Preparation Instructions for the CAMLOG /CONELOG Implant System
|
|
|
- Rudolf Howard
- 5 years ago
- Views:
Transcription
1 J Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization of the instruments and prosthetic components of the CAMLOG and CONELOG Implant Systems. Always observe the current validated legal national regulations and regulations on hygiene relating to dental/medical practices and hospitals. This applies in particular to the guidelines regarding effective prion inactivation. For sterilizer and disinfector As part of your responsibility for the sterility of the products in application, please observe the following: In general, use only adequately validated methods specific to the equipment and product for cleaning/disinfection and sterilization. Regularly check and service the equipment used (thermal disinfector, sterilizer). Observe the validated parameters in each cycle. For U.S.: The validated procedures require the use of FDA-cleared sterilizers, sterilization trays, sterilization wraps, biological indicators, chemical indicators, and other sterilization accessories labeled for the sterilization cycle recommended. The health care facility should monitor the sterilizer for the facility according to an FDA recognized sterility assurance standard such as ANSI/AAMI ST79:2006. Important information: If not otherwise specified in the instruction manual, reusable CAMLOG and CONELOG products may be reprocessed as long as they are maintained in working condition according to the instruction manuals and processing procedures. CAMLOG and CONELOG products intended for single use must not be reused because safe preparation and/or functional safety cannot be ensured. 1 Instruments The instruments used in the CAMLOG and CONELOG Implant Systems are supplied non-sterile unless they are explicitly marked as sterile. They must be cleaned, disinfected and sterilized before first use and every use. Thorough cleaning and disinfection is indispensable for effective sterilization. In application, care should be taken that contaminated instruments are collected separately and are not returned to the instrument tray to prevent contamination of the loaded instrument tray. After cleaning and disinfection, place the instruments back into the instrument tray. Then sterilize the fully loaded instrument tray. Two procedures are described in the following for cleaning and disinfection prior to sterilization: a mechanical procedure under point 1.1 and a manual procedure under point 1.2. Wherever possible, the mechanical procedure described under point 1.1 should be employed. PDF EN 1
2 When choosing a combined cleaning and disinfecting agent for initial disinfection and for manual cleaning and disinfection, one should make sure that: it is suitable for cleaning and disinfecting dental instruments. it is suitable for ultrasonic cleaning (no foam development) it has a proven efficacy for disinfection (e. g. VAH/DGHM listed, CE marking for EU, or EPA registered and FDA compliant for U.S.). it is compatible with the materials of the products to be cleaned and disinfected it does not contain aldehyde (otherwise blood, secretions, tissue remains, etc. may stick). The concentrations and application times as well as instructions for post-rinsing specified by the manufacturer must be observed. Caution! The procedure below must be observed exactly. 1.1 Mechanical cleaning and thermal disinfection Initial disinfection: Immediately after use, immerse all instruments in a bath containing combined cleaning and disinfecting agent. This serves for user safety and prevents the contaminants from drying. The cleaning and disinfecting agent used for pre-treatment is only for personal protection and may not take the place of the disinfection step to be performed after cleaning Disassembly: Completely disassemble all instruments consisting of several parts (see 1.4 Disassembly and Assembly of instruments). Remove depth stops from the drills Pre-treatment after successful initial disinfection and disassembly Remove coarse contaminants from the instruments within a maximum of 2 hours after use Remove contaminants from instruments under running water using a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. Brush until no visible contaminants remain. Some instruments have cavities. Remove all residues in these. Contamination should no longer be visible Remove contaminants in the lumen of the internally irrigated drills, taps and drill extensions with the cleaning needle (Art. No J ) Rinse the lumen of the internally irrigated drills, taps and drill extensions with water at least three times using a cleaning cannula (Art. No. J ) and a syringe (at least 10 ml). Contamination should no longer be visible Full rinse of the instruments for at least one minute under deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml) Rinse the lumen of the internally irrigated drills, taps and drill extensions with deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml) at least five times using the cleaning cannula (Art. No. J ) and a syringe (at least 10 ml) Disinfection of the pre-cleaned products in the thermal disinfector: Thermal disinfector (cleaning and disinfection device) When choosing the thermal disinfector (cleaning and disinfecting device), care should be taken that: In general, the thermal disinfector has a proven efficacy (CE marking, compliant with EN ISO in Europe or FDA clearance in the U.S.) and is validated specifically for the equipment and product. A proven program for thermal disinfection is used (A0 value > 3000 or for older devices at least 5 min. at 90 C / 194 F). The program used is suitable for the instruments and includes sufficient rinsing cycles. Only deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml) is used for rinsing. The air used for drying is filtered. The thermal disinfector (cleaning and disinfection device) is regularly checked and serviced. PDF EN 2
3 Use only thermal disinfection that requires no disinfecting agent. Avoid using any rinse aids. There is a risk of disinfectant residues on the instruments when chemical disinfection is used Cleaning agents When using cleaning agents, care should be taken that: In general, the cleaning agents are suitable for cleaning instruments made of metals and plastics. The chemicals used are compatible with the instruments. We recommend the use of cleaning agents that require no neutralizing agents Worksteps 1. Place the disassembled and pretreated instruments in the thermal disinfector using a small parts basket. The instruments are not to come into contact with one another. 2. Start the program. 3. Remove the instruments from the thermal disinfector after program end. 4. Dry the instruments if necessary. Use compressed dry air, free of oil and with a low bacterial count. We also recommend the use of a sterile filter. 5. Inspect the instruments for signs of corrosion, surface damage, chipping or contamination. Remove damaged instruments from use. Re-clean and disinfect instruments which are still contaminated. Observe the maximum number of times drills may be used as prescribed in the instruction manuals. 6. Assembly: Reassemble all disassembled instruments (see 1.4 Disassembly and Assembly of instruments). Install the depth stops on the drills. 7. Packaging: Pack instruments for sterilization promptly. We recommend using disposable sterilization packaging with CE marking in Europe or FDA clearance in the U.S. It must be ensured that the sterilization packaging is suitable for steam sterilization (constant temperature of at least 141 C / 286 F, sufficient vapor permeability) and that the products are adequately protected against mechanical damage. General note The proof of general suitability for effective mechanical cleaning and disinfection was provided by an independent accredited testing laboratory taking into account the above-described procedure. A thermal disinfector (cleaning and disinfecting device) G 7836 CD (Manufacturer: Miele & Cie. GmbH & Co., Gütersloh) and neodisher medizym as the cleaning agent (Dr. Weigert GmbH & Co. KG, Hamburg) were used. The procedure described above was taken into account. 1.2 Manual cleaning and disinfection Initial disinfection: Immediately after use, immerse all instruments in a bath containing combined cleaning and disinfecting agent. This serves for user safety and prevents the contaminants from drying. The cleaning and disinfecting agent used for pre-treatment is only for personal protection and may not take the place of the disinfection step to be performed later after cleaning Disassembly: Completely disassemble all instruments consisting of several parts (see 1.4 Disassembly and Assembly of instruments) as well as removing all depth stops from the drills Preliminary cleaning: Remove coarse contaminants from the instruments within a maximum of 2 hours after use. For this purpose use a disposable cloth, running water, and a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. Remove residues in areas with difficult access using an appropriate instrument. Remove firmly bonded tissue residues in the cooling channels of internally cooled instruments with the cleaning needle (Art. No. J ). The cooling channels in the internally cooled instruments and the drill extensions must be rinsed out at least three times with water using the cleaning cannula (Art. No. J ) and a syringe (at least 10 ml). PDF EN 3
4 1.2.4 Combined cleaning and disinfection: Place the instruments in a bath of freshly prepared combined cleaning and disinfecting solution for the scheduled application time, making sure they are completely covered. The instruments are not to come into contact with one another. In case of persistent contamination use an ultrasonic unit. To completely remove residue, brush off the instruments with a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. The cooling channels in the internally cooled instruments and the drill extensions must be rinsed out at least five times with the combined cleaning and disinfection solution using the cleaning cannula (Art. No. J ) and a syringe (at least 10 ml). Some instruments have cavities. Use a soft brush to remove residue in these cavities (no metal bristles or steel wool). The brush must be used exclusively for this purpose only Rinsing: Remove the instruments, rinse them completely for at least one minute under deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ ml). Take particular care in rinsing the area that have limited access. The cooling channels of internally cooled instruments and the drill extensions must be rinsed out at least five times using the cleaning cannula (Art. No. J ) and a syringe (at least 10 ml) Drying: Dry the instruments. Use a loose lint free disposable cloth and compressed dry air, free of oil and with a low bacterial count. We also recommend the use of a sterile filter Inspection: Inspect the instruments for signs of corrosion, surface damage, chipping or contamination. Remove damaged instruments from use. Clean and disinfect still contaminated instruments again. Observe the maximum number of times drills may be used as prescribed in the instruction manuals Assembly: Reassemble all disassembled instruments (see 1.4 Disassembly and Assembly of instruments). Install the depth stops on the drills Packaging: Pack instruments for sterilization promptly. We recommend using disposable sterilization packaging with CE marking in Europe or FDA clearance in the U.S. It must be ensured that the sterilization packaging is suitable for steam sterilization (constant temperature of at least 141 C / 286 F, sufficient vapor permeability) and that the products are adequately protected against mechanical damage. 1.3 Sterilization Permissible steam sterilization procedures are fractionated vacuum procedures (with sufficient product drying). Other sterilization procedures (including steam gravity sterilization) are not allowed. The sterilization time (exposure time at sterilization temperature) is at least 20 minutes at a minimum of 121 C / 250 F or at least 4 minutes at a minimum of 132 C / 270 F (not valid for U.S.). For U.S.: The sterilization time (exposure time at sterilization temperature) is at least 4 minutes at a minimum of 132 C / 270 F. A minimum drying time of 30 minutes is recommended for each of the cycles described above. Care should be taken that: The maximum steam sterilization temperature is 138 C / 280 F. The surgical trays do not touch the walls of the steam sterilizer because high local temperatures over 160 C / 320 F may lead to deformation of plastic. The steam sterilizers used have CE marking and comply with the requirements of EN or EN 285 in Europe or have FDA clearance in the U.S. In general, use only adequately validated methods specific to the equipment and product for sterilization according to ISO Regularly check and service the sterilizers used. Observe the validated parameters in each cycle. Warning! All non-sterile packed CAMLOG and CONELOG products must not be sterilized in the CAMLOG original packaging! PDF EN 4
5 1.4 Disassembly and Assembly of instruments The following instruments must be disassembled prior to cleaning and disinfection: Cardanic driver for screw implants (Art. No. J ). Disassemble the cardanic driver in three parts by loosening the clamping screw with a screw driver hex. After reassembling, the clamping screw must be tightened carefully by hand with a screw driver hex. Drivers for bar abutments (for CAMLOG and CONELOG Ø 3.3/3.8/4.3 mm: Art. No. J /J530021; Ø 5.0/6.0 mm: Art. No. J /J ). To disassemble the drivers for bar abutments remove the bolt by turning out. Reassemble by turning in. Drivers for impression posts and healing caps for bar abutments (for CAMLOG and CONELOG Ø 3.3/3.8/4.3 mm: Art. No. J ; Ø 5.0/6.0 mm: Art. No. J ). To disassemble the drivers for impression posts and healing caps for bar abutments remove the bolt by turning it counter-clockwise. Reassemble by turning clockwise. Adapters for screw implants, long, for CAMLOG SCREW-LINE and ROOT-LINE implants (Ø 3.3 mm: Art. No. K ; Ø 3.8 mm: Art. No. K ; Ø 4.3 mm: Art. No. K ). Adapters for screw implants, long, for CONELOG SCREW-LINE implants (Ø 3.3 mm: Art. No. C ; Ø 3.8/4.3 mm: Art. No. C ). To disassemble the adapter for screw implants, long, remove the bolt by turning it counter-clockwise. Reassemble by turning clockwise. Torque wrench (Art. No. J ). For disassembling, maintenance and reassembling of the torque wrench, follow the Instruction Manual - Torque wrench, Art. No. J Prosthetic Components The prosthetic components of the CAMLOG and CONELOG Implant System are supplied non-sterile unless they are explicitly marked as sterile. They must be used one time only and solely on one patient. They must be cleaned and disinfected before and after each use on a patient, (e.g. for shipping to the dental laboratory). We recommend additional sterilization. Thorough cleaning and disinfection is indispensable for effective sterilization. Two cleaning/disinfection procedures are described: a mechanical procedure under point 2.1 and a manual procedure under point 2.2. The mechanical procedure described under point 2.1 is to be preferred. PDF EN 5
6 When choosing a combined cleaning and disinfecting agent for initial disinfection and for manual cleaning and disinfection, one should make sure that: it is suitable for cleaning and disinfecting dental instruments. it is suitable for ultrasonic cleaning (no foam development) it has a proven efficacy for disinfection (e. g. VAH/DGHM listed, CE marking for EU, or EPA registered and FDA compliant for U.S.). it is compatible with the materials of the products to be cleaned and disinfected it does not contain aldehyde (otherwise blood, secretions, tissue remains, etc. may stick). The concentrations and application times specified by the manufacturer must be observed. Caution! Plastic components of the CAMLOG or the CONELOG Implant System must not be sterilized (only cleaned and disinfected), except for the plastic components made out of PEEK. Plastic components made out of PEEK can be sterilized. Prosthetic components connected with a non-sterilizable material cannot be sterilized (just cleaned and disinfected). 2.1 Mechanical cleaning and disinfection Initial disinfection: Immediately after use in the patient's mouth, immerse all prosthetic components in a bath with combined cleaning and disinfecting agent. This serves for user safety and prevents the contaminants from drying. The cleaning and disinfecting agent used for pre-treatment is only for personal protection and may not take the place of the disinfection step to be performed after cleaning Preliminary cleaning Remove coarse contaminants from the prosthetic components (after use in the patient's mouth or contamination in the dental laboratory) within a maximum of 2 hours after use Remove contaminants from the prosthetic components under running water using a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. Brush until no visible contaminants remain. Some prosthetic components have cavities. Remove all residues in these. Contamination should no longer be visible Full rinse of the prosthetic components for one minute under deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml) Disinfection of the pre-cleaned products in the thermal disinfector: Thermal disinfector (cleaning and disinfection device) When choosing the thermal disinfector, care should be taken that: In general, the thermal disinfector has a proven efficacy (CE marking, conforming to EN ISO in Europe or FDA clearance in the U.S.) and is validated specifically for the equipment and product. A proven program for thermal disinfection is used (A0 value > 3000 or for older devices at least 5 min. at 90 C / 194 F). The program used is suitable for the prosthetic components and includes sufficient rinsing cycles. Only deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml) is used for rinsing. The air used for drying is filtered. The thermal disinfector is regularly checked and serviced. Use only thermal disinfection that requires no disinfecting agent. Avoid using any rinse aids. There is a risk of disinfectant residues on the prosthetic components when chemical disinfection is used. PDF EN 6
7 Cleaning agents When using cleaning agents, care should be taken that: in general, the cleaning agents are suitable for cleaning prosthetic components made of metals and plastics. the chemicals used are compatible with the prosthetic components. We recommend the use of cleaning agents that require no neutralizing agents Worksteps 1. Place the pre-treated prosthetic components in the thermal disinfector using a small parts basket. The prosthetic components are not to come into contact with one another. 2. Start the program. 3. Remove the prosthetic components from the thermal disinfector after program end. 4. Dry the prosthetic components if necessary. Use compressed dry air, free of oil and with a low bacterial count. We also recommend the use of a sterile filter. 5. Inspect the prosthetic components for signs of corrosion, surface damage, chipping or contamination. Remove prosthetic components from use. Re-clean and disinfect prosthetic components which are still contaminated. 6. Packaging: Pack prosthetic components for sterilization promptly. We recommend using disposable sterilization packaging with CE marking in Europe or FDA clearance in the U.S. It must be ensured that the sterilization packaging is suitable for steam sterilization (constant temperature of at least 141 C / 286 F, sufficient vapor permeability) and that the products are adequately protected against mechanical damage. General note The proof of general suitability for effective mechanical cleaning and disinfection was provided by an independent accredited testing laboratory taking into account the above-described procedure. A thermal disinfector (cleaning and disinfecting device) G 7836 CD (Manufacturer: Miele & Cie. GmbH & Co., Gütersloh) and neodisher medizym as the cleaning agent (Dr. Weigert GmbH & Co. KG, Hamburg) were used. The procedure described above was taken into account. 2.2 Manual cleaning and disinfection Initial disinfection: Immediately after use in the patient's mouth, immerse all prosthetic components in a bath with combined cleaning and disinfecting agent. This serves for user safety and prevents the contaminants from drying. The cleaning and disinfecting agent used for pretreatment is only for personal protection and may not take the place of the disinfection step to be performed later after cleaning Preliminary cleaning: Remove coarse contaminants from the prosthetic components (after use in the patient's mouth or following contamination in the dental laboratory) within a maximum of 2 hours after use Remove contaminants from the prosthetic components under running water using a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. Brush until no visible contaminants remain. Some prosthetic components have cavities. Remove all residues in these. Contamination should no longer be visible Full rinse of the prosthetic components for at least one minute under deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ml). PDF EN 7
8 2.2.3 Combined cleaning and disinfection: Place the prosthetic components for cleaning and disinfection in the fresh combined cleaning and disinfecting solution for the scheduled application time making sure they are completely covered. The prosthetic components are not to come into contact with one another. In case of persistent contamination use an ultrasonic unit. Brush off the prosthetic components using a soft brush (no metal bristles or steel wool). The brush must be used exclusively for this purpose only. Remove all residues completely. Remove all debris contained in the areas with limited access. Observe the concentrations and application times and the guidelines for rinsing specified by the manufacturer of the combined cleaning and disinfecting agent, as well as the instructions for post-rinsing Rinsing: Remove the prosthetic components, rinse them for at least one minute under deionized water with a low bacterial count (maximum 10 bacteria/ml) and low endotoxin count (maximum 0.25 endotoxin units/ ml). Take particular care in rinsing the area that have limited access Drying: Dry the prosthetic components. Use a loose lint free disposable cloth and compressed dry air, free of oil and with a low bacterial count. We also recommend the use of a sterile filter Inspection: Inspect the prosthetic components for signs of corrosion, surface damage, chipping or contamination. Damaged prosthetic components cannot be used. Re-clean and disinfect prosthetic components which are still contaminated Packaging: Pack prosthetic components for sterilization promptly. We recommend using disposable sterilization packaging with CE marking in Europe or FDA clearance in the U.S. It must be ensured that the sterilization packaging is suitable for steam sterilization (constant temperature of at least 141 C / 286 F, sufficient vapor permeability) and that the products are adequately protected against mechanical damage. 2.3 Sterilization The sterilization time (exposure time at sterilization temperature) is at least 20 minutes at a minimum of 121 C / 250 F or at least 4 minutes at a minimum of 132 C / 270 F. Permissible steam sterilization procedures are fractionated vacuum procedures (with sufficient product drying) and gravitation procedures. Other sterilization procedures are not allowed (not valid for U.S.). For U.S.: Permissible steam sterilization procedures are fractionated vacuum procedures (with sufficient product drying). Other sterilization procedures are not allowed. The sterilization time (exposure time at sterilization temperature) is at least 4 minutes at a minimum of 132 C / 270 F. A minimum drying time of 30 minutes is recommended for each of the cycles described above. Care should be taken that: the maximum steam sterilization temperature is 138 C / 280 F. the steam sterilizers used have CE marking and meet the requirements of EN or EN 285 (Europe) or have FDA clearance (U.S.). In general, use only adequately validated methods specific to the equipment and product for sterilization according to ISO Regularly check and service the sterilizers used. Observe the validated parameters in each cycle. Warning! All non-sterile packed CAMLOG and CONELOG products must not be sterilized in the CAMLOG original packaging! PDF EN 8
9 3 Contact Headquarters CAMLOG Biotechnologies AG Margarethenstrasse 38 CH-4053 Basel Switzerland Tel. +41 (0) 61/ Fax +41 (0) 61/ Manufacturer ALTATEC GmbH Maybachstraße 5 D Wimsheim Germany PDF EN 9
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care
 a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
INSTRUCTIONS FOR CLEANING, DISINFECTION AND STERILIZATION OF DFS INSTRUMENTS
 Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
a perfect fit SURGERY CAMLOG /CONELOG SCREW-LINE Surgery set and drills Osteotomy sets ALTApin set Sinus set Complementary surgical instruments
 a perfect fit SURGERY CAMLOG /CONELOG SCREW-LINE Surgery set and drills Osteotomy sets ALTApin set Sinus set Complementary surgical instruments SURGERY SET CAMLOG /CONELOG SCREW-LINE THE SURGERY SET AND
a perfect fit SURGERY CAMLOG /CONELOG SCREW-LINE Surgery set and drills Osteotomy sets ALTApin set Sinus set Complementary surgical instruments SURGERY SET CAMLOG /CONELOG SCREW-LINE THE SURGERY SET AND
Cleaning and sterilization instructions for Astra Tech Implant System EV
 Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Instructions for use 0483 Permanent and temporary YASARGIL Aneurysm and Vessel Clips
 1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
 EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
INFORMATION TO THE PRODUCT RANGES. Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS
 INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
UNICLIP DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM FOR DENTAL USE ONLY. FISDR / F EN / 11 / updated 06/2016 1/7
 UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS. a perfect fit
 a perfect fit IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS Open and closed impression-taking Impression-taking for option platform switching Bite registration Temporary
a perfect fit IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS Open and closed impression-taking Impression-taking for option platform switching Bite registration Temporary
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Hygienic Reprocessing
 Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
TONTARRA Medizintechnik GmbH. Surgical Instruments in Demand. Laminectomy Punches and Rongeurs
 TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
Aesculap Spine Instructions for use
 Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
ProUltra Ultrasonic Non Surgical Endo Tips
 ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ATHLET VBR. The real winner. VBR in PEEK-OPTIMA PRODUCT INFORMATION
 ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
The Multi-unit Abutments of the CE-Series are indicated for Nobel Biocare / NobelReplace Tapered. The current implant diameters have to be attended.
 Instructions for Use Multi-Unit Abutment with Screw Description: Multi-Unit Abutment with screws for series CC-Series, CE-Series, CV- Series, CF-Series, CH-Series, CK-Series, CL-Series, CN-Series, CR-Series,
Instructions for Use Multi-Unit Abutment with Screw Description: Multi-Unit Abutment with screws for series CC-Series, CE-Series, CV- Series, CF-Series, CH-Series, CK-Series, CL-Series, CN-Series, CR-Series,
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE
 FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE 1 2 LOGICAL LAYOUT 1 Example: C1 Implant Ø4.20 / 11.5L ; Soft Bone Ø4.20 / 11.5L MG-D0624 MG-D1124 MG-D1133 MG-D1137 2 Example: Implant C1 Ø4.20
FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE 1 2 LOGICAL LAYOUT 1 Example: C1 Implant Ø4.20 / 11.5L ; Soft Bone Ø4.20 / 11.5L MG-D0624 MG-D1124 MG-D1133 MG-D1137 2 Example: Implant C1 Ø4.20
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
High-end solution STEP-BY-STEP USER GUIDE
 High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
1) INDICATIONS FOR USE These products have to be used only in hospital environments, clinics or dental offices by qualified dental personnel.
 ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
Cleaning and sterilization. Guidelines for Nobel Biocare products including MRI information
 Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
Zimmer Biomet Instrument Kit System
 Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
Astra Tech Implant System. Manual and product catalog. Repair procedures. Removal and retrieval of screw fragment, abutment and implant
 Astra Tech Implant System Manual and product catalog Repair procedures Removal and retrieval of screw fragment, abutment and implant Astra Tech Implant System CONTENTS Introduction 5 Remove/retrieve on
Astra Tech Implant System Manual and product catalog Repair procedures Removal and retrieval of screw fragment, abutment and implant Astra Tech Implant System CONTENTS Introduction 5 Remove/retrieve on
book Seite 1 Freitag, 21. April : fjmi^kq=omwn. Operating instructions. båöäáëü. lééê~íáåö=fåëíêìåíáçåë
 6278761.book Seite 1 Freitag, 21. April 2017 3:54 15 fjmi^kq=omwn Operating instructions båöäáëü lééê~íáåö=fåëíêìåíáçåë 6278761.book Seite 2 Freitag, 21. April 2017 3:54 15 Table of contents Operating
6278761.book Seite 1 Freitag, 21. April 2017 3:54 15 fjmi^kq=omwn Operating instructions båöäáëü lééê~íáåö=fåëíêìåíáçåë 6278761.book Seite 2 Freitag, 21. April 2017 3:54 15 Table of contents Operating
Instructions for Use DENTAL IMPLANTS
 HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
TITANIUM BASES CAD/CAM FOR CROWN AND BRIDGE RESTORATIONS CAD/CAM PROSTHETICS ON CAMLOG AND CONELOG IMPLANTS
 TITANIUM BASES CAD/CAM FOR CROWN AND BRIDGE RESTORATIONS CAD/CAM PROSTHETICS ON CAMLOG AND CONELOG IMPLANTS TABLE OF CONTENTS GENERAL SYSTEM INFORMATION 2 CAMLOG AND CONELOG TITANIUM BASES CAD/CAM PRODUCT
TITANIUM BASES CAD/CAM FOR CROWN AND BRIDGE RESTORATIONS CAD/CAM PROSTHETICS ON CAMLOG AND CONELOG IMPLANTS TABLE OF CONTENTS GENERAL SYSTEM INFORMATION 2 CAMLOG AND CONELOG TITANIUM BASES CAD/CAM PRODUCT
Instructions for Use. Contra-angle handpieces. Endea Endo Cursor EB-62 Endea EB-75 / EB-79. Contra-angle handpieces. Endo NiTi WD-73 M / WD-74 M
 Instructions for Use Contra-angle handpieces Endea Endo Cursor EB-62 Endea EB-75 / EB-79 Contra-angle handpieces Endo NiTi WD-73 M / WD-74 M Contents W&H Symbols... 3 1. Introduction... 4 5 2. Before use
Instructions for Use Contra-angle handpieces Endea Endo Cursor EB-62 Endea EB-75 / EB-79 Contra-angle handpieces Endo NiTi WD-73 M / WD-74 M Contents W&H Symbols... 3 1. Introduction... 4 5 2. Before use
SelectTech 4.1 Bench Assembly / Owner s Manual
 SelectTech 4.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4
SelectTech 4.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
SimpleLine II Surgical / Prosthesis Manual
 SimpleLine II Surgical / Prosthesis Manual SimpleLine II SURGICAL MANUAL Surgical Drill Sequence 04 Fixture Installation 05 Fixture Connection 06 Surgical Kit Maintenance 08 Warnings 09 04 SimpleLine ll
SimpleLine II Surgical / Prosthesis Manual SimpleLine II SURGICAL MANUAL Surgical Drill Sequence 04 Fixture Installation 05 Fixture Connection 06 Surgical Kit Maintenance 08 Warnings 09 04 SimpleLine ll
care NCDC-Cleanser
 Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
AS Medizintechnik GmbH Sattlerstraße Tuttlingen Germany
 Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for Use / Technical Description Arcadius XP C Instruments Page 1 of 28 USA Aesculap Implant Systems Arcadius XP C Instruments Page
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for Use / Technical Description Arcadius XP C Instruments Page 1 of 28 USA Aesculap Implant Systems Arcadius XP C Instruments Page
isy indications and techniques
 This is isy 3 The isy Implant System demonstrates its unique talents in numerous indications. Its intelligence allows the clinician to focus on what is most common in their everyday implant practice. isy
This is isy 3 The isy Implant System demonstrates its unique talents in numerous indications. Its intelligence allows the clinician to focus on what is most common in their everyday implant practice. isy
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
SelectTech 4.1 Bench. Assembly Manual
 SelectTech 4.1 Bench Assembly Manual Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4 Specifications...5 Before Assembly...5 Parts...6 Hardware...7 Tools...7
SelectTech 4.1 Bench Assembly Manual Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4 Specifications...5 Before Assembly...5 Parts...6 Hardware...7 Tools...7
Safety and esthetics with
 Safety and esthetics with dental implants A guide for patients Dear reader, Implant restorations follow nature's example. You can have the functions of natural teeth completely restored and thus maintain
Safety and esthetics with dental implants A guide for patients Dear reader, Implant restorations follow nature's example. You can have the functions of natural teeth completely restored and thus maintain
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
The intelligent system.
 The intelligent system. Contents This is isy The intelligent system 5 Cost efficient implant sets 7 Total flexibility 9 Purposeful simplicity 11 The digital future 13 Quality without compromise 15 isy
The intelligent system. Contents This is isy The intelligent system 5 Cost efficient implant sets 7 Total flexibility 9 Purposeful simplicity 11 The digital future 13 Quality without compromise 15 isy
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
PROTAPER GOLD TM Treatment
 PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
THE. Curve THE CURVE STRIPPING TOOL
 THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Zimmer Biomet Instrument Kit System
 Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
Zimmer Biomet Instrument Kit System Reference Guide Tapered Screw-Vent (TSV ) Implant System Trabecular Metal Dental Implants. mmd Eztetic Dental Implants Staging Block NP Surgical Module for. mmd Eztetic
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
FOR PROFESSIONALS. Product Catalogue. Cochlear Vistafix 3 System A BONE ANCHORED PROSTHETIC SOLUTION
 FOR PROFESSIONALS Product Catalogue Cochlear Vistafix 3 System A BONE ANCHORED PROSTHETIC SOLUTION 1 The Vistafix 3 System consists of: Cochlear Vistafix VXI300 Implant (Vistafix 3 Implant) Cochlear Vistafix
FOR PROFESSIONALS Product Catalogue Cochlear Vistafix 3 System A BONE ANCHORED PROSTHETIC SOLUTION 1 The Vistafix 3 System consists of: Cochlear Vistafix VXI300 Implant (Vistafix 3 Implant) Cochlear Vistafix
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
S5 Rotary Files. only. Manufacturer. Packing unit. Batch code. For professional use only (0)
 EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
Low Row. User manual E S S E N T I A L S T R E N G T H
 E L E M E N T E S S E N T I A L S T R E N G T H User manual 1 The identification plate of and manufacturer, affixed on the back panel of the weight stack, gives the following details: A B C D E Name and
E L E M E N T E S S E N T I A L S T R E N G T H User manual 1 The identification plate of and manufacturer, affixed on the back panel of the weight stack, gives the following details: A B C D E Name and
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
NARROW DIAMETER implant
 N NARROW IAMETER implant N Precision dental solutions C-Tech Implant is a dynamic company with aggressive growth, producing components and product lines primarily for dental implantology. International
N NARROW IAMETER implant N Precision dental solutions C-Tech Implant is a dynamic company with aggressive growth, producing components and product lines primarily for dental implantology. International
CURVES. Anatomically Adaptable IPR strips. Brochure Instructions for use Processing instructions
 CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
TJF-160F/VF Cleaning and Disinfection Checklist
 TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
Convex Array Transducer
 User Guide Type 8823 Convex Array Transducer English BB1740-C June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com Email:
User Guide Type 8823 Convex Array Transducer English BB1740-C June 2012 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkmed.com Email:
Straumann ProClean Cassette. Uncompromised hygiene. Ready for action.
 Straumann ProClean Cassette Uncompromised hygiene. Ready for action. 2 100% high-grade stainless steel cassette for uncompromised performance. The unique Straumann ProClean Cassettes are made solely of
Straumann ProClean Cassette Uncompromised hygiene. Ready for action. 2 100% high-grade stainless steel cassette for uncompromised performance. The unique Straumann ProClean Cassettes are made solely of
MANUAL FOR USE NO. 5 FLEXIBLE INTRAMEDULLARY REAMER REV ( GB ) non - sterile
 MANUAL FOR USE NO. 5 FLEXIBLE INTRAMEDULLARY REAMER REV. 10.10.2013 ( GB ) non - sterile Before using the intramedullary reamer. please ensure that it is functioning properly and free of any defects. In
MANUAL FOR USE NO. 5 FLEXIBLE INTRAMEDULLARY REAMER REV. 10.10.2013 ( GB ) non - sterile Before using the intramedullary reamer. please ensure that it is functioning properly and free of any defects. In
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
INFECTION CONTROL IN PRACTICE. Continuing Education
 INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
Dentium Surgery Kit Dental Implant Surgical Instrument Set
 www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
English. Fealite Nasal Pillows System. User Manual
 Fealite Nasal Pillows System User Manual 0123 Table of Contents Fealite Nasal Pillows System 1 Intended Use 1 Medical Information 1 Parts of the Fealite 3 Fitting the Fealite 5 Using Tube Retainer (Optional)
Fealite Nasal Pillows System User Manual 0123 Table of Contents Fealite Nasal Pillows System 1 Intended Use 1 Medical Information 1 Parts of the Fealite 3 Fitting the Fealite 5 Using Tube Retainer (Optional)
Smarter Thinking. Simpler Design. Prima Plus. Surgical Manual
 Smarter Thinking. Simpler Design. Prima Plus Surgical Manual TABLE OF CONTENTS PRIMA PLUS IMPLANT SURGICAL MANUAL SURGERY Prima Plus Characteristics 4 Surgical Considerations 5 Prima Plus Surgical Sequence
Smarter Thinking. Simpler Design. Prima Plus Surgical Manual TABLE OF CONTENTS PRIMA PLUS IMPLANT SURGICAL MANUAL SURGERY Prima Plus Characteristics 4 Surgical Considerations 5 Prima Plus Surgical Sequence
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
A82-LIFE-Dog Nebulizer NBB02
 A82-LIFE-Dog Nebulizer NBB02 פיית אוויר A82-LIFE-Dog Nebulizer NBB02 www.mfcl.co.il COMPRESSOR NEBULIZER USER S MANUAL A82-LIFE-Dog Nebulizer NBB02 ILLUSTRATION OF PARTS A. NEBULIZER COMPRESSOR
A82-LIFE-Dog Nebulizer NBB02 פיית אוויר A82-LIFE-Dog Nebulizer NBB02 www.mfcl.co.il COMPRESSOR NEBULIZER USER S MANUAL A82-LIFE-Dog Nebulizer NBB02 ILLUSTRATION OF PARTS A. NEBULIZER COMPRESSOR
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
User Manual Complete Prosthetic Kit (CPK)
 User Manual Complete Prosthetic Kit (CPK) The Complete Prosthetic Kit is designed to enable easy impression and transfer technique and to prevent the need to remove and to re-tighten the abutments. Advantages
User Manual Complete Prosthetic Kit (CPK) The Complete Prosthetic Kit is designed to enable easy impression and transfer technique and to prevent the need to remove and to re-tighten the abutments. Advantages
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
RATCHETS RATCHET ACCESSORIES DENTAL PRODUCTS PRODUCT CATALOG A company of the KLINGEL GROUP
 RATCHETS RATCHET ACCESSORIES DENTAL PRODUCTS PRODUCT CATALOG 0 A company of the KLINGEL GROUP Contents Universal ratchet without torque function General information All ratchets are available with different
RATCHETS RATCHET ACCESSORIES DENTAL PRODUCTS PRODUCT CATALOG 0 A company of the KLINGEL GROUP Contents Universal ratchet without torque function General information All ratchets are available with different
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Convex Array Transducer User Guide. Types 8567-S and 8667
 Convex Array Transducer User Guide Types 8567-S and 8667 English BB0889-D August 2006 WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com Email: info@bkmed.dk
Convex Array Transducer User Guide Types 8567-S and 8667 English BB0889-D August 2006 WORLD HEADQUARTERS Mileparken 34 DK-2730 Herlev Denmark Tel.:+45 44528100 / Fax:+45 44528199 www.bkmed.com Email: info@bkmed.dk
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Straight Octagon Abutment for Tissue Level Implant
 Cover screw in place The presentation that follows lists only one combination of parts. Obviously the clinical situation may call for substitution of another part on this slide Prosthetic instruments needed
Cover screw in place The presentation that follows lists only one combination of parts. Obviously the clinical situation may call for substitution of another part on this slide Prosthetic instruments needed
High Frequency Linear Array Transducer
 User Guide Type 8870 High Frequency Linear Array Transducer English BB1980-C May 2016 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkultrasound.com
User Guide Type 8870 High Frequency Linear Array Transducer English BB1980-C May 2016 For Professional Users Only BK MEDICAL Mileparken 34 2730 Herlev Denmark Tel.:+45 4452 8100 / Fax:+45 4452 8199 www.bkultrasound.com
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Chapter 5: Checking and Maintaining Ultrasound Equipment
 Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
NARROW DIAMETER implant
 ND NARROW DIAMETER implant TABLE OF CONTENTS ND - NARROW DIAMETER implant Implant characteristics page 04 Dental implant page 05 Open Tray Impression Transfer page 06 Titanium Abutments page 07 O-Ball
ND NARROW DIAMETER implant TABLE OF CONTENTS ND - NARROW DIAMETER implant Implant characteristics page 04 Dental implant page 05 Open Tray Impression Transfer page 06 Titanium Abutments page 07 O-Ball
INDEX. Pag. 12. Pag. 05. Pag. 06. Pag. 13. Pag. 06. Pag. 14. Pag. 15. Pag. 15. Pag. 15. Pag. 15. Pag. 08. Pag. 16. Pag. 09. Pag.
 PROSTHESIS MANUAL INDEX PRESENTATION Pag. 05 CEMENT-SCREWED PROSTHESIS Pag. 12 INTRODUCTION PROSTHESIS MANUAL WARNINGS Pag. 06 Pag. 06 IMPRESSION TAKING: NECESSARY MATERIAL PROSTHESIS: NECESSARY MATERIAL
PROSTHESIS MANUAL INDEX PRESENTATION Pag. 05 CEMENT-SCREWED PROSTHESIS Pag. 12 INTRODUCTION PROSTHESIS MANUAL WARNINGS Pag. 06 Pag. 06 IMPRESSION TAKING: NECESSARY MATERIAL PROSTHESIS: NECESSARY MATERIAL
Gewässer Umwelt Schutz
 Gewässer Umwelt Schutz GmbH CUW Oil protector Assembly instructions 2 Foreword Always study this assembly instruction manual before Installation and initial operation. This assembly instruction manual
Gewässer Umwelt Schutz GmbH CUW Oil protector Assembly instructions 2 Foreword Always study this assembly instruction manual before Installation and initial operation. This assembly instruction manual
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Zavation Z-Link Cervical
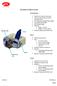 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
