BS EN ISO 14971: Risk Analysis and Risk Management Report
|
|
|
- Clifton Wilkins
- 6 years ago
- Views:
Transcription
1 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) Fax: + 44 (0) BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Maxflex Pop On Disc Classification: Class 2a Medical Device Risk Management Report 1. PRODUCTS Product: Maxflex pop on discs Description: Stoddard Maxflex pop on discs are a Synthetic moulded disc abrasive loaded attached to a central brass ring. They are two component products. 2. RISK MANAGEMENT PLAN The risk management plan is designed to identify and analyse the risks associated with the devices detailed above during their intended usage life cycle. The process verifies as far as is possible the risks and control solutions cited and establishes criteria for risk acceptability. The process includes review of risk management at appropriate stages and as a result of post market surveillance. Risk assessment has been conducted retrospectively on established products but is also conducted as an essential part of the design and development of new or revised products or product types. The process is conducted to comply with the guidance in BS EN ISO Risk management is conducted by the quality management team with guidance as necessary from other departments within the company. 3. RISK ANALYSIS 3.1. INTENDED USES The Dental surgeon or Hygienist uses Stoddard Maxflex pop on discs for the Prophy cleaning or polishing of natural teeth and filled surfaces in the mouth. Rotary power is provided by a handpiece which can be air or electrically driven CONSTRUCTION These rotary instruments are a Synthetic moulded disc, abrasive loaded attached to a central brass ring. They are two component products. Please refer to Maxflex Pop On Disc product specification and data sheet. Issue 2 03/2014 Page 1 of 6
2 3.3. HAZARD IDENTIFICATION 1. The materials used in construction of these devices are inert in the context of their method of use and therefore present no hazards. 2. Improper use of the rotary instrument by the action of the operator. 3. If the instrument became detached from the handpiece during use injury to the tooth or gums or other parts of the buccal cavity or ingestion or inhalation of the instrument could result 3.4. ESTIMATION OF RISK 1. Synthetic Polymer: - While estimating any toxicological risks, the nature of the raw material and its possible prior use, source, processing and biological safety test data is considered. 2. Under normal conditions of use the hazard presented by rotary instruments are limited by the action of the operator. For the purpose of this risk assessment these are presumed to be adequately controlled through the education, experience and skill of the operator. The inevitable trauma of dental treatment using rotary instruments is reduced by the use of torque limiting features on dental handpieces, and the use of anaesthetic and analgesic agents. 3. If the instrument became detached from the handpiece during use injury to the tooth or gums or other parts of the buccal cavity or ingestion or inhalation of the instrument could result. There have been no reported incidents of these instruments becoming detached from the handpiece as a result of a defect in the instrument. Such incidents are generally caused by failure of the handpiece collet. The level of risk is considered acceptable. 4. RISK EVALUATION 1. Synthetic Polymer is a man made product and is for single-use (transient) only. There is no evidence of allergic reaction to these products and no allergic reaction to these products has been reported to the company in the last 20 years. 2. The risks presented by these products in normal conditions are considered to be well controlled by proper use or under the supervision of a suitably qualified dental surgeon or hygienist. 3. In view of the very low risk associated with the instrument becoming detached from the handpiece, the level of risk is considered acceptable. 5. RISK REDUCTION After evaluation the level of risk is considered acceptable and therefore under these circumstances risk reduction is not considered necessary OPTION ANALYSIS RISK REDUCTION BY DESIGN PROTECTION AGAINST RISK The quality control measures in place during and following the manufacture of Maxflex Pop On Discs are designed to control the incidence of failure, breakage or bending of the product. These have been detailed in the relevant parts of the QA system manuals INFORMATION The directions for use for these products specify conditions of use (intended uses, application speeds etc), which are considered adequate to allow safe and effective use of the devices. Issue 2 03/2014 Page 2 of 6
3 5.2. RISK CONTROL MEASURES No further risk control measures other than those described above are considered necessary RESIDUAL RISK Under proper professional use in accordance with the directions for use it is believed that, apart from those controlled risks cited above, there are no significant residual risks with these products RISK/BENEFIT ANALYSIS None considered necessary 5.5. COLLATERAL HAZARDS There are collateral hazards associated with reprocessing. These are well documented and have been the subject of extensive research in recent years in response to the challenges of hepatitis, HIV and CJD. These instruments are for single-use and should be sterilised before first single-use, the directions for use relating to these products are based on validated reprocessing (sterilisation) methods designed to control the risk from contaminated instruments. 6. RISK EVALUATION SUMMARY These instruments are generally low risk products with no uncontrollable residual risks in proper use. No further risk analysis or control is considered necessary unless there is a significant change in knowledge or legislation that may highlight unbeknown or undocumented hazards. 7. POST PRODUCTION ANALYSIS A procedure is established and maintained to review information gained about the medical device or similar devices in the postproduction phase. The Quality Management Team evaluates this information. Analysis of customer complaints indicates that there have been few incidents or defects with these products that could have harmed the patient or end-user or resulted in any clinical problem. The details of the complaints history are contained in the relevant technical file. Risk Analysis Question Answer C.2.1 What is the intended use and how is the medical device to be used? For oral prophylaxis tooth polishing. For use by or under the supervision of a qualified dental surgeon or hygienist. C.2.2 Is the Medical device intended to be implanted? The device is not intended to be implanted C.2.3 Is the medical device intended to be in contact with the patient Yes. It is used in the patient s mouth. or other persons? C.2.4 What materials or components are utilised in the medical device or are used with, or are in contact with the medical device? Refer to Product specification and Safety Data sheet. C.2.5 Is energy delivered to or extracted from the patient? No. C.2.6 Are substances delivered to or extracted from the patient? No. C.2.7 Are Biological materials processed by the medical device for No. subsequent re-use, transfusion or transplantation? C.2.8 Is the medical device supplied sterile or intended to be sterilised No by the user, or are any other microbiological controls applicable? C.2.9 Is the medical device intended to be routinely cleaned and disinfected by the user? No. They are single use Issue 2 03/2014 Page 3 of 6
4 C.2.10 Is the medical device intended to modify the patient No. environment? C.2.11 Are measurements taken? No. C.2.12 Is the medical device interpretative? No. C.2.13 Is the medical device intended for use in conjunction with other medical devices, medicines or other medical technologies? This Instrument is used with Dental Handpieces and Mandrels. C.2.14 Are there unwanted outputs of energy or substances? No. C.2.15 Is the medical device susceptible to environmental influences? Yes They should be stored at room temperature, away from light. C.2.16 Does the medical device influence the environment? No. C.2.17 Are there essential consumables or accessories associated with the medical device? No. C.2.18 Is maintenance or calibration necessary? No. C.2.19 Does the medical device contain software? No. C.2.20 Does the medical device have a restricted shelf life? Yes - 5 years. C.2.21 Are there any delayed or long-term use effects? No. C.2.22 To what mechanical forces will the medical device be Rotary Action. subjected? C.2.23 What determines the lifetime of the medical device? They are single use. C.2.24 Is the medical device single use? Yes. C.2.25 Is safe decommissioning or disposal of the medical device necessary? Yes it is recommended for single use only and suitable disposal is required after use. C.2.26 Does installation or use of the medical device require special training or special skills? Yes - For use by or under the supervision of a qualified dental surgeon or hygienist. C.2.27 How will information for safe use be provided? Refer to Instructions for use. C.2.28 Will new manufacturing processes need to be established or No. introduced? C.2.29 Is the successful application of medical device critically Yes. dependent on human factors such as the user interface? C.2.30 Does the medical device use an alarm system? No. C.2.31 In what way(s) might the medical device be deliberately Use by unqualified personnel. misused? C.2.32 Does the medical device hold data critical to patient care? No. C.2.33 Is the medical device intended to be mobile or portable? No. C.2.34 Does the use of the medical device depend on essential performance? Yes. Issue 2 03/2014 Page 4 of 6
5 Risk Evaluation Hazard Type Energy Environmental Incorrect use Functional failure Contributing Factors Rotary Power see incorrect use Metallic component parts Improper use of the rotary instrument by the action of the operator. Instrument becomes detached from the handpiece Possible effect Severity Probability Score Risk control mechanism Injury to the tooth or gums or other parts of the oral cavity due to excessive pressure. Inappropriate waste disposal Injury to the tooth or gums or other parts of the oral cavity Injury to the tooth or gums or other parts of the oral cavity or ingestion or inhalation of the instrument could result. S2 S1 3 Instruments are only for use by or under the supervision of a suitably qualified person. Single Use Device Incineration and recycling is recommended. S2 S1 3 Misuse of rotary instruments is limited by the action of the operator. Presumed to be adequately controlled through the education, experience and skill of the operator. S2 S1 3 In view of the very low risk associated with the instrument becoming detached from the handpiece, the level of risk is considered acceptable. Connection to the hand piece is made or supervised by a suitably qualified person. Severity after applying control Probability after applying control Score after applying control Issue 2 03/2014 Page 5 of 6
6 Explanation of calculation of Risk Evaluation of Products: For the calculation of Risk evaluation the level of acceptable risk has been set by the Company at a score of 10. The Risk Score is calculated by multiplying the score factors from the Severity and Probability, tables listed below: - Risk of Harm Table (Severity level) Common Term Description Rating Score Significant Death or loss of function or structure S3 5 Moderate Reversible or minor injury S2 3 Negligible Will not cause injury or will injure slightly S1 1 Risk of Harm Table (Probability) Common Term Description Rating Score High Likely to happen, often, frequent S3 5 Medium Can happen, but not frequently S2 3 Low Unlikely to happen, rare, remote S1 1 Example: - A product, which is deemed to be a significant risk of harm, but is unlikely to occur would be given a score of 5 X 1 = 5, which is taken from the tables above. i.e. Significant severity resulting in death or loss of function or structure (Score 5) but its probability is low, unlikely to happen, rare, remote (Score 1) Conclusion of Risk Evaluation. From the calculation of risk shown above and with the severity of potential injury assessed after the application of the risk control mechanism the score is reduced from 10 points to 4 points, which is deemed perfectly acceptable and is well within the target acceptable risk of 10 points. Issue 2 03/2014 Page 6 of 6
BS EN ISO 14971: Risk Analysis and Risk Management Report
 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Tungsten Carbide
BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Tungsten Carbide
BS EN ISO 14971: Risk Analysis and Risk Management Report
 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Gates and
BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Gates and
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
OPERATING INSTRUCTIONS. Orascoptic DK
 User Manual Orascoptic DK is a versatile, 3-in-1 dental device that employs a handheld LED light source and three unique, interchangeable diagnostic instruments. Table of Contents 1. Orascoptic DK Kit
User Manual Orascoptic DK is a versatile, 3-in-1 dental device that employs a handheld LED light source and three unique, interchangeable diagnostic instruments. Table of Contents 1. Orascoptic DK Kit
CURVES. Anatomically Adaptable IPR strips. Brochure Instructions for use Processing instructions
 CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
A Guide to Food Hygiene Regulations in the UK
 A Guide to Food Hygiene Regulations in the UK Business Information Factsheet BIF193 February 2016 Introduction UK food hygiene regulations (the Regulations) consist of four separate sets of national legislation:
A Guide to Food Hygiene Regulations in the UK Business Information Factsheet BIF193 February 2016 Introduction UK food hygiene regulations (the Regulations) consist of four separate sets of national legislation:
Policy Title: Single-Use (Disposable) Devices Policy Number: 13. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Single-Use (Disposable) Devices Policy Number: 13 5. ROLES AND RESPONSIBILITIES: 5.1. All dental healthcare personnel have responsibility to conform and respect all aspects of this policy.
Policy Title: Single-Use (Disposable) Devices Policy Number: 13 5. ROLES AND RESPONSIBILITIES: 5.1. All dental healthcare personnel have responsibility to conform and respect all aspects of this policy.
Convex Array Transducer User Guide. Types 8567-S and 8667
 Convex Array Transducer User Guide Types 8567-S and 8667 English BB0889-D August 2006 WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com Email: info@bkmed.dk
Convex Array Transducer User Guide Types 8567-S and 8667 English BB0889-D August 2006 WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com Email: info@bkmed.dk
Policy Title: Clinical Asepsis Policy Policy Number :19. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Clinical Asepsis Policy Policy Number :19 6.4.9. Take/send instruments and handpieces to the decontamination/sterilization area. 6.4.10. Remove and dispose of the disposable gown (if used)
Policy Title: Clinical Asepsis Policy Policy Number :19 6.4.9. Take/send instruments and handpieces to the decontamination/sterilization area. 6.4.10. Remove and dispose of the disposable gown (if used)
Midwest E Motor. Directions For Use. Doc. No
 Midwest E Motor Directions For Use CAUTION: US Federal law restricts this device to sale by or on the order of a licensed dental professional. Doc. No. 87513-0814 All trademarks referred to are the property
Midwest E Motor Directions For Use CAUTION: US Federal law restricts this device to sale by or on the order of a licensed dental professional. Doc. No. 87513-0814 All trademarks referred to are the property
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
THE. Curve THE CURVE STRIPPING TOOL
 THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
ORIFICE FLOW METERS WITH CHAMBERS MEASURING RINGS
 Bulletin E79 rev0 02/03/03 ORIFICE FLOW METERS WITH CHAMBERS MEASURING RINGS POP-U-S SERIES FEATURES UNI PN6 flanges: iron Chamber measuring rings: iron Disc: AISI30 Max. operating temperature: 00 C Max.
Bulletin E79 rev0 02/03/03 ORIFICE FLOW METERS WITH CHAMBERS MEASURING RINGS POP-U-S SERIES FEATURES UNI PN6 flanges: iron Chamber measuring rings: iron Disc: AISI30 Max. operating temperature: 00 C Max.
Totally lightweight, totally flexible, totally titanium.
 CAD/CAM SYSTEMS INSTRUMENTS HYGIENE SYSTEMS TREATMENT CENTERS IMAGING SYSTEMS T1 LINE THE LIGHTWEIGHT TITANIUM INSTRUMENTS Totally lightweight, totally flexible, totally titanium. T h e D e n t a l C o
CAD/CAM SYSTEMS INSTRUMENTS HYGIENE SYSTEMS TREATMENT CENTERS IMAGING SYSTEMS T1 LINE THE LIGHTWEIGHT TITANIUM INSTRUMENTS Totally lightweight, totally flexible, totally titanium. T h e D e n t a l C o
High Frequency Linear Array Transducer
 User Guide Type 8870 High Frequency Linear Array Transducer English BB1980-C May 2016 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkultrasound.com
User Guide Type 8870 High Frequency Linear Array Transducer English BB1980-C May 2016 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkultrasound.com
ISO/TS TECHNICAL SPECIFICATION. Dental materials Testing of adhesion to tooth structure
 TECHNICAL SPECIFICATION ISO/TS 11405 Second edition 2003-02-01 Dental materials Testing of adhesion to tooth structure Produits dentaires Essai d'adhésion à la structure de la dent Reference number ISO/TS
TECHNICAL SPECIFICATION ISO/TS 11405 Second edition 2003-02-01 Dental materials Testing of adhesion to tooth structure Produits dentaires Essai d'adhésion à la structure de la dent Reference number ISO/TS
Convex Array Transducer
 User Guide Type 8802 Convex Array Transducer English BB0334-I August 2012 For Professional Users Only WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com
User Guide Type 8802 Convex Array Transducer English BB0334-I August 2012 For Professional Users Only WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com
Description: Prosthetic Components for dental restoration under CE-Series, CL- Series, CN-Series, CH-Series, CF-Series, CR-Series, CS-Series.
 Instructions for use Components for dental restoration. Description: Components for dental restoration under CE-Series, CL- Series, CN-Series, CH-Series, CF-Series, CR-Series, CS-Series. Indications: Abutment
Instructions for use Components for dental restoration. Description: Components for dental restoration under CE-Series, CL- Series, CN-Series, CH-Series, CF-Series, CR-Series, CS-Series. Indications: Abutment
Material Safety Data Sheet
 Material Safety Data Sheet CEDARWOOD Date: July 2013 1. Product and Supplier Description Trade Name: Cedarwood INCI Name: Cedrus Atlantica bark oil CAS Number: 92201-55-3 EC (EINECS) NO.: 295-985-9 Supplier:
Material Safety Data Sheet CEDARWOOD Date: July 2013 1. Product and Supplier Description Trade Name: Cedarwood INCI Name: Cedrus Atlantica bark oil CAS Number: 92201-55-3 EC (EINECS) NO.: 295-985-9 Supplier:
Convex Array Transducer
 User Guide Type 8823 Convex Array Transducer English BB1740-C June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com Email:
User Guide Type 8823 Convex Array Transducer English BB1740-C June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com Email:
POS Perkins Statewide Articulation Agreement Documentation Coversheet
 POS Perkins Statewide Articulation Agreement Documentation Coversheet Student Name: Secondary School Name: Secondary School Address: CTE Program of Study: CIP # CIP Program Name Grade 9 1. CAREER AND TECHNICAL
POS Perkins Statewide Articulation Agreement Documentation Coversheet Student Name: Secondary School Name: Secondary School Address: CTE Program of Study: CIP # CIP Program Name Grade 9 1. CAREER AND TECHNICAL
Home Address: City: Zip Code. Phone: Cell Home: Work: Fax #: Personal Reference Name: Relationship: Address: Phone #
 Fullerton Dental Assistant School 2720 N. Harbor Blvd #110 Fullerton, CA 92835 School #714-882-5518 Fax (714)637-1163 WWW.DentalAssistantFullerton.com Student: D.O.B Home Address: City: Zip Code Social
Fullerton Dental Assistant School 2720 N. Harbor Blvd #110 Fullerton, CA 92835 School #714-882-5518 Fax (714)637-1163 WWW.DentalAssistantFullerton.com Student: D.O.B Home Address: City: Zip Code Social
Coronal Polish (CP) Exam Outline and Suggested References
 Coronal Polish (CP) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable duties to dental assistants.
Coronal Polish (CP) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable duties to dental assistants.
AS Medizintechnik GmbH Sattlerstraße Tuttlingen Germany
 Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
book Seite 1 Freitag, 21. April : fjmi^kq=omwn. Operating instructions. båöäáëü. lééê~íáåö=fåëíêìåíáçåë
 6278761.book Seite 1 Freitag, 21. April 2017 3:54 15 fjmi^kq=omwn Operating instructions båöäáëü lééê~íáåö=fåëíêìåíáçåë 6278761.book Seite 2 Freitag, 21. April 2017 3:54 15 Table of contents Operating
6278761.book Seite 1 Freitag, 21. April 2017 3:54 15 fjmi^kq=omwn Operating instructions båöäáëü lééê~íáåö=fåëíêìåíáçåë 6278761.book Seite 2 Freitag, 21. April 2017 3:54 15 Table of contents Operating
Prostate Biplane Transducer
 User Guide Type 8808e Prostate Biplane Transducer English BB1856-D June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com
User Guide Type 8808e Prostate Biplane Transducer English BB1856-D June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com
PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS
 PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS Date: August 2017 Time: 3 HOURS (10:00 13:00) TOTAL: 100 marks SPECIAL INSTRUCTIONS Answer all the questions.
PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS Date: August 2017 Time: 3 HOURS (10:00 13:00) TOTAL: 100 marks SPECIAL INSTRUCTIONS Answer all the questions.
Syllabus for International Dental Assistants. Prepare and maintain the dental surgery, instruments, and equipment for clinical dental procedures
 Syllabus for International Dental Assistants UNIT1 Prepare and maintain the dental surgery, instruments, and equipment for clinical dental procedures This section concerns the preparation and maintenance
Syllabus for International Dental Assistants UNIT1 Prepare and maintain the dental surgery, instruments, and equipment for clinical dental procedures This section concerns the preparation and maintenance
Linear Array Transducer
 User Guide Type 8809 Linear Array Transducer English BB 0911-H August 2012 For Professional Users Only WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com
User Guide Type 8809 Linear Array Transducer English BB 0911-H August 2012 For Professional Users Only WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com
D.T. LIGHT-POST. Bisco. Instructions for Use. Radiopaque Translucent Fiber Post System. Double Taper
 Bisco D.T. LIGHT-POST Double Taper 0459 Radiopaque Translucent Fiber Post System Instructions for Use IN-083R7 Rev. 8/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368
Bisco D.T. LIGHT-POST Double Taper 0459 Radiopaque Translucent Fiber Post System Instructions for Use IN-083R7 Rev. 8/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368
SOLDER Revision Date: 30/05/2003
 Page 1 of 5 MSDS No.: 0135 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND THE COMPANY / UNDERTAKING 1.1. PRODUCTS IDENTIFICATION: Solder 1.2. USE OF SUBSTANCE: No information supplied 1.3. COMPANY:
Page 1 of 5 MSDS No.: 0135 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND THE COMPANY / UNDERTAKING 1.1. PRODUCTS IDENTIFICATION: Solder 1.2. USE OF SUBSTANCE: No information supplied 1.3. COMPANY:
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
Material Safety Data Sheet
 Material Safety Data Sheet DAVANA Date: July 2013 1. Product and Supplier Description Trade Name: Davana INCI Name: Artemisia Pallens Flower Oil Supplier: Aromantic Ltd 17 Tytler Street Forres Moray IV36
Material Safety Data Sheet DAVANA Date: July 2013 1. Product and Supplier Description Trade Name: Davana INCI Name: Artemisia Pallens Flower Oil Supplier: Aromantic Ltd 17 Tytler Street Forres Moray IV36
Material Safety Data Sheet according to 91/155/EEC - ISO
 Material Safety Data Sheet according to 91/155/EEC - ISO 11014-1 Page 1 of 6 OMNISEAL 1K PUR WHITE SDS no. : 180161 Revision: 09.03.2005 printing date: 27.05.2005 1. Identification of the substance/preparation
Material Safety Data Sheet according to 91/155/EEC - ISO 11014-1 Page 1 of 6 OMNISEAL 1K PUR WHITE SDS no. : 180161 Revision: 09.03.2005 printing date: 27.05.2005 1. Identification of the substance/preparation
COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE (CVMP) GUIDELINE ON USER SAFETY FOR IMMUNOLOGICAL VETERINARY MEDICINAL PRODUCTS
 European Medicines Agency Veterinary Medicines and Inspections London, 23 April 2007 Doc. Ref. EMEA/CVMP/IWP/54533/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE (CVMP) GUIDELINE ON USER SAFETY
European Medicines Agency Veterinary Medicines and Inspections London, 23 April 2007 Doc. Ref. EMEA/CVMP/IWP/54533/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE (CVMP) GUIDELINE ON USER SAFETY
Propex IQ Apex Locator. Getting Started Guide Just a few images to help you set up your apex locator easily and get the most out of it.
 Propex IQ Apex Locator Getting Started Guide Just a few images to help you set up your apex locator easily and get the most out of it. User manual 1 Package contents Propex IQ Apex Locator Propex IQ Clamp
Propex IQ Apex Locator Getting Started Guide Just a few images to help you set up your apex locator easily and get the most out of it. User manual 1 Package contents Propex IQ Apex Locator Propex IQ Clamp
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 16635-2 First edition 2014-09-15 Dentistry Dental rubber dam instruments Part 2: Clamp forceps Médecine bucco-dentaire Instruments de digue dentaire en caoutchouc Partie 2: Pinces
INTERNATIONAL STANDARD ISO 16635-2 First edition 2014-09-15 Dentistry Dental rubber dam instruments Part 2: Clamp forceps Médecine bucco-dentaire Instruments de digue dentaire en caoutchouc Partie 2: Pinces
a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210
 a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 for SMEs a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 Copyright protected document All rights reserved.
a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 for SMEs a practical guide ISO 13485:2016 Medical devices Advice from ISO/TC 210 Copyright protected document All rights reserved.
care NCDC-Cleanser
 Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
Instructions for Use. Contra-angle handpieces. Endea Endo Cursor EB-62 Endea EB-75 / EB-79. Contra-angle handpieces. Endo NiTi WD-73 M / WD-74 M
 Instructions for Use Contra-angle handpieces Endea Endo Cursor EB-62 Endea EB-75 / EB-79 Contra-angle handpieces Endo NiTi WD-73 M / WD-74 M Contents W&H Symbols... 3 1. Introduction... 4 5 2. Before use
Instructions for Use Contra-angle handpieces Endea Endo Cursor EB-62 Endea EB-75 / EB-79 Contra-angle handpieces Endo NiTi WD-73 M / WD-74 M Contents W&H Symbols... 3 1. Introduction... 4 5 2. Before use
piezosteril 6 - piezolight 6
 97050228 Rev. 06 15.04 piezosteril 6 - piezolight 6 ENGLISH...2 EN ENGLISH 1. INTENDED USE... 3 2. IMPORTANT WARNINGS... 3 3. SYMBOLS... 4 4. TECHNICAL SPECIFICATIONS... 4 5. CONNECTION TO THE POWER SUPPLY
97050228 Rev. 06 15.04 piezosteril 6 - piezolight 6 ENGLISH...2 EN ENGLISH 1. INTENDED USE... 3 2. IMPORTANT WARNINGS... 3 3. SYMBOLS... 4 4. TECHNICAL SPECIFICATIONS... 4 5. CONNECTION TO THE POWER SUPPLY
19/08/2014. What is Injection Safety?
 Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Allergy Reliever. User Manual. AR 1 Series
 Allergy Reliever User Manual AR 1 Series Content Introduction Parts Warnings Using the Allergy Reliever Battery Information Specification Maintenance and Cautions Explanation of Symbols on Unit 2 3 4 5
Allergy Reliever User Manual AR 1 Series Content Introduction Parts Warnings Using the Allergy Reliever Battery Information Specification Maintenance and Cautions Explanation of Symbols on Unit 2 3 4 5
Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations Brussels,
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations Brussels,
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE. Yuan Xu 18- JAN- 2018
 BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
Goldspeed EVO S1 / S1-L / M5-L /
 97050865 Rev. 00 15.05 Goldspeed EVO S1 / S1-L / M5-L / D1 / D1-L / R20-L / E16 ENGLISH..2 EN ENGLISH 1. INTENDED USE... 3 2. IMPORTANT WARNINGS... 3 3. SYMBOLS... 4 4. TECHNICAL SPECIFICATIONS... 4 5.
97050865 Rev. 00 15.05 Goldspeed EVO S1 / S1-L / M5-L / D1 / D1-L / R20-L / E16 ENGLISH..2 EN ENGLISH 1. INTENDED USE... 3 2. IMPORTANT WARNINGS... 3 3. SYMBOLS... 4 4. TECHNICAL SPECIFICATIONS... 4 5.
Pulpdent Corporation Revision Date: May 6, 2014 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
Titanium Base abutments plus screw and associated restorative and dental laboratory components.
 Instructions for use Titanium Base abutments plus screw and associated restorative and dental laboratory components. Description: Titanium Base with screw for Individual abutments under CE-Series, CL-
Instructions for use Titanium Base abutments plus screw and associated restorative and dental laboratory components. Description: Titanium Base with screw for Individual abutments under CE-Series, CL-
Tissue Transglutaminase (ttg; expressed in Baculovirus/Sf9)
 MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH) DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_tTG_BV_15200_140818_Rev01.docx Tel. +49 761 47979-0 Revision
MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH) DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_tTG_BV_15200_140818_Rev01.docx Tel. +49 761 47979-0 Revision
Safety Data Sheet Product Name: Heparin / PF4 Serum Panel - For In Vitro Diagnostic Use Only
 Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET Document ID : 13-0502-1 Issue date : 23/02/2005 Version : 1.00 Supersedes date : NIL Document status : Issued Total No. of Pages : 6 This MSDS has been prepared by PSK Pharma
MATERIAL SAFETY DATA SHEET Document ID : 13-0502-1 Issue date : 23/02/2005 Version : 1.00 Supersedes date : NIL Document status : Issued Total No. of Pages : 6 This MSDS has been prepared by PSK Pharma
Workshop on the reprocessing of medical devices. 5. December Brussels. Nikou Ghassemieh Managing Director
 Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
book Seite 1 Mittwoch, 26. Juli : qq=ifkb=l=^áê=jçíçê. Operating Instructions / T4 LINE motor kit. båöäáëü. lééê~íáåö=fåëíêìåíáçåë
 6397959.book Seite 1 Mittwoch, 26. Juli 2017 2:52 14 qq=ifkb=l=^áê=jçíçê Operating Instructions / T4 LINE motor kit båöäáëü lééê~íáåö=fåëíêìåíáçåë 6397959.book Seite 2 Mittwoch, 26. Juli 2017 2:52 14 Table
6397959.book Seite 1 Mittwoch, 26. Juli 2017 2:52 14 qq=ifkb=l=^áê=jçíçê Operating Instructions / T4 LINE motor kit båöäáëü lééê~íáåö=fåëíêìåíáçåë 6397959.book Seite 2 Mittwoch, 26. Juli 2017 2:52 14 Table
Material Safety Data Sheet
 1 Material Safety Data Sheet MSDS0061 revised 18-MAY-2002 "PERSPEX" CLEAR CAST ACRYLIC SHEET CHEMICAL PRODUCT/COMPANY IDENTIFICATION "PERSPEX" IS A REGISTERED TRADEMARK OF LUCITE INTERNATIONAL INC. Material
1 Material Safety Data Sheet MSDS0061 revised 18-MAY-2002 "PERSPEX" CLEAR CAST ACRYLIC SHEET CHEMICAL PRODUCT/COMPANY IDENTIFICATION "PERSPEX" IS A REGISTERED TRADEMARK OF LUCITE INTERNATIONAL INC. Material
3. Guarantee conditions
 Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Safety Data Sheet according to (EC) No 1907/ ISO
 Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 6 3880 Adhesive Electrically Conductive SDS no. : 168456 Revision: 12.06.2008 printing date: 04.05.2009 1. Identification of the
Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 6 3880 Adhesive Electrically Conductive SDS no. : 168456 Revision: 12.06.2008 printing date: 04.05.2009 1. Identification of the
SECTION 1: Identification of the substance/mixture and of the company/undertaking
 Page 1 of 10 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product Identifier Trade name / designation: Additional information: The substance does not require registration
Page 1 of 10 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product Identifier Trade name / designation: Additional information: The substance does not require registration
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Ministry of Health ul. Miodowa 15 00-952 Warszawa Poland To : European Commission GROWTH Directorate-General 200 Rue
Notification of a Body in the framework of a technical harmonization directive From : Ministry of Health ul. Miodowa 15 00-952 Warszawa Poland To : European Commission GROWTH Directorate-General 200 Rue
Implant Locator. User Manual
 Implant Locator User Manual Table of Contents Introduction..... 2 1. Indications for use..... 3 2. Contraindications... 3 3. Warnings........ 3 4. Precautions........ 3 5. Adverse Reactions.... 3 6. Step-by-Step
Implant Locator User Manual Table of Contents Introduction..... 2 1. Indications for use..... 3 2. Contraindications... 3 3. Warnings........ 3 4. Precautions........ 3 5. Adverse Reactions.... 3 6. Step-by-Step
Distributed by REF INSTRUCTIONS FOR USE
 Distributed by REF 1600911-001 INSTRUCTIONS FOR USE Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 TAblE Of contents I. MEANING OF SYMBOLS II. SCOPE OF USE III. GENERAL SAFETY INFORMATION IV. TECHNICAL CHARACTERISTICS
Distributed by REF 1600911-001 INSTRUCTIONS FOR USE Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 TAblE Of contents I. MEANING OF SYMBOLS II. SCOPE OF USE III. GENERAL SAFETY INFORMATION IV. TECHNICAL CHARACTERISTICS
2. STRAUMANN PRODUCTS COVERED BY THE STRAUMANN GUARANTEE. Abutment attached to an implant* Replacement with equivalent ceramic abutment**
 Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
This safety data sheet conforms to Regulation (EC) No. 1272/2008. Version
 This safety data sheet conforms to Regulation (EC) No. 1272/2008. Version 1-05.10.17 1.0 IDENTIFICATION OF THE SUBSTANCE/ PREPARATION AND COMPANY 1.1 PRODUCT IDENTIFIERS Product name: FR-DB4 1.2 RELEVANT
This safety data sheet conforms to Regulation (EC) No. 1272/2008. Version 1-05.10.17 1.0 IDENTIFICATION OF THE SUBSTANCE/ PREPARATION AND COMPANY 1.1 PRODUCT IDENTIFIERS Product name: FR-DB4 1.2 RELEVANT
Safety manual for hearing instruments
 Safety manual for hearing instruments Content Safety information 3 Intended use 3 Explanation of symbols 3 General warnings 4 BTE or RIC or custom models 12 For infants, small children and mentally disabled
Safety manual for hearing instruments Content Safety information 3 Intended use 3 Explanation of symbols 3 General warnings 4 BTE or RIC or custom models 12 For infants, small children and mentally disabled
Guidance Document Infectious Substances
 Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
SLEEP IMPROVING WRISTBAND. Item No Owner s Guide
 SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
Intelligent design is apparent from the detail
 W&H oral surgery Intelligent design is apparent from the detail 2 W&H Implantmed The intelligent drive unit from W&H What does it take to make an intelligent piece of equipment like the comprehensively
W&H oral surgery Intelligent design is apparent from the detail 2 W&H Implantmed The intelligent drive unit from W&H What does it take to make an intelligent piece of equipment like the comprehensively
ProPocket TM. User Guide
 ProPocket TM User Guide A1 A Introduction Dear customer, Your hearing instruments are equipped with wireless technology and can therefore be controlled by your ProPocket. These instruction describes how
ProPocket TM User Guide A1 A Introduction Dear customer, Your hearing instruments are equipped with wireless technology and can therefore be controlled by your ProPocket. These instruction describes how
1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION. Material for temporary filling and rebuilding root canals
 Page 1 of 7 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: BIO MTA + Application: Manufacturer s name: Material for temporary filling and rebuilding root canals Wojciech Pawłowski 37-450
Page 1 of 7 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: BIO MTA + Application: Manufacturer s name: Material for temporary filling and rebuilding root canals Wojciech Pawłowski 37-450
MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH),
 MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH), amended by (EU) 2015/830 DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_Phlp2-0101_51400_160222_Rev01.docx
MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH), amended by (EU) 2015/830 DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_Phlp2-0101_51400_160222_Rev01.docx
SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF COMPANY.
 SAFETY DATA SHEET Armillatox Limited The Colliery Industrial Estate Main Road Morton Alfreton Derbyshire DE55 6HL England Tel: +44(0)J773 590566 Fax : +44(0)1773 590681 DATE :- 1 st JUNE 2003 DATASHEET
SAFETY DATA SHEET Armillatox Limited The Colliery Industrial Estate Main Road Morton Alfreton Derbyshire DE55 6HL England Tel: +44(0)J773 590566 Fax : +44(0)1773 590681 DATE :- 1 st JUNE 2003 DATASHEET
ISO 13485:2016 Medical Devices Training Handbook
 ISO 13485:2016 Medical Devices Training Handbook Presented By Maria Mylonas Page 1 of 16 pages How to use this handbook The handbook is organized to focus on particular skills and revisions. These lessons
ISO 13485:2016 Medical Devices Training Handbook Presented By Maria Mylonas Page 1 of 16 pages How to use this handbook The handbook is organized to focus on particular skills and revisions. These lessons
MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH),
 MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH), amended by (EU) 2015/830 DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_Glym5-0101_50600_151013_Rev01.docx
MATERIAL SAFETY DATA SHEET according to Regulation (EC) No 1907/2006 (REACH), amended by (EU) 2015/830 DIARECT AG Bötzinger Str. 29 B 79111 Freiburg, Germany Version: MSDS_Glym5-0101_50600_151013_Rev01.docx
PRODUCT INFORMATION SHEET
 PRODUCT INFORMATION SHEET FEATURES & APPLICATIONS FOOD SAFE SANITISER Unico Limited, North Main Street, Carronshore, Falkirk. FK2 8HT Tel: 0845 27 86426 Fax: 01324 573401 Unico Food Safe Sanitiser is a
PRODUCT INFORMATION SHEET FEATURES & APPLICATIONS FOOD SAFE SANITISER Unico Limited, North Main Street, Carronshore, Falkirk. FK2 8HT Tel: 0845 27 86426 Fax: 01324 573401 Unico Food Safe Sanitiser is a
Withdrawn at December 31, Guideline DKD-R 6-2. Calibration of Measuring Devices for Vacuum DEUTSCHER KALIBRIERDIENST. Part 1.
 DEUTSCHER KALIBRIERDIENST Guideline DKD-R 6-2 Part 1 Calibration of Measuring Devices for Vacuum Fundamentals Edition 03/2002 Page 1 of 6 Published by the Accreditation Body of the Deutscher Kalibrierdienst
DEUTSCHER KALIBRIERDIENST Guideline DKD-R 6-2 Part 1 Calibration of Measuring Devices for Vacuum Fundamentals Edition 03/2002 Page 1 of 6 Published by the Accreditation Body of the Deutscher Kalibrierdienst
SAFETY DATA SHEET THE BIG CHEESE RAT AND MOUSE KILLER ALL-WEATHER BLOCK BAIT. BAIT (HSE No.: 8794)
 SAFETY DATA SHEET THE BIG CHEESE RAT AND MOUSE KILLER ALL-WEATHER BLOCK (HSE No.: 8794). IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND THE COMPANY Product name Company THE BIG CHEESE RAT AND MOUSE
SAFETY DATA SHEET THE BIG CHEESE RAT AND MOUSE KILLER ALL-WEATHER BLOCK (HSE No.: 8794). IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND THE COMPANY Product name Company THE BIG CHEESE RAT AND MOUSE
1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION
 Page 1 of 6 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: Application: Manufacturer: ENDO-SOLution Liquid for root canal preparation Wojciech Pawłowski 37-450 Stalowa Wola ul. Kwiatkowskiego
Page 1 of 6 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: Application: Manufacturer: ENDO-SOLution Liquid for root canal preparation Wojciech Pawłowski 37-450 Stalowa Wola ul. Kwiatkowskiego
PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS
 PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS Date: 18 April 2017 Time: 3 HOURS (10:00 13:00) TOTAL: 100 marks SPECIAL INSTRUCTIONS Answer all the questions.
PROFESSIONAL BOARD FOR DENTAL THERAPY AND ORAL HYGIENE BOARD EXAMINATION FOR DENTAL ASSISTANTS Date: 18 April 2017 Time: 3 HOURS (10:00 13:00) TOTAL: 100 marks SPECIAL INSTRUCTIONS Answer all the questions.
Pulpdent Corporation Revision Date: May 1, 2017
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
PIC/S GUIDANCE ON CLASSIFICATION OF GMP DEFICIENCIES
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 040-1 3 Appendices 1 January 2019 PIC/S GUIDANCE ON CLASSIFICATION OF GMP DEFICIENCIES PIC/S January 2019 Reproduction
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 040-1 3 Appendices 1 January 2019 PIC/S GUIDANCE ON CLASSIFICATION OF GMP DEFICIENCIES PIC/S January 2019 Reproduction
Dental Benefits Summary
 DMO Annual Deductible Individual Family Preventive Services 100% Basic Services 90% Major Services 60% Annual Benefit Maximum Office Visit Copay $5 Orthodontic Services Orthodontic Deductible Orthodontic
DMO Annual Deductible Individual Family Preventive Services 100% Basic Services 90% Major Services 60% Annual Benefit Maximum Office Visit Copay $5 Orthodontic Services Orthodontic Deductible Orthodontic
Sterilization of health care products Radiation. Part 3: Guidance on dosimetric aspects of development, validation and routine control
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-3 Second edition 2017-06 Sterilization of health care products Radiation Part 3: Guidance on dosimetric aspects of development, validation
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-3 Second edition 2017-06 Sterilization of health care products Radiation Part 3: Guidance on dosimetric aspects of development, validation
PROPHYflex 4 Turn air polishing into pure joy.
 PROPHYflex 4 Turn air polishing into pure joy. Two handpieces in one perfect for supragingival and subgingival applications. The new PROPHYflex 4 is an all-in-one miracle device in your hand. Whether you
PROPHYflex 4 Turn air polishing into pure joy. Two handpieces in one perfect for supragingival and subgingival applications. The new PROPHYflex 4 is an all-in-one miracle device in your hand. Whether you
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
The College of Dental Surgeons of Saskatchewan Radiation and Imaging Standard
 The College of Dental Surgeons of Saskatchewan Radiation and Imaging Standard Legislation Radiation safety has long been a priority in Saskatchewan. This province, the first in Canada to have radiation
The College of Dental Surgeons of Saskatchewan Radiation and Imaging Standard Legislation Radiation safety has long been a priority in Saskatchewan. This province, the first in Canada to have radiation
Dental Benefit Summary
 DMO Passive PPO Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered Percent 100% 80% Basic Service Covered Percent 100% 50% Major Service Covered Percent 60% 50% Annual Benefit
DMO Passive PPO Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered Percent 100% 80% Basic Service Covered Percent 100% 50% Major Service Covered Percent 60% 50% Annual Benefit
Safety Data Sheet according to (EC) No 1907/ ISO
 Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 7 Loctite V5004 Part B sds no. : 328930 Revision: 11.06.2010 printing date: 03.01.2012 1. Identification of the substance/preparation
Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 7 Loctite V5004 Part B sds no. : 328930 Revision: 11.06.2010 printing date: 03.01.2012 1. Identification of the substance/preparation
E-STATIS Motor. Operator's Manual. E-STATIS Operator s Manual Copyright 2009 SciCan. All rights reserved.
 Motor Operator's Manual CAUTION: Federal law restricts this device to sale by or on the order of a dentist. www.scican.com Operator s Manual Copyright 2009 SciCan. All rights reserved. Doc. No. 2.000.3441
Motor Operator's Manual CAUTION: Federal law restricts this device to sale by or on the order of a dentist. www.scican.com Operator s Manual Copyright 2009 SciCan. All rights reserved. Doc. No. 2.000.3441
3D Printing Technology ----Applications in Dentistry
 3D Printing Technology ----Applications in Dentistry Xingzhe Ding April 25, 2015 Agenda Introduction of - 3D printing technology - Dentistry Applications of 3D Printing in Dentistry Challenges and Opportunities
3D Printing Technology ----Applications in Dentistry Xingzhe Ding April 25, 2015 Agenda Introduction of - 3D printing technology - Dentistry Applications of 3D Printing in Dentistry Challenges and Opportunities
E-STATIS. Operator's Manual. Motor. E-STATIS Operator s Manual Copyright 2009 SciCan. All rights reserved.
 E-STATIS Motor Operator's Manual CAUTION: Federal law restricts this device to sale by or on the order of a dentist. www.scican.com E-STATIS Operator s Manual Copyright 2009 SciCan. All rights reserved.
E-STATIS Motor Operator's Manual CAUTION: Federal law restricts this device to sale by or on the order of a dentist. www.scican.com E-STATIS Operator s Manual Copyright 2009 SciCan. All rights reserved.
SAFETY DATA SHEET. Section 1: Identification of the substance/mixture and of the company/undertaking. Section 2: Hazards identification
 Page: 1 Compilation date: 18/03/2014 Revision No: 1 Section 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name: Product code: ANTQCRM200 1.2.
Page: 1 Compilation date: 18/03/2014 Revision No: 1 Section 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name: Product code: ANTQCRM200 1.2.
Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO
 Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO PPO Max Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered
Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO PPO Max Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered
Innovative revolutionary approach to root canal preparation
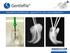 Innovative revolutionary approach to root canal preparation The promise Maintaining the anatomic structure of the canal Symmetric removal of the dentine layer Filing the canal walls rather then drilling
Innovative revolutionary approach to root canal preparation The promise Maintaining the anatomic structure of the canal Symmetric removal of the dentine layer Filing the canal walls rather then drilling
Safety data sheet. Cement is irritating to the eyes, respiratory tract and mucous membranes.
 1 Identification of the product and the company: Identification of the product: Trade name: Fine Microcement Recommended uses Decorative coating. 2 Information on ingredients Chemical description: Mixture
1 Identification of the product and the company: Identification of the product: Trade name: Fine Microcement Recommended uses Decorative coating. 2 Information on ingredients Chemical description: Mixture
