(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
|
|
|
- Brook Small
- 5 years ago
- Views:
Transcription
1 (19) United States US A1 (12) Patent Application Publication (10) Pub. No.: US 2013/ A1 Tsukamoto et al. (43) Pub. Date: (54) MEASURING DEVICE AND MEASURING (52) U.S. Cl. METHOD CPC... G0IN 21/78 ( ) (75) Inventors: Akira Tsukamoto, Otsu-shi (JP); Jun USPC /14; 435/288.7 Okamoto, Otsu-shi (JP) (73) Assignee: TOYOBO CO.,LTD., Osaka-shi, Osaka (57) ABSTRACT (JP) (21) Appl. No.: 13/824,107 (22) PCT Filed: Sep. 26, 2011 (86). PCT No.: S371 (c)(1), (2), (4) Date: PCT/UP2011/ Mar. 15, 2013 (30) Foreign Application Priority Data Sep. 27, 2010 (JP) Publication Classification (51) Int. Cl. GOIN 2L/78 ( ) The device enables measurement of both of blood glucose level and hemoglobin Alc level, being Small-sized and thus portable, allowing the measurement of a small amount of a specimen and, being usable in POC. The device comprises: a test-piece-mounting section (1) for detachably mounting test pieces (200) on which the blood specimen is to be spotted; a light-emitting section (2) for emitting light for irradiating the test pieces; a light-receiving section (3) for receiving reflected lights from the test pieces; and an operating section (4) for calculating the blood glucose level and hemoglobin Alc level of the blood specimen on the basis of photometric values obtained from the light-receiving section. For measuring the blood glucose level of the blood specimen, test piece (A) carries a composition reacting with glucose and showing color change. Test piece (B) carries a composition reacting with glycated hemoglobin and showing color change. OO
2 Patent Application Publication Sheet 1 of 7 US 2013/ A1 FG, 2
3 Patent Application Publication Sheet 2 of 7 US 2013/ A1 FG. 4 2(2b) 3.3b) 1. / i
4 Patent Application Publication Sheet 3 of 7 US 2013/ A (200a200b) Y
5 Patent Application Publication Sheet 4 of 7 US 2013/ A1 e G s
6 Patent Application Publication Sheet 5 of 7 US 2013/ A1 e s
7 Patent Application Publication Sheet 6 of 7 US 2013/ A1
8 Patent Application Publication Sheet 7 of 7 US 2013/ A1 F.G. 13
9 MEASURING DEVICE AND MEASURING METHOD TECHNICAL FIELD The present invention relates to a measuring device that can measure both a blood glucose leveland a hemoglobin Alic level and a measuring method using the measuring device. More specifically, a measuring device according to the present invention is a small, simple measuring device usable at a so-called point of care (POC). BACKGROUND ART In recent years, point-of-care (POC) tests have been attracting attention. POC tests are tests performed near patients or tests performed by patients themselves and include, for example, self-check tests performed by patients in their homes, tests performed at bedsides in hospitals, and tests performed in places other than central laboratories. Since doctors and patients can check the test results immedi ately, POC tests allow actions to be quickly taken and are expected to greatly contribute to improvement of the quality of treatment. Measuring devices used in POC tests (POC devices) are required to be small enough to be portable and perform tests with a small amount of specimen (for example, blood) Devices for self-monitoring of blood glucose (SMBG) are an example of POC devices that are in wide spread use. It is important to check the blood glucose level daily for diagnosis, prevention, and treatment of diabetes, and patients are recommended to measure the blood glucose level themselves for self management of their conditions. For this purpose, POC devices that are simple and capable of quickly measuring the blood glucose level by using several microli ters of blood (whole blood) are extremely useful. Conven tionally, POC devices for measuring the blood glucose level have used a method (enzyme colorimetric method) in which glucose contained in blood is caused to react with an enzyme and develop color. The level of color development is detected in terms of absorbance and converted into an amount of glucose. In recent years, however, devices using an oxygen electrode method, for which the device structure is relatively simple, have become popular. In the oxygen electrode method, glucose contained in blood is caused to react with an enzyme and generate a current, and the value of the generated current is converted into an amount of glucose Hemoglobin A1c (glycated hemoglobin), which is one of glycated proteins, is known as another index used for diagnosis, etc., of diabetes. Hemoglobin Alc is an index that reflects a one to two months history of the blood glucose level of a living body, and shows a long-term average of the blood glucose level. Therefore, for accurate diagnosis and treat ment, the hemoglobin A1c level is preferably checked in addition to the blood glucose level, which varies by large amounts in the short term. However, known methods for measuring the hemoglobin Alc level are difficult to apply to the above-described POC devices, and measurement of the hemoglobin A1c level has been performed only in central laboratories of hospitals and inspection institutes More specifically, in an example of a known method for measuring the hemoglobin Alc level, the hemoglobin Alc level is measured in terms of a glycation rate oran amount of glycation by, for example, a high performance liquid chro matography (HPLC) method or an immunization method. However, in the HPLC method, a large specialized machine is required to only measure a hemoglobin Alc level. In the immunization method, the measurement accuracy decreases unless, for example, contamination of a measurement cell is Sufficiently controlled. Owing to these circumstances, mea surement of the hemoglobin A1c level by these methods has been performed only in hospitals and inspection institutes As another example of a method for measuring the hemoglobin A1c level, PTL 1 discloses a method using a redox reaction caused by an enzyme. In this method for measuring the hemoglobin Alc level using a redox reaction caused by an enzyme, first, a sample containing hemoglobin A1c is treated with fructosyl-amino acid oxidase (FAOD) so that the FAOD acts on a glycated portion of the hemoglobin Alc. Accordingly, hydrogen peroxide, the amount of which corresponds to an amount of hemoglobin Alc, is generated. Then, peroxidase (POD) and a reducing agent are added so that a redox reaction occurs between the hydrogen peroxide and the reducing agent with the POD serving as a catalyst. The amount of generated hydrogen peroxide can be measured by using a reducing agent that develops color when oxidized and detecting the level of color development of the obtained reaction liquid interms of absorbance. As a result, the amount of hemoglobin Alc can be measured However, the method described in PTL 1 is a so called wet measuring method in which the absorbance of the reaction liquid obtained by the redox reaction is measured. Therefore, it is necessary to prepare the reaction liquid, and complex management and maintenance are required. For this reason, it is difficult to apply this method to the above-de scribed POC tests, in particular, to self-check tests performed by patients. PTL 2 proposes a method for measuring the hemoglobin Alc level by using a glycated hemoglobin mea Surement cassette to minimize the operational burden on the operator and reduce the measurement time. However, this method is also a wet measuring method, and is difficult to apply to the above-described POC tests, in particular, to self check tests performed by patients A hemoglobin A1c level measuring method appli cable to POC tests is proposed in PTL 3. This method is a combination of the immunization method and a colorimetric method in which the level of color development is detected in terms of absorbance. However, since this method is based on the immunization method, it is necessary to prepare a diluent obtained by hemolyzing a blood specimen (whole blood) in advance. Therefore, the measurement according to this method is more complex compared to the measurement using the devices for self-monitoring of blood glucose (SMBG). with which the measurement can be performed by simply using whole blood In recent years, greater importance has been placed on the hemoglobin Alc level in diagnosis, prevention, and treatment of diabetes. For example, in the United States, where diagnosis of diabetes has conventionally been per formed on the basis of a fasting blood sugar or the result of a glucose tolerance test, the American Diabetes Association (ADA) announced that the hemoglobin A1c level may be used as a new diagnostic criterion in June, In Japan, it has been recommended since Jul. 1, 2010 that hemoglobin A1c be included in essential test items for diagnosis of diabetes in addition to conventional test items such as the blood glucose level. Accordingly, it is preferable that not only the blood glucose level but also the hemoglobin A1c level be able to be
10 measured by patients themselves for Selfmanagement of their conditions or be able to be measured more easily and quickly at medical sites The above-described blood glucose level and hemo globin A1c level are both used in diagnosis of diabetes. In particular, the blood glucose level and the hemoglobin A1c level are both included in the essential test items for diagnosis of diabetes in Japan. Therefore, for convenience, for example, these measurement items are preferably measurable with a single device A glycosylated hemoglobin/glucose analyzer ADAMS (registered trade mark) Hybrid AH-8280 is com mercially available from Arkray, Inc. as a device capable of measuring the blood glucose level and the hemoglobin Alc level at the same time. This device, however, measures the blood glucose level by a glucose oxidase electrode method and the hemoglobin A1c level by the HPLC method, and two different measurement principles are used. Therefore, the device has a complex inner structure and a large size, and cannot be used at a POC, even though the device has an advantage in that the installation space therefor is Smaller than that in the conventional case where the blood glucose level and the hemoglobin A1c level are measured with differ ent devices. In addition, Banalyst (registered trade mark) Ace', which has been developed by Rohm Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., and Ushio, Inc., is also com mercially available as a device capable of measuring the blood glucose level and the hemoglobin A1c level at the same time. This device, however, measures the blood glucose level by an enzyme method and the hemoglobin A1c level by the immunization method, such as a latex agglutination method, and so also in this device, two different measurement prin ciples are used. Therefore, the device has a complex structure, and is difficult to apply to POC tests, in particular, to self check tests performed by patients PTL 4 proposes self-testing blood sugar testing means for simultaneously measuring a current blood glucose level and a blood sugar marker that reflects the blood glucose level in the past. However, no actual device that can measure the blood glucose level and the hemoglobin A1c level is disclosed. In recent years, devices for self-monitoring of blood glucose (SMBG) capable of measuring not only the blood glucose level but also other test items have been devel oped and become commercially available. For example, Benecheck (registered trade mark) PLUS manufactured by General Life Biotechnology Co., Ltd. in Taiwan can measure not only the blood glucose level but also a cholesterol level and an uric acid level by the oxygen electrode method. How ever, this device also cannot measure the hemoglobin Alc level together with the blood glucose level. Patent Literature CITATION LIST 0013 PTL 1: International Publication No. 2003/ PTL 2: Japanese Unexamined Patent Application Publication No PTL3: U.S. Unexamined Patent Application Publi cation No. 2005/ PTL 4: Japanese Unexamined Patent Application Publication No SUMMARY OF INVENTION Technical Problem The present invention has been made in light of the above-described circumstances, and an object of the present invention is to provide a measuring device that can measure both a blood glucose leveland a hemoglobin A1c level, that is Small enough to be portable, that can perform a measurement with a small amount of specimen, and that is usable at a POC, and a measuring method using the measuring device. Solution to Problem A measuring device according to the present inven tion, which achieves the above-described object, is a device for measuring a blood glucose level and a hemoglobin Alc level of a blood specimen and includes a light-emitting sec tion that emits illuminating light toward the blood specimen; a light-receiving section that receives reflected light from the blood specimen; and an operating section that calculates each of the blood glucose level and the hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section. The light-emitting section is capable of emitting two or more types of light having different wavelengths. The light-emitting section is preferably capable of emitting three types of light having different wavelengths. In this case, one of the three types of light having different wavelengths is light having a specific wavelength that is absorbed by a portion whose color has changed in response to glucose contained in the blood specimen and the remaining two of the three types of light having different wavelengths are two types of light having different specific wavelengths that are absorbed by respective portions whose colors have changed in response to hemoglobin and glycated hemoglobin contained in the blood specimen. The measuring device pref erably further includes a test-piece-mounting section that detachably mounts a test piece to which the blood specimen is applied Another measuring device according to the present invention is a device for measuring a blood glucose level and a hemoglobin Alc level of a blood specimen and includes a test-piece-mounting section that detachably mounts a test piece to which the blood specimen is applied; a light-emitting section that emits illuminating light toward the test piece; a light-receiving section that receives reflected light from the test piece; and an operating section that calculates each of the blood glucose level and the hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section. A test piece (A) that carries a composition (a) that reacts with glucose and changes color is mounted as the test piece to measure the blood glucose level of the blood specimen, and a test piece (B) that carries a composition (b) that reacts with glycated hemoglobin and changes color is mounted as the test piece to measure the hemoglobin A1c level of the blood specimen. In this measur ing device, similar to the above-described measuring device, the light-emitting section is preferably capable of emitting three types of light having different wavelengths In the above-described measuring device according to the present invention, the blood glucose level and the hemoglobin A1c level are both measured by a method based on the same measurement principle in which the test piece is caused to change color in response to a reaction, and the level of the color change is detected by using reflection of light.
11 Therefore, the size of the device can be reduced. Since the test piece carries a composition that reacts with glucose or gly cated hemoglobin contained in the blood specimen, the above-described reaction for causing the test piece to change color can be caused by applying a few drops (about several microliters) of blood specimen (whole blood) directly to the test piece without diluting the blood specimen. The above described measuring device according to the present inven tion can be used as a POC device. The blood glucose level can be measured when the test piece (A) is mounted as the test piece and the hemoglobin A1c level can be measured when the test piece (B) is mounted as the test piece The test pieces (A) and (B) according to the present invention are not necessarily formed separately, and may be formed as a single test piece. For example, the test piece to which the blood specimen is applied may include both a region in which the composition (a) is carried (which corre sponds to the test piece (A)) and a region in which the com position (b) is carried (which corresponds to the test piece (B)) The measuring device according to the present invention measures both the blood glucose level and the hemoglobin Alc level on the basis of a measurement prin ciple in which a color change is caused and the level of the color change is detected in terms of the level of reflection of light. For this purpose, it is important that the light-emitting section be capable of emitting two types of light having different wavelengths. In other words, to measure the blood glucose level and the hemoglobin Alc level with a single device on the basis of the above-described measurement prin ciple, at least two types of light having different wavelengths are required, the wavelengths including a wavelength for detecting a color change caused by glucose to measure the blood glucose level and a wavelength for detecting a color change caused by glycated hemoglobin to measure the hemo globin A1c level In a measuring device according to a preferred embodiment of the present invention, the hemoglobin A1c level is measured by detecting a color change caused by hemoglobin and a color change caused by glycated hemoglo bin and calculating the hemoglobin A1c level on the basis of the detection results. In this case, the color change caused by hemoglobin and the color change caused by glycated hemo globin are preferably detected by using reflection of light having different wavelengths, and the two different wave lengths (the wavelength for detecting the color change caused by hemoglobin and the wavelength for detecting the color change caused by glycated hemoglobin) preferably differ from the wavelength for detecting the color change caused by glucose. When the light-emitting section is capable of emit ting three types of light having different wavelengths, the color change caused by hemoglobin, the color change caused by glycated hemoglobin, and the color change caused by glucose can be detected by using reflection of light having different wavelengths. In the case where two of the color change caused by hemoglobin, the color change caused by glycated hemoglobin, and the color change caused by glucose can be detected with the same wavelength, the light-emitting section may be capable of emitting two types of light having different wavelengths The above-described light-emitting section capable of emitting two or more types of light having different wave lengths may include two or more light-emitting elements, each of which is capable of emitting light having a single wavelength. The light-emitting section preferably includes at least one light-emitting element capable of emitting two or more types of light having different wavelengths (multiple wavelength light-emitting element). In this case, the number of light-emitting elements included in the device can be reduced, and the size of the device can be reduced accord ingly Most preferably, the light-emitting section includes a light-emitting element capable of emitting three types of light having different wavelengths. In this case, the color change caused by glucose, the color change caused by gly cated hemoglobin, and the color change caused by hemoglo bin can be detected with light having different wavelengths by using a single light-emitting element It is also preferable that the light-emitting section capable of emitting two or more or three types of light having different wavelengths include three light-emitting elements (each of which may either be, for example, a light-emitting element capable of emitting light having a single wavelength or a multiple-wavelength light-emitting element), one of which emits light toward one side of the test piece and the remaining two of which emit light toward the other side of the test piece. More specifically, for example, two light-emitting elements may be arranged one on each side (each of front and back sides) of the test piece so that one of the light-emitting elements emits light toward the front surface of the test piece and the other light-emitting element emits light toward the back Surface of the test piece. In this case, two types of light having different wavelengths can be simultaneously emitted toward the test piece, so that the measurement speed can be increased The light-emitting section preferably includes at least one light-emitting element having a peak wavelength of 600 nm or more and a luminous intensity of 1000 mcd or more, more preferably 2000 mcd or more, and still more preferably 3000 mcd or more. In this case, the light at the high wavelength side can be reliably received (detected) and the measurement accuracy can be increased In the measuring device according to the present invention, preferably, two of the test-piece-mounting sections are provided, the test piece (A) being mounted by one of the two test-piece-mounting sections and the test piece (B) being mounted by the other of the two test-piece-mounting sections, and the test pieces (A) and (b) have different shapes or sizes. In other words, when a test-piece-mounting section for mea Suring the blood glucose level and a test-piece-mounting sec tion for measuring the hemoglobin A1c level are provided, the test pieces to be mounted by the respective test-piece-mount ing sections preferably have different shapes or sizes. In this case, insertion of the wrong one of the test pieces (A) and (B) can be prevented In the measuring device according to the present invention, preferably, the test-piece-mounting section has a test-piece-receiving hole, and the test-piece-receiving hole has an edge portion that guides the test piece (A) and an edge portion that guides the test piece (B), which has a shape different from a shape of the test piece (A). In this case, the size of the device can be reduced since only one test-piece mounting section is provided, and insertion of the wrong one of the test pieces (A) and (B) can be prevented A measuring method of the present invention, which achieves the above-described object, includes measuring both a blood glucose level and a hemoglobin A1c level of a blood specimen by using a measuring device including a
12 light-emitting section capable of emitting two or more types (in particular, three types) of light having different wave lengths. Another measuring method according to the present invention uses a measuring device including a test-piece mounting section that detachably mounts a test piece to which a blood specimen is applied, a light-emitting section that emits illuminating light toward the test piece, a light-receiv ing section that receives reflected light from the test piece, and an operating section that each of a blood glucose level and a hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section. A test piece (A) that carries a composition (a) that reacts with glucose and changes color is used as the test piece when the blood glucose level of the blood specimen is to be measured, and a test piece (B) that carries a composition (b) that reacts with glycated hemoglobin and changes color is used as the test piece when the hemoglobin A1c level of the blood speci men is to be measured. The measuring method includes applying the blood specimen to the test piece, emitting the illuminating light toward the test piece with the light-emitting section, receiving the reflected light from the test piece with the light-receiving section, and calculating the blood glucose level or the hemoglobin A1c level with the operating section on the basis of the obtained photometric value. With the measuring method according to the present invention, the blood glucose level and the hemoglobin A1c level can both be appropriately measured by two simple processes of applying the blood specimen to the test piece and mounting the test piece In the measuring method according to the present invention, in the case where the measuring device includes one of the test-piece-mounting section, preferably, the test piece (A) is mounted by the one test-piece-mounting section when the blood glucose level of the blood specimen is to be measured and the test piece (B) is mounted by the one test piece-mounting section when the hemoglobin A1c level of the blood specimen is to be measured. When only one test piece-mounting section is provided and the test piece corre sponding to the measurement item (the blood glucose level or the hemoglobin A1c level) is mounted by the test-piece mounting section as appropriate, the size of the device can be reduced According to the present invention, preferably, the composition (a) carried by the test piece (A) contains glucose oxidase, peroxidase, and a redox color reagent, and the com position (b) carried by the test piece (B) contains protease, fructosyl-amino acid oxidase, peroxidase, and a redox color reagent. In this case, the color of the test piece can be reliably and efficiently changed by glucose and glycated hemoglobin contained in the blood specimen. Advantageous Effects of Invention 0033 According to the present invention, the blood glu cose level and the hemoglobin A1c level can both be mea sured with a single device that can be used at a POC. More specifically, the present invention has an advantage that patients can measure the hemoglobin Alc level together with the blood glucose level simply by using a few drops of blood as the specimen, and POC tests for diabetes can be easily performed not only at hospitals but also at homes In particular, since the measuring device according to the present invention measures both the blood glucose level and the hemoglobin A1c level on the basis of the same mea Surement principle, simplification and size reduction of the device can be easily achieved. The measuring method accord ing to the present invention is a so-called dry measuring method in which the blood specimen is applied to the test piece and Subjected to the measurement. Therefore, it is not necessary to perform dilution, centrifugal separation, equip ment cleaning, etc., which are essential in wet measuring methods, such as the conventional methods for measuring the hemoglobin A1c level based on the immunization method or the enzyme colorimetric method. Since the measuring method according to the present invention is a dry measuring method, the measurement can be performed by using a small amount of specimen, and the test piece to which the blood specimen is applied can be easily handled. In addition, the test piece is disposable, and can be easily disposed of after use. This is also advantageous from the viewpoint of hygiene and infection prevention. BRIEF DESCRIPTION OF DRAWINGS 0035 FIG. 1 is a perspective view of a measuring device according to an embodiment of the present invention and test pieces to be mounted into the measuring device FIG. 2 is a schematic sectional view of the measur ing device illustrated in FIG. 1 taken along line X-X when a test piece (A) is mounted in the measuring device FIG. 3 is a schematic sectional view of the measur ing device illustrated in FIG. 1 taken along line y-y when a test piece (B) is mounted in the measuring device FIG. 4 is a schematic diagram illustrating a measur ing device according to another embodiment of the present invention, and, similar to FIG.3, is a schematic sectional view of the measuring device illustrated in FIG. 1 taken along line y-y when a test piece (B) is mounted into the measuring device FIG. 5 is a schematic sectional view of a light emitting section and a light-receiving section of a measuring device according to another embodiment of the present inven tion FIG. 6 is a schematic sectional view of a light emitting section and a light-receiving section of a measuring device according to another embodiment of the present inven tion FIG. 7 is a perspective view of a blood collection needle that can be used to collect a blood specimen to be Subjected to a measurement according to an embodiment of the present invention FIG. 8 is a perspective view of a measuring device and a test piece to be mounted into the measuring device according to another embodiment of the present invention FIG. 9 shows perspective views of a measuring device and test pieces to be mounted into the measuring device according to another embodiment of the present inven tion FIG. 10 shows perspective views of test-piece-re ceiving holes formed intest-piece-mounting sections of mea Suring devices according to other embodiments of the present invention FIG. 11 is a perspective view of a measuring device and test pieces to be mounted onto the measuring device according to another embodiment of the present invention FIG. 12 is a perspective view of a measuring device and a test piece to be mounted onto the measuring device according to another embodiment of the present invention.
13 0047 FIG. 13 is a schematic sectional view of a light emitting section and a light-receiving section of a measuring device according to another embodiment of the present inven tion. DESCRIPTION OF EMBODIMENTS 0048 (Measuring Device) A measuring device according to the present inven tion measures a blood glucose level and a hemoglobin Alc level of a blood specimen by using a predetermined test piece or test pieces. Hereinafter, the measuring device according to the present invention will be described with reference to the drawings FIG. 1 is a perspective view of a measuring device 100, which is a measuring device according to an embodi ment of the present invention, and test pieces 200 to be mounted into the measuring device 100. FIG. 2 is a schematic sectional view of the measuring device 100 illustrated in FIG. 1 taken along line X-X when a test piece (A) (200a) for mea Suring a blood glucose level is mounted in the measuring device 100 as one of the test pieces 200. FIG. 3 is a schematic sectional view of the measuring device 100 illustrated in FIG. 1 taken along line y-y when a test piece (B) (200b) for mea Suring a hemoglobin Alc level is mounted in the measuring device 100 as another one of the test pieces Referring to FIGS. 1 to 3, the measuring device 100 according to the present invention includes test-piece-mount ing sections 1 into which the test pieces 200, to which the blood specimen is applied, are detachably mounted; a light emitting section 2 that emits illuminating light toward the test pieces 200; a light-receiving section 3 that receives reflected light from the test pieces 200; and an operating section 4 that calculates a blood glucose level and a hemoglobin Alc level of the blood specimen on the basis of photometric values obtained from the light-receiving section Although the measuring device 100 illustrated in FIGS. 1 to 3 includes two test-piece-mounting sections 1, the number of test-piece-mounting sections 1 may instead be one, as illustrated in FIG.8. When the test pieces 200 are mounted into the measuring device 100 illustrated in FIGS. 1 to 3 which includes the two test-piece-mounting sections 1, one test-piece-mounting section 1a receives the test piece (A) (200a) for measuring the blood glucose level, which will be described below, and the other test-piece-mounting section 1b receives the test piece (B) (200b) for measuring the hemo globin A1c level, which will be described below. In the case where only one test-piece-mounting section 1 is provided, the test pieces 200 may be selectively mounted as appropriate so that the test-piece-mounting section 1 receives the test piece (A) when the blood glucose level is to be measured and receives the test piece (B) when the hemoglobin A1c level is to be measured. Although two test-piece-mounting sections 1 are preferably provided to increase the measurement speed, the number of test-piece-mounting sections 1 is preferably only one from the viewpoint of reducing the size of the device In the measuring device 100 illustrated in FIGS. 1 to 3 and in the configuration illustrated in FIG. 8, each test piece-mounting section 1 has a rectangular test-piece-receiv ing hole. However, in the case where there is only one test piece-receiving hole in the test-piece-mounting section 1, the test-piece-receiving hole is preferably shaped so as to have an edge portion that guides the test piece (A) for measuring the blood glucose level and an edge portion that guides the test piece (B) for measuring the hemoglobin A1c level. For example, the test-piece-receiving hole may be cross-shaped as illustrated in FIG. 9, T-shaped as illustrated in FIG. 10(a), or L-shaped as illustrated in FIG. 10(b). In the case where only one test-piece-mounting section 1 is provided to reduce the size of the device, if for example, the test-piece-receiving hole has a simple rectangular shape as illustrated in FIG. 8, the test piece (A) and the test piece (B) are necessarily formed in the same shape. In Such a case, there is a risk that a wrong test piece will be inserted, for example, the test piece (B) for measuring the hemoglobin A1c level will be inserted when the blood glucose level is to be measured. However, when the test-piece-receiving hole is shaped as illustrated in FIGS. 9 and 10, the test piece (A) and the test piece (B) may beformed in obviously different shapes. This contributes to preventing the insertion of the wrong test piece In the case where the test-piece-receiving hole in the test-piece-mounting section 1 is cross-shaped as illustrated in FIG.9, a test piece 200a illustrated in FIG. 9(a), for example, which has a Small dimension (thickness) in the Vertical direc tion of the device is preferably selected as the test piece (A) for measuring the blood glucose level, and a test piece illus trated in FIG.9(b), for example, which has a large dimension (thickness) in the vertical direction of the device is preferably selected as the test piece (B) for measuring the hemoglobin A1c level. The reason for this is as follows. That is, although the test piece (A) for measuring the blood glucose level and the test piece (B) for measuring the hemoglobin A1c level have a multilayer structure in which a plurality of layers are stacked, the test piece (A) for measuring the blood glucose level generally have a smaller number of layers and can be more easily reduced in thickness compared to the test piece (B) for measuring the hemoglobin A1c level In the measuring device 100 illustrated in FIG. 1, the test-piece-mounting sections 1 (1a and 1b) are formed in a side surface of a main body of the device. However, locations of the test-piece-mounting sections 1 are not particularly limited. For example, as illustrated in FIGS. 11 and 12, the test-piece-mounting sections 1 may be formed in the top surface of the main body of the device, and the test pieces may be placed onto the test-piece-mounting sections 1 from above The light-emitting section 2 includes one, two, or more light-emitting elements, and is arranged so that illumi nating light can be emitted toward the test pieces 200 mounted in the test-piece-mounting sections 1 (more specifi cally, toward blood-specimen-applying portions 5 of the test pieces 200 to which the blood specimen is applied) (in the drawings, the dotted lines show the trajectories of light). In the case where a plurality of light-emitting elements are pro vided, all of the light-emitting elements may be arranged on one side (front side or back side) of the test pieces 200. Alternatively, the light-emitting elements may be arranged on both sides (front side and back side) of the test pieces 200. All of the light-emitting elements are preferably arranged on one side of the test pieces 200, as illustrated in FIG. 13, from the viewpoint of reducing the thickness and size of the device. Preferably, the light-emitting elements are arranged on the back side of the test pieces Although the light-emitting section 2 may include any number of light-emitting elements as described above, the light-emitting section 2 is preferably capable of emitting three types of light having different wavelengths. Although the measurement principle of the device according to the
14 present invention will be described in detail below, the device according to the present invention basically measures the hemoglobin Alc level by detecting a color change caused by hemoglobin and a color change caused by glycated hemoglo bin and calculating the hemoglobin A1c level on the basis of the result of the detection. The color change caused by hemo globin and the color change caused by glycated hemoglobin are preferably detected by using reflection of light having different wavelengths. For this purpose, the light-emitting section 2 is preferably capable of emitting two types of light having different wavelengths. In addition, a color change caused by glucose that is detected to measure the blood glu cose level is preferably detected by using reflection of light having a wavelength different from those of the light used to detect the color changes caused by hemoglobin and glycated hemoglobin. Therefore, the light-emitting section 2 is pref erably capable of emitting three types of light having different wavelengths In order for the light-emitting section 2 to be capable of emitting three types of light having different wavelengths as described above, the light-emitting section 2 preferably includes at least one light-emitting element capable of emit ting two or more types of light having different wavelengths (multiple-wavelength light-emitting element). In the case where a multiple-wavelength light-emitting element is used as at least one of the light-emitting elements included in the light-emitting section 2 So that three types of light having different wavelengths can be emitted, the number of light emitting elements included in the device can be reduced. As a result, the size of the device can be reduced The light-emitting section 2 that is capable of emit ting three types of light having different wavelengths prefer ably includes at least two light-emitting elements (each of which may either be, for example, a light-emitting element capable of emitting light having a single wavelength or a multiple-wavelength light-emitting element). In Such a case, the hemoglobin A1c level can be measured by simultaneously emitting two types of light having different wavelengths toward the test piece. As a result, the measurement speed can be increased In the measuring device 100 illustrated in FIGS. 1 to 3, the light-emitting section 2 includes a light-emitting ele ment 2a illustrated in FIG.2 and a light-emitting element 2bb' illustrated in FIG. 3. The light-emitting element 2a emits illuminating light toward the blood-specimen-applying por tion 5 of the test piece (A) (200a) for measuring the blood glucose level that is mounted in the test-piece-mounting sec tion 1a. The light-emitting element 2bb' emits illuminating light toward the blood-specimen-applying portion 5 of the test piece (B) (200b) for measuring the hemoglobin A1c level that is mounted in the test-piece-mounting section 1b. The light-emitting element 2bb' is a multiple-wavelength light emitting element, and emits light having a wavelength for detecting a color change caused by hemoglobin and light having a wavelength for detecting a color change caused by glycated hemoglobin. The light-emitting element 2a is not limited as long as the light-emitting element 2a can emit light having a wavelength for detecting a color change caused by glucose Instead of the light-emitting element 2bb' illustrated in FIG. 3, the light-emitting section 2 may include two light emitting elements 2b and 2b' as illustrated in FIG. 4. In the configuration illustrated in FIG. 4, the two light-emitting elements 2b and 2b' are arranged one on each side (each of front and back sides) of the test piece 200b. One light-emit ting element 2b emits light toward the front surface of the test piece 200b, and the other light-emitting element 2b' emits light toward the back surface of the test piece 200b. In the configuration illustrated in FIGS. 1, 2, and 4, two light-emit ting elements 2a and 2b' are arranged at one side of the device (back side of the test piece) and one light-emitting element 2b is arranged at the other side of the device (front side of the test piece) across the test piece. Since the light-emitting elements are arranged at one and the other sides of the device, the size of the device can be reduced Alternatively, as illustrated in FIG. 13, the light emitting section 2 may include three light-emitting elements 2a, 2b, and 2b' on one side of the test piece The light-emitting section 2 preferably includes one, two, or more light-emitting elements that emit light having peak wavelengths in the range of 450 nm to 780 nm, preferably in the ranges of 450 nm to 550 nm and 600 nm to 700 nm, and more preferably in the ranges of 450 nm to 500 nm and 630 nm to 700 nm. Accordingly, the test piece that has changed color can be irradiated with light having a wave length Suitable for detecting the color change. For example, light having a wavelength in the range of 450 nm to 550 nm, more preferably 450 to 500 nm, may be emitted to detect a color change caused by hemoglobin, and light having wave lengths in the range of 600 nm to 700 nm, more preferably 630 nm to 700 nm, may be emitted to detect a color change caused by glucose and a color change caused by glycated hemoglo bin. Examples of the light-emitting elements include a blue light-emitting diode, a green light-emitting diode, and a red light-emitting diode The light-emitting section 2 preferably includes light-emitting elements having a luminous intensity of 1000 mcd or more, more preferably includes light-emitting ele ments having a luminous intensity of 2000 mcd or more, and still more preferably includes light-emitting elements having a luminous intensity of 3000 mcd or more. At least one of the light-emitting elements has a peak wavelength in the range of 600 nm or more and 700 nm or less and a luminous intensity of 1000mcd or more, more preferably 2000 mcd or more, and still more preferably 3000 mcd or more. In other words, among the light-emitting elements included in the light-emit ting section 2, the light-emitting element having a peak wave length in the range of 600 nm or more and 700 nm or less has a luminous intensity of 1000 mcd or more, more preferably 2000 mcd or more, and still more preferably 3000 mcd or more. In this case, the light at the high wavelength side can be reliably received (detected) so that the accuracy of measure ment of, in particular, the hemoglobin Alc level is expected to be increased. More preferably, the light-emitting element having a peak wavelength in the range of 630 nm or more and 700 nm or less has a luminous intensity of 1000 mcd or more, more preferably 2000 mcd or more, and still more preferably 3000 mcd or more The light-receiving section 3 includes one, two, or more light-receiving elements, and is arranged so that light emitted from the light-emitting section 2 (light-emitting ele ments) and reflected by the blood-specimen-applying por tions 5 of the test pieces 200 can be received. The light receiving section3 may either include a single light-receiving element for each light-emitting element or two or three light receiving elements for each light-emitting element. A Suitable number of light-receiving elements may be arranged in accor dance with the corresponding light-emitting element. The
15 light-receiving section 3 preferably includes a light-receiving element capable of receiving two or more types of light hav ing different wavelengths (multiple-wavelength light-receiv ing element) so that the size of the device can be reduced In the measuring device 100 illustrated in FIGS. 1 to 3, the light-receiving section 3 includes a light-receiving ele ment3a illustrated in FIG. 2 that receives light emitted from the light-emitting element 2a and a light-receiving element 3bb' illustrated in FIG. 3 that receives light emitted from the light-emitting element 2bb'. In this case, the light-receiving element 3bb', which corresponds to the light-emitting ele ment 2bb' that is a multiple-wavelength light-emitting ele ment, is a multiple-wavelength light-receiving element. When, for example, light having a wavelength for detecting a color change caused by hemoglobin is emitted from the light emitting element 2bb', the light-receiving element 3bb' receives this light. When light having a wavelength for detect ing a color change caused by glycated hemoglobin is emitted from the light-emitting element 2bb', the light-receiving ele ment 3bb receives this light The light-receiving section 3 included in the mea suring device 100 illustrated in FIGS. 1 to 3 may include light-receiving elements 3b and 3b' illustrated in FIG. 5 instead of the light-receiving element 3bb' illustrated in FIG. 3. In the configuration illustrated in FIG. 5, the light-receiving elements 3b and 3b' are provided for the light-emitting ele ment 2b, which is a multiple-wavelength light-emitting ele ment. The light-receiving elements 3b and 3b' respectively receive light having a wavelength for detecting a color change caused by hemoglobin and light having a wavelength for detecting a color change caused by glycated hemoglobin In the case where the light-emitting section 2 is structured as illustrated in FIG. 4, the light-receiving section 3 includes a light-receiving element 3b that receives light reflected by the front surface of the test piece 200b and a light-receiving element 3b' that receives light reflected by the back surface of the test piece 200b, as illustrated in FIG Alternatively, as illustrated in FIG. 13, the light receiving section 3 may include a multiple-wavelength light receiving element 3abb' that receives light emitted from the three light-emitting elements 2a, 2b, and 2b' The light-receiving elements included in the light receiving section 3 are not limited as long as the light emitted from the corresponding light-emitting elements can be received. The light-receiving elements may be, for example, photodiodes In the case where the measuring device includes a single test-piece-mounting section 1, the light-emitting sec tion 2 and the light-receiving section 3 may, for example, be structured as illustrated in FIG. 6. More specifically, a single light-emitting element 2a and a single light-receiving ele ment3a may be arranged on the frontside of the test piece 200 and a single light-emitting element 2bb', which is a multiple wavelength light-emitting element, and two light-receiving elements 3b and 3b' are arranged on the back side of the test piece 200. In this case, when the blood glucose level is to be measured, light having a wavelength for detecting a color change caused by glucose is emitted by the light-emitting element 2a and received by the light-receiving element 3a. When the hemoglobin A1c level is to be measured, light having a wavelength for detecting a color change caused by hemoglobin and light having a wavelength for detecting a color change caused by glycated hemoglobin are emitted by the light-emitting element 2bb'at different times and received by the light-receiving elements 3b and 3b', respectively Alternatively, two light-emitting elements and three light-receiving elements may be arranged as illustrated in FIG. 6, and the light-emitting element 2a and the light-receiv ing element 3a may be configured to respectively emit and receive one of light having a wavelength for detecting a color change caused by hemoglobin and light having a wavelength for detecting a color change caused by glycated hemoglobin. In this case, a color change caused by hemoglobin and a color change caused by glycated hemoglobin can be simulta neously detected when the hemoglobin A1c level is to be measured. Therefore, the measurement speed can be increased. In this case, one of the two light-receiving ele ments 3b and 3b' illustrated in FIG. 6 receives light having a wavelength for detecting a color change caused by glucose, and the other receives light having a wavelength for detecting a color change caused by hemoglobin or glycated hemoglo bin. When the color change cased by glucose is compared with the color change caused by hemoglobin or glycated hemoglobin, the color changed by hemoglobin or glycated hemoglobin has a lower reflectance. Therefore, preferably, the light-receiving element 3b, which is closer to the test piece 200, receives the light having a wavelength for detecting a color change caused by hemoglobin or glycated hemoglobin and the light-receiving element 3b', which is farther from the test piece 200, receives the light having a wavelength for detecting a color change caused by glucose The operating section 4 is electrically connected to the light-receiving section 3 so that electrical signals can be transmitted (circuit for transmitting the electrical signals is shown by bold arrows in the figures). The operating section 4 includes a CPU (storage unit) that calculates the blood glu cose level and the hemoglobin A1c level of the blood speci men on the basis of photometric values transmitted from the light-receiving section 3 as electrical signals As in the measuring device 100 illustrated in FIG. 1, a measuring device according to the present invention may include a power switch 6 and a monitor 7 that is electrically connected to the operating section 4 and displays the mea Surement results on the basis of electric signals transmitted from the operating section 4 (circuit for transmitting the elec trical signals is shown by bold arrows in the figures). The measuring device according to the present invention may further include a USB connector used to transmit the mea Surement results to a storage device. Such as a PC. Such an USB connector is convenient because the measurement results of the blood glucose level, which is particularly fre quently measured, may be recorded and managed by using a PC or the like. The measuring device according to the present invention may additionally have structures and members (for example, a sound generator) of conventionally known POC devices (such as SMBG devices) as appropriate. (0075. In the measuring device 100 illustrated in FIG. 1, it is assumed that the test pieces 200 are mounted into the device after the blood specimen is applied to the test pieces 200. Alternatively, however, a specimen-introducing hole may be formed in an upper section of the device, and the blood specimen may be dropped into the specimen introducing hole and guided to test pieces that are mounted in the device in advance The measuring device according to the present invention may be used as a POC device. Therefore, the mea suring device 100 has a length of 100 mm or less, a width of
16 70 mm or less, and a thickness of 30 mm or less, for example, and the weight thereof is 150 g or less, more preferably 100 g or less (Test Piece) The test piece is mounted into the measuring device according to the present invention each time a measurement is performed. The test piece (A), which carries a composition (a) that reacts with glucose and changes color, is mounted when the blood glucose level is to be measured. The test piece (B), which carries a composition (b) that reacts with glycated hemoglobin and changes color, is mounted when the hemo globin A1c level of the blood specimen is to be measured The test piece (each of the test piece (A) and the test piece (B)) includes a base plate that carries a composition (composition (a) or composition (b)) that is configured to react with glucose or hemoglobin Alic contained in the blood specimen and change color and that contains a predetermined enzyme and a redox color reagent. The base plate may be formed of, for example, polyesters, polyamides, polyether sulfones or celluloses. The enzyme is selected from, for example, glucose oxidase (GOD), peroxidase (POD), fructo syl-amino acid oxidase (FAOD), and protease. The redox color reagent includes, for example, 4-aminoantipyrine (4AA) as a coupler and a Trinder reagent, such as N-ethyl-N- (2-hydroxy-3-sulfopropyl)-m-toluidine sodium salt (TOOS; absorption wavelength max=555 nm), N-ethyl-N-(2-hy droxy-3-sulfopropyl)-3,5-dianiline (MAOS; absorption wavelength max=630 nm), or N-ethyl-N-(2-hydroxy-3-sul fopropyl)-3,5-dimethoxyaniline (DAOS: absorption wave length max=593 nm) as a phenolic hydrogen donor; or leuco dye DA-67 (absorption wavelength max=666 nm) or DA-64 (absorption wavelength max=727 nm) other than Trinder reagents The composition (a) carried by the test piece (A) preferably contains glucose oxidase (GOD), peroxidase (POD), and a redox color reagent. The composition (b) car ried by the test piece (B) preferably contains protease, fruc tosyl-amino acid oxidase (FAOD), peroxidase (POD), and a redox color reagent. When the compositions contain these components, the test pieces reliably and efficiently react with glucose and glycated hemoglobin contained in the blood specimen and change color The shapes and sizes of the test pieces 200 (test piece (A) and test piece (B)) are not particularly limited. When, for example, two test-piece-mounting sections are provided as illustrated in FIG. 1 and the test pieces (A) and (B) are mounted into the respective test-piece-mounting sec tions, the test pieces (A) and (B) are preferably designed to have different shapes or sizes. Thus, in the case where the test-piece-mounting section for measuring the blood glucose level and the test-piece-mounting section for measuring the hemoglobin A1c level are provided, the test pieces to be mounted into the respective test-piece-mounting sections may have different shapes or sizes. In such a case, insertion of the wrong one of the test pieces (A) and (B) can be prevented The test pieces 200 may have the shape of, for example, a rectangular, circular, or elliptical thin plate. The test pieces 200 are preferably as small as possible from the viewpoint of reducing the size of the device. For example, when the test pieces 200 have the shape of a rectangular thin plate, the test pieces 200 have a length of 30 mm or less, a width of 20 mm or less, and a thickness of 5 mm or less Each of the test pieces 200 (test piece (A) and test piece (B)) may be provided with a bar code or an IC chip that allows the measurement item corresponding to the test piece to be recognized. In particular, in the case where the measur ing device includes only one test-piece-mounting section 1. the measurement item can be reliably recognized and light having a wavelength corresponding to the measurement item can be reliably emitted when the test piece mounted into the measuring device has a bar code or an IC chip that allows the measurement item to be recognized. I0084 (Measurement Principle) I0085. The measuring device according to the present invention measures both the blood glucose level and the hemoglobin Alc level by causing a reaction that causes a test piece to change color and detecting the level of the color change by using reflection of light (so-called enzyme colori metric method). Since the blood glucose level and the hemo globin A1c level are measured on the basis of the same mea Surement principle, a reduction in size of the device is achieved. The measurement principle will now be described Measurement of Blood Glucose Level I0087 To measure the blood glucose level, first, glucose contained in the blood specimen is caused to react with the composition (a) So that the test piece (A) changes color. For example, in the case where the composition (a) contains the above-described preferred components, glucose contained in the blood specimen applied to the test piece reacts with glu cose oxidase contained in the composition (a), so that glu conolactone and hydrogen peroxide are generated. The hydrogen peroxide reacts with the redox color reagent and peroxidase, so that the test piece (A) changes color. Subse quently, the portion whose color has changed is irradiated with light having a specific wavelength, and the reflected light is measured to detect the level of the color change. The wavelength of the irradiating light may be determined in accordance with the redox color reagent that is used. The blood glucose level is calculated on the basis of the thus obtained photometric value. The detailed calculating method may be similar to that of a conventionally known blood glu cose level measurement technology using the enzyme colo rimetric method (see, for example, Japanese Unexamined Patent Application Publication No ). I0088. In the blood glucose level measurement, the above described reaction for causing the test piece to change color occurs inside the test piece that carries the composition. Therefore, the reaction immediately occurs when the blood specimen (whole blood) is applied to the test piece, and the test piece normally changes color within several seconds after the application of the blood specimen. Therefore, according to the measuring device of the present invention, the measure ment speed can be increased. I0089. An example of a procedure for measuring the blood glucose level will now be described i) First, to measure a reference value (blank value), the test piece 200 is irradiated with light ad having a specific absorption wavelength of the dye before the blood specimen is applied to the test piece 200. The light 1 having the absorption wavelength of the dye is used to detect that the blood specimen has been absorbed by the test piece 200 and a color change has occurred ii) After the blood specimen has been absorbed by the test piece and a color change has been detected, the light-emitting section 2 (light-emitting element) emits the light 1 for a certain time at certain intervals. The light 1 is reflected by the test piece 200 and received by the light receiving section 3 (light-receiving element). The intensity
17 (photometric value) of the light 1 received by the light receiving section3 is transmitted to the operating section 4 as an electrical signal and is stored in a storage unit included in the operating section iii) The operating section 4 is configured to perform a step of calculating a reflectance R (%) on the basis of the intensity (photometric value) of the received light 1. Accordingly, the reflectance R is calculated iv) Subsequently, the operating section 4 converts the calculated reflectance R into a K/S value on the basis of the Kubelka-Munk formula (following Equation (1)). Equation 1 (1-R) (1) 0094 (In Equation (1), R represents the reflectance, K represents the absorption coefficient, and S represents the scattering coefficient) 0095 v) The relationship between the blood glucose level and the K/Svalue (standard curve) is stored in the storage unit of the operating section 4 in advance, and the blood glucose level is calculated on the basis of the standard curve. The calculated blood glucose level is transmitted to the display monitor 7 as an electrical signal and displayed In the blood glucose level measurement, the above described reaction for causing the test piece to change color occurs inside the test piece that carries the composition. Therefore, the reaction immediately occurs when the blood specimen (whole blood) is applied to the test piece, and the test piece normally changes color within several seconds after the application of the blood specimen. Therefore, according to the measuring device of the present invention, the measure ment speed can be increased Measurement of Hemoglobin A1c Level To measure the hemoglobin A1c level, concentra tions of hemoglobin Alc (glycated hemoglobin) and hemo globin in the blood specimen are determined, and the hemo globin A1c level is calculated on the basis of the determined concentrations. 0099] To determine the concentration of glycated hemo globin, first, glycated hemoglobin contained in the blood specimen is caused to react with the composition (b) so that the test piece (B) changes color. For example, in the case where the composition (b) contains the above-described pre ferred components, glycated dipeptide (fructosyl Valyl histi dine) at N-end of hemoglobin B chain in the blood specimen is cut off by the action of protease, and the glycated dipeptide reacts with fructosyl peptide oxidase, so that hydrogen per oxide is generated. The hydrogen peroxide reacts with the redox color reagent and peroxidase, so that the test piece (B) changes color. Subsequently, the portion whose color has changed is irradiated with light having a specific wavelength, and the reflected light is measured to detect the level of the color change. The wavelength of the irradiating light may be determined in accordance with the redox color reagent that is used. The concentration of glycated hemoglobin can be deter mined on the basis of the thus-obtained photometric value The concentration of hemoglobin can be determined by measuring the hemoglobin contained in the blood speci men in terms of for example, the absorbance of light having a wavelength of 475 nm The hemoglobin A1c level expressed in terms of molar ratio (mmol/mol), that is, the International Federation of Clinical Chemistry (IFCC) value, can be calculated on the basis of the above-described concentration of glycated hemo globin (Hb A1c concentration) and the above-described con centration of hemoglobin (total Hb concentration) from the following Equation (2). IFCC value (mmol/mol)=(hba1c concentration)f(total Hb concentration)x1000 (2) 0102 The hemoglobin A1c level may also be expressed in terms of percentage as the Japan Diabetes Society (JDS) value. The JDS value may be obtained by converting the IFCC value obtained by the above Equation (2) into the JDS value by using the following Equation (3). JDS value (%)=0.0963x(IFCC value)+1.62 (3) 0103 Here, (Hb A1c concentration)/(total Hb concentra tion) in the above Equation (2) is an example of a process of calculating the hemoglobin A1c level, and it is not always necessary to perform an intermediate calculation In the hemoglobin A1c level measurement, all of the above-described reactions for causing the test piece to change color occur inside the test piece that carries the composition. Therefore, the reaction immediately occurs when the blood specimen (whole blood) is applied to the test piece, and the test piece normally changes color within several seconds after the application of the blood specimen. Therefore, according to the measuring device of the present invention, the measure ment speed can be increased An example of a procedure for measuring the hemo globin A1c level will now be described I) First, to measure a reference value (blank value), the test piece 200 is irradiated with light 2 having a specific absorption wavelength of the dye (specific absorption wave length of glycated hemoglobin) and lightw3 having a specific absorption wavelength of hemoglobin. The light W2 having the absorption wavelength of the dye is used to detect that the blood specimen has been absorbed by the test piece 200 and a color change has occurred II) After the blood specimen has been absorbed by the test piece and a color change has been detected, the light-emitting section 2 (light-emitting element) emits the light 2 and light 3 for a certain time at certain intervals. The light W2 and light 3 are reflected by the test piece 200 and received by the light-receiving section 3 (light-receiving ele ment). The intensities (photometric values) of the light 2 and light 3 received by the light-receiving section 3 are trans mitted to the operating section 4 as electrical signals and are stored in the storage unit included in the operating section III) The operating section 4 is configured to perform a step of calculating reflectances R (%) on the basis of the intensities (photometric values) of the received light W2 and light 3. Accordingly, the reflectance R (R2) of the light 2 and the reflectance R (R3) of the light 3 are calculated IV) Subsequently, the operating section 4 converts the calculated reflectance R (R2) and the reflectance R (R3) of light 3 into a K/S value (2) and a K/S value (3), respectively, on the basis of the above-described Kubelka-Munk formula (Equation (1)) V) Subsequently, the operating section 4 calculates the K/S Ratio from the K/S value (3) corresponding to hemo globin and the K/S value (2) corresponding to the dye by using the following Equation (4). K/S Ratio=(K/S value (2))/(K/Svalue (3)) Eq. (4)
18 0111 VI). The relationship between the hemoglobin Alc level and the K/S Ratio (standard curve) is stored in the storage unit of the operating section 4 in advance, and the hemoglobin A1c level is calculated on the basis of the stan dard curve. The calculated hemoglobin A1c level is transmit ted to the display monitor 7 as an electrical signal and dis played The above-described calculations may be per formed by two typical methods for enzyme analysis, that is, an end point assay and a rate assay (initial velocity assay). Each of the measurement of blood glucose level and the measurement of hemoglobin A1c level may be performed by either of these methods. The measurement of blood glucose level and the measurement of hemoglobin A1c level are pref erably both performed by the rate assay from the viewpoint of increasing the measurement speed. In the case where the rate assay is used, when, for example, the hemoglobin Alc level is measured, the hemoglobin A1c level may be calculated on the basis of the photometric values at intervals of 10 to 20 seconds for a time period of 20 to 300 seconds from the time of detection of the occurrence of a color change, preferably at intervals of 10 seconds for a time period of 20 to 60 seconds and at intervals of 20 seconds for a time period of 60 to 300 seconds from the time of detection of the occurrence of color change. When the blood glucose level is to be measured, the blood glucose level may be calculated on the basis of the photometric value at, for example, intervals of 10 seconds for a time period of 60 seconds from the time of detection of the occurrence of a color change (Measuring Method) According to a measuring method of the present invention, the blood glucose level and the hemoglobin A1c level of the blood specimen are both measured by using a measuring device including a light-emitting section capable of emitting two types of light having different wavelengths. More specifically, the above-described measuring device according to the present invention is used. The above-de scribed test piece (A) is used as a test piece when the blood glucose level of the blood specimen is to be measured, and the above-described test piece (B) is used as a test piece when the hemoglobin A1c level of the blood specimen is to be mea sured. Since the predetermined test pieces are selectively mounted in accordance with the measurement item, the blood glucose level and the hemoglobin A1c level can both be measured by a single device. In the case where a measuring device including a single test-piece-mounting section is used, the test piece (A) is mounted into the single test-piece-mount ing section when the blood glucose level of the blood speci men is to be measured and the test piece (B) is mounted into the single test-piece-mounting section when the hemoglobin A1c level of the blood specimen is to be measured For example, when the measuring device 100 illus trated in FIGS. 1 to 3 is used, a blood specimen is applied to one of the test pieces 200, and the test piece 200 is mounted into the corresponding test-piece-mounting section 1. The light-emitting section 2 emits light toward the mounted test piece 200 (toward the blood-specimen-applying portion 5 to be exact), and the light reflected by the test piece 200 is received by the light-receiving section 3. The operating sec tion 4 calculates the blood glucose level or the hemoglobin A1c level on the basis of the obtained photometric value. In this method, the only processes that are actually performed by an operator include turning on and off the power Switch 6, mounting the test piece into the device, and applying the blood specimento the test piece. Therefore, the operator is not required to perform complex processes such as a front-end process for the specimen, and the measurement is very simple According to the present invention, whole blood is used as the blood specimen subjected to the measurement. The amount of blood specimen required for each of the mea Surement of blood glucose level and the measurement of hemoglobin A1c level is normally 10 LIL or less, preferably 5 LL or less, more preferably 3 ui or less, and still more pref erably 1 u, or less. According to the device of the present invention, even when the amount of blood specimen is very small as described above, the blood glucose level and the hemoglobin A1c level can be accurately measured. However, if the amount of blood specimen is too small, there is a risk that the accuracy of the measurement values will be reduced. Therefore, the amount of blood specimen is preferably 0.01 ul or more, more preferably 0.05 ul or more, and still more preferably 0.1 ul or more The blood specimen subjected to the measurement according to the present invention may be collected by using, for example, a blood collection needle 10 illustrated in FIG.7. The blood collection needle 10 includes a main body 11 and a replaceable needle 12that is detachably attached to the main body 11. The needle 12 projects outward when a button 13 is pressed. When the button 13 is pressed while the needle 12 of the blood collection needle 10 is pointed toward, for example, a finger, the needle 12 projects outward and the finger bleeds. A few drops of blood are obtained in this manner, and the obtained blood is applied to the test piece 200. EXAMPLES Example A blood glucose level and a hemoglobin A1c level were measured by using the measuring device 100 illustrated in FIGS. 1 to (Measurement of Blood Glucose Level) The power switch 6 of the measuring device 100 was turned on in advance so that the light-emitting element 2a and the light-receiving element 3a were operatively associ ated with each other and light having a wavelength of 630 nm was emitted from the light-emitting element 2a. A test piece carrying a composition (a) containing glucose oxidase (GOD) (produced by Toyobo Co., Ltd.), peroxidase (POD) (produced by Toyobo Co., Ltd.), and 4-aminoantipyrine/Nethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dianiline (4AA MAOS) as a redox color reagent was prepared as the test piece (A), and about 5 ul of blood specimen (whole blood) was applied to the test piece (A). As a result, the color of the test piece (A) was changed from white to blue within several seconds. The test piece (A) whose color had been changed was immediately inserted into the test-piece-receiving hole 1a in the measuring device 100. In the measuring device 100, the light having the wavelength of 630 nm was reflected by the test piece (A) and received by the light-receiving element 3a, and the obtained photometric value was transmitted to the operating section 4. The blood glucose level was calculated by the CPU included in the operating section 4. The photo metric value obtained when the test piece (A) that does not have the blood specimen (whole blood) applied thereto is irradiated with similar light was input to the operating section 4 of the measuring device 100 in advance as the blank value.
19 The photometric value was transmitted to the operating sec tion 4 at intervals of 10 seconds for a time period of 60 seconds from the start of the measurement, and the measure ment result was calculated by the rate assay. The thus-ob tained measurement result was displayed on the display monitor 7. The power switch 6 was turned off after the mea Surement (Measurement of Hemoglobin A1c Level) 0122) The power switch 6 of the measuring device 100 was turned on in advance so that the light-emitting element 2bb', which was a multiple-wavelength light-emitting ele ment, and the light-receiving element 3bb', which was a mul tiple-wavelength light-receiving element, were operatively associated with each other and light having a wavelength of 475 nm was emitted from the light-emitting element 2bb'. A test piece carrying a composition (b) containing protease (produced by Toyobo Co., Ltd.), fructosyl-amino acid oxi dase (FAOD) (produced by Toyobo Co., Ltd.), peroxidase (POD) (produced by Toyobo Co., Ltd.), and leuco dye DA-67 as a redox color reagent was prepared as the test piece (B), and about 5 ul of blood specimen (whole blood) was applied to the test piece (B). As a result, the color of the test piece (B) was changed from white to blue within several seconds. The test piece (B) whose color had been changed was immediately inserted into the test-piece-receiving hole 1b in the measuring device 100. In the measuring device 100, first, the light having the wavelength of 475 nm was reflected by the test piece (B) and received by the light-receiving element 3bb'. Subse quently, light having a wavelength of 660 nm was emitted from the light-emitting element 2bb', reflected by the test piece (B), and received by the light-receiving element 3bb'. The obtained photometric values were transmitted to the operating section 4, and the hemoglobin Alc level was cal culated by the CPU included in the operating section 4. The photometric value obtained when the test piece (B) that does not have the blood specimen (whole blood) applied thereto is irradiated with similar light was input to the operating section 4 of the measuring device 100 in advance as the blank value. The photometric values were transmitted to the operating section 4 at intervals of 10 seconds for a time period of 300 seconds from the start of the measurement, and the measure ment result was calculated by the rate assay. The thus-ob tained measurement result was displayed on the display monitor 7. The power switch 6 was turned off after the mea Surement. Example A blood glucose level and a hemoglobin A1c level were measured by using the measuring device 100 illustrated in FIGS. 1 to (Measurement of Blood Glucose Level) The power switch 6 of the measuring device 100 was turned on in advance so that the light-emitting element 2a and the light-receiving element 3a were operatively associ ated with each other and light having a wavelength of 550 nm was emitted from the light-emitting element 2a. A test piece carrying a composition (a) containing glucose oxidase (GOD) (produced by Toyobo Co., Ltd.), peroxidase (POD) (produced by Toyobo Co., Ltd.), and 4-aminoantipyrine/Nethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidine sodium salt (4AA-TOOS) as a redox color reagent was prepared as the test piece (A), and about 5 ul of blood specimen (whole blood) was applied to the test piece (A). As a result, the color of the test piece (A) was changed from white to purple red within several seconds. The test piece (A) whose color had been changed was immediately inserted into the test-piece receiving hole 1a in the measuring device 100. In the mea suring device 100, the light having the wavelength of 550 nm was reflected by the test piece (A) and received by the light receiving element3a, and the obtained photometric value was transmitted to the operating section 4. The blood glucose level was calculated by the CPU included in the operating section 4. The photometric value obtained when the test piece (A) that does not have the blood specimen (whole blood) applied thereto is irradiated with similar light was input to the oper ating section 4 of the measuring device 100 in advance as the blank value. The photometric value was transmitted to the operating section 4 at intervals of 10 seconds for a time period of 60 seconds from the start of the measurement, and the measurement result was calculated by the rate assay. The thus-obtained measurement result was displayed on the dis play monitor 7. The power switch 6 was turned off after the measurement (Measurement of Hemoglobin A1c Level) I0127. The power switch 6 of the measuring device 100 was turned on in advance so that the light-emitting element 2bb', which was a multiple-wavelength light-emitting ele ment, and the light-receiving element 3bb', which was a mul tiple-wavelength light-receiving element, were operatively associated with each other and light having a wavelength of 540 nm was emitted from the light-emitting element 2bb'. A test piece carrying a composition (b) containing protease (produced by Toyobo Co., Ltd.), fructosyl-amino acid oxi dase (FAOD) (produced by Toyobo Co., Ltd.), peroxidase (POD) (produced by Toyobo Co., Ltd.), and 4-aminoantipy rine/n-ethyl-n-(2-hydroxy-3-sulfopropyl)-3,5-dianiline (4AA-MAOS) as a redox color reagent was prepared as the test piece (B), and about 5 ul of blood specimen (whole blood) was applied to the test piece (B). As a result, the color of the test piece (B) was changed from white to blue within several seconds. The test piece (B) whose color had been changed was immediately inserted into the test-piece-receiv ing hole 1b in the measuring device 100. In the measuring device 100, first, the light having the wavelength of 540 nm was reflected by the test piece (B) and received by the light receiving element 3bb'. Subsequently, light having a wave length of 630 nm was emitted from the light-emitting element 2bb', reflected by the test piece (B), and received by the light-receiving element 3bb'. The obtained photometric val ues were transmitted to the operating section 4, and the hemo globin A1c level was calculated by the CPU included in the operating section 4. The photometric value obtained when the test piece (B) that does not have the blood specimen (whole blood) applied thereto is irradiated with similar light was input to the operating section 4 of the measuring device 100 in advance as the blank value. The photometric values were transmitted to the operating section 4 at intervals of 10 sec onds for a time period of 300 seconds from the start of the measurement, and the measurement result was calculated by the rate assay. The thus-obtained measurement result was displayed on the display monitor 7. The power switch 6 was turned off after the measurement. I0128. The measuring device and the measuring method according to the present invention have been specifically described with reference to the drawings. However, the present invention is not limited to the illustrated examples, and may be implemented with modifications as appropriate within the spirit of the present invention described above and
20 12 below. Such modifications are also included in the technical Scope of the present invention. REFERENCE SIGNS LIST I measuring device I test piece I test-piece-mounting section I light-emitting section light-receiving section operating section I0135) 5 blood-specimen-applying portion ( power switch I display monitor blood collection needle I main body of blood collection needle needle button 1. A measuring device for measuring a blood glucose level and a hemoglobin Alc level of a blood specimen, the mea Suring device comprising: a light-emitting section that emits illuminating light toward the blood specimen; a light-receiving section that receives reflected light from the blood specimen; and an operating section that calculates each of the blood glu cose level and the hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section, wherein the light-emitting section is capable of emitting two or more types of light having different wavelengths. 2. The measuring device according to claim 1, wherein the light-emitting section is capable of emitting three types of light having different wavelengths. 3. The measuring device according to claim 2, wherein one of the three types of light having different wavelengths is light having a specific wavelength that is absorbed by a portion whose color has changed in response to glucose contained in the blood specimen and the remaining two of the three types of light having different wavelengths are two types of light having different specific wavelengths that are absorbed by respective portions whose colors have changed in response to hemoglobin and glycated hemoglobin contained in the blood specimen. 4. The measuring device according to claim 1, further comprising a test-piece-mounting section that detachably mounts a test piece to which the blood specimen is applied. 5. A measuring device for measuring a blood glucose level and a hemoglobin Alc level of a blood specimen, the mea Suring device comprising: a test-piece-mounting section that detachably mounts a test piece to which the blood specimen is applied; a light-emitting section that emits illuminating light toward the test piece; a light-receiving section that receives reflected light from the test piece; and an operating section that calculates each of the blood glu cose level and the hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section, wherein a test piece (A) that carries a composition (a) that reacts with glucose and changes color is mounted as the test piece to measure the blood glucose level of the blood specimen, and a test piece (B) that carries a composition (b) that reacts with glycated hemoglobin and changes color is mounted as the test piece to measure the hemo globin A1c level of the blood specimen. 6. The measuring device according to claim 5, wherein the light-emitting section is capable of emitting three types of light having different wavelengths. 7. The measuring device according to claim 1, wherein the light-emitting section includes at least one light-emitting ele ment capable of emitting two or more types of light having different wavelengths. 8. The measuring device according to claim 7, wherein the light-emitting section includes a light-emitting element capable of emitting three types of light having different wave lengths. 9. The measuring device according to claim 5, wherein the light-emitting section includes three light-emitting elements, one of which emits light toward one side of the test piece and the remaining two of which emit light toward the other side of the test piece. 10. The measuring device according to claim 5, wherein the light-emitting section includes a light-emitting element having a peak wavelength of 600 nm or more and a luminous intensity of 1000 mcd or more. 11. The measuring device according to claim 5, wherein two of the test-piece-mounting sections are provided, and the test piece mounted by one of the two test-piece-mounting sections and the test piece mounted by the other of the two test-piece-mounting sections have different shapes or sizes. 12. The measuring device according to claim 5, wherein the test-piece-mounting section has a test-piece-receiving hole, and the test-piece-receiving hole has an edge portion that guides the test piece (A) and an edge portion that guides the test piece (B), which has a shape different from a shape of the test piece (A). 13. A measuring method comprising measuring both a blood glucose level and a hemoglobin A1c level of a blood specimen by using a measuring device including a light emitting section capable of emitting two or more types of light having different wavelengths. 14. A measuring method using a measuring device includ ing a test-piece-mounting section that detachably mounts a test piece to which a blood specimen is applied, a light-emitting section that emits illuminating light toward the test piece, a light-receiving section that receives reflected light from the test piece, and an operating section that calculates each of a blood glucose level and a hemoglobin A1c level of the blood specimen on the basis of a photometric value obtained from the light-receiving section, wherein a test piece (A) that carries a composition (a) that reacts with glucose and changes color is used as the test piece when the blood glucose level of the blood speci men is to be measured, and a test piece (B) that carries a composition (b) that reacts with glycated hemoglobin and changes color is used as the test piece when the hemoglobin A1c level of the blood specimen is to be measured, and wherein the measuring method comprises applying the blood specimen to the test piece, emitting the illuminat ing light toward the test piece with the light-emitting section, receiving the reflected light from the test piece with the light-receiving section, and calculating the
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008022721 OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0227210 A1 Smith (43) Pub. Date: Sep. 18, 2008 (54) HOME TEST FOR GLYCATED ALBUMIN IN Publication Classification
US 2008022721 OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0227210 A1 Smith (43) Pub. Date: Sep. 18, 2008 (54) HOME TEST FOR GLYCATED ALBUMIN IN Publication Classification
TEPZZ 85_Z 4A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION
 (19) TEPZZ 8_Z 4A_T (11) EP 2 81 034 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.03.1 Bulletin 1/13 (21) Application number: 1022.2 (1) Int Cl.: A61C /02 (06.01) A61C 19/04 (06.01)
(19) TEPZZ 8_Z 4A_T (11) EP 2 81 034 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.03.1 Bulletin 1/13 (21) Application number: 1022.2 (1) Int Cl.: A61C /02 (06.01) A61C 19/04 (06.01)
(12) United States Patent
 USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(12) United States Patent (10) Patent No.: US 6,691,578 B1
 USOO6691578B1 (12) United States Patent (10) Patent No.: US 6,691,578 B1 Puskas (45) Date of Patent: Feb. 17, 2004 (54) MEASUREMENT SYSTEMS FOR 4,710,233 A 12/1987 Hohmann et al.... 205/701 ULTRASOUND
USOO6691578B1 (12) United States Patent (10) Patent No.: US 6,691,578 B1 Puskas (45) Date of Patent: Feb. 17, 2004 (54) MEASUREMENT SYSTEMS FOR 4,710,233 A 12/1987 Hohmann et al.... 205/701 ULTRASOUND
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 2004.0024334A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0024334 A1 Boncompte (43) Pub. Date: Feb. 5, 2004 (54) ULTRASOUND ENDOMASSAGE DEVICE (76) Inventor: Joan
(19) United States US 2004.0024334A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0024334 A1 Boncompte (43) Pub. Date: Feb. 5, 2004 (54) ULTRASOUND ENDOMASSAGE DEVICE (76) Inventor: Joan
United States Patent (19)
 United States Patent (19) Figone et al. 11 Patent Number: 4,834,212 (45) Date of Patent: May 30, 1989 54) (76) 21) 22 (51) (52) (58) SOUND MUFFLER FOR COVERING THE MOUTH Inventors: Moira J. Figone; Frank
United States Patent (19) Figone et al. 11 Patent Number: 4,834,212 (45) Date of Patent: May 30, 1989 54) (76) 21) 22 (51) (52) (58) SOUND MUFFLER FOR COVERING THE MOUTH Inventors: Moira J. Figone; Frank
IHIH IIII. United States Patent (19) 11 Patent Number: 5,417, Date of Patent: May 23, Kawai et al.
 United States Patent (19) Kawai et al. 54 METHOD FOR EXTRACTING AMARGIN LINE FOR DESIGNING AN ARTIFICIAL CROWN 75) Inventors: Masaharu Kawai, Kanagawa; Katsuya Miyoshi, Tokyo; Masami Baba, Saitama, all
United States Patent (19) Kawai et al. 54 METHOD FOR EXTRACTING AMARGIN LINE FOR DESIGNING AN ARTIFICIAL CROWN 75) Inventors: Masaharu Kawai, Kanagawa; Katsuya Miyoshi, Tokyo; Masami Baba, Saitama, all
(12) United States Patent
 USOO7672702B2 (12) United States Patent Hwang et al. (54) NONINVASIVE IN VIVO MEASURING SYSTEMAND NONINVASIVE IN VIVO MEASURING METHOD BY CORRECTING INFLUENCE OF HEMOGLOBIN (75) Inventors: In Duk Hwang,
USOO7672702B2 (12) United States Patent Hwang et al. (54) NONINVASIVE IN VIVO MEASURING SYSTEMAND NONINVASIVE IN VIVO MEASURING METHOD BY CORRECTING INFLUENCE OF HEMOGLOBIN (75) Inventors: In Duk Hwang,
...d. EEEEEEE/EE/EE. (12) Patent Application Publication (10) Pub. No.: US 2008/ A1. (19) United States
 (19) United States US 200802684.85A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0268485 A1 Guarino et al. (43) Pub. Date: Oct. 30, 2008 (54) ICONIC DISPLAY OF MARKERS FOR A METER (76) Inventors:
(19) United States US 200802684.85A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0268485 A1 Guarino et al. (43) Pub. Date: Oct. 30, 2008 (54) ICONIC DISPLAY OF MARKERS FOR A METER (76) Inventors:
(12) United States Patent (10) Patent No.: US 7.690,305 B2
 US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
(12) United States Patent
 US007094007B2 (12) United States Patent Satran et al. (10) Patent No.: (45) Date of Patent: Aug. 22, 2006 (54) TANGENTIAL CUTTING INSERT AND MILLING CUTTER (75) Inventors: Amir Satran, Kfar Vradim (IL);
US007094007B2 (12) United States Patent Satran et al. (10) Patent No.: (45) Date of Patent: Aug. 22, 2006 (54) TANGENTIAL CUTTING INSERT AND MILLING CUTTER (75) Inventors: Amir Satran, Kfar Vradim (IL);
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
(12) United States Patent
 (12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
(12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 2009.02451 05A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0245105 A1 HO (43) Pub. Date: Oct. 1, 2009 (54) METHOD FOR NETWORK TRANSMISSION (30) Foreign Application
(19) United States US 2009.02451 05A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0245105 A1 HO (43) Pub. Date: Oct. 1, 2009 (54) METHOD FOR NETWORK TRANSMISSION (30) Foreign Application
(12) United States Patent
 US007043040B2 (12) United States Patent Westerkull (10) Patent No.: (45) Date of Patent: May 9, 2006 (54) HEARING AID APPARATUS (75) Inventor: Patrick Westerkull, Hovås (SE) (73) Assignee: P&B Research
US007043040B2 (12) United States Patent Westerkull (10) Patent No.: (45) Date of Patent: May 9, 2006 (54) HEARING AID APPARATUS (75) Inventor: Patrick Westerkull, Hovås (SE) (73) Assignee: P&B Research
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
(19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
The Small-Size Electrode Type Blood Glucose Meter Antsense III and Point of Care Testing
 Feature Article The Small-Size Electrode Type Blood Glucose Meter Antsense III and Point of Care Testing Yoichi Ohmori Antsense III is a light, compact and portable electrode type blood glucose meter which
Feature Article The Small-Size Electrode Type Blood Glucose Meter Antsense III and Point of Care Testing Yoichi Ohmori Antsense III is a light, compact and portable electrode type blood glucose meter which
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(12) United States Patent
 USOO948.0458B2 (12) United States Patent Chiang et al. (10) Patent No.: (45) Date of Patent: Nov. 1, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (62) (30) ULTRASONIC POSITONING DEVICE FOR EPIDURAL SPACE
USOO948.0458B2 (12) United States Patent Chiang et al. (10) Patent No.: (45) Date of Patent: Nov. 1, 2016 (54) (71) (72) (73) (*) (21) (22) (65) (62) (30) ULTRASONIC POSITONING DEVICE FOR EPIDURAL SPACE
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 US 2005.0020415A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0020415 A1 Reno (43) Pub. Date: (54) ISOMETRIC EXERCISE EQUIPMENT WITH Publication Classification PORTABLE
US 2005.0020415A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0020415 A1 Reno (43) Pub. Date: (54) ISOMETRIC EXERCISE EQUIPMENT WITH Publication Classification PORTABLE
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 20070207515A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0207515 A1 Matsumoto et al. (43) Pub. Date: (54) METHOD OF MULTIQUANTIFICATION FOR CHOLESTEROL OF LOW-DENSITY
(19) United States US 20070207515A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0207515 A1 Matsumoto et al. (43) Pub. Date: (54) METHOD OF MULTIQUANTIFICATION FOR CHOLESTEROL OF LOW-DENSITY
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
E. }2. E. 156/578 X mountant on the Slide to Spread evenly over the Slide with a
 USOO.5989386A United States Patent (19) 11 Patent Number: 5,989,386 Elliott (45) Date of Patent: Nov. 23, 1999 54 COVERSLIPPICK-UP AND LAYDOWN 4,428,793 1/1984 Sato et al.. APPARATUS 4,455,188 6/1984 Stormby.
USOO.5989386A United States Patent (19) 11 Patent Number: 5,989,386 Elliott (45) Date of Patent: Nov. 23, 1999 54 COVERSLIPPICK-UP AND LAYDOWN 4,428,793 1/1984 Sato et al.. APPARATUS 4,455,188 6/1984 Stormby.
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
-34. (12) United States Patent. 52-e-E. (10) Patent No.: US 8,718,306 B2. (45) Date of Patent: May 6, 2014 / / / Gommel et al.
 USOO871 8306B2 (12) United States Patent Gommel et al. (54) HEARING DEVICE WITH A DETACHABLY COUPLED EARPIECE (75) Inventors: Uli Gommel, Erlangen (DE); Thomas Lotter, Heroldsberg (DE) (73) Assignee: Siemens
USOO871 8306B2 (12) United States Patent Gommel et al. (54) HEARING DEVICE WITH A DETACHABLY COUPLED EARPIECE (75) Inventors: Uli Gommel, Erlangen (DE); Thomas Lotter, Heroldsberg (DE) (73) Assignee: Siemens
Items in the package:
 Intended Use: The EasyLife Hb Monitoring System is designed for in vitro diagnostic use only (external use only), and is suitable for self-testing. The system is for healthcare professionals and persons
Intended Use: The EasyLife Hb Monitoring System is designed for in vitro diagnostic use only (external use only), and is suitable for self-testing. The system is for healthcare professionals and persons
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0135622 A1 SHMOKAWA et al. US 201701 35622A1 (43) Pub. Date: May 18, 2017 (54) (71) (72) (73) (21) (22) (86) (30) URINE FLOW
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0135622 A1 SHMOKAWA et al. US 201701 35622A1 (43) Pub. Date: May 18, 2017 (54) (71) (72) (73) (21) (22) (86) (30) URINE FLOW
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
(12) United States Patent (10) Patent No.: US 6,555,061 B1
 USOO6555061B1 (12) United States Patent (10) Patent No.: Leong et al. (45) Date of Patent: Apr. 29, 2003 (54) MULTI-LAYER REAGENT TEST STRIP 4,9,346 A 6/1990 Phillips et al.... 4/14 5,200,148 A 4/1993
USOO6555061B1 (12) United States Patent (10) Patent No.: Leong et al. (45) Date of Patent: Apr. 29, 2003 (54) MULTI-LAYER REAGENT TEST STRIP 4,9,346 A 6/1990 Phillips et al.... 4/14 5,200,148 A 4/1993
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0287886 A1 Patti US 20140287886A1 (43) Pub. Date: Sep. 25, 2014 (54) (71) (72) (21) (22) (60) PROTECTOR FOR EXERCISE BAR Applicant:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0287886 A1 Patti US 20140287886A1 (43) Pub. Date: Sep. 25, 2014 (54) (71) (72) (21) (22) (60) PROTECTOR FOR EXERCISE BAR Applicant:
1, 2021,22) (12) Patent Application Publication (10) Pub. No.: US 2011/ A1. (19) United States. Lee et al. (43) Pub. Date: Sep.
 (19) United States US 20110224481A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0224481 A1 Lee et al. (43) Pub. Date: (54) SPHENO-TEMPORAL BONE CONDUCTION COMMUNICATION EOUIPMENT AND/OR
(19) United States US 20110224481A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0224481 A1 Lee et al. (43) Pub. Date: (54) SPHENO-TEMPORAL BONE CONDUCTION COMMUNICATION EOUIPMENT AND/OR
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
TEPZZ _ 849 A_T EP A1 (19) (11) EP A1. (12) EUROPEAN PATENT APPLICATION published in accordance with Art.
 (19) TEPZZ _ 849 A_T (11) EP 3 138 493 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 3(4) EPC (43) Date of publication: 08.03.17 Bulletin 17/ (21) Application number: 786271. (22)
(19) TEPZZ _ 849 A_T (11) EP 3 138 493 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 3(4) EPC (43) Date of publication: 08.03.17 Bulletin 17/ (21) Application number: 786271. (22)
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070050.058A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0050058 A1 Zuziak et al. (43) Pub. Date: Mar. 1, 2007 (54) PLACEMAT FOR CALCULATING AND Related U.S. Application
US 20070050.058A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0050058 A1 Zuziak et al. (43) Pub. Date: Mar. 1, 2007 (54) PLACEMAT FOR CALCULATING AND Related U.S. Application
Piersch (45) Date of Patent: Jun. 29, 1993
 United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
United States Patent (19) Hensel
 United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
United States Patent (19) Groesch et al.
 United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Triunfol
 United States Patent (19) Triunfol 11 Patent Number: () Date of Patent: Sep. 9, 1986 (54) DEVICE FOR COLLECTING FLUID DISCHARGED FROM FEMALE ORGANS 76) Inventor: David Triunfol, 2001 N. 72 Ct., Elmwood
United States Patent (19) Triunfol 11 Patent Number: () Date of Patent: Sep. 9, 1986 (54) DEVICE FOR COLLECTING FLUID DISCHARGED FROM FEMALE ORGANS 76) Inventor: David Triunfol, 2001 N. 72 Ct., Elmwood
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O063621A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0063621 A1 NAUMANN (43) Pub. Date: (54) CONNECTOR FOR HEARING INSTRUMENT, HEARING INSTRUMENT AND HEARING INSTRUMENT
(19) United States US 2012O063621A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0063621 A1 NAUMANN (43) Pub. Date: (54) CONNECTOR FOR HEARING INSTRUMENT, HEARING INSTRUMENT AND HEARING INSTRUMENT
Protn-Latex. For Determination of Protein in Latex. (Cat. # Latex, Latex) think proteins! think G-Biosciences
 415PR-02 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Protn-Latex For Determination of Protein in Latex (Cat. # 786-20Latex, 786-21Latex)
415PR-02 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Protn-Latex For Determination of Protein in Latex (Cat. # 786-20Latex, 786-21Latex)
(12) United States Patent
 US009 138575B2 (12) United States Patent Osypka (54) (71) BALLOON CATHETER Applicant: Peter Osypka Stiftung, Grenzach-Wyhlen (DE) (72) Inventor: Peter Osypka, Grenzach-Wyhlen (DE) (73) Assignee: Peter
US009 138575B2 (12) United States Patent Osypka (54) (71) BALLOON CATHETER Applicant: Peter Osypka Stiftung, Grenzach-Wyhlen (DE) (72) Inventor: Peter Osypka, Grenzach-Wyhlen (DE) (73) Assignee: Peter
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 2004O23O254A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0230254A1 Harrison et al. (43) Pub. Date: Nov. 18, 2004 (54) HYBRID IMPLANTABLE COCHLEAR STIMULATOR HEARING
(19) United States US 2004O23O254A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0230254A1 Harrison et al. (43) Pub. Date: Nov. 18, 2004 (54) HYBRID IMPLANTABLE COCHLEAR STIMULATOR HEARING
(12) United States Patent (10) Patent No.: US 6,276,248 B1. Cranna (45) Date of Patent: Aug. 21, 2001
 USOO6276248B1 (12) United States Patent (10) Patent No.: Cranna () Date of Patent: Aug. 21, 2001 (54) BAND SAW BLADE HAVING REDUCED 5,331,876 * 7/1994 Hayden, Sr.... 83/851 NOISE AND UNIFORM TOOTH LOADING
USOO6276248B1 (12) United States Patent (10) Patent No.: Cranna () Date of Patent: Aug. 21, 2001 (54) BAND SAW BLADE HAVING REDUCED 5,331,876 * 7/1994 Hayden, Sr.... 83/851 NOISE AND UNIFORM TOOTH LOADING
(12) United States Patent Li et a].
![(12) United States Patent Li et a]. (12) United States Patent Li et a].](/thumbs/82/84922623.jpg) US008700126B2 (12) United States Patent Li et a]. (10) Patent N0.: (45) Date of Patent: Apr. 15, 2014 (54) SYSTEM AND METHOD FOR COMPUTER AIDED SEPTAL DEFECT DIAGNOSIS AND SURGERY FRAMEWORK (75) Inventors:
US008700126B2 (12) United States Patent Li et a]. (10) Patent N0.: (45) Date of Patent: Apr. 15, 2014 (54) SYSTEM AND METHOD FOR COMPUTER AIDED SEPTAL DEFECT DIAGNOSIS AND SURGERY FRAMEWORK (75) Inventors:
CPC... 46th Au (2013 on 46th 44 Brett P. Belden. Foley & Lardner LLP
 US009301731B2 (12) United States Patent (10) Patent No.: US 9,301,731 B2 Henderson et al. (45) Date of Patent: Apr. 5, 2016 (54) ULTRASOUND SYSTEMANDTRANSDUCER 2: E: ck 358. ihal. 1 361,679.41 mith et
US009301731B2 (12) United States Patent (10) Patent No.: US 9,301,731 B2 Henderson et al. (45) Date of Patent: Apr. 5, 2016 (54) ULTRASOUND SYSTEMANDTRANSDUCER 2: E: ck 358. ihal. 1 361,679.41 mith et
(12) United States Patent (10) Patent No.: US 9,039,574 B2
 US0090395.74B2 (12) United States Patent (10) Patent No.: US 9,039,574 B2 Wilson (45) Date of Patent: May 26, 2015 (54) EXERCISE RING USPC... 482/24, 44 50, 79, 91, 92, 121-128, 482/131, 139 (76) Inventor:
US0090395.74B2 (12) United States Patent (10) Patent No.: US 9,039,574 B2 Wilson (45) Date of Patent: May 26, 2015 (54) EXERCISE RING USPC... 482/24, 44 50, 79, 91, 92, 121-128, 482/131, 139 (76) Inventor:
1 ADJUSTMENT THRESHOLD-N. United States Patent (19) Donehoo et al. 5,660,184. Aug. 26, 1997 PATIENT'S HEARTBEAT PACEMAKEREVENT QRS DETECTION THRESHOLD
 United States Patent (19) Donehoo et al. US00566O184A 11 Patent Number: 45 Date of Patent: 5,660,184 Aug. 26, 1997 54 PACEMAKER PULSE DETECTION AND ARTIFACT REJECTION 75 Inventors: Robert F. Donehoo, Lutz;
United States Patent (19) Donehoo et al. US00566O184A 11 Patent Number: 45 Date of Patent: 5,660,184 Aug. 26, 1997 54 PACEMAKER PULSE DETECTION AND ARTIFACT REJECTION 75 Inventors: Robert F. Donehoo, Lutz;
(12) United States Patent (10) Patent No.: US 6,224,174 B1
 USOO6224174B1 (12) United States Patent (10) Patent No.: US 6,224,174 B1 Mu (45) Date of Patent: *May 1, 2001 (54) POSTER DISPLAY APPARATUS 3.817,393 6/1974 Neilsen... 211/50 4,164,309 8/1979 Staats...
USOO6224174B1 (12) United States Patent (10) Patent No.: US 6,224,174 B1 Mu (45) Date of Patent: *May 1, 2001 (54) POSTER DISPLAY APPARATUS 3.817,393 6/1974 Neilsen... 211/50 4,164,309 8/1979 Staats...
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060074454A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0074454 A1 Freeberg (43) Pub. Date: (54) METHODS AND SYSTEMS FOR SELECTION OF CARDAC PACING ELECTRODE CONFIGURATIONS
(19) United States US 20060074454A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0074454 A1 Freeberg (43) Pub. Date: (54) METHODS AND SYSTEMS FOR SELECTION OF CARDAC PACING ELECTRODE CONFIGURATIONS
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090145840A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0145840 A1 Tanaka et al. (43) Pub. Date: (54) BLOOD CELL SEPARATION MEMBRANE AND BLOOD RETENTION TOOLUSING
(19) United States US 20090145840A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0145840 A1 Tanaka et al. (43) Pub. Date: (54) BLOOD CELL SEPARATION MEMBRANE AND BLOOD RETENTION TOOLUSING
(12) United States Patent
 USOO9381093B1 (12) United States Patent Morris et al. (10) Patent No.: (45) Date of Patent: *Jul. 5, 2016 (54) (71) (72) (73) (*) (21) (22) (63) (51) (52) (58) LOCKING DEVICE FOR FXATION MECHANISM OF MEDICAL
USOO9381093B1 (12) United States Patent Morris et al. (10) Patent No.: (45) Date of Patent: *Jul. 5, 2016 (54) (71) (72) (73) (*) (21) (22) (63) (51) (52) (58) LOCKING DEVICE FOR FXATION MECHANISM OF MEDICAL
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
(12) United States Patent (10) Patent No.: US 9,314,183 B2
 US009314183B2 (12) United States Patent (10) Patent No.: US 9,314,183 B2 Chi et al. (45) Date of Patent: Apr. 19, 2016 (54) TRANSDUCER ASSEMBLIES FOR DRY (52) U.S. Cl. APPLICATIONS OF TRANSDUCERS CPC...
US009314183B2 (12) United States Patent (10) Patent No.: US 9,314,183 B2 Chi et al. (45) Date of Patent: Apr. 19, 2016 (54) TRANSDUCER ASSEMBLIES FOR DRY (52) U.S. Cl. APPLICATIONS OF TRANSDUCERS CPC...
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0113348 A1 Douskey et al. US 2015O113348A1 (43) Pub. Date: Apr. 23, 2015 (54) (71) (72) (73) (21) (22) IMPLEMENTING MISR COMPRESSION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0113348 A1 Douskey et al. US 2015O113348A1 (43) Pub. Date: Apr. 23, 2015 (54) (71) (72) (73) (21) (22) IMPLEMENTING MISR COMPRESSION
III. United States Patent (19) Sheiban 5,226,889. Jul. 13, and at least a pair of inflatable balloons carried on the
 United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
(19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
(12) United States Patent
 (12) United States Patent US009629579B2 (10) Patent No.: Volkmuth et al. (45) Date of Patent: Apr. 25, 2017 (54) LANCING DEVICE FOR TAKING BLOOD (58) Field of Classification Search SAMPLES CPC... A61 B
(12) United States Patent US009629579B2 (10) Patent No.: Volkmuth et al. (45) Date of Patent: Apr. 25, 2017 (54) LANCING DEVICE FOR TAKING BLOOD (58) Field of Classification Search SAMPLES CPC... A61 B
inm sity aawaayawasa United States Patent (19) -l', all \ 2 27 Bl Cinquin 11 Patent Number: 5,865,171 (45) Date of Patent: Feb. 2, g co m 2 BO
 United States Patent (19) Cinquin 54 NEBULIZER WITH PRESSURE SENSOR 75 Inventor: Gérard Cinquin, Villeneuve-sur-Lot, France 73 Assignee: System Assistance Medical, Villeneuve-Sur-Lot, France 21 Appl. No.:
United States Patent (19) Cinquin 54 NEBULIZER WITH PRESSURE SENSOR 75 Inventor: Gérard Cinquin, Villeneuve-sur-Lot, France 73 Assignee: System Assistance Medical, Villeneuve-Sur-Lot, France 21 Appl. No.:
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 (19) United States US 20100142740A1 (12) Patent Application Publication (10) Pub. No.: US 2010/014274.0 A1 Roerup (43) Pub. Date: Jun. 10, 2010 (54) HEARING AID WIRELESS Related U.S. Application Data COMMUNICATION
(19) United States US 20100142740A1 (12) Patent Application Publication (10) Pub. No.: US 2010/014274.0 A1 Roerup (43) Pub. Date: Jun. 10, 2010 (54) HEARING AID WIRELESS Related U.S. Application Data COMMUNICATION
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 (19) United States US 20100140264A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0140264 A1 Hernandez (43) Pub. Date: Jun. 10, 2010 (54) CONTROLLING THE RATED BURST PRESSURE OF A RUPTURE
(19) United States US 20100140264A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0140264 A1 Hernandez (43) Pub. Date: Jun. 10, 2010 (54) CONTROLLING THE RATED BURST PRESSURE OF A RUPTURE
(12) United States Patent
 USOO879424.4B2 (12) United States Patent Hammel et al. (10) Patent No.: US 8,794,244 B2 (45) Date of Patent: Aug. 5, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ELECTRONIC RECHARGEABLESMOKING
USOO879424.4B2 (12) United States Patent Hammel et al. (10) Patent No.: US 8,794,244 B2 (45) Date of Patent: Aug. 5, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ELECTRONIC RECHARGEABLESMOKING
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 20080081975A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0081975 A1 Agashe et al. (43) Pub. Date: (54) SYSTEM AND METHOD FOR DETECTION Publication Classification OF
US 20080081975A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0081975 A1 Agashe et al. (43) Pub. Date: (54) SYSTEM AND METHOD FOR DETECTION Publication Classification OF
(12) United States Patent (10) Patent No.: US 7, B2
 US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
US A United States Patent (19) 11 Patent Number: 6,049,743 Baba (45) Date of Patent: Apr. 11, 2000
 US006049743A United States Patent (19) 11 Patent Number: Baba (45) Date of Patent: Apr. 11, 2000 54 METHOD OF DESIGNING DENTAL Dental Materials and Appliances, vol. 8, Special Issue 14 PROSTHESIS MODEL
US006049743A United States Patent (19) 11 Patent Number: Baba (45) Date of Patent: Apr. 11, 2000 54 METHOD OF DESIGNING DENTAL Dental Materials and Appliances, vol. 8, Special Issue 14 PROSTHESIS MODEL
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160135916A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0135916 A1 RAKC et al. (43) Pub. Date: May 19, 2016 (54) ARTICULATING ARM LIMITER FOR FI6M I3/02 (2006.01)
(19) United States US 20160135916A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0135916 A1 RAKC et al. (43) Pub. Date: May 19, 2016 (54) ARTICULATING ARM LIMITER FOR FI6M I3/02 (2006.01)
(12) United States Patent (10) Patent No.: US 6,334,451 B1
 USOO6334451B1 (12) United States Patent (10) Patent o.: US 6,334,451 B1 Yang (45) Date of Patent: Jan. 1, 2002 (54) TOOTHBRUSH WITH FILLIG OF 1,340,043 A 5/1920 Grace... 132/311 TOOTHPASTE 1,451,941. A
USOO6334451B1 (12) United States Patent (10) Patent o.: US 6,334,451 B1 Yang (45) Date of Patent: Jan. 1, 2002 (54) TOOTHBRUSH WITH FILLIG OF 1,340,043 A 5/1920 Grace... 132/311 TOOTHPASTE 1,451,941. A
BeneCheck BK6-12M. Plus Multi-Monitoring Meter and Strips
 BeneCheck BK6-12M Plus Multi-Monitoring Meter and Strips The BeneCheck BK6-12M multi-monitoring system is an easy to use, handheld device which allows you to check your Total Cholesterol, as well as Blood
BeneCheck BK6-12M Plus Multi-Monitoring Meter and Strips The BeneCheck BK6-12M multi-monitoring system is an easy to use, handheld device which allows you to check your Total Cholesterol, as well as Blood
(12) United States Patent (10) Patent No.: US 9.289,276 B2
 US009289276B2 (12) United States Patent (10) Patent No.: US 9.289,276 B2 Fisker et al. (45) Date of Patent: *Mar. 22, 2016 (54) TOOLS FOR CUSTOMIZED DESIGN OF (52) U.S. Cl. DENTAL RESTORATIONS CPC... A61C
US009289276B2 (12) United States Patent (10) Patent No.: US 9.289,276 B2 Fisker et al. (45) Date of Patent: *Mar. 22, 2016 (54) TOOLS FOR CUSTOMIZED DESIGN OF (52) U.S. Cl. DENTAL RESTORATIONS CPC... A61C
Rat Hemoglobin A1c (HbA1c) Kit Instructions
 V.3 Crystal Chem Rat Hemoglobin A1c (HbA1c) Kit Instructions For the quantitative determination of hemoglobin A1c (HbA1c) in rat whole blood Catalog #80300 96 Assays For research use only. Not for use
V.3 Crystal Chem Rat Hemoglobin A1c (HbA1c) Kit Instructions For the quantitative determination of hemoglobin A1c (HbA1c) in rat whole blood Catalog #80300 96 Assays For research use only. Not for use
(12) United States Patent
 (12) United States Patent Kimber et al. USOO6565529B1 (10) Patent No.: US 6,565,529 B1 (45) Date of Patent: May 20, 2003 (54) TAMPEREVIDENT SYRINGE DESIGN (75) Inventors: Michael Browning Kimber, New South
(12) United States Patent Kimber et al. USOO6565529B1 (10) Patent No.: US 6,565,529 B1 (45) Date of Patent: May 20, 2003 (54) TAMPEREVIDENT SYRINGE DESIGN (75) Inventors: Michael Browning Kimber, New South
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1. Giannelli (43) Pub. Date: Sep. 4, 2003
 US 20030166439A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0166439 A1 Giannelli (43) Pub. Date: Sep. 4, 2003 (54) ROWING MACHINE Publication Classification (76) Inventor:
US 20030166439A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0166439 A1 Giannelli (43) Pub. Date: Sep. 4, 2003 (54) ROWING MACHINE Publication Classification (76) Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 2013 0036989A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0036989 A1 PESALE (43) Pub. Date: Feb. 14, 2013 (54) ENVIRONMENTALLY-FRIENDLY, FORM-FITTING CANINE GARMENT
(19) United States US 2013 0036989A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0036989 A1 PESALE (43) Pub. Date: Feb. 14, 2013 (54) ENVIRONMENTALLY-FRIENDLY, FORM-FITTING CANINE GARMENT
III. United States Patent (19) Hess et al. 11 Patent Number: 5,584,802 (45) Date of Patent: Dec. 17, 1996
 United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
76 Inventors: late Stella YErin, 5,479,944 1/1996 Petruson /858
 USOO593.1799A United States Patent (19) 11 Patent Number: 5,931,799 Guastella et al. (45) Date of Patent: Aug. 3, 1999 54 PARASEPTAL SPLINT FOR USE IN 4,340,040 7/1982 Straith... 606/204.45 SURGICAL NASAL
USOO593.1799A United States Patent (19) 11 Patent Number: 5,931,799 Guastella et al. (45) Date of Patent: Aug. 3, 1999 54 PARASEPTAL SPLINT FOR USE IN 4,340,040 7/1982 Straith... 606/204.45 SURGICAL NASAL
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015.0245822A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0245822 A1 KM et al. (43) Pub. Date: Sep. 3, 2015 (54) GEL PATCH FOR PROBE AND ULTRASONIC Publication Classification
(19) United States US 2015.0245822A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0245822 A1 KM et al. (43) Pub. Date: Sep. 3, 2015 (54) GEL PATCH FOR PROBE AND ULTRASONIC Publication Classification
(12) United States Patent
 US00895.3827B2 (12) United States Patent Sacha et al. (10) Patent No.: (45) Date of Patent: US 8,953,827 B2 Feb. 10, 2015 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) HEARNGAD WITH INTEGRATED
US00895.3827B2 (12) United States Patent Sacha et al. (10) Patent No.: (45) Date of Patent: US 8,953,827 B2 Feb. 10, 2015 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) HEARNGAD WITH INTEGRATED
(12) (10) Patent No.: US 7,112,065 B2 Kopelman et al. (45) Date of Patent: Sep. 26, 2006
 United States Patent US007 112065B2 (12) (10) Patent No.: US 7,112,065 B2 Kopelman et al. (45) Date of Patent: Sep. 26, 2006 (54) METHOD FOR DEFINING A FINISH LINE 5,372,502 A 12/1994 Massen et al. OF
United States Patent US007 112065B2 (12) (10) Patent No.: US 7,112,065 B2 Kopelman et al. (45) Date of Patent: Sep. 26, 2006 (54) METHOD FOR DEFINING A FINISH LINE 5,372,502 A 12/1994 Massen et al. OF
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
y 2. (12) United States Patent VbOt W N 35 (10) Patent No.: US 8,855,348 B2 HAD CIRCUIT ACTIVE TELECOIL (45) Date of Patent: Oct.
 USOO8855348B2 (12) United States Patent Johnson (54) TELECOIL INA DETACHABLE DIRECT AUDIO INPUT ACCESSORY (75) Inventor: Andrew Joseph Johnson, Edina, MN (US) (73) Assignee: Starkey Laboratories, Inc.,
USOO8855348B2 (12) United States Patent Johnson (54) TELECOIL INA DETACHABLE DIRECT AUDIO INPUT ACCESSORY (75) Inventor: Andrew Joseph Johnson, Edina, MN (US) (73) Assignee: Starkey Laboratories, Inc.,
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0088207 A1 Johnston et al. US 2002008.8207A1 (43) Pub. Date: (54) (76) (21) (22) (62) SYSTEM, METHOD AND APPARATUS FOR FILLING
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0088207 A1 Johnston et al. US 2002008.8207A1 (43) Pub. Date: (54) (76) (21) (22) (62) SYSTEM, METHOD AND APPARATUS FOR FILLING
United States Patent (19)
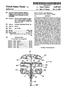 United States Patent (19) Turkel et al. 54) COAXAL BONE MARROW BIOPSY CORNG AND ASPRATING NEEDLE ASSEMBLY AND METHOD OF USE THEREOF 75) Inventors: David H. Turkel; Thomas O. Bales, both of Miami; Frank
United States Patent (19) Turkel et al. 54) COAXAL BONE MARROW BIOPSY CORNG AND ASPRATING NEEDLE ASSEMBLY AND METHOD OF USE THEREOF 75) Inventors: David H. Turkel; Thomas O. Bales, both of Miami; Frank
(12) United States Patent (10) Patent N0.: US 8,702,568 B2 Klabunde et a]. 45 Date of Patent: A r
![(12) United States Patent (10) Patent N0.: US 8,702,568 B2 Klabunde et a]. 45 Date of Patent: A r (12) United States Patent (10) Patent N0.: US 8,702,568 B2 Klabunde et a]. 45 Date of Patent: A r](/thumbs/90/103685815.jpg) USOO87068B2 (12) United States Patent (10) Patent N0.: Klabunde et a]. Date of Patent: A r. 22 2014 a (54) EXERCISE SYSTEM AND A METHOD FOR (52) US. Cl. COMMUNICATION USPC..... 482/8; 482/ 1; 482/9; 482/901
USOO87068B2 (12) United States Patent (10) Patent N0.: Klabunde et a]. Date of Patent: A r. 22 2014 a (54) EXERCISE SYSTEM AND A METHOD FOR (52) US. Cl. COMMUNICATION USPC..... 482/8; 482/ 1; 482/9; 482/901
(12) United States Patent
 (12) United States Patent USOO823 1533B2 (10) Patent No.: US 8,231,533 B2 Buchalter (45) Date of Patent: Jul. 31, 2012 (54) ULTRASOUND COUPLING DEVICE 2003/0149359 A1 8/2003 Smith... 600/437 2003/O195420
(12) United States Patent USOO823 1533B2 (10) Patent No.: US 8,231,533 B2 Buchalter (45) Date of Patent: Jul. 31, 2012 (54) ULTRASOUND COUPLING DEVICE 2003/0149359 A1 8/2003 Smith... 600/437 2003/O195420
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160337769A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0337769 A1 Siddhartha et al. (43) Pub. Date: (54) A METHOD FOR FITTING A HEARING (52) U.S. Cl. DEVICE AS WELL
(19) United States US 20160337769A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0337769 A1 Siddhartha et al. (43) Pub. Date: (54) A METHOD FOR FITTING A HEARING (52) U.S. Cl. DEVICE AS WELL
Technical Data Sheet SONOFLOW CO.55 V1.0
 Technical Data Sheet SONOFLOW CO.55 V1.0 The sensor series SONOFLOW CO.55 V1.0 designed as clamp-on-sensors detect the flow rate of liquids in plastic tubes of different diameters or materials within a
Technical Data Sheet SONOFLOW CO.55 V1.0 The sensor series SONOFLOW CO.55 V1.0 designed as clamp-on-sensors detect the flow rate of liquids in plastic tubes of different diameters or materials within a
(12) United States Patent
 USOO894762OB2 (12) United States Patent Kuo et al. (54) BROADBAND CHOLESTERIC LIQUID CRYSTAL FILM, METHOD FOR FABRICATING THE SAME, POLARIZATION DEVICE, AND HIGH LIGHT EFFICIENCY LIQUID CRYSTAL DISPLAY
USOO894762OB2 (12) United States Patent Kuo et al. (54) BROADBAND CHOLESTERIC LIQUID CRYSTAL FILM, METHOD FOR FABRICATING THE SAME, POLARIZATION DEVICE, AND HIGH LIGHT EFFICIENCY LIQUID CRYSTAL DISPLAY
United States Patent (19)
 United States Patent (19) Fung (54) DENTAL CROWN 76) Inventor: John Fung, 627 George Street, Sydney, NSW 2000, Australia (21) Appl. No.: 969,186 (22) PCT Filed: Jul. 5, 1991 (86). PCT No.: PCT/AU91/00300
United States Patent (19) Fung (54) DENTAL CROWN 76) Inventor: John Fung, 627 George Street, Sydney, NSW 2000, Australia (21) Appl. No.: 969,186 (22) PCT Filed: Jul. 5, 1991 (86). PCT No.: PCT/AU91/00300
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 2013 O136285A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0136285 A1 Naumann (43) Pub. Date: May 30, 2013 (54) INFLATABLE EAR PIECE WITH PRESSURE Publication Classification
(19) United States US 2013 O136285A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0136285 A1 Naumann (43) Pub. Date: May 30, 2013 (54) INFLATABLE EAR PIECE WITH PRESSURE Publication Classification
United States Patent (19) Annoni
 United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE UTILITY APPLICATION FOR UNITED STATES PATENT FOR
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE UTILITY APPLICATION FOR UNITED STATES PATENT FOR Fully-Implantable Cochlear Implant with Nasal Insertion Capability Inventor(s): Hugh Emmanuel 24 Wallalong
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE UTILITY APPLICATION FOR UNITED STATES PATENT FOR Fully-Implantable Cochlear Implant with Nasal Insertion Capability Inventor(s): Hugh Emmanuel 24 Wallalong
(12) (10) Patent No.: US 9,468,773 B1. Anderson et al. (45) Date of Patent: Oct. 18, 2016
 United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
(12) United States Patent (10) Patent No.: US 6,461,697 B1
 USOO646.1697B1 (12) United States Patent (10) Patent No.: US 6,461,697 B1 Slat et al. (45) Date of Patent: Oct. 8, 2002 (54) POLYETHYLENE TEREPHTHALATE 4,149,645 A 4/1979 Valyi MULTI-LAYER PREFORM USED
USOO646.1697B1 (12) United States Patent (10) Patent No.: US 6,461,697 B1 Slat et al. (45) Date of Patent: Oct. 8, 2002 (54) POLYETHYLENE TEREPHTHALATE 4,149,645 A 4/1979 Valyi MULTI-LAYER PREFORM USED
