The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma.
|
|
|
- Godfrey Lucas
- 5 years ago
- Views:
Transcription
1 Northeastern University From the SelectedWorks of Arun Iyer Spring April, 2011 The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma. Arun K. Iyer, Northeastern University Available at:
2 Biomaterials 32 (2011) 2605e2613 Contents lists available at ScienceDirect Biomaterials journal homepage: The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma Arun K. Iyer a, Yang Su b, Jinjin Feng a, Xiaoli Lan a, Xiaodong Zhu b, Yue Liu b, Dongwei Gao a, Youngho Seo a, Henry F. VanBrocklin a,c, V. Courtney Broaddus c,d, Bin Liu b,c, Jiang He a,c, * a Center for Molecular and Functional Imaging, Department of Radiology and Biomedical Imaging, University of California at San Francisco, San Francisco, CA 94143, USA b Department of Anesthesia, University of California at San Francisco, San Francisco, CA 94110, USA c UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA 94143, USA d Lung Biology Center, Department of Medicine, University of California at San Francisco, San Francisco, CA 94143, USA article info abstract Article history: Received 8 November 2010 Accepted 30 November 2010 Available online 20 January 2011 Keywords: Immunoliposomes scfv antibody Mesothelioma SPECT/CT imaging Tumor targeting Immunoliposomes (ILs) anchored with internalizing human antibodies capable of targeting all subtypes of mesothelioma can be useful for targeted imaging and therapy of this malignant disease. The objectives of this study were to evaluate both the in vitro and in vivo tumor targeted internalization of novel internalizing human single chain antibody (scfv) anchored ILs on both epithelioid (M28) and sarcomatoid (VAMT-1) subtypes of human mesothelioma. ILs were prepared by post-insertion of mesothelioma-targeting human scfv (M1) onto preformed liposomes and radiolabeled with 111 In ( 111 In-IL-M1), along with control non-targeted liposomes ( 111 In-CL). Incubation of 111 In-IL-M1 with M28, VAMT-1, and a control non-tumorigenic cell line (BPH-1) at 37 C for 24 h revealed efficient binding and rapid internalization of ILs into both subtypes of tumor cells but not into the BPH-1 cells; internalization accounted for approximately 81e94% of total cell accumulation in mesothelioma cells compared to 37 e55% in control cells. In tumor-bearing mice intravenous (i.v.) injection of 111 In-IL-M1 led to remarkable tumor accumulation: 4% and 4.7% injected dose per gram (% ID/g) for M28 and VAMT-1 tumors, respectively, 48 h after injection. Furthermore, tumor uptake of 111 In-IL-M1 in live xenograft animal models was verified by single photon emission computed tomography (SPECT/CT). In contrast, i.v. injection of 111 In-CL in tumor-bearing mice revealed very low uptake in both subtypes of mesothelioma, 48 h after injection. In conclusion, M1 scfv-anchored ILs showed selective tumor targeting and rapid internalization into both epithelioid and sarcomatoid subtypes of human mesothelioma, demonstrating its potential as a promising vector for enhanced tumor drug targeting. Ó 2010 Elsevier Ltd. All rights reserved. 1. Introduction Malignant mesothelioma is a deadly cancer without a current curative treatment [1,2]. Mesothelioma develops from the mesotheliumda protective membrane that covers many of the body s internal organs, decades after exposure to asbestos [3e5]. Histologically, mesothelioma is categorized into three major subtypes: epithelioid, sarcomatoid and biphasic (or mixed) mesothelioma [6e8]. The diagnosis of mesothelioma is often delayed because the symptoms are similar to other conditions. Mesothelioma in general has * Corresponding author. Center for Molecular and Functional Imaging, Department of Radiology and Biomedical Imaging, University of California at San Francisco, 185 Berry Street, Suite 350, San Francisco, CA 94143, USA. Tel.: þ ; fax: þ address: Jiang.He@ucsf.edu (J. He). a poor prognosis. The response to treatment is limited and varies with the subtype and stage of the disease; for instance patients diagnosed with the more aggressive sarcomatoid mesothelioma are often less likely to respond to treatment than those with epithelioid mesothelioma [9,10]. With ongoingexposure to asbestos in the communityand with populations already known to be at risk, there is an urgent unmet need to develop new strategies for early diagnosis and treatment of mesothelioma, irrespective of their subtype or origin. One promising approach for treating mesothelioma is by utilizing nanotechnology to develop multifunctional nanocarriers [11]. Among the several nanocarriers that are available for drug delivery, liposomes have been extensively studied and are FDAapproved as a safe material for drug delivery applications [12]. In this regard, PEGylated liposomes are ideally suited because of their favorable pharmacokinetic profiles and long plasma half-life in vivo [13,14]. Also, nanosized liposomes can take advantage of the /$ e see front matter Ó 2010 Elsevier Ltd. All rights reserved. doi: /j.biomaterials
3 2606 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e2613 enhanced permeability and retention (EPR)-effect for tumor drug targeting making them versatile carriers for targeted anti-cancer therapy [15,16]. Moreover, liposome s can be easily tailored to encapsulate therapeutic payloads as well as surface functionalized with multifunctional agents such as targeting ligands, antibodies, peptides and/or radiotracers for simultaneous imaging/detection and therapeutic applications [11,17e19]. In order to develop multifunctional immunoliposomes (ILs), as a therapy aimed at mesothelioma, the first step would involve development of ligands or of antibodies that can selectively target overexpressed receptors or antigens on mesothelioma tumor cells. Along these lines, we have identified a panel of internalizing human single chain (scfv) antibodies that can not only target cell surface antigens associated with both epithelioid and sarcomatoid subtypes of human mesothelioma [20] but also internalize rapidly into mesothelioma tumor cells. Also, we showed that these scfvs bind to mesothelioma tumors ex vivo, thereby recognizing clinically represented tumor antigens [20] and offer the potential to target tumor cells selectively [21]. More importantly, we have recently identified melanoma cell adhesion molecule (MCAM/CD146/ MUC18) as a target antigen for mesothelioma tumor cells by screening the yeast surface human cdna display library with mesothelioma-targeting phage antibody [21]. MCAM was overexpressed in more than 80% of epithelioid and sarcomatous mesothelioma tissues, but was not highly expressed in normal mesothelial tissues [21]. Recently, we also showed that one of the scfv identified by phage display library (M40) radiolabeled with 99m Tc ( 99m Tc-M40) could selectively target and internalize rapidly into both epithelioid and sarcomatoid mesothelioma in vitro and in vivo and the tumors could be clearly visualized by small animal- SPECT/CT (Iyer et al., unpublished results). In the current study, we have utilized the targeting and rapid internalizing function of one the scfv (M1) by conjugating them to liposomes, to form target-internalizing ILs. As a further translation of these ILs for clinical development, we have investigated both the in vitro and in vivo tumor targeting and imaging of the internalizing human single chain antibody fragment (M1 scfv) anchored ILs radiolabeled with 111 In ( 111 In-IL-M1) on both epithelioid (M28) and sarcomatoid (VAMT-1) subtypes of human mesothelioma. 2. Materials and methods 2.1. Materials All the lipids and their derivatives such as 1-hexadecanoyl-2-(9Z-octadecenoyl)-snglycero-3-phosphocholine (POPC), (1,2dimyristoyl-sn-glycero-3-phospho-ethanolam ine-n-diethylene-triamine-pentaaceticacid) (DMPE-DTPA), 1,2-distearoyl-sn-glycero-3- phospho-ethanolamine-n-[amino(polyethyleneglycol)-2000] (DSPE-mPEG 2000 ), and 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-[maleimide(polyethyleneglycol)- 2000] (DSPE-PEG MAL) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol obtained from SigmaeAldrich Chemical Co., (St. Louis, MO) was recrystallized in methanol before use. Dry chloroform, DMSO (anhydrous) and phosphate buffer saline (10 PBS) were purchased from SigmaeAldrich Chemical Co.,(St. Louis, MO). 2-mercaptoethanolamine (2-MEA) was obtained from Pierce Biotechnology (Rockford, IL). A mini-extruder was purchased from Avanti Polar Lipids Inc., (Alabaster, AL), with Whatman Ò polycarbonate membrane filter accessories for extrusion of the liposomes. PD-10 columns (Sephadex G-25) and Sepharose GL-4B-10 were purchased from GE Healthcare (Buckinghamshire, UK). 111 InCl 3 was obtained from Perkin Elmer life Science Inc., (Boston, MA) Production of M1 scfv targeting mesothelioma To produce soluble scfvs (M1), genes encoding the scfvs were spliced into an expression vector imparting a c-myc epitope and a hexahistidine tag at the carboxy terminus [22]. To produce soluble cysteine tagged scfvs (M1-CH), a second vector was used to impart a cysteine residue and a hexahistidine tag at the carboxy terminus [22]. Antibody fragments were harvested from the bacterial periplasmic space and purified by immobilized metal affinity chromatography and gel filtration as described before [22]. Following overnight dialysis in phosphate buffer saline (PBS), the antibody purity was determined by gel electrophoresis and its concentration was determined using a UV spectrometer (NanoDrop Products/ Thermo Scientific, Wilmington, DE) by quantifying the absorbance at 280 nm Preparation of liposomes Liposomes were prepared by thin lipid film hydration followed by sonication and extrusion as reported by MacDonald et al., [23] with modifications. Briefly, liposomes composed of a room temperature transition lipid (POPC), cholesterol, DSPE-mPEG 2000, and DMPE-DTPA, were combined in a molar ratio of 200:133:2:0.3 respectively. A thin lipid film was formed by evaporating the solvent on a rotary evaporator (Labrota 4000, Heidolph Instruments GmbH, Schwabach, Germany) under vacuum in a 10 ml round bottom flask. The lipid layer was further subjected to overnight drying in a vacuum desiccator. Each liposome batch consisted of 50 mmol/l phospholipid and was rehydrated in 1 PBS (ph 7.4). Hydration of the lipid films was done at room temperature, involving vigorous vortexing (Vortex- Genie, Scientific Industries, Bohemia, NY) for 15 min, followed by sonication (2510 Bransonic, Branson Ultrasonics, Danbury, CT) for 1 h. The resulting multi-lamellar liposomes were repeatedly extruded (11 times) at room temperature through Whatman Ò polycarbonate membranes filters of gradually decreasing pore size of 0.8, 0.4, 0.2, 0.1 and 0.08 mm respectively, using a mini-extruder, yielding unilamellar liposomes of w100 nm diameter. The hydrodynamic diameter of the liposomes was determined by dynamic light scattering (Malvern Instruments, Southborough, MA) and the z potentials were determined with a Malvern Zetasizer IV (Malvern Instruments, Southborough, MA). The phospholipid concentration of the liposome was determined by a phosphorous assay as described [24] Preparation of scfv-anchored immunoliposomes Reduction of M1 scfv The reduction of cysteine residues in the scfv (M1-CH) was performed using a standard procedure provided by the manufacturer (Pierce Biotechnology, Rockford, IL). Briefly, 100 ml (6 mg/ml) of M1-CH, was mixed with 1.5 ml EDTA (0.5 M, ph 8.5) and 11 ml of a 2-MEA (20 mmol/l, 6 mg/100 ml stock solution). The reaction was allowed to proceed at 37 C for 1.5 h. The efficiency of cysteine reduction was assayed using Ellmann s reagent, and was typically >90%. Subsequently, the reduced antibody was purified using Sephadex G-25 column chromatography (PD-10, GE Healthcare), equilibrated with 0.1 M NaHCO 3 /1 mm EDTA buffer (ph 8.0). The purified fraction was concentrated by using a centrifugal filtration device (Millipore, Milford, MA) with 10 kda molecular weight cut-off (MWCO) filter, at 3000 rpm for 15 min, and the final concentration of scfv was determined using a UV spectrometer (NanoDrop Products/Thermo Scientific, Wilmington, DE), as well as a BCA protein assay kit (Pierce Biotechnology, Rockford, IL) Conjugation of reduced M1-CH scfv with Mal-PEG DSPE The reduced M1-CH scfv were conjugated to Mal-PEG-DSPE as described [25,26]. Briefly, a stock solution of MAL-PEG DSPE (20 mg/ml) was first prepared using dry DMSO. A 5 ml MAL-PEG DSPE stock solution was reacted with 100 ml (200 mg) of the reduced ScFv in 0.1 M NaHCO 3 /1 mm EDTA buffer (ph 8.0), corresponding to 5:1 M excess of maleimide groups to that of reduced scfv. The conjugation reaction was carried out for 1.5 h at room temperature. Subsequently, Sephadex G-25 column chromatography (PD-10, GE Healthcare) was performed to purify the conjugate and exchange the buffer to 1 PBS (ph 7.4). Purified fractions were pooled and concentrated by using Amicon Ò Ultra-4 centrifugal filtration device (Millipore, Milford, MA) with a 10 kda MWCO filter. This process resulted in formation of an active surface for conjugation of the scfvs to the liposomes Preparation of immunoliposomes To obtain ILs, the insertion of the scfv conjugated PEG (DSPE-PEG M1) onto preformed liposomes was performed by employing a post-insertion technique [27]. ILs were prepared with varied antibody density ranging from 1 to 200 antibody molecules per vesicle. For this purpose varied concentration of liposome (lipid) in (0.01 M PBS) was incubated with fixed amount of DSPE-PEG M1 scfv for 1 h (as shown in Table 1), under mild rotation using a rotary evaporator (Labrota 4000, Heidolph Instruments GmbH, Schwabach, Germany) without applying vacuum. As a result, the conjugates become attached to the outer lipid layer of the vesicles via Table 1 Concentration of liposome (lipid) to scfv ratios to obtain varied number of scfv per vesicle is shown. Lipid conc. No. of vesicles a ScFv conc. No. of ScFv 150 mm (100 ml) mg mm (100 ml) mg mm (100 ml) mg mm (100 ml) mg mm (100 ml) mg No of scfv/vesicle a Based on an assumption that 100 nm size liposomes form unilamellar vesicles.
4 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e hydrophobic DSPE domains. The purification of scfv-anchored ILs from the unconjugated scfvs (DSPE-PEG M1) was performed using a Sepharose CL-4B-10 (GE Healthcare) gel chromatography using 1 PBS as the mobile phase Radiolabeling of control liposome and immunoliposomes with 111 In For the purpose of radiolabeling the liposomes, typically 0.5e1.0 mci (18.5e37 MBq) of 111 In was used. 15 ml (0.05 N HCl) of 111 InCl 3 (Perkin Elmer Inc., Boston, MA) was taken in an eppendorf tube and 9 ml of 0.5 M sodium acetate was added to adjust the ph to w5.5e6.0. Then 100 ml of the control (non-targeted) liposomes or ILs was added to the eppendorf tube and incubated for 1 h at room temperature. Subsequently, Sephadex G-25 (GE Healthcare) column chromatography using PBS as the mobile phase was performed to purify the 111 In labeled control liposomes or ILs. Both size exclusion chromatography (Waters Corp., Milford, MA) and radio-thin layer chromatography (TLC) (Bioscan Radio-TLC Imaging Scanner, Bioscan Inc., Washington DC) were used to characterize the 111 In labeled CLs ( 111 In-CL) or ILs ( 111 In-IL-M1) for labeling efficiency, purity as well as for assessing the in vitro stability of the radiolabeled liposomes in buffers and serum. In the case of radio-tlc, the macromolecular 111 In-CL or 111 In-IL-M1 remain at the original loading position whereas the unbound radioligand migrates with the solvent front. The labeling efficiency was estimated from the ratio of radioactivity at the origin compared to the total applied In vitro cell binding and internalization studies 1 million M28, VAMT-1, or control non-tumorigenic (BPH-1) cells were suspended in RPMI-1640 media with 10% fetal bovine serum at 37 C. Approximately 150 kbq 111 In labeled ILs ( 111 In-IL-M1) in a final concentration ranging from 1 nmol/l to 80 nmol/l were added to the cell suspensions and incubated at 37 Cin5%CO 2 for 24 h. After 24 h, the cells were resuspended, washed twice with ice-cold PBS (ph 7.2) and then washed twice with ice-cold glycine buffer (0.05 mol/l glycine solution, 150 mmol/l NaCl, ph adjusted to 2.8 with 1 N HCl) to distinguish between cell surface-bound (acid releasable) and internalized (acid resistant) radioligand. Finally, cells were lysed with 1 N NaOH at 37 C for 10 min. The radioactivity in the cells were measured by using a gamma-counter (Wizard, Perkin Elmer, Milwaukee, WI) and expressed as the percentage of applied activity normalized to 1 million cells Animal studies Animal procedures were performed according to a protocol approved by University of California, San Francisco, Institutional Animal Care and Use Committee Xenograft model Six-week-old male nu/nu mice were purchased from Charles River Laboratories (Wilmington, MA). For tumor inoculation, 1 million M28 and VAMT-1 cells in 200 ml of PBS were administered subcutaneously to seed tumor growth into the right and left shoulders of the animal respectively. Growing tumors were palpated, and the diameters were measured by a vernier caliper. The experiments were commenced when tumor size reached w3e5 mm in diameter Biodistribution studies Tumor-bearing nude mice in groups of 4 animals were injected intravenously (i.v.) with 18.5e37 MBq (0.5e1.0 mci) of 111 In-IL-M1 or 111 In-CL. The mice were euthanized and dissected at 24 h or 48 h after injection of ILs or CLs. Blood, tumor, and major organs were harvested and weighed. The radioactivity in the tissues was measured using a gamma-counter (Wizard, Perkin Elmer, Milwaukee, WI). The results are presented as percentage injected dose per gram (% ID/g) of tissue/organ SPECT/CT imaging Mice were imaged with a dedicated small animal-spect/ct system (FLEX X- SPECT/X-O, Gamma Medica-Ideas, Inc., Northridge, CA). For anatomical correlation, the CT was first performed after 18.5e37 MBq of 111 In labeled liposomes was injected intravenously. As a general procedure, the mouse was placed in the mouse bed and positioned in front of a 2.0 mm diameter pinhole collimator designed for high energy photons (171 and 245 kev) emitted by 111 In. The spatial resolution and sensitivity of the pinhole SPECT system had been characterized from phantom studies and for this imaging geometry, the system spatial resolution was 2.0 mm with a sensitivity of 4600 counts/min/mbq (170 counts/min/mci). A planar image of the line source attached to the holder was acquired for post-acquisition correction of misalignment error. SPECT imaging was initiated 24e48 h after injection of the radiolabeled liposomes, and 64 projection views were acquired over a 360 angle rotation in a matrix. The acquisition time ranged from 30 s/projection to 60 s/projection for a total of 0.5e1 h approximately Statistical analysis Allquantitativedataare reportedasmean SD. Statisticalanalysis wasperformed using a Student s t-test and with more than two data sets, ANOVAwas used to compare results. Data were considered as statistically significant for P values of 0.05 or less. 3. Results 3.1. Preparation of liposomes and immunoliposomes For the preparation of liposomes and ILs the use of room temperature transition POPC lipid was advantageous because the extrusion process to form unilamellar liposomes could be carried out at room temperature. The process of sonication and extrusion of lipid films resulted in formation of unilamellar vesicles of w100 nm size. Measurement of the hydrodynamic diameter of the control non-targeted liposome (CL) by dynamic light scattering showed that the size was nm (Table 2). For the preparation of ILs a post-insertion technique was used [27]. The cysteine residues on the scfv were first reduced for facilitating the conjugation of the scfv to DSPE-PEG-maleimide. The efficiency of cysteine reduction was >90% as assayed by Ellmann s reagent [28]. The conjugation of reduced scfv to DSPE-PEG-maleimide resulted in an active surface for insertion of scfv onto the liposome vesicle. Typically 80e85% of the added scfv conjugate (DSPE-PEG-M1) was incorporated into ILs as observed by BCA protein assay, performed after insertion and purification of the ILs. As seen in Table 2, the hydrodynamic size of the ILs increased from w100 nm (for the control non-targeted liposomes) to nm, for liposomes inserted with w50 scfv per vesicle. The size further increased to nm when the scfv number was increased to 100 per vesicle indicating the successful attachment of the scfvs to the liposomes thereby forming targeted ILs. For facilitating radiolabeling of CLs and ILs, a DTPA-conjugated lipid (DMPE-DTPA) was incorporated during liposome fabrication similar to the one reported earlier [29]. The radiolabeling efficiency was >98% for both the 111 In-CL and 111 In-IL-M1 as determined by radio-tlc and size exclusion chromatography (SEC), indicating the successful labeling of the liposomes. Also, SEC revealed that the liposomal formulation were stable and eluted as a single peak when incubated in 0.01 M phosphate buffer saline (ph 7.4) or 10% serum at 37 C for up to 36 h (data not shown). The hydrodynamic size of the liposomes also remained unaltered (w100 nm) during storage for up to 1 week at room temperature or up to one month at 4 Cas determined by dynamic light scattering (data not presented) In vitro cell binding and internalization studies In order to determine the in vitro cell binding and internalization potentials of the M1 ScFv conjugated ILs, varied density of ScFv per vesicle was inserted onto 111 In labeled liposomes (as shown in Table 1.) and incubated with a sarcomatoid mesothelioma (VAMT-1) cell line which previously showed rapid and selective binding and internalization of a 99m Tc-labeled M1 scfv during a preliminary random antibody library screening [20]. From the results (Fig. 2), it can be seen that at the tested concentration of liposomes (from 1 nm to 60 nm of lipid), as the density of ScFv per vesicle increased, the internalization of the 111 In labeled M1-ILs also increased until about 50e60 ScFv per vesicle and above this threshold number, the internalization of ILs plateaued, and the binding/internalization remained almost constant (Fig. 2). Table 2 Hydrodynamic size and zeta potentials of non-targeted and M1 scfv targeted immunoliposome are shown. IL-M1 (50) and IL-M1 (100) are the M1 scfv targeted immunoliposome inserted with 50 and 100 scfv per vesicle respectively. Liposome formulation Hydrodynamic diameter (nm) Zeta potentials (mv) Non-targeted control liposome IL-M1 (50) IL-M1 (100)
5 2608 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e2613 Fig. 1. A schematic of multifunctional sterically stabilized long circulating immunoliposome. The figure shows two modes of conjugating the scfv antibody to the liposome; in Type A immunolipsome the scfv antibody is directly conjugated onto the liposome whereas in Type B immunoliposome, the scfv antibody is conjugated onto the liposome via a flexible spacer (such as DSPE-PEG). In the next study we investigated the effect of concentration of lipid (liposome) on both the binding and internalization of 111 In-IL- M1 (50) having an optimal scfv density of w50 per vesicle in both epithelioid (M28) and sarcomatoid mesothelioma (VAMT-1) cells. The results were compared with a control non-tumorigenic cell line (BPH-1) as shown in Fig. 3. It can be seen from Fig. 3 that after 24 h incubation of 111 In-IL-M1 (50) at all the lipid concentrations tested (from 1 nm to 60 nm), the total amount (% of total) bound and the total amount internalized was much higher (>85%) for both the mesothelioma cells in comparison to the control non-tumorigenic (BPH-1) cells (<35%). It is also interesting to note that for the case of mesothelioma cells, the ratio of internalized component versus total bound component (% of total radioactivity) was w90 (Fig. 4B) indicating that a majority of the ILs that bound to the tumor cells were rapidly internalized into the cells Small animal-spect/ct imaging and biodistribution of 111 In labeled immunoliposomes The 111 In-IL-M1 (50) formulation containing an optimal number of w50 scfv per vesicle was used to investigate the in vivo tumor % Radioactivityinternalized of total Optimal number~50 scfv/vesicle [ 111 In-IL-M1(50)] Lipid 60nM Lipid 40nM Lipid 20nM Lipid 10nM Lipid 1nM Number of ScFv per vesicle Fig. 2. In vitro internalization of 111 In-IL-M1 in VAMT-1 cells. The internalization of 111 In-IL-M1 was tested with varied number of M1 scfv per liposome ranging from 1 to 200 scfv per vesicle and at varied lipid concentrations from 1 nm to 60 nm as shown, on VAMT-1 cells incubated at 37 C for 24 h (n ¼ 4). targeted imaging and biodistribution of mice bearing both epithelioid (M28) and sarcomatoid (VAMT-1) mesothelioma tumor xenografts. The grafting of the two different subtypes of mesothelioma tumors (M28 and VAMT-1) in a single animal was useful in visualizing (and comparing) the tumor uptake of the radiolabeled ILs in both the tumor subtypes simultaneously, using SPECT/CT. The result of SPECT/CT imaging using live xenograft mice model is shown in Fig. 5. It can be seen from the image taken 24 h post injection that the 111 In-IL-M1 (50) showed marked uptake into both M28 and VAMT-1 mesothelioma tumors (Video S1). There was also high uptake in the liver and the spleen. The imaging results corroborated the ex vivo biodistribution studies performed 24 and 48 h after injection of the 111 In-IL-M1 (50) (Table 3, Figs. 6 and 7). It can be seen from Table 3 and Figs. 6 and 7 that 111 In-IL-M1 (50) was cleared from most normal organs (except the liver and the spleen), giving M28 and VAMT-1 tumors to blood ratios of 31:1 and 36:1 and tumor to muscle ratios of 47:1, 55:1 respectively at 48 h after injection. More importantly, at 48 h after injection there was markedly high and almost comparable tumor uptake of and % ID/g of tissue for M28 and VAMT-1 tumors respectively, which were much higher than all organs/tissues studied (except the liver ) (Table 3 and Fig. 6). In contrast, the control study using non-targeted liposome ( 111 In-CL) exhibited tumor uptake of only and % ID/g for M28 and VAMT-1 tumors respectively (Table 1, Fig. 7). Supplementary video related to this article can be found at doi: /j.biomaterials Discussion Malignant mesothelioma is a deadly tumor that currently has no curative treatment option [2,30]. A few earlier studies using non-targeted liposomes were found useful for treating mesothelioma in the clinic [31e34]. Moreover, current developments using immunoliposomes (ILs) have shown promise for targeted anti-cancer therapies [35e40]. In this report we have focused our attention on developing ILs anchored with internalizing (M1) scfvs and investigated both their in vitro and in vivo tumor targeted binding and internalization towards two different subtypes of human mesothelioma, derived from epithelioid (M28) and sarcomatoid (VAMT-1) origins. The composition of the liposomes and the method of their fabrication, as well as their stability are some of the important parameters that have to be tailored for particular drug delivery application [41e43]. In this regard, our liposomes showed good in vitro stability in PBS or serum at 37 C up to 72 h and their size remained unaltered (w100 nm) on storage up to 1 month at 4 C (data not shown). For the IL preparation, we used a method of first conjugating the scfv at the distal end of PEG 2000 chains (before insertion onto the surface of liposomes) instead of direct labeling of the scfv onto the liposomes (Fig. 1), based on favorable results reported earlier [44e46]. For the scfv conjugation reaction, ideally we want to achieve close to 100% coupling efficiencies without altering its binding affinity. We thus engineered the scfv to contain a cysteine tag that could conveniently be reduced and conjugated to DSPE- PEG functionalized with a maleimide linker (DSPE-PEG MAL) [47]. We used a simple yet effective post-insertion technique for conjugating the DSPE-PEG scfv onto the surface of the liposomes, to form targeted ILs [48]. In this regard, our room temperature insertion technique helped preserve the affinity of the scfvs, contrary to the post-insertion method which usually involves exposing the antibody to elevated temperatures (usually w60 C for 1 h), where the potential exists, for detrimental effects on
6 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e Fig. 3. In vitro cell binding (A) and internalization (B) curves. Binding and internalization of 111 In-IL-M1 (50) on M28 (black), VAMT-1 (grey) and control BPH-1 cells (blue) at 37 C after 24 h incubation period are shown (n ¼ 3). the antibody, such as reduction in its binding affinity for its target antigen [27]. It has been predicted [49] and experimentally demonstrated that the apparent affinity of multivalent ILs can be several fold higher than that of their monovalent counterparts [50]. We thus first optimized the scfv density on the surface of liposomes (Table 1 and Fig. 2). We observed that increasing the antibody density on the liposome surface increased the binding affinity, and in turn the internalizing ability of the ILs to the target cells (Fig. 2), consistent with what was observed for similar systems [51,52]. While IL Fig. 4. Comparative IL uptake in tumor and normal cells. A. Comparison of in vitro binding and internalization of 111 In-IL-M1 (50) in mesothelioma tumor cells (M28, VAMT-1) and normal (BPH-1) cells; B. The percentage radioactivity internalized (of the total) into tumor cells and normal cells are shown. The cells were incubated with 111 In-IL-M1 (50) with a lipid concentration of 20 nm at 37 C for 24 h.
7 2610 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e2613 Fig. 5. SPECT/CT Imaging. SPECT/CT fused image of 111 In-IL-M1 (50) taken 24 h after injection; A) 3- Dimensional Reconstruction; B) Coronal view; C) Transverse view. The uptake of 111 In-IL-M1 (50) in both epithelioid (M28) and sarcomatoid (VAMT-1) mesothelioma tumors at 24 h is clearly seen. binding to cells with different amounts of surface antigen may vary, antibody densities of w50 scfv per vesicle was found optimal in our case (Fig. 2). Our current findings and prior reports indicate that larger amounts of bound antibody may not be necessary for efficient target binding of scfvs to cells [53,54]. More interestingly, a detailed in vitro cell binding and internalization study using the optimized ILs ( 111 In-IL-M1 (50) ) revealed not only efficient and selective binding but also rapid internalization of the ILs by both epithelioid (M28) and sarcomatoid (VAMT-1) mesothelioma cells, but not by the non-targeted BPH-1 cells (Figs. 3 and 4). In contrast, we observed that the in vitro cell binding and internalization of 111 In-CL under varied lipid concentrations was comparably much lower (<40%) in all the three (M28, VAMT-1 and BPH-1) cell lines, under identical conditions, even after 48 h of incubation (data not shown). Although the findings from the in vitro cell studies using 111 In-IL- M1 (50) are very encouraging, it is essential to test the ILs in vivo on animal tumor models for successful translation into clinics. As a next step in this direction, we tested the 111 In-IL-M1 (50) on xenograft mice models bearing both epithelioid (M28) and sarcomatoid (VAMT-1) mesothelioma. The live animal imaging using SPECT/CT and ex vivo Table 3 Biodistribution of 111 In-IL-M1 (50) (at 24 and 48 h after injection) in nude mice bearing both epithelioid (M28) and sarcomatoid (VAMT-1) subtypes of mesothelioma tumors (n ¼ 4). A control study performed using non-targeted 111 In-CL is also shown (n ¼ 3). Data are %ID/g SD. Organ 111 In-IL-M1 (50) Control 111 In-CL 24 h 48 h 48 h Liver Heart Kidney Lung Spleen Pancreas Stomach Sm. Int lg. Int Muscle Fat Blood Tumor (M28) Tumor (VAMT-1) biodistribution results corroborated the in vitro findings. For instance, we observed that the uptake of 111 In-IL-M1 (50) in both M28 and VAMT-1 tumors was strikingly high in the time frame of our study, higher than all other organs/tissues studied (except the liver ) (Table 3 and Fig. 5) and the tumors could be clearly visualized by SPECT/CT (with values of and % ID/g for M28 and VAMT-1 tumors respectively at 48 h after injection). From Figs. 6 and 7, it can be noted that for the time frame from 24 h to 48 h the clearance of the 111 In-IL-M1 (50) from most of the non-target organs and tissues occurred rapidly whereas the tumor uptake remained high, even at 48 h time point. This result may be attributed to the efficient binding and rapid internalization of the 111 In-IL-M1 (50) into the tumor cells. It should be noted that we intentionally used a low % of PEG (w1%) in our liposome formulations in order to distinguish the internalizing property and the active tumor targeting ability of the 111 In-IL-M1 (50) in comparison to the 111 In-CL. From our results it is clear that the 111 In- IL-M1 (50) showed much higher tumor targeted uptake in comparison to 111 In-CL (Figs. 5, 6 and 7, Table 3). It can also be observed that the lower % PEGylation of 111 In-IL-M1 (50) may have attributed at least in part for their faster clearance from the blood as early as 24 h (Fig. 6), thus providing a good contrast for early tumor imaging by SPECT/CT (Fig. 5). However the lower % PEGylation also resulted in shorter plasma circulation half-life of 111 In-IL-M1 (50). Hence, further optimization of the ILs using higher % PEG may render longer plasma residence time to the ILs thereby facilitating their preferential accumulation in the target tumors by passive targeting strategies or the EPR-effect [15,16]. Furthermore, the stealth ILs may also help evade the RES, thereby reducing its non-target uptake in organs such as the liver and the spleen [55,56]. Although we have addressed some of the critical issues of the ILs in vitro in cell cultures and in vivo on animal models, there still remain several questions regarding their effective use in the clinical settings. For instance while our cell studies and animal model demonstrates the ability of the ILs to target and internalize into the cells and implanted tumors in vivo, their ability to reach the target tissue/cell in the clinical setting may vary or depend on whether the target is accessible from the vasculature [53,57,58]. Also their in vivo stability and half-life, tendency to evoke immune response or ability to effectively evade RES and reach their target organ/ tissue are some of the other important factors that needs to be evaluated.
8 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e Fig. 6. Biodistribution of ILs. Biodistribution of 111 In-IL-M1 (50) at 24 h and 48 h in mice bearing both epithelioid (M28) and sarcomatoid (VAMT-1) tumors are shown (n ¼ 4). Inset: The tumor to blood and tumor to muscle ratios of 111 In-IL-M1 (50) at 24 h and 48 h in M28 and VAMT-1 tumors are shown. Fig. 7. Comparison of targeted and non-targeted liposome uptake in vivo. 48 h biodistribution of targeted ILs ( 111 In-IL-M1 (50) ) versus control non-targeted immunoliposome ( 111 In- CL) in mice bearing both epithelioid (M28) and sarcomatoid (VAMT-1) tumors are shown (n ¼ 3).
9 2612 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e Conclusion The preset study reveals that our internalizing M1 human scfvs anchored immunoliposomes showed selective tumor targeting and rapid internalization into both epithelioid (M28) and sarcomatoid (VAMT-1) subtypes of human mesothelioma in vitro and in vivo demonstrating its potentials as a promising vector for enhanced targeting of liposomal drugs and radionuclides for imaging and therapy of this malignant disease. Acknowledgements The authors wish to thank Prof. Francis C. Szoka, Jr., Department of Biopharmaceutical Sciences, UCSF, for permitting use of his facility for dynamic light scattering and zeta potential measurements. This work is partially supported by NIH R01 CA and the American Cancer Society (IRG ) to Jiang He, and R01 CA and R01 CA to Bin Liu. Appendix Figure with essential color discrimination. Figs. 1e5 in this article is difficult to interpret in black and white. The full color images can be found in the on-line version, at doi: /j. biomaterials References [1] Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT, et al. A Phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther 2010;18:852e60. [2] Curran D, Sahmoud T, Therasse P, Van Meerbeeck J, Postmus P, Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European organization for research and treatment of cancer experience. J Clin Oncol 1998;16:145e52. [3] Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379e87. [4] Smith A, Wright C. Chrysotile asbestos is the main cause of pleural mesothelioma. Am J Ind Med 1996;30:252e66. [5] Dunleavey R. Malignant mesothelioma: risk factors and current management. Nurs Times 2004;100:40e3. [6] Churg A, Roggli V, Galateau-Salle F, Cagle P, Gibbs A, Hasleton P, et al. Mesothelioma. In: Travis W, Brambilla E, Muller-Hermelink H, Harris C, editors. Pathology and genetics tumours of the lung, pleura, thymus and heart. Lyon: IARC; p. 125e36. [7] Attanoos R, Gibbs A. Pathology of malignant mesothelioma. Histopathology 1997;30:403e18. [8] Ho L, Sugarbaker D, Skarin A. Malignant pleural mesothelioma. Cancer Treat Res 2001;105:327e73. [9] Butchart E. Contemporary management of malignant pleural mesothelioma. Oncologist 1999;4:488e500. [10] Klebe S, Brownlee N, Mahar A, Burchette J, Sporn T, Vollmer R, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470e9. [11] Torchilin V. Multifunctional nanocarriers. Adv Drug Deliv Rev 2006;58:1532e55. [12] Farokhzad O, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev 2006;58:1456e9. [13] Papahadjopoulos D, Allen T, Gabizon A, Mayhew E, Matthay K, Huang S, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A 1991;88:11460e4. [14] Allen T. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol Sci 1994;15:215e20. [15] Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000;65:271e84. [16] Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006;11:812e8. [17] Nielsen U, Kirpotin D, Pickering E, Hong K, Park J, Refaat Shalaby M, et al. Therapeutic efficacy of anti-erbb2 immunoliposomes targeted by a phage antibody selected for cellular endocytosis. Biochim Biophys Acta 2002;1591:109e18. [18] Kweon S, Lee HJ, Hyung WJ, Suh J, Lim JS, Lim SJ. Liposomes coloaded with iopamidol/lipiodol as a RES-targeted contrast agent for computed tomography imaging. Pharm Res 2010;27:1408e15. [19] Jiang J, Yang SJ, Wang JC, Yang LJ, Xu ZZ, Yang T, et al. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing sirna or doxorubicin. Eur J Pharm Biopharm 2010;76:170e8. [20] An F, Drummond D, Wilson S, Kirpotin D, Nishimura S, Broaddus V, et al. Targeted drug delivery to mesothelioma cells using functionally selected internalizing human single-chain antibodies. Mol Cancer Ther 2008;7: 569e78. [21] Bidlingmaier S, He J, Wang Y, An F, Feng J, Barbone D, et al. Identification of MCAM/CD146 as the target antigen of a human monoclonal antibody that recognizes both epithelioid and sarcomatoid types of mesothelioma. Cancer Res 2009;69:1570e7. [22] Liu B, Conrad F, Cooperberg M, Kirpotin D, Marks J. Mapping tumor epitope space by direct selection of single-chain Fv antibody libraries on prostate cancer cells. Cancer Res 2004;64:704e10. [23] MacDonald R, MacDonald R, Menco B, Takeshita K, Subbarao N, Hu L. Smallvolume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1991;1061:297e303. [24] Bartlett G. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466e8. [25] Kirpotin D, Park J, Hong K, Zalipsky S, Li W, Carter P, et al. Sterically stabilized anti-her2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry 1997;36:66e75. [26] Park J, Hong K, Kirpotin D, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res 2002;8:1172e81. [27] Iden D, Allen T. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta 2001;1513:207e16. [28] Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70e7. [29] Mougin-Degraef M, Bourdeau C, Jestin E, Saï-Maurel C, Bourgeois M, Saëc PR, et al. Doubly radiolabeled liposomes for pretargeted radioimmunotherapy. Int J Pharm 2007;344:110e7. [30] Dhalluin X, Scherpereel A. Treatment of malignant pleural mesothelioma: current status and future directions. Monaldi Arch Chest Dis 2010;73:79e85. [31] Skubitz K. Phase II trial of pegylated-liposomal doxorubicin (doxil) in mesothelioma 1. Cancer Invest 2002;20:693e9. [32] Steele J, O Doherty C, Shamash J, Evans M, Gower N, Tischkowitz M, et al. Phase II trial of liposomal daunorubicin in malignant pleural mesothelioma. Ann Oncol 2001;12:497e9. [33] Baas P, Van Meerbeeck J, Groen H, Schouwink H, Burgers S, Daamen S, et al. Caelyx TM in malignant mesothelioma: a phase II EORTC study. Ann Oncol 2000;11:697e700. [34] Lu C, Perez-Soler R, Piperdi B, Walsh G, Swisher S, Smythe W, et al. Phase II study of a liposome-entrapped cisplatin analog (L-NDDP) administered intrapleurally and pathologic response rates in patients with malignant pleural mesothelioma. J Clin Oncol 2005;23:3495e501. [35] Park J, Hong K, Kirpotin D, Papahadjopoulos D, Benz CC. Immunoliposomes for cancer treatment. Adv Pharmacol 1997;40:399e435. [36] Kontermann R. Immunoliposomes for cancer therapy. Curr Opin Mol Ther 2006;8:39e45. [37] Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, et al. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood 2010;116:2554e8. [38] Moase E, Qi W, Ishida T, Gabos Z, Longenecker B, Zimmermann G, et al. Anti- MUC-1 immunoliposomal doxorubicin in the treatment of murine models of metastatic breast cancer. Biochim Biophys Acta 2001;1510:43e55. [39] Park J, Hong K, Carter P, Asgari H, Guo L, Keller G, et al. Development of antip185her2 immunoliposomes for cancer therapy. Proc Natl Acad Sci U S A 1995;92:1327e31. [40] Bendas G. Immunoliposomes: a promising approach to targeting cancer therapy. BioDrugs 2001;15:215e24. [41] Szoka Jr F, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng 1980;9:467e508. [42] Templeton N, Lasic D. New directions in liposome gene delivery. Mol Biotechnol 1999;11:175e80. [43] Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci 2001;6:66e77. [44] Hansen C, Kao G, Moase E, Zalipsky S, Allen T. Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta 1995;1239:133e44. [45] Allen T, Agrawal A, Ahmad I, Hansen C, Zalipsky S. Antibody-mediated targeting of long-circulating (stealth) liposomes. J Liposome Res 1994;4:1e25. [46] Maruyama K, Takizawa T, Yuda T, Kennel S, Huang L, Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly (ethylene glycol) s conjugated at their distal terminals to monoclonal antibodies. Biochim Biophys Acta 1995;1234:74e80. [47] Martin F, Hubbell W, Papahadjopoulos D. Immunospecific targeting of liposomes to cells: a novel and efficient method for covalent attachment of Fab fragments via disulfide bonds. Biochemistry 1981;20:4229e38. [48] Moreira J, Ishida T, Gaspar R, Allen T. Use of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicity. Pharm Res 2002;19:265e9. [49] Scatchard G. The attraction of proteins for small molecules. Ann NY Acad Sci 1949;51:660e72.
10 A.K. Iyer et al. / Biomaterials 32 (2011) 2605e [50] Sullivan S, Huang L. Preparation and characterization of heat-sensitive immunoliposomes. Biochim Biophys Acta 1985;812:116e26. [51] Babbitt B, Huang L. Effects of valency on thermodynamic parameters of specific membrane interaction. Biochemistry 1985;24:2186e94. [52] Heath T, Fraley R, Bentz J, Voss Jr EW, Herron J, Papahadjopoulos D. Antibodydirected liposomes determination of affinity constants for soluble and liposome-bound antifluorescein. Biochim Biophys Acta 1984;770:148e58. [53] Maruyama K, Kennel S, Huang L. Lipid composition is important for highly efficient target binding and retention of immunoliposomes. Proc Natl Acad Sci U S A 1990;87:5744e8. [54] Ahmad I, Allen T. Antibody-mediatedspecific bindingand cytotoxicity of liposomeentrapped doxorubicin to lung cancer cells in vitro. Cancer Res 1992;52:4817e20. [55] Mishra S, Webster P, Davis M. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol 2004;83:97e111. [56] Hong R, Huang C, Tseng Y, Pang V, Chen S, Liu J, et al. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice. Clin Cancer Res 1999;5:3645e52. [57] Torchilin V, Klibanov A, Huang L, O Donnell S, Nossiff N, Khaw B. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. FASEB J 1992;6:2716e9. [58] De Menezes L, Daniel E, Pilarski L, Allen T. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res 1998;58:3320e30.
USE OF THE POST-INSERTION METHOD FOR THE FORMATION OF LIGAND-COUPLED LIPOSOMES
 CELLULAR & MOLECULAR BIOLOGY LETTERS Volume 7, (2002) pp 889 894 http://www.cmbl.org.pl Received 15 May 2002 Accepted 25 July 2002 Short Communication USE OF THE POST-INSERTION METHOD FOR THE FORMATION
CELLULAR & MOLECULAR BIOLOGY LETTERS Volume 7, (2002) pp 889 894 http://www.cmbl.org.pl Received 15 May 2002 Accepted 25 July 2002 Short Communication USE OF THE POST-INSERTION METHOD FOR THE FORMATION
Supporting Information. Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively
 S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
Development of lysolipid-based thermosensitive liposomes for delivery of high. molecular weight proteins
 Development of lysolipid-based thermosensitive liposomes for delivery of high molecular weight proteins Xin Zhang a, Paul F. Luckham* a, Alun D. Hughes b, Simon Thom b, and Xiao Yun Xu a a Department of
Development of lysolipid-based thermosensitive liposomes for delivery of high molecular weight proteins Xin Zhang a, Paul F. Luckham* a, Alun D. Hughes b, Simon Thom b, and Xiao Yun Xu a a Department of
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
Supporting Information
 Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
DELFIA Tb-DTPA ITC Chelate & Terbium Standard
 AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
UV Tracer TM Maleimide NHS ester
 UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
Essential Medium, containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Huvec were cultured in
 Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade
 AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard. Product Number: AD0013
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
Tivadar Orban, Beata Jastrzebska, Sayan Gupta, Benlian Wang, Masaru Miyagi, Mark R. Chance, and Krzysztof Palczewski
 Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
The Immunoassay Guide to Successful Mass Spectrometry. Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013
 The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
Supporting Information. Lipids for Liposome Shielding by 18 F-Radiolabeling. and Positron Emission Tomography
 Supporting Information Comparison of Linear and Hyperbranched Polyether Lipids for Liposome Shielding by 18 F-Radiolabeling and Positron Emission Tomography Karolin Wagener, Matthias Worm, Stefanie Pektor,
Supporting Information Comparison of Linear and Hyperbranched Polyether Lipids for Liposome Shielding by 18 F-Radiolabeling and Positron Emission Tomography Karolin Wagener, Matthias Worm, Stefanie Pektor,
BabyBio IMAC columns DATA SHEET DS
 BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
A ph-dependent Charge Reversal Peptide for Cancer Targeting
 Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supplementary Information for
 Supplementary Information for Acid-Resistant Mesoporous Metal Organic Framework Toward Oral Insulin Delivery: Protein Encapsulation, Protection & Release Yijing Chen, Peng Li, Justin A. Modica, Riki J.
Supplementary Information for Acid-Resistant Mesoporous Metal Organic Framework Toward Oral Insulin Delivery: Protein Encapsulation, Protection & Release Yijing Chen, Peng Li, Justin A. Modica, Riki J.
RESEARCH ARTICLE e-issn:
 Available online at www.ijtpls.com International Journal of Trends in Pharmacy and Life Sciences Vol. 1, Issue: 5, 2015: 587-592 PREPARATION AND EVALUATION OF LIPOSOMES CONTAINING ZIDOVUDINE CH.B.V.V.L.S.Latha,KVR.
Available online at www.ijtpls.com International Journal of Trends in Pharmacy and Life Sciences Vol. 1, Issue: 5, 2015: 587-592 PREPARATION AND EVALUATION OF LIPOSOMES CONTAINING ZIDOVUDINE CH.B.V.V.L.S.Latha,KVR.
Translating Molecular Detection into a Temperature Test Using. Target-Responsive Smart Thermometer
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Information Translating Molecular Detection into a Temperature Test Using Target-Responsive
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Information Translating Molecular Detection into a Temperature Test Using Target-Responsive
Transfection mechanisms of polyplexes, lipoplexes. DLD-1 colorectal cancer cells
 Supporting Information Transfection mechanisms of polyplexes, lipoplexes and stealth liposomes in α 5 β 1 integrin bearing DLD-1 colorectal cancer cells Maroof M. Adil, Zachary S. Erdman and Efrosini Kokkoli*
Supporting Information Transfection mechanisms of polyplexes, lipoplexes and stealth liposomes in α 5 β 1 integrin bearing DLD-1 colorectal cancer cells Maroof M. Adil, Zachary S. Erdman and Efrosini Kokkoli*
Focused ultrasound a novel tool for liposome formulation
 Focused ultrasound a novel tool for liposome formulation Introduction Liposomes are excellent carriers of active pharmaceutical ingredients and cosmetic agents. Their vesicular structure, housed by lipid
Focused ultrasound a novel tool for liposome formulation Introduction Liposomes are excellent carriers of active pharmaceutical ingredients and cosmetic agents. Their vesicular structure, housed by lipid
Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles. from Uptake by Liver Endothelial Cells for Long Blood Circulation
 Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation Hao Zhou, Zhiyuan Fan, Peter Y. Li, Junjie Deng,, Dimitrios C. Arhontoulis,
Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation Hao Zhou, Zhiyuan Fan, Peter Y. Li, Junjie Deng,, Dimitrios C. Arhontoulis,
Liposomes. Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology
 Liposomes Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Course Outline Definition of liposomes Classification of liposomes Structure of liposomes Preparation of liposomes
Liposomes Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Course Outline Definition of liposomes Classification of liposomes Structure of liposomes Preparation of liposomes
Screening Conditions for NMR of Integral Membrane Proteins Updated 1/2015
 Screening Conditions for NMR of Integral Membrane Proteins Updated 1/2015 Charles R. Sanders, Vanderbilt University chuck.sanders@vanderbilt.edu phone: 615-833-2586 Background Reading Solution NMR of membrane
Screening Conditions for NMR of Integral Membrane Proteins Updated 1/2015 Charles R. Sanders, Vanderbilt University chuck.sanders@vanderbilt.edu phone: 615-833-2586 Background Reading Solution NMR of membrane
SUPPORTING INFORMATION. Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for. Enhanced Cancer-Directed Uptake in Melanoma: A Comparison
 SUPPORTING INFORMATION Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies Feng Chen 1, Kai Ma 2, Li Zhang
SUPPORTING INFORMATION Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies Feng Chen 1, Kai Ma 2, Li Zhang
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate)
 Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
THE PREPARATION OF LIPOSOMES DERIVED FROM MIXED MICELLES OF LECITHIN ADDED BY SODIUM CHOLATE, FOLLOWED BY DIALYSING USING HEMOFLOW HIGH FLUX F60S
 MAKARA, KESEHATAN, VOL. 8, NO. 2, DESEMBER 24: 4-2 THE PREPARATION OF LIPOSOMES DERIVED FROM MIXED MICELLES OF LECITHIN ADDED BY SODIUM CHOLATE, FOLLOWED BY DIALYSING USING HEMOFLOW HIGH FLUX F6S Erni
MAKARA, KESEHATAN, VOL. 8, NO. 2, DESEMBER 24: 4-2 THE PREPARATION OF LIPOSOMES DERIVED FROM MIXED MICELLES OF LECITHIN ADDED BY SODIUM CHOLATE, FOLLOWED BY DIALYSING USING HEMOFLOW HIGH FLUX F6S Erni
9( )- Hydroxyoctadecadienoic Acid ELISA
 Package Insert 9( )- Hydroxyoctadecadienoic Acid ELISA 96 Wells For Research Use Only v. 1.0 Eagle Biosciences, Inc. 82 Broad Street, Suite 383, Boston, MA 02110 Phone: 866-419-2019 Fax: 617-419-1110 INTRODUCTION
Package Insert 9( )- Hydroxyoctadecadienoic Acid ELISA 96 Wells For Research Use Only v. 1.0 Eagle Biosciences, Inc. 82 Broad Street, Suite 383, Boston, MA 02110 Phone: 866-419-2019 Fax: 617-419-1110 INTRODUCTION
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supporting Information. Ligand-mediated Coating of Liposomes with Human Serum Albumin
 Supporting Information Ligand-mediated Coating of Liposomes with Human Serum Albumin Hikari Sato, 1 Elnaz Nakhaei, 1 Takahito Kawano, 2,3 Masaharu Murata, 2,3 Akihiro Kishimura, 1,4,5,6 Takeshi Mori, 1,4,5,
Supporting Information Ligand-mediated Coating of Liposomes with Human Serum Albumin Hikari Sato, 1 Elnaz Nakhaei, 1 Takahito Kawano, 2,3 Masaharu Murata, 2,3 Akihiro Kishimura, 1,4,5,6 Takeshi Mori, 1,4,5,
Antibody H6-11 for Prostate Cancer Imaging
 Supplementary Information Preclinical Evaluation of a Novel Monoclonal Antibody H6-11 for Prostate Cancer Imaging Hongjun Jin, a Mai Xu, a Prashanth K. Padakanti, a Yongjian Liu, a Suzanne Lapi, a and
Supplementary Information Preclinical Evaluation of a Novel Monoclonal Antibody H6-11 for Prostate Cancer Imaging Hongjun Jin, a Mai Xu, a Prashanth K. Padakanti, a Yongjian Liu, a Suzanne Lapi, a and
Supplementary Information
 Supplementary Information Molecular imaging of brain localization of liposomes in mice using MALDI mass spectrometry Annabelle Fülöp 1,2, Denis A. Sammour 1,2, Katrin Erich 1,2, Johanna von Gerichten 4,
Supplementary Information Molecular imaging of brain localization of liposomes in mice using MALDI mass spectrometry Annabelle Fülöp 1,2, Denis A. Sammour 1,2, Katrin Erich 1,2, Johanna von Gerichten 4,
Supplementary Data. Different volumes of ethanol or calcium solution were slowly added through one of four
 Supplementary Data METHODS Liposome preparation Different volumes of ethanol or calcium solution were slowly added through one of four methods: Method I, no ethanol or calcium solution; Method II, exactly
Supplementary Data METHODS Liposome preparation Different volumes of ethanol or calcium solution were slowly added through one of four methods: Method I, no ethanol or calcium solution; Method II, exactly
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
SUPPLEMENTARY DATA. Materials and Methods
 SUPPLEMENTARY DATA Materials and Methods HPLC-UV of phospholipid classes and HETE isomer determination. Fractionation of platelet lipid classes was undertaken on a Spherisorb S5W 150 x 4.6 mm column (Waters
SUPPLEMENTARY DATA Materials and Methods HPLC-UV of phospholipid classes and HETE isomer determination. Fractionation of platelet lipid classes was undertaken on a Spherisorb S5W 150 x 4.6 mm column (Waters
Measuring Lipid Composition LC-MS/MS
 Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Drug Release from Liposomes: Role of Mechanism Based Models. Bradley D. Anderson University of Kentucky
 Drug Release from Liposomes: Role of Mechanism Based Models Bradley D. Anderson University of Kentucky l i p o s o m e Why liposomes? Si OH N O N AR-67 O H O O Xiang and Anderson, Adv. Drug Delivery Rev.
Drug Release from Liposomes: Role of Mechanism Based Models Bradley D. Anderson University of Kentucky l i p o s o m e Why liposomes? Si OH N O N AR-67 O H O O Xiang and Anderson, Adv. Drug Delivery Rev.
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
# Supplementary Material (ESI) for Molecular BioSystems # This journal is The Royal Society of Chemistry 2005
 Supporting Information Multifunctional Polymeric Micelles with Folate-Mediated Cancer Cell Targeting and ph-triggered Drug Releasing Properties for Active Intracellular Drug Delivery Younsoo Bae, Woo-Dong
Supporting Information Multifunctional Polymeric Micelles with Folate-Mediated Cancer Cell Targeting and ph-triggered Drug Releasing Properties for Active Intracellular Drug Delivery Younsoo Bae, Woo-Dong
Preparation of SG3249 antibody-drug conjugates
 Preparation of SG3249 antibody-drug conjugates Conjugate A: Herceptin-SG3249 (ConjA) Antibody (15 mg, 100 nanomoles) was diluted into 13.5 ml of a reduction buffer containing 10 mm sodium borate ph 8.4,
Preparation of SG3249 antibody-drug conjugates Conjugate A: Herceptin-SG3249 (ConjA) Antibody (15 mg, 100 nanomoles) was diluted into 13.5 ml of a reduction buffer containing 10 mm sodium borate ph 8.4,
Protocol for purification of recombinant protein from 300 ml yeast culture
 Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES (AUGUST 2015)
 August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
Radiopharmacy. Prof. Dr. Çetin ÖNSEL. CTF Nükleer Tıp Anabilim Dalı
 Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Europium Labeling Kit
 Europium Labeling Kit Catalog Number KA2096 100ug *1 Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Europium Labeling Kit Catalog Number KA2096 100ug *1 Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
LC-Based Lipidomics Analysis on QTRAP Instruments
 LC-Based Lipidomics Analysis on QTRAP Instruments Junhua Wang and Paul RS Baker SCIEX LC-Based Lipidomics Analysis Topics Covered Lipid extraction techniques Hydrophilic Interaction Chromatography (HILIC)
LC-Based Lipidomics Analysis on QTRAP Instruments Junhua Wang and Paul RS Baker SCIEX LC-Based Lipidomics Analysis Topics Covered Lipid extraction techniques Hydrophilic Interaction Chromatography (HILIC)
Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation)
 Product Manual Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation) Catalog Number MET-5032 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Product Manual Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation) Catalog Number MET-5032 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Student Handout. This experiment allows you to explore the properties of chiral molecules. You have
 Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
New Horizons in radionuclide therapy. John Buscombe Royal Free Hospital
 New Horizons in radionuclide therapy John Buscombe Royal Free Hospital Date for you diary Interested in Radionuclide imaging and therapy using antibodies and peptides IRIST 2008 Krakow Poland 18-21 June
New Horizons in radionuclide therapy John Buscombe Royal Free Hospital Date for you diary Interested in Radionuclide imaging and therapy using antibodies and peptides IRIST 2008 Krakow Poland 18-21 June
Biodegradable Zwitterionic Nanogels with Long. Circulation for Antitumor Drug Delivery
 Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
4. Determination of fat content (AOAC, 2000) Reagents
 94 ANALYTICAL METHODS 1. Determination of moisture content (AOAC, 2000) 1. Dry the empty dish and lid in the oven at 105 C for 3 h and transfer to desiccator to cool. Weigh the empty dish and lid. 2. Weigh
94 ANALYTICAL METHODS 1. Determination of moisture content (AOAC, 2000) 1. Dry the empty dish and lid in the oven at 105 C for 3 h and transfer to desiccator to cool. Weigh the empty dish and lid. 2. Weigh
Effect of number of extrusion passes on liposome nanoparticle size
 CENG 176B, Spring 2016 Drews, Zhang, Yang, Xu, and Fenning Section B01 (W/F), Team 07: Double-O Seven, Lab 5: Effect of number of extrusion passes on liposome nanoparticle size Part I: Part II: Part III:
CENG 176B, Spring 2016 Drews, Zhang, Yang, Xu, and Fenning Section B01 (W/F), Team 07: Double-O Seven, Lab 5: Effect of number of extrusion passes on liposome nanoparticle size Part I: Part II: Part III:
Exosomes Immunocapture and Isolation Tools: Immunobeads
 Product Insert Exosomes Immunocapture and Isolation Tools: Immunobeads HBM-BOLF-##/##-# HBM-BOLC-##/##-# HBM-BTLF-##/##-# Overall Exosome capture from Human Biological fluids Overall Exosome capture from
Product Insert Exosomes Immunocapture and Isolation Tools: Immunobeads HBM-BOLF-##/##-# HBM-BOLC-##/##-# HBM-BTLF-##/##-# Overall Exosome capture from Human Biological fluids Overall Exosome capture from
The radiation absorbed doses to the tumor and normal tissues in CD1 athymic mice with
 MATERIALS AND METHODS Radiation Dosimetry Estimates The radiation absorbed doses to the tumor and normal tissues in CD1 athymic mice with MDA-MB-231/H2N xenografts following injection of 111 In-DTPA-Fab-PEG
MATERIALS AND METHODS Radiation Dosimetry Estimates The radiation absorbed doses to the tumor and normal tissues in CD1 athymic mice with MDA-MB-231/H2N xenografts following injection of 111 In-DTPA-Fab-PEG
An Orthogonal Array Optimization of Lipid-like Nanoparticles for. mrna Delivery in Vivo
 Supporting Information An rthogonal Array ptimization of Lipid-like Nanoparticles for mrna Delivery in Vivo Bin Li, Xiao Luo, Binbin Deng, Junfeng Wang, David W. McComb, Yimin Shi, Karin M.L. Gaensler,
Supporting Information An rthogonal Array ptimization of Lipid-like Nanoparticles for mrna Delivery in Vivo Bin Li, Xiao Luo, Binbin Deng, Junfeng Wang, David W. McComb, Yimin Shi, Karin M.L. Gaensler,
B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small cell lung cancer
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed by natural abundance 1 H 13 C solid-state NMR
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the wner Societies 28 Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the wner Societies 28 Supplementary Information: Liquid-liquid phase coexistence in lipid membranes observed
Supporting Information File S2
 Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation)
 Product Manual OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation) Catalog Number STA-358 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Product Manual OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation) Catalog Number STA-358 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Protein MultiColor Stable, Low Range
 Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
Plasmonic blood glucose monitor based on enzymatic. etching of gold nanorods
 Plasmonic blood glucose monitor based on enzymatic etching of gold nanorods Xin Liu, Shuya Zhang, Penglong Tan, Jiang Zhou, Yan Huang, Zhou Nie* and Shouzhuo Yao State Key Laboratory of Chemo/Biosensing
Plasmonic blood glucose monitor based on enzymatic etching of gold nanorods Xin Liu, Shuya Zhang, Penglong Tan, Jiang Zhou, Yan Huang, Zhou Nie* and Shouzhuo Yao State Key Laboratory of Chemo/Biosensing
Protein directed assembly of lipids
 Protein directed assembly of lipids D. Nordin, O. Yarkoni, L. Donlon, N. Savinykh, and D.J. Frankel SUPPLEMENTARY MATERIAL Materials and Methods Supported bilayer preparation 1,2-dioleoyl-sn-glycero-3-phosphocholine
Protein directed assembly of lipids D. Nordin, O. Yarkoni, L. Donlon, N. Savinykh, and D.J. Frankel SUPPLEMENTARY MATERIAL Materials and Methods Supported bilayer preparation 1,2-dioleoyl-sn-glycero-3-phosphocholine
A Liposome-based Nanostructure for Aptamer Directed Delivery. Supporting Information (SI)
 A Liposome-based Nanostructure for Aptamer Directed Delivery Huaizhi Kang, Meghan B. O'Donoghue, Haipeng Liu and Weihong Tan* Center for Research at the Bio/Nano Interface, Department of Chemistry and
A Liposome-based Nanostructure for Aptamer Directed Delivery Huaizhi Kang, Meghan B. O'Donoghue, Haipeng Liu and Weihong Tan* Center for Research at the Bio/Nano Interface, Department of Chemistry and
Isolation of five carotenoid compounds from tangerine tomatoes
 Isolation of five carotenoid compounds from tangerine tomatoes Thesis Thomas Haufe Advisor: Steven J. Schwartz, Ph.D Department of Food Science and Technology Presented in fulfillment of the requirements
Isolation of five carotenoid compounds from tangerine tomatoes Thesis Thomas Haufe Advisor: Steven J. Schwartz, Ph.D Department of Food Science and Technology Presented in fulfillment of the requirements
Light and X-ray triggered liposomal gene/drug delivery system for cancer therapy
 Light and X-ray triggered liposomal gene/drug delivery system for cancer therapy Wei Deng, 1 Wenjie Chen, 2 Sandhya Clement, 1 Anna Guller, 1 Alexander Engel, 3 Ewa Goldys 1 1 The Graduate School of Biomedical
Light and X-ray triggered liposomal gene/drug delivery system for cancer therapy Wei Deng, 1 Wenjie Chen, 2 Sandhya Clement, 1 Anna Guller, 1 Alexander Engel, 3 Ewa Goldys 1 1 The Graduate School of Biomedical
Heparin Sodium ヘパリンナトリウム
 Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
Title Revision n date
 A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions.
 Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions. Magdalena Bajgrowicz 1,2, Jerzy Detyna 2, Marek Langner 1 1 Institute of Biomedical Engineering and Instrumentation,
Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions. Magdalena Bajgrowicz 1,2, Jerzy Detyna 2, Marek Langner 1 1 Institute of Biomedical Engineering and Instrumentation,
Total Phosphatidic Acid Assay Kit
 Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
JOURNAL OF APPLIED PHARMACEUTICAL RESEARCH LIGAND CONJUGATED LIPOSOMAL DRUG DELIVERY SYSTEM FOR ENHANCED BRAIN UPTAKE OF AMPICILLIN
 10 Journal of Applied Pharmaceutical Research 2016, 4 (4): 10 15 JOURNAL OF APPLIED PHARMACEUTICAL RESEARCH ISSN No. 2348 0335 www.japtronline.com LIGAND CONJUGATED LIPOSOMAL DRUG DELIVERY SYSTEM FOR ENHANCED
10 Journal of Applied Pharmaceutical Research 2016, 4 (4): 10 15 JOURNAL OF APPLIED PHARMACEUTICAL RESEARCH ISSN No. 2348 0335 www.japtronline.com LIGAND CONJUGATED LIPOSOMAL DRUG DELIVERY SYSTEM FOR ENHANCED
Block Copolymer Assemblies for Delivering Drugs and Bioimaging Agents
 Block Copolymer Assemblies for Delivering Drugs and Bioimaging Agents J. S. Riffle, N. Pothayee, R. Zhang, N. Hu, S. Roy- Chaudrury & R. M. Davis Macromolecules and Interfaces Laboratory Virginia Tech
Block Copolymer Assemblies for Delivering Drugs and Bioimaging Agents J. S. Riffle, N. Pothayee, R. Zhang, N. Hu, S. Roy- Chaudrury & R. M. Davis Macromolecules and Interfaces Laboratory Virginia Tech
Lipid Peroxidation Assay
 Package Insert Lipid Peroxidation Assay 96 Wells For Research Use Only v. 1.0 Eagle Biosciences, Inc. 82 Broad Street, Suite 383, Boston, MA 02110 Phone: 866-419-2019 Fax: 617-419-1110 INTRODUCTION Lipid
Package Insert Lipid Peroxidation Assay 96 Wells For Research Use Only v. 1.0 Eagle Biosciences, Inc. 82 Broad Street, Suite 383, Boston, MA 02110 Phone: 866-419-2019 Fax: 617-419-1110 INTRODUCTION Lipid
Chapter 3. Need for Present Study
 Need for Present Study Chapter 3 Chemotherapy, the use of cytotoxic drugs to kill cancerous cells remains the most common approach for cancer therapy. In conventional chemotherapy most of the anticancer
Need for Present Study Chapter 3 Chemotherapy, the use of cytotoxic drugs to kill cancerous cells remains the most common approach for cancer therapy. In conventional chemotherapy most of the anticancer
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Supplementary Figures
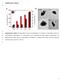 Supplementary Figures 7 8 Supplementary Figure Intracellular fusion and degradation. (a) Changes of intracellular metal ion concentrations. Intracellular ion concentrations were normalized by total protein
Supplementary Figures 7 8 Supplementary Figure Intracellular fusion and degradation. (a) Changes of intracellular metal ion concentrations. Intracellular ion concentrations were normalized by total protein
Supporting information
 S1 Supporting information Biodegradable Injectable Polymer Systems Exhibiting Temperature-Responsive Irreversible Sol-to-Gel Transition by Covalent Bond Formation Yasuyuki YOSHIDA 1,2, Keisuke KAWAHARA
S1 Supporting information Biodegradable Injectable Polymer Systems Exhibiting Temperature-Responsive Irreversible Sol-to-Gel Transition by Covalent Bond Formation Yasuyuki YOSHIDA 1,2, Keisuke KAWAHARA
Compact Gamma Camera for Detection of Prostate Cancer
 Compact Gamma Camera for Detection of Prostate Cancer Presented at: Human Interest Panel Federal Laboratory Consortium Annual Conference Nashville, Tennessee Brookhaven National Laboratory and Hybridyne
Compact Gamma Camera for Detection of Prostate Cancer Presented at: Human Interest Panel Federal Laboratory Consortium Annual Conference Nashville, Tennessee Brookhaven National Laboratory and Hybridyne
Special Considerations for Tracers Based on Proteins or Protein Fragments
 Special Considerations for Tracers Based on Proteins or Protein Fragments 10 June, 2017 Serge K. Lyashchenko, Pharm.D. Assistant Attending, Memorial Hospital, Memorial Sloan Kettering Cancer Center Assistant
Special Considerations for Tracers Based on Proteins or Protein Fragments 10 June, 2017 Serge K. Lyashchenko, Pharm.D. Assistant Attending, Memorial Hospital, Memorial Sloan Kettering Cancer Center Assistant
Physicochemical Properties of PEG-Grafted Liposomes
 1238 Chem. Pharm. Bull. 50(9) 1238 1244 (2002) Vol. 50, No. 9 Physicochemical Properties of PEG-Grafted Liposomes Supaporn SRIWONGSITANONT and Masaharu UENO* Faculty of Pharmaceutical Sciences, Toyama
1238 Chem. Pharm. Bull. 50(9) 1238 1244 (2002) Vol. 50, No. 9 Physicochemical Properties of PEG-Grafted Liposomes Supaporn SRIWONGSITANONT and Masaharu UENO* Faculty of Pharmaceutical Sciences, Toyama
Guideline for the Development of Liposome Drug Products
 参考 ( ガイドライン英訳 ) Guideline for the Development of Liposome Drug Products (March 2016, MHLW, Japan) Table of Contents 1. Introduction... 3 2. Scope... 3 3. Chemistry, manufacturing, and controls... 4 3.1
参考 ( ガイドライン英訳 ) Guideline for the Development of Liposome Drug Products (March 2016, MHLW, Japan) Table of Contents 1. Introduction... 3 2. Scope... 3 3. Chemistry, manufacturing, and controls... 4 3.1
Formulation and Evaluation of Acyclovir Liposomes
 Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
TITLE: Antibody - Pretargeted Cytokine Therapy of Cancer. CONTRACTING ORGANIZATION: Fox Chase Cancer Center Philadelphia, Pennsylvania 19111
 k AD Award Number: DAMD17-99-1-9183 TITLE: Antibody - Pretargeted Cytokine Therapy of Cancer PRINCIPAL INVESTIGATOR: Louis Weiner, M.D. CONTRACTING ORGANIZATION: Fox Chase Cancer Center Philadelphia, Pennsylvania
k AD Award Number: DAMD17-99-1-9183 TITLE: Antibody - Pretargeted Cytokine Therapy of Cancer PRINCIPAL INVESTIGATOR: Louis Weiner, M.D. CONTRACTING ORGANIZATION: Fox Chase Cancer Center Philadelphia, Pennsylvania
Preparation of Liposome Containing Bacteriorhodopsin with Natural. Preferred Orientation of Its Transient Photoresponse
 ISSN 0582-9879 ACTA BIOCHIMICA et BIOPHYSICA SINICA 2003, 35(4): 391-395 CN 31-1300/Q Preparation of Liposome Containing Bacteriorhodopsin with Natural Preferred Orientation of Its Transient Photoresponse
ISSN 0582-9879 ACTA BIOCHIMICA et BIOPHYSICA SINICA 2003, 35(4): 391-395 CN 31-1300/Q Preparation of Liposome Containing Bacteriorhodopsin with Natural Preferred Orientation of Its Transient Photoresponse
Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants
 Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants D. L. Marathe, B. N. Pandey and K. P. Mishra Radiation Biology Division, Bhabha Atomic Research Centre,
Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants D. L. Marathe, B. N. Pandey and K. P. Mishra Radiation Biology Division, Bhabha Atomic Research Centre,
His n. Vivapure Metal Chelate Mega spin columns. Technical data and operating instructions. For in vitro use only.
 His n Vivapure Metal Chelate Mega spin columns Technical data and operating instructions. For in vitro use only. columns - for the purification of proteins with poly-histidine tags Storage conditions columns
His n Vivapure Metal Chelate Mega spin columns Technical data and operating instructions. For in vitro use only. columns - for the purification of proteins with poly-histidine tags Storage conditions columns
U VESTAR, INC. DTIC. I l l11111lIII lli i ll111. ELECTE t4j May 21, 1992 JUN ', AD-A o
 ', AD-A251 817 o I 1111111 1l11 1111l11111lIII lli i ll111 U VESTAR, INC. DTIC ELECTE t4j May 21, 1992 JUN 0 81992 C R. Carter, CMDR, USN S A Scientific Officer Naval Medical Research and Development Command
', AD-A251 817 o I 1111111 1l11 1111l11111lIII lli i ll111 U VESTAR, INC. DTIC ELECTE t4j May 21, 1992 JUN 0 81992 C R. Carter, CMDR, USN S A Scientific Officer Naval Medical Research and Development Command
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
FOCUS Global Fractionation
 139PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS Global Fractionation (Cat. # 786 018) think proteins! think G-Biosciences www.gbiosciences.com
139PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS Global Fractionation (Cat. # 786 018) think proteins! think G-Biosciences www.gbiosciences.com
IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS
 22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids
 User Guide Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 5.6 Contents 1. Storage 3 2. Product Components 3 3. Product
User Guide Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 5.6 Contents 1. Storage 3 2. Product Components 3 3. Product
Investigating Lipids
 Investigating Lipids In today s culture, there is a stigma associated with the word fat. While it is true that too much fat can lead to health problems, fats (or lipids) play very important roles in biology.
Investigating Lipids In today s culture, there is a stigma associated with the word fat. While it is true that too much fat can lead to health problems, fats (or lipids) play very important roles in biology.
Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General
 Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General For the detection and quantitation of human OxLDL in plasma, serum or other biological fluid samples Cat. No. KT-959 For Research Use Only.
Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General For the detection and quantitation of human OxLDL in plasma, serum or other biological fluid samples Cat. No. KT-959 For Research Use Only.
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in
 Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids
 Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 1.1 1 Contents 1. Storage 3 2. Kit contents 3 3. Product Information
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 1.1 1 Contents 1. Storage 3 2. Kit contents 3 3. Product Information
EXPERIMENT 13: Isolation and Characterization of Erythrocyte
 EXPERIMENT 13: Isolation and Characterization of Erythrocyte Day 1: Isolation of Erythrocyte Steps 1 through 6 of the Switzer & Garrity protocol (pages 220-221) have been performed by the TA. We will be
EXPERIMENT 13: Isolation and Characterization of Erythrocyte Day 1: Isolation of Erythrocyte Steps 1 through 6 of the Switzer & Garrity protocol (pages 220-221) have been performed by the TA. We will be
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information ovel pseudo[2]rotaxanes constructed by selfassembly of dibenzyl
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information ovel pseudo[2]rotaxanes constructed by selfassembly of dibenzyl
