Efficiency and tolerability of 5- fluorouracil-based adjuvant chemotherapy in elderly patients with colorectal carcinoma
|
|
|
- Elinor Banks
- 5 years ago
- Views:
Transcription
1 Turkish Journal of Cancer Volume 34, No.4, Efficiency and tolerability of 5- fluorouracil-based adjuvant chemotherapy in elderly patients with colorectal carcinoma LHAN ÖZTOP 1, ARZU YAREN 1, IfiIL SOMAL 2, OKTAY TARHAN 1, U UR YILMAZ 1 1 Dokuz Eylül University, Institute of Oncology, Department of Medical Oncology, 2 Atatürk Research and Training Hospital, Department of Medical Oncology, zmir-turkey ABSTRACT The benefit and tolerability of adjuvant chemotherapy for surgically treated colorectal cancer is less well defined in elderly patients. We reviewed data of 51 patients who received adjuvant chemotherapy for colorectal cancer between October 1993 and December 2002, and were 65 year-old at the first cycle of chemotherapy. Fifty-one elderly patients with colorectal cancer received 5-FU based adjuvant chemotherapy. Twenty-four patients (47.0%) had stage II, 25 (49.0%) had stage III, and 2 (3.9%) had stage IV disease (completely resected liver metastasis). Fourteen patients (27.5%) were treated with bolus 5-FU, while 37 patients (72.5%) were treated with infusional 5-FU. The most commonly observed grade 3-4 toxicities were myelosuppresion (17.6%) and diarrhea (15.6%), and they were more frequently seen in patients who received 5-FU bolus regimens (p<0.001 and p<0.005, respectively). The 3-year DFS and OS rates were 77.7% and 83.5%, respectively. Twenty-nine patients who were 70-year-old were also evaluated. The most commonly grade 3-4 toxicities observed in this age group were myelosuppresion (6.9%) and diarrhea (6.9%), and they occurred in patients who received 5-FU bolus regimens. In survival analyses, the 3-year DFS and OS were 80% and 80.7%, respectively. In elderly patients, the use of 5- FU based adjuvant chemotherapy for colorectal cancer was well tolerated, and advanced age is not an obstacle for the adjuvant chemotherapy of colorectal cancer. [Turk J Cancer 2004;34(4): ] KEY WORDS: Colon cancer, elderly patients, 5-fluorouracil INTRODUCTION The incidence of colorectal cancer increases with advancing age, and in elderly patients with this disease, the cancer is still the major cause of death. Randomized clinical trials demonstrated that adjuvant chemotherapy with 5- fluorouracil (5-FU) based regimens was able to reduce the risk of disease recurrence and mortality in patients with stage III colorectal cancer. Elderly patients were not adequately included to these trials because of traditional age limits of inclusion criteria. Meanwhile, adjuvant chemotherapy in colorectal cancer has minimal toxicity which permits the administration of the planned treatment to the vast majority of patients and reduces the risk of death by one third, as compared with surgery alone (1-4). There is no reason to consider that elderly patients with early colorectal cancer are deprived of a similar benefit in terms of disease control and survival. Due to the low toxicity profile of regimens used in the adjuvant treatment of colorectal cancer, patients with advanced age can receive the adequate treatment, but there are limited data on the risks and benefits of cancer treatment in older patients, because patients of this age group were less involved in clinical trials (5-9).
2 140 Adjuvant 5-FU Based Chemotherapy in Elderly Patients with Colorectal Carcinoma The improvement of preventive and therapeutic health services as well as of socio-economic conditions resulted in an important prolongation of life expectancy in developed countries. As a result of this, the proportion of elderly people has increased as a whole. These persons with advanced age are nowadays in better conditions, and a better health care is delivered to them. Thus, patients with colorectal cancer in advanced age are left without treatment in a lesser degree. In accordance with this trend in developed countries, no age limit was adopted in decisions for adjuvant treatment of patients with surgically treated early stage colorectal cancer and adjuvant chemotherapy was proposed to each one irrespective of age in our department. In order to evaluate the feasibility and tolerability of the adjuvant treatment of elderly patients in early stage colorectal cancer, the records of all patients treated during the last ten years in our department of medical oncology were analyzed and reported below. MATERIALS AND METHODS We reviewed the data of patients with colorectal cancer aged 65 years or older referred to our department of medical oncology for adjuvant treatment following curative surgery from October 1993 to December This included patients with stage II, III and also stage IV with completely resected liver metastasis. The choice of chemotherapy regimen changed during the period and were based on 5- FU as bolus (weekly 5-FU, 5-FU + Levamisol, Mayo) or late as bolus plus infusion (LV5FU2) (Table 1). The first chemotherapy cycle was initiated within two to eight weeks following the surgery. Basic criteria for the administration of chemotherapy were adequate blood counts (WBC 4.000/mm 3, Hb 11 g/dl, PLT /mm 3 ) and serum biochemistry (creatinine 1.5 mg/dl, AST and ALT 2 x upper limit of normal (ULN), bilirubin 2.0 mg/dl) as well as good performance status (ECOG 2) and absence of significant systemic disease. For each patient, the stage of disease (TNM), degree of tumor differentiation, co-morbidities and the type of chemotherapy regimen were recorded. The chemotherapy toxicity and the outcome of the disease and patients were analyzed. The toxicity of chemotherapy was graded according to the Common Toxicity Criteria of World Health Organization (10) and the highest grade observed was recorded for each kind of toxicity. Table 1 The chemotherapy regimens administered to the patients Mayo Clinic regimen 5-FU 425 mg/m 2 (IV bolus) d1-5 Folinic acid 20 mg/m 2 (IV bolus) d1-5 (To be repeated every 4 weeks during 6 months) LV5FU2 regimen 5-FU 400 mg/m 2 (IV bolus) d mg/m 2 (IV 22 h inf) d1+2 Folinic acid 200 mg/m 2 (2 h inf) d1+2 (To be repeated every 2 weeks) 5-FU + Levamisole 5-FU 425 mg/m 2 (IV bolus) d1-5 then weekly x 1 y Levamisole 50 mg t.i.d. PO d1-3 three days per week, every other week Weekly 5-FU 5-FU 425 mg/m 2 (IV bolus) d1 (Weekly)
3 Öztop et al. 141 Statistical Methods Comparisons of the distribution of qualitative parameters among subgroups were made using the chi-square test or Fisher s exact test when it is more adequate. Means were compared with student t-test. Disease-free survival (DFS) and overall survival (OS) were estimated with Kaplan-Meier method. The DFS was defined as the time from the first day of chemotherapy until the first evidence of disease recurrence, and OS was defined as the time from the first day of chemotherapy until death. The survival was also analysed as adjusted survival (AS) censoring non-cancer related deaths at the time of death. Subgroups were compared with log-rank test. SPSS 10.0 for Windows was used for all statistical analyses. The most commonly observed grade 3 and 4 toxicities were myelosuppresion (17.6%) and diarrhea (15.6%), and they were more frequently seen in patients who received 5-FU as bolus regimens (p<0.001 and p<0.005, respectively). Nausea and vomiting were also more frequent in the bolus group (p<0.05) (Table 3). Grade 3-4 myelosupression was also more frequently observed in female patients (6 of 19; 31.5%) compared to males Table 2 Characteristics of elderly (65 and older) patients with colorectal carcinoma who received adjuvant chemotherapy after surgical resection RESULTS Adjuvant chemotherapy for colorectal cancer following surgical resection was started from October 1993 to December 2002 in 155 patients of all ages. Fifty-one among these patients were aged sixty-five years or older at the time of start the chemotherapy and they are the subject of this analysis. The total number of patients in this age group referred after surgery was 55, but four of them did not receive chemotherapy; three patients refused the treatment while one patient was left without chemotherapy because of inadequate performance status. These four patients were not included to this analysis. About two third of the patients were male and the median age was 70 (range years). Approximately half of the patients had stage II disease (47.0%). This analysis included also two patients who received adjuvant chemotherapy after resection of liver metastases at the same time as primary tumor. Only 13 patients (25.5%) had rectal cancer and they also received radiation therapy before or after surgical resection. Characteristics of 51 patients who were analyzed are summarized in table 2. All patients received 5-FU based adjuvant chemotherapy. Fourteen patients (27.5%) were treated with different regimens of bolus 5-FU ± folinic acid, while 37 patients (72.5%) were treated with 5-FU as bolus combined by longer infusion, and high dose folinic acid (LV5FU2). No initial dose reduction was performed for advanced age. Patient characteristics Number of patients (%) Median age (year) 70 Sex Male 32 (62.7) Female 19 (37.3) Performance status 0 12 (23.5) 1 29 (56.8) 2 10 (19.7) Stage II 24 (47.0) III 25 (49.0) IV (metastectomy) 2 (4.0) Comorbidity 0 34 (66.6) 1 10 (19.6) 2 7 (13.8) Localisation of cancer Colon 38 (74.5) Rectum 13 (25.5) Tumor grade Well differentiated 7 (13.7) Moderately differentiated 33 (64.7) Poorly differentiated 3 (5.9) Unkown 8 (15.7) Chemotherapy Bolus 14 (27.5) Infusional 37 (72.5)
4 142 Adjuvant 5-FU Based Chemotherapy in Elderly Patients with Colorectal Carcinoma (3 of 32: 9.4%), but the difference did not reach statistical significance (p>0.05). A Dose reductions were performed in 4 (7.8%) patients as a result of hematological toxicity and in one patient, the chemotherapy was interrupted at month 2 because of hematological toxicity in spite of dose reductions. The chemotherapy dose intensity was defined as the ratio of the dose planned to the dose delivered. The median dose intensity was 92%. The patients who were alive at the end of analysis were followed for a median duration of 26.5 months (range: 2-114). The 3-year DFS and OS were 77.7% and 83.5% respectively (Figure 1). Nine patients were dead during the follow-up and four of these deaths were not related to colorectal cancer. When the survival data was adjusted censoring non-cancer deaths, the median survival was 74 months and 3-year adjusted survival (AS) was 92.5%. Sex, presence of comorbidity, location of tumor, differentiation of tumor, stage, and type of chemotherapy regimens did not have statistically significant effect on DFS, OS and adjusted survival. Twenty nine patients who were seventy years or older were also evaluated separately. The median age was 74 and most of patients (24 of 29 patients) had good performance status (ECOG 0-1). The most commonly grade 3-4 toxicities observed in this age group were myelosuppresion (6.9%) and diarrhea (6.9%), and they occurred in patients who received 5-FU bolus regimens. In survival analyses, the 3-year DFS, OS and AS were 80.0%, 80.7 % and 92.7%, respectively. DISCUSSION The adjuvant chemotherapy for surgically treated colorectal cancer in fifty-one patients being 65 years or older at the beginning of first cycle, were analyzed retrospectively. B Figure 1. Kaplan-Meier estimates for (A) overall and (B) disease free survival among elderly (65 and older) patients who received adjuvant chemotherapy as 5-FU Different, but all 5-FU based chemotherapy regimens were administered. The treatment was well tolerated with only one disruption of treatment because of toxicity. There was no treatment related mortality. The 3-year disease free survival, overall survival and cancer related survival were found 77.7%, 83.5% and 92.5%, respectively. This figure was 80.0%, 80.7% and 92.7%, respectively in patients who were seventy years and older. Table 3 Grade 3-4 toxicity of adjuvant chemotherapy in 65 age and older patients All patients Myelosuppression Diarrhea Stomatitis Handfoot Whole group 17.6% 15.6% 7.8% 3.9% Bolus 5-FU 15.6% 13.6% 7.8% - Bolus + Infusional 5-FU 2.0% 2.0% - 3.9%
5 Öztop et al. 143 These data concern patients of relatively new and developing medical oncology department and most of patients were seen in last years of the analysis period. This fact explains why median follow up is as short as 26.3 months beside an analysis period of 10 years. Otherwise, the inclusion of stage II patients contributed to the good prognosis of the whole group. The adjuvant treatment of colorectal cancer concerns also the radiotherapy when the tumor is localized in the distal rectum. In this context, the use of the radiotherapy in patients is also to be evaluated. This was not done in this analysis because of small number of patients who received adjuvant radiotherapy and lack of adequate data. It has been demonstrated that adjuvant chemotherapy can reduce the risk of disease recurrence and death in patients with stage III colon carcinoma by approximately 40% and 33%, respectively (4). The benefit of adjuvant chemotherapy in stage II is controversial. The choice of the physicians in this department was in favor of the administration of adjuvant chemotherapy also in stage II. Treatment regimens found efficacious in this context are generally well tolerated. Moreover, these studies demonstrated that adjuvant therapy can be administered with minimal toxicity and that the vast majority of patients are able to complete treatment (11,12). Although elderly persons make the majority of the population with cancer, older patients have been less likely to receive standard cancer treatments, even when such treatments are potentially curative and usually well tolerated (8,13,14). Newcomb and Carbone showed that, nearly 50% of patients who have various malignancies at an age under 65 were offered chemotherapy as a treatment option, but only 35% of those over 65 were given the same option. The rate of patients who rejected the chemotherapy was higher among older patients because of fear from side effects (15). In our study, 51 of 55 patients (92.7%) referred in our department were given adjuvant chemotherapy and only three of them refused the treatment despite the opinion of the physician. This high rate of patient compliance despite the age can be explained by the fact that, most patients had good performance status and especially in our country, patients leave the treatment decision to the physician. Clinicians have rational arguments not to use treatments with a high rate of toxicity in patients with advanced age. These include coexisting morbid conditions, lower level of tolerance against adverse effects, declining functional and mental status with advanced age and lack of social support. However, most people older than 75 are independent, and their life expectancy without cancer is 10 to 12 years in western countries and this is increasing towards this level in Turkey (16). Because colorectal cancer typically recurs mostly within five years after diagnosis, it is reasonable to consider adjuvant chemotherapy to prevent recurrence in elderly patients including octogenarians. In a pooled analysis of individual patient data from seven phase III randomised trials, in which the effects of postoperative 5-FU plus leucovorin (five trials) or plus levamisole (two trials) were compared with the effects of surgery alone in elderly patients with stage II or III colon cancer. It was concluded that adjuvant treatment had a significant positive effect on both overall survival and time to tumor recurrence. The 5-year OS was 71% for those who received adjuvant therapy, as compared with 64% for those untreated (8). Another population-based cohort study resulted in favor of a survival benefit of adjuvant 5-FU in elderly patients with stage III colon cancer (17). Furthermore, the survival benefit did not appear to diminish with patient age. Other studies also reported similar survival rates (18-20). The survival analysis in our study showed that the 3- year DFS and OS were 77.7% and 83.5%, respectively. These results were similar to the studies reported elsewhere. Many studies have shown that age was not associated significantly with survival (4,8, 18-22). Conversely, in some studies, there was a trend in favor of an improved clinical outcome for the elderly. In addition to this, Moertel et al. (4) found that older patients with colon carcinoma had a greater survival benefit from 5-FU based adjuvant therapy compared to younger patients. In some studies, patients with advanced age receiving 5-FU based chemotherapy for colorectal cancer experienced increased grade 3-4 toxicities as leukopenia and mucositis (9,23,24). However, a meta-analysis of 19 trials have found no convincing evidence that chemotherapy was more toxic or less beneficial for elderly cancer patients who were aged 70 years and over (12). A secondary analysis of several phase II trials in advanced cancer also found no evidence of increased toxicity in those over age 65 (25). We have found that the most commonly observed grade 3-4 toxicities were myelosupression (17.6%) and diarrhea
6 144 Adjuvant 5-FU Based Chemotherapy in Elderly Patients with Colorectal Carcinoma (15.6%) and they were more frequently seen in patients who received 5-FU bolus regimens. These rates are comparable to rates varying from 9 to 30% observed elsewhere during the adjuvant treatment of colorectal cancer. The toxicity is in general less severe when 5-FU was administered as longer duration infusion (17,18,24,25). We have also compared these patients age 65 years with the 104 patients age < 65 years who received adjuvant chemotherapy in the same context during the study period of our department. In younger group, forty-four patients (42.3%) had stage II and 60 (57.7%) had stage III. Thirtyseven patients (35.6%) were treated with bolus 5-FU, while 67 patients (64.4%) were treated with infusional 5-FU. Grade 3-4 hematological toxicity and diarrhea were observed more frequently in bolus group than infusional group (7.3 vs 2.6% and 6.8 vs 3.2%, respectively). The dose reduction was performed in 6 patients in both group. In survival analysis, there was not any significant difference between bolus and infusional treatment group. The 3-year diseasefree survival and overall survival rates were 76.4% and 79.7% in whole group. Although both age group had a similar survival rates, the hematological toxicity and diarrhea were observed more frequently in patients age 65 years than the patients age < 65 years. The association of gender and toxicity with 5-FU-based adjuvant chemotherapy for patients with colon carcinoma, especially in older patients, has been controversial. The North Central Cancer Treatment Group (NCCTG) recently reported a meta-analysis of 6 NCCTG-cancer-control trials involving 786 patients receiving 5-FU based chemotherapy for colorectal carcinoma (26). Including the effect of age in a logistic regression analysis showed that women who received 5-FU based therapy experienced greater severe toxicity than men. Altered pharmacokinetics in female patients as gender-related differences in dihydropyrimidine dehydrogenase (DPD) activity have been described by Milano and colleagues (27) and are likely to be responsible for the increased toxicity seen in females. We have also found that female patients have experienced grade 3-4 toxicites than male patients, however this was not statistically significant. In our analysis, patients aged 70 years or older were analyzed separately. The survival data and toxicity rates were not much different than those of the whole group. So, when the age limit is increased, there is no change implying different therapeutic decisions. Our analysis showed that elderly patients with colorectal cancer can receive 5-FU based adjuvant chemotherapy with a mild increase in toxicity compared to their younger counterparts. So, we believe that older patients with stage II or III colorectal cancer or resectable metastatic disease should be both offered adjuvant chemotherapy, especially infusional regimens of 5-FU are preferable because of lower toxicity rates unless there are no medical and psychosocial contraindications.
7 Öztop et al. 145 References 1. Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322: Efficacy of adjuvant fluorouracil and folinic acid in colon cancer: International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345: O Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15: Moertel CG, Flemming TR, Macdonald JS, et al. 5-FU plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995;122: Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer 1994;74(Suppl): Hutchins LF, Unger JM, Crowley JJ, et al. Underpresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341: Monfardini S, Sorio R, Boes GH, et al. Entry and evaluation of elderly patients in European Organization for Research and Treatment of Cancer (EORTC) new-drug development studies. Cancer 1995;76: Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345: Popescu RA, Norman A, Ross PJ, et al. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 1999;17: Rothenberg ML, Meropol NJ, Poplin EA, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: Summary findings of an independent panel. J Clin Oncol 2001;19: Zoniboni A, Labianca R, Marsoni S, et al. GIVIO-SITAC 01:a randomized trial of adjuvant 5-fluorouracil and folinic acid administered to patients with colon carcinoma-long term results and evaluation of the indicators of health-related quality of life. Gruppo Italiano Valutazione Interventi in Oncologia. Studio Italiano Terapia Adjuvante Colon. Cancer 1998;82: Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly: The experience of the Eastern Cooperative Oncology Group. Cancer 1983;52: Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 2001;93: Sundararajan V, Grann VR, Jacobson JS, et al. Variations in the use of adjuvant chemotherapy for node-positive colorectal cancer in the elderly: A population-based study. Cancer J 2001;7: Newcomb PA, Carbone PP. Cancer treatment and age: Patient perspectives. J Natl Cancer Inst 1993;85: Kane RL, Ouslander JG, Abrass IB. Essentials of clinical geriatrics. 4th ed. New York: McGraw-Hill, 1999: Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 2002;20: Fata F, Mirza A, Wood GC, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: A 10-year experience of the Geisinger Medical Center. Cancer 2002;94: Ronucci L, Fante R, Losi, et al. Survival for colon and rectal cancer in a population-based cancer registry. Eur J Cancer 1996;32: Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 1988;80: International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol 1999;17: Gastrointestinal Tumor Study Group. Adjuvant therapy of colon cancer: results of a prospectively randomized trial. N Engl J Med. 1984;310: Stein BN, Petrelli NJ, Douglas HO, et al. Age and sex are independent predictors of 5-fluorouracil toxicity: analysis of a large-scale phase III trial. Cancer 1995;75: Zalcberg J, Kerr D, Seymour L, et al. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Eur J Cancer 1998;34: Giovanazzi-Bannon S, Rademaker A, Lai G, et al: Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: An Illinois Cancer Center Study. J Clin Oncol 1994;12: Sloan J, Goldberg R, Sargent D, et al. Women experience greater toxicity with 5-FU based chemotherapy for colorectal cancer: a North Centarl Cancer Treatment Group (NCCTG) meta-analysis. Proc Am Soc Clin Oncol 2000;19: Milano G, Etienne MC, Cassuto-Viguier E, et al. Influence of sex and age on fluorouracil clearence. J Clin Oncol 1992;10:
Efficacy and Toxicity of Adjuvant Chemotherapy in Elderly Patients with Colon Carcinoma
 1931 Efficacy and Toxicity of Adjuvant Chemotherapy in Elderly Patients with Colon Carcinoma A 10-Year Experience of the Geisinger Medical Center Farid Fata, M.D. 1 Ayoub Mirza, M.D. 2 G. Craig Wood, M.S.
1931 Efficacy and Toxicity of Adjuvant Chemotherapy in Elderly Patients with Colon Carcinoma A 10-Year Experience of the Geisinger Medical Center Farid Fata, M.D. 1 Ayoub Mirza, M.D. 2 G. Craig Wood, M.S.
Current Status of Adjuvant Therapy for Colorectal Cancer
 Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD
 LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
Comparative Efficacy of Adjuvant Chemotherapy in Patients With Dukes B Versus Dukes C Colon Cancer: Results From
 Comparative Efficacy of Adjuvant Chemotherapy in Patients With Dukes B Versus Dukes C Colon Cancer: Results From Four National Surgical Adjuvant Breast and Bowel Project Adjuvant Studies (C-01, C-02, C-03,
Comparative Efficacy of Adjuvant Chemotherapy in Patients With Dukes B Versus Dukes C Colon Cancer: Results From Four National Surgical Adjuvant Breast and Bowel Project Adjuvant Studies (C-01, C-02, C-03,
Management of Advanced Colorectal Cancer in Older Patients
 Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Factors associated with delayed time to adjuvant chemotherapy in stage iii colon cancer
 Curr Oncol, Vol. 21, pp. 181-186 doi: http://dx.doi.org/10.3747/co.21.1963 DELAYED TIME TO ADJUVANT CHEMOTHERAPY ORIGINAL ARTICLE Factors associated with delayed time to adjuvant chemotherapy in stage
Curr Oncol, Vol. 21, pp. 181-186 doi: http://dx.doi.org/10.3747/co.21.1963 DELAYED TIME TO ADJUVANT CHEMOTHERAPY ORIGINAL ARTICLE Factors associated with delayed time to adjuvant chemotherapy in stage
Weekly 5-fluorouracil and leucovorin: achieving lower toxicity with higher dose-intensity in adjuvant chemotherapy after colorectal cancer resection
 Original article Annals of Oncology 15: 568 573, 2004 DOI: 10.1093/annonc/mdh134 Weekly 5-fluorouracil and leucovorin: achieving lower toxicity with higher dose-intensity in adjuvant chemotherapy after
Original article Annals of Oncology 15: 568 573, 2004 DOI: 10.1093/annonc/mdh134 Weekly 5-fluorouracil and leucovorin: achieving lower toxicity with higher dose-intensity in adjuvant chemotherapy after
Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal patients in the adjuvant setting
 ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
Irinotecan (CPT-11) in Patients with Advanced Colon Carcinoma Relapsing after 5-Fluorouracil-Leucovorin Combination
 Clinical Report Chemotherapy 2002;48:94 99 Irinotecan (CPT-11) in Patients with Advanced Colon Carcinoma Relapsing after 5-Fluorouracil-Leucovorin Combination N.B. Tsavaris a A. Polyzos b K. Gennatas c
Clinical Report Chemotherapy 2002;48:94 99 Irinotecan (CPT-11) in Patients with Advanced Colon Carcinoma Relapsing after 5-Fluorouracil-Leucovorin Combination N.B. Tsavaris a A. Polyzos b K. Gennatas c
Adjuvant chemotherapy outcomes in patients over 65 years with early stage colorectal carcinoma
 JBUON 2014; 19(4): 906-912 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Adjuvant chemotherapy outcomes in patients over 65 years with early
JBUON 2014; 19(4): 906-912 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Adjuvant chemotherapy outcomes in patients over 65 years with early
Adjuvant Chemotherapy for Patients with Resected Dukes C and High-risk B2 Colon Cancer with Fluorouracil and Levamisole
 733 Adjuvant Chemotherapy for Patients with Resected Dukes C and High-risk B2 Colon Cancer with Fluorouracil and Levamisole E Au,*FAMS, M Med (Int Med), MRCP, P T Ang,**FAMS, FACP, FRCP (Edin), F Seow-Choen,***FAMS,
733 Adjuvant Chemotherapy for Patients with Resected Dukes C and High-risk B2 Colon Cancer with Fluorouracil and Levamisole E Au,*FAMS, M Med (Int Med), MRCP, P T Ang,**FAMS, FACP, FRCP (Edin), F Seow-Choen,***FAMS,
ADJUVANT CHEMOTHERAPY FOR RESECTED COLON CANCER IN ELDERLY PATIENTS
 A POOLED ANALYSIS OF ADJUVANT CHEMOTHERAPY FOR RESECTED COLON CANCER IN ELDERLY PATIENTS DANIEL J. SARGENT, PH.D., RICHARD M. GOLDBERG, M.D., STACY D. JACOBSON, M.D., JOHN S. MACDONALD, M.D., ROBERTO LABIANCA,
A POOLED ANALYSIS OF ADJUVANT CHEMOTHERAPY FOR RESECTED COLON CANCER IN ELDERLY PATIENTS DANIEL J. SARGENT, PH.D., RICHARD M. GOLDBERG, M.D., STACY D. JACOBSON, M.D., JOHN S. MACDONALD, M.D., ROBERTO LABIANCA,
A clinical study of adjuvant chemotherapy in younger and elder rectal cancer patientsa
 A clinical study of adjuvant chemotherapy in younger and elder rectal cancer patientsa The role of postoperative chemotherapy (CT) is still unclear and the evidence for recommendations of adjuvant therapy
A clinical study of adjuvant chemotherapy in younger and elder rectal cancer patientsa The role of postoperative chemotherapy (CT) is still unclear and the evidence for recommendations of adjuvant therapy
Disclosures. Clinical and molecular features to guide adjuvant therapy. Personalized Medicine - Decision Tools -
 Disclosures Clinical and molecular features to guide adjuvant therapy Daniel Sargent Professor of Biostatistics & Oncology Mayo Clinic Consulting activities Amgen Pfizer Roche/Genentech Sanofi-Aventis
Disclosures Clinical and molecular features to guide adjuvant therapy Daniel Sargent Professor of Biostatistics & Oncology Mayo Clinic Consulting activities Amgen Pfizer Roche/Genentech Sanofi-Aventis
Stage III Colon Cancer Susquehanna Cancer Center Warren L Robinson, MD, FACP May 9, 2007
 Stage III Colon Cancer Susquehanna Cancer Center 1997-21 Warren L Robinson, MD, FACP May 9, 27 Stage III Colon Cancer Susquehanna Cancer Center 1997-21 Colorectal cancer is the third most common cancer
Stage III Colon Cancer Susquehanna Cancer Center 1997-21 Warren L Robinson, MD, FACP May 9, 27 Stage III Colon Cancer Susquehanna Cancer Center 1997-21 Colorectal cancer is the third most common cancer
NCCP Chemotherapy Regimen
 QUASAR (Modified) Fluorouracil (370mg/m 2 ) and Folinic Acid (50mg) Weekly INDICATIONS FOR USE: Regimen INDICATION ICD10 Code Treatment of metastatic colorectal cancer C18 00428a Adjuvant treatment of
QUASAR (Modified) Fluorouracil (370mg/m 2 ) and Folinic Acid (50mg) Weekly INDICATIONS FOR USE: Regimen INDICATION ICD10 Code Treatment of metastatic colorectal cancer C18 00428a Adjuvant treatment of
Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant Therapy of Locally Advanced Rectal Cancer
 Efficacy of Modified De Gramont and FOLFOX4 Regimens for Locally Advanced Rectal Cancer RESEARCH COMMUNICATION Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant
Efficacy of Modified De Gramont and FOLFOX4 Regimens for Locally Advanced Rectal Cancer RESEARCH COMMUNICATION Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant
Update on Chemotherapy for Advanced Colorectal Cancer
 Review Article [1] March 02, 2001 By Daniel G. Haller, MD [2] Efforts to improve the length and quality of life, as well as to expand treatment options, for patients with metastatic colorectal cancer have
Review Article [1] March 02, 2001 By Daniel G. Haller, MD [2] Efforts to improve the length and quality of life, as well as to expand treatment options, for patients with metastatic colorectal cancer have
NCCP Chemotherapy Regimen
 Modified Roswell Park (Fluorouracil 500mg/m 2 and Folinic Acid 50mg weekly x 6) Regimen INDICATIONS FOR USE: Regimen INDICATION ICD10 Code Treatment of metastatic colorectal cancer C18 00427a Adjuvant
Modified Roswell Park (Fluorouracil 500mg/m 2 and Folinic Acid 50mg weekly x 6) Regimen INDICATIONS FOR USE: Regimen INDICATION ICD10 Code Treatment of metastatic colorectal cancer C18 00427a Adjuvant
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
Original article. Introduction. Patients and methods
 Original article Annals of Oncology 16: 767 772, 2005 doi:10.1093/annonc/mdi159 Published online 7 April 2005 Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands
Original article Annals of Oncology 16: 767 772, 2005 doi:10.1093/annonc/mdi159 Published online 7 April 2005 Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands
Original article. E. Mitry 1 *, J.-Y. Douillard 2, E. Van Cutsem 3, D. Cunningham 4, E. Magherini 5, D. Mery-Mignard 5, L. Awad 5 & P.
 Original article Annals of Oncology 15: 1013 1017, 2004 DOI: 10.1093/annonc/mdh267 Predictive factors of survival in patients with advanced colorectal cancer: an individual data analysis of 602 patients
Original article Annals of Oncology 15: 1013 1017, 2004 DOI: 10.1093/annonc/mdh267 Predictive factors of survival in patients with advanced colorectal cancer: an individual data analysis of 602 patients
OWa 22 80) :IEZ
 Clinical Study Report: 20025409 Part 2 Date: 22 September 2008 OWa 22 80) 06 --- :IEZ Page 1 SYNOPSIS Name of the Sponsor: Name of Finished Product: Name of Active Ingredient: Immunex Corporation Panitumumab
Clinical Study Report: 20025409 Part 2 Date: 22 September 2008 OWa 22 80) 06 --- :IEZ Page 1 SYNOPSIS Name of the Sponsor: Name of Finished Product: Name of Active Ingredient: Immunex Corporation Panitumumab
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress?
 Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
Case Conference. Craig Morgenthal Department of Surgery Long Island College Hospital
 Case Conference Craig Morgenthal Department of Surgery Long Island College Hospital Neoadjuvant versus Adjuvant Radiation Therapy in Rectal Carcinoma Epidemiology American Cancer Society statistics for
Case Conference Craig Morgenthal Department of Surgery Long Island College Hospital Neoadjuvant versus Adjuvant Radiation Therapy in Rectal Carcinoma Epidemiology American Cancer Society statistics for
Phase I study of postoperative radiotherapy with concomitant weekly irinotecan, 5- fluorouracil and folinic acid in locally advanced rectal cancer.
 Journal of BUON 9: 255-261, 2004 2004 Zerbinis Medical Publications. Printed in Greece ORIGINAL ARTICLE Phase I study of postoperative radiotherapy with concomitant weekly irinotecan, 5- fluorouracil and
Journal of BUON 9: 255-261, 2004 2004 Zerbinis Medical Publications. Printed in Greece ORIGINAL ARTICLE Phase I study of postoperative radiotherapy with concomitant weekly irinotecan, 5- fluorouracil and
Surgical Management of Advanced Stage Colon Cancer. Nathan Huber, MD 6/11/14
 Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
Adjuvant therapy in older adults: controversies and challenges - Colorectal cancer -
 International Society of Geriatric Oncology Lisbon October 23 rd 25t h 2014 Adjuvant therapy in older adults: controversies and challenges - Colorectal cancer - Claus-Henning Köhne Klinik für Onkologie
International Society of Geriatric Oncology Lisbon October 23 rd 25t h 2014 Adjuvant therapy in older adults: controversies and challenges - Colorectal cancer - Claus-Henning Köhne Klinik für Onkologie
Chemotherapy of colon cancers
 Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
ORIGINAL ARTICLE. M. Bakogeorgos, G. Mountzios, G. Kotsantis, P. Economopoulou, N. Fytrakis, N. Kentepozidis. Summary.
 JBUON 2013; 18(3): 629-634 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Chemotherapy compliance, tolerance and efficacy in elderly and non-elderly
JBUON 2013; 18(3): 629-634 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Chemotherapy compliance, tolerance and efficacy in elderly and non-elderly
Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto
 Milano 05.10.2018 Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto Salvatore Corallo U.O.C. Oncologia Medica IRCCS Istituto Nazionale dei Tumori Milano CRC in elderly patients
Milano 05.10.2018 Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto Salvatore Corallo U.O.C. Oncologia Medica IRCCS Istituto Nazionale dei Tumori Milano CRC in elderly patients
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First?
 Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
The impact of operation center and the prognostic factors on the outcome of patients with stage II and stage III colorectal cancer
 Turkish Journal of Cancer Volume 38, No. 4, 28 175 The impact of operation center and the prognostic factors on the outcome of patients with stage II and stage III colorectal cancer ABDULLAH BÜYÜKÇELİK
Turkish Journal of Cancer Volume 38, No. 4, 28 175 The impact of operation center and the prognostic factors on the outcome of patients with stage II and stage III colorectal cancer ABDULLAH BÜYÜKÇELİK
Age and factors associated with access and time to postoperative adjuvant chemotherapy in colon cancer: a French epidemiological study
 Original Article Age and factors associated with access and time to postoperative adjuvant chemotherapy in colon cancer: a French epidemiological study Jean Capsec 1, Carole Lefebvre 1, Fabienne Chupé
Original Article Age and factors associated with access and time to postoperative adjuvant chemotherapy in colon cancer: a French epidemiological study Jean Capsec 1, Carole Lefebvre 1, Fabienne Chupé
Northwestern University, Division of Hematology/Oncology, Chicago, Illinois, USA. Key Words. Colon cancer Stage II Adjuvant chemotherapy
 The Oncologist Dialogues in Oncology Adjuvant Therapy in Stage II Colon Cancer: Current Approaches LISA BADDI, AL BENSON III Northwestern University, Division of Hematology/Oncology, Chicago, Illinois,
The Oncologist Dialogues in Oncology Adjuvant Therapy in Stage II Colon Cancer: Current Approaches LISA BADDI, AL BENSON III Northwestern University, Division of Hematology/Oncology, Chicago, Illinois,
Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers
 日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
RESEARCH ARTICLE. Joanne Chiu 1, Vikki Tang 1, Roland Leung 1, Hilda Wong 1, Kin Wah Chu 2, Jensen Poon 3, Richard J Epstein 4, Thomas Yau 1,3,5 *
 DOI:http://dx.doi.org/10.7314/APJCP.2013.14.11.6585 Adjuvant XELOX in Asian Patients with Colorectal Cancer RESEARCH ARTICLE Efficacy and Tolerability of Adjuvant Oral Capecitabine plus Intravenous Oxaliplatin
DOI:http://dx.doi.org/10.7314/APJCP.2013.14.11.6585 Adjuvant XELOX in Asian Patients with Colorectal Cancer RESEARCH ARTICLE Efficacy and Tolerability of Adjuvant Oral Capecitabine plus Intravenous Oxaliplatin
Low-dose capecitabine (Xeloda) for treatment for gastrointestinal cancer
 Med Oncol (2014) 31:870 DOI 10.1007/s12032-014-0870-2 ORIGINAL PAPER Low-dose capecitabine (Xeloda) for treatment for gastrointestinal cancer Jasmine Miger Annika Holmqvist Xiao-Feng Sun Maria Albertsson
Med Oncol (2014) 31:870 DOI 10.1007/s12032-014-0870-2 ORIGINAL PAPER Low-dose capecitabine (Xeloda) for treatment for gastrointestinal cancer Jasmine Miger Annika Holmqvist Xiao-Feng Sun Maria Albertsson
Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy
 Korean J Hepatobiliary Pancreat Surg 2011;15:152-156 Original Article Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy Suzy Kim 1,#, Kyubo
Korean J Hepatobiliary Pancreat Surg 2011;15:152-156 Original Article Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy Suzy Kim 1,#, Kyubo
ADJUVANT CHEMOTHERAPY...
 Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Peritoneal Involvement in Stage II Colon Cancer
 Anatomic Pathology / PERITONEAL INVOLVEMENT IN STAGE II COLON CANCER Peritoneal Involvement in Stage II Colon Cancer A.M. Lennon, MB, MRCPI, H.E. Mulcahy, MD, MRCPI, J.M.P. Hyland, MCh, FRCS, FRCSI, C.
Anatomic Pathology / PERITONEAL INVOLVEMENT IN STAGE II COLON CANCER Peritoneal Involvement in Stage II Colon Cancer A.M. Lennon, MB, MRCPI, H.E. Mulcahy, MD, MRCPI, J.M.P. Hyland, MCh, FRCS, FRCSI, C.
Sponsor / Company: Sanofi Drug substance(s): Docetaxel (Taxotere )
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Targeted Therapies in Metastatic Colorectal Cancer: An Update
 Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
GASTRIC & PANCREATIC CANCER
 GASTRIC & PANCREATIC CANCER ASCO HIGHLIGHTS 2005 Fadi Sami Farhat, MD Head of Hematology Oncology Division Hammoud Hospital University Medical Center Saida Lebanon Tel: +961 3 753 155 E-Mail: drfadi@drfadi.org
GASTRIC & PANCREATIC CANCER ASCO HIGHLIGHTS 2005 Fadi Sami Farhat, MD Head of Hematology Oncology Division Hammoud Hospital University Medical Center Saida Lebanon Tel: +961 3 753 155 E-Mail: drfadi@drfadi.org
Irinotecan. Class:Camptothecin. Indications : _Cervical cancer. _CNS tumor. _Esophageal cancer. _Ewing s sarcoma. _Gastric cancer
 Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Adjuvant therapy in colon cancer: which treatment in 2005?
 Annals of Oncology 16 (Supplement 4): iv69 iv73, 2005 doi:10.1093/annonc/mdi911 Adjuvant therapy in colon cancer: which treatment in 2005? F. Di Costanzo* & L. Doni Medical Oncology Unit, Department of
Annals of Oncology 16 (Supplement 4): iv69 iv73, 2005 doi:10.1093/annonc/mdi911 Adjuvant therapy in colon cancer: which treatment in 2005? F. Di Costanzo* & L. Doni Medical Oncology Unit, Department of
/m 2 Oxaliplatin 85 1 Q2W 1-3 Leucovorin Q2W 5-FU Q2W 5-FU Q2W
 癌症診療指引33 Adjuvant therapy of colon cancer mfolfox6 Oxaliplatin 85 1 Q2W 1-3 FOLFOX4 Oxaliplatin 85 1 Q2W 9 Leucovorin 200 1-2 Q2W 5-FU 400 1-2 Q2W 5-FU 600 1-2 Q2W FLOX Oxaliplatin 85 1,15,29 Q8W 4 Leucovorin
癌症診療指引33 Adjuvant therapy of colon cancer mfolfox6 Oxaliplatin 85 1 Q2W 1-3 FOLFOX4 Oxaliplatin 85 1 Q2W 9 Leucovorin 200 1-2 Q2W 5-FU 400 1-2 Q2W 5-FU 600 1-2 Q2W FLOX Oxaliplatin 85 1,15,29 Q8W 4 Leucovorin
Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia
 Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia Temario Generalidades Adyuvancia en colon y recto FU / Capecitabina
Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia Temario Generalidades Adyuvancia en colon y recto FU / Capecitabina
Medicinae Doctoris. One university. Many futures.
 Medicinae Doctoris The Before and The After: Can chemotherapy revise the trajectory of gastric and esophageal cancers? Dr. David Dawe MD, FRCPC Medical Oncologist Assistant Professor Disclosures None All
Medicinae Doctoris The Before and The After: Can chemotherapy revise the trajectory of gastric and esophageal cancers? Dr. David Dawe MD, FRCPC Medical Oncologist Assistant Professor Disclosures None All
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using
 BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer
 Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer Gabriela M. Vargas, MD Kristin M. Sheffield, PhD, Abhishek Parmar, MD, Yimei Han, MS, Kimberly M. Brown,
Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer Gabriela M. Vargas, MD Kristin M. Sheffield, PhD, Abhishek Parmar, MD, Yimei Han, MS, Kimberly M. Brown,
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Follow this and additional works at: Part of the Neoplasms Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2015 Will the Addition of Oxaliplatin to 5-Fluorouracil
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2015 Will the Addition of Oxaliplatin to 5-Fluorouracil
Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain
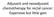 Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain Outline The problem Adjuvant therapy Neoadjuvant therapy Options Conclusion The problem 30 years ago: Local recurrence
Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain Outline The problem Adjuvant therapy Neoadjuvant therapy Options Conclusion The problem 30 years ago: Local recurrence
CLINICAL INVESTIGATION
 Research Article CLINICAL INVESTIGATION Research on the treatment of metastatic colon cancer patients treated by FOLFOXIRI: Efficacy and toxicity of first-line treatment in stage IV metastatic colorectal
Research Article CLINICAL INVESTIGATION Research on the treatment of metastatic colon cancer patients treated by FOLFOXIRI: Efficacy and toxicity of first-line treatment in stage IV metastatic colorectal
Cetuximab with Chemotherapy as Treatment for Stage III Colon or Metastatic Colorectal Cancer
 Cetuximab with Chemotherapy as Treatment for Stage III Colon or Metastatic Colorectal Cancer Cetuximab with Chemotherapy (CT) as First-Line Treatment for Metastatic Colorectal Cancer (mcrc): Analysis of
Cetuximab with Chemotherapy as Treatment for Stage III Colon or Metastatic Colorectal Cancer Cetuximab with Chemotherapy (CT) as First-Line Treatment for Metastatic Colorectal Cancer (mcrc): Analysis of
Advances in gastric cancer: How to approach localised disease?
 Advances in gastric cancer: How to approach localised disease? Andrés Cervantes Professor of Medicine Classical approach to localised gastric cancer Surgical resection Pathology assessment and estimation
Advances in gastric cancer: How to approach localised disease? Andrés Cervantes Professor of Medicine Classical approach to localised gastric cancer Surgical resection Pathology assessment and estimation
Reference No: Author(s) Approval date: 12/05/16. Committee. June Operational Date: Review:
 Reference No: Title: Author(s) Systemic Anti-Cancer Therapy (SACT) Guidelines for Biliary Tract Cancer (BTC) Dr Colin Purcell, Consultant Medical Oncologist on behalf of the GI Oncologists Group, Cancer
Reference No: Title: Author(s) Systemic Anti-Cancer Therapy (SACT) Guidelines for Biliary Tract Cancer (BTC) Dr Colin Purcell, Consultant Medical Oncologist on behalf of the GI Oncologists Group, Cancer
Jonathan Dickinson, LCL Xeloda
 Xeloda A blockbuster in the making Jonathan Dickinson, LCL Xeloda Xeloda unique tumor-activated mechanism Delivering more cancer-killing agent straight into cancer Highly effective comparable efficacy
Xeloda A blockbuster in the making Jonathan Dickinson, LCL Xeloda Xeloda unique tumor-activated mechanism Delivering more cancer-killing agent straight into cancer Highly effective comparable efficacy
Updates on the Conflict of Postoperative Radiotherapy Impact on Survival of Young Women with Cancer Breast: A Retrospective Cohort Study
 International Journal of Medical Research & Health Sciences Available online at www.ijmrhs.com ISSN No: 2319-5886 International Journal of Medical Research & Health Sciences, 2017, 6(7): 14-18 I J M R
International Journal of Medical Research & Health Sciences Available online at www.ijmrhs.com ISSN No: 2319-5886 International Journal of Medical Research & Health Sciences, 2017, 6(7): 14-18 I J M R
Radiation Therapy for Resectable Colon Cancer
 Review Article [1] February 01, 2006 By Brian G. Czito, MD [2], Johanna C. Bendell, MD [3], and Christopher G. Willett, MD [4] Colon cancer is a major public health problem. The primary treatment is resection.
Review Article [1] February 01, 2006 By Brian G. Czito, MD [2], Johanna C. Bendell, MD [3], and Christopher G. Willett, MD [4] Colon cancer is a major public health problem. The primary treatment is resection.
Chemotherapy for metastatic colon cancer: No effect on survival when the dose is reduced due to side effects
 Munker et al. BMC Cancer (2018) 18:455 https://doi.org/10.1186/s12885-018-4380-z RESEARCH ARTICLE Open Access Chemotherapy for metastatic colon cancer: No effect on survival when the dose is reduced due
Munker et al. BMC Cancer (2018) 18:455 https://doi.org/10.1186/s12885-018-4380-z RESEARCH ARTICLE Open Access Chemotherapy for metastatic colon cancer: No effect on survival when the dose is reduced due
Tumors in the Randomized German AIO study KRK-0306
 FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First- Line Treatment for Patients with Metastatic Colorectal Cancer (mcrc): Analysis of Patients with KRAS-Mutated Tumors in the Randomized German
FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First- Line Treatment for Patients with Metastatic Colorectal Cancer (mcrc): Analysis of Patients with KRAS-Mutated Tumors in the Randomized German
Adjuvant Chemotherapy
 State-of-the-art: standard of care for resectable NSCLC Adjuvant Chemotherapy JY DOUILLARD MD PhD Professor of Medical Oncology Integrated Centers of Oncology R Gauducheau University of Nantes France Adjuvant
State-of-the-art: standard of care for resectable NSCLC Adjuvant Chemotherapy JY DOUILLARD MD PhD Professor of Medical Oncology Integrated Centers of Oncology R Gauducheau University of Nantes France Adjuvant
Pharmacologyonline 1: (2010)
 THE EFFECT OF USING COMBINATION CHEMOTHERAPY IN COLORECTAL CANCER IN INDIA: A SINGLE INSTITUTE SURVEY Adiga Sachidananda*, Meena Kumari K**, Bairy KL***, Mohan Babu A**, Vadiraja BM+, Vidyasagar MS++ *Associate
THE EFFECT OF USING COMBINATION CHEMOTHERAPY IN COLORECTAL CANCER IN INDIA: A SINGLE INSTITUTE SURVEY Adiga Sachidananda*, Meena Kumari K**, Bairy KL***, Mohan Babu A**, Vadiraja BM+, Vidyasagar MS++ *Associate
Oncologic Outcomes of Stage IIIA Colon Cancer for Different Chemotherapeutic Regimens
 Original Article Journal of the Korean Society of J Korean Soc Coloproctol 2012;28(5):259-264 http://dx.doi.org/10.3393/jksc.2012.28.5.259 pissn 2093-7822 eissn 2093-7830 Oncologic Outcomes of Stage IIIA
Original Article Journal of the Korean Society of J Korean Soc Coloproctol 2012;28(5):259-264 http://dx.doi.org/10.3393/jksc.2012.28.5.259 pissn 2093-7822 eissn 2093-7830 Oncologic Outcomes of Stage IIIA
De-Escalate Trial for the Head and neck NSSG. Dr Eleanor Aynsley Consultant Clinical Oncologist
 De-Escalate Trial for the Head and neck NSSG Dr Eleanor Aynsley Consultant Clinical Oncologist 3 HPV+ H&N A distinct disease entity Leemans et al., Nature Reviews, 2011 4 Good news Improved response to
De-Escalate Trial for the Head and neck NSSG Dr Eleanor Aynsley Consultant Clinical Oncologist 3 HPV+ H&N A distinct disease entity Leemans et al., Nature Reviews, 2011 4 Good news Improved response to
Chemotherapy options and outcomes in older adult patients with colorectal cancer
 Critical Reviews in Oncology/Hematology 72 (2009) 155 169 Chemotherapy options and outcomes in older adult patients with colorectal cancer Muhammad W. Saif a,, Stuart M. Lichtman b a Yale University School
Critical Reviews in Oncology/Hematology 72 (2009) 155 169 Chemotherapy options and outcomes in older adult patients with colorectal cancer Muhammad W. Saif a,, Stuart M. Lichtman b a Yale University School
Outcome of rectal cancer after radiotherapy with a long or short waiting period before surgery, a descriptive clinical study
 Original Article Outcome of rectal cancer after radiotherapy with a long or short waiting period before surgery, a descriptive clinical study Elmer E. van Eeghen 1, Frank den Boer 2, Sandra D. Bakker 1,
Original Article Outcome of rectal cancer after radiotherapy with a long or short waiting period before surgery, a descriptive clinical study Elmer E. van Eeghen 1, Frank den Boer 2, Sandra D. Bakker 1,
THE RESULTS OF A NATIONAL INstitutes
 ORIGINAL CONTRIBUTION Adjuvant Chemotherapy for Stage III Colon Cancer Implications of Race/Ethnicity, Age, and Differentiation J. Milburn Jessup, MD Andrew Stewart, MS Frederick L. Greene, MD Bruce D.
ORIGINAL CONTRIBUTION Adjuvant Chemotherapy for Stage III Colon Cancer Implications of Race/Ethnicity, Age, and Differentiation J. Milburn Jessup, MD Andrew Stewart, MS Frederick L. Greene, MD Bruce D.
Adjuvant Treatment of Colorectal Cancer
 Adjuvant Treatment of Colorectal Cancer Adjuvant Treatment of Colorectal Cancer Brian M. Wolpin, MD; Jeffrey A. Meyerhardt, MD, MPH; Harvey J. Mamon, MD, PhD; Robert J. Mayer, MD Dr. Wolpin is Instructor
Adjuvant Treatment of Colorectal Cancer Adjuvant Treatment of Colorectal Cancer Brian M. Wolpin, MD; Jeffrey A. Meyerhardt, MD, MPH; Harvey J. Mamon, MD, PhD; Robert J. Mayer, MD Dr. Wolpin is Instructor
Cancer Treatment in the Elderly. Jeffrey A. Bubis, DO, FACOI, FACP Clay County, Baptist South, and Palatka
 Cancer Treatment in the Elderly Jeffrey A. Bubis, DO, FACOI, FACP Clay County, Baptist South, and Palatka Patients 65 and older are the fastest growing segment of the US population By 2030, it will comprise
Cancer Treatment in the Elderly Jeffrey A. Bubis, DO, FACOI, FACP Clay County, Baptist South, and Palatka Patients 65 and older are the fastest growing segment of the US population By 2030, it will comprise
Radiotherapy for rectal cancer. Karin Haustermans Department of Radiation Oncology
 Radiotherapy for rectal cancer Karin Haustermans Department of Radiation Oncology O U T L I N E RT with TME surgery? Neoadjuvant or adjuvant RT? 5 x 5 Gy or long-course CRT? RT with new drugs? Selection
Radiotherapy for rectal cancer Karin Haustermans Department of Radiation Oncology O U T L I N E RT with TME surgery? Neoadjuvant or adjuvant RT? 5 x 5 Gy or long-course CRT? RT with new drugs? Selection
Panitumumab After Resection of Liver Metastases From Colorectal Cancer in KRAS Wild-type Patients
 1 von 5 23.11.2011 10:52 Home Search Study Topics Glossary Full Text View Tabular View No Study Results Posted Related Studies Panitumumab After Resection of Liver Metastases From Colorectal Cancer in
1 von 5 23.11.2011 10:52 Home Search Study Topics Glossary Full Text View Tabular View No Study Results Posted Related Studies Panitumumab After Resection of Liver Metastases From Colorectal Cancer in
Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials
 Original article Annals of Oncology 15: 1330 1338, 2004 doi:10.1093/annonc/mdh344 Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis
Original article Annals of Oncology 15: 1330 1338, 2004 doi:10.1093/annonc/mdh344 Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis
Carcinoma del retto: Highlights
 Carcinoma del retto: Highlights Stefano Cordio Struttura Complessa di Oncologia Medica ARNAS Garibaldi Catania Roma 17 Febbraio 2018 Disclosures Advisory Committee, research funding and speakers bureau
Carcinoma del retto: Highlights Stefano Cordio Struttura Complessa di Oncologia Medica ARNAS Garibaldi Catania Roma 17 Febbraio 2018 Disclosures Advisory Committee, research funding and speakers bureau
trial update clinical
 clinical trial update by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UMPC Cancer Centers In order to provide the most up-to-date and efficacious care to their patients, oncologists must
clinical trial update by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UMPC Cancer Centers In order to provide the most up-to-date and efficacious care to their patients, oncologists must
Gastrointestinal Adverse Effects in Advanced Colorectal Carcinoma Patients Treated with Different Schedules of FOLFOX
 DOI:http://dx.doi.org/10.7314/APJCP.2014.15.19.8089 RESEARCH ARTICLE Gastrointestinal Adverse Effects in Advanced Colorectal Carcinoma Patients Treated with Different Schedules of FOLFOX Nusrat Bano 1
DOI:http://dx.doi.org/10.7314/APJCP.2014.15.19.8089 RESEARCH ARTICLE Gastrointestinal Adverse Effects in Advanced Colorectal Carcinoma Patients Treated with Different Schedules of FOLFOX Nusrat Bano 1
The International Duration Evaluation of Adjuvant Chemotherapy study: implications for clinical practice
 Editorial The International Duration Evaluation of Adjuvant Chemotherapy study: implications for clinical practice Marwan Fakih Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive
Editorial The International Duration Evaluation of Adjuvant Chemotherapy study: implications for clinical practice Marwan Fakih Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/24307 holds various files of this Leiden University dissertation Author: Broek, Colette van den Title: Optimisation of colorectal cancer treatment Issue
Cover Page The handle http://hdl.handle.net/1887/24307 holds various files of this Leiden University dissertation Author: Broek, Colette van den Title: Optimisation of colorectal cancer treatment Issue
Individual- and trial-level surrogacy in colorectal cancer
 Stat Methods Med Res OnlineFirst, published on February 19, 2008 as doi:10.1177/0962280207081864 SMM081864 2008/2/5 page 1 Statistical Methods in Medical Research 2008; 1 9 Individual- and trial-level
Stat Methods Med Res OnlineFirst, published on February 19, 2008 as doi:10.1177/0962280207081864 SMM081864 2008/2/5 page 1 Statistical Methods in Medical Research 2008; 1 9 Individual- and trial-level
Study population The study population comprised patients with completely resected Stage III colon cancer.
 Cost-effectiveness of oxaliplatin and capecitabine in the adjuvant treatment of stage III colon cancer Eggington S, Tappenden P, Pandor A, Paisley S, Saunders M, Seymour M, Sutcliffe P, Chilcott J Record
Cost-effectiveness of oxaliplatin and capecitabine in the adjuvant treatment of stage III colon cancer Eggington S, Tappenden P, Pandor A, Paisley S, Saunders M, Seymour M, Sutcliffe P, Chilcott J Record
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine
 BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer
 original article Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer Thierry André, M.D., Corrado Boni, M.D., Lamia Mounedji-Boudiaf, M.D., Matilde Navarro, M.D., Josep Tabernero,
original article Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer Thierry André, M.D., Corrado Boni, M.D., Lamia Mounedji-Boudiaf, M.D., Matilde Navarro, M.D., Josep Tabernero,
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine
 BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Evaluation of efficacy and toxicity of systemic chemotherapy of combined epirubicin, cisplatin and bolus 5- fluorouracil for hepatobiliary tumors
 Turkish Journal of Cancer Volume 36, No.2, 2006 69 Evaluation of efficacy and toxicity of systemic chemotherapy of combined epirubicin, cisplatin and bolus 5- fluorouracil for hepatobiliary tumors GÜZ
Turkish Journal of Cancer Volume 36, No.2, 2006 69 Evaluation of efficacy and toxicity of systemic chemotherapy of combined epirubicin, cisplatin and bolus 5- fluorouracil for hepatobiliary tumors GÜZ
BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion
 BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact Physician: GIAJFL Gastrointestinal
BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact Physician: GIAJFL Gastrointestinal
Long Term Outcomes of Preoperative versus
 RESEARCH ARTICLE Long Term Outcomes of Preoperative versus Postoperative Concurrent Chemoradiation for Locally Advanced Rectal Cancer: Experience from Ramathibodi Medical School in Thailand Pichayada Darunikorn
RESEARCH ARTICLE Long Term Outcomes of Preoperative versus Postoperative Concurrent Chemoradiation for Locally Advanced Rectal Cancer: Experience from Ramathibodi Medical School in Thailand Pichayada Darunikorn
S u p p o r t e d b y a n i n d e p e n d e n t E d u c a t i o n a l G r a n t f r o m B a y e r
 EXPERTS KNOWLEDGE SHARE with Prof. Köhne, Dr. Modest and Dr. Vecchione Madrid (Spain) Sunday September 10 th 2017 S u p p o r t e d b y a n i n d e p e n d e n t E d u c a t i o n a l G r a n t f r o m
EXPERTS KNOWLEDGE SHARE with Prof. Köhne, Dr. Modest and Dr. Vecchione Madrid (Spain) Sunday September 10 th 2017 S u p p o r t e d b y a n i n d e p e n d e n t E d u c a t i o n a l G r a n t f r o m
Is There a New Standard of Care for Adjuvant Therapy in Colon Cancer? When is 3 Months Enough?
 Is There a New Standard of Care for Adjuvant Therapy in Colon Cancer? When is 3 Months Enough? Jeffrey Meyerhardt, MD, MPH Dana-Farber Cancer Institute Boston, MA 1 Disclosure Ad Board: Genentech Honorarium:
Is There a New Standard of Care for Adjuvant Therapy in Colon Cancer? When is 3 Months Enough? Jeffrey Meyerhardt, MD, MPH Dana-Farber Cancer Institute Boston, MA 1 Disclosure Ad Board: Genentech Honorarium:
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
Fluorouracil/Leucovorin/Interferon α-2a in patients with advanced colorectal cancer. Effects of maintenance therapy on remission duration.
 Chapter 8 Fluorouracil/Leucovorin/Interferon α-2a in patients with advanced colorectal cancer. Effects of maintenance therapy on remission duration. Jan Buter, Harm A.M. Sinnige, Dirk Th. Sleijfer, Elisabeth
Chapter 8 Fluorouracil/Leucovorin/Interferon α-2a in patients with advanced colorectal cancer. Effects of maintenance therapy on remission duration. Jan Buter, Harm A.M. Sinnige, Dirk Th. Sleijfer, Elisabeth
Hyponatremia in small cell lung cancer is associated with a poorer prognosis
 Original Article Hyponatremia in small cell lung cancer is associated with a poorer prognosis Wenxian Wang 1, Zhengbo Song 1,2, Yiping Zhang 1,2 1 Department of Chemotherapy, Zhejiang Cancer Hospital,
Original Article Hyponatremia in small cell lung cancer is associated with a poorer prognosis Wenxian Wang 1, Zhengbo Song 1,2, Yiping Zhang 1,2 1 Department of Chemotherapy, Zhejiang Cancer Hospital,
EXCLUSIONS: Suspected dihydropyrimidine dehydrogenase (DPD) deficiency (see Precautions)
 BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
Exploring and Validating Surrogate Endpoints in Colorectal Cancer. Center, Pittsburgh, USA
 Page 1 Exploring and Validating Surrogate Endpoints in Colorectal Cancer Tomasz Burzykowski, PhD 1,2, Marc Buyse, ScD 1,3, Greg Yothers PhD 4, Junichi Sakamoto, PhD 5, Dan Sargent, PhD 6 1 Center for Statistics,
Page 1 Exploring and Validating Surrogate Endpoints in Colorectal Cancer Tomasz Burzykowski, PhD 1,2, Marc Buyse, ScD 1,3, Greg Yothers PhD 4, Junichi Sakamoto, PhD 5, Dan Sargent, PhD 6 1 Center for Statistics,
DALLA CAPECITABINA AL TAS 102
 DALLA CAPECITABINA AL TAS 102 Milano 29 settembre 2016 LE PROSPETTIVE NELLA RICERCA Armando Santoro Humanitas Cancer Center THE 1,2.AND 3 LINE CHEMOTHERAPY IN CRC M BEVACIZUMAB AFLIBERCET RAS wt RAS mu
DALLA CAPECITABINA AL TAS 102 Milano 29 settembre 2016 LE PROSPETTIVE NELLA RICERCA Armando Santoro Humanitas Cancer Center THE 1,2.AND 3 LINE CHEMOTHERAPY IN CRC M BEVACIZUMAB AFLIBERCET RAS wt RAS mu
