Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata
|
|
|
- Lee Montgomery
- 6 years ago
- Views:
Transcription
1 Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata Julian Mackay-Wiggan,, Angela M. Christiano, Raphael Clynes JCI Insight. 2016;1(15):e Clinical Medicine Dermatology BACKGROUND. Alopecia areata (AA) is a common autoimmune disease with a lifetime risk of 1.7%; there are no FDA-approved treatments for AA. We previously identified a dominant IFN-g transcriptional signature in cytotoxic T lymphocytes (CTLs) in human and mouse AA skin and showed that treatment with JAK inhibitors induced durable hair regrowth in mice by targeting this pathway. Here, we investigated the use of the oral JAK1/2 inhibitor ruxolitinib in the treatment of patients with moderate-to-severe AA. METHODS. We initiated an open-label clinical trial of 12 patients with moderate-to-severe AA, using oral ruxolitinib, 20 mg twice per day, for 3 6 months of treatment followed by 3 months follow-up off drug. The primary endpoint was the proportion of subjects with 50% or greater hair regrowth from baseline to end of treatment. RESULTS. Nine of twelve patients (75%) demonstrated a remarkable response to treatment, with average hair regrowth of 92% at the end of treatment. Safety parameters remained largely within normal limits, and no serious adverse effects were reported. Gene expression profiling revealed treatment-related downregulation of inflammatory markers, including signatures for CTLs and IFN response genes and upregulation of hair-specific markers. CONCLUSION. [ ] Find the latest version:
2 Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata Julian Mackay-Wiggan, 1 Ali Jabbari, 1 Nhan Nguyen, 1 Jane E. Cerise, 1 Charlotte Clark, 1 Grace Ulerio, 1 Megan Furniss, 1 Roger Vaughan, 2 Angela M. Christiano, 1,3 and Raphael Clynes 1 1 Department of Dermatology, 2 Department of Biostatistics, 3 Department of Genetics and Development, Columbia University, New York, New York, USA. BACKGROUND. Alopecia areata (AA) is a common autoimmune disease with a lifetime risk of 1.7%; there are no FDA-approved treatments for AA. We previously identified a dominant IFN-γ transcriptional signature in cytotoxic T lymphocytes (CTLs) in human and mouse AA skin and showed that treatment with JAK inhibitors induced durable hair regrowth in mice by targeting this pathway. Here, we investigated the use of the oral JAK1/2 inhibitor ruxolitinib in the treatment of patients with moderate-to-severe AA. METHODS. We initiated an open-label clinical trial of 12 patients with moderate-to-severe AA, using oral ruxolitinib, 20 mg twice per day, for 3 6 months of treatment followed by 3 months follow-up off drug. The primary endpoint was the proportion of subjects with 50% or greater hair regrowth from baseline to end of treatment. RESULTS. Nine of twelve patients (75%) demonstrated a remarkable response to treatment, with average hair regrowth of 92% at the end of treatment. Safety parameters remained largely within normal limits, and no serious adverse effects were reported. Gene expression profiling revealed treatment-related downregulation of inflammatory markers, including signatures for CTLs and IFN response genes and upregulation of hair-specific markers. CONCLUSION. In this pilot study, 9 of 12 patients (75%) treated with ruxolitinib showed significant scalp hair regrowth and improvement of AA. Larger randomized controlled trials are needed to further assess the safety and efficacy of ruxolitinib in the treatment of AA. TRIAL REGISTRATION. Clinicaltrials.gov NCT Authorship note: J. Mackay-Wiggan, A. Jabbari, A.M. Christiano, and R. Clynes contributed equally to this work. Conflict of interest: A.M. Christiano and R. Clynes report personal and other fees from Aclaris Therapeutics, outside of the scope of this work. Additionally, they are inventors for patent US2011/59029 Methods for Treating Hair Loss Disorders, licensed to Aclaris Therapeutics. Submitted: August 3, 2016 Accepted: August 23, 2016 Published: September 22, 2016 Reference information: JCI Insight. 2016;1(15):e FUNDING. Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award (CUMC CTSA). Introduction Alopecia areata (AA) is a major medical problem and is among the most prevalent autoimmune disease in the US, with a lifetime risk of 1.7% (1). AA affects both sexes across all ethnicities and represents the second most common form of human hair loss, second only to androgenetic alopecia (2). AA usually presents with patchy hair loss. One-third of these patients will experience spontaneous remissions within the first year. However, many patients disease will progress to alopecia totalis (AT, total scalp hair loss) or alopecia universalis (AU, loss of all body hair). Persistent moderate-to-severe AA causes significant disfigurement and psychological distress in affected individuals (3). In clinical practice, there are no evidence-based treatments for AA (4), yet various treatments are offered, most commonly topical and intralesional steroids, which have limited efficacy. Our recent mechanistic studies demonstrated a dominant role for type I cellular immunity in AA pathogenesis, mediated by IFN-γ producing NKG2D-bearing CD8 + cytotoxic T lymphocytes (CTLs) (5). The central role of type I cellular immunity is also reflected in the transcriptional landscape of AA lesional skin in humans and mice, which is dominated by IFN response genes and a CTL signature. These findings provided 1
3 Table 1. Demographic and treatment time variables overall and by responder status Total (n = 12) Responder (n = 9) Nonresponder (n = 3) P value Age (mean yr [SD]) (14.41) (15.27) (14.15) 0.63 Sex (% female) (n = 7) (n = 5) (n = 2) >0.99 Race (n [%]) White 6 (50) 6 (66.67) 0 (0) Black 2 (16.67) 2 (22.22) 0 (0) Hispanic 3 (25) 0 (0) 3 (100) Asian 1 (8.33) 1 (11.11) 0 (0) Baseline SALT score (26.01) (27.96) 65.0 (24.33) >0.99 (mean [SD]) Treatment time (mean wk [SD]) (4.74) (3.07) (2.31) the rationale for therapeutically targeting JAK1/2 kinases in AA, and, indeed, we showed that treatment with JAK inhibitors reversed AA in C3H/HeJ mice and eliminated the type I inflammatory response in the skin (6). On the basis of our preclinical findings, we initiated a phase II efficacy signal-seeking clinical trial in moderate-to-severe AA, assessing the clinical and immunopathological response to treatment with oral ruxolitinib, a JAK1/2 inhibitor currently FDA approved for the treatment of myeloproliferative disorders. Results Efficacy. This study was an open-label clinical trial to investigate ruxolitinib (Jakafi, Incyte Pharmaceuticals), 20 mg orally twice daily, in the treatment of moderate-to-severe AA (Table 1 and Figure 1A). All patients received ruxolitinib for 3 6 months, followed by a 3-month observational phase to assess treatment response durability. Nine of twelve patients (75%) had significant hair regrowth and achieved the primary outcome of at least 50% regrowth (Table 2). The mean baseline severity of alopecia tool (SALT) score of 65.8% ± 28.0% decreased to a score of 24.8% ± 22.9% at 3 months and to a score of 7.3% ± 13.5% at the end of 6 months of treatment (P < 0.005, Table 2). As a group, the responders exhibited a 92% reduction in hair loss from baseline (Figure 1, B D; Figure 2; and Supplemental Table 1; supplemental material available online with this article; DS1), with 7 of the 9 responders achieving over 95% regrowth by end of treatment. Regrowth was seen in responders as soon as 4 weeks after study medication was initiated and initially presented as variably subtle patchy areas of regrowth, consisting of pigmented terminal hairs, with the exception of one patient (subject 4) with concurrent vitiligo, who exhibited primarily gray hair regrowth. Table 2. Description of variables at baseline and end of treatment, among all subjects, responders only, or nonresponders only SALT score (mean [± SD]) P value vs. baseline Regrowth (% mean [± SD]) P value vs. baseline Visual mean (± SD) P value vs. baseline Total (n = 12) Responders (n = 9) Nonresponders (n = 3) Baseline End of treatment Baseline End of treatment Baseline End of treatment 65.6 (± 26.0) 21.7 (± 30.3) 65.8 (± 27.96) 7.3 (± 13.5) 65.0 (± 24.33) 64.7 (± 24.91) < (± 42.6) < (± 28.1) (n = 11) 30.1 (± 29.8) (n = 10) 0.34 < (± 13.5) < (± 27.2) 25.1 (± 29.7) (n = 8) 0.07 > (± 1.6) > (± 30.4) (n = 2) 50.0 (± 28.3) (n = 2) 0.5 Comparisons between baseline and end of treatment were performed using paired Wilcoxon exact sign test for continuous variables or Wilcoxon exact signed-rank test for ordinal variables. 2
4 Figure 1. Hair regrowth during and following discontinuation of ruxolitinib treatment. (A) Patient enrollment flow chart. (B) Severity of alopecia tool (SALT) scores for individual patients during (solid lines) and following cessation of (dashed lines) ruxolitinib treatment. (C) Percentage regrowth for individual patients during and following cessation of ruxolitinib treatment. (D) Predicted (black lines) and actual patient regrowth trajectories (blue lines) from regression models presented in Supplemental Table 1. Of note, the areas of vitiligo in this patient were also noted to improve with ruxolitinib treatment (7). Hair regrowth for all responders increased steadily, with substantial increases each month, resulting in the majority (8 of 9) of responders achieving at least 50% regrowth by the week 12 visit. Responding patients with evidence of regrowth at 3 months continued treatment until they had either achieved 95% 100% regrowth or completed 6 months of treatment. Durability of responses was assessed in the 3-month follow-up period off treatment. Three of nine responders noted shedding, beginning at week 3 following ruxolitinib discontinuation, and had marked hair loss at week 12 off drug (Supplemental Figure 1); however, hair loss did not reach baseline levels (Figure 1, B and C). Six of nine responders reported increased shedding without major hair loss. Biomarker and clinical correlative studies. Gene expression profiling was performed on skin biopsies 3
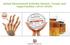
5 Figure 2. Clinical photographs of responder AA patients on ruxolitinib. Pairs of photographs for subjects 1, 2, 3, 4, 8, 9, 10, 11, and 12 are shown as labeled. Photographs labeled a in each pair were taken at baseline, and those labeled b were taken at the end of treatment with ruxolitinib. taken at baseline and following 12 weeks of treatment, with additional optional biopsies performed earlier in the treatment course. Baseline scalp samples exhibited a distinct gene expression profile when compared with samples taken from unaffected patients (Figure 3A and Supplemental Table 2). Following ruxolitinib treatment, gene expression profiles of AA patient scalp samples clustered more closely with healthy control scalp samples than with baseline AA samples (Figure 3B), indicating global normalization of the AA pathogenic response. Gene expression profiles attributed to the IFN, CTL, and hair keratin (KRT) signatures were assessed in the trivariate AA disease activity index (ALADIN, Figure 3C), a summary index of the AA pathogenic inflammatory response and hair regrowth (8). Importantly, eventual AA responders clustered together on the ALADIN matrix at baseline, sharing high IFN and CTL scores (Figure 3, C and D). Notably, baseline samples from eventual AA nonresponders exhibited relatively low IFN and CTL scores (Figure 3, D and E) that were not statistically different than normal control samples. Furthermore, in our cohort of AA patients on ruxolitinib treatment, the CTL and IFN signature scores were capable of distinguishing eventual nonresponders and responders at baseline (P < and P < for CTL and IFN scores, respectively). Consistent with on-target activity of treatment, skin samples taken following 12 weeks of treatment from responding patients exhibited much lower IFN and CTL scores and clustered much more closely to skin samples taken from normal control patients on the ALADIN matrix (Figure 3, D and E). Decreased IFN and CTL scores in biopsies after treatment were demonstrable as early as 2 weeks after the initiation of treatment (Figure 3F). Adverse events. Ruxolitinib was well tolerated and safely administered in all 12 patients. There were no serious adverse effects, and no patients required discontinuation of therapy. Observed adverse effects were infrequent and included 3 minor bacterial skin infections (in the same patient), 9 episodes of upper respiratory tract infection/allergy symptoms in 7 patients, 1 urinary tract infection, 1 case of mild pneumonia, 1 conjunctival hemorrhage following a surgical procedure, and mild gastrointestinal symptoms. One patient developed lowered hemoglobin, which resolved with dose modification. 4
6 Figure 3. Biomarkers based on skin gene expression correlate with clinical response. (A) Heatmap and clustering dendrogram of samples from patients at baseline (n responders = 9, n nonresponders = 2) and week 12 of treatment (n responders = 9, n nonresponders = 1) and healthy controls (n = 6) using differentially expressed genes between baseline responder and healthy control samples (Supplemental Table 2). Black, normal subjects; red, AA responder patient at baseline; purple, AA responder patient after 12 weeks treatment; yellow, AA nonresponder patient at baseline; blue, AA nonresponder patient after 12 weeks treatment. (B) Principal components plots of samples taken from subjects at 12 weeks after treatment and at baseline. Principal components are labeled PC1, PC2, and PC3. (C) Heatmap of AA disease activity index (ALADIN) genes. (D) Three-dimensional plot of ALADIN signatures. (E) ALADIN component signature scores. Left panel, cytotoxic T lymphocyte (CTL) signature scores; middle panel, IFN signature scores; right panel, hair keratin (KRT) signature scores. R, responders; NR, nonresponders; HC, healthy controls. *P < 0.05, **P < 0.01, ***P < Samples were compared using the Kruskal-Wallis test, followed by the Wilcoxon ranked-sum test. The Wilcoxon signed-rank test was used to compare week 0 and week 12 samples. (F) ALADIN component scores from skin samples of AA patients were determined at baseline, week 12, and, in some cases, intermediate or after treatment time points. Red, responder patients; blue, nonresponder patients; black, HC patients. Discussion In this proof-of-concept study, 20 mg ruxolitinib twice per day for 3 6 months induced significant hair regrowth in 9 of 12 patients, an overall 75% rate of response to ruxolitinib in the treatment of AA. In contrast, the expected spontaneous remission rates (occurrence of hair regrowth, without treatment) in patients with moderate-to-severe AA is less than 12% based on two randomized controlled trials with similar subject populations (9, 10). Even the most severe forms of alopecia, AT/AU, responded, indicating that the autoimmune process remains pathogenically active and remains reversible with JAK inhibition. Hair regrowth was evident within 1 month in responders and progressed at a rapid rate. Responses were near complete by 6 months of treatment in 8 of 9 responders, suggesting that 6 months of therapy is sufficient to induce maximal clinical remissions in the majority of responders. In this 9-month study, ruxolitinib was well tolerated. The safety signals in this small study of AA patients, who are otherwise healthy, compare favorably with the prior clinical experience of ruxolitinib in patients with myeloproliferative 5
7 disorders, in which adverse events, particularly those that are hematologically related, are understandably more frequent (11 13), and are consistent with findings from use of tofacitinib in the treatment of patients with psoriasis (14 19). The occurrence of hair shedding in responders following drug cessation suggests that maintenance therapy may be required to sustain remissions. Further clinical evaluation will be required to assess longer durations of therapy and/or investigations of alternative dosing schedules. Larger placebo controlled studies would be required to fully assess the benefit/risk profile of ruxolitinib in AA. Transcriptional profiling of paired baseline and on-treatment scalp biopsies was both mechanistically and clinically informative. Baseline skin samples from responders had high inflammatory ALADIN IFN and CTL scores, with near normalization after 12 weeks of treatment, indicative of JAK1/2 inhibitor mediated suppression of the autoreactive CD8 T cell response. Indeed, early ALADIN normalization, as early as week 2 following initiating treatment (Figure 3F), may be predictive of favorable week 12 clinical outcomes. Conversely, nonresponder samples exhibited low baseline IFN/CTL scores and clustered relatively closely to normal patient samples on the ALADIN matrix, suggestive of alternative inflammatory or noninflammatory etiologies of hair loss in these nonresponders (Supplemental Figure 2). One nonresponder had both AA and androgenic alopecia, another nonresponder s alopecia was consistent with AA histologically but appeared to be a rare diffuse form of the disease, and the final nonresponder exhibited an ophiasis AA pattern. Given our sample size, it is difficult to draw significant conclusions about the efficacy of ruxolitinib in these types of patients. Nevertheless, baseline ALADIN signatures, a measure of type I cellular immunity, may be useful as a predictive biomarker for response to JAK inhibitor treatment, although this needs confirmation in larger well-powered studies, given the caveats described. Recent single-case reports have described clinical responses in AA patients treated with other JAK inhibitors, including tofacitinib (20), ruxolitinib (21), and baricitinib (22). In addition to this paper and Crispin et al. (23), these proof-of-concept data demonstrate immunopathological reversibility of the type I inflammatory response that underlies AA, even in patients with longstanding or more severe forms of disease, providing a strong rationale for clinical development of oral and/or topical JAK inhibitors for the treatment of AA. Methods Study design, oversight, and participants Study assessments and outcomes. The study s primary efficacy endpoint was the proportion of responders at end of treatment, defined as those subjects achieving at least 50% regrowth compared with baseline assessed by the SALT score, a standardized, validated method for estimating hair loss in AA (23). Secondary efficacy endpoints included hair regrowth as a continuous variable. Additionally, quality-of-life measures (Dermatology Quality of Life Index and Skindex) were done at regular prespecified intervals but did not show statistical differences in comparisons performed (data not shown). To assess response durability, responders were followed for 3 months after treatment was completed. Safety analysis was included as a secondary endpoint for all subjects who received at least one dose of ruxolitinib and was monitored as described above at monthly visits. Biomarker assessment and clinical correlative studies Biopsies and peripheral blood were obtained at baseline and after 12 weeks for immune monitoring and molecular studies. Several patients provided additional biopsies at intermediate time points during the course of treatment, and one patient provided an additional sample at week 24. Tissues specimens were fixed and stored in PAXgene Tissue Containers (Qiagen). Total RNA was extracted from skin biopsy specimens harvested during the course of the clinical trial using the PAXgene tissue mirna kit (Qiagen). Library prep was performed for microarray analysis using the Ovation RNA Amplification System V2 and Biotin Encore kits (NuGen Technologies Inc.). Samples were subsequently hybridized to Human Genome U133 Plus 2.0 chips (Affymetrix) and scanned at the Yale Center for Genome Analysis. Library prep and microarray hybridization of RNA extracted from skin biopsies from 3 healthy controls were performed together with the samples from the treated patients for a total of 31 samples. Gene expression analyses included calculation of ALADIN scores, differential expression analysis of the expression levels for the identification of gene expression signatures, principal component analysis, and statistical analysis of the ALADIN scores. Microarray data from the 31 samples have been deposited in GEO under accession number GSE
8 Statistics overview Statistical analysis of clinical data. All variables were examined for distributional assumptions and checked for accuracy and for out of range values. Based on our a priori definition, we classified a patient as a responder if the patient experienced 50% or greater hair regrowth from baseline, based on the SALT score at end of treatment. We examined the overall distribution of demographic factors and looked at possible differences between responders and nonresponders, testing for significance using Fisher s exact test (2 sided) for categorical variables and Mann-Whitney U test for continuous variables. We then examined the change among baseline, end of treatment, and end of study scores for relevant variables overall and for responders and nonresponders employing either a Mann-Whitney U test or Wilcoxon signed-rank test. To estimate the extent of regrowth across time, we considered both a generalized estimating equation and a mixed model approach to model the repeated-measures data and opted for the latter, given the strong normality assumption of generalized estimating equations and our relatively small sample size. For these mixed models, we first modeled regrowth from baseline to end of treatment, where time (in weeks) was the independent variable, and then, to assess maintenance of the observed effect, modeled regrowth from end of treatment to end of study, where time again was the independent variable. In both models, we specified compound symmetry as the initial covariance structure. For all applicable measures, a P value of less than or equal to 0.05 was considered significant. Statistical analysis of gene expression data and ALADIN score In order to examine possible differences among each of the CTL, IFN, and KRT ALADIN scores in responders compared with nonresponders, in responders compared with normal controls, and in nonresponders compared with normal controls, we tested for differences among the 3 groups using a Kruskal-Wallis test, followed by Wilcoxon rank-sum tests, as implemented in the coin package in R. We then examined the change between baseline and ALADIN scores at 12 weeks for responders using Wilcoxon signed-rank test, as implemented in the coin package in R. We further tested for differences in the 3 scores between responders at 12 weeks and normal controls. ALADIN scores are defined such that mean CTL, IFN, and KRT scores are equal to 0, resulting in mean overall (all patients) scores, responder-only scores, and nonresponder-only scores, corresponding to the mean differences between these and the normal controls. Statistically significant differences were observed between overall scores at baseline and normal controls in CTL (P < ), IFN (P < 0.005), and KRT (P < ) scores; between responders-only scores at baseline and normal controls in CTL (P < ), IFN (P < ), and KRT (P < ); between overall scores at week 12 and normal controls in CTL (P < 0.04) and IFN (P < ); and between responders-only and normal controls in KRT (P < ) at α = No statistically significant difference was observed in IFN scores at baseline overall versus normal controls or in CTL and IFN responders-only scores at week 12 versus normal controls. No statistically significant differences were observed between nonresponders at baseline and normal controls in any of the 3 groups of ALADIN scores. Changes in ALADIN scores within individual patients were assessed between baseline and week 12. Statistically significant differences were observed between baseline and week 12 overall in the CTL and IFN scores, with KRT scores reaching marginal significance (α = 0.05). CTL scores declined from 8.30 to 1.51 (P < 0.004), IFN scores declined from to 0.37 (P < 0.004), and KRT scores increased from to (P = 0.054) (Table 2). Among responders only, CTL scores declined from 9.37 to 1.6 (P < 0.008), IFN scores declined from to 0.24 (P < 0.008), and KRT scores increased from to (P = 0.039). Statistically significant differences were observed between responders and nonresponders in CTL and IFN scores at baseline (mean score difference = 5.91 and 40.11, P < and 0.036, respectively) but not in KRT scores (mean score difference = 19.74, P = 0.22). Additional methods and statistical analysis can be found in the Supplemental Methods. Study approval The study was conceived and conducted by the investigative team at Columbia University. All authors had access to the data and attest to its accuracy and, for the fidelity of this report, to the study protocol. This study was conducted in accordance with Good Clinical Practice, as defined by the International Conference on Harmonization, and in accordance with the ethical principles underlying European Union directive 2001/20/EC and the US Code of Federal Regulations, title 21, part 50 (21CFR50). Prior to study 7
9 initiation, the study was reviewed, and approval was obtained from the Columbia University IRB for the protocol and all study-related materials. Freely given written informed consent was obtained from every subject before screening or study-related procedures. Written consent included authorization of the use of photography of patients. Monitoring for regulatory compliance and adherence to the IRB-approved protocol was performed by the Columbia University Clinical Trials Office and the Department of Surgery Regulatory Team. The study was registered on clinicaltrials.gov prior to initiation. Author contributions JMW, AJ, AMC, and RC conceived of and designed the study. JMW, NN, CC, GU, and MF evaluated the subjects and/or collected the clinical data. AJ and JEC collected the experimental data. JMW, AJ, NN, JEC, RV, AMC, and RC analyzed the data. JMW, AJ, NN, AMC, and RC wrote the manuscript. All authors reviewed and approved the final version. Acknowledgments This work was supported in part by funding from the Locks of Love Foundation, the Alopecia Areata Initiative, the NIH/US Public Health Service (grants from NIAMS, including U01AR and R21AR061881), and the Columbia Skin Disease Research Center (NIAMS P30AR044535). AJ is supported by the Irving Institute for Clinical and Translational Research/CUMC CTSA and NIH/NIAMS (K08AR We are grateful to the AA patients who participated in this study. We thank the National Alopecia Areata Registry and the National Alopecia Areata Foundation for their support of this study. Photographic equipment was provided by Canfield Scientific Inc. Address correspondence to: Julian Mackay-Wiggan, 161 Fort Washington Avenue, Room 1264, New York, New York 10032, USA. Phone: ; Or to: Angela Christiano, 1150 St. Nicholas Avenue, Room 307, New York, New York 10032, USA. Phone: ; 1. Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through Mayo Clin Proc. 1995;70(7): Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62(2): , quiz Bickers DR, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3): Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;(2):CD Petukhova L, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302): Xing L, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9): Harris JE, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74(2): Jabbari A, et al. Molecular signatures define alopecia areata subtypes and transcriptional biomarkers. EBioMedicine. 2016;7: Strober BE, et al. Alefacept for severe alopecia areata: a randomized, double-blind, placebo-controlled study. Arch Dermatol. 2009;145(11): Price VH, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58(3): Martí-Carvajal AJ, Anand V, Solà I. Janus kinase-1 and Janus kinase-2 inhibitors for treating myelofibrosis. Cochrane Database Syst Rev. 2015;(4):CD Verstovsek S, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100(4): Palandri F, et al. Safety and efficacy of ruxolitinib in myelofibrosis patients without splenomegaly. Br J Haematol. 2016;174(1): Papp KA, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167(3): Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014: Boy MG, et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129(9):
10 17. Strober B, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169(5): Tofacitinib. Drugs R D. 2010;10(4): Bachelez H, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993): Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134(12): Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90(1): Jabbari A, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2(4): Crispin MK, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e Olsen E, et al. Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol. 1999;40(2 Pt 1):
Snapshot Dx Quiz: September 2018 Detailed Answers
 Snapshot Dx Quiz: September 2018 Detailed Answers Cynthia X. Wang, BA 1 Milan J. Anadkat, MD 1,2 1 Washington University School of Medicine, St. Louis, Missouri 2 Division of Dermatology, St. Louis, Missouri
Snapshot Dx Quiz: September 2018 Detailed Answers Cynthia X. Wang, BA 1 Milan J. Anadkat, MD 1,2 1 Washington University School of Medicine, St. Louis, Missouri 2 Division of Dermatology, St. Louis, Missouri
Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata
 Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata Milène Kennedy Crispin,, Anthony E. Oro, Brett A. King JCI Insight. 2016;1(15):e89776. https://doi.org/10.1172/jci.insight.89776.
Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata Milène Kennedy Crispin,, Anthony E. Oro, Brett A. King JCI Insight. 2016;1(15):e89776. https://doi.org/10.1172/jci.insight.89776.
What s New in Alopecia Areata
 AN UPDATE ON THE LAST 12 MONTHS What s New in Alopecia Areata PhD FRCPC Dermatologist Assistant Professor, University of Toronto Getting information out to Physicians Five new studies to discuss Can cholesterol
AN UPDATE ON THE LAST 12 MONTHS What s New in Alopecia Areata PhD FRCPC Dermatologist Assistant Professor, University of Toronto Getting information out to Physicians Five new studies to discuss Can cholesterol
JAK Inhibitor CTP-543 Achieves Primary Endpoint in Phase 2 Trial in Alopecia Areata An interim analysis of 4 and 8 mg BID
 American Academy of Dermatology Annual Meeting March 2, 2019 JAK Inhibitor CTP-543 Achieves Primary Endpoint in Phase 2 Trial in Alopecia Areata An interim analysis of 4 and 8 mg BID James Cassella, PhD;
American Academy of Dermatology Annual Meeting March 2, 2019 JAK Inhibitor CTP-543 Achieves Primary Endpoint in Phase 2 Trial in Alopecia Areata An interim analysis of 4 and 8 mg BID James Cassella, PhD;
Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study
 Report Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study Laita Bokhari, MPhil Med, and Rodney Sinclair, MBBS, MD, FACD Sinclair
Report Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study Laita Bokhari, MPhil Med, and Rodney Sinclair, MBBS, MD, FACD Sinclair
Alopecia areata: Workup and treatment
 Alopecia areata: Workup and treatment Carolyn Goh Assistant Clinical Professor David Geffen School of Medicine at UCLA Department of Medicine-Dermatology February 2018 DISCLOSURE OF RELATIONSHIPS WITH
Alopecia areata: Workup and treatment Carolyn Goh Assistant Clinical Professor David Geffen School of Medicine at UCLA Department of Medicine-Dermatology February 2018 DISCLOSURE OF RELATIONSHIPS WITH
Breakthrough Drugs in Dermatology. Mark Lebwohl, MD
 Breakthrough Drugs in Dermatology Mark Lebwohl, MD Sol and Clara Kest Professor And Chairman Kimberly and Eric J. Waldman Department of Dermatology Icahn School of Medicine at Mount Sinai LIFE CHANGING
Breakthrough Drugs in Dermatology Mark Lebwohl, MD Sol and Clara Kest Professor And Chairman Kimberly and Eric J. Waldman Department of Dermatology Icahn School of Medicine at Mount Sinai LIFE CHANGING
Brazilian Experience of the Treatment of Alopecia Universalis with the Novel Antirheumatic Therapy Tofacitinib: A Case Series
 Rheumatol Ther (2017) 4:503 508 DOI 10.1007/s40744-017-0069-z CASE SERIES Brazilian Experience of the Treatment of Alopecia Universalis with the Novel Antirheumatic Therapy Tofacitinib: A Case Series Morton
Rheumatol Ther (2017) 4:503 508 DOI 10.1007/s40744-017-0069-z CASE SERIES Brazilian Experience of the Treatment of Alopecia Universalis with the Novel Antirheumatic Therapy Tofacitinib: A Case Series Morton
ALOPECIA AREATA RESEARCH SUMMIT Forging the Future
 ALOPECIA AREATA RESEARCH SUMMIT Forging the Future PRELIMINARY PROGRAM Tuesday, December 4 Wednesday, December 5, 2018 Lerner Hall, Columbia University, New York City, USA Summit Co-Chairs Jerry Shapiro,
ALOPECIA AREATA RESEARCH SUMMIT Forging the Future PRELIMINARY PROGRAM Tuesday, December 4 Wednesday, December 5, 2018 Lerner Hall, Columbia University, New York City, USA Summit Co-Chairs Jerry Shapiro,
ALOPECIA AREATA RESEARCH SUMMIT Forging the Future
 ALOPECIA AREATA RESEARCH SUMMIT Forging the Future PRELIMINARY PROGRAM Tuesday, December 4 Wednesday, December 5, 2018 Lerner Hall, Columbia University, New York City, USA Summit Co-Chairs Jerry Shapiro,
ALOPECIA AREATA RESEARCH SUMMIT Forging the Future PRELIMINARY PROGRAM Tuesday, December 4 Wednesday, December 5, 2018 Lerner Hall, Columbia University, New York City, USA Summit Co-Chairs Jerry Shapiro,
Establishing and Prioritising Research Questions for the Treatment of Alopecia Areata: The Alopecia Areata Priority Setting Partnership
 Establishing and Prioritising Research Questions for the Treatment of Alopecia Areata: The Alopecia Areata Priority Setting Partnership Summary Background Alopecia areata is a common hair loss disorder
Establishing and Prioritising Research Questions for the Treatment of Alopecia Areata: The Alopecia Areata Priority Setting Partnership Summary Background Alopecia areata is a common hair loss disorder
Company Overview. Dr. Neal Walker President and CEO. September Copyright 2016 Aclaris Therapeutics. All rights reserved.
 Company Overview Dr. Neal Walker President and CEO Copyright 2016 Aclaris Therapeutics. All rights reserved. A-11 Disclaimer This presentation contains forward-looking statements, including statements
Company Overview Dr. Neal Walker President and CEO Copyright 2016 Aclaris Therapeutics. All rights reserved. A-11 Disclaimer This presentation contains forward-looking statements, including statements
Aclaris Therapeutics R&D and Investor Day October 4, 2017 Genetics and Immunology of Alopecia Areata
 Aclaris Therapeutics R&D and Investor Day October 4, 2017 Genetics and Immunology of Alopecia Areata Genetics and Immunology of Alopecia Areata Angela M. Christiano, Ph.D. Julian Mackay-Wiggan & Raphael
Aclaris Therapeutics R&D and Investor Day October 4, 2017 Genetics and Immunology of Alopecia Areata Genetics and Immunology of Alopecia Areata Angela M. Christiano, Ph.D. Julian Mackay-Wiggan & Raphael
Pediatric Dermatology. Aniza Giacaman. Hospital Universitari Son Espases, Palma de Mallorca
 Pediatric Dermatology Aniza Giacaman. Hospital Universitari Son Espases, Palma de Mallorca Optimal management of Pediatric Morphea Dr. Yvonne Chiu Pediatric morphea is different from adult morphea More
Pediatric Dermatology Aniza Giacaman. Hospital Universitari Son Espases, Palma de Mallorca Optimal management of Pediatric Morphea Dr. Yvonne Chiu Pediatric morphea is different from adult morphea More
MANIFEST Phase 2 Enhancement / Expansion
 MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients
 IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients with 4 weeks of treatment in a Phase 2a trial A. B. Kimball 1, J. Krueger 2, T. Sullivan
IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients with 4 weeks of treatment in a Phase 2a trial A. B. Kimball 1, J. Krueger 2, T. Sullivan
Transient efficacy of Tofacitinib in Alopecia Areata Universalis
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2016 Transient efficacy of Tofacitinib in Alopecia Areata Universalis Anzengruber,
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2016 Transient efficacy of Tofacitinib in Alopecia Areata Universalis Anzengruber,
Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls
 Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls U033 July 30 th 2017 7:30-8:30AM Paradi Mirmirani, MD The Permanente Medical Group, Vallejo, CA Regional Director, Hair Disorders
Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls U033 July 30 th 2017 7:30-8:30AM Paradi Mirmirani, MD The Permanente Medical Group, Vallejo, CA Regional Director, Hair Disorders
STUDY. Histopathologic Features of Alopecia Areata
 Histopathologic Features of Alopecia Areata A New Look David A. Whiting, MD, FRCP(Edin) STUDY Background: A peribulbar lymphocytic infiltrate is the expected histologic feature of alopecia areata, but
Histopathologic Features of Alopecia Areata A New Look David A. Whiting, MD, FRCP(Edin) STUDY Background: A peribulbar lymphocytic infiltrate is the expected histologic feature of alopecia areata, but
CLINICAL POLICY DEPARTMENT: Medical Management DOCUMENT NAME: JakafiTM REFERENCE NUMBER: NH.PHAR.98
 PAGE: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Stifel Healthcare Conference John Scarlett, M.D. Chief Executive Officer November 19, 2014
 Stifel Healthcare Conference 2014 John Scarlett, M.D. Chief Executive Officer November 19, 2014 1 forward-looking statements Except for statements of historical fact, the statements during this presentation
Stifel Healthcare Conference 2014 John Scarlett, M.D. Chief Executive Officer November 19, 2014 1 forward-looking statements Except for statements of historical fact, the statements during this presentation
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
JERRY SHAPIRO, MD, FAAD PROFESSOR
 The Ronald O. Perelman Department of Dermatology Novel treatments for hair regrowth Where are we? JERRY SHAPIRO, MD, FAAD PROFESSOR AAD Chicago 2018 Disclosures Consultant/Investigator for: Aclaris Samumed
The Ronald O. Perelman Department of Dermatology Novel treatments for hair regrowth Where are we? JERRY SHAPIRO, MD, FAAD PROFESSOR AAD Chicago 2018 Disclosures Consultant/Investigator for: Aclaris Samumed
RE: Docket No. FDA-2017-N-3067 for Patient-Focused Drug Development for Alopecia Areata
 November 3, 2017 Anna K. Abram, Deputy Commissioner for Policy, Planning, Legislation, and Analysis Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville,
November 3, 2017 Anna K. Abram, Deputy Commissioner for Policy, Planning, Legislation, and Analysis Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville,
Jefferies 2016 Healthcare Conference. Reid Huber, PhD Chief Scientific Officer
 Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
 Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses Inc. All rights reserved.
Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses Inc. All rights reserved.
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
2.0 Synopsis. Adalimumab R&D/04/118. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
The role of current biologic therapies in psoriasis
 : An Update on and IL-17 Inhibitors Joanna Dong, BA; Gary Goldenberg, MD PRACTICE POINTS The newest biologics for treatment of moderate to severe plaque psoriasis are and IL-17 inhibitors with unprecedented
: An Update on and IL-17 Inhibitors Joanna Dong, BA; Gary Goldenberg, MD PRACTICE POINTS The newest biologics for treatment of moderate to severe plaque psoriasis are and IL-17 inhibitors with unprecedented
COMPANY OVERVIEW. Dr. Neal Walker President and CEO. January Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1
 COMPANY OVERVIEW Dr. Neal Walker President and CEO January 2017 Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1 Disclaimer This presentation contains forward-looking statements, including
COMPANY OVERVIEW Dr. Neal Walker President and CEO January 2017 Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1 Disclaimer This presentation contains forward-looking statements, including
Global Rheumatoid Arthritis Market: Trends and Opportunities ( )
 Global Rheumatoid Arthritis Market: Trends and Opportunities (2013-2018) Global Rheumatoid Arthritis Market Report Scope of the report The report titled Global Rheumatoid Arthritis Market: Trends and Opportunities
Global Rheumatoid Arthritis Market: Trends and Opportunities (2013-2018) Global Rheumatoid Arthritis Market Report Scope of the report The report titled Global Rheumatoid Arthritis Market: Trends and Opportunities
JAK2 Inhibitors for Myeloproliferative Diseases
 JAK2 Inhibitors for Myeloproliferative Diseases Srdan (Serge) Verstovsek M.D., Ph.D. Associate Professor Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Myeloproliferative
JAK2 Inhibitors for Myeloproliferative Diseases Srdan (Serge) Verstovsek M.D., Ph.D. Associate Professor Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Myeloproliferative
Autoimmunity and Primary Immune Deficiency
 Autoimmunity and Primary Immune Deficiency Mark Ballow, MD Division of Allergy & Immunology USF Morsani School of Medicine Johns Hopkins All Children s Hospital St Petersburg, FL The Immune System What
Autoimmunity and Primary Immune Deficiency Mark Ballow, MD Division of Allergy & Immunology USF Morsani School of Medicine Johns Hopkins All Children s Hospital St Petersburg, FL The Immune System What
COMPANY OVERVIEW. Dr. Neal Walker President and CEO. June Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1
 COMPANY OVERVIEW Dr. Neal Walker President and CEO Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 Disclaimer Any statements contained in this presentation that do not describe historical
COMPANY OVERVIEW Dr. Neal Walker President and CEO Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 Disclaimer Any statements contained in this presentation that do not describe historical
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Adalimumab M Clinical Study Report Final R&D/16/0603
 Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Individualized dosing for Jakafi (ruxolitinib)
 Individualized dosing for Jakafi (ruxolitinib) Indications and Usage Polycythemia vera Jakafi is indicated for treatment of patients with polycythemia vera who have had an inadequate response to or are
Individualized dosing for Jakafi (ruxolitinib) Indications and Usage Polycythemia vera Jakafi is indicated for treatment of patients with polycythemia vera who have had an inadequate response to or are
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/04/900. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
ALOPECIA AREATA (AA) IS A
 STUDY Alefacept for Severe Alopecia Areata A Randomized, Double-blind, Placebo-Controlled Study Bruce E. Strober, MD, PhD; Kavita Menon, MD; Amy McMichael, MD; Maria Hordinsky, MD; Gerald Krueger, MD;
STUDY Alefacept for Severe Alopecia Areata A Randomized, Double-blind, Placebo-Controlled Study Bruce E. Strober, MD, PhD; Kavita Menon, MD; Amy McMichael, MD; Maria Hordinsky, MD; Gerald Krueger, MD;
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
A Comparative Study of Oral Cyclosporine and Betamethasone Minipulse Therapy in the Treatment of Alopecia Areata
 Cyclosporine and Steroid Minipulse Therapy in AA pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 5, 2016 http://dx.doi.org/10.5021/ad.2016.28.5.569 ORIGINAL ARTICLE A Comparative Study of Oral
Cyclosporine and Steroid Minipulse Therapy in AA pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 5, 2016 http://dx.doi.org/10.5021/ad.2016.28.5.569 ORIGINAL ARTICLE A Comparative Study of Oral
Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis. Francesco Passamonti Università degli Studi dell Insubria, Varese
 Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis Francesco Passamonti Università degli Studi dell Insubria, Varese DIPSS during f-up IPSS at diagnosis Diagnose MF and genotype
Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis Francesco Passamonti Università degli Studi dell Insubria, Varese DIPSS during f-up IPSS at diagnosis Diagnose MF and genotype
Stifel Nicolaus 2013 Healthcare Conference. John Scarlett, M.D. Chief Executive Officer September 11, 2013
 Stifel Nicolaus 2013 Healthcare Conference John Scarlett, M.D. Chief Executive Officer September 11, 2013 1 forward-looking statements Except for the historical information contained herein, this presentation
Stifel Nicolaus 2013 Healthcare Conference John Scarlett, M.D. Chief Executive Officer September 11, 2013 1 forward-looking statements Except for the historical information contained herein, this presentation
PFIZER INC. GENERIC DRUG NAME and/or COMPOUND NUMBER: 5% Spironolactone Cream/ PF
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Latest updates in Myeloproliferative Neoplasms. Elizabeth Hexner, MD, MSTR
 Latest updates in Myeloproliferative Neoplasms Elizabeth Hexner, MD, MSTR Disclosures Nothing to disclose Agenda/Goals Treatment goals in PV Indications for cytoreduction in patients polycythemia vera
Latest updates in Myeloproliferative Neoplasms Elizabeth Hexner, MD, MSTR Disclosures Nothing to disclose Agenda/Goals Treatment goals in PV Indications for cytoreduction in patients polycythemia vera
Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia
 doi:./j.46-88.2.78.x Journal of Dermatology 22; 9: 27 2 ORIGINAL ARTICLE Evaluation of efficacy and safety of finasteride mg in 77 Japanese men with androgenetic alopecia Akio SATO, Akira TAKEDA 2 Tokyo
doi:./j.46-88.2.78.x Journal of Dermatology 22; 9: 27 2 ORIGINAL ARTICLE Evaluation of efficacy and safety of finasteride mg in 77 Japanese men with androgenetic alopecia Akio SATO, Akira TAKEDA 2 Tokyo
Ann Dermatol Vol. 27, No. 6,
 IK Yeo, et al Ann Dermatol Vol. 27, No. 6, 2015 http://dx.doi.org/10.5021/ad.2015.27.6.676 ORIGINAL ARTICLE Comparison of High-Dose Corticosteroid Pulse Therapy and Combination Therapy Using Oral Cyclosporine
IK Yeo, et al Ann Dermatol Vol. 27, No. 6, 2015 http://dx.doi.org/10.5021/ad.2015.27.6.676 ORIGINAL ARTICLE Comparison of High-Dose Corticosteroid Pulse Therapy and Combination Therapy Using Oral Cyclosporine
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
General practitioners knowledge and practices related to alopecia areata in Qassim region, Saudi Arabia Abdullateef A. Alzolibani
 24 Original article General practitioners knowledge and practices related to alopecia areata in Qassim region, Saudi Arabia Abdullateef A. Alzolibani Department of Dermatology, College of Medicine, Qassim
24 Original article General practitioners knowledge and practices related to alopecia areata in Qassim region, Saudi Arabia Abdullateef A. Alzolibani Department of Dermatology, College of Medicine, Qassim
Principal Investigator. General Information. Conflict of Interest. Certification Published on The YODA Project (
 Principal Investigator First Name: Liana Last Name: Fraenkel Degree: MD, MPH Primary Affiliation: Yale University School of Medicine E-mail: christine.ramsey@gmail.com Phone number: 610-613-6745 Address:
Principal Investigator First Name: Liana Last Name: Fraenkel Degree: MD, MPH Primary Affiliation: Yale University School of Medicine E-mail: christine.ramsey@gmail.com Phone number: 610-613-6745 Address:
Clinical Study Report SLO-AD-1 Final Version DATE: 09 December 2013
 1. Clinical Study Report RANDOMIZED, OPEN, PARALLEL GROUP, PHASE IIIB STUDY ON THE EVALUATION OF EFFICACY OF SPECIFIC SUBLINGUAL IMMUNOTHERAPY IN PAEDIATRIC PATIENTS WITH ATOPIC DERMATITIS, WITH OR WITHOUT
1. Clinical Study Report RANDOMIZED, OPEN, PARALLEL GROUP, PHASE IIIB STUDY ON THE EVALUATION OF EFFICACY OF SPECIFIC SUBLINGUAL IMMUNOTHERAPY IN PAEDIATRIC PATIENTS WITH ATOPIC DERMATITIS, WITH OR WITHOUT
ustekinumab (Stelara )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Quality of life in patients with alopecia areata attending dermatology department in a tertiary care centre - A cross-sectional study
 Original Article Quality of life in patients with alopecia areata attending dermatology department in a tertiary care centre - A cross-sectional study Fasalul Abideen, Anoop Thy Valappil, Pretty Mathew,
Original Article Quality of life in patients with alopecia areata attending dermatology department in a tertiary care centre - A cross-sectional study Fasalul Abideen, Anoop Thy Valappil, Pretty Mathew,
Evolving Guidelines of MPNs Where do new options fit in your treatment plan?
 Evolving Guidelines of MPNs Where do new options fit in your treatment plan? 34 th Chemotherapy Foundation Symposia Innovative Cancer Therapy for Tomorrow Ruben A. Mesa, MD, FACP Professor and Chair, Division
Evolving Guidelines of MPNs Where do new options fit in your treatment plan? 34 th Chemotherapy Foundation Symposia Innovative Cancer Therapy for Tomorrow Ruben A. Mesa, MD, FACP Professor and Chair, Division
USTEKINUMAB and BRIAKINUMAB
 USTEKINUMAB and BRIAKINUMAB Andrew Blauvelt, M.D. Professor, Dept. of Dermatology and Dept. of Molecular Microbiology & Immunology Oregon Health & Science University Chief, Dermatology Service Veteran
USTEKINUMAB and BRIAKINUMAB Andrew Blauvelt, M.D. Professor, Dept. of Dermatology and Dept. of Molecular Microbiology & Immunology Oregon Health & Science University Chief, Dermatology Service Veteran
Combining semantic triple stores across knowledge domains
 TITLE OF PRESENTATION Combining semantic triple stores across knowledge domains Matthew Clark (m.clark@elsevier.com), Frederik van den Broek, Anton Yuryev, Maria Shkrob, Sherri Matis-Mitchell, Timothy
TITLE OF PRESENTATION Combining semantic triple stores across knowledge domains Matthew Clark (m.clark@elsevier.com), Frederik van den Broek, Anton Yuryev, Maria Shkrob, Sherri Matis-Mitchell, Timothy
Bank of America Merrill Lynch 2016 Health Care Conference
 Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Guselkumab (plaque psoriasis)
 IQWiG Reports Commission No. A18-24 Guselkumab (plaque psoriasis) Addendum to Commission A17-60 1 Addendum Commission: A18-24 Version: 1.0 Status: 27 April 2018 1 Translation of addendum A18-24 Guselkumab
IQWiG Reports Commission No. A18-24 Guselkumab (plaque psoriasis) Addendum to Commission A17-60 1 Addendum Commission: A18-24 Version: 1.0 Status: 27 April 2018 1 Translation of addendum A18-24 Guselkumab
Tuberous Sclerosis Complex Research Program
 Tuberous Sclerosis Complex Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military,
Tuberous Sclerosis Complex Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military,
T. R. Golub, D. K. Slonim & Others 1999
 T. R. Golub, D. K. Slonim & Others 1999 Big Picture in 1999 The Need for Cancer Classification Cancer classification very important for advances in cancer treatment. Cancers of Identical grade can have
T. R. Golub, D. K. Slonim & Others 1999 Big Picture in 1999 The Need for Cancer Classification Cancer classification very important for advances in cancer treatment. Cancers of Identical grade can have
Alopecia Areata.(Author Abstract)(Disease/Disorder Overview): An Article From: Dermatology Nursing [HTML] [Digital] By Paul T. Martinelli;Philip R.
![Alopecia Areata.(Author Abstract)(Disease/Disorder Overview): An Article From: Dermatology Nursing [HTML] [Digital] By Paul T. Martinelli;Philip R. Alopecia Areata.(Author Abstract)(Disease/Disorder Overview): An Article From: Dermatology Nursing [HTML] [Digital] By Paul T. Martinelli;Philip R.](/thumbs/89/98607659.jpg) Alopecia Areata.(Author Abstract)(Disease/Disorder Overview): An Article From: Dermatology Nursing [HTML] [Digital] By Paul T. Martinelli;Philip R. Cohen;Keith E. Schulze;Bruce R. Nelson If you are searching
Alopecia Areata.(Author Abstract)(Disease/Disorder Overview): An Article From: Dermatology Nursing [HTML] [Digital] By Paul T. Martinelli;Philip R. Cohen;Keith E. Schulze;Bruce R. Nelson If you are searching
OVERALL CLINICAL BENEFIT
 noted that there are case reports of a rebound effect upon discontinuation of ruxolitinib (Tefferi 2012), although this was not observed in either the COMFORT I or COMFORT II studies. Therefore, perc considered
noted that there are case reports of a rebound effect upon discontinuation of ruxolitinib (Tefferi 2012), although this was not observed in either the COMFORT I or COMFORT II studies. Therefore, perc considered
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-14-1-0546 TITLE: Assessing EphA2 and Ephrin-A as Novel Diagnostic and Prognostic Biomarkers of Prostate Cancer PRINCIPAL INVESTIGATOR: Carvell Tran Nguyen, MD PhD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-14-1-0546 TITLE: Assessing EphA2 and Ephrin-A as Novel Diagnostic and Prognostic Biomarkers of Prostate Cancer PRINCIPAL INVESTIGATOR: Carvell Tran Nguyen, MD PhD CONTRACTING ORGANIZATION:
Prurisol: A New Small Molecule under investigation for the treatment of Psoriasis
 Prurisol: A New Small Molecule under investigation for the treatment of Psoriasis Krishna Menon, RCM, PhD, VMD, President of Research and Chief Scientific Officer MEDICAL DERMATOLOGY THERAPEUTIC R&D AND
Prurisol: A New Small Molecule under investigation for the treatment of Psoriasis Krishna Menon, RCM, PhD, VMD, President of Research and Chief Scientific Officer MEDICAL DERMATOLOGY THERAPEUTIC R&D AND
DISCLOSURES WHAT S NEW AND EXCITING FROM JAAD
 WHAT S NEW AND EXCITING FROM JAAD Bruce H. Thiers, MD, Editor, JAAD Professor, Medical University of South Carolina Department of Dermatology and Dermatologic Surgery DISCLOSURES PFIZER VALEANT EFFECT
WHAT S NEW AND EXCITING FROM JAAD Bruce H. Thiers, MD, Editor, JAAD Professor, Medical University of South Carolina Department of Dermatology and Dermatologic Surgery DISCLOSURES PFIZER VALEANT EFFECT
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata
 Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
Clinical Policy Title: Alopecia areata
 Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next Review Date:
Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next Review Date:
Association of vitamin D level with alopecia areata
 Original Article Association of vitamin D level with alopecia areata Soheila Nassiri, MD 1 Zahra Saffarian, MD 1 Shima Younespour 2 1. Skin Research Center, Shahid Beheshti University of Medical Sciences,
Original Article Association of vitamin D level with alopecia areata Soheila Nassiri, MD 1 Zahra Saffarian, MD 1 Shima Younespour 2 1. Skin Research Center, Shahid Beheshti University of Medical Sciences,
Should Intermediate-I risk PMF be transplanted immediately or later? Position: Later
 Should Intermediate-I risk PMF be transplanted immediately or later? Position: Later Vikas Gupta, MD, FRCP, FRCPath Associate Professor Department of Medicine Leukemia/BMT Programs Princess Margaret Cancer
Should Intermediate-I risk PMF be transplanted immediately or later? Position: Later Vikas Gupta, MD, FRCP, FRCPath Associate Professor Department of Medicine Leukemia/BMT Programs Princess Margaret Cancer
FDA SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) CLINICAL SECTION CHECKLIST OFFICE OF DEVICE EVALUATION
 FDA SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) CLINICAL SECTION CHECKLIST OFFICE OF DEVICE EVALUATION Note to FDA PMA Reviewers: The Summary of Safety and Effectiveness (SSED) is a document mandated
FDA SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) CLINICAL SECTION CHECKLIST OFFICE OF DEVICE EVALUATION Note to FDA PMA Reviewers: The Summary of Safety and Effectiveness (SSED) is a document mandated
Accelerate Your Research with Conversant Bio
 Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Sponsor. Novartis Pharmaceuticals Corporation Generic Drug Name. Agomelatine Therapeutic Area of Trial. Major depressive disorder Approved Indication
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
Immunotherapy for Breast Cancer. Aurelio B. Castrellon Medical Oncology Memorial Healthcare System
 Immunotherapy for Breast Cancer Aurelio B. Castrellon Medical Oncology Memorial Healthcare System Conflicts Research support : Cascadian therapeutics, Puma biotechnology, Odonate therapeutics, Pfizer,
Immunotherapy for Breast Cancer Aurelio B. Castrellon Medical Oncology Memorial Healthcare System Conflicts Research support : Cascadian therapeutics, Puma biotechnology, Odonate therapeutics, Pfizer,
(tofacitinib) are met.
 Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Janus kinase inhibitors for autoimmune disorders
 THERAY REVIEW Janus kinase inhibitors for autoimmune disorders STEVE CHALIN Three janus kinase () inhibitors baricitinib (Olumiant) and tofacitinib (Xeljanz) for rheumatoid arthritis, and ruxolitinib (Jakavi)
THERAY REVIEW Janus kinase inhibitors for autoimmune disorders STEVE CHALIN Three janus kinase () inhibitors baricitinib (Olumiant) and tofacitinib (Xeljanz) for rheumatoid arthritis, and ruxolitinib (Jakavi)
Assessment of the Tobacco Heating System (THS) 2.2, A Candidate Modified Risk Tobacco Product: From Concept to Early Clinical Data
 Assessment of the Tobacco Heating System (THS) 2.2, A Candidate Modified Risk Tobacco Product: From Concept to Early Clinical Data Global Forum on Nicotine 2015 Bruce D. Clark PhD Philip Morris International
Assessment of the Tobacco Heating System (THS) 2.2, A Candidate Modified Risk Tobacco Product: From Concept to Early Clinical Data Global Forum on Nicotine 2015 Bruce D. Clark PhD Philip Morris International
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
Centocor Ortho Biotech Services, LLC
 SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
Phase 1 Study of the Safety and Efficacy of MRG-106, a Synthetic Inhibitor of microrna-155, in CTCL Patients
 ASH Annual Meeting, December 11, 2017 Atlanta, Georgia, U.S.A. Phase 1 Study of the Safety and Efficacy of MRG-106, a Synthetic Inhibitor of microrna-155, in CTCL Patients Christiane Querfeld, Francine
ASH Annual Meeting, December 11, 2017 Atlanta, Georgia, U.S.A. Phase 1 Study of the Safety and Efficacy of MRG-106, a Synthetic Inhibitor of microrna-155, in CTCL Patients Christiane Querfeld, Francine
Association between serum interleukin-17a and clinical response to tofacitinib and etanercept in moderate to severe psoriasis
 Original article CED Clinical and Experimental Dermatology Association between serum interleukin-17a and clinical response to tofacitinib and etanercept in moderate to severe psoriasis L. Fitz, 1 W. Zhang,
Original article CED Clinical and Experimental Dermatology Association between serum interleukin-17a and clinical response to tofacitinib and etanercept in moderate to severe psoriasis L. Fitz, 1 W. Zhang,
Clinico-epidemiological study of alopecia areata
 Original Article Clinico-epidemiological study of alopecia areata Arun Achar, Sanjay K Rathi*, Leishiwon Kumrah**, Rabindranath Biswas, Samiran Bisai Department of Dermatology, NRS Medical College, Kolkata
Original Article Clinico-epidemiological study of alopecia areata Arun Achar, Sanjay K Rathi*, Leishiwon Kumrah**, Rabindranath Biswas, Samiran Bisai Department of Dermatology, NRS Medical College, Kolkata
University of Groningen. Hidradenitis suppurativa Dickinson-Blok, Janine Louise
 University of Groningen Hidradenitis suppurativa Dickinson-Blok, Janine Louise IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Hidradenitis suppurativa Dickinson-Blok, Janine Louise IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era
 Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
This was a multinational, multicenter study conducted at 14 sites in both the United States (US) and Europe (EU).
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
Autoimmune Diseases. Betsy Kirchner CNP The Cleveland Clinic
 Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
The MOVE Trial Summary. Palovarotene FAQs. 1. What is the MOVE Trial?
 MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
Title: Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles
 Author's response to reviews Title: Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles Authors: Julia Tchou (julia.tchou@uphs.upenn.edu) Andrew V Kossenkov (akossenkov@wistar.org)
Author's response to reviews Title: Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles Authors: Julia Tchou (julia.tchou@uphs.upenn.edu) Andrew V Kossenkov (akossenkov@wistar.org)
Supplemental Figure 1. Gating strategies for flow cytometry and intracellular cytokinestaining
 Supplemental Figure 1. Gating strategies for flow cytometry and intracellular cytokinestaining of PBMCs. Forward scatter area (FSC-A) versus side scatter area (SSC-A) was used to select lymphocytes followed
Supplemental Figure 1. Gating strategies for flow cytometry and intracellular cytokinestaining of PBMCs. Forward scatter area (FSC-A) versus side scatter area (SSC-A) was used to select lymphocytes followed
Comparison of Triple Negative Breast Cancer between Asian and Western Data Sets
 2010 IEEE International Conference on Bioinformatics and Biomedicine Workshops Comparison of Triple Negative Breast Cancer between Asian and Western Data Sets Lee H. Chen Bioinformatics and Biostatistics
2010 IEEE International Conference on Bioinformatics and Biomedicine Workshops Comparison of Triple Negative Breast Cancer between Asian and Western Data Sets Lee H. Chen Bioinformatics and Biostatistics
Etanercept for Treatment of Hidradenitis
 Home Search Browse Resources Help What's New About Purpose Etanercept for Treatment of Hidradenitis This study is currently recruiting patients. Sponsors and Collaborators: University of Pennsylvania Amgen
Home Search Browse Resources Help What's New About Purpose Etanercept for Treatment of Hidradenitis This study is currently recruiting patients. Sponsors and Collaborators: University of Pennsylvania Amgen
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
The Changing Landscape of Alopecia Areata: The Therapeutic Paradigm
 Adv Ther (2017) 34:1594 1609 DOI 10.1007/s12325-017-0542-7 REVIEW The Changing Landscape of Alopecia Areata: The Therapeutic Paradigm Yael Renert-Yuval. Emma Guttman-Yassky Received: February 20, 2017
Adv Ther (2017) 34:1594 1609 DOI 10.1007/s12325-017-0542-7 REVIEW The Changing Landscape of Alopecia Areata: The Therapeutic Paradigm Yael Renert-Yuval. Emma Guttman-Yassky Received: February 20, 2017
TITLE: MicroRNA in Prostate Cancer Racial Disparities and Aggressiveness
 AWARD NUMBER: W81XWH-13-1-0477 TITLE: MicroRNA in Prostate Cancer Racial Disparities and Aggressiveness PRINCIPAL INVESTIGATOR: Cathryn Bock CONTRACTING ORGANIZATION: Wayne State University REPORT DATE:
AWARD NUMBER: W81XWH-13-1-0477 TITLE: MicroRNA in Prostate Cancer Racial Disparities and Aggressiveness PRINCIPAL INVESTIGATOR: Cathryn Bock CONTRACTING ORGANIZATION: Wayne State University REPORT DATE:
Product: Omecamtiv Mecarbil Clinical Study Report: Date: 02 April 2014 Page 1
 Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
