Redipen MIU 1 INJ MSD Irinotecan IRINOTAS mg 1 INJ EUROLAB Irinotecan IRINOTECAN SAFELINE mg 1 INJ SAFELINE (PTY) LTD Irinotecan CIPLA-IRINOTECAN mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Irinotecan ACCORD IRINOTECAN mg 1 INJ ACCORD HEALTHCARE Irinotecan MYLAN IRINOTECAN mg 1 INJ MYLAN Irinotecan IRINOTAS mg 1 INJ EUROLAB Irinotecan IRINOTECAN SAFELINE mg 1 INJ SAFELINE (PTY) LTD Irinotecan CIPLA-IRINOTECAN mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS
6 Irinotecan ACCORD IRINOTECAN mg 1 INJ ACCORD HEALTHCARE Irinotecan MYLAN IRINOTECAN mg 1 INJ MYLAN Ketoconazole SANDOZ KETOCONAZOLE mg 30 ORAL SANDOZ Ketoconazole KETAZOL mg 30 ORAL ASPEN PHARMACARE Ketoconazole KETAZOL mg 10 ORAL ASPEN PHARMACARE Lanreotide SOMATULINE AUTOGEL mg 1 INJ SANOFI AVENTIS Lanreotide SOMATULINE AUTOGEL mg 1 INJ SANOFI AVENTIS Lanreotide SOMATULINE AUTOGEL mg 1 INJ SANOFI AVENTIS Lapatinib TYKERB mg 70 ORAL GLAXO SMITHKLINE Letrozole FEMZOLE mg 30 ORAL ADCOCK CONSUMER Letrozole ACCORD LETROZOLE mg 30 ORAL ACCORD HEALTHCARE Leuprorelin LUCRIN DEPOT S/DOSE mg 1 INJ ABBVIE PTY LTD Leuprorelin LUCRIN VIAL mg 1 INJ ABBVIE PTY LTD Leuprorelin with Atrigel ELIGARD mg 1 INJ KEY ONCOLOGICS Leuprorelin with Atrigel ELIGARD mg 1 INJ KEY ONCOLOGICS Leuprorelin with Atrigel ELIGARD mg 1 INJ KEY ONCOLOGICS Liposomal Doxorubicin DOXOLIP (10 ml) mg 1 INJ LITHA PHARMA Liposomal Doxorubicin DOXOPEG mg 1 INJ KEY ONCOLOGICS Lomustine CEENU (SECTION 21) mg 20 ORAL EQUITY PHARMACEUTICALS Lomustine CEENU (SECTION 21) mg 20 ORAL EQUITY PHARMACEUTICALS Medroxyprogesterone PROVERA mg 100 ORAL PFIZER LABORATORIES Medroxyprogesterone PROVERA mg 100 ORAL PFIZER LABORATORIES Melphalan ALKERAN mg 1 INJ ASPEN PHARMACARE Melphalan ALKERAN mg 25 ORAL ASPEN PHARMACARE Mercaptopurine PURINETHOL mg 25 ORAL ASPEN PHARMACARE Methotrexate METHACOR mg 1 INJ ACCORD HEALTHCARE Methotrexate ABITREXATE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Methotrexate ABITREXATE VIAL 20ML mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS
7 Methotrexate ABITREXATE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Methotrexate ABITREXATE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Methotrexate ABITREXATE mg 100 ORAL CIPLA-MEDPRO PHARMACEUTICALS Mitomycin MITOMYCIN-C mg 1 INJ ASPEN PHARMACARE Mitomycin MITOMYCIN-C mg 1 INJ ASPEN PHARMACARE Mitoxantrone MITOXANTRONE-HEXAL mg 1 INJ SANDOZ Nilotinib TASIGNA mg 112 ORAL NOVARTIS Nilotinib TASIGNA mg 112 ORAL NOVARTIS Octreotide SANDOSTATIN mg 5 INJ NOVARTIS Octreotide SANDOSTATIN mg 5 INJ NOVARTIS Octreotide SANDOSTATIN mg 1 INJ NOVARTIS Octreotide SANDOSTATIN LAR PREFILL mg 1 INJ NOVARTIS Octreotide SANDOSTATIN LAR PREFILL mg 1 INJ NOVARTIS Octreotide SANDOSTATIN LAR PREF mg 1 INJ NOVARTIS Oxaliplatin OXALIPLATIN PCH mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Oxaliplatin INTAS OXALIPLATIN mg 1 INJ EUROLAB Oxaliplatin ACCORD OXALIPLATIN mg 1 INJ ACCORD HEALTHCARE Oxaliplatin OXALIWIN RTU mg 1 INJ ZENTIVA Oxaliplatin OXALIPLATIN PCH mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Oxaliplatin INTAS OXALIPLATIN mg 1 INJ EUROLAB Oxaliplatin ACCORD OXALIPLATIN mg 1 INJ ACCORD HEALTHCARE Oxaliplatin OXALIWIN RTU mg 1 INJ ZENTIVA Paclitaxel SANDOZ PACLITAXEL mg 1 INJ SANDOZ Paclitaxel CIPLA-PACLITAXEL mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Paclitaxel PAXITAS mg 1 INJ EUROLAB Paclitaxel ACCORD PACLITAXEL mg 1 INJ ACCORD HEALTHCARE Paclitaxel SANDOZ PACLITAXEL mg 1 INJ SANDOZ Paclitaxel CIPLA-PACLITAXEL mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS
8 Paclitaxel PAXITAS mg 1 INJ EUROLAB Paclitaxel ACCORD PACLITAXEL mg 1 INJ ACCORD HEALTHCARE Paclitaxel SANDOZ PACLITAXEL mg 1 INJ SANDOZ Paclitaxel CIPLA-PACLITAXEL mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Paclitaxel PAXITAS mg 1 INJ EUROLAB Paclitaxel ACCORD PACLITAXEL mg 1 INJ ACCORD HEALTHCARE Pazopanib VOTRIENT mg 30 ORAL NOVARTIS Pazopanib VOTRIENT mg 30 ORAL NOVARTIS Pemetrexed ALIMTA mg 1 INJ ELI-LILLY SA (PTY) LTD Procarbazine Prozin 50mg CAP ICON mg 50 ORAL Ando Pharma Rituximab MABTHERA mg 2 INJ ROCHE PRODUCTS Rituximab MABTHERA mg 1 INJ ROCHE PRODUCTS Sorafenib NEXAVAR mg 60 ORAL BAYER PHARMA Streptozocin ZANOSAR (SECTION 21) mg 1 INJ EQUITY PHARMACEUTICALS Sunitinib SUTENT mg 28 ORAL PFIZER LABORATORIES Sunitinib SUTENT mg 28 ORAL PFIZER LABORATORIES Sunitinib SUTENT mg 28 ORAL PFIZER LABORATORIES Tamoxifen KESSAR mg 30 ORAL PFIZER LABORATORIES Tamoxifen TAMOXIHEXAL mg 30 ORAL SANDOZ Tamoxifen TAMOPLEX mg 30 ORAL CIPLA-MEDPRO PHARMACEUTICALS Tamoxifen NEOPHEDAN mg 30 ORAL ASPEN PHARMACARE Temozolomide ACCORD TEMOZOLOMIDE mg 5 ORAL ACCORD HEALTHCARE Temozolomide TEMINTAS mg 5 ORAL EUROLAB Temozolomide ACCORD TEMOZOLOMIDE mg 5 ORAL ACCORD HEALTHCARE Temozolomide TEMINTAS mg 5 ORAL EUROLAB Temozolomide ACCORD TEMOZOLOMIDE mg 5 ORAL ACCORD HEALTHCARE Temozolomide TEMINTAS mg 5 ORAL EUROLAB Temozolomide TEMODAL mg 5 ORAL MSD
9 Temozolomide TEMODAL mg 5 ORAL MSD Temozolomide TEMODAL mg 5 ORAL MSD Temozolomide ACCORD TEMOZOLOMIDE mg 5 ORAL ACCORD HEALTHCARE Temozolomide TEMINTAS mg 5 ORAL EUROLAB Teniposide Vumon ICON mg 1 INJ Bristol Myers Squibb Thalidomide THALIDOMIDE PHARMION mg 28 ORAL KEY ONCOLOGICS Tioguanine LANVIS mg 25 ORAL ASPEN PHARMACARE Topotecan HYCAMTIN mg 5 INJ GLAXO SMITHKLINE Topotecan HYCAMTIN mg 10 ORAL GLAXO SMITHKLINE Topotecan HYCAMTIN mg 10 ORAL GLAXO SMITHKLINE Trastuzumab HERCEPTIN MULTIDOSE mg 1 INJ ROCHE PRODUCTS Tretinoin VESANOID mg 100 ORAL PHARMACO DISTRIBUTION (PTY)LTD Vinblastine VINBLASTINE RTU mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Vincristine P&U VINCRISTINE CSV mg 5 INJ PFIZER LABORATORIES Vincristine Accord Vincristine mg 1 INJ ACCORD HEALTHCARE Vincristine P&U VINCRISTINE CSV mg 5 INJ PFIZER LABORATORIES Vinorelbine VINOREL mg 1 INJ PHARMAPLAN Vinorelbine SANDOZ VINORELBINE mg 1 INJ SANDOZ Vinorelbine CIPLA-VINORELBINE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Vinorelbine NAVELBINE mg 10 INJ TEMA MEDICAL Vinorelbine SANDOZ VINORELBINE mg 1 INJ SANDOZ Vinorelbine CIPLA-VINORELBINE mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS Vinorelbine NAVELBINE mg 10 INJ TEMA MEDICAL Vinorelbine VINOREL mg 1 INJ PHARMAPLAN Vinorelbine NAVELBINE ORAL mg 1 ORAL TEMA MEDICAL Vinorelbine NAVELBINE ORAL mg 1 ORAL TEMA MEDICAL Zoledronic Acid ZOMABON mg 1 INJ ACTOR PHARMA
10 Zoledronic Acid ZOLAPOR mg 1 INJ ACTIVO HEALTH T/A ACTIVO PHARMAC Zoledronic Acid ZOMEDRON mg 1 INJ CIPLA-MEDPRO PHARMACEUTICALS
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Formulary Chemotherapy Agents: (Current as of 6/2018) Therapeutic Class
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Anastrozole. Active Ingredient. Brand Name: Arimidex Strength: 1 mg Dosage Form: Tablets Pack Size: 28 Tabs Company: AstraZeneca
 Super Specialty Drugs by Anastrozole Arimidex 1 mg 28 Tabs AstraZeneca Armotraz 1 mg 10 Tabs Cipla Anastrozole Anabrez 1 mg 100 Tabs Sun Decitabine Dacogen 50 mg 1 Vial Eisai Inc. Erlotinib Erlocip-150
Super Specialty Drugs by Anastrozole Arimidex 1 mg 28 Tabs AstraZeneca Armotraz 1 mg 10 Tabs Cipla Anastrozole Anabrez 1 mg 100 Tabs Sun Decitabine Dacogen 50 mg 1 Vial Eisai Inc. Erlotinib Erlocip-150
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Introduction to Antineoplastic Prescribing
 Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
FREEDOM OF INFORMATION SOUTH EAST SCOTLAND CANCER NETWORK
 Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
1 17 ACITRETIN 10MG CAP 20, ,000 14,000 4, ACITRETIN 25MG CAP 50, ,000 35,000 10,000
 ردیف کد ژنریک نام ژنریک مبلغ پوشش وزارت بهداشت مبلغ پوشش بیمه مبلغ پرداخت بیمار درصد پرداخت بیمار قیمت اعالمی وزارت بهداشت نام تجاری 1 17 ACITRETIN 10MG CAP 20,000 10.0 2,000 14,000 4,000 2 18 ACITRETIN
ردیف کد ژنریک نام ژنریک مبلغ پوشش وزارت بهداشت مبلغ پوشش بیمه مبلغ پرداخت بیمار درصد پرداخت بیمار قیمت اعالمی وزارت بهداشت نام تجاری 1 17 ACITRETIN 10MG CAP 20,000 10.0 2,000 14,000 4,000 2 18 ACITRETIN
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Who is Bearing the Cost?
 THE COS ST T OF CANCE R DRUGS IN CANADA PA R T 2 Who is Bearing the Cost? KONG KHOO, ROSEMARY COLUCCI, WILLIAM HRYNIUK, ROBERT KAMINO, TANIA REDINA AND COLLEEN SAVAGE Introduction Last year we reported
THE COS ST T OF CANCE R DRUGS IN CANADA PA R T 2 Who is Bearing the Cost? KONG KHOO, ROSEMARY COLUCCI, WILLIAM HRYNIUK, ROBERT KAMINO, TANIA REDINA AND COLLEEN SAVAGE Introduction Last year we reported
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
InterChina Insight. Partnering up to fight cancer. Strategies for the China oncology market. InterChina Consulting. By Rick Woo April 03, 2013
 Strategies for the China oncology market By Rick Woo InterChina Consulting 英特华投资咨询有限公司 Beijing Shanghai Madrid Washington DC Strategy M&A Advisory IMAP China 2013 InterChina Consulting All Rights Reserved
Strategies for the China oncology market By Rick Woo InterChina Consulting 英特华投资咨询有限公司 Beijing Shanghai Madrid Washington DC Strategy M&A Advisory IMAP China 2013 InterChina Consulting All Rights Reserved
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Bladder Cancer (Urothelial) Pathways
 Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Overview of Cancer. Laura Bingell RN Transition Center Nurse for MFP (607)
 Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
8. Malignant disease and immunosuppression
 1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Bladder Cancer Pathways (Urothelial)
 Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
Supplementary Figures
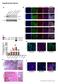 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
An introduction to the Essential Medicines concept: balancing innovation with public health priorities
 An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
Trust Guideline for Prevention and Control of Chemotherapy and Radiotherapy Induced Nausea and Vomiting in Adults
 A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
Hazard Meds: Navigating the Warning Beacons
 Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
DRUG PROPERTIES YOU NEED TO KNOW
 jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Appendix 2. Adjuvant Regimens. AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2
 Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
REGULATORY INFORMATION
 REGULATORY*INFORMATION/*CANCER*MEDICINES* Summary'of'regulatory'status'of'the'medicines' As#recommended#in#the#EML#Secretariat s#instructions#regarding#applications#for#the#addition#of#new# medicines#
REGULATORY*INFORMATION/*CANCER*MEDICINES* Summary'of'regulatory'status'of'the'medicines' As#recommended#in#the#EML#Secretariat s#instructions#regarding#applications#for#the#addition#of#new# medicines#
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
8. Malignant disease and immunosuppression
 1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
Job title: Consultant Pharmacist/Advanced Practice Pharmacist
 Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper)
 A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients
 Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Ovarian Cancer. compendia TREATMENT OF
 drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Cancer Drugs CANCER DRUGS NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS
 Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
North of England Cancer Drugs Approval Group (NECDAG) Cumulative Table of Decisions August 2012
 Notes: This document is updated every 2 to 3 months; please check our website www.cancernorth.nhs.uk for latest version. This document does not all contain Interim Cancer Drugs Fund s, i.e. decisions made
Notes: This document is updated every 2 to 3 months; please check our website www.cancernorth.nhs.uk for latest version. This document does not all contain Interim Cancer Drugs Fund s, i.e. decisions made
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
8. Malignant disease and immunosuppression
 1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
DRUGS YOU NEED TO KNOW
 jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
A themed review of patient safety incidents involving anti-cancer medicines 1 November June 2008
 A themed review of patient safety incidents involving anti-cancer medicines 1 November 2003 30 June 2008 October 2010 Full Report National Patient Safety Agency 2010. Copyright and other intellectual property
A themed review of patient safety incidents involving anti-cancer medicines 1 November 2003 30 June 2008 October 2010 Full Report National Patient Safety Agency 2010. Copyright and other intellectual property
An assessment of the price of oncology drugs in Ukraine 2015
 An assessment of the price of oncology drugs in Ukraine 2015 Prashant Yadav University of Michigan October 2016 The views and opinions expressed in this report are those of the author and should not be
An assessment of the price of oncology drugs in Ukraine 2015 Prashant Yadav University of Michigan October 2016 The views and opinions expressed in this report are those of the author and should not be
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Leukemia. Treatment of. compendia. Associated ICD-9-CM Codes: Drug & Administration. managedcareoncology.com
 Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Objectives. Principles of Cancer Treatment. Cancer Treatment Modalities. Cancer Treatment Modalities
 Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL NOTIFICATION OF REGISTRATION OF MEDICINES BY THE REGISTRAR IN TERMS OF SECTION 17 OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 36/7.5/0281 PHARMACARE LIMITED
MEDICINES CONTROL COUNCIL NOTIFICATION OF REGISTRATION OF MEDICINES BY THE REGISTRAR IN TERMS OF SECTION 17 OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 36/7.5/0281 PHARMACARE LIMITED
Guidelines for the Management of Extravasation (Version 5 May 2012)
 Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Regimens highlighted in red contain an expensive drug that is not currently publicly funded for the regimen and treatment intent.
 Palliative Breast Cancer Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for breast cancer used in the palliative setting. It is expected that the prescribing oncologist
Palliative Breast Cancer Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for breast cancer used in the palliative setting. It is expected that the prescribing oncologist
Fundamentals of Pharmacology for Veterinary Technicians Chapter 20
 Figure 20-1 Figure 20-2 Figure 20-3A Figure 20-3B Figure 20-4 Table 20-1 Antineoplastic Agents Used for Chemotherapy in Animals Drug Type Drug Category Examples Cell-Cycle Effect CCNS Alkylating agents:
Figure 20-1 Figure 20-2 Figure 20-3A Figure 20-3B Figure 20-4 Table 20-1 Antineoplastic Agents Used for Chemotherapy in Animals Drug Type Drug Category Examples Cell-Cycle Effect CCNS Alkylating agents:
CancerPACT Cancer Patients Alliance for Clinical Trials
 TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
MEDICINES CONTROL COUNCIL
 Notification of of Medicines MEDICINES CONTROL COUNCIL NOTIFICATION OF REGISTRATION OF MEDICINES BY THE REGISTRAR IN TERMS OF SECTION 17 OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965)
Notification of of Medicines MEDICINES CONTROL COUNCIL NOTIFICATION OF REGISTRATION OF MEDICINES BY THE REGISTRAR IN TERMS OF SECTION 17 OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965)
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Lisa M. Hess Diane Michael Daniel S. Mytelka Julie Beyrer Astra M. Liepa Steven Nicol
 Gastric Cancer (2016) 19:607 615 DOI 10.1007/s10120-015-0486-z ORIGINAL ARTICLE Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective
Gastric Cancer (2016) 19:607 615 DOI 10.1007/s10120-015-0486-z ORIGINAL ARTICLE Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective
CANCER PREVENTION AND ACCESS TO MEDICINES. Gracemarie Bricalli ESMO Head of International Affairs
 CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
