Academic Year Fourth European Course Evaluation of medicinal products in children
|
|
|
- Martina Webster
- 6 years ago
- Views:
Transcription
1 Academic Year Fourth European Course Evaluation of medicinal products in children Preliminary PROGRAMME Co-ordination : Jean-Marc Husson, Gérard Pons, Jean-Paul Langhendries, Agnès Saint-Raymond, Jean-Marie Maloteaux, Behrouz Kassaï. DAY 1 M 0 Thursday 9 February h00-8h30 : 8h30-9h00 : Registration / Welcome to participants Introduction to the course - Catholic University of Louvain - European diploma in pharmaceutical medicine - Endic paediatricians and pharmacologists DAY 1 M 1 (7h30) Thursday 9 February 2006 SPECIFIC ASPECTS OF PAEDIATRIC PHARMACOLOGY 1) 9h00-10h30 : Jean-Paul Langhendries, Saint Vincent Clinic, Liège and UCL, Belgium (1h30). - Differences between adults and children : growth, development and maturation of the child. - Impact of demographic data, prevalence of diseases and public health in children. 2) 10h30-11h30 : Christopher Milne, Tufts University, Boston, USA (1h00) - Impact of pharmacoeconomics on children therapy. 11h30-12h00 BREAK 3) 12h00-13h00 : Anders Rane, Huddinge University, Huddinge, Sweden (1h00) - Pharmacokinetics and pharmacodynamics (PK/PD) changes in children during maturation and diseases. 13h00-14h00 LUNCH 4) 14h00-15h00 : Evelyne Jacqz-Aigrain, Robert Debré Hospital, Paris, France (1h00) - Prospects of pharmacogenomics in paediatric pharmacology. 5) 15h00-16h00 : Hannsjörg Seyberth, University of Marburg, Marburg, Germany (1h00) - Main diseases unique to children requiring a specific drug evaluation. 16h00-16h30 BREAK 6) 16h30-17h30 : Maurizio Bonati or Antonio Clavenna, Instituto Mario Negri, Milan, Italy (1h00) - Impact of compliance and medication errors/misuse in children, the role of proper information to children and parents. 7) 17h30-18h30 : Gérard Pons, Saint Vincent de Paul Hospital, Paris, France (1h00) - Extrapolability to children of side effects in adults. - Potential long term side effects of drugs related to exposure during growth and maturation.
2 DAY 2 M 2 (7h00) Friday 10 February 2006 SPECIFIC ISSUES RELATED TO DRUG DEVELOPMENT AND DRUG USE IN CHILDREN 1) 8h30-9h30 : Imti Choonara or Helen Sammons, University of Nottingham, Derby, UK (1h00) - Extent of unlicenced and off-label use of medicinal products in children. 2) 9h30-10h30 : Natalie Hoog- Labouret, AFSSAPS, Saint Denis, France (1h00) - The role of Medicine Agencies in recommendations on drug use in children.the AFSSAPS Paediatric Expert Group. 10h30-11h00 BREAK 3) 11h00-12h00 : Tony Nunn, Royal Liverpool Children s NHS Trust, Liverpool, UK (1h00) - The needs for pharmaceutical forms of medicinal products adapted to children. 4) 12h00-13h00 : Stephen Evans, London School of Hygiene & Tropical Medicine, UK (1h00) - Manage the specific aspects of pharmacovigilance in children. 13h00-14h00 LUNCH 5) 14h00-15h00 : Daniel Vasmant, Sanofi-Aventis Pharma S.A., Paris, France (1h00) - Rationale, barriers and opportunities for developing a pediatric medicine : a research based pharmaceutical industry approach. 6) 15h00 16h00 : Ronald Kurz, University of Graz, Austria (1h00) - Specific ethical issues concerning clinical trials in children including the use of placebo and obtention of consent form. - Main cultural differences within Europe. 16h00-16h30 BREAK 7) 16h30-17h30 : José Grosswasser, University Hospital Reine Fabiola, Brussels, Belgium (1h00) - Problems related to drug investigation in children faced by an Ethical Committee
3 DAY 3 M 3 (7h00) Saturday 11 February 2006 SPECIFIC ASPECTS OF CLINICAL TRIALS AND POSTMARKETING SURVEILLANCE IN CHILDREN 1) 8h30-10h00 : Agnès Saint-Raymond, EMEA, London, UK (1h30) - Directive on GCP and clinical trials (basic and appended texts) : specific issues concerning clinical research in children. - ICH E 11 note for guidance. - Forthcoming EU and existing US legislative incentives. 2) 10h00-11h00 : Josep Torrent-Farnell, EMEA/COMP, UK and Autonomous University of Barcelona, Spain (1h00) - Rare diseases, orphan drug and how to develop an orphan drug : Academic and EMEA/COMP viewpoints. 11h00-11h30 BREAK 3) 11h30-12h30 : TBF Heidelberg, Germany (1h00) - Specific requirements for investigator/centre to qualify for drug evaluation in children. 12h30-13h30 LUNCH 4) 13h30-14h30 : Susan Trainor, Brussels, Belgium (1h00) - Quality assurance system and site management organization/smo for the investigational team and other partners. 5) 14h30-16h00 : Beatriz Silva - Lima, University of Lisbon, Portugal and EMEA/CPMP/Safety WP, UK (45mn) - Required preclinical studies for the marketing authorization of a new medicinal product to be used in children : the authorities viewpoint. Gerd Bode, Altana Pharma, Hamburg, Germany (45mn) - Preclinical studies for a new drug application in children : industry strategy including the CTD. 16h00-16h30 BREAK 6) 16h30 17h30 : Gérard Pons, Saint Vincent de Paul Hospital, Paris, France (1h00) - Explain and implement the methodological and technical specifications of clinical trials in children, including placebo effect, choice and assessment of good endpoints.
4 DAY 4 M 4 (7h30) Wednesday 29 March 2006 PRE/PERINATAL DRUG EVALUATION AND USE 1) 8h15-9h15 : Klaus Olejniczak, BfArM, Bonn, Germany (1h00) - Drug evaluation by Regulatory Authorities and specific issues linked to their use during pregnancy : viewpoint of the toxicologist. 2) 9h15-10h15 : Sandra Kweder, FDA, Rockville, USA (1h00) - Drug evaluation by Regulatory Authorities and specific issues linked to their use during pregnancy : product information/labelling. - Questioning clinical trials on medicinal products possible during pregnancy? 10h15-10h45 BREAK 3) 10h45-11h45 : Donald Mattison, National Institute of Child Health and Human Development, Rockville, MD, USA (1h00) - Principles and methodological issues on the evaluation of placental drug transfer. 4) 11h45-12h45 : Elisabeth Elefant, Trousseau Hospital, Paris, France (1h00) - Explain the treatment of pregnant women. - Risk of drug exposure at different stages of pregnancy : consequences for drug use in pregnant women. 5) 12h45-13h15 : Moderator Jean-Marc Husson, Eudipharm, Lyon, France (30 mn) Panel discussion with Elisabeth Elefant, Donald Mattison, Sandra Kweder, Klaus Olejniczack, Gérard Pons, on - Risk of drug exposure at different stages of pregnancy 13h15-14h15 LUNCH 6) 14h15-14h45 : Gérard Pons, Saint Vincent de Paul Hospital, Paris, France (30 mn) - Principles and methodological issues on the evaluation of drug transfer into breast milk. Consequences for breast fed children. 7) 14h45-15h45 : Michael D. Reed, Rainbow Babies & Children s Hospital, Cleveland, U.S.A. (1h00) - Different clinical situations related to foetal drug therapy. Methodological issues on drug evaluation in these situations. 15h45-16h15 BREAK 8) 16h15-17h45 : Corinne Hubinont, St Luc Hospital, Brussels, Belgium (1h30) - Foetal drug therapy : two clinical situations : available evidence-based data.
5 DAY 5 M 5 (7h30) Thursday 30 March 2006 DRUG EVALUATION IN VARIOUS SPECIFIC THERAPEUTIC AREAS 1) 8h30-10h00 : Catherine Chiron, Necker Hospital, Paris, France (1h30 mn) - Specific aspects of epilepsia in children, differences with adults. 2) 10h00-11h30 : Eric Boccard (Bristol Myers Squibb), Paris, France (1h30) - Specific aspects of pain in children, differences with adults. 11h30 12h00 BREAK 3) 12h00-13h30 : Academics TBF (1h30) - Specific aspects of cystic fibrosis in children, differences with adults, symptomatic treatment, genetherapy. 13h30-14h30 LUNCH 4) 14h30-16h00 : Gilles Vassal, Institut Gustave Roussy, Villejuif, Paris, France (1h30) - Specific aspects of evaluation in cancer diseases in children. 16h00 16h30 BREAK 5) 16h30-18h00 : Etienne Sokal, U.C.L., Brussels, Belgium (1h30) - Specific aspects of immunosuppressive agents in children, differences with adults.
6 DAY 6 M 6 (6h00) Friday 31 March 2006 DRUG EVALUATION IN A SPECIFIC THERAPEUTIC AREA IN CHILDREN. STATE OF THE ART. PROTOCOL DESIGN (WORKSHOP TO BE CONTINUED...) 1) 9h00-11h00 : Bart van Overmeire, Antwerpen University Hospital, Belgium (2h00) - Specific aspects of drug evaluation in ductus arteriosus in neonates. 11h00-11h30 BREAK 2) 11h30-13h30 : Bart van Overmeire, Antwerpen University Hospital, Belgium and TBF (2h00) Practical training on the design of one specific protocol with its CRF in a therapeutic area : ductus arteriosus in neonates. - Position of the specific problem : state of the art through evidence based medicine on drug evaluation in ductus arteriosus. - Identification of all ethical, methodological, regulatory issues relevant to the design of the study protocol/crf. 13h30-14h30 LUNCH 3) 14h30-16h30 : TBF (2h00) - Practical training on the design of one specific protocol with its CRF, on drug in ductus arteriosus. DAY 7 M 7 (3h45) Saturday 1st April 2006 DRUG EVALUATION IN A SPECIFIC THERAPEUTIC AREA IN CHILDREN. STATE OF THE ART. PROTOCOL DESIGN (WORKSHOP CONTINUED...) Co-ordination by Bart van Overmeire & TBF Gérard Pons and Jean-Marc Husson (2h00) 8h30-10h30 - Drafting the protocol and the CRF by different students working groups, continued 10h30-11h00 BREAK 11h00-12h30 - Reports by different students working groups (1h30). 12 h30-12h45 : Conclusions on the meeting : Gérard Pons, Jean-Marc Husson (15mn).
Academic Year Eighth European Course Evaluation of Medicinal Products in Children
 - I.M.I. Innovative Medicine Initiative Pharmatrain Academic Year 2009-2010 Eighth European Course Evaluation of Medicinal Products in Children VENUE : 27, rue du Faubourg St Jacques, 75014 Paris, France
- I.M.I. Innovative Medicine Initiative Pharmatrain Academic Year 2009-2010 Eighth European Course Evaluation of Medicinal Products in Children VENUE : 27, rue du Faubourg St Jacques, 75014 Paris, France
Ethical Challenges in Clinical Research at Both Ends of Life
 Preliminary Programme, 24 February 2010 v5 EFGCP-EUCROF Joint Workshop on Ethical Challenges in Clinical Research at Both Ends of Life Common Lessons to be learnt from Paediatric & Geriatric Clinical Development
Preliminary Programme, 24 February 2010 v5 EFGCP-EUCROF Joint Workshop on Ethical Challenges in Clinical Research at Both Ends of Life Common Lessons to be learnt from Paediatric & Geriatric Clinical Development
25 th Anniversary of the European Society for Developmental Perinatal and Paediatric Pharmacology
 25 th Anniversary of the European Society for Developmental Perinatal and Paediatric Pharmacology 1988-2013 A short report to Pharmacology International, the semi-annual newsletter of the International
25 th Anniversary of the European Society for Developmental Perinatal and Paediatric Pharmacology 1988-2013 A short report to Pharmacology International, the semi-annual newsletter of the International
The EFGCP Children s Medicines Working Party 3 rd Annual Conference EU Paediatric Regulation: First European Experiences & Strategic Outlook
 The EFGCP Children s Medicines Working Party 3 rd Annual Conference EU Paediatric Regulation: First European Experiences & Strategic Outlook 9 October 2007 - Management Centre Europe, Brussels, Belgium
The EFGCP Children s Medicines Working Party 3 rd Annual Conference EU Paediatric Regulation: First European Experiences & Strategic Outlook 9 October 2007 - Management Centre Europe, Brussels, Belgium
Development of Paediatric Medicines: From Learning to Adapting
 Preliminary Programme Joint EFGCP / DIA / EMA Medicines for Children Conference on Development of Paediatric Medicines: From Learning to Adapting 26 & 27 September 2012 De Vere Venues Canary Wharf, London,
Preliminary Programme Joint EFGCP / DIA / EMA Medicines for Children Conference on Development of Paediatric Medicines: From Learning to Adapting 26 & 27 September 2012 De Vere Venues Canary Wharf, London,
BDA IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS NOVEMBER 2013 WORKSHOP ADVANCE PROGRAMME
 BDA WORKSHOP IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS 18-19 NOVEMBER 2013 PARIS, FRANCE BIOTHERAPY DEVELOPMENT ASSOCIATION encca European Network for Cancer Research in Children
BDA WORKSHOP IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS 18-19 NOVEMBER 2013 PARIS, FRANCE BIOTHERAPY DEVELOPMENT ASSOCIATION encca European Network for Cancer Research in Children
 Final Programme EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions, Daily Challenges, Ways Forward
Final Programme EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions, Daily Challenges, Ways Forward
Striving for Professionalism in IITs
 EFGCP Workshop on Striving for Professionalism in IITs 26 & 27 February 2014 Courtyard Hotel, Brussels, Belgium Organised by European Forum for Good Clinical Practice In Collaboration with www.efgcp.eu
EFGCP Workshop on Striving for Professionalism in IITs 26 & 27 February 2014 Courtyard Hotel, Brussels, Belgium Organised by European Forum for Good Clinical Practice In Collaboration with www.efgcp.eu
Masterclass on Clinical Research: How to design a clinical trial
 Masterclass on Clinical Research: How to design a clinical trial DAY 1 (Tuesday, 5 March 2013) - Core skills for clinical research 09:30-10:30 Registration and Coffee 10:30 10:35 Welcome Rupert Pearse
Masterclass on Clinical Research: How to design a clinical trial DAY 1 (Tuesday, 5 March 2013) - Core skills for clinical research 09:30-10:30 Registration and Coffee 10:30 10:35 Welcome Rupert Pearse
ITCC-P4 International Workshop
 ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
ESPACOMP SCIENTIFIC MEETING PROGRAM
 Version 29.06.2018 SDG 22th ESPACOMP conference - Dublin, Ireland (European Society for Patient Adherence, COMpliance and Persiste nce) THURSDAY, NOVEMBER 29TH TO SATURDAY, DECEMBER 1ST, 2018 ESPACOMP
Version 29.06.2018 SDG 22th ESPACOMP conference - Dublin, Ireland (European Society for Patient Adherence, COMpliance and Persiste nce) THURSDAY, NOVEMBER 29TH TO SATURDAY, DECEMBER 1ST, 2018 ESPACOMP
 Preliminary Programme Version 9 September 2010 EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions,
Preliminary Programme Version 9 September 2010 EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions,
EDQM and European Pharmacopoeia: State-of-the-art Science for Tomorrow s Medicines
 EDQM and European Pharmacopoeia: State-of-the-art Science for Tomorrow s Medicines International Conference organised by the European Directorate for the Quality of Medicines & HealthCare (EDQM), Council
EDQM and European Pharmacopoeia: State-of-the-art Science for Tomorrow s Medicines International Conference organised by the European Directorate for the Quality of Medicines & HealthCare (EDQM), Council
Medicines for children in Europe: an update
 Medicines for children in Europe: an update José Ramet Secretary-general U.E.M.S. Section of Paediatrics Formerly CESP What the paediatricians know Studies in adults not sufficient Specificity disease
Medicines for children in Europe: an update José Ramet Secretary-general U.E.M.S. Section of Paediatrics Formerly CESP What the paediatricians know Studies in adults not sufficient Specificity disease
This meeting has been made possible by an independent grant from Roche.
 This meeting has been made possible by an independent grant from Roche. This event has been created in collaboration between the University of Milan and ecancer. MILAN SUMMIT ON PRECISION MEDICINE AGENDA
This meeting has been made possible by an independent grant from Roche. This event has been created in collaboration between the University of Milan and ecancer. MILAN SUMMIT ON PRECISION MEDICINE AGENDA
The EU PIP - a step in Pediatric Drug Development. Thomas Severin Bonn,
 The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
PHOTO. VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe
 www.eurordis.org VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe Lucca ( Italy ) March 13-14, 2008 PHOTO www.eurordis.org
www.eurordis.org VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe Lucca ( Italy ) March 13-14, 2008 PHOTO www.eurordis.org
Calendar of Events. 1 2 June 2006 RAPS 2006 European Conference: EU Regulatory Response to Innovation and Global Competitiveness
 Calendar of Events 31 May 2 June 2006 The Validation Manager Venue: Vienna, Austria. 1 2 June 2006 RAPS 2006 European Conference: EU Regulatory Response to Innovation and Global Competitiveness Venue:
Calendar of Events 31 May 2 June 2006 The Validation Manager Venue: Vienna, Austria. 1 2 June 2006 RAPS 2006 European Conference: EU Regulatory Response to Innovation and Global Competitiveness Venue:
 MEETING PROSPECTUS www.virology-education.com CONTENT Introduction... 3 Workshop description... 4 Background...4 Meeting Objectives...4 Learning Objectives...4 Format...4 Unique Meeting Features...4 Target
MEETING PROSPECTUS www.virology-education.com CONTENT Introduction... 3 Workshop description... 4 Background...4 Meeting Objectives...4 Learning Objectives...4 Format...4 Unique Meeting Features...4 Target
Good Laboratory Practice. EU-Serbia screening meeting Brussels, 19 June 2014
 Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Paediatrics: Paediatric Investigation Plan National Agency Assessor s Point of View
 Paediatrics: Paediatric Investigation Plan National Agency Assessor s Point of View Presented by: Dr Ljiljana Milosevic-Kapetanovic Afssaps, France EC Twinning Project 2006-2009 -Relation between ALIMS/MoH
Paediatrics: Paediatric Investigation Plan National Agency Assessor s Point of View Presented by: Dr Ljiljana Milosevic-Kapetanovic Afssaps, France EC Twinning Project 2006-2009 -Relation between ALIMS/MoH
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs
 Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, june 28, 200 A European collaboration between pediatric rheumatology centers regarding
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, june 28, 200 A European collaboration between pediatric rheumatology centers regarding
EURORDIS SUMMER SCHOOL EXPRESS YOURSELF!
 EXPERT PATIENTS AND RESEARCHERS EURORDIS SUMMER SCHOOL EXPRESS YOURSELF! CASTELLDEFELS, BARCELONA, SPAIN JUNE 11-15, 2018 A capacity building programme for patient representatives & researchers on medicines
EXPERT PATIENTS AND RESEARCHERS EURORDIS SUMMER SCHOOL EXPRESS YOURSELF! CASTELLDEFELS, BARCELONA, SPAIN JUNE 11-15, 2018 A capacity building programme for patient representatives & researchers on medicines
JOSEFIEN VENDRIK, QUALITY MANAGER IRENE JONGENELEN, COÖRDINATOR CLINICAL SUPPLIES MANAGEMENT/ SENIOR CLINICAL STUDY MANAGER
 Food for Thought JOSEFIEN VENDRIK, QUALITY MANAGER IRENE JONGENELEN, COÖRDINATOR CLINICAL SUPPLIES MANAGEMENT/ SENIOR CLINICAL STUDY MANAGER CLINOPS DAG, 12 APRIL 2018 HISTORY OF RESEARCH AND DEVELOPMENT
Food for Thought JOSEFIEN VENDRIK, QUALITY MANAGER IRENE JONGENELEN, COÖRDINATOR CLINICAL SUPPLIES MANAGEMENT/ SENIOR CLINICAL STUDY MANAGER CLINOPS DAG, 12 APRIL 2018 HISTORY OF RESEARCH AND DEVELOPMENT
ACCELERATE. 5 th ACCELERATE Paediatric Oncology Conference SIOP. Brussels, Belgium 2 3 March 2017 CDDF ADVANCE PROGRAMME
 ACCELERATE INNOVATION FOR CHILDREN AND ADOLESCENTS WITH CANCER ACCELERATE PLATFORM (CDDF-ITCC-SIOPE MULTI-STAKEHOLDER PLATFORM) 5 th ACCELERATE Paediatric Oncology Conference Brussels, Belgium 2 3 March
ACCELERATE INNOVATION FOR CHILDREN AND ADOLESCENTS WITH CANCER ACCELERATE PLATFORM (CDDF-ITCC-SIOPE MULTI-STAKEHOLDER PLATFORM) 5 th ACCELERATE Paediatric Oncology Conference Brussels, Belgium 2 3 March
Survey Responses to the EUROPEAN COCOA FORUM 2016
 Survey Responses to the EUROPEAN COCOA FORUM The survey was sent to all delegates, speakers and moderators who took part in the European Cocoa Forum in Dubrovnik, and aimed at gathering feedback on the
Survey Responses to the EUROPEAN COCOA FORUM The survey was sent to all delegates, speakers and moderators who took part in the European Cocoa Forum in Dubrovnik, and aimed at gathering feedback on the
The Early Nutrition Academy (ENA) was established in the context of sustainability actions of
 1st Announcement Early Nutrition Academy Symposium on Nutritional Programming, from theory to practice April 18 th April 20 th 2012, Reus (Spain) Symposium Venue: School of Medicine, Universitat Rovira
1st Announcement Early Nutrition Academy Symposium on Nutritional Programming, from theory to practice April 18 th April 20 th 2012, Reus (Spain) Symposium Venue: School of Medicine, Universitat Rovira
GLP in the European Union Ecolabel detergents, GLP and accreditation
 GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
TREAT-NMD and the Role of the Industry in Orphan Diseases. Dr. Stefanie Possekel Santhera Pharmaceuticals
 TREAT-NMD and the Role of the Industry in Orphan Diseases Dr. Stefanie Possekel Santhera Pharmaceuticals TREAT-NMD EU-funded infrastructure to accelerate therapy development in neuromuscular diseases Clinical
TREAT-NMD and the Role of the Industry in Orphan Diseases Dr. Stefanie Possekel Santhera Pharmaceuticals TREAT-NMD EU-funded infrastructure to accelerate therapy development in neuromuscular diseases Clinical
LIST OF RARE CANCERS AND ITS RATIONALE
 Forth Eastern European Conference for Rare Diseases and Orphan Drugs Together for Integrative approach to Rare Diseases 13-14 June 2009 Plovdid, Bulgaria LIST OF RARE CANCERS AND ITS RATIONALE Annalisa
Forth Eastern European Conference for Rare Diseases and Orphan Drugs Together for Integrative approach to Rare Diseases 13-14 June 2009 Plovdid, Bulgaria LIST OF RARE CANCERS AND ITS RATIONALE Annalisa
FIP GUIDELINES Pharmaceutical Research in Paediatric Patients
 Adopted by the FIP Council as an FIP statement of policy in Vienna in 2000, transformed to guidelines in 2014 FIP GUIDELINES Pharmaceutical Research in Paediatric Patients Introduction For many medicines,
Adopted by the FIP Council as an FIP statement of policy in Vienna in 2000, transformed to guidelines in 2014 FIP GUIDELINES Pharmaceutical Research in Paediatric Patients Introduction For many medicines,
MICROBIOME A NEW REVOLUTION FOR PERSONALIZED MEDICINE WORLD ACADEMIC COLLOQUIUM PARIS October 19-20, 2017 IN CHRONIC DISEASES?
 WORLD ACADEMIC COLLOQUIUM PARIS 2017 MICROBIOME A NEW REVOLUTION FOR PERSONALIZED MEDICINE IN CHRONIC DISEASES? October 19-20, 2017 16, rue Bonaparte 75006 PARIS International COLLOQUIUM organized by With
WORLD ACADEMIC COLLOQUIUM PARIS 2017 MICROBIOME A NEW REVOLUTION FOR PERSONALIZED MEDICINE IN CHRONIC DISEASES? October 19-20, 2017 16, rue Bonaparte 75006 PARIS International COLLOQUIUM organized by With
Data Collection on Adverse Events of Anti-HIV Drugs. The D:A:D Study. Signe W. Worm MD, PhD, D:A:D Study Coordinator >
 Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
LYON. FRANCE NOVEMBER / 2013 TRANSLATIONAL RESEARCH IN CHRONIC VIRAL HEPATITIS - BRIDGING BASIC SCIENCE AND CLINICAL RESEARCH
 LYON. FRANCE NOVEMBER 29-30 / 2013 TRANSLATIONAL RESEARCH IN CHRONIC VIRAL HEPATITIS - BRIDGING BASIC SCIENCE https://events.easl.eu/eventportal/ Information/MLYON2013/HOME.aspx Abstract submission deadline:
LYON. FRANCE NOVEMBER 29-30 / 2013 TRANSLATIONAL RESEARCH IN CHRONIC VIRAL HEPATITIS - BRIDGING BASIC SCIENCE https://events.easl.eu/eventportal/ Information/MLYON2013/HOME.aspx Abstract submission deadline:
Table Of Content. European Surveillance of Congenital Anomalies (Phase 3)... 3 Summary... 4 Coordinator, Leader contact and partners...
 Table Of Content European Surveillance of Congenital Anomalies (Phase 3)... 3 Summary... 4 Coordinator, Leader contact and partners... 5 Registry of Congenital Anomalies... 5 CNR Institute of Clinical
Table Of Content European Surveillance of Congenital Anomalies (Phase 3)... 3 Summary... 4 Coordinator, Leader contact and partners... 5 Registry of Congenital Anomalies... 5 CNR Institute of Clinical
*+,-""."!"/"0"0"0"123)(.!!
 First Announcement *+,-""."!"/"0"0"0"123)(.!! ".456789:"!6;%?#%/$0#4'$7%@%:(?285$#!"#$%&'(%)%*'(%%+,-.
First Announcement *+,-""."!"/"0"0"0"123)(.!! ".456789:"!6;%?#%/$0#4'$7%@%:(?285$#!"#$%&'(%)%*'(%%+,-.
February 9th-10th Park City, Utah, USA
 INTERNATIONAL LIVER TRANSPLANTATION SOCIETY February 9th-10th Park City, Utah, USA ILTS.ORG www.ilts.org 1 ILTS Headquarters K.I.T. Group GmbH Association & Conference Management Kurfürstendamm 71 10709
INTERNATIONAL LIVER TRANSPLANTATION SOCIETY February 9th-10th Park City, Utah, USA ILTS.ORG www.ilts.org 1 ILTS Headquarters K.I.T. Group GmbH Association & Conference Management Kurfürstendamm 71 10709
ACCELERATE. 6 TH ACCELERATE Paediatric Oncology Conference. Brussels, Belgium 8-9 February 2018 PROGRAMME ACCELERATE MULTISTAKEHOLDER PLATFORM
 ACCELERATE INNOVATION FOR CHILDREN AND ADOLESCENTS WITH CANCER ACCELERATE MULTISTAKEHOLDER PLATFORM 6 TH ACCELERATE Paediatric Oncology Conference Brussels, Belgium 8-9 February 2018 PROGRAMME PROGRAMME
ACCELERATE INNOVATION FOR CHILDREN AND ADOLESCENTS WITH CANCER ACCELERATE MULTISTAKEHOLDER PLATFORM 6 TH ACCELERATE Paediatric Oncology Conference Brussels, Belgium 8-9 February 2018 PROGRAMME PROGRAMME
Clinical Evidence in the Daily Work of an Anthroposophic Hospital Dr. med. Harald Matthes, Medical Director, Havelhöhe Hospital in Berlin
 Programme Venue: Time: Chair: Presentations: Members Salon, European Parliament 8:00 9:30 am Marian Harkin MEP Research Evidence in Homeopathy: Overview of published clinical investigations Robert Mathie
Programme Venue: Time: Chair: Presentations: Members Salon, European Parliament 8:00 9:30 am Marian Harkin MEP Research Evidence in Homeopathy: Overview of published clinical investigations Robert Mathie
Pharmaceutical, Medical and Health-related Government and Regulatory bodies around the world.
 1 International International Conference on Harmonization (ICH) World Health Organization (WHO) 2 Argentina National Administration of Drugs, Food and medical Technology. Australia s Department of health
1 International International Conference on Harmonization (ICH) World Health Organization (WHO) 2 Argentina National Administration of Drugs, Food and medical Technology. Australia s Department of health
2008 Public Status Report on the Implementation of the European Risk Management Strategy. Executive Summary
 European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
Response to European Commission report on the Paediatric Regulation: consultation document
 Response to European Commission report on the Paediatric Regulation: consultation document Introduction February 2017 1 The Nuffield Council on Bioethics is an independent UK body that examines and reports
Response to European Commission report on the Paediatric Regulation: consultation document Introduction February 2017 1 The Nuffield Council on Bioethics is an independent UK body that examines and reports
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs
 Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, december 4, 2009 A European collaboration between pediatric rheumatology centers
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, december 4, 2009 A European collaboration between pediatric rheumatology centers
Comparison of two HCV-RNA assays assessing early response to simeprevir+pegifn/rbv to select patients suitable to shorten therapy to 12 weeks
 Comparison of two HCV-RNA assays assessing early response to simeprevir+pegifn/rbv to select patients suitable to shorten therapy to 12 weeks C Sarrazin, 1 M Buti, 2 C Moreno, 3 M Gschwantler, 4 GR Foster,
Comparison of two HCV-RNA assays assessing early response to simeprevir+pegifn/rbv to select patients suitable to shorten therapy to 12 weeks C Sarrazin, 1 M Buti, 2 C Moreno, 3 M Gschwantler, 4 GR Foster,
Screening for hepatitis B and C among migrants in the European Union
 Screening for hepatitis B and C among migrants in the European Union Conference on Migrants and Health 12 th May 2016, Lisbon, Portugal Amena Ahmad Hamburg University of Applied Sciences Germany Email:
Screening for hepatitis B and C among migrants in the European Union Conference on Migrants and Health 12 th May 2016, Lisbon, Portugal Amena Ahmad Hamburg University of Applied Sciences Germany Email:
The Paediatric Committee (PDCO)
 www.eurordis.org The Paediatric Committee (PDCO) Fernando de Andres-Trelles (UCM, PDCO, AEMPS) Barcelona, June 2013 1 Some of the slides based on EMA sources, gratefully acknowledged* but opinions are
www.eurordis.org The Paediatric Committee (PDCO) Fernando de Andres-Trelles (UCM, PDCO, AEMPS) Barcelona, June 2013 1 Some of the slides based on EMA sources, gratefully acknowledged* but opinions are
EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH)
 12 June 2017 EMA/848554/2016 Rev.1 Human Medicines Research and Development Support Division EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH) 12-13 June 2017, meeting
12 June 2017 EMA/848554/2016 Rev.1 Human Medicines Research and Development Support Division EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH) 12-13 June 2017, meeting
AIFA UNICRI FDA TRAINING COURSE. on GCP Inspectorates and GCP Inspections
 AIFA UNICRI FDA TRAINING COURSE on GCP Inspectorates and GCP Inspections Civil Service Training Centre (CSTC) P.O.Box M49, Cantonments, Accra, Ghana 7-11 July 2014 AGENDA Monday 7 July 2014 8.30 9.00 Registration
AIFA UNICRI FDA TRAINING COURSE on GCP Inspectorates and GCP Inspections Civil Service Training Centre (CSTC) P.O.Box M49, Cantonments, Accra, Ghana 7-11 July 2014 AGENDA Monday 7 July 2014 8.30 9.00 Registration
February 9th-10th Park City, Utah, USA
 INTERNATIONAL LIVER TRANSPLANTATION SOCIETY Immunosuppression in February 9th-10th Park City, Utah, USA ILTS.ORG www.ilts.org 1 ILTS Headquarters K.I.T. Group GmbH Association & Conference Management Kurfürstendamm
INTERNATIONAL LIVER TRANSPLANTATION SOCIETY Immunosuppression in February 9th-10th Park City, Utah, USA ILTS.ORG www.ilts.org 1 ILTS Headquarters K.I.T. Group GmbH Association & Conference Management Kurfürstendamm
Workshop: Challenges in the Design and Evaluation of Bioequivalence Studies Course No.: 363, 8-10 March 1999, Frankfurt/Main, Germany
 Surf- ARBEITSGEMEINSCHAFT FUR PHARMAZEUTISCHE VERFAHRENSTECHNIK E.V. INTERNATIONAL ASSOCIATION FOR PHARMACEUTICAL TECHNOLOGY ASSOCIATION INTERNATIONALE POUR LA TECHNOLOGIE PHARMACEUTIQUE ARBEITSGEMEINSCHAFT
Surf- ARBEITSGEMEINSCHAFT FUR PHARMAZEUTISCHE VERFAHRENSTECHNIK E.V. INTERNATIONAL ASSOCIATION FOR PHARMACEUTICAL TECHNOLOGY ASSOCIATION INTERNATIONALE POUR LA TECHNOLOGIE PHARMACEUTIQUE ARBEITSGEMEINSCHAFT
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017
 WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
EPIDEMIOLOGY. Accurate, in-depth information for understanding and assessing targeted markets
 EPIDEMIOLOGY Accurate, in-depth information for understanding and assessing targeted markets WHY ACCURATE MARKET FORECASTS Start with accurate understanding Epidemiology is the study of disease patterns
EPIDEMIOLOGY Accurate, in-depth information for understanding and assessing targeted markets WHY ACCURATE MARKET FORECASTS Start with accurate understanding Epidemiology is the study of disease patterns
ESSO Advanced Course on Primary Liver Tumours
 ESSO Advanced Course on Primary Liver Tumours 24-25 April 2014 LIVERPOOL (UK) Endorsed by ESSO Advanced Course on Primary Liver Tumours Chair Hassan Malik, University Hospital Aintree NHS Trust, Liverpool,
ESSO Advanced Course on Primary Liver Tumours 24-25 April 2014 LIVERPOOL (UK) Endorsed by ESSO Advanced Course on Primary Liver Tumours Chair Hassan Malik, University Hospital Aintree NHS Trust, Liverpool,
The Early Nutrition Academy (ENA) was established in the context of sustainability actions of
 Early Nutrition Academy Symposium on Nutritional Programming, from theory to practice April 18 th April 20 th 2012, Reus (Spain) Symposium Venue: School of Medicine, Universitat Rovira I Virgili The Early
Early Nutrition Academy Symposium on Nutritional Programming, from theory to practice April 18 th April 20 th 2012, Reus (Spain) Symposium Venue: School of Medicine, Universitat Rovira I Virgili The Early
WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) CHAIRPERSON: Barbara van Zwieten-Boot
 European Medicines Agency London, 17 December 2009 EMA/CHMP/EWP/248088/2009 Rev. 1 WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) 2010 CHAIRPERSON: Barbara van Zwieten-Boot 1. MEETINGS SCHEDULED FOR 2010
European Medicines Agency London, 17 December 2009 EMA/CHMP/EWP/248088/2009 Rev. 1 WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) 2010 CHAIRPERSON: Barbara van Zwieten-Boot 1. MEETINGS SCHEDULED FOR 2010
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Meeting report, September 2005
 European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 24 October 2005 Doc. Ref. EMEA//322553/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS () Meeting report, 19-20 September
European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 24 October 2005 Doc. Ref. EMEA//322553/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS () Meeting report, 19-20 September
Version 1.11 of 20/07/2018
 Version 1.11 of 20/07/2018 NICE JANUARY 17-19, 2019 6 th International Workshop on Lung Health New approaches to respiratory diseases PRESIDENTS: F. BLASI, G.W. CANONICA CHAIRMEN: S. CENTANNI, J.C. VIRCHOW,
Version 1.11 of 20/07/2018 NICE JANUARY 17-19, 2019 6 th International Workshop on Lung Health New approaches to respiratory diseases PRESIDENTS: F. BLASI, G.W. CANONICA CHAIRMEN: S. CENTANNI, J.C. VIRCHOW,
Member State Marketing Authorisation Holder Invented name Strength Pharmaceutical Form Route of administration
 ANNEX I LIST OF THE INVENTED NAMES, PHARMACEUTICAL FORMS, STRENGTHS OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION AND MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES CHMP/309507/2007 1/6 EMEA
ANNEX I LIST OF THE INVENTED NAMES, PHARMACEUTICAL FORMS, STRENGTHS OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION AND MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES CHMP/309507/2007 1/6 EMEA
Access to innovative oncology medicines in Europe January 2014 Bonn, Germany BDA WORKSHOP BIOTHERAPY DEVELOPMENT ASSOCIATION
 BDA WORKSHOP Access to innovative oncology medicines in Europe 16-17 January 2014 Bonn, Germany BIOTHERAPY DEVELOPMENT ASSOCIATION Content Meeting Objectives 2 Organising Committee 2 Who should attend?
BDA WORKSHOP Access to innovative oncology medicines in Europe 16-17 January 2014 Bonn, Germany BIOTHERAPY DEVELOPMENT ASSOCIATION Content Meeting Objectives 2 Organising Committee 2 Who should attend?
Clinical Pharmacology and Therapeutics
 Clinical Pharmacology and Therapeutics Updated on 23 Feb 2017 I) OBJECTIVES 1. To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism
Clinical Pharmacology and Therapeutics Updated on 23 Feb 2017 I) OBJECTIVES 1. To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS
 COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS 2016-2017 Introduction This summary provides an overview of the activities carried out by
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS 2016-2017 Introduction This summary provides an overview of the activities carried out by
The European network for paediatric research. Carlo Giaquinto University of Padova Padova
 The European network for paediatric research Carlo Giaquinto University of Padova Padova Types of Paediatric networks (examples) Clinical Trials networks: National networks (MCRN, FinPedMed, CICPed, PaedNet
The European network for paediatric research Carlo Giaquinto University of Padova Padova Types of Paediatric networks (examples) Clinical Trials networks: National networks (MCRN, FinPedMed, CICPed, PaedNet
Drug trials in children: problems and the way forward
 Drug trials in children: problems and the way forward Sharon Conroy, 1 John McIntyre, 1 Imti Choonara 1 & Terence Stephenson 2 1 Academic Division of Child Health, University of Nottingham, Derbyshire
Drug trials in children: problems and the way forward Sharon Conroy, 1 John McIntyre, 1 Imti Choonara 1 & Terence Stephenson 2 1 Academic Division of Child Health, University of Nottingham, Derbyshire
Improving new drug development for paediatric cancer: the EMA and PDCO vision
 Dr. Dirk Mentzer, MD, PhD Consultant General Paediatrics Head of Pharmacovigilance unit Chair of PDCO at EMA Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany Disclaimer The
Dr. Dirk Mentzer, MD, PhD Consultant General Paediatrics Head of Pharmacovigilance unit Chair of PDCO at EMA Paul-Ehrlich-Institut Federal Institute for Vaccines and Biomedicines, Germany Disclaimer The
Feedback from the Member States questionnaire
 Annex 6 Feedback from the Member States questionnaire Reinhilde A.R. Schoonjans Special meeting of the EFSA Advisory Forum on GMO risk assessment in Europe 13 November 2007, Brussels Topics 1. Highlights
Annex 6 Feedback from the Member States questionnaire Reinhilde A.R. Schoonjans Special meeting of the EFSA Advisory Forum on GMO risk assessment in Europe 13 November 2007, Brussels Topics 1. Highlights
Behavioural indicators in men who have sex with men
 Behavioural indicators in men who have sex with men Dr. Brenda Spencer, IUMSP, Lausanne 13 th HIV/AIDS Think Tank Meeting First presented by Prof. Jonathan Elford, City University London European Parliament,
Behavioural indicators in men who have sex with men Dr. Brenda Spencer, IUMSP, Lausanne 13 th HIV/AIDS Think Tank Meeting First presented by Prof. Jonathan Elford, City University London European Parliament,
SUMMARY COMPARISON OF NATIONAL PLANS AND PRACTICES. Domenica Taruscio
 Presentation to the European Conference Rare Diseases Luxembourg, 21-22 June, 2005 SUMMARY COMPARISON OF NATIONAL PLANS AND PRACTICES Domenica Taruscio taruscio@iss.it http://www.cnmr.iss.it Co-ordinator
Presentation to the European Conference Rare Diseases Luxembourg, 21-22 June, 2005 SUMMARY COMPARISON OF NATIONAL PLANS AND PRACTICES Domenica Taruscio taruscio@iss.it http://www.cnmr.iss.it Co-ordinator
Market surveillance of medical devices
 Market surveillance of medical devices A joint action on market surveillance of medical devices to reinforce public health protection Information for healthcare professionals Introduction The European
Market surveillance of medical devices A joint action on market surveillance of medical devices to reinforce public health protection Information for healthcare professionals Introduction The European
International Symposium on the Ehlers-Danlos Syndromes
 It is with great pleasure that we invite you to participate in the International Symposium on the Ehlers-Danlos Syndromes September 26 29, 2018 The conference will take place in beautiful Ghent, Belgium,
It is with great pleasure that we invite you to participate in the International Symposium on the Ehlers-Danlos Syndromes September 26 29, 2018 The conference will take place in beautiful Ghent, Belgium,
CURRENT AND FUTURE CHALLENGES OF INNOVATIVE ONCOLOGY DRUG DEVELOPMENT
 CDDF Cancer Drug Development Forum CDDF 8 TH ALPINE CONFERENCE CURRENT AND FUTURE CHALLENGES OF INNOVATIVE ONCOLOGY DRUG DEVELOPMENT INNSBRUCK, AUSTRIA 2-4 MARCH 2015 Workshop objectives The goal of the
CDDF Cancer Drug Development Forum CDDF 8 TH ALPINE CONFERENCE CURRENT AND FUTURE CHALLENGES OF INNOVATIVE ONCOLOGY DRUG DEVELOPMENT INNSBRUCK, AUSTRIA 2-4 MARCH 2015 Workshop objectives The goal of the
EPAG UPDATE. Lenja Wiehe. European Patient Advocacy Groups Manager, EURORDIS
 EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
Standardised Lab Manual for bioanalytical support in clinical studies : Introducing the harmonised Lab Manual. Introduction to the Workshop
 Standardised Lab Manual for bioanalytical support in clinical studies : Introducing the harmonised Lab Manual Introduction to the Workshop Philip Timmerman (for EBF) 05 February 2014 NH Sablon Hotel, Brussels
Standardised Lab Manual for bioanalytical support in clinical studies : Introducing the harmonised Lab Manual Introduction to the Workshop Philip Timmerman (for EBF) 05 February 2014 NH Sablon Hotel, Brussels
TEDDY. Teddy Network of Excellence. Annagrazia ALTAVILLA. Ph.D. Sciences Ethics LL.M. Health Law. diterranée
 Teddy Network of Excellence Annagrazia ALTAVILLA TEDDY Task-force in Europe for Drug Development for the Young Ph.D. Sciences Ethics LL.M. Health Law Associated Senior Lecturer Université de la MéditerranM
Teddy Network of Excellence Annagrazia ALTAVILLA TEDDY Task-force in Europe for Drug Development for the Young Ph.D. Sciences Ethics LL.M. Health Law Associated Senior Lecturer Université de la MéditerranM
Organized by. Scientific comittee
 July 11 & 12, 2019 Institut Pasteur Organized by Arun Sanyal Virginia Commonwealth University School of Medicine, Richmond, Virginia, USA Lawrence Serfaty Hôpital Hautepierre Hôpitaux Universitaires de
July 11 & 12, 2019 Institut Pasteur Organized by Arun Sanyal Virginia Commonwealth University School of Medicine, Richmond, Virginia, USA Lawrence Serfaty Hôpital Hautepierre Hôpitaux Universitaires de
PRELIMINARY PROGRAMME
 0 PRELIMINARY PROGRAMME OVERVIEW The aim of the symposium is to provide quality education, based on practical clinical experience, on all aspects of the nurse s role related to the management of thyroid
0 PRELIMINARY PROGRAMME OVERVIEW The aim of the symposium is to provide quality education, based on practical clinical experience, on all aspects of the nurse s role related to the management of thyroid
Residual Cancer. May 3-5, International Symposium. on Minimal. Le Corum. > Preliminary. Program MONTPELLIER, FRANCE
 International Symposium th on Minimal Residual Cancer > Preliminary Program May 3-5, 2018 Le Corum MONTPELLIER, FRANCE Organizers: www.ismrc2018.com > Committee & Speakers > Welcome to Montpellier Scientific
International Symposium th on Minimal Residual Cancer > Preliminary Program May 3-5, 2018 Le Corum MONTPELLIER, FRANCE Organizers: www.ismrc2018.com > Committee & Speakers > Welcome to Montpellier Scientific
European Medicines Agency decision
 EMA/38339/2017 European Medicines Agency decision P/0021/2017 of 3 February 2017 on the acceptance of a modification of an agreed paediatric investigation plan for dupilumab (EMEA- 001501-PIP02-13-M02)
EMA/38339/2017 European Medicines Agency decision P/0021/2017 of 3 February 2017 on the acceptance of a modification of an agreed paediatric investigation plan for dupilumab (EMEA- 001501-PIP02-13-M02)
Annual Academic Meeting (Incorporating the Annual Blair Bell Research Society Competition) Joint RCOG/Blair Bell Research Society
 Annual Academic Meeting (Incorporating the Annual Blair Bell Research Society Competition) Joint RCOG/Blair Bell Research Society Thursday 17 Friday 18 January 2019 Location: RCOG Overview The Annual Academic
Annual Academic Meeting (Incorporating the Annual Blair Bell Research Society Competition) Joint RCOG/Blair Bell Research Society Thursday 17 Friday 18 January 2019 Location: RCOG Overview The Annual Academic
Table Of Content. Outputs... 7
 Table Of Content European Cooperation on Development and Implementation of Cancer Screening and Prevention Guidelines... 2 Summary... 3 Coordinator, Leader contact and partners... 5 Scientific Institute
Table Of Content European Cooperation on Development and Implementation of Cancer Screening and Prevention Guidelines... 2 Summary... 3 Coordinator, Leader contact and partners... 5 Scientific Institute
Pharmacovigilance Methods and Post-Authorisation Safety Studies
 Pharmacovigilance Methods and Post-Authorisation Safety Studies Alex Dodoo Director, WHO Collaborating Centre for Advocacy and Training in Pharmacovigilance Accra, Ghana Objectives At the end of this module
Pharmacovigilance Methods and Post-Authorisation Safety Studies Alex Dodoo Director, WHO Collaborating Centre for Advocacy and Training in Pharmacovigilance Accra, Ghana Objectives At the end of this module
ICH Topic E 5 (R1) Questions and Answers Ethnic Factors in the Acceptability of Foreign Clinical Data. Step 5 QUESTIONS AND ANSWERS (CPMP/ICH/289/95)
 European Medicines Agency June 2006 CPMP/ICH/5746/03 ICH Topic E 5 (R1) Questions and Answers Ethnic Factors in the Acceptability of Foreign Clinical Data Step 5 QUESTIONS AND ANSWERS (CPMP/ICH/289/95)
European Medicines Agency June 2006 CPMP/ICH/5746/03 ICH Topic E 5 (R1) Questions and Answers Ethnic Factors in the Acceptability of Foreign Clinical Data Step 5 QUESTIONS AND ANSWERS (CPMP/ICH/289/95)
Safety Pharmacology Post Meeting Survey 2014
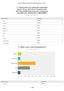 Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
5 th PVRI Annual Drug Discovery & Development Symposium for Pulmonary Hypertension. The pathway to breakthrough therapies WELCOME TO BETHESDA
 Insights from leaders in academia, industry & regulatory... Promising targets ready for clinical development... PVRI Innovative drug discovery for faster approval... Pulmonary vascular diseases... Right
Insights from leaders in academia, industry & regulatory... Promising targets ready for clinical development... PVRI Innovative drug discovery for faster approval... Pulmonary vascular diseases... Right
Vaccine Clinical. Cancer. Group. Working. Trial. Final Workshop, 10 November 2005
 Cancer Vaccine Clinical Trial Working Group Final Workshop, 10 November 2005 CVCTWG Issues Cancer vaccines have unique developmental challenges. Some potential solutions exist. Not all potential solutions
Cancer Vaccine Clinical Trial Working Group Final Workshop, 10 November 2005 CVCTWG Issues Cancer vaccines have unique developmental challenges. Some potential solutions exist. Not all potential solutions
Decentralised Procedure. Public Assessment Report. Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack
 Decentralised Procedure Public Assessment Report Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack Memantin-ratiopharm Starterpackung 5 mg /10 mg /15 mg /20 mg Filmtabletten ;10 mg/20 mg Filmtabletten
Decentralised Procedure Public Assessment Report Memantin AbZ 10 mg/20 mg Filmtabletten ; Starterpack Memantin-ratiopharm Starterpackung 5 mg /10 mg /15 mg /20 mg Filmtabletten ;10 mg/20 mg Filmtabletten
European Medicines Agency decision
 EMA/761887/2015 European Medicines Agency decision P/0307/2015 of 21 December 2015 on the acceptance of a modification of an agreed paediatric investigation plan for odanacatib (EMEA- 001123-PIP01-11-M03)
EMA/761887/2015 European Medicines Agency decision P/0307/2015 of 21 December 2015 on the acceptance of a modification of an agreed paediatric investigation plan for odanacatib (EMEA- 001123-PIP01-11-M03)
Regulatory update on guidelines relevant to paediatric formulations
 Regulatory update on guidelines relevant to paediatric formulations Workshop on Paediatric Formulations II London, 8 November 2011 Presented by: Piotr Kozarewicz Scientific Administrator Quality of Medicines
Regulatory update on guidelines relevant to paediatric formulations Workshop on Paediatric Formulations II London, 8 November 2011 Presented by: Piotr Kozarewicz Scientific Administrator Quality of Medicines
PRELIMINARY PROGRAMME
 PRELIMINARY PROGRAMME 26 May 2017 ITALIAN LANGUAGE SYMPOSIUM PHARMACOLOGY IN INTERVENTIONAL CARDIOLOGY DAPT AFTER PCI 10.00 CONTROVERSY 1 - P2Y12 INHIBITION AFTER PCI IN STABLE PATIENTS Clopidogrel remains
PRELIMINARY PROGRAMME 26 May 2017 ITALIAN LANGUAGE SYMPOSIUM PHARMACOLOGY IN INTERVENTIONAL CARDIOLOGY DAPT AFTER PCI 10.00 CONTROVERSY 1 - P2Y12 INHIBITION AFTER PCI IN STABLE PATIENTS Clopidogrel remains
The health economic landscape of cancer in Europe
 1 Approval number The health economic landscape of cancer in Europe Bengt Jönsson, Professor Emeritus of Health Economics Stockholm School of Economics 2 Disclaimer This presentation was developed by Professor
1 Approval number The health economic landscape of cancer in Europe Bengt Jönsson, Professor Emeritus of Health Economics Stockholm School of Economics 2 Disclaimer This presentation was developed by Professor
EUROPEAN MEDICINES AGENCY DECISION. of 22 September 2009
 European Medicines Agency Doc. Ref. EMEA/583696/2009 P/190/2009 EUROPEAN MEDICINES AGENCY DECISION of 22 September 2009 on the agreement of a Paediatric Investigation Plan and on the granting of a deferral
European Medicines Agency Doc. Ref. EMEA/583696/2009 P/190/2009 EUROPEAN MEDICINES AGENCY DECISION of 22 September 2009 on the agreement of a Paediatric Investigation Plan and on the granting of a deferral
COMMISSION OF THE EUROPEAN COMMUNITIES
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 22.12.2008 COM(2008) 882 final REPORT FROM THE COMMISSION TO THE COUNCIL, THE EUROPEAN PARLIAMENT, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 22.12.2008 COM(2008) 882 final REPORT FROM THE COMMISSION TO THE COUNCIL, THE EUROPEAN PARLIAMENT, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE
Child mortality of children aged 5-15 years in the UK and Sweden: a comparison
 Child mortality of children aged 5-15 years in the UK and Sweden: a comparison Parag Tambe, Helen M Sammons, Imti Choonara Academic Unit of Child Health, University of Nottingham, Derbyshire Children s
Child mortality of children aged 5-15 years in the UK and Sweden: a comparison Parag Tambe, Helen M Sammons, Imti Choonara Academic Unit of Child Health, University of Nottingham, Derbyshire Children s
rare diseases research through National Plans and Strategies
 Recommendations to support rare diseases research through National Plans and Strategies Dr Domenica Taruscio EUROPLAN Coordinator Director of the Italian Centre for Rare Diseases, Italian National Institute
Recommendations to support rare diseases research through National Plans and Strategies Dr Domenica Taruscio EUROPLAN Coordinator Director of the Italian Centre for Rare Diseases, Italian National Institute
PULMONARY HYPERTENSION. by CLINICAL CASES. Archiginnasio Palace
 Under the Aegis of European Board for Accreditation in Cardiology PULMONARY HYPERTENSION by CLINICAL CASES An educational event of the University of Bologna Master Degree in Pulmonary Vascular Diseases
Under the Aegis of European Board for Accreditation in Cardiology PULMONARY HYPERTENSION by CLINICAL CASES An educational event of the University of Bologna Master Degree in Pulmonary Vascular Diseases
EUROPEAN AEROSOL PRODUCTION
 Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
ESSO Advanced Course on Breast Cancer Surgery
 ESSO Advanced Course on Breast Cancer Surgery 08-10 October 2015 LISBON In Partnership with ESSO Advanced Course on Breast Cancer Surgery Chairs Marjut Leidenius, Helsinki University Central Hospital,
ESSO Advanced Course on Breast Cancer Surgery 08-10 October 2015 LISBON In Partnership with ESSO Advanced Course on Breast Cancer Surgery Chairs Marjut Leidenius, Helsinki University Central Hospital,
1. To provide an update of the diagnosis, pathophysiology, outcome, screening and management of pregnancies complicated by IUGR.
 FETAL GROWTH 2016 Thursday November 17 Saturday November 19, 2016 Salter Auditorium, Hospital for Sick Children Sadowski Auditorium, Mount Sinai Hospital www.fetalgrowth2016.ca Dear Colleagues: We are
FETAL GROWTH 2016 Thursday November 17 Saturday November 19, 2016 Salter Auditorium, Hospital for Sick Children Sadowski Auditorium, Mount Sinai Hospital www.fetalgrowth2016.ca Dear Colleagues: We are
HCC and the Hallmarks of Cancer: steps towards novel therapeutic strategies
 EASL HCC SUMMIT 2017 2-5 February 2017, Geneva, Switzerland Scientific Committee: Basic Programme: Tom Luedde, Germany Helen Reeves, United Kingdom Clinical Programme: Alejandro Forner, Spain Franco Trevisani,
EASL HCC SUMMIT 2017 2-5 February 2017, Geneva, Switzerland Scientific Committee: Basic Programme: Tom Luedde, Germany Helen Reeves, United Kingdom Clinical Programme: Alejandro Forner, Spain Franco Trevisani,
MS MasterClass Specialist Neurologists (MS4.1) Module 1 14,15 & 16 March Halifax Hall, Sheffield
 14,15 & 16 March 2018 - Halifax Hall, Sheffield Day 1-14 March 2018 10:15 Registration and refreshments 10:30 Introductions, pre-course analysis and course structure 11:30 Getting the diagnosis right and
14,15 & 16 March 2018 - Halifax Hall, Sheffield Day 1-14 March 2018 10:15 Registration and refreshments 10:30 Introductions, pre-course analysis and course structure 11:30 Getting the diagnosis right and
