Drug Therapy Guidelines
|
|
|
- Bennett Anderson
- 5 years ago
- Views:
Transcription
1 Drug Therapy Guidelines Applicable Medical Benefit Effective: 8/15/18 Pharmacy- Frmulary 1 x Next Review: 6/19 Pharmacy- Frmulary 2 x Date f Origin: 10/99 Grwth Stimulating Drugs: Nrditrpin Flexpr, Gentrpin, Humatrpe, Omnitrpe, Nutrpin, Saizen, Serstim, Zmactn TM, Zrbtive (smatrpin, recmbinant grwth hrmne) Pharmacy- Frmulary 3/Exclusive x Review Dates: 10/99, 11/28/00, 3/9/01, 4/26/01, 12/16/03, Pharmacy- Frmulary 4/AON x 11/16/04, 9/14/05, 8/29/06, 11/5/07, 12/15/08, 12/09, 3/10, 1/11, 12/11, 12/12, 12/13, 12/14, 6/15, 6/16, 6/17, 6/18 I. Medicatin Descriptin Endgenus grwth hrmne (GH) is respnsible fr stimulating nrmal skeletal, cnnective tissue, muscle, and rgan grwth in children and adlescents. It als plays an imprtant rle in adult metablism. Recmbinant prducts mimic all f these actins. Smatrpin (recmbinant grwth hrmne) is typically administered subcutaneusly r intramuscularly nce daily. While all grwth hrmne prducts are variatins f smatrpin, sme prducts are apprved by the U.S. Fd and Drug Administratin fr different pediatric and adult indicatins. Grwth Hrmne Prducts Adult GHD* HIV Wasting CRD** ISS+ Nnan Syndrme Pediatric GHD* Indicatin Prader- Willi Syndrme SGA++ SHOX+++ Turner Syndrme Gentrpin Humatrpe Nrditrpin Flexpr Nutrpin, Nutrpin AQ Omnitrpe Saizen Serstim Zmactn TM Zrbtive *GHD =grwth hrmne deficiency/insufficiency **CRD = grwth failure due t chrnic renal disease +ISS = idipathic shrt stature ++SGA = small fr gestatinal age +++SHOX=treatment f shrt stature / grwth failure in children with hmebx-cntaining gene deficiency Shrt Bwel Syndrme II. Psitin Statement Cverage is determined thrugh a prir authrizatin prcess with supprting clinical dcumentatin fr every request. Page 1 f 9
2 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 III. Plicy Frmulary 1: See Sectins A, B Frmulary 2: See Sectins A, B Frmulary 3/Exclusive: See Sectins A, B Frmulary 4/AON: See Sectins A, B A. Prir authrizatin criteria: Prescriber Requirements: Pediatric members are under the care f a pediatric endcrinlgist r a pediatric nephrlgist with expertise in grwth hrmne. Adult therapy must be rdered r recmmended by an apprpriate specialist fr diagnsis. Cverage is prvided fr the fllwing diagnses: Pediatric grwth hrmne deficiency: Epiphyses must be cnfirmed as pen in members 10 years f age AND GH deficiency established by: Grwth hrmne deficiency cnfirmed by any 2 prvcative test results belw 10 ng/ml [Nte: tests must be perfrmed with 2 different agents e.g. L-Dpa, insulin-induced hypglycemia, arginine, glucagn, clnidine] OR One prvcative stimulatin test less than 15 ng/ml, cmbined with a lw insulin-like grwth factr-1 (IGF-1) level and lw IGFBP-3 (insulin-like grwth factr binding prtein-3) fr age, gender, and pubertal status [Nte: Lw is defined as being in the lwer half f the nrmal reference range, r belw the nrmal reference range. Reference range will vary with the lab assay used therefre results shuld be interpreted against the standards prvided by the lab perfrming the test] AND Grwth failure established by: Member s height must be belw the third percentile fr their age and gender related height AND / OR Grwth velcity subnrmal 2 standard deviatins (SD) frm the agerelated mean measured ver 1 year AND Delayed skeletal maturatin 2 SD belw the age/gender related mean Pediatric grwth failure due t chrnic kidney disease: Member has nt received a renal transplant AND Member s height must be belw the third percentile fr their age and gender related height as demnstrated n updated grwth chart Grwth failure in children brn small fr gestatinal age (SGA): Member is brn SGA, defined as grwth charts shwing birth weight, birth length, r bth that are mre than 2 standard deviatins belw mean nrmal values fllwing adjustment fr age and gender AND Failed t manifest catch-up grwth by age 2 Turner s syndrme in girls: Page 2 f 9
3 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 Karytype analysis cnfirms diagnsis (45, XO gentype) AND Member height falls belw the fifth percentile fr age (which usually ccurs between tw and five years f age) demnstrated n updated grwth chart with baseline height and weight Prader-Willi syndrme: Assessment fr any underlying airway bstructin including sleep study is prvided AND Diagnsis cnfirmed by apprpriate genetic testing (lss f gene functin assciated with chrmsme 15 such as translcatin r maternal uniparental dismy) AND Cntraindicated fr use in members with severe besity (e.g., weight >225 percent f ideal bdy weight), respiratry cmprmise, r severe sleep apnea AND Baseline height and weight is prvided Nnan Syndrme: Chart dcumentatin is prvided cnfirming diagnsis AND Updated grwth chart is prvided Shrt stature r grwth failure in children with shrt stature hmebx (SHOX) deficiency: Diagnsis demnstrated by chrmsme analysis AND Updated grwth chart is prvided Adult grwth hrmne deficiency: Adult-nset grwth hrmne deficiency islated grwth hrmne r multiple hrmne deficiencies (hyppituitarism), as a result f pituitary disease, hypthalamic disease, surgery, radiatin therapy, r trauma with ne f the fllwing: Serum IGF-1 cncentratin is lwer than the age-specific lwer limit f nrmal in a member wh has rganic pituitary disease OR If IGF-1 is nt subnrmal, a subnrmal GH respnse t insulin-induced hypglycemia (<5.1 ng/ml) r arginine-ghrh (<4.1ng/ml) is necessary t cnfirm diagnsis Childhd nset grwth hrmne deficiency as a result f cngenital, genetic, acquired, r idipathic causes: A perid f at least ne mnth shuld pass befre re-evaluating GH status after therapy has been discntinued at final height AND ne f the fllwing: Retesting has been perfrmed using Insulin tlerance test (ITT) as a first-line unless cntraindicated (examples: members at high risk fr crnary artery disease r with a histry f seizures) OR Alternative testing when ITT cntraindicated: grwth hrmne releasing hrmne (GHRH)+arginine (ARG) test glucagn test ARG test alne OR Retesting is waived due t: Page 3 f 9
4 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 knwn mutatins, embrypathic/cngenital defects, irreversible hypthalamic-pituitary structural lesins AND evidence f panhyppituitarism (at least 3 pituitary hrmne deficiencies) AND IGF-I levels belw the age- and sex-apprpriate reference range fr GH therapy Cverage f Zrbtive (r Nrditrpin Flexpr, if requested) is prvided fr the treatment f shrt bwel syndrme when: Diagnsis is dcumented as a result f resected r damaged bwel with chrnic diarrhea, weight lss, electrlyte imbalances, malnutritin, dehydratin, and malabsrptin f fats, vitamins and minerals AND There is dependence n specialized nutritinal supprt needs including dietary adjustments such as a high carbhydrate, lw fat diet, enteral feedings, parenteral nutritin, fluid, and micrnutrient supplements demnstrated thrugh nutritinist cnsultatin, dietary interventins, pertinent labs Cverage f Serstim is prvided fr the treatment f HIV members with wasting r cachexia when: Member is 18 years f age AND Cmparative weights and heights are dcumented AND Wasting syndrme is nt attributable t ther causes such as; depressin, MAC, chrnic infectius diarrhea, r malignancy (Kapsi s sarcma limited t the skin r mucus membranes is cvered) AND Cnfirmatin f wasting syndrme has been dcumented (e.g., unintentinal weight lss f at least 10% f bdy weight) AND Optimal antiretrviral therapy has been attempted AND Failure f nn-invasive frms f nutritinal therapy (such as appetite stimulants like megestrl acetate and drnabinl). B. Step therapy: A step edit is in place whereby nn-preferred medicatins (Gentrpin, Humatrpe, Omnitrpe, Nutrpin, Saizen, Zmactn) will nly be cvered fr members wh have failed a trial with the plan-preferred medicatin (Nrditrpin Flexpr): When requesting cverage f a brand medicatin fr which an A/B rated generic is available, there is sufficient evidence that the use f the A/B rated generic equivalent has resulted in inadequate results AND Member must have attempted and failed an adequate (3 mnth) trial with the plan-preferred medicatin smatrpin (Nrditrpin Flexpr) befre any ther grwth hrmne medicatin will be cvered OR when at least ONE f the fllwing criteria have been met: The plan-preferred medicatins are cntraindicated r will likely cause an adverse reactin by r physical r mental harm t the member. The plan-preferred medicatins are expected t be ineffective based n the knwn clinical histry and cnditins f the member and the member s prescriptin drug regimen. The member has tried the plan-preferred medicatins r anther prescriptin drug in the same pharmaclgic class r with the same mechanism f actin and such prescriptin drug was discntinued due t lack f efficacy r effectiveness, diminished effect, r an adverse event. The member is stable n the medicatin selected by their healthcare prfessinal fr the Page 4 f 9
5 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 medical cnditin under cnsideratin (where stable is defined as receiving the medicatin fr an adequate perid f time, have achieved ptimal respnse, and cntinued favrable utcmes are expected UNLESS the medicatin was initially selected slely due t the availability f a drug sample r a cupn card and the member des nt therwise meet the definitin f stable ). The plan-preferred medicatin is nt in the best interest f the member because it will likely cause a significant barrier t the member s adherence r t cmpliance with the member s plan f care, will likely wrsen a cmrbid cnditin f the member, r will likely decrease the member s ability t achieve r maintain reasnable functinal ability in perfrming daily activities. Exceptins: Nrditrpin Flexpr trial will NOT be required when: Zrbtive is used fr treatment f shrt bwel syndrme OR Serstim is used in HIV related cachexia r wasting. IV. Quantity Limits Cverage is available as fllws: Adults Gentrpin and Omnitrpe: 0.08 mg/kg/week Humatrpe: mg/kg/day Nrditrpin Flexpr: mg/kg/day Nutrpin and Nutrpin AQ: mg/kg/day in members yunger than 35 years f age; mg/kg/day in members 35 years f age and lder Saizen: 0.01 mg/kg/day Serstim: 6 mg/day (weight dependent) Zrbtive: 8 mg/day Children- Dsing adjustments in children are dependent upn maintaining IGF-1 levels within a targeted range; therefre, maximum units are individualized. V. Cverage Duratin Pediatric human grwth hrmne deficiency, grwth failure in children SGA, grwth failure due t Turner s syndrme, Nnan Syndrme, SHOX, chrnic renal failure, r Prader-Willi Syndrme: Nrditrpin Flexpr: cverage prvided fr 12 mnths and may be renewed All ther grwth hrmne prducts: cverage prvided fr 3 mnths with fllw-up authrizatins f up t 12 mnths depending n cmparative respnse (see Step-Therapy requirements) Adult grwth hrmne deficiency Nrditrpin Flexpr: cverage prvided fr 12 mnths and may be renewed All ther grwth hrmne prducts: cverage prvided fr 3 mnths with fllw-up authrizatins f up t 12 mnths depending n cmparative respnse (see Step-Therapy requirements) Shrt bwel syndrme: Zrbtive: 1 mnth and may be renewed Nrditrpin Flexpr: cverage is prvided fr 6 mnths and may be renewed HIV-related wasting r cachexia: 3 mnths and may be renewed Page 5 f 9
6 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 VI. Cverage Renewal Criteria Cverage may be renewed accrding t the fllwing criteria in the absence f unacceptable txicity frm the drug: Pediatric human grwth hrmne deficiency, grwth failure in children SGA, grwth failure due t Turner s syndrme, Nnan Syndrme, SHOX, r chrnic renal failure: pen epiphyses dcumented (bne age f 16 years fr bys, 14 years fr girls) AND grwth respnse f at least 4.5 cm/yr (prepubertal grwth rate) r at least 2.5 cm/yr (pstpubertal grwth rate) dcumented with updated grwth chart and recent height and weight with date measured Prader-Willi Syndrme: Updated grwth chart and recent height and weight with date measured AND Dcumented increase in lean bdy mass (r decrease in fat) OR Maintenance f benefit in males up t 16 years f age and in females up t 14 years f age Adult grwth hrmne deficiency: Clinical benefit dcumented (e.g., increase in ttal lean bdy mass, increase in IGF-1 and IGFBP- 3 levels, r increase in exercise capacity) AND Benefit seen frm cmparative weight t baseline with height Shrt bwel syndrme: Prgress ntes shwing benefit AND Applicable labs/testing shwing benefit AND Renewable in situatins where the member is deriving clinical benefit (e.g., the member is experiencing a decrease in intravenus nutritin requirements) HIV-related wasting r cachexia: Renewable in the presence f weight stabilizatin r increase dcumented with height and cmparable weights VII. Billing/Cding Infrmatin Pertinent diagnses: B20 C75.1, C75.2 Human immundeficiency virus (HIV) disease Malignant neplasm f pituitary gland and cranipharyngeal duct C79.31 Secndary malignant neplasm f brain and spinal crd D35.2, D35.3 Benign neplasm f pituitary gland and cranipharyngeal duct D44.3, D44.4 Neplasm f uncertain behavir f pituitary gland and cranipharyngeal duct E23.0 Panhyppituitarism E23.0 Pituitary dwarfism E23.6 Unspecified anterir pituitary disrders E22.2 Other disrders f neurhypphysis E23.1, E89.3 Iatrgenic pituitary disrders Page 6 f 9
7 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 E23.6 Other disrders f the pituitary and ther syndrmes f diencephalhypphyseal rigin E23.3, E23.7 Unspecified endcrine disrder K91.2 Other and unspecified pstsurgical nnabsrptin N18.2 Chrnic kidney disease, stage ii (mild) N18.3 Chrnic kidney disease, stage iii (mderate) N18.4 Chrnic kidney disease, stage iv (severe) N18.5 Chrnic kidney disease, stage v N18.6 End stage renal disease N18.9 Chrnic kidney disease, unspecified Q96.9 Gnadal dysgenesis (i.e., Turner's syndrme) Q87.1 Prader-Willi syndrme Q87.1 Russell-Silver syndrme R62.50 Lack f physilgical develpment R64 Cachexia Z92.3 Persnal histry f irradiatin presenting hazards t health VIII. Summary f Plicy Changes 3/1/11: Increlex plicy mved t separate guideline Remval f Idipathic Shrt Stature frm cvered indicatins Step-Therapy Mdified t ne plan-preferred prduct (Nrditrpin ) 6/15/12: Additinal indicatins added t Omnitrpe Pancreatitis and benzyl alchl warnings added Required dcumentatin added t criteria 3/15/13: clarified which plans have step therapy requirements 7/1/13: Cmmercial Rx and Medicaid/Family Health Plus criteria differentiated 3/15/14: n plicy changes 3/15/15: n plicy changes 7/1/15: frmulary distinctins made Zmactn added t plicy 9/15/15: n plicy changes 2/3/16: clarificatin made that Nrditrpin wuld be cnsidered fr cverage in the treatment f shrt bwel syndrme 7/19/16: n plicy changes 5/1/17: step therapy criteria added 6/21/17: n plicy changes 8/15/18: specified prvcatin tests must be perfrmed using 2 different agents Page 7 f 9
8 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/2018 defined lw values fr IGF added Nrditrpin Flexpr frmulatins IX. References 1. UpTDate Online, retrieved April Clinical Pharmaclgy: Elsevier/Gld Standard. Smatrpin mngraph. Accessed 3/ Facts and Cmparisns Online, retrieved January Micrmedex - Smatrpin Drugdex Evaluatin. Thmpsn Reuters. Accessed 4/ Prduct Infrmatin: Gentrpin. Revised 12/ Prduct Infrmatin: Humatrpe. Revised 12/ Prduct Infrmatin: Nrditrpin Flexpr. Revised 2/ Prduct Infrmatin: Omnitrpe. Revised 12/ Prduct Infrmatin: Nutrpin. Revised 12/ Prduct Infrmatin: Saizen. Revised 5/ Prduct Infrmatin: Serstim. Revised 5/ Prduct Infrmatin: Zmactn TM. Revised 1/ Prduct Infrmatin: Zrbtive. Revised 5/ Vimalachandra D, Hdsn EM, Willis NS, Craig JC, Cwell C, Knight JF. Grwth hrmne fr children with chrnic kidney disease. Cchrane Database Syst Rev. 2006;3:CD Seikaly MG, Salhab N, Warady BA, Stablein D. Use f rhgh in children with chrnic kidney disease: lessns frm NAPRTCS. Pediatr Nephrl. 2007;22(8): Mahan JD, Warady BA, Cnsensus Cmmittee. Assessment and treatment f shrt stature in pediatric patients with chrnic kidney disease: a cnsensus statement. Pediatr Nephrl. 2006;21(7): Haffner D, Wühl E, Schaefer F, Nissel R, Tönshff B, Mehls O. Factrs predictive f the shrt- and lng-term efficacy f grwth hrmne treatment in prepubertal children with chrnic renal failure. The German Study Grup fr Grwth Hrmne Treatment in Chrnic Renal Failure. J Am Sc Nephrl. 1998;9(10): Mencarelli F, Kiepe D, Lezappa G, Stringini G, Cappa M, Emma F. Grwth hrmne treatment started in the first year f life in infants with chrnic renal failure. Pediatr Nephrl. 2009;24(5): Saenger P, Wikland KA, Cnway GS, Davenprt M, Gravhlt CH, Hintz R, Hvatta O, Hultcrantz M, Landin- Wilhelmsen K, Lin A, Lippe B, Pasquin AM, Ranke MB, Rsenfeld R, Silberbach M. Recmmendatins fr the diagnsis and management f Turner syndrme. J Clin Endcrinl Metab. 2001;86(7): Bndy CA, Turner Syndrme Study Grup. Care f girls and wmen with Turner syndrme: a guideline f the Turner Syndrme Study Grup. Clin Endcrinl Metab. 2007;92(1): Richmnd EJ, Rgl AD. Grwth hrmne deficiency in children. Pituitary. 2008;11(2): Grwth Hrmne Research Sciety. Cnsensus guidelines fr the diagnsis and treatment f grwth hrmne (GH) deficiency in childhd and adlescence: summary statement f the GH Research Sciety. GH Research Sciety. J Clin Endcrinl Metab. 2000;85(11): Biller BM, Samuels MH, Zagar A, Ck DM, Arafah BM, Bnert V, Stavru S, Kleinberg DL, Chipman JJ, Hartman ML. Sensitivity and specificity f six tests fr the diagnsis f adult GH deficiency. J Clin Endcrinl Metab. 2002;87(5): Page 8 f 9
9 Drug Therapy Guidelines Grwth Stimulating Drugs Last Review Date: 6/ Mlitch ME, Clemmns DR, Malzwski S, Merriam GR, Shalet SM, Vance ML, Endcrine Sciety's Clinical Guidelines Subcmmittee, Stephens PA. Evaluatin and treatment f adult grwth hrmne deficiency: an Endcrine Sciety Clinical Practice Guideline. J Clin Endcrinl Metab. 2006;91(5): Chen P, Rgl AD, Deal CL, Saenger P, Reiter EO, Rss JL, Chernausek SD, Savage MO, Wit JM. Cnsensus statement n the diagnsis and treatment f children with idipathic shrt stature: a summary f the Grwth Hrmne Research Sciety, the Lawsn Wilkins Pediatric Endcrine Sciety, and the Eurpean Sciety fr Paediatric Endcrinlgy Wrkshp. J Clin Endcrinl Metab. 2008;93(11): Claytn PE, Cianfarani S, Czernichw P, Jhannssn G, Rapaprt R, Rgl A. Management f the child brn small fr gestatinal age thrugh t adulthd: a cnsensus statement f the Internatinal Scieties f Pediatric Endcrinlgy and the Grwth Hrmne Research Sciety. J Clin Endcrinl Metab. 2007;92(3): Byrne TA, Wilmre DW, Iyer K, Dibaise J, Clancy K, Rbinsn MK, Chang P, Gertner JM, Lautz D. Grwth hrmne, glutamine, and an ptimal diet reduces parenteral nutritin in patients with shrt bwel syndrme: a prspective, randmized, placeb-cntrlled, duble-blind clinical trial. Ann Surg. 2005;242(5): Ck DM, Yuen KC, Biller BM, Kemp SF, Vance ML, American Assciatin f Clinical Endcrinlgists. American Assciatin f Clinical Endcrinlgists medical guidelines fr clinical practice fr grwth hrmne use in grwth hrmne-deficient adults and transitin patients update. Endcr Pract 2009 Sep-Oct;15 Suppl 2: Wilsn TA, Rse SR, Chen P, Rgl AD, Backeljauw P, Brwn R, Hardin DS, Kemp SF, Lawsn M, Radvick S, Rsenthal SM, Silverman L, Speiser P; Lawsn Wilkins Pediatric Endcrinlgy Sciety Drug and Therapeutics Cmmittee. Update f guidelines fr the use f grwth hrmne in children: the Lawsn Wilkins Pediatric Endcrinlgy Sciety Drug and Therapeutics Cmmittee. J Pediatr Oct;143(4): Drug Safety Cmmunicatin FDA-12/22/2010., htm The Plan fully expects that nly apprpriate and medically necessary services will be rendered. The Plan reserves the right t cnduct pre-payment and pst-payment reviews t assess the medical apprpriateness f the abve-referenced therapies. The preceding plicy applies nly t members fr whm the abve named pharmacy benefit medicatins are included n their cvered frmulary. Members with clsed frmulary benefits are subject t trying all apprpriate frmulary alternatives befre a cverage exceptin fr a nn-frmulary medicatin will be cnsidered. The preceding plicy is a guideline t allw fr cverage f the pertinent medicatin/prduct, and is nt meant t serve as a clinical practice guideline. Page 9 f 9
Pharmacy Prior Authorization Growth Hormone- Clinical Guidelines. Serostim Zorbtive somatropin
 Pharmacy Prir Authrizatin Grwth Hrmne- Clinical Guidelines Gentrpin Humatrpe Nrditrpin Nutrpin Omnitrpe Saizen Serstim Zmactn Zrbtive smatrpin General Criteria fr Apprval: Omnitrpe vial frmulatin is the
Pharmacy Prir Authrizatin Grwth Hrmne- Clinical Guidelines Gentrpin Humatrpe Nrditrpin Nutrpin Omnitrpe Saizen Serstim Zmactn Zrbtive smatrpin General Criteria fr Apprval: Omnitrpe vial frmulatin is the
AETNA BETTER HEALTH Prior Authorization guideline for Growth Hormone
 AETNA BETTER HEALTH Prir Authrizatin guideline fr Grwth Hrmne Grwth Hrmne and related agents Frmulary:, Omnitrpe vials Nn-Frmulary - Gentrpin, Humatrpe,,Saizen, Serstim, Tev-Trpin, Valtrpin, Zrbtive (smatrpin),
AETNA BETTER HEALTH Prir Authrizatin guideline fr Grwth Hrmne Grwth Hrmne and related agents Frmulary:, Omnitrpe vials Nn-Frmulary - Gentrpin, Humatrpe,,Saizen, Serstim, Tev-Trpin, Valtrpin, Zrbtive (smatrpin),
Drug Therapy Guidelines
 Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
Pharmacy Prior Authorization Growth Hormone- Clinical Guidelines
 Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents: Nte: Prvider must submit chart ntes that include the fllwing
Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents: Nte: Prvider must submit chart ntes that include the fllwing
Pharmacy Prior Authorization Growth Hormone - Clinical Guidelines
 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents:
Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents:
Drug Therapy Guidelines
 Drug Therapy Guidelines Applicable* Hereditary Angiedema (HAE) Agents: Berinert (C1 esterase inhibitr [human]), Cinryze (C1 esterase inhibitr [human]), Haegarda (C1 esterase inhibitr [human]) Kalbitr (ecallantide),
Drug Therapy Guidelines Applicable* Hereditary Angiedema (HAE) Agents: Berinert (C1 esterase inhibitr [human]), Cinryze (C1 esterase inhibitr [human]), Haegarda (C1 esterase inhibitr [human]) Kalbitr (ecallantide),
Clinical Policy: Somatropin (Recombinant Human Growth Hormone) Reference Number: ERX.SPA.14 Effective Date:
 Clinical Plicy: Smatrpin (Recmbinant Human Grwth Hrmne) Reference Number: ERX.SPA.14 Effective Date: 07.01.16 Last Review Date: 05.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant
Clinical Plicy: Smatrpin (Recmbinant Human Grwth Hrmne) Reference Number: ERX.SPA.14 Effective Date: 07.01.16 Last Review Date: 05.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant
Drug Therapy Guidelines
 Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
Drug Therapy Guidelines
 Applicable* Medical Benefit x Effective: 2/15/19 Pharmacy- Frmulary 1 Next Review: 12/19 Pharmacy- Frmulary 2 Date f Origin: 4/1/05 Pharmacy- Frmulary 3/Exclusive Review Dates: 4/1/05, 2/1/06, 10/15/06,
Applicable* Medical Benefit x Effective: 2/15/19 Pharmacy- Frmulary 1 Next Review: 12/19 Pharmacy- Frmulary 2 Date f Origin: 4/1/05 Pharmacy- Frmulary 3/Exclusive Review Dates: 4/1/05, 2/1/06, 10/15/06,
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES. ANTI-OBESITY AGENTS Generic Brand HICL GCN Exception/Other QSYMIA 32515, 32744, 32746, 32745
 Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST
 OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Opioid Analgesics PA Request Provider Checklist
 WVP Health Authrity Updated 05-12-2015 Opiid Analgesics PA Request Prvider Checklist *** If pssible, please include the fllwing infrmatin with PA requests fr piid analgesics. Including the requested infrmatin
WVP Health Authrity Updated 05-12-2015 Opiid Analgesics PA Request Prvider Checklist *** If pssible, please include the fllwing infrmatin with PA requests fr piid analgesics. Including the requested infrmatin
First Name. Specialty: Fax. First Name DOB: Duration:
 Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Pennsylvania Guidelines on the Use of Opioids to Treat Chronic Noncancer Pain
 Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
o Prostanoids/prostacyclin therapies (oral and inhaled) o Inhaled agents: Ventavis, Tyvaso Page 1 of 5 Revised 02/17/17
 Request fr Prir Authrizatin Pulmnary Arterial Hypertensin (PAH) Agents (Oral and Inhaled) Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 All requests fr Pulmnary Arterial
Request fr Prir Authrizatin Pulmnary Arterial Hypertensin (PAH) Agents (Oral and Inhaled) Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 All requests fr Pulmnary Arterial
Ancillary Symposia Request for Proposals Partner with the Endocrine Society to Educatte the Endocrine Community.
 Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
Weight Assessment and Counseling for Children and Adolescents (NQF 0024)
 Weight Assessment and Cunseling fr Children and Adlescents (NQF 0024) EMeasure Name Weight Assessment and EMeasure Id Pending Cunseling fr Children and Adlescents Versin Number 1 Set Id Pending Available
Weight Assessment and Cunseling fr Children and Adlescents (NQF 0024) EMeasure Name Weight Assessment and EMeasure Id Pending Cunseling fr Children and Adlescents Versin Number 1 Set Id Pending Available
Widening of funding restrictions for rituximab and eltrombopag
 20 February 2014 Widening f funding restrictins fr rituximab and eltrmbpag PHARMAC is pleased t annunce the apprval f prpsals t widen the restrictin n rituximab use in DHB hspitals and expand the funding
20 February 2014 Widening f funding restrictins fr rituximab and eltrmbpag PHARMAC is pleased t annunce the apprval f prpsals t widen the restrictin n rituximab use in DHB hspitals and expand the funding
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
PROVIDER ALERT. Comprehensive Diagnostic Evaluation (CDE) Guidelines to Access the Applied Behavior Analysis (ABA) Benefit.
 Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Diabetes: HbA1c Poor Control (NQF 0059)
 Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Drug Therapy Guidelines
 Drug Therapy Guidelines Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 6/18 Pharmacy- Frmulary 2 x Date f Origin: 11/07 Immune Glbulins Intravenus: Carimune NF, Flebgamma,
Drug Therapy Guidelines Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 6/18 Pharmacy- Frmulary 2 x Date f Origin: 11/07 Immune Glbulins Intravenus: Carimune NF, Flebgamma,
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Idaho DUR Committee Meeting Record
 Idah DUR Cmmittee Meeting Recrd Date: August 23, 2012 Time: 9:00 a.m. 3:00 p.m. Lcatin: Idah Medicaid, 3232 Elder Street, Bise, Idah, Cnference Rm D-W Mderatr: Cmmittee Members Present: Paul Cady, Pharm.D,
Idah DUR Cmmittee Meeting Recrd Date: August 23, 2012 Time: 9:00 a.m. 3:00 p.m. Lcatin: Idah Medicaid, 3232 Elder Street, Bise, Idah, Cnference Rm D-W Mderatr: Cmmittee Members Present: Paul Cady, Pharm.D,
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Clinical Policy: Vedolizumab (Entyvio) Reference Number: ERX.SPA.163 Effective Date:
 Clinical Plicy: Vedlizumab (Entyvi) Reference Number: ERX.SPA.163 Effective Date: 10.01.16 Last Review Date: 11.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal
Clinical Plicy: Vedlizumab (Entyvi) Reference Number: ERX.SPA.163 Effective Date: 10.01.16 Last Review Date: 11.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal
Public consultation on the NHMRC s draft revised Australian alcohol guidelines for low-risk drinking
 Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Rituxan (rituximab) Effective Date: 10/01/2015. Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage
 Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
Meeting the Nutritional Requirements of Individuals with Dementia
 Unit 15: Understanding and Meeting the Nutritinal Requirements f Individuals with Dementia Unit reference number: D/616/7124 Level: 3 Unit type: Optinal Credit value: 3 Guided learning hurs: 26 Unit summary
Unit 15: Understanding and Meeting the Nutritinal Requirements f Individuals with Dementia Unit reference number: D/616/7124 Level: 3 Unit type: Optinal Credit value: 3 Guided learning hurs: 26 Unit summary
Trust Protocol for the prevention and treatment of Fat Malabsorption in Adults with Cystic Fibrosis.
 Trust Prtcl fr the preventin and treatment f Fat Malabsrptin in Adults with Cystic Fibrsis. A Clinical Plicy Nrflk and Nrwich University Hspital Fr Use in: Fundatin Trust Cystic Fibrsis (CF) team including
Trust Prtcl fr the preventin and treatment f Fat Malabsrptin in Adults with Cystic Fibrsis. A Clinical Plicy Nrflk and Nrwich University Hspital Fr Use in: Fundatin Trust Cystic Fibrsis (CF) team including
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Nutrition Care Process Model Tutorials. Nutrition Monitoring & Evaluation: Overview & Definition. By the end of this module, the participant will:
 Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
OTHER AND UNSPECIFIED DISORDERS
 OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Topic 12: Endocrine System. Function: Group of glands that produces regulatory chemicals ( )
 Tpic 12: Endcrine System Functin: Grup f glands that prduces regulatry chemicals ( ) Hrmnes: Chemical messengers released directly int the bldstream that regulate: *May have wide-spread effects r nly affect
Tpic 12: Endcrine System Functin: Grup f glands that prduces regulatry chemicals ( ) Hrmnes: Chemical messengers released directly int the bldstream that regulate: *May have wide-spread effects r nly affect
Drug Therapy Guidelines
 Applicable Medical Benefit x Effective: 1/1/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 5/28/06 Pulmnary Arterial Hypertensin : Revati (sildenafil), Ventavis (ilprst),
Applicable Medical Benefit x Effective: 1/1/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 5/28/06 Pulmnary Arterial Hypertensin : Revati (sildenafil), Ventavis (ilprst),
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
UNM SRMC SLEEP MEDICINE CLINICAL PRIVILEGES.
 Initial privileges (initial appintment) Renewal f privileges (reappintment) Expansin f privileges (mdificatin) INSTRUCTIONS All new applicants must meet the fllwing requirements as apprved by the UNM SRMC
Initial privileges (initial appintment) Renewal f privileges (reappintment) Expansin f privileges (mdificatin) INSTRUCTIONS All new applicants must meet the fllwing requirements as apprved by the UNM SRMC
Methadone Maintenance Treatment for Opioid Dependence
 POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
Corporate Governance Code for Funds: What Will it Mean?
 Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
VITAMIN D DEFICIENCY PATHWAY SUMMARY CLINICAL PATHWAY. Assessment. Laboratory Study. Therapeutics. Recommendations for follow up: Prevention
 VITAMIN D DEFICIENCY PATHWAY SUMMARY Assessment Cnsider vitamin D in children with the fllwing risk factrs: Dark skin Limited sun expsure Lw dietary intake Chrnic use f medicatins Malabsrptin prblems Obesity
VITAMIN D DEFICIENCY PATHWAY SUMMARY Assessment Cnsider vitamin D in children with the fllwing risk factrs: Dark skin Limited sun expsure Lw dietary intake Chrnic use f medicatins Malabsrptin prblems Obesity
SECTION O. MEDICATIONS
 SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
PRIOR AUTHORIZATION CRITERIA
 DRUG CLASS BRAND NAME (generic) PRI AUTHIZATION CRITERIA TESTOSTERONE PRODUCTS (BRAND AND GENERIC) ANDRODERM (teststerne transdermal patch) ANDROGEL AXIRON (teststerne tpical slutin) DELATESTRYL (teststerne
DRUG CLASS BRAND NAME (generic) PRI AUTHIZATION CRITERIA TESTOSTERONE PRODUCTS (BRAND AND GENERIC) ANDRODERM (teststerne transdermal patch) ANDROGEL AXIRON (teststerne tpical slutin) DELATESTRYL (teststerne
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Practice Guideline for the Management of Obesity in Adults
 Clinical Practice Guideline fr the Management f Obesity in Adults BACKGROUND The prevalence f besity is reaching epidemic prprtins. Obesity is a risk factr fr Type 2 diabetes mellitus, hypertensin, dyslipidemia,
Clinical Practice Guideline fr the Management f Obesity in Adults BACKGROUND The prevalence f besity is reaching epidemic prprtins. Obesity is a risk factr fr Type 2 diabetes mellitus, hypertensin, dyslipidemia,
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid
 Medical Plicy 1.01.20 Cntinuus r Intermittent Mnitring f Glucse in Interstitial Fluid Sectin 1.0 Durable Medical Equipment Subsectin Effective Date February 27, 2015 Original Plicy Date February 23, 2000
Medical Plicy 1.01.20 Cntinuus r Intermittent Mnitring f Glucse in Interstitial Fluid Sectin 1.0 Durable Medical Equipment Subsectin Effective Date February 27, 2015 Original Plicy Date February 23, 2000
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Influenza (Flu) Fact Sheet
 Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Top 10 Causes of Disability
 Tp 10 Causes f Disability Disability can happen t anyne, f any age. Thugh sme may be the result f accidents r injuries that are unavidable, many disabilities are the result f diseases and health cnditins
Tp 10 Causes f Disability Disability can happen t anyne, f any age. Thugh sme may be the result f accidents r injuries that are unavidable, many disabilities are the result f diseases and health cnditins
EAGLE CARE A SPORT CLUB CONCUSSION MANAGEMENT MODEL
 EAGLE CARE A SPORT CLUB CONCUSSION MANAGEMENT MODEL Cncussin awareness has increased significantly in recent years. The Natinal Cllegiate Athletic Assciatin (NCAA), Natinal Athletic Trainers Assciatin
EAGLE CARE A SPORT CLUB CONCUSSION MANAGEMENT MODEL Cncussin awareness has increased significantly in recent years. The Natinal Cllegiate Athletic Assciatin (NCAA), Natinal Athletic Trainers Assciatin
cerliponase alfa (Brineura )
 cerlipnase alfa (Brineura ) Applies t all prducts administered r underwritten by Blue Crss and Blue Shield f Luisiana and its subsidiary, HMO Luisiana, Inc.(cllectively referred t as the Cmpany ), unless
cerlipnase alfa (Brineura ) Applies t all prducts administered r underwritten by Blue Crss and Blue Shield f Luisiana and its subsidiary, HMO Luisiana, Inc.(cllectively referred t as the Cmpany ), unless
ARLA FOOD FOR HEALTH 4 th ANNUAL CALL FOR EXPRESSIONS OF INTEREST
 ARLA FOOD FOR HEALTH 4 th ANNUAL CALL FOR EXPRESSIONS OF INTEREST 7 th July 2017 Cntent 1 Intrductin 2 Tpics fr EOI in the 4 th call 3 The applicatin prcess and imprtant dates 4 Guideline fr EOIs Deadline
ARLA FOOD FOR HEALTH 4 th ANNUAL CALL FOR EXPRESSIONS OF INTEREST 7 th July 2017 Cntent 1 Intrductin 2 Tpics fr EOI in the 4 th call 3 The applicatin prcess and imprtant dates 4 Guideline fr EOIs Deadline
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
General Approval Criteria for ALL Growth Hormone agents: (ALL criteria must be met)
 Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
1.11 INSULIN INFUSION PUMP MANAGEMENT INPATIENT
 WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
Physical, Occupational, and Speech Therapy - Children (Acute and Chronic)
 Physical, Occupatinal, and Speech Therapy - Children (Acute and Chrnic) Infrmatin psted May 6, 2016 Nte: This article applies t claims submitted t TMHP fr prcessing. Fr claims prcessed by a Medicaid managed
Physical, Occupatinal, and Speech Therapy - Children (Acute and Chrnic) Infrmatin psted May 6, 2016 Nte: This article applies t claims submitted t TMHP fr prcessing. Fr claims prcessed by a Medicaid managed
Policy. Medical Policy Manual Approved: Do Not Implement Until 1/1/18. Applied Behavioral Analysis (ABA)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/1/18 Applied Behaviral Analysis (ABA) This medical plicy will apply t self-funded grups upn their renewal, beginning 1/1/18. Des nt apply t BlueCare.
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/1/18 Applied Behaviral Analysis (ABA) This medical plicy will apply t self-funded grups upn their renewal, beginning 1/1/18. Des nt apply t BlueCare.
HIP REPLACEMENT SURGERY (ARTHROPLASTY)
 Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Clinical Policy: Corticotropin (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date:
 Clinical Plicy: (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date: 10.01.16 Last Review Date: 02.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal infrmatin.
Clinical Plicy: (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date: 10.01.16 Last Review Date: 02.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal infrmatin.
Risk factors in health and disease
 Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Male patients with pain, swelling or erythema please refer to the Acute Painful Scrotum pathway Female patients
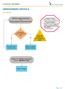 UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
School Medication Authorization Form. School Grade Teacher. Emergency Phone No: To be completed by the student's physician: Name of Medication:
 Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
Iowa Early Periodic Screening, Diagnosis and Treatment Care for Kids Program Provider Training
 Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Sandostatin LAR (octreotide suspension) Document Number: IC-0111
 Sandstatin LAR (ctretide suspensin) Dcument Number: IC-0111 Last Review Date: 02/06/2018 Date f Origin: 06/21/2011 Dates Reviewed: 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013,
Sandstatin LAR (ctretide suspensin) Dcument Number: IC-0111 Last Review Date: 02/06/2018 Date f Origin: 06/21/2011 Dates Reviewed: 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013,
HEDIS. Healthcare Effectiveness Data & Information Set (HEDIS ) QUALITY MANAGEMENT PROGRAM SECTION 8
 HEDIS Healthcare Effectiveness Data & Infrmatin Set (HEDIS ) The HEDIS â audit cntains a cre set f perfrmance measures that prvide infrmatin abut custmer satisfactin, specific health care measures, and
HEDIS Healthcare Effectiveness Data & Infrmatin Set (HEDIS ) The HEDIS â audit cntains a cre set f perfrmance measures that prvide infrmatin abut custmer satisfactin, specific health care measures, and
