Paradoxical heat sensation in healthy subjects: peripherally conducted by Aδ or C fibres?
|
|
|
- Abraham Randall
- 5 years ago
- Views:
Transcription
1 Brain (1999), 122, Paradoxical heat sensation in healthy subjects: peripherally conducted by Aδ or C fibres? Ehud Susser, 1 Elliot Sprecher 2 and David Yarnitsky 1,2 1 Technion Faculty of Medicine, Israel Institute of Correspondence to: Dr David Yarnitsky, Neurology, Technology, 2 Department of Neurology, Rambam Medical Rambam Medical Centre, Haifa, Israel Centre and Technion Faculty of Medicine, Haifa, Israel Summary Paradoxical heat sensation upon cooling of the skin has been reported in central as well as in peripheral neurological conditions. In our study, we examined this phenomenon in 35 naive healthy test subjects, of whom 23 experienced paradoxical heat sensation under test conditions. We measured the peripheral conduction velocities of cold sensation, warm sensation and of paradoxical heat sensation by using a quantitative sensory testing model of indirect peripheral conduction velocity measurement. This was based on comparison of measurements at a proximal and a distal site using two measurement methods, one inclusive and the other exclusive of reaction time. We found that the conduction Keywords: paradoxical heat sensation; quantitative sensory testing; conduction velocity; reaction time Abbreviations: ANOVA analysis of variance; HPC heat, pinch, cold; PHS paradoxical heat sensation; QST quantitative sensory testing Introduction Paradoxical heat sensation (PHS) upon cooling of the skin was reported by Goldsheider as early as 1912, and has been found in 10 12% of healthy individuals (Hämäläinen et al., 1982; Hansen et al., 1996). Preheating was found to increase the incidence of this phenomenon markedly, to 35% of healthy subjects (Hämäläinen et al., 1982). More recent studies have demonstrated the phenomenon of PHS in apparently diverse neurological disorders, of both peripheral and central origin. It was found in 42% of patients with uraemic polyneuropathy (Yosipovitch et al., 1995) and in 63% of patients with probable or definite multiple sclerosis (Hansen et al., 1996), and experimentally in normal subjects under compression ischaemia block (Wahren et al., 1989; Yarnitsky and Ochoa, 1990). Other studies have further demonstrated this ostensible aetiological diversity. The triple cold syndrome (Ochoa and Yarnitsky, 1994) is a syndrome of cold hypoaesthesia, cold limbs and cold hyperalgesia, described in patients with peripheral polyneuropathies or mononeuropathies of various aetiologies. The cold hyperalgesia has been described as a Oxford University Press 1999 velocity of paradoxical heat sensation (0.70 m/s) was similar to that of warm sensation (0.68 m/s), and that the conduction velocity of cold sensation ( m/s) was considerably faster. Thus, we conclude that paradoxical heat sensation in healthy subjects is conducted peripherally via slow unmyelinated C fibres and not via the faster Aδ fibres. Consequently, we propose that paradoxical heat sensation is encoded via the heat sensing pathway, in accordance with the labelled-line code theory. The mechanisms proposed suggest a malfunctioning coldsensing pathway disinhibiting the heat-sensing pathway, at peripheral, central or both levels, thus facilitating a paradoxical heat sensation. burning sensation induced by cooling stimuli, a description compatible with PHS. The Thunberg grill illusion (Craig and Bushnell, 1994), demonstrated in healthy subjects, describes the sensation of paradoxical strong or painful heat elicited by touching interlaced mildly warm and cool bars. This apparent aetiological diversity suggests different hypotheses pertaining to the mechanism of PHS. Primarily, it is necessary to investigate the way in which the stimulus is encoded. PHS could be transmitted via Aδ cold-specific fibres, but would be sensed as heat due to central modulation, thus behaving in accordance with the pattern code theory of stimulus encoding. Conversely, PHS could be transmitted through C fibres which normally mediate sensations evoked by high temperature, thus behaving in accordance with the labelled-line code theory of stimulus encoding. We investigated PHS in healthy subjects by using a quantitative sensory testing (QST) model of indirect measurement of peripheral conduction velocity. We established the peripheral conduction velocities of PHS, warm sensation and of cold sensation. Consequently, by comparing these results
2 240 E. Susser et al. we were able to determine whether PHS is conducted peripherally via C fibres or via Aδ fibres. Methods Subjects The study was performed on 35 paid healthy subjects (16 men and 19 women) aged years (median 22 years). All subjects filled in a health questionnaire and gave informed consent according to the Declaration of Helsinki. The study was approved by the Ethics Committee of The Technion Medical School, Haifa. Subjects with any sign of a neurological disorder, diabetes, renal disease or neoplastic disease were disqualified, as were subjects taking any medication which might have interfered with the study, e.g. analgesics. Quantitative thermal sensory testing All tests were performed using a thermal sensory analyser (TSA 2001; Medoc, Ramat Yishai, Israel). This consists of a probe (of interchangeable size) including a Peltier element and two thermistors, a subject feedback unit and a computer. The Peltier element cools and heats the contact plate while its temperature is measured by the two thermistors in the probe. The adaptation temperature in all tests was set to 32 C. The temperature range was limited to 0 50 C in order to prevent skin injury. Measurement accuracy of the methods employed was 0.1 C. In order to calculate the reaction time indirectly for each site, we used two methods of threshold measurement: the method of limits, inclusive of reaction time, and the method of levels, excluding it (Yarnitsky, 1997). The method of limits as performed in this study was a modification of the classical method of limits. Testing algorithms Levels A series of temperature ramps was administered to the subject s skin, and after each one the subject replied whether he sensed anything, and if so, of what quality (hot or cold). The first predetermined temperature ramp was a step of 3.0 C ( 3.0 C for warm sensation, 3.0 C for cold sensation and PHS), and this was halved at each turn temperature (the peak temperature of the stimulus at which the step-to-step stimulus change switches its direction, i.e. both the preceding and succeeding stimuli were either higher or lower than the one at the turning point). Thus, if the subject replied that the step was sensed (Y), the next step was halved (i.e. in this case to 1.5 C). However, if the subject replied that he sensed nothing (N), the next temperature ramp was increased to twice the initial step (i.e. in this case to 6.0 C), until the stimulus was sensed. At that point, the stimulus was lowered by a step half the size of the previous step, until the next turn point, where the step size was halved again, and so on. This went on until the step reached a predetermined size, in our case 0.1 C. The average of the last N and Y responses was taken as the equivalent sensation threshold. Limits A ramp of increasing or decreasing temperature was given, and the subject, using his feedback unit, pressed a button the instant he sensed something. After pressing the button, he told us what the sensation was. Such transients were given five times for each test, and their average was taken as the threshold. Thresholds obtained by this method are higher than those by the previous one, due to reaction time. Two probes were used, a large one with an area of cm (13.5 cm 2 ) and a small one with an area of cm (2.56 cm 2 ). The large probe was used for testing the modalities of cold sensation and warm sensation, the rate of stimulus temperature change being 1.0 C/s, as routinely done in clinical QST applications. The small probe was used for PHS and cold sensation, and differed from the routine clinical application in having a high rate of temperature change (4.0 C/s). For PHS only, preheating was employed, giving five stimuli of heat pain at threshold intensity immediately prior to the measurement. Each probe was placed on two sites on the subject s dominant lower limb: (i) anterior and superior to the lateral malleolus; and (ii) the proximal anterior thigh. The distance between these two sites (d 1 2 ) was measured. The testing sequence Each set of tests consisted of measuring the thresholds of the above sensations using limits and levels at both sites. The subjects were blinded to the type of sensation that was being tested. When tested for PHS, the sites were preheated prior to each test, as previously described. We defined PHS as a subjective feeling of heat upon cooling in 67% of all cold stimuli given with the small probe (on average around 25 such stimuli). The test sequence was randomized for the factors of probe size, starting site and starting method, except that (i) cold sensation was always tested before warm sensation in order to avoid the preheating effect, and (ii) small-probe warm sensation was not tested. The overall test time was h per subject. Determination of reaction time Reaction time was determined indirectly for each site by calculating the difference between the thermal thresholds obtained by the two methods. This temperature difference was divided by the predetermined rate of stimulus temperature change (rate), thus yielding the time between the administration of the stimulus of sufficient intensity and eventual perception of the sensation (Yarnitsky and Ochoa, 1991) (equation 1).
3 RT levels ( C) limits ( C) (1) ( C/s) where RT reaction time in seconds. Determination of conduction velocity The peripheral conduction velocity was determined using a model of indirect measurement, which was based on the work of Fowler et al. (1988), incorporating the reaction time determination method reported by Yarnitsky and Ochoa (1991), as described above. We determined the reaction time of both sites as described above in equation 1, and the distance between the two sites (d 1 2 ). We then extracted the peripheral conduction time by calculating the difference between RT 1 and RT 2 ( t). Thus, conduction velocity was calculated according to the following formula (equation 2): Conduction velocity d 1 2 / t (2) where conduction velocity is expressed in metres per second, d 1 2 in metres and t in seconds. The calculation might slightly underestimate the velocity because of the normal curvature of nerves. This model assumes equal central processing time for the two sites. Statistical analysis We calculated the mean values of t and d 1 2 for all subjects, and their respective standard deviations. We then calculated the conduction velocity by dividing the mean d 1 2 by the mean t. The statistical analysis consisted of a repeated measures (mixed model) analysis of variance (ANOVA) run under SAS (SAS Institute, Cary, NC, USA) Procedure MIXED, with sensory modality as the repeated variable and peripheral conduction time as the dependent variable. An appropriate covariance structure was chosen, based on Akaike s Information Criterion, from among the following: compound symmetry; heterogeneous compound symmetry; Huynh- Feldt; and Variance Components/Simple. Scheffe s test was used for post hoc comparisons among all pairwise combinations of modalities. Results Paradoxical heat sensation PHS was reported by 23 subjects (66%) using the previously described protocol. All these subjects reported sensations of heat upon cooling of the skin in 67% (mean 90%) of the stimuli given. Upon further analysis of the results regarding factors such as probe size, rate of stimulus temperature change and preheating, we found that PHS occurred in only 6% of the Paradoxical heat sensation: C or Aδ fibres? 241 tested subjects using a large probe, a slow rate of stimulus temperature change and without preheating. This percentage increased to 26% when we used a smaller probe and a faster rate of stimulus temperature change, but still without preheating. When preheating was applied to the tested area using the smaller probe and the faster rate of stimulus temperature change, this percentage further increased to 66%. The need to control for non-paradoxical sensation under these circumstances led to the measurement of cold sensation conduction with the small probe at the fast rate, without preheating. For a small number of subjects, outlying results ( 4 SD above mean) were excluded: one outlying result was excluded for cold sensation and two for warm sensation. Other exclusions consisted of two more subjects who had PHS upon attempts to produce cold sensation. Thresholds, reaction times and conduction velocities The mean thresholds of the tested sensations are shown in Table 1, as are the distances and the calculated reaction times of the tested sites. As expected, the thresholds obtained by the method of limits were higher than those obtained by the method of levels, and the calculated reaction times for the distal site were longer than those for the proximal site. Mixed model ANOVA, using a heterogeneous compound symmetry covariance structure (which Akaike s Information Criterion indicated was the most appropriate for these data), indicated an overall effect of sensory modality on peripheral conduction time [F(3,65) 15.95, P ]. Scheffe s test indicated that the extracted peripheral conduction time ( t) of PHS was significantly longer than that of cold sensation (P ) and of small-probe cold sensation (P 0.03). In addition, there was no difference between cold sensation and small-probe cold sensation and between PHS and warm sensation, while cold sensation and warm sensation did differ statistically (Scheffe s test, P ). The mean t of cold sensation was 0.07 s and that of small-probe cold sensation 0.08 s, while that of warm sensation was 0.86 s and that of PHS was 0.84 s. Thus, the subsequent calculated peripheral conduction velocities of cold sensation (8.01 and 7.74 m/s for large and small probes, respectively) were significantly faster than those of the similar and slower conduction velocities of warm sensation (0.68 m/s) and PHS (0.70 m/s). We consequently concluded that the peripheral conduction velocity of PHS is similar to that of warm sensation. Analysis of the sex subgroups did not show any significant differences throughout the study. Discussion It is commonly accepted that cold sensation is conducted peripherally by myelinated cold-specific Aδ fibres and that
4 242 E. Susser et al. Table 1 Thresholds, rate, calculated reaction times, % distances and conduction velocities between the sites with respective standard deviations Sensation No. of Surface area Rate Site 1 (lateral malleolus) Site 2 (proximal anterior thigh) d 1 2 t CV tested subjects of probe (cm 2 ) ( C/s) (thresholds and calculated RT SD) (thresholds and calculated RT SD) (m) (s) (m/s) Levels Limits RT 1 Levels Limits RT 2 ( C) ( C) (s) ( C) ( C) (s) Cold Small probe, cold Warm PHS Preheating values for PHS* *Preheating was done by giving five stimuli of heat pain threshold using the method of limits. This was done prior to each test. The maximum temperature was limited to 50 C to avoid skin damage.
5 warm sensation is conducted by unmyelinated warm-specific C fibres (Darian-Smith, 1984; Fowler et al., 1988; Martin and Jessell, 1991; Yarnitsky and Ochoa, 1991). Recent studies have demonstrated that C polymodal nociceptors participate in the mediation of painful low temperature stimuli (cats: Craig and Bushnell, 1994; primates: Simone et al., 1994; man: Campero et al., 1996). The conduction velocity of these fibres has been studied by several strategies. Neurophysiological studies have shown the conduction velocity of Aδ cold fibres to be 6.3 m/s (primates: Hensel and Iggo, 1971) and 14.5 m/s (monkey: Darian-Smith et al., 1973), and the conduction velocity of C warm fibres to be 0.7 (primates: Hensel and Iggo, 1971) and 0.8 (monkey: Duclaux and Kenshalo, 1980). By using microneurography in humans, Torebjörk (1974) found velocities of m/s for C nociceptor fibres, and Adriaensen et al. (1983) found a range of m/s for Aδ fibres of both low and high mechanical thresholds. For pain-related somatosensory evoked potentials following laser CO 2 stimulation, human Aδ pain primary afferents were shown to have a conduction velocity of 9.0 m/s (Kakigi et al., 1991). In the few QST studies that have been performed, the conduction velocities of Aδ fibres and C fibres were found to be 2.6 and 1.6 m/s, respectively (Yarnitsky and Ochoa, 1991) and 2.1 and 0.2 m/s, respectively (Fowler et al., 1988). The textbook conduction velocities are, thus, 5 30 m/s for myelinated Aδ fibres and m/s for C fibres (Martin and Jessell, 1991). The conduction velocities found here for warm sensation fall well within expected limits. The conduction velocities for cold sensation, however, deviate from those reported by the previous psychophysical reports, probably because of methodological drawbacks in those papers; Fowler et al. (1988) used reaction times to thermal stimuli at distal and proximal sites, without considering the precise threshold at each site. Yarnitsky and Ochoa compared thresholds of reaction time-inclusive and -exclusive methods for a specific site, assuming a certain value for central processing time. In the present method, these two drawbacks are taken care of. The present study found results which are consistent with the neurophysiological literature. The spinal conduction of both thermal sensations is through the spinothalamic tract (Darian-Smith, 1984; Lindsay et al., 1991). There is considerably less information about the central processing of thermal sensations. It has been shown recently that the pathway starting with peripheral Aδ fibres reaches cold cells in the thalamus, and that the pathway starting with C fibres reaches heat-pinch-cold cells (HPC cells) in the thalamus, via lamina I at the dorsal horn (Craig and Bushnell, 1994). The anatomical location of these cells was suggested to be the posterior part of the ventral medial nucleus for cold cells and the caudal part of medial dorsal nucleus for the HPC cells (Craig, 1994). A low-temperature stimulus activates, according to these authors, both the Aδ fibre cold cell and the C fibre HPC cell pathways and thus generates a dual message with a cold component and a heat or pain component. Normally, the Aδ fibres inhibit the C Paradoxical heat sensation: C or Aδ fibres? 243 fibres peripherally when the two primary afferents meet at the dorsal horn [gate control equivalent (Lindsay et al., 1991)], and centrally at the thalamic level, where the cold cells inhibit the HPC cells so that only the cold component of the sensation is perceived (Craig and Bushnell, 1994) (Fig. 1A). The phenomenon of PHS raises fundamental issues about the nature of thermal sensation. Principally, it is necessary to elucidate the very nature of the way in which stimuli are encoded. Hypotheses pertaining to the encoding of PHS are presented below. A pattern code-encoding modality in which PHS is conducted via the Aδ cold cell pathway but is felt as heat because of an altered pattern of firing of the normally cold-sensing receptor Firing pattern had been suggested to be a determinant in the generation of thermal sensations (Emmers, 1976; Schingnitz and Werner, 1980). The conduction velocity of PHS would be similar to that of cold sensation in the QST model of conduction velocity measurement (Fig. 1B). A labelled-line code modality in which PHS is conducted via the C fibre HPC cell pathway as a result of disinhibition It is currently accepted that almost all sensory coding is done via the labelled-line code (Lindblom and Ochoa, 1986; Martin, 1991). This would suggest that PHS has to be conducted through a heat-sensing pathway, the C fibre- HPC pathway, since the sensory pathways are committed. Disinhibition could be either peripheral or central. The peripheral hypothesis Malfunctioning Aδ fibres disinhibit C fibre nociceptors by a mechanism of gate control in the dorsal horn of the spine, thus facilitating PHS (Yarnitsky and Ochoa, 1990; Lindsay et al., 1991; Ochoa and Yarnitsky, 1994; Yosipovitch et al., 1995) (Fig. 1C). This theory is supported by experiments on selective conduction blockade of the Aδ fibres using a sphygmomanometer cuff. As a result, the subjects felt a hot burning sensation upon further cooling stimuli (Yarnitsky and Ochoa, 1990). The peripheral hypothesis is in accordance with the finding of PHS in patients with uraemic polyneuropathy and in patients with the triple cold syndrome. The central hypothesis Malfunctioning cold cells in the thalamus disinhibit the HPC cells in the thalamus, thus facilitating PHS (Craig, 1994; Craig and Bushnell, 1994; Hansen et al., 1996) (Fig. 1C). The central hypothesis is in accordance with the finding of PHS in patients with multiple sclerosis.
6 244 E. Susser et al. In both the peripheral and the central hypothesis, the PHS is ultimately delivered via the C fibre HPC pathway. Therefore, the indirectly measured peripheral conduction velocity of PHS would be similar to that of warm sensation in both cases. Fig. 1 The thermal sensation model and the principal hypotheses of the mechanism of PHS. (A) The thermal sensation model. Heat is conducted from the C fibres, through the spinothalamic tract to the HPC cells in the thalamus, via lamina I at the dorsal horn. Cold, along with its accompanying noxious component, is conducted through both pathways, but the Aδ fibres inhibit the C fibres, and the cold cells inhibit the HPC cells. Thus, only the cold component is perceived. (Based on Craig and Bushnell, 1994.) (B) PHS is sensed via the Aδ cell fibre-cold pathway due to an altered pattern of firing, consistent with a pattern code modality. (C) PHS is sensed via the C fibre HPC cell pathway as a result of disinhibition, consistent with a labelled-line code modality. 1 the malfunctioning location in the peripheral hypothesis; 2 the malfunctioning location in the central hypothesis. (D) PHS is sensed peripherally via the Aδ fibres but is transmitted onto the heat-sensing pathway centrally as a result of a crossover due to a neuromodulator, as in the theory of secondary hyperalgesia. An additional model in which PHS is conducted peripherally by Aδ cold fibres but is transmitted onto the C fibre HPC pathway centrally Findings regarding the phenomenon of secondary hyperalgesia in normal skin surrounding a local injury may be applicable to a separate type of understanding of PHS. It has been shown (although controversy exists regarding these findings) that secondary hyperalgesia is conducted peripherally by Aβ fibres, which normally do not mediate painful sensations. The message conveyed by these fibres is transformed in the dorsal horn onto wide-dynamic range and high-threshold cells in the spinothalamic tract, thus producing a sensation of pain (LaMotte, 1992). Analogously, this model can be suggested for PHS, where the low-temperature stimulus activates Aδ primary afferents, which, in turn, transmit the message abnormally to the heat-sensing pathway in the CNS, although no experimental evidence exists for this model (Fig. 1D). In this case, the peripheral conduction velocity of PHS would be similar to that of cold sensation. In this study, our aim was to produce PHS in a maximum number of subjects in order to amass as much data as we could in order to calculate the peripheral conduction velocity. As stated in the Introduction, we know that preheating the designated area increases the incidence of PHS to ~35%. Through our preliminary tests we have also come to realize that this percentage can be further increased by the use of a probe with a smaller area, and by raising the rate of change of the stimulus temperature. These conditions are somewhat unexpected, since the higher density of cold than of warm receptors predicts the opposite. It might be that temporal summation properties are different for the two types of fibre, such that the warm message is further augmented under these conditions. Thus, we defined the testing sequence described in the Methods section. The result was conclusive. The peripheral conduction velocity of PHS was found to be almost equal to that of warm sensation. Thus, we propose that PHS is conducted peripherally by C fibres, and not by Aδ fibres. Consequently, we conclude that PHS is encoded via the C fibre HPC cell heat-sensing pathway, in accordance with the labelled-line code theory (Fig. 1C). The present study cannot determine where in the sensory pathway the disinhibition takes place peripherally or centrally. These two possibilities are not necessarily mutually exclusive and can thus coexist. Thus, peripheral neuropathies may cause higher incidences of PHS through Aδ fibre dysfunction, and multiple sclerosis may cause the same symptom through a completely different mechanism of central thalamic cold-cell dysfunction.
7 As stated above, the nature of the method used in our study does not enable us to distinguish which one of these mechanisms, peripheral or central, is responsible for PHS in healthy subjects. This is due to the indirect nature of the peripheral conduction velocity measurement used in quantitative sensory testing. Nonetheless, our study suggests that PHS may be a peripheral phenomenon in healthy subjects; the dependence of PHS on probe size and the rate of temperature change corresponds to the relatively small number of Aδ fibres in the peripheral nerve a smaller probe and a shorter stimulus duration reduce the amount of Aδ evoked activity to less than the minimum required for sensation. Another issue not resolved by this conduction velocity-based study is the type of C fibre conducting the PHS. On the one hand, the low thresholds of PHS suggest non-nociceptive fibres, but on the other hand evidence from the cuff experiments (Yarnitsky and Ochoa 1990), from Simone (1994) and from Craig and Bushnell (1994) suggests that it is the C nociceptors that mediate PHS. An additional consideration is the abundance of nociceptors compared with warm-specific fibres. The appearance of PHS after preheating also favours the latter possibility, via a possible mechanism of nociceptor sensitization. In summary, the peripheral conduction velocity of PHS was found to be statistically equivalent to that of warm sensation using a QST model of indirect conduction velocity measurement. We thus conclude that PHS is encoded via the C fibre HPC pathway in accordance with the labelled-line code theory. Since PHS can be an early expression of the uraemic polyneuropathy (Yosipovitch et al., 1995) and potentially other polyneuropathies, further studies into its mechanism in such patients will be of clinical value. Acknowledgements This study was performed as part of the requirements for Ehud Susser s MD degree from the Technion Faculty of Medicine, Israel Institute of Technology. References Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Response properties of thin myelinated (A-δ) fibers in human skin nerves. J Neurophysiol 1983; 49: Campero M, Serra J, Ochoa JL. C polymodal nociceptors activated by noxious low temperature in human skin. J Physiol (Lond) 1996; 497: Craig AD. Spinal and supraspinal processing of specific pain and temperature. In: Boivie J, Hansson P, Lindblom U, editors. Touch, temperature, and pain in health and disease: mechanisms and assessments. Progress in pain research and management, Vol. 3. Seattle: IASP Press; p Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science 1994; 265: Paradoxical heat sensation: C or Aδ fibres? 245 Darian-Smith I. Thermal sensibility. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology. Sect. I, Vol. 3, Pt. 2. Bethesda, MD: American Physiological Society; p Darian-Smith I, Johnson KO, Dykes R. Cold fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol 1973; 36: Duclaux R, Kenshalo DR Sr. Response characteristics of cutaneous warm receptors in the monkey. J Neurophysiol 1980; 43: Emmers R, Thalamic mechanisms that process a temporal pulse code for pain. Brain Res 1976; 103: Fowler CJ, Sitzoglou K, Ali Z, Halonen P. The conduction velocities of peripheral nerve fibres conveying sensations of warming and cooling. J Neurol Neurosurg Psychiatry 1988; 51: Goldscheider A. Beiträge zur Lahre von der Hautsensibilität. II. Uber die Empfindung der Hitze. Z Klin Med 1912; 75: Hämäläinen H, Vartiainen M, Karvanen L, Järvilehto T. Paradoxical heat sensations during moderate cooling of the skin. Brain Res 1982; 251: Hansen C, Hopf HC, Treede RD. Paradoxical heat sensation in patients with multiple sclerosis. Brain 1996; 119: Hensel H, Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflügers Arch 1971; 329: 1 8. Kakigi R, Endo C, Neshige R, Kuroda Y, Shibasaki H. Estimation of conduction velocity of Aδ fibres in humans. Muscle Nerve 1991; 14: LaMotte RH. Neurophysiological mechanisms of cutaneous secondary hyperalgesia in the primate. In: Willis WD, editor. Hyperalgesia and allodynia. New York: Raven Press; p Lindblom U, Ochoa J. Somatosensory function and dysfunction. In: Asbury AK, McKhann GM, McDonald WI, editors. Diseases of the nervous system. Philadelphia: W.B. Saunders; p Lindsay KW, Bone I, Callander R. Neurology and neurosurgery illustrated, 2nd ed. Edinburgh: Churchill Livingstone; p Martin JH. Coding and processing of sensory information. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science, 3rd ed. East Norwalk, CT: Appleton & Lange; p Martin JH, Jessell TM. Modality coding in the somatic sensory system. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science, 3rd ed. East Norwalk, CT: Appleton & Lange; p Ochoa JL, Yarnitsky D. The triple cold syndrome: cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain 1994; 117: Schingnitz G, Werner J. Thalamic burst firing a neuronal code for temperature? Brain Res 1980; 195: Simone DA, Gilchrist HD, Kajander KC. Responses of primate cutaneous nociceptors evoked by noxious cold [abstract]. Soc Neurosci Abstr 1994; 20: Torebjörk HE. Afferent C units responding to mechanical, thermal
8 246 E. Susser et al. and chemical stimuli in human non glabrous skin. Acta Physiol Scand 1974; 92: Wahren LK, Torebjörk HE, Jorum E. Central suppression of coldinduced C fibre pain by myelinated fibre input. Pain 1989; 38: Yarnitsky D. Quantitative sensory testing [see comments]. [Review]. Muscle Nerve 1997; 20: Comment in: Muscle Nerve 1997; 20: Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain 1990; 113: Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems: psychophysical thresholds, reaction times and peripheral conduction velocities. Brain 1991; 114: Yosipovitch G, Yarnitsky D, Mermelstein V, Sprecher E, Reiss J, Witenberg C, et al. Paradoxical heat sensation in uremic polyneuropathy. Muscle Nerve 1995; 18: Received March 21, Revised June 9, Second revision August 14, Accepted October 5, 1998
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatosensory modalities!
 Somatosensory modalities! The somatosensory system codes five major sensory modalities:! 1. Discriminative touch! 2. Proprioception (body position and motion)! 3. Nociception (pain and itch)! 4. Temperature!
Somatosensory modalities! The somatosensory system codes five major sensory modalities:! 1. Discriminative touch! 2. Proprioception (body position and motion)! 3. Nociception (pain and itch)! 4. Temperature!
Thermal detection thresholds of Aδ- and C-fibre afferents activated by brief CO 2 laser pulses applied onto the human hairy skin.
 Thermal detection thresholds of Aδ- and C-fibre afferents activated by brief CO 2 laser pulses applied onto the human hairy skin. Maxim Churyukanov 1,2, Léon Plaghki 1, Valéry Legrain 1,3, André Mouraux
Thermal detection thresholds of Aδ- and C-fibre afferents activated by brief CO 2 laser pulses applied onto the human hairy skin. Maxim Churyukanov 1,2, Léon Plaghki 1, Valéry Legrain 1,3, André Mouraux
SENSORY FUNCTIONS OF THE SKIN OF HUMANS
 SENSORY FUNCTIONS OF THE SKIN OF HUMANS SENSORY FUNCTIONS OF THE SKIN OF HUMANS EDITED BY DAN R. KENSHALO Florida State University Tallahassee, Florida PLENUM PRESS NEW YORK AND LONDON Library of Congress
SENSORY FUNCTIONS OF THE SKIN OF HUMANS SENSORY FUNCTIONS OF THE SKIN OF HUMANS EDITED BY DAN R. KENSHALO Florida State University Tallahassee, Florida PLENUM PRESS NEW YORK AND LONDON Library of Congress
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Overview of Questions
 Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Bi/CNS/NB 150: Neuroscience. November 11, 2015 SOMATOSENSORY SYSTEM. Ralph Adolphs
 Bi/CNS/NB 150: Neuroscience November 11, 2015 SOMATOSENSORY SYSTEM Ralph Adolphs 1 Menu for today Touch -peripheral -central -plasticity Pain 2 Sherrington (1948): senses classified as --teloreceptive
Bi/CNS/NB 150: Neuroscience November 11, 2015 SOMATOSENSORY SYSTEM Ralph Adolphs 1 Menu for today Touch -peripheral -central -plasticity Pain 2 Sherrington (1948): senses classified as --teloreceptive
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D.
 Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS. Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement
 Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement 2 as 20 Experts published and leaders in their respective field 12 month lead in
Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement 2 as 20 Experts published and leaders in their respective field 12 month lead in
A Review of Neuropathic Pain: From Diagnostic Tests to Mechanisms
 DOI 10.1007/s40122-017-0085-2 REVIEW A Review of Neuropathic Pain: From Diagnostic Tests to Mechanisms Andrea Truini Received: September 19, 2017 Ó The Author(s) 2017. This article is an open access publication
DOI 10.1007/s40122-017-0085-2 REVIEW A Review of Neuropathic Pain: From Diagnostic Tests to Mechanisms Andrea Truini Received: September 19, 2017 Ó The Author(s) 2017. This article is an open access publication
EVALUATION AND CHARACTERISATION OF THE THERMAL GRILL APPARATUS FOR SPINAL CORD INJURY PATIENTS
 EVALUATION AND CHARACTERISATION OF THE THERMAL GRILL APPARATUS FOR SPINAL CORD INJURY PATIENTS by Diane Kostka A thesis submitted in conformity with the requirements for the degree of Master of Health
EVALUATION AND CHARACTERISATION OF THE THERMAL GRILL APPARATUS FOR SPINAL CORD INJURY PATIENTS by Diane Kostka A thesis submitted in conformity with the requirements for the degree of Master of Health
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain is always subjective
 Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Human cutaneous C fibres activated by cooling, heating and menthol
 J Physiol 587.23 (2009) pp 5633 5652 5633 Human cutaneous C fibres activated by cooling, heating and menthol M. Campero 1, T. K. Baumann 2,H.Bostock 3 and J. L. Ochoa 4 1 Facultad de Medicina, Clínica
J Physiol 587.23 (2009) pp 5633 5652 5633 Human cutaneous C fibres activated by cooling, heating and menthol M. Campero 1, T. K. Baumann 2,H.Bostock 3 and J. L. Ochoa 4 1 Facultad de Medicina, Clínica
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be?
 Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
Our senses provide us with wonderful capabilities. If you had to lose one, which would it be? Neurological disorders take away sensation without a choice! http://neuroscience.uth.tmc.edu/s2/chapter04.html
PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus. MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive
 PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN MANAGEMENT in the CANINE PATIENT
 PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Chapter 14: The Cutaneous Senses
 Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
Pain and Touch. Academic Press. Edited by Lawrence Kruger. Department of Neurobiology University of California, Los Angeles Los Angeles, California
 Pain and Touch Edited by Lawrence Kruger Department of Neurobiology University of California, Los Angeles Los Angeles, California San Diego New York Sydney Academic Press London Boston Tokyo Toronto Contributors
Pain and Touch Edited by Lawrence Kruger Department of Neurobiology University of California, Los Angeles Los Angeles, California San Diego New York Sydney Academic Press London Boston Tokyo Toronto Contributors
How strong is it? What is it? Where is it? What must sensory systems encode? 9/8/2010. Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
San Francisco Chronicle, June 2001
 PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
SOMATOSENSORY SYSTEMS
 SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
Coding of Sensory Information
 Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Mechanism of Pain Production
 Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
Mechanism of Pain Production Pain conducting nerve fibers are small myelinated (A-delta) or unmyelinated nerve fibers (C-fibers). Cell bodies are in the dorsal root ganglia (DRG) or sensory ganglia of
What it Takes to be a Pain
 What it Takes to be a Pain Pain Pathways and the Neurophysiology of pain Dennis S. Pacl, MD, FACP, FAChPM Austin Palliative Care/ Hospice Austin A Definition of Pain complex constellation of unpleasant
What it Takes to be a Pain Pain Pathways and the Neurophysiology of pain Dennis S. Pacl, MD, FACP, FAChPM Austin Palliative Care/ Hospice Austin A Definition of Pain complex constellation of unpleasant
Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin
 Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin SERGEY G. KHASABOV, 1 DAVID M. CAIN, 2 DINH THONG, 3 PATRICK W. MANTYH, 1 AND DONALD A.
Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin SERGEY G. KHASABOV, 1 DAVID M. CAIN, 2 DINH THONG, 3 PATRICK W. MANTYH, 1 AND DONALD A.
Biomechanics of Pain: Dynamics of the Neuromatrix
 Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article
 CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain Schilder A et
CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain Schilder A et
Sensory information processing, somato-sensory systems
 mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014
 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
SOMATOSENSORY SYSTEMS AND PAIN
 SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
Testing the gate-control theory of pain in man
 Journal of Neurology, Neurosurgery, and Psychiatry, 1974, 37, 1366-1372 Testing the gate-control theory of pain in man P. W. NATHAN' AND P. RUDGE From the National Hospital, Queen Square, London SYNOPSIS
Journal of Neurology, Neurosurgery, and Psychiatry, 1974, 37, 1366-1372 Testing the gate-control theory of pain in man P. W. NATHAN' AND P. RUDGE From the National Hospital, Queen Square, London SYNOPSIS
Posterior White Column-Medial Lemniscal Pathway
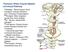 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Pain. Types of Pain. Types of Pain 8/21/2013
 Pain 1 Types of Pain Acute Pain Complex combination of sensory, perceptual, & emotional experiences as a result of a noxious stimulus Mediated by rapidly conducting nerve pathways & associated with increased
Pain 1 Types of Pain Acute Pain Complex combination of sensory, perceptual, & emotional experiences as a result of a noxious stimulus Mediated by rapidly conducting nerve pathways & associated with increased
*Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord.
 *somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
*somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
Sensory Assessment of Regional Analgesia in Humans
 REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
NERVOUS SYSTEM. Academic Resource Center. Forskellen mellem oscillator og krystal
 NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
INTERACTIVE QUESTIONS
 INTERACTIVE QUESTIONS Pathophysiology What is pain? Pathophysiology Does everyone feel pain the same way? Pathophysiology From a practical point of view, how do you classify pain? Pathophysiology What
INTERACTIVE QUESTIONS Pathophysiology What is pain? Pathophysiology Does everyone feel pain the same way? Pathophysiology From a practical point of view, how do you classify pain? Pathophysiology What
Cutaneous thermal thresholds in patients with
 Journal of Neurology, Neurosurgery, and Psychiatry 1991;54:877-881 Department of Clinical Neurophysiology, The National Hospital for Nervous Diseases, London, UK S J M Smith Z Ali C J Fowler Correspondence
Journal of Neurology, Neurosurgery, and Psychiatry 1991;54:877-881 Department of Clinical Neurophysiology, The National Hospital for Nervous Diseases, London, UK S J M Smith Z Ali C J Fowler Correspondence
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Lateral view of human brain! Cortical processing of touch!
 Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile
 Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Psychophysical laws. Legge di Fechner: I=K*log(S/S 0 )
 Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Motor Control, Pain, Somatic Dysfunction, Core Stability. Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA
 Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA How do we move? Decision to move (Executive or Cognitive
Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA How do we move? Decision to move (Executive or Cognitive
What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle.
 What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle. Nociception The detection of tissue damage or impending tissue damage, but There can be tissue damage without
What is pain?: An unpleasant sensation. What is an unpleasant sensation?: Pain. - Aristotle. Nociception The detection of tissue damage or impending tissue damage, but There can be tissue damage without
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
OVERVIEW. Today. Sensory and Motor Neurons. Thursday. Parkinsons Disease. Administra7on. Exam One Bonus Points Slides Online
 OVERVIEW Today Sensory and Motor Neurons Thursday Parkinsons Disease Administra7on Exam One Bonus Points Slides Online 7 major descending motor control pathways from Cerebral Cortex or Brainstem
OVERVIEW Today Sensory and Motor Neurons Thursday Parkinsons Disease Administra7on Exam One Bonus Points Slides Online 7 major descending motor control pathways from Cerebral Cortex or Brainstem
AAEM PRACTICE TOPIC IN ELECTRODIAGNOSTIC MEDICINE
 AAEM PRACTICE TOPIC IN ELECTRODIAGNOSTIC MEDICINE American Association of Electrodiagnostic Medicine 421 First Avenue S.W., Suite 300 East, Rochester, MN 55902 (507/288-0100) ABSTRACT: The development
AAEM PRACTICE TOPIC IN ELECTRODIAGNOSTIC MEDICINE American Association of Electrodiagnostic Medicine 421 First Avenue S.W., Suite 300 East, Rochester, MN 55902 (507/288-0100) ABSTRACT: The development
Spinal Cord Injury Pain. Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018
 Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
1. Tactile sensibility. Use a wisp of cotton-wool or a fine camel-fir brush. If it is desired to test the sensibility or the skin to light touch over
 SENSORY EXAMINATION 1. Tactile sensibility. Use a wisp of cotton-wool or a fine camel-fir brush. If it is desired to test the sensibility or the skin to light touch over a hairy part, it is essential to
SENSORY EXAMINATION 1. Tactile sensibility. Use a wisp of cotton-wool or a fine camel-fir brush. If it is desired to test the sensibility or the skin to light touch over a hairy part, it is essential to
1. Somatosensory Pathways
 1. Somatosensory Pathways Objectives 1. Describe the general characteristics of sensory pathways 2. Understand the general organization and numbered areas of spinal cord gray matter 3. Understand dermatomes
1. Somatosensory Pathways Objectives 1. Describe the general characteristics of sensory pathways 2. Understand the general organization and numbered areas of spinal cord gray matter 3. Understand dermatomes
OBSERVATIONS ON THE REACTION TIME TO CUTANEOUS
 J. Neurol. Neurosurg. Psychiat., 1955, 18, 120. OBSERVATIONS ON THE REACTION TIME TO CUTANEOUS THERMAL STIMULI BY P. P. LELE* and D. C. SINCLAIR From the Department of Anatomy, University of Oxford The
J. Neurol. Neurosurg. Psychiat., 1955, 18, 120. OBSERVATIONS ON THE REACTION TIME TO CUTANEOUS THERMAL STIMULI BY P. P. LELE* and D. C. SINCLAIR From the Department of Anatomy, University of Oxford The
راما ندى أسامة الخضر. Faisal Muhammad
 22 راما ندى أسامة الخضر Faisal Muhammad Revision Last time we started talking about sensory receptors, we defined them and talked about the mechanism of their reaction. Now we will talk about sensory receptors,
22 راما ندى أسامة الخضر Faisal Muhammad Revision Last time we started talking about sensory receptors, we defined them and talked about the mechanism of their reaction. Now we will talk about sensory receptors,
Sensory conduction of the sural nerve in polyneuropathy'
 Jourtial of Neurology, Neurosurgery, anid Psychiatry, 1974, 37, 647-652 Sensory conduction of the sural nerve in polyneuropathy' DAVID BURKE, NEVELL F. SKUSE, AND A. KEITH LETHLEAN From the Unit of Clinical
Jourtial of Neurology, Neurosurgery, anid Psychiatry, 1974, 37, 647-652 Sensory conduction of the sural nerve in polyneuropathy' DAVID BURKE, NEVELL F. SKUSE, AND A. KEITH LETHLEAN From the Unit of Clinical
Somatosensory System. Steven McLoon Department of Neuroscience University of Minnesota
 Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
MANAGEMENT OF CRPS. Brachial Neuralgia n = 81 patients. CRPS type II n = 126 patients CONTENTS. Neuropathic Pain. Introduction.
 MANAGEMENT OF CRPS Paradoxical Hypoaesthetic Skin, Painful to Touch: A Target to relieve Neuropathic Pain Spicher, C.J. et al. (2010) Paris: Sauramps medical Paradoxical Hypoaesthetic Skin, Painful to
MANAGEMENT OF CRPS Paradoxical Hypoaesthetic Skin, Painful to Touch: A Target to relieve Neuropathic Pain Spicher, C.J. et al. (2010) Paris: Sauramps medical Paradoxical Hypoaesthetic Skin, Painful to
Peripheral Nervous System
 Peripheral Nervous System 1 Sensory Receptors Sensory Receptors and Sensation Respond to changes (stimuli) in the environment Generate graded potentials that can trigger an action potential that is carried
Peripheral Nervous System 1 Sensory Receptors Sensory Receptors and Sensation Respond to changes (stimuli) in the environment Generate graded potentials that can trigger an action potential that is carried
Superficial Cold And Heat Application During Transcutaneous Electrical Stimulation May Not Change Perceived Sensation Of Pain
 ISPUB.COM The Internet Journal of Pain, Symptom Control and Palliative Care Volume 9 Number 1 Superficial Cold And Heat Application During Transcutaneous Electrical Stimulation May Not Change H Jelinek,
ISPUB.COM The Internet Journal of Pain, Symptom Control and Palliative Care Volume 9 Number 1 Superficial Cold And Heat Application During Transcutaneous Electrical Stimulation May Not Change H Jelinek,
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
Anesthesiology, V 92, No 3, Mar 2000
 691 Anesthesiology 2000; 92:691 8 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Heterogenous Patterns of Sensory Dysfunction in Postherpetic Neuralgia Suggest Multiple
691 Anesthesiology 2000; 92:691 8 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Heterogenous Patterns of Sensory Dysfunction in Postherpetic Neuralgia Suggest Multiple
Effects of morphine on the experimental illusion of pain produced by a thermal grill.
 Effects of morphine on the experimental illusion of pain produced by a thermal grill. Delphine Kern, Frédéric Plantevin, Didier Bouhassira To cite this version: Delphine Kern, Frédéric Plantevin, Didier
Effects of morphine on the experimental illusion of pain produced by a thermal grill. Delphine Kern, Frédéric Plantevin, Didier Bouhassira To cite this version: Delphine Kern, Frédéric Plantevin, Didier
By the end of this lecture the students will be able to:
 UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
PAIN MODULATION. numerical value. adjectives. DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH
 PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
Thalamo-Cortical Pain Mechanisms Prof. Fred A. Lenz
 Thalamo-Cortical Pain Mechanisms Hopkins Hospital, Baltimore, USA F.H. Baker, D.S. Borett, T. Chase, D. Chau, H. Chen, R. Coghill, N.E. Crone, R.G Cutler, M.R. DeLong, P.M. Dougherty, J. Dostrovsky, I.M.
Thalamo-Cortical Pain Mechanisms Hopkins Hospital, Baltimore, USA F.H. Baker, D.S. Borett, T. Chase, D. Chau, H. Chen, R. Coghill, N.E. Crone, R.G Cutler, M.R. DeLong, P.M. Dougherty, J. Dostrovsky, I.M.
-12. -Osama Asa ad Khader. -Ameera Almomani. - Loay Zgoul
 -12 -Osama Asa ad Khader -Ameera Almomani - Loay Zgoul In this lecture we are going to talk about dermatomes, visceral sensation and pain disorders. Sorry for any mistakes though I tried my best to include
-12 -Osama Asa ad Khader -Ameera Almomani - Loay Zgoul In this lecture we are going to talk about dermatomes, visceral sensation and pain disorders. Sorry for any mistakes though I tried my best to include
Signal. Differential Ability of Human Cutaneous Nociceptors Mechanical Pain and to Produce Vasodilatation
 The Journal of Neuroscience, March 1994, 14(3): 1756-1765 Differential Ability of Human Cutaneous Nociceptors Mechanical Pain and to Produce Vasodilatation to Signal Martin Koltzenburg and Hermann 0. HandwerkeP
The Journal of Neuroscience, March 1994, 14(3): 1756-1765 Differential Ability of Human Cutaneous Nociceptors Mechanical Pain and to Produce Vasodilatation to Signal Martin Koltzenburg and Hermann 0. HandwerkeP
Center for Sensory-Motor Interaction (SMI), Laboratory for Musculoskeletal Pain and Motor Control, Aalborg University, Denmark
 14RC2 Assessment and mechanisms of musculo-skeletal pain Thomas Graven-Nielsen, Lars Arendt-Nielsen Center for Sensory-Motor Interaction (SMI), Laboratory for Musculoskeletal Pain and Motor Control, Aalborg
14RC2 Assessment and mechanisms of musculo-skeletal pain Thomas Graven-Nielsen, Lars Arendt-Nielsen Center for Sensory-Motor Interaction (SMI), Laboratory for Musculoskeletal Pain and Motor Control, Aalborg
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Mechanosensation. Central Representation of Touch. Wilder Penfield. Somatotopic Organization
 Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
Analysis of Paradoxical Phenomenon in Thermal Sensation
 Analysis of Paradoxical Phenomenon in Thermal Sensation Keisuke Arai arai@rm.is.ritsumei.ac.jp Satoshi Hashiguchi hasiguti@rm.is.ritsumei.ac.jp Fumihisa Shibata fshibata@is.ritsumei.ac.jp Asako Kimura
Analysis of Paradoxical Phenomenon in Thermal Sensation Keisuke Arai arai@rm.is.ritsumei.ac.jp Satoshi Hashiguchi hasiguti@rm.is.ritsumei.ac.jp Fumihisa Shibata fshibata@is.ritsumei.ac.jp Asako Kimura
Conditioned Pain Modulation Induced by Non-noxious Thermal Conditioning Stimulus
 34 J Meikai Dent Med 461, 34 39, 2017 conditioned pain modulation Conditioned pain modulationcpmconditioning stimulus : CS CS CPM CPM CS pressure pain threshold : PPT3 CS CS 12 PPT CPM 22.547.9CPM P0.01
34 J Meikai Dent Med 461, 34 39, 2017 conditioned pain modulation Conditioned pain modulationcpmconditioning stimulus : CS CS CPM CPM CS pressure pain threshold : PPT3 CS CS 12 PPT CPM 22.547.9CPM P0.01
RESPONSE VARIABILITY TO REPEATED MECHANICAL STIMULATION OF THE SKIN IN THE DORSAL COLUMN SYSTEM
 ACTA NEUROBIOL. EXP. 1986, 46: 179-186 RESPONSE VARIABILITY TO REPEATED MECHANICAL STIMULATION OF THE SKIN IN THE DORSAL COLUMN SYSTEM Antti PERTOVAARA, Timo TUKEVA and Timo HUOPANIEMI Department of Physiology,
ACTA NEUROBIOL. EXP. 1986, 46: 179-186 RESPONSE VARIABILITY TO REPEATED MECHANICAL STIMULATION OF THE SKIN IN THE DORSAL COLUMN SYSTEM Antti PERTOVAARA, Timo TUKEVA and Timo HUOPANIEMI Department of Physiology,
Basic Neuroscience. Sally Curtis
 The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
The Physiology of Pain Basic Neuroscience Sally Curtis sac3@soton.ac.uk The behaviour of humans is a result of the actions of nerves. Nerves form the basis of Thoughts, sensations and actions both reflex
Reasons for thinking so, according to von Frey:
 Reasons for thinking so, according to von Frey: The experiment consisted of sticking a pig bristle against the eye. Even with fairly light contact, the bristle was painful...... and a warm and cold bristle
Reasons for thinking so, according to von Frey: The experiment consisted of sticking a pig bristle against the eye. Even with fairly light contact, the bristle was painful...... and a warm and cold bristle
Neuropathic pain, pain matrix dysfunction, and pain syndromes
 Neuropathic pain, pain matrix dysfunction, and pain syndromes MSTN121 - Neurophysiology Session 3 Department of Myotherapy Session objectives Describe the mechanism of nociceptive chronic pain. Define
Neuropathic pain, pain matrix dysfunction, and pain syndromes MSTN121 - Neurophysiology Session 3 Department of Myotherapy Session objectives Describe the mechanism of nociceptive chronic pain. Define
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System
 The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
MYOFASCIAL PAIN. Dr. Janet Travell ( ) credited with bringing MTrPs to the attention of healthcare providers.
 Myofascial Trigger Points background info Laurie Edge-Hughes BScPT, MAnimSt (Animal Physio), CAFCI, CCRT History lesson Dr. Janet Travell (1901 1997) credited with bringing MTrPs to the attention of healthcare
Myofascial Trigger Points background info Laurie Edge-Hughes BScPT, MAnimSt (Animal Physio), CAFCI, CCRT History lesson Dr. Janet Travell (1901 1997) credited with bringing MTrPs to the attention of healthcare
Unmyelinated tactile afferents signal touch and project to insular cortex
 Unmyelinated tactile afferents signal touch and project to insular cortex H. Olausson 1, Y. Lamarre 2, H. Backlund 1,3, C. Morin 4, B.G. Wallin 1, G. Starck 5, S. Ekholm 6, I. Strigo 4, K. Worsley 7, Å.B.
Unmyelinated tactile afferents signal touch and project to insular cortex H. Olausson 1, Y. Lamarre 2, H. Backlund 1,3, C. Morin 4, B.G. Wallin 1, G. Starck 5, S. Ekholm 6, I. Strigo 4, K. Worsley 7, Å.B.
UNDERSTANDING AND DEALING WITH PAIN. Lorimer Moseley Talk. Disclosure. Patient C.C. Patient C.C. Goals 06/12/2017
 UNDERSTANDING AND DEALING WITH PAIN DAVID V. SMITH MD CHKD SPORTS MEDICINE Lorimer Moseley Talk https://www.youtube.com/watch?v=gwdwldihjs&feature=youtu.be ALEXANDRA LARAMEE, LCSW CHKD BEHAVIORAL HEALTH
UNDERSTANDING AND DEALING WITH PAIN DAVID V. SMITH MD CHKD SPORTS MEDICINE Lorimer Moseley Talk https://www.youtube.com/watch?v=gwdwldihjs&feature=youtu.be ALEXANDRA LARAMEE, LCSW CHKD BEHAVIORAL HEALTH
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
