Can eculizumab be discontinued in ahus? Case report and review of the literature
|
|
|
- Maximillian Horton
- 6 years ago
- Views:
Transcription
1 Clinical Case Report Medicine Can eculizumab be discontinued in ahus? Case report and review of the literature Tuncay Sahutoglu, MD a,, Taner Basturk, MD a, Tamer Sakaci, MD a, Yener Koc, MD a, Elbis Ahbap, MD a, Mustafa Sevinc, MD a, Ekrem Kara, MD b, Cuneyt Akgol, MD a, Feyza Bayraktar Caglayan, MD a, Abdulkadir Unsal, MD a, Mohamed R. Daha, MD c Abstract Background: The management of atypical hemolytic uremic syndrome (ahus) has evolved into better control of thrombotic microangiopathy (TMA) and recovery of renal functions since the recent introduction of the terminal complement cascade blocker, eculizumab, into clinical use. Better characterization of genotype phenotype relations has become possible with genetic and clinical studies. However, these advances brought up some important issues, such as the possibility and timing of discontinuation of eculizumab and strategy of follow-up that need to be enlightened. Case Summary: One of our ahus cases with a novel complement factor H mutation, who developed unusual laboratory findings (thrombocytopenia and mild creatinine elevation without other features of TMA) following discontinuation of eculizumab was presented. Literature and case reports relevant to discontinuation of eculizumab in ahus patients were reviewed. Conclusion: Limited experience suggests that the risk of recurrence of TMA following discontinuation of eculizumab is relatively low for patients with MCP mutations, homozygous CFHR3/R1 deletions, anti-cfh antibodies, CFI mutations, and no identifiable mutations, whereas there is a major risk for patients with CFH mutations. Early detection of TMA recurrence and prompt retreatment with eculizumab seem to be efficient in controlling of TMA and restoration of kidney functions. Abbreviations: ADAMTS13 = A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, ahus = atypical hemolytic uremic syndrome, ALT = alanine transaminase, AST = aspartate transaminase, CFB = complement factor B, CFH = complement factor H, CFHR = complement factor H-related proteins, CFI = complement factor I, CPK = creatine phosphokinase, GFR = glomerular filtration rate, LDH = lactate dehydrogenase, MCP = membrane cofactor protein, THBD = thrombomodulin, TMA = thrombotic microangiopathy. Keywords: ahus, eculizumab, mutation of complement factors OPEN 1. Introduction Hemolytic uremic syndrome (HUS) is a systemic microvascular disease, with thrombotic microangiopathy (TMA) is its fundamental histologic pathology and kidney injury is the most prominent clinical finding. [1] Recent advances in understanding of the pathophysiology of thrombotic microangiopathy and laboratory tests have eased the differential diagnosis between the 4 groups of causes of this entity, namely thrombotic Editor: Pavlos Malindretos. The authors declare that there is not any financial or nonfinancial conflict of interests to be disclosed. a Department of Nephrology, Sisli Hamidiye Etfal Educational and Research Hospital, Istanbul, b Department of Internal Medicine, Recep Tayyip Erdogan University, Rize, Turkey, c Department of Nephrology, C3-P, Leiden University Medical Center, Leiden, The Netherlands. Correspondence: Tuncay Sahutoglu, Department of Nephrology, Sisli Hamidiye Etfal Educational and Research Hospital, Halaskargazi Cad. Etfal Sk., Sisli, Istanbul, Turkey ( tu_cay83@yahoo.com). Copyright 2016 the Author(s). Published by Wolters Kluwer Health, Inc. All rights reserved. This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Medicine (2016) 95:31(e4330) Received: 2 March 2016 / Received in final form: 20 June 2016 / Accepted: 23 June thrombocytopenic purpura, secondary HUS, shigella toxin-related HUS, and atypical HUS (complement mediated HUS, ahus). [2] Discovery of the distinct role of complement dysregulation as the primary cause of ahus and the success of treatment of ahus with terminal complement cascade blocker eculizumab have opened a new era in the management of this debilitating disease. [3,4] Indeed, treatment of patients on long-term plasma therapy and progressive ahus patients with eculizumab resulted in remission of TMA in majority of cases, clinically significant gain in glomerular filtration rate (GFR), and even recovery from dialysis. [5] However, the episodic nature of the disease, exceptional cost, and potential side effects of eculizumab have raised a new and yet to be cleared questions. How long should eculizumab be continued once TMA has been taken under control and glomerular filtration rate has been stabilized? How should the strategy of follow-up of TMA be done while off treatment? We herein present a patient with ahus who was treated with and discontinued eculizumab and review the literature relevant to the discontinuation of eculizumab in ahus patients. 2. Case A previously healthy, 33-year-old white female was presented with headache and fever for 3 days. She did not used to smoke or consume alcohol. She gave 3 live healthy births and 1 year ago bilateral leg swellings and high blood pressure were noticed close to her last delivery, but medical investigation was not performed and her symptoms disappeared soon after the delivery. Her 1
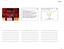
2 Medicine mother succumbed to a sudden disease, which was characterized by acute renal and neurological injuries, but further information was not available. On physical examination, she was good on appearance, and temperature, blood pressure, and pulse rate were 38 C, 160/100 mmhg, and 110 bpm, respectively. Bilateral minimal pretibial edema was noticed. The laboratory tests were consistent with thrombotic microangiopathy and severe renal dysfunction (leukocytes 5800 cells/ mm 3, urea 255 mg/dl, creatinine 11.8 mg/dl, uric acid 8.7mg/ dl, Na 133 meq/l, K 4.9 meq/l, AST 43 U/L, ALT 105 U/L, LDH 1248 U/L, total bilirubin 0.03 mg/dl, CPK 37 U/L, C- reactive protein <3 mg/l, 2 3 leukocytes and 8 10 erythrocytes per high power field and 3+ proteinuria in urinalysis, 24 hours proteinuria 2.4 g, serum haptoglobin <10 mg/dl, Coomb tests negative, reticulocytes 3.68%, and 5% schistocytes per field in peripheral blood film). Plasma ADAMTS13 levels and activity were within the normal limits. Antinuclear antibody was negative, C3 level was 80mg/dL (85 200), and C4 level was within the normal range. Left renal agenesis and enlarged right kidney ( mm) were detected by urinary ultrasonography. Genetic analysis revealed a novel mutation in exon 21 of complement factor H (CFH) (c.3454t>a; p.c1152s), and the same mutation was later identified in her asymptomatic 3 (males) of 4 siblings. Daily plasma exchange using 40 ml/kg fresh frozen plasma and on-demand hemodialysis were started. Markers of thrombotic microangiopathy did not consistently normalize during 22 sessions of plasma exchange; therefore, PE was replaced by eculizumab within 2 weeks of vaccination against Neisseria meningitides (900 mg/week for 4 weeks, 1200 mg every other week from the 5th week on). Thrombocytopenia and elevated LDH normalized within 1 month along with gradual improvement in renal functions and the need for dialysis was eliminated within 2 months of eculizumab treatment (Fig. 1A, B). Eculizumab was discontinued after 1 year of treatment, during which creatinine nadir was 1.35 mg/dl, and the patient was set to follow-up. Thrombocytes dropped and remained below the lower limit of normal from the 7th month (January 6, 2015) of followup on, but LDH levels remained around the upper limit of normal (Fig. 1C). Multiple peripheral blood films, serum haptoglobin levels, and reticulocyte counts were found normal, except for thrombocytopenia, since detection of thrombocytopenia. Levels of creatinine slightly increased but remained <2 mg/dl except for a few occasions, whereas the levels of proteinuria remained <0.5 g/day (385 mg/day at last visit) (Fig. 1D). Informed consent was obtained from the patient. 3. Discussion We have reported an ahus case caused by CHF mutation and successfully treated with and discontinued eculizumab with an unusual course of follow-up. The outcome of patients who discontinue eculizumab treatment used to be either stable or relapse of TMA mostly along with acute deterioration in renal functions. [6] The present case, however, developed only thrombocytopenia and mild increase in creatinine levels above nadir (from 1.35 mg/dl or 51 ml/min/1.73m 2 to 1.65 mg/dl or 40 ml/min/1.73 m 2 at the last visit), whereas the proteinuria remained <0.5 g/day. These features may appear like insignificantly faint at first glance, but it should be noticed that the estimated GFR decreased by 11 ml/min/1.73m 2 in 16 months of follow-up. Kidney biopsy could add valuable inputs for further characterization of these findings, but solitary kidney presented a relative contraindication. Factors that have been associated with thrombocytopenia were sought, but none was identified. Therefore, we concluded that thrombocytopenia could either be due to subclinical smoldering ahus or immune thrombocytopenia. Retreatment with eculizumab could be tried and interesting findings could be derived regarding its effects on slightly increased creatinine levels and thrombocytopenia, but the absence of other components of TMA and the excessive price of the drug lead to disapproval by the insurance system. The efficacy of eculizumab in the treatment of ahus has been shown in a number of case reports and in the phase 2 study reported by Legendre et al. [5] But once satisfactory aims of a treatment have been reached, new goals dawn on the horizon, and safe discontinuation of eculizumab is probably one of the most important issues today in ahus. This is because ahus is characterized by episodic attacks of disease that is thought to represent an environmental trigger upon a genetically suitable patient. [3] Asymptomatic relatives with the exact mutations in complement pathway genes and TMA-free periods interspersed between episodes of disease attacks in ahus patients are supportive of this concept. These findings suggest that there might be some periods of time where complement inhibition is unnecessary. However, randomized controlled trials on the safety and outcomes of discontinuation of eculizumab have not been published yet, but the phase 2 study of eculizumab in ahus, case reports/series, and bench studies could provide with some insights. The 2-year extension of phase 2 eculizumab study revealed that hematological normalization, which was one of the primary end points, was achieved in 76%/90%, 88%/90%, and 88%/90% of patients, whereas gains in GFR were +33/+6, +25/+7, and +37/+8 ml/min/1.73 m 2 in acute progressive and chronic TMA patients within 26 weeks, 1 year, and 2 years of eculizumab treatment, respectively. [7] The differences in changes of GFR between 26 weeks and 1 or 2 years of eculizumab treatment were not statistically significant in both of the subgroups. [7] These results suggest that there is little benefit if any in terms of renal functions with extending the duration of eculizumab treatment after TMA has resolved and creatinine levels have been stabilized, but it also should be kept in mind that all patients, except for 2, had GFR <60mL/min/1.73 m 2 at study entry; therefore, GFR changes may need to be interpreted according to chronic kidney disease. The underlying genetic mutations have been shown as important indicators of clinical outcomes and recurrence rates, with complement factor H, I, and C3 abnormalities having the highest rates of recurrence after transplantation. [1,8] However, these reports belong to the time before widespread availability of eculizumab in the treatment of ahus; therefore, relapse rates, strategy of follow-up, and outcomes after attempted withdrawal of eculizumab have not been clarified. The study by Legendre et al provided limited data about discontinuation of eculizumab, that was one of 4 patients developed TMA on the 80 th day and retreatment with eculizumab resulted in control of hemolysis with gradual renal improvement. [7] Therefore, we aimed to analyze the outcome of patients who discontinued eculizumab in more details and 7 studies with a total of 24 cases were identified that can be reviewed in this regard (Table 1). In this analysis, eculizumab was restarted in all cases 8 patients (33%) who relapsed within an average of 4.5 months (range months) of follow-up since its discontinuation and patientmonths of drug-free follow-up were gained in return. Among them, 1 patient with no identified mutation lost her limitedly 2
3 Figure 1. (A) Creatinine levels decrease initially with plasma exchange and hemodialysis, but rise again under plasma exchange treatment. Treatment with eculizumab induces steady decline in creatinine levels and later allows to discontinue hemodialysis. (B) Thrombocyte counts and lactate dehydrogenase (LDH) levels change initially toward normal ranges, but return to abnormal levels under plasma exchange and hemodialysis. Treatment with eculizumab results in consistent normalization of both thrombocyte counts and LDL levels. (C) The course of LDH levels and thrombocyte count during off treatment follow-up shows that thrombocyte counts drop and remain <150,000 cells/ml since the 7th month of discontinuation of eculizumab, whetreas LDH levels remain mostly just below the upper limit of normal. (D) Creatinine levels during off treatment follow-up swing around 1.6mg/dL, which is 0.25mg/dL higher than the nadir level of 1.35mg/dL under eculizumab treatment. functioning (creatinine mg/dl before discontinuation) second kidney transplant, 1 patient had unreported outcome data, and 6 patients had favorable outcomes (all patients were dialysis-free, reported creatinine levels of 5 patients remained below or nearly equal to the levels before discontinuation of eculizumab). The overall relapse rates in patients with combined complement abnormalities were 100% in the presence of CFH mutation and 50% in homozygous deletions of CFHR3/R1 plus anti-cfh antibodies. The frequencies of relapses in patients with isolated complement abnormalities were 60% for CFH mutation, 25% for CFI, and 0% for MCP, homozygous CFHR3/R1 deletions, and anti-cfh antibodies. When the Table 1 was 3
4 Medicine Figure 1. (Continued ). analyzed in further details according to the underlying complement abnormalities, the following findings were revealed. Relapse was reported in 1 of 5 patients (20%) without detected complement abnormalities (patient 1) after 5 months of discontinuation of eculizumab with a consequence of graft lost, whereas the other 4 patients (patients 5, 19, 21, 24)who were being followed-up for a mean of 11 months ( ) after drug discontinuation had favorable outcomes with no relapse. CFH mutation was reported in 7 patients (patients 4, 6, 7, 8, 9, 10, 18) and 5 of them (71%) (3 of the 5 with isolated CFH mutations and 2 of the 2 with combined complement abnormalities) relapsed within a mean of 2.5 months (0.9 6) after discontinuation of eculizumab; the outcomes of 3 patients who were reported by Ardissino et al were good, whereas 1 patient remained free of dialysis (patient 4) and the outcome of the other patient (patient 7) was not reported. CFI mutation was reported in 5 (patients 2, 10, 11, 12, 13) patients and relapse was detected in 2 of them (1 of 1 with combined and 1 of the 4 with isolated complement abnormalities; patients 10 and 13, respectively) after 0.9 (patient 10) and 17.3 (patient 13) months of eculizumab discontinuation; the outcomes were good in both. The outcomes of 3 patients (all with isolated complement abnormalities, patients 3, 16, 23) with MCP mutations were good and no relapse was reported after a mean of 13.6 months ( ) of eculizumab discontinuation. The only patient (patient 10) who carried THBD mutation in combination with CFH and CFI mutations relapsed after 0.9 month of eculizumab discontinuation with a good outcome. Homozygous deletions of CFHR3/R1 were reported in 5 patients (patients 14, 17, 18, 20, 22) and 2 of them (patients 18, 22) relapsed (2 of 3 patients with combined complement abnormalities) after 1.2 and 0.7 months of eculizumab discontinuations and their outcomes were good. Anti-CFH antibodies were detected in 4 patients (patients 15, 17, 18, 22) and relapse was reported in 2 cases (2 of the 3 with combined complement abnormalities, patients 18 and 22) after 1.2 and 0.7 months of eculizumab discontinuations with good outcomes in both. The only case who had permanent renal damage following relapse has some important features to be pointed out. [9] Her risk of recurrence could be foreseen very high as she had already lost her native and 1st transplanted kidney functions under plasma exchange therapy after the initial episode and first recurrence of TMA, respectively. Furthermore, the second TMA relapse, which occurred 3.5 months after her 2nd kidney transplantation, was initially treated with plasma exchange and eculizumab was added 4
5 Table 1 Summary of ahus cases who were treated with and discontinued eculizumab. Study Patient number Age at onset of ahus, sex Kidney (native or transplanted) Complement abnormality Duration of eculizumab treatment before discontinuation (months) Method of follow-up TM recurrence, time, mo Retreatment with eculizumab Outcome (creatinine, proteinuria) Alachkar et al, 2012 [9] 1 24, F Transplant 2 nd None 1.9 Unreported Yes, 5 Yes Graft loss Caycı et al, 2012 [18] 2 10,F Native CFI 0.75 Unreported No, 4 No Good (0.5mg/dL, unreported) Gulleroglu et al, 2013 [19] 3 6, F Native MCP 1.25 Unreported No, 9 No Good (normal renal functions, unreported) Carr et al, 2013 [20] 4 20, F Native CFH 9 Unreported Yes, 6 Yes Dialysis-free (values unreported) Pu et al, 2014 [21] 5 85, F Native None 3 Unreported No, 12 No Good (normal renal functions) Wetzels et al, 2015 [22] 6 30, F Unreported CFH 35 Unreported No, 17 No Stable 7 21, F Unreported CFH 4.5 Unreported Yes, 3 Yes Unreported 8 43, F CFH 4 Unreported No, 11 No Stable Ardissino et al, 2015 [10] 9 4.3, M Unreported CFH 6.7 Home-based urine Yes, 1.5 Yes Good (0.57 mg/dl, not detectable) dipstick test thrice weekly to detect microscopic hematuria , F Unreported CFH+CFI +THBD 14.4 Yes, 0.9 Yes Good (1.16 mg/dl, 1.12mg/mg) , M Unreported CFI 1.5 No, 40 No Good (0.78 mg/dl, 0.22 mg/mg) , F Unreported CFI 10.4 No, 28.2 No Good (2.11 mg/dl, 1.30 mg/mg) , M Unreported CFI 5.6 Yes, 17.3 Yes Good (0.45 mg/dl, 0.31mg/mg) , F Unreported CFHR3/R1 del/del 13.4 No, 23.7 No Good (0.24 mg/dl, 3.81mg/mg) , M Unreported Anti-CFH+ 5.5 No, 31.4 No Good (1.13 mg/dl, 0.15mg/mg) , F Unreported MCP 0.5 No, 31.2 No Good (0.54 mg/dl, 0.20mg/mg) , M Unreported Anti-CFH+CFHR3/R1 del/del 2.6 No, 25.8 No Good (0.8mg/dL, 0.19mg/mg) , F Unreported CFH+CFHR3/R1 del/del+anti-cfh 0.9 Yes, 1.2 Yes Good (0.8mg/dL, 0.07mg/mg) 19 44, F Unreported None 4.1 No, 24.7 No Good (0.84 mg/dl, not detectable) 20 52, F Unreported CFHR3/R1 del/del 8.3 No, 8.9 No Good (2.3 mg/dl, not detectable) 21 60, M Unreported None 4.5 No, 7.2 No Good (1.1mg/dL, not detectable) , F Unreported Anti-CFH+CFHR3/R1 del/del 0.8 Yes, 0.7 Yes Good (0.83mg/dL, 0.74 mg/mg) , M Unreported MCP 0.5 No, 0.7 No Good (0.68mg/dL, 0.16 mg/mg) , F Unreported None 0.6 No, 0.4 No Good (0.81mg/dL, 0.33 mg/mg) ahus=atypical hemolytic uremic syndrome, CFI=complement factor I, CFH=complement factor H, CFHR=complement factor H-related proteins, MCP=membrane cofactor protein, THBD=thrombomodulin, TMA=thrombotic microangiopathy. 5
6 Medicine later, after which partial renal recovery and complete remission of TMA could be obtained. The 3rd relapse, which occurred 5 months following discontinuation of eculizumab, was triggered by pneumonia and intervening endovascular procedure caused further renal injury while renal recovery was ongoing. In comparison, home-based urine dipstick test 3 times a week was incorporated into routine follow-up and permanent damage was not reported in any case by Ardissino et al despite the fact that significant acute renal injury was present in all 3 of their relapsed patients as they were presented in a previous report. [10,6] Favorable outcomes of the latter study might be because of early detection of relapse and restarting of eculizumab. However, there is not any established strategy yet to be adopted in this regard, but according to Ardissino et al, [6] home-based urine dipstick test was able to identify all of the 3 cases who relapsed with only 2 false positive results. Although not validated, it is an easy and cheap test that is probably worth to be performed, which could save a few days/weeks before the development symptoms or date of next outpatient appointment. However, urine dipstick test and other fundamental laboratory indicators of TMA can only be detected after significant endothelial or renal injury has occurred, which is not ideal. A recent study by Noris et al showed that an in vitro human microvascular endothelial cell model could indicate complement activation in the fluid phase and adequacy of complement inhibition, which might be capable of predicting pending disease flare up. [11] If such a model can be validated, standardized, and made widely accessible/affordable, it could reveal the status of complement activity, which can be used to evaluate the adequacy of complement inhibition and timing of drug discontinuation and reinstitution. The paucity of available evidence precludes making strong suggestions regarding the strategy of discontinuation of eculizumab. Furthermore, the types of mutations in each culprit complement factor are not uniform, and as different mutations in a single gene can result in different genotype phenotype consequences, one should anticipate that carriers of different types of mutation in a single gene could have different clinical phenotypes. [3] However, the aforementioned experiences suggest that discontinuation of eculizumab and close follow-up in patients with isolated MCP mutations, homozygous CFHR3/R1 deletions, or anti-cfh antibodies seem to be safe once TMA has resolved and renal functions have been stabilized. Patients with no identifiable complement abnormality, CFI mutations, or homozygous CFHR3/R1 deletions plus anti-cfh antibodies are under considerable risk of recurrence. CFH mutations in isolation or in combination with other complement abnormalities have a very high risk of recurrence and patients with limited renal functions should probably have lifelong eculizumab treatment, whereas the termination of treatment in cases with normal renal functions should be left on the discretion of physicians and patients. The treatment of patients with anti-cfh antibodies presents significant differences than others. Anti-CFH antibodies have been shown to be pathogenic and thought to develop because of homozygous deletions of CFHR3/R1 proteins in majority of cases. [12] A combination of plasma exchange plus immunosuppressive regimen has been shown to induce and maintain remission with low serum anti-cfh antibody levels. [13,14,15,16] Therefore, it suggests that after low titers of anti-cfh antibodies have been attained, discontinuation of eculizumab can be considered if it is used, and the monitoring of anti-cfh antibody titters should be included in the follow-up if available. Experience about discontinuation of eculizumab in patients with CFB and C3 mutations has not been published, but these 2 mutations lead to end-stage renal disease in about 60% to 70% of patients and recurrence risk after kidney transplantation was found significantly high. [1,17] Therefore, approach to patients with these mutations should be similar to those with CFH mutations. 4. Conclusions Evidence to guide the discontinuation of eculizumab in patients with ahus is weak. However, limited experience suggests that patients with MCP mutations, homozygous CFHR3/R1 deletions, anti-cfh antibodies, no identifiable mutations and CFI mutations carry a low risk, whereas CFH mutations pose a major risk of recurrence of TMA following discontinuation of eculizumab in patients with ahus. Preinjury markers of complement/tma activation are needed, as early detection of TMA recurrence and prompt retreatment with eculizumab seem to be efficient in controlling of TMA and restoration of normal renal functions. Therefore, the decision on discontinuation of eculizumab should be customized individually for patients. References [1] Noris M, Remuzzi G. Atypical hemolytic uremic syndrome. N Engl J Med 2009;361: [2] Noris M, Remuzzi G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and c3 glomerulopathy: Core Curriculum Am J Kidney Dis 2015;66: [3] Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol 2013;33: [4] Nürnberger J, Philipp T, Witzke O, et al. Eculizumab for atypical hemolytic uremic syndrome. N Engl J Med 2009;360: [5] Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic uremic syndrome. N Engl J Med 2013;368: [6] Ardissino G, Tetsa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 2014;64: [7] Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 2015;87: [8] Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial ahus and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010;5: [9] Alachkar N, Bagnasco SM, Montgomery RA. Eculizumab for the treatment of two recurrences of atypical hemolytic uremic syndrome in a kidney allograft. Transpl Int 2012;25:e93 5. [10] Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 2015;66: [11] Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in ahus and how to monitor eculizumab therapy. Blood 2014;124: [12] Loirat C, Frémeaux-Bacchi V. Anti-factor H autoantibody-associated hemolytic uremic syndrome: the earlier diagnosed and treated, the better. Kidney Int 2014;85: [13] Boyer O, Balzamo E, Charbit M, et al. Pulse cyclophosphamide therapy and clinical remission in atypical hemolytic uremic syndrome with anticomplement factor H autoantibodies. Am J Kidney Dis 2010;55: [14] Sana G, Dragon-Durey MA, Charbit M, et al. Long-term remission of atypical HUS with anti-factor H antibodies after cyclophosphamide pulses. Pediatr Nephrol 2014;29: [15] Sinha A, Gulati A, Saini S, et al. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int 2014;85: [16] Dragon-Durey M-A, Sethi SK, Bagga A, et al. Clinical features of antifactor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol 2010;21: [17] Le Quintrec M, Zuber J, Moulin B, et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients 6
7 with atypical hemolytic and uremic syndrome. Am J Transplant 2013;13: [18] Caycı FS, Cakar N, Hancer VS, et al. Eculizumab therapy in a child with hemolytic uremic syndrome and CFI mutation. Pediatr Nephrol 2012;27: [19] Gulleroglu K, Fidan K, Hancer VS, et al. Neurologic involvement in atypical hemolytic uremic syndrome and successful treatment with eculizumab. Pediatr Nephrol 2013;28: [20] Carr R, Cataland SR. Relapse of ahus after discontinuation of therapy with eculizumab in a patient with ahus and factor H mutation. Ann Hematol 2013;92: [21] Pu JJ, Sido A. Successful discontinuation of eculizumab therapy in a patient with ahus. Ann Hematol 2014;93: [22] Wetzels JFM, van de Kar NCAJ. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis 2015;65:342. 7
M.Weitz has documented that he has no relevant financial relationships to disclose or conflict of interest to resolve.
 M.Weitz has documented that he has no relevant financial relationships to disclose or conflict of interest to resolve. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic
M.Weitz has documented that he has no relevant financial relationships to disclose or conflict of interest to resolve. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic
Atypical Hemolytic Uremic Syndrome: When the Environment and Mutations Affect Organ Systems. A Case Report with Review of Literature
 Atypical Hemolytic Uremic Syndrome: When the Environment and Mutations Affect Organ Systems. A Case Report with Review of Literature Mouhanna Abu Ghanimeh 1, Omar Abughanimeh 1, Ayman Qasrawi 1, Abdulraheem
Atypical Hemolytic Uremic Syndrome: When the Environment and Mutations Affect Organ Systems. A Case Report with Review of Literature Mouhanna Abu Ghanimeh 1, Omar Abughanimeh 1, Ayman Qasrawi 1, Abdulraheem
DRUG NAME: Eculizumab Brand(s): Soliris DOSAGE FORM/ STRENGTH: 10 mg/ml (300 mg per vial)
 Preamble: A confirmed diagnosis of atypical hemolytic uremic syndrome (ahus) is required for eculizumab funding. The information below is to provide clinicians with context for how a diagnosis of ahus
Preamble: A confirmed diagnosis of atypical hemolytic uremic syndrome (ahus) is required for eculizumab funding. The information below is to provide clinicians with context for how a diagnosis of ahus
Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Dialysis
 SP281 Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Johan Vande Walle, 1 Larry A. Greenbaum, 2 Camille L. Bedrosian, 3 Masayo Ogawa, 3 John F. Kincaid, 3 Chantal
SP281 Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Johan Vande Walle, 1 Larry A. Greenbaum, 2 Camille L. Bedrosian, 3 Masayo Ogawa, 3 John F. Kincaid, 3 Chantal
When a patient presents with TMA, identify the underlying cause for the appropriate diagnosis... IS IT TTP OR IS IT ahus?
 When a patient presents with TMA, identify the underlying cause for the appropriate diagnosis... IS IT TTP OR IS IT ahus? ADAMTS13 activity >5% RULES OUT a diagnosis of severe ADAMTS13 deficiency (TTP)
When a patient presents with TMA, identify the underlying cause for the appropriate diagnosis... IS IT TTP OR IS IT ahus? ADAMTS13 activity >5% RULES OUT a diagnosis of severe ADAMTS13 deficiency (TTP)
Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Dialysis
 SA-PO546 Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Johan Vande Walle, 1 Larry A. Greenbaum, 2 Camille L. Bedrosian, 3 Masayo Ogawa, 3 John F. Kincaid,
SA-PO546 Safety and Efficacy of Eculizumab in Pediatric Patients With ahus, With or Without Baseline Johan Vande Walle, 1 Larry A. Greenbaum, 2 Camille L. Bedrosian, 3 Masayo Ogawa, 3 John F. Kincaid,
ahus A PATIENT S GUIDE To learn more about ahus, visit Copyright 2011, Alexion Pharmaceuticals, Inc. All rights reserved.
 To learn more about ahus, visit www.ahussource.com ahus A PATIENT S GUIDE Copyright 2011, Alexion Pharmaceuticals, Inc. All rights reserved. SOL 1169 BECOME EMPOWERED By learning more and taking control
To learn more about ahus, visit www.ahussource.com ahus A PATIENT S GUIDE Copyright 2011, Alexion Pharmaceuticals, Inc. All rights reserved. SOL 1169 BECOME EMPOWERED By learning more and taking control
Recent advances in pathogenesis & treatment of ahus
 Recent advances in pathogenesis & treatment of ahus Miquel Blasco Pelicano Nephrology and Kidney Transplant Unit Hospital Clínic, Barcelona Atypical Hemolytic Uremic Syndrome (ahus) Ultra-rare disease:
Recent advances in pathogenesis & treatment of ahus Miquel Blasco Pelicano Nephrology and Kidney Transplant Unit Hospital Clínic, Barcelona Atypical Hemolytic Uremic Syndrome (ahus) Ultra-rare disease:
New insights in thrombotic microangiopathies : TTP and ahus
 New insights in thrombotic microangiopathies : TTP and ahus Dr Catherine LAMBERT Hematology Cliniques universitaires Saint-Luc Catherine.lambert@uclouvain.be New insights in thrombotic microangiopathies
New insights in thrombotic microangiopathies : TTP and ahus Dr Catherine LAMBERT Hematology Cliniques universitaires Saint-Luc Catherine.lambert@uclouvain.be New insights in thrombotic microangiopathies
Spectrum of complement-mediated thrombotic microangiopathies after kidney transplantation
 Spectrum of complement-mediated thrombotic microangiopathies after kidney transplantation Marius Miglinas Vilnius university hospital: Nephrology center, Center of Rare Kidney Diseases Vilnius university
Spectrum of complement-mediated thrombotic microangiopathies after kidney transplantation Marius Miglinas Vilnius university hospital: Nephrology center, Center of Rare Kidney Diseases Vilnius university
Hemolytic uremic syndrome
 Hemolytic uremic syndrome Doyeun Oh Department of Internal Medicine CHA University School of Medicine Disclosures for Doyeun Oh Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau
Hemolytic uremic syndrome Doyeun Oh Department of Internal Medicine CHA University School of Medicine Disclosures for Doyeun Oh Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau
Beyond Plasma Exchange: Targeted Therapy for Thrombotic Thrombocytopenic Purpura
 Beyond Plasma Exchange: Targeted Therapy for Thrombotic Thrombocytopenic Purpura Kristen Knoph, PharmD, BCPS PGY2 Pharmacotherapy Resident Pharmacy Grand Rounds April 25, 2017 2016 MFMER slide-1 Objectives
Beyond Plasma Exchange: Targeted Therapy for Thrombotic Thrombocytopenic Purpura Kristen Knoph, PharmD, BCPS PGY2 Pharmacotherapy Resident Pharmacy Grand Rounds April 25, 2017 2016 MFMER slide-1 Objectives
TMA CASE STUDY. Pamela Harmon, RN & Keturah Tomlin, RN Toronto General Hospital Apheresis Unit
 TMA CASE STUDY Pamela Harmon, RN & Keturah Tomlin, RN Toronto General Hospital Apheresis Unit Cumulative fraction of patients free of events ahus is a catastrophic disease that can result in sudden & progressive
TMA CASE STUDY Pamela Harmon, RN & Keturah Tomlin, RN Toronto General Hospital Apheresis Unit Cumulative fraction of patients free of events ahus is a catastrophic disease that can result in sudden & progressive
Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome
 Clinical Kidney Journal, 2016, 1 10 doi: 10.1093/ckj/sfw115 Original Article ORIGINAL ARTICLE Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome
Clinical Kidney Journal, 2016, 1 10 doi: 10.1093/ckj/sfw115 Original Article ORIGINAL ARTICLE Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome
TMA in HUS and TTP: new insights
 TMA in HUS and TTP: new insights Daan Dierickx University Hospitals Leuven, Department of Hematology, Belgium 20th Annual Meeting Belgian Society on Thrombosis and Haemostatis Antwerpen, 22 th November
TMA in HUS and TTP: new insights Daan Dierickx University Hospitals Leuven, Department of Hematology, Belgium 20th Annual Meeting Belgian Society on Thrombosis and Haemostatis Antwerpen, 22 th November
Eculizumab as Prophylactic Therapy in Atypical Hemolytic Uremic Syndrome in Adult Living-Related Kidney Transplantation
 Anti-C5 as Prophylactic Therapy in Atypical Hemolytic Uremic Syndrome in Living- Related Kidney Transplantation Authors: Miquel Blasco 1, Santiago Rodríguez de Córdoba 2, Fritz Diekmann 1, Mercedes Saiz
Anti-C5 as Prophylactic Therapy in Atypical Hemolytic Uremic Syndrome in Living- Related Kidney Transplantation Authors: Miquel Blasco 1, Santiago Rodríguez de Córdoba 2, Fritz Diekmann 1, Mercedes Saiz
What is meant by Thrombotic Microangiopathy (TMA)?
 What is meant by Thrombotic Microangiopathy (TMA)? Thrombotic Microangiopathy (TMA) is a group of disorders characterized by injured endothelial cells, microangiopathic hemolytic anemia (MAHA), with its
What is meant by Thrombotic Microangiopathy (TMA)? Thrombotic Microangiopathy (TMA) is a group of disorders characterized by injured endothelial cells, microangiopathic hemolytic anemia (MAHA), with its
Clinical Study Eculizumab Therapy Leads to Rapid Resolution of Thrombocytopenia in Atypical Hemolytic Uremic Syndrome
 Hindawi Publishing Corporation Advances in Hematology Volume 214, Article ID 295323, 7 pages http://dx.doi.org/1.1155/214/295323 Clinical Study Therapy Leads to Rapid Resolution of Thrombocytopenia in
Hindawi Publishing Corporation Advances in Hematology Volume 214, Article ID 295323, 7 pages http://dx.doi.org/1.1155/214/295323 Clinical Study Therapy Leads to Rapid Resolution of Thrombocytopenia in
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lapeyraque A-L, Malina M, Fremeaux-Bacchi V, et al. Eculizumab
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lapeyraque A-L, Malina M, Fremeaux-Bacchi V, et al. Eculizumab
Risk factors of chronic renal failure after atypical Hemolytic Uremic Syndrome under plasmatherapy
 Risk factors of chronic renal failure after atypical Hemolytic Uremic Syndrome under plasmatherapy Professeur Eric Rondeau Urgences néphrologiques et Transplantation rénale Hôpital Tenon, Paris WWA SFH
Risk factors of chronic renal failure after atypical Hemolytic Uremic Syndrome under plasmatherapy Professeur Eric Rondeau Urgences néphrologiques et Transplantation rénale Hôpital Tenon, Paris WWA SFH
Hemolytic uremic syndrome: Investigations and management
 Hemolytic uremic syndrome: Investigations and management SAWAI Toshihiro M.D., Ph.D. Department of Pediatrics, Shiga University of Medical Science Otsu, JAPAN AGENDA TMA; Thrombotic micro angiopathy STEC-HUS;
Hemolytic uremic syndrome: Investigations and management SAWAI Toshihiro M.D., Ph.D. Department of Pediatrics, Shiga University of Medical Science Otsu, JAPAN AGENDA TMA; Thrombotic micro angiopathy STEC-HUS;
Specialised Services Policy: CP98 Eculizumab for Atypical Haemolytic Uraemic Syndrome (ahus)
 Specialised Services Policy: CP98 Eculizumab for Atypical Haemolytic Uraemic Syndrome (ahus) Document Author: Assistant Director for Evidence, Evaluation and Effectiveness Executive Lead: Medical Director
Specialised Services Policy: CP98 Eculizumab for Atypical Haemolytic Uraemic Syndrome (ahus) Document Author: Assistant Director for Evidence, Evaluation and Effectiveness Executive Lead: Medical Director
Soliris and You. Your Guide To Living With ahus. INDICATION & IMPORTANT SAFETY INFORMATION FOR SOLIRIS (eculizumab)
 INDICATION & IMPORTANT SAFETY INFORMATION FOR SOLIRIS (eculizumab) INDICATION What is SOLIRIS? SOLIRIS is a prescription medicine called a monoclonal antibody. SOLIRIS is used to treat: adults and children
INDICATION & IMPORTANT SAFETY INFORMATION FOR SOLIRIS (eculizumab) INDICATION What is SOLIRIS? SOLIRIS is a prescription medicine called a monoclonal antibody. SOLIRIS is used to treat: adults and children
DIAGNOSTIC CHALLENGES IN THROMBOTIC MICROANGIOPATHIES
 DIAGNOSTIC CHALLENGES IN THROMBOTIC MICROANGIOPATHIES Summary of Presentations from the Alexion-Sponsored Symposium, held at the 51 st ERA EDTA Congress, Amsterdam, the Netherlands, on 1 st June 2014 Chairperson
DIAGNOSTIC CHALLENGES IN THROMBOTIC MICROANGIOPATHIES Summary of Presentations from the Alexion-Sponsored Symposium, held at the 51 st ERA EDTA Congress, Amsterdam, the Netherlands, on 1 st June 2014 Chairperson
Accepted Manuscript. No more thrombotic thrombocytopenic purpura/hemolytic uremic syndrome please. Yeong-Hau H. Lien MD, PhD S (18)
 Accepted Manuscript No more thrombotic thrombocytopenic purpura/hemolytic uremic syndrome please Yeong-Hau H. Lien MD, PhD PII: S0002-9343(18)30965-3 DOI: https://doi.org/10.1016/j.amjmed.2018.10.009 Reference:
Accepted Manuscript No more thrombotic thrombocytopenic purpura/hemolytic uremic syndrome please Yeong-Hau H. Lien MD, PhD PII: S0002-9343(18)30965-3 DOI: https://doi.org/10.1016/j.amjmed.2018.10.009 Reference:
Soliris (eculizumab) Inhibits TMA and Improves Renal Function in Pediatric and Adult Patients with atypical Hemolytic Uremic Syndrome (ahus)
 Soliris (eculizumab) Inhibits TMA and Improves Renal Function in Pediatric and Adult Patients with atypical Hemolytic Uremic Syndrome (ahus) New Data from the Largest Prospective Trial of Adult Patients
Soliris (eculizumab) Inhibits TMA and Improves Renal Function in Pediatric and Adult Patients with atypical Hemolytic Uremic Syndrome (ahus) New Data from the Largest Prospective Trial of Adult Patients
C3 Glomerulonephritis versus C3 Glomerulopathies?
 Washington University School of Medicine Digital Commons@Becker Kidneycentric Kidneycentric 2016 C3 Glomerulonephritis versus C3 Glomerulopathies? T. Keefe Davis Washington University School of Medicine
Washington University School of Medicine Digital Commons@Becker Kidneycentric Kidneycentric 2016 C3 Glomerulonephritis versus C3 Glomerulopathies? T. Keefe Davis Washington University School of Medicine
Thrombotic Microangiopathy (TMA) The Clinical Facets of TMA
 International Consensus on Management Atypical Hemolytic Uremic Syndrome in Children Loirat C. et al. Pediatr Nephrol 31: 15-39, 2016 Ruth A. McDonald, MD Professor and Vice Chair Clinical Affairs Department
International Consensus on Management Atypical Hemolytic Uremic Syndrome in Children Loirat C. et al. Pediatr Nephrol 31: 15-39, 2016 Ruth A. McDonald, MD Professor and Vice Chair Clinical Affairs Department
Introduction. Arif Asif 1 Ali Nayer 2 Christian S. Haas 3
 J Nephrol (2017) 30:347 362 DOI 10.1007/s40620-016-0357-7 REVIEW Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment
J Nephrol (2017) 30:347 362 DOI 10.1007/s40620-016-0357-7 REVIEW Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment
Soliris (eculizumab) DRUG.00050
 Market DC Soliris (eculizumab) DRUG.00050 Override(s) Prior Authorization Approval Duration 1 year Medications Soliris (eculizumab) APPROVAL CRITERIA Paroxysmal Nocturnal Hemoglobinuria I. Initiation of
Market DC Soliris (eculizumab) DRUG.00050 Override(s) Prior Authorization Approval Duration 1 year Medications Soliris (eculizumab) APPROVAL CRITERIA Paroxysmal Nocturnal Hemoglobinuria I. Initiation of
Medical Policy. MP Eculizumab (Soliris) Related Policies None. Last Review: 01/24/2019 Effective Date: 04/25/2019 Section: Prescription Drug
 Medical Policy Last Review: 01/24/2019 Effective Date: 04/25/2019 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for informational purposes only and are not
Medical Policy Last Review: 01/24/2019 Effective Date: 04/25/2019 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for informational purposes only and are not
Introduction to pathogenesis and treatment of thrombotic microangiopathies (TMA)
 Introduction to pathogenesis and treatment of thrombotic microangiopathies (TMA) JM.Campistol, Nephrology and Renal Transplant Department, Hospital Clinic, University of Barcelona, Barcelona, Spain. jmcampis@clinic.cat
Introduction to pathogenesis and treatment of thrombotic microangiopathies (TMA) JM.Campistol, Nephrology and Renal Transplant Department, Hospital Clinic, University of Barcelona, Barcelona, Spain. jmcampis@clinic.cat
R. Coward has documented that he has received cooperative grants from Takeda and Novo Nordisk
 R. Coward has documented that he has received cooperative grants from Takeda and Novo Nordisk Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy Lindsay Keir Richard
R. Coward has documented that he has received cooperative grants from Takeda and Novo Nordisk Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy Lindsay Keir Richard
A 60 year old woman with altered mental status and thrombotic microangiopathy. Josh Veatch
 A 60 year old woman with altered mental status and thrombotic microangiopathy Josh Veatch Previously healthy 60 year old woman 2 3 months of fatigue following a URI, transient episodes being out of it
A 60 year old woman with altered mental status and thrombotic microangiopathy Josh Veatch Previously healthy 60 year old woman 2 3 months of fatigue following a URI, transient episodes being out of it
A PATIENT S JOURNEY. Learning about atypical hemolytic uremic syndrome (ahus)
 A PATIENT S JOURNEY Learning about atypical hemolytic uremic syndrome (ahus) Begin your path to empowerment Being diagnosed with ahus can be overwhelming. You may have many questions: What is ahus? How
A PATIENT S JOURNEY Learning about atypical hemolytic uremic syndrome (ahus) Begin your path to empowerment Being diagnosed with ahus can be overwhelming. You may have many questions: What is ahus? How
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Soliris (eculizumab) Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Soliris (eculizumab) Prime Therapeutics will review Prior Authorization requests
Soliris (eculizumab) Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Soliris (eculizumab) Prime Therapeutics will review Prior Authorization requests
w ahus pathology is linked to dysregulation of the alternative complement pathway.
 ahus - Pathogenesis, Etiology, and Clinical Advances Craig B. Langman MD The Isaac A Abt MD Professor of Kidney Diseases Feinberg School of Medicine, Northwestern University Head, Kidney Diseases The Ann
ahus - Pathogenesis, Etiology, and Clinical Advances Craig B. Langman MD The Isaac A Abt MD Professor of Kidney Diseases Feinberg School of Medicine, Northwestern University Head, Kidney Diseases The Ann
HUS and TTP Testing. Kenneth D. Friedman, M.D. Director, Hemostasis Reference Lab BloodCenter of Wisconsin, Milwaukee WI
 HUS and TTP Testing Kenneth D. Friedman, M.D. Director, Hemostasis Reference Lab BloodCenter of Wisconsin, Milwaukee WI Disclosures Relevant Financial Relationships Consultant: Ablynx, Bayer, CSL Behring,
HUS and TTP Testing Kenneth D. Friedman, M.D. Director, Hemostasis Reference Lab BloodCenter of Wisconsin, Milwaukee WI Disclosures Relevant Financial Relationships Consultant: Ablynx, Bayer, CSL Behring,
Hemolytic Uremic Syndrome
 Hemolytic Uremic Syndrome Francesco Emma Division of Nephrology and Dialysis Bambino Gesù Children s Hospital, IRCCS Rome, Italy Hemolytic Uremic Syndrome (HUS) microangiopathic hemolytic anemia thrombocytopenia
Hemolytic Uremic Syndrome Francesco Emma Division of Nephrology and Dialysis Bambino Gesù Children s Hospital, IRCCS Rome, Italy Hemolytic Uremic Syndrome (HUS) microangiopathic hemolytic anemia thrombocytopenia
ahus: Facts, Controversies & Treatment Updates Patrick D. Brophy MD Pediatric Nephrology, Dialysis & Transplant University of Iowa
 ahus: Facts, Controversies & Treatment Updates Patrick D. Brophy MD Pediatric Nephrology, Dialysis & Transplant University of Iowa DISCLOSURE STATEMENT I, Patrick Brophy disclose the following relationships.
ahus: Facts, Controversies & Treatment Updates Patrick D. Brophy MD Pediatric Nephrology, Dialysis & Transplant University of Iowa DISCLOSURE STATEMENT I, Patrick Brophy disclose the following relationships.
Dr. E.SUDHA (Fellow in Pediatric Nephrology) DEPT OF PEDIATRIC NEPHROLOGY & DIALYSIS Dr.MEHTA CHILDRENS HOSPITAL
 Dr. E.SUDHA (Fellow in Pediatric Nephrology) DEPT OF PEDIATRIC NEPHROLOGY & DIALYSIS Dr.MEHTA CHILDRENS HOSPITAL CASE HISTORY 4 yrs old previously well boy Born to 2 nd degree consanguinity Fever x 5 days
Dr. E.SUDHA (Fellow in Pediatric Nephrology) DEPT OF PEDIATRIC NEPHROLOGY & DIALYSIS Dr.MEHTA CHILDRENS HOSPITAL CASE HISTORY 4 yrs old previously well boy Born to 2 nd degree consanguinity Fever x 5 days
Challenges in Renal Apheresis. Mark E. Williams MD, FACP, FASN Director, Renal Apheresis Beth Israel Deaconess Medical Center Harvard Medical School
 Challenges in Renal Apheresis Mark E. Williams MD, FACP, FASN Director, Renal Apheresis Beth Israel Deaconess Medical Center Harvard Medical School Outline Principles of Separation ASFA Guidelines Renal
Challenges in Renal Apheresis Mark E. Williams MD, FACP, FASN Director, Renal Apheresis Beth Israel Deaconess Medical Center Harvard Medical School Outline Principles of Separation ASFA Guidelines Renal
HUS-MPGN-TTP. & related disorders
 ES-PCR 4 th International Conference HUS-MPGN-TTP & related disorders Current diagnosis and therapy of hemolytic uremic syndrome (HUS), membranoproliferative glomerulonephritis (MPGN), thrombotic thrombocytopenic
ES-PCR 4 th International Conference HUS-MPGN-TTP & related disorders Current diagnosis and therapy of hemolytic uremic syndrome (HUS), membranoproliferative glomerulonephritis (MPGN), thrombotic thrombocytopenic
Novel aspects of atypical haemolytic uraemic syndrome and the role of eculizumab
 Nephrol Dial Transplant (2014) 29: iv131 iv141 doi: 10.1093/ndt/gfu235 Full Review Novel aspects of atypical haemolytic uraemic syndrome and the role of eculizumab Jacobien C. Verhave 1, Jack F.M. Wetzels
Nephrol Dial Transplant (2014) 29: iv131 iv141 doi: 10.1093/ndt/gfu235 Full Review Novel aspects of atypical haemolytic uraemic syndrome and the role of eculizumab Jacobien C. Verhave 1, Jack F.M. Wetzels
Pathology of Complement Mediated Renal Disease
 Pathology of Complement Mediated Renal Disease Mariam Priya Alexander, MD Associate Professor of Pathology GN Symposium Hong Kong Society of Nephrology July 8 th, 2017 2017 MFMER slide-1 The complement
Pathology of Complement Mediated Renal Disease Mariam Priya Alexander, MD Associate Professor of Pathology GN Symposium Hong Kong Society of Nephrology July 8 th, 2017 2017 MFMER slide-1 The complement
A CAREGIVER S JOURNEY
 A CAREGIVER S JOURNEY Learning about atypical hemolytic uremic syndrome (ahus) Begin your path to effective advocacy and support Caring for someone who is diagnosed with ahus can be overwhelming. You may
A CAREGIVER S JOURNEY Learning about atypical hemolytic uremic syndrome (ahus) Begin your path to effective advocacy and support Caring for someone who is diagnosed with ahus can be overwhelming. You may
Thrombocytopenia is not mandatory to diagnose haemolytic and uremic syndrome
 Sallée et al. BMC Nephrology 2013, 14:3 RESEARCH ARTICLE Open Access Thrombocytopenia is not mandatory to diagnose haemolytic and uremic syndrome Marion Sallée 1, Khalil Ismail 1, Fadi Fakhouri 2, Henri
Sallée et al. BMC Nephrology 2013, 14:3 RESEARCH ARTICLE Open Access Thrombocytopenia is not mandatory to diagnose haemolytic and uremic syndrome Marion Sallée 1, Khalil Ismail 1, Fadi Fakhouri 2, Henri
An international consensus approach to the management of atypical hemolytic uremic syndrome in children
 DOI 10.1007/s00467-015-3076-8 REVIEW An international consensus approach to the management of atypical hemolytic uremic syndrome in children Chantal Loirat & Fadi Fakhouri & Gema Ariceta & Nesrin Besbas
DOI 10.1007/s00467-015-3076-8 REVIEW An international consensus approach to the management of atypical hemolytic uremic syndrome in children Chantal Loirat & Fadi Fakhouri & Gema Ariceta & Nesrin Besbas
Soliris Medical Policy Prior Authorization Program Summary
 Soliris Medical Policy Prior Authorization Program Summary Precertification/Prior Authorization may be required under certain plans. Please verify each member s benefits. OBJECTIVE The intent of the Soliris
Soliris Medical Policy Prior Authorization Program Summary Precertification/Prior Authorization may be required under certain plans. Please verify each member s benefits. OBJECTIVE The intent of the Soliris
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
 Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen D, Delmas Y, Douglas K, Furman R, Gaber O, Goodship T, Herthelius M, Hourmant M, Legendre C, Remuzzi G, Sheerin N, Trivelli A, Loirat C. Efficacy
Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen D, Delmas Y, Douglas K, Furman R, Gaber O, Goodship T, Herthelius M, Hourmant M, Legendre C, Remuzzi G, Sheerin N, Trivelli A, Loirat C. Efficacy
Kidney disease associated with autoimmune disease
 Kidney disease associated with autoimmune disease Masaomi Nangaku Division of Nephrology and Endocrinology the University of Tokyo Graduate School of Medicine, Japan M-type Phospholipase A2 Receptor as
Kidney disease associated with autoimmune disease Masaomi Nangaku Division of Nephrology and Endocrinology the University of Tokyo Graduate School of Medicine, Japan M-type Phospholipase A2 Receptor as
Thrombotic Microangiopathies (TMA) / TTP/HUS/αHUS Pathology & Molecular. Genetics
 Thrombotic Microangiopathies (TMA) / TTP/HUS/αHUS Pathology & Molecular Genetics Helen Liapis, M.D. Senior Consultant Arkana Labs Professor of Pathology & Immunology. retired Washington University School
Thrombotic Microangiopathies (TMA) / TTP/HUS/αHUS Pathology & Molecular Genetics Helen Liapis, M.D. Senior Consultant Arkana Labs Professor of Pathology & Immunology. retired Washington University School
ahus: recent insights and management
 ahus: recent insights and management Steven Van Laecke MD, PhD Renal Division, Ghent University Hospital, Belgium 13 th BANTAO Sarajevo October 8 2017 The complement is everywhere I could benefit from
ahus: recent insights and management Steven Van Laecke MD, PhD Renal Division, Ghent University Hospital, Belgium 13 th BANTAO Sarajevo October 8 2017 The complement is everywhere I could benefit from
Case Report Late Onset Cobalamin Disorder and Hemolytic Uremic Syndrome: A Rare Cause of Nephrotic Syndrome
 Hindawi Case Reports in Pediatrics Volume 217, Article ID 27946, 4 pages https://doi.org/1.11/217/27946 Case Report Late Onset Cobalamin Disorder and Hemolytic Uremic Syndrome: A Rare Cause of Nephrotic
Hindawi Case Reports in Pediatrics Volume 217, Article ID 27946, 4 pages https://doi.org/1.11/217/27946 Case Report Late Onset Cobalamin Disorder and Hemolytic Uremic Syndrome: A Rare Cause of Nephrotic
1. INSTRUCTIONS 2. DEFINITION OF HUS
 CQ_IBK_aHUS_01 / version 25/11/09 European Paediatric Research Group for HUS and related disorders Case questionnaire for diarrhoea negative/vtec (STEC) negative cases acute phase 1. INSTRUCTIONS Please
CQ_IBK_aHUS_01 / version 25/11/09 European Paediatric Research Group for HUS and related disorders Case questionnaire for diarrhoea negative/vtec (STEC) negative cases acute phase 1. INSTRUCTIONS Please
Coding... 5 Benefit Application... 5 Description of Services... 6 Clinical Evidence... 7
 TABLE OF CONTENTS Product Variations.... 1 Policy Statement.... 1 Related Policies.... 4 Policy Guidelines..... 4 Coding.... 5 Benefit Application........ 5 Description of Services..... 6 Clinical Evidence.......
TABLE OF CONTENTS Product Variations.... 1 Policy Statement.... 1 Related Policies.... 4 Policy Guidelines..... 4 Coding.... 5 Benefit Application........ 5 Description of Services..... 6 Clinical Evidence.......
THE MULTIPLE FACETS OF THROMBOTIC MICROANGIOPATHIES
 THE MULTIPLE FACETS OF THROMBOTIC MICROANGIOPATHIES Summary of Presentations from the Alexion-Sponsored Symposium, held at the 19 th EHA Congress, Milan, Italy, on 12 th June 2014 Chairperson Pier Mannuccio
THE MULTIPLE FACETS OF THROMBOTIC MICROANGIOPATHIES Summary of Presentations from the Alexion-Sponsored Symposium, held at the 19 th EHA Congress, Milan, Italy, on 12 th June 2014 Chairperson Pier Mannuccio
Atypical hemolytic uremic syndrome (ahus) is a progressive
 www.kidney-international.org Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome OPEN Larry A. Greenbaum 1, Marc Fila 2, Gianluigi Ardissino 3, Samhar
www.kidney-international.org Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome OPEN Larry A. Greenbaum 1, Marc Fila 2, Gianluigi Ardissino 3, Samhar
Hemolytic uremic syndrome with simultaneous Shiga toxin producing Escherichia coli and complement abnormalities
 McCoy and Weaver BMC Pediatrics 2014, 14:278 CASE REPORT Open Access Hemolytic uremic syndrome with simultaneous Shiga toxin producing Escherichia coli and complement abnormalities Nicole McCoy 1 and Donald
McCoy and Weaver BMC Pediatrics 2014, 14:278 CASE REPORT Open Access Hemolytic uremic syndrome with simultaneous Shiga toxin producing Escherichia coli and complement abnormalities Nicole McCoy 1 and Donald
PREGNANCY ASSOCIATED THROMBOTIC THROMBOCYTOPENIC PURPURA AND ACUTE KIDNEY INJURY
 VII, 2013, 2 33 A, PREGNANCY ASSOCIATED THROMBOTIC THROMBOCYTOPENIC PURPURA AND ACUTE KIDNEY INJURY M. Lubomirova Clinic of Nephrology, University Hospital Aleksandrovska So a : ( ), /HELLP, (AFLP) (TTP)
VII, 2013, 2 33 A, PREGNANCY ASSOCIATED THROMBOTIC THROMBOCYTOPENIC PURPURA AND ACUTE KIDNEY INJURY M. Lubomirova Clinic of Nephrology, University Hospital Aleksandrovska So a : ( ), /HELLP, (AFLP) (TTP)
CASE REPORT. Introduction. Case Report
 doi: 10.2169/internalmedicine.0228-17 Intern Med Advance Publication http://internmed.jp CASE REPORT A Rare Case of Lupus Nephritis Presenting as Thrombotic Microangiopathy with Diffuse Pseudotubulization
doi: 10.2169/internalmedicine.0228-17 Intern Med Advance Publication http://internmed.jp CASE REPORT A Rare Case of Lupus Nephritis Presenting as Thrombotic Microangiopathy with Diffuse Pseudotubulization
Untying the Knot of Thrombotic Thrombocytopenic Purpura and Atypical Hemolytic Uremic Syndrome
 REVIEW Untying the Knot of Thrombotic Thrombocytopenic Purpura and Atypical Hemolytic Uremic Syndrome Han-Mou Tsai, MD imah Hematology Associates, New Hyde Park, NY. ABSTRACT Patients presenting with microangiopathic
REVIEW Untying the Knot of Thrombotic Thrombocytopenic Purpura and Atypical Hemolytic Uremic Syndrome Han-Mou Tsai, MD imah Hematology Associates, New Hyde Park, NY. ABSTRACT Patients presenting with microangiopathic
ISTITUTO DI RICERCHE FARMACOLOGICHE MARIO NEGRI CLINICAL RESEARCH CENTER ALDO E FOR CELE RARE DACCO DISEASES ALDO E CELE DACCO
 ISTITUTO DI RICERCHE FARMACOLOGICHE MARIO NEGRI CENTRO MARIO DI NEGRI RICERCHE INSTITUTE CLINICHE FOR PHARMACOLOGICAL PER LE MALATTIE RESEARCH RARE CLINICAL RESEARCH CENTER ALDO E FOR CELE RARE DACCO DISEASES
ISTITUTO DI RICERCHE FARMACOLOGICHE MARIO NEGRI CENTRO MARIO DI NEGRI RICERCHE INSTITUTE CLINICHE FOR PHARMACOLOGICAL PER LE MALATTIE RESEARCH RARE CLINICAL RESEARCH CENTER ALDO E FOR CELE RARE DACCO DISEASES
HHS Public Access Author manuscript Int J Med Pharm Case Reports. Author manuscript; available in PMC 2016 April 28.
 Atypical Hemolytic Uremic Syndrome and Chronic Ulcerative Colitis Treated with Eculizumab Tennille N. Webb 1, Heidi Griffiths 2, Yosuke Miyashita 1, Riha Bhatt 3, Ronald Jaffe 4, Michael Moritz 1, Johannes
Atypical Hemolytic Uremic Syndrome and Chronic Ulcerative Colitis Treated with Eculizumab Tennille N. Webb 1, Heidi Griffiths 2, Yosuke Miyashita 1, Riha Bhatt 3, Ronald Jaffe 4, Michael Moritz 1, Johannes
Guideline. Abstract. Key words
 bs_bs_banner Pediatrics International (2014) 56, 1 5 doi: 10.1111/ped.12274 Guideline Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the Joint Committee of the Japanese Society
bs_bs_banner Pediatrics International (2014) 56, 1 5 doi: 10.1111/ped.12274 Guideline Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the Joint Committee of the Japanese Society
Thrombotic microangiopathy and indications for therapeutic plasma exchange
 SPIN DOCTORS:APHERESIS FOR HEMATOLOGISTS Thrombotic microangiopathy and indications for therapeutic plasma exchange Jill Adamski 1 1 Department of Laboratory Medicine and Pathology, Mayo Clinic Arizona,
SPIN DOCTORS:APHERESIS FOR HEMATOLOGISTS Thrombotic microangiopathy and indications for therapeutic plasma exchange Jill Adamski 1 1 Department of Laboratory Medicine and Pathology, Mayo Clinic Arizona,
Thrombotic Thrombocytopenic Purpura and the Role of ADAMTS-13
 Thrombotic Thrombocytopenic Purpura and the Role of ADAMTS-13 Mark Cunningham,MD Director, Hematology Laboratory Department of Pathology University of Kansas Medical Center College of American Pathologists
Thrombotic Thrombocytopenic Purpura and the Role of ADAMTS-13 Mark Cunningham,MD Director, Hematology Laboratory Department of Pathology University of Kansas Medical Center College of American Pathologists
Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial
 Original Investigation Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial Fadi Fakhouri, MD, PhD, 1 Maryvonne Hourmant, MD,
Original Investigation Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial Fadi Fakhouri, MD, PhD, 1 Maryvonne Hourmant, MD,
List of authors and affiliations :
 List of authors and affiliations : Fadi Fakhouri 1, Marc Fila 2, François Provot 3, Yahsou Delmas 2, Christelle Barbet 5, Valérie Châtelet 6, Cédric afat 7, Mathilde Cailliez 8, Julien Hogan 9, Aude Servais
List of authors and affiliations : Fadi Fakhouri 1, Marc Fila 2, François Provot 3, Yahsou Delmas 2, Christelle Barbet 5, Valérie Châtelet 6, Cédric afat 7, Mathilde Cailliez 8, Julien Hogan 9, Aude Servais
Acquired Drivers of Disaese ahus and autoantibodies: their role in disease and their impact on patient management
 Acquired Drivers of Disaese ahus and autoantibodies: their role in disease and their impact on patient management Veronique Fremeaux-Bacchi Cordeliers Research Center and Européen Georges Pompidou Hospital,
Acquired Drivers of Disaese ahus and autoantibodies: their role in disease and their impact on patient management Veronique Fremeaux-Bacchi Cordeliers Research Center and Européen Georges Pompidou Hospital,
Extrarenal involvement in pediatric ahus Kosan C et al
 Case Report J Ped. Nephrology 2014;2(4):154-157 http://journals.sbmu.ac.ir/jpn Atypical Hemolytic Uremic Syndrome with Severe Extrarenal Manifestations: a Case Report How to Cite This Article: KOŞAN C,
Case Report J Ped. Nephrology 2014;2(4):154-157 http://journals.sbmu.ac.ir/jpn Atypical Hemolytic Uremic Syndrome with Severe Extrarenal Manifestations: a Case Report How to Cite This Article: KOŞAN C,
Two Patients With History of STEC-HUS, Posttransplant Recurrence and Complement Gene Mutations
 American Journal of Transplantation 2013; 13: 2201 2206 Wiley Periodicals Inc. Case Report C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi:
American Journal of Transplantation 2013; 13: 2201 2206 Wiley Periodicals Inc. Case Report C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi:
Thrombotic thrombocytopenic purpura: a look at the future
 Thrombotic thrombocytopenic purpura: a look at the future Andrea Artoni, MD Ph.D. Angelo Bianchi Bonomi Hemophilia and Thrombosis Center IRCCS Ca Granda Ospedale Maggiore Policlinico Milan, Italy andrea.artoni@policlinico.mi.it
Thrombotic thrombocytopenic purpura: a look at the future Andrea Artoni, MD Ph.D. Angelo Bianchi Bonomi Hemophilia and Thrombosis Center IRCCS Ca Granda Ospedale Maggiore Policlinico Milan, Italy andrea.artoni@policlinico.mi.it
Pathogenic Variants in Complement Genes and Risk of Atypical Hemolytic Uremic Syndrome Relapse after Eculizumab Discontinuation
 Article Pathogenic s in Complement Genes and Risk of Atypical Hemolytic Uremic Syndrome Relapse after Eculizumab Discontinuation Fadi Fakhouri, Marc Fila, François Provôt, Yahsou Delmas, Christelle Barbet,
Article Pathogenic s in Complement Genes and Risk of Atypical Hemolytic Uremic Syndrome Relapse after Eculizumab Discontinuation Fadi Fakhouri, Marc Fila, François Provôt, Yahsou Delmas, Christelle Barbet,
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
Correspondence should be addressed to Camino García Monteavaro;
 Hindawi Publishing Corporation Case Reports in Nephrology Volume 2016, Article ID 7471082, 7 pages http://dx.doi.org/10.1155/2016/7471082 Case Report Adjustment of Eculizumab Dosage Pattern in Patients
Hindawi Publishing Corporation Case Reports in Nephrology Volume 2016, Article ID 7471082, 7 pages http://dx.doi.org/10.1155/2016/7471082 Case Report Adjustment of Eculizumab Dosage Pattern in Patients
2015. This manuscript version is made available under the CC-BY-NC-ND 4.0 license
 Iqbal Z, Wood K, Carter V, Goodship TH, Brown AL, Sheerin NS. Thrombotic microangiopathy as a cause of chronic kidney transplant dysfunction: a case report demonstrating successful treatment with Eculizumab.
Iqbal Z, Wood K, Carter V, Goodship TH, Brown AL, Sheerin NS. Thrombotic microangiopathy as a cause of chronic kidney transplant dysfunction: a case report demonstrating successful treatment with Eculizumab.
A 23 year old Caucasian male presented with shortness of breath, hypertension, bloody sputum, and a history of drug abuse (confirmed by urinalysis).
 A 23 year old Caucasian male presented with shortness of breath, hypertension, bloody sputum, and a history of drug abuse (confirmed by urinalysis). He was found to have severe kidney injury requiring
A 23 year old Caucasian male presented with shortness of breath, hypertension, bloody sputum, and a history of drug abuse (confirmed by urinalysis). He was found to have severe kidney injury requiring
Initial management of TMA syndromes
 Initial management of TMA syndromes Elie Azoulay, Saint-Louis Hospital, Medical Intensive Care Unit Paris Diderot Sorbonne University Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique
Initial management of TMA syndromes Elie Azoulay, Saint-Louis Hospital, Medical Intensive Care Unit Paris Diderot Sorbonne University Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique
Familial DDD associated with a gain-of-function mutation in complement C3.
 Familial DDD associated with a gain-of-function mutation in complement C3. Santiago Rodríguez de Córdoba, Centro de investigaciones Biológicas, Madrid Valdés Cañedo F. and Vázquez- Martul E., Complejo
Familial DDD associated with a gain-of-function mutation in complement C3. Santiago Rodríguez de Córdoba, Centro de investigaciones Biológicas, Madrid Valdés Cañedo F. and Vázquez- Martul E., Complejo
Complement and the atypical hemolytic uremic syndrome in children
 Pediatr Nephrol (2008) 23:1957 1972 DOI 10.1007/s00467-008-0872-4 EDUCATIONAL REVIEW Complement and the atypical hemolytic uremic syndrome in children Chantal Loirat & Marina Noris & Véronique Fremeaux-Bacchi
Pediatr Nephrol (2008) 23:1957 1972 DOI 10.1007/s00467-008-0872-4 EDUCATIONAL REVIEW Complement and the atypical hemolytic uremic syndrome in children Chantal Loirat & Marina Noris & Véronique Fremeaux-Bacchi
Diagnosis and Treatment of Common TMAs
 Diagnosis and Treatment of Common TMAs K. Pavenski, MD FRCPC St. Michael s Hospital June 13, 2014 Disclosures I have a relevant conflict of interest - Alexion Pharmaceuticals Inc. Participated in advisory
Diagnosis and Treatment of Common TMAs K. Pavenski, MD FRCPC St. Michael s Hospital June 13, 2014 Disclosures I have a relevant conflict of interest - Alexion Pharmaceuticals Inc. Participated in advisory
PNH ahus. Dosing and Administration. For Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic Uremic Syndrome (ahus) patients
 For Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic Uremic Syndrome (ahus) patients PNH ahus Dosing and Administration Soliris is indicated for the treatment of patients with paroxysmal
For Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic Uremic Syndrome (ahus) patients PNH ahus Dosing and Administration Soliris is indicated for the treatment of patients with paroxysmal
* Renal insufficiencies
 Thrombotic Thrombocytopenic Purpura Behzad Poopak, DCLS PhD. Tehran medical Branch Islamic Azad university bpoopak@yahoo.com Case Summary Ms. X, a 35-year year-old woman Complained of weakness, low grade
Thrombotic Thrombocytopenic Purpura Behzad Poopak, DCLS PhD. Tehran medical Branch Islamic Azad university bpoopak@yahoo.com Case Summary Ms. X, a 35-year year-old woman Complained of weakness, low grade
THROMBOTIC MICROANGIOPATHY. Jun-Ki Park 7/19/11
 THROMBOTIC MICROANGIOPATHY Jun-Ki Park 7/19/11 TMAs are microvascular occlusive disorders characterized by systemic or intrarenal aggregation of platelets, thrombocytopenia, and mechanical injury to erythrocytes.
THROMBOTIC MICROANGIOPATHY Jun-Ki Park 7/19/11 TMAs are microvascular occlusive disorders characterized by systemic or intrarenal aggregation of platelets, thrombocytopenia, and mechanical injury to erythrocytes.
Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic Uremic Syndrome
 original article Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic Uremic Syndrome C.M. Legendre, C. Licht, P. Muus, L.A. Greenbaum, S. Babu, C. Bedrosian, C. Bingham, D.J. Cohen, Y. Delmas,
original article Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic Uremic Syndrome C.M. Legendre, C. Licht, P. Muus, L.A. Greenbaum, S. Babu, C. Bedrosian, C. Bingham, D.J. Cohen, Y. Delmas,
Medication Prior Authorization Form
 Policy Number: 1054 Policy History Approve Date: 06/01/2018 Effective Date: 06/01/2018 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
Policy Number: 1054 Policy History Approve Date: 06/01/2018 Effective Date: 06/01/2018 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
STUDY SYNOPSIS: Protocol number: CDX
 STUDY SYNOPSIS: Protocol number: Title: Investigators Investigational Treatment: Indication: Number of Patients: Number of Study Centers: CDX1135-01 A Pilot, Open-label, Multicenter Clinical Trial of CDX-1135
STUDY SYNOPSIS: Protocol number: Title: Investigators Investigational Treatment: Indication: Number of Patients: Number of Study Centers: CDX1135-01 A Pilot, Open-label, Multicenter Clinical Trial of CDX-1135
Eculizumab in ahus: where do we stand in Prof. Fadi Fakhouri Dept. of nephrology and immunology, CHU de Nantes.
 Eculizumab in ahus: where do we stand in 2016 Prof. Fadi Fakhouri Dept. of nephrology and immunology, CHU de Nantes. INSERM UMR S-1064 Thrombotic microangiopathy: evolving concepts ST-HUS Coagulation mediated-tma
Eculizumab in ahus: where do we stand in 2016 Prof. Fadi Fakhouri Dept. of nephrology and immunology, CHU de Nantes. INSERM UMR S-1064 Thrombotic microangiopathy: evolving concepts ST-HUS Coagulation mediated-tma
Case report 24 th Summer School of Internal Medicine 2015
 Case report 24 th Summer School of Internal Medicine 2015 Goldmannová D., Horák P., Skácelová M. IIIrd Internal Clinic - endocrinology, diabetology, rheumatology, nephrology University hospital Olomouc,
Case report 24 th Summer School of Internal Medicine 2015 Goldmannová D., Horák P., Skácelová M. IIIrd Internal Clinic - endocrinology, diabetology, rheumatology, nephrology University hospital Olomouc,
RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT. J. H. Helderman,MD,FACP,FAST
 RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT J. H. Helderman,MD,FACP,FAST Vanderbilt University Medical Center Professor of Medicine, Pathology and Immunology Medical Director, Vanderbilt Transplant
RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT J. H. Helderman,MD,FACP,FAST Vanderbilt University Medical Center Professor of Medicine, Pathology and Immunology Medical Director, Vanderbilt Transplant
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: eculizumab_soliris 8/2014 4/2018 4/2019 4/2018 Description of Procedure or Service Paroxysmal nocturnal hemoglobinuria
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: eculizumab_soliris 8/2014 4/2018 4/2019 4/2018 Description of Procedure or Service Paroxysmal nocturnal hemoglobinuria
1/26/12. Selected Topics in Pediatric Hematology/Oncology COMPLEMENTOLOGY OBJECTIVES. Classically Different Topics but not so much
 1/26/12 OBJECTIVES Selected Topics in Pediatric Hematology/Oncology COMPLEMENTOLOGY Chatchawin Assanasen MD Recognize implications of complement pathway diseases Signs and symptoms of PNH and ahus Complications
1/26/12 OBJECTIVES Selected Topics in Pediatric Hematology/Oncology COMPLEMENTOLOGY Chatchawin Assanasen MD Recognize implications of complement pathway diseases Signs and symptoms of PNH and ahus Complications
PNH ahus gmg. Dosing and Administration Guide
 Injection for Intravenous Use PNH ahus gmg For Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic Uremic Syndrome (ahus), and generalized Myasthenia Gravis (gmg) patients Dosing and Administration
Injection for Intravenous Use PNH ahus gmg For Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic Uremic Syndrome (ahus), and generalized Myasthenia Gravis (gmg) patients Dosing and Administration
Unravelling the pathophysiology of HUS in light of recent discoveries on complement activation
 Unravelling the pathophysiology of HUS in light of recent discoveries on complement activation Marina Noris November 13, 2018 1 THROMBOTIC MICROANGIOPATHY Histology lesions: Swelling and detachment of
Unravelling the pathophysiology of HUS in light of recent discoveries on complement activation Marina Noris November 13, 2018 1 THROMBOTIC MICROANGIOPATHY Histology lesions: Swelling and detachment of
Microangiopatia trombotica (MAT) e Sindrome emolitico-uremica atipica (SEUa): Basi patogenetiche, inquadramento diagnostico e principi del
 Microangiopatia trombotica (MAT) e Sindrome emolitico-uremica atipica (SEUa): Basi patogenetiche, inquadramento diagnostico e principi del trattamento Vincenzo Montinaro U.O. Nefrologia Azienda Ospedaliera
Microangiopatia trombotica (MAT) e Sindrome emolitico-uremica atipica (SEUa): Basi patogenetiche, inquadramento diagnostico e principi del trattamento Vincenzo Montinaro U.O. Nefrologia Azienda Ospedaliera
A Case of Escherichia coli Hemolytic Uremic Syndrome in a 10-Year-Old Male With Severe Neurologic Involvement Successfully Treated With Eculizumab
 741144HICXXX10.1177/2324709617741144Journal of Investigative Medicine High Impact Case ReportsRasa et al research-article20172017 Case Report A Case of Escherichia coli Hemolytic Uremic Syndrome in a 10-Year-Old
741144HICXXX10.1177/2324709617741144Journal of Investigative Medicine High Impact Case ReportsRasa et al research-article20172017 Case Report A Case of Escherichia coli Hemolytic Uremic Syndrome in a 10-Year-Old
Haemolytic uraemic syndrome the story of a whodunit
 Haemolytic uraemic syndrome the story of a whodunit Paul Warwicker Lancashire Teaching Hospitals NHS Trust RCP Kidney for the General Physician Conference Nov 17 Renal thrombotic microangiopathy (TMA)
Haemolytic uraemic syndrome the story of a whodunit Paul Warwicker Lancashire Teaching Hospitals NHS Trust RCP Kidney for the General Physician Conference Nov 17 Renal thrombotic microangiopathy (TMA)
A Rational Approach to Evaluation of Thrombotic Microangiopathy
 A Rational Approach to Evaluation of Thrombotic Microangiopathy An Algorithmic Approach C. Christopher Hook, MD for the Complement Alternative Pathway Thrombotic Micro- Angiopathy (CAP-TMA) Disease-Oriented
A Rational Approach to Evaluation of Thrombotic Microangiopathy An Algorithmic Approach C. Christopher Hook, MD for the Complement Alternative Pathway Thrombotic Micro- Angiopathy (CAP-TMA) Disease-Oriented
Atypical hemolytic uremic syndrome. Diana Karpman Department of Pediatrics Lund University
 Atypical hemolytic uremic syndrome Diana Karpman Department of Pediatrics Lund University Image courtesy of Dr. Sabine Leh, Haukeland University Hospital, Bergen Norway Hemolytic Uremic Syndrome Non-immune
Atypical hemolytic uremic syndrome Diana Karpman Department of Pediatrics Lund University Image courtesy of Dr. Sabine Leh, Haukeland University Hospital, Bergen Norway Hemolytic Uremic Syndrome Non-immune
