Ovalbumin removal in egg-based influenza vaccine production using Capto Core 700
|
|
|
- Bertha Walker
- 5 years ago
- Views:
Transcription
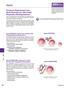
1 Application note AA Vaccines Ovalbumin removal in egg-based influenza vaccine production using Capto Core 7 This application note demonstrates the performance of Capto Core 7 chromatography medium (resin) as an alternative for ovalbumin removal in the production of egg-based influenza vaccine. Ovalbumin removal and haemagglutinin (HA) recovery were determined after the Capto Core 7 step in combination with ultrafiltration and diafiltration (UF/DF). The process performance was compared with that of zonal ultracentrifugation (UC), a technique traditionally used for ovalbumin removal in influenza vaccine purification. The results show that an influenza purity that meets the regulatory requirements was achieved with both methods. The two methods were also compared in terms of productivity and equipment cost at different scales. The process economic model demonstrates higher productivity at all scales and a more cost-effective production at process scale for the Capto Core 7 process, when compared with the zonal UC process. Introduction Influenza virus causes both seasonal epidemics and occasionally severe worldwide pandemics. Vaccination is an effective prevention strategy for protection of large populations. To meet the annual needs, efficient and cost-effective vaccine production is required. Investing in a domestic influenza vaccine production could further increase the availability and the ability for many countries to meet the urgent needs in a pandemic situation. Today, influenza vaccine is primarily produced in embryonated hen eggs and the purification processes has been essentially unchanged for the past decades. Industrial processes for manufacturing of influenza vaccine are commonly based on a combination of zonal UC and different filtration steps. However, modernization of legacy vaccine processes can help meet the requirements for increased productivity and costefficiency in the production of influenza vaccine. Fig 1. Schematic illustration of the purification processes for egg-based influenza virus. Ovalbumin removal and HA recovery in the Capto Core 7 (CC7) processes were compared with those in the Zonal UC process (marked in dark blue). The purification steps included in calculation of process economy are marked with dotted lines. For egg-based vaccines, ovalbumin is the main impurity, corresponding to approximately 6% of the total protein content (1). Ovalbumin needs to be removed, as it can cause severe allergic reactions. According to WHO, the recommended ovalbumin content should be below 1 µg ovalbumin/vaccine dose (2). In this work, static and dynamic binding capacities of Capto Core 7 for ovalbumin were determined. Furthermore, the performance of Capto Core 7, in terms of ovalbumin removal and HA recovery, was compared with that of zonal UC (Fig 1). The experimental work was performed in collaboration with Intravacc, an institute with a long history gelifesciences.com
2 in influenza vaccine product and process development. In addition, a model for calculation of process economy was designed to compare productivity and capital cost at pilot and process scales for the two purification methods. Capto Core 7 chromatography media The Capto Core 7 medium consists of an inactive shell and a ligand-activated core. The octylamine ligands in the core of the bead are multimodal, being both hydrophobic and positively charged. The core is surrounded by an inert outer shell, consisting of cross-linked agarose. Pores in the shell allow smaller molecules (M r < 7 ) to enter and be captured in the core, while larger entities, such as virus particles, pass in the flowthrough (Fig 2). Fig 2. Schematic view of Capto Core 7 showing a bead with an inert shell and a ligand-containing core. Pores in the shell allow small proteins and impurities (colored green, yellow, and blue) to enter and be captured in the core, while virus particles (red) and other larger entities (M r > 7 ) pass in the flowthrough. Material and methods Determination of static binding capacity (SBC) To investigate the effect of sample ph and ionic strength on protein binding, the SBC of Capto Core 7 for ovalbumin (Sigma-Aldrich Corp., St. Louis, MO, USA) was evaluated in PreDictor filter plates filled with 1 µl medium per well (available on demand). For ovalbumin sample preparation and for equilibration of the PreDictor plates, 2 mm phosphate, ph 6.5, 7., 7.5, and 8. as well as 2 mm Tris, ph 7.5, 8., and 8.5 buffers with either 15 mm or 1 M NaCl were used (Fig 3). The amount of ovalbumin recovered in the combined flowthrough and wash solution was compared with the amount of ovalbumin in the sample and the difference was stated as the SBC. All experiments were performed in triplicate (Fig 3). Determination of dynamic binding capacity (DBC) The DBC of Capto Core 7 for ovalbumin was evaluated in duplicate, on three separate Tricorn 5/5 columns (column volume (CV) approximately 1 ml), using the ÄKTA explorer 1 system. For ovalbumin sample preparation and column equilibration, 2 mm Tris-HCl, 15 mm NaCl, ph 7.5 was used. All columns were loaded with ovalbumin (3 mg /ml) at a flow velocity of 1 cm/h, equivalent to a residence time of 3 min. DBC was determined at 1% breakthrough (DBC1%), the point where 1% of unbound ovalbumin was detected in the flowthrough. Propagation of virus in hen eggs Pilot-scale virus inoculations (approximately 1 eggs) were performed with two different strains of influenza: A/Uruguay/716/27 (H3N2) and A/Puerto Rico/8/1934 (H1N1). Each virus batch was prepared using embryonated hen eggs cultivated in 36 C and infected at day 11. The allantoic fluid was harvested 72 h post infection (approximately 1 L pooled volume). Clarification of allantoic fluid Clarification was performed by continuous centrifugation using a disk stack separator (GEA Westfalia Separator Nederland B. V., Cuijk, The Netherlands) followed by filtration using two parallel 1 inch 2. μm ULTA Prime filter capsules. The part of the allantoic fluid intended for chromatographic experiments was additionally filtered through a.6 μm ULTA prime GF to protect the column from possible aggregates. Capto Core 7 in well Equilibration with buffer 2 5 µl Ovalbumin addition Incubation on rocking table (11 rpm, 1 h) Wash with buffer, 2 µl Centrifugation Centrifugation Waste UV plate UV plate Absorbance measurement at 254 and 28 nm Fig 3. Schematic illustration of the evaluation of SBC of Capto Core 7 medium for ovalbumin in PreDictor plates AA
3 Zonal UC process An ekii centrifuge (Alfa Wassermann, Woerden, The Netherlands) with K3rotor (3.2 L) was used for zonal UC at 2 C. A sucrose gradient was created using.125 M citrate buffer, ph 7.8 and a 6% sucrose solution. The clarified allantoic fluid was loaded at an initial flow rate of 4.8 L/h with a constant speed of 15 rpm (16 35 g), which gradually was increased to 18 L/h and 35 rpm (89 g) over 7 h. After centrifugation, a peristaltic pump was used to collect the virus-containing fraction at a sucrose content between 35% and 47%. In the zonal UC process, 7 L allantoic fluid was processed, resulting in a final volume of 471 ml, equivalent to a concentration factor of 149. Capto Core 7 process UF/DF A hollow fiber cartridge with.85 m 2 filter area, 1 mm lumen diameter, 75 nominal molecular weight cut-off (NMWC) (model number UPF -75E-4X2MA) was connected to a 54U pump (Watson-Marlow Pumps Group, Cornwall, UK). The filter was run at a shear rate of 35 s -1 and an approximate flux of 35 L/m 2 /h. The process material was first concentrated 2 and then 1-fold diafiltered for buffer exchange to phosphate buffered saline (PBS). The filter load was 88 L/m 2 for the A/Uruguay/716/27 (H3N2) strain and 112 L/m 2 for the A/Puerto Rico/8/1934 (H1N1) strain. Capto Core 7 chromatography For chromatography using Capto Core 7 medium, a HiScreen column (4.7 ml CV, 1 cm bed height) was used in the experiments including influenza strain A/Uruguay/ 716/27 (H3N2), whereas a HiScale 16 column (15 ml CV, 7.5 cm bed height) was used in the experiments including the A/Puerto Rico/8/1934 (H1N1) strain. Both columns were operated using the ÄKTA explorer 1 system. Columns were equilibrated with PBS. A flow velocity corresponding to 6 min residence time was selected. Sample (1 CV) from UF/DF was loaded on the columns and the flowthrough was collected. In addition, a reversed Capto Core 7 process was performed by loading 12.5 CV of clarified allantoic fluid on a Capto Core 7 HiScale 5/2 column (2 ml CV, 1 cm bed height) before the UF/DF procedure (performed as above). Between runs, the columns were washed with PBS followed by cleaning in place (CIP) with 1 M NaOH + 27% 1-propanol. The total contact time for CIP was 3 min. Analytical methods Haemagglutinin (HA) HA concentration was determined by a single-radial immunodiffusion (SRID) assay according to the method described by Wood et al. (3). All reagents were supplied by NIBSC, Potters Bar, London, UK. SDS-PAGE analysis SDS-PAGE analysis was performed according to the method described by Harvey et al. (4). Virus was inactivated by β-propiolactone treatment. Virus proteins were deglycosylated by incubation with PNGase F enzyme (New England Biolabs, Ipswich, USA) at 37ºC overnight. SDS- PAGE analysis of 5 µg of sample, taken before and after deglycosylation, was performed using the Gold precast gel system (Lonza, Basel, Switzerland). All samples were mixed with water and loading dye and denatured in the presence of dithiothreitol by heating to 1ºC for 5 min prior to loading on the gel. Gel separation was performed at 14 V for 1 h. The gels were stained using the Colloidal Blue Stain Kit (Invitrogen, Bleiswijk, The Netherlands) and scanned using an in-gel imaging system (Isogen, De Meern, The Netherlands). Gel spots from each major band were excised for protein identification by liquid chromatography-mass spectrometry (LC-MS) analysis. The gel spots were destained with acetonitrile in ammonium bicarbonate prior to in-gel tryptic digestion overnight. The dried digestion products were subjected to nano-scale LC-MS as described by Meiring et al. (5) and the acquired data was compared against the protein database UniprotKB/SwissProt ( Ovalbumin Ovalbumin concentration was measured using a commercially available ELISA kit (Seramun Diagnostica, Wolzig, Germany). Results and discussion SBC of Capto Core 7 The SBC of Capto Core 7 for ovalbumin was reduced only to a minor extent by increasing the ph and conductivity (Fig 4). Increasing the ph of the phosphate buffer from 6.5 to 8. or of the Tris buffer from 7.5 to 8.5 resulted in a SBC reduction to 88% of the maximum capacity. The SBC was shown to exceed 16 mg ovalbumin/ml medium for all conditions tested, which in many cases can eliminate the need for sample adjustment (ph or conductivity) prior to load. SBC (mg/ml) mm phosphate + 15 mm NaCl 2 mm phosphate + 1 mm NaCl 2 mm Tris + 15 mm NaCl 2 mm Tris + 1 mm NaCl ph 6.5 ph 7. ph 7.5 ph 8. ph 7.5 ph 8. ph 8.5 Fig 4. SBC of Capto Core 7 for ovalbumin in different ph and conductivities AA 3
4 DBC of Capto Core 7 The DBC of Capto Core 7 at 1% breakthrough (DBC1%) and 3 min residence time was 14.1 mg ovalbumin/ml medium in duplicate runs on three separate columns (Table 1). The results are in agreement with the SBC data, indicating strong binding of ovalbumin to the Capto Core 7 medium. Table 1. DBC1% of Capto Core 7 for ovalbumin (average of duplicate runs performed on three separate columns) Experiment DBC (mg/ml) average 14.1 HA recovery in pilot-scale experiments HA recovery (%) for the zonal UC process was 8%. However, for the Capto Core 7 process, comprising both the Capto Core 7 chromatography and the UF/DF step, the HA recovery was between 57% and 69% (Table 2). Table 2. Comparison of results from influenza virus purification using zonal UC and Capto Core 7 (average of duplicate runs) A/Uruguay/ 716/27 (H3N2) Zonal UC UF/DF + Capto Core 7 Capto Core 7 + UF/DF HA recovery (%) 8* Ovalbumin log reduction factor Ovalbumin (ng)/dose (45 µg HA) A/Puerto Rico/8/1934 (H1N1) HA recovery (%) 8* Ovalbumin log reduction factor Ovalbumin (ng)/dose (45 µg HA) * The HA amount obtained in the SRID assay after zonal UC was estimated to 8% of the amount in the clarified harvest. Ovalbumin removal in pilot-scale experiments Ovalbumin was reduced 12 times (log reduction factor 4.1) both by the zonal UC process and the Capto Core 7 process. The results were similar for both influenza strains investigated. Figure 5 shows ovalbumin removal in the Capto Core 7 process for A/Puerto Rico/8/1934 (H1N1), when performing UF/DF prior to the Capto Core 7 step. Approximately 8% of the ovalbumin was removed by UF/DF. The Capto Core 7 step removed an additional 99.9% of the remaining ovalbumin, giving less than.15% ovalbumin in the final sample. Ovalbumin and HA remaining, log scale (%) µm filtration (start) UF/DF Capto Core 7 Ovalbumin (g) HA-SRID (g) Fig 5. Ovalbumin removal from clarified allantoic fluid, containing the A/Puerto Rico/8/1934 (H1N1) influenza strain, by UF/DF followed by a Capto Core 7 chromatography step. In the zonal UC process, the ovalbumin content was reduced from an initial concentration of 63 5 ng/ml to 61 ng/ml for the A/Uruguay/716/27 (H3N2) strain, resulting in an amount of ovalbumin per dose (45 µg HA) of 12 ng. For the A/Puerto Rico/8/1934 (H1N1) strain, the amount of ovalbumin per dose was 59 ng. These results are significantly lower than the WHO target of 1 ng ovalbumin per 45 µg HA (2). In the Core 7 process (UF/DF followed by chromatography), ovalbumin was reduced from 51 2 ng/ml to 7 ng/ml. The resulting average amount of ovalbumin per dose was 11 ng, which is similar to the results achieved for the A/Uruguay/ 716/27 (H3N2) strain using zonal centrifugation. For the A/Puerto Rico/8/1934 (H1N1) strain, the ovalbumin amount per dose was 82 ng. When the Capto Core 7 process was performed in the reverse order (chromatography followed by UF/DF), the ovalbumin amount per dose was lower, approximately 54 ng for A/Puerto Rico/8/1934 (H1N1) and 25 ng for A/Uruguay/716/27 (H3N2). For A/Puerto Rico/8/1934 (H1N1), the ovalbumin removal was less efficient when concentrating the allantoic fluid before loading onto the chromatography column. This outcome might be attributed to impurity aggregation caused by concentration in the UF/DF step, which might lead to less efficient impurity removal. As summarized in Table 2, performing UF/DF prior to chromatography improves ovalbumin removal for A/Uruguay/ 716/27 (H3N2). For A/Puerto Rico/8/1934 (H1N1), ovalbumin removal was more efficient in the reversed process, performing chromatography prior to UF/DF. For both influenza strains, the most efficient ovalbumin removal obtained with the Capto Core 7 process are comparable with the results from the zonal UC process. A chromatogram showing ovalbumin removal in the Capto Core 7 process for A/Uruguay/716/27 (H3N2) is displayed in Figure AA
5 A 28 (mau) Product (virus) Volume (ml) Fig 6. Impurity removal for A/Uruguay/716/27 (H3N2) using Capto Core 7 HiScreen column. Virus particles passed in the flowthrough, while ovalbumin and other smaller impurities were captured in the bead core and subsequently eluted in the CIP procedure. SDS-PAGE SDS-PAGE analysis gives a qualitative picture of proteins present in a sample. Glycosylated proteins generate smearing bands in SDS-PAGE gel analysis. As HA, like many other virus proteins, is glycosylated, samples were subjected to deglycosylation prior to SDS-PAGE analysis to obtain more distinct gel bands. Analysis by SDS-PAGE revealed an almost identical banding pattern when comparing material derived from the zonal UC and Capto Core 7 processes, indicating a similar virus purity profile for both processes (Fig 7). Protein (M r 1 3 ) Protein (M r 1 3 ) HA (82) NP (56 and 52) HA1 (51) M1 (26) HA2 (24 ) Lane 1: zonal UC Lane 2: zonal UC (PNGase F treatment) Lane 3: UF/DF + Capto Core 7 Lane 4: UF/DF + Capto Core 7 (PNGase F treatment) Lane 5: Capto Core 7 + UF/DF Lane 6: Capto Core 7 + UF/DF (PNGase F treatment) NP (57, 54, 46, and 42) Cond. (ms/cm) Deglycosylated HA1 (35 and 31) M1 (26) Deglycosylated HA2 (24) Process economic model To compare costs for ovalbumin removal using Capto Core 7 versus zonal UC, a side-by-side comparison of the different techniques was made. The calculations compare hardware investments, process time, and costs for disposables, labor, and buffers. The results are stated as costs per dose, cost per batch, and doses/process time. It was assumed that similar process steps were required before and after the ovalbumin removal steps included in the comparison. The calculation assumes virus production in 1, 1, and 3 eggs, equivalent with 1 L, 1 L, and 3 L harvested allantoic fluid, respectively. Based on the obtained HA recovery shown in this work using either the zonal UC or the Capto Core 7 process, the recoveries in the process economic model were estimated to 9% after clarification, 8% after the Capto Core 7 or the zonal UC step, and 8% after UF/DF (6) (Fig 8). 8% 8% 9% 8% 8% Fig 8. Estimated recoveries for the purification steps included in the process economic model. Costs for equipment and consumables, including filters, buffer bags, chromatography media, columns, filterand chromatography skids, were all based on pricing of GE Healthcare products. Single-use bags were selected for sample and buffer storage up to 5 L. For larger volumes, stainless steel vessels were selected. Facility-related costs, QC/QA costs, and system validation costs were not included. Additionally, it was not considered that the purified influenza virus will have different concentrations after the two purification procedures, which would impact equipment size further down the process until the final formulation step. General assumptions and costs are summarized in Table 3. The model gives an indication of advantages and disadvantages of the different purification techniques. Fig 7. SDS-PAGE analysis of whole, inactivated A/Puerto Rico/8/1934 (H1N1) virus concentrates, before and after deglycosylation. Weak bands corresponding to neuraminidase (NA) and nonstructural proteins (NSP) were also detected in each lane (not indicated). Hemagglutinin = HA, hemagglutinin 1 = HA1, hemagglutinin 2 = HA2, nucleoproteins = NP, matrix protein = M AA 5
6 Table 3. General assumptions and process costs General assumptions Annual production Buffer storage Material Dose Buffer cost Labor cost/person Depreciation 8 batches/year single use < 5 L stainless steel > 5 L influenza virus from allantoic fluid, 3 µg HA/mL 15 µg/strain/dose, pandemic situation 2 USD/L (incl. buffers, cleaning solutions) 1 USD/h (incl. hands-on preparations and process monitoring) 1 years Interest rate 1% UF/DF Filter membrane Hollow fiber 5 C Reuse of fiber 1 times Flux 25 L/m 2 /h Shear rate 5 s -1 Filter load ~ 1 L/m 2 Capto Core 7 Investigated scale (L) Process time (h) 11 to to to 13 UF/DF system UniFlux 3 UniFlux 4 UniFlux 4 Capto Core 7 volume.2 L 2 L 6 L Columns AxiChrom 5/3 column AxiChrom 1/3 column AxiChrom 1/3 column System ÄKTApilot system ÄKTAprocess system ÄKTAprocess system Equilibration 7* CV Sample load 11 mg ovalbumin/ml medium (8% of ovalbumin DBC) Sample residence time 4 min Wash 5 CV CIP 1* CV Reuse of chromatography medium 2 times Zonal centrifugation Investigated scales (L) Process time (h) Ultracentrifuge ekii centrifuge (Alfa Wassermann) with rotor K3 (3.2 L) No. of ultracentrifuges No. of cycles/uc Load/run 1 L 2 L 2 L Load flow rate 25 L/h 25 L/h 25 L/h UF/DF system QuixStand bench top system UniFlux 1 UniFlux 1 * Required volumes of equilibration buffer and CIP solution depend on buffers/solution type and must be investigated for each specific case and feed. Volumes could be significantly reduced following an optimization of the process. It was assumed that ovalbumin contributes to approximately 6% of the total protein content (1) and that 8% of the ovalbumin from the clarified harvest was removed in the UF/DF step prior to the chromatography step AA
7 Process economic outcome As shown in Figures 9 to 11, the total operational cost/batch is lower for the zonal UC process than for the Capto Core 7 process at 1 L pilot scale. At 1 L and 3 L scales, however, when multiple ultracentrifuges are required for zonal UC, the total operational cost is lower for the Capto Core 7 process than for the zonal UC process. The operational cost/ dose is lower for the Capto Core 7 process at all scales. Cost/run (normalized) Cost/run (normalized) $/dose Hardware Consumables Buffers Hardware Consumables Buffers UF/DF and Capto Core 7 Labour Labour Zonal Ultracentrifugation and UF/DF Total operating cost Total operating cost 1 L 1 L 3 L 3 L 1 L 1 L Fig 9. Operational costs (normalized) at different scales for the combination of UF/DF and Capto Core 7 chromatography. 3 L 1 L 1 L Fig 1. Operational costs (normalized) at different scales for the combination of zonal UC and UF/DF. Fig 11 Operational cost (USD)/dose for the Capto Core 7 and the zonal UC processes at 1 L, 1 L, and 3 L scales. As shown in Figure 12, the productivity of the Capto Core 7 process is twice as high for the 1 L scale and up to four times higher at 3 L scale, as compared with the zonal UC process. #doses/process time (h) UF/DF and Capto Core 7 Zonal Ultracentrifugation and UF/DF 1 L 1 L 3 L Fig 12. Productivity stated as the number of doses/process time (h) for Capto Core 7 and zonal UC processes at 1 L, 1 L, and 3 L scales. The process time is 11 to 13 h for the Capto Core 7 process regardless of process scale. Substantially longer process time is required for the zonal UC process, which is even more evident at larger scales. Process time for the zonal UC process at the 1 L, 1 L, and 3 L scales are 24, 3, and 49 h, respectively. Accordingly, the labor cost is substantially higher for the zonal UC process than for the Capto Core 7 process. In summary, the productivity is higher and operational cost/ dose is lower for the Capto Core 7 process at all scales. At process scale (3 L), the Capto Core 7 process can deliver four times as many doses/h at half the operational cost. Conclusion The main focus of this study was to investigate the performance of Capto Core 7 chromatography medium as ovalbumin scavenger in the purification of egg-based influenza virus. The result was compared with the traditionally used zonal UC technique. Both methods generated a virus purity that meets regulatory requirements. SDS-PAGE analysis shows an almost identical banding pattern when comparing samples from Capto Core 7 and zonal UC process steps, indicating a similar impurity removal. Capto Core 7 exhibits high protein binding capacity over wide ph and conductivity ranges and can thus be used at different stages of a purification process without the need of sample conditioning prior to the run. As shown in this work, the Capto Core 7 step can be incorporated before or after the UF/DF steps with similar results. However, by reducing the sample volume in a UF/DF step prior to the chromatography step, less Capto Core 7 medium is required. Running unconcentrated feed on the Capto Core 7 column, on the other hand, can improve impurity removal. This strategy can, for example, be used in cases were the impurities tend to aggregate when concentrated during UF/DF, leading to less efficient purification in the subsequent chromatographic step AA 7
8 The Capto Core 7 process can be a viable alternative to zonal UC for ovalbumin removal and can offer substantial cost benefits at larger production scales where time and productivity are key considerations. Capto Core 7 exhibits high loading capacity and the purification process can be performed at high flow rates. The combination of these two factors has a strong influence on the overall process economy. A Capto Core 7 step in combination with UF/DF can deliver twice as many doses per process hour at a 1 L process scale and a four-fold number of doses per process hour at 3 L scale compared with zonal UC in combination with UF/DF. As the combination of Capto Core 7 and UF/DF is easily implemented and scaled, this process for ovalbumin removal could be an alternative to take into consideration for actors interested in expanding domestic influenza virus production capacity for rapid response to pandemic outbreaks. References 1. Huntington, J. A. and Stein, P.E. Structure and properties of ovalbumin. Journal of Chromatography B 756, (21) 2. WHO Technical Report Series No. 927 (25) 3. Wood, J. M., Schild, G. C., Newman, R. W., and Seagroatt, V. An improved singleradial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand 5, (1977) 4. Harvey, R., Wheeler, J.X., Wallis, C.L., Robertson, J.S., Engelhardt, O.G. Quantitation of haemagglutinin in H5N1 influenza virusses reveals low haemagglutinin content of vaccine virus NIBRG-14 (H5N1). Vaccine 26, (28) 5. Meiring, H. D., van der Heeft, E., ten Hove, G. J., and de Jong, A. P. J. M. Nanoscale LC-MS(n): technical design and applications to peptide and protein analysis. Journal of Separation Science 25, (22) 6. Application note: Concentration and diafiltration of cell-derived, live influenza virus using 75 C hollow fiber filter cartridge. GE Healthcare, , Edition AA (214) Ordering information Product Size Code number Capto Core 7 25 ml Capto Core 7 1 ml Capto Core 7 1 L Capto Core 7 5 L Prepacked columns Size Code number HiTrap Capto Core ml HiScreen Capto Core ml Related literature Code number Capto Core 7, data file The use of Capto Core 7 and Capto Q ImpRes in the purification of human papilloma virus like particles, application note Purification of influenza A/H1N1 using multimodal Capto Core 7, application note For local office contact information, visit GE Healthcare Bio-Sciences AB Björkgatan Uppsala Sweden GE and GE monogram are trademarks of General Electric Company. ÄKTA, ÄKTApilot, ÄKTAprocess, AxiChrom, BioProcess, Capto, HiScreen, HiScale, PreDictor, QuickStand, Tricorn, ULTA, and UniFlux are trademarks of General Electric Company or one of its subsidiaries. BioSolve is a trademark of BioPharm Services. All other third party trademarks are the property of their respective owner. 214 General Electric Company All rights reserved. First published Sep. 214 All goods and services are sold subject to the terms and conditions of sale of the company within GE Healthcare which supplies them. A copy of these terms and conditions is available on request. Contact your local GE Healthcare representative for the most current information. GE Healthcare UK Limited Amersham Place, Little Chalfont Buckinghamshire, HP7 9NA UK GE Healthcare Europe, GmbH Munzinger Strasse 5 D Freiburg Germany GE Healthcare Bio-Sciences Corp. 8 Centennial Avenue, P.O. Box 1327 Piscataway, NJ USA GE Healthcare Japan Corporation Sanken Bldg., , Hyakunincho Shinjuku-ku, Tokyo Japan AA 9/214
Use of Capto ViralQ for the removal of genomic DNA from influenza virus produced in MDCK cells
 GE Healthcare Application note 28-9769-69 AA Vaccines Use of Capto ViralQ for the removal of genomic DNA from influenza virus produced in MDCK cells We have developed a chromatographic method to remove
GE Healthcare Application note 28-9769-69 AA Vaccines Use of Capto ViralQ for the removal of genomic DNA from influenza virus produced in MDCK cells We have developed a chromatographic method to remove
High-throughput process development and scale-up of an intermediate purification step for recombinant insulin
 GE Healthcare Life Sciences Application note 29-18-56 AA Process development High-throughput process development and scale-up of an intermediate purification step for recombinant insulin This Application
GE Healthcare Life Sciences Application note 29-18-56 AA Process development High-throughput process development and scale-up of an intermediate purification step for recombinant insulin This Application
Concentration and diafiltration of cell-derived, live influenza virus using 750 C hollow fiber filter cartridge
 GE Healthcare Life Sciences Application note 29-928-26 AA Cross flow filtration Concentration and diafiltration of cell-derived, live influenza virus using 75 C hollow fiber filter cartridge This application
GE Healthcare Life Sciences Application note 29-928-26 AA Cross flow filtration Concentration and diafiltration of cell-derived, live influenza virus using 75 C hollow fiber filter cartridge This application
Superose 6 Increase columns
 Data file 9-99- AA Size-exclusion chromatography Superose Increase columns Superose Increase prepacked columns (Fig ) are designed for rapid separation and analysis of proteins and other biomolecules by
Data file 9-99- AA Size-exclusion chromatography Superose Increase columns Superose Increase prepacked columns (Fig ) are designed for rapid separation and analysis of proteins and other biomolecules by
GE Healthcare Life Sciences. Quality matters. Whatman TM filters for air monitoring
 GE Healthcare Life Sciences Quality matters Whatman TM filters for air monitoring Quality matters Why does quality matter? Particulate testing Examples include PM 10 & PM 2.5 particulate monitoring. Quality
GE Healthcare Life Sciences Quality matters Whatman TM filters for air monitoring Quality matters Why does quality matter? Particulate testing Examples include PM 10 & PM 2.5 particulate monitoring. Quality
Amersham ECL Prime Western blotting reagent
 GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
BabyBio IMAC columns DATA SHEET DS
 BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
Biacore biosensor assays for
 GE Healthcare pplication note 28-977-57 Vaccines iacore biosensor assays for quantitation of influenza virus and HCP iacore biosensor technology is used for the quantitation of virus haemagglutinin (H)
GE Healthcare pplication note 28-977-57 Vaccines iacore biosensor assays for quantitation of influenza virus and HCP iacore biosensor technology is used for the quantitation of virus haemagglutinin (H)
Application Note USD 2916 (1) Purification of Influenza Virus by Ion Exchange Chromatography on Mustang Q XT Membranes
 Application Note USD 2916 (1) Purification of Influenza Virus by Ion Exchange Chromatography on Mustang Q XT Membranes Fast Purification Conditions Screening on AcroPrep Advance 96-Well Filter Plates Summary
Application Note USD 2916 (1) Purification of Influenza Virus by Ion Exchange Chromatography on Mustang Q XT Membranes Fast Purification Conditions Screening on AcroPrep Advance 96-Well Filter Plates Summary
Sephadex G-25 media and prepacked formats
 Data File 28-9137-87 AC Desalting/buffer exchange and gel filtration Sephadex G-25 media and prepacked formats Gel filtration has successfully been employed for size based separations of macromolecules
Data File 28-9137-87 AC Desalting/buffer exchange and gel filtration Sephadex G-25 media and prepacked formats Gel filtration has successfully been employed for size based separations of macromolecules
Influenza virus capture using membrane chromatography: Improving selectivity by matrix design and pseudo-affinity ligand interactions
 Engineering Conferences International ECI Digital Archives Vaccine Technology VII Proceedings 6-17-2018 Influenza virus capture using membrane chromatography: Improving selectivity by matrix design and
Engineering Conferences International ECI Digital Archives Vaccine Technology VII Proceedings 6-17-2018 Influenza virus capture using membrane chromatography: Improving selectivity by matrix design and
Sephadex G-25 resins and prepacked formats
 GE Healthcare Desalting/buffer exchange and size exclusion chromatography Sephadex G-25 resins and prepacked formats Size exclusion chromatography (SEC) or as it is also commonly named, gel filtration
GE Healthcare Desalting/buffer exchange and size exclusion chromatography Sephadex G-25 resins and prepacked formats Size exclusion chromatography (SEC) or as it is also commonly named, gel filtration
Propagation of influenza virus in Vero cells using Cytodex TM microcarriers in WAVE Bioreactor TM systems
 Propagation of influenza virus in Vero cells using Cytodex TM microcarriers in WAVE Bioreactor TM systems Ann-Christin Magnusson, Therese Lundström, *Christian Kaisermayer and Mats Lundgren GE Healthcare
Propagation of influenza virus in Vero cells using Cytodex TM microcarriers in WAVE Bioreactor TM systems Ann-Christin Magnusson, Therese Lundström, *Christian Kaisermayer and Mats Lundgren GE Healthcare
Column chromatography for the development of pandemic and seasonal influenza vaccines
 Column chromatography for the development of pandemic and seasonal influenza vaccines Olga Chervyakova Research Institute for Biological Safety Problems, RK ME&S 5 th Meeting with International Partners
Column chromatography for the development of pandemic and seasonal influenza vaccines Olga Chervyakova Research Institute for Biological Safety Problems, RK ME&S 5 th Meeting with International Partners
Prepacked chromatography columns for ÄKTA systems
 Prepacked chromatography columns for ÄKTA systems Selection guide gelifesciences.com Select the chromatography technique of your choice below This guide gives you an overview of prepacked columns from
Prepacked chromatography columns for ÄKTA systems Selection guide gelifesciences.com Select the chromatography technique of your choice below This guide gives you an overview of prepacked columns from
Influenza A H1N1 HA ELISA Pair Set
 Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
Annex 5. Generic protocol for the calibration of seasonal and pandemic influenza antigen working reagents by WHO essential regulatory laboratories
 Annex 5 Generic protocol for the calibration of seasonal and pandemic influenza antigen working reagents by WHO essential regulatory laboratories Abbreviations 262 1. Introduction 262 2. Essential regulatory
Annex 5 Generic protocol for the calibration of seasonal and pandemic influenza antigen working reagents by WHO essential regulatory laboratories Abbreviations 262 1. Introduction 262 2. Essential regulatory
Data File. Sephadex ion exchange media. Ion exchange chromatography. Introduction. Sephadex ion exchangers General description
 A m e r s h a m B i o s c i e n c e s Sephadex ion exchange media Data File Ion exchange chromatography Based on well documented and well proven Sephadex base matrix Simple and economical to use Very high
A m e r s h a m B i o s c i e n c e s Sephadex ion exchange media Data File Ion exchange chromatography Based on well documented and well proven Sephadex base matrix Simple and economical to use Very high
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin
 SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard. Product Number: AD0013
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
DELFIA Tb-DTPA ITC Chelate & Terbium Standard
 AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
An Affordable Pandemic Influenza Vaccine Solution for Developing Countries (and Developed Countries Too)
 An Affordable Pandemic Influenza Vaccine Solution for Developing Countries (and Developed Countries Too) James M. Robinson, PE VP Technical and Quality Operations Novavax, Inc. New Cells for New Vaccines
An Affordable Pandemic Influenza Vaccine Solution for Developing Countries (and Developed Countries Too) James M. Robinson, PE VP Technical and Quality Operations Novavax, Inc. New Cells for New Vaccines
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade
 AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
Introduction of Tangential Flow Filtration (TFF) Karen Chan 16 May 2017
 Introduction of Tangential Flow Filtration (TFF) Karen Chan 16 May 2017 What you will learn TFF principles and applications in mammalian cell processes TFF vocabulary definitions and key process parameters
Introduction of Tangential Flow Filtration (TFF) Karen Chan 16 May 2017 What you will learn TFF principles and applications in mammalian cell processes TFF vocabulary definitions and key process parameters
Use of online cell counting for micronucleus and neurite outgrowth assays on IN Cell Analyzer 2000
 GE Healthcare Application note 28-9673-96 AA IN Cell Analyzer 2000 Use of online cell counting for micronucleus and neurite outgrowth assays on IN Cell Analyzer 2000 Key words: IN Cell Analyzer online
GE Healthcare Application note 28-9673-96 AA IN Cell Analyzer 2000 Use of online cell counting for micronucleus and neurite outgrowth assays on IN Cell Analyzer 2000 Key words: IN Cell Analyzer online
MagCapture Exosome Isolation Kit PS Q&A
 MagCapture Exosome Isolation Kit PS Q&A Specifications and performance P.1 Comparison of the conventional method P.2 Operation methods and composition P.4 Amount of starting sample P.5 Analysis after exosomes
MagCapture Exosome Isolation Kit PS Q&A Specifications and performance P.1 Comparison of the conventional method P.2 Operation methods and composition P.4 Amount of starting sample P.5 Analysis after exosomes
CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES (AUGUST 2015)
 August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
Standards and beyond: challenges of application of old methods to next generation products. Elena Semenova, Penny Post, Tim Fields, Manon Cox
 Standards and beyond: challenges of application of old methods to next generation products Elena Semenova, Penny Post, Tim Fields, Manon Cox March 25, 2014 Recombinant Flu vaccines Propagation of the influenza
Standards and beyond: challenges of application of old methods to next generation products Elena Semenova, Penny Post, Tim Fields, Manon Cox March 25, 2014 Recombinant Flu vaccines Propagation of the influenza
ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006)
 September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
Critical process for vaccine manufacturing and process validation
 Critical process for vaccine manufacturing and process validation Inactivation Influenza Vaccine Production Project The Government Pharmaceutical Organization Presented by Ms RATSAMIKORN SINGCHAREON and
Critical process for vaccine manufacturing and process validation Inactivation Influenza Vaccine Production Project The Government Pharmaceutical Organization Presented by Ms RATSAMIKORN SINGCHAREON and
SeQuant ZIC -HILIC For all who expect more...
 SeQuant ZIC -ILIC For all who expect more... The better choice for PLC and LC/MS of all types of polar and hydrophilic compounds EMD Millipore Corp. is a subsidiary of Merck KGaA, Darmstadt, Germany Poor
SeQuant ZIC -ILIC For all who expect more... The better choice for PLC and LC/MS of all types of polar and hydrophilic compounds EMD Millipore Corp. is a subsidiary of Merck KGaA, Darmstadt, Germany Poor
Purification of Insulin with YMC-Triart Prep C8. Increased Output at Reduced Costs
 Purification of Insulin with YMC-Triart Prep C8 Increased Output at Reduced Costs 2 Introduction YMC bulk material along with phases from Daiso and Kromasil took part in a study by a major peptide manufacturer.
Purification of Insulin with YMC-Triart Prep C8 Increased Output at Reduced Costs 2 Introduction YMC bulk material along with phases from Daiso and Kromasil took part in a study by a major peptide manufacturer.
Ultracentrifugation: Probably the best downstream processing tool for Large Scale Viral Vaccines.
 Ultracentrifugation: Probably the best downstream processing tool for Large Scale Viral Vaccines. Sandra Meriño Global Project Director Alfa Wassermann BV Zonal Ultracentrifugation 1966 2001 2006 1968
Ultracentrifugation: Probably the best downstream processing tool for Large Scale Viral Vaccines. Sandra Meriño Global Project Director Alfa Wassermann BV Zonal Ultracentrifugation 1966 2001 2006 1968
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
SeQuant ZIC -HILIC For all who expect more...
 SeQuant ZIC -ILIC For all who expect more... The better choice for PLC and LC/MS of all types of polar and hydrophilic compounds EMD Millipore is a division of Merck KGaA, Darmstadt, Germany Poor retention
SeQuant ZIC -ILIC For all who expect more... The better choice for PLC and LC/MS of all types of polar and hydrophilic compounds EMD Millipore is a division of Merck KGaA, Darmstadt, Germany Poor retention
TiO 2 Mag Sepharose. SepharoseTM. GE Healthcare. Simple handling
 GE Healthcare Data file 28-9539-44 AA Protein sample preparation TiO 2 Mag Sepharose TiO 2 Mag Sepharose magnetic beads use titanium dioxide (TiO 2 )-based chromatography to simplify capture and enrichment
GE Healthcare Data file 28-9539-44 AA Protein sample preparation TiO 2 Mag Sepharose TiO 2 Mag Sepharose magnetic beads use titanium dioxide (TiO 2 )-based chromatography to simplify capture and enrichment
VaTx1 VaTx2 VaTx3. VaTx min Retention Time (min) Retention Time (min)
 a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Biochemical Techniques 06 Salt Fractionation of Proteins. Biochemistry
 . 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
. 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
LC Columns - Exceed the limit. A premium inert range of LC columns delivering optimal peak shape. ProteCol -P PEEK lined
 ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set
 Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Enzymatic Removal of N- and O-glycans using PNGase F or the Protein Deglycosylation Mix
 be INSPIRED drive DISCOVERY stay GENUINE APPLICATION NOTE Enzymatic Removal of N- and O-glycans using PNGase F or the Protein Deglycosylation Mix Alicia Bielik and Paula Magnelli, New England Biolabs,
be INSPIRED drive DISCOVERY stay GENUINE APPLICATION NOTE Enzymatic Removal of N- and O-glycans using PNGase F or the Protein Deglycosylation Mix Alicia Bielik and Paula Magnelli, New England Biolabs,
Tunable Hydrophobicity in DNA Micelles Anaya, Milena; Kwak, Minseok; Musser, Andrew J.; Muellen, Klaus; Herrmann, Andreas; Müllen, Klaus
 University of Groningen Tunable Hydrophobicity in DNA Micelles Anaya, Milena; Kwak, Minseok; Musser, Andrew J.; Muellen, Klaus; Herrmann, Andreas; Müllen, Klaus Published in: Chemistry DOI: 10.1002/chem.201001816
University of Groningen Tunable Hydrophobicity in DNA Micelles Anaya, Milena; Kwak, Minseok; Musser, Andrew J.; Muellen, Klaus; Herrmann, Andreas; Müllen, Klaus Published in: Chemistry DOI: 10.1002/chem.201001816
Babu Antharavally, Ryan Bomgarden, and John Rogers Thermo Fisher Scientific, Rockford, IL
 A Versatile High-Recovery Method for Removing Detergents from Low-Concentration Protein or Peptide Samples for Mass Spectrometry Sample Preparation and Analysis Babu Antharavally, Ryan Bomgarden, and John
A Versatile High-Recovery Method for Removing Detergents from Low-Concentration Protein or Peptide Samples for Mass Spectrometry Sample Preparation and Analysis Babu Antharavally, Ryan Bomgarden, and John
Trypsin Digestion Mix
 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name 239PR Trypsin Digestion Mix Provides optimal buffered conditions for in gel trypsin digestion
G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name 239PR Trypsin Digestion Mix Provides optimal buffered conditions for in gel trypsin digestion
PosterREPRINT INTRODUCTION. 2-D PAGE of Mouse Liver Samples. 2-D PAGE of E.coli Samples. Digestion / Cleanup. EXPERIMENTAL 1-D PAGE of BSA Samples
 INTRODUCTION Identification and characterization of low abundance proteins separated by 2D gel electrophoresis is complicated by two important factors; the use of suitable staining techniques for the visualization
INTRODUCTION Identification and characterization of low abundance proteins separated by 2D gel electrophoresis is complicated by two important factors; the use of suitable staining techniques for the visualization
High-Throughput Quantitative LC-MS/MS Analysis of 6 Opiates and 14 Benzodiazepines in Urine
 High-Throughput Quantitative LC-MS/MS Analysis of and 14 Benzodiazepines in Urine Bill Yu, Kristine Van Natta, Marta Kozak, Thermo Fisher Scientific, San Jose, CA Application Note 588 Key Words Opiates,
High-Throughput Quantitative LC-MS/MS Analysis of and 14 Benzodiazepines in Urine Bill Yu, Kristine Van Natta, Marta Kozak, Thermo Fisher Scientific, San Jose, CA Application Note 588 Key Words Opiates,
Core-Shell Technology for Proteins and Peptides
 Core-Shell Technology for Proteins and Peptides Better BioSeparations on HPLC and UHPLC Systems www.phenomenex.com/aeris Aeris Core-Shell Technology Core-Shell Particles Precision Engineered for Protein
Core-Shell Technology for Proteins and Peptides Better BioSeparations on HPLC and UHPLC Systems www.phenomenex.com/aeris Aeris Core-Shell Technology Core-Shell Particles Precision Engineered for Protein
In-Solution Digestion for proteomics
 In-Solution Digestion for proteomics Guidelines for sample preparation (How to protect your samples from contamination with keratin) 1. Try to avoid any contact of samples and solutions with dust, skin
In-Solution Digestion for proteomics Guidelines for sample preparation (How to protect your samples from contamination with keratin) 1. Try to avoid any contact of samples and solutions with dust, skin
Influenza or flu is a
 Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
IFPMA IVS ITF Background Paper The WHO System for Influenza Surveillance and Supply of Viruses for the Standardized Production of Influenza Vaccines
 The WHO System for Influenza Surveillance and Supply of Viruses for the Standardized Production of Influenza Vaccines BACKGROUND Chemin Louis-Dunant 15, P.O., Box 195, 1211 Geneva 20, Switzerland Tel:
The WHO System for Influenza Surveillance and Supply of Viruses for the Standardized Production of Influenza Vaccines BACKGROUND Chemin Louis-Dunant 15, P.O., Box 195, 1211 Geneva 20, Switzerland Tel:
SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine
 SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine J. Jones, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 709 Key Words SPE, SOLA
SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine J. Jones, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 709 Key Words SPE, SOLA
In vitro human regulatory T cell expansion
 - 1 - Human CD4 + CD25 + regulatory T cell isolation, Workflow in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation of T
- 1 - Human CD4 + CD25 + regulatory T cell isolation, Workflow in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation of T
Exosomes Immunocapture and Isolation Tools: Immunobeads
 Product Insert Exosomes Immunocapture and Isolation Tools: Immunobeads HBM-BOLF-##/##-# HBM-BOLC-##/##-# HBM-BTLF-##/##-# Overall Exosome capture from Human Biological fluids Overall Exosome capture from
Product Insert Exosomes Immunocapture and Isolation Tools: Immunobeads HBM-BOLF-##/##-# HBM-BOLC-##/##-# HBM-BTLF-##/##-# Overall Exosome capture from Human Biological fluids Overall Exosome capture from
Peptide sequencing using chemically assisted fragmentation (CAF) and Ettan MALDI-ToF Pro mass spectrometry
 application note Ett MALDI-ToF Pro Peptide sequencing using chemically assisted fragmentation (CAF) d Ett MALDI-ToF Pro mass spectrometry Key words: MALDI-ToF, PSD, peptide sequencing, chemically assisted
application note Ett MALDI-ToF Pro Peptide sequencing using chemically assisted fragmentation (CAF) d Ett MALDI-ToF Pro mass spectrometry Key words: MALDI-ToF, PSD, peptide sequencing, chemically assisted
The Investigation of Factors Contributing to Immunosuppressant Drugs Response Variability in LC-MS/MS Analysis
 The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit
 2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
Doubling the throughput of long chromatographic methods by using a novel Dual LC workflow
 APPLICATIN NTE 7601 Doubling the throughput of long chromatographic methods by using a novel Dual LC workflow Authors Sylvia Grosse, Mauro De Pra, Frank Steiner Thermo Fisher Scientific, Germering, Germany
APPLICATIN NTE 7601 Doubling the throughput of long chromatographic methods by using a novel Dual LC workflow Authors Sylvia Grosse, Mauro De Pra, Frank Steiner Thermo Fisher Scientific, Germering, Germany
Application of μmacs Streptavidin MicroBeads for the analysis of HIV-1 directly from patient plasma
 Excerpt from MACS&more Vol 8 1/2004 Application of μmacs Streptavidin MicroBeads for the analysis of HIV-1 directly from patient plasma L. Davis Lupo and Salvatore T. Butera HIV and Retrovirology Branch,
Excerpt from MACS&more Vol 8 1/2004 Application of μmacs Streptavidin MicroBeads for the analysis of HIV-1 directly from patient plasma L. Davis Lupo and Salvatore T. Butera HIV and Retrovirology Branch,
TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010)
 June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection. EPL-BAS Method No.
 Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Protocol for purification of recombinant protein from 300 ml yeast culture
 Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
Aeris. Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations. Aeris PEPTIDE. Aeris WIDEPORE
 Aeris Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations Aeris is a specialized line of reversed phase core-shell UHPLC columns, built exclusively for the ultra-high performance
Aeris Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations Aeris is a specialized line of reversed phase core-shell UHPLC columns, built exclusively for the ultra-high performance
[ CARE AND USE MANUAL ] GlycoWorks Single Use Sample Preparation Kit CONTENTS
![[ CARE AND USE MANUAL ] GlycoWorks Single Use Sample Preparation Kit CONTENTS [ CARE AND USE MANUAL ] GlycoWorks Single Use Sample Preparation Kit CONTENTS](/thumbs/76/74042273.jpg) GlycoWorks Single Use Sample Preparation Kit CONTENTS I. INTRODUCTION General Guideline of Sample Preparation from Glycoprotein to enrich FLR Labeled Glycans Using Reductive Amination Reaction. II. STORAGE
GlycoWorks Single Use Sample Preparation Kit CONTENTS I. INTRODUCTION General Guideline of Sample Preparation from Glycoprotein to enrich FLR Labeled Glycans Using Reductive Amination Reaction. II. STORAGE
PNGase F Instruction Manual
 PNGase F Instruction Manual Catalog Number 170-6883 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 4006094 Rev A Table of Contents Section 1 Introduction...1 Section 2 Kit Components and
PNGase F Instruction Manual Catalog Number 170-6883 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 4006094 Rev A Table of Contents Section 1 Introduction...1 Section 2 Kit Components and
Purification and Fluorescent Labeling of Exosomes Asuka Nanbo 1*, Eri Kawanishi 2, Ryuji Yoshida 2 and Hironori Yoshiyama 3
 Purification and Fluorescent Labeling of Exosomes Asuka Nanbo 1*, Eri Kawanishi 2, Ryuji Yoshida 2 and Hironori Yoshiyama 3 1 Graduate School of Medicine, Hokkaido University, Sapporo, Japan; 2 Graduate
Purification and Fluorescent Labeling of Exosomes Asuka Nanbo 1*, Eri Kawanishi 2, Ryuji Yoshida 2 and Hironori Yoshiyama 3 1 Graduate School of Medicine, Hokkaido University, Sapporo, Japan; 2 Graduate
TECHNICAL BULLETIN. R 2 GlcNAcβ1 4GlcNAcβ1 Asn
 GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
ProteaseMAX Surfactant, Trypsin Enhancer
 Technical Bulletin ProteaseMAX Surfactant, Trypsin Enhancer INSTRUCTIONS FOR USE OF PRODUCTS V2071 AND V2072. PRINTED IN USA. Revised 1/10 ProteaseMAX Surfactant, Trypsin Enhancer All technical literature
Technical Bulletin ProteaseMAX Surfactant, Trypsin Enhancer INSTRUCTIONS FOR USE OF PRODUCTS V2071 AND V2072. PRINTED IN USA. Revised 1/10 ProteaseMAX Surfactant, Trypsin Enhancer All technical literature
Bioanalytical Quantitation of Biotherapeutics Using Intact Protein vs. Proteolytic Peptides by LC-HR/AM on a Q Exactive MS
 Bioanalytical Quantitation of Biotherapeutics Using Intact Protein vs. Proteolytic Peptides by LC-HR/AM on a Q Exactive MS Jenny Chen, Hongxia Wang, Zhiqi Hao, Patrick Bennett, and Greg Kilby Thermo Fisher
Bioanalytical Quantitation of Biotherapeutics Using Intact Protein vs. Proteolytic Peptides by LC-HR/AM on a Q Exactive MS Jenny Chen, Hongxia Wang, Zhiqi Hao, Patrick Bennett, and Greg Kilby Thermo Fisher
APPLICATIONS Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns
 Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns Michael McGinley, Deborah Jarrett, and Jeff Layne Phenomenex, Inc., 411 Madrid Avenue, Torrance, CA 91 USA Aeris WIDEPORE
Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns Michael McGinley, Deborah Jarrett, and Jeff Layne Phenomenex, Inc., 411 Madrid Avenue, Torrance, CA 91 USA Aeris WIDEPORE
GE Healthcare Life Sciences. Methodology Guideline AA. Quantification of influenza hemagglutinin using Biacore T200
 GE Healthcare Life Sciences Methodology Guideline 29-0136-74 AA Biacore systems Quantification of influenza hemagglutinin using Biacore T200 Table of Contents 1. Introduction... 3 1.1. Steps recommended
GE Healthcare Life Sciences Methodology Guideline 29-0136-74 AA Biacore systems Quantification of influenza hemagglutinin using Biacore T200 Table of Contents 1. Introduction... 3 1.1. Steps recommended
Ion Exchange Chromatography COSMOGEL IEX Series Columns
 Ion Echange Chromatography COSMOGEL IEX Series Columns Available in 3 different ion-echange modes Anion-echange type, Cation-echange type, Amphoteric ion-echange type Available for 3 different application
Ion Echange Chromatography COSMOGEL IEX Series Columns Available in 3 different ion-echange modes Anion-echange type, Cation-echange type, Amphoteric ion-echange type Available for 3 different application
In vitro human regulatory T cell expansion
 - 1 - Human CD4 + CD25 + CD127 dim/- regulatory T cell Workflow isolation, in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation
- 1 - Human CD4 + CD25 + CD127 dim/- regulatory T cell Workflow isolation, in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation
In vitro human regulatory T cell suppression assay
 Human CD4 + CD25 + regulatory T cell isolation, in vitro suppression assay and analysis In vitro human regulatory T cell suppression assay Introduction Regulatory T (Treg) cells are a subpopulation of
Human CD4 + CD25 + regulatory T cell isolation, in vitro suppression assay and analysis In vitro human regulatory T cell suppression assay Introduction Regulatory T (Treg) cells are a subpopulation of
Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set
 Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK40103 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK40103 To achieve the best assay results, this manual must be read carefully before using this product and the assay
For Research Use Only Ver
 INSTRUCTION MANUAL Quick-RNA Miniprep Kit Catalog Nos. R1054 & R1055 Highlights High-quality total RNA (including small RNAs) from a wide range of samples. You can opt to isolate small and large RNAs in
INSTRUCTION MANUAL Quick-RNA Miniprep Kit Catalog Nos. R1054 & R1055 Highlights High-quality total RNA (including small RNAs) from a wide range of samples. You can opt to isolate small and large RNAs in
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/4/e1500980/dc1 Supplementary Materials for The crystal structure of human dopamine -hydroxylase at 2.9 Å resolution Trine V. Vendelboe, Pernille Harris, Yuguang
advances.sciencemag.org/cgi/content/full/2/4/e1500980/dc1 Supplementary Materials for The crystal structure of human dopamine -hydroxylase at 2.9 Å resolution Trine V. Vendelboe, Pernille Harris, Yuguang
INSTRUCTION MANUAL. RNA Clean & Concentrator -5 Catalog Nos. R1015 & R1016. Highlights. Contents
 INSTRUCTION MANUAL Catalog Nos. R1015 & R1016 Highlights Quick (5 minute) method for cleaning and concentrating RNA. Ideal for purification of RNA from aqueous phase following an acid phenol extraction.
INSTRUCTION MANUAL Catalog Nos. R1015 & R1016 Highlights Quick (5 minute) method for cleaning and concentrating RNA. Ideal for purification of RNA from aqueous phase following an acid phenol extraction.
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set
 Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A. Real-Time RT-PCR Detection.
 For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
2D-LC as an Automated Desalting Tool for MSD Analysis
 2D-LC as an Automated Desalting Tool for MSD Analysis Direct Mass Selective Detection of a Pharmaceutical Peptide from an MS-Incompatible USP Method Application Note Biologics and Biosimilars Author Sonja
2D-LC as an Automated Desalting Tool for MSD Analysis Direct Mass Selective Detection of a Pharmaceutical Peptide from an MS-Incompatible USP Method Application Note Biologics and Biosimilars Author Sonja
EASIMIP TM PATULIN Product Code: P250 / P250B
 EASIMIP TM PATULIN Product Code: P250 / P250B Molecularly imprinted polymer columns for use in conjunction with HPLC. For in vitro use only. P250B/V5/03.09.18 www.r-biopharm.com Contents Page Test Principle...
EASIMIP TM PATULIN Product Code: P250 / P250B Molecularly imprinted polymer columns for use in conjunction with HPLC. For in vitro use only. P250B/V5/03.09.18 www.r-biopharm.com Contents Page Test Principle...
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry. Supporting Information
 Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual)
 HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual) BACKGROUND Human Immunodeficiency Virus ( HIV ) can be divided into two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to
HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual) BACKGROUND Human Immunodeficiency Virus ( HIV ) can be divided into two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to
RITONAVIRI COMPRESSI RITONAVIR TABLETS. Final text for addition to The International Pharmacopoeia (July 2012)
 July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
Comparison of a UPLC Method across Multiple UHPLC Systems
 Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Trypsin Mass Spectrometry Grade
 058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
Agilent Anion-Exchange Media for Proteins - Loading vs Resolution - Effect of Flow Rate and Example Protein Separations
 Agilent Anion-Exchange Media for Proteins - Loading vs Resolution - Effect of Flow Rate and Example Protein Separations Technical Overview Introduction PL-SAX is a hydrophilic strong anion-exchange chromatographic
Agilent Anion-Exchange Media for Proteins - Loading vs Resolution - Effect of Flow Rate and Example Protein Separations Technical Overview Introduction PL-SAX is a hydrophilic strong anion-exchange chromatographic
Title Revision n date
 A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS. (January 2012) DRAFT FOR COMMENT
 January 2012 RESTRICTED DRAFT PROPOSAL FOR The International Pharmacopoeia LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS (January 2012) DRAFT FOR COMMENT This document was provided by a quality control expert.
January 2012 RESTRICTED DRAFT PROPOSAL FOR The International Pharmacopoeia LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS (January 2012) DRAFT FOR COMMENT This document was provided by a quality control expert.
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit
 Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
