GUIDELINES FOR THE MANAGEMENT OF
|
|
|
- Agnes Robbins
- 6 years ago
- Views:
Transcription
1 GUIDELINES FOR THE MANAGEMENT OF RENAL CANCER Date of endorsement: July 2011 Authors: Mr. RD Mills & Mr. WH Turner Ref: AngCN-SSG-U3 Page 1 of 14 Approved and Published: Aug 2011
2 Title: Guidelines for the Management of Renal Cancer Authors: Mr. RD Mills & Mr. WH Turner Document management Document ratification and history Approved by: Review period: Urology NSSG Joint Chair and Network CEO 2 years (or earlier in the light of new evidence) Date placed on electronic library: Aug 2011 Authors: Mr R.D. Mills and Mr W.T. Turner Document Owner: Anglia Cancer Network Tel: Version number as approved and published: 2 Unique identifier no.: AngCN-SSG-U3 For comments / amendments to these guidelines, please contact: Name Hospital Tel. No Rob Mills NNUH Robert.mills@nnuh.nhs.uk William Turner Addenbrookes William.turner@addenbrookes.nhs.uk For copies of guidelines, please refer to the Anglia Cancer Network website: Monitoring the effectiveness of the Process a) Process for Monitoring compliance and Effectiveness - Review of compliance as determined by audit. Any non compliance to be presented by PQ Manager to the AngCN Business Meeting on an annual basis the minutes of this meeting are retained for a minimum of five years. b) Standards/Key Performance Indicators This process forms part of a quality system working to, but not accredited to, International Standard BS EN ISO 9001:2008. The effectiveness of the process will be monitored in accordance with the methods given in the quality manual, AngCN-QM Equality and Diversity Statement This document complies with the Suffolk PCT Equality and Diversity statement an EqIA assessment is available on request to Anglia Cancer Network PQ Manager, Gibson Centre, Exning Road, Newmarket, CB8 7JG. Disclaimer It is your responsibility to check against the electronic library that this printed out copy is the most recent issue of this document. Page 2 of 14 Approved and Published: Aug 2011
3 CONTENTS Introduction...4 Referrals...4 Staging...4 Treatment...4 Partial nephrectomy... 5 Radical nephrectomy... 5 Adrenalectomy... 5 Lymphadenectomy... 6 Cytoreductive surgery... 6 High risk or metastatic disease... 6 Classification... 6 Follow up...6 Following radical nephrectomy... 6 Following partial nephrectomy... 7 Patients with hereditary renal cancer... 7 Recurrent disease...7 Local recurrence... 7 Distant recurrence... 7 Appendices...8 Appendix 1 TNM Classification 2002 (6th Edition)... 9 T Primary Tumour... 9 N Regional Lymph Nodes... 9 M1 Distant Metastasis... 9 ptnm Pathological Classification Appendix 2 Fuhrman Pathological Grading System Appendix Appendix 4 Kidney Cancer Pathway Page 3 of 14 Approved and Published: Aug 2011
4 Introduction The purpose of this manual is to collate the numerous guidelines that exist, into a working manual for the management of renal cancer within the Anglia Cancer Network. It should act as a summary guide for the management of patients with renal cancer based on the available published evidence. Its scope is to aid all health practitioners involved with the patient from primary care and referral through treatment to follow up. However, as constant modifications are being made, these guidelines should only be used to give an indication of current management. They should not be used to treat patients without checking that changes have not been made. These guidelines have been developed by discussion between clinicians within the Anglia Cancer Network Urology Site Specific Group but without the establishment of a formal guideline development group. These guidelines have been endorsed by the Anglia Cancer Network Urology Site Specific Group. They will be reviewed and updated on an annual basis or more frequently as required. The guidelines are intended as a working document for daily practice. For more detailed sources, and for educational material, please refer to guidelines produced by eg, NICE, BAUS, EAU, AUA. Referrals For referrals from Primary and Secondary Care, see AngCN kidney cancer pathway and DH suspected cancer guidance. Staging Haemoglobin, ESR or CRP, creatinine, electrolytes, bone and liver profile. CT of chest, abdomen and pelvis Bone scintigram if alkaline phosphatase elevated or bone pain. Nuclear medicine studies may be considered prior to partial nephrectomy. If clarification of venous involvement is required, consider US or MR Brain CT only if symptoms of cerebral metastases. 3D reconstruction of CT may be considered prior to partial nephrectomy. Biopsy is not normally necessary before surgery but should be considered in cases where imaging is unclear or where surgery is not appropriate and biological treatment is being considered. Treatment As a result of accumulated evidence confirming the oncological safety of nephronsparing surgery, and confirming the risk of CKD (with its inherent cardiovascular risks) following elective radical nephrectomy, and consistent with the 2009 AUA guidelines, all patients with presumed T1 tumours (solid mass < 7cm) should be considered for partial nephrectomy. Those deemed suitable for partial nephrectomy should then be discussed with the Specialist Multi-disciplinary Team at Addenbrooke s or the Norfolk and Norwich University Hospital. Page 4 of 14 Approved and Published: Aug 2011
5 Other patients with complex renal cancer (defined below) should also be discussed with the Specialist Multi-disciplinary Team at Addenbrooke s or the Norfolk and Norwich University Hospital. Those whose tumours have or may have extended to the renal vein, IVC or the heart Those with nodes of greater than 2 cm or with limited distant metastatic disease which might be amenable to resection Those who have bilateral disease or who will require dialysis Patients with von Hippel-Lindau disease or hereditary papillary tumours Patients with non-renal cell cancer, other than those with upper tract TCC, eg sarcoma Partial nephrectomy Indications Tumour less than 7 cm with normal contralateral kidney Tumour in anatomically or functionally solitary kidney Bilateral synchronous presumed renal cell cancer Possible metachronous cancers (hereditary renal cancer) Relative indications Systemic disease that might affect renal function in the future, eg diabetes, stone disease Patients who do not need to be discussed with an SMDT should be managed locally by radical surgery, or conservatively, if: not suitable surgical candidates (eg very frail or very elderly) patient preference (active monitoring) Partial nephrectomy should take place at either Addenbrooke s or the Norfolk and Norwich University Hospital. Radical nephrectomy Patients with T2 or T3a disease, and with an apparently normal contralateral kidney should be considered, in their LMDT, for radical nephrectomy. This can be an open operation or a laparoscopic nephrectomy. The latter may be contraindicated if the tumour is very large, there is extensive nodal enlargement or the renal vein is involved Adrenalectomy Adrenalectomy is indicated where there is high risk of adrenal metastasis, evidenced by large (T3) tumours in the upper half of the kidney or where the adrenal is suspicious for metastasis on pre-operative imaging. Page 5 of 14 Approved and Published: Aug 2011
6 Lymphadenectomy Left to individual surgeon preference. Cytoreductive surgery The role of nephrectomy in metastatic disease is controversial. The decision about such surgery should be made jointly at an SMDT and should involve clinical oncologists with specific expertise in renal cancer. High risk or metastatic disease Patients with high risk surgical pathology or with metastatic disease should be referred to an oncologist with specific expertise in renal cancer. Palliative nephrectomy for local symptoms is sometimes indicated in patients with metastatic disease. For selected patients renal embolisation may have a role. For patients whose metastatic disease is asymptomatic a period of observation may be indicated. When a patient has not undergone surgical resection, biopsy should be considered before immunotherapy is offered. Classification For details of pathology guidelines for renal cancer, see the ACN Pathology guidelines. Tumour should be classified using the 2002 TNM classification system. (6th Edition) (see Appendix 1). The Fuhrman pathological grading system is favoured (see Appendix 2). Histopathology reporting should accord with the Royal College of Pathologists guidelines. In addition, reporting should allow prognostication by the Leibovich (Mayo) scoring system (see Appendix 2). Follow up All stages six weeks post surgery visit to: exclude complications of surgery physical examination, serum haemoglobin, creatinine, with alkaline phosphatase optional. Following radical nephrectomy Page 6 of 14 Approved and Published: Aug 2011
7 Further follow-up depends on classification of recurrence risk: Low recurrence risk (T1, Fuhrman 1 or 2) o no further follow-up Intermediate recurrence risk (T2, Fuhrman 1 or 2) o 6 monthly visits with FBC, C & E and CXR to 3 years, with CT chest/abdomen at 6, 12, 24 and 36 months (omit CXR at these points) High recurrence risk (all T3 or 4, all Fuhrman 3 or 4) o 6 monthly visits with FBC, C & E and CXR to 5 years, with CT chest/abdomen at 6, and 12, months, then annually to 5 years (omit CXR at these points) Following partial nephrectomy 6 monthly visits with FBC, C & E and CXR to 5 years, with CT chest/abdomen at 6, and 12, months, then annually to 5 years (omit CXR at these points) Patients with hereditary renal cancer follow up also in the medical genetics unit. Recurrent disease Local recurrence discussion in SMDT with clinical oncologists with specific expertise in renal cancer restaging consider period of observation to assess disease cadence then consider excision if isolated recurrence Distant recurrence discussion in SMDT with clinical oncologists with specific expertise in renal cancer restaging consider period of observation to assess disease natural history then consider excision if isolated recurrence if widespread recurrence, discuss with clinical oncologists with specific expertise in renal cancer Page 7 of 14 Approved and Published: Aug 2011
8 Appendices Appendix 1 TNM Classification 2002 (6th Edition) Appendix 2 Fuhrman Pathological Grading System and Leibovich Prognosis Score Appendix 3 Clinical Trials Appendix 4 Kidney Cancer Pathway Page 8 of 14 Approved and Published: Aug 2011
9 Appendix 1 TNM Classification 2002 (6th Edition) T Primary Tumour TX T0 T1 Primary tumour cannot be assessed No evidence of primary tumour Tumour 7cm or less in greatest dimension, limited to the kidney T1a T1b Tumour 4cm or less Tumour more than 4cm but not more than 7cm T2 T3 Tumour more than 7cm in greatest dimension, limited to the kidney Tumour extends into major veins or directly invades adrenal gland or perinephric tissues but not beyond Gerota fascia T3a T3b T3c Tumour directly invades adrenal gland or peri-nephric tissues 1 but not beyond Gerota fascia Tumour grossly extends into renal veins(s) 2 or vena cava or its wall below diaphragm Tumour grossly extends into renal veins(s) 2 or vena cava or its wall above diaphragm T4 Tumour directly invades beyond Gerota fascia Notes: 1 Includes renal sinus (peripelvic) fat 2 Includes segmental (muscle-containing) branches N Regional Lymph Nodes NX N0 N1 N2 Regional lymph nodes cannot be assessed No regional lymph node metastasis Metastasis in a single regional lymph node Metastasis in more than one regional lymph node M1 Distant Metastasis MX M0 M1 Distant metastasis cannot be assessed No distant metastasis Distant metastasis Page 9 of 14 Approved and Published: Aug 2011
10 ptnm Pathological Classification The pt, pn and pm categories correspond to the T, N and M categories. pn0 Histological examination of a regional lymphadenectomy specimen will ordinarily include 8 or more lymph nodes. If the lymph nodes are negative but the number ordinarily examined is not met, classify as pn0. G - Histopathological Grading GX Grade of differentiation cannot be assessed G1 Well differentiated G2 Moderately differentiated G3-4 Poorly differentiated/undifferentiated Stage Grouping Stage I T1 N0 M0 Stage II T2 N0 M0 Stage III T3 N0 M0 T1, T2, T3 N1 M0 Stage IV T4 N0, N1 M0 Any T N2 M0 Any T Any N M1 Summary Kidney T1 7cm; limited to the kidney T1a T1b 4cm > 4cm T2 T3 > 7cm; limited to the kidney Adrenal or perinephric invasion; major veins T3a T3b T3c Adrenal or perinephric invasion Renal veins(s); vena cava below diaphragm Vena cava above diaphragm T4 N1 N2 Beyond Gerota fascia Single More than one Page 10 of 14 Approved and Published: Aug 2011
11 Appendix 2 Fuhrman Pathological Grading System Grade Nuclear size Nuclear shape Nucleoli Other features 1 10um Round and uniform Inconspicuous or absent 2 15um Irregular Present Examined at x400 magnification 3 20um Obviously irregular Large Examined at x100 magnification 4 20+um Bizarre or multilobulated Large Clumped chromatin, spindle cells Leibovich Score Table 1 Scoring Algorithm to Predict Metastases after Radical Nephrectomy in Patients with Clear Cell Renal Cell Carcinoma Feature Score Primary tumor status (pathologic T stage) a pt1a 0 pt1b 2 pt2 3 pt3a 4 pt3b 4 pt3c 4 pt4 4 Regional lymph node status (N stage) a pnx 0 pn0 0 pn1 2 pn2 2 Tumor size (cm) < Nuclear grade Histologic tumor necrosis No 0 Yes 1 a According to the 2002 American Joint Committee on Cancer TNM staging system.[19] Page 11 of 14 Approved and Published: Aug 2011
12 Table 2. Estimated Metastases Free Survival after Radical Nephrectomy in Patients with Clear Cell Renal Cell Carcinoma by Score Estimated metastasis free survival rate (% ± SE) Year 1 Year 3 Year 5 Year 7 Year 10 No. of patients Score (%) Rate No. Rate No. Rate No. Rate No. Rate No (22.0) 99.7 ± (19.2) 99.3 ± (9.7) 96.1 ± (14.7) 90.2 ± (12.0) 86.1 ± (10.9) 66.9 ± (5.6) 59.3 ± (5.9) 39.7 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± SE: standard error. Page 12 of 14 Approved and Published: Aug 2011
13 Appendix 3 The Manual for Cancer Services 2004 states that one of the responsibilities of the MDT Lead Clinician is To ensure mechanisms are in place to support entry of eligible patients into clinical trials. The infrastructure to support clinical trials activity is the responsibility of the West Anglia, Norfolk & Waveney and Ipswich locality of the Mid-Anglia Cancer Research Networks (WACRN, NWCRN and MACRN) which provide facilities enabling patient entry into clinical trials in all of its units. The Anglia Cancer Network Urology SSG is committed to participation in high quality research studies and clinical trials. Whenever possible, patients should be considered for inclusion in local and national research studies and clinical trials. There is an NSSG agreed single list of clinical trials and research studies supported by the individual MDTs. This is updated at least annually. Further details and protocols are available as follow: Trust Contact Telephone Addenbrooke s West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network Bedford West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network Hinchingbrooke West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network Ipswich Anglia East Cancer To be To be confirmed Network confirmed James Paget Anglia East Cancer Natalie.barber@nnuh.nhs.uk Network King s Lynn West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network Norfolk & Norwich Anglia East Cancer Natalie.barber@nnuh.nhs.uk Network Papworth West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network Peterborough West Anglia Cancer Roy.harris@addenbrookes.nhs.uk Research Network West Suffolk West Anglia Cancer Research Network Roy.harris@addenbrookes.nhs.uk Page 13 of 14 Approved and Published: Aug 2011
14 Appendix 4 Kidney Cancer Pathway KIDNEY CANCER PATHWAY (E&W) Final Oncology OPA Decision to Treat (DTT) Systemic Therapy Radiotherapy Recurrence detected Urgent GP suspected cancer referral (2WW) OPA/ Haematuria Clinic Staging CT / MRI Where indicated DMSA (Unit) LOCAL MULTIDISCIPLINARY TEAM (LMDT) MEETING Decision to refer to SMDT OPA Patient informed of diagnosis Decision to Treat (DTT) SPECIALIST MULTIDISCIPLINARY (SMDT) MEETING Uro-Oncology Clinic (UOC) Decision to Treat (DTT) Cryotherapy Venous inv Surgery Partial Nephrectomy Active Monitoring L/SMDT / OPA to assess fitness for subsequent treatment Earliest clinically appropriate date, decision to treat (DTT) Follow Up Metasteses excision Radiotherapy Renal Oncology Where indicated DMSA (Unit) Palliative Radiotherapy Palliative Care Other Referrals i.e. non urgent 18ww referral Palliative Care Nephrectomy Active Monitoring Palliative Care Consultant upgrade points e.g. referral meets NICE criteria; at first seen, during or after diagnostic tests; on or before MDT date & decision to treat date +62 days from these upgrade point dates Consider Clinical Trial and Follow Up Referral to extended MDT services at any point in pathway e.g. Palliative care specialists, Specialist Nurses and AHPs, education about adverse effects of treatment; access to help and advice from all specialists; staff alert to psychosocial needs; therapists/local healthcare teams, Community Matron, Pharmacists (medicines usage review), Social Services, Mental Health Services, Housing benefits (Social Care and other agencies), GPs, Expert Patient Programme, Smoking Cessation, Alcohol Service (NORCAS), Voluntary organisations, information on prescription, Homeshield (over 60s) Day 0 (Referral) By Day 14 (1st seen) By Day 28 (LMDT Meeting) By Day 42 (DTT) By Day 62 (treatment) Day 0 (DTT) By Day 31 (2nd treatment) Key: Unit / Centre Centre Access to specialist services GFOCW Elapsed time for follow up or presentation of recurrence, mets or predetermined gap between treatments Page 14 of 14 Approved and Published: Aug 2011
GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER
 GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER For approvals and version control see Document Management Record on page 9 Ref: AngCN-SSG-U4 Page 1 of 11 Approved and Published: Aug 2012 TABLE OF CONTENTS
GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER For approvals and version control see Document Management Record on page 9 Ref: AngCN-SSG-U4 Page 1 of 11 Approved and Published: Aug 2012 TABLE OF CONTENTS
Brain and CNS tumours Presentation pathway
 Brain and CNS tumours Presentation pathway Ref: AngCN-SSG-BC16 Page 1 of 8 1 Background and Scope This presentation pathway deals with the pathway of referral from all aspects of primary care to hospitals
Brain and CNS tumours Presentation pathway Ref: AngCN-SSG-BC16 Page 1 of 8 1 Background and Scope This presentation pathway deals with the pathway of referral from all aspects of primary care to hospitals
BLADDER CANCER PATHWAY (E&W) Version 1
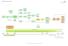 BLADDER CANCER PATHWAY (E&W) Version 1 Flexi cysto FU (via cystoscopy) ptag1/2 and OP instillations MMC ptag3/cis BCG BCG cancer One-stop Haematuria Clinic TURBT Histology presented Decision to refer to
BLADDER CANCER PATHWAY (E&W) Version 1 Flexi cysto FU (via cystoscopy) ptag1/2 and OP instillations MMC ptag3/cis BCG BCG cancer One-stop Haematuria Clinic TURBT Histology presented Decision to refer to
GUIDELINES ON RENAL CELL CARCINOMA
 GUIDELINES ON RENAL CELL CARCINOMA B. Ljungberg (chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction This EAU guideline was prepared to help urologists
GUIDELINES ON RENAL CELL CARCINOMA B. Ljungberg (chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction This EAU guideline was prepared to help urologists
GUIDELINES ON RENAL CELL CANCER
 20 G. Mickisch (chairman), J. Carballido, S. Hellsten, H. Schulze, H. Mensink Eur Urol 2001;40(3):252-255 Introduction is characterised by a constant rise in incidence over the last 50 years, with a predominance
20 G. Mickisch (chairman), J. Carballido, S. Hellsten, H. Schulze, H. Mensink Eur Urol 2001;40(3):252-255 Introduction is characterised by a constant rise in incidence over the last 50 years, with a predominance
Guidelines on Renal Cell
 Guidelines on Renal Cell Carcinoma (Text update March 2009) B. Ljungberg (Chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction Renal cell carcinoma
Guidelines on Renal Cell Carcinoma (Text update March 2009) B. Ljungberg (Chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction Renal cell carcinoma
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Chemotherapy Training and Assessment Policy. For Medical Prescribers and Pharmacy Verifiers
 Chemotherapy Training and Assessment Policy For Medical Prescribers and Pharmacy Verifiers For approvals and version control see Document Management Record on page 6 Doc Ref: AngCN-CCG-C36 Approved and
Chemotherapy Training and Assessment Policy For Medical Prescribers and Pharmacy Verifiers For approvals and version control see Document Management Record on page 6 Doc Ref: AngCN-CCG-C36 Approved and
Manchester Cancer. Guidelines for the management of renal cancer
 Guidelines for the management of renal cancer Approved by the urology pathway board September 2014 To be reviewed September 2016 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Guidelines for the management of renal cancer Approved by the urology pathway board September 2014 To be reviewed September 2016 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Guidance for the Network Review of Chemotherapy Errors
 Guidance for the Network Review of Chemotherapy Errors For approvals and version control see Document Management Record on page 8 Doc Ref: AngCN-CCG-C31 Approved and published: March 2013 Page 1 of 8 Table
Guidance for the Network Review of Chemotherapy Errors For approvals and version control see Document Management Record on page 8 Doc Ref: AngCN-CCG-C31 Approved and published: March 2013 Page 1 of 8 Table
RENAL CANCER GUIDELINES
 Greater Manchester and Cheshire Cancer Network RENAL CANCER GUIDELINES Agreed by Urology CSG: July 2010 Review Date: July 2012 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Greater Manchester and Cheshire Cancer Network RENAL CANCER GUIDELINES Agreed by Urology CSG: July 2010 Review Date: July 2012 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Ref No: AngCN-SSG-Sa9. H:\Cancer Network\Tumour Site\Sarcoma\Peer Review\Active\AngCN-SSG- Sa9_v2_Anglia_Configuration_Sarcoma_Services.
 Anglia Cancer Network Configuration of Sarcoma Services Please note this document has only been partially approved. For further details, approvals and version control please see Document Management Record
Anglia Cancer Network Configuration of Sarcoma Services Please note this document has only been partially approved. For further details, approvals and version control please see Document Management Record
West Midlands Sarcoma Advisory Group
 West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Specialist Sarcoma Multi Disciplinary Team for Suspected Bone Sarcoma Version History Version Date Brief Summary
West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Specialist Sarcoma Multi Disciplinary Team for Suspected Bone Sarcoma Version History Version Date Brief Summary
Bladder Cancer Guidelines
 Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Skin SSG (Anglia East & Anglia West)
 Guidelines for Referrals between Skin LMDT and SMDT Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist Approved by: Anglia
Guidelines for Referrals between Skin LMDT and SMDT Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist Approved by: Anglia
MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER
 10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
1/25/13 Right partial nephrectomy followed by completion right radical nephrectomy.
 History and Physical Case Scenario 1 45 year old white male presents with complaints of nausea, weight loss, and back pain. A CT of the chest, abdomen and pelvis was done on 12/8/12 that revealed a 12
History and Physical Case Scenario 1 45 year old white male presents with complaints of nausea, weight loss, and back pain. A CT of the chest, abdomen and pelvis was done on 12/8/12 that revealed a 12
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Appendix 4 Urology Care Pathways
 Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
West Midlands Sarcoma Advisory Group
 West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Sarcoma Specialist Multi Disciplinary Team for Suspected Sarcoma of Soft Tissue Extremities (limbs and trunk
West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Sarcoma Specialist Multi Disciplinary Team for Suspected Sarcoma of Soft Tissue Extremities (limbs and trunk
Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction
 Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Guidelines for the Management of Suspected Sarcoma in Primary Care
 Guidelines for the Management of Suspected Sarcoma in Primary Care Author: Anglia Cancer Network Sarcoma SSG Document Approved Date: 16-Dec-10 Review Date: December 2012 Ref Code: AngCN-SSG-Sa1 Status:
Guidelines for the Management of Suspected Sarcoma in Primary Care Author: Anglia Cancer Network Sarcoma SSG Document Approved Date: 16-Dec-10 Review Date: December 2012 Ref Code: AngCN-SSG-Sa1 Status:
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Kidney Case 1 SURGICAL PATHOLOGY REPORT
 Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
Guidelines for the Management of Bladder Cancer
 Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
Kidney Q&A 5/5/16 Q1: Can we please get that clarification sent with the presentation and Q&A? Also a start date for that clarification
 Kidney Q&A 5/5/16 Q1: Can we please get that clarification sent with the presentation and Q&A? Also a start date for that clarification A1: Yes. See below. I don't think it will have a start date. Clarification
Kidney Q&A 5/5/16 Q1: Can we please get that clarification sent with the presentation and Q&A? Also a start date for that clarification A1: Yes. See below. I don't think it will have a start date. Clarification
NAACCR Webinar Series 1
 NAACCR 2009 2010 Webinar Series Collecting Cancer Data: Kidney 1 Questions Please use the Q&A panel to submit your questions Send questions to All Panelist 2 Fabulous Prizes 3 NAACCR 2009 2010 Webinar
NAACCR 2009 2010 Webinar Series Collecting Cancer Data: Kidney 1 Questions Please use the Q&A panel to submit your questions Send questions to All Panelist 2 Fabulous Prizes 3 NAACCR 2009 2010 Webinar
3. Guidelines for Reporting Bladder Cancer, Prostate Cancer and Renal Tumours
 60 3. Guidelines for Reporting Bladder Cancer, Prostate Cancer and Renal Tumours Compilation and editing and of this volume: Prof. Chandu de Silva (Consultant Histopathologist) List of contributors Consultant
60 3. Guidelines for Reporting Bladder Cancer, Prostate Cancer and Renal Tumours Compilation and editing and of this volume: Prof. Chandu de Silva (Consultant Histopathologist) List of contributors Consultant
3.1 Investigations for Patients Presenting with Haematuria Table 1
 3.1 Investigations for Patients Presenting with Haematuria Table 1 Patients at risk of bacterial endocarditis should be given antibiotic prophylaxis as per local guidelines. Patients with heart valve replacements
3.1 Investigations for Patients Presenting with Haematuria Table 1 Patients at risk of bacterial endocarditis should be given antibiotic prophylaxis as per local guidelines. Patients with heart valve replacements
Renal cell carcinoma (RCC)
 Renal cell carcinoma (RCC) Introduction The most common solid renal tumor. Accounts for 2 3% of all adult malignancies. It is the 3 rd most common urological tumor in men and the 2 nd in women. It is th
Renal cell carcinoma (RCC) Introduction The most common solid renal tumor. Accounts for 2 3% of all adult malignancies. It is the 3 rd most common urological tumor in men and the 2 nd in women. It is th
Cancer of Unknown Primary Service
 Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
GUIDELINES ON PROSTATE CANCER
 10 G. Aus (chairman), C. Abbou, M. Bolla, A. Heidenreich, H-P. Schmid, H. van Poppel, J. Wolff, F. Zattoni Eur Urol 2001;40:97-101 Introduction Cancer of the prostate is now recognized as one of the principal
10 G. Aus (chairman), C. Abbou, M. Bolla, A. Heidenreich, H-P. Schmid, H. van Poppel, J. Wolff, F. Zattoni Eur Urol 2001;40:97-101 Introduction Cancer of the prostate is now recognized as one of the principal
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
3/23/2017. Disclaimer. Common Changes in TNM Staging. Overview. Overview. Understanding Terminology. Overview
 Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
Skin SSG (Anglia East & Anglia West)
 Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist Approved by: Anglia Cancer Network Skin NSSG Approved on: Reviewed and
Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist Approved by: Anglia Cancer Network Skin NSSG Approved on: Reviewed and
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for Management of Penile Cancer
 Guidelines for Management of Penile Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Sections 3, 5, 6 and 16 updated. Page 1 of 10 1. Scope
Guidelines for Management of Penile Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Sections 3, 5, 6 and 16 updated. Page 1 of 10 1. Scope
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
Guideline for the Diagnosis of Breast Cancer
 Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM)
 INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
Male genital tract tumors. SiCA. Division of Urology, Department of Surgery, Faculty of Medicine Siriraj Hospital.
 Male genital tract tumors Division of Urology, Department of Surgery, Faculty of Medicine Siriraj Hospital. adenocarcinoma Prostate Cancer most common male cancer in western countries more detected in
Male genital tract tumors Division of Urology, Department of Surgery, Faculty of Medicine Siriraj Hospital. adenocarcinoma Prostate Cancer most common male cancer in western countries more detected in
IMAGING GUIDELINES - COLORECTAL CANCER
 IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
A06/S(HSS)b Ex-vivo partial nephrectomy service (Adult)
 A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
Staging Issues: Lung Cancer & Mesothelioma. Mick Peake Clinical Lead, NCIN Chair, Lung SSCRG
 Staging Issues: Lung Cancer & Mesothelioma Mick Peake Clinical Lead, NCIN Chair, Lung SSCRG Staging systems Non-Small Cell Lung Cancer (>85%): UICC TNM v6 used until 1.1.10 transition since then to v7
Staging Issues: Lung Cancer & Mesothelioma Mick Peake Clinical Lead, NCIN Chair, Lung SSCRG Staging systems Non-Small Cell Lung Cancer (>85%): UICC TNM v6 used until 1.1.10 transition since then to v7
The new TNM staging for renal cell carcinoma: what and why the urologists want to know.
 The new TNM staging for renal cell carcinoma: what and why the urologists want to know. Poster No.: C-1132 Congress: ECR 2011 Type: Educational Exhibit Authors: Y. Y. Lim, A. Hattab, A. Bradley ; Manchester/UK,
The new TNM staging for renal cell carcinoma: what and why the urologists want to know. Poster No.: C-1132 Congress: ECR 2011 Type: Educational Exhibit Authors: Y. Y. Lim, A. Hattab, A. Bradley ; Manchester/UK,
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER
 MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012
 Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Section Activity Activity Description Details Reference(s)
 Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with Renal Cell Carcinoma AA Cancer Centre Referrals Not routine pre-op referral indicated
Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with Renal Cell Carcinoma AA Cancer Centre Referrals Not routine pre-op referral indicated
Vincenzo Ficarra 1,2,3. Associate Editor BJU International
 Partial Nephrectomy for RCC Vincenzo Ficarra 1,2,3 1 Director Department of Urology University of Udine, Italy 2 Associate Editor BJU International 3 Scientific Director OLV Robotic Surgery Institute,
Partial Nephrectomy for RCC Vincenzo Ficarra 1,2,3 1 Director Department of Urology University of Udine, Italy 2 Associate Editor BJU International 3 Scientific Director OLV Robotic Surgery Institute,
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Head and Neck Tumours Pages 20, p27, p34, p38, p41, and p49 ly pn2a Metastasis in a single ipsilateral lymph node, less than 3cm in greatest dimension with
UICC TNM 8 th Edition Errata ions are in italics Head and Neck Tumours Pages 20, p27, p34, p38, p41, and p49 ly pn2a Metastasis in a single ipsilateral lymph node, less than 3cm in greatest dimension with
Kidney. Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin.
 Kidney Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin. Procedures Incisional Biopsy (Needle or Wedge) Partial Nephrectomy
Kidney Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin. Procedures Incisional Biopsy (Needle or Wedge) Partial Nephrectomy
Renal Parenchymal Neoplasms
 Renal Parenchymal Neoplasms د. BENIGN TUMORS : Benign renal tumors include adenoma, oncocytoma, angiomyolipoma, leiomyoma, lipoma, hemangioma, and juxtaglomerular tumors. Renal Adenomas : The adenoma is
Renal Parenchymal Neoplasms د. BENIGN TUMORS : Benign renal tumors include adenoma, oncocytoma, angiomyolipoma, leiomyoma, lipoma, hemangioma, and juxtaglomerular tumors. Renal Adenomas : The adenoma is
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Cancer of Unknown Primary Network Site Specific Group. Clinical Guidelines
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012
 Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do?
 Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs. Gynaecological sarcomas Version 1
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
Radical removal of the kidney (radical nephrectomy): procedure-specific information
 PATIENT INFORMATION Radical removal of the kidney (radical nephrectomy): procedure-specific information What is the evidence base for this information? This leaflet includes advice from consensus panels,
PATIENT INFORMATION Radical removal of the kidney (radical nephrectomy): procedure-specific information What is the evidence base for this information? This leaflet includes advice from consensus panels,
GUIDELINEs ON PROSTATE CANCER
 GUIDELINEs ON PROSTATE CANCER (Text update March 2005: an update is foreseen for publication in 2010. Readers are kindly advised to consult the 2009 full text print of the PCa guidelines for the most recent
GUIDELINEs ON PROSTATE CANCER (Text update March 2005: an update is foreseen for publication in 2010. Readers are kindly advised to consult the 2009 full text print of the PCa guidelines for the most recent
EAU GUIDELINES ON RENAL CELL CARCINOMA
 EAU GUIDELINES ON RENAL ELL ARINOMA (Limited text update March 2016) B. Ljungberg (hair), K. Bensalah, A. Bex (Vice-chair), S. anfield, R.H. Giles (Patient Organisation Representative), M. Hora, M.A. Kuczyk,
EAU GUIDELINES ON RENAL ELL ARINOMA (Limited text update March 2016) B. Ljungberg (hair), K. Bensalah, A. Bex (Vice-chair), S. anfield, R.H. Giles (Patient Organisation Representative), M. Hora, M.A. Kuczyk,
UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT
 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr Prasad Bollina, NHS Lothian
SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr Prasad Bollina, NHS Lothian
PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY
 Lancashire & South Cumbria PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY 2017 Guidelines developed by the Urology NSSG (Lancashire & South Cumbria) Reviewed: April 2017 Review date:
Lancashire & South Cumbria PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY 2017 Guidelines developed by the Urology NSSG (Lancashire & South Cumbria) Reviewed: April 2017 Review date:
Sex: 女 Age: 51 Occupation: 無 Admission date:92/07/22
 Sex: 女 Age: 51 Occupation: 無 Admission date:92/07/22 Chief complaint Unknown fever for one month Hand tremor and left huge renal tumor was noted Present illness Suffered from fever for one month, hand
Sex: 女 Age: 51 Occupation: 無 Admission date:92/07/22 Chief complaint Unknown fever for one month Hand tremor and left huge renal tumor was noted Present illness Suffered from fever for one month, hand
Carcinoma of the Renal Pelvis and Ureter Histopathology
 Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
Thyroid INTRODUCTION ANATOMY SUMMARY OF CHANGES
 AJC 7/14/06 1:19 PM Page 67 Thyroid C73.9 Thyroid gland SUMMARY OF CHANGES Tumor staging (T) has been revised and the categories redefined. T4 is now divided into T4a and T4b. Nodal staging (N) has been
AJC 7/14/06 1:19 PM Page 67 Thyroid C73.9 Thyroid gland SUMMARY OF CHANGES Tumor staging (T) has been revised and the categories redefined. T4 is now divided into T4a and T4b. Nodal staging (N) has been
Work Programme/Service Delivery Plan 2010/2013
 Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Guidelines for the Management of Head and Neck Cancer
 Guidelines for the Management of Head and Neck Cancer Version: 2 Ref: AngCN-SSG-NH5 Contents 1. Introduction... 3 2. General Principles... 3 3. Site Specific Guidelines... 4 3.1 Oral Cavity... 4 3.2 Oropharynx...
Guidelines for the Management of Head and Neck Cancer Version: 2 Ref: AngCN-SSG-NH5 Contents 1. Introduction... 3 2. General Principles... 3 3. Site Specific Guidelines... 4 3.1 Oral Cavity... 4 3.2 Oropharynx...
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Solitary Contralateral Adrenal Metastases after Nephrectomy for Renal Cell Carcinoma
 Original Report ISSN 1537-744X; DOI 10.1100/tsw.2004.39 Solitary Contralateral Adrenal after Nephrectomy for Renal Cell Carcinoma Nikolaos Antoniou, M.D. and Demetrios Karanastasis, M.D. General Hospital
Original Report ISSN 1537-744X; DOI 10.1100/tsw.2004.39 Solitary Contralateral Adrenal after Nephrectomy for Renal Cell Carcinoma Nikolaos Antoniou, M.D. and Demetrios Karanastasis, M.D. General Hospital
Skin SSG (Anglia East & Anglia West)
 Guidelines for the Management of Skin Cancer in Specific Anatomical Sites Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist
Guidelines for the Management of Skin Cancer in Specific Anatomical Sites Skin SSG (Anglia East & Anglia West) Author: Dr Jennifer Garioch, Consultant Dermatologist Dr Pamela Todd, Consultant Dermatologist
Oral cancer: Prognosis & Treatment. Dr. Hani Al Sheikh Radhi
 Oral cancer: Prognosis & Treatment Dr. Hani Al Sheikh Radhi Prognostic factors in Oral caner TNM staging T stage N stage M stage Site Histological Factors Vascular & Perineural Invasion Surgical Margins
Oral cancer: Prognosis & Treatment Dr. Hani Al Sheikh Radhi Prognostic factors in Oral caner TNM staging T stage N stage M stage Site Histological Factors Vascular & Perineural Invasion Surgical Margins
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS. Service Specification No.
 B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date of Review B14/S/b Penile
B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date of Review B14/S/b Penile
UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT
 Urological Cancer Audit 2009 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr
Urological Cancer Audit 2009 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr
Colorectal NSSG. Constitution
 Colorectal NSSG Constitution For approvals and version control see Document Management Record on page 10 Ref No: AngCN-SSG-C7 Page 1 of 10 Table of Contents 1 Membership of the Colorectal NSSG (1A-201d)...3
Colorectal NSSG Constitution For approvals and version control see Document Management Record on page 10 Ref No: AngCN-SSG-C7 Page 1 of 10 Table of Contents 1 Membership of the Colorectal NSSG (1A-201d)...3
1. Written information to patient /GP: fax ASAP to GP & offer copy of consultation letter.
 Skin Cancer follow up guidelines If NEW serious diagnosis given: 1. Written information to patient /GP: fax ASAP to GP & offer copy of consultation letter. 2. Free prescription information details. 3.
Skin Cancer follow up guidelines If NEW serious diagnosis given: 1. Written information to patient /GP: fax ASAP to GP & offer copy of consultation letter. 2. Free prescription information details. 3.
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
Partial Nephrectomy Planning: Everybody s s doing it, you can to
 Partial Nephrectomy Planning: Everybody s s doing it, you can to Brian R. Herts, MD Associate Professor of Radiology Head, Abdominal Imaging, Imaging Institute & Staff, The Glickman Urological and Kidney
Partial Nephrectomy Planning: Everybody s s doing it, you can to Brian R. Herts, MD Associate Professor of Radiology Head, Abdominal Imaging, Imaging Institute & Staff, The Glickman Urological and Kidney
Thyroid Gland. Protocol applies to all malignant tumors of the thyroid gland, except lymphomas.
 Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
COSD & Source of Referral
 COSD & Source of Referral A Brief guide October 2014 Michael Sharpe Data Improvement Manager National Cancer Registration Service What is COSD? Cancer and Outcomes Services Dataset Clinical dataset for
COSD & Source of Referral A Brief guide October 2014 Michael Sharpe Data Improvement Manager National Cancer Registration Service What is COSD? Cancer and Outcomes Services Dataset Clinical dataset for
North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma
 THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
6. Cervical Lymph Nodes and Unknown Primary Tumors of the Head and Neck
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Management of Locally Reccurent Renal Cell Carcinoma. Jose A. Karam, MD, FACS Assistant Professor Department of Urology
 Management of Locally Reccurent Renal Cell Carcinoma Jose A. Karam, MD, FACS Assistant Professor Department of Urology DefiniAons Defini&ve treatment Aiming for cure Abla&on therapy Radiofrequency abla&on
Management of Locally Reccurent Renal Cell Carcinoma Jose A. Karam, MD, FACS Assistant Professor Department of Urology DefiniAons Defini&ve treatment Aiming for cure Abla&on therapy Radiofrequency abla&on
Clinical/Surgical trials that will change my practice
 Clinical/Surgical trials that will change my practice Mr Jim M Adshead Herts and Beds Urological Cancer Centre, Lister Hospital What s changed and where do I feel we are clutching at straws? Regional Specialist
Clinical/Surgical trials that will change my practice Mr Jim M Adshead Herts and Beds Urological Cancer Centre, Lister Hospital What s changed and where do I feel we are clutching at straws? Regional Specialist
Prognostic factors of genitourinary tumors: Do we have to care?
 Prognostic factors of genitourinary tumors: Do we have to care? Jae Y. Ro, MD, PhD Professor and Director of Surgical Pathology The Methodist Hospital, Weill Medical College of Cornell University, Houston,
Prognostic factors of genitourinary tumors: Do we have to care? Jae Y. Ro, MD, PhD Professor and Director of Surgical Pathology The Methodist Hospital, Weill Medical College of Cornell University, Houston,
Small Renal Mass Guidelines. Clif Vestal, MD USMD Arlington, Texas
 Small Renal Mass Guidelines Clif Vestal, MD USMD Arlington, Texas Evaluation/Diagnosis 1. Obtain high quality, multiphase, cross-sectional abdominal imaging to optimally characterize/stage the renal mass.
Small Renal Mass Guidelines Clif Vestal, MD USMD Arlington, Texas Evaluation/Diagnosis 1. Obtain high quality, multiphase, cross-sectional abdominal imaging to optimally characterize/stage the renal mass.
Chirurgie beim oligo-metastatischen NSCLC
 24. Ärzte-Fortbildungskurs in Klinischer Onkologie 20.-22. Februar 2014, Kantonsspital St. Gallen Chirurgie beim oligo-metastatischen NSCLC Prof. Dr. med. Walter Weder Klinikdirektor Thoraxchirurgie, UniversitätsSpital
24. Ärzte-Fortbildungskurs in Klinischer Onkologie 20.-22. Februar 2014, Kantonsspital St. Gallen Chirurgie beim oligo-metastatischen NSCLC Prof. Dr. med. Walter Weder Klinikdirektor Thoraxchirurgie, UniversitätsSpital
National Cancer Peer Review Sarcoma. Julia Hill Acting Deputy National Co-ordinator
 National Cancer Peer Review Sarcoma Julia Hill Acting Deputy National Co-ordinator Improving Outcomes Guidance The Intentions of Improving Outcomes for People with Sarcoma Changes in the provision of care
National Cancer Peer Review Sarcoma Julia Hill Acting Deputy National Co-ordinator Improving Outcomes Guidance The Intentions of Improving Outcomes for People with Sarcoma Changes in the provision of care
Integrated Cancer Services Action Plan. Colchester Hospital University NHS Foundation Trust 31 March 2014
 Integrated Cancer Services Action Plan Colchester Hospital University NHS Foundation Trust 31 March KEY Implemented, clearly evidenced and externally approved On Track to deliver Some issues narrative
Integrated Cancer Services Action Plan Colchester Hospital University NHS Foundation Trust 31 March KEY Implemented, clearly evidenced and externally approved On Track to deliver Some issues narrative
