New Medicines Committee Briefing May 2015
|
|
|
- Loraine Emmeline Bailey
- 5 years ago
- Views:
Transcription
1 New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed for use within: Summary: Primary Care Secondary Care Picato is a macrocyclic diterpene ester licenced for the cutaneous treatment of nonhypertrophic AK in adults. Picato was launched in the UK in January 2013 and available in 2 strengths, 150 microgram/gram (for face/scalp) and 500 microgram/gram (for trunk/extremities). NICE Evidence summary reviewed Picato and stating that local decision makers will need to consider its place alongside other available treatments for AK as there are no direct head-tohead studies with other treatments. SMC and RDTC have recommended use of Picato for treatment of non-hyperkeratotic, nonhypertrophic AK. MTRAC recommended that Picato is suitable for prescribing in primary care where there is a confident diagnosis of AK. Primary Care Dermatology Society (PCDS) guideline on AK recommends ingenol for the treatment of Grade I, Grade II and Field change AK. 4 phase III double-blind, randomised, vehicle-gel controlled trials of patients with AK associated ingenol with significantly higher rates of complete lesion clearance in the treatment area, compared with vehicle gel. Picato is a black triangle drug ( ) and is monitored intensively by the CHM and MHRA. 1
2 Formulary application: Dermatology department: Consultant submitting application: Dr Nicholas Craven (Consultant Dermatologist) Clinical Director supporting application: Dr Craven has requested for Picato to be considered for inclusion in the North Staffordshire Joint Formulary for the treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis in adults. Dr Craven estimates that 20 patients will be treated with Picato for 2-3 days treatment at a cost of 65 per patient. He in his application that the cost will be balanced by the savings made in using Picato as an alternative to Solaraze gel, Efudix cream, imiquimod or cryotherapy in patients for whom these other options would be less appropriate. He noted that the advantage of Picato over the other treatment options on the formulary included: fewer applications and shorter treatment course; shorter time for LSRs to resolve from start of treatment; cost savings; less application site pain; greater efficacy on trunk/extremities; two dose strengths tailored to treatment site; exact dosing with single-use tubes; absence of photo-effects; absence of measurable systemic absorption and acceptance/recommendation for use without restriction by the SMC and AWMSG. There are no direct comparison trials between ingenol mebutate gel and any of these three products. Background: AK is a common sun-induced scaly or hyperkeratotic lesion, which has the potential to become malignant. NICE estimates that over 23% of the UK population aged over 60 years have AK. Although the risk of AK transforming into a squamous cell carcinoma (SCC) is very low, the risk increases over time and with larger numbers of lesions. The presence of ten AK is associated with a 14% risk of developing an SCC within five years. 1 AK are a consequence of cumulative long-term sun-exposure, they are uncommon in patients <45 years with the incidence increasing with age. AK lesions are normally asymptomatic and commonly affect the face (especially nose and forehead), forearms, backs of the hands, rims of the ears, bald scalps and below the knees. If lesions show recent growth, pain/ tenderness, bleeding or ulceration are suggestive of transformation into an SCC. 1 Some studies also indicate a high spontaneous regression rate in the order of 15-25% for AKs over a 1 year period and a low rate of malignant transformation of less than one in 1000 per annum. 2,3 Its main treatment options are: 1,2,3 Close observation: o Emollients and sun screens 2
3 Cryotherapy Surgical excision Topical agents o Efudix, Actikerall, Aldara / Zyclara, Picato, Solaraze Photodynamic therapy Imiquimod 5%, 5-fluorouracil and diclofenac sodium 3% gel are well established therapies although they do have a number of commonly reported adverse effects such as contact dermatitis, erythema and application site reactions including pain, swelling, inflammation and pruritus. Newer treatment options include Actikerall and imiquimod 3.75% Ingenol mebutate is a new topical agent whose novel mode of action is not fully understood but is believed to involve a dual effect on AK lesions: induction of local lesion cell death and a proinflammatory response characterised by infiltration of immune-competent cells. Two strengths are licensed for the treatment of AK: 150 microgram/gram for treating lesions of the face or scalp, and 500 microgram/gram for treating the trunk or extremities. The treatment course is once daily application for 2 consecutive days for the trunk or extremities, and once daily for 3 consecutive days for the face or scalp. Current formulary status: Sunscreen preparations Spectraban (UVB) RoC Sante Soleil (UVB) Uvistat (UVB) Prescriptions in primary care must be endorsed ACBS Prescriptions in primary care must be endorsed ACBS Prescriptions in primary care must be endorsed ACBS Photodamage Solaraze (diclofenac sodium 3% in sodium hyaluronate gel) Efudix (fluorouracil 5% cream) Aldara (imiquimod 5% cream) Aldara (imiquimod 5% cream) st line for superficial basal cell carcinoma Restriction: Initiation by a consultant dermatologist or GP with a special interest 2 nd line for superficial basal cell carcinoma Restriction: Initiation by a consultant dermatologist or GP with a special interest For Bowen s Disease [unlicensed indication] Restriction: To be initiated by a dermatology consultant 3
4 Actikerall (5-fluorouracil 0.5% and salicylic acid 10% cutaneous solution) Metvix (methyl-5-aminolevulinate cream) Therapeutic class and mode of action: 4 The mechanism of action of ingenol mebutate for use in actinic keratosis is not clearly understood. In vivo and in vitro models have shown a dual mechanism of action for the effects of ingenol mebutate: 1) Induction of local lesion cell death 2) Promoting an inflammatory response characterised by local production of proinflammatory cytokines and chemokines and infiltration of immunocompetent cells. Licensed indication: Picato is indicated for the cutaneous treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis in adults. Dosage and administration: The contents of one tube should be applied to the affected areas once daily for 2 days Safety and adverse effects: Contraindications: Hypersensitivity to the active substance or to any of the excipients listed Caution: Eye exposure: Contact with the eyes should be avoided. If accidental exposure occurs, the eyes should be flushed immediately with large amounts of water, and the patient should seek medical care as soon as possible. 4
5 Ingestion: Picato must not be ingested. If accidental ingestion occurs the patient should drink plenty of water and seek medical care. General: Administration of Picato is not recommended until the skin is healed for treatment with any previous medical product or surgical treatment and should not be applied to open wounds or damaged skin where the skin barrier is compromised. It should not be used near the eyes, on the side of the nostrils, on the inside of the ears or on the lips. Local skin responses: Local skin responses such as erythema, flaking/scaling, and crusting should be expected to occur after cutaneous application. Localised skin responses are transient and typically occur within 1 day of treatment initiation and peak in intensity up to 1 week following application. Localised skin response typically resolves within 2 weeks of treatment initiation when treating areas on the face and scalp and within 4 weeks of treatment initiation when treating areas of the trunk and extremities. Treatment effect may not be adequately assessed until resolution of local skin responses. Sun exposure Studies have been conducted to assess the effects of UV irradiation of the skin following single and multiple applications. It did not demonstrate any potential for photo irritation or phot allergic effects. However due to the nature of the disease excessive exposure to sunlight including lamps and tanning beds should be avoided or minimised. Management of actinic keratosis: Lesions clinically atypical for actinic keratosis or suspicious for malignancy should be biopsied to determine appropriate treatment. Pregnancy There is no data from the use of ingenol mebutate in pregnant women. The risk to humans receiving cutaneous treatment is considered unlikely as it is not absorbed systemically. Animal studies showed slight embryo-foetal toxicity. As a precaution it is preferable to avid its use during pregnancy. Breast-feeding No effects on the breastfed new-born/ infant are anticipated as it is not absorbed systemically. 5
6 Fertility No fertility studies have been performed. Undesirable effects: The most frequently reported adverse reactions are local skin responses including erythema, flaking/scaling, crusting, swelling, vesiculation, postulation and erosion/ulceration at the application site. Following application most patients >95% experienced one or more local skin responses. The incidence of local skin responses that occurred at an incidence >1% in both the 'face/scalp' and the 'trunk/extremities', respectively are: application site erythema (94% and 92%), application site exfoliation (85% and 90%), application site scab (80% and 74%), application site swelling (79% and 64%), application site vesicles (13% and 20%), application site pustules (43% and 23%) and application site erosion (31% and 25%). Severe local skin responses occurred with an incidence of 29% on the face and scalp and with an incidence of 17% on the trunk and extremities. The incidence of severe local skin responses that occurred at an incidence >1% in both the 'face/scalp' and the 'trunk/extremities', respectively are: application site erythema (24% and 15%), application site exfoliation (9% and 8%), application site scab (6% and 4%), application site swelling (5% and 3%) and application site pustules (5% and 1%). For additional information refer to the Summaries of Product Characteristics. Drug Interactions: The systemic pharmacokinetic profile of ingenol mebutate and its metabolites has not been characterised in humans due to the absence of quantifiable whole blood levels following cutaneous administration. No systemic absorption was detected at or above the lower limit of detection (0.1 ng/ml) when Picato 500 mcg/g from 4 tubes was applied to an area of 100 cm 2 on the dorsal forearm in actinic keratosis patients once daily for 2 consecutive days. In vitro study results demonstrate that ingenol mebutate does not inhibit or induce human cytochrome P450 isoforms. Presentation: Single-dose laminate tubes with inner layer of High Density Polyethylene (HDPE) and aluminum as the barrier layer. Caps of HDPE. Picato 500 mcg/g gel is available in a carton containing 2 tubes with 0.47 g of gel each. 6
7 Special precautions for storage: Picato is to be stored in a refrigerator (2 C-8 C). Tubes should be discarded after first opening. Guidance: National Institute for Health and care Excellence (NICE) NO Scottish Medicines Consortium (SMC): 5 YES The SMC has accepted the use of Picato in NHS Scotland for the treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis in adults. SMC indicated that the evidence for the use of ingenol mebutate in the treatment of AK is from four similarly designed randomised, double-blinded, vehicle-controlled, parallel-group phase III studies. Two of the studies investigated the efficacy and safety of ingenol mebutate when applied to lesions on the face or scalp and two when applied to the trunk or extremities. Observational extension studies gathered evidence of safety, durability of response and recurrence rate over one year of follow-up. There was significantly greater proportion of adults with AK achieving complete clearance when treated with ingenol mebutate gel compared with vehicle control in all the trials. All Wales Medicines Strategy Group (AWSG) 6 YES The AWSG recommended Picato as an option for use within NHS Wales for the cutaneous treatment of non-hyperkeratotic, non-hypertrophic AK in adults. Regional Drug and Therapeutic Centre (RDTC) 7 Yes RDTC that Picato appeared to be well tolerated due to the treatment course being only 2 or 3 days. Patients are more likely to complete treatment compared to longer and more complex treatment regimens. There was higher proportion of patients completing the trials compared with completion rates seen in trials for both imiquimod and Efudix. There was similar adverse effect with other treatments but shorter durations of treatment made Picato a preferred option as adverse effects resolved quicker. RDTC summarised trial data available for the newer treatments as shown in the table below: 7
8 Table 1 7 Trial design* Treatmen t area Patients with complete clearance (Primary endpoint) Confirm ed by histolog y Partial clearance rates (Secondary endpoint) Patients with no recurren ce at follow up Patients experiencin g local skin reactions Timeframe for adverse events Ingenol Mebutat e 150mcg/ g 9 57 day follow up Ingenol mebutat e vs. placebo n= 547 Face/scalp 42.2% vs 3.7% P< % vs. 7.4% (p<0.001) 46.1% General administrati on site condition 19% vs. 2.6% Peaked at day 4, declined to baseline by day 29 Ingenol Mebutat e 500mcg/ g 9 57 day follow up ingenol mebutat e vs. placebo Trunk/ Extremitie s 34.1% vs 4.7% P< % vs. 6.9% 44% General administrati on site condition Peaked at day 4, declined to baseline by day 29 n=479 (p<0.001) 12% vs. 2.6% 5-FU/SA 8 week follow up 5-FU/SA vs. diclofena c 3% Face/fore head or bald scalp 35.6% vs. 6.3% P<0.001 Complete clearance 55.4% vs. 22.6% (p<0.001) No follow-up >70% vs. 25% Most reported as clear by week 20 n=470 Imiquimo d 3.75% 8 week follow-up Imiquimo d Face or balding scalp 35.6% vs. 6.3% P< % vs. 22.6% (p<0.001) 40.5% 33.8% vs. 1.3% Baseline erythema score reached at week 10 8
9 MTRAC: 8 YES MTRAC recommended that Ingenol mebutate gel is suitable for restricted prescribing under defined conditions in primary care where there is a confident diagnosis of AK. MTRAC recommended that commissioners may wish to bear the following in mind when considering the commissioning of ingenol mebuatate gel: The treatment course with ingenol mebutate gel is shorter than for other topical treatments for AK, with localised skin responses peaking after completion of treatment. This may be an advantage in terms of patient convenience and compliance. The primary care dermatology society guidance on AK also notes the shorter recovery phase associated with this treatment. Patients with AK will frequently require re-treatment. At the present time, clinical data regarding repeated use of ingenol mebutate gel are not available. There is an absence of head-to-head studies comparing ingenol mebutate gel with other treatments. MTRAC also noted that although the treatment period is shorter than for other topical treatments, ingenol mebutate gel should have a lower place in the management of AK given the currently limited clinical experience and the availability of other more established treatments. British Association of Dermatologist (BAD): 2 NO The BAD guidelines for the management of AK pre-date ingenol mebutate gel. BAD are currently working on a new guideline for the management of AK. BAD recommend that no therapy or emollient is a reasonable option for mild AK lesions. Sun block, when applied twice daily for 7 months may prevent the development of AKs. Most patients when treatment is required can be managed in primary care. The guideline state that the data comparing individual treatments are not sufficient quality to justify making a single recommendation, especially since there are newer options available. Decision should be based on the clinical presentation, efficacy, morbidity, availability and cost of relevant treatments and patient preference. In addition to medical treatment all patients should be advised to regularly use a broad spectrum sunscreen (UVA and UVB) with an SPF of >15. 9
10 Primary Care Dermatology Society (PCDS): 1 YES The PCDS guideline on management of AK (syn. solar keratosis) (2014) provided the following advice regarding the use of ingenol mebutate gel for the treatment of areas of AK field change the size of a palm or most of the forehead: a new treatment similar outcomes to Aldara cream and Efudix cream, but in addition a very short treatment period and recovery phase when compared to the other topical treatments The guideline recommendations the use of Picato in the treatment of Grade I AK lesions, and a strong recommendation for its use to treat Grade II AK lesions. PCDS state that due to the very large number of patients who have AK it is important that the majority of patients should be managed in the community, and preferably by GPs otherwise consultant and GPwSI clinics will become overburdened, and patients with more serious skin problems will wait longer to be seen by a specialist. They recommend that patients with uncertainty of diagnosis or patients with more widespread/ severe AK should be referred to consultants or GPwSI. They recommend the following treatment steps for AK: 1. General measures- appropriate for all patients a. Education regarding AK and sun screens + emollients 2. Observation 3. Lesion specific treatment i.e. a few lesions or large numbers that are widely distributed (i.e. dotted around the face, scalp and hand etc) a. Cryotherapy, Efudix, Actikerall 4. Field change a. For smaller areas of field change (e.g. an area the size of a palm or most of the forehead) consider the following treatments: i. Aldara 5%, Efudix, Picato gel. b. For larger areas of field change consider the following treatments: i. Solaraze gel, Zyclara gel NICE Evidence Summary New Medicine (ESNM): 3 YES NICE in an evidence summary on ingenol reviewed 4 phase III, double-blind, randomised, placebo controlled trials of patients with AK. 458 patients were randomised in the 2 trunk/extremities studies while 547 patients were randomised in the 2 face/scalp studies. 10
11 Ingenol was associated with significant higher rates of complete lesion clearance compared to placebo. In a pooled analyses of these studies, ingenol mebutate gel was more effective than placebo for the primary outcome of complete clearance of all visible AK, in the target treatment area (42.2% - face/scalp and 34.1% - trunk/extremities) in comparison to placebo-gel (3.7% - face/scalp and 4.7% - trunk/extremities). The 4 studies that contributed to the pooled analysis also individually showed a statistically higher rate of complete clearance with ingenol mebutate gel, compared with placebo (p<0.001 in all studies). In both the pooled analyses and individual studies, ingenol mebutate gel was also associated with higher rates of partial clearance of lesions (p<0.01 in all cases) as well as greater median percentage reductions in lesion numbers. The most common treatment related adverse events were skin responses at the site of application. The simple dosing regimen of ingenol mebutate gel and the short duration of the treatment course compared with other self-applied, field-directed agents (with side effects peaking after treatment is completed) may be beneficial in terms of adherence to treatment. This is supported by the high study-completion rates (>98%) in the phase III studies. However, clinical data on the use of more than 1 course of ingenol mebutate gel for 2 or 3 consecutive days, or treatment of more than 1 area of 25cm 2, are not available. NICE recommended that local decision makers should consider ingenol together with other established treatments while bearing in mind that there are still no head-to-head studies of ingenol with other treatments as well as no long term data. Cochrane Review: 9 YES 83 RCT were included in the review, with 10,036 participants. The RCTs covered 18 topical treatments, 1 oral, 2 mechanical interventions and 3 chemical interventions, including photodynamic therapy (PDT). Most of the studies lacked descriptions of some methodological details, such as the generation of the randomisation sequence or allocation concealment, and half of the studies had a high risk of reporting bias. Study comparison was difficult because of the multiple parameters used to report efficacy and safety outcomes, as well as statistical limitations. No data was found on the possible reduction of squamous cell carcinoma. The primary outcome 'participant complete clearance' significantly favoured four fielddirected treatments compared to vehicle or placebo: 3% diclofenac in 2.5% hyaluronic acid (RR 2.46, 95% CI 1.66 to 3.66; 3 studies with 420 participants), 0.5% 5-fluorouracil (RR 8.86, 95% CI: 3.67 to 21.44; 3 studies with 522 participants), 5% imiquimod (RR 7.70, 95% CI 4.63 to 12.79; 9 studies with1871 participants), and 0.025% to 0.05% ingenol mebutate (RR 4.50, 95% CI 2.61 to 7.74; 2 studies with 456 participants). It also significantly favoured the treatment of individual lesions with photodynamic therapy (PDT) compared to placebo-pdt with the following photosensitisers: aminolevulinic acid (ALA) 11
12 (blue light: RR 6.22, 95% CI 2.88 to 13.43; 1 study with 243 participants, aminolevulinic acid (ALA) (red light: RR 5.94, 95% CI 3.35 to 10.54; 3 studies with 422 participants), and methyl aminolevulinate (MAL) (red light: RR 4.46, 95% CI 3.17 to 6.28; 5 studies with 482 participants). ALA-PDT was also significantly favoured compared to cryotherapy (RR 1.31, 95% CI 1.05 to 1.64). The corresponding comparative risks in terms of number of participants completely cleared per 1000 were as follows: 313 with 3% diclofenac compared to 127 with 2.5% hyaluronic acid; 136 with 0.5% 5-fluorouracil compared to 15 with placebo; 371 with 5% imiquimod compared to 48 with placebo; 331 with ingenol mebutate compared to 73 with vehicle; 527 to 656 with ALA/MAL-PDT treatment compared to 89 to 147 for placebo-pdt; and 580 with ALA-PDT compared to 443 with cryotherapy. 5% 5-fluorouracil efficacy was not compared to placebo, but it was comparable to 5% imiquimod (RR 1.85, 95% Cl 0.41 to 8.33). A significant number of participants withdrew because of adverse events with 144 participants affected out of 1000 taking 3% diclofenac in 2.5% hyaluronic acid, compared to 40 participants affected out of 1000 taking 2.5% hyaluronic acid alone, and 56 participants affected out of 1000 taking 5% imiquimod compared to 21 participants affected out of 1000 taking placebo. Based on investigator and participant evaluation, imiquimod treatment and photodynamic therapy resulted in better cosmetic outcomes than cryotherapy and 5-fluorouracil. For individual lesions, photodynamic therapy appears more effective and has a better cosmetic outcome than cryotherapy. For field-directed treatments, diclofenac, 5-fluorouracil, imiquimod, and ingenol mebutate had similar efficacy, but their associated adverse events and cosmetic outcomes are different. More direct comparisons between these treatments are needed to determine the best therapeutic approach. Efficacy: Ingenol mebutate gel for actinic keratosis 10 This is a multicentre, randomised, parallel-group, double-blind, vehicle-controlled study. Patients with AK were randomly assigned to receive ingenol mebutate gel or placebo for self-application on the face or scalp or on trunk or extremities. Inclusive criteria included patients aged 18 years, with four to eight clinically typical, visible and discrete AK of 25-cm 2 contiguous field on the face or scalp or on the trunk or extremities. Exclusive criteria included patients recently treated with other treatments for AK and the presence, within the target treatment area, of: hypertrophic and hyperkeratotic lesions, cutaneous horns, or lesions that had not responded to repeated cryosurgery. Lebwohl et al investigated the efficacy and safety of ingenol a new topical field therapy for AK for face and scalp (150 microgram/gram) and trunk and extremities (500 microgram/gram) treatment of the face or scalp was once daily application to the 25cm contiguous field for 3 consecutive days while the trunk or extremities was for 2 consecutive days 12
13 The primary end point was complete clearance of all clinically visible AK in the target treatment area on day 57. Secondary outcome was the partial clearance rate which was defined as a reduction of 75% or more in the number of clinically visible AK lesions in the target treatment area at day 57. Safety end points included skin response at the site of treatment application assessed by a local skin response scale. The scale graded 6 different responses on a scale of 0 to 4 with higher score indicating greater severity. Below is the result of the studies in a table: Table 2 Summary of pooled analyses of studies of the face/scalp and the trunk/extremities 3 : Pooled analysis of face/scalp studies Placebo (vehicle) gel Ingenol mebutate gel 150 micrograms/gram Efficacy (ITT analysis): n=270 n=277 Primary outcome: % patients with complete clearance of lesions % patients with partial clearance of actinic keratosis lesions Median % reduction in number of lesions(n) 3.7% (10/270) 42.2% (117/277) Secondary outcomes: 7.4% (20/270) 63.9% (177/277) 0% (range 100% to 100%) (n=269) 83% (range 50% to 100%) (n=273) Safety: n=271 n=274 Mean maximum composite local-skinresponse score (±SD) 1.8 (±1.6) 9.1 (±4.1) % patients reporting any adverse event 22.1% (60/271) 37.2% (102/274) % patients with adverse event(s) at application site % patients with adverse event(s) leading to early discontinuation 2.6% (7/271) 19.0% (52/274) 0.4% (1/271) 0.4% (1/274) Pooled analysis of trunk/extremities studies Placebo (vehicle) gel Ingenol mebutate gel 500 micrograms/gram Efficacy (ITT analysis): n=232 n=226 Primary outcome: % patients with complete clearance of lesions % patients with partial clearance of actinic keratosis lesions Median % reduction in number of lesions (n) 4.7% (11/232) h 34.1% (77/226) Secondary outcomes: 6.9% (16/232) h 49.1% (111/226) 0% (range 33% to 100%) (n=229) 75% (range 25% to 100%) (n=220) Safety: n=232 n=225 Analysis p<0.001 NNT=3 p<0.001 NNT=2 Analysis p<0.001 NNT=4 p<0.001 NNT=3 13
14 Mean maximum composite local skin response score (±SD) 1.6 (±1.5) 6.8 (±3.5) % patients reporting any adverse event 27.2% (63/232) 33.3% (75/225) % patients with adverse event(s) at application site % patients with adverse event(s) leading to early discontinuation 2.6% (6/232) 12.0% (27/225) 0.9% (2/232) 0.9% (2/225) A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe 11 A meta-analysis was completed in order to provide comparative efficacy of available treatment modalities for mild to moderate AK on the face or scalp. These treatments included two 5- aminolaevulinic acid photodynamic therapies (ALA-PDT), applied as gel (BF-200 ALA) or patch; methyl-aminolevulinate photodynamic therapy (MAL-PDT); three modalities with imiquimod (IMI), applied as a 4 week or 16 week course with 5% imiquimod, or a 2-3 week course with 3.75% imiquimod; cryotherapy; diclofenac 3% in 2.5% hyaluronic acid; 0.5% 5-fluorouracil (5-FU); and ingenol mebutate (IMB). The Cochrane review in March 2011 (which included 83 trials) was used in identifying studies included in the meta-analysis. According to Vegter and Tolley no further on-going new trials or studies were identified outside of this Cochrane review upon extensive literature search, up until Jan Two dermatology consultants then analysed these trials for inclusion within the metaanalysis. Trials were excluded, if there were any intra-individual designs, immunosuppressed patients, combination preparation usage and any trials studying dose variations of a single treatment. Trials were included which reported intention to treat data, where evaluation of efficacy was a minimum of one month after the end of treatment but no more than 1 year posttreatment, with study participants with mild to moderate AK on face or scalp defined as 5 and 20 lesions, and also an allowance of multiple treatment courses were included (with data regarding clearance rates after the second course only included). The primary endpoint of the meta-analysis was total clearance of all of a patient s lesions. For treatments that allowed for multiple treatment courses, the clearance rates after the second course were used in the meta-analysis. The authors that network meta-analysis provided a valid statistical alternative to direct head-to-head studies as well as the ability to rank treatments according to SUCRA scores. A total of 25 trials of 5,562 patients were included in the meta-analysis. All treatments were significantly better than placebo. Out of the ten different treatment modalities, BF-200 ALA showed the highest efficacy compared to placebo, followed by IMI 5% 4 weeks and 5-FU 0.5%. Ingenol mebutate was the sixth most efficacious treatment compared to placebo. The results of the meta-analysis are summarised in the table below
15 Table 3: Efficacy of AK treatments for total patients clearance Direct meta-analysis Network meta-analysis Probability to be best SUCRA Placebo 1 (reference) 1 (reference) 0.0% 0.0% MAL-PDT 14.3 ( ) 16.5 ( ) 1.1% 57.2% BF-200 ALA 40.1 ( ) 45.9 ( ) 64.8% 92.1% ALA-PDT patch 16.7 ( ) 18.1 ( ) 6.7% 62.8% Cryotherapy 7.3 ( ) 8.0 ( ) 0.3% 30.6% Imiquimod 5% (16 weeks) Imiquimod 5% 21.7 ( ) 23.8 ( ) 10.1% 74.2% 17.5 ( ) 17.6 ( ) 3.9% 60.9% (4 weeks) Diclofenac 3% 3.4 ( ) 4.3 ( ) 0.0% 14.0% 5-FU 0.5% 20.5 ( ) 20.7 ( ) 7.2% 66.8% Ingenol mebutate Imiquimod 3.75% (4 weeks) 16.8 ( ) 16.4 ( ) 5.5% 58.1% 8.5 ( ) 8.7 ( ) 0.6% 33.2% The authors concluded from the meta-analysis that BF-200 ALA gel, provided the greatest response in complete patient clearance of AKs on the face and scalp ranking its NMA with the highest probability of being the most efficacious treatment. Long-term Follow-up Study of Ingenok Mebutate Gel for the Treatment of Actinic Keratoses 12 A long term follow up study of patients who had achieved complete clearance of AK in 4 studies was conducted during a 12 month period. Patients were originally treated with ingenol mebutate gel 0.015%, daily for 3 consecutive days for AK on the face or scalp or with ingenol mebutate gel, 0.05% daily for 2 consecutive days for AK on the trunk or extremities. These patients had to have achieved complete clearance in a pre-specified 25-cm 2 area at day 57 of treatment for participation in this follow up study. They were then revisited at months 3, 6, 9 and 12 after day 57 visit of the previous study. The primary end point for this study was to assess the recurrence rate of AK and safety associated with treatment with ingenol in the target treatment area. Patients were treated in an outpatients setting and was said to have achieved sustained clearance if their target treatment area remained clear after 12 months. 15
16 A total of 108 patients with complete clearance of face or scalp lesions in the original trial and 76 patients with complete clearance of trunk or extremity lesions in the original trial were enrolled in the 12 month observational follow up study. Of these 100 patients (face or scalp) and 71 patients (trunk or extremities) completed 12 months. The sustained lesion reduction rates compared with baseline were 87.2% for the face or scalp and 86.8% for the trunk and extremities. There were no safety concerns during the follow-up period. In conclusion, the authors that ingenol mebutate gel produced clinically relevant sustained clearance and long term lesion reduction. Cost Analysis: Cost analysis: Drug Strength Dose Pack Size Solaraze 3% diclofenac gel with hyaluronic acid Cost at UHNS per pack (exc VAT) Cost in primary care per pack* (exc Estimated quantity per course BD for 60 to 90 days 50g x 50g tubes Cost at UHNS per course (exc VAT) Cost in primary care per course* (exc VAT) Efudix 5% 5-fluorouracil OD or BD for 3 to 4 weeks 40g x 40g tube Actikerall 0.5% 5-fluorouracil/ 10% salicylic acid solution Od for 6 to 12 weeks 25ml x 25 ml bottles Aldara Picato 5% imiquimod cream Ingenol mebutate gel 150mcg/gram 3 nights a week for 4 weeks.after a 4 week treatment-free period, if lesions persist repeat treatment for additional 4 weeks Applied OD for 3 days to face/scalp 12 sachets 3x0.47g single use tubes x 4 week treatment 12 sachets. 2x 4 week treatments, 24 sachets tubes Picato Ingenol mebutate gel 500mcg/gram Applied OD for 2 days to the trunk/ extremities 2 x 0.47g single use tubes tubes Note: A comparison of costs for topical medicines licensed for the treatment of hyperkaratotic AK may not be misleading as usage will depend on the surface area and number of lesions to be treated. 16
17 References 1 Primary Care Dermatology Society (PCDS). Actinic Keratosis (syn. Solar Keratosis). Last updated 23/11/14. Available at [accessed: 2/4/15] 2 British Association of Dermatologists (BAD). Guidelines for the management of acitinic keratoses. British journal of dermatology , p National Institute for Health and Care Excellence (NICE), Evidence summary: new medicines. ESNM:14 Actinic keratosis: ingenol mebutate gel. March Accessed via: 01/05/15. 4 Summary of Product Characteristics (SPC). Picato 500mcg/g Gel. Leo Laboratories Ltd. Last up dated 19/12/13. Available at: [Accessed on 2/4/15] 5 Scottish Medicines consortium (SMC). Ingenol mebutate (Picato ) Avialable at: 3_amended_ _for_website.pdf [accessed on 01/05/15]. 6 All Wales Medicines Strategy Group (AWMSG). Ingenol mebutate (Picato ) Available at: 7 Regional Drug and Therapeutic Centre (Newcastle). Evaluation Report Newer Topical Therapies for actinic keratosis. April 2013Medical 8 Midlands Therapeutics Review and Advisory Committee (MTRAC). Commissioning support Ingenol mebutate gel (Picato ) for the treatment of actinic keratosis. June Gupta AK, PaquetM, Villanueva E, BrintnellW. Interventions for actinic keratoses. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No.: CD DOI: / CD pub2. 10 Lebwhol, M. Swanson N, Anderson LL et al. Ingenol mebutate gel for actinic keratosis. New England Journal of Medicine. 2012; 366: rmation) l Vegter. S, Tolley. K. A Network Meta-analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe. PLoS ONE 2014; 9(6): e Doi: /journal.pone Lebwohl M, Shumack S, Gold LS et al. Long-term Follow-up Study of Ingenol Mebutate Gel for the Treatment of Actinic Keratoses. JAMA Dermatol. 2013; 149(6): Produced by Tanesha Mistry Specialist Clinical Pharmacist University Hospital of North Staffordshire Telephone: Tanesha.Mistry@uhns.nhs.uk Produced for use within the NHS. Not to be reproduced for commercial purposes. 17
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A.
 fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
AWMSG SECRETARIAT ASSESSMENT REPORT. Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel. Reference number: 1392 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Diagnosis and Management of Actinic Keratosis (AKs)
 Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
AWMSG SECRETARIAT ASSESSMENT REPORT. 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel. Reference number: 1074 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel Reference number: 1074 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
AWMSG SECRETARIAT ASSESSMENT REPORT 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel Reference number: 1074 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
Richard Turner Consultant Dermatologist
 Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Skin lesions The Good and the Bad. Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry
 Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Opinion 26 June 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
Clinical Trial Report Synopsis. Efficacy and Safety of LEO in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up
 Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Developing the next generation of dermatology products to treat serious skin diseases
 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 26 November 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
PICATO (ingenol mebutate) gel
 PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee
 I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
Corporate Medical Policy
 Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
TOPICAL TREATMENT OF ACTINIC KERATOSIS
 TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
Clinical Study Report Synopsis
 This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses
 Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Skin disorders. Basal cell carcinoma December 2009 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig ...
 December 29 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig.................................................. ABSTRACT INTRODUCTION: (BCC) is the most common form of skin cancer, predominantly affecting
December 29 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig.................................................. ABSTRACT INTRODUCTION: (BCC) is the most common form of skin cancer, predominantly affecting
PRODUCT INFORMATION METVIX
 PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare
 camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Medical Policy. MP Dermatologic Applications of Photodynamic Therapy
 Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
Aldara. Aldara (imiquimod) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Ingenol Mebutate: A Succinct Review of a Succinct Therapy
 Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Actikerall 5 mg/g mg/g Cutaneous Solution. Fluorouracil + Salicylic acid
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Actikerall 5 mg/g + 100 mg/g Cutaneous Solution Fluorouracil + Salicylic acid Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Actikerall 5 mg/g + 100 mg/g Cutaneous Solution Fluorouracil + Salicylic acid Read all of this leaflet carefully before you start using this
Actinic Keratoses and Bowen s disease
 Actinic Keratoses and Bowen s disease Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Actinic Keratoses and Bowen s disease Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest risk.
 Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects *
 Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
Service Line: Rapid Response Service Version: 1.0 Publication Date: September 15, 2017 Report Length: 33 Pages
 CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Imiquimod for the Treatment of Actinic Keratosis: A Review of Clinical Effectiveness and Cost-Effectiveness Service Line: Rapid Response Service
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Imiquimod for the Treatment of Actinic Keratosis: A Review of Clinical Effectiveness and Cost-Effectiveness Service Line: Rapid Response Service
Have a Voice in Your Choice!
 Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
SMC briefing note. The following medicines were accepted for use: The following medicine has not been recommended for use: About SMC.
 Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
Monthly briefings are produced in order to help members of the media and other interested people understand the work and advice of the Scottish Medicines Consortium. The detailed advice for each medicine
Package leaflet: Information for the user. Ameluz 78 mg/g gel 5-aminolaevulinic acid
 B. PACKAGE LEAFLET Package leaflet: Information for the user Ameluz 78 mg/g gel 5-aminolaevulinic acid Read all of this leaflet carefully before you start using this medicine because it contains important
B. PACKAGE LEAFLET Package leaflet: Information for the user Ameluz 78 mg/g gel 5-aminolaevulinic acid Read all of this leaflet carefully before you start using this medicine because it contains important
Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally
 University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
Cutaneous Malignancies: A Primer COPYRIGHT. Marissa Heller, M.D.
 Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Swiss clinical practice guidelines on field cancerization of the skin
 Published 24 December 2014, doi:10.4414/smw.2014.14026 Cite this as: Swiss clinical practice guidelines on field cancerization of the skin Günther Hofbauer a, Mark Anliker b, Wolf-Henning Boehncke c, Christoph
Published 24 December 2014, doi:10.4414/smw.2014.14026 Cite this as: Swiss clinical practice guidelines on field cancerization of the skin Günther Hofbauer a, Mark Anliker b, Wolf-Henning Boehncke c, Christoph
Core Safety Profile. Pharmaceutical form(s)/strength: solution 1%, spray 1%, cream 1%, gel 1% SK/H/PSUR/0005/001 Date of FAR:
 Core Safety Profile Active substance: Terbinafine Pharmaceutical form(s)/strength: solution 1%, spray 1%, cream 1%, gel 1% P-RMS: SK/H/PSUR/0005/001 Date of FAR: 08.06.2012 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Terbinafine Pharmaceutical form(s)/strength: solution 1%, spray 1%, cream 1%, gel 1% P-RMS: SK/H/PSUR/0005/001 Date of FAR: 08.06.2012 4.3 Contraindications Hypersensitivity
30 Actinic Keratosis (Solar Keratosis)
 30 Actinic Keratosis (Solar Keratosis) CLINICAL APPLICATION QUESTIONS A 65-year-old white man is seen at your office for multiple scaling lesions over his face, ears, neck, and the V of the chest. These
30 Actinic Keratosis (Solar Keratosis) CLINICAL APPLICATION QUESTIONS A 65-year-old white man is seen at your office for multiple scaling lesions over his face, ears, neck, and the V of the chest. These
Living Beyond Cancer Skin Cancer Detection and Prevention
 Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Opinion 6 March 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
Periocular skin cancer
 Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ. Övermark, Meri.
 https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
Dermatological Manifestations in the Elderly. Sanjay Siddha Staff Dermatologist UHN & MSH
 Dermatological Manifestations in the Elderly Sanjay Siddha Staff Dermatologist UHN & MSH Disclosure No actual or potential conflicts of interest or commercial relationships to declare Objectives Recognize
Dermatological Manifestations in the Elderly Sanjay Siddha Staff Dermatologist UHN & MSH Disclosure No actual or potential conflicts of interest or commercial relationships to declare Objectives Recognize
New Medicines Committee Briefing November 2011
 New Medicines Committee Briefing November 2011 Paliperidone long-acting injection (Xeplion ) for schizophrenia Paliperidone palmitate long-acting injection (Xeplion ) is to be reviewed for use within:
New Medicines Committee Briefing November 2011 Paliperidone long-acting injection (Xeplion ) for schizophrenia Paliperidone palmitate long-acting injection (Xeplion ) is to be reviewed for use within:
AUSTRALIAN PRODUCT INFORMATION APOC-5FU CREAM (FLUOROURACIL)
 AUSTRALIAN PRODUCT INFORMATION APOC-5FU CREAM (FLUOROURACIL) 1 NAME OF THE MEDICINE Fluorouracil 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM APOC-5FU cream is intended for
AUSTRALIAN PRODUCT INFORMATION APOC-5FU CREAM (FLUOROURACIL) 1 NAME OF THE MEDICINE Fluorouracil 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM APOC-5FU cream is intended for
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Clinical characteristics
 Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe
 A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe Stefan Vegter 1,2 *, Keith Tolley 3 1 University of Groningen, Department of Pharmacy,
A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe Stefan Vegter 1,2 *, Keith Tolley 3 1 University of Groningen, Department of Pharmacy,
SOLARAZE 3% gel. (diclofenac sodium)
 SOLARAZE 3% gel (diclofenac sodium) NAME OF THE MEDICINE Solaraze (diclofenac sodium) Gel 3% contains the active ingredient, diclofenac sodium, in a clear, transparent, colourless to light orange gel base.
SOLARAZE 3% gel (diclofenac sodium) NAME OF THE MEDICINE Solaraze (diclofenac sodium) Gel 3% contains the active ingredient, diclofenac sodium, in a clear, transparent, colourless to light orange gel base.
Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel: Report of Two Cases
 Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
Photodynamic Therapy (PDT) Basics and clinical applications
 Photodynamic Therapy () Basics and clinical applications D. Roseeuw, S. T kint Department of Dermatology UZBrussel - VUB GOAL of : selective destruction of targeted abnormal cells Light O 2 Photosensitiser
Photodynamic Therapy () Basics and clinical applications D. Roseeuw, S. T kint Department of Dermatology UZBrussel - VUB GOAL of : selective destruction of targeted abnormal cells Light O 2 Photosensitiser
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD
 A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
Area Drug and Therapeutics Committee Prescribing Supplement No 3 November 2003
 Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Dr A Anzarut, MSc, CIP, MD, FRCSC
 Dr A Anzarut, MSc, CIP, MD, FRCSC Plastic and Cosmetic Surgery ASSH Fellowship trained hand surgeon 201 2763 Beverly Street, Duncan, British Columbia Tel: (250) 597 2064 Fax: (250) 597-1297 PATIENT INFORMATION
Dr A Anzarut, MSc, CIP, MD, FRCSC Plastic and Cosmetic Surgery ASSH Fellowship trained hand surgeon 201 2763 Beverly Street, Duncan, British Columbia Tel: (250) 597 2064 Fax: (250) 597-1297 PATIENT INFORMATION
1) Photodynamic therapy with topical 5 aminolevulinic acid is considered medically necessary and is covered for the treatment of:
 Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
ESKATA TM (hydrogen peroxide) topical solution Initial U.S. Approval: 2017
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA TM safely and effectively. See full prescribing information for ESKATA TM. ESKATA TM (hydrogen
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA TM safely and effectively. See full prescribing information for ESKATA TM. ESKATA TM (hydrogen
ESKATA (hydrogen peroxide) topical solution Initial U.S. Approval: 2017
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA safely and effectively. See full prescribing information for ESKATA. ESKATA (hydrogen peroxide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA safely and effectively. See full prescribing information for ESKATA. ESKATA (hydrogen peroxide)
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
Nonmelanoma skin cancers
 Skin cancer Philip Clarke Nonmelanoma skin cancers Treatment options Background Australia has one of the highest skin cancer rates in the world. Early detection and treatment of skin cancer is vital to
Skin cancer Philip Clarke Nonmelanoma skin cancers Treatment options Background Australia has one of the highest skin cancer rates in the world. Early detection and treatment of skin cancer is vital to
OCCG SERVICE SPECIFICATION (2017/18)
 OCCG SERVICE SPECIFICATION (2017/18) Primary Care Service for Skin Cancers: Dermatology Shared Care Monitoring for Melanoma, Lichen Sclerosus and Squamos Cell Carcinoma 1. Background For patients who have
OCCG SERVICE SPECIFICATION (2017/18) Primary Care Service for Skin Cancers: Dermatology Shared Care Monitoring for Melanoma, Lichen Sclerosus and Squamos Cell Carcinoma 1. Background For patients who have
Keratolytics and Other Topical Dermatological Agents
 DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
Summary of Product Characteristics
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Terbinafine Dermapharm, 10 mg/g, cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of cream contains 10 mg of terbinafine hydrochloride.
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Terbinafine Dermapharm, 10 mg/g, cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of cream contains 10 mg of terbinafine hydrochloride.
Diclofenac 3% Gel, Diclofenac 1.5% and 2% Topical Solution
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd
 Scottish Medicines Consortium Resubmission levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd 11 January 2008 The Scottish Medicines
Scottish Medicines Consortium Resubmission levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd 11 January 2008 The Scottish Medicines
Patient Group Direction for Doxycycline (Tetracycline) Version: 01 Start Date: October 2015 Expiry Date: October 2018
 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
Clinical Study Topical Colchicine Gel versus Diclofenac Sodium Gel for the Treatment of Actinic Keratoses: A Randomized, Double-Blind Study
 Advances in Medicine Volume 2016, Article ID 5918393, 6 pages http://dx.doi.org/10.1155/2016/5918393 Clinical Study Topical Colchicine Gel versus Diclofenac Sodium Gel for the Treatment of Actinic Keratoses:
Advances in Medicine Volume 2016, Article ID 5918393, 6 pages http://dx.doi.org/10.1155/2016/5918393 Clinical Study Topical Colchicine Gel versus Diclofenac Sodium Gel for the Treatment of Actinic Keratoses:
Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA
 Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA A Practical Approach: Field Treatment of AKs with PDT Why it s essential to treat visible and invisible
Insert to September 2018 A PRACTICAL APPROACH: Field Treatment of AKs with PDT SUPPORTED BY BIOFRONTERA A Practical Approach: Field Treatment of AKs with PDT Why it s essential to treat visible and invisible
New Medicine Review. Racecadotril for the symptomatic treatment of acute diarrhoea (adults and children over 3 months)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) April 2013 Review date: April 2016 Bulletin 180: Racecadotril for the symptomatic treatment of acute diarrhoea in adults and children over 3 months
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) April 2013 Review date: April 2016 Bulletin 180: Racecadotril for the symptomatic treatment of acute diarrhoea in adults and children over 3 months
2 SYNOPSIS. Study code : MC 9308 FR.
 MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
MC9308 FR Study 19 December 2000 Page 15 of142 2 SYNOPSIS Study code : MC 9308 FR. Title: A comparative study of calcipotriol ointment in combination with narrow-band UVB (TL-01) phototherapy and placebo
Learning Objectives. Tanning. The Skin. Classic Features. Sun Reactive Skin Type Classification. Skin Cancers: Preventing, Screening and Treating
 Learning Objectives Skin Cancers: Preventing, Screening and Treating Robert A. Baldor, MD, FAAFP Professor, Family Medicine & Community Health University of Massachusetts Medical School Distinguish the
Learning Objectives Skin Cancers: Preventing, Screening and Treating Robert A. Baldor, MD, FAAFP Professor, Family Medicine & Community Health University of Massachusetts Medical School Distinguish the
1. NAME OF THE MEDICINAL PRODUCT. Lamisil 1% cutaneous solution. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Lamisil 1% cutaneous solution. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: 10 mg terbinafine hydrochloride per 1 g solution (1% w/w). Excipient(s) with
1. NAME OF THE MEDICINAL PRODUCT Lamisil 1% cutaneous solution. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: 10 mg terbinafine hydrochloride per 1 g solution (1% w/w). Excipient(s) with
Extreme dermatoheliosis: How to approach the severely sun damaged patient
 Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Clinical Policy: Benign Skin Lesion Removal Reference Number: CP.MP.HN150
 Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
ABSTRACT ORIGINAL RESEARCH
 Dermatol Ther (Heidelb) (2017) 7:81 96 DOI 10.1007/s13555-016-0161-2 ORIGINAL RESEARCH Efficacy and Safety of 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Actinic Keratosis:
Dermatol Ther (Heidelb) (2017) 7:81 96 DOI 10.1007/s13555-016-0161-2 ORIGINAL RESEARCH Efficacy and Safety of 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Actinic Keratosis:
ORIGINAL ARTICLE. Abstract
 DOI: 10.1111/jdv.13211 JEADV ORIGINAL ARTICLE A randomized trial comparing simultaneous vs. sequential field treatment of actinic keratosis with ingenol mebutate on two separate areas of the head and body
DOI: 10.1111/jdv.13211 JEADV ORIGINAL ARTICLE A randomized trial comparing simultaneous vs. sequential field treatment of actinic keratosis with ingenol mebutate on two separate areas of the head and body
Scottish Medicines Consortium
 Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
Actinic keratosis (AK): Dr Sarma s simple guide
 Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Scottish Medicines Consortium
 Scottish Medicines Consortium esomeprazole, 40mg vial of powder for solution for intravenous injection or infusion (Nexium I.V. ) No. (578/09) AstraZeneca 09 October 2009 The Scottish Medicines Consortium
Scottish Medicines Consortium esomeprazole, 40mg vial of powder for solution for intravenous injection or infusion (Nexium I.V. ) No. (578/09) AstraZeneca 09 October 2009 The Scottish Medicines Consortium
The no t-so-simple cutaneous wart
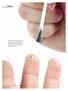 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
PRODUCT INFORMATION WARTEC SOLUTION
 PRODUCT INFORMATION WARTEC SOLUTION NAME OF THE MEDICINE WARTEC Solution contains podophyllotoxin as the active ingredient. CAS number: 518-28-5 DESCRIPTION WARTEC Solution is a blue solution for topical
PRODUCT INFORMATION WARTEC SOLUTION NAME OF THE MEDICINE WARTEC Solution contains podophyllotoxin as the active ingredient. CAS number: 518-28-5 DESCRIPTION WARTEC Solution is a blue solution for topical
Page: Pre-test screening for eligible subjects was performed during the 28 days before the anticipated study start (Day 1).
 This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
New Medicines Committee Briefing July Minims Povidone Iodine 5% w/v Eye Drops, Solution
 New Medicines Committee Briefing July 2013 Minims Povidone Iodine 5% w/v Eye Drops, Solution Minims Povidone Iodine 5% w/v Eye Drops is to be reviewed for use within: Primary Care Secondary Care Summary:
New Medicines Committee Briefing July 2013 Minims Povidone Iodine 5% w/v Eye Drops, Solution Minims Povidone Iodine 5% w/v Eye Drops is to be reviewed for use within: Primary Care Secondary Care Summary:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ameluz 78 mg/g gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram (g) gel contains 78 mg of 5-aminolaevulinic acid (as
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ameluz 78 mg/g gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram (g) gel contains 78 mg of 5-aminolaevulinic acid (as
NHS. Photodynamic therapy for non-melanoma skin tumours (including premalignant and primary non-metastatic skin lesions)
 NHS National Institute for Health and Clinical Excellence Issue date: February 2006 Photodynamic therapy for non-melanoma skin (including premalignant and primary non-metastatic skin lesions) Understanding
NHS National Institute for Health and Clinical Excellence Issue date: February 2006 Photodynamic therapy for non-melanoma skin (including premalignant and primary non-metastatic skin lesions) Understanding
See spot change: Lesion identification and management in primary care ERIN HENNESSEY DNP, APRN, FNP-C
 See spot change: Lesion identification and management in primary care ERIN HENNESSEY DNP, APRN, FNP-C Learning objectives Discuss malignant skin lesions commonly seen in primary care. Identify common treatments
See spot change: Lesion identification and management in primary care ERIN HENNESSEY DNP, APRN, FNP-C Learning objectives Discuss malignant skin lesions commonly seen in primary care. Identify common treatments
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit:
 Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Single Technology Appraisal (STA) Vismodegib for treating basal cell carcinoma [ID1043]
![Single Technology Appraisal (STA) Vismodegib for treating basal cell carcinoma [ID1043] Single Technology Appraisal (STA) Vismodegib for treating basal cell carcinoma [ID1043]](/thumbs/90/101981882.jpg) Single Technology Appraisal (STA) Vismodegib for treating basal cell carcinoma [ID1043] Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please note: Comments
Single Technology Appraisal (STA) Vismodegib for treating basal cell carcinoma [ID1043] Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please note: Comments
Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine
![Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine](/thumbs/83/88391058.jpg) APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
Rivaroxaban film coated tablets are available in 2 strengths for this indication: 15mg and 20mg.
 Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 204 : Brimonidine tartrate gel (Mirvaso ) for the treatment of facial erythema of rosacea in
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 204 : Brimonidine tartrate gel (Mirvaso ) for the treatment of facial erythema of rosacea in
SQUAMOUS CELL CARCINOMA
 SQUAMOUS CELL CARCINOMA What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinomas of the skin. It tells you what they are, what causes
SQUAMOUS CELL CARCINOMA What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinomas of the skin. It tells you what they are, what causes
Scottish Medicines Consortium
 Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
