TITLE: TRANSURETHRAL MICROWAVE THERMOTHERAPY FOR THE TREATMENT OF BENIGN PROSTATIC HYPERPLASIA
|
|
|
- Moris Stevenson
- 5 years ago
- Views:
Transcription
1 TITLE: TRANSURETHRAL MICROWAVE THERMOTHERAPY FOR THE TREATMENT OF BENIGN PROSTATIC HYPERPLASIA AUTHOR: Leah Karliner, MD, MAS Assistant Professor of Medicine Division of General Internal Medicine Department of Medicine University of California San Francisco PUBLISHER: California Technology Assessment Forum DATE OF PUBLICATION: 10/28/2009 PLACE OF PUBLICATION: San Francisco, CA 1
2 TRANSURETHRAL MICROWAVE THERMOTHERAPY FOR THE TREATMENT OF BENIGN PROSTATIC HYPERPLASIA A Technology Assessment INTRODUCTION The California Technology Assessment Forum is requested to review the scientific evidence for the use of Transurethral Microwave Thermotherapy for the Treatment of Benign Prostatic Hyperplasia, with a particular question of difference in efficacy dependent on prostate size. BACKGROUND Benign prostatic hyperplasia (BPH) and resultant lower urinary tract symptoms are very common, with 60% of year old US men reporting at least one symptom (nocturia, incomplete emptying or hesitancy), and 75% of men 70 years or older reporting at least one symptom. 1 In a primary care clinical setting, 30% of men over 50 report moderate to severe symptoms, with two-thirds of these men reporting being bothered by their symptoms. 2 Oral medication with alpha1-blockers or 5alphareductase inhibition are considered first line treatment of bothersome symptoms. 3 For those men at highest risk of symptomatic progression (large prostate, poor peak flow rate, high post-void residual), dual therapy with both classes of medication may be indicated. 4 Not all men achieve adequate response to medical treatment for BPH, however, and many go on to have a more invasive treatment. The most common surgical treatment to which all other invasive treatments are compared is transurethral resection of the prostate or TURP. While TURP is considered very effectively for rapid symptom improvement and for its persistent effects, it requires hospitalization, carries the risks of anesthesia, bleeding, urethral scarring and erectile dysfunction (ED). 5 Thus, there has been an effort to develop minimally invasive interventions to treat symptomatic BPH. The most prominent of these is Transurethral Microwave Thermotherapy or TUMT. TUMT is an outpatient procedure requiring topical anesthesia, oral analgesics and rarely sedation. It involves the positioning of a microwave antenna within the prostate after introduction via a urinary catheter. Early (low energy) TUMT heated intraprostatic temperatures to C. More 2
3 recent practice is to use high energy TUMT which heats the prostate above 45 C to temperatures up to 70 C, while at the same time using urethral cooling to keep the urethral temperature below 45 C. 6 The Cochrane Collaboration published a systematic review on the topic in 2009 and concluded that TUMT was a reasonable alternative to TURP and alpha-blockade, however TURP was more clinically effective. 7 We undertook a similar review of the evidence, paying particular attention to reported prostate size in the trials. TECHNOLOGY ASSESSMENT (TA) TA Criterion 1: The technology must have final approval from the appropriate government regulatory bodies. There are many manufacturers of devices for this technology. marketing through the FDA Premarket Approval (PMA) process. All have received approval for TA Criterion 1 is met. TA Criterion 2: The scientific evidence must permit conclusions concerning the effectiveness of the technology regarding health outcomes. The Medline, Embase, and Cochrane clinical trials database, Cochrane reviews database and the Database of Abstracts of Reviews of Effects (DARE) were searched for relevant references through August (See appendix for search terms) Of 420 potentially relevant citations, we found 34 papers from 20 unique randomized controlled studies (RCT) to include in this assessment. (See Figure below for study selection details) Of these 20 studies, 10 were RCTs of TUMT versus a sham control, 8-24 eight were RCTs of TUMT versus transurethral resection of the prostate (TURP), one was an RCT of TUMT versus alpha-blockade, 39, 40 and one was an RCT of TUMT versus TUMT plus alpha-reductase inhibition. 41 3
4 Figure 1: Study Selection 639 potentially relevant references screened 219 excluded because they were duplicates 420 abstracts for assessment 55 manuscripts for full text review 34 manuscripts from 20 unique randomized controlled trials included in assessment: 10 compare TUMT to Sham 8 compare TUMT to TUR 1 compares TUMT to alpha-blockade 1 compares TUMT to TUMT plus alphablockade 365 excluded (observational data only, editorials, reviews, not directly relevant. duplicates) 21 manuscripts excluded (observational data only, editorials, reviews, not directly relevant. duplicates) Level of Evidence: 1, 2 TA Criterion 2 is met. TA Criterion 3: The technology must improve net health outcomes. TUMT vs. Sham Control (Table 1) All ten of the RCTs comparing TUMT to a sham control - in which the patients receiving the sham intervention underwent instrumentation similar to the actual intervention without the application of microwave thermotherapy were small. Most reported results at three to six months after the intervention. One reported five-year follow-up; however this was on a very small subset of the original participants and an overall extremely small number of patients (n=15). 22 Among the ten studies, eight different thermotherapy devices were used. Although not all of the studies stated prostate size/volume as an inclusion or exclusion criterion, those that did had varying criteria, 4
5 including prostates between 30-80grams, 8, mL, mL, g, g, 18 or simply excluding those patients with enlarged or predominantly enlarged median lobes , 19, 20, 24 On the whole it does appear that the included patients had moderately severe obstructive symptoms based on low peak flow rates and high scores on symptom scales, but not so severe as to cause post-void residual volumes greater than 350mL. Not all studies listed their exclusion criteria; however, among those that did, there was apparent uniformity in excluding patients with prostate cancer, prostatitis, urethral stricture, intravesical pathology such as stones or mass, neurogenic bladder dysfunction, active urinary tract infection, prior prostate surgery, coagulopathy, metallic implant or cardiac pacemaker, or a short prostatic urethra (<25mm). In all ten studies the sham group showed significant improvement from baseline in both obstructive symptoms, as measured on a variety of different validated symptom scales, as well as on urodynamic measures (usually peak flow rate) when studied. All but three of the studies10, 19, 24 demonstrated significantly greater improvement in obstructive symptoms for the TUMT group than for the sham group. However, differences in improvement of urodynamics varied across studies. In one of the higher quality studies which recruited from multiple centers that had 12-month follow-up, reported their loss-to follow-up rate, and conservatively treated those lost to follow-up as nonresponders, there was no difference in peak flow rate improvement between TUMT and sham treated patients. 8, 9 However, in another reasonably high quality study which used double blinding neither patients nor physicians evaluating the outcomes were aware of study assignment investigators reported a substantially higher proportion of the TUMT group with a significant increase in peak flow rate compared to the sham group (58% vs. 27%; p<.01). 13 Of the three negative studies, one had unclear inclusion criteria for obstruction, 24 another did not present the baseline data by treatment group and thus it is not possible to assess the adequacy of randomization. 19 The third negative study was reasonably large (n=200 overall, although the number in each group is unclear), was only single blinded (patients). 10 5
6 Table 1. Published Randomized Controlled Trials of TUMT vs. Sham Control. N=10 Unique Studies Study Participants / prostate Device / max weight or volume / other inclusion criteria tissue temperature Abbou 1994, 1995 TUMT n=66; Sham n=31 >age 50 Prostate 30-80g Voiding difficulty 3 months Albala 2002 N=200; randomized 2:1 TUMT:Sham Age Prostate volume mL AUA symptom score >13 Bother score > 11 Peak flow rate <12mL/s Thermex II Prostcare BSD-50 / 45 C TherMatrx TMx / C Results Quality Comments (single or multi-center, randomization, blinding, follow-up) TUMT 50% decrease Madsen score vs. Sham 17% decrease (p<.01) No difference in Peak flow rate change (14% vs. 17%). TUMT AUA symptom score decreased from 22.5 to 12.4 vs. Sham from 22.8 to 17 at 3 months ( p>.05 ; actual p-value not given) Recruitment from 7 centers; single treatment center. TUMT group had somewhat lower Madsen Score and residual volume at baseline. 12-month follow-up. Withdrawals (17% TUMT; 38% sham) all treated as non-responders. Treatment in 7 offices. Patients blinded. After 3 months allowed cross-over to treatment group. Follow-up for treatment group only for 12 months. Bdesha 1993, randomized, 2 lost to follow-up. TUMT n=22; Sham n=18 WHO symptom score >14 Residual volume 50 or peak flow rate <15mL/s Large glands (>40mm) excluded Blute 1996 TUMT N=78; Sham N=37 Madsen symptom score >8 Residual volume mL Peak flow rate <10 Prostate length 3.5-5cm Leo Microthermer / C Prostatron / temperature not given TUMT group 82% responded vs. Sham 17% (p<.001) TUMT 58% increase in peak flow vs. Sham 27% (p<.01) TUMT 55% decrease in Madsen score vs. Sham 28% (p<.001) Single center. Patients, investigator and evaluators blinded. 3-month follow-up. Patients and evaluating physicians blinded. 3-month follow-up. Too much loss to follow-up for 12-month outcomes. 6
7 Brehmer 1999 N=44: TUMT30min =14 TUMT 60min=16 Sham=14 Peak flow rate <12mL/s Prostate weight 50g Larson 1998 Randomized 3:1 TUMT N=125 Sham N=44 AUA symptom score 9 Peak flow rate 12mL/s No enlarged median lobe Prostate weight 100g TUMT 3.2% increase in residual volume vs. Sham 3.7% (p>.05) ECP / 46 C ICS symptom questionnaire no significant difference between groups. Urologix Targis / 44.5 C Improvement in both TUMT groups (compared with sham) in flow rate, daytime and nocturnal frequency. TUMT AUA symptom scores 50% decrease at 6 months compared with sham 32% decrease (p<.01). Decrease in symptoms not significantly related to prostate volume. Patients blinded; no mention of evaluator or investigator blinding. Single site; underpowered study. 4-month follow-up Multi-center recruitment and treatment 5 centers. Double blinded. Mean prostate volume 17% greater in sham group at baseline, but AUA symptom scores not different. 6-month follow-up Nawrocki 1997 TUMT N=38 Sham N=40 No treatment N=42 Peak flow rate <15mL/s Residual volume <350mL No enlarged middle lobe; no other mention prostate size Prostasoft v. 2 / temp not stated Percent decrease in symptoms similar for those with moderate and severe symptoms at baseline. Change in minimal urethral opening pressure and in AUA symptom score not significantly different between TUMT and sham; both interventions better than no treatment. Single site. Double blinded for two active & sham treatment groups. No standard table 1 to evaluate adequacy of randomization. 6-month follow-up. Roehrborn 1998 Trachtenberg 1998 Tan 2005 TUMT N=147 Sham N=73 Age 55 AUA symptom score 13 Peak flow rate 12mL/s Prostate volume mL Dornier Urowave / 50 C Both groups had equally improved AUA symptom score and peak flow rates at 1 month, with further improvement at 3 months, remaining steady at 6 months for TUMT group compared to Sham (p<.05). Multicenter trial. Double blinded. Randomization adequate. 5-year follow-up report (Tan et all 2005) is on very small subset (N=15) of original sample from a single site. 7
8 Venn 1995 TUMT N=48 Sham N=48 Madsen score >8 Urodynamic evidence of bladder outlet obstruction Predominantly lateral lobe enlargement Microwave Engineering Designs / 46 C No significant difference found between treatment and sham groups on Madsen score reduction or peak flow rate at 3 or 6 months. Single center. Single blinded. Unclear inclusion criteria. 6-month considerable differential loss to follow-up (42/48 TUMT; 20/48 Sham). Ogden 1993 de la Rosette 1994 de Wildt 1996 Francisca 1997 TUMT N=47 Sham =46 Age >45 Madsen score > 8 Peak flow rate <15mL/s Residual volume <350mL No prominent isolated middle lobe Neither device nor max temp stated TUMT group with higher proportion >50% decrease in Madsen score at 3 months (62% vs. 18%; p=.002); >50% increase in peak flow rate (36% vs. 11%; p<.002); >50% decrease in post-void residual volume (49% vs. 22%; p=.002). Two- centers. Individual results reported in Ogden & de la Rosette / Francisca; combine results reported in de Wildt. Randomization adequate. After 3-months allowed crossover of sham patients to TUMT group, so cannot make comparison after 3-months. AUA WHO ICS American Urologic Association World Health Organization International Continence Society 8
9 TUMT vs. TURP (Table 2) All of the eight trials of TUMT vs. TURP were relatively small with fewer than 100 people in each group. Most were single site, with the exception of two of the more recent studies. 34, 36-38, 42 Understandably, none of the patients were blinded to surgical assignment; however, none of the studies appear to have blinded investigators evaluating or adjudicating outcomes either. While exact inclusion criteria differed from study to study, they all included men with high symptom scores, impaired peak flow rates, moderate post-void residual volumes, medium length prostatic urethras, and varying size prostate glands. Three studies included men with any prostate volume above 30mL, 31-33, 36 three limited to between mL, 25, 29, 30, 34, 37, 38 and two did not state prostate size as an inclusion or exclusion criteria , 35 None specified a large median lobe as an absolute exclusion criterion. All studies used either the Prosatron/Prostasoft or the ProstaLund devices. All eight studies found both TUMT and TURP to be effective at achieving clinically and statistically significantly reduced symptom scores and with the exception of one smaller study which found no peak flow rate improvement in the TURP group 25 increased peak flow rates compared to baseline. On the whole, TURP achieved lower symptom scores and higher peak flow rates than TUMT; these differences persisted at six and 12 month follow-up. For example in one of the larger trials conducted in the Netherlands with the Prostatron device by Floratos et al, the TUMT group achieved an average increase in peak flow rate from 9.2mL/s at baseline to 15.1mL/s at 12- months, compared with an increase from 7.8mL/s to 24.5mL/s in the TURP group. This effect declined somewhat for the TUMT group at 24 and 36 months to 14.5 and 11.9mL/s respectively, while it did not decline in the TURP group (23.0 and 24.7mL/s). Likewise, both groups improved in their symptom scores (mean IPSS), with a decrease for the TUMT group from 20 to 8, 9, and 12 at 12, 24 and 36 months respectively and a greater and more persistent decrease for the TURP group from 20 to 3,4 and 3. At three years post-treatment 14 (18%) of the TUMT group had undergone another treatment for BPH, for the most part related to treatment failure; whereas, eight (12%) of the TURP group had undergone another treatment such as TURP or laser therapy, for the most part related to a complication of TURP such as ureteral stricture or bladder neck sclerosis. 31 9
10 While the details of the results differ across studies, this study by Floratos et al is representative of the results of the TUMT vs. TURP RCT s taken together TUMT provides symptomatic and urinary flow improvement for men with moderately severe obstructive symptoms, however not as great or as long-lasting as TURP. This seems to be similar for the Prostatron and ProstaLund devices, with the larger of the two ProstaLund device studies by Wagrell et al reporting a five-year retreatment rate of 10% for the TUMT group (both medical and surgical treatments for BPH), and of 4% for the TURP group (alpha-blockade and urethral stricture treatment). 34, 37, 38 10
11 Table 2. Published Randomized Controlled Trials of TUMT vs. TURP. N=8 Unique Studies Study Participants / prostate Device / max weight or volume / other inclusion criteria tissue temperature Ahmed 1997 TUMT N=30 TURP N=30 Groups matched for age Age 55 years AUA symptom score 12 Peak flow rate <15mL/s Residual volume<300ml Prostate Volume mL Excluded patients with prior drug treatment for BPH Prostasoft v minute session/ rectal temp 43.5 C Results Quality Comments (single or multi-center, randomization, blinding, follow-up) Both groups with significant and equal decrease in AUA symptom score; TUMT 18.5 to 5.3; TURP 18.4 to 5.2). TURP with significant improvement in peak flow rate (9.5 to 14.6; p=.001); TUMT with no improvement (10.1 to 9.1; p>.05). TURP with significant improvement in post void residual volume (109.1 to 32.5; p<.001); TUMT with insignificant increase (94.4 to 104.9; p>.05). Single site. No apparent blinding. No loss to follow-up. 6-month follow-up. D Ancona 1997, 1998 TUMT N=31 TURP N=21 Age 45 years Prostate volume mL Prostate length 25-50mm Madsen score 8 Peak flow rate 15mL/s Residual volume 300mL Posatron v.2.5 / max temp not given / mean energy applied 152kJ Both groups had improvement in symptom scores and peak flow rate at three months which then stabilized at 6-months without further change at 1 year and slight increase at 2.5 years, with TURP group consistently doing a little better than TUMT group. TURP group 78% had 50% improvement in symptom scores vs.68% of TUMT group (no p-value given); similarly 100% TURP group had improvement in uroflowmetry vs. 68% TUMT group (no p-value given). Single site. No apparent blinding. Randomization adequate. At 12-month follow-up TUMT group 27/31; TURP group 27/21. At 2.5 year follow-up TUMT group 17/31; TURP group 12/21. In TUMT group, 2 had undergone TURP at 6-month f/u and 4 more had undergone TURP at 2.5 year follow-up. Dahlstrand 1993, 1994, 1995 TUMT N=39 TURP N=44 Prostatron / 44.5 C TUMT and TURP groups with equal and significant reduction in Madsen symptom score and residual volume at Single site. Randomization blinded but no other mention of blinding. 11
12 Age 45 years Prostate volume not stated Prostate length 35-50mm Madsen score >8 Peak flow rate <15mL/s Residual volume 350mL 8 weeks, with significantly more improvement in the TURP group at 3, 6, 12 and 24 months. TUMT and TURP groups both with significant improvement in peak flow rate at all time periods; TURP group with significantly greater improvement than TUMT group at all time periods. Minimal loss to follow-up over 2 years. Francisca 1999, 2000 TUMT N=74 TURP N=73 Age 45 years Prostate volume 30mL Prostatic urethra length 25mm Madsen score 8 Peak flow rate <15mL/s Residual volume 350mL Prostasoft 2.5 / rectal temp 43.5 C Madsen score improved for both groups, but more for TURP compared to TUMT at 1 year (87% improvement vs. 63%; no p-value given). Peak flow rate results were similarly improved for both groups, but more for TURP. Both groups experienced improvement in QOL overall, with no significant difference in the total score between groups. TURP group improved significantly more on both general and specific perception of urinary difficulties scale (p<.01 for both). Single site. No mention of blinding. Randomization adequate. Excluded patients in both groups with poor outcomes: in TUMT group 8 patients excluded, 3 of whom had adverse outcomes & received surgical treatment; in TURP group 17 patients excluded, 2 of whom had adverse outcomes & received further treatment. Not clear that QOL questionnaire had been validated previously; although reliability data is given for this study. TUMT group with more ejaculation associated with orgasm at 3 and 12 months compared with TURP group (67% vs. 37% at 12 months; p=.006). TUMT group more satisfied with sexual functioning at 3 months than TURP group (55% very satisfied vs. 26%; p=.02) 12
13 Floratos 2001 TUMT N=78 TURP N=66 Age 45 years Prostate volume 30mL Prostatic urethra length 25mm Madsen score 8 Peak flow rate <15mL/s Residual volume 350mL Nielsen 2002 TUMT N=46 TURP or TUIP N=24 (ILC N=48) Age 50 years Prostate volume not stated Prostatic urethra length 25mm I-PSS 7 Peak flow rate 12mL/s Residual volume 350mL Prostatron / temp not stated, but average energy delivered 140kJ Prostasoft v2.0 and v2.5 / temp not stated Both groups with significant improvement on I-PSS, QOL, peak flow rate, with more improvement in the TURP group on all parameters at 1, 2, and 3 years. Additionally, while some effect was maintained for 3 years in both groups, improvement in the TURP group was more persistent. No p- values were given for comparison between groups. All groups had significant improvement on I-PSS at 1, 3, and 6 months, with significantly more improvement in TURP group at 1 and 3 months and equal improvement at 6 months. All groups had significant improvement in peak flow rate, with the TURP group achieving the highest flow rates, significantly higher than TUMT group at 6 months. Single site. No mention of blinding. Randomization adequate. During 36 month follow-up TUMT group: 14 underwent another treatment usually related to treatment failure, 7 lost to followup; TURP group 8 underwent another treatment usually related to a complication of TURP, 11 lost to followup Single site. No mention of blinding. Randomization adequate. Intention to treat analysis; one patient randomized to TUMT inadvertently had a TURP; one patient randomized to TURP declined surgery. 2 patients randomized to TURP were excluded due to diagnosis of prostate cancer. 6-month follow-up. 13
14 Schlein 2006 TUMT N=61 TURP or prostate enucleation N=59 Age 45 years Prostate volume >30mL Prostatic urethra length 35mm Residual volume 300mL Indwelling catheter /intermittent catheterization for 1 month ProstaLund CoreTherm / The majority of both groups were catheter free at 3 months with no significant change at 6 months (TUMT 79% vs. TURP 88% at 6-months; p=.2). Mean IPSS scores for both groups were in the mild symptom range at 3 and 6 months, with scores for the TURP group being significantly lower than the TUMT group; however no baseline scores are given and improvement from baseline is not reported. Multisite. No mention of blinding. No traditional Table 1 to assess randomization adequacy. 12 of the TUMT patients had prostate volumes >100mL at study entry. 6-month follow-up. Wagrell 2002, 2004 TUMT N=100 TURP N=46 Age 45 years Prostate volume mL Peak flow rate<13ml/s Residual volume 300mL I-PSS 13 ProstaLund CoreTherm/ 55 C Both groups improved significantly, with an increase in peak flow rate and decrease in IPSS score at 3 months; these improvements persisted at 12 months. There was no statistical difference between the two groups. For those remaining in the study at 2, 3 and 5 years: Peak flow rate improvement persisted at 2, 3, and 5 years for both groups. The TURP group had slightly higher rates, but the difference was not statistically significant at any time point (at 5-years TUMT 11.4mL/s vs. TURP 13.6mL/s; p=0.2) Results were similar for decrease in IPSS. Retreatment: at 5-years, 10% of the Multisite. No mention of blinding Randomization adequate. Utilized a wash-out period of 6-weeks for alpha- 43cptor blockers or finasteride. Dropout: 154 initially randomized; 5 TURP group and 3 TUMT group withdrew prior to treatment 2- year results reflect 79/100 TUMT and 39/46 TURP groups. 3-year results reflect 69/100 TUMT and 36/46 TURP groups. 5-year results reflect 62/100TUMT and 34/46 TURP groups. Standard procedure of indwelling catheter x 14 days after TUMT & 3-7 days after TURP Individualized treatment time based on temperature and estimated coagulation necrosis. 14
15 TUMT group had undergone additional BPH treatment medical & surgical; 4% of the TURP group had undergone additional treatment including 1 alphablocker and 1 urethral stricture treatment. QOL I-PSS Quality of life International Prostate Symptom Scoree 15
16 Adverse Events in the TUMT vs. TURP trials (Table 3) A review of the adverse events in the TUMT vs. TURP RCTs demonstrates that both the early and late adverse effects are greater with TURP. In particular, there is higher risk of blood loss, hematuria, urethral stricture, meatal stenosis, bladder neck sclerosis and sexual side effects including retrograde ejaculation and erectile dysfunction with TURP. There is little to no report of these side effects occurring anew after the procedure in the TUMT group. There is very little report of incontinence in either group. TUMT, on the other hand, seems to confer more early risk of urinary tract infection, irritative voiding symptoms and longer-post-procedure indwelling catheter times. All studies gave prophylactic antibiotics at the time of the procedure and for variable lengths of time post-procedure for the TUMT groups. Table 3. Adverse events reported in the TUMT vs. TURP RCTs. Study TURP adverse events TUMT adverse events Ahmed 1997 Blood transfusions in 4 patients; 4- week indwelling catheters 2 patients; severe UTI 1 patient; mild UTI 2 patients; meatal narrowing 2 patients; bladder neck stenosis 1 patient. Immediate post-procedure worsening of symptoms & dysuria; blood stained urethral discharge & constipation x 24 hours. All self-cathed 3 required indwelling Sexual dysfunction: 4/19 sexually catheters; severe UTI 1 patient active men with ED; 12/19 with Sexual dysfunction: 4/18 sexually retrograde ejaculation. active men with retrograde ejaculation. D Ancona 1997, 1998 Average hospital admission days = 4. Indwelling catheter 4-5 days 4% UTI 19% Irritative voiding symptoms 14% hematuria requiring treatment No mention of sexual side effects Dahlstrand 1993, 1994, 1995 Francisca 1999, patients with post-operative bleeding requiring re-operation 4 patients with UTI 3 patients with dysuria 2 patients with urethral strictures 2 patients with meatal stenoses 4 new cases of retrograde ejaculation; no ED 2 patients with urethral stricture requiring urethrotomy Sexual side effects in results Table 2 No peri-procedure events reported No ED. No hospital admissions Indwelling catheter 6-35 days 16% UTI 29% Irritative voiding symptoms 0 hematuria requiring treatment No mention of sexual side effects 5 patients with UTI 5 patients with dysuria No new cases of retrograde ejaculation; no ED 2 patients with persistent urinary retention treated with further surgery 1 patient with urethral stricture requiring urethrotomy Sexual side effects in results Table 2 No peri-procedure events reported 16
17 Floratos 2001 Nielsen 2002 Schlein 2006 Wagrell 2002, deaths of unrelated cause 3 patients with bladder neck sclerosis 2 urethral strictures 1 stress urinary incontinence No periprocedure events reported Early 2 (9%) patients had blood loss requiring transfusion 3 (14%) patients had UTI re-retention in 1 (5%) of patients No persistent retention after treatment Late 1 (5%) patient with urethral stricture 1 (5%) patient with stress incontinence Sexual 7 (50%) patients with retrograde ejaculation 1 (14%) sexually active patient with ED Overall 22% with UTI 1 patient with serious UTI 1 patient with hematuria 1 patient with bleeding 1 patient with bladder neck sclerosis 1 patient with stroke Serious 4 patients with hematuria 1 patient with UTI 1 patient with TURP syndrome 1 patient with urosepsis 1 patient with clot retention 2 deaths of unrelated cause No periprocedure events reported Early No blood transfusions 14 (30%) had UTI re-retention in 3 (7%) of patients Persistent retention in 1 (2%) of patients Late No urethral stricture No stress incontinence Sexual 6 (22%) patients with retrograde ejaculation 2 (9%) sexually active patients with ED Overall 33% with UTI 1 patient with hematuria Serious 1 patient with hematuria 1 patient with urine retention UTI Mild 13% with micturition urgency 13% with urinary retention 20% with UTI 39% with hematuria 11% with impotence 13% with transient incontinence Urinary tract infection Mild 37% with micturition urgency 19% with urinary retention 18% with UTI 13% with hematuria 6% with impotence 3% with transient incontinence TUMT and Alpha-blockade/Alpha-reductase inhibition A single trial has compared TUMT (N=51) to medical management (N=52) with an alpha-blocker medicine. 39, 40 Inclusion criteria were similar to that in the TUMT vs. TURP trials. This study used the Targis device and compared it to oral medication treatment with 5-10mg of terazosin and found that while the initial response (first two weeks) was greater for alpha-blockade, there was equal 17
18 response at six weeks, and the improvement was greater for TUMT by 12 weeks. TUMT achieved at least 50% improvement in symptom score (IPSS) and peak flow rate for a much greater proportion of patients than did alpha-blockade at six months (for IPSS 78.4% vs. 32.7%; for peak flow rate 64.7% vs. 9.6%; p<.0005 for both comparisons). This difference appeared to be sustained at 18 months. The same authors conducted a trial comparing TUMT to TUMT plus alphareductase inhibition with tamsulosin to evaluate whether alpha-blockade could accelerate the improvements resulting from TUMT. 41 This study of 81 patients found that addition of prophylactic tamsulosin 0.4mg/day two weeks prior to TUMT and continuing for six weeks after TUMT improved symptom scores (IPSS) but not peak flow rates at two and six weeks post-procedure. Both groups achieved equal improvement at 12 weeks. The symptomatic improvement for the TUMT plus tamsulosin group was already present at the time of the TUMT procedure, indicating that it was likely independent of and not additive to the effect of TUMT. TA Criterion 3 is met. TA Criterion 4: The technology must be as beneficial as any established alternatives. As noted above in the discussion of the TUMT vs. TURP trials, compared to the gold-standard of TURP, TUMT is somewhat less effective with somewhat less sustained improvements. However, TUMT also is an outpatient procedure that does not require parenteral anesthesia and which poses fewer serious short-term risks and long-term adverse side-effects than TURP. Thus, the benefits TUMT does attain are done so with less risk. TA Criterion 4 is met. TA Criterion 5: The improvement must be attainable outside of the investigational setting. 18
19 While most of the studies conducted to date have been at single centers, there have been many observational studies and RCTs conducted throughout the United States and Europe (primarily in the Netherlands) in outpatient surgical centers. It appears that this procedure, while like any procedure requires training, is relatively simple and many of the operative dependent issues (e.g. maximum temperature applied) are controlled by software in the newer devices. TA Criterion 5 is met. CONCLUSION In summary, while somewhat inconsistent in inclusion criteria, outcome measures and quality, the bulk of the studies comparing TUMT to sham-tumt demonstrate clinical improvement at three and six months for men with moderately severe obstruction due to BPH that is above and beyond a placebo effect. In addition, the studies comparing TUMT to TURP demonstrate that TUMT provides symptomatic and urinary flow improvement for men with moderately severe obstructive symptoms, however not as great or as long-lasting as TURP. This seems to be similar for the two devices used in these trials (Prostatron and ProstaLund). However, TUMT also is an outpatient procedure that does not require parenteral anesthesia and which poses fewer serious short-term risks and long-term adverse side-effects than TURP. Thus, the benefits TUMT does attain are done so with less risk. There is some additional data indicating that prophylactic alpha-blockade prior to TUMT and in the early post-procedure period provides improved symptomatic relief in the first few months after TUMT when irritative bladder symptoms and need for catheterization are most common. It remains unclear if TUMT is equally effective for men with very large prostates (larger than 100mL or 100g) as for men with smaller prostates. While some of the trials of TUMT vs. TURP may have included a small number of men with very large prostates, they were underpowered to examine this issue, and none of the trials with sham TUMT included this group. RECOMMENDATION It is recommended that transurethral microwave thermotherapy meets CTAF criteria 1-5 for safety, effectiveness and improvement in health outcomes for the treatment of benign prostatic 19
20 hyperplasia in men with moderately severe obstructive symptoms and prostate volume of mL, with no history of prostate procedures, and without prostate cancer,. October 28, 2009 This is the first assessment of this technology to be reviewed by CTAF The CTAF panel voted unanimously to approve the recommendation as written. 20
21 RECOMMENDATIONS OF OTHERS Blue Cross Blue Shield Association (BCBSA) The BCBSA Technology Evaluation Center has not conducted a formal assessment of this technology. Centers for Medicare and Medicaid Services (CMS) No specific National Coverage Decision regarding this technology was found in a search of the CMS web site. California Urological Association (CUA) The CUA has provided an opinion regarding the use of this technology. A representative was not available to attend the meeting. American Urological Association (AUA) The AUA is in the process of updating its guideline and expects publication in ABBREVIATIONS USED IN THIS REVIEW BPH ED TUMT FDA PMA DARE RCT TURP AUA WHO ICS QOL I-PSS UTI Benign prostatic hypertrophy Erectile dysfunction Transurethral microwave thermotherapy Food and Drug Administration Pre-market approval Database of Abstracts of Reviews of Effects Randomized controlled trial Transurethral resection of the prostate American Urologic Assocation World Health Organization International Continence Society Quality of Life International prostate symptom score Urinary tract infection 21
22 APPENDIX: Search strategy PubMed: Search Most Recent Queries Time Result #16 Search #10 OR #15 19:09: #15 Search #11 AND #13 AND (in process[sb] OR publisher[sb] OR 19:09:03 21 pubmednotmedline[sb]) AND eng[la] #13 Search microwave* OR thermotherap* OR tumt OR microwave 19:08: thermal* OR minimally invasiv* #11 Search prostatic hyperplas*[ti] OR prostatic hypertroph*[ti] OR 19:06: bph[ti] #10 Search #8 NOT #9 19:03: #9 Search #8 Limits: Animals 19:03:06 24 #8 Search #5 OR #6 AND ENG [LA] 19:01: #7 Search #5 OR #6 19:01: #6 Search #3 AND TREATMENT OUTCOME[MH] AND FOLLOW- 19:01:14 51 UP STUDIES[MH] #5 Search #3 AND #4 19:00: #4 Search Limits: Clinical Trial, Meta-Analysis, Randomized Controlled 19:00: Trial, Comparative Study, Consensus Development Conference, Consensus Development Conference, NIH, Controlled Clinical Trial, Evaluation Studies, Multicenter Study, Research Support, N I H, Extramural, Research Support, N I H, Intramural, Research Support, Non U S Gov't, Research Support, U S Gov't, Non P H S, Research Support, U S Gov't, P H S, Technical Report, English #3 Search #1 AND #2 18:40: #2 Search hyperthermia, induced[mh] OR microwaves[mh] OR 18:39: diathermy[mh] OR thermotherapy[tiab] OR tumt[tiab] OR microwave thermal* OR (minimally invasive* AND (microwave OR microwaves OR thermotherap* OR thermal)) #1 Search prostatic hyperplasia/therapy OR prostatic hyperplasia[majr] 18:37: Embase Search Queries Access the EMBASE.com Info site if you have questions about this message or other features of this service. Please do not reply to this . No. Query Results #16 #13 OR #14 OR # #15 #10 OR #11 AND (random*:ab,ti OR systematic:ab,ti) 110 #14 #10 OR #11 AND 'treatment outcome'/exp AND ('clinical
23 study'/exp OR 'major clinical study'/exp OR 'controlled study'/exp) #13 #10 OR #11 AND ([cochrane review]/lim OR [controlled clinical trial]/lim OR [meta analysis]/lim OR [randomized controlled trial]/lim OR [systematic review]/lim) 115 #12 #10 OR # #11 #8 NOT #9 574 #10 #3 AND #6 AND [english]/lim AND [animals]/lim AND [humans]/lim #9 #3 AND #6 AND [english]/lim AND [animals]/lim 30 #8 #3 AND #6 AND [english]/lim 604 #7 #3 AND #6 804 #6 #4 OR # #5 'microwave radiation'/de OR 'microwave radiation' OR 'thermotherapy'/de OR 'thermotherapy' OR 'tumt'/de OR tumt OR 'microwave thermal' OR ('minimally invasive' AND (microwave* OR thermotherap* OR thermal)) #4 'transurethral microwave thermotherapy'/exp 289 #3 #1 OR # #2 'prostate hypertrophy'/exp/mj #1 'prostate hypertrophy'/exp/dm_su,dm_th,dm_dm 6080 EMBASE.com provides access to more than 23 million validated biomedical and pharmacological records from EMBASE and MEDLINE Cochrane Library #1 (prostatic hyperplasia or prostatic hypertrophy or bph) and (microwave or microwaves or thermotherap* or tumt or (microwave and thermal)) 104 edit delete 23
24 REFERENCES 1. Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology. Jun 2002;59(6): Collins MF, Friedman RH, Ash A, Hall R, Moskowitz MA. Underdetection of clinical benign prostatic hyperplasia in a general medical practice. J Gen Intern Med. Sep 1996;11(9): O'Leary MP. Lower urinary tract symptoms/benign prostatic hyperplasia: maintaining symptom control and reducing complications. Urology. Sep 2003;62(3 Suppl 1): Trachtenberg J. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in relation to the patient's risk profile for progression. BJU Int. Jun 2005;95 Suppl 4: AUA. Benign Prostatic Hyperplasia (BPH): A Patient's Guide: American Urological Association. 6. Rubenstein J, McVary KT. Transurethral Microwave Thermotherapy of the Prostate (TUMT). emedicine; Hoffman RM, Monga M, Elliot SP, Macdonald R, Wilt TJ. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2007(4):CD Abbou CC, Colombel M, Payan C, et al. The efficacy of microwave induced hyperthermia in the treatment of BPH: the Paris public hospitals' experience. Prog Clin Biol Res. 1994;386: Abbou CC, Payan C, Viens-Bitker C, et al. Transrectal and transurethral hyperthermia versus sham treatment in benign prostatic hyperplasia: a double-blind randomized multicentre clinical trial. The French BPH Hyperthermia. Br J Urol. Nov 1995;76(5): Albala DM, Fulmer BR, Turk TM, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx J Endourol. Feb 2002;16(1): Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow RO. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ. May ;306(6888): Bdesha AS, Bunce CJ, Snell ME, Witherow RO. A sham controlled trial of transurethral microwave therapy with subsequent treatment of the control group. J Urol. Aug 1994;152(2 Pt 1): Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. J Endourol. Dec 1996;10(6): Brehmer M, Wiksell H, Kinn A. Sham treatment compared with 30 or 60 min of thermotherapy for benign prostatic hyperplasia: a randomized study. BJU Int. Aug 1999;84(3): De la Rosette JJMCH, De Wildt MJAM, Alivizatos G, Froeling FMJA, Debruyne FMJ, Blute ML. Transurethral microwavethermotherapy(tumt) in benign prostatic hyperplasia: Placebo versustumt. Urology. 1994;44(1): De Wildt MJ, Hubregtse M, Ogden C, Carter SS, Debruyne FM, De la Rosette JJ. A 12-month study of the placebo effect in transurethral microwave thermotherapy. Br J Urol. Feb 1996;77(2): Francisca EA, d'ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de la Rosette JJ. Quality of life assessment in patients treated with lower energy thermotherapy (Prostasoft 2.0): results of a randomized transurethral microwave thermotherapy versus sham study. J Urol. Nov 1997;158(5): Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, 24
25 sham-controlled, prospective, double-blind, multicenter clinical trial. Urology. May 1998;51(5): Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. Br J Urol. Mar 1997;79(3): Ogden CW, Reddy P, Johnson H, Ramsay JW, Carter SS. Sham versus transurethral microwave thermotherapy in patients with symptoms of benign prostatic bladder outflow obstruction. Lancet. Jan ;341(8836): Roehrborn CG, Preminger G, Newhall P, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, shamcontrolled trial. Urology. Jan 1998;51(1): Tan AH, Nott L, Hardie WR, Chin JL, Denstedt JD, Razvi H. Long-term results of microwave thermotherapy for symptomatic benign prostatic hyperplasia. J Endourol. Dec 2005;19(10): Trachtenberg J, Roehrborn CG. Updated results of a randomized, double-blind, multicenter shamcontrolled trial of microwave thermotherapy with the Dornier Urowave in patients with symptomatic benign prostatic hyperplasia. Urowave Investigators Group. World J Urol. 1998;16(2): Venn SN, Montgomery BS, Sheppard SA, et al. Microwave hyperthermia in benign prostatic hypertrophy: a controlled clinical trial. Br J Urol. Jul 1995;76(1): Ahmed M, Bell T, Lawrence WT, Ward JP, Watson GM. Transurethral microwave thermotherapy (Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. Br J Urol. Feb 1997;79(2): Dahlstrand C, Geirsson G, Fall M, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: preliminary results of a randomized study. Eur Urol. 1993;23(2): Dahlstrand C, Walden M, Geirsson G, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for symptomatic benign prostatic obstruction: a prospective randomized study with a 2-year follow-up. Br J Urol. Nov 1995;76(5): Dahlstrand C, Walden M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Prog Clin Biol Res. 1994;386: D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, de la Rosette JJ. High energy thermotherapy versus transurethral resection in the treatment of benign prostatic hyperplasia: results of a prospective randomized study with 1 year of followup. J Urol. Jul 1997;158(1): D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, De La Rosette JJ. Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. Br J Urol. Feb 1998;81(2): Floratos DL, Kiemeney LA, Rossi C, Kortmann BB, Debruyne FM, de La Rosette JJ. Long-term followup of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. J Urol. May 2001;165(5): Francisca EA, d'ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de La Rosette JJ. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. Eur Urol. Nov 2000;38(5): Francisca EA, d'ancona FC, Meuleman EJ, Debruyne FM, de la Rosette JJ. Sexual function following high energy microwave thermotherapy: results of a randomized controlled study comparing transurethral microwave thermotherapy to transurethral prostatic resection. J Urol. Feb 1999;161(2):
26 34. Mattiasson A, Wagrell L, Schelin S, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology. Jan 2007;69(1):91-96; discussion Norby B, Nielsen HV, Frimodt-Moller PC. Transurethral interstitial laser coagulation of the prostate and transurethral microwave thermotherapy vs transurethral resection or incision of the prostate: results of a randomized, controlled study in patients with symptomatic benign prostatic hyperplasia. BJU Int. Dec 2002;90(9): Schelin S, Geertsen U, Walter S, et al. Feedback microwave thermotherapy versus TURP/prostate enucleation surgery in patients with benign prostatic hyperplasia and persistent urinary retention: a prospective, randomized, controlled, multicenter study. Urology. Oct 2006;68(4): Wagrell L, Schelin S, Nordling J, et al. Feedback microwave thermotherapy versus TURP for clinical BPH--a randomized controlled multicenter study. Urology. Aug 2002;60(2): Wagrell L, Schelin S, Nordling J, et al. Three-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology. Oct 2004;64(4): Djavan B, Roehrborn CG, Shariat S, Ghawidel K, Marberger M. Prospective randomized comparison of high energy transurethral microwave thermotherapy versus alpha-blocker treatment of patients with benign prostatic hyperplasia. J Urol. Jan 1999;161(1): Djavan B, Seitz C, Roehrborn CG, et al. Targeted transurethral microwave thermotherapy versus alpha-blockade in benign prostatic hyperplasia: outcomes at 18 months. Urology. Jan 2001;57(1): Djavan B, Shariat S, Fakhari M, et al. Neoadjuvant and adjuvant alpha-blockade improves early results of high-energy transurethral microwave thermotherapy for lower urinary tract symptoms of benign prostatic hyperplasia: a randomized, prospective clinical trial. Urology. Feb 1999;53(2): Sall M, Bruskewitz RC. Prostatic urethral strictures after transurethral microwave thermal therapy for benign prostatic hyperplasia. Urology. Dec 1997;50(6):
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Name of Policy: Transurethral Microwave Thermotherapy
 Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
NOTE: This policy is not effective until April 1, Transurethral Water Vapor Thermal Therapy of the Prostate
 NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
Clinically and Statistically Significant Changes Seen in Sham Surgery Arms of Randomized, Controlled Benign Prostatic Hyperplasia Surgery Trials
 Clinically and Statistically Significant Changes Seen in Sham Surgery Arms of Randomized, Controlled Benign Prostatic Hyperplasia Surgery Trials Charles Welliver,*, Michael Kottwitz, Paul Feustel and Kevin
Clinically and Statistically Significant Changes Seen in Sham Surgery Arms of Randomized, Controlled Benign Prostatic Hyperplasia Surgery Trials Charles Welliver,*, Michael Kottwitz, Paul Feustel and Kevin
Heat versus drugs in the treatment of benign prostatic hyperplasia
 B. DJAVAN et al. Heat versus drugs in the treatment of benign prostatic hyperplasia B. DJAVAN, C. SEITZ and M. MARBERGER Department of Urology, University of Vienna, Austria SUMMARY The improvement in
B. DJAVAN et al. Heat versus drugs in the treatment of benign prostatic hyperplasia B. DJAVAN, C. SEITZ and M. MARBERGER Department of Urology, University of Vienna, Austria SUMMARY The improvement in
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia.
 Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
The Enlarged Prostate Symptoms, Diagnosis and Treatment
 The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
Subject: Temporary Prostatic Stent and Prostatic Urethral Lift
 02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Chapter 3: Results of the Treatment Outcomes Analyses
 Chapter 3: Results of the Treatment Outcomes Analyses Introduction To determine the appropriateness of individual therapies, as well as to develop practice recommendations, the American Urological Association
Chapter 3: Results of the Treatment Outcomes Analyses Introduction To determine the appropriateness of individual therapies, as well as to develop practice recommendations, the American Urological Association
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia.
 Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
Cooled ThermoTherapy TM
 Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Last Review Status/Date: December Summary
 Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Management of LUTS after TURP and MIT
 Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
European Urology 44 (2003) 89 93
 European Urology European Urology 44 (2003) 89 93 Long-Term Evaluation of Transurethral Needle Ablation of the Prostate (TUNA) fortreatment of Symptomatic Benign Prostatic Hyperplasia: Clinical Outcome
European Urology European Urology 44 (2003) 89 93 Long-Term Evaluation of Transurethral Needle Ablation of the Prostate (TUNA) fortreatment of Symptomatic Benign Prostatic Hyperplasia: Clinical Outcome
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
Treating BPH: Comparing Rezum UroLift and HoLEP
 Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Overview. Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia. Iain McAuley September 15, 2014
 Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Benign Prostatic Hyperplasia (BPH):
 Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Benign Prostatic Hyperplasia (BPH)
 Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment
 A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment Claus G. Roehrborn Professor and Chairman Medical 3-9 mo. Min. Invas. 3-9 mo. Surgical 3-9 mo. Medical 1-16 mo. Min. Invas. 1-16 mo.
A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment Claus G. Roehrborn Professor and Chairman Medical 3-9 mo. Min. Invas. 3-9 mo. Surgical 3-9 mo. Medical 1-16 mo. Min. Invas. 1-16 mo.
MODULE 3: BENIGN PROSTATIC HYPERTROPHY
 MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
Rezūm procedure for the Prostate
 Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Managing urinary morbidity after brachytherapy. Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester
 Managing urinary morbidity after brachytherapy Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester Themes Can we predict urinary morbidity? Prevention of urinary morbidity
Managing urinary morbidity after brachytherapy Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester Themes Can we predict urinary morbidity? Prevention of urinary morbidity
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Prostatic Urethral Lift
 Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
APPENDIX CLINICAL INPUT RESPONSES
 CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments
 Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Prostatic Urethral Lift. Summary
 Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Diagnostic approach to LUTS in men. Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center
 Diagnostic approach to LUTS in men Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center Classification of LUTS Storage symptoms Voiding symptoms Post micturition
Diagnostic approach to LUTS in men Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center Classification of LUTS Storage symptoms Voiding symptoms Post micturition
Related Policies None
 Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
EAU GUIDELINES POCKET EDITION 3
 EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
 available at www.sciencedirect.com journal homepage: www.europeanurology.com How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
available at www.sciencedirect.com journal homepage: www.europeanurology.com How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of insertion of prostatic urethral lift implants to treat lower urinary tract symptoms
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of insertion of prostatic urethral lift implants to treat lower urinary tract symptoms
Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist
 Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist Lower Urinary Tract Symptoms Storage Symptoms Frequency, urgency, incontinence, Nocturia Voiding Symptoms Hesitancy, poor flow, intermittency,
Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist Lower Urinary Tract Symptoms Storage Symptoms Frequency, urgency, incontinence, Nocturia Voiding Symptoms Hesitancy, poor flow, intermittency,
Prostatic Urethral Lift Corporate Medical Policy
 Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Public Statement: Medical Policy Statement:
 Medical Policy Title: Radiofrequency Ablation ARBenefits Approval: 10/26/2011 of Tumors Effective Date: 01/01/2012 Document: ARB0300 Revision Date: Code(s): 20982 Ablation, bone tumor(s), radiofrequency,
Medical Policy Title: Radiofrequency Ablation ARBenefits Approval: 10/26/2011 of Tumors Effective Date: 01/01/2012 Document: ARB0300 Revision Date: Code(s): 20982 Ablation, bone tumor(s), radiofrequency,
Executive Summary. Non-drug local procedures for treatment of benign prostatic hyperplasia 1. IQWiG Reports - Commission No.
 IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
Related Policies None
 Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
P ROLIEVE. Thermodilatation System. The Prolieve System Patient Information is Directed to You, the Patient. A Transurethral Microwave Therapy Device
 P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
What is Benign Prostatic Hyperplasia (BPH)?
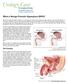 What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
Transurethral microwave thermotherapy
 Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Prostatic Urethral Lift
 Protocol Prostatic Urethral Lift (701151) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization No Review Dates: 05/17, 01/18 Preauthorization is not required. The following
Protocol Prostatic Urethral Lift (701151) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization No Review Dates: 05/17, 01/18 Preauthorization is not required. The following
Can men with prostates sized 80 ml or larger be managed conservatively?
 Original Article - Lower Urinary Tract Dysfunction Investig Clin Urol 2017;58:359-364. pissn 2466-0493 eissn 2466-054X Can men with prostates sized 80 ml or larger be managed conservatively? Alvin Lee,
Original Article - Lower Urinary Tract Dysfunction Investig Clin Urol 2017;58:359-364. pissn 2466-0493 eissn 2466-054X Can men with prostates sized 80 ml or larger be managed conservatively? Alvin Lee,
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH
 MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
50% of men. 90% of men PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS. Want more information? What are the symptoms?
 PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
Increasing Awareness, Diagnosis, and Treatment of BPH, LUTS, and EP
 Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA
 St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS
 Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia.
 Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny Published: 2006-01-01 Link to publication Citation for published version (APA): Schelin, S. (2006).
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny Published: 2006-01-01 Link to publication Citation for published version (APA): Schelin, S. (2006).
Chapter 4: Research and Future Directions
 Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
Chapter 2: Methodology
 Chapter 2: Methodology TABLE OF CONTENTS Introduction... 2 Study Selection and Data Abstraction... 2 Data Synthesis... 8 Guideline Development and Approvals... 9 Conflict of Interest... 10 Copyright 2010
Chapter 2: Methodology TABLE OF CONTENTS Introduction... 2 Study Selection and Data Abstraction... 2 Data Synthesis... 8 Guideline Development and Approvals... 9 Conflict of Interest... 10 Copyright 2010
Benign Prostatic Hyperplasia. Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary
 Benign Prostatic Hyperplasia Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied,
Benign Prostatic Hyperplasia Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied,
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011
 Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients
 Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2018/10 Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients N. Narayanamoorthy,
Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2018/10 Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients N. Narayanamoorthy,
PATIENT INFORMATION 2017 NeoTract, Inc. All rights reserved. Printed in the USA. MAC Rev A
 PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
Wednesday 25 June Poster Session 9: BPH 1 Chairmen: M. Speakman and D. Rosario
 Wednesday 25 June 14.30 15.30 Poster Session 9: BPH 1 Chairmen: M. Speakman and D. Rosario P080 Unreliable residual volume measurement after increased water load diuresis G. ALIVIZATOS, A. SKOLARIKOS,
Wednesday 25 June 14.30 15.30 Poster Session 9: BPH 1 Chairmen: M. Speakman and D. Rosario P080 Unreliable residual volume measurement after increased water load diuresis G. ALIVIZATOS, A. SKOLARIKOS,
Alpha antagonists from initial concept to routine clinical practice
 european urology 50 (2006) 635 642 available at www.sciencedirect.com journal homepage: www.europeanurology.com Editorial 50th Anniversary Alpha antagonists from initial concept to routine clinical practice
european urology 50 (2006) 635 642 available at www.sciencedirect.com journal homepage: www.europeanurology.com Editorial 50th Anniversary Alpha antagonists from initial concept to routine clinical practice
All about the Prostate
 MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms
 Case Report INJ 2010;14:125-129 An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms Joo-Yong Lee, Dong-Hyuk Kang, Hee-Young Park, Jung-Soo Park, Young-Woo Son, Hong-Sang
Case Report INJ 2010;14:125-129 An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms Joo-Yong Lee, Dong-Hyuk Kang, Hee-Young Park, Jung-Soo Park, Young-Woo Son, Hong-Sang
OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1
 PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
Management of Voiding Problems in Older Men. Dr. John Fenn Consultant, QEH 10 th October, 2005
 Management of Voiding Problems in Older Men Dr. John Fenn Consultant, QEH 10 th October, 2005 Voiding Problems Poor stream Hesitancy Straining Incomplete emptying Intermittent micturition Terminal dribbling
Management of Voiding Problems in Older Men Dr. John Fenn Consultant, QEH 10 th October, 2005 Voiding Problems Poor stream Hesitancy Straining Incomplete emptying Intermittent micturition Terminal dribbling
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA. A Minimally Invasive Innovative Treatment
 PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
Introduction to PAE. Rational / Animal. Francisco Cesar Carnevale MD, PhD. University of Sao Paulo Medical School - Brazil
 Introduction to PAE Rational / Animal Francisco Cesar Carnevale MD, PhD University of Sao Paulo Medical School - Brazil Francisco Carnevale, M.D. No relevant financial relationship reported Bladder Outlet
Introduction to PAE Rational / Animal Francisco Cesar Carnevale MD, PhD University of Sao Paulo Medical School - Brazil Francisco Carnevale, M.D. No relevant financial relationship reported Bladder Outlet
TITLE: Plasma Vaporization of the Prostate for Treatment of Benign Prostatic Hypertrophy: A Review of Clinical and Cost-Effectiveness, and Safety
 TITLE: Plasma Vaporization of the Prostate for Treatment of Benign Prostatic Hypertrophy: A Review of Clinical and Cost-Effectiveness, and Safety DATE: 05 March 2014 CONTEXT AND POLICY ISSUES Benign prostatic
TITLE: Plasma Vaporization of the Prostate for Treatment of Benign Prostatic Hypertrophy: A Review of Clinical and Cost-Effectiveness, and Safety DATE: 05 March 2014 CONTEXT AND POLICY ISSUES Benign prostatic
Victoria Sharp, MD, MBA, FAAFP. Clinical Professor of Urology and Family Medicine
 Victoria Sharp, MD, MBA, FAAFP Clinical Professor of Urology and Family Medicine Victoria Sharp, MD, MBA, FAAFP Market Chief Medial Officer AmeriHealth Caritas Family of Companies Office phone: (515) 330-3740
Victoria Sharp, MD, MBA, FAAFP Clinical Professor of Urology and Family Medicine Victoria Sharp, MD, MBA, FAAFP Market Chief Medial Officer AmeriHealth Caritas Family of Companies Office phone: (515) 330-3740
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water vapour ablation for lower urinary tract symptoms caused by
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water vapour ablation for lower urinary tract symptoms caused by
Index. urologic.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
The Journal of International Medical Research 2005; 33:
 The Journal of International Medical Research 2005; 33: 562 573 A Comparative Study on the Safety and Efficacy of Tamsulosin and Alfuzosin in the Management of Symptomatic Benign Prostatic Hyperplasia:
The Journal of International Medical Research 2005; 33: 562 573 A Comparative Study on the Safety and Efficacy of Tamsulosin and Alfuzosin in the Management of Symptomatic Benign Prostatic Hyperplasia:
Key words: Lower Urinary Tract Symptoms (LUTS), Prostatic Hyperplasia, Alpha-1 Adrenoceptor Antagonists, Tamsulosin, Terazosin.
 The Professional Medical Journal DOI: 10.17957/TPMJ/17.4102 ORIGINAL PROF-4102 PROSTATIC HYPERPLASIA; COMPARISON BETWEEN TAMSULOSIN AND TERAZOSIN FOR EFFICACY IN MEDICAL MANAGEMENT OF LOWER URINARY TRACT
The Professional Medical Journal DOI: 10.17957/TPMJ/17.4102 ORIGINAL PROF-4102 PROSTATIC HYPERPLASIA; COMPARISON BETWEEN TAMSULOSIN AND TERAZOSIN FOR EFFICACY IN MEDICAL MANAGEMENT OF LOWER URINARY TRACT
Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia
 Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia Lance A. Mynderse, MD* and Thayne R. Larson, MD Address *Department of Urology, Mayo Foundation, 200 First Street, SW, Rochester,
Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia Lance A. Mynderse, MD* and Thayne R. Larson, MD Address *Department of Urology, Mayo Foundation, 200 First Street, SW, Rochester,
Original Article. Introduction. Methods. Abstract
 Blackwell Science, LtdOxford, UKIJUInternational Journal of Urology0919-81722003 Blackwell Publishing Asia Pty LtdNovember 20031011587594Original ArticleUsefulness of a1-blockers in BPHI Ikemoto et al.
Blackwell Science, LtdOxford, UKIJUInternational Journal of Urology0919-81722003 Blackwell Publishing Asia Pty LtdNovember 20031011587594Original ArticleUsefulness of a1-blockers in BPHI Ikemoto et al.
Abstract. Key words Trial without catheter, Acute urinary retention, Benign prostatic hyperplasia, Introduction
 The role of sustained-released alfuzosin in the treatment of acute urinary retention Mohamed Fawzi Ahmed. Department of Surgery, Ninevah College of Medicine, University of Mosul. Abstract To see whether
The role of sustained-released alfuzosin in the treatment of acute urinary retention Mohamed Fawzi Ahmed. Department of Surgery, Ninevah College of Medicine, University of Mosul. Abstract To see whether
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water jet ablation for lower urinary tract symptoms caused by benign
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water jet ablation for lower urinary tract symptoms caused by benign
Office Management of Benign Prostatic Enlargement
 Focus on CME at McGill University Office Management of Benign Prostatic Enlargement Symptomatic benign prostate enlargement is a common medical problem encountered in our aging society. Watchful waiting,
Focus on CME at McGill University Office Management of Benign Prostatic Enlargement Symptomatic benign prostate enlargement is a common medical problem encountered in our aging society. Watchful waiting,
RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA
 American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
The Evolution of Combination Therapy. US men eligible for BPH treatment * with projected population changes
 The Management of BPH & The Impact of Combination Therapy Results Combination of Avodart and Tamsulosin (CombAT) Medical Therapy of Prostate Symptoms (MTOPS) Dr. Jack Barkin, md, fics, facs, dabu, Mcert
The Management of BPH & The Impact of Combination Therapy Results Combination of Avodart and Tamsulosin (CombAT) Medical Therapy of Prostate Symptoms (MTOPS) Dr. Jack Barkin, md, fics, facs, dabu, Mcert
NON-Neurogenic Chronic Urinary Retention AUA White Paper
 NON-Neurogenic Chronic Urinary Retention AUA White Paper Great Lakes SUNA Inside Urology March 16, 2018 Michelle J. Lajiness FNP-BC Nurse Practitioner DMC Urology Incidence Really unknown Lack consensus
NON-Neurogenic Chronic Urinary Retention AUA White Paper Great Lakes SUNA Inside Urology March 16, 2018 Michelle J. Lajiness FNP-BC Nurse Practitioner DMC Urology Incidence Really unknown Lack consensus
Shrestha A, Chalise PR, Sharma UK, Gyawali PR, Shrestha GK, Joshi BR. Department of Surgery, TU Teaching Hospital, Maharajgunj, Kathmandu, Nepal
 Original Article Intravesical Prostatic Protrusion is better than Prostate Volume in Predicting Symptom Severity in Benign Prostatic Hyperplasia: A Prospective Clinical Study Shrestha A, Chalise PR, Sharma
Original Article Intravesical Prostatic Protrusion is better than Prostate Volume in Predicting Symptom Severity in Benign Prostatic Hyperplasia: A Prospective Clinical Study Shrestha A, Chalise PR, Sharma
Therapeutic Strategies for Managing BPH Progression
 european urology supplements 5 (2006) 997 1003 available at www.sciencedirect.com journal homepage: www.europeanurology.com Therapeutic Strategies for Managing BPH Progression John M. Fitzpatrick a, *,
european urology supplements 5 (2006) 997 1003 available at www.sciencedirect.com journal homepage: www.europeanurology.com Therapeutic Strategies for Managing BPH Progression John M. Fitzpatrick a, *,
ISSN: (Print) (Online) Journal homepage:
 Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/iaan19 CHANGE IN INTERNATIONAL PROSTATE SYMPTOM SCORE AFTER TRANSURETHRAL
Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/iaan19 CHANGE IN INTERNATIONAL PROSTATE SYMPTOM SCORE AFTER TRANSURETHRAL
A Comparative Study of Trans Urethral Resection Versus Trans Urethral Incision for Small Size Obstructing Prostate
 ORIGINAL ARTICLE A Comparative Study of Trans Urethral Resection Versus Trans Urethral Incision for Small Size Obstructing Prostate ABSTRACT Rafique Ahmed Sahito, Abdul Jabbar Pirzada, Masood Ahmed Qureshi,
ORIGINAL ARTICLE A Comparative Study of Trans Urethral Resection Versus Trans Urethral Incision for Small Size Obstructing Prostate ABSTRACT Rafique Ahmed Sahito, Abdul Jabbar Pirzada, Masood Ahmed Qureshi,
Management of LUTS. Simon Woodhams February 2012
 Management of LUTS Simon Woodhams February 2012 The management of lower urinary tract symptoms (LUTS) in men Implementing NICE guidance May 2010 NICE clinical guideline 97 Background Lower urinary tract
Management of LUTS Simon Woodhams February 2012 The management of lower urinary tract symptoms (LUTS) in men Implementing NICE guidance May 2010 NICE clinical guideline 97 Background Lower urinary tract
Title of Research Thesis:
 Eastern Michigan University By Fatai Osinowo Adviser s Name: Dr. Stephen Sonstein, PhD Title of Research Thesis: A sub-analyses from the Benign Prostatic Hyperplasia (BPH) Registry and Patient survey:
Eastern Michigan University By Fatai Osinowo Adviser s Name: Dr. Stephen Sonstein, PhD Title of Research Thesis: A sub-analyses from the Benign Prostatic Hyperplasia (BPH) Registry and Patient survey:
Initial Experience of High Power Diode Laser for Vaporization of Prostate Muhammad Rafiq Zaki, Mujahid Hussain, Tahir Mehmood, Murtaza Hiraj
 Original Article Initial Experience of High Power Diode Laser for Vaporization of Prostate Muhammad Rafiq Zaki, Mujahid Hussain, Tahir Mehmood, Murtaza Hiraj Abstract Objectives: Prospective evaluation
Original Article Initial Experience of High Power Diode Laser for Vaporization of Prostate Muhammad Rafiq Zaki, Mujahid Hussain, Tahir Mehmood, Murtaza Hiraj Abstract Objectives: Prospective evaluation
Indications for Use Targis System Description Contraindications for Targis Treatment Warnings Precautions
 Instructions for Use Targis System Control Unit, Model 4000 and Model 5000 Series Targis Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag CAUTION: Federal (U.S.A.) law restricts
Instructions for Use Targis System Control Unit, Model 4000 and Model 5000 Series Targis Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag CAUTION: Federal (U.S.A.) law restricts
Prostate Artery Embolization
 Prostate Artery Embolization in the office interventional suite Robert J. Kennedy, M.D. Interventional & Vascular Center Melbourne, Florida The speaker has no financial conflicts of interest to disclose.
Prostate Artery Embolization in the office interventional suite Robert J. Kennedy, M.D. Interventional & Vascular Center Melbourne, Florida The speaker has no financial conflicts of interest to disclose.
GUIDELINES ON NON-NEUROGENIC MALE LUTS INCLUDING BENIGN PROSTATIC OBSTRUCTION
 GUIDELINES ON NON-NEUROGENIC MLE LUTS INCLUDING BENIGN PROSTTIC OBSTRUCTION (Text update March 2015) S. Gravas (Chair), T. Bach,. Bachmann, M. Drake, M. Gacci, C. Gratzke, S. Madersbacher, C. Mamoulakis,
GUIDELINES ON NON-NEUROGENIC MLE LUTS INCLUDING BENIGN PROSTTIC OBSTRUCTION (Text update March 2015) S. Gravas (Chair), T. Bach,. Bachmann, M. Drake, M. Gacci, C. Gratzke, S. Madersbacher, C. Mamoulakis,
IS IRRIGATION NECESSARY AFTER MONOPOLAR TURP? OUR 11 YEARS EXPERIENCE
 IS IRRIGATION NECESSARY AFTER MONOPOLAR TURP? OUR 11 YEARS EXPERIENCE Prasannakumar K, Venkatesh Krishnamoorthy, Maneesh Sinha, Krishna Prasad T, Pradeepa MG Abstract Objective: This study was conducted
IS IRRIGATION NECESSARY AFTER MONOPOLAR TURP? OUR 11 YEARS EXPERIENCE Prasannakumar K, Venkatesh Krishnamoorthy, Maneesh Sinha, Krishna Prasad T, Pradeepa MG Abstract Objective: This study was conducted
DOWNLOAD OR READ : TREATMENT OF BENIGN PROSTATIC HYPERPLASIA PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : TREATMENT OF BENIGN PROSTATIC HYPERPLASIA PDF EBOOK EPUB MOBI Page 1 Page 2 treatment of benign prostatic hyperplasia treatment of benign prostatic pdf treatment of benign prostatic
DOWNLOAD OR READ : TREATMENT OF BENIGN PROSTATIC HYPERPLASIA PDF EBOOK EPUB MOBI Page 1 Page 2 treatment of benign prostatic hyperplasia treatment of benign prostatic pdf treatment of benign prostatic
EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT
 Basrah Journal Of Surgery EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT MB, ChB, FIBMS, Assistant Professor
Basrah Journal Of Surgery EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT MB, ChB, FIBMS, Assistant Professor
Original Article - Lasers in Urology. Min Ho Lee, Hee Jo Yang, Doo Sang Kim, Chang Ho Lee, Youn Soo Jeon
 www.kjurology.org http://dx.doi.org/10.4111/kju.2014.55.11.737 Original Article - Lasers in Urology http://crossmark.crossref.org/dialog/?doi=10.4111/kju.2014.55.11.737&domain=pdf&date_stamp=2014-11-16
www.kjurology.org http://dx.doi.org/10.4111/kju.2014.55.11.737 Original Article - Lasers in Urology http://crossmark.crossref.org/dialog/?doi=10.4111/kju.2014.55.11.737&domain=pdf&date_stamp=2014-11-16
