ORIGINAL INVESTIGATION. A Randomized Multicenter Trial of Crotalinae Polyvalent Immune Fab (Ovine) Antivenom
|
|
|
- Lewis Edwards
- 6 years ago
- Views:
Transcription
1 ORIGINAL INVESTIGATION A Randomized Multicenter Trial of Crotalinae Polyvalent Immune Fab (Ovine) Antivenom for the Treatment for Crotaline Snakebite in the United States Richard C. Dart, MD, PhD; Steven A. Seifert, MD; Leslie V. Boyer, MD; Richard F. Clark, MD; Edward Hall, MD; Patrick McKinney, MD; Jude McNally, RPh; Craig S. Kitchens, MD; Steven C. Curry, MD; Gregory M. Bogdan, PhD; Suzanne B. Ward, PharmD; R. Stephen Porter, PharmD Background: Current therapy for crotaline snakebite includes antivenin (Crotalidae) polyvalent, an antivenom with numerous adverse effects. We compared the efficacy and safety of 2 dosing regimens with a new antivenom, Crotalinae polyvalent immune Fab (Fab AV). Methods: A single dose of Fab AV alone (as-needed [PRN] group) was compared with an initial dose plus repeated treatments during 18 hours (scheduled group) in a multicenter randomized trial. The study included patients with minimal or moderate envenomation by a crotaline snake within the preceding 6 hours, aged 10 years or older, in whom worsening of the envenomation syndrome was observed before Fab AV treatment. After treatment with Fab AV to achieve initial control, patients were randomized to the scheduled or PRN treatment group. Scheduled group patients received additional doses of Fab AV every 6 hours for 3 doses. The PRN group received no planned additional doses of antivenom. Results: The mean severity score of the 31 patients decreased from 4.35 to 2.39 points (P.001); there was no difference between scheduled and PRN groups. No patient in the scheduled group received unplanned Fab AV doses, but 8 of 16 patients in the PRN group received unplanned doses (P=.002). Acute reactions occurred in 6 patients (19%), and serum sickness occurred in 6 (23%) of 26 patients who returned for follow-up. Conclusions: In the first randomized trial of antivenom in the United States, Fab AV effectively terminated venom effects. Since the unplanned use of Fab AV in the PRN group was common, the treatment regimen may require more than 1 initial dose. Arch Intern Med. 2001;161: The affiliations of the authors appear in the acknowledgment section at the end of the article. VENOMOUS snakebite is an important health problem. Several thousand victims suffer snakebite each year in the United States. 1 There are approximately 6 deaths per year from snakebite. 2 In addition, 15% to 40% of patients develop long-term sequelae of envenomation ranging from minor limb disfigurement to death. 3 In the United States, rattlesnakes, water moccasins, and copperhead snakes cause nearly all medically important snakebites. Currently, the therapy for snakebite is limited to general supportive care of local tissue injury, coagulopathy, and hypotension as well as the administration of antivenom to selected patients. The only antivenom available in the United States is antivenin (Crotalidae) polyvalent (Wyeth-Ayerst Laboratories, Philadelphia, Pa), a hyperimmune horse serum product that neutralizes many components of crotaline snake venom. Although effective, its use has been limited by the frequency of adverse effects. Acute reactions occur in 20% to 25% of patients; the severity of reaction ranges from minor rashes to life-threatening anaphylaxis. 4-6 Death caused by overwhelming acute bronchospasm and hypotension has occurred. Equally troublesome is the frequent occurrence of serum sickness, a delayed type III hypersensitivity reaction that occurs in 50% to 75% of patients treated with the Wyeth-Ayerst Laboratories product. 4,7 Serum sickness may cause fever, diffuse rash, intense urticaria, arthralgia, hematuria, and constitutional symptoms that persist for several days and often prevent normal activities unless treated with antihistamines and systemic administration of corticosteroids. A new medication has been developed for the treatment of crotaline snakebite. Crotalinae polyvalent immune Fab (ovine) (Therapeutic Antibodies Inc, Nashville, Tenn) is produced in a manner simi- 2030
2 PATIENTS AND METHODS PATIENTS Inclusion criteria were (1) minimal or moderate envenomation (defined herein) by a North American crotaline snake within the 6 hours preceding administration of the study drug, (2) age 10 years or older, and (3) progression of the envenomation syndrome. Progression was defined as worsening under direct observation of an evaluation measure used in grading the envenomation: local injury, coagulation abnormality, or systemic symptoms or signs. Minimal envenomation was defined as swelling, pain, and ecchymosis limited to the immediate bite site; no systemic symptoms and signs; and normal coagulation measures with no clinical evidence of bleeding. Moderate envenomation was defined as swelling, pain, and ecchymosis involving less than 1 extremity (if on the trunk, head, or neck, extending less than 50 cm); systemic symptoms and signs may be present but not life-threatening (may include but are not limited to nausea, vomiting, oral paresthesia or unusual tastes, mild hypotension [systolic blood pressure 90 mm Hg], mild tachycardia [heart rate 150 beats per minute], and tachypnea); and coagulation measures may be abnormal, but without clinical evidence of bleeding (minor hematuria, gum bleeding, and nosebleeds are allowed if not deemed severe by the investigator). Exclusion criteria were (1) lack of progression of the envenomation, (2) severe venom poisoning (swelling, pain, and ecchymosis involving more than an entire extremity or threatening the airway; markedly abnormal systemic symptoms and signs, including severe alteration of mental status, severe hypotension, severe tachycardia, tachypnea, or respiratory insufficiency; and abnormal coagulation measures with serious bleeding or severe threat of bleeding), (3) bite by the copperhead snake (Agkistrodon contortrix), (4) infusion of more than 1 vial of antivenin (Crotalidae) polyvalent before enrollment, (5) history of hypersensitivity to a sheep-derived product, (6) use of corticosteroids or any experimental medication in the 4 weeks preceding study enrollment, (7) pregnancy or lactation, (8) previous enrollment in the study, (9) inability to give informed consent, or (10) the presence of any disease that would interfere with patient examination. STUDY DESIGN This study was a prospective, randomized, controlled, openlabel comparative trial (Figure) performed at 7 sites (Table 1). The study was performed between June 11, 1994, and November 1, Written informed consent was obtained and the study was approved by the institutional review board at each site. After enrollment and standardized initial assessment (history, physical examination, and laboratory tests), each patient received an intravenous dose of 6 or 12 vials of Fab AV to achieve initial control. Initial control of the envenomation syndrome was prospectively defined as cessation of progression of all components of envenomation: local effects, systemic effects, and coagulopathy. This included complete termination of swelling progression and complete reversal of systemic effects. Coagulopathy had to return to normal or near-normal values. Because the study was not blinded, patients were randomized to a treatment group only after initial control was achieved to distribute evenly any bias in the investigator s assessment of when initial control had been achieved. Randomization was assigned individually by means of a predetermined randomization procedure administered by a central call center. Treatment with Fab AV was administered in 2 phases. First, a dose of 6 vials was administered to obtain initial control. If necessary, a second dose of 6 vials was allowed to achieve initial control. If initial control could not be achieved with 12 vials of Fab AV and within 6 hours of initial treatment, treatment was deemed to have failed. After initial control was achieved, patients were randomized to the as-needed (PRN) or scheduled group for the second phase. The second phase involved clinical monitoring for progression or resolution of venom effect. Patients in the PRN group received no further Fab AV unless the investigator detected progression of one of the signs of envenomation: local effects, systemic effects, or coagulopathy. Additional Fab AV could then be administered without limit in 2-vial increments. Patients in the scheduled Continued on next page lar to digoxin immune Fab (Digibind; Glaxo Wellcome Inc, Research Triangle Park, NC). Individual flocks of sheep are immunized with 1 of 4 crotaline venoms (Western diamondback, Eastern diamondback, and Mojave rattlesnakes and the cottonmouth). The immune serum from each flock is digested with papain to produce antibody fragments (Fab), which are then purified by chromatographic methods to remove the immunogenic Fc portion of the antibody and the nonneutralizing components of ovine serum. The 4 monospecific antivenoms thereby produced are combined to form the final antivenom product. The new antivenom averaged 5.2 times (range, times) more potent than antivenin (Crotalidae) polyvalent on the basis of weight in a mouse lethality model. 8 A prospective open-label trial of the new antivenom in 11 patients found that all patients improved after treatment and no acute or delayed allergic reactions occurred. 9 However, progression of limb swelling was found in 3 patients, with time of onset ranging from 6 to 19 hours. To suppress local recurrence, an improved dosing schedule was devised. The purpose of this study was to compare the efficacy and safety of Crotalinae polyvalent immune Fab (Fab AV) with the use of 2 dosing regimens. We believe this to be the first randomized trial of antivenom in North America. RESULTS There were 31 patients enrolled (Figure). No patients were excluded or dropped from the study. The study groups were similar in age, weight, sex and racial composition, initial severity score, range of severity scores, and the amount of Fab AV required to achieve initial control of the envenomation (Table 1). Progression of local swelling was present in all patients before treatment. 2031
3 Recurrence was defined as the return of any venom effect after that abnormality had resolved. Thus, return of progression of swelling after its initial arrest had been documented was described as a local recurrence, while return of thrombocytopenia, hypofibrinogenemia, progroup received 2 vials of Fab AV every 6 hours for a total of 3 doses (18 hours) and could receive additional doses if the investigator detected worsening of the envenomation. The severity score was determined at 1, 6, and 12 hours after the establishment of initial control. Swelling measurements were performed every 2 hours from 6 to 12 hours and then every 12 hours until 36 hours after initial control. Safety evaluations, including laboratory tests, were performed at 1, 2, 6, 12, 24, and 96 hours and at 14 days. All patients were discharged within 36 hours. STUDY DRUG Each vial of lyophilized Crotalinae polyvalent immune Fab (ovine) contained 750 mg of Fab and sodium phosphate buffer. The Fab preparation has been described previously. 8 Each vial was prepared for infusion by reconstitution in 10 ml of sterile water. The initial dose of 6 vials was diluted in normal saline to a final volume of 250 ml and infused during 60 minutes. Two-vial doses were administered during 30 to 60 minutes. EVALUATIONS AND DEFINITIONS To assess efficacy, each patient was examined at baseline, on achievement of initial control, and at 1, 6, and 12 hours after initial control was achieved. Initial control was defined as the cessation of worsening of all evaluation measures as assessed by the investigator. The primary assessment tool was the snakebite severity score, a previously tested measure that quantifies the clinical effects of venom. 10 A secondary measure, the investigator s clinical assessment, was used to confirm the severity score and ascertain its clinical relevance. To perform each assessment, the investigator assigned the patient s response to 1 of 4 categories: (1) clinical response: pretreatment signs and symptoms associated with the bite improved or progression was arrested after treatment with antivenom; (2) partial response: pretreatment signs and symptoms associated with the bite site worsened, but at a slower rate than anticipated; (3) clinical nonresponse: the patient s condition was not favorably affected by the administration of study drug; or (4) not evaluable. Evaluations for acute hypersensitivity reactions were performed by the investigator at baseline, during study drug administration, and 1, 2, 6, and 12 hours after initial control. An acute reaction was defined broadly as any apparent adverse response that occurred during or within 2 hours of Fab AV infusion. Evaluations for delayed hypersensitivity reactions (serum sickness) were performed at 2, 4, 7, and 14 days after discharge from the hospital. Delayed reactions were defined as any adverse event involving 1 of the following: cutaneous eruption, arthralgia, gastrointestinal tract complaints, or lymphadenopathy occurring more than 24 hours after antivenom administration. In addition, each patient completed a diary for recording of symptoms that occurred between visits. STATISTICAL ANALYSIS Sample size calculations were calculated using data from a pilot study. 9 A sample size of 28 patients was calculated to achieve 80% power to detect a severity score difference of 1.0 point between groups, assuming an level of.05 and using 2-sided independent-group t test. A 5% dropout rate was postulated, producing a planned study size of 30 patients. The primary assessment of efficacy was the snakebite severity score. 10 Efficacy was defined as a stable or decreasing severity score during the 12-hour evaluation period after establishment of initial control. Because of the discrete nature of the data, severity scores were compared nonparametrically over time by means of the Friedman test. Severity scores between the 2 dosing regimens were compared at each time point by means of the 2-sided Mann-Whitney test. The 6 components of the severity score were also compared over time by the Friedman test. Statistical significance was defined as a probability less than 5% (P.05). All tests were performed with commercially available statistical software (Arcus Quickstat Biomedical Version 6.2; Longman Software, Cambridge, England). The proportion of patients in each regimen who developed local swelling or coagulopathy recurrence was compared by Fisher exact test. The total number of vials used in each dosing regimen was compared by a 2-tailed unpaired t test. Safety was assessed by means of descriptive statistics and individual case analysis and review. EFFICACY EVALUATION All patients were included in the efficacy evaluation. Initial control of the envenomation syndrome was achieved in all patients. The mean total severity score began to decrease on the infusion of Fab AV in all patients and continued to decrease in both groups through the evaluation period (Table 2). The mean severity score of the 31 patients decreased from 4.35 to 2.39 points (P.001); there was no statistical difference between the scheduled and PRN groups. The decrease in the severity of illness after Fab AV infusion, as represented by the severity score, was composed entirely of reductions in the coagulation, central nervous system, gastrointestinal tract, cardiac, and pulmonary components (data not shown). The local wound component did not decrease or increase after treatment. There were also no differences in the individual component scores between the PRN and scheduled groups. At the follow-up visits, local venom effects were described as improved throughout the study period. Investigators also categorized the time needed to reconstitute Fab AV into 4 categories (0-10, 10-20, 20-30, or 30 minutes). Overall, for 27 of the patients, 6 (22%) took 0 to 10 minutes for reconstitution, 13 (48%) took greater than 10 to 20 minutes, and 8 (30%) took greater than 20 to 30 minutes; the time was not recorded for 4 patients. RECURRENCE PHENOMENON 2032
4 PRN Group n=16 Completed Efficacy Evaluation 1, 6, 12 h n=16 Completed Follow-up Evaluations 2, 4, 7, 14 d n=13 Enrollment N=31 Initial Control Using Investigational Antivenom n=31 Yes Randomization to Maintenance Phase n=31 n=15 Completed Efficacy Evaluation 1, 6, 12 h n=15 Completed Follow-up Evaluations 2, 4, 7, 14 d n=13 Schematic diagram of study. PRN indicates as needed. longation of prothrombin time, or elevation of levels of fibrin split products was described as a coagulopathy recurrence. The issue of coagulopathy has been addressed in detail previously. 11 The planned dose of Fab AV in the scheduled group was a total of 12 to 18 vials (6 or 12 vials for initial control and then 2 vials every 6 hours for 3 doses). The dose in the PRN group was 6 to 12 vials with no planned doses after initial control. As a safety precaution, the protocol allowed the investigator to administer additional rescue doses of Fab AV to patients in either group if clinically needed. No patient in the scheduled group received additional Fab AV, while 8 patients (50%) in the PRN group received additional doses for recurrence of local wound progression during the first 12 hours (P=.002; Table 3). Despite the use of different dosing regimens, the total amount of study drug administered was not statistically different between groups: a mean (± SD) total of 13.0±3.9 vials was used in the scheduled group, while 11.1±4.5 vials were used in the PRN group. Each episode of local recurrence was successfully terminated with 2 additional vials of Fab AV. There were no recurrences of local effects after discharge from the hospital. SAFETY EVALUATION Treatment Failure n=0 No Table 1. Demographic Data of Enrolled Patients* Variable Total Patients (N = 31) (n = 15) PRN Group (n = 16) Age, y Mean ± SD 37.3 ± ± ± 18.1 Range Body weight, 74.4 ± ± ± 14.9 mean ± SD, kg Sex, No. M:F 24:7 12:3 12:4 Race, No. White Hispanic Asian Native American Bite location Finger/hand Arm Toe/foot Leg Clavicle Site of enrollment Albuquerque, NM Denver, Colo Gainesville, Fla Thomasville, Ga Phoenix, Ariz San Diego, Calif Tucson, Ariz Envenomation grade at entry Minimal Moderate No. of vials of Fab AV to achieve initial control, No. (%) 3 1 (3) 1 (7) (65) 10 (67) 10 (68) (32) 4 (27) 6 (38) *PRN indicates as needed; Fab AV, Crotalinae polyvalent immune Fab (ovine). See Patients subsection of Patients and Methods section for severity grading. Patient received only 3 vials because of moderate allergic reaction. Six patients (19%) developed an acute reaction during Fab AV infusion; 3 occurred in each study group (Table 4). As categorized by the investigator, there were 4 mild cases of transient urticaria. There were 2 moderate cases: 1 in a patient who developed wheezing and dyspnea and 1 in a patient who developed cough and urticaria. Two patients received no treatment, and all other patients responded promptly to treatment. Five patients did not return for the 14-day visit. Of the remaining 26 patients, 6 (23%) developed serum sickness (Table 5). Two cases were rated as mild, 2 were moderate, and 2 were severe. The worst case of serum sickness involved diffuse hives and urticaria, which was treated successfully on an outpatient basis with antihistamines and a short course of prednisone. There were no reported sequelae of serum sickness at the 28-day follow-up visit. Notably, the first 5 patients who developed serum sickness were treated with a batch of Fab AV that was later found to contain excess Fc fragments retained from a flawed step in the manufacturing process. A new purification step was introduced, and only 1 (6%) of the subsequent 16 patients developed serum sickness. In contrast, the rate of acute reactions was similar among batches of Fab AV. The Fab AV was administered concomitantly with opioid, minor analgesic, antiemetic, and anxiolytic medications in nearly all patients (90%). No drug-drug or drug-disease interactions were reported. Preexisting patient conditions included hypertension in 3 patients, hypothyroidism in 2 patients, and renal insufficiency, diabetes, congestive heart failure and hypertension in 1 pa- 2033
5 Table 2. Comparison of Total Severity Scores for PRN and s* Period PRN Group (n = 16) tient. A total of 3 patients with a history of asthma were enrolled; 2 developed an early reaction during Fab AV infusion. COMMENT (n = 15) Baseline Mean ± SD 4.7 ± ± 1.3 Median Range End of Fab AV infusion Mean ± SD 3.3 ± ± 1.4 Median Range After initial control achieved 1h Mean ± SD 3.2 ± ± 1.3 Median Range h Mean ± SD 2.6 ± ± 1.5 Median Range h Mean ± SD 2.4 ± ± 1.1 Median Range *PRN indicates as needed; Fab AV, Crotalinae polyvalent immune Fab (ovine). Dosing regimen scores at each time point are not statistically different (P.05, Mann-Whitney test). Baseline scores are statistically different from 12-h scores (P.001, Friedman test). Table 3. Recurrence of Local Venom Effects No. of Recurrences per Patient PRN Group* (n = 16) No. (%) of Patients (n = 15) Total (N = 31) 1 2 (12.5) 0 2 (6.5) 2 4 (25.0) 0 4 (12.9) 3 2 (12.5) 0 2 (6.5) Total 8 (50.0) 0 8(25.8) *One patient did not return and could not be contacted for evaluation. P =.002 compared with scheduled group. Crotaline snakebite is a dynamic disease process. By definition, a crotaline envenomation begins with minimal clinical effects (fang marks and perhaps localized pain and swelling). In many cases, however, the initial minimal injury worsens to threaten limb or life. Every envenomation that is destined to become severe must therefore progress through the grades of minimal and moderate. In untreated cases, death has occurred within hours from shock and multiple-organ injury. Conversely, the venom effects may worsen in an indolent manner during many hours or may fail to worsen at all, if no venom was actually injected (the dry bite ). The variable nature of venomous snakebite renders clinical evaluation difficult. If therapy is applied to a bite that is not destined to worsen, it may erroneously appear to be an effective treatment, while a massive envenomation could overwhelm all forms of therapy, causing an otherwise effective therapy to appear ineffective. To address these difficulties, we used a comparative trial design with inclusion criteria designed to maximize the probability that the patient had suffered a clinically important envenomation. Patients were enrolled within 6 hours of envenomation, while they still had minimal or moderate grade envenomations and evidence of clinical worsening at the time of antivenom administration. Patients with severe envenomation were excluded because extensive injury may obscure antivenom effect. For example, if the maximal amount of swelling has already occurred, then the antivenom would be expected to have minimal clinical effect. The primary limitation of this study is the open label design. We attempted to minimize this effect by using a randomized design and a validated severity score with specific definitions to limit and distribute investigator bias. However, the potential bias of open-label studies may manifest in forms for which we could not compensate. Another limitation is the exclusion of patients with severe envenomation, which was desired by the regulatory agency. While an antivenom effective in less severe cases would also be expected to be useful in severe envenomations, further evaluation in this patient group is needed. Crotalinae polyvalent immune Fab (ovine) successfully achieved initial control of the envenomation syndrome in all study patients, and both groups continued to improve during the 12-hour evaluation period. As judged by the snakebite severity score, the 2 treatment strategies (PRN vs scheduled) showed similar effectiveness. However, additional unscheduled doses to recover control of recurrent local swelling were administered to one half of patients in the PRN group, while no patient in the scheduled group required unplanned doses. This increased the actual total dose administered to the PRN group and caused the 2 groups to receive similar total amounts of antivenom, despite the fact that the dosing regimen for the scheduled group required a minimum dose of 12 to 18 vials while the PRN group required only 6 to 12 vials. This result indicates that repeated doses of Fab AV will likely be needed to provide adequate clinical treatment of envenomation. The effect of Fab antivenom was most noticeable in the components of the severity score involving the coagulation system, central nervous system, gastrointestinal tract, and cardiovascular system. Each of these measures decreased during the initial infusion of Fab AV and continued to decrease throughout the evaluation period. Thus, venom-induced dysfunction in these areas appears to be a dynamic process that can be terminated and reversed by antivenom. In contrast, the local injury component of the score did not improve. This observation may be explained by the fact that ecchymosis and edema formation involve hemorrhage, cell swelling, and cell death, processes that are less reversible or only slowly 2034
6 Table 4. Adverse Events During Infusion of Crotalinae Polyvalent Immune Fab (Ovine) Severity Description Group* Treatment Mild Isolated urticaria PRN None, resolved spontaneously Mild Isolated urticaria PRN None, resolved spontaneously Mild Isolated urticaria Scheduled Diphenhydramine hydrochloride, cimetidine, methylprednisolone acetate Mild Isolated urticaria PRN Cimetidine, methylprednisolone Moderate Allergic reaction (cough and urticaria) in patient with history of reactive airway disease Scheduled Infusion was stopped; patient s symptoms responded promptly to intravenous administration of diphenhydramine and ranitidine hydrochloride; reaction recurred when infusion was restarted; no further antivenom was administered Moderate *PRN indicates as needed. Allergic reaction (hives, dyspnea, and wheezing) in patient with history of reactive airway disease Scheduled Infusion was stopped; patient s symptoms responded promptly to diphenhydramine, famotidine, and albuterol therapy; additional antivenom was infused without further reaction by using precaution of epinephrine infusion Table 5. Delayed Adverse Events (Serum Sickness) Severity Description Group* Treatment Mild Pruritus, rash, arthralgia (patient received test dose Scheduled None, resolved spontaneously of horse serum) Mild Pruritus, rash, arthralgia PRN None, resolved spontaneously Moderate Rash PRN Diphenhydramine hydrochloride Moderate Pruritus, rash, anorexia Scheduled Diphenhydramine, methylprednisolone acetate Severe Hives, urticaria PRN Diphenhydramine, hydroxyzine hydrochloride, methylprednisolone Severe Rash, pruritus Scheduled Methylprednisolone, hydroxyzine *PRN indicates as needed. reversible. 12 It is important that the snakebite severity score did not worsen. Because we did not include an untreated control group, it is impossible to assert that the severity scores of these patients would have worsened. However, an increase in the score would be the expected course for patients who met the inclusion criteria for this study. This study alters understanding of crotaline snakebite in other ways as well. As noted above, local manifestations recurred in 8 of 16 patients. While this was easily managed by the administration of additional Fab AV, this phenomenon has not been described previously. A related finding is much more striking. In addition to recurrence of local effects, return of coagulation abnormalities was noted in many patients who exhibited a coagulopathy at presentation. Although the information has not entered the mainstream medical literature, anecdotal evidence of this phenomenon has been reported sporadically for nearly 20 years. 13 A retrospective analysis of a large database of crotaline snake envenomations indicates that the phenomenon also occurs after the use of the currently available antivenom, antivenin (Crotalidae) polyvalent. 14 The pathophysiology underlying either local or coagulopathy recurrence is unknown. However, there are at least 4 potential explanations. First, the snake may have injected sufficient venom to overwhelm the neutralization capacity of the antivenom. A related concept involves the half-life of the antivenom; the elimination halflife of ovine Fab is relatively short for an antibody, 12 to 30 hours Perhaps all venom in the blood was neutralized initially, but continued to be absorbed from the bite site. Unbound Fab AV would be eliminated renally. As the antivenom was eliminated, the point would be reached where further venom absorbed from the bite site could not be neutralized by the unbound Fab AV remaining in the blood. Renewed venom effect could then occur. Second, it is possible that the venom Fab AV complex dissociated, allowing the venom injury to redevelop. The antigen-antibody complex in snake venom poisoning may be predisposed to this phenomenon. Many of the venom components have molecular weights of to daltons. When bound to a Fab with a molecular weight of daltons, the complex would be too large for renal excretion, and persistence in the circulation would be expected. A similar phenomenon of recurrence has been documented with digoxin immune Fab, where recurrence of free (unbound) digoxin levels has been documented as early as 12 to 24 hours after administration. 18 Because of the high affinity of digoxin immune Fab for digoxin, however, it has been concluded that the cause is much more likely to be redistribution of digoxin. 18 Third, the composition of venom components absorbed from the bite site may change over time. Since 2035
7 crotaline snake venom has dozens of components, perhaps those that are absorbed later differ from components absorbed earlier and are not bound as well by antivenom. The components with delayed absorption might then produce effects that could not be terminated by antivenom administration. This explanation seems unlikely because the recurrent coagulopathy caused the same abnormalities as the original coagulopathy and was successfully treated with additional doses of antivenom in most of the patients with recurrence. Fourth, human antisheep antibodies may have developed. If the patient rapidly produced antibodies against the ovine Fab, these antibodies might have interfered with the binding of Fab to venom components. This is not likely because recurrence occurred within hours to days, and this type of response requires 7 to 19 days to develop. 19 We believe the most likely explanation is that venom continues to be absorbed from the bite site for days after injection. Animal and clinical data support this conclusion. 17,20,21 The recurrence of venom effect, therefore, should be responsive to further antivenom administration. This was the case in all patients with local recurrence and nearly all patients with coagulopathy recurrence. Finally, the reconstitution profile for the new antivenom may be clinically important. The antivenom currently available, antivenin (Crotalidae) polyvalent, requires at least 30 to 60 minutes to enter solution. This can become a serious clinical problem when swelling occurs in critical areas such as the airway or the patient manifests systemic effects such as hypotension. An antivenom that could be administered within 15 to 30 minutes would be desirable under these conditions. The administration of Crotalinae polyvalent immune Fab (ovine) was consistently associated with prompt improvement in the patient s condition. Antivenom dosage was contingent on an individual patient s response. On the basis of our data, the recommended initial dose is 6 vials, with further 6-vial doses until initial control of the envenomation syndrome has been achieved. After initial control has been established, additional 2-vial doses every 6 hours for 3 doses may be needed. Additional 2-vial doses may be needed on the basis of close follow-up for late recurrences of coagulopathy, especially if these effects were present during hospitalization. Accepted for publication March 13, From the Rocky Mountain Poison and Drug Center, Denver Health Authority, Denver, Colo (Drs Dart and Bogdan); University of Colorado Health Sciences Center, Denver (Dr Dart); Department of Emergency Medicine, Kino Community Hospital, Tucson, Ariz (Dr Seifert); Department of Pediatrics, University of Arizona, Tucson (Dr Boyer); Division of Medical Toxicology, University of California San Diego Medical Center and the San Diego Division of the California Poison Control System (Dr Clark); Department of Surgery, John D. Archbold Memorial Hospital, Thomasville, Ga (Dr Hall); Department of Emergency Medicine and the New Mexico Poison Center, University of New Mexico Health Sciences Center, Albuquerque (Dr McKinney); Arizona Poison and Drug Information Center, School of Pharmacy, University of Arizona, Tucson (Mr McNally); Department of Medicine, Veterans Affairs Hospital, Gainesville, Fla (Dr Kitchens); Department of Medical Toxicology, Good Samaritan Regional Medical Center, Phoenix, Ariz, and Division of Clinical Medicine, University of Arizona College of Medicine, Tucson (Dr Curry); and Therapeutic Antibodies Inc, Nashville, Tenn (Drs Ward and Porter). This research was supported by a grant from Protherics Inc, formerly Therapeutic Antibodies Inc. Corresponding author and reprints: Richard C. Dart, MD, PhD, Rocky Mountain Poison and Drug Center, 1010 Yosemite Cir, Denver, CO ( rdart@rmpdc.org). REFERENCES 1. Russell FE. Identification and distribution of North American venomous snakes. In: Snake Venom Poisoning. Great Neck, NY: Scholium International Inc; 1984: Langley RL, Morrow WE. Deaths resulting from animal attacks in the United States. Wild Environ Med. 1997;8: Dart RC. Sequelae of pit viper envenomation. In: Campbell JA, Brodie ED Jr, eds. Biology of the Pit Vipers. Tyler, Tex: Selva Publishing; 1992: Jurkovich GJ, Luterman A, McCullar K, Ramenofsky ML, Curreri WP. Complications of Crotalidae antivenin treatment. J Trauma. 1988;28: Dart RC, Horowitz RS. Use of antibodies as antivenoms: a primitive solution for a complex problem? In: Bon C, Goyffon M, eds. Envenomings and Their Treatments. Paris, France: Institut Pasteur; 1996: Parrish HM. Incidence of treated snakebites in the United States. Public Health Rep. 1966;81: Corrigan P, Russell FE, Wainschel J. Clinical reactions to antivenin. Toxicon. 1978;16 (suppl 1): Consroe P, Egen NB, Russell FE, et al. Comparison of a new ovine antigen binding fragment (Fab) for United States Crotalidae with the commercial antivenin for protection against venom-induced lethality in mice. Am J Trop Med Hyg. 1995; 53: Dart RC, Seifert SA, Carroll L, et al. Affinity-purified, mixed monospecific crotalid antivenom ovine Fab for the treatment of crotalid venom poisoning. Ann Emerg Med. 1997;30: Dart RC, Garcia RA, Hurlbut KM, Boren JL. Development of a severity score for the assessment of crotalid snakebite. Ann Emerg Med. 1996;27: Boyer LV, Seifert SA, Clark RF, et al. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Intern Med. 1999;159: Ohsaka A. Hemorrhagic, necrotizing and edema-forming effects of snake venoms. In: Lee C-Y, ed. Snake Venoms. New York, NY: Springer-Verlag; 1979: Hardy DL, Jeter M, Corrigan JJ. Envenomation by the Northern blacktail rattlesnake (Crotalus molossus molossus): report of two cases and the in vitro effects of the venom on fibrinolysis and platelet aggregation. Toxicon. 1982; 20: Bogdan GM, Dart RC, Falbo SC, McNally J, Spaite D. Recurrent coagulopathy after antivenom treatment of crotalid snakebite. South Med J. 2000;93: Scherrmann JM, Pepin S. Biodynamics of antigen-antibody neutralization in vivo. In: Bon C, Goyffon M, editors. Envenomings and Their Treatments. Paris, France: Institut Pasteur; 1996: SjöströmL.Fab and Venom Concentrations in Blood and Urine Samples Following Administration of ViperaTAbTM. Nashville, Tenn: Therapeutic Antibodies Inc; Seifert SA, Boyer LV, Dart RC, Porter RS, Sjöström L. Relationship of venom effects to venom antigen and antivenom serum concentrations in a patient with Crotalus atrox envenomation treated with a Fab antivenom. Ann Emerg Med. 1997; 30: Ujhelyi MR, Rober S. Pharmacokinetic aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet. 1995;28: Chatenoud L. Humoral immune response against OKT3. Transplant Proc. 1993; 25(2, suppl 1): Meyer WP, Habib AG, Onayade AA, et al. First clinical experiences with a new ovine Fab Echis ocellatus snakebite antivenom in Nigeria: randomized comparative trial with Institut Pasteur serum (IPSER) Africa antivenom. Am J Trop Med Hyg. 1997;56: Burgess JL, Dart RC, Egen NB, Mayersohn M. Effects of constriction bands on rattlesnake venom absorption: a pharmacokinetic study. Ann Emerg Med. 1992; 21:
SNAKEBITE / CROTALID ANTIVENOMS
 DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
ORIGINAL INVESTIGATION. Recurrent and Persistent Coagulopathy Following Pit Viper Envenomation
 ORIGINAL INVESTIGATION Recurrent and Persistent Coagulopathy Following Pit Viper Envenomation Leslie V. Boyer, MD; Steven A. Seifert, MD; Richard F. Clark, MD; Jude T. McNally, RPh; Saralyn R. Williams,
ORIGINAL INVESTIGATION Recurrent and Persistent Coagulopathy Following Pit Viper Envenomation Leslie V. Boyer, MD; Steven A. Seifert, MD; Richard F. Clark, MD; Jude T. McNally, RPh; Saralyn R. Williams,
CROFAB Crotalidae Polyvalent Immune Fab (Ovine) Lyophilized Powder for Solution for Injection For Intravenous Use Only. Initial U.S.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CROFAB safely and effectively. See full prescribing information for CROFAB. CROFAB Crotalidae Polyvalent
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CROFAB safely and effectively. See full prescribing information for CROFAB. CROFAB Crotalidae Polyvalent
Merkblatt 8 - E CROFAB TM CROTALIDAE POLYVALENT IMMUNE FAB (OVINE)
 Serum-Depot Berlin e. V. Merkblatt 8 - E CROFAB TM CROTALIDAE POLYVALENT IMMUNE FAB (OVINE) Englisch (Stand: 2000) DESCRIPTION CroFab TM [Crotalidae Polyvalent Immune Fab (Ovine)] is a sterile, nonpyrogenic,
Serum-Depot Berlin e. V. Merkblatt 8 - E CROFAB TM CROTALIDAE POLYVALENT IMMUNE FAB (OVINE) Englisch (Stand: 2000) DESCRIPTION CroFab TM [Crotalidae Polyvalent Immune Fab (Ovine)] is a sterile, nonpyrogenic,
SNAKEBITE / CROTALID ENVENOMATION
 DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
Product Information BROWN SNAKE ANTIVENOM AUST R 74897
 Product Information APPROVED NAME BROWN SNAKE ANTIVENOM AUST R 74897 DESCRIPTION BROWN SNAKE ANTIVENOM is prepared from the plasma of horses immunised with the venom of the brown snake (Pseudonaja textilis).
Product Information APPROVED NAME BROWN SNAKE ANTIVENOM AUST R 74897 DESCRIPTION BROWN SNAKE ANTIVENOM is prepared from the plasma of horses immunised with the venom of the brown snake (Pseudonaja textilis).
American Journal oftoxicology
 American Journal of Toxicology Sliesoraitis S et al. American Journals of Toxicology 2015, 1:1-7 American Journal oftoxicology http://ivyunion.org/index.php/ajt Page 1 of 7 Vol 1 Article ID 20150679, 7
American Journal of Toxicology Sliesoraitis S et al. American Journals of Toxicology 2015, 1:1-7 American Journal oftoxicology http://ivyunion.org/index.php/ajt Page 1 of 7 Vol 1 Article ID 20150679, 7
Brandon. A 38-year-old victim. Pit viper envenomation. Bitten at: Tyler, Texas Treated at: Emergency room at regional medical center
 Brandon Pit viper envenomation A 38-year-old victim Bitten at: Tyler, Texas Treated at: Emergency room at regional medical center History 0 1 hour after bite A man was bitten on a toe on his right foot
Brandon Pit viper envenomation A 38-year-old victim Bitten at: Tyler, Texas Treated at: Emergency room at regional medical center History 0 1 hour after bite A man was bitten on a toe on his right foot
Key Points. Snakebites. Background
 Snakebites Guideline developed by Branson Bolden, MD, in collaboration with the ANGELS team, August 16, 2013. Last revised by Branson Bolden, MD, August 30, 2016. Key Points Pit viper (rattlesnake, cottonmouth,
Snakebites Guideline developed by Branson Bolden, MD, in collaboration with the ANGELS team, August 16, 2013. Last revised by Branson Bolden, MD, August 30, 2016. Key Points Pit viper (rattlesnake, cottonmouth,
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ANASCORP safely and effectively. See full prescribing information for ANASCORP. ----------------------DOSAGE
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ANASCORP safely and effectively. See full prescribing information for ANASCORP. ----------------------DOSAGE
ANTIVIPMYN TREATMENT PACKAGE
 The Ontario Massasauga Rattlesnake Antivenom Depot Dr. T. J. Fargher, MB. Ch.B., F.C.P (SA), F.R.C.P(C) Medical Director 705-746-9321 ANTIVIPMYN TREATMENT PACKAGE FOR EASTERN MASSASAUGA RATTLESNAKE BITES
The Ontario Massasauga Rattlesnake Antivenom Depot Dr. T. J. Fargher, MB. Ch.B., F.C.P (SA), F.R.C.P(C) Medical Director 705-746-9321 ANTIVIPMYN TREATMENT PACKAGE FOR EASTERN MASSASAUGA RATTLESNAKE BITES
POLYVALENT SNAKE ANTIVENOM Product Information 1(5) Product Information POLYVALENT SNAKE ANTIVENOM (AUSTRALIA - PAPUA NEW GUINEA) AUST R 74899
 POLYVALENT SNAKE ANTIVENOM Product Information 1(5) Product Information NAME OF THE MEDICINE POLYVALENT SNAKE ANTIVENOM (AUSTRALIA - PAPUA NEW GUINEA) AUST R 74899 DESCRIPTION POLYVALENT SNAKE ANTIVENOM
POLYVALENT SNAKE ANTIVENOM Product Information 1(5) Product Information NAME OF THE MEDICINE POLYVALENT SNAKE ANTIVENOM (AUSTRALIA - PAPUA NEW GUINEA) AUST R 74899 DESCRIPTION POLYVALENT SNAKE ANTIVENOM
CBC with Differential. PHYSICIAN SIGNATURE DATE TIME DRUG ALLERGIES WT: KG
 DRUG AND TREATMENT Non Categorized SUB ED Snakebite Protocol (SUB)* Non Categorized ***(NOTE)*** This plan is designed to be used as part of a larger plan, not independently. Please do NOT order individually.
DRUG AND TREATMENT Non Categorized SUB ED Snakebite Protocol (SUB)* Non Categorized ***(NOTE)*** This plan is designed to be used as part of a larger plan, not independently. Please do NOT order individually.
Specific treatment: Antivenom (AV) Therapy
 Specific treatment: Antivenom (AV) Therapy It is never too late to give antivenom provided the indications are present: Only if features of systemic envenoming are present for bites of snakes in the red
Specific treatment: Antivenom (AV) Therapy It is never too late to give antivenom provided the indications are present: Only if features of systemic envenoming are present for bites of snakes in the red
Risk of immediate effects from F(ab)2 bivalent antivenin in Taiwan
 Wilderness and Environmental Medicine, 11, 163-167 (2000) ORIGINAL RESEARCH Risk of immediate effects from F(ab)2 bivalent antivenin in Taiwan JIH-CHANG CHEN, MD; MICHAEL J. BULLARD, MD, PRCP; TE-FA CHID,
Wilderness and Environmental Medicine, 11, 163-167 (2000) ORIGINAL RESEARCH Risk of immediate effects from F(ab)2 bivalent antivenin in Taiwan JIH-CHANG CHEN, MD; MICHAEL J. BULLARD, MD, PRCP; TE-FA CHID,
PRODUCT INFORMATION DIGIBIND INJECTION
 PRODUCT INFORMATION DIGIBIND INJECTION NAME OF THE MEDICINE Digibind, Digoxin immune Fab (Ovine), is a sterile lyophilized powder of antigen binding fragments (Fab) derived from specific antidigoxin antibodies
PRODUCT INFORMATION DIGIBIND INJECTION NAME OF THE MEDICINE Digibind, Digoxin immune Fab (Ovine), is a sterile lyophilized powder of antigen binding fragments (Fab) derived from specific antidigoxin antibodies
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Blood products and plasma substitutes
 Blood products and plasma substitutes Plasma substitutes Dextran 70 and polygeline are macromolecular substances which are metabolized slowly; they may be used to expand and maintain blood volume in shock
Blood products and plasma substitutes Plasma substitutes Dextran 70 and polygeline are macromolecular substances which are metabolized slowly; they may be used to expand and maintain blood volume in shock
STATE TOXINOLOGY SERVICES Toxinology Dept., Women s & Children s Hospital, North Adelaide SA 5006 AUSTRALIA
 Family Viperidae www.toxinology.com record number Common name Western Diamond Rattlesnake SN0419 Scientific name combined Global region in which snake is found North America + Central America CLINICAL
Family Viperidae www.toxinology.com record number Common name Western Diamond Rattlesnake SN0419 Scientific name combined Global region in which snake is found North America + Central America CLINICAL
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT DigiFab, 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each glass vial
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT DigiFab, 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each glass vial
LEMTRADA REMS Education Program for Healthcare Facilities
 For Healthcare Facilities LEMTRADA REMS Education Program for Healthcare Facilities This Educational Piece Includes Information About: The LEMTRADA REMS Program requirements to implement in your healthcare
For Healthcare Facilities LEMTRADA REMS Education Program for Healthcare Facilities This Educational Piece Includes Information About: The LEMTRADA REMS Program requirements to implement in your healthcare
Allergic reactions anaphylaxis *** CME Version *** Aaron J. Katz, AEMT-P, CIC
 Allergic reactions anaphylaxis *** CME Version *** Aaron J. Katz, AEMT-P, CIC www.es26medic.net Some terms Allergic reaction Exaggerated immune system response to an allergen Allergen The thing that causes
Allergic reactions anaphylaxis *** CME Version *** Aaron J. Katz, AEMT-P, CIC www.es26medic.net Some terms Allergic reaction Exaggerated immune system response to an allergen Allergen The thing that causes
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
Chapter 8. Learning Objectives. Learning Objectives 9/11/2012. Anaphylaxis. List symptoms of anaphylactic shock
 Chapter 8 Anaphylaxis Learning Objectives List symptoms of anaphylactic shock Discuss role of immune system in fighting antigens Define allergic response Learning Objectives Describe body s response to
Chapter 8 Anaphylaxis Learning Objectives List symptoms of anaphylactic shock Discuss role of immune system in fighting antigens Define allergic response Learning Objectives Describe body s response to
DERBY-BURTON LOCAL CANCER NETWORK FILENAME Peruse.DOC CONTROLLED DOC NO: CCPG R29
 Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Airway Compromise After First Rattlesnake Envenomation
 Wilderness and Environmental Medicine, 15, 188 193 (2004) CASE REPORT Airway Compromise After First Rattlesnake Envenomation Daniel E. Brooks, MD; Kimberlie A. Graeme, MD From the Department of Medical
Wilderness and Environmental Medicine, 15, 188 193 (2004) CASE REPORT Airway Compromise After First Rattlesnake Envenomation Daniel E. Brooks, MD; Kimberlie A. Graeme, MD From the Department of Medical
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
Target Audience: Emergency Medicine Residents (junior and senior level postgraduate learners), Medical Students
 Rattlesnake Bite Authors: Laura Morrison, MD / Ryan Chuang, MD Reviewers: Shawn M. Varney, MD Target Audience: Emergency Medicine Residents (junior and senior level postgraduate learners), Medical Students
Rattlesnake Bite Authors: Laura Morrison, MD / Ryan Chuang, MD Reviewers: Shawn M. Varney, MD Target Audience: Emergency Medicine Residents (junior and senior level postgraduate learners), Medical Students
Case Presentation #4: A Pretty Worm
 Case Presentation #4: Presented by Brent R. King, MD, FAAP, FACEP A three year-old child arrives via EMS with ptosis, stridor, hypersalivation, hypotonia, and poor respiratory effort. The symptoms began
Case Presentation #4: Presented by Brent R. King, MD, FAAP, FACEP A three year-old child arrives via EMS with ptosis, stridor, hypersalivation, hypotonia, and poor respiratory effort. The symptoms began
October 2016 Newsletter
 October 2016 Newsletter Website About Us Legislative Issues News Calendar NOTICE OF RENEWALS OF PHARMACIST AND NUCLEAR PHARMACIST LICENSES AND NOTICE REGARDING ACTIVE - RENEWAL PENDING STATUS Meetings
October 2016 Newsletter Website About Us Legislative Issues News Calendar NOTICE OF RENEWALS OF PHARMACIST AND NUCLEAR PHARMACIST LICENSES AND NOTICE REGARDING ACTIVE - RENEWAL PENDING STATUS Meetings
Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate).
 VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
PM-03 PED ALLERGY/ANAPHYLAXIS. Protocol SECTION: PM-03 PROTOCOL TITLE: PED ALLERGY/ANAPHYLAXIS REVISED: 01MAY2018
 SECTION: PROTOCOL TITLE: REVISED: 01MAY2018 BLS SPECIFIC CARE: See General Pediatric Care Protocol PM-1 - Determine patient s color category on length based resuscitation tape (Broselow Tape) Epi Pen Protocol
SECTION: PROTOCOL TITLE: REVISED: 01MAY2018 BLS SPECIFIC CARE: See General Pediatric Care Protocol PM-1 - Determine patient s color category on length based resuscitation tape (Broselow Tape) Epi Pen Protocol
Does the traditional snakebite severity score correctly classify envenomated patients?
 Clin Exp Emerg Med 216;3(1):34-4 http://dx.doi.org/1.15441/ceem.16.123 Does the traditional snakebite severity score correctly classify envenomated patients? Seungho Kang, Jeongmi Moon, Byeongjo Chun Department
Clin Exp Emerg Med 216;3(1):34-4 http://dx.doi.org/1.15441/ceem.16.123 Does the traditional snakebite severity score correctly classify envenomated patients? Seungho Kang, Jeongmi Moon, Byeongjo Chun Department
GAZYVA Dosing and Administration Guide
 GAZYVA Dosing and Administration Guide Indications GAZYVA is a CD20-directed cytolytic antibody and is indicated: In combination with chemotherapy followed by GAZYVA monotherapy in patients achieving at
GAZYVA Dosing and Administration Guide Indications GAZYVA is a CD20-directed cytolytic antibody and is indicated: In combination with chemotherapy followed by GAZYVA monotherapy in patients achieving at
Cycle 1 PERTuzumab (day 1) and trastuzumab (day 2) loading doses: Drug Dose BC Cancer Administration Guideline
 BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
DIGIFAB DIGOXIN IMMUNE FAB (OVINE)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIGIFAB safely and effectively. See full prescribing information for DIGIFAB. DigiFab, Digoxin Immune
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIGIFAB safely and effectively. See full prescribing information for DIGIFAB. DigiFab, Digoxin Immune
Immunological transfusion reactions
 Immunological transfusion reactions Immunological transfusion reactions can be hemolytic or non-hemolytic in nature. Both types can be separated into acute (those occurring immediately after transfusion)
Immunological transfusion reactions Immunological transfusion reactions can be hemolytic or non-hemolytic in nature. Both types can be separated into acute (those occurring immediately after transfusion)
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab
 BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
Princess Alexandra Hospital Emergency Department. Clinical Module. Clinical features of envenoming: Major toxin syndromes 1 :
 Princess Alexandra Hospital Emergency Department Clinical Module Toxicology Review Officer: Toxicology registrar Version no: 1 Approval date: February 2017 Review date: February 2019 Approving Officer
Princess Alexandra Hospital Emergency Department Clinical Module Toxicology Review Officer: Toxicology registrar Version no: 1 Approval date: February 2017 Review date: February 2019 Approving Officer
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for
Envenomations by Melissa C. Janse, MD. What you may encounter locally What to do
 Envenomations by Melissa C. Janse, MD What you may encounter locally What to do Local Envenomations Venomous snakes (Copperhead, Various rattlers, Coral snake, Cottonmouth) Hymenoptera (bees, wasps, and
Envenomations by Melissa C. Janse, MD What you may encounter locally What to do Local Envenomations Venomous snakes (Copperhead, Various rattlers, Coral snake, Cottonmouth) Hymenoptera (bees, wasps, and
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities
 Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging
 ABSTRACT FOR SPS POSTER CASE PRESENTATION K Singer Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging Introduction: Children undergoing radiologic imaging frequently
ABSTRACT FOR SPS POSTER CASE PRESENTATION K Singer Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging Introduction: Children undergoing radiologic imaging frequently
MabThera. SC. The wait is over. MabThera delivered in just 5 minutes. SC= subcutaneous injection
 MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
The First rfviii WITH A PROLONGED HALF-LIFE
 Visit ELOCTATEpro.com for more information The First rfviii WITH A PROLONGED HALF-LIFE Indications ELOCTATE [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, antihemophilic
Visit ELOCTATEpro.com for more information The First rfviii WITH A PROLONGED HALF-LIFE Indications ELOCTATE [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, antihemophilic
BOLSTRAN Injection (Omalizumab)
 Published on: 27 Feb 2017 BOLSTRAN Injection (Omalizumab) Composition Active Substance Omalizumab is a humanized monoclonal antibody manufactured from a mammalian cell line. One vial of BOLSTRAN 150 mg
Published on: 27 Feb 2017 BOLSTRAN Injection (Omalizumab) Composition Active Substance Omalizumab is a humanized monoclonal antibody manufactured from a mammalian cell line. One vial of BOLSTRAN 150 mg
CEREZYME Genzyme. 70 mg (52 mg) (18 mg)
 CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT DigiFab (referred to as DIGIFAB) 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT DigiFab (referred to as DIGIFAB) 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Study design: Multicenter, randomized, controlled, cross-over, blinded PK comparison
 Brand Name 1, 2 : Rixubis Generic Name 1, 2 : Coagulation factor IX recombinant Manufacturer 5 : Baxter Drug Class 1, 2, 3 : Antihemophilic agent Labeled Uses 1, 2 : Hemophilia B hemorrhage, routine prophylaxis,
Brand Name 1, 2 : Rixubis Generic Name 1, 2 : Coagulation factor IX recombinant Manufacturer 5 : Baxter Drug Class 1, 2, 3 : Antihemophilic agent Labeled Uses 1, 2 : Hemophilia B hemorrhage, routine prophylaxis,
Digoxin Immune Fab (Ovine)
 Digoxin Immune Fab (Ovine) CATEGORIES: Ingredients: Digoxin Immune Fab (Ovine). Indications: Toxicity, digoxin. Off-label Indications: Not clinically relevant: Glycoside Toxicity; Glycoside Toxicity (diagnosis);
Digoxin Immune Fab (Ovine) CATEGORIES: Ingredients: Digoxin Immune Fab (Ovine). Indications: Toxicity, digoxin. Off-label Indications: Not clinically relevant: Glycoside Toxicity; Glycoside Toxicity (diagnosis);
Management of Common Bites and Stings
 Management of Common Bites and Stings Nicholas E. Kman, MD The Ohio State University Department of Emergency Medicine Mary Jo Bowman, M.D. Nationwide Children's Hospital Columbus, Ohio Case 29 yo man was
Management of Common Bites and Stings Nicholas E. Kman, MD The Ohio State University Department of Emergency Medicine Mary Jo Bowman, M.D. Nationwide Children's Hospital Columbus, Ohio Case 29 yo man was
VACCINE-RELATED ALLERGIC REACTIONS
 VACCINE-RELATED ALLERGIC REACTIONS Management of Anaphylaxis IERHA Immunization Program September 2016 VACCINE-RELATED ADVERSE EVENTS Local reactions pain, edema, erythema Systemic reactions fever, lymphadenopathy
VACCINE-RELATED ALLERGIC REACTIONS Management of Anaphylaxis IERHA Immunization Program September 2016 VACCINE-RELATED ADVERSE EVENTS Local reactions pain, edema, erythema Systemic reactions fever, lymphadenopathy
TEXAS CHILDREN S HOSPITAL EVIDENCE-BASED OUTCOMES CENTER Evaluation & Management of Suspected U.S. Pit Viper Snakebites Evidence Summary
 TEXAS CHILDREN S HOSPITAL EVIDENCE-BASED OUTCOMES CENTER Evaluation & Management of Suspected U.S. Pit Viper Snakebites Evidence Summary DATE: April 2017 Inclusion Criteria Patients
TEXAS CHILDREN S HOSPITAL EVIDENCE-BASED OUTCOMES CENTER Evaluation & Management of Suspected U.S. Pit Viper Snakebites Evidence Summary DATE: April 2017 Inclusion Criteria Patients
ADULT DRUG REFERENCE Drug Indication Adult Dosage Precautions / Comments
 ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities
 Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN)
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
Core Safety Profile. Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% Date of FAR:
 Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Immunocompetence The immune system responds appropriately to a foreign stimulus
 Functions of the immune system Protect the body s internal environment against invading organisms Maintain homeostasis by removing damaged cells from the circulation Serve as a surveillance network for
Functions of the immune system Protect the body s internal environment against invading organisms Maintain homeostasis by removing damaged cells from the circulation Serve as a surveillance network for
Transfusion Reactions
 Transfusion Reactions From A to T Provincial Blood Coordinating Program Daphne Osborne MN PANC (C) RN We want you to know Definition Appropriate actions Classification Complete case studies Transfusion
Transfusion Reactions From A to T Provincial Blood Coordinating Program Daphne Osborne MN PANC (C) RN We want you to know Definition Appropriate actions Classification Complete case studies Transfusion
Who Should Be Premediciated for Contrast-Enhanced Exams?
 Who Should Be Premediciated for Contrast-Enhanced Exams? Jeffrey C. Weinreb, MD,FACR Yale University School of Medicine jeffrey.weinreb@yale.edu Types of Intravenous Contrast Media Iodinated Contrast Agents
Who Should Be Premediciated for Contrast-Enhanced Exams? Jeffrey C. Weinreb, MD,FACR Yale University School of Medicine jeffrey.weinreb@yale.edu Types of Intravenous Contrast Media Iodinated Contrast Agents
Chemotherapy must not be started unless the following drugs have been given:
 BC Cancer Protocol Summary for Second-Line Therapy for Metastatic or Locally Advanced Gastric or Gastroesophageal Junction Cancer Using Weekly PACLitaxel and Ramucirumab Protocol Code: Tumour Group: Contact
BC Cancer Protocol Summary for Second-Line Therapy for Metastatic or Locally Advanced Gastric or Gastroesophageal Junction Cancer Using Weekly PACLitaxel and Ramucirumab Protocol Code: Tumour Group: Contact
BRAJACTT. Protocol Code. Breast. Tumour Group. Dr. Karen Gelmon. Contact Physician
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Manufacturer: Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc.
 Brand Name: Mylotarg Generic Name: gentuzumab ozogamicin Manufacturer: Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc. Drug Class: CD33-directed antibody-drug conjugate Uses: Labeled Uses: Newly-diagnosed
Brand Name: Mylotarg Generic Name: gentuzumab ozogamicin Manufacturer: Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc. Drug Class: CD33-directed antibody-drug conjugate Uses: Labeled Uses: Newly-diagnosed
BRAVTPCARB. Protocol Code: Breast. Tumour Group: Dr. Karen Gelmon. Contact Physician:
 BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
As you begin this build-up phase there are several factors to keep in mind concerning your treatment:
 SLIT IMMUNOTHERAPY INSTRUCTIONS David R. Scott, M.D./William A. Scott, M.D. Congratulations on beginning your new sublingual immunotherapy (SLIT) prescription! The initial 30 doses are advancement doses.
SLIT IMMUNOTHERAPY INSTRUCTIONS David R. Scott, M.D./William A. Scott, M.D. Congratulations on beginning your new sublingual immunotherapy (SLIT) prescription! The initial 30 doses are advancement doses.
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION
 GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
ABACAVIR HYPERSENSITIVITY REACTION
 ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
Student Health Center
 Referring Allergist Agreement Your patient is requesting that the University of Mary Washington Student Health Center (UMWSHC) administer allergy extracts provided by your office. Consistent with our policies
Referring Allergist Agreement Your patient is requesting that the University of Mary Washington Student Health Center (UMWSHC) administer allergy extracts provided by your office. Consistent with our policies
PFIZER INC. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Cerebyx / Fosphenytoin Sodium
 PFIZER INC These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Atrovent Administration
 Atrovent Administration ICEMA Training 2007 Sherri Shimshy RN OBJECTIVES Describe the pharmacology of Atrovent Identify the indications for use of Atrovent in the Adult Population Identify the indications
Atrovent Administration ICEMA Training 2007 Sherri Shimshy RN OBJECTIVES Describe the pharmacology of Atrovent Identify the indications for use of Atrovent in the Adult Population Identify the indications
Active ingredients: Metoclopramide Hydrochloride mg Equivalent to metoclopramide hydrochloride anhydrous mg
 Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
PRODUCT INFORMATION 1 ABOUT THIS GUIDE DOSAGE FORM AND STRENGTH STORAGE AND HANDLING
 DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
Recombinant Factor VIIa for Treatment of Gastrointestinal Hemorrhage Following Rattlesnake Envenomation
 Wilderness and Environmental Medicine, 20, 156 160 (2009) CASE REPORT Recombinant Factor VIIa for Treatment of Gastrointestinal Hemorrhage Following Rattlesnake Envenomation Anne-Michelle Ruha, MD; Steven
Wilderness and Environmental Medicine, 20, 156 160 (2009) CASE REPORT Recombinant Factor VIIa for Treatment of Gastrointestinal Hemorrhage Following Rattlesnake Envenomation Anne-Michelle Ruha, MD; Steven
ULYRICE. Protocol Code. Lymphoma. Tumour Group. Dr. Laurie Sehn. Contact Physician
 BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
WARNING, CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS,
 Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
Snake bite: a current approach to management
 Snake bite: a current approach to management Geoffrey K Isbister, Senior Research Fellow, Tropical Toxinology Unit, Menzies School of Health Research, Charles Darwin University, Northern Territory, Clinical
Snake bite: a current approach to management Geoffrey K Isbister, Senior Research Fellow, Tropical Toxinology Unit, Menzies School of Health Research, Charles Darwin University, Northern Territory, Clinical
Rattlesnake Envenomation
 CE Article #3 Rattlesnake Envenomation Laura Najman, DVM, DACVECC Ravi Seshadri, DVM, DACVECC, DAVBP Advanced Critical Care and Internal Medicine Tustin, California ABSTRACT: Snake envenomation has been
CE Article #3 Rattlesnake Envenomation Laura Najman, DVM, DACVECC Ravi Seshadri, DVM, DACVECC, DAVBP Advanced Critical Care and Internal Medicine Tustin, California ABSTRACT: Snake envenomation has been
PRIMARY CARE PRACTICE GUIDELINES
 Community Mgmt Team - 1 of 6 1. OUTCOME To identify anaphylaxis in the primary care setting and provide an evidence informed emergency response utilizing the most current provincial and federal practice
Community Mgmt Team - 1 of 6 1. OUTCOME To identify anaphylaxis in the primary care setting and provide an evidence informed emergency response utilizing the most current provincial and federal practice
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case
 Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Drew University Health Service 36 Madison Avenue Madison, New Jersey Tel: Fax:
 Dear Student, Enclosed you will find our policies, procedures and student consent form for your allergy immunotherapy. We ask that you read them carefully, sign the consent form, and take the physician
Dear Student, Enclosed you will find our policies, procedures and student consent form for your allergy immunotherapy. We ask that you read them carefully, sign the consent form, and take the physician
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin)
 SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
Prospective Study of Recovery from Copperhead Snake Envenomation: An Observational Study
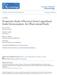 University of Kentucky UKnowledge Emergency Medicine Faculty Publications Emergency Medicine 5-15-2015 Prospective Study of Recovery from Copperhead Snake Envenomation: An Observational Study Eric J. Lavonas
University of Kentucky UKnowledge Emergency Medicine Faculty Publications Emergency Medicine 5-15-2015 Prospective Study of Recovery from Copperhead Snake Envenomation: An Observational Study Eric J. Lavonas
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Emergency Department Guideline. Anaphylaxis
 Emergency Department Guideline Inclusion criteria: 1. Acute onset of an illness (minutes to hours) with a AND (b OR c): a. Skin and/or mucosa (pruritus, flushing, hives, angioedema) b. Respiratory compromise
Emergency Department Guideline Inclusion criteria: 1. Acute onset of an illness (minutes to hours) with a AND (b OR c): a. Skin and/or mucosa (pruritus, flushing, hives, angioedema) b. Respiratory compromise
Dosing and Administration Guide for ARZERRA
 Dosing and Administration Guide for ARZERRA INDICATIONS for ARZERRA (ofatumumab) In combination with chlorambucil, for the treatment of previously untreated patients with chronic lymphocytic leukemia (CLL)
Dosing and Administration Guide for ARZERRA INDICATIONS for ARZERRA (ofatumumab) In combination with chlorambucil, for the treatment of previously untreated patients with chronic lymphocytic leukemia (CLL)
GOOVIPPC. Protocol Code: Gynecology. Tumour Group: Paul Hoskins. Contact Physician: James Conklin. Contact Pharmacist:
 BCCA Protocol Summary for Primary Treatment of Stage III less than or equal to 1 cm Visible Residual Invasive Epithelial Ovarian Cancer or Stage I Grade 3 or Stage II Grade 3 Papillary Serous Ovarian Cancer
BCCA Protocol Summary for Primary Treatment of Stage III less than or equal to 1 cm Visible Residual Invasive Epithelial Ovarian Cancer or Stage I Grade 3 or Stage II Grade 3 Papillary Serous Ovarian Cancer
