Four-Year Efficacy of RTS,S/AS01E and Its Interaction with Malaria Exposure
|
|
|
- Jacob Lamb
- 6 years ago
- Views:
Transcription
1 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Four- Efficacy of RTS,S/AS1E and Its Interaction with Malaria Exposure Ally Olotu, M.B., Ch.B., Gregory Fegan, Ph.D., Juliana Wambua, B.Sc., George Nyangweso, B.Sc., Ken O. Awuondo, H.N.D., Amanda Leach, M.R.C.P.C.H., Marc Lievens, M.Sc., Didier Leboulleux, M.D., Patricia Njuguna, M.B., Ch.B., Norbert Peshu, M.B., Ch.B., Kevin Marsh, F.R.C.P., and Philip Bejon, Ph.D. A BS TR AC T BACKGROUND The candidate malaria vaccine RTS,S/AS1E has entered phase 3 trials, but data on long-term outcomes are limited. METHODS For 4 years, we followed children who had been randomly assigned, at 5 to 17 months of age, to receive three doses of RTS,S/AS1E vaccine (223 children) or rabies vaccine (224 controls). The end point was clinical malaria (temperature of 37.5 C and Plasmodium falciparum parasitemia density of >25 parasites per cubic millimeter). Each child s exposure to malaria was estimated with the use of the distance-weighted local prevalence of malaria. RESULTS Over a period of 4 years, 118 of 223 children who received the RTS,S/AS1E vaccine and 138 of 224 of the controls had at least 1 episode of clinical malaria. Vaccine efficacies in the intention-to-treat and per-protocol analyses were 29.9% (95% confidence interval [CI], 1.3 to 45.3; P =.5) and 32.1% (95% CI, 11.6 to 47.8; P =.4), respectively, calculated by Cox regression. Multiple episodes were common, with 551 and 618 malarial episodes in the RTS,S/AS1E and control groups, respectively; vaccine efficacies in the intention-to-treat and per-protocol analyses were 16.8% (95% CI, 8.6 to 36.3; P =.18) and 24.3% (95% CI, 1.9 to 41.6; P =.4), respectively, calculated by the Andersen Gill extension of the Cox model. For every vaccinated children, 65 cases of clinical malaria were averted. Vaccine efficacy declined over time (P =.4) and with increasing exposure to malaria (P =.1) in the per-protocol analysis. Vaccine efficacy was 43.6% (95% CI, 15.5 to 62.3) in the first year but was.4% (95% CI, 32.1 to 45.3) in the fourth year. Among children with a malaria-exposure index that was average or lower than average, the vaccine efficacy was 45.1% (95% CI, 11.3 to 66.), but among children with a malaria-exposure index that was higher than average it was 15.9% (95% CI, 11. to 36.4). From the Centre for Geographic Medicine Research, Kenya Medical Research Institute (KEMRI) Wellcome Trust Research Programme, Kilifi, Kenya (A.O., G.F., J.W., G.N., K.O.A., P.N., N.P., K.M., P.B.); the Centre for Clinical Vaccinology and Tropical Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom (G.F., K.M., P.B.); GlaxoSmithKline Biologicals, Wavre, Belgium (A.L., M.L.); and the Program for Appropriate Technology in Health (PATH) Malaria Vaccine Initiative, Bethesda, MD (D.L.). Address reprint requests to Dr. Olotu at the KEMRI Wellcome Trust Research Programme, P.O. Box 23, Kilifi, Kenya, or at aolotu@kemri-wellcome.org. N Engl J Med 213;368: DOI: 1.156/NEJMoa Copyright 213 Massachusetts Medical Society. CONCLUSIONS The efficacy of RTS,S/AS1E vaccine over the 4-year period was 16.8%. Efficacy declined over time and with increasing malaria exposure. (Funded by the PATH Malaria Vaccine Initiative and Wellcome Trust; ClinicalTrials.gov number, NCT ) n engl j med 368;12 nejm.org march 21,
2 T h e n e w e ngl a nd j o u r na l o f m e dic i n e Malaria remains an important cause of illness and death among children in sub-saharan Africa. 1 RTS,S is the most advanced candidate malaria vaccine and has entered phase 3 trials. 2 The variation in vaccine efficacy over time will be critical to public health policy decisions concerning the introduction of the vaccine. We previously conducted a phase 2 proof-of-concept trial of RTS,S/AS1E in Kilifi, Kenya, and Korogwe, Tanzania, to evaluate its safety and efficacy against episodes of Plasmodium falciparum malaria in children 5 to 17 months old. 3 At the end of the double-blind phase (mean duration of follow-up, 7.9 months), efficacy against the first malarial episode was 53% (95% confidence interval [CI], 28 to 69; P<.1), 3 and at 15 months, it was 46% (95% CI, 24 to 61; P<.1). 4 Here we present data on efficacy after 4 years of follow-up in Kilifi, Kenya. Reductions in estimated vaccine efficacy over time may reflect a waning of the vaccine-induced protective immune responses to sporozoites, delayed acquisition of natural immunity to bloodstage parasites in the RTS,S/AS1E group because of reduced exposure to blood-stage parasites in the presence of vaccine-induced immunity to sporozoites, or an artifact in survival analysis caused by microheterogeneity in malaria exposure within the cohort. 5 To adjust for variations in malaria exposure within our cohort, we used the distance-weighted local prevalence of malaria as a marker of a person s exposure to malaria, which we refer to as the malaria exposure index. 6 This exposure index is a relative measure of the intensity of malaria exposure and is distinct from absolute measures, such as the prevalence of asymptomatic parasitemia or the entomologic inoculation rate. These absolute measures are frequently used to assess exposure at the population level, but using them to assess individual exposure would be very labor-intensive. ME THODS STUDY DESIGN The details of the study design have been described previously 3,4 and are provided in the Supplementary Appendix and the study protocol (including the statistical analysis plan), which are available with the full text of this article at NEJM.org. The original randomized, controlled trial was conducted in Kilifi, Kenya, and in Korogwe, Tanzania. Here we present the Kilifi data from randomization to 48 months after the third dose was administered. The original study was sponsored by GlaxoSmithKline Biologicals, which monitored the trial and managed the database for the first 12 months of follow-up. Extended follow-up after 12 months was led by the investigators and sponsored by the Kenya Medical Research Institute Wellcome Trust Research Programme. GlaxoSmithKline Biologicals was responsible for reporting safety events to the regulatory authorities. For details of the roles of the sponsors and investigators, see the Supplementary Appendix. PARTICIPANTS Children who underwent randomization in the initial study in Kilifi 3 were eligible for enrollment in this extended follow-up study. The original study and its extensions were approved by the Kenya Medical Research Institute National Ethics Review Committee, the Western Institutional Review Board, and the Oxford Tropical Research Ethics Committee. Written informed consent for the extension study was obtained from the parents or guardians of all the children with the use of approved consent forms provided in Swahili or Giriama. Nonliterate parents indicated consent by using a thumbprint, and a signature was obtained from a literate witness. STUDY PROCEDURES Details of the study procedures are described in the Supplementary Appendix. In brief, we used active and passive surveillance methods to identify cases of clinical malaria. We collected blood samples at specified time points to look for antibodies to P. falciparum circumsporozoite repeat region (anti-circumsporozoite antibodies) and asymptomatic parasitemia. MALARIA EXPOSURE The malaria exposure index was calculated as the distance-weighted proportion of asymptomatic or symptomatic cases of malaria within a 1-km radius of each child over a 6-month interval, with the use of data from 87 children under active surveillance in the same study area as the vaccinated cohort. 6 Children were categorized as having lower or higher exposure according to whether they were at or below the cohort mean or above the cohort mean, respectively (see the Supplementary Appendix for details) n engl j med 368;12 nejm.org march 21, 213
3 Four- Efficacy of RTS,S/AS1E STATISTICAL ANALYSIS The end point was clinical malaria (temperature of 37.5 C and P. falciparum parasitemia of more than 25 parasites per cubic millimeter). The intention-to-treat cohort included all children who had undergone randomization and received at least one dose of vaccine. The per-protocol cohort included children who had received three doses of vaccine according to the study protocol and for whom surveillance data were available from 2 weeks after the third dose was administered. Data from each participant were censored at 4 years of follow-up. The sample was limited by the original number of children who had undergone randomization in Kilifi. On the basis of the observed cumulative incidence of clinical malaria of 6% over a period of 4 years, the study had 85% power to detect a vaccine efficacy of 3% at the 5% significance level. Cox proportional-hazard models were used for the analysis of first malarial episodes. Multiple episodes were analyzed by means of negative binomial regression, with clustering to adjust for repeated measures, and by means of the Andersen Gill extension of the Cox regression model for multiple-event analysis. 7 Vaccine efficacy was defined as 1 minus the hazard ratio or the incidence-rate ratio. Waning of vaccine efficacy was assessed by means of time-dependent interactions between the logarithm of failure time (or year of followup) and RTS,S/AS1E vaccination. Plots of adjusted vaccine efficacy over time were produced from the regression coefficients in the Cox and Andersen Gill regression models. The reduction in the incidence of malaria that was attributable to the vaccine was calculated as the difference between the incidence of malaria in the control group and the incidence in the RTS,S/AS1E group in the intention-to-treat cohort and was expressed as the number of cases averted per children per year of follow-up. The cumulative number of averted cases was calculated by summing the number of averted cases for each year. Between-group differences in the prevalence of asymptomatic P. falciparum parasitemia were assessed with the use of Fisher s exact test. We calculated imputed weekly anti-circumsporozoite antibody titers by using a fractional polynomial regression model of time and crosssectional anti-circumsporozoite antibody titers. The Cox regression model with spline functions was used to assess the relation between imputed anti-circumsporozoite antibodies as a time-varying covariate and protection against malaria. Data were analyzed with the use of Stata software, version 12. (StataCorp). For details of the statistical analysis, see the Supplementary Appendix. R ESULT S STUDY PARTICIPANTS Of the 447 children eligible for randomization in Kilifi, 223 were randomly assigned to receive the RTS,S/AS1E vaccine and 224 to receive the rabies vaccine. A total of 32 children (72%) completed 4 years of follow-up (Fig. S1 in the Supplementary Appendix). A total of 415 children (29 children in the RTS,S/AS1E group and 26 in the control group) received all three planned doses of vaccine according to the study protocol and were included in the per-protocol analysis. Baseline characteristics were similar in the two groups. The median duration of follow-up was 47.5 months (47.7 months in the RTS,S/AS1E group and 47.1 months in the control group), with no significant difference between the two groups (Table 1). EFFICACY AGAINST THE FIRST OR ONLY MALARIA EPISODE In the intention-to-treat cohort, 118 first or only episodes of clinical malaria meeting the primary case definition were documented in the RTS,S/ AS1E group, as compared with 138 episodes in the control group, for an unadjusted efficacy of 29.9% (95% CI, 1.3 to 45.3; P =.5) by Cox regression. In the per-protocol cohort, 111 and 13 first or only episodes of clinical malaria were documented in the RTS,S/AS1E group and the control group, respectively, for an adjusted vaccine efficacy of 32.1% (95% CI, 11.6 to 47.8; P =.4) by Cox regression (Table 2). The time-dependent Cox regression model showed weak evidence of nonproportionality of the hazard associated with the RTS,S/AS1E vaccine as compared with the rabies vaccine (hazard ratio, 1.33; 95% CI,.98 to 1.81; P =.7). A plot of adjusted vaccine efficacy over time showed a nonsignificant waning of efficacy (Fig. 1). EFFICACY AGAINST ALL EPISODES We used two different analyses to examine efficacy against all malarial episodes, both of which allowed for possible variations in efficacy and the n engl j med 368;12 nejm.org march 21,
4 T h e n e w e ngl a nd j o u r na l o f m e dic i n e Table 1. Demographic and Clinical Characteristics at Baseline and Follow-up in the Per-Protocol Cohort.* Characteristic RTSS/AS1E Vaccine (N = 29) Rabies Vaccine (N = 26) Total (N = 415) Follow-up time mo Median th 95th percentile Age at vaccination mo Median 1.± ± ±3.5 Range Sex no. (%) Female 16 (5.7) 12 (49.5) 28 (5.1) Male 13 (49.3) 14 (5.5) 27 (49.9) Geographic area no. (%) 1 59 (28.2) 44 (21.4) 13 (24.8) 2 48 (23.) 54 (26.2) 12 (24.6) 3 5 (23.9) 56 (27.2) 16 (25.5) 4 52 (24.9) 52 (25.2) 14 (25.1) Distance to dispensary no. (%) <5 km 154 (73.7) 144 (69.9) 298 (71.8) 5 1 km 55 (26.3) 62 (3.1) 117 (28.2) Exposure index Median.37±.21.37±.24.37±.23 Unknown no. (%) 7 (3.3) 3 (1.5) 1 (2.4) Bed-net use no./total no. (%) 1 154/24 (75.5) 148/199 (74.4) 32/43 (74.9) 2 94/23 (46.3) 89/189 (47.1) 183/392 (46.7) 3 47/174 (27.) 34/145 (23.4) 81/319 (25.4) 4 46/156 (29.5) 37/139 (26.6) 83/295 (28.1) * Plus minus values are medians ±SD. There were no significant between-group differences. Geographic area 1 corresponded to Bodoi, Bomani, and Junju; area 2 to Gongoni, Kolewa, Mapawa, and Mwembetsungu; area 3 to Chodari, Kadzinuni, Kapecha, and Pingilikani; and area 4 to Bokini, Dindiri, Makata, and Ng ombeni. The exposure index is the distance-weighted local prevalence of malaria and was calculated for each participant as the mean exposure over the entire follow-up period. Bed-net use was a time-varying covariate in the statistical models. malaria exposure index over time: a modified Coxregression model (namely, the Andersen Gill model) and a negative binomial regression model, which fitted the data significantly better than the Poisson model (chi-square = with 1 df by the likelihood-ratio test of overdispersion, P<.1) (Fig. S2 in the Supplementary Appendix). In the intention-to-treat cohort, we recorded 551 and 618 episodes of clinical malaria among 223 children in the RTS,S/AS1E group and among 224 in the control group, respectively. On the basis of these numbers, the unadjusted efficacy against multiple episodes was 16.8% (95% CI, 8.6 to 36.3; P =.18) by the Andersen Gill model and 18.6% (95% CI, 7.2 to 38.3; P =.14) by the negative binomial regression model. In the per-protocol cohort, there were 475 and 518 episodes of clinical malaria among 29 children in the RTS,S/AS1E group and among 26 in the control group, respectively. On the basis of these numbers, the adjusted vaccine efficacy against all episodes was 24.3% (95% CI, 1.9 to 41.6; P =.4) by the Andersen Gill model and 23.5% (95% CI,.7 to 41.9; P =.6) by the negative binomial regression model (Table 2). Vaccine efficacy was lower at later time points, 1114 n engl j med 368;12 nejm.org march 21, 213
5 Four- Efficacy of RTS,S/AS1E Table 2. Efficacy of RTS,S/AS1E Vaccine against Episodes of Plasmodium falciparum Clinical Malaria.* Variable RTS,S/AS1E Vaccine Rabies Vaccine Efficacy (95% CI) P Value No. of Participants No. of Events Person-yr at Risk Event Rate No. of Participants No. of Events Person-yr at Risk Intention-to-treat cohort First or only episode, Cox regression >25 parasites/mm (1.3 to 45.3).5 > parasites/mm (6.8 to 42.3).1 All episodes, Andersen Gill Cox regression >25 parasites/mm ( 8.6 to 36.3).18 > parasites/mm ( 8.8 to 36.2).18 All episodes, negative binomial regression >25 parasites/mm ( 7.2 to 38.3).14 > parasites/mm ( 6.8 to 36.9).14 Per-protocol cohort First or only episode, Cox regression >25 parasites/mm (11.6 to 47.8).4 > parasites/mm (15 to 48.7).1 All episodes, Andersen Gill Cox regression >25 parasites/mm (1.9 to 41.6).4 > parasites/mm (1.6 to 41.5).4 All episodes, negative binomial regression >25 parasites/mm (.7 to 41.9).6 > parasites/mm (.9 to 41.3).4 Event Rate * Vaccine efficacy was defined as 1 minus the hazard ratio or 1 minus the incidence rate ratio. Efficacy was adjusted for age, bed-net use, geographic area, malaria exposure index, and distance to dispensary in the classic Cox model. In the negative binomial and Andersen Gill Cox models, no adjustment was made for the malaria exposure index because of its interaction effect with vaccine efficacy. Efficacy estimates were unadjusted in the intention-to-treat analysis. n engl j med 368;12 nejm.org march 21,
6 T h e n e w e ngl a nd j o u r na l o f m e dic i n e A Entire Cohort Kaplan Meier Plot Efficacy of RTS,S/AS1E against First or Only Episode Efficacy of RTS,S/AS1E against All Episodes 1. Cumulative Incidence RTS,S/AS1E vaccine P=.2 by log-rank test B Cohort with Low Exposure Index Kaplan Meier Plot Efficacy of RTS,S/AS1E against First or Only Episode Efficacy of RTS,S/AS1E against All Episodes 1. Cumulative Incidence RTS,S/AS1E vaccine P=.2 by log-rank test C Cohort with High Exposure Index Kaplan Meier Plot Efficacy of RTS,S/AS1E against First or Only Episode Efficacy of RTS,S/AS1E against All Episodes Cumulative Incidence RTS,S/AS1E vaccine P=.1 by log-rank test Figure 1. Kaplan Meier Curves and Vaccine Efficacy over Time in the Per-Protocol Cohort. Kaplan Meier plots of the cumulative incidence of malaria and corresponding vaccine efficacy over time are shown for the entire cohort (Panel A) and for the cohorts with low and high malaria exposure indexes (Panels B and C, respectively). Clinical falciparum malaria was defined as the presence of fever (temperature 37.5 C) and a Plasmodium falciparum density of more than 25 parasites per cubic millimeter. A log (time) interaction model was used to produce the fit for the vaccine-efficacy plots. In these plots, the solid line indicates the point estimates of efficacy and the dotted lines indicate 95% confidence intervals. Vaccine efficacy was truncated at % as the lower limit; hence, the lower limit of the confidence interval is visible only at the start of monitoring. The lower limit of the 95% confidence interval for vaccine efficacy against the first or only episode in the cohort with a low exposure index is not visible because it is below and has been truncated. as indicated by significant interaction between vaccine efficacy and follow-up time (hazard ratio for malaria in the RTS,S/AS1E group as compared with the control group, 1.28; 95% CI, 1.8 to 1.51; P =.4 by the Andersen Gill model; incidence-rate ratio in year 4, 1.65; 95% CI, 1.3 to 2.63; P =.4 by the negative binomial regression model) (Table S1 in the Supplementary Appendix). Efficacy was lower among children with a high malaria exposure index than among those 1116 n engl j med 368;12 nejm.org march 21, 213
7 Four- Efficacy of RTS,S/AS1E with a low malaria exposure index, as shown by the interaction between vaccination group and the malaria-exposure index (hazard ratio in the RTS,S/AS1E group, 5.17; 95% CI, 1.98 to 13.47; P =.1 by the Andersen Gill model; incidencerate ratio, 2.48; 95% CI, 1.18 to 5.21; P =.2 by the negative binomial regression model). Over time, the effect of malaria exposure on the risk of clinical malaria declined, as indicated by the interaction between the malaria-exposure index and follow-up time (hazard ratio in the RTS,S/AS1E group,.71; 95% CI,.51 to.98; P =.4 by the Andersen Gill model; incidencerate ratio in year 3,.35; 95% CI,.15 to.82; P =.2; and incidence-rate ratio in year 4,.35; 95% CI,.16 to.79; P =.1 by the negative binomial regression model). There was no interaction between bed-net use and vaccination group (hazard ratio for malaria in the RTS,S/AS1E group,.95; 95% CI,.64 to 1.4; P =.78). On the basis of the negative binomial regression model, stratified efficacy estimates according to year of follow-up were 46.2% (95% CI, 21.2 to 63.4; P =.1) for year 1, 24.7% (95% CI, 19.1 to 52.3; P =.23) for year 2, 22.% (95% CI, 17. to 48.; P =.23) for year 3, and 1.2% (95% CI, 46.8 to 31.2; P =.95) for year 4 (Table S2 in the Supplementary Appendix). Vaccine efficacy was 45.1% (95% CI, 11.3 to 66.) among children with a malaria exposure index that was average or lower than average but 15.9% (95% CI, 11. to 36.4) among children with a malaria exposure index that was higher than average (Table S2 in the Supplementary Appendix). On the basis of the Andersen Gill model, vaccine efficacy was 43.6% (95% CI, 15.5 to 62.3) in year 1 and.4% (95% CI, 32.1 to 45.3) in year 4 after vaccination (Fig. 1). The decline in estimated vaccine efficacy was more rapid among children with a higher malaria exposure index than among those with a lower malaria exposure index (Fig. 1), although terms for three-way interactions among vaccination group, malariaexposure index, and time (to determine whether vaccine efficacy declined more rapidly at high exposure than at low exposure) were not significant (hazard ratio for malaria in the RTS,S/AS1E group and low malaria exposure index, 1.56; 95% CI,.81 to 2.99; P =.18 by the Andersen Gill model; incidence-rate ratio, 1.28; 95% CI,.76 to 2.14; P =.35 by the negative binomial regression model). CLINICAL MALARIA EPISODES AVERTED The incidence of clinical malaria episodes increased in both groups during follow-up (Fig. 2A, 2B, and 2C). In the intention-to-treat cohort, the estimated numbers of malaria cases per children that were averted in the 4 successive years of follow-up were 26, 22, 18, and 1, respectively, for a total of 65 cases averted over a period of 4 years (Fig. 2D). The numbers of cases averted over the 4-year follow-up period among children with a low malaria exposure index and those with a high malaria exposure index were 62 and 78 cases per children, respectively (Fig. 2D). CROSS-SECTIONAL SURVEY ANALYSIS The prevalence of asymptomatic P. falciparum parasitemia was significantly lower among children in the RTS,S/AS1E group than among those in the control group at all cross-sectional surveys except at 8, 25, and 49 months (9% vs. 24% at 12 months, P =.5; 1% vs. 6% at 15 months, P =.3; and 9% vs. 2% at 38 months, P =.8); the prevalence was similar in the two groups at 8 months (1% and 4%; P =.6), 25 months (4% and 7%; P =.21), and 49 months (7% and 5%, respectively; P =.47 (Table S3 in the Supplementary Appendix). There were no significant between-group differences in the mean hemoglobin concentration. ANTI-CIRCUMSPOROZOITE ANTIBODY TITERS AND PROTECTION Antibody titers waned over time but remained significantly higher in the RTS,S/AS1E group than in the control group throughout the 4 years (Fig. 3A), with geometric mean titers of 17.2 enzymelinked immunosorbent assay units (EU) per milliliter versus 2.1 EU per milliliter (P =.1) at 38 months after the final vaccination. There was no significant difference in the geometric mean peak titers according to the malaria exposure index (P =.66). As previously described, 4 we found a nonlinear association between the imputed anticircumsporozoite antibody titer and protection from clinical malaria when we included data from the full 4-year follow-up period (Fig. 3B). DISCUSSION During 4 years of follow-up, RTS,S/AS1E was associated with 29.9% and 16.8% efficacy against first and all episodes of P. falciparum clinical ma- n engl j med 368;12 nejm.org march 21,
8 T h e n e w e ngl a nd j o u r na l o f m e dic i n e A Entire Cohort 1. RTS,S/AS1E vaccine B Cohort with High Exposure Index RTS,S/AS1E vaccine 1.5 Incidence (no. of episodes/person-yr) Incidence (no. of episodes/person-yr) C Cohort with Low Exposure Index RTS,S/AS1E vaccine.6 D Malaria Cases Averted Incidence (no. of episodes/person-yr).4.2 Cumulative No. of Cases Cohort with high exposure index Entire cohort Cohort with low exposure index Figure 2. Incidence of Malaria in the Entire Cohort and According to Exposure Level, and Vaccine-Attributable Reduction in Cases of Malaria in the Intention-to-Treat Cohort. Panel A shows the incidence of malaria according to year of follow-up in the entire cohort, Panel B shows the incidence in the cohort with a high exposure index, and Panel C shows the incidence in the cohort with a low exposure index. Panel D shows the cumulative number of malaria cases averted in the entire cohort and in the high-exposure and low-exposure cohorts. laria, respectively, among children vaccinated at 5 to 17 months of age in a country where malaria is endemic. Both negative binomial regression and Andersen Gill models showed significant variations in vaccine efficacy over time and according to the level of malaria exposure (as measured by means of our malaria exposure index). The efficacy estimate during follow-up is surrounded by considerable uncertainty. On the one hand, the estimate suggests possible sustained efficacy during 4 years of follow-up. On the other hand, the significant interaction between time and vaccine efficacy argues against the hypothesis that efficacy is constant over time. Furthermore, the upper limits of the confidence intervals for efficacy in year 4 exclude an efficacy of more than 31% overall and an efficacy of more than 15% among children with a high exposure index. The waning of vaccine efficacy observed with our data is unlikely to be the consequence of heterogeneity in malaria exposure, because reductions in efficacy over time were similar whether first or all episodes were analyzed and also because they persisted even after the exposure index was included in the model. We identified significant interactions between time since vaccination and vaccine efficacy and between level of exposure to malaria and vaccine efficacy. These interactions indicate how vaccine efficacy varies in the presence of other covariates. For instance, in the negative binomial regression model, the theoretical efficacy of vaccination at an exposure index of and in the first year is given by an incidence-rate ratio of.37 (i.e., 63% efficacy). As the exposure index rises to 1, vaccine efficacy can be calculated by multiplying the incidence-rate ratio of.37 by the interaction 1118 n engl j med 368;12 nejm.org march 21, 213
9 Four- Efficacy of RTS,S/AS1E Figure 3. Anti-circumsporozoite Antibody Titers in the RTS,S/AS1E Group in the Per-Protocol Cohort. Panel A shows anti-circumsporozoite antibody titers during follow-up in children who received the RTS,S/ AS1E vaccine. Anti-circumsporozoite antibody titers in the control group were consistently low or undetectable throughout follow-up and are not shown. The horizontal line within each box represents the median, the top and bottom of each box represent the 25th and 75th percentiles, respectively, and the I bars represent the highest and lowest values within 1.5 times the interquartile range. The circles denote outliers. The horizontal line under the baseline values indicates that the values for the median and interquartile range were identical. Panel B shows the association between imputed anti-circumsporozoite antibody titers and the hazard ratio for clinical malaria episodes among children who received the RTS,S/ AS1E vaccine, according to a Cox regression model with cubic splines and with a baseline titer of 1. enzymelinked immunosorbent assay unit (EU) per milliliter as the reference. The dotted lines indicate the 95% confidence interval. There was no significant variation in risk between 1 EU per milliliter and EU per milliliter (i.e., the confidence intervals include a hazard ratio of 1.); at values above EU per milliliter, however, there was a reduced risk of clinical malaria with increasing antibody titers. A Anti-circumsporozoite Antibody Titer (EU/ml) B 1, 1, Baseline Month term of the incidence-rate ratio of 2.48 (i.e.,.91, or 9% efficacy). The graphs in Figures 2 and 3 may make these variations in efficacy intuitively evident. In contrast, a long-term follow-up study evaluating the efficacy of the related RTS,S/AS2 vaccine in Mozambican children showed no evidence of the efficacy against a first episode waning over time. 8 One reason for the difference in these findings may be that in our study the waning efficacy was more readily apparent on analysis of all episodes in the Andersen Gill survival models than it was with first or only episodes in the Cox regression model, and the former analysis was not used in the study in Mozambique. In addition, whereas the incidence of clinical malaria was sustained (and, in fact, increased) during follow-up of our cohort, it fell over time in Mozambique, reducing the power to identify waning efficacy. The Andersen Gill regression model includes all malaria episodes (1169 total episodes vs. 243 first episodes in our trial) and, in particular, includes all the second, third, and fourth episodes that occurred later during follow-up but would be censored in a Cox regression analysis. Other differences between the two cohorts were higher rates of transmission in Mozambique than in Kilifi (entomologic inoculation rate of 38 vs. 22 and parasite prevalence of 2% vs. 15%), higher median age at vaccination Hazard Ratio Anti-circumsporozoite Antibody Titer (EU/ml) in Mozambique than in Kilifi (35.9 months vs. 11. months), and different adjuvants (AS2 in Mozambique vs. AS1 in Kilifi). Increases in the incidence of malaria during the follow-up period reflect an increase in transmission in our study area (i.e., the Junju Pingilikani area). The mean value of the malaria exposure index increased from.27 (95% CI,.26 to.28) in the first year to.43 (95% CI,.42 to.44) in the fourth year. This increase in the malaria exposure indexes in the Junju Pingilikani area is in contrast with overall declines in malaria transmission in Kilifi, 9 suggesting that there is marked regional heterogeneity in transmission within the district. 1,11 Estimates of vaccine efficacy were significantly lower among children with a high malaria expo- n engl j med 368;12 nejm.org march 21,
10 Four- Efficacy of RTS,S/AS1E sure index than among those with a low exposure index, suggesting that vaccine efficacy may appear to wane because the acquisition of natural immunity to blood-stage parasites among children who received the RTS,S/AS1E vaccine was slower than that among controls, owing to reduced exposure to blood-stage parasites in the RTS,S/AS1E group. Alternatively, vaccine efficacy may wane because anti-circumsporozoite antibody levels fall over time (Fig. 3A). Anti-circumsporozoite antibodies may mediate protection and were associated with a reduced risk of clinical malaria in our study (Fig. 3B). Efficacy estimates immediately after RTS,S/AS1E vaccination in the cohort with a high malaria exposure index were lower than those in the cohort with a low malaria exposure index, possibly owing to a heavy sporozoite challenge that overcomes the vaccine-induced immunity. 12,13 Despite waning efficacy over time and with a higher level of exposure to malaria, vaccination with RTS,S/AS1E resulted in an overall reduction in the number of episodes of clinical malaria over a 4-year follow-up period; in total, 65 cases were averted per vaccinated children. Whereas vaccine efficacy determined from survival models provides a useful estimate of the biologic effect of vaccination, 14 there is concern that such estimates may be misleading in public health terms. 15 The absolute reductions in risk that are attributable to the vaccine provide a further metric in evaluating the possible public health effects. 16 Although the confidence intervals were wide, our findings suggest that efficacy against asymptomatic parasitemia may persist longer than efficacy against clinical malaria. A similar pattern has been noted by others. 8 A pre-erythrocytic vaccine might, in theory, achieve such an effect if blood-stage immunity was lower in persons who received the RTS,S/AS1E vaccine, as compared with those who received the rabies vaccine, thus resulting in a greater likelihood of clinical disease due to any given infection. In conclusion, RTS,S/AS1E was associated with a reduction in the incidence of first and of all episodes of P. falciparum clinical malaria, but vaccine efficacy waned during the 4 years of follow-up. Both the waning of vaccine-induced immunity and the more rapid acquisition of blood-stage immunity in the controls than in the children who received the RTS,S/AS1E vaccine may have contributed to the waning of efficacy over time, and the latter may explain the variations in efficacy according to the level of malaria exposure. Supported by the Program for Appropriate Technology in Health Malaria Vaccine Initiative and the Wellcome Trust. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. We thank the parents of the study participants and the village and district authorities for their cooperation; the data and safety monitoring board, chaired by Prof. Malcolm Molyneux, and the local safety monitors; Dr. Jay Berkley in Kilifi; Janet Musembi, Omar Ngoto, Ester Kache, and Steven Chakaya (study clinicians); Dorothy Mwachiro (community liaison officer); and all the community field assistants who conducted active surveillance in the field. REFERENCES 1. World malaria report. Geneva: World Health Organization, The RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS1 malaria vaccine in African children. N Engl J Med 211;365: Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS1E vaccine against malaria in children 5 to 17 months of age. N Engl J Med 28;359: Olotu A, Lusingu J, Leach A, et al. Efficacy of RTS,S/AS1E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis 211;11:12-9. [Erratum, Lancet Infect Dis 211;11:159.] 5. White MT, Griffin JT, Drakeley CJ, Ghani AC. Heterogeneity in malaria exposure and vaccine response: implications for the interpretation of vaccine efficacy trials. Malar J 21;9: Olotu A, Fegan G, Wambua J, et al. Estimating individual exposure to malaria using local prevalence of malaria infection in the field. PLoS One 212;7(3): e Cheung YB, Xu Y, Tan SH, Cutts F, Milligan P. Estimation of intervention effects using first or multiple episodes in clinical trials: the Andersen-Gill model re-examined. Stat Med 21;29: [Erratum, Stat Med 21;29:368.] 8. Sacarlal J, Aide P, Aponte JJ, et al. Long-term safety and efficacy of the RTS,S/AS2A malaria vaccine in Mozambican children. J Infect Dis 29;2: Okiro EA, Hay SI, Gikandi PW, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J 27; 6: Kreuels B, Kobbe R, Adjei S, et al. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis 28;197: Greenwood BM. The microepidemiology of malaria and its importance to malaria control. Trans R Soc Trop Med Hyg 1989;83:Suppl: Rickman LS, Jones TR, Long GW, et al. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am J Trop Med Hyg 199;43: Beier JC, Davis JR, Vaughan JA, Noden BH, Beier MS. Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi. Am J Trop Med Hyg 1991;44: Halloran ME, Haber M, Longini IM Jr, Struchiner CJ. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol 1991;133: Duncan CJ, Hill AV. What is the efficacy of the RTS,S malaria vaccine? BMJ 211;343:d Greenwood B. Interpreting vaccine efficacy. Clin Infect Dis 25;4: Copyright 213 Massachusetts Medical Society. 112 n engl j med 368;12 nejm.org march 21, 213
abstract n engl j med 374;26 nejm.org June 30,
 The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
Status of RTS,S/AS01 malaria vaccine candidate
 Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation:
 RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation: Results from a phase 3 randomized controlled trial in Lilongwe, Malawi Larry Han Senior Honors Thesis Department of
RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation: Results from a phase 3 randomized controlled trial in Lilongwe, Malawi Larry Han Senior Honors Thesis Department of
BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC
 BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC Introduction Date: 12 August 2012. Author: WHO Secretariat with input from JTEG Chair The most advanced vaccine candidate against
BRIEFING ON RTS,S/AS01 MALARIA VACCINE FOR THE SEPTEMBER 2012 MEETING OF MPAC Introduction Date: 12 August 2012. Author: WHO Secretariat with input from JTEG Chair The most advanced vaccine candidate against
Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data
 Efficacy of malaria vaccines: individual-participant pooled analysis of phase 2 data Philip Bejon, Michael T White, Ally Olotu, Kalifa Bojang, John P A Lusingu, Nahya Salim, Nekoye N Otsyula, Selidji T
Efficacy of malaria vaccines: individual-participant pooled analysis of phase 2 data Philip Bejon, Michael T White, Ally Olotu, Kalifa Bojang, John P A Lusingu, Nahya Salim, Nekoye N Otsyula, Selidji T
Abstract. Conclusions RTS,S/AS01E shows promise as a candidate malaria vaccine. (ClinicalTrials.gov number, NCT )
 The new england journal of medicine established in 1812 december 11, 2008 vol. 359 no. 24 Efficacy of RTS,S/AS01E Vaccine against Malaria in Children 5 to 17 Months of Age Philip Bejon, Ph.D., John Lusingu,
The new england journal of medicine established in 1812 december 11, 2008 vol. 359 no. 24 Efficacy of RTS,S/AS01E Vaccine against Malaria in Children 5 to 17 Months of Age Philip Bejon, Ph.D., John Lusingu,
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018
 Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation
 Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation Malaria vaccine development and a role for conjugates Ashley J Birkett, PhD Director, Research & Development New Cells, New Vaccines
Enhancing Immunogenicity of Recombinant Vaccines: Chemical Conjugation Malaria vaccine development and a role for conjugates Ashley J Birkett, PhD Director, Research & Development New Cells, New Vaccines
Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya
 MAJOR ARTICLE Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya Tabitha W. Mwangi, 1 Amanda Ross, 1,2,a Robert W. Snow, 1,2 and Kevin Marsh 2 1 Kenya
MAJOR ARTICLE Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya Tabitha W. Mwangi, 1 Amanda Ross, 1,2,a Robert W. Snow, 1,2 and Kevin Marsh 2 1 Kenya
Copyright White et al. Open Access article distributed under the terms of CC BY.
 Immunogenicity of the RTS,S/AS0 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial Michael T White, Robert Verity,
Immunogenicity of the RTS,S/AS0 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial Michael T White, Robert Verity,
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS,S/AS01
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS,S/AS01
The RTS,S Clinical Trials Partnership " * Abstract
 Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites The RTS,S Clinical Trials
Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites The RTS,S Clinical Trials
Gradual acquisition of immunity to severe malaria with increasing
 Gradual acquisition of immunity to severe malaria with increasing exposure Jamie T Griffin 1*, T Déirdre Hollingsworth 2,3,4*, Hugh Reyburn 5, Chris J Drakeley 5, Eleanor M Riley 5, Azra C Ghani 1 1 MRC
Gradual acquisition of immunity to severe malaria with increasing exposure Jamie T Griffin 1*, T Déirdre Hollingsworth 2,3,4*, Hugh Reyburn 5, Chris J Drakeley 5, Eleanor M Riley 5, Azra C Ghani 1 1 MRC
Long term Efficacy of a Pre-erythrocytic malaria vaccine and correlates of. protection in children residing in a malaria endemic country
 Long term Efficacy of a Pre-erythrocytic malaria vaccine and correlates of protection in children residing in a malaria endemic country A thesis submitted for degree of Doctor of Philosophy. Ally Olotu
Long term Efficacy of a Pre-erythrocytic malaria vaccine and correlates of protection in children residing in a malaria endemic country A thesis submitted for degree of Doctor of Philosophy. Ally Olotu
Malaria parasite vaccine development Strategies & Targets
 Malaria parasite vaccine development Strategies & Targets Tulane University Ahmed Aly Most malaria disease deaths are among children and pregnant women A child or a pregnant woman dies of malaria nearly
Malaria parasite vaccine development Strategies & Targets Tulane University Ahmed Aly Most malaria disease deaths are among children and pregnant women A child or a pregnant woman dies of malaria nearly
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital
 Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
Supplementary Online Content
 Supplementary Online Content Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B 1. JAMA. 9;3(19):119-1. etable 1. Circulating Levels of B
Supplementary Online Content Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B 1. JAMA. 9;3(19):119-1. etable 1. Circulating Levels of B
JTEG s Summary of RTS,S/ AS01 Clinical Trial Data
 JTEG s Summary of RTS,S/ AS01 Clinical Trial Data Peter Smith On behalf of the Joint Technical Expert Group (JTEG) based on Cohen et al 2010 RTS,S/AS01 Malaria Vaccine l Based on large segment of P. falciparum
JTEG s Summary of RTS,S/ AS01 Clinical Trial Data Peter Smith On behalf of the Joint Technical Expert Group (JTEG) based on Cohen et al 2010 RTS,S/AS01 Malaria Vaccine l Based on large segment of P. falciparum
Downloaded from:
 Bejon, P; Cook, J; Bergmann-Leitner, E; Olotu, A; Lusingu, J; Mwacharo, J; Vekemans, J; Njuguna, P; Leach, A; Lievens, M; Dutta, S; von Seidlein, L; Savarese, B; Villafana, T; Lemnge, MM; Cohen, J; Marsh,
Bejon, P; Cook, J; Bergmann-Leitner, E; Olotu, A; Lusingu, J; Mwacharo, J; Vekemans, J; Njuguna, P; Leach, A; Lievens, M; Dutta, S; von Seidlein, L; Savarese, B; Villafana, T; Lemnge, MM; Cohen, J; Marsh,
Biostatistics II
 Biostatistics II 514-5509 Course Description: Modern multivariable statistical analysis based on the concept of generalized linear models. Includes linear, logistic, and Poisson regression, survival analysis,
Biostatistics II 514-5509 Course Description: Modern multivariable statistical analysis based on the concept of generalized linear models. Includes linear, logistic, and Poisson regression, survival analysis,
APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015
 APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015 PREPARED BY THE JOINT TECHNICAL EXPERT GROUP ON MALARIA VACCINES (JTEG) AND WHO SECRETARIAT 1 Appendix 1. Clinical studies conduc
APPENDICES BACKGROUND PAPER ON THE RTS,S/AS01 MALARIA VACCINE SEPTEMBER 2015 PREPARED BY THE JOINT TECHNICAL EXPERT GROUP ON MALARIA VACCINES (JTEG) AND WHO SECRETARIAT 1 Appendix 1. Clinical studies conduc
RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting
 RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting October 2009 This document is submitted to SAGE upon request from WHO for the sole purpose of publication in the Yellow book in preparation
RTS,S/AS01 Candidate Malaria vaccine Summary for the SAGE meeting October 2009 This document is submitted to SAGE upon request from WHO for the sole purpose of publication in the Yellow book in preparation
Bariatric Surgery versus Intensive Medical Therapy for Diabetes 3-Year Outcomes
 The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
Supplement for: CD4 cell dynamics in untreated HIV-1 infection: overall rates, and effects of age, viral load, gender and calendar time.
 Supplement for: CD4 cell dynamics in untreated HIV-1 infection: overall rates, and effects of age, viral load, gender and calendar time. Anne Cori* 1, Michael Pickles* 1, Ard van Sighem 2, Luuk Gras 2,
Supplement for: CD4 cell dynamics in untreated HIV-1 infection: overall rates, and effects of age, viral load, gender and calendar time. Anne Cori* 1, Michael Pickles* 1, Ard van Sighem 2, Luuk Gras 2,
ASSESSMENT OF HUMORAL AND CELLULAR IMMUNE RESPONSES OF THE RTS,S/AS02D MALARIA VACCINE CANDIDATE ADMINISTERED TO
 ASSESSMENT OF HUMORAL AND CELLULAR IMMUNE RESPONSES OF THE RTS,S/AS02D MALARIA VACCINE CANDIDATE ADMINISTERED TO INFANTS LIVING IN A MALARIA ENDEMIC AREA IN MOZAMBIQUE Pedro Carlos Paulino Aide A research
ASSESSMENT OF HUMORAL AND CELLULAR IMMUNE RESPONSES OF THE RTS,S/AS02D MALARIA VACCINE CANDIDATE ADMINISTERED TO INFANTS LIVING IN A MALARIA ENDEMIC AREA IN MOZAMBIQUE Pedro Carlos Paulino Aide A research
Parasite Burden and Severity of Malaria in Tanzanian Children
 The new england journal of medicine original article Parasite Burden and Severity of Malaria in Tanzanian Children Bronner P. Gonçalves, M.D., Chiung-Yu Huang, Ph.D., Robert Morrison, M.Sc., Sarah Holte,
The new england journal of medicine original article Parasite Burden and Severity of Malaria in Tanzanian Children Bronner P. Gonçalves, M.D., Chiung-Yu Huang, Ph.D., Robert Morrison, M.Sc., Sarah Holte,
Understandable Statistics
 Understandable Statistics correlated to the Advanced Placement Program Course Description for Statistics Prepared for Alabama CC2 6/2003 2003 Understandable Statistics 2003 correlated to the Advanced Placement
Understandable Statistics correlated to the Advanced Placement Program Course Description for Statistics Prepared for Alabama CC2 6/2003 2003 Understandable Statistics 2003 correlated to the Advanced Placement
Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman
 Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman Copyright John-Michael Maas, Darby Communications Agenda Malaria Disease burden Prevention and vaccine
Development of a Malaria Vaccine for Sub-Saharan African Children December 3, 2009 Lode Schuerman Copyright John-Michael Maas, Darby Communications Agenda Malaria Disease burden Prevention and vaccine
Clinical Analysis Report For Study Sample Study
 Clinical Analysis Report For Study Sample Study Automated report generated by WWARN May 9, 2014 Falciparum class org.wwarn.csr.clinicalstudyreport Version 1.020140508-1558 1 Contents 1 Executive Summary
Clinical Analysis Report For Study Sample Study Automated report generated by WWARN May 9, 2014 Falciparum class org.wwarn.csr.clinicalstudyreport Version 1.020140508-1558 1 Contents 1 Executive Summary
Downloaded from:
 Bejon, P; Mwacharo, J; Kai, O; Mwangi, T; Milligan, P; Todryk, S; Keating, S; Lang, T; Lowe, B; Gikonyo, C; Molyneux, C; Fegan, G; Gilbert, SC; Peshu, N; Marsh, K; Hill, AV (2006) A Phase 2b Randomised
Bejon, P; Mwacharo, J; Kai, O; Mwangi, T; Milligan, P; Todryk, S; Keating, S; Lang, T; Lowe, B; Gikonyo, C; Molyneux, C; Fegan, G; Gilbert, SC; Peshu, N; Marsh, K; Hill, AV (2006) A Phase 2b Randomised
Safety and Immunogenicity of RTS,S/AS02D Malaria Vaccine in Infants
 The new england journal of medicine original article Safety and Immunogenicity of RTS,S/AS02D Malaria Vaccine in Infants Salim Abdulla, M.D., Ph.D., Rolf Oberholzer, M.D., Omar Juma, M.D., Sulende Kubhoja,
The new england journal of medicine original article Safety and Immunogenicity of RTS,S/AS02D Malaria Vaccine in Infants Salim Abdulla, M.D., Ph.D., Rolf Oberholzer, M.D., Omar Juma, M.D., Sulende Kubhoja,
Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database
 open access Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database Yana Vinogradova, 1 Carol Coupland, 1 Peter Brindle, 2,3 Julia Hippisley-Cox
open access Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database Yana Vinogradova, 1 Carol Coupland, 1 Peter Brindle, 2,3 Julia Hippisley-Cox
COMPARISON OF THE SURVIVAL OF SHIPPED AND LOCALLY TRANSPLANTED CADAVERIC RENAL ALLOGRAFTS
 COMPARISON OF THE SURVIVAL OF SHIPPED AND LOCALLY TRANSPLANTED CADAVERIC RENAL ALLOGRAFTS A COMPARISON OF THE SURVIVAL OF SHIPPED AND LOCALLY TRANSPLANTED CADAVERIC RENAL ALLOGRAFTS KEVIN C. MANGE, M.D.,
COMPARISON OF THE SURVIVAL OF SHIPPED AND LOCALLY TRANSPLANTED CADAVERIC RENAL ALLOGRAFTS A COMPARISON OF THE SURVIVAL OF SHIPPED AND LOCALLY TRANSPLANTED CADAVERIC RENAL ALLOGRAFTS KEVIN C. MANGE, M.D.,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bucholz EM, Butala NM, Ma S, Normand S-LT, Krumholz HM. Life
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bucholz EM, Butala NM, Ma S, Normand S-LT, Krumholz HM. Life
Unit 1 Exploring and Understanding Data
 Unit 1 Exploring and Understanding Data Area Principle Bar Chart Boxplot Conditional Distribution Dotplot Empirical Rule Five Number Summary Frequency Distribution Frequency Polygon Histogram Interquartile
Unit 1 Exploring and Understanding Data Area Principle Bar Chart Boxplot Conditional Distribution Dotplot Empirical Rule Five Number Summary Frequency Distribution Frequency Polygon Histogram Interquartile
Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
 Tropical Medicine and International Health doi:10.1111/j.1365-3156.2007.01977.x volume 13 no 1 pp 83 90 january 2008 Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
Tropical Medicine and International Health doi:10.1111/j.1365-3156.2007.01977.x volume 13 no 1 pp 83 90 january 2008 Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
UK Liver Transplant Audit
 November 2012 UK Liver Transplant Audit In patients who received a Liver Transplant between 1 st March 1994 and 31 st March 2012 ANNUAL REPORT Advisory Group for National Specialised Services Prepared
November 2012 UK Liver Transplant Audit In patients who received a Liver Transplant between 1 st March 1994 and 31 st March 2012 ANNUAL REPORT Advisory Group for National Specialised Services Prepared
Sample size calculation for multicentre efficacy trials of blood-stage malaria antigens
 Bosomprah Malaria Journal 2013, 12:253 METHODOLOGY Open Access Sample size calculation for multicentre efficacy trials of blood-stage malaria antigens Samuel Bosomprah Abstract Background: Sample size
Bosomprah Malaria Journal 2013, 12:253 METHODOLOGY Open Access Sample size calculation for multicentre efficacy trials of blood-stage malaria antigens Samuel Bosomprah Abstract Background: Sample size
Downloaded from:
 Lusingu, J; Olotu, A; Leach, A; Lievens, M; Vekemans, J; Olivier, A; Benns, S; Olomi, R; Msham, S; Lang, T; Gould, J; Hallez, K; Guerra, Y; Njuguna, P; Awuondo, KO; Malabeja, A; Abdul, O; Gesase, S; Dekker,
Lusingu, J; Olotu, A; Leach, A; Lievens, M; Vekemans, J; Olivier, A; Benns, S; Olomi, R; Msham, S; Lang, T; Gould, J; Hallez, K; Guerra, Y; Njuguna, P; Awuondo, KO; Malabeja, A; Abdul, O; Gesase, S; Dekker,
Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review)
 Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review) Meremikwu MM, Donegan S, Esu E This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review) Meremikwu MM, Donegan S, Esu E This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
International Measles Incidence and Immunization Coverage
 SUPPLEMENT ARTICLE International Measles Incidence and Immunization Coverage Robert Hall and Damien Jolley Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine,
SUPPLEMENT ARTICLE International Measles Incidence and Immunization Coverage Robert Hall and Damien Jolley Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Gonçalves BP, Huang C-Y, Morrison R, et al. Parasite burden
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Gonçalves BP, Huang C-Y, Morrison R, et al. Parasite burden
Average Household Size and the Eradication of Malaria
 For General Release Summary of: Average Household Size and the Eradication of Malaria By Lena Huldén, Ross McKitrick and Larry Huldén Journal of the Royal Statistical Society Series A, October 2013 Online
For General Release Summary of: Average Household Size and the Eradication of Malaria By Lena Huldén, Ross McKitrick and Larry Huldén Journal of the Royal Statistical Society Series A, October 2013 Online
CHL 5225 H Advanced Statistical Methods for Clinical Trials. CHL 5225 H The Language of Clinical Trials
 CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis
 Comfort et al. Malaria Journal 2014, 13:383 RESEARCH Open Access Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis Alison B Comfort
Comfort et al. Malaria Journal 2014, 13:383 RESEARCH Open Access Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis Alison B Comfort
APPENDIX D REFERENCE AND PREDICTIVE VALUES FOR PEAK EXPIRATORY FLOW RATE (PEFR)
 APPENDIX D REFERENCE AND PREDICTIVE VALUES FOR PEAK EXPIRATORY FLOW RATE (PEFR) Lung function is related to physical characteristics such as age and height. In order to assess the Peak Expiratory Flow
APPENDIX D REFERENCE AND PREDICTIVE VALUES FOR PEAK EXPIRATORY FLOW RATE (PEFR) Lung function is related to physical characteristics such as age and height. In order to assess the Peak Expiratory Flow
Kath Maitland Imperial College London & KEMRI / Wellcome Trust Programme, Kilifi, Kenya
 Severe malaria: management with few resources Kath Maitland Imperial College London & KEMRI / Wellcome Trust Programme, Kilifi, Kenya Plasmodium falciparum malaria In African children
Severe malaria: management with few resources Kath Maitland Imperial College London & KEMRI / Wellcome Trust Programme, Kilifi, Kenya Plasmodium falciparum malaria In African children
The RTS,S Clinical Trials Partnership* A bs tr ac t
 The new england journal of medicine established in 1812 november 17, 2011 vol. 365 no. 20 First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children The RTS,S Clinical Trials Partnership*
The new england journal of medicine established in 1812 november 17, 2011 vol. 365 no. 20 First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children The RTS,S Clinical Trials Partnership*
Supplementary figures
 Weight, kg Lumefantrine concentration μg/ml Supplementary figures 8 7 6 5 4 3 2 model data 1 0 0 5 10 15 Days Supplementary Figure 1. Plasma lumefantrine concentrations in African children [1] and model-fitted
Weight, kg Lumefantrine concentration μg/ml Supplementary figures 8 7 6 5 4 3 2 model data 1 0 0 5 10 15 Days Supplementary Figure 1. Plasma lumefantrine concentrations in African children [1] and model-fitted
Statistical Analysis Plan (SAP)
 Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Progress and Challenges in Malaria Vaccines
 Progress and Challenges in Malaria Vaccines Vasee Moorthy MD PhD Initiative for Vaccine Research, WHO Geneva 1 SKIN STAGE VIDEOS 1 Hour: IgG 2 1 Hour: IgG 6 Days: CD8 7 Days: IgG Passive: IgG NATURAL IMMUNITY
Progress and Challenges in Malaria Vaccines Vasee Moorthy MD PhD Initiative for Vaccine Research, WHO Geneva 1 SKIN STAGE VIDEOS 1 Hour: IgG 2 1 Hour: IgG 6 Days: CD8 7 Days: IgG Passive: IgG NATURAL IMMUNITY
SEVERE MALNUTRITION HAS A HIGH
 ORIGINAL CONTRIBUTION Assessment of Severe Malnutrition Among Hospitalized Children in Rural Kenya Comparison of Weight for Height and Mid Upper Arm Circumference James Berkley, MD Isaiah Mwangi, MTropPaeds
ORIGINAL CONTRIBUTION Assessment of Severe Malnutrition Among Hospitalized Children in Rural Kenya Comparison of Weight for Height and Mid Upper Arm Circumference James Berkley, MD Isaiah Mwangi, MTropPaeds
The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Results from the Africa Centre s population cohort
 The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Results from the Africa Centre s population cohort Frank Tanser Presentation at 8 th International Workshop on HIV Treatment,
The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Results from the Africa Centre s population cohort Frank Tanser Presentation at 8 th International Workshop on HIV Treatment,
Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine
 Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine Thomas Smith 1,2 *, Amanda Ross 1,2, Nicolas Maire 1,2, Nakul Chitnis 1,2, Alain Studer 1,2, Diggory Hardy 1,2,
Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine Thomas Smith 1,2 *, Amanda Ross 1,2, Nicolas Maire 1,2, Nakul Chitnis 1,2, Alain Studer 1,2, Diggory Hardy 1,2,
Ad35.CS.01 RTS,S/AS01 prime boost second generation malaria vaccine candidate
 Ad35.CS.01 /AS01 prime boost second generation malaria vaccine candidate Johan Vekemans MD, PhD Malaria vaccines Global Vaccine Development GSK Biologicals NIH 2012 1 The need for a malaria vaccine: targets
Ad35.CS.01 /AS01 prime boost second generation malaria vaccine candidate Johan Vekemans MD, PhD Malaria vaccines Global Vaccine Development GSK Biologicals NIH 2012 1 The need for a malaria vaccine: targets
Immunity to febrile malaria in children: an analysis that distinguishes immunity from lack of exposure.
 IAI Accepts, published online ahead of print on 1 February 00 Infect. Immun. doi:./iai.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
IAI Accepts, published online ahead of print on 1 February 00 Infect. Immun. doi:./iai.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
Analysis of Immunity to Febrile Malaria in Children That Distinguishes Immunity from Lack of Exposure
 INFECTION AND IMMUNITY, May 2009, p. 1917 1923 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01358-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Analysis of Immunity
INFECTION AND IMMUNITY, May 2009, p. 1917 1923 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01358-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Analysis of Immunity
Abstract. Introduction
 Efficiency of Household Reactive Case Detection for Malaria in Rural Southern Zambia: Simulations Based on Cross-Sectional Surveys from Two Epidemiological Settings Kelly M. Searle 1, Timothy Shields 2,
Efficiency of Household Reactive Case Detection for Malaria in Rural Southern Zambia: Simulations Based on Cross-Sectional Surveys from Two Epidemiological Settings Kelly M. Searle 1, Timothy Shields 2,
Statistical approaches to antibody data analysis for populations on the path of malaria elimination
 Statistical approaches to antibody data analysis for populations on the path of malaria elimination Nuno Sepúlveda, nuno.sepulveda@lshtm.ac.uk LSHTM, 28 th April 2017 Malaria Plasmodium parasite species
Statistical approaches to antibody data analysis for populations on the path of malaria elimination Nuno Sepúlveda, nuno.sepulveda@lshtm.ac.uk LSHTM, 28 th April 2017 Malaria Plasmodium parasite species
are expected to begin inoculating at least 2000 African infants in the largest trial ever undertaken of an experimental vaccine for malaria.
 The NEW ENGLAND JOURNAL of MEDICINE Perspective november 3, 2005 Betting on a Malaria Vaccine Susan Okie, M.D. Sometime within the next two years, clinical researchers are expected to begin inoculating
The NEW ENGLAND JOURNAL of MEDICINE Perspective november 3, 2005 Betting on a Malaria Vaccine Susan Okie, M.D. Sometime within the next two years, clinical researchers are expected to begin inoculating
Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study
 Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study Julia Hippisley-Cox, 1,2 Carol Coupland 1 1 Division of Primary Care, University
Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study Julia Hippisley-Cox, 1,2 Carol Coupland 1 1 Division of Primary Care, University
Safety, Immunogenicity and Duration of Protection of the RTS,S/AS02 D Malaria Vaccine: One Year Follow-Up of a Randomized Controlled Phase I/IIb Trial
 Safety, Immunogenicity and Duration of Protection of the RTS,S/AS02 D Malaria Vaccine: One Year Follow-Up of a Randomized Controlled Phase I/IIb Trial Pedro Aide 1,3 *, John J. Aponte 1,2, Montse Renom
Safety, Immunogenicity and Duration of Protection of the RTS,S/AS02 D Malaria Vaccine: One Year Follow-Up of a Randomized Controlled Phase I/IIb Trial Pedro Aide 1,3 *, John J. Aponte 1,2, Montse Renom
Supplementary Online Content
 Supplementary Online Content Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patientlevel data. Published online
Supplementary Online Content Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patientlevel data. Published online
Table S1. Number of eligible individuals by cohort, HIV-CAUSAL and CNICS Collaborations,
 Cohort Table S1. Number of eligible individuals by cohort, HIV-CAUSAL and CNICS Collaborations, 2000-2013 No. of antiretroviraltherapy naïve individuals No. initiated cart regimen in 2000 or later No.
Cohort Table S1. Number of eligible individuals by cohort, HIV-CAUSAL and CNICS Collaborations, 2000-2013 No. of antiretroviraltherapy naïve individuals No. initiated cart regimen in 2000 or later No.
A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants The RTS,S Clinical Trials Partnership A BS TR AC T The authors are
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants The RTS,S Clinical Trials Partnership A BS TR AC T The authors are
Predicting the Impact of a Nonsterilizing Vaccine against Human Immunodeficiency Virus
 JOURNAL OF VIROLOGY, Oct. 2004, p. 11340 11351 Vol. 78, No. 20 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.20.11340 11351.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Predicting
JOURNAL OF VIROLOGY, Oct. 2004, p. 11340 11351 Vol. 78, No. 20 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.20.11340 11351.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Predicting
8/10/2015. Introduction: HIV. Introduction: Medical geography
 Introduction: HIV Incorporating spatial variability to generate sub-national estimates of HIV prevalence in SSA Diego Cuadros PhD Laith Abu-Raddad PhD Sub-Saharan Africa (SSA) has by far the largest HIV
Introduction: HIV Incorporating spatial variability to generate sub-national estimates of HIV prevalence in SSA Diego Cuadros PhD Laith Abu-Raddad PhD Sub-Saharan Africa (SSA) has by far the largest HIV
Measuring the path toward malaria elimination
 Measuring the path toward malaria elimination Rigorous and achievable targets and milestones can be developed from standard malaria surveillance data. By Thomas S. Churcher, 1 Justin M. Cohen, 2 Joseph
Measuring the path toward malaria elimination Rigorous and achievable targets and milestones can be developed from standard malaria surveillance data. By Thomas S. Churcher, 1 Justin M. Cohen, 2 Joseph
ArcGIS and the fight against malaria: Using GIS and remote sensing to compare malaria control interventions in Tanzania
 SUMMARY PAPER ArcGIS and the fight against malaria: Using GIS and remote sensing to compare malaria control interventions in Tanzania Application for the 2017 Esri Young Scholar Award Applicant name Emily
SUMMARY PAPER ArcGIS and the fight against malaria: Using GIS and remote sensing to compare malaria control interventions in Tanzania Application for the 2017 Esri Young Scholar Award Applicant name Emily
Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa
 Opinion Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa David L. Smith 1, Simon I. Hay 2,3, Abdisalan M. Noor 3,4 and Robert W. Snow 3,4 1 Department of Biology
Opinion Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa David L. Smith 1, Simon I. Hay 2,3, Abdisalan M. Noor 3,4 and Robert W. Snow 3,4 1 Department of Biology
Cost-effectiveness analysis of inhaled zanamivir in the treatment of influenza A and B in high-risk patients Griffin A D, Perry A S, Fleming D M
 Cost-effectiveness analysis of inhaled zanamivir in the treatment of influenza A and B in high-risk patients Griffin A D, Perry A S, Fleming D M Record Status This is a critical abstract of an economic
Cost-effectiveness analysis of inhaled zanamivir in the treatment of influenza A and B in high-risk patients Griffin A D, Perry A S, Fleming D M Record Status This is a critical abstract of an economic
Malaria Vaccine Implementation Programme Framework for Policy Decision
 Malaria Vaccine Implementation Programme Framework for Policy Decision Mary J Hamel, IVR SAGE 17 April 2018 1 Questions for SAGE on the Framework for Policy Decision 1. Does SAGE agree with the approach?
Malaria Vaccine Implementation Programme Framework for Policy Decision Mary J Hamel, IVR SAGE 17 April 2018 1 Questions for SAGE on the Framework for Policy Decision 1. Does SAGE agree with the approach?
Statistical Models for Censored Point Processes with Cure Rates
 Statistical Models for Censored Point Processes with Cure Rates Jennifer Rogers MSD Seminar 2 November 2011 Outline Background and MESS Epilepsy MESS Exploratory Analysis Summary Statistics and Kaplan-Meier
Statistical Models for Censored Point Processes with Cure Rates Jennifer Rogers MSD Seminar 2 November 2011 Outline Background and MESS Epilepsy MESS Exploratory Analysis Summary Statistics and Kaplan-Meier
Malaria Vaccine Pipeline
 Malaria Vaccine Pipeline Perspectives and Challenges Carla Botting World Vaccine Congress Asia 2008 3 June 2008 Discussion Points Scope of the problem: the burden and challenge of malaria Malaria vaccine
Malaria Vaccine Pipeline Perspectives and Challenges Carla Botting World Vaccine Congress Asia 2008 3 June 2008 Discussion Points Scope of the problem: the burden and challenge of malaria Malaria vaccine
STATISTICAL APPENDIX
 STATISTICAL APPENDIX MODELS We present here some mathematical details of the model used in the adjusted analyses. The general exponential regression model of survival is a proportional hazards model of
STATISTICAL APPENDIX MODELS We present here some mathematical details of the model used in the adjusted analyses. The general exponential regression model of survival is a proportional hazards model of
A Fully Magnetically Levitated Left Ventricular Assist Device. Final Report of the MOMENTUM 3 Trial
 A Fully Magnetically Levitated Left Ventricular Assist Device Final Report of the MOMENTUM 3 Trial Mandeep R. Mehra, MD, Nir Uriel, MD, Joseph C. Cleveland, Jr., MD, Daniel J. Goldstein, MD, National Principal
A Fully Magnetically Levitated Left Ventricular Assist Device Final Report of the MOMENTUM 3 Trial Mandeep R. Mehra, MD, Nir Uriel, MD, Joseph C. Cleveland, Jr., MD, Daniel J. Goldstein, MD, National Principal
Supplementary Figures
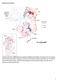 Supplementary Figures Supplementary Fig. 1: Map of the ten countries included in the analysis. Showing the first sub-national administrative boundaries in light grey, and DHS survey locations representing
Supplementary Figures Supplementary Fig. 1: Map of the ten countries included in the analysis. Showing the first sub-national administrative boundaries in light grey, and DHS survey locations representing
Malaria. Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania
 Malaria Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania Despite 42% reduction since 2000, a child dies every minute in Africa from malaria 207 million malaria cases in 2012,
Malaria Dr. Salim Abdulla, Director Ifakara Health Institute, Dar-es-salaam, Tanzania Despite 42% reduction since 2000, a child dies every minute in Africa from malaria 207 million malaria cases in 2012,
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
38 Current Concepts in
 38 Current Concepts in Management of Falciparum Malaria Abstract: Artemisinin based Combination Therapy (ACT) is the preferred agent to treat drug resistance uncomplicated Plasmodium Falciparum (PF) Malaria.
38 Current Concepts in Management of Falciparum Malaria Abstract: Artemisinin based Combination Therapy (ACT) is the preferred agent to treat drug resistance uncomplicated Plasmodium Falciparum (PF) Malaria.
Appendix: Supplementary material [posted as supplied by author]
![Appendix: Supplementary material [posted as supplied by author] Appendix: Supplementary material [posted as supplied by author]](/thumbs/87/96959307.jpg) Appendix: Supplementary material [posted as supplied by author] Part 1 Defining a recorded 10 year CVD risk score All available recorded 10-year risk scores in the CPRD were identified during the study
Appendix: Supplementary material [posted as supplied by author] Part 1 Defining a recorded 10 year CVD risk score All available recorded 10-year risk scores in the CPRD were identified during the study
Severe Anemia in Young Children After High and Low Malaria Transmission Seasons in the Kassena- Nankana District of Northern Ghana
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 2000 Severe Anemia in Young Children After High and Low Malaria Transmission
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 2000 Severe Anemia in Young Children After High and Low Malaria Transmission
How to design intranational deferral Malaria in Greece
 How to design intranational deferral Malaria in Greece Constantina Politis Coordinating Haemovigilance Centre (SKAE) Hellenic Centre for Disease Control and Prevention (KEELPNO) Malaria - Key Facts Malaria
How to design intranational deferral Malaria in Greece Constantina Politis Coordinating Haemovigilance Centre (SKAE) Hellenic Centre for Disease Control and Prevention (KEELPNO) Malaria - Key Facts Malaria
Determining the size of a vaccine trial
 17th Advanced Vaccinology Course Annecy 26 th May 2016 Determining the size of a vaccine trial Peter Smith London School of Hygiene & Tropical Medicine Purposes of lecture Introduce concepts of statistical
17th Advanced Vaccinology Course Annecy 26 th May 2016 Determining the size of a vaccine trial Peter Smith London School of Hygiene & Tropical Medicine Purposes of lecture Introduce concepts of statistical
Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings.
 Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease Epidemiology,
Comparison of the cost effectiveness of LLINs, SMC, the RTS,S vaccine and RTS,S plus IPTi in African settings. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease Epidemiology,
FINDINGS from a clinical trial (Protocol B-06) conducted
 1456 THE NEW ENGLAND JOURNAL OF MEDICINE Nov. 30, 1995 REANALYSIS AND RESULTS AFTER 12 YEARS OF FOLLOW-UP IN A RANDOMIZED CLINICAL TRIAL COMPARING TOTAL MASTECTOMY WITH LUMPECTOMY WITH OR WITHOUT IRRADIATION
1456 THE NEW ENGLAND JOURNAL OF MEDICINE Nov. 30, 1995 REANALYSIS AND RESULTS AFTER 12 YEARS OF FOLLOW-UP IN A RANDOMIZED CLINICAL TRIAL COMPARING TOTAL MASTECTOMY WITH LUMPECTOMY WITH OR WITHOUT IRRADIATION
HIV and Malaria Interactions
 HIV and Malaria Interactions Dr. Moses R. Kamya Chair and Professor of Medicine Makerere University College of Health Sciences Kampala, Uganda INTEREST, Mombasa, Kenya 8 th -11 th May 2012 1 Outline Brief
HIV and Malaria Interactions Dr. Moses R. Kamya Chair and Professor of Medicine Makerere University College of Health Sciences Kampala, Uganda INTEREST, Mombasa, Kenya 8 th -11 th May 2012 1 Outline Brief
A Methodological Issue in the Analysis of Second-Primary Cancer Incidence in Long-Term Survivors of Childhood Cancers
 American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
Downloaded from:
 Abreha, T; Alemayehu, B; Assefa, A; Awab, GR; Baird, JK; Bezabih, B; Cheah, PY; Day, NP; Devine, A; Dorda, M; Dondorp, AM; Girma, S; Hien, TT; Jima, D; Kassa, M; Kebende, A; Khu, NH; Leslie, T; Ley, B;
Abreha, T; Alemayehu, B; Assefa, A; Awab, GR; Baird, JK; Bezabih, B; Cheah, PY; Day, NP; Devine, A; Dorda, M; Dondorp, AM; Girma, S; Hien, TT; Jima, D; Kassa, M; Kebende, A; Khu, NH; Leslie, T; Ley, B;
Generalized Linear Models of Malaria Incidence in Jubek State, South Sudan
 Science Journal of Applied Mathematics and Statistics 2017; 5(4): 134-138 http://www.sciencepublishinggroup.com/j/sjams doi: 10.11648/j.sjams.20170504.12 ISSN: 2376-9491 (Print); ISSN: 2376-9513 (Online)
Science Journal of Applied Mathematics and Statistics 2017; 5(4): 134-138 http://www.sciencepublishinggroup.com/j/sjams doi: 10.11648/j.sjams.20170504.12 ISSN: 2376-9491 (Print); ISSN: 2376-9513 (Online)
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA,
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative
Relationships between sickle cell trait, malaria, and educational outcomes in Tanzania
 Croke et al. BMC Infectious Diseases (2017) 17:568 DOI 10.1186/s12879-017-2644-x RESEARCH ARTICLE Open Access Relationships between sickle cell trait, malaria, and educational outcomes in Tanzania Kevin
Croke et al. BMC Infectious Diseases (2017) 17:568 DOI 10.1186/s12879-017-2644-x RESEARCH ARTICLE Open Access Relationships between sickle cell trait, malaria, and educational outcomes in Tanzania Kevin
A Field Trial to Assess a Blood-Stage Malaria Vaccine
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Field Trial to Assess a Blood-Stage Malaria Vaccine Mahamadou A. Thera, M.D., M.P.H., Ogobara K. Doumbo, M.D., Ph.D., Drissa Coulibaly,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Field Trial to Assess a Blood-Stage Malaria Vaccine Mahamadou A. Thera, M.D., M.P.H., Ogobara K. Doumbo, M.D., Ph.D., Drissa Coulibaly,
Data Analysis Using Regression and Multilevel/Hierarchical Models
 Data Analysis Using Regression and Multilevel/Hierarchical Models ANDREW GELMAN Columbia University JENNIFER HILL Columbia University CAMBRIDGE UNIVERSITY PRESS Contents List of examples V a 9 e xv " Preface
Data Analysis Using Regression and Multilevel/Hierarchical Models ANDREW GELMAN Columbia University JENNIFER HILL Columbia University CAMBRIDGE UNIVERSITY PRESS Contents List of examples V a 9 e xv " Preface
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney
Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine
 Original Article Genetic Diversity and Protective Efficacy of the RTS,S/AS1 Malaria Vaccine D.E. Neafsey, M. Juraska, T. Bedford, D. Benkeser, C. Valim, A. Griggs, M. Lievens, S. Abdulla, S. Adjei, T.
Original Article Genetic Diversity and Protective Efficacy of the RTS,S/AS1 Malaria Vaccine D.E. Neafsey, M. Juraska, T. Bedford, D. Benkeser, C. Valim, A. Griggs, M. Lievens, S. Abdulla, S. Adjei, T.
The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Findings from the Africa Centre s population cohort
 The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Findings from the Africa Centre s population cohort Frank Tanser Presentation at the South African Clinicians Society Conference
The population impacts of ART scale-up in rural KwaZulu-Natal, South Africa: Findings from the Africa Centre s population cohort Frank Tanser Presentation at the South African Clinicians Society Conference
