AGROWING BODY OF LITERAture
|
|
|
- Randolph Garrison
- 5 years ago
- Views:
Transcription
1 ORIGINAL CONTRIBUTION JAMA-EXPRESS Low-Dose Aspirin in the Primary Prevention of Cancer The Women s Health Study: A Randomized Controlled Trial Nancy R. Cook, ScD I-Min Lee, MBBS, ScD J. Michael Gaziano, MD David Gordon, MA Paul M Ridker, MD JoAnn E. Manson, MD, DrPH Charles H. Hennekens, MD, DrPH Julie E. Buring, ScD AGROWING BODY OF LITERAture supports a protective effect of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) on the development of cancer. In experimental animal models, NSAIDs have been shown to suppress tumor growth. 1,2 Observational epidemiological investigations suggest a strong inverse association, with risk reductions as high as 2% to 5% for various cancer sites, 3 including but not limited to colorectal cancer, 4,5 breast cancer, 6 and gastric cancer. 3 In addition, polyp prevention trials have consistently demonstrated a beneficial effect of aspirin and other NSAIDs on recurrence of colorectal adenomas. 5 Aspirin is thought to influence cancer risk primarily through its effect on cyclooxygenase (COX) activity. Aspirin, as well as other NSAIDs, inhibits the COX enzymes, which convert arachidonic acid into prostaglandins. 7 The proposed mechanism for aspirin s effect See also pp 56 and 15. Context Basic research and observational evidence as well as results from trials of colon polyp recurrence suggest a role for aspirin in the chemoprevention of cancer. Objective To examine the effect of aspirin on the risk of cancer among healthy women. Design, Setting, and Participants In the Women s Health Study, a randomized 2 2 factorial trial of aspirin and vitamin E conducted between September 1992 and March 24, US women aged at least 45 years and initially without previous history of cancer, cardiovascular disease, or other major chronic illness were randomly assigned to receive either aspirin or aspirin placebo and followed up for an average of 1.1 years. Intervention A dose of 1 mg of aspirin (n=19 934) or aspirin placebo (n=19 942) administered every other day. Main Outcome Measures Confirmed newly diagnosed invasive cancer at any site, except for nonmelanoma skin cancer. Incidence of breast, colorectal, and lung cancer were secondary end points. Results No effect of aspirin was observed on total cancer (n=2865; relative risk [RR], 1.1; 95% confidence interval [CI], ; P =.87), breast cancer (n=123; RR,.98; 95% CI, ; P=.68), colorectal cancer (n=269; RR,.97; 95% CI, ; P=.83), or cancer of any other site, with the exception of lung cancer for which there was a trend toward reduction in risk (n=25; RR,.78; 95% CI, ; P=.8). There was also no reduction in cancer mortality either overall (n=583; RR,.95; 95% CI, ; P=.51) or by site, except for lung cancer mortality (n=14; RR,.7; 95% CI,.5-.99; P=.4). No evidence of differential effects of aspirin by follow-up time or interaction with vitamin E was found. Conclusions Results from this large-scale, long-term trial suggest that alternate day use of low-dose aspirin (1 mg) for an average 1 years of treatment does not lower risk of total, breast, colorectal, or other site-specific cancers. A protective effect on lung cancer or a benefit of higher doses of aspirin cannot be ruled out. JAMA. 25;294: Author Affiliations: Divisions of Preventive Medicine (Drs Cook, Lee, Gaziano, Ridker, Manson, and Buring, and Mr Gordon), Cardiovascular Medicine (Drs Gaziano and Ridker), and Aging (Drs Gaziano and Buring), Department of Medicine, Brigham and Women s Hospital, Harvard Medical School, Boston, Mass; Department of Epidemiology, Harvard School of Public Health, Boston (Drs Cook, Lee, Ridker, Manson, and Buring); Veterans Affairs Boston Healthcare System (Dr Gaziano); Department of Ambulatory Care and Prevention, Harvard Medical School, Boston (Dr Buring); and the Departments of Medicine and Epidemiology and Public Health, University of Miami School of Medicine, Miami, Fla, and Department of Biomedical Science, Center of Excellence, Florida Atlantic University, Boca Raton (Dr Hennekens). Corresponding Author: Nancy R. Cook, ScD, Department of Medicine, Brigham and Women s Hospital, Harvard Medical School, 9 Commonwealth Ave E, Boston, MA 2215 (ncook@rics.bwh.harvard.edu). 25 American Medical Association. All rights reserved. (Reprinted) JAMA, July 6, 25 Vol 294, No. 1 47
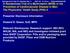
2 Figure 1. Flow Diagram of the Aspirin Component of the Women s Health Study (WHS) on cancer lies with its effect on the cyclooxygenase 2 (COX-2) enzyme, which is linked to inflammation and related to tumor growth through its effect on apoptosis, cell migration, and angiogenesis. 1,8 Aspirin may also have an effect through its potential role as an antioxidant, 7 or through modulation of estrogen biosynthesis. 9 In contrast with the observational evidence on cancer incidence, data from randomized trials, which provide more definitive results due to their ability to minimize bias and confounding, have been far more limited. 1,11 The Physicians Health Study (PHS) found no effect on colorectal cancer of 325 mg of aspirin administered every other day over a 5-year randomized period 11 or in posttrial follow-up. 12 In addition, no randomized trial has yet assessed the impact of aspirin on the development of breast cancer. The Women s Health Study (WHS) was a randomized, double-blind, placebo-controlled, 2 2 factorial trial evaluating the balance of benefits and risks of 1 mg of aspirin every other day and 6 IU of vitamin E every other day (see accompanying article on page 1.7 Million Women Invited to Participate Completed Baseline Questionnaire Entered the Run-in Phase Randomized Excluded After Run-in (Noncompliance, Unwillingness, or Ineligibility) Assigned to Receive Aspirin Assigned to Receive Placebo Status on March 31, Alive 669 Dead 118 Unknown Vital Status Status on March 31, Alive 693 Dead 116 Unknown Vital Status Included in Primary Analysis Included in Primary Analysis *The numbers of deaths are higher than those in Table 2 because this figure includes all reported deaths in the WHS, whereas the deaths in Table 2 include only reported deaths confirmed by the Endpoints Committee or by a death certificate. 56), in the primary prevention of cardiovascular disease 13 and cancer in a cohort of healthy female health care professionals over an average duration of 1.1 years. This article reports the findings for the aspirin component with regard to cancer risk. METHODS Study Design and Participants The WHS is a randomized 2 2 factorial trial evaluating the effects of lowdose aspirin (1 mg every other day, provided by Bayer HealthCare) and vitamin E (6 IU every other day, provided by the Natural Source Vitamin E Association) in the primary prevention of cardiovascular disease and cancer. The trial initially contained a beta carotene component, which was terminated January 18, 1996, after an average of 2 years of follow-up. 14 Written informed consent was obtained from all participants. The trial was approved by the institutional review board of Brigham and Women s Hospital, Boston, Mass, and monitored by an external data and safety monitoring board. Detailed methods of the design have been described previously. 13,15 In brief, from September 1992 to May 1995, letters of invitation to participate in the trial and baseline health questionnaires were mailed to more than 1.7 million female health care professionals throughout the United States (FIGURE 1). Women were eligible if they were aged at least 45 years without previous history of cancer (except nonmelanoma skin cancer), cardiovascular disease, or other major chronic illness; no history of adverse effects to aspirin; not taking aspirin or NSAIDs more than once a week (or willing to forgo their use during the trial); not taking anticoagulants or corticosteroids; and not taking individual supplements of vitamin A, vitamin E, or beta carotene more than once a week. The women who were willing and eligible entered a 3-month run-in period using both placebo aspirin and placebo vitamin E to identify likely long-term compliers to pill taking. A total of women remained willing and eligible, were compliant during run-in, and were randomized into the trial ( to aspirin and to placebo). Randomization used blocks of size 16 within 5-year age strata and took place from April 3, 1993, through January 24, Participants were sent an annual supply of monthly calendar packs containing active agents or placebo. Every 6 months for the first year, then every 12 months subsequently, participants were sent questionnaires seeking information on compliance, adverse effects, occurrence of relevant clinical end points, and risk factors. Study medications and end point ascertainment were continued in blinded fashion through the scheduled end of the trial on March 31, 24. Follow-up and validation of reported end points were completed in February 25. Morbidity and mortality follow-up were 97.2% and 99.4% complete, respectively. Compliance, defined as taking at least two thirds of the study aspirin or aspirin placebo, was 76% at 5 years and 67% at 1 years, with an average of 73% throughout the trial. Compliance was slightly but statistically significantly lower in the active aspirin group, with 48 JAMA, July 6, 25 Vol 294, No. 1 (Reprinted) 25 American Medical Association. All rights reserved.
3 proportions averaging about 1% lower from 24 months onward. Use of outside aspirin for 4 or more days per month averaged 12% during the followup, with no statistically significant difference by aspirin assignment. End Point Definition All participants were followed up for the occurrence of cancer or cardiovascular events. Following a report of cancer by questionnaire or death certificate, written consent for medical record review was requested from the participant, or next of kin if deceased, and medical records were obtained from hospitals or treating physicians. All relevant information was reviewed by the WHS Endpoints Committee composed of physicians blinded to treatment assignment. Reports of cancer were confirmed on the basis of pathology or cytology reports (96.8%) or, rarely, based on strong clinical and radiological or laboratory marker evidence (eg, elevated CA-125) when a pathology or cytology review was not conducted. The primary cancer end point for the WHS was any invasive cancer, excluding nonmelanoma skin cancer. The incidence of breast, colorectal, and lung cancer were secondary end points for the aspirin component of the trial. Only confirmed cancer end points were included in these analyses. In addition, a total of 588 women self-reported a diagnosis of colon polyp occurring following randomization. Medical records were obtained and reviewed for a random subset of these women. Of 558 postrandomization polyp reports reviewed, 295 were confirmed for a total confirmation rate of 53% (55% in the aspirin group and 51% in the placebo group). Because confirmation of these reports is incomplete, preliminary data on self-reported colon polyps only are presented. The use of colonoscopy or sigmoidoscopy for screening purposes during the past year, as reported on the 12-month questionnaire among women without cancer, was similar by aspirin assignment (5.1% vs 5.2% in active vs placebo, respectively). As reported on the final questionnaire, the use of colonoscopy for screening purposes from the beginning of the trial was similar by aspirin assignment (41.% vs 41.7% in active vs placebo, respectively), as was use of sigmoidoscopy (22.5% vs 23.6%, respectively). Statistical Analysis Primary analyses were based on the intent-to-treat principle, including all randomized women, as randomized. Kaplan-Meier survival curves were used to estimate incidence over time, and the log-rank test was computed to compare curves. Cox proportional hazards regression models 16 were used to estimate the relative risks (RRs) and 95% confidence intervals (CIs) comparing women randomized to active aspirin vs aspirin placebo, adjusting for age and randomized vitamin E and beta carotene assignments. Tests of proportionality used an interaction term for aspirin the logarithm of follow-up time. The RR by 2-year follow-up periods was used to investigate patterns in the effect over time. All analyses were conducted with SAS version 8.2 (SAS Institute, Cary, NC), and a 2-sided significance level of =.5 was used (P.5). Analyses were conducted for the primary end point of total invasive cancer as well as for site-specific cancers. If a woman developed more than 1 primary cancer (excluding nonmelanoma skin cancer), the first following randomization was used in these analyses. In secondary analyses, the effects of aspirin on the combination of invasive and in situ cancers, estrogen- and progesterone-receptor positive breast cancer, colon cancer site and stage (Dukes classification 17 ), and small cell and non small cell lung cancer were examined. Additional analyses examined the effect of aspirin on cancer risk according to known risk factors for cancer at baseline, including age, body mass index (calculated as weight in kilograms divided by the square of height in meters), smoking (current, past, or never), alcohol use ( 1or 1 drinks per week), and family history of cancer (history of breast, colorectal, or ovarian cancer in a parent or sibling). Participants were asked at baseline if they ever smoked 1 or more cigarettes in their lifetime. Responses included yes, currently smoke (current); yes, smoked in the past but quit (past); or no (never). Subgroups defined by physical activity (estimated energy expenditure per week from leisure activities of 1 or 1 kcal per week), menopausal status and hormone therapy (premenopausal, postmenopausal with or without hormone therapy, or uncertain menopausal status), and other randomized assignments were also examined. Tests for effect modification used a 1-degree interaction term in the Cox proportional hazards regression model in the case of ordinal subgroups, or a multidegree of freedom test for unordered categories. To examine the effect of actual as opposed to assigned aspirin use, separate sensitivity analyses according to compliance were conducted. Women were censored if and when they stopped taking at least two thirds of their study medication, whether active aspirin or aspirin placebo. To allow for a lag in effect, additional analyses censored women 2 years after they limited compliance. The effect of aspirin among those women who consistently reported good compliance during the first 2 years or 5 years of the trial on cancers occurring following these periods was also examined. RESULTS No statistically significant differences in baseline characteristics were observed between the aspirin and placebo groups (TABLE 1). 13,15 Mean (SD) age at trial entry was 54.6 (7.) years, 5235 women (13%) were current smokers, women (36%) were past smokers, and 2 34 women (51%) were never smokers. Mean (SD) body mass index was 26. (5.1), and women (54%) were postmenopausal of whom (55%) were current, 2745 (13%) were past, and 6959 (32%) were never users of hormone therapy, with 3 women missing hormone informa- 25 American Medical Association. All rights reserved. (Reprinted) JAMA, July 6, 25 Vol 294, No. 1 49
4 tion. A total of 746 women (18%) reported a family history of breast, colorectal, or ovarian cancer in a parent or sibling. During an average 1.1 years of follow-up, 2865 cases of invasive cancer, excluding nonmelanoma skin cancer, were confirmed by the study end points committee (741 events per 1 person-years) (TABLE 2). Of these cases, 123 (43%) were breast cancer, 269 (9%) were colorectal cancer, and 25 (7%) were lung cancer. There was no effect of aspirin on incidence of the primary end point of total cancer (RR, 1.1; 95% CI, ; P=.87) or of cancer at other sites, including breast (RR,.98; 95% CI, ; P=.68) or colorectal cancer (RR,.97; 95% CI, ; P=.83), although there was a trend toward reduction in lung cancer in the active aspirin group that was not statistically significant (RR,.78; 95% CI, ; P=.8). There were no statistically significant effects on other cancer sites considered. In addition to those cases listed, there were 11 cases of Hodgkin lymphoma (7 vs 4 in aspirin vs placebo groups) and 122 cases of non-hodgkin lymphoma (RR,.97; 95% CI, ; P=.85). There were 7 confirmed cases of esophageal Table 1. Baseline Characteristics of Women by Randomized Aspirin Assignment* Characteristics Aspirin (n = ) Placebo (n = ) Total (N = ) P Value Age, y Mean (SD) 54.6 (7.) 54.6 (7.) 54.6 (7.) (6.2) (6.2) (6.2) (29.5) 5878 (29.5) (29.5) (1.3) 249 (1.3) 497 (1.3) BMI Mean (SD) 26.1 (5.1) 26. (5.) 26. (5.1) (5.8) 9937 (5.8) (5.8) 25 to (3.9) 654 (31.) (3.9) (18.3) 3551 (18.2) 7126 (18.2) Smoking status Current 258 (13.) 2655 (13.3) 5235 (13.1) Past 7167 (36.) 798 (35.6) (35.8).58 Never (51.1) (51.) 2 34 (51.1) Alcohol use, drinks/wk (58.3) (58.2) (58.3) (41.7) 8338 (41.8) (41.7).74 Physical activity, kcal/wk (65.7) (66.3) (66.) (34.3) 6632 (33.7) (34.).17 Menopausal status and use of HT Premenopausal 5478 (27.5) 5495 (27.6) (27.6) Uncertain 3521 (17.7) 3628 (18.2) 7149 (18.) Postmenopausal and current HT 637 (3.4) 5911 (29.7) (3.).4 Postmenopausal and no HT 485 (24.4) 4854 (24.4) 974 (24.4) Family history of cancer Yes 3529 (17.7) 3517 (17.6) 746 (17.7) No (82.3) (82.4) (82.3).86 Randomized to receive vitamin E Yes 9966 (5.) 9971 (5.) (5.) No 9968 (5.) 9971 (5.) (5.).99 Randomized to receive beta carotene Yes 9967 (5.) 9972 (5.) (5.) No 9967 (5.) 997 (5.) (5.).99 Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by the square of height in meters; HT, hormone therapy. *Data are presented as No. (%) unless otherwise indicated. Because of rounding, percentages may not all total 1. See Methods section of the text for descriptions of smoking status, alcohol use, and physical activity. History of breast, colorectal, or ovarian cancer in a parent or sibling. cancer (2 in aspirin and 5 in placebo groups), and 151 confirmed cases of cancer occurring at other sites not listed in Table 2 (78 vs 73 in aspirin vs placebo groups, respectively). No difference was observed by aspirin assignment when cases of carcinoma in situ were considered in addition to invasive cancer, with a total of 3241 confirmed invasive or in situ cancers (RR, 1.; 95% CI, ; P=.94). When these cancers were further divided by site, there were 1535 cases in the breast (RR,.98; 95% CI, ; P=.69) and 298 cases in the colon or rectum (RR,.97; 95% CI, ). Of the invasive breast cancer cases, 82 were estrogen-receptor positive and progesterone-receptor positive, 125 were estrogen-receptor positive and progesterone-receptor negative, and 25 were estrogen-receptor negative and progesterone-receptor positive. None of these subtypes was statistically significantly affected by aspirin assignment (RR,.98; 95% CI, ; P=.77 for estrogenreceptor positive/progesteronereceptor positive; RR,.81; 95% CI, ; P=.24 for estrogen-receptor positive/progesterone-receptor negative; and RR,.79; 95% CI, ; P=.55 for estrogen-receptor negative/ progesterone-receptor positive). There was no statistically significant effect of aspirin by site of colon cancer, including 112 proximal cancers (RR,.86; 95% CI, ; P=.44) or 95 distal cancers (RR,.94; 95% CI, ; P=.75), or colorectal cancer stage, including 89 cases of Dukes stage A (RR,.93; 95% CI, ; P=.74), 66 cases of Dukes stage B (RR,.94; 95% CI, ; P=.79), and 113 cases of Dukes stage C (RR, 1.5; 95% CI, ; P=.79). In addition, there were 251 self-reports of colon polyps in the aspirin group and 2578 in the placebo group, leading to an RR of.97 (95% CI, ; P=.27). When lung cancer cases were classified by cell type, there was no statistically significant effect on non small cell cancer (RR,.85; 95% CI, ; P=.35), with a marginally statistically significant reduction in small 5 JAMA, July 6, 25 Vol 294, No. 1 (Reprinted) 25 American Medical Association. All rights reserved.
5 cell cancers (12 vs 22; RR,.54; 95% CI, ; P=.9). No difference was observed by aspirin assignment in overall cancer mortality during the trial (RR,.95; 95% CI, ; P=.51), with 583 such deaths confirmed (Table 2). There was also no difference in deaths due to breast cancer (n=63) or colorectal cancer (n=65). Among the 14 deaths due to lung cancer, however, there was a statistically significant reduction among those women assigned to active aspirin vs placebo (58 vs 82; RR,.7; 95% CI,.5-.99; P=.4). There were no other differences by aspirin assignment in confirmed deaths at other cancer sites considered. In addition, there was no difference in total mortality. Cumulative incidence curves (FIGURE 2) suggested no variation in the effect of randomized aspirin over time on total cancer (log-rank P=.83). In addition, plots of the RR by 2-year follow-up intervals (FIGURE 3) did not suggest an increasing effect with duration of use for total, breast, colorectal, or lung cancer. Tests for proportionality of the hazard ratio over time indicated no statistically significant trend over time for total cancer (P =.16), breast cancer (P=.87), colorectal cancer (P=.69), lung cancer (P=.94), or cancer death (P=.24). There were no effects on total cancer after excluding the first 2 years (RR, 1.; 95% CI, ; P=.97) or first 5 years (RR,.98; 95% CI, ; P=.73) of followup. For breast cancer, the RR after excluding the first 2 years was.98 (95% CI, ; P=.74), and the RR after excluding the first 5 years was.96 (95% CI, ; P=.63); for colorectal cancer, the RR was.97 (95% CI, ; P=.82) after 2 years and 1.13 (95% CI, ; P =.46) after 5 years; and for cancer mortality, the RR was.94 (95% CI, ; P=.45) after 2 years and.96 (95% CI, ; P=.7) after 5 years. For lung cancer, the RR after excluding the first 2 years was.76 (95% CI, ; P=.7) and the RR after excluding the first 5 years was.79 (95% CI, ; P=.23); and for lung cancer mortality, the RR was.71 (95% CI,.5-1.2; P=.6) after 2 years and.68 (95% CI, ; P=.8) after 5 years. When effect modification of the impact of aspirin on total cancer by baseline risk factors was considered (TABLE 3), there was no statistically significant difference in effect by age, body mass index, alcohol use, physical activity, menopausal status and hormone therapy, family history of cancer, or by the other randomized interventions, vitamin E or beta carotene. There was, however, a statistically significant difference in the aspirin effect by smoking status, but in an unexpected direc- Table 2. Confirmed Invasive Cancer Cases by Randomized Aspirin Assignment No. of Events Aspirin Placebo Relative Risk Cancer Site (n = ) (n = ) (95% Confidence Interval) P Value Total* ( ).87 Breast ( ).68 Colorectal ( ).83 Colon ( ).57 Rectal (.7-2.4).5 Lung ( ).8 Stomach ( ).99 Pancreas ( ).21 Uterine ( ).14 Ovary ( ).79 Kidney ( ).25 Bladder ( ).69 Melanoma ( ).87 Thyroid ( ).2 Brain ( ).59 Leukemia/lymphoma ( ).32 Leukemia ( ).1 Lymphoma ( ).94 Multiple myeloma ( ).28 Any cancer death ( ).51 Any death ( ).32 *Excluding nonmelanoma skin cancer. Figure 2. Cumulative Incidence of Total Invasive Cancer (Excluding Nonmelanoma Skin Cancer) by Randomized Aspirin Assignment Cumulative Incidence No. at Risk Aspirin Placebo Aspirin Placebo Log-Rank P = American Medical Association. All rights reserved. (Reprinted) JAMA, July 6, 25 Vol 294, No. 1 51
6 tion. Never smokers at baseline had a significant increase in risk among those participants assigned to aspirin, although past smokers had a marginally significant decrease in risk, with no effect among current smokers, leading to a statistically significant interaction by smoking (P=.2). This effect was primarily observed for breast cancer, where the interaction was marginal (P=.9); among never smokers, women assigned to aspirin had an increased risk (RR, 1.11; 95% CI, ; P=.21), but among past smokers they had a decreased risk (RR,.84; 95% CI,.7-1.1; P=.7), with no effect among current smokers (RR,.93; 95% CI, ; P=.63). No statistically significant effect modification by smoking was observed for colorectal or lung cancer. In particular, for lung cancer, the RRs were.83 (95% CI, ; P=.3) with 117 cases among current smokers,.69 (95% CI, ; P=.13) with 7 cases among past smokers, and 1. (95% CI, ; P =.99) with 18 cases among never smokers. In sensitivity analyses by compliance, there were no effects on total cancer in analyses censoring women at the time they stopped taking at least two thirds of their study pills (RR, 1.3; 95% CI, ; P=.55) or in an analysis censoring women 2 years after they stopped taking their study pills (RR, 1.2; 95% CI, ; P=.56). Results for breast, colorectal, or lung cancer also did not show any significance. Among women who consistently took at least two thirds of their study aspirin or aspirin placebo during the first 2 years of the trial, there was no effect on total (RR, 1.2; 95% CI, ; P=.69), breast (RR,.99; 95% CI, ; P=.88), colorectal (RR, 1.7; 95% CI, ; P=.63), or lung (RR,.83; 95% CI, ; P=.27) cancers occurring after 2 years of follow-up. The same was true among women who complied with study medication use during the first 5 years of intervention. COMMENT No overall effect of aspirin administered at a dose of 1 mg every other day on total cancer was found among nearly 4 women randomized to aspirin or aspirin placebo and followed Figure 3. Relative Risks (RRs) and 95% Confidence Intervals (CIs) Comparing Active vs Placebo Aspirin Assignment for Total, Breast, Colorectal, and Lung Cancer by 2-Year Follow-up Intervals RR (95% CI) RR (95% CI) Total Cancer Colorectal Cancer RR (95% CI) RR (95% CI) Breast Cancer Lung Cancer up for an average of 1 years. This was true for several cancer sites considered, including breast cancer, by far the most common cancer type in this cohort, and colorectal cancer. A marginally statistically significant protective effect on lung cancer was observed, which was significant for lung cancer deaths. No apparent effect modification by duration of aspirin use was found, and estimates were virtually identical after excluding the first 2 or 5 years of follow-up. The strongest evidence of an aspirin benefit from observational studies has emerged for breast and colorectal cancer. Although no randomized trials have previously examined the effect of aspirin on breast cancer, a meta-analysis estimated an RR for aspirin of.7 among 4 case-control studies, and an RR of.79 among 6 cohort studies, the latter with considerable heterogeneity. 6 Results by dose or duration of use were inconsistent across these studies. In a nested case-control study, 18 the largest reduction in breast cancer occurred among those women using the lowest dose of 75 mg. In contrast, data from the Women s Health Initiative cohort study 19 found no reduction among women taking 81 mg of aspirin daily, but did find reductions with higher doses, other NSAIDs, and a trend with longer duration. Finally, in a population-based casecontrol study, larger decreases in risk were found among regular users and those participants taking aspirin at least 7 times a week. 9 In that study, reductions were restricted to hormonereceptor positive breast cancer, a finding that was not replicated in the WHS. Of the 2 randomized trials assessing colorectal cancer incidence, 11,2 no reduced risk was found. The PHS demonstrated no effect of 325 mg of aspirin every other day on colorectal cancer among male physicians initially free of cancer either during the 5-year trial period 11 or in longer-term observational follow-up. 12 The Aspirin/ Folate Polyp Prevention Study 2 was designed to examine colorectal adenomas but reported 6 cases of colorectal cancer, 1 in the placebo group, 2 in the 52 JAMA, July 6, 25 Vol 294, No. 1 (Reprinted) 25 American Medical Association. All rights reserved.
7 low-dose aspirin group, and 3 in the high-dose aspirin group. In a metaanalysis of 14 mostly observational studies of cancer incidence, all but 2 found a beneficial association with aspirin, with a summary RR of A protective effect is supported by trials of recurrent colorectal adenoma, 2-22 with a summary RR estimate of It has been suggested that the lack of effect on colorectal cancer observed in the 2 trials could be due to the natural history of disease requiring a longer duration. 5 Among male health care professionals, the reduction in risk became stronger with longer and more consistent use of aspirin. 23 In the Nurses Health Study, there was a strong gradient in risk reduction with increasing years of aspirin use, with the largest benefit observed among those participants using aspirin for at least 2 years. 24 Among those participants using aspirin for 1 to 19 years, comparable in length with the WHS, the RR was.7, but not statistically significant. A duration effect, however, is not supported by the 1- year follow-up in the WHS or the observational posttrial follow-up in the PHS. 12 Only 1 other randomized trial of aspirin has examined the impact on cancer incidence. In a British openlabeled trial 1 of 5 mg of aspirin daily among 5139 male physicians followed up for 6 years with two thirds randomized to daily aspirin, the overall risk of death due to cancer was decreased by 18%, with no difference in the incidence of nonfatal malignant neoplasms. Lung cancer deaths, however, were reduced by 36%, an observation that was deemed unanticipated and likely due to data fluctuations. 1 Although the PHS found no effect on colorectal cancer, 11 unpublished data on lung cancer deaths, which were based on small numbers, were compatible with those found in the WHS and the British trial. 1 In the PHS, there was a nonsignificant 22% reduction in lung cancer mortality in the active aspirin group during the trial period (14 vs 18, P=.48; J. M. Gaziano, written communication, 25). There was also a 13% reduction in lung cancer incidence (25 vs 29, P=.61), with no reduction in total cancer incidence or mortality. Observational evidence for an effect of aspirin on lung cancer has been mixed. A meta-analysis of 5 studies, including 2 case-control and 3 cohort studies, found an RR of.84 (95% CI, ) for aspirin and lung cancer, but with statistically significant heterogeneity across studies. 3 Several large cohort studies 8,25-28 have found differential but inconsistent results by sex. Although some studies found a stronger association for non small cell lung cancer, 27 this was also inconsistent. 28 Thus, although results from the British trial, 1 the PHS, and the WHS are compatible with a reduction in risk of lung cancer, particularly lung cancer mortality, evidence for such an effect remains uncertain and may simply reflect the play of chance. Table 3. Effect of Randomized Aspirin Assignment on Confirmed Total Cancer by Subgroups No. of Events Characteristics Aspirin (n = ) Placebo (n = ) Relative Risk (95% Confidence Interval) P Value P for Interaction Age, y ( ) ( ) ( ).42 BMI ( ) to ( ) ( ).37 Smoking*.2 Current ( ).81 Past (.79-1.).6 Never ( ).3 Alcohol use, drinks/wk* ( ) ( ).87 Physical activity, kcal/wk* ( ) ( ).97 Menopausal status and use of HT Premenopausal ( ).66 Uncertain ( ).55 Postmenopausal and current HT ( ).96 Postmenopausal and no HT ( ).93 Family history of cancer.37 Yes ( ).46 No ( ).59 Randomized to receive.67 vitamin E Yes ( ).85 No ( ).67 Randomized to receive beta carotene Yes ( ).18 No ( ).26 Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by the square of height in meters; HT, hormone therapy. *See Methods section of the text for descriptions of smoking status, alcohol use, and physical activity. History of breast, colorectal, or ovarian cancer in a parent or sibling American Medical Association. All rights reserved. (Reprinted) JAMA, July 6, 25 Vol 294, No. 1 53
8 In subgroup analyses, no difference in effects by a number of cancer risk factors, including age, body mass index, or family history of cancer, was observed. For smoking, there was an increased risk of total cancer with active aspirin among never smokers, a protective effect among past smokers, and no effect among current smokers, an interaction largely due to modification of the effect on breast cancer. This result was unanticipated and could be due to chance, particularly given the number of comparisons made. Proinflammatory tobacco carcinogens, however, could potentially increase aspirin s effectiveness in cancer chemoprevention. 29 Although at least 1 previous study has suggested larger effects of aspirin on lung cancer among smokers, 3 this has not been consistent. 28 Modification of aspirin s effect on breast cancer by smoking has not previously been reported to our knowledge. The issue of adequate dose remains an unanswered question. Low-dose aspirin has been shown to permanently inhibit platelet aggregation, 31 and the dose used in the WHS, 1 mg every other day, has been found to substantially reduce levels of both thromboxane and prostacyclin in men and women. 32 Aspirin, however, has been found to be a more potent inhibitor of platelet COX-1 than of COX-2 activity in other cells, 7,31 which may be influenced by dose. Low-dose aspirin appears relatively specific for COX-1, although higher doses ( 1 g/d) inhibit both COX-1 and COX-2 and may have a stronger anti-inflammatory effect. 8 In addition, the required dosing interval for COX-2 inhibition may be shorter, due to the rapid resynthesis of the enzyme. 31 COX-2 expression has been found to be increased in colorectal neoplasia, 8 as well as in breast tumor cell tissue, 33 suggesting that higher and/or more frequent doses of aspirin may be more effective in cancer prevention. It is thus possible that the low everyother-day dose of 1 mg used in this trial could have limited aspirin s effect on COX-2 activity and cancer chemoprevention. In general, the effects of aspirin observed in studies of colon polyps appear to strengthen with increasing dose, and a meta-analysis found a lower risk of adenomatous polyps only at higher doses of aspirin and other NSAIDs. 34 However, the dose-response effect has not been consistent. Although some studies have found a reduction in risk of colorectal adenoma or cancer only with higher doses, 4,34-36 in the Aspirin/Folate Polyp Prevention Study, 2 effects on colorectal adenomas were observed for lowdose (81 mg daily) but not for higherdose (325 mg daily) aspirin. In addition, in studies among healthy subjects using colorectal mucosal prostaglandin E 2 levels as a biomarker, a daily dose of 81 mg of aspirin was sufficient to suppress these levels after a 28-day period, 37,38 and the extent of reduction of prostaglandin E 2 was not greater at higher doses. 38 Although the low alternate-day dose of aspirin used in the WHS could possibly explain the lack of effect on cancer, evidence for a dose-response effect remains inconsistent. The strength of the WHS and other randomized trials is a reduction in possible biases that remain in observational studies. Case-control studies, in particular, could have protopathic bias, in which, for example, early and undiagnosed symptoms of the disease affect the use of aspirin. In general, however, findings for breast and colorectal cancer from case-control and cohort studies tend to be consistent. 3,4,6 Confounding by indication is also reduced in intent-to-treat analyses. Conditions that lead to self-selected use of aspirin could be related to the subsequent development of cancer. 39 In the PHS, posttrial self-selected use of aspirin was associated with several risk factors for both cancer and cardiovascular disease as well as gastrointestinal symptoms. 12,4 Randomized trials reduce the possibility of bias by uncontrolled or unmeasured confounders. The findings from the WHS suggest that aspirin at a dose of 1 mg every other day is not effective in reducing risk of cancer in healthy women, although a beneficial effect on lung cancer cannot be ruled out. This large study of almost 4 women had a duration of 1 years of treatment and followup, which was the longest of any trial completed to date, and should be sufficient to detect long-term effects. To determine whether higher doses of aspirin taken daily would be effective in cancer prevention requires direct randomized trial data. Such data would need to be considered in the context of risk of gastrointestinal adverse effects 13 before recommending higherdose aspirin for cancer chemoprevention among low-risk individuals. Author Contributions: Dr Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Cook, Gaziano, Gordon, Ridker, Manson, Hennekens, Buring. Acquisition of data: Lee, Gaziano, Gordon, Hennekens, Buring. Analysis and interpretation of data: Cook, Lee, Gaziano, Ridker, Manson, Hennekens, Buring. Drafting of the manuscript: Cook. Critical revision of the manuscript for important intellectual content: Cook, Lee, Gaziano, Gordon, Ridker, Manson, Hennekens, Buring. Statistical analysis: Cook. Obtained funding: Hennekens, Buring. Administrative, technical, or material support: Lee, Gaziano, Gordon, Ridker, Manson, Hennekens, Buring. Study supervision: Cook, Lee, Gaziano, Gordon, Ridker, Manson, Hennekens, Buring. Financial Disclosures: Dr Cook has served as a consultant to Bayer. Dr Gaziano has served as a consultant to and received grant support from Bayer and McNeil. Dr Ridker has received grant support from Bayer. Dr Hennekens has served as a consultant to Bayer and McNeil and received grant support from Bayer. Funding/Support: This study was supported by grants HL and CA from the National Heart, Lung, and Blood Institute and the National Cancer Institute, Bethesda, Md. Aspirin and aspirin placebo were provided by Bayer Healthcare. Vitamin E and vitamin E placebo were provided by the Natural Source Vitamin E Association. Role of the Sponsors: Neither Bayer Healthcare nor the Natural Source Vitamin E Association provided any input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Data and Safety Monitoring Board: Lawrence Cohen, Rory Collins, Theodore Colton, David DeMets, I. Craig Henderson, Andrea La Croix, Ross Prentice, and Nanette Wenger (chair), and Mary Frances Cotch, Frederick Ferris, Lawrence Friedman, Peter Greenwald, Natalie Kurinij, Marjorie Perloff, Eleanor Schron, and Alan Zonderman (ex-officio members). Acknowledgment: We are indebted to the participants in the Women s Health Study for their dedicated and conscientious collaboration; to the entire staff of the Women s Health Study, under the leadership of David Gordon, Maria Andrade, Susan Burt, Mary Breen, Marilyn Chown, Lisa Fields-Johnson, Georgina Friedenberg, Inge Judge, Jean MacFadyen, Geneva McNair, Laura Pestana, Philomena Quinn, Claire Ridge, Harriet Samuelson, Fred Schwerin, and 54 JAMA, July 6, 25 Vol 294, No. 1 (Reprinted) 25 American Medical Association. All rights reserved.
9 Marty Van Denburgh; to Christine Albert, Michelle Albert, Gavin Blake, Claudia Chae, Wendy Chen, Richard Doll, Carlos Kase, Tobias Kurth, Richard Peto, Aruna Pradhan, Kathryn Rexrode, Bernard Rosner, Jacqueline Suk, and Shumin Zhang for their assistance in the design and conduct of the trial; and especially to James Taylor for chairing the Endpoints Committee. REFERENCES 1. Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst. 1998;9: Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part II). J Natl Cancer Inst. 1998;9: Gonzalez-Perez A, Garcia Rodriguez LA, Lopez- Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 23;3: Garcia Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 21;12: Asano TK, McLeod RS. Nonsteroidal antiinflammatory drugs and aspirin for the prevention of colorectal adenomas and cancer: a systematic review. Dis Colon Rectum. 24;47: Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 21;84: Awtry EH, Loscalzo J. Aspirin. Circulation. 2;11: Thun MJ. Beyond willow bark: aspirin in the prevention of chronic disease. Epidemiology. 2; 11: Terry MB, Gammon MD, Zhang FF, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 24;291: Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ. 1988;296: Gann PH, Manson JAE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85: Sturmer T, Glynn RJ, Lee IM, Manson JAE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post-trial follow-up from the Physicians Health Study. Ann Intern Med. 1998;128: Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 25;352: Lee IM, Cook NR, Manson JAE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women s Health Study. J Natl Cancer Inst. 1999;91: Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women s Health Study. J Womens Health Gend Based Med. 2;9: Cox DR. Regression models and life tables (with discussion). J Roy Stat Soc [Ser B]. 1972;34: Anderson JR, ed. Muir s Textbook of Pathology. 12th ed. London, England: Edward Arnold; Garcia Rodriguez LA, Gonzalez-Perez A. Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer. 24;91: Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women s Health Initiative. Cancer Res. 23;63: Baron JA, Cole BF, Sandler RS, et al. A randomized trial to prevent colorectal adenomas. N Engl J Med. 23;348: Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC Trial. Gastroenterology. 23;125: Sandler RS, Halabie S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 23;348: Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121: Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333: Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5: Paganini-Hill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases: a cohort study of the elderly. BMJ. 1989;299: Akhmedkhanov A, Toniolo P, Zeleniuch- Jacquotte A, Koenig KL, Shore RE. Aspirin and lung cancer in women. Br J Cancer. 22;87: Holick CN, Michaud DS, Leitzman MF, Willett WC, Giovannucci EL. Aspirin use and lung cancer in men. Br J Cancer. 23;89: Harris RE, Beebe-Donk J, Schuller HM. Chemoprevention of lung cancer by non-steroidal antiinflammatory drugs among cigarette smokers. Oncol Rep. 22;9: Muscat JE, Chen SQ, Richie JP Jr, et al. Risk of lung carcinoma among users of nonsteroidal antiinflammatory drugs. Cancer. 23;97: Patrono C, Coller B, Dalen J, et al. Platelet-active drugs: the relationship among dose, effectiveness, and side effects. Chest. 21;119:39S-63S. 32. Ridker PM, Hennekens CH, Tofler GH, Lipinska I, Buring JE. Anti-platelet effects of 1 mg alternate day oral aspirin: a randomized, double-blind, placebocontrolled trial of regular and enteric coated formulations in men and women. J Cardiovasc Risk. 1996; 3: Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;9: Garcia Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal adenoma among long-term users of nonsteroidal antiinflammatory drugs: a pooled analysis of published studies and a new populationbased study. Epidemiology. 2;11: Chan AT, Giovannucci EL, Schernhammer ES, et al. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med. 24;14: Tangrea JA, Albert PS, Lanza E, et al. Nonsteroidal anti-inflammatory drug use is associated with reduction in recurrence of advanced and nonadvanced colorectal adenomas (United States). Cancer Causes Control. 23;14: Krishnan K, Ruffin MT, Normolle D, et al. Colonic mucosal prostaglandin E 2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 21;1: Sample D, Wargovich M, Fischer SM, et al. A dosefinding study of aspirin for chemoprevention using rectal mucosal prostaglandin E 2 levels as a biomarker. Cancer Epidemiol Biomarkers Prev. 22;11: Baron JA. Epidemiology of non-steroidal antiinflammatory drugs and cancer. In: Dannenberg AJ, DuBois RN, eds. COX-2. Vol 37. Basel, Switzerland: Karger; 23: Cook NR, Hebert PR, Manson JAE, Buring JE, Hennekens CH. Self-selected aspirin use post-trial and subsequent cardiovascular disease and mortality in the Physicians Health Study. Arch Intern Med. 2;16: American Medical Association. All rights reserved. (Reprinted) JAMA, July 6, 25 Vol 294, No. 1 55
journal of medicine The new england A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women abstract
 The new england journal of medicine established in 1812 march 31, 2005 vol. 352 no. 13 A Randomized Trial of Low-Dose in the Primary Prevention of Cardiovascular Disease in Women Paul M Ridker, M.D., Nancy
The new england journal of medicine established in 1812 march 31, 2005 vol. 352 no. 13 A Randomized Trial of Low-Dose in the Primary Prevention of Cardiovascular Disease in Women Paul M Ridker, M.D., Nancy
Dietary Fatty Acids and the Risk of Hypertension in Middle-Aged and Older Women
 07/14/2010 Dietary Fatty Acids and the Risk of Hypertension in Middle-Aged and Older Women First Author: Wang Short Title: Dietary Fatty Acids and Hypertension Risk in Women Lu Wang, MD, PhD, 1 JoAnn E.
07/14/2010 Dietary Fatty Acids and the Risk of Hypertension in Middle-Aged and Older Women First Author: Wang Short Title: Dietary Fatty Acids and Hypertension Risk in Women Lu Wang, MD, PhD, 1 JoAnn E.
Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency
 British Journal of Cancer (2007) 97, 1295 1299 All rights reserved 0007 0920/07 $30.00 www.bjcancer.com Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency D Feskanich*,1,
British Journal of Cancer (2007) 97, 1295 1299 All rights reserved 0007 0920/07 $30.00 www.bjcancer.com Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency D Feskanich*,1,
ORIGINAL INVESTIGATION. Self-Selected Posttrial Aspirin Use and Subsequent Cardiovascular Disease and Mortality in the Physicians Health Study
 ORIGINAL INVESTIGATION Self-Selected Posttrial Aspirin Use and Subsequent Cardiovascular Disease and Mortality in the Physicians Health Study Nancy R. Cook, ScD; Patricia R. Hebert, PhD; JoAnn E. Manson,
ORIGINAL INVESTIGATION Self-Selected Posttrial Aspirin Use and Subsequent Cardiovascular Disease and Mortality in the Physicians Health Study Nancy R. Cook, ScD; Patricia R. Hebert, PhD; JoAnn E. Manson,
RECENT RANDOMIZED INTERVENtion
 ORIGINAL CONTRIBUTION Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer Andrew T. Chan, MD, MPH Edward L. Giovannucci, MD, ScD Jeffrey A. Meyerhardt, MD, MPH
ORIGINAL CONTRIBUTION Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer Andrew T. Chan, MD, MPH Edward L. Giovannucci, MD, ScD Jeffrey A. Meyerhardt, MD, MPH
FOLATE, VITAMIN B6, AND VITAMIN
 ORIGINAL CONTRIBUTION Effect of Combined Folic Acid, Vitamin B 6, and Vitamin B 12 on Cancer Risk in Women A Randomized Trial Shumin M. Zhang, MD, ScD Nancy R. Cook, ScD Christine M. Albert, MD, MPH J.
ORIGINAL CONTRIBUTION Effect of Combined Folic Acid, Vitamin B 6, and Vitamin B 12 on Cancer Risk in Women A Randomized Trial Shumin M. Zhang, MD, ScD Nancy R. Cook, ScD Christine M. Albert, MD, MPH J.
ORIGINAL INVESTIGATION. Alcohol Consumption and Mortality in Men With Preexisting Cerebrovascular Disease
 ORIGINAL INVESTIGATION Alcohol Consumption and Mortality in Men With Preexisting Cerebrovascular Disease Vicki A. Jackson, MD; Howard D. Sesso, ScD; Julie E. Buring, ScD; J. Michael Gaziano, MD Background:
ORIGINAL INVESTIGATION Alcohol Consumption and Mortality in Men With Preexisting Cerebrovascular Disease Vicki A. Jackson, MD; Howard D. Sesso, ScD; Julie E. Buring, ScD; J. Michael Gaziano, MD Background:
Headache, migraine and risk of brain tumors in women: prospective cohort study
 Kurth et al. The Journal of Headache and Pain (2015) 16:17 DOI 10.1186/s10194-015-0501-0 RESEARCH ARTICLE Open Access Headache, migraine and risk of brain tumors in women: prospective cohort study Tobias
Kurth et al. The Journal of Headache and Pain (2015) 16:17 DOI 10.1186/s10194-015-0501-0 RESEARCH ARTICLE Open Access Headache, migraine and risk of brain tumors in women: prospective cohort study Tobias
PHYSICAL INACTIVITY AND BODY
 ORIGINAL CONTRIBUTION Relationship of Physical Activity vs Body Mass Index With Type 2 Diabetes in Women Amy R. Weinstein, MD, MPH Howard D. Sesso, ScD, MPH I. Min Lee, MBBS, ScD Nancy R. Cook, ScD JoAnn
ORIGINAL CONTRIBUTION Relationship of Physical Activity vs Body Mass Index With Type 2 Diabetes in Women Amy R. Weinstein, MD, MPH Howard D. Sesso, ScD, MPH I. Min Lee, MBBS, ScD Nancy R. Cook, ScD JoAnn
Copyright, 1996, by the Massachusetts Medical Society
 Copyright, 1996, by the Massachusetts Medical Society Volume 334 MAY 2, 1996 Number 18 LACK OF EFFECT OF LONG-TERM SUPPLEMENTATION WITH BETA CAROTENE ON THE INCIDENCE OF MALIGNANT NEOPLASMS AND CARDIOVASCULAR
Copyright, 1996, by the Massachusetts Medical Society Volume 334 MAY 2, 1996 Number 18 LACK OF EFFECT OF LONG-TERM SUPPLEMENTATION WITH BETA CAROTENE ON THE INCIDENCE OF MALIGNANT NEOPLASMS AND CARDIOVASCULAR
LOW FOLATE INTAKE HAS INcreased
 ORIGINAL CONTRIBUTION A Prospective Study of Folate Intake and the Risk of Breast Cancer Shumin Zhang, MD, ScD David J. Hunter, MBBS, ScD Susan E. Hankinson, ScD Edward L. Giovannucci, MD, ScD Bernard
ORIGINAL CONTRIBUTION A Prospective Study of Folate Intake and the Risk of Breast Cancer Shumin Zhang, MD, ScD David J. Hunter, MBBS, ScD Susan E. Hankinson, ScD Edward L. Giovannucci, MD, ScD Bernard
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 J UNE 19, 1997 NUMBER 25 POSTMENOPAUSAL HORMONE THERAPY AND MORTALITY FRANCINE GRODSTEIN, SC.D., MEIR
The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 J UNE 19, 1997 NUMBER 25 POSTMENOPAUSAL HORMONE THERAPY AND MORTALITY FRANCINE GRODSTEIN, SC.D., MEIR
ORIGINAL INVESTIGATION
 ORIGINAL INVESTIGATION A Randomized Factorial Trial of Vitamins C and E and Beta Carotene in the Secondary Prevention of Cardiovascular Events in Women Results From the Women s Antioxidant Cardiovascular
ORIGINAL INVESTIGATION A Randomized Factorial Trial of Vitamins C and E and Beta Carotene in the Secondary Prevention of Cardiovascular Events in Women Results From the Women s Antioxidant Cardiovascular
A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II
 A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II Presenter Disclosure Information Howard D. Sesso, ScD, MPH Relevant Disclosures:
A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II Presenter Disclosure Information Howard D. Sesso, ScD, MPH Relevant Disclosures:
DESPITE UNCERTAINTY REGARDing
 ORIGINAL CONTRIBUTION JAMA-EXPRESS Vitamins E and C in the Prevention of Cardiovascular Disease in Men The Physicians Health Study II Randomized Controlled Trial Howard D. Sesso, ScD, MPH Julie E. Buring,
ORIGINAL CONTRIBUTION JAMA-EXPRESS Vitamins E and C in the Prevention of Cardiovascular Disease in Men The Physicians Health Study II Randomized Controlled Trial Howard D. Sesso, ScD, MPH Julie E. Buring,
HOMOCYSTEINE LEVELS HAVE
 ORIGINAL CONTRIBUTION Effect of Folic Acid and B Vitamins on Risk of Cardiovascular Events and Total Mortality Among Women at High Risk for Cardiovascular Disease A Randomized Trial Christine M. Albert,
ORIGINAL CONTRIBUTION Effect of Folic Acid and B Vitamins on Risk of Cardiovascular Events and Total Mortality Among Women at High Risk for Cardiovascular Disease A Randomized Trial Christine M. Albert,
Although the association between blood pressure and
 Two-Year Changes in Blood Pressure and Subsequent Risk of Cardiovascular Disease in Men Howard D. Sesso, ScD, MPH; Meir J. Stampfer, MD, DrPH; Bernard Rosner, PhD; J. Michael Gaziano, MD, MPH; Charles
Two-Year Changes in Blood Pressure and Subsequent Risk of Cardiovascular Disease in Men Howard D. Sesso, ScD, MPH; Meir J. Stampfer, MD, DrPH; Bernard Rosner, PhD; J. Michael Gaziano, MD, MPH; Charles
Aspirin prevents stroke but not MI in women; Vitamin E has no effect on CV disease or cancer
 MEDICAL GRAND ROUNDS CME CREDIT JULIE E. BURING, ScD* Professor of Medicine, Harvard Medical School; Division of Preventive Medicine, Brigham and Women s Hospital, Boston; principal investigator, Women
MEDICAL GRAND ROUNDS CME CREDIT JULIE E. BURING, ScD* Professor of Medicine, Harvard Medical School; Division of Preventive Medicine, Brigham and Women s Hospital, Boston; principal investigator, Women
Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials
 JNCI Journal of the National Cancer Institute Advance Access published February 10, 2009 ARTICLE Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials Bernard F.
JNCI Journal of the National Cancer Institute Advance Access published February 10, 2009 ARTICLE Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials Bernard F.
FREE RADICALS CAN CAUSE LIPID
 ORIGINAL CONTRIBUTION JAMA-EXPRESS in the Primary Prevention of Cardiovascular Disease and Cancer The Women s Health Study: A Randomized Controlled Trial I-Min Lee, MBBS, ScD Nancy R. Cook, ScD J. Michael
ORIGINAL CONTRIBUTION JAMA-EXPRESS in the Primary Prevention of Cardiovascular Disease and Cancer The Women s Health Study: A Randomized Controlled Trial I-Min Lee, MBBS, ScD Nancy R. Cook, ScD J. Michael
YOUNG ADULT MEN AND MIDDLEaged
 BRIEF REPORT Favorable Cardiovascular Profile in Young Women and Long-term of Cardiovascular and All-Cause Mortality Martha L. Daviglus, MD, PhD Jeremiah Stamler, MD Amber Pirzada, MD Lijing L. Yan, PhD,
BRIEF REPORT Favorable Cardiovascular Profile in Young Women and Long-term of Cardiovascular and All-Cause Mortality Martha L. Daviglus, MD, PhD Jeremiah Stamler, MD Amber Pirzada, MD Lijing L. Yan, PhD,
NONNARCOTIC ANALGESICS ARE
 ORIGINAL CONTRIBUTION Analgesic Use and Renal Function in Men Kathryn M. Rexrode, MD Julie E. Buring, ScD Robert J. Glynn, ScD Meir J. Stampfer, MD Linda D. Youngman, PhD J. Michael Gaziano, MD, MPH Context
ORIGINAL CONTRIBUTION Analgesic Use and Renal Function in Men Kathryn M. Rexrode, MD Julie E. Buring, ScD Robert J. Glynn, ScD Meir J. Stampfer, MD Linda D. Youngman, PhD J. Michael Gaziano, MD, MPH Context
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1999, by the Massachusetts Medical Society VOLUME 340 J ANUARY 21, 1999 NUMBER 3 DIETARY FIBER AND THE RISK OF COLORECTAL CANCER AND ADENOMA IN WOMEN CHARLES
The New England Journal of Medicine Copyright, 1999, by the Massachusetts Medical Society VOLUME 340 J ANUARY 21, 1999 NUMBER 3 DIETARY FIBER AND THE RISK OF COLORECTAL CANCER AND ADENOMA IN WOMEN CHARLES
Chemoprevention of Colorectal Cancer: Where We Stand
 Chemoprevention of Colorectal Cancer: Where We Stand Andrew T. Chan, MD, MPH Division of Gastroenterology Massachusetts General Hospital 2 nd World Congress on Controversies in Gastroenterology Xi an,
Chemoprevention of Colorectal Cancer: Where We Stand Andrew T. Chan, MD, MPH Division of Gastroenterology Massachusetts General Hospital 2 nd World Congress on Controversies in Gastroenterology Xi an,
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH. Ross L. Prentice. Fred Hutchinson Cancer Research Center
 COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
The Framingham Coronary Heart Disease Risk Score
 Plasma Concentration of C-Reactive Protein and the Calculated Framingham Coronary Heart Disease Risk Score Michelle A. Albert, MD, MPH; Robert J. Glynn, PhD; Paul M Ridker, MD, MPH Background Although
Plasma Concentration of C-Reactive Protein and the Calculated Framingham Coronary Heart Disease Risk Score Michelle A. Albert, MD, MPH; Robert J. Glynn, PhD; Paul M Ridker, MD, MPH Background Although
Aspirin Intake and Survival After Breast Cancer Michelle D. Holmes, Wendy Y. Chen, Lisa Li, Ellen Hertzmark, Donna Spiegelman, and Susan E.
 VOLUME 28 NUMBER 9 MARCH 20 2010 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Aspirin Intake and Survival After Breast Cancer Michelle D. Holmes, Wendy Y. Chen, Lisa Li, Ellen Hertzmark, Donna
VOLUME 28 NUMBER 9 MARCH 20 2010 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Aspirin Intake and Survival After Breast Cancer Michelle D. Holmes, Wendy Y. Chen, Lisa Li, Ellen Hertzmark, Donna
Personalized Aspirin Therapy
 Personalized Aspirin Therapy Nadir Arber, MD, MSc, MHA Head - Integrated Cancer Prevention Center Tel Aviv Medical Centre and Tel Aviv University Heidelberg 2014 CRC is Preventable Early detection Chemoprevention
Personalized Aspirin Therapy Nadir Arber, MD, MSc, MHA Head - Integrated Cancer Prevention Center Tel Aviv Medical Centre and Tel Aviv University Heidelberg 2014 CRC is Preventable Early detection Chemoprevention
Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes FRANK B. HU, MD 1,2,3 MEIR J. STAMPFER,
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes FRANK B. HU, MD 1,2,3 MEIR J. STAMPFER,
Rotating night shift work and risk of psoriasis in US women
 Rotating night shift work and risk of psoriasis in US women The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published
Rotating night shift work and risk of psoriasis in US women The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published
Aspirin Use and Pancreatic Cancer Mortality in a Large United States Cohort
 Aspirin Use and Pancreatic Cancer Mortality in a Large United States Cohort Eric J. Jacobs, Cari J. Connell, Carmen Rodriguez, Alpa V. Patel, Eugenia E. Calle, Michael J. Thun Background: Results from
Aspirin Use and Pancreatic Cancer Mortality in a Large United States Cohort Eric J. Jacobs, Cari J. Connell, Carmen Rodriguez, Alpa V. Patel, Eugenia E. Calle, Michael J. Thun Background: Results from
Information Services Division NHS National Services Scotland
 Cancer in Scotland April 2017 First published in June 2004, revised with each National Statistics publication Next due for revision October 2017 Information Services Division NHS National Services Scotland
Cancer in Scotland April 2017 First published in June 2004, revised with each National Statistics publication Next due for revision October 2017 Information Services Division NHS National Services Scotland
Epidemiological studies indicate that a parental or family
 Maternal and Paternal History of Myocardial Infarction and Risk of Cardiovascular Disease in Men and Women Howard D. Sesso, ScD, MPH; I-Min Lee, MBBS, ScD; J. Michael Gaziano, MD, MPH; Kathryn M. Rexrode,
Maternal and Paternal History of Myocardial Infarction and Risk of Cardiovascular Disease in Men and Women Howard D. Sesso, ScD, MPH; I-Min Lee, MBBS, ScD; J. Michael Gaziano, MD, MPH; Kathryn M. Rexrode,
A Proposed Randomized Trial of Cocoa Flavanols and Multivitamins in the Prevention of Cardiovascular Disease and Cancer
 A Proposed Randomized Trial of Cocoa Flavanols and Multivitamins in the Prevention of Cardiovascular Disease and Cancer JoAnn E. Manson, MD, DrPH Howard D. Sesso, ScD, MPH Brigham and Women's Hospital
A Proposed Randomized Trial of Cocoa Flavanols and Multivitamins in the Prevention of Cardiovascular Disease and Cancer JoAnn E. Manson, MD, DrPH Howard D. Sesso, ScD, MPH Brigham and Women's Hospital
The COSMOS Trial. (COcoa Supplement and Multivitamins Outcomes Study) JoAnn E. Manson, MD, DrPH Howard D. Sesso, ScD, MPH
 COSMOS Trial The COSMOS Trial (COcoa Supplement and Multivitamins Outcomes Study) JoAnn E. Manson, MD, DrPH Howard D. Sesso, ScD, MPH Brigham and Women's Hospital Harvard Medical School Garnet L. Anderson,
COSMOS Trial The COSMOS Trial (COcoa Supplement and Multivitamins Outcomes Study) JoAnn E. Manson, MD, DrPH Howard D. Sesso, ScD, MPH Brigham and Women's Hospital Harvard Medical School Garnet L. Anderson,
Cancers attributable to excess body weight in Canada in D Zakaria, A Shaw Public Health Agency of Canada
 Cancers attributable to excess body weight in Canada in 2010 D Zakaria, A Shaw Public Health Agency of Canada Introduction Cancer is a huge burden in Canada: Nearly 50% of Canadians are expected to be
Cancers attributable to excess body weight in Canada in 2010 D Zakaria, A Shaw Public Health Agency of Canada Introduction Cancer is a huge burden in Canada: Nearly 50% of Canadians are expected to be
ORIGINAL INVESTIGATION. An Update on Aspirin in the Primary Prevention of Cardiovascular Disease
 ORIGINAL INVESTIGATION An Update on in the Primary Prevention of Cardiovascular Disease Rachel S. Eidelman, MD; Patricia R. Hebert, PhD; Steven M. Weisman, PhD; Charles H. Hennekens, MD, DrPH Background:
ORIGINAL INVESTIGATION An Update on in the Primary Prevention of Cardiovascular Disease Rachel S. Eidelman, MD; Patricia R. Hebert, PhD; Steven M. Weisman, PhD; Charles H. Hennekens, MD, DrPH Background:
Pinkal Desai MD MPH Weill Cornell Medical College New York, NY WHI Annual Meeting, May 5-6, 2016
 Pinkal Desai MD MPH Weill Cornell Medical College New York, NY WHI Annual Meeting, May 5-6, 2016 Reproductive hormones interact with the immune system Human lymphocytes (B and T) and some lymphoma and
Pinkal Desai MD MPH Weill Cornell Medical College New York, NY WHI Annual Meeting, May 5-6, 2016 Reproductive hormones interact with the immune system Human lymphocytes (B and T) and some lymphoma and
Electronic Supplementary Material
 Electronic Supplementary Material Effects of chemopreventive agents on the incidence of recurrent colorectal adenomas: A systematic review with network meta-analysis of randomized controlled trials Sajesh
Electronic Supplementary Material Effects of chemopreventive agents on the incidence of recurrent colorectal adenomas: A systematic review with network meta-analysis of randomized controlled trials Sajesh
I t is established that regular light to moderate drinking is
 32 CARDIOVASCULAR MEDICINE Taking up regular drinking in middle age: effect on major coronary heart disease events and mortality S G Wannamethee, A G Shaper... See end of article for authors affiliations...
32 CARDIOVASCULAR MEDICINE Taking up regular drinking in middle age: effect on major coronary heart disease events and mortality S G Wannamethee, A G Shaper... See end of article for authors affiliations...
Impact of Screening Colonoscopy on Outcomes in Colon Cancer Surgery
 Impact of Screening Colonoscopy on Outcomes in Colon Cancer Surgery The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Impact of Screening Colonoscopy on Outcomes in Colon Cancer Surgery The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Alcohol and Mortality from All Causes
 Biol Res 37: 183-187, 2004 BR 183 Alcohol and Mortality from All Causes SERGE RENAUD 1, DOMINIQUE LANZMANN-PETITHORY 1, RENÉ GUEGUEN 2 and PASCALE CONARD 2 1 Emile Roux Hospital, Public Assistance of Paris
Biol Res 37: 183-187, 2004 BR 183 Alcohol and Mortality from All Causes SERGE RENAUD 1, DOMINIQUE LANZMANN-PETITHORY 1, RENÉ GUEGUEN 2 and PASCALE CONARD 2 1 Emile Roux Hospital, Public Assistance of Paris
Mortality in relation to alcohol consumption: a prospective study among male British doctors
 IJE vol.34 no.1 International Epidemiological Association 2005; all rights reserved. International Journal of Epidemiology 2005;34:199 204 Advance Access publication 12 January 2005 doi:10.1093/ije/dyh369
IJE vol.34 no.1 International Epidemiological Association 2005; all rights reserved. International Journal of Epidemiology 2005;34:199 204 Advance Access publication 12 January 2005 doi:10.1093/ije/dyh369
Postmenopausal hormone therapy and cancer risk
 International Congress Series 1279 (2005) 133 140 www.ics-elsevier.com Postmenopausal hormone therapy and cancer risk P. Kenemans*, R.A. Verstraeten, R.H.M. Verheijen Department of Obstetrics and Gynaecology,
International Congress Series 1279 (2005) 133 140 www.ics-elsevier.com Postmenopausal hormone therapy and cancer risk P. Kenemans*, R.A. Verstraeten, R.H.M. Verheijen Department of Obstetrics and Gynaecology,
Aspirin Use and Risk of Atrial Fibrillation in the Physicians' Health Study
 Aspirin Use and Risk of Atrial Fibrillation in the Physicians' Health Study The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation
Aspirin Use and Risk of Atrial Fibrillation in the Physicians' Health Study The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation
ASPIRIN IN THE TREATMENT AND PREVENTION OF CARDIOVASCULAR DISEASE
 Annu. Rev. Public Health. 1997. 18:37 49 Copyright c 1997 by Annual Reviews Inc. All rights reserved ASPIRIN IN THE TREATMENT AND PREVENTION OF CARDIOVASCULAR DISEASE Charles H. Hennekens Departments of
Annu. Rev. Public Health. 1997. 18:37 49 Copyright c 1997 by Annual Reviews Inc. All rights reserved ASPIRIN IN THE TREATMENT AND PREVENTION OF CARDIOVASCULAR DISEASE Charles H. Hennekens Departments of
Beyond Controlling for Confounding: Design Strategies to Avoid Selection Bias and Improve Efficiency in Observational Studies
 September 27, 2018 Beyond Controlling for Confounding: Design Strategies to Avoid Selection Bias and Improve Efficiency in Observational Studies A Case Study of Screening Colonoscopy Our Team Xabier Garcia
September 27, 2018 Beyond Controlling for Confounding: Design Strategies to Avoid Selection Bias and Improve Efficiency in Observational Studies A Case Study of Screening Colonoscopy Our Team Xabier Garcia
9/29/2015. Primary Prevention of Heart Disease: Objectives. Objectives. What works? What doesn t?
 Primary Prevention of Heart Disease: What works? What doesn t? Samia Mora, MD, MHS Associate Professor, Harvard Medical School Associate Physician, Brigham and Women s Hospital October 2, 2015 Financial
Primary Prevention of Heart Disease: What works? What doesn t? Samia Mora, MD, MHS Associate Professor, Harvard Medical School Associate Physician, Brigham and Women s Hospital October 2, 2015 Financial
General practice. Abstract. Subjects and methods. Introduction. examining the effect of use of oral contraceptives on mortality in the long term.
 Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners oral contraception study Valerie Beral, Carol Hermon, Clifford
Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners oral contraception study Valerie Beral, Carol Hermon, Clifford
In the Clinic: Annals Sweta Kakaraparthi 1/23/15
 In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
Is aspirin ready for colorectal cancer chemoprevention and adjuvant therapy?
 Is aspirin ready for colorectal cancer chemoprevention and adjuvant therapy? Robert Benamouzig Service de Gastroentérologie Hôpital Avicenne Bobigny, France Aspirin and Colorectal carcinogenesis Aspirin
Is aspirin ready for colorectal cancer chemoprevention and adjuvant therapy? Robert Benamouzig Service de Gastroentérologie Hôpital Avicenne Bobigny, France Aspirin and Colorectal carcinogenesis Aspirin
Statin use does not prevent recurrent adenomatous polyp formation in a VA population
 Indian J Gastroenterol (2010) 29:106 111 DOI 10.1007/s12664-010-0032-1 ORIGINAL ARTICLE Statin use does not prevent recurrent adenomatous polyp formation in a VA population Nikki Parker-Ray & Jehad Barakat
Indian J Gastroenterol (2010) 29:106 111 DOI 10.1007/s12664-010-0032-1 ORIGINAL ARTICLE Statin use does not prevent recurrent adenomatous polyp formation in a VA population Nikki Parker-Ray & Jehad Barakat
Diet, obesity, lifestyle and cancer prevention:
 Diet, obesity, lifestyle and cancer prevention: Epidemiologic perspectives Graham A Colditz, MD DrPH Niess-Gain Professor Chief, November, 2017 Outline Review evidence on contribution of diet, obesity,
Diet, obesity, lifestyle and cancer prevention: Epidemiologic perspectives Graham A Colditz, MD DrPH Niess-Gain Professor Chief, November, 2017 Outline Review evidence on contribution of diet, obesity,
BECAUSE OF THE BENEFIT OF
 ORIGINAL INVESTIGATION Dairy Food, Calcium, and Risk of Cancer in the NIH-AARP Diet and Health Study Yikyung Park, ScD; Michael F. Leitzmann, MD; Amy F. Subar, PhD; Albert Hollenbeck, PhD; Arthur Schatzkin,
ORIGINAL INVESTIGATION Dairy Food, Calcium, and Risk of Cancer in the NIH-AARP Diet and Health Study Yikyung Park, ScD; Michael F. Leitzmann, MD; Amy F. Subar, PhD; Albert Hollenbeck, PhD; Arthur Schatzkin,
What is the Impact of Cancer on African Americans in Indiana? Average number of cases per year. Rate per 100,000. Rate per 100,000 people*
 What is the Impact of Cancer on African Americans in Indiana? Table 13. Burden of Cancer among African Americans Indiana, 2008 2012 Average number of cases per year Rate per 100,000 people* Number of cases
What is the Impact of Cancer on African Americans in Indiana? Table 13. Burden of Cancer among African Americans Indiana, 2008 2012 Average number of cases per year Rate per 100,000 people* Number of cases
Fat, Fiber, Meat and the Risk of Colorectal Adenomas
 American Journal of Gastroenterology ISSN 0002-9270 C 2005 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2005.00336.x Published by Blackwell Publishing Fat, Fiber, Meat and the Risk of Colorectal
American Journal of Gastroenterology ISSN 0002-9270 C 2005 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2005.00336.x Published by Blackwell Publishing Fat, Fiber, Meat and the Risk of Colorectal
Childhood Cancer Survivor Study Analysis Concept Proposal
 Title: Multiple Subsequent Neoplasms Working Group and Investigators: Childhood Cancer Survivor Study Analysis Concept Proposal This proposed publication will be within the Second Malignancy Working Group
Title: Multiple Subsequent Neoplasms Working Group and Investigators: Childhood Cancer Survivor Study Analysis Concept Proposal This proposed publication will be within the Second Malignancy Working Group
EPIDEMIOLOGY AND BIOSTATISTICS. Aspirin Use and Risk of Cataract in Posttrial Follow-up of Physicians Health Study I
 EPIDEMIOLOGY AND BIOSTATISTICS Aspirin Use and Risk of Cataract in Posttrial Follow-up of Physicians Health Study I William G. Christen, ScD; Umed A. Ajani, MBBS; Debra A. Schaumberg, ScD; Robert J. Glynn,
EPIDEMIOLOGY AND BIOSTATISTICS Aspirin Use and Risk of Cataract in Posttrial Follow-up of Physicians Health Study I William G. Christen, ScD; Umed A. Ajani, MBBS; Debra A. Schaumberg, ScD; Robert J. Glynn,
Several studies have reported that people with periodontal
 Oral Health and Peripheral Arterial Disease Hsin-Chia Hung, DDS, DrPH; Walter Willett, MD, DrPH; Anwar Merchant, DMD, DrPH; Bernard A. Rosner, PhD; Alberto Ascherio, MD, DrPH; Kaumudi J. Joshipura, ScD
Oral Health and Peripheral Arterial Disease Hsin-Chia Hung, DDS, DrPH; Walter Willett, MD, DrPH; Anwar Merchant, DMD, DrPH; Bernard A. Rosner, PhD; Alberto Ascherio, MD, DrPH; Kaumudi J. Joshipura, ScD
Folate, vitamin B 6, and vitamin B 12 are cofactors in
 Research Letters Dietary Folate and Vitamin B 6 and B 12 Intake in Relation to Mortality From Cardiovascular Diseases Japan Collaborative Cohort Study Renzhe Cui, MD; Hiroyasu Iso, MD; Chigusa Date, MD;
Research Letters Dietary Folate and Vitamin B 6 and B 12 Intake in Relation to Mortality From Cardiovascular Diseases Japan Collaborative Cohort Study Renzhe Cui, MD; Hiroyasu Iso, MD; Chigusa Date, MD;
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL
 CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL 1. Study title: Subsequent neoplasms among survivors of childhood cancer not previously treated with radiation 2. Working group and investigators:
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL 1. Study title: Subsequent neoplasms among survivors of childhood cancer not previously treated with radiation 2. Working group and investigators:
Downloaded from:
 Ellingjord-Dale, M; Vos, L; Tretli, S; Hofvind, S; Dos-Santos-Silva, I; Ursin, G (2017) Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening
Ellingjord-Dale, M; Vos, L; Tretli, S; Hofvind, S; Dos-Santos-Silva, I; Ursin, G (2017) Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening
ESPEN Congress Florence 2008
 ESPEN Congress Florence 2008 Severe obesity - Session organised in conjunction with ASPEN Long term mortality in cohorts of severely obese subjects D. Mirabelli (Italy) Long-term mortality in cohorts of
ESPEN Congress Florence 2008 Severe obesity - Session organised in conjunction with ASPEN Long term mortality in cohorts of severely obese subjects D. Mirabelli (Italy) Long-term mortality in cohorts of
INFLAMMATION HAS BEEN HYPOTHesized
 ORIGINAL CONTRIBUTION C-Reactive Protein and the Risk of Incident Colorectal Cancer Thomas P. Erlinger, MD, MPH Elizabeth A. Platz, ScD, MPH Nader Rifai, PhD Kathy J. Helzlsouer, MD, MHS INFLAMMATION HAS
ORIGINAL CONTRIBUTION C-Reactive Protein and the Risk of Incident Colorectal Cancer Thomas P. Erlinger, MD, MPH Elizabeth A. Platz, ScD, MPH Nader Rifai, PhD Kathy J. Helzlsouer, MD, MHS INFLAMMATION HAS
Fruit and vegetable intake and the risk of cataract in women 1 3
 Fruit and vegetable intake and the risk of cataract in women 1 3 William G Christen, Simin Liu, Debra A Schaumberg, and Julie E Buring ABSTRACT Background: Prospective data on cataract in relation to total
Fruit and vegetable intake and the risk of cataract in women 1 3 William G Christen, Simin Liu, Debra A Schaumberg, and Julie E Buring ABSTRACT Background: Prospective data on cataract in relation to total
Copyright, 1995, by the Massachusetts Medical Society
 Copyright, 1995, by the Massachusetts Medical Society Volume 332 JUNE 15, 1995 Number 24 THE USE OF ESTROGENS AND PROGESTINS AND THE RISK OF BREAST CANCER IN POSTMENOPAUSAL WOMEN GRAHAM A. COLDITZ, M.B.,
Copyright, 1995, by the Massachusetts Medical Society Volume 332 JUNE 15, 1995 Number 24 THE USE OF ESTROGENS AND PROGESTINS AND THE RISK OF BREAST CANCER IN POSTMENOPAUSAL WOMEN GRAHAM A. COLDITZ, M.B.,
The Role of Observational Studies. Edward Giovannucci, MD, ScD Departments of Nutrition and Epidemiology
 The Role of Observational Studies Edward Giovannucci, MD, ScD Departments of Nutrition and Epidemiology Disclosure Information As required, I would like to report that I have no financial relationships
The Role of Observational Studies Edward Giovannucci, MD, ScD Departments of Nutrition and Epidemiology Disclosure Information As required, I would like to report that I have no financial relationships
ORIGINAL INVESTIGATION. C-Reactive Protein Concentration and Incident Hypertension in Young Adults
 ORIGINAL INVESTIGATION C-Reactive Protein Concentration and Incident Hypertension in Young Adults The CARDIA Study Susan G. Lakoski, MD, MS; David M. Herrington, MD, MHS; David M. Siscovick, MD, MPH; Stephen
ORIGINAL INVESTIGATION C-Reactive Protein Concentration and Incident Hypertension in Young Adults The CARDIA Study Susan G. Lakoski, MD, MS; David M. Herrington, MD, MHS; David M. Siscovick, MD, MPH; Stephen
(i) Is there a registered protocol for this IPD meta-analysis? Please clarify.
 Reviewer: 4 Additional Questions: Please enter your name: Stefanos Bonovas Job Title: Researcher Institution: Humanitas Clinical and Research Institute, Milan, Italy Comments: The authors report the results
Reviewer: 4 Additional Questions: Please enter your name: Stefanos Bonovas Job Title: Researcher Institution: Humanitas Clinical and Research Institute, Milan, Italy Comments: The authors report the results
The Relation of Surgery for Prostatic Hypertrophy to Carcinoma of the Prostate
 American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
Table Cohort studies of consumption of alcoholic beverages and cancer of the colorectum
 Akhter et al. (2007), Japan, Miyagi Study [data also included in the pooled analysis, Mizoue et al. (2008)] of 21 199 men living in the Miyagi region recruited in 1990; aged 40 64 years; followed-up until
Akhter et al. (2007), Japan, Miyagi Study [data also included in the pooled analysis, Mizoue et al. (2008)] of 21 199 men living in the Miyagi region recruited in 1990; aged 40 64 years; followed-up until
BNORC: Contribution over 25 years to evidence on obesity and cancer
 BNORC: Contribution over 25 years to evidence on obesity and cancer Graham A Colditz, MD DrPH Niess-Gain Professor Chief, Boston July 10, 2017 https://tinyurl.com/ybmnqorq Economic costs of diabetes:
BNORC: Contribution over 25 years to evidence on obesity and cancer Graham A Colditz, MD DrPH Niess-Gain Professor Chief, Boston July 10, 2017 https://tinyurl.com/ybmnqorq Economic costs of diabetes:
Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers
 Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers Ross Prentice Fred Hutchinson Cancer Research Center and University of Washington AICR, November 5, 2009 Outline
Low-Fat Dietary Pattern Intervention Trials for the Prevention of Breast and Other Cancers Ross Prentice Fred Hutchinson Cancer Research Center and University of Washington AICR, November 5, 2009 Outline
Obesity and Breast Cancer Risk
 Program on Breast Cancer Environmental Risk Factors Fact Sheet #56 August 2007 TOPICS Measurement of obesity BMI and breast cancer risk Weight gain and loss and breast cancer risk Body fat distribution
Program on Breast Cancer Environmental Risk Factors Fact Sheet #56 August 2007 TOPICS Measurement of obesity BMI and breast cancer risk Weight gain and loss and breast cancer risk Body fat distribution
Original Contribution. A Prospective Study of Night Shift Work, Sleep Duration, and Risk of Parkinson s Disease
 American Journal of Epidemiology Copyright ª 2006 by the Johns Hopkins Bloomberg School of Public Health All rights reserved; printed in U.S.A. Vol. 163, No. 8 DOI: 10.1093/aje/kwj096 Advance Access publication
American Journal of Epidemiology Copyright ª 2006 by the Johns Hopkins Bloomberg School of Public Health All rights reserved; printed in U.S.A. Vol. 163, No. 8 DOI: 10.1093/aje/kwj096 Advance Access publication
Contraception and cancerepidemiological
 Contraception and cancerepidemiological evidence Hormonal contraception Combined Progestogen-only Philip C Hannaford University of Aberdeen Breast cancer and combined oral Re-analysis of individual data
Contraception and cancerepidemiological evidence Hormonal contraception Combined Progestogen-only Philip C Hannaford University of Aberdeen Breast cancer and combined oral Re-analysis of individual data
Folate and prevention of neural tube defects: Tracking red blood cell concentrations will help guide policy decisions about fortification
 Folate and prevention of neural tube defects: Tracking red blood cell concentrations will help guide policy decisions about fortification Derrick Bennett, University of Oxford, UK 8 October, 2014 IXth
Folate and prevention of neural tube defects: Tracking red blood cell concentrations will help guide policy decisions about fortification Derrick Bennett, University of Oxford, UK 8 October, 2014 IXth
Current Use of Unopposed Estrogen and Estrogen Plus Progestin and the Risk of Acute Myocardial Infarction Among Women With Diabetes
 Current Use of Unopposed Estrogen and Estrogen Plus Progestin and the Risk of Acute Myocardial Infarction Among Women With Diabetes The Northern California Kaiser Permanente Diabetes Registry, 1995 1998
Current Use of Unopposed Estrogen and Estrogen Plus Progestin and the Risk of Acute Myocardial Infarction Among Women With Diabetes The Northern California Kaiser Permanente Diabetes Registry, 1995 1998
A Randomized Trial of Low-Dose Aspirin in the Prevention of Clinical Type 2 Diabetes in Women
 A Randomized Trial of Low-Dose Aspirin in the Prevention of Clinical Type 2 Diabetes in Women The Harvard community has made this article openly available. Please share how this access benefits you. Your
A Randomized Trial of Low-Dose Aspirin in the Prevention of Clinical Type 2 Diabetes in Women The Harvard community has made this article openly available. Please share how this access benefits you. Your
2018 Texas Cancer Registry Annual Report
 2018 Texas Cancer Registry Annual Report As Required by Texas Health and Safety Code Section 82.007 November 2018 Table of Contents Executive Summary... 1 1. Introduction... 2 2. Background... 3 Cancer
2018 Texas Cancer Registry Annual Report As Required by Texas Health and Safety Code Section 82.007 November 2018 Table of Contents Executive Summary... 1 1. Introduction... 2 2. Background... 3 Cancer
Estimated Minnesota Cancer Prevalence, January 1, MCSS Epidemiology Report 04:2. April 2004
 MCSS Epidemiology Report 04:2 Suggested citation Perkins C, Bushhouse S.. Minnesota Cancer Surveillance System. Minneapolis, MN, http://www.health.state.mn.us/divs/hpcd/ cdee/mcss),. 1 Background Cancer
MCSS Epidemiology Report 04:2 Suggested citation Perkins C, Bushhouse S.. Minnesota Cancer Surveillance System. Minneapolis, MN, http://www.health.state.mn.us/divs/hpcd/ cdee/mcss),. 1 Background Cancer
CANCER FACTS & FIGURES For African Americans
 CANCER FACTS & FIGURES For African Americans Pennsylvania, 2006 Pennsylvania Cancer Registry Bureau of Health Statistics and Research Contents Data Hightlights...1 Pennsylvania and U.S. Comparison...5
CANCER FACTS & FIGURES For African Americans Pennsylvania, 2006 Pennsylvania Cancer Registry Bureau of Health Statistics and Research Contents Data Hightlights...1 Pennsylvania and U.S. Comparison...5
Menopausal hormone therapy currently has no evidence-based role for
 IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
IN PERSPECTIVE HT and CVD Prevention: From Myth to Reality Nanette K. Wenger, M.D. What the studies show, in a nutshell The impact on coronary prevention Alternative solutions Professor of Medicine (Cardiology),
Measures of Obesity and Cardiovascular Risk Among Men and Women
 Journal of the American College of Cardiology Vol. 52, No. 8, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2008.03.066
Journal of the American College of Cardiology Vol. 52, No. 8, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2008.03.066
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
Fruit and vegetable intake and risk of cardiovascular disease: the Women s Health Study 1,2
 Fruit and vegetable intake and risk of cardiovascular disease: the Women s Health Study 1,2 Simin Liu, JoAnn E Manson, I-Min Lee, Stephen R Cole, Charles H Hennekens, Walter C Willett, and Julie E Buring
Fruit and vegetable intake and risk of cardiovascular disease: the Women s Health Study 1,2 Simin Liu, JoAnn E Manson, I-Min Lee, Stephen R Cole, Charles H Hennekens, Walter C Willett, and Julie E Buring
Final Report 22 January 2014
 Final Report 22 January 2014 Cohort Study of Pioglitazone and Cancer Incidence in Patients with Diabetes Mellitus, Follow-up 1997-2012 Kaiser Permanente Division of Research Assiamira Ferrara, MD, Ph.D.
Final Report 22 January 2014 Cohort Study of Pioglitazone and Cancer Incidence in Patients with Diabetes Mellitus, Follow-up 1997-2012 Kaiser Permanente Division of Research Assiamira Ferrara, MD, Ph.D.
Blood pressure and risk of developing type 2 diabetes mellitus: The Women s Health Study
 European Heart Journal (2007) 28, 2937 2943 doi:10.1093/eurheartj/ehm400 Clinical research Hypertension Blood pressure and risk of developing type 2 diabetes mellitus: The Women s Health Study David Conen
European Heart Journal (2007) 28, 2937 2943 doi:10.1093/eurheartj/ehm400 Clinical research Hypertension Blood pressure and risk of developing type 2 diabetes mellitus: The Women s Health Study David Conen
Smoking and Mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC)
 Smoking and Mortality SECTION 6 Smoking and Mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Kotaro Ozasa Abstract In the JACC study, risk of death with all cancers and
Smoking and Mortality SECTION 6 Smoking and Mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Kotaro Ozasa Abstract In the JACC study, risk of death with all cancers and
ORIGINAL INVESTIGATION. The Impact of Diabetes Mellitus on Mortality From All Causes and Coronary Heart Disease in Women
 The Impact of Mellitus on Mortality From All Causes and Coronary Heart Disease in Women 20 Years of Follow-up ORIGINAL INVESTIGATION Frank B. Hu, MD; Meir J. Stampfer, MD; Caren G. Solomon, MD; Simin Liu,
The Impact of Mellitus on Mortality From All Causes and Coronary Heart Disease in Women 20 Years of Follow-up ORIGINAL INVESTIGATION Frank B. Hu, MD; Meir J. Stampfer, MD; Caren G. Solomon, MD; Simin Liu,
Supplementary Online Content
 Supplementary Online Content Li S, Chiuve SE, Flint A, et al. Better diet quality and decreased mortality among myocardial infarction survivors. JAMA Intern Med. Published online September 2, 2013. doi:10.1001/jamainternmed.2013.9768.
Supplementary Online Content Li S, Chiuve SE, Flint A, et al. Better diet quality and decreased mortality among myocardial infarction survivors. JAMA Intern Med. Published online September 2, 2013. doi:10.1001/jamainternmed.2013.9768.
Prevention of cancer the influence of diet
 Prevention of cancer the influence of diet Food Network, 2010 Anne Tjønneland Institut for Epidemiologisk Kræftforskning, Kræftens Bekæmpelse Risk of cancer Indien / USA Aldersspecifik brystkræftincidens
Prevention of cancer the influence of diet Food Network, 2010 Anne Tjønneland Institut for Epidemiologisk Kræftforskning, Kræftens Bekæmpelse Risk of cancer Indien / USA Aldersspecifik brystkræftincidens
SESSION 3 11 AM 12:30 PM
 SESSION 3 11 AM 12:30 PM for the Primary Prevention of Cardiovascular Disease: A Personalized Approach SPEAKER Samia Mora MD, MHS Presenter Disclosure Information The following relationships exist related
SESSION 3 11 AM 12:30 PM for the Primary Prevention of Cardiovascular Disease: A Personalized Approach SPEAKER Samia Mora MD, MHS Presenter Disclosure Information The following relationships exist related
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity
 Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
Association Between Consumption of Beer, Wine, and Liquor and Plasma Concentration of High-Sensitivity C-Reactive Protein in Women Aged 39 to 89 Years
 Association Between Consumption of Beer, Wine, and Liquor and Plasma Concentration of High-Sensitivity C-Reactive Protein in Women Aged 39 to 89 Years Emily B. Levitan, MS a,e, Paul M. Ridker, MD, MPH
Association Between Consumption of Beer, Wine, and Liquor and Plasma Concentration of High-Sensitivity C-Reactive Protein in Women Aged 39 to 89 Years Emily B. Levitan, MS a,e, Paul M. Ridker, MD, MPH
Risk Factors for Mortality in the Nurses Health Study: A Competing Risks Analysis
 American Journal of Epidemiology ª The Author 2010. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail:
American Journal of Epidemiology ª The Author 2010. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail:
The Impact of Diabetes Mellitus and Prior Myocardial Infarction on Mortality From All Causes and From Coronary Heart Disease in Men
 Journal of the American College of Cardiology Vol. 40, No. 5, 2002 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)02044-2
Journal of the American College of Cardiology Vol. 40, No. 5, 2002 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)02044-2
Information Services Division NHS National Services Scotland
 Cancer in Scotland October 2012 First published in June 2004, revised with each National Statistics publication Next due for revision April 2013 Information Services Division NHS National Services Scotland
Cancer in Scotland October 2012 First published in June 2004, revised with each National Statistics publication Next due for revision April 2013 Information Services Division NHS National Services Scotland
State of the art pharmacoepidemiological study designs for post-approval risk assessment
 State of the art pharmacoepidemiological study designs for post-approval risk assessment Cardiac Safety Research Consortium Think Tank Round Table Meeting Thursday, March 6, 2014 Jennifer L. Lund, PhD
State of the art pharmacoepidemiological study designs for post-approval risk assessment Cardiac Safety Research Consortium Think Tank Round Table Meeting Thursday, March 6, 2014 Jennifer L. Lund, PhD
