Earlens Sitting Hybrid Impression Procedure System
|
|
|
- Buddy Day
- 5 years ago
- Views:
Transcription
1 Earlens Sitting Hybrid Impression Procedure System 1. Introduction Earlens Hybrid Impression Procedure and System Description Precautions Indication for Use Contraindications Clinical Study Results Operating Instructions Graphic Symbols Contained in Device Labeling
2 1. Introduction CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE. Rx ONLY 2. Earlens Hybrid Impression Procedure and System Description Instruments and materials: Impression Return Box w/ Label Topical Light Mineral Oil (OIL02) Impression Dispensers (IDG01 only) Medial Impression Material Cartridge (blue, low viscosity material) Medial Impression Mixing Tip Lateral Impression Material Cartridge (pink, high viscosity material) Lateral Impression Mixing Tip Additional standard otological office supplies are required, which include: Ear Specula: multiple sizes Wax curette Triangular tipped metal cotton applicator Small suction tips (recommend 5 Gauge, 3 Gauge, and 20 Gauge) Thin curved elevator Spatula Gauze pads: 4x4 Cotton balls EPA registered hospital disinfectant wipes Tray (small disposable dish for holding mineral oil) 3. Precautions Before using the Earlens Sitting Hybrid Impression Procedure and System, read and make sure you understand each of the following safety precautions. It is important that the physician use the specified impression materials in the proper sequence when performing this deep impression, and that the instructions are followed. No substitutions for materials in the impression system are allowed. The impression procedure should be performed by a physician with relevant experience, such as an ENT or Otologist. When using any instruments in the ear canal and particularly when the mixing tip is submerged, be careful to avoid damaging the ear canal or tympanic membrane. Do not perform the impression procedure if the Impression Dispenser or any other component appears to be damaged or expired. Do not use the lateral impression material in the medial section of the ear canal. This material is not indicated for use in deep canal impressions and should be used exclusively in the lateral section of the ear. 4. Indication for Use The Earlens Sitting Hybrid Impression Procedure and System is intended for use in individuals 18 years and older to generate an ear canal impression. 2
3 5. Contraindications Perforated tympanic membrane Otitis externa Visible hematoma, bleeding or effusion Hypersensitivity to contact resulting in bleeding or hematoma at the discretion of the physician Bony exostosis, atresia, or excessively restrictive anatomy that would cause difficulty removing the impression material or inability to view the tympanic membrane at the discretion of the physician 6. Clinical Study Results 6.1. Study Overview Earlens conducted an impression study to collect ear impressions from many different ear canal anatomies and to gain experience with the deep canal impression procedure itself. 6.2 Study Demographics A total of 78 subjects had bilateral ear canal impressions taken (37 female, 41 male). The total number of ears that had impressions taken was 154 (2 subjects had unilateral impressions). Subject age ranged from 32 to 82 years with an average of 66.1 years. The subjects were seen across three clinical sites. 6.3 Outcomes Number of Impressions A total of 200 ear impressions were taken on 154 ears (78 subjects). Fourteen subjects (18%) required more than one clinic visit to obtain adequate ear impressions. Multiple impressions were required on some subject ears due to the presence of voids in the impression of anatomical areas that are critical to the custom-build of the Tympanic Lens. The average number of impressions per subject was 2.6, or 1.3 per ear. Thirty-eight percent of subjects required three or more impressions; 17% required four or more impressions. Comfort During Procedure Based on a subject survey, a total of 84% of impressions were rated as either no discomfort or mild discomfort. Ear Canal Status While 80% of the study ears were observed to be normal (unremarkable otoscopic inspection) after the impression procedure, the remaining ears were reported with minor skin contact findings. The most common observation was a micro-hematoma (16%) while petechia (1%), ecchymosis (2%) and abrasion (1%) was reported less frequently. All otologic findings resolved without treatment and without sequelae. Micro-hematomae and ecchymoses can take 2-4 weeks to resolve. Abrasion and petechia, depending on extent and location, can be a more minor finding and may or may not require delay of subsequent otologic procedures, including Tympanic Lens placement. 7. Operating Instructions 1. Provide an overview to the patient about the impression process and what to expect, including: o The ear will be inspected for any contraindications. o The ear will be cleaned, any wax or skin debris present will be removed and the ear canal will be lubricated with mineral oil. From the cleaning procedure there may be sensations such as a cough reflex, tickle, or general discomfort. o The ear canal and conchal bowl will be filled with impression material. The patient should remain still and try and hold their mouth closed in a resting position without clenching teeth. The patient should avoid talking while the impression material is curing in the ear. 3
4 The patient may feel a sensation of fullness when the impression material is being applied to the ear canal. o The patient will be in both the supine and seated position during the procedure. o In a supine position the medial blue material is inserted into the medial ear canal. After eight minutes, the medial impression material will set-up and cure. During this time, the sensation of hearing may be diminished due to the presence of the impression material in the ear canal. o After the medial impression, the patient will be asked to move to a seated position and lateral (pink) impression material will be inserted into the ear canal. This material will cure over three minutes. It is important that the patient doesn t talk or otherwise move their jaw during this curing time. o The impression is then removed. This may cause a sensation of pressure or discomfort. There is a possibility of discomfort, bruising, abrasion and/or minor bleeding of the ear canal during this procedure. 2. Perform an otologic exam and inspect the external auditory canal and the tympanic membrane for the presence of any contraindications. o Do not proceed with the impression procedure if any of the contraindications are present. 3. Clean cerumen and epithelial debris from the ear canal, anterior sulcus, and tympanic membrane. Use a cotton wisp and the provided mineral oil. o Do not use any water based cleaning fluids. o Do not proceed with the impression if bleeding or if a hematoma occurs as a result of the cleaning process. 4. Wipe out the ear canal, TM and anterior sulcus with a clean cotton wisp to remove any excess oil. Repeat with a new cotton wisp until it comes out dry. o Avoid excessive pooling of mineral oil, which may cause defects in the quality of the impression. 5. Prepare the Impression Dispensers, Impression Material Cartridges, and Mixing Tips for dispensing: o Test the actuation of both Impression Dispensers, ensuring that the handles smoothly ratchet the piston forward. Do not use an Impression Dispenser if it appears to be damaged. o Load the Medial Impression Material Cartridge into one Impression Dispenser. The lower viscosity, blue, medial impression material is intended to be put deep in the ear canal and in contact with the tympanic membrane. o Load the Lateral Impression Material Cartridge into the other Impression Dispenser. The higher viscosity pink, lateral impression material is intended to be deposited lateral to the medial impression in the outer ear canal. o o o IMPORTANT: Do not use the lateral impression material in the medial section of the ear canal. This material is not indicated for use in deep canal impressions and should be used exclusively in the lateral section of the ear and is NOT intended to be in direct contact with the tympanic membrane. Remove the caps from the impression cartridges. Prior to attaching the Mixing Tip, prime the Medial Impression Cartridge by dispensing a small amount of medial impression material onto a piece of gauze or an equivalent, then wiping any excess material off of the end of the cartridge. Confirm that both materials (blue and white) are advancing equally from the tip. IMPORTANT: Ensure there is not mixing of the two materials (blue and white) between the outlets of the cartridge after wiping. Prior to attaching the Mixing Tip, prime the Lateral Impression Cartridge by dispensing a small amount of medial impression material onto a piece of gauze or an equivalent, then wiping any excess material off of the end of the cartridge. IMPORTANT: Ensure there is not mixing of the two materials (pink and white) between the outlets of the cartridge after wiping. 4
5 o Attach the single use Mixing Tips to the respective impression material cartridges. Orient the curved tip on the Medial Impression Mixing Tip for the right or left ear. Note: It is important not to move or rotate the Medial Mixing Tip around the Medial Impression Material Cartridge once attached. 6. First position the patient in the supine position. Then position the patient s head for making the impression, being sure that there is an adequate view of the tympanic membrane, and dispense the medial (blue) impression material into the ear: o With the speculum in place, place the distal end of the mixing tip between the umbo and the inferior anterior annular area. o Start a timer. Slowly dispense the impression material into the anterior sulcus and onto the tympanic membrane. Note: It is important not to move the tip of the dispenser until the tympanic membrane is fully covered keeping the Mixing Tip submerged. Too rapid of a flow may trap air in the anterior inferior sulcus and in the pars flaccida area, which will yield an incomplete impression. As a guide, allow approximately seconds to fully cover the tympanic membrane, dispensing material at a slow and steady pace. o Once the tympanic membrane and pars flaccida are covered with the impression material, slowly withdraw the tip laterally, keeping the tip slightly submerged and proceed to more rapidly fill the external auditory canal up to a level slightly medial of smooth skin/glandular skin transition on the anterior / superior aspect of the canal. The medial impression material should not be filled beyond the smooth skin/glandular transition. o Stop dispensing the medial impression material. o Allow the medial impression material to cure for a minimum of eight minutes after the completion of the medial impression. The patient should remain still and hold the mouth closed in a resting position without clenching teeth. The patient should avoid talking while the impression material is curing in the ear. 7. Reposition the subject to a sitting position with head and back as vertical as possible and begin the lateral impression. The patient should remain still, with head facing forward, and hold the mouth closed in a resting position without clenching teeth. o Purge the air from the Lateral Impression Material Cartridge by squeezing the handle until a small amount of the impression material exits the end of the cartridge. Wipe the tip clean. o Attach the Mixing Tip. o Position the Lateral Mixing Tip into the canal against the existing medial impression material. The distal end of the Lateral Mixing Tip should be directed towards the superior aspect of the existing medial impression material (blue) that was previously dispensed into the ear canal. o While dispensing the lateral impression material, ensure the distal end of the Mixing Tip remains buried in the lateral impression material as the remaining aspects of the canal are filled. Slowly withdraw the tip laterally as the canal is filled and maintain a consistent dispensing speed. o Once you have completely filled the canal, advance the material in a clockwise direction for the right ear and counterclockwise direction for the left, filling the concha bowl first. Fill the canal and concha bowl with the Lateral Impression Material (pink) to the level of the scapha, covering the tragus and filling the concha cymba. Optionally, create a pile of the impression material that protrudes laterally from the concha bowl for ease of impression removal. 5
6 o Allow the lateral impression material to cure for a minimum of three minutes in the sitting position. The patient should remain still, with head facing forward, and try and hold the mouth closed in a resting position without clenching teeth. The patient should avoid talking while the impression material is curing in the ear. 8. Reset the Impression Dispensers by depressing the lever on the back of the piston and pulling proximally, detach the Impression Material Cartridge and Mixing Tip from each Impression Dispenser. o Discard the single-use Mixing Tips, and discard the Impression Material Cartridges, if desired. It is acceptable to save the impression material cartridges for use in another impression if there is adequate unused material left. Note: The Impression Dispenser has a useful life of 300 patients. 9. With the subject in an upright sitting position remove the fully cured impression. Loosen the impression from the conchal skin and external auditory canal and break the seal between the impression material and the ear canal wall. Carefully remove the cured impression material from the ear. o Ask the patient to open and close their jaw as if they are trying to pop their ears o While grasping the impression and pulling the pinna posteriorly to straighten the canal, slowly rotate the impression toward the patient s nose (anterior direction) while gently increasing the amount of pull force applied. Gentle twisting forces may be applied as the impression is slowly removed from the ear canal. o Ask the patient to indicate whether they experience a feeling of a pressure drop or an increase in hearing. This is a sign that an air leak path down to the tympanic membrane has been formed. 10. Inspect the ear canal and tympanic membrane. 11. Inspect the impression to see if it is complete and acceptable or requires a repeat attempt. Flaws that may require a repeat attempt include the following (see Figures 1, 2, 3): o Inclusion of cerumen or epithelial plaques in the umbo and anterior sulcus areas. o Voids, flaws and bubbles in the area of the umbo or anterior sulcus areas indicating an incomplete impression in the tympanic membrane region. o Voids, flaws, and bubbles at the transition or junction between the medial and lateral impression material. Also ensure the transition point is not past the smooth skin/glandular skin transition. o Voids, flaws, and bubbles indicating an incomplete impression in the ear canal and conchal bowl region. Small bubbles and voids are acceptable in the ear canal and conchal bowl regions. 12. If the impression is unacceptable, repeat the procedure a second time, beginning with Step 4. o Do not repeat the procedure if any of the contraindications have developed. o Do not exceed two attempts per ear per day. 13. Alternatively, if only the medial aspect of the impression is unacceptable, repeat the deployment of the medial impression material (blue) portion of procedure to obtain a short medial impression: o Repeat Step 5 (Dispenser Preparation) for the Medial Impression Material Dispenser. o Follow Step 6 to dispense the medial impression material (blue) into the ear up to the level of the anterior bulge, then stop. o Allow the impression material to cure for a minimum of eight minutes. o Gently loosen the impression material using a right angle pick to break the seal between the impression material and the ear canal wall, then carefully remove the cured impression material from the ear with a right angle pick. o Repeat Step 10 (Ear Canal Inspection) and Step 11 (Impression Inspection). o Note: Avoid applying excessive force in the medial direction when loosening and removing the short medial impression. Do not repeat the procedure if any of the contraindications have developed. Do not exceed two attempts per ear per day 14. Repeat all steps for the opposite ear (if necessary). 6
7 15. Once all impressions for a single patient are completed, and if impression material is going to be used on a subsequent patient, replace the clean cap on the cartridge. Wipe down the dispenser and impression material cartridge (if re-use is planned) with an EPA registered hospital disinfecting wipe to prevent any possible cross contamination. Dispose of all single-use items. 16. Prepare and send the impression(s) to Earlens by placing all impressions taken in the supplied Impression Return Box and labeling the container with patient identification. Figure 1: Medial Sulcus Impression Examples an ideal medial impression features a complete sulcus which is needed to prepare an adequate contact platform for the Lens. Figure 2: Medial & Lateral Junction Impression Examples an ideal junction features a smooth transition between the medial and lateral materials with no voids. Figure 3: Lateral Impression Examples Figure 3: Lateral Impression Examples an ideal lateral impression has no folds or voids and is smooth. 7
8 8. Graphic Symbols Contained in Device Labeling Symbol Description Reference Symbol Description Reference Consult instructions for use Temperature limit Use-by date Caution Batch code Catalog number CE conformity marking MDD 93/42/EEC, Annex XII Caution: Federal law restricts this device to sale by or on the order of a (licensed healthcare practitioner) FDA Final Rule 81 FR Humidity limitation Manufactured by (Ref ): Earlens Corporation. 4045A Campbell Ave. Menlo Park, CA European Authorized Representative (Ref ): Medimark Europe SARL 11, Rue Emile Zola, B.P F Grenoble Cedex 2 France 2018 Earlens Corp. All rights reserved. 8
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Wireless Earlens Light-Driven Hearing Aid Physician Instructions
 Wireless Earlens Light-Driven Hearing Aid Physician Instructions 1. Introduction... 2 2. Wireless Earlens Light-Driven Hearing Aid Device Description... 2 3. Indications for Use... 3 4. Fitting Range...
Wireless Earlens Light-Driven Hearing Aid Physician Instructions 1. Introduction... 2 2. Wireless Earlens Light-Driven Hearing Aid Device Description... 2 3. Indications for Use... 3 4. Fitting Range...
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial
 Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use
 INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use Instructions for Use Read and follow this Instructions for Use so that you inject TYMLOS pen the right way. Call
INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use Instructions for Use Read and follow this Instructions for Use so that you inject TYMLOS pen the right way. Call
Effective Date: August 31, To provide guidelines for the proper administration of ear irrigation
 SUBJECT: EAR IRRIGATION 1. PURPOSE: COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 406 Effective Date: August 31, 2006 To provide guidelines for the proper
SUBJECT: EAR IRRIGATION 1. PURPOSE: COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 406 Effective Date: August 31, 2006 To provide guidelines for the proper
How to Use ENBREL : Vial Adapter Method
 How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER
 INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
For more information visit or contact hearx:
 USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
Ear Irrigation Guideline
 Ear Irrigation Guideline INTRODUCTION CERUMEN MANAGEMENT Cerumen, or wax as it is commonly known, is a normal secretion of the ceruminous glands in the outer meatus. It is slightly acidic, giving bactericidal
Ear Irrigation Guideline INTRODUCTION CERUMEN MANAGEMENT Cerumen, or wax as it is commonly known, is a normal secretion of the ceruminous glands in the outer meatus. It is slightly acidic, giving bactericidal
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use
 Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
For use only with INSTRUCTIONS FOR USE
 TM For use only with INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Follistim Pen is a precision device. It is very important that you read and follow all directions
TM For use only with INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Follistim Pen is a precision device. It is very important that you read and follow all directions
PRO-V IDS Kit. Bisco. Instructions for Use. Provisional Restorative System
 Bisco PRO-V IDS Kit Provisional Restorative System Instructions for Use U.S. Patent: 7,748,980; 8,106,110 IN-199R3 Rev. 5/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000
Bisco PRO-V IDS Kit Provisional Restorative System Instructions for Use U.S. Patent: 7,748,980; 8,106,110 IN-199R3 Rev. 5/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000
SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector
 SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
Otoscopic Examination
 Year Group: BVSc4 + Document number: CSL_P09 Equipment for this station: Otoscope dog ear model Otoscope Otoscope speculum Equipment list: Considerations for this station: Please ensure the otoscope is
Year Group: BVSc4 + Document number: CSL_P09 Equipment for this station: Otoscope dog ear model Otoscope Otoscope speculum Equipment list: Considerations for this station: Please ensure the otoscope is
9/26/2012. Counseling & Troubleshooting
 9/26/2012 Counseling & Troubleshooting Key clinical messages Patient selection and candidacy are vital to happy Lyric patients Follow candidacy guidelines Select motivated patients Adhere to the proven
9/26/2012 Counseling & Troubleshooting Key clinical messages Patient selection and candidacy are vital to happy Lyric patients Follow candidacy guidelines Select motivated patients Adhere to the proven
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
TruGuard Custom Tongue and Jaw Positioner Instructions for Use
 TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
Flexible Fiberoptic Exam
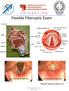 Flexible Fiberoptic Exam True Vocal Cords abducted True Vocal Cords adducted *Fiberoptic endoscopy, image is true. Cerumen Removal Position Patient -Explain Procedure Visualize Canal/Landmarks Determine
Flexible Fiberoptic Exam True Vocal Cords abducted True Vocal Cords adducted *Fiberoptic endoscopy, image is true. Cerumen Removal Position Patient -Explain Procedure Visualize Canal/Landmarks Determine
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector
 Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector How do I prepare and give an injection with Enbrel Single-use Prefilled SureClick Autoinjector? This section
Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector How do I prepare and give an injection with Enbrel Single-use Prefilled SureClick Autoinjector? This section
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Inspection Window. Syringe Body
 Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Lilly. Disposable Insulin Delivery Device User Manual
 1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
RESINOMER Dual- Bisco. Instructions for Use. Cured Amalgam Bonding/Luting System
 Bisco RESINOMER Dual- Cured Amalgam Bonding/Luting System 0459 Instructions for Use IN-029R9 Rev. 10/17 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 1-847-534-6000 1-800-247-3368 Caution:
Bisco RESINOMER Dual- Cured Amalgam Bonding/Luting System 0459 Instructions for Use IN-029R9 Rev. 10/17 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 1-847-534-6000 1-800-247-3368 Caution:
Contents.
 Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Connecting the Video Otoscope... 5 Connect the LEMO Cable... 6 Wiring Diagram... 7 Specula Adapter... 8 Trouble Shooting
Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Connecting the Video Otoscope... 5 Connect the LEMO Cable... 6 Wiring Diagram... 7 Specula Adapter... 8 Trouble Shooting
Unit # 10 B Assessment of Ears
 In The Name of God (A PROJECT OF NEW LIFE HEALTH CARE SOCIETY KARACHI) Unit # 10 B Assessment of Ears Shahzad Bashir RN, BScN, DCHN, MScN (Std. DUHS) Instructor New Life College of Nursing Updated, January
In The Name of God (A PROJECT OF NEW LIFE HEALTH CARE SOCIETY KARACHI) Unit # 10 B Assessment of Ears Shahzad Bashir RN, BScN, DCHN, MScN (Std. DUHS) Instructor New Life College of Nursing Updated, January
Sharps Container (not included) 1 Gather and check supplies. Gather supplies
 Instructions for Use BENLYSTA (ben-list-ah) (belimumab) injection, for subcutaneous use Prefilled Syringe Read this Instructions for Use before you start to use BENLYSTA and each time you refill your prescription.
Instructions for Use BENLYSTA (ben-list-ah) (belimumab) injection, for subcutaneous use Prefilled Syringe Read this Instructions for Use before you start to use BENLYSTA and each time you refill your prescription.
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe
 Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
SARASOTA MEMORIAL HOSPITAL. NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) 12/18 12/18 1 of 7 RESPONSIBILITY:
 SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
User Manual Important: First read the Medication Guide that comes inside your FORTEO carton.
 FORTEO (for-tay-o) teriparatide (rdna origin) injection 1 User Manual Important: First read the Medication Guide that comes inside your FORTEO carton. Before you use your new FORTEO delivery device, please
FORTEO (for-tay-o) teriparatide (rdna origin) injection 1 User Manual Important: First read the Medication Guide that comes inside your FORTEO carton. Before you use your new FORTEO delivery device, please
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
ACE ALL-BOND TE. Bisco CE Universal Dental Adhesive System. Dual- Cured
 Bisco CE 0459 ACE ALL-BOND TE Universal Dental Adhesive System Dual- Cured Licensed under 1 or more of the following U.S. Patents: 7,748,980, 5,789,610, 5,270,351 & 5,401,783. See also U.S. Patent 5,348,988.
Bisco CE 0459 ACE ALL-BOND TE Universal Dental Adhesive System Dual- Cured Licensed under 1 or more of the following U.S. Patents: 7,748,980, 5,789,610, 5,270,351 & 5,401,783. See also U.S. Patent 5,348,988.
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Instructions for Use. For use with. 10 mg vial
 Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
Instruction Guide. Today is the day you change the way you live with dentures. How to Apply DenSureFit Soft Silicone Reline Material
 Instruction Guide Today is the day you change the way you live with dentures. How to Apply DenSureFit Soft Silicone Reline Material Dentist-grade soft silicone denture reline materialnow available in an
Instruction Guide Today is the day you change the way you live with dentures. How to Apply DenSureFit Soft Silicone Reline Material Dentist-grade soft silicone denture reline materialnow available in an
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe. Plunger rod Used plunger rod
 Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
SDS 4.0 Tips and Tricks
 SDS 4.0 Tips and Tricks Content Important information 3 SDS 4.0 Fit and Go Box overview 4 Selecting the receiver length 5 Choosing the correct earpiece for the receiver 6 Attaching / removing the retention
SDS 4.0 Tips and Tricks Content Important information 3 SDS 4.0 Fit and Go Box overview 4 Selecting the receiver length 5 Choosing the correct earpiece for the receiver 6 Attaching / removing the retention
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
INSTALLATION MANUAL. VIDEO Camera, Probe and Lightsource OTOSCOPES.
 INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
Recommended Procedure. Taking an aural impression
 Date: February Due for review: February 2018 Page2 General foreword This document presents a by the British Society of Audiology (). A provides a reference standard for the conduct of an audiological intervention
Date: February Due for review: February 2018 Page2 General foreword This document presents a by the British Society of Audiology (). A provides a reference standard for the conduct of an audiological intervention
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel.
 Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
PLEASE READ THIS USER MANUAL BEFORE USE
 USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
Cap Clip Rubber Seal Plunger Pen Body Dose Window
 Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
Instructions for Use. HUMALOG KwikPen. insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen
 1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Do not Do not YOUR KIT CONTAINS:
 ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
Breezhaler. Open to see clear capsule. How do I use my Breezhaler? Care of my Breezhaler
 Breezhaler 3 Pull cap off Press buttons once and release Breathe in rapidly and steadily. Hold. Open. Place capsule in chamber. Close (click). Breathe out 4 Open to see clear capsule. If not all clear
Breezhaler 3 Pull cap off Press buttons once and release Breathe in rapidly and steadily. Hold. Open. Place capsule in chamber. Close (click). Breathe out 4 Open to see clear capsule. If not all clear
Patient Information Publications Warren Grant Magnuson Clinical Center National Institutes of Health
 Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Pollen Slide Mounting Protocol
 Pollen Slide Mounting Protocol Materials: Syn-Matrix mounting medium Microcentrifuge Microscope slides Slide coverslips (18mm x 18mm) Coverslip podium (see Figure 1) Capillary tubes Dissecting microscope
Pollen Slide Mounting Protocol Materials: Syn-Matrix mounting medium Microcentrifuge Microscope slides Slide coverslips (18mm x 18mm) Coverslip podium (see Figure 1) Capillary tubes Dissecting microscope
LESSON ASSIGNMENT , Irrigate an Obstructed Ear. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 6 Irrigate an Obstructed Ear. TEXT ASSIGNMENT Paragraphs 6-1 through 6-9. TASK TAUGHT LESSON OBJECTIVES 081-833-0059, Irrigate an Obstructed Ear. After completing this lesson,
LESSON ASSIGNMENT LESSON 6 Irrigate an Obstructed Ear. TEXT ASSIGNMENT Paragraphs 6-1 through 6-9. TASK TAUGHT LESSON OBJECTIVES 081-833-0059, Irrigate an Obstructed Ear. After completing this lesson,
Instructions for Use. Welcome!
 Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
SDS 4.0 Reference Guide
 SDS 4.0 Reference Guide Content Important information 2 SDS 4.0 Fit and Go Box overview 3 Selecting the receiver length 4 Choosing the correct earpiece for the receiver 5 Attaching / removing the retention
SDS 4.0 Reference Guide Content Important information 2 SDS 4.0 Fit and Go Box overview 3 Selecting the receiver length 4 Choosing the correct earpiece for the receiver 5 Attaching / removing the retention
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Integrating the LenSx Laser Laser into your Clinic: Our advice for those considering it
 Integrating the LenSx Laser Laser into your Clinic: Our advice for those considering it (DAY) Facilitator: (Name) LenSx Laser Important Safety Information Caution United States Federal Law restricts this
Integrating the LenSx Laser Laser into your Clinic: Our advice for those considering it (DAY) Facilitator: (Name) LenSx Laser Important Safety Information Caution United States Federal Law restricts this
IO considerations. Daniel Dunham
 IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
CHAIR ASSEMBLY & CARE GUIDE
 CHAIR ASSEMBLY & CARE GUIDE For maximum effectiveness and safety, read instructions and important tips before using the Pilates PRO Chair. important safety information assembly instructions use and care
CHAIR ASSEMBLY & CARE GUIDE For maximum effectiveness and safety, read instructions and important tips before using the Pilates PRO Chair. important safety information assembly instructions use and care
PLEASE READ THESE INSTRUCTIONS BEFORE USE
 Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
INSTRUCTIONS FOR USE. INBRIJA (in-brih-jah) (levodopa inhalation powder) For Oral Inhalation Only
 INSTRUCTIONS FOR USE INBRIJA (in-brih-jah) (levodopa inhalation powder) For Oral Inhalation Only Read and follow these instructions before you start using INBRIJA and each time you get a refill. There
INSTRUCTIONS FOR USE INBRIJA (in-brih-jah) (levodopa inhalation powder) For Oral Inhalation Only Read and follow these instructions before you start using INBRIJA and each time you get a refill. There
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel.
 Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml
 EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Tips for Training New Customers
 Tips for Training New Customers Good Habits: Test Strips Recap vial immediately and tightly after removing a single test strip. Never remove the desiccant from the vial. Do not lay test strips out ahead
Tips for Training New Customers Good Habits: Test Strips Recap vial immediately and tightly after removing a single test strip. Never remove the desiccant from the vial. Do not lay test strips out ahead
BAK/C Cervical Anterior Interbody Fusion System
 Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Advanced Catheterisation Trainer User Guide
 Advanced Catheterisation Trainer User Guide Also for Advanced Male Catheterisation Trainer Part No: 605 Advanced Female Catheterisation Trainer Part No: 6055 Designed and manufactured by Limbs & Things
Advanced Catheterisation Trainer User Guide Also for Advanced Male Catheterisation Trainer Part No: 605 Advanced Female Catheterisation Trainer Part No: 6055 Designed and manufactured by Limbs & Things
UDELL DENTAL LABORATORY Instructions for Use PREAT Precision Attachments
 Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions.
 1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
limbsandthings.com Advanced Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
HUMULIN 70/30 KwikPen
 1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
IMPORTANT: PLEASE READ. Don t
 PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Phonak Insight. Clinical and Medical Review. Jacob Johnson, M.D. Medical Director, Phonak Lyric. The History of Lyric. March 2015
 Phonak Insight March 2015 Clinical and Medical Review Jacob Johnson, M.D. Medical Director, Phonak Lyric Lyric is a unique hearing solution that created a new extended wear hearing device category when
Phonak Insight March 2015 Clinical and Medical Review Jacob Johnson, M.D. Medical Director, Phonak Lyric Lyric is a unique hearing solution that created a new extended wear hearing device category when
Instructions for Use. BASAGLAR KwikPen. insulin glargine injection (100 units/ml, 3 ml pen)
 Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Otology Workshop Basic
 April 21, 2017 Chicago Otology Basic workshop Jeffrey Fichera, PhD, PA C Updated 2/09/2017 Otology Workshop Basic Clear Instruction Live Demonstration Learn by doing Hands On Practice Identify normal,
April 21, 2017 Chicago Otology Basic workshop Jeffrey Fichera, PhD, PA C Updated 2/09/2017 Otology Workshop Basic Clear Instruction Live Demonstration Learn by doing Hands On Practice Identify normal,
To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status.
 Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
For use only with Puregon cartridge (follitropin beta injection) INSTRUCTIONS FOR USE
 For use only with Puregon cartridge (follitropin beta injection) 0344 INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Puregon Pen is a precision device. It is very
For use only with Puregon cartridge (follitropin beta injection) 0344 INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Puregon Pen is a precision device. It is very
Device Technical File
 Device Technical File ContactPro Matrix System Instructions for Use 1.0 Product Description A. For placement of restorations in the posterior area of the oral cavity, the Microbrush ContactPro Sectional
Device Technical File ContactPro Matrix System Instructions for Use 1.0 Product Description A. For placement of restorations in the posterior area of the oral cavity, the Microbrush ContactPro Sectional
INSTRUCTION MANUAL. Orascoptic 3225 Deming Way, Suite 190 Middleton, WI USA T:
 INSTRUCTION MANUAL Orascoptic 3225 Deming Way, Suite 190 Middleton, WI 53562 USA T: 800.369.3698 www.orascoptic.com ORASCOPTIC DK LED LIGHT SOURCE INSTRUCTIONS OPERATION AND FIRST TIME USE: Remove the
INSTRUCTION MANUAL Orascoptic 3225 Deming Way, Suite 190 Middleton, WI 53562 USA T: 800.369.3698 www.orascoptic.com ORASCOPTIC DK LED LIGHT SOURCE INSTRUCTIONS OPERATION AND FIRST TIME USE: Remove the
Patient Instruction Booklet
 Patient Instruction Booklet TAP 3 ThermAcryl TAP 3 TL TAP 3 Revision E, 2014 PRTD17 Table of Contents Important Safeguards Important Safeguards... 3 SAVE THESE INSTRUCTIONS 2 Introduction... Warnings...
Patient Instruction Booklet TAP 3 ThermAcryl TAP 3 TL TAP 3 Revision E, 2014 PRTD17 Table of Contents Important Safeguards Important Safeguards... 3 SAVE THESE INSTRUCTIONS 2 Introduction... Warnings...
USER MANUAL Rev. 1.15
 QUANTUM QSP 7 TM BEACH CHAIR SHOULDER POSITIONER (BCP) USER MANUAL Rev. 1.15 Please read all the following instructions carefully to ensure proper functionality and to avoid injury or damage to your device.
QUANTUM QSP 7 TM BEACH CHAIR SHOULDER POSITIONER (BCP) USER MANUAL Rev. 1.15 Please read all the following instructions carefully to ensure proper functionality and to avoid injury or damage to your device.
TJF-160F/VF Cleaning and Disinfection Checklist
 TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL
 Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
Clostridium difficile Specimen Collection
 Clostridium difficile Specimen Collection Collect stool specimens into a clean, airtight container with no preservative. All stool specimens should be tested as soon as possible. Ideally, stool specimens
Clostridium difficile Specimen Collection Collect stool specimens into a clean, airtight container with no preservative. All stool specimens should be tested as soon as possible. Ideally, stool specimens
Junctional Emergency Treatment Tool Item#: NSN#:
 Junctional Emergency Treatment Tool Item#: 30-0088 NSN#: 6515-01-616-5841 35 Tedwall Ct. Greer, SC 29650 Anatomy of the JETT (Junctional Emergency Treatment Tool) Instructions Overview The Junctional Emergency
Junctional Emergency Treatment Tool Item#: 30-0088 NSN#: 6515-01-616-5841 35 Tedwall Ct. Greer, SC 29650 Anatomy of the JETT (Junctional Emergency Treatment Tool) Instructions Overview The Junctional Emergency
01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation.
 Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
