Received 13 September 2010/Accepted 22 December 2010
|
|
|
- Karen Hutchinson
- 6 years ago
- Views:
Transcription
1 JOURNAL OF VIROLOGY, Mar. 2011, p Vol. 85, No X/11/$12.00 doi: /jvi Copyright 2011, American Society for Microbiology. All Rights Reserved. Efficacy of Vaccination with Different Combinations of MF59-Adjuvanted and Nonadjuvanted Seasonal and Pandemic Influenza Vaccines against Pandemic H1N1 (2009) Influenza Virus Infection in Ferrets Judith M. A. van den Brand, 1 Joost H. C. M. Kreijtz, 1 Rogier Bodewes, 1 Koert J. Stittelaar, 1,2 Geert van Amerongen, 1 Thijs Kuiken, 1 James Simon, 2 Ron A. M. Fouchier, 1 Giuseppe Del Giudice, 3 Rino Rappuoli, 3 Guus F. Rimmelzwaan, 1,2 and Albert D. M. E. Osterhaus 1,2 * Department of Virology, Erasmus Medical Center, Rotterdam, Netherlands 1 ; ViroClinics Biosciences BV, Rotterdam, Netherlands 2 ; and Novartis Vaccines and Diagnostics, Siena, Italy 3 Received 13 September 2010/Accepted 22 December 2010 Serum antibodies induced by seasonal influenza or seasonal influenza vaccination exhibit limited or no cross-reactivity against the 2009 pandemic swine-origin influenza virus of the H1N1 subtype (ph1n1). Ferrets immunized once or twice with MF59-adjuvanted seasonal influenza vaccine exhibited significantly reduced lung virus titers but no substantial clinical protection against ph1n1-associated disease. However, priming with MF59-adjuvanted seasonal influenza vaccine significantly increased the efficacy of a pandemic MF59- adjuvanted influenza vaccine against ph1n1 challenge. Elucidating the mechanism involved in this priming principle will contribute to our understanding of vaccine- and infection-induced correlates of protection. Furthermore, a practical consequence of these findings is that during an emerging pandemic, the implementation of a priming strategy with an available adjuvanted seasonal vaccine to precede the eventual pandemic vaccination campaign may be useful and life-saving. * Corresponding author. Mailing address: Department of Virology, Erasmus Medical Center, P.O. Box 2040, 3000 CA Rotterdam, Netherlands. Phone: Fax: J.M.A.V.D.B. and J.H.C.M.K. contributed equally to this study. Published ahead of print on 5 January The first influenza pandemic of the 21st century caused by the swine origin influenza virus of the H1N1 subtype (ph1n1) emerged in Mexico in 2009 and caused an as-yet-unknown number of clinical and fatal cases (28). Due to its rapid spread and the apparent absence of preexisting immunity in especially young people, there was an urgent need for a safe and effective vaccine (6). Early in the pandemic phase it became clear that seasonal influenza virus infection or vaccination with seasonal influenza vaccines did not or only marginally induce antibodies that cross-reacted with ph1n1 (5, 14). The vaccines used against seasonal influenza are so-called conventional nonadjuvanted vaccines that display suboptimal immunogenicity and reduced protection due to periodic antigenic drifts (4, 18). To enhance immunogenicity and/or broaden the immune response, there are several options: the use of alternative routes for antigen delivery, the administration of higher antigen doses, the selection of more conserved vaccine antigens, or the addition of an adjuvant to the vaccine (9). There are several adjuvants under development, most of them based on oil-in-water emulsions. MF59 is such an adjuvant that has been well characterized and is used in a seasonal influenza vaccine that has been registered in many European and other countries since The adjuvant is an oil-in-water emulsion that contains 9.75 mg of squalene, mg of polysorbate 80, mg of sorbitan trioleate, sodium citrate dihydrate, and citric acid monohydrate (19). MF59 has been shown to potentiate the immunogenicity of seasonal and pandemic vaccines at all ages (20). It was the first adjuvant to be shown to successfully allow dose sparing with an H5-based vaccine and to widen the breadth of cross-clade neutralization by anti-ha antibodies (19, 25). In addition, more recently MF59 was shown to expand the repertoire of B-cell epitopes recognized by anti-ha cross-neutralizing antibodies (16). MF59-adjuvanted swine origin H1N1 vaccine has been widely used in many European and other countries during the past pandemic (7) and is now utilized for the trivalent vaccine for the season which contains the new H1N1 strain. For all of these reasons, it was relevant to ask what contribution MF59 could have given to a potential effect of vaccination of seasonal H1N1 on subsequent vaccination with the swine-origin H1N1 vaccine (8). In preclinical and clinical studies it thus has been demonstrated that the adjuvant MF59 has an antigen-sparing effect and broadens the intra-subtypic antibody response against influenza viruses upon vaccination (1, 2, 10, 16). Therefore, we investigated the potential of an MF59-adjuvanted trivalent seasonal influenza vaccine to elicit protection against ph1n1 infection in ferrets, since in this vaccine a virus strain is represented that shares an ancestor with ph1n1 (15). Recently, we have shown that immunization with an MF59-adjuvanted seasonal influenza vaccine did prime ferrets for the protective antibody response induced upon a second immunization with the MF59 adjuvanted ph1n1 vaccine (8). To obtain a more detailed understanding of the impact of different vaccination strategies, we analyzed here to what extent thus-vaccinated ferrets would be protected from ph1n1 replication in the upper and lower respiratory tracts and from ph1n1 infectionassociated disease by evaluating the gross pathology and histopathological changes of their lungs. 2851
2 2852 VAN DEN BRAND ET AL. J. VIROL. TABLE 1. Clinical parameters, percentage of lung tissue affected, and relative lung weights of ferrets 4 days after infection with ph1n1 in experiment 1 Immunization % Wt loss (SD) Presence of fever MATERIALS AND METHODS % Affected lung area (SD) % Relative lung wt a (SD) MF (4.2) Yes 36.7 (10.3) 1.67 (0.22) svacmf (7.8) Yes 28.3 (7.5) 1.59 (0.30) (one dose) svacmf59 (two doses) 11.4 (2.6) Yes 18.3 (13.3) 1.60 (0.38) a The relative lung weight is expressed as a percentage of the total body weight. Vaccines. In the present study we used commercially available seasonal trivalent vaccine with (svacmf59) or without (svac) MF59 as an adjuvant. Both vaccines contained envelope subunits (hemagglutinin [HA] and neuraminidase [NA]) (15 g of HA) from the influenza viruses A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Brisbane/60/2008. The subunit vaccine based on pandemic influenza virus A/California/4/2009 (H1N1) was used as a prototype ph1n1 vaccine (15 g of HA), which was also used with (pvacmf59) or without (pvac) MF59 as an adjuvant. The ferrets received 0.5 ml of vaccine containing 0.25 ml of antigen and 0.25 ml of MF59. The adjuvant MF59 and phosphatebuffered saline (PBS) were also used to immunize control animals. Viruses. Influenza virus A/Netherlands/602/2009 (A/NL/602/09) (ph1n1) was isolated from a 3-year-old child that was the first laboratory-confirmed case of the pandemic influenza virus in the Netherlands (17). The virus was isolated in embryonated chicken eggs and subsequently passaged once in Madin-Darby canine kidney (MDCK) cells. The infectious virus titer (50% tissue culture infective dose [TCID 50 ]) was determined as described previously (22). Ferret studies. Healthy outbred male and female ferrets (Mustela putorius furo) between 6 and 12 months of age and seronegative for antibodies against circulating influenza viruses H1N1, H3N2, and ph1n1 by hemagglutination inhibition (HI) assay were used. Two weeks prior to the start of the experiment, the animals were anesthetized by using a cocktail of ketamine (Alfasan, Woerden, Netherlands) and domitor (Orion Pharma, Espoo, Finland), and a temperature logger (DST Micro-T Ultra small temperature logger; Star-Oddi, Reykjavik, Iceland) was placed in their peritoneal cavities. This device recorded body temperature of the animals every 15 min. The animals were maintained in standard housing and provided with commercial food pellets and water ad libitum. For the challenge inoculation, the animals were placed in BSL-3 isolator units. An independent animal ethics committee of the Erasmus Medical Center (DEC consult) approved the experimental protocol before the start of the experiments. Immunizations and infection: experiment 1. Three groups of six ferrets each were immunized intramuscularly in a 3-week interval regimen. Animals received the following immunizations: two doses of MF59, one dose of svacmf59, or two doses of svacmf59 (Table 1). At 4 weeks after the last immunization the animals were challenged intratracheally with ph1n1 at a dose of 10 6 TCID 50 in a volume of 3 ml. On days 0, 2, and 4 after infection, nose and throat swabs were taken from the animals under anesthesia. At 4 days after challenge, the ferrets were euthanized by exsanguination under anesthesia. Immunizations and infection: experiment 2. For the second experiment seven groups of six animals each received two immunizations at an interval of 4 weeks with different combinations of vaccines (Table 2). Animals that were primed with adjuvanted seasonal vaccine (svacmf59) were boosted with pandemic vaccine without (pvac) or with (pvacmf59) adjuvant (groups 1 and 2). Animals primed with unadjuvanted seasonal vaccine (svac) were boosted with svac, pvac, or pvacmf59 (groups 3, 4, and 5). The animals in groups 6 and 7 received a mock vaccination with PBS and subsequently an immunization with pvacmf59 and PBS, respectively. A group that would receive PBS and subsequently svacmf59 was not included in experiment 2 since results similar to those observed in groups 3 and 7 of experiment 2 could be expected by extrapolation of the results from experiment 1. At 4 weeks after the second immunization, the animals were challenged intratracheally with ph1n1 at a dose of 10 6 TCID 50. On days 0, 1, 2, 3, and 4 after infection nose and throat swabs were taken from the animals, and on day 4 the animals were euthanized by exsanguination under total anesthesia. TABLE 2. Vaccination parameters in experiment 2 a First immunization Second immunization Antigen Adjuvant Antigen Adjuvant 1 svac MF59 pvac 2 svac MF59 pvac MF59 3 svac svac 4 svac pvac 5 svac pvac MF59 6 PBS pvac MF59 7 PBS PBS PBS PBS a Animals were immunized intramuscularly with an interval of 4 weeks. svac, seasonal vaccine, subunits (0.25 ml of antigen, 15 g of HA) from influenza viruses A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Brisbane/60/2008; pvac, pandemic vaccine, subunits (0.25 ml of antigen, 15 g of HA) from virus strain A/California/04/2009 (H1N1); MF59, 0.25 ml. Serology. Serum samples collected prior to the first and second immunizations and prior to infection were stored at 20 C. They were tested for the presence of anti-ha antibodies by using an HI assay with 1% turkey erythrocytes (21). Subsequently, the sera were tested for the presence of virus neutralizing antibodies by using a micro virus neutralization (VN) assay (11). Sera were tested for the presence of antibodies reactive with influenza viruses A/NL/602/09 (ph1n1), A/Brisbane/59/2007 (H1N1), and A/Uruguay/716/2007 (H3N2) in the HI assay and in the VN assay (experiment 2 sera only). For this purpose, a reverse genetics virus was used with the HA and NA of the 2009 H1N1 virus and the remaining six gene segments of influenza virus A/Puerto Rico/8/34 (A/PR/8/34). The antibody titers obtained with this virus were comparable to those obtained with the wild-type ph1n1 virus (data not shown). The sera of ferrets infected with the homologous strain were used as control sera. These sera were also tested against other swine influenza H1N1 viruses: A/swine/Iowa/15/30, A/swine/Netherlands/ 25/80, A/Netherlands/386/86 (derived from a zoonotic infection [23]), and influenza H5N1 virus A/Vietnam/1194/04. Postinfection sera from ferrets infected with these viruses were used as positive controls. Virus replication in the upper and lower respiratory tracts. Samples of all lobes of the right lung and the accessory lobe were collected and snap-frozen by using a dry ice-ethanol bath and stored at 70 C until further processing. Lung samples were weighed and subsequently homogenized with a FastPrep-24 (MP Biomedicals, Eindhoven, Netherlands) in Hanks balanced salt solution containing 0.5% lactalbumin, 10% glycerol, 200 U of penicillin/ml, 200 g of streptomycin/ml, 100 U of polymyxin B sulfate/ml, 250 g of gentamicin/ml, and 50 U of nystatin/ml (ICN Pharmaceuticals, Zoetermeer, Netherlands) and centrifuged briefly before dilution. After collection, nose and throat swabs were stored at 70 C in the same medium used for the processing of the lung samples. Quadruplicate 10-fold serial dilutions of lung and swab supernatants were used to determine the virus titers in confluent layers of MDCK cells as described previously (22). Histopathology and immunohistochemistry. The animals were necropsied according to a standard protocol. The trachea was clamped off so that the lungs would not deflate upon opening the pleural cavity. This allowed an accurate visual quantification of the areas of affected lung parenchyma. Samples for histological examination of the left lung and trachea were taken and stored in 10% neutral-buffered formalin (lungs after inflation with formalin), embedded in paraffin, sectioned at 4 m, and stained with hematoxylin and eosin (H&E) for examination by light microscopy. Samples were taken in a standardized way and not guided by changes observed in the gross pathology. Histopathology was described, and semiquantitative assessment of influenza virus-associated inflammation in the lung was performed as described previously (Table 4) (26). For the extent of alveolitis and alveolar damage we used the following scoring scale: 0, 0%; 1, 25%; 2, 25 to 50%; and 3, 50%. The severity of alveolitis was scored as follows: 0, no inflammatory cells; 1, few inflammatory cells; 2, moderate numbers of inflammatory cells; and 3, many inflammatory cells. We also scored for presence of alveolar edema (no 0, yes 1), alveolar hemorrhage (no 0, yes 1), and type II pneumocyte hyperplasia (no 0, yes 1). For the severity of bronchiolitis, bronchitis, and tracheitis we used the following scale: 0, no inflammatory cells; 1, few inflammatory cells; 2, moderate numbers of inflammatory cells; and 3, many inflammatory cells. Finally, the extent of peribronchial, peribronchiolar, and perivascular infiltrates were scored as follows: 0, none; 1, one to two cells thick; 2, three to ten cells thick; and 3, more than ten cells thick. Slides
3 VOL. 85, 2011 EFFICACY OF VACCINATION STRATEGIES FOR PANDEMIC H1N FIG. 1. Virus replication in the upper and lower respiratory tracts of ferrets. Virus replication was determined in the nose (A) and throat (B) on days 2 (f) and4(u) p.i. and expressed as the TCID 50 /ml. (C) Lung virus titers were determined on day 4 p.i. and expressed as the TCID 50 /g of lung. (An asterisk indicates that the mean titer is significantly lower than that in the MF59-immunized group; 0.5 TCID 50 is the limit of detection of the assay.) were examined without knowledge of the identity of the animals. The cumulative scores for size and severity of inflammation provided the total score per animal. Immunohistochemistry. For the detection of influenza A virus antigen, sequential slides were stained with a monoclonal antibody (clone HB65 IgG2a [American Type Culture Collection]) against the nucleoprotein of influenza A virus. Goat anti-mouse IgG2a HRP (Southern Biotech, Birmingham, AL) was used as a secondary antibody. Peroxidase was revealed with a diaminobenzidine substrate, resulting in a deep red precipitate in the nuclei of infected cells and a less intense red staining of their cytoplasm. The sections were counterstained with hematoxylin. After determining the cell types expressing viral antigen, semiquantitative assessment of influenza virus antigen expression in the lungs was performed as reported previously (26). For the alveoli, 25 arbitrarily chosen, 20 objective, fields of lung parenchyma in each lung section were examined by light microscopy for the presence of influenza virus nucleoprotein, without the knowledge of the identity of the animals. The cumulative scores for each animal were presented as number of positive fields per 100 fields. For the bronchi and bronchioles, the percentage of positively staining bronchial and bronchiolar epithelium was determined on every slide, and the average of the four slides was taken to provide the score per animal. Statistical analysis. Differences between groups were analyzed statistically using the Student t test. Differences were considered significant at P RESULTS Experiment 1: efficacy of MF59-adjuvanted seasonal influenza vaccine. Ferrets immunized once or twice with svac- MF59 developed HI antibodies against the seasonal H1N1 strain A/Brisbane/59/07 with a geometric mean titer (GMT) of 45 (standard deviation [SD] 6) and 968 (SD 3), respectively. After a single immunization four of six animals developed detectable antibody titers, whereas after two immunizations all animals tested positive. The A/Brisbane/59/07-specific antibodies did not cross-react with ph1n1 in the HI assay. Upon infection with ph1n1 virus animals from both vaccinated and the control MF59 groups developed clinical signs such as lethargy, loss of appetite, and dyspnea from day 2 onward. All animals lost weight after infection ranging from 12.9 (SD 4.2) to 14.0% (SD 7.8) and 11.4% (SD 2.6) for the animals immunized with MF59, svac, and svacmf59 (two immunizations), respectively (Table 1). Virus was detected in the nose on day 2 postinfection (p.i.) in at least one of six animals, and by day 4 all animals had detectable virus in the nose with GMTs of (SD ), (SD ), and (SD ) for the three groups, respectively (Fig. 1A). All animals had detectable virus titers in their throats on day 2 and 4 (Fig. 1B). The differences in virus titers in the upper respiratory tract between the respective study groups were not statistically significant. On day 4 p.i. virus titers in the lungs of ferrets that had received two immunizations with svac-mf59 were significantly lower (P 0.003) than those in the MF59- immunized group (Fig. 1C). The reduced virus replication correlated with a reduction in gross pathological changes of the lungs (Table 1). Experiment 2: efficacy of pandemic vaccine and effects of priming and MF59 usage. (i) Serology. After primary immunization with seasonal influenza vaccine or PBS, animals received a second immunization with seasonal vaccine, pandemic vaccine, or PBS. Both vaccines were used with or without MF59, and all groups that had received pvac in combination with MF59 developed antibody titers against the homologous strain, as detected in the HI and VN assays. In the svacmf59-pvac group and the svac-pvac animals (groups 1 and 4) the mean HI antibody titers against the pandemic virus were 48.8 (SD 5.6) and 26.2 (SD 4.8), and the animals had VN antibody titers of 46.5 (SD 3.6) and 18.3 (SD 4.0), respectively. Animals that had received pvac- MF59 as a second immunization appeared to have higher antibody titers, especially when they had first been immunized with svac-mf59 (group 2). The mean antibody titers in this group were (SD 1.9) compared to (SD 2.3) and (SD 2.4) in the PBS-pVacMF59 (group 6) and svac-pvacmf59 (group 5) animals, respectively. The VN titers for these groups were (SD 1.6), 97.9 (SD 4.8), and (SD 2.4), respectively. In the svac-svac animals (group 3), only a serological response against the seasonal influenza A/H1N1 virus was detected, whereas no virus-specific antibodies were detected in sera from the PBS-PBS animals (group 7). Postimmunization sera were tested for cross-reactive antibody responses against multiple swine influenza viruses of the H1N1 subtype and H5N1 virus. Cross-reactive antibody titers were detected against two swine influenza H1N1 strains from the Netherlands: A/Netherlands/386/86 (derived from a zoonotic event) and A/swine/Netherlands/25/80 (Table 3). The ranking of GMTs in the HI assay for the different groups was identical to that of the GMTs against the homologous influenza virus. No cross-reactivity was seen with influenza virus A/swine/Iowa/15/30 H1N1 or with A/Vietnam/1194/04 H5N1. (ii) Clinical signs. From day 1 p.i. onward, all infected animals developed symptoms including lethargy, loss of appetite, and dyspnea. These symptoms were mild in the svacmf59- pvacmf59 animals (group 2). To quantify clinical outcome, animals were weight before infection, and on day 4 p.i. and their body temperatures were recorded during the course of the experiment. The greatest weight loss was observed in animals from groups 3, 4, 6, and 7 (12 to 17%), whereas animals
4 2854 VAN DEN BRAND ET AL. J. VIROL. TABLE 3. HI and VN antibody titers against swine origin influenza A/H1N1 viruses and H5N1 virus GMT (SD) a Immunization Swine origin H1N1 A/NL/602/09 b A/NL/386/86 A/NL/25/80 A/Iowa/15/30 H5N1 (A/VN/ 1194/04) First Second HI VN HI VN HI VN HI VN HI VN 1 svacmf59 pvac 2 svacmf59 pvacmf (2) 767 (2) 181 (2) 18 (3) 80 (2) 16 (2) svac svac (1) svac pvac 26 (5) 18 (4) (3) 10 6 (1) svac pvacmf (2) 229 (2) 45 (4) (3) 7 (2) PBS pvacmf (2) 98 (5) 28 (3) (3) PBS PBS a Antibody titers are expressed as the geometric mean titer (GMT) with the standard deviation in parentheses. A/NL/602/09, A/Netherlands/602/2009; A/NL/386/86, A/Netherlands/386/1986; A/NL/25/80, A/Netherlands/25/1980; A/Iowa/15/30, A/swine/Iowa/15/30; A/VN/1194/04, A/Vietnam/1194/04. b Data were obtained as described by Del Giudice et al. (8). from groups 1, 2, and 5 had reduced weight loss with median percentages of 10.8 (range, 0 to 17), 7.5 (2 to 16), and 10.1 (1 to 22), respectively (Table 4). The weight losses in the svacmf59-pvac or pvacmf59 animals (groups 1 and 2) were lower than those of the other groups. Body temperature rose during day 1 p.i. in all animals (Fig. 2). The svacmf59- pvacmf59 animals (group 2) displayed a reduced temperature increase and did not develop a sustained fever in contrast to the other groups. (iii) Virus replication in the respiratory tract. Nose and pharyngeal swabs were collected daily to determine the kinetics of virus replication in the upper respiratory tract. Not all nasal swabs tested positive or animals became positive only after day 3 or 4 p.i. However, those that were positive showed an increase in virus titers in time with maximum titers on day 4 p.i. of (SD ), (SD ), (SD ), and (SD ) for groups 1, 3, 4, and 7, respectively (Fig. 3A). Lower virus titers (SD ) and (SD ), respectively were found in the ferrets that had been immunized with svac or PBS and subsequently with pvacmf59 (groups 5 and 6). Except for one ferret with a low titer on day 3 p.i., no virus was detectable in nasal swabs from animals that had been immunized with svac-mf59, followed by pvacmf59 (group 2). In pharyngeal swabs of virtually all animals of groups 1, 3, 4, 5, 6, and 7 virus was detected from day 1 p.i. onward, with titers increasing until day 4 p.i., resulting in mean titers of (SD ), (SD ), (SD ), (SD ), (SD ), and (SD ), respectively (Fig. 3B). All of the svacmf59- pvacmf59 animals (group 2) tested negative on day 4 p.i., and most of them also tested negative on day 2 p.i. On day 4 p.i. the highest virus titers were found in the lungs of ferrets that had been immunized twice with svac, (SD ), or PBS, (SD ) (groups 3 and 7; Table 4). Animals that had received svacmf59 or svac, followed by pvac (groups 1 and 4) showed lower virus titers of (SD ) and (SD ), respectively. The svacpvacmf59-immunized animals (group 6) had significantly lower virus titers (SD 0) in their lungs than those of the other groups. Moreover, no virus was detected in the lungs of animals that had been immunized with svacmf59 or PBS, and subsequently received pvacmf59 (groups 2 and 6). (iv) Pathology. In all ferrets, the primary macroscopic lesions were found in the lungs and consisted of pulmonary consolidation, characterized by reddening and slightly increased firmness of the lung parenchyma. The extent of pulmonary consolidation was assessed by visual quantification of the affected lung area and the relative lung weight. The aver- TABLE 4. Weight loss, percentage of lung tissue affected, relative lung weights, and virus titers in the lungs of ferrets 4 days after infection with ph1n1 Immunization Wt loss (%) Affected lung tissue (%) Relative lung wt (%) First Second Median Range Median Range Median Range Lung virus titers (TCID 50 /g of lung) a 1 svacmf59 pvac c (1.9) 4 2 svacmf59 pvacmf Neg d 0 3 svac svac (0.6) 6 4 svac pvac (1.8) 4 5 svac pvacmf (0.0) 1 6 PBS pvacmf Neg 0 7 PBS PBS (0.8) 6 a Data were obtained as described by Del Giudice et al. (8) and are given as a log 10 values. b n, The number of animals positive for virus isolation from the lungs of six animals per group. c Weight loss data were available for 5 of 6 animals. d Neg, negative (i.e., all animals tested negative for virus replication in the lungs). Mean (SD) n b
5 VOL. 85, 2011 EFFICACY OF VACCINATION STRATEGIES FOR PANDEMIC H1N FIG. 2. Mean body temperature of ferrets. The mean body temperatures in ferrets (n 6 per group) were measured by a temperature logger (DST Micro-T Ultra small temperature logger; Star-Oddi, Reykjavik, Iceland) placed in the peritoneal cavity. Black bars indicate the median. (In group 3, data from four temperature loggers could be retrieved, and in group 1 and groups 6 and 7 data from five temperature loggers could be retrieved. The data from the other loggers could not be retrieved.) age percentage of affected lung tissue was lowest in ferrets first vaccinated with svac with or without MF59 or with PBS and subsequently with pvac with or without MF59 (groups 1, 2, 4, 5, and 6). The ferrets vaccinated twice with svac (group 3) and the sham-vaccinated animals (group 7) had the highest percentages of affected lung tissue (Table 4). However, a slightly different pattern was found for the relative lung weights: only animals that had first received svac with or without MF59 and subsequently pvac with MF59 (groups 2 and 5) had low relative lung weights. Other macroscopic lesions related to challenge with influenza virus were enlargement of the tracheobronchial lymph nodes (lymphadenopathy) and splenomegaly. This was considered to be an effect of the immune response following influenza virus infection of the respiratory tract. No notable lesions were found in other tissues. The macroscopically observed pulmonary consolidation corresponded with necrotizing broncho-interstitial pneumonia at microscopic examination (Fig. 4). This broncho-interstitial pneumonia was characterized by the presence of inflammatory cells in alveolar and bronchiolar lumina and walls and necrosis of alveolar and bronchiolar epithelium. The inflammatory cells consisted mainly of neutrophils and macrophages with variable amounts of intraluminal edema fluid, fibrin, and erythrocytes. Type II pneumocyte hyperplasia was observed as a reaction to alveolar epithelial cell damage. Mild to severe interstitial edema was seen in perivascular and peribronchiolar areas. The tracheobronchial submucosal glands in many animals exhibited infiltration of neutrophils and fewer macrophages and lymphocytes with associated epithelial cell necrosis. Less severe inflammation and epithelial necrosis were observed in bronchi and trachea. There were no indications for concurrent infections. The histological parameters that were scored are summarized in Table 5. For all parameters but one, the svacmf59- pvacmf59 animals (group 2) had the lowest scores for alveolar lesions. The most severe lesions were found in the sham-vaccinated animals (group 7). Again, the svacmf59- pvacmf59 animals (group 2) had the lowest scores for bronchiolitis and bronchitis. These scores were lower in the PBSpVacMF59 and control animals (groups 6 and 7) than in the other groups (groups 1, 3, 4, and 5). The colocalization of perivascular and peribronchiolar cuffing with lymphocytes at areas of alveolar inflammation in lung sections suggests that the lymphocytic infiltration was a response to the alveolar inflammation. (v) Immunohistochemistry. By immunohistochemistry, influenza virus antigen expression was visible as diffuse to granular red staining, most often stronger in the nucleus than in the cytoplasm (Fig. 4). Influenza virus antigen expression was closely associated with the presence of histological lesions at different levels of the lower respiratory tract. Antigen expression was seen predominantly in type I and II pneumocytes, alveolar macrophages, bronchiolar epithelial cells, bronchial epithelial cells, and tracheal and bronchial epithelial cells of submucosal glands. Semiquantitative scoring showed that there was no influenza virus antigen expression in the alveoli and bronchi and expression in only few cells in the bronchioles of the svacmf59-pvacmf59 animals (group 2) (Table 6). The highest expression at all levels of the respiratory tract was seen in the control animals (group 7), followed by the PBSpVacMF59 animals (group 6), as well as the svac-svac and svac-pvac animals (groups 3 and 4). FIG. 3. Virus titer kinetics in the upper respiratory tract of ferrets. Virus titers were determined daily in the noses (A) and throats (B) of the infected animals (n 6 per group). Nasal and pharyngeal swabs that tested negative were assigned a value of 0.5. (0.5 is the limit of detection of the assay.)
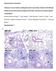
6 2856 VAN DEN BRAND ET AL. J. VIROL. Downloaded from FIG. 4. Lung pathology and virus replication on day 4 p.i. Histopathology and immunohistochemistry analyses were performed 4 days after infection with ph1n1. Representative slides were selected for the protected animals from group 2 (immunized with svacmf59 and pvacmf59). For intermediate protection, representative slides were chosen from group 1 (immunized with svacmf59 and pvac) and represent group 1 and groups 3 to 6. The unprotected animals were from group 7; these were immunized twice with PBS and developed severe pneumonia upon challenge infection. Based on the histopathology of the alveoli (first row, H&E stained) there was only mild inflammation in the svacmf59-pvacmf59- immunized animals (group 2) with few inflammatory cells. The inflammation was intermediate in animals of group 1 and groups 3 to 6, with thickening of the septa with moderate infiltration of neutrophils, pneumocyte type II hyperplasia, and mild necrosis, and a severe pneumonia was seen in the PBS control group with severe necrosis and intra-alveolar flooding with edema fluid and inflammatory cells. Based on immunohistochemistry of the alveoli (second row), there was no antigen expression in svacmf59-pvacmf59-immunized animals, intermediate expression of predominantly pneumocytes type II in group 1 and groups 3 to 6, and abundant expression in the PBS control group. In the bronchioles (third row) there was a mild to moderate bronchiolitis with neutrophils and macrophages and necrosis in svacmf59-pvacmf59-immunized animals and in the animals in group 1 and groups 3 to 6, and there was severe bronchiolitis with severe necrosis in the animals in group 7. Based upon immunohistochemistry (fourth row), there was no antigen expression in svacmf59-pvacmf59-immunized animals, mild expression in bronchiolar epithelial cells in group 1 and groups 3 to 6, and moderate expression in the PBS control group. Magnifications: 400, alveoli; 200, bronchioles. on May 9, 2018 by guest DISCUSSION The primary aim of vaccination against ph1n1 influenza virus is the reduction of virus replication and the associated spread of the virus, as well as prevention of the disease and pathological changes in the respiratory tract. In the present study we have carefully described these parameters in ferrets after different vaccination regimes using combinations of MF59-adjuvanted and nonadjuvanted seasonal and pandemic influenza vaccines. The most striking finding was that prevaccination with svacmf59 not only induced a strong priming effect on the antibody response elicited by pvacmf59 vaccination but also a strong priming effect on the protection induced with this vaccine against virus replication, associated disease, and pathological changes in the respiratory tract. First, we showed that immunization with svacmf59 alone, administered either once or twice, failed to induce ph1n1 influenza virus-specific antibodies and, at best, afforded mod-
7 VOL. 85, 2011 EFFICACY OF VACCINATION STRATEGIES FOR PANDEMIC H1N First TABLE 5. Semiquantitative scoring for histopathology in lungs of ferrets 4 days after infection with ph1n1 Immunization Second Extent of alveolitis/ alveolar damage Score (1 to 3) % Positive slides Score (1 to 3) Severity of alveolitis Alveolar edema Alveolar hemorrhage Avg a Type II pneumocyte hyperplasia Severity of bronchitis/ bronchiolitis Degree of perivascular/ peribronchial cuffing Severity of tracheitis 1 svacmf59 pvac svacmf59 pvacmf svac svac svac pvac svac pvacmf PBS pvacmf PBS PBS a Scores and values were determined as follows. Extent of alveolitis and alveolar damage: 0, 0%; 1, 25%; 2, 25 to 50%; and 3, 50%. Severity of alveolitis: 0, no inflammatory cells; 1, few inflammatory cells; 2, moderate numbers of inflammatory cells; 3, many inflammatory cells. Alveolar edema: 0, no; 1, yes. Alveolar hemorrhage: 0, no; 1, yes. Type II pneumocyte hyperplasia: 0, no; 1, yes. Severity of bronchiolitis, bronchitis, and tracheitis: 0, no inflammatory cells; 1, few inflammatory cells; 2, moderate numbers of inflammatory cells; 3, many inflammatory cells. Extent of peribronchial, peribronchiolar, and perivascular infiltrates: 0, none; 1, one to two cells thick; 2, three to ten cells thick; 3, more than 10 cells thick. est protection. These findings are in agreement with the failure to induce antibodies cross-reactive with ph1n1 influenza virus in humans with adjuvanted and nonadjuvanted seasonal influenza vaccines (14). Apparently, the HAs of the seasonal and pandemic H1N1 strains are antigenically quite distinct, as had already been shown by using postinfection ferret serum against both strains (13). Although svacmf59 failed to induce cross-reactive antibodies, it did have a significant priming effect on the induction of both specific antibodies and protective responses induced by pvacmf59 against ph1n1 influenza virus. It may be speculated that this priming effect, which apparently should be attributed to the adjuvant, is caused by eliciting cross-reactive T-cell and memory B-cell responses (12, 16). Such responses may indeed contribute to protective and cross-reactive immunological memory, which may be an additional correlate of protection against influenza virus besides specific HI or VN antibodies. Cross-reactive antibody titers against two swine influenza viruses of the H1N1 subtype indicate broadening of the antibody response by MF59 adjuvanted vaccines when used for prime and boost immunizations. This, as well as the absence of intersubtypic cross-reactive antibodies upon vaccination with adjuvanted influenza vaccines, is in line with previous data from studies using different adjuvant systems (3, 16). In the control ferrets (group 7) and those not protected after vaccination, the character of the macroscopic and microscopic lung lesions with associated antigen expression was consistent with that described previously in ph1n1 influenza virus-infected ferrets and humans (26, 27). Immunization with svacmf59, followed by administration of pvacmf59 (group 2), resulted in the strongest reduction of alveolar, bronchiolar, and bronchial lesions, as well as the extent of inflammation. Leaving out the adjuvant in the priming seasonal or the boosting pandemic vaccine strongly reduced the protective efficacy of vaccination against the development of alveolar lesions (groups 1 and 5). Omitting the priming vaccination reduced this protective efficacy of pvacmf59 vaccination even further (group 6). The substantial alveolar damage observed in these animals was apparently not paralleled by the levels of weight loss and increased lung weight. This could probably be explained by the delay between viral clearance and the resolution of histopathological changes. The extent of lymphocytic cuffing and the severity of bronchitis and bronchiolitis were more pronounced in the ferrets of group 1 (svacmf59-pvac) and in those primed with svac regardless the vaccine used for boost- TABLE 6. Semiquantitative assessment of influenza virus antigen expression in the lungs of ferrets 4 days after infection with ph1n1 Score a Immunization Alveoli Bronchioles Bronchi Median Range Median Range Median Range 1 svacmf59 pvac svacmf59 pvacmf59 0 NA NA 3 svac svac svac pvac svac pvacmf PBS pvacmf PBS PBS a For the alveoli, 25 arbitrarily chosen, 20 objective fields of lung parenchyma in each lung section were examined by light microscopy for the presence of influenza viral nucleoprotein, without the knowledge of the identity of the animals. The cumulative scores for each animal are presented as the number of positive fields per 100 fields. For the bronchi and bronchioles, the percentages of positively staining bronchial and bronchiolar epithelium were estimated on every slide, and the average of the four slides was taken to provide the score per animal.
8 2858 VAN DEN BRAND ET AL. J. VIROL. ing (groups 3, 4, and 5) than in the ferrets of groups 2, 6, and 7. It may be speculated that this finding is related to a phenomenon recently described in Canada suggesting that subjects vaccinated with seasonal vaccine would be more at risk to develop severe ph1n1 influenza (24). However, the mechanism underlying this phenomenon is poorly understood, and more research is required to elucidate its postulated immunological basis. Collectively, it may be concluded that the MF59-adjuvanted pandemic influenza vaccine used afforded strong protection against the development of severe ph1n1 influenza-associated lesions and disease in the ferret model. This vaccine-induced protective immunity could further be enhanced by prior vaccination with an MF59-adjuvanted seasonal vaccine. Further studies into the mechanism involved in this priming immune response will yield understanding of novel correlates of infection and vaccine-induced protection. This understanding will especially be important for future quality assessment of novel generations of adjuvanted, vectored, and live influenza virus vaccines that are expected to induce broader protection than the currently used classical seasonal vaccines. Finally, a practical consequence of these findings is that during an emerging pandemic, the implementation of a priming strategy with an available adjuvanted seasonal vaccine that precedes the eventual pandemic vaccination campaign, may be useful and life saving. ACKNOWLEDGMENTS We thank P. van Run, L. Leijten, R. van Lavieren, C. W. I. Verhagen, W. van Aert, and R. C. A. Boom for technical assistance and S. van Trierum and W. Vos for biotechnical assistance. This study was supported in part by EU FP7 EMPERIE project , VIRGO (NGI), and EU FP6 FLUVAC grant Potential conflicts of interest are as follows. K.J.S. and J.S. are full-time employed and G.V.A., G.F.R., and A.D.M.E.O. are partly employed by ViroClinics-Biosciences BV. G.D.G. and R.R. are fulltime employed by Novartis Vaccines and Diagnostics. A.D.M.E.O. is CSO of ViroClinics-Biosciences BV, an affiliate of Erasmus Medical Center that collaborates with pharmaceutical companies. Novartis Vaccines and Diagnostics is financially supported by ViroClinics-Biosciences BV. REFERENCES 1. Ansaldi, F., et al Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 26: Ansaldi, F., et al Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 28: Bosch, B. J., et al Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 84: Carrat, F., and A. Flahault Influenza vaccine: the challenge of antigenic drift. Vaccine 25: Centers for Disease Control and Prevention Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 58: Centers for Disease Control and Prevention Prevention and control of seasonal influenza with vaccines, recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR Morb. Mortal. Wkly. Rep. 58: Clark, T. W., et al Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N. Engl. J. Med. 361: Del Giudice, G., et al Seasonal influenza vaccine provides priming for A/H1N1 immunization. Sci. Transl. Med. 1:12re1. 9. Durando, P., G. Icardi, and F. Ansaldi MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin. Biol. Ther. 10: Forrest, H. L., et al Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine 27: Frank, A. L., J. Puck, B. J. Hughes, and T. R. Cate Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J. Clin. Microbiol. 12: Galli, G., et al Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl. Acad. Sci. U. S. A. 106: Garten, R. J., et al Antigenic and genetic characteristics of swineorigin 2009 A(H1N1) influenza viruses circulating in humans. Science 325: Hancock, K., et al Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361: Igarashi, M., et al Predicting the antigenic structure of the pandemic (H1N1) 2009 influenza virus hemagglutinin. PLoS One 5:e Khurana, S., et al Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra Munster, V. J., et al Pathogenesis and transmission of swine-origin 2009 A (H1N1) influenza virus in ferrets. Science 325: Nichol, K. L The efficacy, effectiveness, and cost-effectiveness of inactivated influenza virus vaccines. Vaccine 21: Nicholson, K. G., et al Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357: O Hagan, D. T., A. Wack, and A. Podda MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin. Pharmacol. Ther. 82: Palmer, D., W. Dowle, M. Coleman, and G. Schild Hemagglutination inhibition test, p In Advanced laboratory techniques for influenza diagnosis: procedural guide. U.S. Department of Health Education, Atlanta, GA. 22. Rimmelzwaan, G. F., M. Baars, E. C. Claas, and A. D. Osterhaus Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus, and immunofluorescence as methods for monitoring influenza virus replication in vitro. J. Virol. Methods 74: Rimmelzwaan, G. F., et al Antigenic and genetic characterization of swine influenza A (H1N1) viruses isolated from pneumonia patients in The Netherlands. Virology 282: Skowronski, D. M., et al Association between the seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 7:e Stephenson, I., et al Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 191: van den Brand, J. M., et al Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J. Infect. Dis. 201: Webb, S. A., I. M. Seppelt, and A. I. Investigators Pandemic (H1N1) 2009 influenza ( swine flu ) in Australian and New Zealand intensive care. Crit. Care Resusc. 11: World Health Organization Pandemic (H1N1) 2009 update 97. World Health Organization, Geneva, Switzerland. /don/2010_04_23a/en/index.html.
Modification of the Ferret Model for Pneumonia From Seasonal Human Influenza A Virus Infection
 Modification of the Ferret Model for Pneumonia From Seasonal Human Influenza A Virus Infection Veterinary Pathology 49(3) 562-568 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Modification of the Ferret Model for Pneumonia From Seasonal Human Influenza A Virus Infection Veterinary Pathology 49(3) 562-568 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
MAJOR ARTICLE. Department of Virology, Erasmus Medical Centre and 2 ViroClinics Biosciences BV, Rotterdam, The Netherlands
 MAJOR ARTICLE Severity of Pneumonia Due to New H1N1 Influenza Virus in Ferrets Is Intermediate between That Due to Seasonal H1N1 Virus and Highly Pathogenic Avian Influenza H5N1 Virus Judith M. A. van
MAJOR ARTICLE Severity of Pneumonia Due to New H1N1 Influenza Virus in Ferrets Is Intermediate between That Due to Seasonal H1N1 Virus and Highly Pathogenic Avian Influenza H5N1 Virus Judith M. A. van
WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update
 WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update 23 July 2009 Introduction This document updates guidance 1 from the World
WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update 23 July 2009 Introduction This document updates guidance 1 from the World
Studying Repeated Immunization in an Animal Model. Kanta Subbarao Laboratory of Infectious Diseases, NIAID
 Studying Repeated Immunization in an Animal Model Kanta Subbarao Laboratory of Infectious Diseases, NIAID Animal models in Influenza Research Commonly used Mice Ferrets Guinea pigs Non human primates Less
Studying Repeated Immunization in an Animal Model Kanta Subbarao Laboratory of Infectious Diseases, NIAID Animal models in Influenza Research Commonly used Mice Ferrets Guinea pigs Non human primates Less
Reduced Antibody Responses to the Pandemic (H1N1) 2009 Vaccine after Recent Seasonal Influenza Vaccination
 CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2011, p. 1519 1523 Vol. 18, No. 9 1556-6811/11/$12.00 doi:10.1128/cvi.05053-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Reduced Antibody
CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2011, p. 1519 1523 Vol. 18, No. 9 1556-6811/11/$12.00 doi:10.1128/cvi.05053-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Reduced Antibody
Active and Passive Immunization for Avian Influenza Virus Infections
 NIAID Active and Passive Immunization for Avian Influenza Virus Infections Kanta Subbarao, MD, MPH Laboratory of Infectious Diseases NIAID, NIH Immortalizing H5 HA-Specific Memory B Cells Collection of
NIAID Active and Passive Immunization for Avian Influenza Virus Infections Kanta Subbarao, MD, MPH Laboratory of Infectious Diseases NIAID, NIH Immortalizing H5 HA-Specific Memory B Cells Collection of
Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines
 Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines 28 May 2009 Introduction This document updates WHO guidance 1 to national
Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines 28 May 2009 Introduction This document updates WHO guidance 1 to national
Clinical Trials of Pandemic Vaccines: Key Issues. John Treanor University of Rochester Rochester, NY
 Clinical Trials of Pandemic Vaccines: Key Issues John Treanor University of Rochester Rochester, NY Inactivated vaccine approach Proven technology Used successfully in 1957 and 1968 Abundant efficacy data
Clinical Trials of Pandemic Vaccines: Key Issues John Treanor University of Rochester Rochester, NY Inactivated vaccine approach Proven technology Used successfully in 1957 and 1968 Abundant efficacy data
Min Levine, Ph. D. Influenza Division US Centers for Disease Control and Prevention. June 18, 2015 NIBSC
 Workshop on Immunoassay Standardization for Universal Flu Vaccines Min Levine, Ph. D. Influenza Division US Centers for Disease Control and Prevention June 18, 2015 NIBSC 1 Multiple Immune Mechanisms Contribute
Workshop on Immunoassay Standardization for Universal Flu Vaccines Min Levine, Ph. D. Influenza Division US Centers for Disease Control and Prevention June 18, 2015 NIBSC 1 Multiple Immune Mechanisms Contribute
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ehrlich HJ, Müller M, Oh HML, et al. A clinical trial of a
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ehrlich HJ, Müller M, Oh HML, et al. A clinical trial of a
Update on influenza monitoring and vaccine development
 Update on influenza monitoring and vaccine development Annette Fox WHO Collaborating Centre for Reference and Research on Influenza at The Peter Doherty Institute for Infection and Immunity 1 Outline Why
Update on influenza monitoring and vaccine development Annette Fox WHO Collaborating Centre for Reference and Research on Influenza at The Peter Doherty Institute for Infection and Immunity 1 Outline Why
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature10831 1. Pathological analyses of H5 avian-human reassortant viruses. The rgca04 infection in the lungs of ferrets mainly caused severe bronchopneumonia with
SUPPLEMENTARY INFORMATION doi:10.1038/nature10831 1. Pathological analyses of H5 avian-human reassortant viruses. The rgca04 infection in the lungs of ferrets mainly caused severe bronchopneumonia with
NASDAQ:NVAX Novavax, Inc. All rights reserved.
 Novavax vaccine induced improved immune responses against homologous and drifted A(H3N2) viruses in older adults compared to egg-based, high-dose, influenza vaccine World Vaccine Congress April 4, 2018
Novavax vaccine induced improved immune responses against homologous and drifted A(H3N2) viruses in older adults compared to egg-based, high-dose, influenza vaccine World Vaccine Congress April 4, 2018
Blocking Interhost Transmission of Influenza Virus by Vaccination in the Guinea Pig Model
 JOURNAL OF VIROLOGY, Apr. 2009, p. 2803 2818 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02424-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Blocking Interhost Transmission
JOURNAL OF VIROLOGY, Apr. 2009, p. 2803 2818 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02424-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Blocking Interhost Transmission
Influenza or flu is a
 Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
Jan C. de Jong, 1 * Walter E.P. Beyer, 1 Abraham M. Palache, 2 Guus F. Rimmelzwaan, 1 and Albert D.M.E. Osterhaus 1 KEY WORDS:
 Journal of Medical Virology 61:94 99 (2000) Mismatch Between the 1997/1998 Influenza Vaccine and the Major Epidemic A(H3N2) Virus Strain as the Cause of an Inadequate Vaccine-Induced Antibody Response
Journal of Medical Virology 61:94 99 (2000) Mismatch Between the 1997/1998 Influenza Vaccine and the Major Epidemic A(H3N2) Virus Strain as the Cause of an Inadequate Vaccine-Induced Antibody Response
FluSure XP TM Update with Regards to 2009 H1N1 Swine Influenza Virus
 FluSure XP TM Update with Regards to 2009 H1N1 Swine Influenza Virus Although the 2009 H1N1 swine influenza virus has not been identified in US swine herds, it should be noted that swine influenza vaccines,
FluSure XP TM Update with Regards to 2009 H1N1 Swine Influenza Virus Although the 2009 H1N1 swine influenza virus has not been identified in US swine herds, it should be noted that swine influenza vaccines,
(the change introduced is to add a risk assessment, missing from the previous version, for small-scale laboratory work with characterized CVV)
 Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza vaccines against avian influenza A(H7N9) virus As of 23 May 2013 (replaces version of 10
Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza vaccines against avian influenza A(H7N9) virus As of 23 May 2013 (replaces version of 10
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/35908 holds various files of this Leiden University dissertation Author: Soema, Peter Title: Formulation of influenza T cell peptides : in search of a universal
Cover Page The handle http://hdl.handle.net/1887/35908 holds various files of this Leiden University dissertation Author: Soema, Peter Title: Formulation of influenza T cell peptides : in search of a universal
Nyamdolgor.U, Usuhgerel.S, Baatarjargal.P, others, Journal of agricultural sciences 15 (02): 51-55, 2015
 51 HISTOPATHOLOGICAL STUDY FOR USING OF POX INACTIVATED VACCINE IN GOATS Nyamdolgor.U 1*, Usuhgerel.S 2, Baatarjargal.P 1, Altanchimeg.A 1, Odbileg.R 1 1-Institute of Veterinary Medicine, MULS, Mongolia
51 HISTOPATHOLOGICAL STUDY FOR USING OF POX INACTIVATED VACCINE IN GOATS Nyamdolgor.U 1*, Usuhgerel.S 2, Baatarjargal.P 1, Altanchimeg.A 1, Odbileg.R 1 1-Institute of Veterinary Medicine, MULS, Mongolia
Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],
![Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],](/thumbs/86/93801604.jpg) Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
INFLUENZA VIRUS. INFLUENZA VIRUS CDC WEBSITE
 INFLUENZA VIRUS INFLUENZA VIRUS CDC WEBSITE http://www.cdc.gov/ncidod/diseases/flu/fluinfo.htm 1 THE IMPACT OF INFLUENZA Deaths: PANDEMICS 1918-19 S p a n is h flu 5 0 0,0 0 0 U S 2 0,0 0 0,0 0 0 w o rld
INFLUENZA VIRUS INFLUENZA VIRUS CDC WEBSITE http://www.cdc.gov/ncidod/diseases/flu/fluinfo.htm 1 THE IMPACT OF INFLUENZA Deaths: PANDEMICS 1918-19 S p a n is h flu 5 0 0,0 0 0 U S 2 0,0 0 0,0 0 0 w o rld
7/14/2014 VACCINE-INDUCED ANTI-HA2 ANTIBODIES PROMOTE VIRUS FUSION AND ENHANCE INFLUENZA VIRUS RESPIRATORY DISEASE (VAERD)
 7/14/214 VACCINATION & ENHANCED DISEASE VACCINE-INDUCED ANTI-HA2 ANTIBODIES PROMOTE VIRUS FUSION AND ENHANCE INFLUENZA VIRUS RESPIRATORY DISEASE (VAERD) HANA GOLDING & SURENDER KHURANA DIVISION OF VIRAL
7/14/214 VACCINATION & ENHANCED DISEASE VACCINE-INDUCED ANTI-HA2 ANTIBODIES PROMOTE VIRUS FUSION AND ENHANCE INFLUENZA VIRUS RESPIRATORY DISEASE (VAERD) HANA GOLDING & SURENDER KHURANA DIVISION OF VIRAL
GSK s Adjuvanted Influenza Vaccines The Taming of the Flu
 GSK s Adjuvanted Influenza Vaccines The Taming of the Flu JITMM, Bangkok, October 2008 Bruce L. Innis, MD Global Clinical Research and Development GlaxoSmithKline Biologicals 1 Annual Burden of Influenza
GSK s Adjuvanted Influenza Vaccines The Taming of the Flu JITMM, Bangkok, October 2008 Bruce L. Innis, MD Global Clinical Research and Development GlaxoSmithKline Biologicals 1 Annual Burden of Influenza
Application of Reverse Genetics to Influenza Vaccine Development
 NIAID Application of Reverse Genetics to Influenza Vaccine Development Kanta Subbarao Laboratory of Infectious Diseases NIAID, NIH Licensed Vaccines for Influenza Principle: Induction of a protective
NIAID Application of Reverse Genetics to Influenza Vaccine Development Kanta Subbarao Laboratory of Infectious Diseases NIAID, NIH Licensed Vaccines for Influenza Principle: Induction of a protective
Comparison of the Pathology Caused by H1N1, H5N1, and H3N2 Influenza Viruses
 Archives of Medical Research 40 (2009) 655e661 REVIEW ARTICLE Comparison of the Pathology Caused by H1N1, H5N1, and H3N2 Influenza Viruses Jeannette Guarner a and Reynaldo Falcón-Escobedo b a Department
Archives of Medical Research 40 (2009) 655e661 REVIEW ARTICLE Comparison of the Pathology Caused by H1N1, H5N1, and H3N2 Influenza Viruses Jeannette Guarner a and Reynaldo Falcón-Escobedo b a Department
Correlates of Protection for Flu vaccines and Assays Overview. by Simona Piccirella, PhD Chief Executive Officer
 Correlates of Protection for Flu vaccines and Assays Overview by Simona Piccirella, PhD Chief Executive Officer Company Overview: VisMederi is an Italian private small enterprise established in 2009 and
Correlates of Protection for Flu vaccines and Assays Overview by Simona Piccirella, PhD Chief Executive Officer Company Overview: VisMederi is an Italian private small enterprise established in 2009 and
Acute respiratory illness This is a disease that typically affects the airways in the nose and throat (the upper respiratory tract).
 Influenza glossary Adapted from the Centers for Disease Control and Prevention, US https://www.cdc.gov/flu/glossary/index.htm and the World Health Organization http://www.wpro.who.int/emerging_diseases/glossary_rev_sept28.pdf?ua=1
Influenza glossary Adapted from the Centers for Disease Control and Prevention, US https://www.cdc.gov/flu/glossary/index.htm and the World Health Organization http://www.wpro.who.int/emerging_diseases/glossary_rev_sept28.pdf?ua=1
Influenza Vaccines in older persons 65+ Paul Van Buynder Professor, Griffith University Chairman, Immunisation Coalition
 Influenza Vaccines in older persons 65+ Paul Van Buynder Professor, Griffith University Chairman, Immunisation Coalition Outline 1. Influenza in older persons 2. Vaccine effectiveness in the elderly including
Influenza Vaccines in older persons 65+ Paul Van Buynder Professor, Griffith University Chairman, Immunisation Coalition Outline 1. Influenza in older persons 2. Vaccine effectiveness in the elderly including
1918 Influenza; Influenza A, H1N1. Basic agent information. Section I- Infectious Agent. Section II- Dissemination
 1918 Influenza; Influenza A, H1N1 Basic agent information Section I- Infectious Agent Risk Group: - RG3 Synonym or Cross reference: - Spanish Flu - 1918 Flu - El Grippe Characteristics: - SELECT AGENT
1918 Influenza; Influenza A, H1N1 Basic agent information Section I- Infectious Agent Risk Group: - RG3 Synonym or Cross reference: - Spanish Flu - 1918 Flu - El Grippe Characteristics: - SELECT AGENT
H5N1 and H7 LAIV-IAV Prime-Boost Studies
 NIAID H5N1 and H7 LAIV-IAV Prime-Boost Studies Kanta Subbarao, MD, MPH NIAID, NIH The LID Pandemic Influenza Vaccine Program Program: CRADA with MedImmune Clinical Trials: Center for Immunization Research,
NIAID H5N1 and H7 LAIV-IAV Prime-Boost Studies Kanta Subbarao, MD, MPH NIAID, NIH The LID Pandemic Influenza Vaccine Program Program: CRADA with MedImmune Clinical Trials: Center for Immunization Research,
Supporting Information
 Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
ph1n1 H3N2: A Novel Influenza Virus Reassortment
 ph1n1 H3N2: A Novel Influenza Virus Reassortment Jonathan Gubbay Medical Microbiologist Public Health Laboratory Public Health Ontario June 16, 2011 ph1n1 H3N2 Reassortment: Talk Overview Explain strain
ph1n1 H3N2: A Novel Influenza Virus Reassortment Jonathan Gubbay Medical Microbiologist Public Health Laboratory Public Health Ontario June 16, 2011 ph1n1 H3N2 Reassortment: Talk Overview Explain strain
Incorporating virologic data into seasonal and pandemic influenza vaccines
 Incorporating virologic data into seasonal and pandemic influenza vaccines Kanta Subbarao WHO Collaborating Centre for Reference and Research on Influenza & Department of Microbiology and Immunology, University
Incorporating virologic data into seasonal and pandemic influenza vaccines Kanta Subbarao WHO Collaborating Centre for Reference and Research on Influenza & Department of Microbiology and Immunology, University
24 26 January 2013, Hong Kong SAR, CHINA. TITLE from VIEW and SLIDE MASTER February 27, 2013
 The first WHO integrated meeting on development and clinical trials of influenza vaccines that induce broadly protective and long-lasting immune responses 24 26 January 2013, Hong Kong SAR, CHINA 1 TITLE
The first WHO integrated meeting on development and clinical trials of influenza vaccines that induce broadly protective and long-lasting immune responses 24 26 January 2013, Hong Kong SAR, CHINA 1 TITLE
Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness
 World Health Organization Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness General information Highly pathogenic avian influenza (HPAI)
World Health Organization Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness General information Highly pathogenic avian influenza (HPAI)
Improving Influenza vaccines: Looking ahead
 Improving Influenza vaccines: Looking ahead Gerd Sutter gerd.sutter@lmu.de ESWI Flu Summit - Brussels 30.09.2015 First 20th century pandemic - The Spanish Flu Courtesy: A. Garcia-Sastre Influenza A/CDC/1918
Improving Influenza vaccines: Looking ahead Gerd Sutter gerd.sutter@lmu.de ESWI Flu Summit - Brussels 30.09.2015 First 20th century pandemic - The Spanish Flu Courtesy: A. Garcia-Sastre Influenza A/CDC/1918
INFLUENZA-2 Avian Influenza
 INFLUENZA-2 Avian Influenza VL 7 Dec. 9 th 2013 Mohammed El-Khateeb Overview 1. Background Information 2. Origin/History 3. Brief overview of genome structure 4. Geographical Distribution 5. Pandemic Nature
INFLUENZA-2 Avian Influenza VL 7 Dec. 9 th 2013 Mohammed El-Khateeb Overview 1. Background Information 2. Origin/History 3. Brief overview of genome structure 4. Geographical Distribution 5. Pandemic Nature
Recipients of Vaccine against the 1976 Swine Flu Have Enhanced Neutralization Responses to the 2009 Novel H1N1 Influenza Virus
 MAJOR ARTICLE Recipients of Vaccine against the 1976 Swine Flu Have Enhanced Neutralization Responses to the 2009 Novel H1N1 Influenza Virus Jonathan A. McCullers, Lee-Ann Van De Velde, Kim J. Allison,
MAJOR ARTICLE Recipients of Vaccine against the 1976 Swine Flu Have Enhanced Neutralization Responses to the 2009 Novel H1N1 Influenza Virus Jonathan A. McCullers, Lee-Ann Van De Velde, Kim J. Allison,
A Live Attenuated H7N7 Candidate Vaccine Virus Induces Neutralizing Antibody That Confers Protection from Challenge in Mice, Ferrets, and Monkeys
 JOURNAL OF VIROLOGY, Nov. 2010, p. 11950 11960 Vol. 84, No. 22 0022-538X/10/$12.00 doi:10.1128/jvi.01305-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. A Live Attenuated H7N7
JOURNAL OF VIROLOGY, Nov. 2010, p. 11950 11960 Vol. 84, No. 22 0022-538X/10/$12.00 doi:10.1128/jvi.01305-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. A Live Attenuated H7N7
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Influenza Virus Genotypes Circulating In Central Greece During And Vaccine Strain Match
 ISPUB.COM The Internet Journal of Microbiology Volume 13 Number 1 Influenza Virus Genotypes Circulating In Central Greece During 2012-2014 And Vaccine Strain Match E Plakokefalos, A Vontas, Z Florou, G
ISPUB.COM The Internet Journal of Microbiology Volume 13 Number 1 Influenza Virus Genotypes Circulating In Central Greece During 2012-2014 And Vaccine Strain Match E Plakokefalos, A Vontas, Z Florou, G
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set
 Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
100 years of Influenza Pandemic and the prospects for new influenza vaccines
 100 years of Influenza Pandemic and the prospects for new influenza vaccines Dr John McCauley Director, WHO Collaborating Centre for Reference and Research on influenza The Francis Crick Institute London
100 years of Influenza Pandemic and the prospects for new influenza vaccines Dr John McCauley Director, WHO Collaborating Centre for Reference and Research on influenza The Francis Crick Institute London
Supporting Information
 Supporting Information Yen et al. 10.1073/pnas.1111000108 SI Materials and Methods Cells. Madin Darby canine kidney (MDCK) cells and human embryonic kidney 293T cells were obtained from the American Type
Supporting Information Yen et al. 10.1073/pnas.1111000108 SI Materials and Methods Cells. Madin Darby canine kidney (MDCK) cells and human embryonic kidney 293T cells were obtained from the American Type
Influenza: The past, the present, the (future) pandemic
 Influenza: The past, the present, the (future) pandemic Kristin Butler, MLS (ASCP) cm Department of Clinical Laboratory Sciences Louisiana Health Sciences Center - Shreveport Fall 2017 Objectives 1) Detail
Influenza: The past, the present, the (future) pandemic Kristin Butler, MLS (ASCP) cm Department of Clinical Laboratory Sciences Louisiana Health Sciences Center - Shreveport Fall 2017 Objectives 1) Detail
Influenza vaccines. Cheryl Cohen
 Influenza vaccines Cheryl Cohen cherylc@nicd.ac.za Overview Burden of influenza and risk groups Clinical presentation, diagnosis and treatment Influenza the virus Currently available influenza vaccines
Influenza vaccines Cheryl Cohen cherylc@nicd.ac.za Overview Burden of influenza and risk groups Clinical presentation, diagnosis and treatment Influenza the virus Currently available influenza vaccines
Patricia Fitzgerald-Bocarsly
 FLU Patricia Fitzgerald-Bocarsly October 23, 2008 Orthomyxoviruses Orthomyxo virus (ortho = true or correct ) Negative-sense RNA virus (complementary to mrna) Five different genera Influenza A, B, C Thogotovirus
FLU Patricia Fitzgerald-Bocarsly October 23, 2008 Orthomyxoviruses Orthomyxo virus (ortho = true or correct ) Negative-sense RNA virus (complementary to mrna) Five different genera Influenza A, B, C Thogotovirus
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit
 2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
GSK s Candidate Influenza A (H5N1) Virus Monovalent Vaccine
 GSK s Candidate Influenza A (H5N1) Virus Monovalent Vaccine Regulatory Pathway for Licensure VRBPAC, February 29, 2012 Katalin Abraham, Director, US Regulatory Affairs GSK Biologicals GSK s Influenza Vaccines
GSK s Candidate Influenza A (H5N1) Virus Monovalent Vaccine Regulatory Pathway for Licensure VRBPAC, February 29, 2012 Katalin Abraham, Director, US Regulatory Affairs GSK Biologicals GSK s Influenza Vaccines
Recommended composition of influenza virus vaccines for use in the influenza season
 Recommended composition of influenza virus vaccines for use in the 2006 2007 influenza season This recommendation relates to the composition of vaccines for the forthcoming influenza season in the northern
Recommended composition of influenza virus vaccines for use in the 2006 2007 influenza season This recommendation relates to the composition of vaccines for the forthcoming influenza season in the northern
بسم هللا الرحمن الرحيم
 - 1 - - - 1 P a g e بسم هللا الرحمن الرحيم This sheet was made from record section 1 all information are included - Introduction Our respiratory tract is divided anatomically to upper (URT),middle and
- 1 - - - 1 P a g e بسم هللا الرحمن الرحيم This sheet was made from record section 1 all information are included - Introduction Our respiratory tract is divided anatomically to upper (URT),middle and
Lars R. Haaheim ( ) PhD, Professor Emeritus, University of Bergen, Norway
 Lars R. Haaheim (1945-2011) PhD, Professor Emeritus, University of Bergen, Norway Licensing requirements a personal perspective John M Wood Summer School on Influenza, Siena August 1-5 2011 National Institute
Lars R. Haaheim (1945-2011) PhD, Professor Emeritus, University of Bergen, Norway Licensing requirements a personal perspective John M Wood Summer School on Influenza, Siena August 1-5 2011 National Institute
Influenza 2009: Not Yet The Perfect Storm
 Influenza 2009: Not Yet The Perfect Storm What s needed for a pandemic strain? Novel virus (little to no immunity) Capable of causing disease in humans Highly pathogenic / virulent Capable of sustained
Influenza 2009: Not Yet The Perfect Storm What s needed for a pandemic strain? Novel virus (little to no immunity) Capable of causing disease in humans Highly pathogenic / virulent Capable of sustained
Orthomyxoviridae and Paramyxoviridae. Lecture in Microbiology for medical and dental medical students
 Orthomyxoviridae and Paramyxoviridae Lecture in Microbiology for medical and dental medical students Orthomyxoviridae and Paramyxoviridae are ss RNA containng viruses Insert Table 25.1 RNA viruses 2 SIZE
Orthomyxoviridae and Paramyxoviridae Lecture in Microbiology for medical and dental medical students Orthomyxoviridae and Paramyxoviridae are ss RNA containng viruses Insert Table 25.1 RNA viruses 2 SIZE
Sponsors. Production Assistants Steven Claas Lynn Leary. Layout David Brown
 Sponsors University of Minnesota College of Veterinary Medicine College of Agricultural, Food and Environmental Sciences Extension Service Swine Center Production Assistants Steven Claas Lynn Leary Layout
Sponsors University of Minnesota College of Veterinary Medicine College of Agricultural, Food and Environmental Sciences Extension Service Swine Center Production Assistants Steven Claas Lynn Leary Layout
Influenza A virus infection predisposes hosts to secondary infection with different
 Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Reagents for the Typing of Human Influenza Isolates 2011
 Reagents for the Typing of Human Influenza Isolates 2011 This product was developed by the Victorian Infectious Diseases Reference Laboratory (VIDRL) in its capacity as a WHO Collaborating Centre for Reference
Reagents for the Typing of Human Influenza Isolates 2011 This product was developed by the Victorian Infectious Diseases Reference Laboratory (VIDRL) in its capacity as a WHO Collaborating Centre for Reference
Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza
 The Journal of Infectious Diseases BRIEF REPORT Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza Kirsty R. Short, 1,2 Rebecca Veeris, 1 Lonneke M. Leijten, 1 Judith
The Journal of Infectious Diseases BRIEF REPORT Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza Kirsty R. Short, 1,2 Rebecca Veeris, 1 Lonneke M. Leijten, 1 Judith
Human Influenza. Dr. Sina Soleimani. Human Viral Vaccine Quality Control 89/2/29. November 2, 2011 HVVQC ١
 Human Influenza Dr. Sina Soleimani Human Viral Vaccine Quality Control 89/2/29 November 2, 2011 HVVQC ١ Presentation outline 1. Introduction 2. Virology 3. Classification 4. Hosts 5. Antigenic Specifications
Human Influenza Dr. Sina Soleimani Human Viral Vaccine Quality Control 89/2/29 November 2, 2011 HVVQC ١ Presentation outline 1. Introduction 2. Virology 3. Classification 4. Hosts 5. Antigenic Specifications
Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY. Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p.
 Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
A paradigm shift in vaccine production for pandemic influenza
 A paradigm shift in vaccine production for pandemic influenza John Steel, Emory University Journal Title: Annals of translational medicine Publisher: AME Publishing Company 2015-07 Type of Work: Article
A paradigm shift in vaccine production for pandemic influenza John Steel, Emory University Journal Title: Annals of translational medicine Publisher: AME Publishing Company 2015-07 Type of Work: Article
This product was developed by the Victorian Infectious Diseases Reference Laboratory (VIDRL) in its capacity as a WHO Collaborating Centre for
 This product was developed by the Victorian Infectious Diseases Reference Laboratory (VIDRL) in its capacity as a WHO Collaborating Centre for Reference and Research on Influenza, with material provided
This product was developed by the Victorian Infectious Diseases Reference Laboratory (VIDRL) in its capacity as a WHO Collaborating Centre for Reference and Research on Influenza, with material provided
Histopathology: pulmonary pathology
 Histopathology: pulmonary pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about these
Histopathology: pulmonary pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about these
BRIEF REPORT. The degree of immunity to pandemic influenza A(H1N1) 2009 (hereafter pandemic H1N1) in humans positively correlates
 BRIEF REPORT Inactivated Seasonal Influenza Vaccines Increase Serum Antibodies to the Neuraminidase of Pandemic Influenza A(H1N1) 2009 Virus in an Age-Dependent Manner Glendie Marcelin, 1 Hilliary M. Bland,
BRIEF REPORT Inactivated Seasonal Influenza Vaccines Increase Serum Antibodies to the Neuraminidase of Pandemic Influenza A(H1N1) 2009 Virus in an Age-Dependent Manner Glendie Marcelin, 1 Hilliary M. Bland,
UNIVERSAL INFLUENZA VIRUS VACCINES Adolfo García Sastre. Icahn School of Medicine at Mount Sinai, New York
 UNIVERSAL INFLUENZA VIRUS VACCINES Adolfo García Sastre Icahn School of Medicine at Mount Sinai, New York INFLUENZA VIRUSES PAx B EPIDEMIOLOGY OF HUMAN INFLUENZA VIRUSES A H1N1 H3N2 1968 H2N2 1957 H1N1
UNIVERSAL INFLUENZA VIRUS VACCINES Adolfo García Sastre Icahn School of Medicine at Mount Sinai, New York INFLUENZA VIRUSES PAx B EPIDEMIOLOGY OF HUMAN INFLUENZA VIRUSES A H1N1 H3N2 1968 H2N2 1957 H1N1
Supplemental Information Dose Response Parameters for Gain of Function Pathogens
 Supplemental Information Dose Response Parameters for Gain of Function Pathogens Infection Dose-Response To quantify the likelihood of an individual or animal becoming infected from exposure to virus,
Supplemental Information Dose Response Parameters for Gain of Function Pathogens Infection Dose-Response To quantify the likelihood of an individual or animal becoming infected from exposure to virus,
Trial of 2009 Influenza A (H1N1) Monovalent MF59-Adjuvanted Vaccine
 The new england journal of medicine original article Trial of 2009 Influenza A (H1N1) Monovalent MF59-Adjuvanted Vaccine Tristan W. Clark, M.R.C.P., Manish Pareek, M.R.C.P., Katja Hoschler, Ph.D., Helen
The new england journal of medicine original article Trial of 2009 Influenza A (H1N1) Monovalent MF59-Adjuvanted Vaccine Tristan W. Clark, M.R.C.P., Manish Pareek, M.R.C.P., Katja Hoschler, Ph.D., Helen
Prevalence of Antibodies against Seasonal Influenza A and B Viruses in Children in Netherlands
 CLINICAL AND VACCINE IMMUNOLOGY, Mar. 2011, p. 469 476 Vol. 18, No. 3 1556-6811/11/$12.00 doi:10.1128/cvi.00396-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Prevalence of
CLINICAL AND VACCINE IMMUNOLOGY, Mar. 2011, p. 469 476 Vol. 18, No. 3 1556-6811/11/$12.00 doi:10.1128/cvi.00396-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Prevalence of
Technology Overview. Summary
 Live Attenuated Influenza Vaccines with Altered NS1 Technology Overview Summary Transformative Technology: Live attenuated influenza vaccines (LAIVs) with precise, genetically stable truncations of the
Live Attenuated Influenza Vaccines with Altered NS1 Technology Overview Summary Transformative Technology: Live attenuated influenza vaccines (LAIVs) with precise, genetically stable truncations of the
RESPIRATORY TRACT INFECTIONS. CLS 212: Medical Microbiology Zeina Alkudmani
 RESPIRATORY TRACT INFECTIONS CLS 212: Medical Microbiology Zeina Alkudmani Lower Respiratory Tract Upper Respiratory Tract Anatomy of the Respiratory System Nasopharynx Oropharynx Respiratory Tract Infections
RESPIRATORY TRACT INFECTIONS CLS 212: Medical Microbiology Zeina Alkudmani Lower Respiratory Tract Upper Respiratory Tract Anatomy of the Respiratory System Nasopharynx Oropharynx Respiratory Tract Infections
Vaccine. Design and Manufacturing. Liting Bi. https://en.wikipedia.org/wiki/vaccine
 Vaccine Design and Manufacturing Liting Bi https://en.wikipedia.org/wiki/vaccine 1 Outline Vaccine Intro. 4 Vaccine Types 2 Manufacturing Methods 2 Tests & Applications Take-home messages 2 https://www.youtube.com/watch?v=t_me5ef0ne4
Vaccine Design and Manufacturing Liting Bi https://en.wikipedia.org/wiki/vaccine 1 Outline Vaccine Intro. 4 Vaccine Types 2 Manufacturing Methods 2 Tests & Applications Take-home messages 2 https://www.youtube.com/watch?v=t_me5ef0ne4
Flu, Avian Flu and emerging aspects (H1N1 resistance)
 EU-CIS Seminar New trends in Infectious Diseases 26 28 November 2008 / Lyon, France Flu, Avian Flu and emerging aspects (H1N1 resistance) Pr. Florence MORFIN FRE 3011 Université Lyon 1 - CNRS Laboratory
EU-CIS Seminar New trends in Infectious Diseases 26 28 November 2008 / Lyon, France Flu, Avian Flu and emerging aspects (H1N1 resistance) Pr. Florence MORFIN FRE 3011 Université Lyon 1 - CNRS Laboratory
Influenza Update. Lisa Grohskopf, MD, MPH Influenza Division, CDC. NAICP Call 6 October 2015
 Influenza Update Lisa Grohskopf, MD, MPH Influenza Division, CDC NAICP Call 6 October 2015 National Center for Immunization and Respiratory Diseases Influenza Division Overview Surveillance update ACIP
Influenza Update Lisa Grohskopf, MD, MPH Influenza Division, CDC NAICP Call 6 October 2015 National Center for Immunization and Respiratory Diseases Influenza Division Overview Surveillance update ACIP
6.2 Pandemic influenza
 6.2 Pandemic influenza See Background Paper 6.2 (BP6_2Pandemic.pdf) Background and developments since 2004 The WHO estimates that annual influenza epidemics account for about 3 to 5 million cases of severe
6.2 Pandemic influenza See Background Paper 6.2 (BP6_2Pandemic.pdf) Background and developments since 2004 The WHO estimates that annual influenza epidemics account for about 3 to 5 million cases of severe
Preparing for the Fall Flu Season. Jonathan Gubbay Medical Microbiologist Public Health Laboratory OAHPP
 Preparing for the Fall Flu Season Laboratory Perspective Jonathan Gubbay Medical Microbiologist Public Health Laboratory OAHPP September 21, 2009 Objectives 1. Review the emergence of Novel Influenza A
Preparing for the Fall Flu Season Laboratory Perspective Jonathan Gubbay Medical Microbiologist Public Health Laboratory OAHPP September 21, 2009 Objectives 1. Review the emergence of Novel Influenza A
Pandemic Influenza influenza epidemic: realization of a worst-case scenario
 Pandemic Influenza October 9, 2006 1918 influenza epidemic: realization of a worst-case scenario First case: Albert Mitchell, Camp Funston, KS, March 11, 1918 Up to 20% of all humans infected 20-50 million
Pandemic Influenza October 9, 2006 1918 influenza epidemic: realization of a worst-case scenario First case: Albert Mitchell, Camp Funston, KS, March 11, 1918 Up to 20% of all humans infected 20-50 million
Antigenic cartography of H5 viruses
 14th joint annual meeting of the national reference laboratories for AI and ND Brussels, April 10, 2008 Antigenic cartography of H5 viruses Ron A.M. Fouchier, PhD. Professor Molecular Virology National
14th joint annual meeting of the national reference laboratories for AI and ND Brussels, April 10, 2008 Antigenic cartography of H5 viruses Ron A.M. Fouchier, PhD. Professor Molecular Virology National
(JPC ) Caprine lungs
 2011-7-2 (JPC 3133973) Caprine lungs Bat Otgontugs Bovine Pathology Contributor: Natoinal Institute Animal Health, Tsukuba, Japan Signalment: 5-year 3-month old female Japanese native breed goat, (Capra
2011-7-2 (JPC 3133973) Caprine lungs Bat Otgontugs Bovine Pathology Contributor: Natoinal Institute Animal Health, Tsukuba, Japan Signalment: 5-year 3-month old female Japanese native breed goat, (Capra
Introduction. In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology.
 Introduction In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology. - Genomics: fasten antigen discovery for complex pathogens
Introduction In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology. - Genomics: fasten antigen discovery for complex pathogens
C E E Z A D. Rational Development of Influenza Vaccines: NDV-based influenza vaccines for poultry and livestock
 C E E Z A D Center of Excellence for Emerging and Zoonotic Animal Diseases A Department of Homeland Security Center of Excellence Rational Development of Influenza Vaccines: NDV-based influenza vaccines
C E E Z A D Center of Excellence for Emerging and Zoonotic Animal Diseases A Department of Homeland Security Center of Excellence Rational Development of Influenza Vaccines: NDV-based influenza vaccines
Influenza. Gwen Clutario, Terry Chhour, Karen Lee
 Influenza Gwen Clutario, Terry Chhour, Karen Lee Overview Commonly referred to as the flu Defined as a highly contagious viral infection where it starts at the upper respiratory tract and attacks the nose,
Influenza Gwen Clutario, Terry Chhour, Karen Lee Overview Commonly referred to as the flu Defined as a highly contagious viral infection where it starts at the upper respiratory tract and attacks the nose,
Overview of the Influenza Virus
 Overview of the Influenza Virus Victor C. Huber, Ph.D. September 24, 2015 victor.huber@usd.edu General Features of Influenza Virus Infections Clinical Features of Influenza Sudden onset of symptoms Incubation
Overview of the Influenza Virus Victor C. Huber, Ph.D. September 24, 2015 victor.huber@usd.edu General Features of Influenza Virus Infections Clinical Features of Influenza Sudden onset of symptoms Incubation
Received 21 March 2011/Returned for modification 19 May 2011/Accepted 19 July 2011
 CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2011, p. 1401 1405 Vol. 18, No. 9 1556-6811/11/$12.00 doi:10.1128/cvi.05046-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Immunogenicity,
CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2011, p. 1401 1405 Vol. 18, No. 9 1556-6811/11/$12.00 doi:10.1128/cvi.05046-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Immunogenicity,
Live Attenuated Influenza Vaccine. I. Background and Seasonal Vaccine
 Live Attenuated Influenza Vaccine I. Background and Seasonal Vaccine Influenza infection stimulates multiple arms of the immune system Systemic antibody to HA and NA, and multiple internal proteins Mucosal
Live Attenuated Influenza Vaccine I. Background and Seasonal Vaccine Influenza infection stimulates multiple arms of the immune system Systemic antibody to HA and NA, and multiple internal proteins Mucosal
Infection dynamics of novel influenza A viruses isolated from Australian pigs
 Infection dynamics of novel influenza A viruses isolated from Australian pigs Joanne Taylor PhD Candidate Supervisors: Dr Frank Wong, Dr David Williams, Dr John Bingham, Dr Joanne Meers 3 rd December 2015
Infection dynamics of novel influenza A viruses isolated from Australian pigs Joanne Taylor PhD Candidate Supervisors: Dr Frank Wong, Dr David Williams, Dr John Bingham, Dr Joanne Meers 3 rd December 2015
Zoonotic potential of non-avian influenza A viruses
 Laboratory of Virology Faculty of Veterinary Medicine Ghent University, Belgium Zoonotic potential of non-avian influenza A viruses Prof. Kristien Van Reeth (1) Several documented cases of influenza virus
Laboratory of Virology Faculty of Veterinary Medicine Ghent University, Belgium Zoonotic potential of non-avian influenza A viruses Prof. Kristien Van Reeth (1) Several documented cases of influenza virus
Sensitivity and Specificity of Serologic Assays for Detection of Human Infection with 2009 Pandemic H1N1 Virus in U.S. Populations
 JOURNAL OF CLINICAL MICROBIOLOGY, June 2011, p. 2210 2215 Vol. 49, No. 6 0095-1137/11/$12.00 doi:10.1128/jcm.00229-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Sensitivity
JOURNAL OF CLINICAL MICROBIOLOGY, June 2011, p. 2210 2215 Vol. 49, No. 6 0095-1137/11/$12.00 doi:10.1128/jcm.00229-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Sensitivity
H7N9 Live Attenuated Influenza Vaccine Is Highly Immunogenic, Prevents Virus Replication, and Protects Against Severe Bronchopneumonia in Ferrets
 The American Society of Gene & Cell Therapy original article H7N9 Live Attenuated Influenza Vaccine Is Highly Immunogenic, Prevents Virus Replication, and Protects Against Severe Bronchopneumonia in Ferrets
The American Society of Gene & Cell Therapy original article H7N9 Live Attenuated Influenza Vaccine Is Highly Immunogenic, Prevents Virus Replication, and Protects Against Severe Bronchopneumonia in Ferrets
PATH Influenza Vaccine Projects
 PATH Influenza Vaccine Projects Overview John W. Boslego, MD John Boslego Director, Vaccine Development Global Program March 25 th, 2014 Influenza Vaccine Project (IVP) at PATH IVP Goal: Advance the development
PATH Influenza Vaccine Projects Overview John W. Boslego, MD John Boslego Director, Vaccine Development Global Program March 25 th, 2014 Influenza Vaccine Project (IVP) at PATH IVP Goal: Advance the development
Review on vectored influenza vaccines. Sarah Gilbert Jenner Institute Oxford
 Review on vectored influenza vaccines Sarah Gilbert Jenner Institute Oxford Viral Vectored Influenza Vaccines Can be used to induce antibodies against HA Will also boost CD4 + T cell responses against
Review on vectored influenza vaccines Sarah Gilbert Jenner Institute Oxford Viral Vectored Influenza Vaccines Can be used to induce antibodies against HA Will also boost CD4 + T cell responses against
Influenza A 6/23/2010. Lisa Winston, MD UCSF / San Francisco General Hospital Divisions of Infectious Diseases and Hospital Medicine
 Influenza Update in a Pandemic Year Nothing to disclose. Lisa Winston, MD UCSF / San Francisco General Hospital Divisions of Infectious Diseases and Hospital Medicine Influenza Biology Influenza Biology
Influenza Update in a Pandemic Year Nothing to disclose. Lisa Winston, MD UCSF / San Francisco General Hospital Divisions of Infectious Diseases and Hospital Medicine Influenza Biology Influenza Biology
INFLUENZA A VIRUS. Structure of the influenza A virus particle.
 INFLUENZA INFLUENZA A VIRUS Structure of the influenza A virus particle. TYPE A VIRUS HAS TWO TYPES OF SPIKES, THE HEMAGGLUTININ (H) AND THE NEURAMINIDASE (N), PROTRUDING FROM THE VIRAL ENVELOPE THE HEMAGGLUTININ
INFLUENZA INFLUENZA A VIRUS Structure of the influenza A virus particle. TYPE A VIRUS HAS TWO TYPES OF SPIKES, THE HEMAGGLUTININ (H) AND THE NEURAMINIDASE (N), PROTRUDING FROM THE VIRAL ENVELOPE THE HEMAGGLUTININ
Influenza virus evolution: A tale of birds, pigs, and other mammals.
 Vaccination: an evolutionary engine for species? Les Pensieres November 26 th, 2013 Influenza virus evolution: A tale of birds, pigs, and other mammals. Albert Osterhaus DVM PhD Professor/Head Department
Vaccination: an evolutionary engine for species? Les Pensieres November 26 th, 2013 Influenza virus evolution: A tale of birds, pigs, and other mammals. Albert Osterhaus DVM PhD Professor/Head Department
Abstract: I. A ims Aim 1:
 Abstract: Previous work from our laboratory demonstrated that obese mice have alterations in antiviral cytokine gene expression when infected with the influenza virus. Since these cytokines play a major
Abstract: Previous work from our laboratory demonstrated that obese mice have alterations in antiviral cytokine gene expression when infected with the influenza virus. Since these cytokines play a major
THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION OF ANTIBODIES IN THE SERUM OF SHEEP.
 Onderstepoort Journal of Veterinary Research, Volume 27, Number 2, October, 1956. The Government Printer. THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION
Onderstepoort Journal of Veterinary Research, Volume 27, Number 2, October, 1956. The Government Printer. THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION
Interaction between Mycoplasma hyopneumoniae and Swine Influenza Virus
 JOURNAL OF CLINICAL MICROBIOLOGY, July 2001, p. 2525 2530 Vol. 39, No. 7 0095-1137/01/$04.00 0 DOI: 10.1128/JCM.39.7.2525 2530.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, July 2001, p. 2525 2530 Vol. 39, No. 7 0095-1137/01/$04.00 0 DOI: 10.1128/JCM.39.7.2525 2530.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved.
VIROLOGY OF INFLUENZA. Subtypes: A - Causes outbreak B - Causes outbreaks C - Does not cause outbreaks
 INFLUENZA VIROLOGY OF INFLUENZA Subtypes: A - Causes outbreak B - Causes outbreaks C - Does not cause outbreaks PATHOGENICITY High pathogenicity avian influenza (HPAI) Causes severe disease in poultry
INFLUENZA VIROLOGY OF INFLUENZA Subtypes: A - Causes outbreak B - Causes outbreaks C - Does not cause outbreaks PATHOGENICITY High pathogenicity avian influenza (HPAI) Causes severe disease in poultry
Influenza Viruses A Review
 Influenza Viruses A Review AVIAN INFLUENZA: INTERSECTORAL COLLABORATION Larnaca, Cyprus 20 22 July 2009 Kate Glynn Scientific and Technical Department, OIE Influenza Viruses C. Goldsmith,1981 Influenza
Influenza Viruses A Review AVIAN INFLUENZA: INTERSECTORAL COLLABORATION Larnaca, Cyprus 20 22 July 2009 Kate Glynn Scientific and Technical Department, OIE Influenza Viruses C. Goldsmith,1981 Influenza
