Rhodococcus equi Pneumonia
|
|
|
- Walter Stanley
- 6 years ago
- Views:
Transcription
1 Rhodococcus equi Pneumonia Steeve Giguère, DVM, PhD, Diplomate ACVIM Rhodococcus equi is the most devastating cause of pneumonia in foals between 3 weeks and 5 months of age. Much progress has been made in recent years in understanding the epizootiology and pathogenesis of R. equi infections. This article reviews recent advances with emphasis on diagnosis, treatment, and prevention of R. equi infections. Author s address: Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL AAEP. 1. Introduction Rhodococcus equi, a Gram-positive facultative intracellular pathogen, is the most devastating cause of pneumonia in foals between 3 weeks and 5 months of age. R. equi has also emerged as a significant opportunistic pathogen in immunosuppressed people, especially those infected with the human immunodeficiency virus. 1,2 Although all horse farms are likely to be infected to various degree with R. equi and antibody is widespread in horse populations, the clinical disease is enzootic and devastating on some farms, sporadic on others, and unrecognized on most. On farms where the disease is enzootic, costs associated with veterinary care, early diagnosis, long-term therapy, and mortality of foals may be very high. In addition to significant immediate costs, R. equi pneumonia has a long-term detrimental effect on the equine industry because foals that recover from the disease are less likely to race as adults. 3 Much progress has been made in recent years in understanding the epizootiology and pathogenesis of R. equi infections. These advances have been valuable in the development of rapid diagnostic techniques and more effective strategies for the control of the disease. This article reviews recent advances with emphasis on diagnosis, treatment, and prevention of R. equi infections. 2. Clinical Manifestations The most common manifestation of R. equi infections in foals is a chronic suppurative bronchopneumonia with extensive abscessation. The slow spread of the lung infection, combined with the remarkable ability of foals to compensate for the progressive loss of functional lung, make early clinical diagnosis difficult. Early clinical signs often only include a mild fever or a slight increase in respiratory rate that may not be apparent unless foals are exercised or stressed by handling. As the pneumonia progresses, clinical signs may include decreased appetite, lethargy, fever, tachypnea, and increased effort of breathing characterized by nostril flaring and increased abdominal effort. Cough and bilateral nasal discharge are inconsistent findings. A smaller percentage of affected foals may present with a more devastating subacute form. These foals may be found dead or, more commonly, present in acute respiratory distress with a high fever and no previous history of clinical respiratory disease. Foals with the subacute form of the disease have a poor prognosis despite appropriate therapy. NOTES Vol. 47 AAEP PROCEEDINGS
2 Extrapulmonary manifestations of rhodococcal infections may also occur. Intestinal lesions are present in approximately 50% of foals with R. equi pneumonia presented for necropsy. 4 However, the majority of foals with R. equi pneumonia do not show clinical signs of intestinal disease. In the same study, only 4% of the foals had intestinal lesions without pneumonia. 4 The intestinal form of R. equi infection is characterized by a multifocal ulcerative enterocolitis and typhlitis over the area of the Peyer s patches with granulomatous or suppurative inflammation of the mesenteric and/or colonic lymph nodes. 4 Occasionally, a single large abdominal abscess (usually in a mesenteric lymph node), often causing adhesion to the large or small bowel, is the only finding. Clinical signs associated with the abdominal form of the disease may include fever, depression, anorexia, weight loss, colic, and diarrhea. 5 Marked gastrointestinal lymphatic obstruction associated with increased protein concentration in the peritoneal fluid and hypoproteinemia may lead to ascites giving foals a pot-bellied appearance. Such foals have a poor prognosis because of the extensive granulomatous inflammation of the colonic mucosa and submucosa and mesenteric lymph nodes. Immune-complex deposition within synovial structures leads to polysynovitis in approximately one-third of cases with R. equi pneumonia. 6 8 The tibiotarsal and stifle joints are most commonly affected. Occasionally all joints are affected. The degree of joint effusion is variable and, in most cases, lameness is not apparent or limited to a stiff gait. Cytological examination of the synovial fluid usually reveals a non-septic mononuclear pleocytosis and bacteriologic culture of the synovial fluid is negative. 8 Histologic examination of the synovial membrane reveals lymphoplasmacytic synovitis. 6,7 Fluoresceinlabeled anti-equine IgG staining of the synovial membrane of three affected foals revealed evidence of immunoglobulin within the synovial membrane. 7 Rheumatoid factors (i.e., antibodies directed against autologous or heterologous Fc portion of immunoglobulin) were also identified in the synovial fluid of a foal with non-septic joint effusion and R. equi pneumonia. 6 Local therapy of the affected joints is not indicated as the effusion usually resolves without any apparent consequences as the primary infection resolves with antimicrobial therapy. The presence of a non-septic polysynovitis in a foal between 3 weeks and 6 months of age is highly suggestive of R. equi infection and deserves further investigation (see section 7. Diagnosis). Immune complex deposition may also contribute to the development of uveitis, anemia and thrombocytopenia in some foals. 9 Bacteremic spread of the organism from the lungs or gastrointestinal tract may occasionally result in septic arthritis or osteomyelitis. However, foals can occasionally develop R. equi septic arthritis or osteomyelitis without apparent lung or other source of infection. The degree of lameness of foals with septic arthritis distinguishes them from foals with immune-mediated polysynovitis. In equivocal cases, bacterial culture and cytological examination of the synovial fluid should be performed. In addition to appropriate antimicrobial therapy (see treatment), foals with R. equi septic arthritis and osteomyelitis often require aggressive local therapy. R. equi vertebral osteomyelitis or diskospondylitis resulting in spinal cord compression has also been reported Other rare extrapulmonary manifestations of R. equi infections in foals include panophthalmitis, guttural pouch empyema, sinusitis, pericarditis, nephritis, and hepatic and renal abscessation. 9 Ulcerative lymphangitis, cellulitis, and subcutaneous abscesses have also been reported. Disease due to R. equi is rare in adult horses. There are a few reports of sporadically occurring illness similar to that observed in foals involving primarily lungs or colon and related lymph nodes, or rarely as a wound infection. In one adult horse, acquired combined immunodeficiency of unknown origin led to R. equi septicemia and lung abscessation. 13 Rarely, the organism has also been isolated from infertile mares and aborted fetuses. 4,14 3. Pathogenesis R. equi is a facultative intracellular pathogen and its infectivity in vitro is limited to cells of the monocytemacrophage lineage. 15 The ability of R. equi to persist in, and eventually destroy, alveolar macrophages seems to be the basis of its pathogenicity. Intracellular persistence correlates with the absence of phagosome-lysosome fusion and phagocytosis of R. equi by equine macrophages is not associated with a functional respiratory burst As opposed to resident macrophages, interferon (IFN)- -activated mouse macrophages produce both reactive oxygen (ROI) and reactive nitrogen (RNI) intermediates. These two radicals combine to form peroxynitrite, which efficiently kills R. equi. 19 Neither ROI nor RNI alone is sufficient to mediate killing of R. equi. 19 Optimal binding of R. equi to mouse macrophages in vitro requires complement and is mediated leukocyte complement receptors type 3 (CR3, CD11b/CD18). 15 Entry of several microorganisms into macrophages after adherence to complement receptors has been shown to allow them to avoid the toxic consequences of the oxidative burst. Opsonization of R. equi with specific antibody is associated with increased phagosome-lysosome fusion and significantly enhances killing of R. equi by equine macrophages suggesting that the mechanism of cellular entry can mediate the fate of the bacteria. 17 As opposed to macrophages, neutrophils from foals and adult horses are fully bactericidal and killing of R. equi is considerably enhanced by specific opsonizing antibody The ability of R. equi to induce disease in foals likely depends on both host and microbial factors. Knowledge of the virulence mechanisms of R. equi were largely speculative until the discovery of the AAEP PROCEEDINGS Vol
3 virulence plasmid. 25,26 Unlike most environmental R. equi, isolates from pneumonic foals typically contain kb plasmids encoding a family of seven closely related virulence-associated proteins designated VapA and VapC to VapH. 25,27 31 VapA is expressed on the bacterial surface and its expression is temperature regulated, occurring between 34 C and 41 C. 32 VapC, -D, and -E are secreted proteins coordinately regulated by temperature with VapA. 33 Plasmid-cured derivatives of virulent R. equi strains lose their ability to replicate and survive in macrophages (Fig. 1). 34 Plasmid-cured derivatives also fail to induce pneumonia and are completely cleared from the lungs of foals two weeks following heavy intrabronchial challenge, confirming the absolute necessity of the large plasmid for the virulence of R. equi (Fig. 2). 34,35 A recombinant plasmid-cured derivative expressing wild-type levels of VapA failed to survive and replicate in macrophages and remained avirulent for foals showing that expression of VapA alone is not sufficient to restore the virulence phenotype. 34 Simultaneous expression of all the vap-like genes may be necessary for virulence. The precise role of each of these genes in the pathophysiology of R. equi infections remains to be determined. Cell wall mycolic acid-containing glycolipids may also contribute to virulence of R. equi. Strains with a longer carbon chain mycolic are more virulent as determined by lethality and granuloma formation in mice than those with shorter chains. 36 Other unexplored candidates as virulence factors include capsular polysaccharides as well as cholesterol oxidase, choline phosphohydrolase, and phospholipase C exoenzymes ( equi factors ). However, both capsule and exoenzymes are produced by virulent as well as by avirulent strains suggesting that their contribution to virulence, if any, is insignificant in comparison to plasmid-mediated functions. 4. Epidemiology R. equi is a soil organism with simple growth requirements. Intestinal carriage in adult herbivores is passive and only represents acquisition from contaminated grass, but the organism multiplies in the intestine of the foal up to about three months of age, reaching numbers up to 10 5 colony forming Fig. 1. Role of the large plasmid in survival and replication of R. equi in macrophages. Mouse peritoneal macrophages were infected in vitro with either the plasmid-containing and VapAexpressing strain 103 or with its plasmid-cured derivative (103 ) using an infectivity ratio of 5 bacteria/macrophage. At 48 h post infection, 103 infected monolayers were heavily infected (A, arrows) whereas monolayers infected with 103 had only a few remaining intracellular bacteria (B). Fig. 2. Pathological findings in foals infected intrabronchially with R. equi strain 103 or 103. (A) Cross section of a cranioventral lung in a foal infected with R. equi 103 showing no lesions on day 14 postinfection. (B) Cross section of a cranioventral lung lobe in a foal infected with R. equi 103 showing multiple well-defined nodular areas of pulmonary consolidation on day 14 postinfection Vol. 47 AAEP PROCEEDINGS
4 units (CFU)/g of feces. 37 Since pneumonic foals swallow infected sputum, the virulent bacteria so swallowed can multiply in their intestine. Therefore, the manure of R. equi affected foals is likely a major source of progressive contamination of the environment with virulent organisms. Under suitable conditions of high summer temperatures, R. equi can multiply in the environment by 10,000-fold in only 2 weeks. 38 A single gram of soil contaminated with foal manure may therefore, under favorable conditions, contain millions of virulent R. equi. Inhalation of dust particles laden with virulent R. equi is the major route of pneumonic infection. Ingestion of the organism is a significant route of exposure, and likely also of immunization, but rarely leads to hematogenously acquired pneumonia unless the foal has multiple exposures to large numbers of bacteria. 39 Although all horse farms are likely infected with R. equi, the incidence of clinical disease varies from farm to farm. This probably reflects differences in environmental and management conditions, as well as differences in virulence of isolate. Although the total numbers of R. equi in the environment may be similar in farms with and without a history of R. equi infections, farms with enzootic disease tend to be more heavily infected with virulent R. equi. 27 In a survey of the prevalence of virulent R. equi at horse breeding farms in Japan, the organism was isolated from almost all soil samples, at numbers of CFU per gram of soil. The vast majority of these isolates did not contain plasmids and were avirulent. Virulent R. equi isolates containing kb plasmids and expressing VapA were cultured from 24 of the 31 farms examined. On those farms, virulent R. equi represented 1.7 to 23.3% of all isolates R. equi Isolates in Immunosuppressed Humans Analysis of 39 isolates from immunocompromised human patients with and without AIDS revealed that only eight contained an kb plasmid, expressed VapA, and were virulent for mice. 41 These results suggested that opportunistic infections in immunocompromised patients can be caused by both mouse-virulent and avirulent R. equi strains. Therefore, the pathogenesis of R. equi infection in immunocompromised human patients appears to be different from the pathogenesis in foals, in which the virulence plasmid is always found. In a recent study characterizing the plasmid content, expression of the virulence associated proteins, and mouse virulence of 19 R. equi isolates from human patients with or without AIDS, a new category of R. equi was defined. Isolates from patients with AIDS tend either to be virulent (10 6 bacteria needed for lethality in mice) and possess a kb plasmid encoding VapA and, or to have intermediate virulence (10 7 bacteria needed for lethality) with one of four distinct large plasmids encoding a 20-kDa antigen (VapB) related to, but distinct from, VapA. Most of the non-aids isolates are avirulent ( 10 8 bacteria needed for lethality) and do not express these antigens. 42 In another study, R. equi was isolated from 73.9% of soil samples collected from 115 parks and 49 yards in Japan. The number of R. equi in those samples ranged from CFU per gram of soil. None of the 1294 isolates from those samples expressed VapA or VapB, suggesting that the human environment is not a significant route of exposure to virulent or intermediately virulent R. equi. 43 In contrast, almost all isolates from the submaxillary lymph nodes of pigs produce VapB and are intermediately virulent to mice, suggesting that pigs or their environment may be the source of infection for some human cases. 44 To the author s knowledge, intermediately virulent isolates (expressing VapB) have never been isolated from foals with naturally acquired R. equi infections. Experimentally, heavy intrabronchial challenge with intermediately virulent R. equi results in pneumonia but at a dose much higher than that required for induction of pneumonia with VapA expressing strains Immunity Antibody-Mediated Immunity Immunity to R. equi pneumonia in foals likely depends on both the antibody and cell-mediated components of the immune system but its exact basis remains to be determined. The age of development of R. equi pneumonia coincides with and may in part be related to the decline of maternally derived antibodies. 46 However, the strongest evidence for a role of antibody in protection against R. equi is the partially protective effect of passively transferred anti R. equi hyperimmune (HI) equine plasma (see section 10). The mechanisms by which HI plasma confers protection are not completely understood. The list of possible effector molecules includes antibody and non-specific factors such as fibronectin, complement components, collectins, cytokines, and acute phase proteins. Opsonization of R. equi with specific antibody has been shown to promote phagocytosis and killing of R. equi by alveolar macrophages, identifying antibody as a critical component of HI plasma. 17 In all the studies evaluating the protective effect of HI plasma, plasma donors were immunized with whole cell vaccines or a mixture of several soluble antigens, making it impossible to determine the role of antibody against defined antigens of R. equi. Recent studies have focused more specifically against the role of antibody against plasmid-encoded virulence-associated proteins (Vap). First, a monoclonal antibody to VapA and serum from horses immunized with partially purified VapA have opsonizing activity. 47 Moreover, purified immunoglobulins obtained from horses vaccinated with partially purified VapA protected mice against intraperitoneal challenge with virulent R. equi compared with mice administered immunoglobulins from non-immunized horses. 48 More recently, in- AAEP PROCEEDINGS Vol
5 travenous administration of purified immunoglobulins obtained from horses immunized with recombinant VapA and VapC to foals was found to reduce the severity of pneumonia following heavy experimental challenge with R. equi. 49 In the same study, the degree of protection conferred by purified anti-vapa and -VapC immunoglobulins was similar to that provided by commercially available HI plasma. 49 Cell-Mediated Immunity Because of the facultative intracellular nature of R. equi, cell-mediated immune mechanisms are thought to be of major importance in resistance. Almost all knowledge of cell-mediated immunity to R. equi infections comes from infection of mice. Deficiencies in the complement component C5, phagocytic cells, and NK cells in mice do not impair the pulmonary clearance of virulent R. equi. 50 In contrast, functional T lymphocytes are absolutely required for the clearance of virulent (plasmid and VapA positive) R. equi in mice However, athymic nude mice (lacking functional T lymphocytes) clear plasmid-cured derivatives from their lungs within one week of infection suggesting that, as opposed to virulent organisms, clearance of avirulent plasmid-negative strains in mice does not require functional lymphocytes and depends mainly on innate defense mechanisms. 52 The two major mechanisms by which T lymphocytes mediate clearance of intracellular pathogens are secretion of cytokines and direct cytotoxicity. Although both CD4 (helper) and CD8 (cytotoxic) T cells contribute to host defense against R. equi in mice, CD4 T lymphocytes play the major role and are absolutely required for complete pulmonary clearance The mouse CD4 Th cells can be divided in 2 subsets based on the cytokines they produce. The Th1 subset produces mainly IFN- and IL-2 and is mainly responsible for macrophage activation and cell-mediated immunity. The Th2 subset produces mainly IL-4, IL-5, and IL-10, which mainly promote humoral immunity. Studies in mice have clearly shown that a Th1 response is sufficient to effect pulmonary clearance of R. equi while a Th2 response is detrimental. 51,56 How these findings in mice relate to the foal remains to be determined. Analogy to human immunodeficiency virus related R. equi pneumonia suggest either that foals are immunocompromised in some way or that infection with virulent R. equi alters immune response in foals. The cytokine response of foals infected with virulent and avirulent R. equi has recently been investigated. Foals infected intrabronchially with a virulence plasmid-containing strain of R. equi showed marked reduction in IFN- mrna expression by bronchial lymph node CD4 T lymphocytes compared to CD4 T cells similarly Vol. 47 AAEP PROCEEDINGS isolated from foals infected with an avirulent plasmid-cured derivative of the same strain. 57 In addition, IL-10, a cytokine known to downregulate a Th1 response in other species, was only expressed in the lungs of foals infected with the virulent strain. 57 These findings suggest that virulent R. equi have an immunomodulating effect important in the pathogenesis of infection. 7. Diagnosis The distinction between lower respiratory tract infections caused by R. equi and that caused by other pathogens is problematic especially on farms with no previous history of R. equi infections. Many diagnostic tests including a complete blood count (CBC), measurement fibrinogen concentrations, radiographs, and serology may help distinguish R. equi pneumonia from that caused by other pathogens. However, bacteriologic culture or polymerase chain reaction (PCR) amplification combined with cytological examination of tracheobronchial aspirate (TBA) are necessary to make a definitive diagnosis of R. equi pneumonia. Clinical Laboratory Tests Hyperfibrinogenemia is the most consistent laboratory finding in foals with R. equi pneumonia, although rare cases may have normal fibrinogen concentrations. Neutrophilic leukocytosis with or without monocytosis is also common. One study showed significantly higher fibrinogen concentrations and white blood cell counts (WBC) in nonsurvivors than in survivors 58 whereas another study showed no difference between the 2 groups. 8 In a review of 40 cases of lung abscesses there was a trend toward higher fibrinogen concentrations and WBC counts for the foals from which R. equi was isolated from a TBA as opposed to foals from which another pathogen was isolated. 59 However, in all these studies there was a considerable overlap in ranges, which preclude the use of fibrinogen concentrations and WBC as diagnostic tests or prognostic indicators for an individual animal. Imaging Techniques Thoracic radiography is useful in evaluating the severity of pneumonia and in assessing response to therapy. A prominent alveolar pattern characterized by ill-defined regional consolidation is the most common radiographic abnormality 58 (Fig. 3). The consolidated lesions are often seen as more discrete nodular and cavitary lesions compatible with pulmonary abscessation (Fig. 3). Although in one study non-survivors tended to have more severe radiographic lesions than survivors, many survivors have very severe radiographic lesions and radiographs should not be used as the sole criterion for prognostication and euthanasia. 8,58 In foals less than 4 months of age radiographic evidence of nodular lung lesions and lymphadenopathy are highly suggestive of R. equi infections. However, in foals of 4 months and older S. zooepidemicus is another common cause of lung abscesses. 59 Ultrasonography is a helpful
6 Fig. 3. Thoracic radiographs from a foal with R. equi pneumonia showing an alveolar pattern with ill-defined regional consolidation compatible with early abscess formation (arrows). diagnostic tool when lung involvement includes peripheral areas but may not be as useful as radiography to evaluate the extent of lung lesions since abscesses with overlying aerated lung will not be detected. However, in most horses and foals with pulmonary abscessation the periphery of the lung is affected enabling the ultrasonographer to successfully image some of the abscesses (Fig. 4). To the author s knowledge, comparison between ultrasonography and radiography has not been critically evaluated in foals with R. equi pneumonia. Nevertheless, ultrasonography is very useful in evaluating the severity of pneumonia and in assessing response to therapy especially for equine practitioners who do not have access to thoracic radiography. A sector scanner equipped with a 7.5 or 5.0 MHz transducer is preferred but the linear scanner commonly used in equine reproduction can also be used. Serology A number of workers have investigated serological approaches to diagnose R. equi infections in foals. The two most commonly used types of assays are agar gel immunodiffusion (AGID), and enzymelinked immunosorbent assay (ELISA). Serologic diagnosis of R. equi infections is problematic because the widespread exposure of foals to this organism at a young age leads to antibody production Fig. 4. Thoracic ultrasonography of the left cranioventral lung field from a foal with R. equi pneumonia. Multiple abscesses were imaged (arrows). AAEP PROCEEDINGS Vol
7 without necessarily producing clinical disease. In addition, maternally derived antibody causes positive reactions with sensitive ELISA assays which further confound the interpretation of the test. We recently evaluated the accuracy of AGID and 3 previously described ELISA assays 46,60,61 by comparing serological results to culture of a tracheobronchial aspirate in 100 foals with pneumonia. The serological tests evaluated were found to either have low sensitivity, low specificity, or both. Improving either sensitivity or specificity of ELISA assays by changing the cut-off value of the tests could only be done at the detriment of the other. Simple reliance on serology as a diagnostic test for R. equi infections results in overdiagnosis of the disease and in missing infections in the early stages. Serologic tests may be more useful at the farm level to detect overall exposure but they have little value in the diagnosis of clinical R. equi infections. Cytology, Culture and PCR Amplification Bacteriologic culture or PCR amplification combined with cytological examination of a tracheobronchial aspirate are, therefore, the only acceptable way of making a definitive diagnosis of R. equi pneumonia. In one study, only 7 (64%) of the 11 foals with positive R. equi culture at necropsy and 57 (64%) of the 89 foals with radiographic evidence of lung abscessation yielded R. equi on culture of a TBA. 62 However, in 2 other studies, all 17 foals in which R. equi was isolated from the lung parenchyma at necropsy, were previously found to yield the organism on culture of a TBA suggesting that culture of a TBA is a consistent and reliable method of diagnosing R. equi pneumonia. 59,63 Larger case series are required to determine the exact sensitivity of TBA for the diagnosis of R. equi pneumonia in foals. Multiple other pathogens are often isolated along with R. equi. Foals without clinical disease exposed to contaminated environments may have R. equi in their trachea as a result of inhalation of contaminated dust. In one study conducted on a farm with enzootic R. equi pneumonia 77 (35%) of 216 foals sampled had positive TBA cultures but no signs of respiratory disease. 64 For this reason, bacteriologic culture of a TBA should be interpreted in the context of cytological evaluation, physical examination and laboratory results. A light growth of R. equi from a foal with no clinical signs of respiratory disease, normal fibrinogen concentrations and WBC count, and no cytological evidence of airway inflammation is likely an incidental finding. In a recent study, the use of PCR amplification based on the VapA gene sequence was found to be a more sensitive mean of identifying R. equi in TBA samples than bacterial culture, especially if the foal sampled is concurrently being treated with antimicrobial Vol. 47 AAEP PROCEEDINGS agents. 65 However, increased sensitivity may also result in a higher incidence of false positive results due to the detection of very small numbers of R. equi present as environmental contaminants. PCR amplification may be done in association with, but should not replace, bacterial culture because it does not permit identification of concurrent bacterial pathogens and in vitro antimicrobial susceptibility testing. Positive R. equi culture from nasal or fecal swabs cannot be taken as evidence of infection. R. equi can be cultured from the feces of normal horses even if they live in farms with no history of R. equi pneumonia. 37,66,67 The quantitative culture of the feces of foals at weekly intervals has been advocated as an aid in early diagnosis of R. equi infections in foals because the bacterial count per gram of feces increased at the same time as clinical signs appeared. 67 However, a single fecal sample from a foal has no diagnostic value because of individual as well as farm to farm variation in the number of R. equi in the feces. 37,66,67 Furthermore, a negative fecal culture may not be helpful in ruling out R. equi infection since, in one study, only 5 (17%) of 30 foals with confirmed R. equi pneumonia had positive fecal cultures. 64 Similarly, bacterial culture and PCR amplification of nasal swabs are poorly sensitive for the diagnosis of R. equi pneumonia. 65,68 8. Treatment A wide variety of antimicrobial agents are effective against R. equi in vitro. However, because R. equi is an intracellular pathogen surviving and replicating in macrophages 69 and it causes granulomatous lesions with thick caseous material, many of these drugs are ineffective in vivo. For example, in one study all 17 foals with R. equi pneumonia treated with the combination of penicillin and gentamicin died despite the fact that all isolates were sensitive to gentamicin. 8 The combination of erythromycin and rifampin has become the treatment of choice for R. equi infections in foals and has dramatically reduced foal mortality since its introduction. 8,62 The two drugs are bacteriostatic against R. equi but highly effective in vitro. 70 Their combination is synergistic both in vitro and in vivo and use in combination reduces the likelihood of resistance to either drug. 70,71 Rifampin and, to a lesser extent, erythromycin are lipid soluble, allowing them to penetrate caseous material. Rifampin is concentrated approximately 2-fold in phagocytic cells and is effective against many intracellular bacteria. 72 Erythromycin is concentrated 10- to 20-fold in granulocytes and alveolar macrophages by an active mechanism but, despite high intracellular concentrations, its intracellular antibacterial activity at best equals that against extracellular bacteria. 72 The concentration of erythromycin within lysosomes may explain the poor efficacy of the drug against intracellular pathogens since many of them, like R. equi, can prevent phagosomelysosome fusion. Alternatively, acidity within the phagolysosome may reduce the efficacy of erythromycin. 72 The recommended dosage regimen for rifampin is 5 mg/kg q 12 h or 10 mg/kg q 24 h orally Rec-
8 ommended dosage of the esters or salts of erythromycin is 25 mg/kg q 6 8 h, orally. 76,77 A third antimicrobial agent may be necessary if another pathogen resistant to erythromycin and rifampin is isolated in significant numbers along with R. equi. The combination of gentamicin or amikacin with erythromycin or rifampin in vitro give significant antagonistic activity against R. equi compared with either drug alone. 70,71 Therefore, administration of an aminoglycoside with either erythromycin or rifampin is not recommended for the treatment of R. equi infections. Resolution of clinical signs, normalization of plasma fibrinogen and radiographic or ultrasonographic resolution of lung lesions are commonly used to guide the duration of therapy which generally ranges between 4 and 9 weeks. Although well tolerated by most foals, erythromycin commonly causes fecal consistency to soften. Most of the time this effect is self-limiting and does not necessitate cessation of therapy but these foals should be monitored carefully because some may develop severe diarrhea. During surges of very hot weather an idiosyncratic reaction characterized by severe hyperthermia and tachypnea has been described in foals treated with erythromycin. 78 Administration of antipyretic drugs and placing the foal in a cold environment will treat this problem. Clostridium difficile enterocolitis has also been observed occasionally in the dams of nursing foals while the foals are being treated with oral erythromycin presumably because coprophagic behavior leads to ingestion of sufficient active erythromycin to perturb the intestinal flora of the mare. 79 Even though the vast majority of R. equi isolates from foals are sensitive to erythromycin and rifampin resistant strains to either drugs have been encountered. Therapy of foals infected with resistant isolates is problematic because of the limited range of alternative effective drugs. Alternative Antimicrobial Agents High doses of the trimethoprim-sulfonamide (TMS) combination (30 mg/kg of combination q 8 12 h, orally) may be effective in foals with mild or early R. equi pneumonia without marked evidence of pulmonary abscessation or for continued therapy in foals responding well to other antimicrobials. 80 However, TMS is unlikely to be as effective as erythromycin and rifampin for the treatment of severe R. equi pneumonia with extensive abscessation because of its poor activity in caseous material and against most intracellular pathogens. Chloramphenicol can also be administered orally and achieves high concentrations within phagocytic cells. The recommended dosage regimen is 50 mg/kg q 6 h orally. However, the fact that only 70% of R. equi isolates are susceptible to this drug and the potential human health risk make this drug a less attractive alternative. Azithromycin and clarithromycin are two newer generation macrolides commonly used in human medicine. Compared to erythromycin, they are more chemically stable, have a greater bioavailibility, and achieve higher concentrations in phagocytic cells and tissues. In people, the incidence and the severity of side effects for these two new macrolides are also considerably decreased when compared to erythromycin. The need for other effective and safer antimicrobial agents for the treatment of R. equi infections in foals makes azithromycin and clarithromycin attractive alternatives. We recently investigated the pharmacokinetics of these two antimicrobial agents in 2- to 4-month-old foals and determined their in vitro minimal inhibitory concentrations (MIC) for common equine pathogens. Based on the pharmacokinetic parameters, the MIC of Rhodococcus equi isolates, and pharmacodynamic parameters correlated with a favorable outcome of infection in other species, azithromycin, at a dose of 10 mg/kg q 24 h orally, or clarithromycin, at a dose of 7.5 mg/kg q 12 h orally would likely be appropriate for the treatment of R. equi infections in foals. Additional studies are required to confirm the efficacy and safety of these dosages in a clinical setting. Until otherwise proven, the combination of erythromycin and rifampin should remain the first line of therapy for R. equi infections in foals. 9. Prognosis Prior to the introduction of the combination erythromycin rifampin as the recommended treatment, the prognosis of R. equi pneumonia was poor with reported mortality rate as high as 80%. Using erythromycin and rifampin, Hillidge reported a successful outcome (as assessed by survival) in 50 (88%) of 57 foals with confirmed R. equi pneumonia. 62 However, until recently there was no information on the impact of R. equi infections on future athletic performance. Recently, the records of 115 foals diagnosed with R. equi pneumonia and treated with erythromycin and rifampin were reviewed. 3 The survival rate was 72%. Death was more likely in foals presented with respiratory distress and the non-survivors had more severe radiographic abnormalities on admission than survivors. Of the survivors, 54% had at least one racing start as opposed to 65% for the control population suggesting that horses contracting R. equi pneumonia as foals are slightly less likely to race. However, racing performance of foals that raced as adults was not significantly different from that of the US racing population Control of R. equi Infections on Farms where the Disease is Enzootic Approaches for the successful control of R. equi infections on enzootic farms depend on 1) a decrease in the size of infective challenge, 2) earlier recognition and therapy of the disease, and 3) passive immunization. These approaches have recently been reviewed in detail. 81 AAEP PROCEEDINGS Vol
9 Decreasing the Size of Infective Challenge The management and environmental factors associated with development of R. equi pneumonia have never been critically investigated. Nevertheless, there seems to be a progressive build up of infection on horse farms with prolonged use. Thus, enzootic farms are likely to be those used for breeding horses for many years, those with heavy concentrations of mares and foals, and those located where summer temperatures are high, where the soil type is sandy, and where dust is extensive. Large numbers of foals kept on bare, dusty, manure-containing paddocks will result in heavy challenge, with clinical disease maintaining virulent bacteria. It is important to house foals in well-ventilated, dust-free areas, and to avoid dirt paddocks and crowding. Pneumonic foals must be isolated, because they are the major source of contamination of the environment with virulent organisms, and their manure composted. Pasture must be rotated to decrease dust formation and consequent inhalation of R. equi. Any sandy or dirt areas should ideally be planted with grass and made off limits to foals or, alternatively, irrigation may be useful in decreasing dust formation and encouraging grass. Manure should be regularly removed from paddocks and composted. Because mares and foals tend to congregate around water sources and under shade in hot summers, reduction in the size of mare foal bands may reduce the destruction of grass and exposure to barren soil. Dispersal of foals onto grass paddocks will reduce dust formation and, therefore, the number of inhaled bacteria. Ingestion of low number of organisms via grazing on contaminated grass may actually have the beneficial effect of immunizing foals orally. 82 Earlier Recognition of the Disease R. equi pneumonia is often not recognized until it is well advanced and, therefore, difficult to treat. Even severely affected foals may appear to suckle and behave normally to a casual observer. Early recognition of the disease with isolation and appropriate treatment of infected foals will reduce losses, limit the spread of virulent organisms in the environment, and decrease the cost of therapy. In one study on a farm with enzootic R. equi problems, twice weekly complete physical examination with auscultation of the lungs was successful in promoting early diagnosis and preventing mortality. 83 Other suggested approaches for early identification of R. equi infected foals on enzootic farms include periodic measurement of fibrinogen concentrations, serological surveillance using AGID, and lung ultrasonography. However, to the author s knowledge, the usefulness of these approaches has never been critically investigated. We recently assessed the value of monthly measurement of WBC counts and fibrinogen concentrations compared to bimonthly serological surveillance using the AGID test for early identification of R. equi infected foals on a large Vol. 47 AAEP PROCEEDINGS breeding farm with enzootic R. equi infections. We monitored 165 foals during the entire breeding season. Diagnosis of R. equi pneumonia was confirmed in pneumonic foals by culture of a tracheobronchial aspirate. WBC count was found to be a more sensitive and specific indicator of early R. equi pneumonia than measurement of fibrinogen concentrations. Foals with leukocyte counts 14,000 cells/ l should either be subjected to more diagnostic tests (e.g., tracheobronchial aspirate) or treated. Foals with leukocyte counts 13,000 but 14,000 cells/ l should be retested weekly until resolution of leukocytosis. The sensitivity and specificity of the AGID assay were respectively 76.2% and 50.5%, limiting the usefulness of this test for early identification of R. equi infected foals on enzootic farms. Passive Immunization Intravenous administration of HI plasma obtained from horses vaccinated against R. equi using various antigens has proved effective in significantly reducing the incidence of R. equi pneumonia in foals following both experimental and natural challenge in some studies. 84,85 However, other studies have failed to identify significant differences in the incidence of naturally acquired R. equi pneumonia between HI plasma recipients and control foals, 86,87 suggesting that the efficacy of a particular HI plasma product cannot be extrapolated to another product prepared in a similar but not identical fashion. In North America, most horse farms relying on HI plasma for the prevention of R. equi pneumonia use commercially available HI plasma products. Hyperimmune plasma obtained from horses vaccinated with R. equi antigens is commercially available from two companies, both currently holding a conditional USDA license for these products. a,b However, to the author s knowledge, the efficacy of these commercially available plasma products in protecting against R. equi pneumonia has, until recently, never been critically investigated. To determine the efficacy of the Lake Immunogenics Inc. HI plasma, a 165 Thoroughbred foals on a farm with enzootic R. equi infections were randomly assigned to two treatment groups (HI plasma or non-treated controls). Transfused foals were given two 950-ml doses of HI plasma by rapid intravenous infusion. The first dose was given between 1 and 10 days of age, and the second dose was given between 30 and 50 days of age. A tracheobronchial aspirate for bacterial culture was performed on every foal with clinical signs of lower respiratory tract disease. The incidence of R. equi pneumonia in foals without failure of passive transfer of immunity was lower in foals transfused with HI plasma (19.1%) than in non-treated controls (30%) (p 0.09). All transfused foals that developed R. equi pneumonia were diagnosed with the disease prior to administration of the second dose of HI plasma. The median age at diagnosis of R. equi pneumonia on this particular farm was 36 days. Therefore,
10 administration of the second dose of HI plasma around 25 days of age may have been more beneficial. A different approach was used to assess the efficacy to the Veterinary Dynamics Inc. HI plasma. b Briefly, seven healthy 3-week-old pony foals received 950-ml of HI plasma 24 h prior to heavy intrabronchial challenge with virulent R. equi. Eleven foals not receiving HI plasma were also infected using an identical protocol. All foals were euthanized 2 weeks post-challenge, their lung lesions were assessed, and the numbers of R. equi in their lungs were enumerated. Compared to the untreated group, lung lesions were less severe and bacterial numbers were significantly lower in transfused foals (p 0.01). 49 Collectively, the results of these independent studies show that the two commercially available anti R. equi HI plasma products are effective in reducing the incidence or severity of R. equi pneumonia in foals. However, the differences in study design preclude conclusions regarding any advantage of one product over the other. The two commercially available anti R. equi HI plasma products evaluated were found to have high anti-vapa antibody titers. These antibodies may be responsible for the protective effect observed (see section 6), although the possibility that antibodies against other antigens or molecules other than antibody contribute to protection cannot be excluded. These studies also emphasize that protection conferred by HI plasma is not complete. For optimal control of the disease on enzootically infected farms, administration of hyperimmune plasma should be combined with other control strategies, such as attempts at decreasing the size of infective challenge and early identification and treatment of infected foals. The ideal time for administration of HI plasma and determination of the minimal effective dose require further research. Administration of HI plasma prior to infection with R. equi is important. 88 However, early administration may result in the decline of passively transferred antibody to a non-protective level at a time when foals are still highly susceptible to R. equi and environmental challenge is high. 86,87 Therefore, intravenous administration of 1lofHIplasma within the first week of life followed by a second administration at approximately 25 days of age, although expensive, may be the best approach on farms with high morbidity rates. A single administration at the beginning of the warm season may be advisable for foals born early during the year in geographic areas where cold winter temperatures reduce environmental challenge. These recommendations are only guidelines and the best time for administration of HI plasma may in fact vary depending on the geographic location of the farm, the size of infective challenge, and the age at which most foals on the farm develop clinical signs. References and Footnotes 1. Donisi A, Suardi MG, Casari S, et al. Rhodococcus equi infection in HIV-infected patients. AIDS 1996;10: Harvey RL, Sunstrum JC. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev Infect Dis 1991;13: Ainsworth DM, Eicker SW, Yeagar AE, et al. Associations between physical examination, laboratory, and radiographic findings and outcome and subsequent racing performance of foals with Rhodococcus equi infection: 115 cases ( ). J Am Vet Med Assoc 1998;213: Zink MC, Yager JA, Smart NL. Corynebacterium equi infections in horses, : A review of 131 cases. Can J Vet Res 1986;27: Baldwin JL, Bertone JJ, Sommer MM, et al. Rhodococcus equi enteritis, colonic lymphadenitis, and peritonitis in three foals with nonresponsive Rhodococcus equi bronchopneumonia. Equine Pract 1992;14: Kenney DG, Robbins SC, Prescott JF, et al. Development of reactive arthritis and resistance to erythromycin and rifampin in a foal during treatment for Rhodococcus equi pneumonia. Equine Vet J 1994;26: Madison JB, Scarratt WK. Immune-mediated polysynovitis in four foals. J Am Vet Med Assoc 1988;192: Sweeney CR, Sweeney RW, Divers TJ. Rhodococcus equi pneumonia in 48 foals: Response to antimicrobial therapy. Vet Microbiol 1987;14: Chaffin MK, Martens RJ. Extrapulmonary disorders associated with Rhodococcus equi pneumonia in foals: Retrospective study of 61 cases ( ), in Proceedings. Am Assoc Equine Pract 1997;43: Chaffin MK, Honnas CM, Crabill MR, et al. Cauda equina syndrome, diskospondylitis, and a paravertebral abscess caused by Rhodococcus equi in a foal. J Am Vet Med Assoc 1995;206: Giguère S, Lavoie JP. Rhodococcus equi vertebral osteomyelitis in 3 Quarter Horse colts. Equine Vet J 1994;26: Olchowy TW. Vertebral body osteomyelitis due to Rhodococcus equi in two Arabian foals. Equine Vet J 1994;26: Freestone JF, Hietala S, Moulton J, et al. Acquired immunodeficiency in a seven-year-old horse. J Am Vet Med Assoc 1987;190: Fitzgerald SD, Yamini B. Rhodococcal abortion and pneumonia in an equine fetus. J Vet Diagn Invest 1995;7: Hondalus MK, Diamond MS, Rosenthal LA, et al. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect Immun 1993;61: Brumbaugh GW, Davis LE, Thurmon JC, et al. Influence of Rhodococcus equi on the respiratory burst of resident alveolar macrophages from adult horses. Am J Vet Res 1990;51: Hietala SK, Ardans AA. Interaction of Rhodococcus equi with phagocytic cells from R. equi-exposed and non-exposed foals. Vet Microbiol 1987;14: Zink MC, Yager JA, Prescott JF, et al. Electron microscopic investigation of intracellular events after ingestion of Rhodococcus equi by foal alveolar macrophages. Vet Microbiol 1987;14: Darrah PA, Hondalus MK, Chen Q, et al. Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect Immun 2000;68: Hietala SK, Ardans AA. Neutrophil phagocytic and serum opsonic response of the foal to Corynebacterium equi. Vet Immunol Immunopathol 1987;14: Martens JG, Martens RJ, Renshaw HW. Rhodococcus (Corynebacterium) equi: bactericidal capacity of neutrophils from neonatal and adult horses. Am J Vet Res 1988;49: Martens RJ, Martens JG, Renshaw HW, et al. Rhodococcus equi: Equine neutrophil chemiluminescent and bactericidal AAEP PROCEEDINGS Vol
11 responses to opsonizing antibody. Vet Microbiol 1987;14: Takai S, Morozumi Y, Higashiyama S, et al. Nitroblue tetrazolium reduction by neutrophils of newborn foals, adult horses, and a foal infected with Rhodococcus (Corynebacterium) equi. Jpn J Vet Sci 1986;48: Yager JA, Foster SF, Zink MC, et al. In vivo bactericidal efficacy of equine polymorphonuclear leukocytes against Corynebacterium equi. Am J Vet Res 1986;47: Takai S, Sekizaki T, Ozawa T, et al. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun 1991;59: Tkachuk-Saad O, Prescott J. Rhodococcus equi plasmids: Isolation and partial characterization. J Clin Microbiol 1991;29: Takai S, Ohbushi S, Koike K, et al. Prevalence of virulent Rhodococcus equi in isolates from soil and feces of horses from horse-breeding farms with and without endemic infections. J Clin Microbiol 1991;29: Takai S, Watanabe Y, Ikeda T, et al. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol 1993;31: Tan C, Prescott JF, Patterson MC, et al. Molecular characterization of a lipid-modified virulence-associated protein of Rhodococcus equi and its potential in protective immunity. Can J Vet Res 1995;59: Sekizaki T, Takai S, Egawa Y, et al. Sequence of the Rhodococcus equi gene encoding the virulence-associated kda antigens. Gene 1995;155: Takai S, Hines SA, Sekizaki T, et al. DNA sequence and comparison of virulence plasmids from Rhodococcus equi ATCC and 103. Infect Immun 2000;68: Takai S, Fukunaga N, Kamisawa K, et al. Expression of virulence-associated antigens of Rhodococcus equi is regulated by temperature and ph. Microbiol Immunol 1996;40: Byrne BA, Prescott JF, Palmer GH, et al. Virulence plasmid of Rhodococcus equi contains inducible gene family encoding secreted proteins. Infect Immun 2001;69: Giguère S, Hondalus MK, Yager JA, et al. Role of the 85- kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi. Infect Immun 1999;67: Wada R, Kamada M, Anzai T, et al. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet Microbiol 1997;56: Gotoh K, Mitsuyama M, Imaizumi S, et al. Mycolic acidcontaining glycolipid as a possible virulence factor of Rhodococcus equi for mice. Microbiol Immunol 1991;35: Takai S, Ohkura H, Watanabe Y, et al. Quantitative aspects of fecal Rhodococcus (Corynebacterium) equi in foals. J Clin Microbiol 1986;23: Barton MD, Hughes KL. Ecology of Rhodococcus equi. Vet Microbiol 1984;9: Johnson JA, Prescott JF, Markham RJ. The pathology of experimental Corynebacterium equi infection in foals following intragastric challenge. Vet Pathol 1983;20: Takai S, Anzai T, Yamaguchi K, et al. Prevalence of virulence plasmids in environmental isolates of Rhodococcus equi from horse-breeding farms in hokkaido. J Equine Sci 1994; 5: Takai S, Sasaki Y, Ikeda T, et al. Virulence of Rhodococcus equi isolates from patients with and without AIDS. J Clin Microbiol 1994;32: Takai S, Imai Y, Fukunaga N, et al. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J Infect Dis 1995;172: Takai S, Fukunaga N, Ochiai S, et al. Isolation of virulent and intermediately virulent Rhodococcus equi from soil and sand on parks and yards in Japan. J Vet Med Sci 1996;58: Takai S, Fukunaga N, Ochiai S, et al. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J Clin Microbiol 1996;34: Takai S, Anzai T, Fujita Y, et al. Pathogenicity of Rhodococcus equi expressing a virulence-associated 20 kda protein (VapB) in foals. Vet Microbiol 2000;76: Hietala SK, Ardans AA, Sansome A. Detection of Corynebacterium equi-specific antibody in horses by enzyme-linked immunosorbent assay. Am J Vet Res 1985;46: Prescott JF, Nicholson VM, Patterson MC, et al. Use of Rhodococcus equi virulence-associated protein for immunization of foals against R. equi pneumonia. Am J Vet Res 1997; 58: Fernandez AS, Prescott JF, Nicholson VM. Protective effect against Rhodococcus equi infection in mice of IgG purified from horses vaccinated with virulence associated protein (VapA)-enriched antigens. Vet Microbiol 1997;56: Hooper-McGrevy K, Giguère S, Wilkie BN, et al. Antibody to virulence-associated proteins A and C in protection against Rhodococcus equi pneumonia in foals. Am J Vet Res 2001; 62: Yager JA, Prescott CA, Kramar DP, et al. The effect of experimental infection with Rhodococcus equi on immunodeficient mice. Vet Microbiol 1991;28: Kanaly ST, Hines SA, Palmer GH. Transfer of a CD4 Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lung. Infect Immun 1996;64: Madarame H, Takai S, Matsumoto C, et al. Virulent and avirulent Rhodococcus equi infection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol Med Microbiol 1997;17: Ross TL, Balson GA, Miners JS, et al. Role of CD4, CD8 and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with Rhodococcus equi. Can J Vet Res 1996;60: Kanaly ST, Hines SA, Palmer GH. Failure of pulmonary clearance of Rhodococcus equi infection in CD4 T-lymphocyte-deficient transgenic mice. Infect Immun 1993;61: Nordmann P, Ronco E, Nauciel C. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect Immun 1992; 60: Kanaly ST, Hines SA, Palmer GH. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect Immun 1995;63: Giguère S, Wilkie BN, Prescott JF. Modulation of cytokine response of pneumonic foals by virulent Rhodococcus equi. Infect Immun 1999;67: Falcon J, Smith BP, O Brien TR, et al. Clinical and radiographic findings in Corynebacterium equi pneumonia of foals. J Am Vet Med Assoc 1985;186: Lavoie JP, Fiset L, Laverty S. Review of 40 cases of lung abscesses in foals and adult horses. Equine Vet J 1994;26: Prescott JF, Fernandez AS, Nicholson VM, et al. Use of a virulence-associated protein based enzyme-linked immunosorbent assay for Rhodococcus equi serology in horses. Equine Vet J 1996;28: Takai S, Kawazu S, Tsubaki S. Enzyme-linked immunosorbent assay for diagnosis of Corynebacterium (Rhodococcus) equi infection in foals. Am J Vet Res 1985;46: Hillidge CJ. Use of erythromycin-rifampin combination in treatment of Rhodococcus equi pneumonia. Vet Microbiol 1987;14: Muller NS, Madigan JE. Methods of implementation of an immunoprophylaxis program for the prevention of Rhodococcus equi pneumonia: Results of a 5-year field study, in Proceedings. Am Assoc Equine Pract 1992;38: Ardans AA, Hietala SK, Spensley MS, et al. Studies of naturally occurring and experimental Rhodococcus equi, in Proceedings. Am Assoc Equine Pract 1986;32: Sellon DC, Besser TE, Vivrette SL, et al. Comparison of nucleic acid amplification, serology, and microbiologic culture for diagnosis of Rhodococcus equi pneumonia in foals. J Clin Microbiol 2001;39: Vol. 47 AAEP PROCEEDINGS
RHODOCOCCUS EQUI. Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation
 RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
Focus on Upper and Lower Respiratory Diseases
 www.ivis.org Proceedings of the American Association of Equine Practitioners - Focus Meeting Focus on Upper and Lower Respiratory Diseases Salt Lake City, UT, USA 2010 Next Focus Meetings: July 24-26,
www.ivis.org Proceedings of the American Association of Equine Practitioners - Focus Meeting Focus on Upper and Lower Respiratory Diseases Salt Lake City, UT, USA 2010 Next Focus Meetings: July 24-26,
Rhodococcus equi Infections (28 Apr 2000)
 In: Recent Advances in Equine Neonatal Care, Wilkins P.A. and Palmer J.E. (Eds.) Publisher: International Veterinary Information Service (www.ivis.org) Rhodococcus equi Infections (28 Apr 2000) S. Giguère
In: Recent Advances in Equine Neonatal Care, Wilkins P.A. and Palmer J.E. (Eds.) Publisher: International Veterinary Information Service (www.ivis.org) Rhodococcus equi Infections (28 Apr 2000) S. Giguère
Development of Hyperimmune Equine Plasma to Virulence Associated Protein A (VapA) of Rhodococcus equi
 Development of Hyperimmune Equine Plasma to Virulence Associated Protein A (VapA) of Rhodococcus equi R. B. Brandon 1, R. Hill 2, R. P. Wilson 1 Objective To develop a new potent Rhodococcus equi vaccine
Development of Hyperimmune Equine Plasma to Virulence Associated Protein A (VapA) of Rhodococcus equi R. B. Brandon 1, R. Hill 2, R. P. Wilson 1 Objective To develop a new potent Rhodococcus equi vaccine
Defense mechanism against pathogens
 Defense mechanism against pathogens Immune System What is immune system? Cells and organs within an animal s body that contribute to immune defenses against pathogens ( ) Bacteria -Major entry points ;open
Defense mechanism against pathogens Immune System What is immune system? Cells and organs within an animal s body that contribute to immune defenses against pathogens ( ) Bacteria -Major entry points ;open
INNATE IMMUNITY TO RHODOCOCCUS EQUI: THE RESPONSE OF ADULT AND JUVENILE EQUINE NEUTROPHILS
 INNATE IMMUNITY TO RHODOCOCCUS EQUI: THE RESPONSE OF ADULT AND JUVENILE EQUINE NEUTROPHILS A Dissertation by JESSICA RACHEL NERREN Submitted to the Office of Graduate Studies of Texas A&M University in
INNATE IMMUNITY TO RHODOCOCCUS EQUI: THE RESPONSE OF ADULT AND JUVENILE EQUINE NEUTROPHILS A Dissertation by JESSICA RACHEL NERREN Submitted to the Office of Graduate Studies of Texas A&M University in
Unit 5 The Human Immune Response to Infection
 Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Chapter 24 The Immune System
 Chapter 24 The Immune System The Immune System Layered defense system The skin and chemical barriers The innate and adaptive immune systems Immunity The body s ability to recognize and destroy specific
Chapter 24 The Immune System The Immune System Layered defense system The skin and chemical barriers The innate and adaptive immune systems Immunity The body s ability to recognize and destroy specific
Medical Bacteriology- Lecture 10. Mycobacterium. Actinomycetes. Nocardia
 Medical Bacteriology- Lecture 10 Mycobacterium Actinomycetes Nocardia 1 Mycobacterium Characteristics - Large, very weakly gram positive rods - Obligate aerobes, related to Actinomycetes - Catalase positive
Medical Bacteriology- Lecture 10 Mycobacterium Actinomycetes Nocardia 1 Mycobacterium Characteristics - Large, very weakly gram positive rods - Obligate aerobes, related to Actinomycetes - Catalase positive
Survival of Foals with Experimentally Induced Rhodococcus equi
 Veterinary Therapeutics Vol. 3, No. 3, Summer 2001 Survival of Foals with Experimentally Induced Rhodococcus equi Infection Given Either Hyperimmune Plasma Containing R. equi Antibody or Normal Equine
Veterinary Therapeutics Vol. 3, No. 3, Summer 2001 Survival of Foals with Experimentally Induced Rhodococcus equi Infection Given Either Hyperimmune Plasma Containing R. equi Antibody or Normal Equine
The Immune System. These are classified as the Innate and Adaptive Immune Responses. Innate Immunity
 The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Bacteriology- lecture 13. Mycobacterium Actinomycetes
 Medical Bacteriology- lecture 13 Mycobacterium Actinomycetes Mycobacterium tuberculosis Large, very weakly gram positive rods, Obligate aerobes, related to Actinomycetes, non spore forming, non motile
Medical Bacteriology- lecture 13 Mycobacterium Actinomycetes Mycobacterium tuberculosis Large, very weakly gram positive rods, Obligate aerobes, related to Actinomycetes, non spore forming, non motile
Immunity and Infection. Chapter 17
 Immunity and Infection Chapter 17 The Chain of Infection Transmitted through a chain of infection (six links) Pathogen: Disease causing microorganism Reservoir: Natural environment of the pathogen Portal
Immunity and Infection Chapter 17 The Chain of Infection Transmitted through a chain of infection (six links) Pathogen: Disease causing microorganism Reservoir: Natural environment of the pathogen Portal
EQUINE VACCINATION AN EDUCATIONAL GUIDE FOR HORSE OWNERS.
 EQUINE VACCINATION AN EDUCATIONAL GUIDE FOR HORSE OWNERS. A HEALTHY HORSE IS A PROPERLY VACCINATED HORSE. A properly designed vaccination regimen may be one of the most important things you can do to help
EQUINE VACCINATION AN EDUCATIONAL GUIDE FOR HORSE OWNERS. A HEALTHY HORSE IS A PROPERLY VACCINATED HORSE. A properly designed vaccination regimen may be one of the most important things you can do to help
Pathogenesis of Infectious Diseases. CLS 212: Medical Microbiology
 Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms. Table 2: Innate Immunity: First Lines of Defense
 I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
EVALUATION OF THE SUSCEPTIBILITY AND HUMORAL IMMUNE RESPONSE OF FOALS TO RHODOCOCCUS EQUI INFECTION
 University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2014 EVALUATION OF THE SUSCEPTIBILITY AND HUMORAL IMMUNE RESPONSE OF FOALS TO RHODOCOCCUS EQUI INFECTION
University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2014 EVALUATION OF THE SUSCEPTIBILITY AND HUMORAL IMMUNE RESPONSE OF FOALS TO RHODOCOCCUS EQUI INFECTION
PATHOGENICITY OF MICROORGANISMS
 PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
Immune system. Aims. Immune system. Lymphatic organs. Inflammation. Natural immune system. Adaptive immune system
 Aims Immune system Lymphatic organs Inflammation Natural immune system Adaptive immune system Major histocompatibility complex (MHC) Disorders of the immune system 1 2 Immune system Lymphoid organs Immune
Aims Immune system Lymphatic organs Inflammation Natural immune system Adaptive immune system Major histocompatibility complex (MHC) Disorders of the immune system 1 2 Immune system Lymphoid organs Immune
Infectious Respiratory Disease in Horses. Age of onset: bacterial pathogens. Equine Viral Respiratory Disease 1/12/2019
 Infectious Respiratory Disease in Horses Beth Davis, DVM, PhD, DACVIM Kansas State University Age of onset: bacterial pathogens YOUNG Perinatal < 1 month Gram negatives Association with FPT 1 6 months
Infectious Respiratory Disease in Horses Beth Davis, DVM, PhD, DACVIM Kansas State University Age of onset: bacterial pathogens YOUNG Perinatal < 1 month Gram negatives Association with FPT 1 6 months
Blood and Immune system Acquired Immunity
 Blood and Immune system Acquired Immunity Immunity Acquired (Adaptive) Immunity Defensive mechanisms include : 1) Innate immunity (Natural or Non specific) 2) Acquired immunity (Adaptive or Specific) Cell-mediated
Blood and Immune system Acquired Immunity Immunity Acquired (Adaptive) Immunity Defensive mechanisms include : 1) Innate immunity (Natural or Non specific) 2) Acquired immunity (Adaptive or Specific) Cell-mediated
STRANGLES (STREPTOCOCCUS EQUI SUBSPECIES EQUI)
 STRANGLES (STREPTOCOCCUS EQUI SUBSPECIES EQUI) Definition Clinical Signs Incubation Period Risk Factors Transmission Diagnostic Sampling, Testing and Handling Post-mortem Shedding of Virus Following Resolution
STRANGLES (STREPTOCOCCUS EQUI SUBSPECIES EQUI) Definition Clinical Signs Incubation Period Risk Factors Transmission Diagnostic Sampling, Testing and Handling Post-mortem Shedding of Virus Following Resolution
ANATOMY OF THE IMMUNE SYSTEM
 Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Western Veterinary Conference 2013
 Western Veterinary Conference 2013 SA283 EMERGING CANINE INFECTIOUS RESPIRATORY DISEASES Stephanie D Janeczko, DVM, MS, Dipl. ABVP (Canine/Feline) ASPCA New York, NY, USA Management of infectious respiratory
Western Veterinary Conference 2013 SA283 EMERGING CANINE INFECTIOUS RESPIRATORY DISEASES Stephanie D Janeczko, DVM, MS, Dipl. ABVP (Canine/Feline) ASPCA New York, NY, USA Management of infectious respiratory
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Yersinia pestis. Yersinia and plague. Dr. Hala Al Daghistani
 Yersinia pestis Dr. Hala Al Daghistani Yersinia species Short, pleomorphic gram-negative rods that can exhibit bipolar staining. Catalase positive, and microaerophilic or facultatively anaerobic. Animals
Yersinia pestis Dr. Hala Al Daghistani Yersinia species Short, pleomorphic gram-negative rods that can exhibit bipolar staining. Catalase positive, and microaerophilic or facultatively anaerobic. Animals
The Streptococci. Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens
 The Streptococci Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens Strong fermenters Facultative anaerobes Non-motile Catalase Negative 1 Classification 1 2 Classification
The Streptococci Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens Strong fermenters Facultative anaerobes Non-motile Catalase Negative 1 Classification 1 2 Classification
Chapter 22. Pulmonary Infections
 Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Chapter 22 Pulmonary Infections Objectives State the incidence of pneumonia in the United States and its economic impact. Discuss the current classification scheme for pneumonia and be able to define hospital-acquired
Nonspecific External Barriers skin, mucous membranes
 Immune system Chapter 36 BI 103 Plant-Animal A&P Levels of Defense Against Disease Nonspecific External Barriers skin, mucous membranes Physical barriers? Brainstorm with a partner If these barriers are
Immune system Chapter 36 BI 103 Plant-Animal A&P Levels of Defense Against Disease Nonspecific External Barriers skin, mucous membranes Physical barriers? Brainstorm with a partner If these barriers are
NOTES: CH 43, part 2 Immunity; Immune Disruptions ( )
 NOTES: CH 43, part 2 Immunity; Immune Disruptions (43.3-43.4) Activated B & T Lymphocytes produce: CELL-MEDIATED IMMUNE RESPONSE: involves specialized T cells destroying infected host cells HUMORAL IMMUNE
NOTES: CH 43, part 2 Immunity; Immune Disruptions (43.3-43.4) Activated B & T Lymphocytes produce: CELL-MEDIATED IMMUNE RESPONSE: involves specialized T cells destroying infected host cells HUMORAL IMMUNE
Fluid movement in capillaries. Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system
 Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity
 1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
Topics. Humoral Immune Response Part II Accessory cells Fc Receptors Opsonization and killing mechanisms of phagocytes NK, mast, eosynophils
 Topics Humoral Immune Response Part II Accessory cells Fc Receptors Opsonization and killing mechanisms of phagocytes NK, mast, eosynophils Immune regulation Idiotypic network 2/15/2005 MICR 415 / 515
Topics Humoral Immune Response Part II Accessory cells Fc Receptors Opsonization and killing mechanisms of phagocytes NK, mast, eosynophils Immune regulation Idiotypic network 2/15/2005 MICR 415 / 515
Chapter 43. Immune System. phagocytosis. lymphocytes. AP Biology
 Chapter 43. Immune System phagocytosis lymphocytes 1 Why an immune system? Attack from outside lots of organisms want you for lunch! animals must defend themselves against unwelcome invaders viruses protists
Chapter 43. Immune System phagocytosis lymphocytes 1 Why an immune system? Attack from outside lots of organisms want you for lunch! animals must defend themselves against unwelcome invaders viruses protists
May 14, Review for final exam (May 21, 2011, 8 AM)
 May 14, 2011 Review for final exam (May 21, 2011, 8 AM) The final exam is comprehensive. Two thirds of the test will cover material from the last one third of the class. The remaining one third of the
May 14, 2011 Review for final exam (May 21, 2011, 8 AM) The final exam is comprehensive. Two thirds of the test will cover material from the last one third of the class. The remaining one third of the
BACTERIAL PATHOGENESIS
 BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
Pathology of pulmonary tuberculosis. Dr: Salah Ahmed
 Pathology of pulmonary tuberculosis Dr: Salah Ahmed Is a chronic granulomatous disease, caused by Mycobacterium tuberculosis (hominis) Usually it involves lungs but may affect any organ or tissue Transmission:
Pathology of pulmonary tuberculosis Dr: Salah Ahmed Is a chronic granulomatous disease, caused by Mycobacterium tuberculosis (hominis) Usually it involves lungs but may affect any organ or tissue Transmission:
M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology
 Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Section 6.1 Defence mechanisms
 Section 6.1 Defence mechanisms Defence mechanisms Non-specific mechanisms that do not distinguish between one type of pathogen and another, but respond to all of them in the same way. These mechanisms
Section 6.1 Defence mechanisms Defence mechanisms Non-specific mechanisms that do not distinguish between one type of pathogen and another, but respond to all of them in the same way. These mechanisms
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Immunology Lecture- 1
 Immunology Lecture- 1 Immunology and Immune System Immunology: Study of the components and function of the immune system Immune System a network collected from cells, tissues organs and soluble factors
Immunology Lecture- 1 Immunology and Immune System Immunology: Study of the components and function of the immune system Immune System a network collected from cells, tissues organs and soluble factors
Streptococcus pneumonia
 Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
Cell-mediated response (what type of cell is activated and what gets destroyed?)
 The Immune System Reading Guide (Chapter 43) Name Per 1. The immune response in animals can be divided into innate immunity and adaptive immunity. As an overview, complete this figure indicating the divisions
The Immune System Reading Guide (Chapter 43) Name Per 1. The immune response in animals can be divided into innate immunity and adaptive immunity. As an overview, complete this figure indicating the divisions
phagocytic leukocyte Immune System lymphocytes attacking cancer cell lymph system
 phagocytic leukocyte Immune System lymphocytes attacking cancer cell lymph system 2006-2007 1) recognizing the presence of an infection; 2) containing the infection and working to eliminate it; 3) regulating
phagocytic leukocyte Immune System lymphocytes attacking cancer cell lymph system 2006-2007 1) recognizing the presence of an infection; 2) containing the infection and working to eliminate it; 3) regulating
Disease causing organisms Resistance Immunity
 Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Tuberculosis Pathogenesis
 Tuberculosis Pathogenesis Renuka Khurana, MD, MPH May 12, 2015 TB for Community Providers May 12, 2015 Phoenix, Arizona EXCELLENCE EXPERTISE INNOVATION Renuka Khurana, MD, MPH has the following disclosures
Tuberculosis Pathogenesis Renuka Khurana, MD, MPH May 12, 2015 TB for Community Providers May 12, 2015 Phoenix, Arizona EXCELLENCE EXPERTISE INNOVATION Renuka Khurana, MD, MPH has the following disclosures
Tuberculosis Intensive
 Tuberculosis Intensive San Antonio, Texas April 3 6, 2012 Tuberculosis Pathogenesis Lynn Horvath, MD April 3, 2012 Lynn Horvath, MD has the following disclosures to make: No conflict of interests No relevant
Tuberculosis Intensive San Antonio, Texas April 3 6, 2012 Tuberculosis Pathogenesis Lynn Horvath, MD April 3, 2012 Lynn Horvath, MD has the following disclosures to make: No conflict of interests No relevant
Bacterial Diseases IMMUNITY TO BACTERIAL INFECTIONS. Gram Positive Bacteria. Gram Negative Bacteria. Many Infectious agents and many diseases
 IMMUNITY TO BACTERIAL INFECTIONS Chapter 18 Bacterial Diseases Many Infectious agents and many diseases Bacteria can Infect any part of the body Cause disease due to Growth of the microbe in a tissue Produce
IMMUNITY TO BACTERIAL INFECTIONS Chapter 18 Bacterial Diseases Many Infectious agents and many diseases Bacteria can Infect any part of the body Cause disease due to Growth of the microbe in a tissue Produce
EQUINE DISEASES AND GUIDELINES
 EQUINE DISEASES AND vaccination GUIDELINES CORE AND RISK VACCINATION GUIDELINES FOR ADULT HORSES ALL VACCINATION PROGRAMS SHOULD BE DEVELOPED IN CONSULTATION WITH A LICENSED VETERINARIAN The American Association
EQUINE DISEASES AND vaccination GUIDELINES CORE AND RISK VACCINATION GUIDELINES FOR ADULT HORSES ALL VACCINATION PROGRAMS SHOULD BE DEVELOPED IN CONSULTATION WITH A LICENSED VETERINARIAN The American Association
Respiratory Tract Disease
 J Barry David, DVM, DACVIM Hagyard Equine Medical Institute Respiratory Tract Disease Foal Rhodococcus equi Foals 1-5 months of age Typical signs of pneumonia Frequently heat intolerant Now observing ARDs
J Barry David, DVM, DACVIM Hagyard Equine Medical Institute Respiratory Tract Disease Foal Rhodococcus equi Foals 1-5 months of age Typical signs of pneumonia Frequently heat intolerant Now observing ARDs
Proceedings of the 10th International Congress of World Equine Veterinary Association
 www.ivis.org Proceedings of the 10th International Congress of World Equine Veterinary Association Jan. 28 Feb. 1, 2008 - Moscow, Russia Next Congress: Reprinted in IVIS with the permission of the Conference
www.ivis.org Proceedings of the 10th International Congress of World Equine Veterinary Association Jan. 28 Feb. 1, 2008 - Moscow, Russia Next Congress: Reprinted in IVIS with the permission of the Conference
Hematopoiesis. Hematopoiesis. Hematopoiesis
 Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Warm-up. Parts of the Immune system. Disease transmission. Disease transmission. Why an immune system? Chapter 43 3/9/2012.
 Warm-up Objective: Explain how antigens react with specific lymphocytes to induce immune response and immunological memory. Warm-up: Which of the following would normally contain blood with the least amount
Warm-up Objective: Explain how antigens react with specific lymphocytes to induce immune response and immunological memory. Warm-up: Which of the following would normally contain blood with the least amount
I. Critical Vocabulary
 I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
Streptococcus equi equi: Fact and Fiction: Strangles and Related Diseases
 Streptococcus equi equi: Fact and Fiction: Strangles and Related Diseases Peter Heidmann DVM MPH Dipl. American College of Vet. Internal Med. Equine Internal Medicine & Disease Prevention Montana Equine
Streptococcus equi equi: Fact and Fiction: Strangles and Related Diseases Peter Heidmann DVM MPH Dipl. American College of Vet. Internal Med. Equine Internal Medicine & Disease Prevention Montana Equine
Topics in Parasitology BLY Vertebrate Immune System
 Topics in Parasitology BLY 533-2008 Vertebrate Immune System V. Vertebrate Immune System A. Non-specific defenses against pathogens 1. Skin - physical barrier a. Tough armor protein KERATIN b. Surface
Topics in Parasitology BLY 533-2008 Vertebrate Immune System V. Vertebrate Immune System A. Non-specific defenses against pathogens 1. Skin - physical barrier a. Tough armor protein KERATIN b. Surface
محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases
 محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases Immunity to infection depends on a combination of innate mechanisms (phagocytosis, complement, etc.) and antigen
محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases Immunity to infection depends on a combination of innate mechanisms (phagocytosis, complement, etc.) and antigen
Neurosciences- Lecture 2 Virus associated meningitis Polio Virus
 Al- Balqa Applied University Faculty of Medicine Neurosciences- Lecture 2 Virus associated meningitis Polio Virus Dr. Hala Al Daghistani The most important Enteroviruses are the three poliovirus serotypes
Al- Balqa Applied University Faculty of Medicine Neurosciences- Lecture 2 Virus associated meningitis Polio Virus Dr. Hala Al Daghistani The most important Enteroviruses are the three poliovirus serotypes
primary : thymus, bone marrow lymphoid tissue secondary : lymph nodes, MALT, spleen spleen: there are 2 main types of tissues in the spleen :
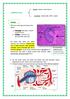 primary : thymus, bone marrow lymphoid tissue secondary : lymph nodes, MALT, spleen spleen: there are 2 main types of tissues in the spleen : 1. white pulp (gray area) : contains lymphoid tissue 2. red
primary : thymus, bone marrow lymphoid tissue secondary : lymph nodes, MALT, spleen spleen: there are 2 main types of tissues in the spleen : 1. white pulp (gray area) : contains lymphoid tissue 2. red
Guidelines for dealing with Strangles outbreaks
 Guidelines for dealing with Strangles outbreaks Dr Kirsten Neil BVSc (Hons) MANZCVS MS Dip ACVIM CMAVA Cert. ISELP Dip ACVSMR Specialist in equine medicine Sporthorse Veterinary Specialists M: 0408 769
Guidelines for dealing with Strangles outbreaks Dr Kirsten Neil BVSc (Hons) MANZCVS MS Dip ACVIM CMAVA Cert. ISELP Dip ACVSMR Specialist in equine medicine Sporthorse Veterinary Specialists M: 0408 769
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep
 The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
Role of the 85-Kilobase Plasmid and Plasmid-Encoded Virulence-Associated Protein A in Intracellular Survival and Virulence of Rhodococcus equi
 INFECTION AND IMMUNITY, July 1999, p. 3548 3557 Vol. 67, No. 7 0019-9567/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Role of the 85-Kilobase Plasmid and Plasmid-Encoded
INFECTION AND IMMUNITY, July 1999, p. 3548 3557 Vol. 67, No. 7 0019-9567/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Role of the 85-Kilobase Plasmid and Plasmid-Encoded
Physiology Unit 3. ADAPTIVE IMMUNITY The Specific Immune Response
 Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
MPI is satisfied the current quarantine measures in place are sufficient to manage the situation.
 Equine Herpes Virus type 1 myeloencephalopathy Update 7 February 2014 MPI has confirmed that Equine Herpes Virus type 1 myeloencephalopathy is present on a stud farm in the Waikato. It has not been reported
Equine Herpes Virus type 1 myeloencephalopathy Update 7 February 2014 MPI has confirmed that Equine Herpes Virus type 1 myeloencephalopathy is present on a stud farm in the Waikato. It has not been reported
NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses ( )
 NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses (43.1-43.2) The lymphatic system is closely associated with the cardiovascular system. LYMPHATIC PATHWAYS Lymphatic capillaries
NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses (43.1-43.2) The lymphatic system is closely associated with the cardiovascular system. LYMPHATIC PATHWAYS Lymphatic capillaries
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Overview: The immune responses of animals can be divided into innate immunity and acquired immunity.
 GUIDED READING - Ch. 43 - THE IMMUNE SYSTEM NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted.
GUIDED READING - Ch. 43 - THE IMMUNE SYSTEM NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted.
Unit 23: Immunity from Disease
 Unit 5 The Human Body Unit 23 Immunity from Disease- Unit 23: Immunity from Disease Name: Period: Page 1 of 51 Unit 5 The Human Body Unit 23 Immunity from Disease- Chapter 23 assignments Pages/Sections
Unit 5 The Human Body Unit 23 Immunity from Disease- Unit 23: Immunity from Disease Name: Period: Page 1 of 51 Unit 5 The Human Body Unit 23 Immunity from Disease- Chapter 23 assignments Pages/Sections
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Gram Positive Coccus Staphylococci Dr. Hala Al Daghistani
 Medical bacteriology Gram Positive Coccus Staphylococci Dr. Hala Al Daghistani The Staphylococci are gram-positive spherical cells, nonmotile, usually arranged in grapelike irregular clusters. Some are
Medical bacteriology Gram Positive Coccus Staphylococci Dr. Hala Al Daghistani The Staphylococci are gram-positive spherical cells, nonmotile, usually arranged in grapelike irregular clusters. Some are
Adaptive Immunity: Specific Defenses of the Host
 17 Adaptive Immunity: Specific Defenses of the Host SLOs Differentiate between innate and adaptive immunity, and humoral and cellular immunity. Define antigen, epitope, and hapten. Explain the function
17 Adaptive Immunity: Specific Defenses of the Host SLOs Differentiate between innate and adaptive immunity, and humoral and cellular immunity. Define antigen, epitope, and hapten. Explain the function
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Innate Immune Response
 The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
Third line of Defense. Topic 8 Specific Immunity (adaptive) (18) 3 rd Line = Prophylaxis via Immunization!
 Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Chapter 35 Active Reading Guide The Immune System
 Name: AP Biology Mr. Croft Chapter 35 Active Reading Guide The Immune System Section 1 Phagocytosis plays an important role in the immune systems of both invertebrates and vertebrates. Review the process
Name: AP Biology Mr. Croft Chapter 35 Active Reading Guide The Immune System Section 1 Phagocytosis plays an important role in the immune systems of both invertebrates and vertebrates. Review the process
1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50%
 BIOL2030 Huaman A&P II -- Exam 3 -- XXXX -- Form A Name: 1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50% 2. Special lymphatic vessels called
BIOL2030 Huaman A&P II -- Exam 3 -- XXXX -- Form A Name: 1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50% 2. Special lymphatic vessels called
LYMPHATIC AND IMMUNE SYSTEMS. Chapter 33
 LYMPHATIC AND IMMUNE SYSTEMS Chapter 33 THE LYMPHATIC SYSTEM The lymphatic system has three main functions Take up excess tissue fluid and return it to the bloodstream Receive fats called lipoproteins
LYMPHATIC AND IMMUNE SYSTEMS Chapter 33 THE LYMPHATIC SYSTEM The lymphatic system has three main functions Take up excess tissue fluid and return it to the bloodstream Receive fats called lipoproteins
Aerosol delivery of Rhodococcus equi specific IgG to the lungs of adult ponies. Alicia K Foley
 Aerosol delivery of Rhodococcus equi specific IgG to the lungs of adult ponies. Alicia K Foley Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment
Aerosol delivery of Rhodococcus equi specific IgG to the lungs of adult ponies. Alicia K Foley Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment
IMMUNE SYSTEM. Biology 2201
 IMMUNE SYSTEM Biology 2201 What is a disease? Other than an injury, any change in the body that interferes with the normal functioning of the body. Two Types of Diseases Non-infectious often called functional
IMMUNE SYSTEM Biology 2201 What is a disease? Other than an injury, any change in the body that interferes with the normal functioning of the body. Two Types of Diseases Non-infectious often called functional
IMMUNE SYSTEM. Biology What is a disease? Other than an injury, any change in the body that interferes with the normal functioning of the body.
 IMMUNE SYSTEM Biology 2201 What is a disease? Other than an injury, any change in the body that interferes with the normal functioning of the body. 1 Two Types of Diseases Non-infectious often called functional
IMMUNE SYSTEM Biology 2201 What is a disease? Other than an injury, any change in the body that interferes with the normal functioning of the body. 1 Two Types of Diseases Non-infectious often called functional
INFO SHEET. E. coli Versus. Environmental Management: Which Has Evolved Faster?
 INFO SHEET E. coli Versus Environmental Management: Which Has Evolved Faster? info.hybrid@hendrix-genetics.com www.hybridturkeys.com Disease is caused by a complex interaction between the disease agent
INFO SHEET E. coli Versus Environmental Management: Which Has Evolved Faster? info.hybrid@hendrix-genetics.com www.hybridturkeys.com Disease is caused by a complex interaction between the disease agent
Shigella and salmonella
 Sulaimani University College of Pharmacy Microbiology Lec. 9 & 10 Shigella and salmonella Dr. Abdullah Ahmed Hama PhD. Microbiology/Molecular Parasitology abdullah.hama@spu.edu.iq 1 Shigella Shigella species
Sulaimani University College of Pharmacy Microbiology Lec. 9 & 10 Shigella and salmonella Dr. Abdullah Ahmed Hama PhD. Microbiology/Molecular Parasitology abdullah.hama@spu.edu.iq 1 Shigella Shigella species
Appendix E1. Epidemiology
 Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
Immune System Notes Innate immunity Acquired immunity lymphocytes, humoral response Skin lysozyme, mucus membrane
 Immune System Notes I. The immune system consists of innate and acquired immunity. A. An animal must defend itself against unwelcome intruders the many potentially dangerous viruses, bacteria, and other
Immune System Notes I. The immune system consists of innate and acquired immunity. A. An animal must defend itself against unwelcome intruders the many potentially dangerous viruses, bacteria, and other
VMC-221: Veterinary Immunology and Serology (1+1) Question Bank
 VMC-221: Veterinary Immunology and Serology (1+1) Objective type Questions Question Bank Q. No. 1 - Fill up the blanks with correct words 1. The British physician, who developed the first vaccine against
VMC-221: Veterinary Immunology and Serology (1+1) Objective type Questions Question Bank Q. No. 1 - Fill up the blanks with correct words 1. The British physician, who developed the first vaccine against
The Immune System is the Third Line of Defense Against Infection. Components of Human Immune System
 Chapter 17: Specific Host Defenses: The Immune Response The Immune Response Immunity: Free from burden. Ability of an organism to recognize and defend itself against specific pathogens or antigens. Immune
Chapter 17: Specific Host Defenses: The Immune Response The Immune Response Immunity: Free from burden. Ability of an organism to recognize and defend itself against specific pathogens or antigens. Immune
2014 Pearson Education, Inc. Exposure to pathogens naturally activates the immune system. Takes days to be effective Pearson Education, Inc.
 The innate immune interact with the adaptive immune system 1. Damage to skin causes bleeding = bradykinin activated, resulting in inflammation 2. Dendritic phagocytose pathogens Adaptive immunity 4. Dendritic
The innate immune interact with the adaptive immune system 1. Damage to skin causes bleeding = bradykinin activated, resulting in inflammation 2. Dendritic phagocytose pathogens Adaptive immunity 4. Dendritic
Chapter 1. Chapter 1 Concepts. MCMP422 Immunology and Biologics Immunology is important personally and professionally!
 MCMP422 Immunology and Biologics Immunology is important personally and professionally! Learn the language - use the glossary and index RNR - Reading, Note taking, Reviewing All materials in Chapters 1-3
MCMP422 Immunology and Biologics Immunology is important personally and professionally! Learn the language - use the glossary and index RNR - Reading, Note taking, Reviewing All materials in Chapters 1-3
HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY. 09-Mar-15. Dr. Muhammad Tariq Javed. RESPIRATORY SYSTEM Lec-1
 RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
Body Defense Mechanisms
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 13 Body Defense Mechanisms Lecture Presentation Anne Gasc Hawaii Pacific University and University of
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 13 Body Defense Mechanisms Lecture Presentation Anne Gasc Hawaii Pacific University and University of
Chapter 38- Immune System
 Chapter 38- Immune System First Line of Defense: Barriers Nonspecific defenses, such as the skin and mucous membranes, are barriers to potential pathogens. In addition to being a physical barrier to pathogens,
Chapter 38- Immune System First Line of Defense: Barriers Nonspecific defenses, such as the skin and mucous membranes, are barriers to potential pathogens. In addition to being a physical barrier to pathogens,
immunity produced by an encounter with an antigen; provides immunologic memory. active immunity clumping of (foreign) cells; induced by crosslinking
 active immunity agglutination allografts immunity produced by an encounter with an antigen; provides immunologic memory. clumping of (foreign) cells; induced by crosslinking of antigenantibody complexes.
active immunity agglutination allografts immunity produced by an encounter with an antigen; provides immunologic memory. clumping of (foreign) cells; induced by crosslinking of antigenantibody complexes.
Tthe main goal of the respiratory system is to
 Respiratory Diseases Tthe main goal of the respiratory system is to transfer oxygen from the air that is breathed to the red blood cells where the oxygen will be transported throughout the body. In addition,
Respiratory Diseases Tthe main goal of the respiratory system is to transfer oxygen from the air that is breathed to the red blood cells where the oxygen will be transported throughout the body. In addition,
Anti-infectious Immunity
 Anti-infectious Immunity innate immunity barrier structures Secretory molecules Phagocytes NK cells Anatomical barriers 1. Skin and mucosa barrier 2.hemo-Spinal Fluid barrier 3. placental barrier Phagocytic
Anti-infectious Immunity innate immunity barrier structures Secretory molecules Phagocytes NK cells Anatomical barriers 1. Skin and mucosa barrier 2.hemo-Spinal Fluid barrier 3. placental barrier Phagocytic
Immune System. Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka
 Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
Chapter 16 Innate Immunity: Nonspecific Defenses of the Host
 Module 10 Chapter 16 Innate Immunity: Nonspecific Defenses of the Host The concept of immunity Immunity: ability to protect against from microbes and their o Aka, Susceptibility: vulnerability or lack
Module 10 Chapter 16 Innate Immunity: Nonspecific Defenses of the Host The concept of immunity Immunity: ability to protect against from microbes and their o Aka, Susceptibility: vulnerability or lack
