Developing the next generation of dermatology products to treat serious skin diseases
|
|
|
- Sheena Hoover
- 5 years ago
- Views:
Transcription
1 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer
2 Forward Looking Statements This presentation contains "forward-looking statements" as defined under the U.S. federal securities laws including, but not limited to, statements regarding Peplin s future clinical development program, the timing of FDA filings and its target market. Actual events could differ materially from those anticipated in the forward-looking statements as a result of certain factors, including but not limited to: the results and duration of clinical trials, the ability to retain and attract key employees, and other events and important factors disclosed in Peplin s filings with the U.S. Securities and Exchange Commission and its disclosures to the ASX. Peplin disclaims any obligation to update any such forward-looking statements after the date of this presentation. 2
3 Peplin Highlights Breakthrough treatment for actinic keratosis and skin cancers Novel mechanisms of action Short duration of therapy Enhanced cosmetic outcome Compelling and consistent clinical data First Phase 3 study completed. Two additional Phase 3 studies enrolling. March 31, 2008: $26.1m in cash Sufficient to complete Phase 3 trials Global product rights 3
4 Investment Thesis Large market for actinic keratosis and other skin diseases High level of patient dissatisfaction with current therapies Long treatment durations Poor patient compliance Local side effects during extended treatment Novel, Phase 3 product with compelling, consistent clinical trial results Strong intellectual property Strong financial position Significant value building milestones over next 2 quarters 4
5 Euphorbia Peplus Common plant European origin Early 1800 s sap used topically to treat dermatological conditions warts, corns, waxy growths, skin cancers Effective home remedy to treat skin cancers (1) 1) Australasian Journal of Dermatology,
6 Peplin History Peplin founded to explore the folklore observation of anticancer activity of the sap of Euphorbia peplus Documented the sap s anti-cancer activity in human skin cancers Selected PEP005 as lead candidate for its Pre-clinical pharmacology Toxicology Purification and formulation technology INDs filed for AK and BCC Completed Phase 3 trial Company headquarters moved from Australia to US Pursued activityguided fractionation to identify key active ingredient 6
7 PEP005 (ingenol mebutate) MOA Dual mode of action Rapid removal of tumor (necrosis) and prevention of the tumor relapse (immune response) 1. Local necrosis (1) Disruption of mitochondrial membrane Mitochondrial breakdown Cellular cytotoxicity (necrosis) 2. Activation of immune system (2) Activation of adhesion molecules (e.g. E-selectin) (3) Recruitment of neutrophils (e.g. IL-8) Production of tumor-specific antibodies 1) Cancer Research 64: , ) J Immunol. 177: , ) Cancer Immunol. Immunother. 57: ,
8 PEP005 (ingenol mebutate) Gel Pipeline Indication Pre-clinical Phase 1 Phase 2 Phase 3 Actinic Keratosis Non-head treatment sites Head treatment sites Basal Cell Carcinoma Squamous cell carcinoma Cutaneous warts Other cancers 8
9 Actinic Keratosis (AK) 1) Dermatol Surg 2007;33: ) Lewin Group, The Burden of Skin Diseases ) US office visits for AK, NAMCS database (Avg. for ) 4) Journal of Dermatological Treatment,2006; 17: Caused by accumulated sun damage Can unpredictably transition to Squamous Cell Carcinoma (SCC) 67% of cases had prior AK diagnosis (4) 1,300 to 2,300 US deaths per year (1) Expensive and difficult treatment Affects more than 58 million Americans (2), increasing with age 5.6 million office visits annually (3) 8.2 million office visit estimates have been published (2) $1.2 billion annual direct cost in US (2) 25% of all treatments involve topical therapies (4) 9
10 Market Opportunity Actinic keratosis is one of the most frequently diagnosed skin disease by US dermatologists Topical treatment in the US Cryotherapy 75% Topical 16% Cryo plus topical 9% Topical treatments during office visits Total office visits (1) Proportion treated Treatment visits Topical treatments (2) Annual topical treatments 5.6 M 90% 5.0 M 25% 1.26 M 1) US office visits for AK, NAMCS database (Aug. for ) 2) Journal of Dermatological Treatment, 2006; 17:
11 In-office Procedures Approach Cryotherapy (liquid nitrogen) Levulan Kerastick (2) (aminolevulinic acid HCl) Major benefits Quick and inexpensive Historically attractive reimbursement Well established modality Single topical application Major short-comings Only discrete lesions Short term localized pain and irritation Potential long term scarring 1 year recurrence rate of 72% (1) Declining reimbursement (3) Irradiation 14 to 18 hours later Burning and stinging Requirement for dedicated equipment Low reimbursement 1) British Journal of Dermatology 2007; 157 (Suppl. 2): ) Product Full Prescribing Information 3) 2008 CMS Medicare Fee Schedule 11
12 Topical Treatments (1) Long durations of treatment Product Course Efficacy: Complete clearance AWP Cost /Tx (3) Side effects Aldara (imiquimod 5%) 2x/wk for 16 wks 45% $793 Erythema, flaking/ scaling/dryness, scabbing/crusting Carac (fluorouracil cream 0.5%) 1x/d for 4 wks 48% $150 Erythema, dryness, burning, erosion, pain Efudex (Fluorouracil cream 5%) Generic 5% 5-FU 2x/day for 4 wks ~50% (2) $745 $494 Burning, crusting, contact dermatitis, erosion, erythema Solaraze (diclofenac sodium 3%) 2x/day for days 14-47% $421 Contact dermatitis, rash, dry skin/ scaling 1) Product Full Prescribing Information 2) Supplement to The Journal of Family Practice, May ) Redbook, March
13 The Peplin Solution PEP005 (ingenol mebutate) Gel for AK: Product Profile Description Patient applied topical gel Course of therapy Once-a-day for two or three consecutive days Packaging Two or three single use mini-tubes Side effect profile Localized erythema, flaking or scaling, crusting, vesicles and swelling. Peaks in 3-8 days, resolves in 2-4 weeks Treatment area Concentration Non-head (2 day) 0.05% (PEP005 Gel) Head (3 day) 0.015% (PEP005 Gel) 13
14 PEP005 (ingenol mebutate) Gel for AK Strong Clinical Data IND filed June 2004 Advancement in AK trials ( ) Single dose Phase 1 (lesion-directed) Two dose Phase 2a (lesion-directed) Two dose Phase 2b (field-directed therapy-body) 2 and 3 day Phase 2a/b (field-directed-body) 2 and 3 day Phase 2a/b (field-directed-face) 2 day Phase 3 (field-directed body) Exposure to PEP005: >950 subjects Four key clinical trials PEP , REGION-Ia: treatment sites on body and scalp PEP & -015: treatment sites on face and face/scalp 14
15 AK Phase 2 (PEP ): Study Design Treatment location Status Number of patients Design Endpoints Body and scalp Patients with 4-8 AK lesions in a 25 cm 2 treatment area on the arm, shoulder, chest, back or scalp Complete 222 Multi-center, double blind, double dummy, randomized, vehicle-controlled Four arms: 3 active + 1 vehicle Concentration Vehicle 0.025% 0.05% 0.05% Group N/A D1,2,3 (Low) DV,2,3 (Medium vehicle D1) D1,2,3 (High) Evaluating safety and efficacy Pt Number
16 AK Phase 2 (PEP ): Efficacy Results AK clearance rates - Intent to Treat 80% 60% 40% 20% 0% Vehicle Low Medium High Complete clearance 75% clearance Medium strength data 0.05% (Two days) Vehicle P-value N=55 N=60 Complete clearance rate 44% 12% Partial clearance rate 62% 22% <
17 AK Phase 2 (PEP ): Safety Time course of local skin response 17
18 AK Phase 3: Study Design Treatment location Status Number of patients Design Endpoints Non-Head (trunk and extremities) Patients with 4-8 AK lesions in a 25 cm 2 treatment area Complete (May 2009) 250 total patients Randomized, double-blind, vehicle-controlled conducted at multiple sites Special Protocol Assessment (SPA) 2 days of treatment Concentration = 0.05% Primary: Complete clearance rate Secondary: Partial clearance rate (clearance of majority of AK lesions within the area) 18
19 REGION-I Efficacy Results REGION-I Clearance Results 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Vehicle PEP005 Overall Arm Back Back of Hand Chest Complete Clearance Partial Clearance Median % Reduction 19
20 REGION-I Safety Results 6 5 REGION-I: Local Skin Responses PEP005 Gel Vehicle 4 LSRs Days 20
21 REGION-I vs. PEP Complete clearance rate (arms/shoulders/chest/back) 014 Active 31% (27/86) vs Vehicle 6% (5/85) 006 Active 45% (19/42) vs Vehicle 14% (6/43) Partial clearance rate (arms/shoulders/chest/back) 014 Active 52% (27/86) vs Vehicle 9% (5/85) 006 Active 67% (28/42) vs Vehicle 21% (9/43) Of interest is the much higher vehicle responses seen in the 006 study vs the 014 study 21
22 AK Phase 2b (PEP ): Study Design Treatment location Status No. of patients Design Head (face and scalp) Field-treatment Complete, January Randomized, double-blind, vehicle-controlled, multiple sites 6 active arms: 0.005%, 0.010% or 0.015% at 2 or 3 days of treatment vs. vehicle at 2 or 3 days Enpoints Concentration Group ITT (1) Pt No. Group Vehicle 2 day Rx 33 3 day Rx 0.005% 2 day Rx 33 3 day Rx 0.01% 2 day Rx 34 3 day Rx 0.015% 2 day Rx 33 3 day Rx Primary: Complete clearance rate ITT (1) Pt No Secondary: Partial clearance rate (clearance of majority of AK lesions within the area) 1) ITT = Intent to Treat 22
23 PEP Efficacy Results (2-Day) Day Clearance Rates - Intent to Treat 0.0 Vehicle Low Medium High Complete clearance 75% clearance High strength data (0.015% x 2 days) Active N=33 Vehicle N=33 P-value Complete clearance rate 36.4% 0.0% <0.001 Partial clearance rate 51.5% 3.0% <0.001 Median % reduction* 75% 0.0% n/a *Calculated within per protocol population, not ITT 23
24 PEP Efficacy Results (3-Day) Day Clearance Rates - Intent to Treat 0.0 Vehicle Low Medium High Complete clearance 75% clearance High strength data (0.015% x 3 days) Active N=32 Vehicle N=33 P-value Complete clearance rate 50.0% 9.0% <0.001 Partial clearance rate 71.9% 12.1% <0.001 Median % reduction* 84.5% 0.0% n/a *Calculated within per protocol population, not ITT 24
25 AK Phase 2b Study (PEP ): Safety Composite Local Skin Response Mean Composite LSR D Vehicle 2D 0.005% 2D 0.010% 2D 0.015% 3D Vehicle 3D 0.005% 3D 0.010% 3D 0.015% Day 25
26 Non-Melanoma Skin Cancer Most prevalent cancer worldwide Basal cell carcinoma (BCC): 80% Squamous cell carcinoma (SCC): 16% 1.2 million cases in US in 2004 (1) US$1.4 billion in 2004 (1) Incidence: 3-8% worldwide increase annually since 1964 (2) Gold standard is surgical excision 1) Lewin Group The Burden of Skin Diseases ) J Am Acad Dermatol 2007; 56:
27 PEP005 (ingenol mebutate) Gel for sbcc Tumor directed application 1 or 2 treatments Higher concentration than AK treatment Benefits over surgery Reduced pain, scarring Improved cosmesis No sutures, infections Proof of concept in PEP study 70% histology confirmed lesion clearance at 12 weeks (p=0.02) with 2 day treatment of 0.05% PEP005 27
28 sbcc Phase 2 (PEP ): Study Design Up to 110 patients in the US Open label, maximally tolerated dose study 2 dosing regimens: q.d. for one day or q.d. for two days, a week apart Ten concentrations from 0.025% to 0.25% PEP005 (ingenol mebutate) Gel Physician applied spot-therapy to 4 to 15 mm sbcc lesion on trunk Punch biopsy at screening for histologic diagnosis Full excision of lesion at 3 months post-treatment Study is ongoing 28
29 Milestones Initiate Non-head AK Phase 3 (REGION-Ia) Results Head AK Phase 2b End of Phase 2 meeting with the FDA Results Non-head AK Phase 3 (REGION-Ia) Initiation of Head AK Phase 3 program Initiation of Non-head AK Phase 3 (REGION-Ib) Completion of AK development program File New Drug Application Sept 2008 Jan Q Q Q Q 2009 YE 2009 Mid
30 Clear Route to Market Highly targeted sales approach Small specialty sales force can cover key customers 10,500 practicing dermatologists in the US 6,000 treat 90% of all the AK cases Effectively covered with sales force of reps Reimbursement is established Existing reimbursement scheme for AK treatments Payers receptive, anxious to get their money s worth Operating PEP005 manufacturing plant GMP manufacturing plant established in Queensland 30
31 Investment Thesis Large market for actinic keratosis and other skin diseases High level of patient dissatisfaction with current therapies Long treatment durations Poor patient compliance Local side effects during extended treatment Novel, Phase 3 product with compelling, consistent clinical trial results Strong intellectual property Strong financial position Significant value-building milestones over next 2 quarters 31
32 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer
Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses
 Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Diagnosis and Management of Actinic Keratosis (AKs)
 Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Company Overview. Dr. Neal Walker President and CEO. September Copyright 2016 Aclaris Therapeutics. All rights reserved.
 Company Overview Dr. Neal Walker President and CEO Copyright 2016 Aclaris Therapeutics. All rights reserved. A-11 Disclaimer This presentation contains forward-looking statements, including statements
Company Overview Dr. Neal Walker President and CEO Copyright 2016 Aclaris Therapeutics. All rights reserved. A-11 Disclaimer This presentation contains forward-looking statements, including statements
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Ingenol Mebutate: A Succinct Review of a Succinct Therapy
 Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
Richard Turner Consultant Dermatologist
 Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 26 November 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
PICATO (ingenol mebutate) gel
 PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
Opinion 26 June 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
Aldara. Aldara (imiquimod) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Clinical Trial Report Synopsis. Efficacy and Safety of LEO in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up
 Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Innovation In Ophthalmics
 Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
AWMSG SECRETARIAT ASSESSMENT REPORT. Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel. Reference number: 1392 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
The future of Skin Cancer Treatment
 The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
METVIX PDT ON THE MARKET IN GERMANY AND UK
 METVIX PDT ON THE MARKET IN GERMANY AND UK PhotoCure ASA First Quarter Report 2003 Highlights: The launches of Metvix PDT by Galderma in February in Germany and in May in the UK, have triggered milestone
METVIX PDT ON THE MARKET IN GERMANY AND UK PhotoCure ASA First Quarter Report 2003 Highlights: The launches of Metvix PDT by Galderma in February in Germany and in May in the UK, have triggered milestone
Clinical Study Report Synopsis
 This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
TOPICAL TREATMENT OF ACTINIC KERATOSIS
 TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel: Report of Two Cases
 Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee
 I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
Advancement in a natural, topical, anti-aging therapy for Non-Melanoma skin cancers.
 Advancement in a natural, topical, anti-aging therapy for Non-Melanoma skin cancers. Simon Agius September 10th, 2010 Citing research by Dr. Bill Elliot Cham as published: Solasodine Rhamnosyl Glycosides
Advancement in a natural, topical, anti-aging therapy for Non-Melanoma skin cancers. Simon Agius September 10th, 2010 Citing research by Dr. Bill Elliot Cham as published: Solasodine Rhamnosyl Glycosides
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS
 BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
PhotoCure ASA Presentation First quarter 2005 May 3, 2005
 PhotoCure ASA Presentation First quarter 2005 May 3, 2005 Obtaining milestones and building value Hexvix Approval in 27 EU/EEA countries, working on national approvals Ongoing phase III study for extended
PhotoCure ASA Presentation First quarter 2005 May 3, 2005 Obtaining milestones and building value Hexvix Approval in 27 EU/EEA countries, working on national approvals Ongoing phase III study for extended
Corporate Overview. February 2018 NASDAQ: CYTR
 Corporate Overview February 2018 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES. ACTUAL RESULTS COULD DIFFER
Corporate Overview February 2018 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES. ACTUAL RESULTS COULD DIFFER
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD
 A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest risk.
 Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
PhotoCure ASA. Presentation. Results 1 Quarter 2004
 PhotoCure ASA Presentation Results 1 Quarter 2004 7 th of May 2004 Progress towards sustained profitability Continued product roll out Metvix European sales continue to grow Final US approval for Actinic
PhotoCure ASA Presentation Results 1 Quarter 2004 7 th of May 2004 Progress towards sustained profitability Continued product roll out Metvix European sales continue to grow Final US approval for Actinic
Supernus Pharmaceuticals
 Supernus Pharmaceuticals Investor Presentation March 2017 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking
Supernus Pharmaceuticals Investor Presentation March 2017 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking
Corporate Medical Policy
 Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A.
 fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
July, ArQule, Inc.
 July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
Photocure ASA Executing the Strategy
 Photocure ASA Executing the Strategy NOVEMBER 2012 ERIK DAHL, CFO KATHLEEN DEARDORFF, COO Disclaimer The information included in this Presentation contains certain forward-looking statements that address
Photocure ASA Executing the Strategy NOVEMBER 2012 ERIK DAHL, CFO KATHLEEN DEARDORFF, COO Disclaimer The information included in this Presentation contains certain forward-looking statements that address
Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA
 Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA A Practical Approach: Field Treatment of AKs with PDT Why it s essential to treat visible and invisible
Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA A Practical Approach: Field Treatment of AKs with PDT Why it s essential to treat visible and invisible
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST
 Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
AM-125 : Intranasal Betahistine
 AM-125 : Intranasal Betahistine February 3, 2017 NASDAQ: EARS Forward-looking Statements This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
AM-125 : Intranasal Betahistine February 3, 2017 NASDAQ: EARS Forward-looking Statements This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
New Medicines Committee Briefing May 2015
 New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
Photocure ASA Executing the Strategy
 Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
Skin lesions The Good and the Bad. Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry
 Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference. February 7, 2007
 Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
NASDAQ: CYTR FIGHTING CANCER WITH CUTTING EDGE SCIENCE. Corporate Overview. July 2018
 NASDAQ: CYTR Corporate Overview July 2018 CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES. ACTUAL RESULTS COULD DIFFER MATERIALLY
NASDAQ: CYTR Corporate Overview July 2018 CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES. ACTUAL RESULTS COULD DIFFER MATERIALLY
Dynavax Corporate Presentation
 Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects *
 Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
Extreme dermatoheliosis: How to approach the severely sun damaged patient
 Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
COMPANY OVERVIEW. Dr. Neal Walker President and CEO. January Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1
 COMPANY OVERVIEW Dr. Neal Walker President and CEO January 2017 Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1 Disclaimer This presentation contains forward-looking statements, including
COMPANY OVERVIEW Dr. Neal Walker President and CEO January 2017 Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 1 Disclaimer This presentation contains forward-looking statements, including
Corporate Overview. July 2016 NASDAQ: CYTR
 Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
Corporate Presentation August 6, 2015
 Corporate Presentation August 6, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events
Corporate Presentation August 6, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events
Supernus Pharmaceuticals
 Supernus Pharmaceuticals Jefferies 2016 Healthcare Conference May 2016 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include
Supernus Pharmaceuticals Jefferies 2016 Healthcare Conference May 2016 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include
Diagnostics for the early detection and prevention of colon cancer. Fourth-Quarter 2014 Earnings Call February 24, 2015
 Diagnostics for the early detection and prevention of colon cancer Fourth-Quarter 2014 Earnings Call February 24, 2015 Safe Harbor Statement Certain statements made in this presentation contain forward-looking
Diagnostics for the early detection and prevention of colon cancer Fourth-Quarter 2014 Earnings Call February 24, 2015 Safe Harbor Statement Certain statements made in this presentation contain forward-looking
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
Experts in photodynamic therapy Investor Presentation I September 2018
 Experts in photodynamic therapy nvestor Presentation Page 1 Disclaimer This presentation contains forward-looking statements including, without limitation, statements containing the words expects, future,
Experts in photodynamic therapy nvestor Presentation Page 1 Disclaimer This presentation contains forward-looking statements including, without limitation, statements containing the words expects, future,
35 th Annual J.P. Morgan Healthcare Conference
 35 th Annual J.P. Morgan Healthcare Conference Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts, regarding management's
35 th Annual J.P. Morgan Healthcare Conference Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts, regarding management's
Q3 18 Earnings Supplemental Slides
 (Nasdaq: INSY) Q3 18 Earnings Supplemental Slides November 5, 2018 Safe-Harbor Statement This presentation contains both historical information and forward-looking statements. Forward-looking statements
(Nasdaq: INSY) Q3 18 Earnings Supplemental Slides November 5, 2018 Safe-Harbor Statement This presentation contains both historical information and forward-looking statements. Forward-looking statements
Forward-looking Statement Disclaimer
 Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
N A S D A Q : E V F M
 N A S D A Q : E V F M Jefferies Global Healthcare Conference June 7, 2018 Disclaimer This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform
N A S D A Q : E V F M Jefferies Global Healthcare Conference June 7, 2018 Disclaimer This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
The no t-so-simple cutaneous wart
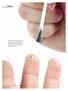 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Titan Pharmaceuticals Overview
 Corporate Presentation July 2014 1 Safe Harbor The presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities
Corporate Presentation July 2014 1 Safe Harbor The presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities
Skin Cancer in Organ Transplant Recipients Challenges and Opportunities
 Disclosure Skin Cancer in Organ Transplant Recipients Challenges and Opportunities Investigator: DUSA Pharmaceuticals, Inc. Investigator: Genentech Consultant: Gerson Lehrman Group Sarah Tuttleton Arron,
Disclosure Skin Cancer in Organ Transplant Recipients Challenges and Opportunities Investigator: DUSA Pharmaceuticals, Inc. Investigator: Genentech Consultant: Gerson Lehrman Group Sarah Tuttleton Arron,
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
34 th Annual J.P. Morgan Healthcare Conference
 JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
Novan Announces Promising Clinical Results with SB414
 Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Keratolytics and Other Topical Dermatological Agents
 DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
Corporate Presentation Asia Investment Series March 2018
 Corporate Presentation Asia Investment Series March 2018 Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward-looking" statements within the meaning of the United
Corporate Presentation Asia Investment Series March 2018 Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward-looking" statements within the meaning of the United
TELECONFERENCE FY February 2015
 TELECONFERENCE FY 2014 5 February 2015 Company disclaimer This presentation contains forward-looking statements that provide our expectations or forecasts of future events such as new product introductions,
TELECONFERENCE FY 2014 5 February 2015 Company disclaimer This presentation contains forward-looking statements that provide our expectations or forecasts of future events such as new product introductions,
UBS Global Healthcare Conference May 19, 2014
 UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
ADAPTIMMUNE INVESTOR PRESENTATION. August 2016
 ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC. August 6, 2015
 Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
37 th Annual J.P. Morgan Healthcare Conference. January 9, 2019
 37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
MARINE Study Results
 TM MARINE Study Results Nasdaq: AMRN www.amarincorp.com 1 Forward-Looking Statement This presentation contains forward-looking statements, including those relating to the Company s product development
TM MARINE Study Results Nasdaq: AMRN www.amarincorp.com 1 Forward-Looking Statement This presentation contains forward-looking statements, including those relating to the Company s product development
Investor Presentation December 2012
 Investor Presentation December 2012 1 Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forwardlooking
Investor Presentation December 2012 1 Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forwardlooking
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Targeting and Treating Cancer
 Targeting and Treating Cancer Mark R. Baker, Chief Executive Officer Jefferies Healthcare Conference June 2015 Disclosure Notice This presentation may contain projections and other forward-looking statements
Targeting and Treating Cancer Mark R. Baker, Chief Executive Officer Jefferies Healthcare Conference June 2015 Disclosure Notice This presentation may contain projections and other forward-looking statements
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
F066: Photodynamic Therapy in Medical and Aesthetic Dermatology
 F066: Photodynamic Therapy in Medical and Aesthetic Dermatology Improving Efficacy and Maintaining Safety of ALA-PDT American Academy of Dermatology 77 th Annual Meeting Washington, DC Maria M. Tsoukas,
F066: Photodynamic Therapy in Medical and Aesthetic Dermatology Improving Efficacy and Maintaining Safety of ALA-PDT American Academy of Dermatology 77 th Annual Meeting Washington, DC Maria M. Tsoukas,
Acorda Acquisition of Civitas Therapeutics. September 24, 2014
 Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva )
 Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Have a Voice in Your Choice!
 Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update. August 2, 2018
 Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Capricor Therapeutics
 Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
LEERINK GLOBAL HEALTHCARE CONFERENCE. Marino Garcia EVP, Chief Strategy Officer February 15, 2017
 LEERINK GLOBAL HEALTHCARE CONFERENCE Marino Garcia EVP, Chief Strategy Officer February 15, 2017 SAFE HARBOR STATEMENT This presentation and any statements made for and during any presentation or meeting
LEERINK GLOBAL HEALTHCARE CONFERENCE Marino Garcia EVP, Chief Strategy Officer February 15, 2017 SAFE HARBOR STATEMENT This presentation and any statements made for and during any presentation or meeting
MANIFEST Phase 2 Enhancement / Expansion
 MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program
 Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results
 Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
DERMATOLOGIC APPLICATIONS OF PHOTODYNAMIC THERAPY
 DERMATOLOGIC APPLICATIONS OF PHOTODYNAMIC THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
DERMATOLOGIC APPLICATIONS OF PHOTODYNAMIC THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
HIGH CLINICAL ACCEPTANCE OF METVIX PDT
 HIGH CLINICAL ACCEPTANCE OF METVIX PDT PhotoCure ASA Second Quarter Report 2002 Highlights: Marketing of Metvix PDT in Europe Progressing as Planned Positive Phase III Clinical Results in the Treatment
HIGH CLINICAL ACCEPTANCE OF METVIX PDT PhotoCure ASA Second Quarter Report 2002 Highlights: Marketing of Metvix PDT in Europe Progressing as Planned Positive Phase III Clinical Results in the Treatment
Creating a Leading Global HBV Therapeutics Company. ARB-1467 Update Call December 12, 2016
 Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Dicerna Pharmaceuticals Overview. Delivering RNAi-Based Breakthrough Therapies
 Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
Jefferies 2016 Healthcare Conference. Reid Huber, PhD Chief Scientific Officer
 Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Building a Premier Oncology Biotech
 Building a Premier Oncology Biotech August 208 Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within
Building a Premier Oncology Biotech August 208 Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within
AXIM Biotechnologies Reports Year End 2017 Results
 March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
