High-Dosage Ascorbic Acid Treatment in Charcot-Marie-Tooth Disease Type 1A Results of a Randomized, Double-Masked, Controlled Trial
|
|
|
- Ernest Norman
- 6 years ago
- Views:
Transcription
1 Research Original Investigation CLINICAL TRIAL High-Dosage Ascorbic Acid Treatment in Charcot-Marie-Tooth Disease Type 1A Results of a Randomized, Double-Masked, Controlled Trial Richard A. Lewis, MD; Michael P. McDermott, PhD; David N. Herrmann, MD; Ahmet Hoke, MD, PhD; Lora L. Clawson, MSN, CRNP; Carly Siskind, MS; Shawna M. E. Feely, MS; Lindsey J. Miller, MS; Richard J. Barohn, MD; Patricia Smith, BS; Elizabeth Luebbe, MS; Xingyao Wu, MD, PhD; Michael E. Shy, MD; for the Muscle Study Group IMPORTANCE No current medications improve neuropathy in subjects with Charcot-Marie-Tooth disease type 1A (CMT1A). Ascorbic acid (AA) treatment improved the neuropathy of a transgenic mouse model of CMT1A and is a potential therapy. A lower dosage (1.5 g/d) did not cause improvement in humans. It is unknown whether a higher dosage would prove more effective. Editorial page 969 Supplemental content at jamaneurology.com OBJECTIVE To determine whether 4-g/d AA improves the neuropathy of subjects with CMT1A. DESIGN A futility design to determine whether AA was unable to reduce worsening on the CMT Neuropathy Score (CMTNS) by at least 50% over a 2-year period relative to a natural history control group. SETTING Three referral centers with peripheral nerve clinics (Wayne State University, Johns Hopkins University, and University of Rochester). PARTICIPANTS One hundred seventy-four subjects with CMT1A were assessed for eligibility; 48 did not meet eligibility criteria and 16 declined to participate. The remaining 110 subjects, aged 13 to 70 years, were randomly assigned in a double-masked fashion with 4:1 allocation to oral AA (87 subjects) or matching placebo (23 subjects). Sixty-nine subjects from the treatment group and 16 from the placebo group completed the study. Two subjects from the treatment group and 1 from the placebo group withdrew because of adverse effects. INTERVENTIONS Oral AA (4 g/d) or matching placebo. MAIN OUTCOMES AND MEASURES Change from baseline to year 2 in the CMTNS, a validated composite impairment score for CMT. RESULTS The mean 2-year change in the CMTNS was 0.21 for the AA group and 0.92 for the placebo group, both better than natural history (+1.33). This was well below 50% reduction of CMTNS worsening from natural history, so futility could not be declared (P >.99). CONCLUSIONS AND RELEVANCE Both treated patients and those receiving placebo performed better than natural history. It seems unlikely that our results support undertaking a larger trial of 4-g/d AA treatment in subjects with CMT1A. TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT JAMA Neurol. 2013;70(8): doi: /jamaneurol Published online June 24, Author Affiliations: Author affiliations are listed at the end of this article. Group Information: The Muscle Study Group members are listed at the end of this article. Corresponding Author: Michael E. Shy, MD, Department of Neurology, Carver College of Medicine, University of Iowa, 200 Hawkins Dr, 1188 Medical Laboratories, Iowa City, IA (michael-shy@uiowa.edu). Section Editor: Ira Shoulson, MD 981
2 Research Original Investigation High-Dosage Ascorbic Acid Treatment in CMT1A Charcot-Marie-Tooth (CMT) disorders are inherited peripheral neuropathies and are among the most common genetic neurological disorders, with a prevalence of 1 in 2400 individuals. 1 Charcot-Marie-Tooth disease type 1A (CMT1A) is caused by a 1.4-megabase duplication on chromosome 17p11.2 2,3 and constitutes approximately 50% of all cases of CMT. 4,5 The peripheral myelin protein 22 gene (PMP22; Gen- Bank AC005703) is contained within the duplication, and the increased level of PMP22 causes the neuropathy. 6 High dosages of ascorbic acid (AA) reduced the messenger RNA (mrna) levels of PMP22, improved function, and increased the numbers of myelinated peripheral nerve axons in the C22 mouse model of CMT1A. 7 Subsequently, multiple clinical trials were undertaken to determine whether AA would slow progression or improve the neuropathy in subjects with CMT1A. Three randomized, placebo-controlled trials assessed patients for 1 year. A small Dutch study of 2-g/d AA 8 and an Australian trial of 30-mg/kg/d AA in children showed no benefit in the primary end point of change in nerve conduction velocity. 9 A French study of 179 adults noted no significant benefit of 1-g/d or 3-g/d AA over 1 year using the CMT Neuropathy Score (CMTNS); however, a benefit from 3 g/d was detected when the physiology component of the CMTNS was removed from the scoring. 10 A trial conducted in Italy and the United Kingdom of 138 adults (aged years) receiving 1.5-g/d AA compared with 133 adults receiving placebo for 2 years showed no effect of AA on the CMTNS. 11 We performed a clinical trial using high-dosage (4-g/d) AA for 2 years in subjects with CMT1A. We used the change in CMTNS as the primary outcome measure based on recommendations from the 136th European Neuromuscular Centre International Workshop on CMT that this was the best currently available primary end point for clinical trials. 12 An AA dosage of 4 g/d was used because this was equivalent to the maximum amount ingested by mice and up to 10 g/d of AA has been well tolerated in humans. 13 We used a futility design with a natural history control group. Randomization and Masking Subjects were randomly assigned with 4:1 allocation to AA (80%) or placebo (20%). The AA was given as 4 g taken as four 500-mg capsules twice daily. Active drug and placebo capsules identical in appearance, taste, and smell were manufactured, packed, and labeled at a central pharmacy (CSP Clinical Research Pharmacy Coordinating Center, US Department of Veterans Affairs). The randomization plan was computer generated and included stratification by site and blocking to enhance preservation of the 4:1 allocation ratio at each site. Only a programmer in the Muscle Study Group Biostatistics Center and the investigational pharmacy had access to the treatment assignments during the trial. Procedures Potentially eligible subjects were evaluated at screening visits that included a history, confirmation of CMT1A genotyping, physical examination, recording of the CMTNS and Neuropathy Impairment Score (NIS), 14 blood sampling for AA levels, complete blood cell count, electrolytes, blood urea nitrogen, creatinine, glycated hemoglobin, calcium, uric acid, and fasting blood glucose. Urinalysis and urinary pregnancy tests were obtained for women of childbearing age. Median or ulnar motor and antidromic sensory nerve conduction studies were performed on the patient s left side unless structural abnormalities necessitated use of the right side. 15 A 36-Item Short Form Health Survey (SF-36) 16 was obtained. If subjects met eligibility criteria, they were randomized and baseline skin biopsy specimens were obtained. Follow-up visits were conducted at 6, 12, 18, and 24 months after baseline, at which time the physical examination, CMTNS, NIS, nerve conduction studies, SF-36, and serum AA levels were obtained. Drug compliance was measured by counting dispensed and returned pills and by serum AA levels. Adverse events were reviewed and vital signs obtained at each visit. The laboratory studies done at screening were repeated at 12 and 24 months and a second skin biopsy was performed at the 24-month (final) visit. Subjects and site investigators were then asked to provide guesses of the subjects treatment assignments. Methods Subjects Subjects with CMT1A between the ages of 13 and 70 years were eligible for the study. Diagnosis of CMT1A was made by detecting the duplication on chromosome 17p11.2, performed by either pulsed-field gel electrophoresis or fluorescence in situ hybridization, or by the subject having a first- or second-degree relative with a documented duplication and having uniform motor conduction slowing of the median or ulnar nerve between 10 and 35 m/s. Written informed consent was provided by subjects aged 18 years or older and by parents or guardians for subjects younger than 18 years (with written assent from the subject). Subjects were excluded if they had criteria listed in etable 1 in Supplement. The institutional review boards at all 3 participating sites approved the study (Wayne State University, Johns Hopkins University, and University of Rochester). We received an Investigational New Drug for the study from the US Food and Drug Administration (74970). Skin Biopsy and Real-Time Polymerase Chain Reaction Analysis After informed consent was obtained, 2 adjacent 2-mm punch biopsy specimens were obtained as previously described. 17 Biopsies were performed in the left volar forearm, 9 cm proximal to the ulnar crease at the wrist. Initial biopsies were performed 0.5 cm below a line connecting the ulnar crease at the wrist to the olecranon at the elbow, and final biopsies were performed 0.5 cm above the line. Real-time polymerase chain reaction was performed. The PMP22 mrna levels were normalized to the 18S ribosomal band, S-100 protein mrna (to normalize to Schwann cell number), and myelin basic protein mrna (to normalize for myelin gene expression). Primers used in the reactions are listed in etable 2 in Supplement. Outcome Measures The primary outcome measure was the change in CMTNS from baseline to year 2. Secondary outcome measures included 2-year changes in the CMT examination score, which was the CMTNS 982 JAMA Neurology August 2013 Volume 70, Number 8 jamaneurology.com
3 High-Dosage Ascorbic Acid Treatment in CMT1A Original Investigation Research minus the 2 electrophysiological items, 18 the NIS, 14 ulnar motor nerve conduction velocity, nerve conduction distal latency, nerve conduction distal compound motor action potential amplitude, SF-36 summary and subscale scores, and PMP22 mrna levels obtained from the skin biopsy specimens. Statistical Analysis To determine whether it would be prudent to test AA in a large confirmatory trial, we used a futility study design with a natural history control. A concurrent placebo group was included to facilitate double masking and to provide a descriptive check of the assumption that the natural history control group would yield changes in the CMTNS that were comparable to those of a concurrent placebo group. 19 The primary statistical analysis compared the mean 2-year worsening (increase) in the CMTNS in the AA group with a prespecified fixed value that represents a 50% reduction from the natural history of a mean increase in CMTNS of 1.33 points over 2 years 19 ; thus, a mean increase of 0.67 points on the CMTNS would represent a 50% reduction from expected. We formulated the statistical problem as that of testing H 0 :μ 0.67vsH 1 : μ > 0.67, where μ is the true mean worsening in CMTNS in the AA group. If H 0 were rejected, ie, if there were sufficient evidence that the mean worsening in the CMTNS from baseline to year 2 was not at least 50% less than that expected, it would be considered futile to pursue testing AA in a future confirmatory trial. This futility study approach to screening out ineffective agents has recently been used in other trials in neurodegenerative diseases The primary analysis estimated the adjusted mean changes in CMTNS in the AA and placebo groups using a mixed-model repeated-measures approach. 23 The model included treatment group, month (6, 12, 18, and 24 months, treated as a categorical variable), the interaction between treatment group and month, site, and the baseline CMTNS as independent variables. The covariance matrix for the within-subjects measurements was specified as unstructured for model fitting. This approach appropriately accounts for missing data when estimating the model parameters under the missing at random assumption. 24 This model was used to construct a t test to compare the mean increase in CMTNS in the AA group with the fixed value of 0.67 using a 1-tailed 10% significance level. Data from the secondary outcome variables (CMT examination score, NIS, electrophysiological tests, SF-36 summary and subscale scores, vital signs, and laboratory test results) were analyzed using the mixed-model repeated-measures approach. Analysis of covariance was used to compare the treatment groups with respect to 2-year changes in log-transformed PMP22 mrna levels, adjusting for center and the baseline level. Comparisons between the AA and placebo groups were chiefly descriptive owing to the small sample size of the placebo group. Adverse events were summarized as the percentage of subjects in each treatment group who experienced the event during the trial. We used χ 2 tests to compare the AA and placebo groups with respect to the percentage of subjects thought (by subjects and by site investigators) to be receiving AA. Data from our natural history study suggest that the standard deviation of the change in CMTNS from baseline to year 2 is approximately 2.2 points. 19 A sample size of 80 subjects in the AAgroupwasplannedtoprovide85%powertodetectthatthe mean worsening in the CMTNS in the AA group was not at least 50% less than that expected, assuming that AA has no effect (ie, that the true mean decline is 1.33 points), using a 1-tailed t test and a significance level of 10%. If AA actually has no effect on the mean change in CMTNS, we had 85% power to detect futility of this agent. To account for a dropout rate of 20%, a sample size of 100 subjects was planned for the AA group. Results Subject Characteristics One hundred ten subjects were recruited and evaluated between April 2007 and July 2011, with 87 patients receiving AA and 23 receiving placebo. Demographic and clinical characteristics were comparable between the treatment groups (Table 1). The mean (SD) age was 42.8 (14.7) years (range, years), and 58% were female. The mean (SD) CMTNS at baseline was 16.5 (4.5), and the mean (SD) AA level was 0.87 (0.47) mg/dl (to convert to micromoles per liter, multiply by 56.78). Subjects in the natural history study (n = 72) had a slightly a lower mean CMTNS and a higher mean NIS at baseline (Table 1). Subject Disposition Sixty-nine subjects (79%) in the AA group and 16 (70%) in the placebo group completed the study. Reasons for subject withdrawal are summarized in Figure 1. Two subjects in the AA group had the study drug temporarily suspended, one because of frequent muscle cramps and the other after an ankle fracture. Two others in the AA group had their dosage reduced, one to 3 g/d and the other to 2 g/d, owing to stomach upset. Primary Outcome Variable The adjusted mean change in the CMTNS over 2 years was 0.21 for the AA group and 0.92 for the placebo group; both performed better than the natural history group (+1.33) (Table 2 and Figure 2). The 90% lower confidence bound for the mean change in CMTNS in the AA group was 0.63, well below that representing a 50% reduction of CMTNS worsening from natural history (+0.67), so futility could not be formally declared (P >.99). The improvement in CMTNS in both treatment groups was apparent within the first 6 months and was sustained throughout the study (Figure 2). The results for the CMT examination score were similar to those of the CMTNS. Secondary Outcome Variables The results for the NIS were similar to those for the CMTNS, with the adjusted mean changes in the AA group (+0.79) and the placebo group ( 1.12) both numerically less than that expected from natural history (+2.73). 21 Results for other secondary outcome variables, including the SF-36 summary and subscale scores and the electrophysiological tests, are shown in Table 2. No treatment group differences were apparent, although the placebo group was small (n = 23). The PMP22 mrna levels were obtained at baseline and at 2 years in a subset of 69 subjects, 55 in the AA group and 14 in the jamaneurology.com JAMA Neurology August 2013 Volume 70, Number 8 983
4 Research Original Investigation High-Dosage Ascorbic Acid Treatment in CMT1A Table 1. Baseline Characteristics of Participating Subjects Mean (SD) Variable Ascorbic Acid (n = 87) Placebo (n = 23) Natural History (n = 72) a Age, y 43.2 (14.7) 41.4 (14.9) 39.8 (18.5) Female, % Time since diagnosis, y 14.2 (13.4) 13.6 (13.3) NA Center, % Wayne State University Johns Hopkins University University of Rochester CMTNS 16.7 (4.4) 15.6 (5.0) 13.2 (6.1) CMTES 11.0 (3.9) 9.9 (4.4) NA NIS 42.0 (15.6) 40.9 (16.1) 62.5 (19.2) SF-36 score Mental component summary 51.1 (7.4) 50.9 (7.9) NA Physical component summary 41.6 (10.2) 41.5 (9.7) NA Ulnar motor nerve CMAP amplitude, mv 3.4 (2.0) 3.4 (2.1) NA Distal motor latency, ms 7.7 (2.1) 8.0 (1.8) NA Nerve conduction velocity, m/s 20.6 (5.6) 19.3 (6.3) NA Ascorbic acid level, mg/dl 0.88 (0.50) 0.83 (0.39) NA Abbreviations: CMAP, compound motor action potential; CMTES, Charcot-Marie-Tooth examination score; CMTNS, Charcot-Marie-Tooth Neuropathy Score; NA, not available; NIS, Neuropathy Impairment Score; SF-36, 36-Item Short Form Health Survey. SI conversion factor: To convert ascorbic acid to micromoles per liter, multiply by a Natural history data are from Shyetal. 19 Figure 1. Flow of Subjects Through the Trial 87 Allocated to intervention 87 Received allocated intervention 69 Completed follow-up 18 Withdrew 8 Lost to follow-up 2 Gastrointestinal adverse events 4 Protocol violation 2 Lack of perceived benefit 1 Pregnancy 1 Difficulty traveling 87 Included in analysis 0 Excluded 174 Assessed for eligibility 110 Randomized 64 Excluded 48 Did not meet eligibility criteria 16 Declined to participate 23 Allocated to intervention 23 Received allocated intervention 16 Completed follow-up 7 Withdrew 1 Fear of developing kidney stones 1 Developed kidney stones 1 Protocol violation 2 Lack of perceived benefit 1 Thought medication was placebo 1 Unspecified reason 23 Included in analysis 0 Excluded placebo group. No treatment effects were detected in these levels (Table 3). In agreement with previous reports, 25 PMP22 mrna levels were not associated with the CMTNS at baseline (r < 0.05) or with the CMTNS (r < 0.12) or AA level (r < 0.16) at 2 years. Mean (SD) compliance with the assigned treatment (based on pill counting) was similar for subjects receiving AA (85.2% [23.0%]) and placebo (85.9% [19.6%]). Mean serum AA levels were significantly higher (P <.002) in subjects receiving AA than in those receiving placebo at all times after the baseline visit (Figure 3). Mean levels increased by approximately 1.0 mg/dl in the AA group and changed little in the placebo group. Forty-eight percent of subjects receiving AA and 33% of subjects receiving placebo thought they had been receiving AA (P =.24). Site investigators guessed that the subject was receiving AA for 76% of subjects in the AA group and 70% of subjects in the placebo group (P =.58). Safety Eighteen serious adverse events (AEs) occurred in 15 subjects (17%) in the AA group compared with 6 serious AEs in 5 subjects (22%) in the placebo group. The serious AEs are summarized in etable 3 in Supplement. No serious AEs were judged to be related to the study drug. The most common AEs were heartburn/gastrointestinal/ vomiting/reflux, musculoskeletal, and upper respiratory tract infections (etable 4 in Supplement). There were no significant differences at 24 months between the treatment groups with respect to changes in vital signs and laboratory test results, including urinary oxalate levels. Discussion This trial was designed to determine whether it would be futile to carry out a larger randomized, double-blind, placebocontrolled trial to examine the efficacy of high-dosage (4- g/d) AA in slowing the progression of CMT1A. Subjects had better outcomes than those reported in natural history studies. Thus, based on the prespecified primary analysis for this trial, high-dosage AA could not be declared futile for further study as a therapy for CMT1A. However, the concurrent placebo group, though small, had better than expected outcomes over a 2-year period. In addition, our results for both the AA and placebo groups were similar to results from the Italian/UK study in which the CMTNS was largely unchanged for both groups after 2 years (Figure 2). From all of the available 984 JAMA Neurology August 2013 Volume 70, Number 8 jamaneurology.com
5 High-Dosage Ascorbic Acid Treatment in CMT1A Original Investigation Research Table 2. Outcome Variables by Treatment Group at Year 2 Adjusted Mean Change a Variable Ascorbic Acid Placebo Treatment Effect (95% CI) CMTNS ( 0.70 to 2.12) CMTES ( 0.61 to 1.95) NIS ( 2.24 to 6.07) SF-36 score MCS ( 3.80 to 4.73) PCS ( 2.99 to 3.77) Physical functioning ( 2.63 to 3.41) Role-physical ( 2.69 to 5.82) Bodily pain ( 7.41 to 1.62) General health ( 0.42 to 6.05) Vitality ( 2.20 to 2.80) Social functioning ( 5.16 to 4.86) Role-emotional ( 4.42 to 6.82) Mental health ( 2.82 to 2.71) Ulnar motor nerve CMAP amplitude, mv ( 0.81 to 0.95) Distal motor latency, ms ( 0.87 to 0.64) NCV, m/s ( 0.88 to 2.61) Abbreviations: CMAP, compound motor action potential; CMTES, Charcot-Marie-Tooth examination score; CMTNS, Charcot-Marie-Tooth Neuropathy Score; MCS, mental component summary; NCV, nerve conduction velocity; NIS, Neuropathy Impairment Score; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey. a Values are adjusted mean changes from baseline to year 2, calculated from a repeated-measures analysis of a covariance model that included treatment group, month (categorical), the interaction between treatment group and month, site, and the baseline value of the outcome variable as independent variables; see text for details. evidence, we conclude that it is unlikely that 4-g/d AA has a clinically meaningful effect on the course of CMT1A over a 2-year period. It is unlikely that the interpretation of our results was hindered by toxic effects from high dosages of AA as most patients tolerated the dosage well and there was little difference between the treatment groups in terms of AEs. It is also unlikely that subject perception of whether they were receiving AA biased our results. The CMTNS is mainly investigator (examination) driven, 15 and similar percentages of subjects were guessed by investigators to be receiving AA in the 2 treatment groups (76% and 70%). More than half of subjects in both groups thought they were receiving placebo. Placebo Groups May Not Reflect Natural History This clinical trial as well as the Italian/UK trial indicate that current natural history data for changes in the CMTNS over 2 years in CMT1A cannot be used in lieu of a placebo group in clinical trials. The factors accounting for the differences between our natural history data for the CMTNS and those from our placebo and active treatment groups in this trial are unclear. Systematic differences between current study participants and those in the natural history group may be partially responsible, although these groups had similar distributions of age, sex, and CMTNS (Table 1). It is also possible that the use of an allocation ratio of 4:1 (AA:placebo) had an influence on the relatively favorable outcomes in this trial given that both investigators and subjects were aware of this allocation ratio. Our findings are consistent with the positive placebo effect seen in therapeutic trials in diabetic neuropathy. 26,27 An important question in this regard is whether the combined results of patients and placebo controls from both the Italian/UK and North American studies could be useful as a historical placebo cohort for future earlyphase clinical trials in patients with CMT1A. Figure 2. Changes in the Charcot-Marie-Tooth Neuropathy Score (CMTNS) Over the 2-Year Study Change in CMTNS Ascorbic acid Placebo French placebo Italian/UK placebo Natural history Month Adjusted mean changes in the CMTNS over 2 years are shown for the ascorbic acid group and placebo group. These are compared with the mean change at 2 years expected from published natural history 19 and observed mean changes in the placebo groups in the French trial 10 and the Italian/UK trial. 11 Bars indicate 1 SEM. Larger (positive) changes indicate greater worsening. CMT1A Progression and Clinical Trials Results from our trial as well as those of others emphasize the facts that CMT1A is a slowly progressive disease and that slowing of progression in adults may not be detectable within a 2-year period, particularly if mild improvement can occur from placebo effect alone. This, in fact, is consistent with a Dutch study that hypothesized that progression in adults with CMT1A might be more related to processes of natural aging than of disease progression itself. 28 If CMT1A is relatively stable in adults, jamaneurology.com JAMA Neurology August 2013 Volume 70, Number 8 985
6 Research Original Investigation High-Dosage Ascorbic Acid Treatment in CMT1A Table 3. Log-Transformed Peripheral Myelin Protein 22 Messenger RNA Levels by Treatment Group Peripheral Myelin Protein 22 mrna Level Baseline 2-y Change a Normalized to Ascorbic Acid Placebo Ascorbic Acid Placebo Treatment Effect (95% CI) 18S RNA band ( 0.80 to 0.52) S-100 protein mrna ( 1.08 to 0.12) MBP mrna ( 1.10 to 0.21) Abbreviations: MBP, myelin basic protein; mrna, messenger RNA. a Values are adjusted mean changes from baseline to year 2, calculated from an analysis of covariance model that included treatment group, site, and the baseline value of the outcome variable as independent variables; see text for details. Figure 3. Adjusted Mean Changes in Ascorbic Acid Levels Over the 2-Year Study Change in Ascorbic Acid Level, mg/dl Ascorbic acid Placebo Month Mean changes in serum ascorbic acid levels are shown for subjects receiving 4-g/d ascorbic acid and those receiving placebo. Bars indicate 1 SEM. Group differences in mean levels were statistically significant (P <.002) at all times. To convert ascorbic acid to micromoles per liter, multiply by then goals for future clinical trials will need to focus on detecting actual improvement in adult patients rather than just slowing disease progression. Alternatively, more sensitive outcome measures will need to be identified for future studies. Additionally, future studies may need to involve children, who were excluded from both the North American and Italian/UK studies. It is likely that much of the progression in CMT1A occurs during the first 2 decades of life. 29,30 The recent publication of the CMT Pediatric Scale provides what will hopefully be a sensitive tool that is normalized for age to measure disability and progression in children with CMT1A. 31 It may be that such a tool will permit the development of clinical trials in children with CMT1A. State of the Field and Lessons Learned Our trial provides an illustration of the potential dangers of using historical controls as the primary basis of comparison in a clinical trial. The problems may be accentuated when the historical controls are not derived from placebo groups of past, similarly designed clinical trials. In addition, when one uses a historical control group in a futility trial, it is helpful to include a concurrent placebo group but an allocation ratio as extreme as 4:1 (treatment:placebo) may not be wise; a ratio closer to 1:1 may be more appropriate to minimize potential placebo effects. 21,22 While it is disappointing that no dosage of AA has proven effective in treating CMT1A, we believe that it is important to not lose sight of what has been accomplished in just a few years because of these trials. Before the AA trials, there was no infrastructure in place to conduct large-scale multicenter trials for any form of CMT. To develop such an infrastructure, future investigators from the Italian/UK, French, Dutch, and North American trials met in Naarden, the Netherlands, to discuss the use of the CMTNS and other outcome measures in the AA trials. 12 As the trials were nearing completion, these same investigators, along with those from Australia and elsewhere, met again in Naarden to discuss ways to improve the CMTNS, 32 which subsequently led to the development of a newer version of the CMTNS 33 and the CMT Pediatric Scale. 31 It is because of the experience gained in the AA trials for CMT1A that the infrastructure is now in place to test new therapies in coordinated international trials. ARTICLE INFORMATION Accepted for Publication: April 16, Published Online: June 24, doi: /jamaneurol Author Affiliations: Department of Neurology, Wayne State University, Detroit, Michigan (Lewis, Siskind, Feely, Miller, Wu, Shy); Department of Biostatistics and Computational Biology, University of Rochester, Rochester, New York (McDermott); Department of Neurology, University of Rochester, Rochester, New York (McDermott, Herrmann, Smith, Luebbe); Department of Neurology, Johns Hopkins School of Medicine, Baltimore, Maryland (Hoke, Clawson); Department of Neurology, University of Kansas, Kansas City (Barohn); currently with the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California (Lewis); currently with the Department of Neurology, Stanford University, Palo Alto, California (Siskind); currently with the Department of Neurology, Carver College of Medicine, University ofiowa,iowacity(feely,wu,shy). Author Contributions: Drs Lewis and Shy were principal investigator and coprincipal investigator of the trial, designed the protocol, and functioned as site principal investigator and coprincipal investigator at Wayne State University. Dr Shy obtained the Investigational New Drug number for the study. Dr Herrmann was physician lead for the Muscle Study Group Coordinating Center and site principal investigator at the University of Rochester and assisted in the development of the protocol. Dr Hoke was site principal investigator at Johns Hopkins School of Medicine. Dr McDermott was the primary biostatistician for the study. Mss Clawson, Siskind, Feely, Miller, Smith, and Luebbe served as study coordinators at the 3 sites. Mss Smith and Luebbe additionally served as lead project managers for the Muscle Study Group Coordinating Center. Dr Barohn was the safety officer for the trial. Dr Wu performed the real-time polymerase chain reaction analysis of the skin biopsy specimens. Study concept and design: Lewis, McDermott, Herrmann, Hoke, Wu, and Shy. Acquisition of data: Lewis, Herrmann, Hoke, Clawson, Siskind, Feely, Miller, Smith, Luebbe, Wu, and Shy. Analysis and interpretation of data: Lewis, McDermott, Herrmann, Hoke, Barohn, Wu, and Shy. Drafting of the manuscript: Lewis, Hoke, Clawson, Smith, Wu, and Shy. Critical revision of the manuscript for important intellectual content: Lewis, McDermott, Herrmann, 986 JAMA Neurology August 2013 Volume 70, Number 8 jamaneurology.com
7 High-Dosage Ascorbic Acid Treatment in CMT1A Original Investigation Research Hoke, Siskind, Feely, Miller, Barohn, Luebbe, Wu, and Shy. Statistical analysis: McDermott and Wu. Obtained funding: Hoke and Shy. Administrative, technical, and material support: Herrmann, Hoke, Siskind, Feely, Miller, Smith, Luebbe, Wu, and Shy. Study supervision: Lewis, Herrmann, Hoke, Siskind, Barohn, Smith, and Shy. Conflict of Interest Disclosures: Dr Lewis has consulted for Baxter, CSL Behring, AxelaCare, Novartis, and Bristol-Myers Squibb. Dr Shy has received grant support from the National Institute of Neurological Disorders and Stroke, Muscular Dystrophy Association, and Charcot-Marie-Tooth Association. Funding/Support: This work was supported by the Muscular Dystrophy Association and the Charcot-Marie-Tooth Association. Wayne State University, the University of Rochester, and Johns Hopkins University were members of the Inherited Neuropathy Consortium of the Rare Disease Clinical Research Network supported by the National Institute of Neurological Disorders and Stroke and Office of Rare Diseases. Muscle Study Group Members: Richard A. Lewis, MD; Michael P. McDermott, PhD; David N. Herrmann, MD; Ahmet Hoke, MD, PhD; Lora L. Clawson, MSN, CRNP; Carly Siskind, MS; Shawna M. E. Feely, MS; Lindsey J. Miller, MS; Richard J. Barohn, MD; Patricia Smith, BS; Elizabeth Luebbe, MS; Xingyao Wu, MD, PhD; Michael E. Shy, MD; Rabi Tawil, MD; Berch Griggs, MD; Colleen Donlin Smith, MA, CCRC; William Martens; Arthur Watts; Steve Bean, PharmD; Eileen Eastwood; Nuran Dilek, MS; and Carrie Irvine. Additional Information: The full trial protocol is available from Dr Shy Additional Contributions: We thank all the subjects who participated in the trial. Lisa Rowe, BA, worked in coordinating subject visits, Karen M. Krajewski, MS, helped in designing the protocol, Richard Kimball, RN, PhD, helped with subject recruitment and evaluations, and Arthur Watts, BS, helped with the statistical analysis. REFERENCES 1. Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth s disease. Clin Genet. 1974;6(2): Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2): Raeymaekers P, Timmerman V, Nelis E, et al; HMSN Collaborative Research Group. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). Neuromuscul Disord. 1991;1(2): Nelis E, Van Broeckhoven C, De Jonghe P, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet. 1996;4(1): Saporta ASD, Sottile SL, Miller LJ, Feely SME, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol. 2011;69(1): Timmerman V, Nelis E, Van Hul W, et al. The peripheral myelin protein gene PMP-22 is contained within the Charcot-Marie-Tooth disease type 1A duplication.nat Genet. 1992;1(3): Passage E, Norreel JC, Noack-Fraissignes P, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med. 2004;10(4): Verhamme C, de Haan RJ, Vermeulen M, Baas F, de Visser M, van Schaik IN. Oral high dose ascorbic acid treatment for one year in young CMT1A patients: a randomised, double-blind, placebo-controlled phase II trial. BMC Med. 2009;7: Burns J, Ouvrier RA, Yiu EM, et al. Ascorbic acid for Charcot-Marie-Tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol. 2009;8(6): Micallef J, Attarian S, Dubourg O, et al. Effect of ascorbic acid in patients with Charcot-Marie-Tooth disease type 1A: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2009;8(12): Pareyson D, Reilly MM, Schenone A, et al; CMT-TRIAAL Group; CMT-TRAUK Group. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial.lancet Neurol. 2011;10(4): Reilly MM, de Jonghe P, Pareyson D. 136th ENMC International Workshop: Charcot-Marie- Tooth disease type 1A (CMT1A)8-10 April 2005, Naarden, the Netherlands. Neuromuscul Disord. 2006;16(6): Weber P, Bendich A, Schalch W. Vitamin C and human health: a review of recent data relevant to human requirements. Int J Vitam Nutr Res. 1996;66(1): Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45(6): Shy ME, Blake J, Krajewski K, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64(7): Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6): Li J, Bai Y, Ghandour K, et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain. 2005;128(pt 5): Shy ME, Siskind C, Swan ER, et al. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68(11): Shy ME, Chen L, Swan ER, et al. Neuropathy progression in Charcot-Marie-Tooth disease type 1A. Neurology. 2008;70(5): Gordon PH, Cheung YK, Levin B, et al; Combination Drug Selection Trial Study Group. A novel, efficient, randomized selection trial comparing combinations of drug therapy for ALS. Amyotroph Lateral Scler. 2008;9(4): Huntington Study Group DOMINO Investigators. A futility study of minocycline in Huntington s disease. Mov Disord. 2010;25(13): NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66(5): Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5(3): Little RA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: John Wiley & Sons; Katona I, Wu X, Feely SM, et al. PMP22 expression in dermal nerve myelin from patients with CMT1A.Brain. 2009;132(pt 7): Tesfaye S, Tandan R, Bastyr EJ III, Kles KA, Skljarevski V, Price KL; Ruboxistaurin Study Group. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007;30(10): Dyck PJ, Norell JE, Tritschler H, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30(10): Verhamme C, van Schaik IN, Koelman JH, de Haan RJ, de Visser M. The natural history of Charcot-Marie-Tooth type 1A in adults: a 5-year follow-up study. Brain. 2009;132(pt 12): Thomas PK, Marques W Jr, Davis MB, et al. The phenotypic manifestations of chromosome 17p11.2 duplication.brain. 1997;120(Pt 3): Shy M, Lupski JR, Chance PF, Klein CJ, Dyck PJ. The hereditary motor and sensory neuropathies: an overview of the clinical, genetic, electrophysiologic and pathologic features. In: Dyck PJTP, ed. Peripheral Neuropathy. 4th ed. Philadelphia, PA: WB Saunders; 2005: Burns J, Ouvrier R, Estilow T, et al. Validation of the Charcot-Marie-Tooth Disease Pediatric Scale as an outcome measure of disability. Ann Neurol. 2012;71(5): Reilly MM, Shy ME, Muntoni F, Pareyson D. 168th ENMC International Workshop: outcome measures and clinical trials in Charcot-Marie-Tooth disease (CMT). Neuromuscul Disord. 2010;20(12): Murphy SM, Herrmann DN, McDermott MP, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16(3): jamaneurology.com JAMA Neurology August 2013 Volume 70, Number 8 987
Charcot-Marie-Tooth disease (CMT) is the eponym
 ORIGINAL ARTICLE Charcot-Marie-Tooth Disease Subtypes and Genetic Testing Strategies Anita S.D. Saporta, MD, 1 Stephanie L. Sottile, BA, 1 Lindsey J. Miller, MS, 1 Shawna M.E. Feely, MS, 1 Carly E. Siskind,
ORIGINAL ARTICLE Charcot-Marie-Tooth Disease Subtypes and Genetic Testing Strategies Anita S.D. Saporta, MD, 1 Stephanie L. Sottile, BA, 1 Lindsey J. Miller, MS, 1 Shawna M.E. Feely, MS, 1 Carly E. Siskind,
Corporate Medical Policy
 Corporate Medical Policy Genetic Testing for the Diagnosis of Inherited Peripheral Neuropathy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_the_diagnosis_of_inherited_peripheral_neuropathy
Corporate Medical Policy Genetic Testing for the Diagnosis of Inherited Peripheral Neuropathy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_the_diagnosis_of_inherited_peripheral_neuropathy
Faculty Academic Appointments 7/2011-8/2016 Assistant Professor 7/2012-8/2016 Instructor Neurology Harvard Medical School
 Vera Fridman, M.D. October 25, 2017 Office Address: Place of Birth: Neuromuscular Division Department of Neurology 12631 East 17 th Ave Mail Stop B185 Aurora, CO 80045 Phone: (303) 724-2188 Fax: (303)
Vera Fridman, M.D. October 25, 2017 Office Address: Place of Birth: Neuromuscular Division Department of Neurology 12631 East 17 th Ave Mail Stop B185 Aurora, CO 80045 Phone: (303) 724-2188 Fax: (303)
Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial
 Sponsored document from Lancet Neurology Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial Davide Pareyson a,*, Mary M Reilly b, Angelo Schenone
Sponsored document from Lancet Neurology Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial Davide Pareyson a,*, Mary M Reilly b, Angelo Schenone
X inactivation in females with X-linked Charcot Marie Tooth disease
 Available online at www.sciencedirect.com Neuromuscular Disorders 22 (2012) 617 621 www.elsevier.com/locate/nmd X inactivation in females with X-linked Charcot Marie Tooth disease Sinéad M. Murphy a,b,,
Available online at www.sciencedirect.com Neuromuscular Disorders 22 (2012) 617 621 www.elsevier.com/locate/nmd X inactivation in females with X-linked Charcot Marie Tooth disease Sinéad M. Murphy a,b,,
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
DSS-1. No financial disclosures
 DSS-1 No financial disclosures Clinical History 9 year old boy with past medical history significant for cerebral palsy, in-turning right foot, left clubfoot that was surgically corrected at 3 years of
DSS-1 No financial disclosures Clinical History 9 year old boy with past medical history significant for cerebral palsy, in-turning right foot, left clubfoot that was surgically corrected at 3 years of
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne
 Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
University of Colorado- Anschutz Medical
 Vera Fridman, M.D. February 28, 2018 Office Address: Neuromuscular Division Department of Neurology University of Colorado Anschutz Medical 12631 East 17 th Ave Mail Stop B185 Aurora, CO 80045 Phone: (303)
Vera Fridman, M.D. February 28, 2018 Office Address: Neuromuscular Division Department of Neurology University of Colorado Anschutz Medical 12631 East 17 th Ave Mail Stop B185 Aurora, CO 80045 Phone: (303)
Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial
 Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial Davide Pareyson, Mary M Reilly, Angelo Schenone, Gian Maria Fabrizi, Tiziana Cavallaro,
Ascorbic acid in Charcot Marie Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial Davide Pareyson, Mary M Reilly, Angelo Schenone, Gian Maria Fabrizi, Tiziana Cavallaro,
Increased Serum Creatine Kinase
 Clinical Chemistry 60:2 301 306 (2014) Clinical Case Study Increased Serum Creatine Kinase Mehdi Mirzazadeh, 1* Negar Maghsoodi, 2 and Jeffrey Barron 3 CASE DESCRIPTION A 38-year-old white male taxi driver
Clinical Chemistry 60:2 301 306 (2014) Clinical Case Study Increased Serum Creatine Kinase Mehdi Mirzazadeh, 1* Negar Maghsoodi, 2 and Jeffrey Barron 3 CASE DESCRIPTION A 38-year-old white male taxi driver
Genetics & CMT Q&A Questions from the CMTA Genetics & CMT Discussion Forum
 Genetics & CMT Q&A Questions from the CMTA Genetics & CMT Discussion Forum Presented by : Shawna Feely, MS, CGC & Carly Siskind MS, LCGC Is CMT a type of Muscular Dystrophy (MD)? Myopathy: a disease of
Genetics & CMT Q&A Questions from the CMTA Genetics & CMT Discussion Forum Presented by : Shawna Feely, MS, CGC & Carly Siskind MS, LCGC Is CMT a type of Muscular Dystrophy (MD)? Myopathy: a disease of
CharcoteMarieeTooth disease: frequency of genetic subtypes and guidelines for genetic testing
 RESEARCH PAPER CharcoteMarieeTooth disease: frequency of genetic subtypes and guidelines for genetic testing Sinead M Murphy, 1,2,3 Matilde Laura, 1,2 Katherine Fawcett, 4,5 Amelie Pandraud, 1,2 Yo-Tsen
RESEARCH PAPER CharcoteMarieeTooth disease: frequency of genetic subtypes and guidelines for genetic testing Sinead M Murphy, 1,2,3 Matilde Laura, 1,2 Katherine Fawcett, 4,5 Amelie Pandraud, 1,2 Yo-Tsen
Motor and sensory nerve conduction studies
 3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Hands-on Course 2 Assessment of peripheral nerves function and structure in suspected peripheral neuropathies
3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Hands-on Course 2 Assessment of peripheral nerves function and structure in suspected peripheral neuropathies
Abstract. Introduction
 Brazilian Journal of Medical and Biological Research (2003) 36: 1403-1407 Thr(118)Met in Charcot-Marie-Tooth disease ISSN 0100-879X 1403 Thr(118)Met amino acid substitution in the peripheral myelin protein
Brazilian Journal of Medical and Biological Research (2003) 36: 1403-1407 Thr(118)Met in Charcot-Marie-Tooth disease ISSN 0100-879X 1403 Thr(118)Met amino acid substitution in the peripheral myelin protein
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: patisiran_onpattro 11/2018 n/a 5/2019 2/2019 Description of Procedure or Service is a double-stranded small
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: patisiran_onpattro 11/2018 n/a 5/2019 2/2019 Description of Procedure or Service is a double-stranded small
Neuromuscular Disorders
 Neuromuscular Disorders 20 (2010) 839 846 Contents lists available at ScienceDirect Neuromuscular Disorders journal homepage: www.elsevier.com/locate/nmd Workshop report 168th ENMC International Workshop:
Neuromuscular Disorders 20 (2010) 839 846 Contents lists available at ScienceDirect Neuromuscular Disorders journal homepage: www.elsevier.com/locate/nmd Workshop report 168th ENMC International Workshop:
The Experimental Therapeutics of Rare Diseases
 The Experimental Therapeutics of Rare Diseases Robert C. Griggs, M.D., FAAN Professor of Neurology, Medicine, Pediatrics, Pathology and Laboratory Medicine, Center for Human Experimental Therapeutics University
The Experimental Therapeutics of Rare Diseases Robert C. Griggs, M.D., FAAN Professor of Neurology, Medicine, Pediatrics, Pathology and Laboratory Medicine, Center for Human Experimental Therapeutics University
Supplementary Online Content
 Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_diagnosis_of_inherited_peripheral_neuropathies 01/01/2019 N/A 01/01/2020 01/01/2019 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_diagnosis_of_inherited_peripheral_neuropathies 01/01/2019 N/A 01/01/2020 01/01/2019 Description
Cross-sectional analysis of a large cohort with X-linked Charcot-Marie-Tooth disease (CMTX1)
 Cross-sectional analysis of a large cohort with X-linked Charcot-Marie-Tooth disease (CMTX1) Francis B. Panosyan, MD, PhD Matilde Laura, MD Alexander M. Rossor, MD Chiara Pisciotta, MD, PhD Giuseppe Piscosquito,
Cross-sectional analysis of a large cohort with X-linked Charcot-Marie-Tooth disease (CMTX1) Francis B. Panosyan, MD, PhD Matilde Laura, MD Alexander M. Rossor, MD Chiara Pisciotta, MD, PhD Giuseppe Piscosquito,
Comparison of diabetes patients with demyelinating diabetic sensorimotor polyneuropathy to those diagnosed with CIDP
 Comparison of diabetes patients with demyelinating diabetic sensorimotor polyneuropathy to those diagnosed with CIDP Samantha K. Dunnigan 1, Hamid Ebadi 1, Ari Breiner 1, Hans D. Katzberg 1, Leif E. Lovblom
Comparison of diabetes patients with demyelinating diabetic sensorimotor polyneuropathy to those diagnosed with CIDP Samantha K. Dunnigan 1, Hamid Ebadi 1, Ari Breiner 1, Hans D. Katzberg 1, Leif E. Lovblom
PREVALENCE AND ORTHOPEDIC MANAGEMENT OF FOOT AND ANKLE DEFORMITIES IN CHARCOT MARIE TOOTH DISEASE
 PREVALENCE AND ORTHOPEDIC MANAGEMENT OF FOOT AND ANKLE DEFORMITIES IN CHARCOT MARIE TOOTH DISEASE MATILDE LAUR A, MD, PhD, 1 DISHAN SINGH, MBChB, FRCS(Orth), 2 GITA RAMDHARRY, PhD, 3 JASPER MORROW, MD,
PREVALENCE AND ORTHOPEDIC MANAGEMENT OF FOOT AND ANKLE DEFORMITIES IN CHARCOT MARIE TOOTH DISEASE MATILDE LAUR A, MD, PhD, 1 DISHAN SINGH, MBChB, FRCS(Orth), 2 GITA RAMDHARRY, PhD, 3 JASPER MORROW, MD,
Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific Journals
 ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
Bristol-Myers Squibb
 A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
A family study of Charcot-Marie-Tooth disease
 Joturnal of Medical Genetics, 1982, 19, 88-93 A family study of Charcot-Marie-Tooth disease A P BROOKS* AND A E H EMERY From the University Department of Human Genetics, Western General Hospital, Edinburgh
Joturnal of Medical Genetics, 1982, 19, 88-93 A family study of Charcot-Marie-Tooth disease A P BROOKS* AND A E H EMERY From the University Department of Human Genetics, Western General Hospital, Edinburgh
Test Information Sheet
 Genetic Testing for Neuropathy: Axonal Charcot-Marie-Tooth Panel Sequencing and Exon-Level Deletion/Duplication Testing of 21 Genes Panel Gene List: AARS, BSCL2, DNM2, DYNC1H1, GARS, GDAP1, GJB1, HINT1,
Genetic Testing for Neuropathy: Axonal Charcot-Marie-Tooth Panel Sequencing and Exon-Level Deletion/Duplication Testing of 21 Genes Panel Gene List: AARS, BSCL2, DNM2, DYNC1H1, GARS, GDAP1, GJB1, HINT1,
Summary ID# Clinical Study Summary: Study B4Z-MC-LYCL
 CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
SYNOPSIS. ER OROS Paliperidone: Clinical Study Report R SCH-301
 SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
419 Church Street East, Penrose, Auckland 1642 P O Box 12063, Penrose, Auckland
 CHARCOT-MARIE-TOOTH DISEASE What is Charcot-Marie-Tooth Disease? First described in 1886 by three physicians Jean-Marie Charcot, Pierre Marie, and Howard Henry Tooth, CharcotMarie-Tooth (CMT) is a group
CHARCOT-MARIE-TOOTH DISEASE What is Charcot-Marie-Tooth Disease? First described in 1886 by three physicians Jean-Marie Charcot, Pierre Marie, and Howard Henry Tooth, CharcotMarie-Tooth (CMT) is a group
Safety and Efficacy of Inotersen in Patients with Hereditary Transthyretin Amyloidosis with Polyneuropathy (NEURO-TTR) Annabel K.
 Safety and Efficacy of Inotersen in Patients with Hereditary Transthyretin Amyloidosis with Polyneuropathy (NEURO-TTR) Annabel K. Wang, MD University of California, Irvine ANA 2017 October 17, 2017 San
Safety and Efficacy of Inotersen in Patients with Hereditary Transthyretin Amyloidosis with Polyneuropathy (NEURO-TTR) Annabel K. Wang, MD University of California, Irvine ANA 2017 October 17, 2017 San
Diabetic Neuropathy: Discordance between Symptoms and Electrophysiological Testing in Saudi Diabetics
 Bahrain Medical Bulletin, Vol.24, No.1, March 2002 Diabetic Neuropathy: Discordance between Symptoms and Electrophysiological Testing in Saudi Diabetics Daad H Akbar, FRCP(UK), Arab Board, Saudi Board
Bahrain Medical Bulletin, Vol.24, No.1, March 2002 Diabetic Neuropathy: Discordance between Symptoms and Electrophysiological Testing in Saudi Diabetics Daad H Akbar, FRCP(UK), Arab Board, Saudi Board
Table of Contents. 1 Architecture of the peripheral nerve P. Young, M. Boentert
 Table of Contents z General part 1 Architecture of the peripheral nerve... 3 P. Young, M. Boentert Introduction... 3 1.1 Cellular components of the PNS... 3 1.2 Architecture of the myelin compartment..
Table of Contents z General part 1 Architecture of the peripheral nerve... 3 P. Young, M. Boentert Introduction... 3 1.1 Cellular components of the PNS... 3 1.2 Architecture of the myelin compartment..
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. GENERIC DRUG NAME / COMPOUND NUMBER: Tofacitinib / CP-690,550
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. GENERIC DRUG NAME / COMPOUND NUMBER: Tofacitinib / CP-690,550
The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
 An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A The Harvard
An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A The Harvard
Disclosure. Entrapment Neuropathies - Overview. Common mononeuropathy sites. Definitions. Common mononeuropathy sites. Common mononeuropathy sites
 Disclosure Entrapment Neuropathies - Overview I receive compensation from Wiley- Blackwell publishers for my work as Editor-in-Chief of Muscle & Nerve Lawrence H. Phillips, II, MD Definitions Mononeuropathy:
Disclosure Entrapment Neuropathies - Overview I receive compensation from Wiley- Blackwell publishers for my work as Editor-in-Chief of Muscle & Nerve Lawrence H. Phillips, II, MD Definitions Mononeuropathy:
PFIZER INC. These results are supplied for informational purpose only. Prescribing decisions should be made based on the approved package insert.
 Public Disclosure Synopsis Protocol A3924 4 November 24 Final PFIZER INC. These results are supplied for informational purpose only. Prescribing decisions should be made based on the approved package insert.
Public Disclosure Synopsis Protocol A3924 4 November 24 Final PFIZER INC. These results are supplied for informational purpose only. Prescribing decisions should be made based on the approved package insert.
2.0 Synopsis. Choline fenofibrate capsules (ABT-335) M Clinical Study Report R&D/06/772. (For National Authority Use Only) Name of Study Drug:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
Measure #1a: Essential Components of Electrodiagnostic (EDX) Evaluation for Median Neuropathy at the Wrist
 Measure #1a: Essential Components of Electrodiagnostic (EDX) Evaluation for Median Neuropathy at the Wrist Measure Description Percentage of patients referred for EDX evaluation of CTS who had adequate
Measure #1a: Essential Components of Electrodiagnostic (EDX) Evaluation for Median Neuropathy at the Wrist Measure Description Percentage of patients referred for EDX evaluation of CTS who had adequate
BRL /RSD-101RLL/1/CPMS-716. Report Synopsis
 Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
CIDP + MMN - how to diagnose and treat. Dr Hadi Manji
 CIDP + MMN - how to diagnose and treat Dr Hadi Manji Outline Introduction CIDP Diagnosis Clinical features MRI Nerve conduction tests Lumbar puncture Nerve biopsy Treatment IV Ig Steroids Plasma Exchnage
CIDP + MMN - how to diagnose and treat Dr Hadi Manji Outline Introduction CIDP Diagnosis Clinical features MRI Nerve conduction tests Lumbar puncture Nerve biopsy Treatment IV Ig Steroids Plasma Exchnage
Summary ID# Clinical Study Summary: Study B4Z-MC-LYBX
 CT Registry ID#7068 Page 1 Summary ID# 7068 Clinical Study Summary: Study B4Z-MC-LYBX A Randomized, Double-Blind Comparison of Hydrochloride and Placebo in Child and Adolescent Outpatients with Attention-
CT Registry ID#7068 Page 1 Summary ID# 7068 Clinical Study Summary: Study B4Z-MC-LYBX A Randomized, Double-Blind Comparison of Hydrochloride and Placebo in Child and Adolescent Outpatients with Attention-
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 Public Disclosure Synopsis Protocol A7772 September 25 Final PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
Public Disclosure Synopsis Protocol A7772 September 25 Final PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
SYNOPSIS. Risperidone-R064766: Clinical Study Report RIS-USA-232 (FOR NATIONAL AUTHORITY USE ONLY)
 SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
Stage I: Rule-Out Dashboard Secondary Findings in Adults
 Stage I: Rule-Out Dashboard GENE/GENE PANEL: DNM2 DISORDER: DNM2-Related Intermediate Charcot-Marie-Tooth Neuropathy HGNC ID: 2974 OMIM ID: 606482 ACTIONABILITY PENETRANCE 1. Is there a qualifying resource,
Stage I: Rule-Out Dashboard GENE/GENE PANEL: DNM2 DISORDER: DNM2-Related Intermediate Charcot-Marie-Tooth Neuropathy HGNC ID: 2974 OMIM ID: 606482 ACTIONABILITY PENETRANCE 1. Is there a qualifying resource,
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Epidermal Nerve Fiber and Schwann cell densities in the distal leg of Nine-banded Armadillos with Experimental Leprosy neuropathy
 Epidermal Nerve Fiber and Schwann cell densities in the distal leg of Nine-banded Armadillos with Experimental Leprosy neuropathy Gigi J Ebenezer 1 Richard Truman 2 David Scollard 2 Michael Polydefkis
Epidermal Nerve Fiber and Schwann cell densities in the distal leg of Nine-banded Armadillos with Experimental Leprosy neuropathy Gigi J Ebenezer 1 Richard Truman 2 David Scollard 2 Michael Polydefkis
Diabetic Neuropathy. Nicholas J. Silvestri, M.D.
 Diabetic Neuropathy Nicholas J. Silvestri, M.D. Types of Neuropathies Associated with Diabetes Mellitus p Chronic distal sensorimotor polyneuropathy p Focal compression neuropathies p Autonomic neuropathy
Diabetic Neuropathy Nicholas J. Silvestri, M.D. Types of Neuropathies Associated with Diabetes Mellitus p Chronic distal sensorimotor polyneuropathy p Focal compression neuropathies p Autonomic neuropathy
Patogenesi e terapia della Neuropatia Motoria Multifocale
 26 Settembre 2014 Patogenesi e terapia della Neuropatia Motoria Multifocale Francesca Gallia Neurologia 2, Ist. Clin. Humanitas Rozzano, Milano Multifocal Motor Neuropathy Rare disorder characterized by:
26 Settembre 2014 Patogenesi e terapia della Neuropatia Motoria Multifocale Francesca Gallia Neurologia 2, Ist. Clin. Humanitas Rozzano, Milano Multifocal Motor Neuropathy Rare disorder characterized by:
Alpha blockers have no role in renal colic
 Alpha blockers have no role in renal colic HARRY WINKLER Director, section of Endourology Kidney stone center Dept.of Urology Sheba Medical Center Financial and Other Disclosures Off-label use of drugs,
Alpha blockers have no role in renal colic HARRY WINKLER Director, section of Endourology Kidney stone center Dept.of Urology Sheba Medical Center Financial and Other Disclosures Off-label use of drugs,
Sponsor Novartis. Generic Drug Name. NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia
 Page 1 Sponsor Novartis Generic Drug Name NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia Approved Indication Investigational. Study Number CA2206 Title A
Page 1 Sponsor Novartis Generic Drug Name NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia Approved Indication Investigational. Study Number CA2206 Title A
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
GENETICS IN HEREDITARY NEUROPATHY
 GENETICS IN HEREDITARY NEUROPATHY ERNESTO ALONSO, SWETHA JASTI,MAZEN M.DIMACHKIE, RICHARD J. BAROHN, MELANIE GLENN, OMAR JAWDAT, JEFFREY STATLAND, LAURA HERBELIN, CONSTANTINE FARMAKIDIS,DUAA JABARI, MAMATHA
GENETICS IN HEREDITARY NEUROPATHY ERNESTO ALONSO, SWETHA JASTI,MAZEN M.DIMACHKIE, RICHARD J. BAROHN, MELANIE GLENN, OMAR JAWDAT, JEFFREY STATLAND, LAURA HERBELIN, CONSTANTINE FARMAKIDIS,DUAA JABARI, MAMATHA
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Therapeutic Area of Trial L-dopa induced dyskinesias in Parkinson s disease (PD-LID) Approved Indication Not approved yet for any
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Therapeutic Area of Trial L-dopa induced dyskinesias in Parkinson s disease (PD-LID) Approved Indication Not approved yet for any
Study No Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable(s):
 Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Page: 17 December 2012 (Study M13-692) 22 October 2013 (Study M13-692)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Study population The study population comprised adult patients fulfilling the following inclusion criteria:
 Cost-utility analysis of intravenous immunoglobulin and prednisolone for chronic inflammatory demyelinating polyradiculoneuropathy McCrone P, Chisholm D, Knapp M, Hughes R, Comi G, Dalakas M C, Illa I,
Cost-utility analysis of intravenous immunoglobulin and prednisolone for chronic inflammatory demyelinating polyradiculoneuropathy McCrone P, Chisholm D, Knapp M, Hughes R, Comi G, Dalakas M C, Illa I,
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine (HOE901) Insulin Glulisine (HMR1964)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Charcot-Marie-Tooth Disease (CMT) Sometimes known as Hereditary Motor and Sensory neuropathy (HMSN) or Peroneal Muscular Atrophy (PMA)
 Version: 2 Published: January 2004 Updated: December 2009 Date of review: November 2011 Author: Dr.Hilton-Jones MD, FRCP, FRCPE Clinical Director, Oxford MDC Muscle and Nerve Centre, and revised by Dr.
Version: 2 Published: January 2004 Updated: December 2009 Date of review: November 2011 Author: Dr.Hilton-Jones MD, FRCP, FRCPE Clinical Director, Oxford MDC Muscle and Nerve Centre, and revised by Dr.
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
Paliperidone: Clinical Protocol R076477SCH4012, CR Amendment INT-1
 Paliperidone: Clinical Protocol R076477SCH4012, CR013771 Amendment INT-1 A Randomized, Double-Blind, Placebo- and Active-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of a Fixed
Paliperidone: Clinical Protocol R076477SCH4012, CR013771 Amendment INT-1 A Randomized, Double-Blind, Placebo- and Active-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of a Fixed
Accelerating Drug Discovery to Transform Patients Lives
 Accelerating Drug Discovery to Transform Patients Lives CMT Overview and ACE-083 Phase II Trial Webinar April 3, 2017 Acceleron Forward-Looking Statements This presentation contains forward-looking statements
Accelerating Drug Discovery to Transform Patients Lives CMT Overview and ACE-083 Phase II Trial Webinar April 3, 2017 Acceleron Forward-Looking Statements This presentation contains forward-looking statements
Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists
 Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists What is NCS/EMG? NCS examines the conduction properties of sensory and motor peripheral nerves. For both
Guide to the use of nerve conduction studies (NCS) & electromyography (EMG) for non-neurologists What is NCS/EMG? NCS examines the conduction properties of sensory and motor peripheral nerves. For both
Neuren s trofinetide successful in proof of concept Phase 2 clinical trial in Fragile X syndrome
 Neuren (NEU) - ASX Announcement 7 December 2015 Neuren s trofinetide successful in proof of concept Phase 2 clinical trial in Fragile X syndrome Highlights: Positive top-line results provide a strong rationale
Neuren (NEU) - ASX Announcement 7 December 2015 Neuren s trofinetide successful in proof of concept Phase 2 clinical trial in Fragile X syndrome Highlights: Positive top-line results provide a strong rationale
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
Pilot Study: Clinical Trial Task Ontology Development. A prototype ontology of common participant-oriented clinical research tasks and
 Pilot Study: Clinical Trial Task Ontology Development Introduction A prototype ontology of common participant-oriented clinical research tasks and events was developed using a multi-step process as summarized
Pilot Study: Clinical Trial Task Ontology Development Introduction A prototype ontology of common participant-oriented clinical research tasks and events was developed using a multi-step process as summarized
UvA-DARE (Digital Academic Repository) The hand in Charcot-Marie-Tooth disease 1A Videler, A.J. Link to publication
 UvA-DARE (Digital Academic Repository) The hand in Charcot-Marie-Tooth disease 1A Videler, A.J. Link to publication Citation for published version (APA): Videler, A. J. (2009). The hand in Charcot-Marie-Tooth
UvA-DARE (Digital Academic Repository) The hand in Charcot-Marie-Tooth disease 1A Videler, A.J. Link to publication Citation for published version (APA): Videler, A. J. (2009). The hand in Charcot-Marie-Tooth
Multifocal motor neuropathy: diagnostic criteria that predict the response to immunoglobulin treatment
 Multifocal motor neuropathy: diagnostic criteria that predict the response to immunoglobulin treatment 7 MMN RM Van den Berg-Vos, H Franssen, JHJ Wokke, HW Van Es, LH Van den Berg Annals of Neurology 2000;
Multifocal motor neuropathy: diagnostic criteria that predict the response to immunoglobulin treatment 7 MMN RM Van den Berg-Vos, H Franssen, JHJ Wokke, HW Van Es, LH Van den Berg Annals of Neurology 2000;
ORIGINAL ARTICLE. STUDY OF CLINICO ELECTROPHYSIOLOGICAL PROFILE OF DIABETIC NEUROPATHY Sachin. G. J, Ravi Vaswani, Shilpa. B.
 STUDY OF CLINICO ELECTROPHYSIOLOGICAL PROFILE OF DIABETIC NEUROPATHY Sachin. G. J, Ravi Vaswani, Shilpa. B. 1. Assistant Professor, Department of Medicine, Vijayanagara Institute of Medical Sciences. Bellary.
STUDY OF CLINICO ELECTROPHYSIOLOGICAL PROFILE OF DIABETIC NEUROPATHY Sachin. G. J, Ravi Vaswani, Shilpa. B. 1. Assistant Professor, Department of Medicine, Vijayanagara Institute of Medical Sciences. Bellary.
Clinical Trial Synopsis TL-OPI-525, NCT#
 Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
CRITICALLY APPRAISED PAPER (CAP)
 CRITICALLY APPRAISED PAPER (CAP) Wu, C., Huang, P., Chen, Y., Lin, K., & Yang, H. (2013). Effects of mirror therapy on motor and sensory recovery in chronic stroke: A randomized controlled trial. Archives
CRITICALLY APPRAISED PAPER (CAP) Wu, C., Huang, P., Chen, Y., Lin, K., & Yang, H. (2013). Effects of mirror therapy on motor and sensory recovery in chronic stroke: A randomized controlled trial. Archives
1/28/2019. OSF HealthCare INI Care Center Team. Neuromuscular Disease: Muscular Dystrophy. OSF HealthCare INI Care Center Team: Who are we?
 Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
AII-type: Select the most appropriate answer
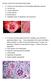 AII-type: Select the most appropriate answer ( )1. Choose one best answer for the following pathologic pictures. A. choroid cyst B. choroid papilloma C. pontine glioma D. ependymoma E. metastatic tumor
AII-type: Select the most appropriate answer ( )1. Choose one best answer for the following pathologic pictures. A. choroid cyst B. choroid papilloma C. pontine glioma D. ependymoma E. metastatic tumor
A COMPARISON OF IMPUTATION METHODS FOR MISSING DATA IN A MULTI-CENTER RANDOMIZED CLINICAL TRIAL: THE IMPACT STUDY
 A COMPARISON OF IMPUTATION METHODS FOR MISSING DATA IN A MULTI-CENTER RANDOMIZED CLINICAL TRIAL: THE IMPACT STUDY Lingqi Tang 1, Thomas R. Belin 2, and Juwon Song 2 1 Center for Health Services Research,
A COMPARISON OF IMPUTATION METHODS FOR MISSING DATA IN A MULTI-CENTER RANDOMIZED CLINICAL TRIAL: THE IMPACT STUDY Lingqi Tang 1, Thomas R. Belin 2, and Juwon Song 2 1 Center for Health Services Research,
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Professor Julie BYLES
 Professor Julie BYLES J Byles, D Sibbritt, C Miller, P Chiarelli. Research Centre for Gender Health and Ageing at the University of Newcastle. Living with Urinary Incontinence: A longitudinal Study of
Professor Julie BYLES J Byles, D Sibbritt, C Miller, P Chiarelli. Research Centre for Gender Health and Ageing at the University of Newcastle. Living with Urinary Incontinence: A longitudinal Study of
Product: Denosumab (AMG 162) Clinical Study Report: month Primary Analysis Date: 21 November 2016 Page 1
 Date: 21 November 2016 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Prolia Name of Active Ingredient: denosumab Title of Study: Randomized, Double-blind,
Date: 21 November 2016 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Prolia Name of Active Ingredient: denosumab Title of Study: Randomized, Double-blind,
Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline
 J Neurol Neurosurg Psychiatry 2001;71:205 209 205 Department of Neurology, Academic Medical Centre, University of Amsterdam, PO Box 22700, 1100 DE Amsterdam, The Netherlands N R Rosenberg P Portegies M
J Neurol Neurosurg Psychiatry 2001;71:205 209 205 Department of Neurology, Academic Medical Centre, University of Amsterdam, PO Box 22700, 1100 DE Amsterdam, The Netherlands N R Rosenberg P Portegies M
Senior Vice President, Medical Affairs and Health Outcomes, 150 South Saunders Road, Lake Forest, IL 60045, Horizon Pharma USA, Inc, USA
 Research Hyperammonemic crises in patients with urea cycle disorders on chronic nitrogen scavenger therapy with either sodium phenylbutyrate or glycerol phenylbutyrate Jeffrey D Kent 1, Robert J Holt,2
Research Hyperammonemic crises in patients with urea cycle disorders on chronic nitrogen scavenger therapy with either sodium phenylbutyrate or glycerol phenylbutyrate Jeffrey D Kent 1, Robert J Holt,2
Running head: VITAMIN C USED IN PREVENTING COMPLEX 1
 Running head: VITAMIN C USED IN PREVENTING COMPLEX 1 Evidence Based Critically Appraised Topic Vitamin C Used in Preventing Complex Regional Pain Syndrome Shelby Marie Frost, RN, BSN, FNP-s University
Running head: VITAMIN C USED IN PREVENTING COMPLEX 1 Evidence Based Critically Appraised Topic Vitamin C Used in Preventing Complex Regional Pain Syndrome Shelby Marie Frost, RN, BSN, FNP-s University
BRL /RSD-101C0F/1/CPMS-716. Report Synopsis
 Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-Term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-Term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Investor Presentation. 3 April 2017
 Investor Presentation 3 April 2017 1 Forward looking statements This presentation contains forward looking statements that involve risks and uncertainties. Although we believe that the expectations reflected
Investor Presentation 3 April 2017 1 Forward looking statements This presentation contains forward looking statements that involve risks and uncertainties. Although we believe that the expectations reflected
Critically Appraised Paper for Efficacy of occupational therapy for patients with Parkinson's disease: A randomized controlled trial
 Dominican University of California Dominican Scholar Occupational Therapy Critically Appraised Papers Series Occupational Therapy 2017 Critically Appraised Paper for Efficacy of occupational therapy for
Dominican University of California Dominican Scholar Occupational Therapy Critically Appraised Papers Series Occupational Therapy 2017 Critically Appraised Paper for Efficacy of occupational therapy for
2.0 Synopsis. Paricalcitol Capsules M Clinical Study Report R&D/15/0380. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
SYNOPSIS. Clinical Study Report AI Addendum #1. Open-label Dosing Phase
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Individual Study Table Referring to the Dossier (For National Authority Use Only) Name of Active Ingredient: Entecavir SYNOPSIS Clinical
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Individual Study Table Referring to the Dossier (For National Authority Use Only) Name of Active Ingredient: Entecavir SYNOPSIS Clinical
In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials
 In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials are comparative, large scale studies that typically
In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials are comparative, large scale studies that typically
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Centocor Ortho Biotech Services, LLC
 SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
For personal use only
 Investor Presentation 25 November 2016 1 Forward looking statements This presentation contains forward looking statements that involve risks and uncertainties. Although we believe that the expectations
Investor Presentation 25 November 2016 1 Forward looking statements This presentation contains forward looking statements that involve risks and uncertainties. Although we believe that the expectations
Association of motor milestones and SMN2 copy and outcome in spinal muscular. atrophy types 0 4
 jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
ORIGINAL CONTRIBUTION
 ORIGINAL CONTRIBUTION Common Misdiagnosis of a Common Neurological Disorder How Are We Misdiagnosing Essential Tremor? Samay Jain, MD; Steven E. Lo, MD; Elan D. Louis, MD, MS Background: As a common neurological
ORIGINAL CONTRIBUTION Common Misdiagnosis of a Common Neurological Disorder How Are We Misdiagnosing Essential Tremor? Samay Jain, MD; Steven E. Lo, MD; Elan D. Louis, MD, MS Background: As a common neurological
Individual Study Table Referring to Part of Dossier: Volume: Page:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: ABT-639 Name of Active Ingredient: ABT-639 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: ABT-639 Name of Active Ingredient: ABT-639 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
Peripheral Neuropathies
 Peripheral Neuropathies ELBA Y. GERENA MALDONADO, MD ACTING ASSISTANT PROFESSOR UNIVERSITY OF WASHINGTON MEDICAL CENTER Objectives Definition Neurophysiology Evaluation of polyneuropathies Cases Summary
Peripheral Neuropathies ELBA Y. GERENA MALDONADO, MD ACTING ASSISTANT PROFESSOR UNIVERSITY OF WASHINGTON MEDICAL CENTER Objectives Definition Neurophysiology Evaluation of polyneuropathies Cases Summary
