Abstract. Hematopathology / HEMATOGONE POPULATIONS
|
|
|
- Bertha Strickland
- 5 years ago
- Views:
Transcription
1 Hematopathology / HEMATOGONE POPULATIONS Benign Hematogone-Rich Lymphoid Proliferations Can Be Distinguished From B-Lineage Acute Lymphoblastic Leukemia by Integration of Morphology, Immunophenotype, Adhesion Molecule Expression, and Architectural Features Lisa M. Rimsza, MD, 1 Richard S. Larson, MD, PhD, 2 Stuart S. Winter, MD, 3 Kathy Foucar, MD, 2 Yap-Yee Chong, MD, 4 Kelly W. Garner, BS, 2 Catherine P. Leith, MBBChir 2 Key Words: Hematogones; Immunophenotype; Flow cytometry; Adhesion molecules; Clot sections, Immunohistochemistry Abstract Distinction of normal B-lymphoid proliferations including precursors known as hematogones from acute lymphoblastic leukemia (ALL) is critical for disease management. We present a multiparameter assessment of 27 bone marrow samples containing at least 25% hematogones (range, 25%-72%) by morphologic review. We used flow cytometry to evaluate B-cell differentiation antigen and adhesion molecule expression and immunohistochemistry on clot sections to evaluate architectural distribution. Flow cytometry revealed that intermediately differentiated cells (CD19+, CD10+) predominated, followed in frequency by CD20+, surface immunoglobulin positive cells, with CD34+, terminal deoxynucleotidyl transferase (TdT)- positive cells as the smallest subset. Adhesion molecules (CD44, CD54) were expressed more heterogeneously compared with expression in acute lymphoblastic leukemia. Immunohistochemistry revealed that CD34+, TdT-positive cells were dispersed without significant clustering, while CD20+ cells exceeded CD34/TdT-positive cells in 24 of 25 cases. This multidisciplinary study demonstrates that hematogone-rich lymphoid proliferations exhibit a spectrum of B-lymphoid differentiation antigen expression with predominance of intermediate and mature B-lineage cells, heterogeneity of adhesion molecule expression, and nonclustered bone marrow architectural distribution. B-lymphocyte progenitor cells, so-called hematogones, and mature B lymphocytes are normal bone marrow constituents, which are more prominent in pediatric bone marrow. Although hematogones show a range in overall cell size, these morphologically distinctive cells characteristically exhibit highly condensed, uniform nuclear chromatin and scant cytoplasm. By using immunophenotype, B- lineage cells can be divided into 3 stages of differentiation from very immature cells (CD34+, terminal deoxynucleotidyl transferase [TdT]-positive) to intermediately differentiated cells (CD10+, CD19+) to mature CD20+, surface immunoglobulin (SIg)-positive B cells. 1-6 Hematogones correspond to the immature and intermediately differentiated B-cell precursors identified immunophenotypically. In some circumstances, the number of hematogones in the bone marrow is increased greatly, especially in children recovering from chemotherapy, aplastic conditions, other forms of bone marrow injury, and nonhematopoietic disorders. 1-4,7 These cellular populations are especially problematic when identified in the bone marrow specimens of children after therapy for acute lymphoblastic leukemia (ALL), since hematogones can resemble malignant lymphoblasts by their morphologic features and by expression of an immature B-cell phenotype. 1,8-12 Accurate distinction of hematogone-rich lymphoid regeneration from leukemic lymphoblasts is clearly critical for patient care. We undertook the present study to assist the practicing pathologist in the decision-making process when hematogones are suspected by morphologic examination. We sought to identify useful architectural and immunophenotypic 66 Am J Clin Pathol 2000;114:66-75 American Society of Clinical Pathologists
2 Hematopathology / ORIGINAL ARTICLE features that most reliably differentiate hematogone-rich lymphoid populations from leukemic lymphoblasts. Previous studies on hematogones in the bone marrow typically have included only a single-parameter assessment; we integrated morphologic examination, multiparameter flow cytometry, and immunohistochemical parameters to determine the features most useful for distinguishing hematogones from lymphoblasts. We analyzed the flow cytometry data and calculated the proportion of immature and mature B cells present, as a reflection of population heterogeneity and maturation. Adhesion molecule expression also was studied to determine whether the single-cell distribution of hematogones in bone marrow clot sections as previously described could be linked with a particular antigenic pattern. 13 Finally, we extended our previous immunohistochemical work 13 on CD34 and TdT staining of hematogone populations in clot sections to include CD20, CD99, and CD3. Materials and Methods Case Selection Twenty-seven bone marrow samples with substantial numbers of hematogones from 21 children were identified retrospectively at the University of New Mexico Department of Pathology (Albuquerque). For 12 samples with florid hematogone proliferations, cryopreserved cell suspensions were available for flow cytometric studies. For 25 samples, adequate material was available for paraffin clot section immunohistochemistry. Seven diagnostic samples from children with B-precursor ALL were used as flow cytometry controls, including diagnostic samples from 3 patients who subsequently had increased hematogone populations that also were included in the study group. The clinical charts of all of the children were reviewed to determine their current disease status. Morphologic Review Wright-Giemsa stained bone marrow aspirate smears were reviewed by 1 or 2 of us (K.F., C.P.L.). The aspirate smear containing the greatest proportion of lymphoid cells was selected, and a bone marrow differential count, including the percentage of bone marrow hematogones, lymphocytes, blasts, and other cells, was performed. The morphologic hallmarks that were used to identify hematogones in this study were their uniform, highly condensed nuclear chromatin with inconspicuous nucleoli and their characteristically scant cytoplasm. Cases in which morphologically identified hematogones constituted 25% or more of bone marrow cells were included in the study. Flow Cytometric Immunophenotyping Five-parameter (3-color) flow cytometry was used for all antigens studied except TdT. Ficoll-separated cryopreserved cell suspensions were defrosted in a mixture of RPMI and 20% fetal calf serum and then washed to remove dimethyl sulfoxide. Following a second wash with phosphate-buffered saline, the cells were incubated with directly conjugated monoclonal antibodies at the manufacturers recommended concentrations for 30 minutes at room temperature, washed twice in phosphate-buffered saline with 0.2% sodium azide, and resuspended in Isoton II (Becton Dickinson, San Jose, CA). Antibodies for flow cytometry included CD3, CD20, CD22, CD34, CD38, CD44, CD54, CD11a, CD49d, and kappa and lambda light chains (Becton Dickinson); CD10, CD19, and CD79b (Pharmingen); and TdT (Supertech, Bethesda, MD). To allow B-cell gating in subsequent analysis, each tube included a pan-b-cell antibody (CD19, CD20, CD22, or CD79b), as well as other antibodies of interest. CD19 was the pan-b-cell antibody used for analysis of adhesion molecule expression, CD22 for CD10 and CD38 expression, CD20 for kappa and lambda expression, and CD79b for CD34 expression. For TdT detection, cells were stained with CD19, permeabilized using the Fix- N-Perm kit (Caltag, Burlingame, CA), and subsequently stained with TdT. Three-color data acquisition was performed using the Lysis II program on a FACScan instrument (Becton Dickinson). Analysis was performed using Paint-a-Gate software (Becton Dickinson). In each case, gating was performed on the low side-scatter cells that expressed the pan-b antigen included in that analysis tube. The proportion of bone marrow B cells expressing different maturation antigens was calculated as the percentage of total CD19+ B cells. Immunohistochemistry Immunohistochemistry was performed on B-5 fixed bone marrow clot sections. Antibodies for immunohistochemistry included CD34 (Qbend10, Immunotech, Westbrook, ME), TdT (Supertech), CD99 (Mic-2, DAKO, Carpinteria, CA), and CD3 (Becton Dickinson). Tissue sections underwent antigen retrieval by microwaving (Classic Collection II, Samsung, Fairfield, NJ) on high power (power setting 10) for 7 minutes followed by 12 minutes on low power (power setting 4) in citrate buffer, ph Slides were stained on an automated immunostainer (Ventana ES, Ventana Medical Systems, Tucson, AZ) using the manufacturer s paraffin protocol. Primary antibodies were followed by the Ventana proprietary mixture of secondary antibodies, streptavidin-horse radish peroxidase, and diaminobenzidine as the color substrate. Slides were counterstained with the manufacturer s hematoxylin and American Society of Clinical Pathologists Am J Clin Pathol 2000;114:
3 Rimsza et al / HEMATOGONE POPULATIONS coverslips applied using an automated instrument (Coveraid, Tissue-Tek, Torrance, CA). The immunohistochemical staining pattern of each case was assessed by 2 or 3 pathologists (L.M.R., C.P.L., and/or K.F.). The number of positively stained cells in 5 high-power fields ( 400) was counted, and the number of positive cells per field was calculated. For cases in which the count in 5 fields exceeded 500 cells, the number of positive cells per field was expressed as >100. In addition, the number of positively stained cells in the largest contiguous group (largest cluster) was noted in each case. Statistical Analysis The Fisher exact test was used to calculate differences in the frequency of expression of different antigens between hematogone-rich cases and ALL control cases. Results Clinical and Morphologic Features Twenty-seven archival bone marrow specimens containing 25% or more hematogones identified by morphologic review from 21 children were identified. The diagnoses for the 27 cases were as follows: ALL in remission, 21 samples from 15 children; pure RBC aplasia, 3 children; regenerative bone marrow after unknown probable viral injury, 1 child; hereditary spherocytosis, 1 child; and amegakaryocytic thrombocytopenia, 1 child. The children were from 6 months to 13.5 years old and included 12 boys and 9 girls Table 1. Clinical chart review revealed that none of the children with a benign diagnosis at the time of the bone marrow aspiration developed ALL. Of the 15 children with hematogone-rich bone marrow specimens that were detected after chemotherapy for ALL, 12 remain in remission, 2 experienced subsequent bone marrow relapse more than 12 months after the hematogone-rich bone marrow (patients 17 and 19), and 1 experienced isolated testicular relapse 8 months later and is now in a second remission (patient 10). By definition, all the bone marrow aspirates included in the study showed at least 25% hematogones (range, 25%- 72%; median, 37%). Mature lymphocytes were much less frequent (range, 0%-12%; median, 6%). The morphologic hallmarks that were used to identify hematogones were their uniform, highly condensed nuclear chromatin with inconspicuous nucleoli and their characteristically scant cytoplasm. Hematogone size was variable; large and small hematogones were identified. Image 1 illustrates the morphologic features of the hematogones and the similarity of large hematogones to some leukemic lymphoblasts. In addition to the percentage of hematogones and lymphocytes in each bone marrow specimen, the percentage of bone marrow blasts (myeloblasts and lymphoblasts) was assessed. The blast count in all cases was less than 5% in all cases (range, 0%-4%; median, 1%). The cellularity in the bone marrow specimens as judged by clot sections ranged from 20% to 95% (median, 70%). Flow Cytometry of B-Cell Populations Containing Hematogones There was a broad range in the total percentage of CD19+ B-lineage cells in the hematogone-rich bone marrow specimens, varying from 20% to more than 80% (median, 43%) of the total cells in the low side-scatter (nongranulated cell) gate. When these B-lineage cells were specifically gated and analyzed for expression of different maturation antigens, we found a range of differentiation among the hematogonerich cases from very immature TdT-positive B-lineage cells to CD20+ SIg-positive B cells. In each case, CD22 expression closely mirrored CD19 expression, indicating that both antigens were expressed on all the B-lineage cells examined. The largest subset of these B-lineage cells included cells that expressed CD10; 10 of 12 cases expressed CD10 in more than two thirds of the CD19+, CD22+ cells Table 2. CD20 also was expressed on a substantial fraction of the B-lineage cells identified; 26% to 95% (median, 56%) of B-lineage cells were CD20+. These CD20+ cells included a majority that expressed polytypic surface light chains, as well as a substantial fraction that were SIg-negative Figure 1. As expected, the intensity of staining with CD20 was lower among the SIg-negative than among the SIg-positive cells. Very immature B cells, expressing TdT and CD34, were the least frequent B-cell population found. TdT-positive, CD19+ cells accounted for only 0% to 18% (median, 9%) of B-lineage cells Figure 2, and in no cases did these very immature B-lineage cells outnumber the proportion of mature CD20+, SIg-positive cells. The percentage of CD34+ cells in the agranular gate, most of which were surface CD79b, closely mirrored that for TdT. In contrast with the spectrum of B-cell differentiation among the hematogone-rich bone marrow specimens, the 7 ALL control specimens analyzed showed a predominance of immature B-lineage cells, with a substantial percentage (39%-100%) of TdT-positive B cells identified (Figure 2). Unlike the hematogone cases, there was a paucity of mature SIg-positive B cells present in these ALL samples (Figure 1). Thus, these ALL cases lacked the maturational spectrum, including the presence of a substantial proportion of mature B cells, which was seen in all the hematogone cases. We found a heterogeneous pattern of adhesion molecule expression on the CD19+ B-lineage cells in the 68 Am J Clin Pathol 2000;114:66-75 American Society of Clinical Pathologists
4 Hematopathology / ORIGINAL ARTICLE Table 1 Clinical and Morphologic Features of 27 Bone Marrow Specimens With 25% or More Hematogones From 21 Pediatric Patients Bone Marrow Differential (%) Patient No./ Sex/Age (y) Bone Marrow Diagnosis Clinical Follow-Up Hematogones Lymphocytes Blasts 1/F/0.5 RBC aplasia Lost to follow-up /M/2 Postviral marrow regeneration Alive and well, 48 mo /F/2 RBC aplasia Lost to follow-up /M/0.5 Hereditary spherocytosis Alive and well, 118 mo /M/13 Amegakaryocytic Lost to follow-up thrombocytopenia 6/M/2 RBC aplasia Alive and well, 111 mo /M/7 ALL in CR CR, 112 mo /F/8 ALL in CR CR, 110 mo /F/6 ALL in CR CR, 73 mo /M/4.5 ALL in CR Relapse * at 8 mo a/F/12.5 ALL in CR CR, 135 mo b/F/13.5 ALL in CR CR, 124 mo a/F/9 ALL-ML in CR CR, 119 mo b/F/9.5 ALL-ML in CR CR, 113 mo a/M/9 ALL/Burkitt lymphoma in CR CR, 120 mo b/M/10 ALL/Burkitt lymphoma in CR CR, 117 mo /F/5 ALL in CR CR, 106 mo /M/4 ALL in CR CR, 122 mo a/M/8 ALL in CR CR, 110 mo b/M/8 ALL in CR CR, 105 mo a/M/6.5 ALL in CR Relapse at 27 mo b/M/7 ALL in CR Relapse, 21 mo /M/3 ALL in CR CR, 93 mo 29 19a/M/3 ALL in CR Relapse at 11 mo b/M/3 ALL in CR Relapse at 9 mo /F/5.5 ALL in CR CR, 121 mo /F/11 ALL in CR CR, 8 mo ALL, acute lymphoblastic leukemia; ALL-ML, ALL with mixed lineage phenotype; CR, complete remission. * Isolated testicular relapse 8 months after bone marrow with increased hematogones. Patient now in CR for 77 months after reinduction. Relapse 27 months after bone marrow with increased hematogones. Patient now in second CR at 87 months. Relapse 11 months after bone marrow with increased hematogones and died of disease 8 months later. Patient was in CR when she was lost to follow-up 8 months after bone marrow with increased hematogones. hematogone-rich bone marrow specimens. In all cases, most (>80%) of the CD19+ cells expressed CD11a and CD49d. In contrast, CD44 and CD54 expression was much more variable; CD44 was expressed on 7% to 78% of CD19+ B cells (median, 35%) and CD54 on 39% to 67% of CD19+ B cells (median, 51%). In addition, in each case, B-cell populations expressing all possible antigen combinations (CD44+ and CD54+, CD44 and CD54+, CD44+ and CD54, CD44 and CD54 ) could be identified. In particular, a CD44, CD54, CD19+ population constituting more than 20% of bone marrow B cells was identified in 11 of 12 cases (Table 2). In 10 of 12 cases, no single CD44-CD54 antigen combination exceeded 50% of the CD19+ B-lineage cells present Figure 3, highlighting the heterogeneity of expression of CD44 and CD54 among hematogone-rich cases. Bone Marrow Architectural Pattern Immunohistochemistry with CD34, TdT, CD20, and CD99/Mic-2 on bone marrow clot sections was used to study the proportion of mature and immature B cells within the bone marrow and their tissue distribution. We noted a wide range in the number of cells per high-power field stained with each antibody Table 3. However, despite the wide range of positive cells found in different cases, in almost all cases, the proportion of mature CD20+ B cells exceeded the number of immature (CD34+ or TdT-positive) cells. In 23 of 25 cases, the number of CD20+ cells exceeded the number of CD34+ cells, with a median of 4.4 times more CD20+ than CD34+ cells in each case. Similarly, the number of CD20+ cells per high-power field exceeded the number of TdT-positive cells in 16 of 18 cases (median, 4.6 times more CD20+ than TdT-positive cells per high-power field). Image 2 illustrates a typical case that shows a relatively higher number of CD20+ than CD34+ or TdT-positive cells in a similar field. These findings concurred with the flow cytometry data for the same antigens; the proportion of CD20+ more mature B-lineage cells in each case exceeded that of the TdT-positive, CD34+, more immature cells (Table 2). Examination of interobserver variability showed that all pathologists found similar relative proportions of CD20-, TdT-, and CD34- staining cells in each case, even if the total number of cells American Society of Clinical Pathologists Am J Clin Pathol 2000;114:
5 Rimsza et al / HEMATOGONE POPULATIONS A B C counted was slightly different. This variability likely reflected interobserver differences, including the choice of bone marrow particles assessed by each pathologist. Immunohistochemistry demonstrated that in hematogone-rich B-cell populations, the most immature cells consistently were distributed diffusely within the bone marrow without clusters of more than 5 contiguous CD34+ or TdT-positive cells (Table 3). Larger clusters of up to 8 contiguous CD20+ cells were identified in a few (3/26) of the study cases. Interobserver agreement on cluster size was good; all 3 pathologists independently agreed that there were no large clusters (>5 cells) of immature CD34+ or TdT-positive cells in any of the hematogone-rich cases. CD3+ T cells were present only in small clusters of 3 or fewer in study cases and ALL control cases. CD99 (Mic-2), which is commonly used in the diagnosis of ALL and primitive neuroectodermal tumors, stained myeloid precursors and Image 1 Morphologic features of bone marrow aspirate smears: hematogones vs acute lymphoblastic leukemia (ALL) blasts. A, Bone marrow aspirate smear from a child with B-cell proliferation containing increased hematogones. These cells exhibit the typical morphologic features of hematogones, including very high nuclear/cytoplasmic (N/C) ratios, smudged and condensed chromatin, and no nucleoli. The size of the hematogone varies from cells as small as lymphocytes to much larger cells. All the hematogones have similar condensed chromatin (Wright, 400). B, Bone marrow aspirate smear from a child with ALL. The malignant lymphoblasts show high N/C ratios, fine stippled chromatin, uniform size, and small nucleoli. The cells are clearly malignant (Wright, 400). C, Bone marrow aspirate smear from a child with ALL. The malignant lymphoblasts show high N/C ratios, more condensed chromatin than that in Image 1B, fairly uniform size, and no nucleoli. Distinction of hematogones from ALL blasts with this type of appearance can be difficult when using morphologic features alone (Wright, 1,000). endothelium, thus making this marker unsuitable for identification of immature lymphoid cells in clot sections. Cell counts for this antigen, therefore, are not included in the data summary. Comparison of B-Lineage Proliferations Containing Hematogones and ALL Our analysis of hematogone-rich bone marrow specimens showed a complete maturational spectrum of B cells, from very immature cells to mature, SIg-positive B cells. In contrast, when we examined 7 control specimens from cases of ALL, we found a predominance of immature B-lineage cells, with a substantial percentage (39%-100%) of TdT-positive B cells (Table 2). Unlike the hematogone cases, there was a paucity of mature SIg-positive B cells in these ALL samples. Thus, the ALL cases 70 Am J Clin Pathol 2000;114:66-75 American Society of Clinical Pathologists
6 Hematopathology / ORIGINAL ARTICLE Table 2 Relative Proportions of B-Lineage Cells at Different Maturational Stages in 12 Bone Marrow Samples With 25% or More Hematogones Compared With 7 ALL Control Specimens From Time of Diagnosis * Patient SIg-Positive and SIg-Negative and TdT-Positive and CD44 /CD54 and No. Total CD20+ CD20+ CD20+ CD10+ and CD22+ CD19+ CD a a b a b ALL controls ND ALL, acute lymphoblastic leukemia; SIg, surface immunoglobulin; TdT, terminal deoxynucleotidyl transferase. * The proportions of B-lineage cells are expressed as the percentage of total CD19+ cells identified. Among the cases with 25% or more hematogones, there was a spectrum of CD10 staining from weak to strong antigen expression. A B A B Lambda Lambda CD19 CD19 Kappa Kappa TdT TdT Figure 1 Dot plots of multiparameter flow cytometry results comparing surface immunoglobulin (SIg) light chain expression on benign and malignant cases. Three-color flow cytometry (kappa, lambda, CD20) was performed. A, In the case with hematogones, gating on CD20+ B cells was performed. This gate includes a large proportion of kappa-positive cells (blue) and lambda-positive cells (pink), as well as CD20+, SIg-negative cells (red). B, For the case of acute lymphoblastic leukemia, blasts were identified by light scattering characteristics and constituted >90% of the population. These blasts failed to stain with CD20, kappa, or lambda (purple). Figure 2 Dot plots of 2-color flow cytometry results comparing CD19 and terminal deoxynucleotidyl transferase (TdT) expression. A, Hematogone-rich bone marrow demonstrated distinct CD19+/TdT-negative and CD19+ /TdT-positive subsets. TdT-negative cells always outnumbered TdT-positive cells by a ratio of at least 4:1. B, In contrast, the acute lymphoblastic leukemia case demonstrated a single population of cells with uniform expression of both antigens. American Society of Clinical Pathologists Am J Clin Pathol 2000;114:
7 Rimsza et al / HEMATOGONE POPULATIONS lacked the maturational spectrum, including the presence of a substantial proportion of mature B cells, that was seen in all cases with hematogones. We analyzed the proportion of B-lineage (CD19+) cells at different stages of maturation among the hematogone-rich cases and the ALL cases to determine whether quantitative measures could be used to assess the differences in the maturation spectrum observed, which would help distinguish hematogone-rich and ALL specimens. When we analyzed the proportion of the more mature B-lineage cells, we found that in 10 of 12 hematogone-rich cases, more than 20% of the B-lineage cells were SIg-positive, in contrast with none of the ALL cases (P <.001). In addition, in all hematogonerich cases, more than 25% of marrow B cells expressed A CD54 CD44 CD44 Figure 3 Dot plot of 3-color flow cytometry results comparing CD44 and CD54 expression on CD19+ B cells. Cells were costained with CD19, and CD19 gating was performed. A, The hematogone-rich bone marrow shows distinct populations of CD19+, CD44, CD54 cells (red), CD19+, CD44, CD54+ cells (green), and CD19+, CD44+, CD54+ cells (black). This sort of heterogeneity of expression was typical of the benign cases. B, In contrast, the acute lymphoblastic leukemia case shows a single uniform pattern of CD19+, weak CD44+, and CD54+ (green). B CD54 CD20 (with or without SIg), in contrast with only 1 of 7 cases of ALL (P <.001) Table 4 (Figure 1). While more mature B cells were present in hematogone-rich than in ALL cases, the converse was true when we examined markers of B-cell immaturity. In the hematogone-rich cases, very immature TdT-positive B-lineage cells made up less than 20% of B-lineage cells, in contrast with the ALL cases in which TdT-positive cells generally predominated (P <.001) (Tables 2 and 4, Figure 2). By using the combination of these 3 parameters (percentages of CD20+ B cells, SIg-positive B cells, and TdT-positive B cells), we were able to discriminate between ALL blasts and hematogone-rich specimens in all cases tested. When we compared the pattern of expression of adhesion molecules in hematogone-rich bone marrow specimens and specimens from ALL cases, we found a greater degree of heterogeneity in the expression of CD44 and CD54 among the hematogone-rich cases than in the ALL control cases. Among the hematogone-rich cases, subsets of B-lineage cells that expressed CD44, CD54, or both were identified, as were subsets of cells that lacked 1 or both antigens. In 10 of 12 cases, no single CD44-CD54 subset constituted more than 50% of the B-lineage population, while in 11 of 12 cases, more than 20% of B cells lacked expression of either CD44 or CD54. In contrast, the pattern of expression of CD44 and CD54 was more homogeneous among the ALL cases. The majority of ALL cases expressed a single predominant phenotype; for example 4 of 6 cases showed a predominant CD44, CD54+ pattern, while 1 was CD44+, CD54+, and only 1 showed a more heterogeneous pattern of antigen expression with no single subset predominating. Interestingly, the significant population (>20% of CD19+ cells) of CD44, CD54 cells found among the hematogone-rich cases was found in only 1 of 6 ALL cases (P <.01) (Tables 2 and 4, Figure 3). When we compared immunohistochemical staining patterns of hematogone-rich bone marrow specimens with ALL cases, findings mirrored our findings by flow cytometry. Among the hematogone-rich cases, there was a higher Table 3 Results of Clot Section Immunohistochemistry of Hematogone-Rich B-Cell Proliferations No. of Positive Cells per High-Power Field Antibody Range Median Architectural Distribution: Largest Cluster * CD TdT CD20 5 to > CD3 0 to > TdT, terminal deoxynucleotidyl transferase. * The number of positively stained cells in the largest contiguous group. 72 Am J Clin Pathol 2000;114:66-75 American Society of Clinical Pathologists
8 Hematopathology / ORIGINAL ARTICLE A B C Image 2 Bone marrow clot section from a child with increased numbers of B cells, including florid hematogone proliferation. A, Numerous CD20+ cells are scattered singly (elsewhere in groups) (CD20, 400). B, Scattered CD34+ cells (never arranged in groups of >5) are present but less frequent than the CD20+ cells seen in Image 2A (CD34, 400). C, Scattered terminal deoxynucleotidyl transferase (TdT)-positive cells showing nuclear staining are present. The positively stained cells are never arranged in groups of >5 contiguous cells. Again, the cells are less frequent than CD20+ cells seen in Image 2A (TdT, 400). Table 4 Comparison of the Phenotypes of Hematogone-Rich Cases With ALL by Flow Cytometry and Immunohistochemistry * Analysis Hematogone-Rich Cases ALL Control Cases P Flow cytometry CD20+ cells >25% of total B-lineage cells 12/12 1/7 <.001 SIg-positive B cells >20% of total B-lineage cells 10/12 0/7 <.001 TdT-positive cells <20% of total B-lineage cells 12/12 0/7 <.001 CD44 /CD54 B cells >20% of total B-lineage cells 11/12 1/6 <.01 Immunohistochemistry No. of CD20+ more than no. of CD34+ per high-power field 23/25 1/7 <.001 ALL, acute lymphoblastic leukemia; SIg, surface immunoglobulin; TdT, terminal deoxynucleotidyl transferase. * Data are given as the percentage of cells expressing the antigen compared with the total CD19+ cells identified. The spectrum of B-cell differentiation in hematogone-rich cases compared with ALL control cases was compared by examining the relative proportion of CD20+, SIg-positive, and TdT-positive B-lineage cells measured by flow cytometry and immunohistochemistry. The CD44 /CD54 phenotype also was compared between the 2 groups. proportion of more mature B-lineage cells, demonstrated by a larger proportion of CD20+ cells compared with TdTpositive, CD34+ cells in almost all marrow specimens (Table 4), while among the ALL control cases, the proportion of immature CD34+ cells was higher compared with CD20+ cells. We found TdT expression by immunohistochemistry in only 3 of 6 ALL cases, possibly owing to problems of antigen preservation, thus making this immature marker less reliable than CD34 in our hands for distinguishing hematogone cases from ALL cases. American Society of Clinical Pathologists Am J Clin Pathol 2000;114:
9 Rimsza et al / HEMATOGONE POPULATIONS When we examined the architectural distribution of immature cells in the hematogone-rich bone marrow specimens, we found the most immature cells consistently were dispersed diffusely within the bone marrow without clusters of more than 5 cells of CD34+ or TdT-positive cells in any of the 25 study cases examined. This is in contrast to findings of previous work on minimal residual disease or early relapse ALL in which clusters of CD34+, TdT-positive cells were found in 6 of 9 cases. 13 Discussion Cells with hematogone morphologic features are seen as part of the benign expansion of normal B-lymphoid progenitor cells present in a variety of aplastic or reactive bone marrow conditions. While a morphologic and immunophenotypic overlap exists between hematogones and leukemic lymphoblasts, we demonstrate that morphologic review combined with evaluation of immunophenotypic characteristics and architectural distribution in the bone marrow can help distinguish hematogone from residual leukemic blasts. Our immunophenotypic studies of the B cells in hematogone-rich bone marrow specimens found a spectrum of B-cell maturation. In each case, there was a relatively small proportion of very immature, TdT-positive, CD34+ B cells, a predominant fraction of CD10+, TdT-negative, SIgnegative cells, some of which expressed CD20, and another substantial fraction of mature SIg-positive B cells. These findings are similar to previous studies evaluating the phenotype of hematogones by flow cytometry. 1-9 In contrast, the ALL samples consistently expressed a more immature, but homogeneous, immunophenotype, with the majority of cases expressing TdT, CD34, or both. These differences in antigen expression between hematogone and ALL blasts have been well described and can be exploited in minimal residual disease studies to detect small numbers of abnormal cells. 8-12,15,16 In the present study, we attempted to develop a reproducible way to quantify the differentiation spectrum of hematogones compared with leukemic blasts. We found that in almost all hematogone-rich cases, more than 25% of the marrow B cells were CD20+, including 20% of marrow B cells that expressed SIg. This was in contrast with ALL cases, which rarely expressed CD20 or SIg on a substantial proportion of B cells. In contrast, the hematogone-rich cases showed a low proportion of TdT-positive, CD34+ B cells (<20% in all cases) compared with the much higher frequency of expression of these antigens in ALL cases. Thus, these simple calculations to derive a maturation index of B-lineage cells in the marrow may help differentiate leukemic blasts from florid hematogone proliferations. Further studies on larger numbers of patients will help determine how consistently useful this method is for the analysis of hematogone-rich bone marrow specimens. Rimsza et al 13 identified clustering of small numbers of leukemic blasts by immunohistochemistry in the bone marrow specimens of patients with ALL in morphologic remission who subsequently experienced relapse. In contrast, our preliminary studies failed to show clustering of hematogone within the bone marrow. Since clustering of ALL blasts is likely due to cell-cell interactions, and since adhesion defects have been described in leukemic cells, 17 we studied our cases with a panel of antibodies that recognized cell adhesion molecules known to be expressed on leukemic cells We found a marked heterogeneity of expression of the adhesion molecules, CD44 (the fibronectin hyaluronic acid receptor) and CD54 (the intercellular adhesion molecule, ICAM-1), among hematogone-rich cases compared with ALL control cases. Of particular interest, we found a significant (P <.01) population of CD44, CD54 B cells, constituting more than 20% of the B-cell population, in all hematogone cases but in only 1 ALL control case. Thus, the presence of this subpopulation seems to be another useful discriminator between hematogones and leukemic blasts. The heterogeneity of adhesion antigen expression on hematogones may explain in part their lack of bone marrow clustering compared with ALL cases. All of the immunohistochemical staining in the present study was performed on bone marrow clot sections, demonstrating the usefulness of this material, which retains bone marrow tissue architecture while avoiding the performance of a core biopsy. In previous work, 6 of 9 cases of early ALL relapse exhibited clusters of more than 5 CD34+ or TdT-positive cells. 13 This is in contrast with the diffusely distributed single cells or small groups of 2 to 3 CD34+ or TdT-positive cells seen in the hematogone-rich cases. 13 The finding of dispersed CD34+, TdT-positive cells without clustering among hematogone-rich cases was confirmed in the present larger study. Others 22 also have studied the pattern of CD34 staining in normal and regenerating bone marrow core biopsy specimens and found that CD34+ cells (of unspecified lineage) were isolated and randomly distributed but never arranged in aggregates; these findings agree with our data. We conclude that the presence or absence of clustering of the immature cells is a useful architectural criterion to distinguish hematogones from leukemic lymphoblasts. In addition, immunohistochemistry on paraffin clot sections revealed an excess of CD20+ cells over CD34+ or TdT-positive cells in the majority of cases, a finding that correlated with the flow cytometric data. Unlike TdT or CD34 assessment, these CD20+ B cells exhibited clustering in hematogone-rich cases. Thus, immunohistochemistry on routinely fixed bone marrow specimens seems 74 Am J Clin Pathol 2000;114:66-75 American Society of Clinical Pathologists
10 Hematopathology / ORIGINAL ARTICLE to provide a useful reproducible way to distinguish hematogones from leukemic blasts. We describe the integration of morphologic features, immunophenotype, and architectural features in the assessment of hematogone-rich B-lineage proliferations and the features that can help differentiate reliably between hematogones and malignant lymphoblasts. The role of adhesion molecules in the architectural clustering or nonclustering of cells (in cases of ALL and hematogone, respectively) is intriguing and may warrant further study. From the Departments of 1 Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, FL; 2 Pathology, University of New Mexico, Albuquerque; 3 Pediatrics, University of New Mexico, Albuquerque; and 4 Pathology, Case Western Reserve University, Cleveland, OH. Funding provided by a Children Miracle Network Telethon Grant (Dr Foucar), University of New Mexico. Address reprint requests to Dr Rimsza: P.O. Box , Dept of Pathology, Immunology and Laboratory Medicine, JHMHSC, College of Medicine, 1600 SW Archer Rd, Gainesville, FL References 1. Longacre TA, Foucar K, Crago S, et al. Hematogones: a multiparameter analysis of bone marrow precursor cells. Blood. 1989;73: Caldwell CW, Poje E, Helikson MA. B-cell precursors in normal pediatric bone marrow. Am J Clin Pathol. 1991;95: Leitenberg D, Rappeport JM, Smith BR. B-cell precursor bone marrow reconstitution after bone marrow transplantation. Am J Clin Pathol. 1994;102: van den Doel L, Pieters R, Huismans D, et al. Immunological phenotype of lymphoid cells in regenerating bone marrow of children after treatment of acute lymphoblastic leukemia. Eur J Haematol. 1988;41: Dworzak MN, Fritsch G, Fleischer C, et al. Multiparameter phenotype mapping of normal and post-chemotherapy B lymphopoiesis in pediatric bone marrow. Leukemia. 1997;11: Washington L, Ansari M, Picker L, et al. Immunophenotypic analysis of B-cell precursors (BCP) in 661 bone marrow (BM) specimens by 4 color flow cytometry (FC) [abstract]. Mod Pathol. 1999;12:148A. 7. Rego EM, Garcia AB, Viana SR, et al. Age-related changes of lymphocyte subsets in normal bone marrow biopsies. Cytometry. 1998;34: Wells DA, Sale GE, Shulman HM, et al. Multidimensional flow cytometry of marrow can differentiate leukemic from normal lymphoblasts and myeloblasts after chemotherapy and bone marrow transplantation. Am J Clin Pathol. 1998;110: Lucio P, Parreira A, van den Beemd M, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-b-all. Leukemia. 1999;13: Ryan DH, Chapple CW, Kossover SA, et al. Phenotypic similarities and differences between CALLA-positive acute lymphoblastic leukemia cells and normal marrow CALLApositive B cell precursors. Blood. 1987;70: Weir EG, Cowan K, LeBeau P, et al. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: implications for residual disease detection. Leukemia. 1999;12: Dworzak MN, Fritsch G, Fleischer C, et al. Comparative phenotype mapping of normal vs malignant pediatric B- lymphopoiesis unveils leukemia-associated aberrations. Exp Hematol. 1998;26: Rimsza LM, Viswanatha DS, Winter SS, et al. The presence of CD34+ cell clusters predicts impending relapse in children with acute lymphoblastic leukemia receiving maintenance chemotherapy. Am J Clin Pathol. 1998; 110: Gown A, Wever N, Battifora H. Micro-wave based antigenic unmasking: a revolutionary technique for routine immunohistochemistry. Appl Immunohistochemistry. 1993;1: Ciudad J, San Miguel J, Lopez-Berges M, et al. Detection of abnormalities in B-cell differentiation pattern is a useful tool to predict relapse in precursor-b-all. Br J Haematol. 1999;104: Farahat N, Lens D, Zomas A, et al. Quantitative flow cytometry can distinguish between normal and leukaemic B- cell precursors. Br J Haematol. 1995;91: Geijtenbeek TBH, van Kooyk Y, van Vliet SJ, et al. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood. 1999;94: Horst E, Meijer C, Radaskiewicz T, et al. Expression of a human homing receptor (CD44) in lymphoid malignancies and related stages of lymphoid development. Leukemia. 1990;4: Reuss-Borst M, Buhring H, Klein G, et al. Adhesion molecules on CD34+ hematopoietic cells in normal human bone marrow and leukemia. Ann Hematol. 1992;65: Dennig D, Lacerda J, Yan Y, et al. ICAM-1 (CD54) expression on B lymphocytes is associated with their costimulatory function and can be increased by coactivation with IL-1 and IL-7. Cell Immunol. 1994;156: Krauter J, Ganser A, Heil G. Effect of stimulation by interferon gamma and tumor necrosis factor alpha on CD54 expression and homotypic aggregation of blasts of acute lymphoblastic leukemias. Ann Hematol. 1998;76: Soligo D, Delia D, Oriani A, et al. Identification of CD34+ cells in normal and pathological bone marrow biopsies by QBEND10 monoclonal antibody. Leukemia. 1991;5: American Society of Clinical Pathologists Am J Clin Pathol 2000;114:
Case 3. Ann T. Moriarty,MD
 Case 3 Ann T. Moriarty,MD Case 3 59 year old male with asymptomatic cervical lymphadenopathy. These images are from a fine needle biopsy of a left cervical lymph node. Image 1 Papanicolaou Stained smear,100x.
Case 3 Ann T. Moriarty,MD Case 3 59 year old male with asymptomatic cervical lymphadenopathy. These images are from a fine needle biopsy of a left cervical lymph node. Image 1 Papanicolaou Stained smear,100x.
CD34/QBEND10 Immunostaining in the Bone Marrow Trephine Biopsy. A Study of CD34-Positive Mononuclear Cells and Megakaryocytes
 CD34/QBEND10 Immunostaining in the Bone Marrow Trephine Biopsy A Study of CD34-Positive Mononuclear Cells and Megakaryocytes Goran Torlakovic, MD; Ruth Langholm, MD; Emina Torlakovic, MD Context. The immunohistochemical
CD34/QBEND10 Immunostaining in the Bone Marrow Trephine Biopsy A Study of CD34-Positive Mononuclear Cells and Megakaryocytes Goran Torlakovic, MD; Ruth Langholm, MD; Emina Torlakovic, MD Context. The immunohistochemical
Significant CD5 Expression on Normal Stage 3 Hematogones and Mature B Lymphocytes in Bone Marrow
 Hematopathology / CD5 Expression on Normal B Cells Significant CD5 Expression on Normal Stage 3 Hematogones and Mature B Lymphocytes in Bone Marrow Franklin S. Fuda, DO, Nitin J. Karandikar, MD, PhD, and
Hematopathology / CD5 Expression on Normal B Cells Significant CD5 Expression on Normal Stage 3 Hematogones and Mature B Lymphocytes in Bone Marrow Franklin S. Fuda, DO, Nitin J. Karandikar, MD, PhD, and
Multiparameter flow cytometry can be used to
 Minimal residual disease testing in Acute Leukemia Anjum Hassan MD Assistant Professor of Pathology and Immunology, Director FISH laboratory in Anatomic Pathology, Washington University in St Louis, School
Minimal residual disease testing in Acute Leukemia Anjum Hassan MD Assistant Professor of Pathology and Immunology, Director FISH laboratory in Anatomic Pathology, Washington University in St Louis, School
Mihaela Onciu, MD, Robert B. Lorsbach, MD, PhD, E. Charlene Henry, HTL(ASCP)QIHC, and Frederick G. Behm, MD. Abstract
 Hematopathology / TDT-POSITIVE CELLS IN LYMPH NODES Terminal Deoxynucleotidyl Transferase Positive Lymphoid Cells in Reactive Lymph Nodes From Children With Malignant Tumors Incidence, Distribution Pattern,
Hematopathology / TDT-POSITIVE CELLS IN LYMPH NODES Terminal Deoxynucleotidyl Transferase Positive Lymphoid Cells in Reactive Lymph Nodes From Children With Malignant Tumors Incidence, Distribution Pattern,
WBCs Disorders 1. Dr. Nabila Hamdi MD, PhD
 WBCs Disorders 1 Dr. Nabila Hamdi MD, PhD ILOs Compare and contrast ALL, AML, CLL, CML in terms of age distribution, cytogenetics, morphology, immunophenotyping, laboratory diagnosis clinical features
WBCs Disorders 1 Dr. Nabila Hamdi MD, PhD ILOs Compare and contrast ALL, AML, CLL, CML in terms of age distribution, cytogenetics, morphology, immunophenotyping, laboratory diagnosis clinical features
The spectrum of flow cytometry of the bone marrow
 The spectrum of flow cytometry of the bone marrow Anna Porwit Lund University Faculty of Medicine Dept. of Clinical Sciences Div. Oncology and Pathology anna.porwit@med.lu.se Disclosure of speaker s interests
The spectrum of flow cytometry of the bone marrow Anna Porwit Lund University Faculty of Medicine Dept. of Clinical Sciences Div. Oncology and Pathology anna.porwit@med.lu.se Disclosure of speaker s interests
FLOW CYTOMETRY PRINCIPLES AND PRACTICE. Toby Eyre Consultant Haematologist Oxford University Hospitals NHS Foundation Trust June 2018
 FLOW CYTOMETRY PRINCIPLES AND PRACTICE Toby Eyre Consultant Haematologist Oxford University Hospitals NHS Foundation Trust June 2018 Aims and Objectives Principles of flow cytometry Preparation Steps involved
FLOW CYTOMETRY PRINCIPLES AND PRACTICE Toby Eyre Consultant Haematologist Oxford University Hospitals NHS Foundation Trust June 2018 Aims and Objectives Principles of flow cytometry Preparation Steps involved
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells. Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital
 Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
VUmc Basispresentatie
 Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
ADx Bone Marrow Report. Patient Information Referring Physician Specimen Information
 ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
NUMERATOR: Patients who had baseline cytogenetic testing performed on bone marrow
 Quality ID #67 (NQF 0377): Hematology: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow National Quality Strategy Domain: Effective Clinical Care
Quality ID #67 (NQF 0377): Hematology: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow National Quality Strategy Domain: Effective Clinical Care
Leukemic Phase of Mantle Cell Lymphoma, Blastoid Variant
 Hematopathology / LEUKEMIC BLASTOID MANTLE CELL LYMPHOMA Leukemic Phase of Mantle Cell Lymphoma, Blastoid Variant Timothy P. Singleton, MD, Margaret M. Anderson, MD, Charles W. Ross, MD, and Bertram Schnitzer,
Hematopathology / LEUKEMIC BLASTOID MANTLE CELL LYMPHOMA Leukemic Phase of Mantle Cell Lymphoma, Blastoid Variant Timothy P. Singleton, MD, Margaret M. Anderson, MD, Charles W. Ross, MD, and Bertram Schnitzer,
Presentation material is for education purposes only. All rights reserved URMC Radiology Page 1 of 98
 Presentation material is for education purposes only. All rights reserved. 2011 URMC Radiology Page 1 of 98 Radiology / Pathology Conference February 2011 Brooke Koltz, Cytopathology Resident Presentation
Presentation material is for education purposes only. All rights reserved. 2011 URMC Radiology Page 1 of 98 Radiology / Pathology Conference February 2011 Brooke Koltz, Cytopathology Resident Presentation
Successful flow cytometric immunophenotyping of body fluid specimens
 Successful flow cytometric immunophenotyping of body fluid specimens Fiona E. Craig, MD Division of Hematopathology Mayo Clinic Arizona 2017 MFMER slide-1 Financial disclosure No conflicts 2017 MFMER slide-2
Successful flow cytometric immunophenotyping of body fluid specimens Fiona E. Craig, MD Division of Hematopathology Mayo Clinic Arizona 2017 MFMER slide-1 Financial disclosure No conflicts 2017 MFMER slide-2
Immunopathology of Lymphoma
 Immunopathology of Lymphoma Noraidah Masir MBBCh, M.Med (Pathology), D.Phil. Department of Pathology Faculty of Medicine Universiti Kebangsaan Malaysia Lymphoma classification has been challenging to pathologists.
Immunopathology of Lymphoma Noraidah Masir MBBCh, M.Med (Pathology), D.Phil. Department of Pathology Faculty of Medicine Universiti Kebangsaan Malaysia Lymphoma classification has been challenging to pathologists.
Hematology Measure #1: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow
 Hematology Measure #1: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow This measure may be used as an Accountability measure Clinical Performance
Hematology Measure #1: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow This measure may be used as an Accountability measure Clinical Performance
Lack of Surface Immunoglobulin Light Chain Expression by Flow Cytometric Immunophenotyping Can Help Diagnose Peripheral B-Cell Lymphoma
 Hematopathology / SURFACE IMMUNOGLOBULIN LIGHT CHAIN NEGATIVE PERIPHERAL B-CELL LYMPHOMA Lack of Surface Immunoglobulin Light Chain Expression by Flow Cytometric Immunophenotyping Can Help Diagnose Peripheral
Hematopathology / SURFACE IMMUNOGLOBULIN LIGHT CHAIN NEGATIVE PERIPHERAL B-CELL LYMPHOMA Lack of Surface Immunoglobulin Light Chain Expression by Flow Cytometric Immunophenotyping Can Help Diagnose Peripheral
EDUCATIONAL COMMENTARY DIFFERENTIATING IMMATURE PERIPHERAL BLOOD CELLS
 Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the
Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the
2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228
 2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228 Vishnu V. B Reddy, MD University of Alabama at Birmingham Birmingham, AL USA 11/03/07
2007 Workshop of Society for Hematopathology & European Association for Hematopathology Indianapolis, IN, USA Case # 228 Vishnu V. B Reddy, MD University of Alabama at Birmingham Birmingham, AL USA 11/03/07
Comparison of myeloperoxidase detection by flow cytometry using two different clones of monoclonal antibodies
 Malaysian J Pathol 2004; 26(2) : 111 116FLOW CYTOMETRY MYELOPEROXIDASE DETECTION Comparison of myeloperoxidase detection by flow cytometry using two different clones of monoclonal antibodies CF Leong MPath,
Malaysian J Pathol 2004; 26(2) : 111 116FLOW CYTOMETRY MYELOPEROXIDASE DETECTION Comparison of myeloperoxidase detection by flow cytometry using two different clones of monoclonal antibodies CF Leong MPath,
Reactive and Neoplastic Lymphocytosis
 Reactive and Neoplastic Lymphocytosis Koranda A. Walsh, VMD, BS Assistant Professor, Clinical Pathobiology University of Pennsylvania School of Veterinary Medicine PLEASE NOTE: These notes are meant as
Reactive and Neoplastic Lymphocytosis Koranda A. Walsh, VMD, BS Assistant Professor, Clinical Pathobiology University of Pennsylvania School of Veterinary Medicine PLEASE NOTE: These notes are meant as
Mixed Phenotype Acute Leukemias
 Mixed Phenotype Acute Leukemias CHEN GAO; AMY M. SANDS; JIANLAN SUN NORTH AMERICAN JOURNAL OF MEDICINE AND SCIENCE APR 2012 VOL 5 NO.2 INTRODUCTION Most cases of acute leukemia can be classified based
Mixed Phenotype Acute Leukemias CHEN GAO; AMY M. SANDS; JIANLAN SUN NORTH AMERICAN JOURNAL OF MEDICINE AND SCIENCE APR 2012 VOL 5 NO.2 INTRODUCTION Most cases of acute leukemia can be classified based
Test Utilization: Chronic Lymphocytic Leukemia
 Test Utilization: Chronic Lymphocytic Leukemia Initial Evaluation Diagnostic Criteria Selection of Tests for Prognosis Response to Therapy Challenges Assessment for persistent disease Paul J. Kurtin, M.D.
Test Utilization: Chronic Lymphocytic Leukemia Initial Evaluation Diagnostic Criteria Selection of Tests for Prognosis Response to Therapy Challenges Assessment for persistent disease Paul J. Kurtin, M.D.
Mantle Cell Lymphoma
 HEMATOPATHOLOGY Original Article Mantle Cell Lymphoma Morphologic Findings in Bone Marrow Involvement JAY WASMAN, MD, 1 NANCY S. ROSENTHAL, MD,' AND DIANE C. FARHI, MD 2 Although mantle cell lymphoma (MCL),
HEMATOPATHOLOGY Original Article Mantle Cell Lymphoma Morphologic Findings in Bone Marrow Involvement JAY WASMAN, MD, 1 NANCY S. ROSENTHAL, MD,' AND DIANE C. FARHI, MD 2 Although mantle cell lymphoma (MCL),
B-Cell Precursors in Normal Pediatric Bone Marrow
 HEMATOPATHOLOGY Original Article B-Cell Precursors in Normal Pediatric Bone Marrow CHARLES W. CALDWELL, M.D., PH.D., EDWARD POJE, M.D., AND MARY A. HELIKSON, M.D. A number of studies have been published
HEMATOPATHOLOGY Original Article B-Cell Precursors in Normal Pediatric Bone Marrow CHARLES W. CALDWELL, M.D., PH.D., EDWARD POJE, M.D., AND MARY A. HELIKSON, M.D. A number of studies have been published
Normal Blood and Bone Marrow Populations
 4 CHAPTER 4 Normal Blood and Bone Marrow Populations It is essential to have a sound understanding of the nature and immunophenotypic characteristics of the normal cell populations encountered in bone
4 CHAPTER 4 Normal Blood and Bone Marrow Populations It is essential to have a sound understanding of the nature and immunophenotypic characteristics of the normal cell populations encountered in bone
CME/SAM. Olga Pozdnyakova, MD, PhD, 1 Svetlana Kondtratiev, MD, 1,2 Betty Li, MS, 1 Karry Charest, 1 and David M. Dorfman, MD, PhD 1.
 Hematopathology / New Mastocytosis Flow Cytometry Approach High-Sensitivity Flow Cytometric Analysis for the Evaluation of Systemic Mastocytosis Including the Identification of a New Flow Cytometric Criterion
Hematopathology / New Mastocytosis Flow Cytometry Approach High-Sensitivity Flow Cytometric Analysis for the Evaluation of Systemic Mastocytosis Including the Identification of a New Flow Cytometric Criterion
Detection of Leukemic Lymphoblasts in CSF Is Instrument-Dependent
 Hematopathology / Detection of Lymphoblasts in CSF Detection of Leukemic Lymphoblasts in CSF Is Instrument-Dependent lison R. Huppmann, MD, 1 Susan R. Rheingold, MD, 2 L. Charles ailey, MD, 2 Marybeth
Hematopathology / Detection of Lymphoblasts in CSF Detection of Leukemic Lymphoblasts in CSF Is Instrument-Dependent lison R. Huppmann, MD, 1 Susan R. Rheingold, MD, 2 L. Charles ailey, MD, 2 Marybeth
CME/SAM. Flow Cytometric Immunophenotyping of Cerebrospinal Fluid Specimens
 Hematopathology / Flow Cytometric Immunophenotyping of CSF Specimens Flow Cytometric Immunophenotyping of Cerebrospinal Fluid Specimens Fiona E. Craig, MD, 1 N. Paul Ohori, MD, 2 Timothy S. Gorrill, MD,
Hematopathology / Flow Cytometric Immunophenotyping of CSF Specimens Flow Cytometric Immunophenotyping of Cerebrospinal Fluid Specimens Fiona E. Craig, MD, 1 N. Paul Ohori, MD, 2 Timothy S. Gorrill, MD,
FLOW CYTOMETRIC ANALYSIS OF NORMAL BONE MARROW
 XI International Conference Hematopoiesis Immunology Budapest, June 6-7, 2014 FLO CYTOMETRIC ANALYSIS OF NORMAL BONE MARRO Bruno Brando and Arianna Gatti Hematology Laboratory and Transfusion Center Legnano
XI International Conference Hematopoiesis Immunology Budapest, June 6-7, 2014 FLO CYTOMETRIC ANALYSIS OF NORMAL BONE MARRO Bruno Brando and Arianna Gatti Hematology Laboratory and Transfusion Center Legnano
VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES COPYRIGHTED MATERIAL SECOND EDITION
 VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES SECOND EDITION COPYRIGHTED MATERIAL CHAPTER ONE HEMATOPOIESIS GENERAL FEATURES All blood cells have a finite life span, but in normal
VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES SECOND EDITION COPYRIGHTED MATERIAL CHAPTER ONE HEMATOPOIESIS GENERAL FEATURES All blood cells have a finite life span, but in normal
Participants Identification No. % Evaluation. Mitotic figure Educational Erythrocyte precursor, abnormal 1 0.
 Cell Identification Mitotic figure 212 99.5 Educational Erythrocyte precursor, abnormal BMD-02 The arrowed cell is a mitotic figure. It was correctly identified by 99.5% of the participants. A cell containing
Cell Identification Mitotic figure 212 99.5 Educational Erythrocyte precursor, abnormal BMD-02 The arrowed cell is a mitotic figure. It was correctly identified by 99.5% of the participants. A cell containing
Posttherapy Surveillance of B-Cell Precursor A c u t e Lymphoblastic Leukemia Value of Polymerase Chain Reaction and Limitations of Flow Cytometry
 Hematopatholocjy / PCR SURVEILLANCE OF TREATED B-CELL PRECURSOR ALL Posttherapy Surveillance of B-Cell Precursor A c u t e Lymphoblastic Leukemia Value of Polymerase Chain Reaction and Limitations of Flow
Hematopatholocjy / PCR SURVEILLANCE OF TREATED B-CELL PRECURSOR ALL Posttherapy Surveillance of B-Cell Precursor A c u t e Lymphoblastic Leukemia Value of Polymerase Chain Reaction and Limitations of Flow
Wedge biopsies of the testis were obtained from 37 boys with ALL. Repeat biopsies were obtained from
 J Clin Pathol 1986;39:1236-1240 Terminal transferase positive cells in testicular biopsy specimens from boys with acute lymphoblastic leukaemia J M CHESSELLS,* J R PINCOTT,* W DANIELS-LAKE*t From the *Departments
J Clin Pathol 1986;39:1236-1240 Terminal transferase positive cells in testicular biopsy specimens from boys with acute lymphoblastic leukaemia J M CHESSELLS,* J R PINCOTT,* W DANIELS-LAKE*t From the *Departments
CCND1-IGH Fusion-Amplification and MYC Copy Number Gain in a Case of Pleomorphic Variant Mantle Cell Lymphoma
 AJCP /CASE REPORT CCND1-IGH Fusion-Amplification and MYC Copy Number Gain in a Case of Pleomorphic Variant Mantle Cell Lymphoma Yuan Miao, MD, 1,2 Pei Lin, MD, 1 Wei Wang, MD, 1 L. Jeffrey Medeiros, MD,
AJCP /CASE REPORT CCND1-IGH Fusion-Amplification and MYC Copy Number Gain in a Case of Pleomorphic Variant Mantle Cell Lymphoma Yuan Miao, MD, 1,2 Pei Lin, MD, 1 Wei Wang, MD, 1 L. Jeffrey Medeiros, MD,
Bone marrow aspiration as the initial diagnostic tool in the diagnosis of leukemia - A case study
 Original Research Article Bone marrow aspiration as the initial diagnostic tool in the diagnosis of leukemia - A case study Priyanka Poonam 1*, N.K. Bariar 2 1 Tutor, Department of Pathology, Patna Medical
Original Research Article Bone marrow aspiration as the initial diagnostic tool in the diagnosis of leukemia - A case study Priyanka Poonam 1*, N.K. Bariar 2 1 Tutor, Department of Pathology, Patna Medical
V. Acute leukemia. Flow cytometry in evaluation of hematopoietic neoplasms: A case-based approach
 V. Acute leukemia Evaluating a sample for an acute leukemia Acute leukemia is a neoplasm of immature myeloid or lymphoid cells characterized by a block in maturation, usually at the stage of an early progenitor
V. Acute leukemia Evaluating a sample for an acute leukemia Acute leukemia is a neoplasm of immature myeloid or lymphoid cells characterized by a block in maturation, usually at the stage of an early progenitor
Immunohistochemistry Can Be Used to Subtype Acute Myeloid Leukemia in Routinely Processed Bone Marrow Biopsy Specimens Comparison With Flow Cytometry
 Hematopathology / IMMUNOHISTOLOGIC EXAMINATION IN DIAGNOSIS OF ACUTE MYELOID LEUKEMIA Immunohistochemistry Can Be Used to Subtype Acute Myeloid Leukemia in Routinely Processed Bone Marrow Biopsy Specimens
Hematopathology / IMMUNOHISTOLOGIC EXAMINATION IN DIAGNOSIS OF ACUTE MYELOID LEUKEMIA Immunohistochemistry Can Be Used to Subtype Acute Myeloid Leukemia in Routinely Processed Bone Marrow Biopsy Specimens
Mast Cell Disease Case 054 Session 7
 Mast Cell Disease Case 054 Session 7 Rodney R. Miles, M.D., Ph.D. Lauren B. Smith, M.D. Cem Akin, M.D. Diane Roulston,, Ph.D. Charles W. Ross, M.D. Departments of Pathology and Internal Medicine University
Mast Cell Disease Case 054 Session 7 Rodney R. Miles, M.D., Ph.D. Lauren B. Smith, M.D. Cem Akin, M.D. Diane Roulston,, Ph.D. Charles W. Ross, M.D. Departments of Pathology and Internal Medicine University
EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION
 EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
Participants Identification No. % Evaluation
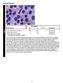 BMD-02 Cell Identification Participants Identification No. % Evaluation Erythrocyte precursor, normal 228 95.8 Educational Erythrocyte, normal 7 3.0 Educational Erythrocyte precursor with megaloblastic
BMD-02 Cell Identification Participants Identification No. % Evaluation Erythrocyte precursor, normal 228 95.8 Educational Erythrocyte, normal 7 3.0 Educational Erythrocyte precursor with megaloblastic
FLOCK Cluster Analysis of Mast Cell Event Clustering by High-Sensitivity Flow Cytometry Predicts Systemic Mastocytosis
 FLOCK Cluster Analysis of Mast Cell Event Clustering by High-Sensitivity Flow Cytometry Predicts Systemic Mastocytosis David M. Dorfman, MD, PhD, Charlotte D. LaPlante, Olga Pozdnyakova, MD, PhD, and Betty
FLOCK Cluster Analysis of Mast Cell Event Clustering by High-Sensitivity Flow Cytometry Predicts Systemic Mastocytosis David M. Dorfman, MD, PhD, Charlotte D. LaPlante, Olga Pozdnyakova, MD, PhD, and Betty
Terminal Deoxynucleotidyl Transferase Positive Cells in Human Tonsils
 Hematopathology / TDT-POSITIVE TONSIL CELLS Terminal Deoxynucleotidyl Transferase Positive Cells in Human Tonsils James A. Strauchen, MD, and Lorraine K. Miller, PhD Key Words: Terminal deoxynucleotidyl
Hematopathology / TDT-POSITIVE TONSIL CELLS Terminal Deoxynucleotidyl Transferase Positive Cells in Human Tonsils James A. Strauchen, MD, and Lorraine K. Miller, PhD Key Words: Terminal deoxynucleotidyl
Mimics of Lymphoma in Routine Biopsies. I have nothing to disclose regarding the information to be reported in this talk.
 Mimics of Lymphoma in Routine Biopsies Patrick Treseler, MD, PhD Professor of Pathology University of California San Francisco I have nothing to disclose regarding the information to be reported in this
Mimics of Lymphoma in Routine Biopsies Patrick Treseler, MD, PhD Professor of Pathology University of California San Francisco I have nothing to disclose regarding the information to be reported in this
Done By : WESSEN ADNAN BUTHAINAH AL-MASAEED
 Done By : WESSEN ADNAN BUTHAINAH AL-MASAEED Acute Myeloid Leukemia Firstly we ll start with this introduction then enter the title of the lecture, so be ready and let s begin by the name of Allah : We
Done By : WESSEN ADNAN BUTHAINAH AL-MASAEED Acute Myeloid Leukemia Firstly we ll start with this introduction then enter the title of the lecture, so be ready and let s begin by the name of Allah : We
Blood Cell Identification Graded
 BCP-21 Blood Cell Identification Graded Case History The patient is a 37-year-old female with a history of multiple sickle cell crises. She now presents with avascular necrosis of the left hip. Laboratory
BCP-21 Blood Cell Identification Graded Case History The patient is a 37-year-old female with a history of multiple sickle cell crises. She now presents with avascular necrosis of the left hip. Laboratory
Hematology Unit Lab 2 Review Material
 Objectives Hematology Unit Lab 2 Review Material - 2018 Laboratory Instructors: 1. Assist students during lab session Students: 1. Review the introductory material 2. Study the case histories provided
Objectives Hematology Unit Lab 2 Review Material - 2018 Laboratory Instructors: 1. Assist students during lab session Students: 1. Review the introductory material 2. Study the case histories provided
Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma
 Anatomic Pathology / CYTOKERATINS 7 AND 20 IN PROSTATE AND BLADDER CARCINOMAS Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma Nader H. Bassily,
Anatomic Pathology / CYTOKERATINS 7 AND 20 IN PROSTATE AND BLADDER CARCINOMAS Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma Nader H. Bassily,
Hematopathology Lab. Third year medical students
 Hematopathology Lab Third year medical students Objectives Identify the lesion Know the specific name of the lesion Know associated disease Know relevant pathologic background Spherocytes: appear small,
Hematopathology Lab Third year medical students Objectives Identify the lesion Know the specific name of the lesion Know associated disease Know relevant pathologic background Spherocytes: appear small,
Case Report A case of EBV positive diffuse large B-cell lymphoma of the adolescent
 Int J Clin Exp Med 2014;7(1):307-311 www.ijcem.com /ISSN:1940-5901/IJCEM1311029 Case Report A case of EBV positive diffuse large B-cell lymphoma of the adolescent Qilin Ao 2, Ying Wang 1, Sanpeng Xu 2,
Int J Clin Exp Med 2014;7(1):307-311 www.ijcem.com /ISSN:1940-5901/IJCEM1311029 Case Report A case of EBV positive diffuse large B-cell lymphoma of the adolescent Qilin Ao 2, Ying Wang 1, Sanpeng Xu 2,
DETERMINATION OF A LYMPHOID PROCESS
 Chapter 2 Applications of Touch Preparation Cytology to Intraoperative Consultations: Lymph Nodes and Extranodal Tissues for Evaluation of Hematolymphoid Disorders INTRODUCTION As discussed in Chap. 1,
Chapter 2 Applications of Touch Preparation Cytology to Intraoperative Consultations: Lymph Nodes and Extranodal Tissues for Evaluation of Hematolymphoid Disorders INTRODUCTION As discussed in Chap. 1,
Cell sorting: A bridge between morphology and flow cytometry. Pr François Mullier, Pr Bernard Chatelain July 19th, 2013
 Cell sorting: A bridge between morphology and flow cytometry Pr François Mullier, Pr Bernard Chatelain July 19th, 2013 1 CELL SORTING: PRINCIPLES D.Davies CELL SORTING: PRINCIPLES Aims Efficiency Purity
Cell sorting: A bridge between morphology and flow cytometry Pr François Mullier, Pr Bernard Chatelain July 19th, 2013 1 CELL SORTING: PRINCIPLES D.Davies CELL SORTING: PRINCIPLES Aims Efficiency Purity
Classification of Hematologic Malignancies. Patricia Aoun MD MPH
 Classification of Hematologic Malignancies Patricia Aoun MD MPH Objectives Know the basic principles of the current classification system for hematopoietic and lymphoid malignancies Understand the differences
Classification of Hematologic Malignancies Patricia Aoun MD MPH Objectives Know the basic principles of the current classification system for hematopoietic and lymphoid malignancies Understand the differences
Case Report Blasts-more than meets the eye: evaluation of post-induction day 21 bone marrow in CBFB rearranged acute leukemia
 Int J Clin Exp Pathol 2014;7(7):4498-4502 www.ijcep.com /ISSN:1936-2625/IJCEP0000851 Case Report Blasts-more than meets the eye: evaluation of post-induction day 21 bone marrow in CBFB rearranged acute
Int J Clin Exp Pathol 2014;7(7):4498-4502 www.ijcep.com /ISSN:1936-2625/IJCEP0000851 Case Report Blasts-more than meets the eye: evaluation of post-induction day 21 bone marrow in CBFB rearranged acute
7 Omar Abu Reesh. Dr. Ahmad Mansour Dr. Ahmad Mansour
 7 Omar Abu Reesh Dr. Ahmad Mansour Dr. Ahmad Mansour -Leukemia: neoplastic leukocytes circulating in the peripheral bloodstream. -Lymphoma: a neoplastic process in the lymph nodes, spleen or other lymphatic
7 Omar Abu Reesh Dr. Ahmad Mansour Dr. Ahmad Mansour -Leukemia: neoplastic leukocytes circulating in the peripheral bloodstream. -Lymphoma: a neoplastic process in the lymph nodes, spleen or other lymphatic
Acute myeloid leukemia. M. Kaźmierczak 2016
 Acute myeloid leukemia M. Kaźmierczak 2016 Acute myeloid leukemia Malignant clonal disorder of immature hematopoietic cells characterized by clonal proliferation of abnormal blast cells and impaired production
Acute myeloid leukemia M. Kaźmierczak 2016 Acute myeloid leukemia Malignant clonal disorder of immature hematopoietic cells characterized by clonal proliferation of abnormal blast cells and impaired production
Hematogones With Lambda Light Chain Restriction in a 4-Year-Old Boy With Burkitt Lymphoma: A Potential Diagnostic Pitfall
 Hematogones With Lambda Light Chain Restriction in a 4-Year-Old Boy With Burkitt Lymphoma: A Potential Diagnostic Pitfall Tesha Guillory, MD, 1 Shiyong Li, MD, PhD, 1 Daniel J. Bergsagel, MD, 2 Elizabeth
Hematogones With Lambda Light Chain Restriction in a 4-Year-Old Boy With Burkitt Lymphoma: A Potential Diagnostic Pitfall Tesha Guillory, MD, 1 Shiyong Li, MD, PhD, 1 Daniel J. Bergsagel, MD, 2 Elizabeth
Bumps on the Neck and Groin of a 2-Year-Old Male. Laboratory Findings: Table 1, Table 2; Figure 1; Image 1, Image 2, Image 3
 Bumps on the Neck and Groin of a 2-Year-Old Male 1 Erikakelly Strand, BS* Clinical History Patient: 2-year-old white male. Chief Complaint: Bumps on neck and groin. History of Present Illness: A 2-year-old
Bumps on the Neck and Groin of a 2-Year-Old Male 1 Erikakelly Strand, BS* Clinical History Patient: 2-year-old white male. Chief Complaint: Bumps on neck and groin. History of Present Illness: A 2-year-old
A.M.W. van Marion. H.M. Lokhorst. N.W.C.J. van de Donk. J.G. van den Tweel. Histopathology 2002, 41 (suppl 2):77-92 (modified)
 chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
Acute Lymphoblastic Leukaemia
 Acute Lymphoblastic Leukaemia Terri Boyer 17 th October 2006 Overview Disease information: Aetiology of ALL proposed theory, contributing factors Symptoms Complications Diagnostic approaches - morphology
Acute Lymphoblastic Leukaemia Terri Boyer 17 th October 2006 Overview Disease information: Aetiology of ALL proposed theory, contributing factors Symptoms Complications Diagnostic approaches - morphology
Multidimensional Flow Cytometry for Detection of Rare Populations in Hematological Malignancies
 TZU CHI MED J March 2009 Vol 21 No 1 available at http://ajws.elsevier.com/tcmj Tzu Chi Medical Journal Original Article Multidimensional Flow Cytometry for Detection of Rare Populations in Hematological
TZU CHI MED J March 2009 Vol 21 No 1 available at http://ajws.elsevier.com/tcmj Tzu Chi Medical Journal Original Article Multidimensional Flow Cytometry for Detection of Rare Populations in Hematological
Ordering Physician CLIENT,CLIENT. Collected REVISED REPORT
 HPWET Hematopathology Consultation, MML Embed Client Hematopathology Consult REVISED INAL DIAGNOSIS Interpretation Peripheral blood, bone marrow aspirate and biopsies, bilateral iliac crests: 1. Normocellular
HPWET Hematopathology Consultation, MML Embed Client Hematopathology Consult REVISED INAL DIAGNOSIS Interpretation Peripheral blood, bone marrow aspirate and biopsies, bilateral iliac crests: 1. Normocellular
Hematology MUHAMMAD M. KHURRAM* SAGHIR A. JAFRI* ABDUL MANNAN** AFTAB NADEEM*** ASIF JAMAL*
 Hematology FREQUENCY OF ABERRANT EXPRESSION OF CD MARKERS IN CASES OF ACUTE LEUKEMIA MUHAMMAD M. KHURRAM* SAGHIR A. JAFRI* ABDUL MANNAN** AFTAB NADEEM*** ASIF JAMAL* SUMMARY: In the present study 100 patients
Hematology FREQUENCY OF ABERRANT EXPRESSION OF CD MARKERS IN CASES OF ACUTE LEUKEMIA MUHAMMAD M. KHURRAM* SAGHIR A. JAFRI* ABDUL MANNAN** AFTAB NADEEM*** ASIF JAMAL* SUMMARY: In the present study 100 patients
Levels of expression of CD19 and CD20 in chronic B cell leukaemias
 364 Academic Department of Haematology and Cytogenetics, The Royal Marsden Hospital and Institute of Cancer Research, London SW3, UK E Matutes N Farahat R Morilla D Catovsky Department of Internal Medicine,
364 Academic Department of Haematology and Cytogenetics, The Royal Marsden Hospital and Institute of Cancer Research, London SW3, UK E Matutes N Farahat R Morilla D Catovsky Department of Internal Medicine,
Mimics of Lymphoma in Routine Biopsies. Mixed follicular and paracortical hyperplasia. Types of Lymphoid Hyperplasia
 Mimics of Lymphoma in Routine Biopsies Patrick Treseler, MD, PhD Professor of Pathology University of California San Francisco Types of Lymphoid Hyperplasia Follicular hyperplasia (B-cells) Paracortical
Mimics of Lymphoma in Routine Biopsies Patrick Treseler, MD, PhD Professor of Pathology University of California San Francisco Types of Lymphoid Hyperplasia Follicular hyperplasia (B-cells) Paracortical
First relapsed childhood ALL Role of chemotherapy
 First relapsed childhood ALL Role of chemotherapy Thirachit Chotsampancharoen, M.D. Division of Pediatric Hematology/Oncology Department of Pediatrics Prince of Songkla University Hat-Yai, Songkhla 25
First relapsed childhood ALL Role of chemotherapy Thirachit Chotsampancharoen, M.D. Division of Pediatric Hematology/Oncology Department of Pediatrics Prince of Songkla University Hat-Yai, Songkhla 25
Diagnostic challenge: Acute leukemia with biphenotypic blasts and BCR-ABL1 translocation
 Case Study Diagnostic challenge: Acute leukemia with biphenotypic blasts and BCR-ABL1 translocation Ling Wang 1 and Xiangdong Xu 1,2,* 1 Department of Pathology, University of California, San Diego; 2
Case Study Diagnostic challenge: Acute leukemia with biphenotypic blasts and BCR-ABL1 translocation Ling Wang 1 and Xiangdong Xu 1,2,* 1 Department of Pathology, University of California, San Diego; 2
Gray Zones and Double Hits Distinguishing True Burkitt Lymphoma from Other High-Grade B-NHLs Burkitt Lymphoma Burkitt-Like Lymphoma DLBCL Patrick Tres
 Gray Zones and Double Hits Distinguishing True Burkitt Lymphoma from Other High-Grade B-NHLs Burkitt Lymphoma Burkitt-Like Lymphoma DLBCL Patrick Treseler, MD, PhD University of California San Francisco
Gray Zones and Double Hits Distinguishing True Burkitt Lymphoma from Other High-Grade B-NHLs Burkitt Lymphoma Burkitt-Like Lymphoma DLBCL Patrick Treseler, MD, PhD University of California San Francisco
Bone Marrow Pathology. Part 1. R.S. Riley, M.D., Ph.D.
 Bone Marrow Pathology Part 1 R.S. Riley, M.D., Ph.D. Bone Marrow Pathology Bone marrow basics Red cell diseases White cell diseases Other diseases Bone Marrow Pathology Bone marrow basics Hematopoiesis
Bone Marrow Pathology Part 1 R.S. Riley, M.D., Ph.D. Bone Marrow Pathology Bone marrow basics Red cell diseases White cell diseases Other diseases Bone Marrow Pathology Bone marrow basics Hematopoiesis
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES
 EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
Acute lymphoblastic leukemia (ALL) represents approximately
 Terminal Deoxynucleotidyl Transferase Negative Acute Lymphoblastic Leukemia Joana Faber, MD; Hagop Kantarjian, MD; W. Mark oberts, MD; Michael Keating, MD; Emil Freireich, MD; Maher Albitar, MD Objective.
Terminal Deoxynucleotidyl Transferase Negative Acute Lymphoblastic Leukemia Joana Faber, MD; Hagop Kantarjian, MD; W. Mark oberts, MD; Michael Keating, MD; Emil Freireich, MD; Maher Albitar, MD Objective.
Participants Identification No. % Evaluation. Mitotic figure Educational Erythrocyte precursor, abnormal/
 Cell Identification BMD-09 Participants Identification No. % Evaluation Mitotic figure 233 96.7 Educational Erythrocyte precursor, abnormal/ 4 1.7 Educational dysplastic nuclear features Erythrocyte precursor
Cell Identification BMD-09 Participants Identification No. % Evaluation Mitotic figure 233 96.7 Educational Erythrocyte precursor, abnormal/ 4 1.7 Educational dysplastic nuclear features Erythrocyte precursor
Flow Cytometric Analysis of Cerebral Spinal Fluid Involvement by Leukemia or Lymphoma
 Flow Cytometric Analysis of Cerebral Spinal Fluid Involvement by Leukemia or Lymphoma Maryalice Stetler-Stevenson, M.D., Ph.D. Flow Cytometry Unit, Laboratory of Pathology, DCS, NCI, NIH DEPARTMENT OF
Flow Cytometric Analysis of Cerebral Spinal Fluid Involvement by Leukemia or Lymphoma Maryalice Stetler-Stevenson, M.D., Ph.D. Flow Cytometry Unit, Laboratory of Pathology, DCS, NCI, NIH DEPARTMENT OF
Megakaryoblastic Leukemia in a Dog A. Hillström 1, H. Tvedten 1, M. Kiupel 2.
 Megakaryoblastic Leukemia in a Dog A. Hillström 1, H. Tvedten 1, M. Kiupel 2. 1 University Animal Hospital, Swedish University of Agricultural Sciences and Strömsholm Referral Animal Hospital, Sweden 2
Megakaryoblastic Leukemia in a Dog A. Hillström 1, H. Tvedten 1, M. Kiupel 2. 1 University Animal Hospital, Swedish University of Agricultural Sciences and Strömsholm Referral Animal Hospital, Sweden 2
Clinical question. Screening tube. Diagnostic panel MRD. Clinical question
 OW CYTOMETRY UPDATES IN LYMPHOPROLIFERATIVE DISORDERS CANCER RESEARCH CENTER IBSAL UNIVERSITY & UNIVERSITY HOSPITAL, SALAMANCA (SPAIN) DISCLOSURES The EuroFlow Scientific Consortium Iamco-chairof receives
OW CYTOMETRY UPDATES IN LYMPHOPROLIFERATIVE DISORDERS CANCER RESEARCH CENTER IBSAL UNIVERSITY & UNIVERSITY HOSPITAL, SALAMANCA (SPAIN) DISCLOSURES The EuroFlow Scientific Consortium Iamco-chairof receives
Children ALL MRD study according to protocol of D. Campana
 Children ALL MRD study according to protocol of D. Campana L.Yu. Grivtsova Laboratory of Haematopoiesis Immunology State N.N.Blokhin Russian Cancer Research Center affiliated to the Russian Academy of
Children ALL MRD study according to protocol of D. Campana L.Yu. Grivtsova Laboratory of Haematopoiesis Immunology State N.N.Blokhin Russian Cancer Research Center affiliated to the Russian Academy of
ABERRANT EXPRESSION OF CD19 AND CD43
 ABERRANT EXPRESSION OF CD19 AND CD43 IN A PATIENT WITH THERAPY-RELATED ACUTE MYELOID LEUKEMIA AND A HISTORY OF MANTLE CELL LYMPHOMA Yen-Chuan Hsieh, 1 Chien-Liang Lin, 2 Chao-Jung Tsao, 2 Pin-Pen Hsieh,
ABERRANT EXPRESSION OF CD19 AND CD43 IN A PATIENT WITH THERAPY-RELATED ACUTE MYELOID LEUKEMIA AND A HISTORY OF MANTLE CELL LYMPHOMA Yen-Chuan Hsieh, 1 Chien-Liang Lin, 2 Chao-Jung Tsao, 2 Pin-Pen Hsieh,
Bone Marrow Specimen (Aspirate and Trephine Biopsy) Proforma
 Bone Marrow Specimen (Aspirate and Trephine Biopsy) Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Sex Male Female Intersex/indeterminate Date
Bone Marrow Specimen (Aspirate and Trephine Biopsy) Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Sex Male Female Intersex/indeterminate Date
HEAMATOLOGICAL INDICES AND BONE MARROW BIOPSY
 HEAMATOLOGICAL INDICES AND BONE MARROW BIOPSY HEMATOCRIT Hematocrit is a measure of the percentage of the total blood volume that is made up by the red blood cells The hematocrit can be determined directly
HEAMATOLOGICAL INDICES AND BONE MARROW BIOPSY HEMATOCRIT Hematocrit is a measure of the percentage of the total blood volume that is made up by the red blood cells The hematocrit can be determined directly
Original Article Acute Leukemias And Aberrant Markers Expression Pak Armed Forces Med J 2018; 68 (3):
 Open Access Original Article Acute Leukemias And Aberrant Markers Expression Pak Armed Forces Med J 2018; 68 (3): 450-54 SPECTRUM OF ACUTE LEUKEMIAS AND ABERRANT MARKERS EXPRESSION BASED ON FLOWCYTOMETRY
Open Access Original Article Acute Leukemias And Aberrant Markers Expression Pak Armed Forces Med J 2018; 68 (3): 450-54 SPECTRUM OF ACUTE LEUKEMIAS AND ABERRANT MARKERS EXPRESSION BASED ON FLOWCYTOMETRY
Patterns of Lymphoid Neoplasia in Peripheral Blood. Leon F. Baltrucki, M.D. Leon F. Baltrucki, M.D. Disclosure
 Patterns of Lymphoid Neoplasia in Peripheral Blood Leon F. Baltrucki, M.D. Leon F. Baltrucki, M.D. Disclosure Dr Baltrucki has received an honorarium for his participation as a faculty presenter in this
Patterns of Lymphoid Neoplasia in Peripheral Blood Leon F. Baltrucki, M.D. Leon F. Baltrucki, M.D. Disclosure Dr Baltrucki has received an honorarium for his participation as a faculty presenter in this
Group of malignant disorders of the hematopoietic tissues characteristically associated with increased numbers of white cells in the bone marrow and
 Group of malignant disorders of the hematopoietic tissues characteristically associated with increased numbers of white cells in the bone marrow and / or peripheral blood Classified based on cell type
Group of malignant disorders of the hematopoietic tissues characteristically associated with increased numbers of white cells in the bone marrow and / or peripheral blood Classified based on cell type
By Dr. Mohamed Saad Daoud
 By Dr. Mohamed Saad Daoud Part I Introduction Types of White Blood Cells Genesis of the White Blood Cells Life Span of the White Blood Cells Dr. Mohamed Saad Daoud 2 Leucocytes Introduction: Infectious
By Dr. Mohamed Saad Daoud Part I Introduction Types of White Blood Cells Genesis of the White Blood Cells Life Span of the White Blood Cells Dr. Mohamed Saad Daoud 2 Leucocytes Introduction: Infectious
CME/SAM. Mixed Phenotype Acute Leukemia
 AJCP / Original Article Mixed Phenotype Acute Leukemia A Study of 61 Cases Using World Health Organization and European Group for the Immunological Classification of Leukaemias Criteria Olga K. Weinberg,
AJCP / Original Article Mixed Phenotype Acute Leukemia A Study of 61 Cases Using World Health Organization and European Group for the Immunological Classification of Leukaemias Criteria Olga K. Weinberg,
Bone marrow morphology in reactive conditions. Kaaren K. Reichard, MD Mayo Clinic Rochester
 Bone marrow morphology in reactive conditions Kaaren K. Reichard, MD Mayo Clinic Rochester reichard.kaaren@mayo.edu Nothing to disclose Conflict of Interest Outline of Presentation Brief introduction General
Bone marrow morphology in reactive conditions Kaaren K. Reichard, MD Mayo Clinic Rochester reichard.kaaren@mayo.edu Nothing to disclose Conflict of Interest Outline of Presentation Brief introduction General
Lymphoma: What You Need to Know. Richard van der Jagt MD, FRCPC
 Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Collected: , PM Sent: , PM Received: , PM Preliminary: , PM. Notification Status: COMPREHENSIVE DIAGNOSIS
 PATIENT Name:HIPAA, Compliant DOB: 03-25-1945 (60 yr) ID#: xxx-xx-0000 Sex: M Tel: xxx-xxx-xxxx SPECIMEN Your No:WS05-xxxx Case No:C05-00xxx Req. No:Txxxxx Collected: 06-08-05, PM Sent: 06-09-05, PM Received:
PATIENT Name:HIPAA, Compliant DOB: 03-25-1945 (60 yr) ID#: xxx-xx-0000 Sex: M Tel: xxx-xxx-xxxx SPECIMEN Your No:WS05-xxxx Case No:C05-00xxx Req. No:Txxxxx Collected: 06-08-05, PM Sent: 06-09-05, PM Received:
Acute Lymphoblastic Leukemia in Elderly Patients The Philadelphia Chromosome May Not Be a Significant Adverse Prognostic Factor
 Hematopathology / ACUTE LYMPHOBLASTIC LEUKEMIA IN ELDERLY PATIENTS Acute Lymphoblastic Leukemia in Elderly Patients The Philadelphia Chromosome May Not Be a Significant Adverse Prognostic Factor Mihaela
Hematopathology / ACUTE LYMPHOBLASTIC LEUKEMIA IN ELDERLY PATIENTS Acute Lymphoblastic Leukemia in Elderly Patients The Philadelphia Chromosome May Not Be a Significant Adverse Prognostic Factor Mihaela
Single and Multiplex Immunohistochemistry
 Single and Multiplex Immunohistochemistry Steve Westra, BS Reagent Product Specialist Leica Biosystems IHC Theory Polyclonal vs Monoclonal Polyclonal reagents Detect a multitude of epitopes Batch to batch
Single and Multiplex Immunohistochemistry Steve Westra, BS Reagent Product Specialist Leica Biosystems IHC Theory Polyclonal vs Monoclonal Polyclonal reagents Detect a multitude of epitopes Batch to batch
Case Report Parotid gland follicular lymphoma lacking both cytoplasmic and surface light chains: a rare case
 Int J Clin Exp Pathol 2014;7(10):7100-7104 www.ijcep.com /ISSN:1936-2625/IJCEP0001717 Case Report Parotid gland follicular lymphoma lacking both cytoplasmic and surface light chains: a rare case Jenny
Int J Clin Exp Pathol 2014;7(10):7100-7104 www.ijcep.com /ISSN:1936-2625/IJCEP0001717 Case Report Parotid gland follicular lymphoma lacking both cytoplasmic and surface light chains: a rare case Jenny
Hematopathology Case Study
 www.medfusionservices.com Hematopathology Case Study CV3515-14 JUNE Clinical Presentation: Clinical Information: A 42 year old male with history of chronic myelogenous leukemia (CML) presents with an elevated
www.medfusionservices.com Hematopathology Case Study CV3515-14 JUNE Clinical Presentation: Clinical Information: A 42 year old male with history of chronic myelogenous leukemia (CML) presents with an elevated
Flow Cytometry. What is flow cytometry?
 Flow Cytometry Flow Cytometry What is flow cytometry? Flow cytometry is a popular laser-based technology to analyze the characteristics of cells or particles. It is predominantly used to measure fluorescence
Flow Cytometry Flow Cytometry What is flow cytometry? Flow cytometry is a popular laser-based technology to analyze the characteristics of cells or particles. It is predominantly used to measure fluorescence
Bone Marrow Morphology after Therapy and Stem Cell Transplantation. Arash Mohtashamian, MD Naval Medical Center, San Diego
 Bone Marrow Morphology after Therapy and Stem Cell Transplantation Arash Mohtashamian, MD Naval Medical Center, San Diego Objectives Bone marrow findings after myeloablative therapy. Effects of recombinant
Bone Marrow Morphology after Therapy and Stem Cell Transplantation Arash Mohtashamian, MD Naval Medical Center, San Diego Objectives Bone marrow findings after myeloablative therapy. Effects of recombinant
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR
 Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
Granular lymphoblast in a case of acute lymphoblastic leukemia: A rare morphology
 www.edoriumjournals.com CLINICAL IMAGES PEER REVIEWED OPEN ACCESS Granular lymphoblast in a case of acute lymphoblastic leukemia: A rare morphology Shehab Fareed Mohamed, Dina Sameh Soliman, Firyal Ibrahim
www.edoriumjournals.com CLINICAL IMAGES PEER REVIEWED OPEN ACCESS Granular lymphoblast in a case of acute lymphoblastic leukemia: A rare morphology Shehab Fareed Mohamed, Dina Sameh Soliman, Firyal Ibrahim
2013 AAIM Pathology Workshop
 2013 AAIM Pathology Workshop John Schmieg, M.D., Ph.D. None Disclosures 1 Pathology Workshop Objectives Define the general philosophy of reviewing pathology reports Review the various components of Bone
2013 AAIM Pathology Workshop John Schmieg, M.D., Ph.D. None Disclosures 1 Pathology Workshop Objectives Define the general philosophy of reviewing pathology reports Review the various components of Bone
Transient Myeloproliferative Disorder and Acute Myeloid Leukemia in Down Syndrome An Immunophenotypic Analysis
 Hematopathology / IMMUNOPHENOTYPE OF TRNSIENT MYELOPROLIFERTIVE DISORDER VS ML IN DOWN SYNDROME Transient Myeloproliferative Disorder and cute Myeloid Leukemia in Down Syndrome n Immunophenotypic nalysis
Hematopathology / IMMUNOPHENOTYPE OF TRNSIENT MYELOPROLIFERTIVE DISORDER VS ML IN DOWN SYNDROME Transient Myeloproliferative Disorder and cute Myeloid Leukemia in Down Syndrome n Immunophenotypic nalysis
Neuroendocrine Lung Tumors Myers
 Diagnosis and Classification of Neuroendocrine Lung Tumors Jeffrey L. Myers, M.D. A. James French Professor Director, Anatomic Pathology & MLabs University of Michigan, Ann Arbor, MI myerjeff@umich.edu
Diagnosis and Classification of Neuroendocrine Lung Tumors Jeffrey L. Myers, M.D. A. James French Professor Director, Anatomic Pathology & MLabs University of Michigan, Ann Arbor, MI myerjeff@umich.edu
HEMATOPATHOLOGY SUMMARY REPORT RL;MMR;
 HEMATOPATHOLOGY SUMMARY REPORT RL;MMR; Page 1 of 1 05/15/20XX HP000000-20XX 05/21/20XX (212) 123-457 (51) 32-3455 (51) 123-457 Age: 78 DOB: 0/05/19XX SS#: 45-45-45 Clinical Information: 78 y/o female with
HEMATOPATHOLOGY SUMMARY REPORT RL;MMR; Page 1 of 1 05/15/20XX HP000000-20XX 05/21/20XX (212) 123-457 (51) 32-3455 (51) 123-457 Age: 78 DOB: 0/05/19XX SS#: 45-45-45 Clinical Information: 78 y/o female with
