Cutaneous T cell lymphoma: update on treatment
|
|
|
- Antony Sullivan
- 6 years ago
- Views:
Transcription
1 Review Cutaneous T cell lymphoma: update on treatment Uwe Wollina, MD Department of Dermatology, Dresden- Friedrichstadt Hospital, Dresden, Germany Correspondence Uwe Wollina, MD Department of Dermatology Krankenhaus Dresden-Friedrichstadt Friedrichstrasse Dresden Germany wollina-uw@khdf.de Funding: None. Conflicts of interest: None. Abstract Background Cutaneous T-cell lymphoma (CTCL) is a myeloproliferative disease with pronounced epidermotropism. The major subtypes of CTCL are mycosis fungoides and Sézary syndrome. Survival is dependent on the histological subtype and clinical stage. Early CTCL has a normal life expectancy, therefore early disease recognition and stage adapted treatment might help to ensure a good prognosis. Methods This is a review of recent advances in CTCL treatment based on literature review. Results Skin targeted therapies are useful for patch and limited plaque disease with phototherapy as the cornerstone of such treatments. More advanced disease will benefit from systemic mono- or combined treatments including drug therapy, extracorporeal photopheresis, and radiotherapy. In practice combined treatments may reduce adverse events and improve response rates. For selected younger patients, stem cell transplantation seems a third-line option. Conclusions The therapeutic spectrum for CTCL has been advanced during the last years, providing the opportunity of tailored treatment for patients. Introduction Cutaneous T cell lymphoma (CTCL) refers to a heterogeneous group of lymphoproliferative disorders characterized by the clonal accumulation of neoplastic T lymphocytes in the skin. 1 The World Health Organization and European Organization for Research and Treatment of Cancer (WHO EORTC) classification of cutaneous lymphomas with primary cutaneous manifestations lists 13 entities (Table 1). The most common subtypes are mycosis fungoides (MF), Sézary syndrome (SS), primary cutaneous anaplastic large cell lymphoma, and lymphomatoid papulosis; these account for approximately 95% of CTCL cases. 2 Because of the expression of homing factors, such as the cutaneous lymphocyte antigen and chemokine receptor (e.g. CXCR), early clinical stages of CTCL are present within the epidermis, with infiltrates particularly along the basal layer and adjacent to Langerhans cells. As the disease progresses, neoplastic cells are detected within dermal infiltrates in increasing numbers, with a consequent loss of epidermotropism. 3 Cutaneous T cell lymphoma has significant negative effects on health-related quality of life (HRQL) in various ways in both the psychological and physical dimensions. 4,5 Diagnosis is based on medical history, clinical examination, and cutaneous histopathology, including immunohistology (Tables 2 and 3). Molecular biology diagnostic techniques are available but are not used routinely in most parts of the world. 1,2 Epidemiology In the years , the overall annual age-adjusted incidence of CTCL in the USA was 6.4 per million persons. Annual incidence increased by )6 per decade. Incidences were higher among African-Americans ( )6 ) than among people of White European extraction ( )6 ) and higher among men ( )6 ) than among women ( )6 ). 5,6 During , the population-based incidence rate reached )6. 7 The most frequent presentation of CTCL is MF, which accounts for about 50% of cases. However, the stage of disease at which a patient first presents with cutaneous lymphomas varies. For example, 80% of patients with MF in Germany present in the early stages (I IIa) of disease, whereas 34% of patients in the USA have reached the tumor stage or have organ involvement at presentation. 8 Substantial geographic variations in incidence have been found. Incidence is correlated with high physician density, high family income, and higher social setting ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
2 1020 Review Cutaneous T cell lymphoma: treatment update Wollina Table 1 World Health Organization and European Organization for Research and Treatment of Cancer classification for primary cutaneous lymphomas and associated frequency and 5-year survival 2 The continuing rise in the incidence of CTCL is substantial, and its cause is unknown. 6,7 A recent study from Kuwait reported an incidence of 0.43 patients per 100,000 inhabitants/year. By contrast with data reported from the Western world, mean age at onset was 35.2 years, and 16% of patients were in their first or second decades of life. 8 Major clinical Subtypes Frequency, % Cutaneous T cell and NK cell lymphomas Indolent mycosis fungoides Follicular mycosis fungoides 4 80 Pagetoid reticulosis <1 100 Granulomatous slack skin <1 100 CD30+ lymphoproliferative diseases Anaplastic large cell 8 95 lymphoma Lymphomatoid papulosis Subcutaneous 1 82 panniculitis-like T cell lymphoma CD4+ small/medium 2 72 pleomorphic T cell lymphoma Aggressive diseases Sézary syndrome 3 24 Cutaneous aggressive <1 18 CD8+ T cell lymphoma Cutaneous c/d T cell <1 lymphoma Cutaneous peripheral T cell 2 16 lymphoma, unspecified Cutaneous NK/T cell lymphoma, nasal-type <1 NK, natural killer. 5-year survival, % Mycosis fungoides represents the most common type of CTCL, accounting for 50% of cases and demonstrating a male predominance of approximately 2 : 1. 1,9 It is typically a chronic disease that progresses slowly and has an indolent evolution. The disease is characterized by the development of patches, plaques, or tumors (Figs. 1 and 2). Between the patches lie islands of uninvolved skin. Patches are highly variable and can give rise to or coexist with plaques, which are raised, palpable skin lesions (Fig. 3). Tumors correlate with deeper infiltration of malignant CD4+ T helper cells into the skin. Tumors are thicker and Table 2 Tumor node metastasis blood (TNMB) classification in mycosis fungoides and Sézary syndrome 10 T (skin) T1 T2 T3 T4 N (nodes) N0 N1 N2 N3 Nx M (viscera) M0 M1 B (blood) B0 B1 B2 1a 1b 2a 2b 1a 1b 2a 2b 0a 0b BSA, body surface area. Limited patch/papules/plaque (<10% of BSA) Patches Patches and plaques Generalized patch/plaque (>10% of BSA) Patches Patches and plaques One or more tumors (diameter 1 cm) Confluence of erythroderma covering >80% of BSA No clinically abnormal peripheral lymph nodes, no biopsy required Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 1 or NCI LN 0 2 Clone negative Clone positive Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 2 or NCI LN 3 Clone negative Clone positive Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 3 4 or NCI LN 4, clone negative or positive Clinically abnormal peripheral lymph nodes, not confirmed by histopathology No visceral organ involvement Visceral organ involvement (organ specified and confirmed by histopathology) Absence of significant involvement: <5% Sézary cells Clone negative Clone positive Low blood burden: >5% Sézary cells, but does not meet criteria for B2 High blood burden: 1000 Sézary cells/ll, clone positive deeper than plaques and may necrotize and ulcerate, leading to opportunistic infections 1 (Figs. 4 and 5). Sézary syndrome accounts for approximately 3 5% of all newly reported cases of CTCL. The disease is characterized by circulating, atypical, malignant T lymphocytes with cerebriform nuclei (Sézary cells), erythroderma and, often, lymphadenopathy. Severe pruritus, ectropion, alopecia, and palmoplantar keratoderma (Fig. 2) are common associated features. Patients with CTCL may eventually develop visceral organ involvement. Staging is based on the TNMB (tumor node metastasis blood) system, which takes into account the extent of skin involvement, the presence of lymph node or visceral dis- International Journal of Dermatology 2012, 51, ª 2012 The International Society of Dermatology
3 Wollina Cutaneous T cell lymphoma: treatment update Review 1021 Table 3 Classification of stages in mycosis fungoides and Sézary syndrome10 Stage Tumor Node Metastasis Blood Ia Ib IIa IIb IIIa IIIb IVa IVa IVb Figure 3 Mixed patch and plaque on the breast in mycosis fungoides Figure 1 Eczematoid patch in early-stage mycosis fungoides Figure 2 Hyperkeratotic plaque in Sézary syndrome ease, and the detection of Sézary cells in the peripheral blood. 10 Although patients with limited patch and plaque disease of <10% of body surface area (BSA) (T1) have a Figure 4 Tumor-stage mycosis fungoides with necrotic ulcerations normal life expectancy, patients who undergo transformation to large cell lymphoma (8 23% of patients) have a poor prognosis with a mean survival of 2 19 months. 11 ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
4 1022 Review Cutaneous T cell lymphoma: treatment update Wollina Table 4 Mean complete response rates in early and advanced cutaneous T cell lymphoma (CTCL) Complete response rates, % Treatment Early CTCL Advanced CTCL Figure 5 Intraoral spread of advanced mycosis fungoides A 31-year, retrospective analysis of 297 patients with MF and SS studied long-term outcomes and identified clinical predictors of outcome in patients with advancedstage disease (n = 92) and large cell transformation (n = 22). The median overall survival (OS) in advancedstage disease was five years. The median OS from diagnosis of large cell transformation was two years. 12,13 Therefore, large cell transformation represents a more serious constellation than advanced-stage disease. Whereas patients in early-stage MF have a normal life expectancy, five-year survival has been estimated at 70% in stage IIa, 48% in stages IIb IIIb, and 36% in stage IV. 13,14 New biomarkers of CTCL were detected in peripheral blood lymphocytes and sera. The a-defensin HNP3 showed sensitivity and specificity for the prediction of CTCL of 96%. 15 A protein set that distinguishes MF patients from healthy controls with a sensitivity of 82.6% and a specificity of 100% was identified (i.e. transthyretin [TTR] and three TTR modifications). Transthyretin can be used as a screening marker. 16 Complications of CTCL One unique feature of CTCL is that the malignant T cell clone expands at the expense of normal T cells, creating an immunodeficiency in numbers of cells A clinically relevant immune failure develops. Patients with CTCL have increased risk for second malignancies, 20 succumb to bacterial infections and septicemia, 21 and suffer from persistent viral infections. 22 These features can be described as immune collapse. The unique immunocompromise that plays such a pivotal role in the demise of patients with CTCL is a target for therapy. Narrowband ultraviolet-b light c. 60 therapy Psoralen + ultraviolet-a light 80 therapy Extracorporeal photopheresis 70 c. 20 Interferon-a Interferon-a + extracorporeal photopheresis Retinoids (classical) c. 30 c. 30 Retinoid treatment with 70 c. 30 psoralen + UVA light therapy Bexarotene (oral) c. 60 c. 60 Gemcitabine 22 Pegylated liposomal encapsulated doxorubicin (Peg-Doxo) Staging and Classification of CTCL (MF and SS) The International Society for Cutaneous Lymphomas (ISCL) and the EORTC Cutaneous Lymphoma Task Force have suggested a revised staging and classification system for both MF and SS (Tables 2 and 3). 10 Treatment of CTCL (with more stage IV patients 6.1%) Alemtuzumab 50 Zanolimumab 10 Histone-acetylase inhibitors 5 Denileukin diftitox 10 Therapeutic choices are based on whether the patient has early (stages Ia IIa) or late (stages IIb IVb) disease. Treatment must protect immune function to prevent immune collapse. This lesson was learned when the initial response to early aggressive treatment protocols was accompanied by rapid relapse and treatment-related morbidity and mortality. 23 Although lesions of patients with early MF are generally well controlled by topical skin-directed therapies or phototherapy, extensive skin or blood involvement, tumors and nodal disease require systemic therapy with biologic response modifiers, skin radiation, and chemotherapy. Often a combination of either sequential or concomitant therapies gives a higher rate of response, but patients with advanced disease often relapse, and curative therapy remains elusive 23 (Table 4). International Journal of Dermatology 2012, 51, ª 2012 The International Society of Dermatology
5 Wollina Cutaneous T cell lymphoma: treatment update Review 1023 Skin-directed therapy Steroids Topical corticosteroids are frequently prescribed to induce the clearing of skin lesions in patients with limited patches (stage Ia). In an investigatory trial, 79 patients were treated daily with topical class I III steroids. Thirtytwo (63%) stage T1 patients and seven (25%) stage T2 patients achieved complete clearance. The duration of response depends on the extent of disease and the thickness of the targeted lesion. 24 In practice, the administration of class II III topical corticosteroids is often combined with phototherapy. Nitrogen mustard Nitrogen mustard (mechlorethamine) has been widely used as a first-line treatment of early-stage MF in many countries. It may be applied locally or to the entire skin once daily. Many investigators have demonstrated the efficacy of topical nitrogen mustard at concentrations of % in an aqueous or ointment base and have reported complete response rates of up to 72% in earlystage MF patients and occasional long-term remissions of >8 years. Skin clearance may require 6 months and is followed by maintenance therapy; however, there is no evidence that prolonged maintenance is beneficial. The most common side effects were irritant contact dermatitis and hypersensitivity reaction. 25 Carmustine Another type of topical chemotherapy that has been employed for MF is the nitrosourea alkylating agent, carmustine (1,3-bis[2-chloroethyl)-I-nitrosourea [BCNU]). Clearance rates in 51 (84%) T1 and 38 (37%) T2 patients appeared to be similar to those achieved with nitrogen mustard. Patients almost always develop erythema at the site of application. Although irritant and/or allergic reactions occur less frequently, the cutaneous toxicity of carmustine may be greater than that of nitrogen mustard; progressing telangiectasias may develop, and bone marrow suppression has been reported to result from systemic absorption. 26 Retinoids Bexarotene 1% gel (Targretin Ò ; Cephalon, Munich, Germany) has been approved by the US Food and Drug Administration (FDA) for early-stage MF. The potential for skin irritation makes it unlikely that bexarotene gel will be used in patients with skin involvement of >10% of BSA. Bexarotene gel is applied sparingly to patches or plaques and is most effective and best tolerated when used twice daily. Topical bexarotene has been evaluated in a dose-escalating trial with concentrations ranging from 0.1% to 1.0%. Median treatment duration with bexarotene gel was 10.5 months. The trial reported a complete response rate of 21%, an overall response rate (ORR) of 63%, and a 75% response rate in treatmentnaive patients. The median duration of remission was 24 months. 27 Phototherapy Phototherapy is one of the mainstay treatment modalities in CTCL. Of the available phototherapies, psoralen plus ultraviolet (UV)-A light therapy (PUVA) with orally administered 8-methoxypsoralen (8-MOP), which sensitizes the skin to UVA irradiation, is a cornerstone of treatment in early-stage disease. The 8-MOP may also be added to either a medical bath (bath-puva) or a topical ointment (cream-puva). Therapy is typically given three times per week until complete remission is achieved. Several studies have confirmed high remission rates in earlystage MF and have reported complete response in up to 71% of patients. 28 Although it is effective in clearing superficial lesions, PUVA is limited in its ability to resolve deeply infiltrated lesions. Disease-free survival rates at 5 and 10 years have been reported to be 56% and 30%, respectively, in stage Ia disease, and 74% and 50%, respectively, in stages Ib and IIa. Relapse status, however, has no effect on long-term outcome. The 15-year survival rates were 82% (Ia) and 58% (Ib/IIa). 29 The most commonly reported acute adverse effects were erythema, pruritus, and nausea, which were usually mild at presentation and generally manageable with dose adjustments of UVA, 8-MOP, or dose interruptions. Gastrointestinal adverse effects were seen only when 8- MOP was given orally. Long-term exposure was associated with an increased risk for chronic photodamage and non-melanoma skin cancer in about one-third of patients. 29 The efficacy of broadband UVB tends to be limited to the patch stage, whereas PUVA is also effective in clearing plaques. The effects of UVB phototherapy were retrospectively evaluated in 37 patients with MF limited to patch/plaque disease. A total of 71% achieved a complete response in a median duration of 22 months. However, 83% of patients with disease limited to patches achieved remission, whereas none of the patients with plaque disease did so. 30 Narrowband (NB) UVB is considered to be less carcinogenic and may offer an alternative treatment option in early-stage MF. Reported response rates in MF stage Ia are 50% in patients with skin types I and II, which is almost twice as high as for broadband UVB. Less favorable results are found in patients with darker skin types. ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
6 1024 Review Cutaneous T cell lymphoma: treatment update Wollina A meta-analysis suggested that in responders to UVB phototherapy, maintenance therapy seems to be superior to intermittent treatment. 31 The situation is somewhat different for PUVA. A recent trial involving 40 patients with early-stage CTCL (stages Ia and Ib) and follow-up of 28 months concluded that PUVA maintenance does not prevent future relapse. 32 Because of its possible longterm adverse effects and the absence of benefit to patients, PUVA maintenance cannot be recommended. An open trial in early-stage CTCL included 95 patients treated with PUVA (83%) and 19 treated with NB-UVB (17%). With PUVA, 59 patients (62%) achieved a complete response and 24 (25%) a partial response. Narrowband UVB led to a complete response in 12 (63.2%) patients and a partial response in five (26%). There were no differences in terms of time to relapse between patients treated with PUVA and those treated with NB- UVB. No major adverse reactions were attributed to the treatment. These results confirm that phototherapy is a safe, effective, and well-tolerated first-line therapy in patients with early-stage CTCL and can achieve prolonged disease-free remission. These findings also suggest that NB-UVB is at least as effective as PUVA in the treatment of early-stage MF. 28,33 New developments in skin-targeted phototherapy include the excimer laser and the use of excimer light. Excimer light and laser are used for unilesional and palmoplantar MF. 19,34,35 In the two largest case series, a total of 19 MF stage Ia and Ib patients were treated by either 308-nm laser (n = 10) or 308-nm (n = 9) excimer light, both of which achieved response rates of 100%. 36,37 No comparative trials have yet been published. Photodynamic therapy Photodynamic therapy (PDT) involves the exposure of tumor cells to a photosensitizing drug and to subsequent irradiation with light. Thus far, PDT has much in common with PUVA but usually targets only limited parts of the body. The photosensitizers used in PDT differ from those applied in PUVA and are used mostly topically and at wavelengths that differ from those in UVA. The most important topical photosensitizers in PDT are 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL). The treatment is of particular value in unilesional MF. A recent review analyzed outcomes in 12 patients with unilateral MF treated with MAL/ALA PDT. The treatment showed a high local clearance rate (20 of 27 treated lesions). 38 In a Phase II placebo-controlled trial, topical hypericin was used with visible light (8 20 J/cm 2 ) twice daily for six weeks. This resulted in a significant improvement in targeted lesions without severe adverse effects. 39 Recent preclinical studies have investigated other compounds for use in the treatment of CTCL. Photodynamic therapy with phthalocyanine Pc4 was shown to induce apoptosis in malignant T cells in vitro. Its peak absorbance is within the red light spectrum at 675 nm, and it is an effective generator of reactive oxygen species when irradiated. 40 Radiation Patients with localized tumors (stage IIb) may require systemic treatment, but most respond very well to localized orthovoltage radiation. Total skin electron beam treatment (TSEBT) may be used in patients with more disseminated cutaneous disease. In TSEBT, ionizing radiation is administered to the entire skin surface, penetrating to the dermis. The standard total dose is 36 Gy delivered with electrons of 4 MeV fractionated over 8 10 weeks. Reported rates of complete remission range from 40% to 98% among patients in stages T1 and T2; however, relapse rates are high when the treatment is used as the sole modality. 41 Nearly all patients develop skin-related side effects, including erythema, telangiectasia, xerosis, nail dystrophy, and/or reversible alopecia. In a more recent trial, 18 patients with advanced and therapy-refractory CTCL in stages IIb IV were treated with TSEBT for the first time. Median daily fractions of 1 Gy were administered up to a median total dose of 25 Gy. The median follow-up period was 11 months. Nine patients (50%) achieved a complete and seven (39%) a limited response. The actuarial one-year progression-free survival was 24%. Four patients (22%) had continuing remission over a median period of six months. The median OS after TSEBT was 12 months, resulting in an actuarial one-year OS of 48%. Treatmentrelated acute effects (grades 1 and 2) were observed in all patients during radiation therapy. Transient grade 3 epitheliolyses developed in five patients (28%), late skin effects (grades 1 and 2) in 16 patients (89%), and hypohidrosis in six patients (33%). 42 Some patients, however, may suffer from delayed chronic skin damage with non-healing ulcerations and poikiloderma that markedly reduces their HRQL and increases the cost of treatment-related complications. 43 Systemic therapy Drug therapy Interferon. Interferon-a (INF-a) is one of the most widely used firstline treatments and probably the most effective single agent in the treatment of CTCL. The available subtypes, International Journal of Dermatology 2012, 51, ª 2012 The International Society of Dermatology
7 Wollina Cutaneous T cell lymphoma: treatment update Review 1025 INF-a-2a (Roferon-A Ò ; F. Hoffmann-La Roche AG, Basel, Switzerland) and INF-a-2b (Intron-A Ò ; Essex Pharma AG, Munich, Germany), do not differ in their activity and have shown a wide range of biologic effects, including antiviral, antiproliferative, and immunomodulatory actions. Overall response rates range from 29% to 74% of patients, with median durations of 4 42 months. 44 The higher response rates and durations occurred in early-stage MF patients who had not received previous therapy. IFN-a is used as a first-line therapy in stage IIb III disease and as a second-line treatment in early disease. Interferon-a is generally given as long-term therapy, although its optimal dose and duration in CTCL have not been established. Various dosages and treatment schedules have been used. Therapy should be initiated at low doses of 1 3 million units (MU) three times weekly and should be gradually escalated to 9 12 MU daily or as tolerated. Combination therapy with IFN-a and PUVA resulted in very high response rates (ORR 92%, complete response 62 70%) and was superior to other combinations with retinoids or extracorporeal photopheresis (ECP). 45,46 Initially, almost all patients develop temporary flu-like symptoms. Chronic side effects can include anorexia, fatigue, depression, alopecia, cytopenia, and impaired liver function. 45 Retinoids Retinoids are vitamin A derivatives that have important effects on cell growth, terminal differentiation, and apoptosis; benefits have been reported in several studies. As they are strongly teratogenic, conception control before, during, and after treatment is necessary in all female patients of child-bearing age. Overall response rates to retinoids (all-trans retinoic acid, 13-cis-retinoic acid, and the synthetic analogs isotretinoin, etretinate, and acitretin) ranged from 44% to 67% and complete response rates from 21% to 35%, with a median response duration of around eight months. Common side effects included skin and mucous membrane dryness. 47 The combination of retinoid treatment with PUVA (RePUVA) achieved a complete response rate of 38% after 48 weeks in a controlled trial. 46 Bexarotene, a new retinoid X receptor (RXR)-selective retinoid, has been approved for the treatment of relapsed or refractory CTCL in early and advanced stages. The approval of bexarotene capsules was based on outcomes in two multicenter, open-label Phase II and III clinical trials involving 152 CTCL patients with early- or advancedstage disease who had failed or were refractory to two or more standard therapies. 48,49 In the early stage trial, 58 patients randomized to doses of 6.5 mg/m 2, 300 mg/m 2, or 650 mg/m 2 daily achieved response rates (defined as a >50% improvement in skin lesions) of 20%, 54%, and 67%, respectively. Median time to response was eight weeks. 48 In the advanced stage trial, response rates of 45% and 55% were observed in 94 patients randomized to daily doses of 300 mg/m 2 or 650 mg/m 2, respectively, giving an ORR of 48%. However, only six of the 94 (6%) patients achieved a complete response. The median duration of response was 10 months. 49 Retrospectively collected comparison data suggest that there may be little difference in efficacy between the RXR-selective retinoid bexarotene and the retinoic acid receptor (RAR)-specific retinoid all-trans retinoic acid. 50 The most common significant side effects experienced in trials in both early- and advanced-stage disease were headaches, hypertriglyceridemia, hypercholesterolemia, central hypothyroidism, and leukopenia; these required additional treatment with lipid-lowering agents such as statins, HMG-CoA reductase inhibitors or fenofibrates, and thyroid hormone replacement. The concomitant use of gemfibrozil (Gemcor Ò ; Upsher-Smith Laboratories, Maple Grove, MN, USA) is contraindicated as the latter increases plasma concentration of bexarotene and triglyceride levels, possibly related to cytochrome P450 3A4 isoenzyme inhibition. In addition, bexarotene is a direct inhibitor of clotting factors IX and X. 51 A combined regimen with PUVA may not increase response rates but has the advantage of decreasing the dosage requirements for both PUVA and bexarotene. 50 Outcomes of one trial have suggested that combined IFNa/bexarotene therapy does not significantly increase response rates. 53 Other modalities that might be combined with bexarotene include ECP and NB-UVB Chemotherapy Single-agent and combination chemotherapies in advanced, refractory, and aggressive forms of CTCL have been associated with high response rates but shortlived durations. Their use is limited to the palliation of symptoms. Options involve single-agent or multi-agent chemotherapy including with steroids, methotrexate, chlorambucil (Leukeran Ò ; Stada Arzneimittel GmbH, Bad Vilbel, Germany), vincristine (Oncovin Ò ; Alkopharma SA, Martigny, Switzerland), doxorubicin (Adriamycin Ò ; Pharmacia SpA, Milan, Italy), pegylated doxorubicin (Doxil Ò [Alza Pharmaceuticals Inc., Mountain View, CA, USA]; CaelyxÔ [Essex Pharma GmbH, Munich, Germany]), cyclophosphamide (Cytoxan Ò ; Bristol-Myers Squibb - Mead Johnson & Co, Somerville, NJ, USA), etoposide (Vepesid Ò ; Bristol-Myers Squibb, New York, NY, USA), gemcitabine (Gemzar Ò ; Eli Lilly & Co., Florence, Italy), nucleoside analogs, and alkylators. Combination regimens include cyclophospha- ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
8 1026 Review Cutaneous T cell lymphoma: treatment update Wollina mide, doxorubicin, vincristine, and prednisone (CHOP) or cis-platinum, vincristine, and prednisolone (CVP) therapy. 18,56 A selection of chemotherapies will be discussed in more detail. Gemcitabine (2,2-difluorodeoxycytidine) is a pyrimidine antimetabolite with structural similarities to cytarabine; however, its pharmacology and mechanism of action differ from those of other pyrimidine analogs in several respects. Gemcitabine serves as a better transport substrate for uptake into cells, is phosphorylated more efficiently to the active gemcitabine triphosphate, inhibits elongation of the DNA chain through a mechanism termed masked chain termination, and competitively inhibits ribonucleotide reductase. The result is impaired DNA synthesis and the induction of apoptosis. 57 Gemcitabine (Gemzar Ò ) has been demonstrated to be active in heavily pretreated patients with Hodgkin s disease, as well as in those with aggressive and indolent non-hodgkin s lymphoma. 58,59 In a Phase II trial in T3 and T4 (N0, M0) CTCL patients, monotherapy with gemcitabine achieved an ORR of 75% and a complete response rate of 22%. 59 Recently, a low-dose gemcitabine regimen of 250 mg/m 2 was suggested as an alternative to standard dosages in tumor-stage MF, but no data from controlled trials are yet available. 60 Doxorubicin is an anthracyclin with antiproliferative activity in a broad range of non-hodgkin s lymphomas. The treatment is limited by severe cardiotoxicity when the cumulative dosage reaches 400 mg/m 2. Pegylated liposomal encapsulated doxorubicin (Peg-Doxo [CaelyxÔ or Doxil Ò ]) circumvents this limitation by utilizing a different method of distributing the compound and favorable pharmacokinetics. 61 The first published trial with Peg-Doxo included six patients with relapsing or recalcitrant CTCL of the MF type (stages Ib IIb). The patients were treated with Peg- Doxo at a dosage of 20 mg/m 2 once per month, a low dosage that has been used for HIV-related Kaposi s sarcoma. The best outcome was complete response in four patients and partial response in two. The final outcome was complete response in four, partial response in one, and progressive disease in one patient (ORR 83%). The responders showed a decrease in lymphocytic infiltrates and activated T lymphocytes in skin biopsy specimens. 62 Further investigations also demonstrated selective changes in glycoconjugates such as galectins. 63 Side effects were seen temporarily, ranging from grades 0 to 3 in severity. The most frequent side effects were mild anemia and lymphopenia. No additional therapy for side effects was required. 62 A retrospective, multicenter study evaluated 34 patients, of whom 27 received Peg-Doxo 20 mg/m 2, five received Peg-Doxo mg/m 2, and two received Peg- Doxo 40 mg/m 2. Peg-Doxo was administered IV every two weeks in six patients, every 2 3 weeks in four patients, and every four weeks in 23 patients. Thirty-four patients received at least one cycle of Peg-Doxo. Fifteen patients achieved a complete response and 15 achieved a partial response, resulting in an ORR of 88%. Overall survival was 17.8 ± 10.5 months (n = 33 patients), eventfree survival was 12.0 ± 9.5 months, and disease-free survival was 13.3 ± 10.5 months (n = 16 patients). Adverse events were seen in 14 of 34 patients (41%). Only six patients had adverse events of grades 3 or A prospective Phase II trial enrolled 19 patients with stage I IV disease. Peg-Doxo was given IV at a dosage of 20 mg/m 2 once per month. Overall and complete response rates of 84% and 42%, respectively, (with no significant differences between stage I IIa and IIb IV patients) and grade III/IV toxicity of 11% were observed. After a maximum follow-up of 46 months, median OS was 34 months at a rate of 44%. 65 In an open multicenter trial, 25 patients with either stage II IV CTCL that had been previously treated unsuccessfully with at least two lines of treatment, or histologically transformed epidermotropic CTCL requiring chemotherapy, were given IV Peg-Doxo once every four weeks at a dose of 40 mg/m 2. At the end of treatment, an objective response was seen in 56% of the patients: five achieved complete and nine achieved partial response. The median length of OS was 43.7 months. In the 14 patients who experienced an objective response, median progression-free survival after the end of treatment was five months. Responses were observed in two subpopulations of patients in whom the prognosis was known to be poorer, comprising patients with SS (ORR 60%) and patients with transformed CTCL (ORR 50%). In addition, this study showed that escalating the dose to 40 mg/m 2 did not seem to improve its effectiveness but did increase toxic effects (especially hematologic toxic effects) compared with the previously tested dose of 20 mg/m Recently, the EORTC Phase II trial of liposomal doxorubicine (CaelyxÔ) was presented at the 52nd Meeting of the American Society of Hematology. The trial involved 49 patients with advanced CTCL (MF type) in stages IIb, IVa, and IVb. The percentage of patients with stage IV disease was higher in this trial than in previous studies. After a mean of five treatment cycles using doses of 20 mg/m 2, the ORR was 41% and complete response rate was 6%. The drug was well tolerated with a maximum of 6% of patients experiencing grade 3 and 4 toxicities. 67 Liposomal daunorubicin was investigated in a pilot trial in three patients with advanced CTCL. The response rate was 100%; one patient achieved a complete response. 68 International Journal of Dermatology 2012, 51, ª 2012 The International Society of Dermatology
9 Wollina Cutaneous T cell lymphoma: treatment update Review 1027 Extracorporeal photopheresis Extracorporeal photopheresis was approved by the FDA for the palliative treatment of patients with CTCL. Circulating mononuclear cells are separated by a leukapheresisbased method, mixed with 8-MOP (UVADEXÔ; J & J Medical Therakos, Exton, PA, USA), exposed to UVA light (1 2 J/cm 2 ) that activates the 8-MOP, thereby causing cross-linking of DNA, and re-infused to the patient. One suggested mechanism of action is that the induction of apoptosis in circulating malignant T lymphocytes and the subsequent release of tumor antigens lead to a systemic antitumor response against the malignant T cell clone, among other effects. Extracorporeal photopheresis can also generate human dendritic cells from monocytes. 69,70 Treatment is empirically given on two consecutive days every days. It usually takes several months for improvement to become evident, but the treatment is of particular benefit in both erythrodermic MF and SS with circulating neoplastic T cells. The first multicenter trial of ECP in CTCL achieved an ORR of 73%. 71 Of the responding patients, 24 had exfoliative erythroderma. The mean time to response was 22 weeks. The authors suggested that optimal candidates for ECP are patients with SS of <2 years duration, a modest tumor burden and circulating neoplastic cells, and almost normal counts of circulating CD8+ T cells and natural killer (NK) cells. Prolonged response rates have been reported for ECP combined with INF-a, bexarotene, granulocyte colony-stimulating factor (G-CSF), or TSEBT Few adverse events related to ECP treatment have been reported; those that have include catheter-related infection and hypotension caused by volume shifts. A literature search analyzed 124 early-stage patients treated with ECP or ECP plus adjuvant therapy during in 16 different reports. Response rates in this patient population to treatment with ECP and ECP plus adjuvant therapy varied from 33% to 88%. 75 A metaanalysis of 562 CTCL patients treated with ECP found an ORR of 62% and a complete response rate of 23%. Of the cases published, only 83 patients were treated solely by ECP; the majority received adjuvant interferon or other treatments. 76 A retrospective analysis of our own data suggested that responders to ECP show a prolonged OS of 29 months. 77 This supports the proposal that ECP may affect a vaccination-like restoration of immune surveillance. In a prospective trial, 14 patients with CTCL stage IIa or IIb were treated twice per month for six months with ECP in combination with subcutaneous IFN-a-2a three times per week at the maximal tolerable dosage ( U). After six months, the best outcome was complete response in four patients, partial response in three, and the achievement of stable disease in seven of 14 patients (ORR 50%). In responders, the time to best response was 4.3 ± 1.4 months. In patients with stage IIa disease, the response rate was 60%, whereas that in patients with stage IIb disease was only 25%. Transient side effects of grades 0 3 were seen. There was no need for additional therapy, but the IFN-a dose was decreased because of side effects. After one year of follow-up, the ORR was 46% (six of 13 patients), accounted for by five of nine patients (56%) with stage IIa disease and one of four (25%) with stage IIb disease. 78 This study suggests that stable responses can be achieved in patients with early CTCL. Future and emerging therapies Skin-directed therapies Toll-like receptor antagonists Toll-like receptor (TLR) antagonists, such as imiquimod (Aldara Ò ; Laboratoires 3M Santé, Pithiviers, France) and CpG-7909, enhance immune responses via the activation of TLR-7, -8, and/or -9. Imiquimod is a topically active imidazoquinoline immunomodulating agent. It works as an indirect antiviral and antitumor agent and stimulates the production of INF-a and various other cytokines. Imiquimod has been used to treat limited plaques in several case reports. 79 Local irritation and inflammation are the most frequent adverse effects. Controlled trials have not yet been performed. 79 Limited clinical evidence suggests that a synergic effect of imiquimod and systemic INF-a-2a treatment may improve clinical response. 80 Phosphocholines Miltefosine is an ether lipid which represents the first agent in its class and targets the membrane of tumor cells. It is an alkylphosphocholine with a long-chain fatty acid-like backbone which mimics normal membrane phospholipids and is thought to interfere in signal transduction pathways such as the phospholipase/protein kinase C pathway. 81 In a recent study, 6% miltefosine (ASTA Medica GmbH, Frankfurt, Germany) was applied to lesions as well as to a 3-cm margin around each lesion. Miltefosine was applied once daily during the first week and then, if no intolerable skin reactions were observed, twice daily during the next seven weeks. After eight weeks of topical application of miltefosine, five patients were considered to have demonstrated a complete and two patients a partial response, and five patients had achieved stable disease. The ORR was 58%. Over a median follow-up of 26 months, two of the complete responders and one patient in partial response progressed; their median length of freedom from progression was 12 months. Three patients (25%) had responses lasting years. 82 ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
10 1028 Review Cutaneous T cell lymphoma: treatment update Wollina Systemic therapies Chemotherapies Forodesine (BCX-1777, immucillin H, 1-[9-deazahypoxanthine]-1,4-dideoxy-1,4-imino-D-ribitol) has carboncarbon linkage between a cyclic amine moiety that replaces ribose and 9-deaza-hypixanthine. The drug is a novel T cell selective immunosuppressive agent which, in the presence of 2-deoxyguanosine (dguo), inhibits human lymphocyte proliferation. Its effect appears to be linked to increased 2-deoxyguanosine levels in plasma which, in turn, is converted to 2-deoxyguanosine triphosphate in target cells and disrupts DNA synthesis. First investigations suggest activity in CTCL. 83 Pralatrexate (FolotynÔ; Allos Therapeutics Inc., Westminster, CO, USA) is a novel folate antagonist. It has been approved by the FDA for single-agent treatment of patients with relapsed or refractory peripheral T cell lymphoma (PTCL). The folate analog inhibits dihydrofolate reductase and was developed to overcome the limitations of the folate analog methotrexate. In preclinical studies, pralatrexate demonstrated superior intracellular transport via the reduced folate carrier and increased accumulation within cells by enhanced polyglutamylation, compared with methotrexate. Preclinical studies in vitro and in models of T cell lymphomas indicated that pralatrexate exhibited antitumor activity superior to the activity of other antifolates. 84 Temozolomide is an oral derivate of dacarbazine. The drug induces DNA damage by methylation of nucleotide bases. In a Phase II trial, 26 patients with MF or SS (stages Ib IVb) were treated with 200 mg temozolomide/ m 2 for five days every 28 days. After a median of four treatments, the ORR was 27% and two patients achieved a complete response. Median OS was 24 months. Toxicities included gastrointestinal and hematologic side effects. 85 Enzastaurin is a protein kinase C-b inhibitor. A multicenter, single-arm, open-label Phase II trial enrolled MF and SS patients with stage Ib IVb disease. Twenty-five patients were treated orally with enzastaurin 250 mg twice daily until disease progression or intolerable toxicity was reached. The median time to progression was 78 days in MF patients and 44 days in SS patients. A major beneficial clinical effect was pruritus relief. 86 Monoclonal antibodies Alemtuzumab (CamPath Ò ; Genzyme, Cambridge, MA, USA) is a humanized immunoglobulin G1 (IgG1) monoclonal antibody (MoAb) which targets the CD52 antigen that is abundantly expressed on normal and malignant B and T cells but not on hematopoietic stem cells. It is FDA-approved for the treatment of chronic lymphocytic leukemia. An update on alemtuzumab reported response rates of >50% and a complete response rate of 32% in two studies conducted with heavily pretreated relapsed or refractory MF and SS patients. 87 Results were most promising in patients with SS. Median response duration was 12 months. This compound, however, can be associated with significant hematologic toxicity and infectious complications, including the reactivation of cytomegalovirus, herpes zoster, tuberculosis, and pulmonary aspergillosis. This may be particularly problematic in heavily pretreated patients. Cytopenias and prolonged immunosuppression require prophylactic antibiotic, antiviral, and antifungal treatment and potential support with G-CSF. One group observed adverse cardiac events such as congestive heart failure associated with alemtuzumab therapy. 87 Zanolimumab (HuMax-CD4; Merck Serono SA, Geneva, Switzerland) is a fully human anti-cd4 MoAb isolated from transgenic mice as a hybridoma clone and subsequently expressed in Chinese hamster ovary (CHO) cells. Two essentially identical prospective, multicenter, open-label, uncontrolled Phase II studies (Hx-CD4-007 and Hx-CD4-008) of zanolimumab have been conducted, of which one enrolled patients with treatment-refractory and persistent early-stage CTCL (MF stages Ib IIa; doses of 280 mg or 560 mg weekly) and one enrolled patients with treatment-refractory and persistent advanced-stage CTCL (MF stages IIb IVb and SS; doses of 280 mg or 980 mg weekly). The study duration was 20 weeks. Overall, 13 of 38 patients with MF (complete response, n =1; clinical complete response, n = 3; partial response, n = 9) and two of nine patients with SS (partial response, n =2) demonstrated an objective response to zanolimumab. The most frequently reported adverse events included lowgrade infections and eczematous dermatitis. 88 Histone-acetylase inhibitors Alterations in histone-acetylation regulatory enzymes (histone acetylases and histone deacetylases [HDACs]) have been identified in various hematologic malignancies. These findings raise the possibility that epigenetic modulation of gene transcription can be used therapeutically in cancer. Acetylation of core nucleosomal histones is associated with transcriptional activation that presumably reflects, in part, the increased accessibility of transcription factors to DNA promoter sequences. Inhibition of HDAC activity causes an accumulation of acetylated proteins, including histones. 89 In several CTCL cell lines, HDAC inhibitors downregulate immunosuppressive interleukin- 10 (IL-10) but increase IL-2 and IL-4 RNA. This supports the argument that these inhibitors act as STAT3 inhibitors. 90 Recent studies suggest that HR23B seems to be a biomarker for the sensitivity of CTCL cells to HDAC inhibitors. 91 International Journal of Dermatology 2012, 51, ª 2012 The International Society of Dermatology
11 Wollina Cutaneous T cell lymphoma: treatment update Review 1029 Vorinostat (Zolinza Ò ; Merck & Co. Inc., Whitehouse Station, NJ, USA), also known as suberoylanilide hydroxamic acid (SAHA), is an orally bioavailable inhibitor of class I and II HDACs. In preclinical studies, vorinostat has been shown to cause accumulation of acetylated histones and to induce cell cycle arrest and apoptosis in CTCL lines. 92 Vorinostat has also shown antitumor activity in lymphoma and solid-tumor models in vivo. 93 Vorinostat is metabolized and excreted following glucoronidation by the uridine diphosphate glucuronosyl-transferase (UGT) enzyme system. Polymorphisms in the gene encoding for this enzyme system, UGT1A1, may be an important predictor of vorinostat toxicity and response levels in individual patients. Vorinostat is not metabolized by and does not inhibit the cytochrome P-450 isoenzyme system, and only two drugs warfarin and valproic acid have been noted to interact with vorinostat or other HDAC inhibitors. 92,93 Phase I trials to evaluate the safety and activity of vorinostat were conducted in patients with advanced CTCL. Both IV and oral formulations were well tolerated. Dose-limiting toxicities observed included gastrointestinal toxicity, anorexia, dehydration, fatigue, and myelosuppression. 94 The maximum tolerated oral doses of vorinostat were determined to be 400 mg/d or 200 mg twice daily administered as continuous dosing, 300 mg twice daily for three consecutive days per week, or 200 mg twice daily or thrice daily for 14 days followed by seven days of rest. 95 A Phase II trial evaluated the activity and safety of vorinostat in patients with refractory CTCL. Group 1 received vorinostat 400 mg daily, group 2 received vorinostat 300 mg twice daily for three days followed by four days of rest, and group 3 received vorinostat 300 mg twice daily for 14 days followed by seven days of rest, followed by an administration of 200 mg twice daily. Treatment continued until disease progression or intolerable toxicity was apparent. Thirty-three patients who had received a median of five prior therapies were enrolled. Eight patients achieved a partial response, including seven with advanced disease and four with SS. The median time to response, duration of response, and time to progression for responders were 11.9 weeks, 15.1 weeks, and 30.2 weeks, respectively. Fourteen of 31 evaluable patients had pruritus relief. The most common drug-related adverse reactions were fatigue, thrombocytopenia, diarrhea, and nausea. The most common grade 3 or 4 drug-related adverse events were thrombocytopenia and dehydration. 96 Vorinostat demonstrated activity in heavily pretreated patients with CTCL. The 400-mg/d regimen achieved the most favorable safety profile, with mostly grade 1 and 2 toxicities. Fewer than 6% of patients experienced more severe toxicities including fatigue, nausea, and thrombocytopenia. 97 A meta-analysis of 12 vorinostat trials in advanced primary CTCL evaluated 74 patients. Responses were assessed according to an overall skin disease score obtained using a severity-weighted assessment tool (SWAT). The rate of objective response in skin disease as assessed by the change in the overall SWAT score from baseline was 30% in patients with stage IIb or higher disease. Median response duration was 168 days; median time to tumor progression was 148 days. 98 Romidepsin, sometimes referred to as depsipeptide, (Istodax Ò ; Celgene, Summit, NJ, USA) is another HDAC approved by the FDA for use in advanced CTCL. It exhibits a considerably stronger direct inhibition in class I HDAC enzymes compared with class II. In addition to histone deacetylation, romidepsin modulates additional targets involved in cancer initiation and progression such as c-myc, Hsp90, and p53. A Phase I trial included patients with advanced or refractory neoplasias. Romidepsin was administered as a four-hour IV infusion on days 1 and 5 of a 21-day cycle. The starting dose of the trial was 1 mg/m 2, and dose escalations proceeded through a total of eight levels to a maximum of 24.9 mg/ m 2. Thirty-seven patients received a total of 88 cycles of treatment in the study. Dose-limiting toxicities included grade 3 fatigue, nausea and vomiting, grade 4 thrombocytopenia, and cardiac arrhythmia. One patient achieved a partial response. 99 In two independent Phase II trials in 167 CTCL patients, the ORR was 34% and the complete response rate was 6%. The most frequent toxicities in these trials were nausea, vomiting, fatigue, anorexia, and dysguesia. Grade 4 toxicities were observed but seldom led to dose limitations. 100 A pivotal multicenter study of romidepsin in treatment-refractory CTCL (stages Ib IVa) enrolled 96 patients. The median time to response was two months; the ORR reached 34% and included five cases of complete response. The median duration of reduced pruritus was six months. 101 A Phase II trial included patients with CTCL who had received no more than two prior cytotoxic regimens. Subsequently, the protocol was expanded to enroll patients who had received more than two such regimens previously. Twenty-seven patients were enrolled in the first cohort, and a total of 71 patients were included in the analysis. These patients had undergone a median of four prior treatments, and 62 patients (87%) had advancedstage disease. Toxicities included nausea, vomiting, fatigue, and transient thrombocytopenia and granulocytopenia. A complete response was achieved in four patients and a partial response in 20 patients, giving an ORR of 34%. The median duration of response was 13.7 months. 102 Panobinostat (LBH589) was evaluated in nine patients with CTCL. The drug was administered orally three times ª 2012 The International Society of Dermatology International Journal of Dermatology 2012, 51,
Therapeutic Management of Early Cutaneous Mycosis Fungoides
 Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Index. derm.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acitretin and cutaneous T-cell lymphomas, 716 719, 722, 725 ADCC. See Antibody-dependent cell-mediated cytotoxicity. Adjunctive therapies and
Note: Page numbers of article titles are in boldface type. A Acitretin and cutaneous T-cell lymphomas, 716 719, 722, 725 ADCC. See Antibody-dependent cell-mediated cytotoxicity. Adjunctive therapies and
Clinical Policy: Vorinostat (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10/11
 Clinical Policy: (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10/11 Last Review Date: 12/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10/11 Last Review Date: 12/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
TARGRETIN (bexarotene) oral capsule & external gel
 TARGRETIN (bexarotene) oral capsule & external gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
TARGRETIN (bexarotene) oral capsule & external gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
CUTANEOUS T-CELL LYMPHOMA PROFORMA
 STATE OF KUWAIT MINISTRY OF HEALTH AS AD ALHAMAD DERMATOLOGY CENTER ALSABAH HOSPITAL دولة الكویت وزارة الصحة مرآز أسعد الحمد للا مراض الجلدیة مستشفى الصباح بسم االله الرحمن الرحيم CUTANEOUS TCELL LYMPHOMA
STATE OF KUWAIT MINISTRY OF HEALTH AS AD ALHAMAD DERMATOLOGY CENTER ALSABAH HOSPITAL دولة الكویت وزارة الصحة مرآز أسعد الحمد للا مراض الجلدیة مستشفى الصباح بسم االله الرحمن الرحيم CUTANEOUS TCELL LYMPHOMA
Overview of Cutaneous Lymphomas: Diagnosis and Staging. Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology
 Overview of Cutaneous Lymphomas: Diagnosis and Staging Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology Definition of Lymphoma A cancer or malignancy that comes from
Overview of Cutaneous Lymphomas: Diagnosis and Staging Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology Definition of Lymphoma A cancer or malignancy that comes from
Michi Shinohara MD Associate Professor University of Washington/Seattle Cancer Care Alliance Dermatology, Dermatopathology
 Michi Shinohara MD Associate Professor University of Washington/Seattle Cancer Care Alliance Dermatology, Dermatopathology Agenda Overview of cutaneous T and B- cell lymphomas Diagnosis, Staging, Prognosis
Michi Shinohara MD Associate Professor University of Washington/Seattle Cancer Care Alliance Dermatology, Dermatopathology Agenda Overview of cutaneous T and B- cell lymphomas Diagnosis, Staging, Prognosis
1/19/2018. Early CTCL: treatment considerations
 Treatment of early stage CTCL Joan Guitart, MD Chicago, IL USA Disclosures: Actelion (grants, consultant), Tetralogic/Medivir (grant, consultant), Solygenix (grant) Therakos (grant) Early CTCL: treatment
Treatment of early stage CTCL Joan Guitart, MD Chicago, IL USA Disclosures: Actelion (grants, consultant), Tetralogic/Medivir (grant, consultant), Solygenix (grant) Therakos (grant) Early CTCL: treatment
Supportive Care in the Management of T-cell Lymphomas
 Supportive Care in the Management of T-cell Lymphomas Erin Kopp, ACNP-BC City of Hope Comprehensive Cancer Center NCCN.org For Clinicians NCCN.org/patients For Patients Objectives Discuss the role of supportive
Supportive Care in the Management of T-cell Lymphomas Erin Kopp, ACNP-BC City of Hope Comprehensive Cancer Center NCCN.org For Clinicians NCCN.org/patients For Patients Objectives Discuss the role of supportive
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Vorinostat (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Clinical Policy: Vorinostat (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Cutaneous T-Cell Lymphoma
 PROVIDING THE LATEST INFORMATION FOR PATIENTS & CAREGIVERS Cutaneous T-Cell Lymphoma Revised 2019 Support for this publication provided by A six-word narrative about living with blood cancer from patients
PROVIDING THE LATEST INFORMATION FOR PATIENTS & CAREGIVERS Cutaneous T-Cell Lymphoma Revised 2019 Support for this publication provided by A six-word narrative about living with blood cancer from patients
Sézary syndrome. Diagnosis and staging
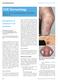 CME DERMATOLOGY Clinical Medicine 2012, Vol 12, No 2: 160 4 Edited by Clive B Archer, consultant dermatologist and lead for Medical Education, St John s Institute of Dermatology, Guy s and St Thomas NHS
CME DERMATOLOGY Clinical Medicine 2012, Vol 12, No 2: 160 4 Edited by Clive B Archer, consultant dermatologist and lead for Medical Education, St John s Institute of Dermatology, Guy s and St Thomas NHS
Corporate Medical Policy
 Corporate Medical Policy Extracorporeal Photopheresis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: extracorporeal_photopheresis 9/2010 8/2017 8/2018 8/2017 Description of Procedure
Corporate Medical Policy Extracorporeal Photopheresis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: extracorporeal_photopheresis 9/2010 8/2017 8/2018 8/2017 Description of Procedure
Disclosures. Advisory Board. Consultant. Investigator. MiRagen, Actelion, Celgene, Therakos. Mindera
 Cutaneous Lymphomas Christiane Querfeld, MD, PhD Director, Cutaneous Lymphoma Program City of Hope ~ How the Experts Treat Hematologic Malignancies Symposium March 10 13, 2017 Disclosures Advisory Board
Cutaneous Lymphomas Christiane Querfeld, MD, PhD Director, Cutaneous Lymphoma Program City of Hope ~ How the Experts Treat Hematologic Malignancies Symposium March 10 13, 2017 Disclosures Advisory Board
Forum 115 Management Issues in Cutaneous Lymphomas. Management of Transformed Mycosis Fungoides
 Forum 115 Management Issues in Cutaneous Lymphomas Management of Transformed Mycosis Fungoides Madeleine Duvic, MD Deputy Chairman -Department of Dermatology Blanche Bender Professor of Cancer Research
Forum 115 Management Issues in Cutaneous Lymphomas Management of Transformed Mycosis Fungoides Madeleine Duvic, MD Deputy Chairman -Department of Dermatology Blanche Bender Professor of Cancer Research
Poteligeo (mogamulizmuab-kpkc)
 Poteligeo (mogamulizmuab-kpkc) Policy Number: 5.02.556 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Poteligeo
Poteligeo (mogamulizmuab-kpkc) Policy Number: 5.02.556 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Poteligeo
Allogeneic Stem Cell Transplantation for Cutaneous T-cell Lymphoma: Updated results from a single center
 Allogeneic Stem Cell Transplantation for Cutaneous T-cell Lymphoma: Updated results from a single center Madeleine Duvic, MD Professor and Deputy Chairman Blanche Bender Professor in Cancer Research Departments
Allogeneic Stem Cell Transplantation for Cutaneous T-cell Lymphoma: Updated results from a single center Madeleine Duvic, MD Professor and Deputy Chairman Blanche Bender Professor in Cancer Research Departments
CUTANEOUS T-CELL LYMPHOMA: SYSTEMIC THERAPY. Anne W. Beaven, MD Associate Professor Director, Lymphoma Program University of North Carolina
 CUTANEOUS T-CELL LYMPHOMA: SYSTEMIC THERAPY Anne W. Beaven, MD Associate Professor Director, Lymphoma Program University of North Carolina CTCL Extranodal Nodal Leukemia Mycosis fungoides Sezary Syndrome
CUTANEOUS T-CELL LYMPHOMA: SYSTEMIC THERAPY Anne W. Beaven, MD Associate Professor Director, Lymphoma Program University of North Carolina CTCL Extranodal Nodal Leukemia Mycosis fungoides Sezary Syndrome
Clinical Trial Outcomes
 l Recent clinical evidence for topical mechlorethamine in mycosis fungoides Mycosis fungoides (MF) is a rare, potentially life-threatening cutaneous T-cell lymphoma characterized by cutaneous homing of
l Recent clinical evidence for topical mechlorethamine in mycosis fungoides Mycosis fungoides (MF) is a rare, potentially life-threatening cutaneous T-cell lymphoma characterized by cutaneous homing of
Synthetic hypericin (SGX301; VIMRxyn) for cutaneous T cell lymphoma, unspecified
 NIHR Innovation Observatory Evidence Briefing: May 2017 Synthetic hypericin (SGX301; VIMRxyn) for cutaneous T cell lymphoma, unspecified NIHRIO (HSRIC) ID: 10761 NICE ID: 8375 LAY SUMMARY Cutaneous T cell
NIHR Innovation Observatory Evidence Briefing: May 2017 Synthetic hypericin (SGX301; VIMRxyn) for cutaneous T cell lymphoma, unspecified NIHRIO (HSRIC) ID: 10761 NICE ID: 8375 LAY SUMMARY Cutaneous T cell
Immunopathogenesis and therapy of cutaneous T cell lymphoma
 Science in medicine Immunopathogenesis and therapy of cutaneous T cell lymphoma Ellen J. Kim, Stephen Hess, Stephen K. Richardson, Sara Newton, Louise C. Showe, Bernice M. Benoit, Ravi Ubriani, Carmela
Science in medicine Immunopathogenesis and therapy of cutaneous T cell lymphoma Ellen J. Kim, Stephen Hess, Stephen K. Richardson, Sara Newton, Louise C. Showe, Bernice M. Benoit, Ravi Ubriani, Carmela
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Istodax) Reference Number: CP.PHAR.314 Effective Date: 01.01.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
Clinical Policy: (Istodax) Reference Number: CP.PHAR.314 Effective Date: 01.01.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
OBSERVATION. Psoralen Plus Long-Wave UV-A (PUVA) and Bexarotene Therapy
 OBSERVATION Psoralen Plus Long-Wave UV-A (PUVA) and Bexarotene Therapy An Effective and Synergistic Combined Adjunct to Therapy for Patients With Advanced Cutaneous T-Cell Lymphoma Karen S. McGinnis, MD;
OBSERVATION Psoralen Plus Long-Wave UV-A (PUVA) and Bexarotene Therapy An Effective and Synergistic Combined Adjunct to Therapy for Patients With Advanced Cutaneous T-Cell Lymphoma Karen S. McGinnis, MD;
Immunosuppressants. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
Novel therapeutic agents for cutaneous T-Cell lymphoma
 Jain et al. Journal of Hematology & Oncology 2012, 5:24 JOURNAL OF HEMATOLOGY & ONCOLOGY REVIEW Novel therapeutic agents for cutaneous T-Cell lymphoma Salvia Jain 1, Jasmine Zain 1 and Owen O Connor 2*
Jain et al. Journal of Hematology & Oncology 2012, 5:24 JOURNAL OF HEMATOLOGY & ONCOLOGY REVIEW Novel therapeutic agents for cutaneous T-Cell lymphoma Salvia Jain 1, Jasmine Zain 1 and Owen O Connor 2*
Indolent Lymphomas. Dr. Melissa Toupin The Ottawa Hospital
 Indolent Lymphomas Dr. Melissa Toupin The Ottawa Hospital What does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with
Indolent Lymphomas Dr. Melissa Toupin The Ottawa Hospital What does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with
Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL)
 Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL) Lymphoid Neoplasms: 1- non-hodgkin lymphomas (NHLs) 2- Hodgkin lymphoma 3- plasma cell neoplasms Non-Hodgkin lymphomas (NHLs) Acute Lymphoblastic Leukemia/Lymphoma
Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL) Lymphoid Neoplasms: 1- non-hodgkin lymphomas (NHLs) 2- Hodgkin lymphoma 3- plasma cell neoplasms Non-Hodgkin lymphomas (NHLs) Acute Lymphoblastic Leukemia/Lymphoma
Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders
 Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders Definition A spectrum of related conditions originating from transformed or activated CD30-positive T-lymphocytes May coexist in individual
Primary Cutaneous CD30-Positive T-cell Lymphoproliferative Disorders Definition A spectrum of related conditions originating from transformed or activated CD30-positive T-lymphocytes May coexist in individual
Cutaneous Lymphoid Proliferations: A Comprehensive Textbook of Lymphocytic Infiltrates of the Skin
 Cutaneous Lymphoid Proliferations: A Comprehensive Textbook of Lymphocytic Infiltrates of the Skin Magro, Cynthia M., MD ISBN-13: 9780471695981 Table of Contents Chapter One: Introduction to the Classification
Cutaneous Lymphoid Proliferations: A Comprehensive Textbook of Lymphocytic Infiltrates of the Skin Magro, Cynthia M., MD ISBN-13: 9780471695981 Table of Contents Chapter One: Introduction to the Classification
Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma
 Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma Kahl BS et al. Cancer 2010;116(1):106-14. Introduction > Bendamustine is a novel alkylating
Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma Kahl BS et al. Cancer 2010;116(1):106-14. Introduction > Bendamustine is a novel alkylating
clinical recommendations
 19 (Supplement 2): ii72 ii76, 2008 doi:10.1093/annonc/mdn095 Primary cutaneous lymphoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up R. Dummer 1 & M. Dreyling 2 On behalf of the
19 (Supplement 2): ii72 ii76, 2008 doi:10.1093/annonc/mdn095 Primary cutaneous lymphoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up R. Dummer 1 & M. Dreyling 2 On behalf of the
Lymphoma: What You Need to Know. Richard van der Jagt MD, FRCPC
 Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Indolent Lymphomas: Current. Dr. Laurie Sehn
 Indolent Lymphomas: Current Dr. Laurie Sehn Why does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with current standard
Indolent Lymphomas: Current Dr. Laurie Sehn Why does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with current standard
2. Sézary syndrome (SS)
 Go Back to the Top To Order, Visit the Purchasing Page for Details Clinical images are available in hardcopy only. Clinical images are available in Clinical images are available in d e f g h i j Fig..36-2
Go Back to the Top To Order, Visit the Purchasing Page for Details Clinical images are available in hardcopy only. Clinical images are available in Clinical images are available in d e f g h i j Fig..36-2
UKCLG Guidelines for Primary Cutaneous Lymphomas
 UKCLG Guidelines for Primary Cutaneous Lymphomas Sean Whi:aker Di Gilson Fiona Child Julia Scarisbrick Tim Illidge Eileen Parry Katy Rezvani Claire Dearden and Stephen Morris Diagnosis, staging, prognosis
UKCLG Guidelines for Primary Cutaneous Lymphomas Sean Whi:aker Di Gilson Fiona Child Julia Scarisbrick Tim Illidge Eileen Parry Katy Rezvani Claire Dearden and Stephen Morris Diagnosis, staging, prognosis
Dermatopathology: The tumor is composed of keratinocytes which show atypia, increase mitoses and abnormal mitoses.
 Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
Extracorporeal Photophoresis
 Ontario Health Technology Assessment Series 2006; Vol. 6, No. 6 Extracorporeal Photophoresis An Evidence-Based Analysis March 2006 Medical Advisory Secretariat Ministry of Health and Long-Term Care 1 Suggested
Ontario Health Technology Assessment Series 2006; Vol. 6, No. 6 Extracorporeal Photophoresis An Evidence-Based Analysis March 2006 Medical Advisory Secretariat Ministry of Health and Long-Term Care 1 Suggested
Update: New Treatment Modalities
 ASH 2008 Update: New Treatment Modalities ASH 2008: Update on new treatment modalities GA101 Improves tumour growth inhibition in mice and exhibits a promising safety profile in patients with CD20+ malignant
ASH 2008 Update: New Treatment Modalities ASH 2008: Update on new treatment modalities GA101 Improves tumour growth inhibition in mice and exhibits a promising safety profile in patients with CD20+ malignant
Large cell immunoblastic Diffuse histiocytic (DHL) Lymphoblastic lymphoma Diffuse lymphoblastic Small non cleaved cell Burkitt s Non- Burkitt s
 Non Hodgkin s Lymphoma Introduction 6th most common cause of cancer death in United States. Increasing in incidence and mortality. Since 1970, the incidence of has almost doubled. Overview The types of
Non Hodgkin s Lymphoma Introduction 6th most common cause of cancer death in United States. Increasing in incidence and mortality. Since 1970, the incidence of has almost doubled. Overview The types of
Title A Phase II study of oral LBH589 in adult patients with refractory cutaneous T-Cell lymphoma
 Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL
 New Evidence reports on presentations given at ASH 2009 Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL From ASH 2009: Non-Hodgkin
New Evidence reports on presentations given at ASH 2009 Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL From ASH 2009: Non-Hodgkin
Targretin. Targretin (bexarotene) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.81 Subject: Targretin Page: 1 of 5 Last Review Date: June 22, 2017 Targretin Description Targretin
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.81 Subject: Targretin Page: 1 of 5 Last Review Date: June 22, 2017 Targretin Description Targretin
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
through the cell cycle. However, how we administer drugs also depends on the combinations that we give and the doses that we give.
 Hello and welcome to this lecture. My name is Hillary Prescott. I am a Clinical Pharmacy Specialist at The University of Texas MD Anderson Cancer Center. My colleague, Jeff Bryan and I have prepared this
Hello and welcome to this lecture. My name is Hillary Prescott. I am a Clinical Pharmacy Specialist at The University of Texas MD Anderson Cancer Center. My colleague, Jeff Bryan and I have prepared this
The complex nature of the immunology of the skin
 Menus for Managing Patients With Cutaneous T-Cell Lymphoma Brian Poligone, MD, PhD,* and Peter Heald, MD In the management of patients with cutaneous T-cell lymphoma (CTCL), there are numerous distinct
Menus for Managing Patients With Cutaneous T-Cell Lymphoma Brian Poligone, MD, PhD,* and Peter Heald, MD In the management of patients with cutaneous T-cell lymphoma (CTCL), there are numerous distinct
Anti-cancer drugs. Introduction : Body : 1) Alkylating Agents
 Anti-cancer drugs Introduction : In this journal I will try to explain what is anti-cancer agents, how they work, how can they inhibit the growth of tumor and what is the advantages and disadvantages of
Anti-cancer drugs Introduction : In this journal I will try to explain what is anti-cancer agents, how they work, how can they inhibit the growth of tumor and what is the advantages and disadvantages of
Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up
 Annals of Oncology 24 (Supplement 6): vi149 vi154, 2013 doi:10.1093/annonc/mdt242 Published online 17 July 2013 Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and
Annals of Oncology 24 (Supplement 6): vi149 vi154, 2013 doi:10.1093/annonc/mdt242 Published online 17 July 2013 Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and
Cutaneous T-cell Lymphoma: Therapy Update
 Cutaneous T-cell Lymphoma: Therapy Update Youn H. Kim Multidisciplinary Cutaneous Lymphoma Group Stanford Comprehensive Cancer Center Stanford University School of Medicine Disclosure of Conflicts of Interest
Cutaneous T-cell Lymphoma: Therapy Update Youn H. Kim Multidisciplinary Cutaneous Lymphoma Group Stanford Comprehensive Cancer Center Stanford University School of Medicine Disclosure of Conflicts of Interest
BJD British Journal of Dermatology. 1.0 Purpose and scope. 2.0 Methodology GUIDELINES. Linked Editorial: Wang and Bagot. Br J Dermatol 2019; 180:
 GUIDELINES BJD British Journal of Dermatology British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018 D. Gilson, 1 S.J.
GUIDELINES BJD British Journal of Dermatology British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018 D. Gilson, 1 S.J.
ISPUB.COM. Primary Cutaneous Anaplastic Large Cell Lymphoma Long-term Management with Low Dose Methotrexate. S Parker INTRODUCTION
 ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Primary Cutaneous Anaplastic Large Cell Lymphoma Long-term Management with Low Dose S Parker Citation S Parker.. The Internet Journal of
ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Primary Cutaneous Anaplastic Large Cell Lymphoma Long-term Management with Low Dose S Parker Citation S Parker.. The Internet Journal of
CLINICAL RESEARCH RESULTS FROM THE ANNUAL MEETINGS OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY AND THE SOCIETY OF NUCLEAR MEDICINE
 FOR IMMEDIATE RELEASE CLINICAL RESEARCH RESULTS FROM THE ANNUAL MEETINGS OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY AND THE SOCIETY OF NUCLEAR MEDICINE Results of Studies of BEXXAR TM Therapy Show Promise
FOR IMMEDIATE RELEASE CLINICAL RESEARCH RESULTS FROM THE ANNUAL MEETINGS OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY AND THE SOCIETY OF NUCLEAR MEDICINE Results of Studies of BEXXAR TM Therapy Show Promise
Guidelines for the management of cutaneous lymphomas (2011): A consensus statement by the Japanese Skin Cancer Society Lymphoma Study Group
 doi: 10.1111/j.1346-8138.2012.01639.x Journal of Dermatology 2013; 40: 2 14 GUIDELINE Guidelines for the management of cutaneous lymphomas (2011): A consensus statement by the Japanese Skin Cancer Society
doi: 10.1111/j.1346-8138.2012.01639.x Journal of Dermatology 2013; 40: 2 14 GUIDELINE Guidelines for the management of cutaneous lymphomas (2011): A consensus statement by the Japanese Skin Cancer Society
Lymphoma (Lymphosarcoma) by Pamela A. Davol
 Lymphoma (Lymphosarcoma) by Pamela A. Davol Cells derived from the bone marrow that mature and take part in cellular immune reactions are called lymphocytes. When lymphocytes undergo transformation and
Lymphoma (Lymphosarcoma) by Pamela A. Davol Cells derived from the bone marrow that mature and take part in cellular immune reactions are called lymphocytes. When lymphocytes undergo transformation and
Narrow band UVB (311 nm), psoralen UVB (311 nm) and PUVA therapy in the treatment of early-stage mycosis fungoides: a right left comparative study
 Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
ISPUB.COM. Management of Co-existing Mycosis Fungoides and Lymphomatoid Papulosis. E Kim PHYSICAL FINDINGS INTRODUCTION INITIAL PRESENTATION
 ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Management of Co-existing Mycosis Fungoides and Lymphomatoid Papulosis E Kim Citation E Kim. Management of Co-existing Mycosis Fungoides
ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Management of Co-existing Mycosis Fungoides and Lymphomatoid Papulosis E Kim Citation E Kim. Management of Co-existing Mycosis Fungoides
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C
 A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
Disclosures WOJCIECH JURCZAK
 Disclosures WOJCIECH JURCZAK ABBVIE (RESEARCH FUNDING), CELGENE (RESEARCH FUNDING); EISAI (RESEARCH FUNDING); GILEAD (RESEARCH FUNDING); JANSEN (RESEARCH FUNDING); MORPHOSYS (RESEARCH FUNDING), MUNDIPHARMA
Disclosures WOJCIECH JURCZAK ABBVIE (RESEARCH FUNDING), CELGENE (RESEARCH FUNDING); EISAI (RESEARCH FUNDING); GILEAD (RESEARCH FUNDING); JANSEN (RESEARCH FUNDING); MORPHOSYS (RESEARCH FUNDING), MUNDIPHARMA
Subject Index. Dry desquamation, see Skin reactions, radiotherapy
 Subject Index Actinic keratosis disseminated disease 42 surgical excision 42 AIDS, see Kaposi s sarcoma Amifostine, skin reaction prophylaxis 111 Basal cell carcinoma, superficial X-ray therapy Bowen s
Subject Index Actinic keratosis disseminated disease 42 surgical excision 42 AIDS, see Kaposi s sarcoma Amifostine, skin reaction prophylaxis 111 Basal cell carcinoma, superficial X-ray therapy Bowen s
HDAC Inhibitors and PARP inhibitors. Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine
 HDAC Inhibitors and PARP inhibitors Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine Histone Acetylation HAT Ac Ac Ac Ac HDAC Ac Ac Ac Ac mrna DACs
HDAC Inhibitors and PARP inhibitors Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine Histone Acetylation HAT Ac Ac Ac Ac HDAC Ac Ac Ac Ac mrna DACs
Lymphomatoid Papulosis. اللمفواني الحطاطي الداء = lymphomatoide Papulose Thursday, 21 October :16 - Last Updated Friday, 03 December :54
 Lymphomatoid Papulosis 1 / 12 Lymphomatoid papulosis (LyP) is a chronic papulonecrotic or papulonodular skin disease with histologic features suggestive of a malignant lymphoma. The disease is characterized
Lymphomatoid Papulosis 1 / 12 Lymphomatoid papulosis (LyP) is a chronic papulonecrotic or papulonodular skin disease with histologic features suggestive of a malignant lymphoma. The disease is characterized
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Development of Mogamulizumab, a defucosylated anti-ccr4 humanized monoclonal antibody
 New Drugs in Hematology Development of Mogamulizumab, a defucosylated anti-ccr4 humanized monoclonal antibody Michinori Ogura, MD, PhD Department of Hematology Tokai Central Hospital Bologna, Royal Hotel
New Drugs in Hematology Development of Mogamulizumab, a defucosylated anti-ccr4 humanized monoclonal antibody Michinori Ogura, MD, PhD Department of Hematology Tokai Central Hospital Bologna, Royal Hotel
Case Report A Severe Case of Lymphomatoid Papulosis Type E Successfully Treated with Interferon-Alfa 2a
 Hindawi Case Reports in Dermatological Medicine Volume 2017, Article ID 3194738, 5 pages https://doi.org/10.1155/2017/3194738 Case Report A Severe Case of Lymphomatoid Papulosis Type E Successfully Treated
Hindawi Case Reports in Dermatological Medicine Volume 2017, Article ID 3194738, 5 pages https://doi.org/10.1155/2017/3194738 Case Report A Severe Case of Lymphomatoid Papulosis Type E Successfully Treated
Sezary Syndrome(SS) and other malignancies. Hernani Cualing MD Hematopathologist IHCFLOW Lab
 Sezary Syndrome(SS) and other malignancies Hernani Cualing MD Hematopathologist IHCFLOW Lab Disclosures IHCFLOW Laboratory:consultant and director NEOGENOMICS: contract consultant USF: contract reviewer
Sezary Syndrome(SS) and other malignancies Hernani Cualing MD Hematopathologist IHCFLOW Lab Disclosures IHCFLOW Laboratory:consultant and director NEOGENOMICS: contract consultant USF: contract reviewer
Gazyva (obinutuzumab)
 STRENGTH DOSAGE FORM ROUTE GPID 1000mg/40mL Vial Intravenous 35532 MANUFACTURER Genentech, Inc. INDICATION(S) Gazyva (obinutuzumab) is a CD20- directed cytolytic antibody and is indicated, in combination
STRENGTH DOSAGE FORM ROUTE GPID 1000mg/40mL Vial Intravenous 35532 MANUFACTURER Genentech, Inc. INDICATION(S) Gazyva (obinutuzumab) is a CD20- directed cytolytic antibody and is indicated, in combination
Biological Therapies for Cancer: Questions and Answers
 Biological Therapies for Cancer: Questions and Answers Key Points Biological therapies use the body s immune system to fight cancer or to lessen the side effects that may be caused by some cancer treatments
Biological Therapies for Cancer: Questions and Answers Key Points Biological therapies use the body s immune system to fight cancer or to lessen the side effects that may be caused by some cancer treatments
Index. neurosurgery.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A A Complimentary Trial of an Immunotherapy Vaccine Against Tumor-specific EGFRvIII (ACTIVATE), 90 91 Active immunotherapy, 5 8, 96. See
Index Note: Page numbers of article titles are in boldface type. A A Complimentary Trial of an Immunotherapy Vaccine Against Tumor-specific EGFRvIII (ACTIVATE), 90 91 Active immunotherapy, 5 8, 96. See
What is the best approach to the initial therapy of PTCL? standards of treatment? Should all
 What is the best approach to the initial therapy of PTCL? standards of treatment? hould all Jia Ruan, M.D., Ph.D. Center for Lymphoma and Myeloma Weill Cornell Medical College New York Presbyterian Hospital
What is the best approach to the initial therapy of PTCL? standards of treatment? hould all Jia Ruan, M.D., Ph.D. Center for Lymphoma and Myeloma Weill Cornell Medical College New York Presbyterian Hospital
Chronic Lymphocytic Leukemia Update. Learning Objectives
 Chronic Lymphocytic Leukemia Update Ashley Morris Engemann, PharmD, BCOP, CPP Clinical Associate Adult Stem Cell Transplant Program Duke University Medical Center August 8, 2015 Learning Objectives Recommend
Chronic Lymphocytic Leukemia Update Ashley Morris Engemann, PharmD, BCOP, CPP Clinical Associate Adult Stem Cell Transplant Program Duke University Medical Center August 8, 2015 Learning Objectives Recommend
BLOOD AND LYMPH CANCERS
 BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
This was a multicenter study conducted at 11 sites in the United States and 11 sites in Europe.
 Protocol CAM211: A Phase II Study of Campath-1H (CAMPATH ) in Patients with B- Cell Chronic Lymphocytic Leukemia who have Received an Alkylating Agent and Failed Fludarabine Therapy These results are supplied
Protocol CAM211: A Phase II Study of Campath-1H (CAMPATH ) in Patients with B- Cell Chronic Lymphocytic Leukemia who have Received an Alkylating Agent and Failed Fludarabine Therapy These results are supplied
Narrow-band UVB PHOTOTHERAPY for Skin Diseases
 Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Sézary syndrome presenting with leonine facies ajd_560
 285..288 Australasian Journal of Dermatology (2009) 50, 285 288 doi: 10.1111/j.1440-0960.2009.00560.x CASE REPORT Sézary syndrome presenting with leonine facies ajd_560 Shano Nassem, 1 Rajesh Kashyap,
285..288 Australasian Journal of Dermatology (2009) 50, 285 288 doi: 10.1111/j.1440-0960.2009.00560.x CASE REPORT Sézary syndrome presenting with leonine facies ajd_560 Shano Nassem, 1 Rajesh Kashyap,
Extracorporeal Photopheresis and Crohn s Disease. Jill Adamski MD, PhD Transfusion Medicine Department of Pathology
 Extracorporeal Photopheresis and Crohn s Disease Jill Adamski MD, PhD Transfusion Medicine Department of Pathology Outline 1. Crohn s Disease (CD) 2. Extracoporeal Photopheresis (ECP) 3. Pilot studies
Extracorporeal Photopheresis and Crohn s Disease Jill Adamski MD, PhD Transfusion Medicine Department of Pathology Outline 1. Crohn s Disease (CD) 2. Extracoporeal Photopheresis (ECP) 3. Pilot studies
TRANSPARENCY COMMITTEE OPINION. 27 January 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
Adverse side effects associated to metronomic chemotherapy
 Adverse side effects associated to metronomic chemotherapy Elisabetta Munzone, MD Division of Medical Senology Istituto Europeo di Oncologia Milano, Italy LDM: the optimal biological dose Although there
Adverse side effects associated to metronomic chemotherapy Elisabetta Munzone, MD Division of Medical Senology Istituto Europeo di Oncologia Milano, Italy LDM: the optimal biological dose Although there
Changing the landscape of treatment in Peripheral T-cell Lymphoma
 Changing the landscape of treatment in Peripheral T-cell Lymphoma Luis Fayad Associate Professor MD Anderson Cancer Center Department of Lymphoma and Myeloma 1 6 What is peripheral 2008 WHO CLASSIFICATION
Changing the landscape of treatment in Peripheral T-cell Lymphoma Luis Fayad Associate Professor MD Anderson Cancer Center Department of Lymphoma and Myeloma 1 6 What is peripheral 2008 WHO CLASSIFICATION
Rayos Prior Authorization Program Summary
 Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
1 Introduction. 1.1 Cancer. Introduction
 Introduction 1 1.1 Cancer 1 Introduction Cancer is the most precarious disease characterized by uncontrolled proliferation of cells without any physiological demands of the organism. Cancer may be defined
Introduction 1 1.1 Cancer 1 Introduction Cancer is the most precarious disease characterized by uncontrolled proliferation of cells without any physiological demands of the organism. Cancer may be defined
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/2010 holds various files of this Leiden University dissertation. Author: Benner, Marchina Frederika Title: Cutaneous CD30-positive lymphoproliferations
Cover Page The handle http://hdl.handle.net/1887/2010 holds various files of this Leiden University dissertation. Author: Benner, Marchina Frederika Title: Cutaneous CD30-positive lymphoproliferations
Intron A. Intron A (interferon alfa-2b) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.07 Subject: Intron A Page: 1 of 6 Last Review Date: June 22, 2018 Intron A Description Intron A (interferon
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.07 Subject: Intron A Page: 1 of 6 Last Review Date: June 22, 2018 Intron A Description Intron A (interferon
Plasma cell myeloma (multiple myeloma)
 Plasma cell myeloma (multiple myeloma) Common lymphoid neoplasm, present at old age (70 years average) Remember: plasma cells are terminally differentiated B-lymphocytes that produces antibodies. B-cells
Plasma cell myeloma (multiple myeloma) Common lymphoid neoplasm, present at old age (70 years average) Remember: plasma cells are terminally differentiated B-lymphocytes that produces antibodies. B-cells
Management of Mycosis Fungoides: Part 2. Treatment
 Review Article [1] October 01, 2003 By Benjamin D. Smith, MD [2] and Lynn D. Wilson, MD, MPH [3] Mycosis fungoides is a low-grade lymphoproliferative disorder of skin-homing CD4+ lymphocytes that may produce
Review Article [1] October 01, 2003 By Benjamin D. Smith, MD [2] and Lynn D. Wilson, MD, MPH [3] Mycosis fungoides is a low-grade lymphoproliferative disorder of skin-homing CD4+ lymphocytes that may produce
ISPUB.COM. Bexarotene in Tumor stage Mycosis Fungoides. R Talpur, M Duvic INITIAL PRESENTATION TREATMENT COURSE INTRODUCTION
 ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Bexarotene in Tumor stage Mycosis Fungoides R Talpur, M Duvic Citation R Talpur, M Duvic. Bexarotene in Tumor stage Mycosis Fungoides. The
ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Bexarotene in Tumor stage Mycosis Fungoides R Talpur, M Duvic Citation R Talpur, M Duvic. Bexarotene in Tumor stage Mycosis Fungoides. The
DRUGS FOR NEOPLASIA. Chapter 37
 DRUGS FOR NEOPLASIA Chapter 37 Keywords- You tell Me! Cancer dz characterized by abnormal, uncontrolled cell division Tumor swelling, abnormal enlargement or mass of tissue Carcinogen any substance or
DRUGS FOR NEOPLASIA Chapter 37 Keywords- You tell Me! Cancer dz characterized by abnormal, uncontrolled cell division Tumor swelling, abnormal enlargement or mass of tissue Carcinogen any substance or
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
ISPUB.COM. Advanced Stage CTCL, PTCL with Cutaneous Involvement. J Messenger, P Porcu INTRODUCTION INITIAL PRESENTATION
 ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Advanced Stage CTCL, PTCL with Cutaneous Involvement J Messenger, P Porcu Citation J Messenger, P Porcu. Advanced Stage CTCL, PTCL with Cutaneous
ISPUB.COM The Internet Journal of Dermatology Volume 7 Number 3 Advanced Stage CTCL, PTCL with Cutaneous Involvement J Messenger, P Porcu Citation J Messenger, P Porcu. Advanced Stage CTCL, PTCL with Cutaneous
Classification of Cutaneous T cell Lymphomas (CTCLs) Hernani Cualing, MD
 Classification of Cutaneous T cell Lymphomas (CTCLs) Hernani Cualing, MD Pathology and Cell Biology, USF IFLOW, Inc. CTCL, MF, and Sézary syndrome In 1806, mycosis fungoides (MF) was first described 1
Classification of Cutaneous T cell Lymphomas (CTCLs) Hernani Cualing, MD Pathology and Cell Biology, USF IFLOW, Inc. CTCL, MF, and Sézary syndrome In 1806, mycosis fungoides (MF) was first described 1
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma
 Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
0BCore Safety Profile. Pharmaceutical form(s)/strength: Cream 1% DK/H/PSUR/0009/005 Date of FAR:
 0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
The World of Dermatology
 The World of Dermatology, F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates, LTD Objectives Cite the advances in management
The World of Dermatology, F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates, LTD Objectives Cite the advances in management
Phase 1 Trial Evaluating MRG-106, a Synthetic Inhibitor of microrna- 155, in Patients with CTCL
 EORTC CLTF 2017 meeting on Cutaneous Lymphomas: Insights & Therapeutic Progress Phase 1 Trial Evaluating MRG-106, a Synthetic Inhibitor of microrna- 155, in Patients with CTCL Christiane Querfeld, Francine
EORTC CLTF 2017 meeting on Cutaneous Lymphomas: Insights & Therapeutic Progress Phase 1 Trial Evaluating MRG-106, a Synthetic Inhibitor of microrna- 155, in Patients with CTCL Christiane Querfeld, Francine
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
TRANSPARENCY COMMITTEE OPINION. 8 November 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 8 November 2006 MABTHERA 100 mg, concentrate for solution for infusion (CIP 560 600-3) Pack of 2 MABTHERA 500 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 8 November 2006 MABTHERA 100 mg, concentrate for solution for infusion (CIP 560 600-3) Pack of 2 MABTHERA 500 mg,
BR for previously untreated or relapsed CLL
 1 Protocol synopsis Title Rationale Study Objectives Multicentre phase II trial of bendamustine in combination with rituximab for patients with previously untreated or relapsed chronic lymphocytic leukemia
1 Protocol synopsis Title Rationale Study Objectives Multicentre phase II trial of bendamustine in combination with rituximab for patients with previously untreated or relapsed chronic lymphocytic leukemia
Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients with CLL: A Randomized, Open-Label, Phase III Trial
 Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients with CLL: A Randomized, Open-Label, Phase III Trial Hallek M et al. Lancet 2010;376:1164-74. Introduction > In patients with CLL, the
Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients with CLL: A Randomized, Open-Label, Phase III Trial Hallek M et al. Lancet 2010;376:1164-74. Introduction > In patients with CLL, the
MabThera. SC. The wait is over. MabThera delivered in just 5 minutes. SC= subcutaneous injection
 MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
Intron A. Intron A (interferon alfa-2b) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Intron A Page: 1 of 6 Last Review Date: June 19, 2015 Intron A Description Intron A (interferon
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Intron A Page: 1 of 6 Last Review Date: June 19, 2015 Intron A Description Intron A (interferon
