Flexible Outpatient. Opportunity. Decision: Dalvance
|
|
|
- Jeffry Smith
- 6 years ago
- Views:
Transcription
1 Flexible Outpatient Opportunity Decision: Dalvance
2 DALVANCE: an opportunity for use at various SITES OF CARE HOME HOME Outpatient Infusion Site ED One 30-minute infusion a week for two weeks provides a full course of therapy in treating adults with acute bacterial skin and skin structure infections (ABSSSI) two-dose regimen: 000 mg followed one week later by 500 mg.* ED-based infusion Observation Hospital Inpatient (average of 5 days ) HOME Outpatient Infusion Site Outpatient Infusion Site Days Indication DALVANCE (dalbavancin) for injection is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible and methicillin-resistant strains), Streptococcus pyogenes, Streptococcus agalactiae, and Streptococcus anginosus group (including S. anginosus, S. intermedius, S. constellatus). Important Safety Information Contraindications DALVANCE is contraindicated in patients with known hypersensitivity to dalbavancin. Warnings and Precautions Hypersensitivity Reactions Serious hypersensitivity (anaphylactic) and skin reactions have been reported with glycopeptide antibacterial agents, including DALVANCE. Exercise caution in patients with known hypersensitivity to glycopeptides due to the possibility of cross-sensitivity. If an allergic reaction occurs, treatment with DALVANCE should be discontinued. In the Phase 3 clinical trials, approximately 25% of patients receiving DALVANCE and the comparator were treated in outpatient settings 2 Potential Outpatient Infusion Settings Hospital Outpatient Department office/practice-based Infusion Center Free-standing Infusion Center Home Infusion Service * in patients with renal impairment whose known creatinine clearance is less than 30 ml/min and who are not receiving regularly scheduled hemodialysis, the recommended two-dose regimen for DALVANCE is 750 mg followed one week later by 375 mg. Please see additional Important Safety Information throughout. Please see full Prescribing Information. 2 3
3 The economic burden of hospitalization in absssi Example of allocation of U.S. costs by department for treating ABSSSI in an inpatient setting,3 Hospital costs and utilization were evaluated using a national hospital database for 203 covering 90,969 hospital discharges with the principal diagnosis of ABSSSI after exclusion criteria were applied. Diagnoses and procedures were categorized according to ICD-9 and CPT codes current at the time. Average length of stay (LOS) was 5 days. The most frequent initial antibiotic treatments included vancomycin, piperacillin/tazobactam, clindamycin, linezolid, daptomycin, and tigecycline. Allocation of Costs for ABSSSI Inpatient Care,3 Costs associated with IV antibiotic treatment for ABSSSI Illustrative Costs Associated With Inpatient and Outpatient Treatment 4 * Resource Cost per unit 4 5 days inpatient vancomycin 7 days outpatient Hospital bed $,853/day $9,265 N/A Vancomycin g bid $.58/day $58 $8 Therapeutic drug monitoring renal function and trough levels $02/blood draw $02 $02 PICC line placement $786/placement $786 N/A Pharmacy Other 0.5% 4.0% 5.8% Room and board PICC line complication management $88/patient $88 N/A Total $0,399 $83 Nursing labor 0.7% Laboratory IV therapy.% Emergency room Diagnostic imaging Central supply Surgery 6.% 3.% 2.7% 3.7% 6.4% * illustrative costs do not include all costs associated with treatment of ABSSSI and do not represent overall cost of care. Average length of stay for ABSSSI patients. Cost of vancomycin updated July 204. Average cost per patient. PICC=Peripherally inserted central catheter 4 5
4 Indication DALVANCE cost considerations Drug Acquisition Cost Associated With DALVANCE Two-Dose Regimen* $,490/500 mg vial First dose * represents DALVANCE wholesale acquisition cost only. This does not include cost of any inpatient hospital stay, outpatient administration costs, other costs associated with the treatment of ABSSSI, price adjustments, or discounts for specific purchasers. 000 mg $2,980 Second dose 500 mg $,490 $4,470 Dalvance: An Outpatient Opportunity Clinician Opportunity Delivers full treatment course with two 30-minute IV infusions given one week apart through a peripheral line and may facilitate treatment in an outpatient setting Patient Opportunity May significantly reduce the number of IV infusions typically required and may facilitate treatment in an outpatient setting Hospital Opportunity May be administered at inpatient and outpatient sites of care to provide flexible therapeutic options in today s changing healthcare delivery environment Two-dose regimen: 000 mg followed one week later by 500 mg For information on dosing in patients with renal impairment, see Use in Specific Populations. DALVANCE (dalbavancin) for injection is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible and methicillin-resistant strains), Streptococcus pyogenes, Streptococcus agalactiae, and Streptococcus anginosus group (including S. anginosus, S. intermedius, S. constellatus). Important Safety Information Contraindications DALVANCE is contraindicated in patients with known hypersensitivity to dalbavancin. Warnings and Precautions Hypersensitivity Reactions Serious hypersensitivity (anaphylactic) and skin reactions have been reported with glycopeptide antibacterial agents, including DALVANCE. Exercise caution in patients with known hypersensitivity to glycopeptides due to the possibility of cross-sensitivity. If an allergic reaction occurs, treatment with DALVANCE should be discontinued. Infusion-Related Reactions Rapid intravenous infusion of DALVANCE can cause reactions, including flushing of the upper body, urticaria, pruritus, and rash. Hepatic Effects ALT elevations with DALVANCE treatment were reported in clinical trials. Clostridium difficile-associated Diarrhea Clostridium difficile-associated diarrhea (CDAD) has been reported with nearly all systemic antibacterial agents, including DALVANCE, with severity ranging from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs. Development of Drug-Resistant Bacteria Prescribing DALVANCE in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. Adverse Reactions The most common adverse reactions in patients treated with DALVANCE were nausea (5.5%), headache (4.7%), and diarrhea (4.4%). Use in Specific Populations There have been no adequate and well-controlled studies with DALVANCE in pregnant or nursing women. DALVANCE should only be used if the potential benefit justifies the potential risk in these populations. In patients with renal impairment whose known creatinine clearance is less than 30 ml/min and who are not receiving regularly scheduled hemodialysis, the recommended two-dose regimen for DALVANCE is 750 mg followed one week later by 375 mg. No dosage adjustment is recommended for patients receiving regularly scheduled hemodialysis, and DALVANCE can be administered without regard to the timing of hemodialysis. Caution should be exercised when prescribing DALVANCE to patients with moderate or severe hepatic impairment (Child-Pugh Class B or C) as no data are available to determine the appropriate dosing in these patients. Please see full Prescribing Information. 6 7
5 Decision: Dalvance Flexible Outpatient Opportunity References:. lapensee KT, Fan W. Economic burden of hospitalization with antibiotic treatment for ABSSSI in the United States: an analysis of the Premier Hospital Database. Presented at ISPOR 202. Washington, DC. June 2-6, 202. (Poster PIN22). 2. boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 204;370: U.S. Premier Hospital database. 4. Data on file. Durata Therapeutics, Inc. Actavis TM and its design are trademarks of Actavis, Inc. or its affiliates. Dalvance and its design are trademarks of Durata Therapeutics Holding C.V., an Actavis affiliate. Actavis 205. All rights reserved. DAV /5
6 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DALVANCE safely and effectively. See full prescribing information for DALVANCE. DALVANCE (dalbavancin) for injection, for intravenous use Initial U.S. Approval: 204 INDICATIONS AND USAGE DALVANCE is indicated for acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible strains of Gram-positive microorganisms. () To reduce the development of drug-resistant bacteria and maintain the effectiveness of DALVANCE and other antibacterial drugs, DALVANCE should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. DOSAGE AND ADMINISTRATION Two-dose regimen: 000 mg followed one week later by 500 mg (2.) Dosage adjustment for patients with creatinine clearance less than 30 ml/min and not receiving regularly scheduled hemodialysis: 750 mg followed one week later by 375 mg (2.2) Administer by intravenous infusion over 30 minutes (2.3) DOSAGE FORMS AND STRENGTHS For injection: 500 mg of lyophilized powder in a single-use vial for reconstitution (3) CONTRAINDICATIONS Hypersensitivity to dalbavancin (4) WARNINGS AND PRECAUTIONS Serious hypersensitivity (anaphylactic) and skin reactions have been reported with glycopeptide antibacterial agents, including DALVANCE; exercise caution in patients with known hypersensitivity to glycopeptides. (5.) Rapid intravenous infusion of glycopeptide antibacterial agents can cause reactions. (5.2) ALT elevations with DALVANCE treatment were reported in clinical trials. (5.3) Clostridium difficile-associated diarrhea (CDAD) has been reported with nearly all systemic antibacterial agents, including DALVANCE. Evaluate if diarrhea occurs. (5.4) ADVERSE REACTIONS The most common adverse reactions in patients treated with DALVANCE were nausea (5.5%), headache (4.7%), and diarrhea (4.4%). To report SUSPECTED ADVERSE REACTIONS, contact Durata Therapeutics, Inc. at or FDA at -800-FDA-088 or USE IN SPECIFIC POPULATIONS Dosage adjustment is required in patients whose creatinine clearance is less than 30 ml/min and who are not receiving regularly scheduled hemodialysis. (8.6) See 7 for PATIENT COUNSELING INFORMATION Revised May 204 FULL PRESCRIBING INFORMATION: CONTENTS INDICATION AND USAGE. Acute Bacterial Skin and Skin Structure Infections.2 Usage 2 DOSAGE AND ADMINISTRATION 2. Recommended Dose Regimen 2.2 Patients with Renal Impairment 2.3 Preparation and Administration 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5. Hypersensitivity Reactions 5.2 Infusion-Related Reactions 5.3 Hepatic Effects 5.4 Clostridium diffi cile- Associated Diarrhea 5.5 Development of Drug- Resistant Bacteria 6 ADVERSE REACTIONS 6. Adverse Reactions in Clinical Trials 7 DRUG INTERACTIONS 7. Drug-Laboratory Test Interactions 7.2 Drug-Drug Interactions 8 USE IN SPECIFIC POPULATIONS 8. Pregnancy: Category C 8.3 Nursing Mothers 8.4 Pediatric Use 8.5 Geriatric Use 8.6 Renal Impairment 8.7 Hepatic Impairment 0 OVERDOSAGE DESCRIPTION 2 CLINICAL PHARMACOLOGY 2. Mechanism of Action 2.2 Pharmacodynamics 2.3 Pharmacokinetics 2.4 Microbiology 3 NONCLINICAL TOXICOLOGY 3. Carcinogenesis, Mutagenesis, Impairment of Fertility 3.2 Animal Toxicology and/or Pharmacology 4 CLINICAL STUDIES 5 REFERENCES 6 HOW SUPPLIED/STORAGE AND HANDLING 7 PATIENT COUNSELING INFORMATION
7 FULL PRESCRIBING INFORMATION DALVANCE (dalbavancin) for injection INDICATION AND USAGE. Acute Bacterial Skin and Skin Structure Infections DALVANCE (dalbavancin) for injection is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillinsusceptible and methicillin-resistant strains), Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus anginosus group (including S. anginosus, S. intermedius, S. constellatus)..2 Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of DALVANCE and other antibacterial agents, DALVANCE should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. 2 DOSAGE AND ADMINISTRATION 2. Recommended Dosage Regimen For treatment of adults with ABSSSI, the recommended two-dose regimen of DALVANCE is 000 mg followed one week later by 500 mg. DALVANCE should be administered over 30 minutes by intravenous infusion [see Dosage and Administration (2.3)]. 2.2 Patients with Renal Impairment In patients with renal impairment whose known creatinine clearance is less than 30 ml/min and who are not receiving regularly scheduled hemodialysis, the recommended two-dose regimen of DALVANCE is 750 mg followed one week later by 375 mg. No dosage adjustment is recommended for patients receiving regularly scheduled hemodialysis, and DALVANCE can be administered without regard to the timing of hemodialysis [see Use in Specific Populations (8.5) and Clinical Pharmacology (2.3)]. 2.3 Preparation and Administration DALVANCE (dalbavancin) for injection must be reconstituted with Sterile Water for Injection, USP, and subsequently diluted only with 5% Dextrose Injection, USP, to a final concentration of mg/ml to 5 mg/ml. Reconstitution: DALVANCE must be reconstituted under aseptic conditions, using 25 ml of Sterile Water for Injection, USP, for each 500 mg vial. To avoid foaming, alternate between gentle swirling and inversion of the vial until its contents are completely dissolved. Do not shake. The reconstituted vial contains 20 mg/ml dalbavancin as a clear, colorless to yellow solution. Reconstituted vials may be stored either refrigerated at 2 to 8 C (36 to 46 F), or at controlled room temperature 20 to 25 C (68 to 77 F). Do not freeze. Dilution: Aseptically transfer the required dose of reconstituted dalbavancin solution from the vial(s) to an intravenous bag or bottle containing 5% Dextrose Injection, USP. The diluted solution must have a final dalbavancin concentration of mg/ml to 5 mg/ml. Discard any unused portion of the reconstituted solution. Once diluted into an intravenous bag or bottle as described above, DALVANCE may be stored either refrigerated at 2 to 8 C (36 to 46 F) or at a controlled room temperature of 20 to 25 C (68 to 77 F). Do not freeze. The total time from reconstitution to dilution to administration should not exceed 48 hours. Like all parenteral drug products, diluted DALVANCE should be inspected visually for particulate matter prior to infusion. If particulate matter is identified, do not use. After reconstitution and dilution, DALVANCE is to be administered via intravenous infusion, using a total infusion time of 30 minutes. Do not co-infuse DALVANCE with other medications or electrolytes. Saline-based infusion solutions may cause precipitation and should not be used. The compatibility of reconstituted DALVANCE with intravenous medications, additives, or substances other than 5% Dextrose Injection, USP has not been established. If a common intravenous line is being used to administer other drugs in addition to DALVANCE, the line should be flushed before and after each DALVANCE infusion with 5% Dextrose Injection, USP. 3 DOSAGE FORMS AND STRENGTHS DALVANCE is supplied in single-use, clear glass vials containing sterile powder (white/off-white to pale yellow) equivalent to 500 mg of anhydrous dalbavancin. 4 CONTRAINDICATIONS DALVANCE is contraindicated in patients with known hypersensitivity to dalbavancin. No data are available on cross-reactivity between dalbavancin and other glycopeptides, including vancomycin. 5 WARNINGS AND PRECAUTIONS 5. Hypersensitivity Reactions Serious hypersensitivity (anaphylactic) and skin reactions have been reported in patients treated with DALVANCE. If an allergic reaction occurs, treatment with DALVANCE should be discontinued. Before using DALVANCE, inquire carefully about previous hypersensitivity reactions to glycopeptides, and due to the possibility of cross-sensitivity, exercise caution in patients with a history of glycopeptide allergy [see Patient Counseling Information (7)]. 5.2 Infusion Related Reactions DALVANCE is administered via intravenous infusion, using a total infusion time of 30 minutes to minimize the risk of infusion-related reactions. Rapid intravenous infusions of DALVANCE can cause reactions that resemble Red-Man Syndrome, including flushing of the upper body, urticaria, pruritus, and/or rash. Stopping or slowing the infusion may result in cessation of these reactions. 5.3 Hepatic Effects In Phase 2 and 3 clinical trials, more DALVANCE- than comparator-treated subjects with normal baseline transaminase levels had post-baseline alanine aminotransferase (ALT) elevation greater than 3 times the upper limit of normal (ULN). Overall, abnormalities in liver tests (ALT, AST, bilirubin) were reported with similar frequency in the DALVANCE and comparator arms [see Adverse Reactions (6.)]. 5.4 Clostridium difficile-associated Diarrhea Clostridium difficile-associated diarrhea (CDAD) has been reported in users of nearly all systemic antibacterial drugs, including DALVANCE, with severity ranging from mild diarrhea to fatal colitis. Treatment with antibacterial agents can alter the normal flora of the colon, and may permit overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile should be discontinued, if possible. Appropriate measures such as fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. 5.5 Development of Drug-Resistant Bacteria Prescribing DALVANCE in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. 6 ADVERSE REACTIONS Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of DALVANCE cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
8 FULL PRESCRIBING INFORMATION DALVANCE (dalbavancin) for injection 6. Adverse Reactions in Clinical Trials Adverse reactions were evaluated for 778 patients treated with DALVANCE and 224 patients treated with comparator antibacterial drugs in seven Phase 2 and Phase 3 clinical trials. A causal relationship between study drug and adverse reactions was not always established. The median age of patients treated with DALVANCE was 47 years, ranging between 6 and 93 years old. Patients treated with DALVANCE were predominantly male (60%) and Caucasian (78%). Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation Serious adverse reactions occurred in 09/778 (6.%) of patients treated with DALVANCE and in 80/224 (6.5%) of patients treated with comparator. DALVANCE was discontinued due to an adverse reaction in 53/778 (3%) patients and the comparator was discontinued due to an adverse reaction in 35/224 (2.8%) patients. Most Common Adverse Reactions The most common adverse reactions in patients treated with DALVANCE were nausea (5.5%), headache (4.7%), and diarrhea (4.4%). The median duration of adverse reactions was 4.0 days in both treatment groups. Table lists selected adverse reactions occurring in more than 2% of patients treated with DALVANCE in clinical trials. Table. Selected Adverse Reactions in Phase 2/3 Trials (Number (%) of Patients) Dalbavancin (N = 778) Comparator* (N = 224) Nausea 98 (5.5) 78 (6.4) Vomiting 50 (2.8) 37 (3) Diarrhea 79 (4.4) 72 (5.9) Headache 83 (4.7) 59 (4.8) Rash 48 (2.7) 30 (2.4) Pruritus 38 (2.) 4 (3.3) *Comparators included linezolid, cefazolin, cephalexin, and vancomycin. The following selected adverse reactions were reported in DALVANCE treated patients at a rate of less than 2% in these clinical trials: Blood and lymphatic system disorders: anemia, hemorrhagic anemia, leucopenia, neutropenia, thrombocytopenia, petechiae, eosinophilia, thrombocytosis Gastrointestinal disorders: gastrointestinal hemorrhage, melena, hematochezia, abdominal pain General disorders and administration site conditions: infusion related reactions Hepatobiliary disorders: hepatotoxicity Immune system disorders: anaphylactoid reaction Infections and infestations: Clostridium difficile colitis, oral candidiasis, vulvovaginal mycotic infection Investigations: hepatic transaminases increased, blood alkaline phosphatase increased, international normalized ratio increased Metabolism and nutrition disorders: hypoglycemia Nervous system disorders: dizziness Respiratory, thoracic and mediastinal disorders: bronchospasm Skin and subcutaneous tissue disorders: urticaria Vascular disorders: flushing, phlebitis, wound hemorrhage, spontaneous hematoma Alanine Aminotransferase (ALT) Elevations Among patients with normal baseline ALT levels, more DALVANCE- than comparator-treated patients had post-baseline ALT elevations greater than 3 times the upper limit of normal (ULN), 2 (0.8%) vs. 2 (0.2%), respectively including three subjects with post-baseline ALT values greater than 0 times ULN. Eight of 2 patients treated with DALVANCE and one comparator patient had underlying conditions which could affect liver enzymes, including chronic viral hepatitis and a history of alcohol abuse. In addition, one DALVANCEtreated subject in a Phase trial had post-baseline ALT elevations greater than 20 times ULN. ALT elevations were reversible in all subjects. No comparator-treated subject with normal baseline transaminases had post-baseline ALT elevation greater than 0 times ULN. 7 DRUG INTERACTIONS 7. Drug-Laboratory Test Interactions Drug-laboratory test interactions have not been reported. 7.2 Drug-Drug Interactions No clinical drug-drug interaction studies have been conducted with DALVANCE. There is minimal potential for drug-drug interactions between DALVANCE and cytochrome P450 (CYP450) substrates, inhibitors, or inducers [see Clinical Pharmacology (2.3)]. 8 USE IN SPECIFIC POPULATIONS 8. Pregnancy: Category C There have been no adequate and well-controlled studies with dalbavancin in pregnant women. DALVANCE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. No evidence of embryo or fetal toxicity was found in the rat or rabbit at a dose of 5 mg/kg/day (.2 and 0.7 times the human dose on an exposure basis, respectively). Delayed fetal maturation was observed in the rat at a dose of 45 mg/kg/day (3.5 times the human dose on an exposure basis). In a rat prenatal and postnatal development study, increased embryo lethality and increased offspring deaths during the first week post-partum were observed at a dose of 45 mg/kg/day (3.5 times the human dose on an exposure basis). 8.3 Nursing Mothers Dalbavancin is excreted in the milk of lactating rats. It is not known whether dalbavancin or its metabolite is excreted in human milk; therefore, caution should be exercised when DALVANCE is administered to a nursing woman. 8.4 Pediatric Use Safety and efficacy in pediatric patients have not been established. 8.5 Geriatric Use Of the 778 patients treated with DALVANCE in Phase 2 and 3 clinical trials, 33 patients (7.7%) were 65 years of age or older. The efficacy and tolerability of DALVANCE were similar to comparator regardless of age. The pharmacokinetics of dalbavancin were not significantly altered with age; therefore, no dosage adjustment is necessary based on age alone. DALVANCE is substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection in this age group. 8.6 Renal Impairment In patients with renal impairment whose known creatinine clearance is less than 30 ml/min and who are not receiving regularly scheduled hemodialysis, the recommended two-dose regimen for DALVANCE is 750 mg followed one week later by 375 mg. No dosage adjustment is recommended for patients receiving regularly scheduled hemodialysis, and DALVANCE can be administered without regard to the timing of hemodialysis [see Dosage and Administration (2.2) and Clinical Pharmacology (2.3)]. 8.7 Hepatic Impairment No dosage adjustment of DALVANCE is recommended for patients with mild hepatic impairment (Child-Pugh Class A). Caution should be exercised when prescribing dalbavancin to patients with moderate or severe hepatic impairment (Child-Pugh Class B or C) as no data are available to determine the appropriate dosing in these patients [see Clinical Pharmacology (2.3)]. 0 OVERDOSAGE Specific information is not available on the treatment of overdose with DALVANCE, as doselimiting toxicity has not been observed in clinical studies. In Phase studies, healthy volunteers have been administered single doses of up to 500 mg, and cumulative doses of up to 4500 mg over a period of up to 8 weeks, with no signs of toxicity nor laboratory results of clinical concern. Treatment of overdose with DALVANCE should consist of observation and general supportive measures. Although no information is available specifically regarding the use of hemodialysis to treat overdose, in a Phase study in patients with renal impairment less than 6% of the recommended dalbavancin dose was removed [see Clinical Pharmacology (2.3)].
9 FULL PRESCRIBING INFORMATION DALVANCE (dalbavancin) for injection DESCRIPTION DALVANCE (dalbavancin) for injection is a lipoglycopeptide synthesized from a fermentation product of Nonomuraea species. Dalbavancin is a mixture of five closely related active homologs (A 0, A, B 0, B, and B 2 ); the component B 0 is the major component of dalbavancin. The homologues share the same core structure and differ in the fatty acid side chain of the N-acylaminoglucuronic acid moiety (R ) structure and/or the presence of an additional methyl group (R 2 ) on the terminal amino group (shown in the figure and table below). Figure. Dalbavancin Structural Formula Table 2. Substitution Patterns for Dalbavancin API Homologs Dalbavancin R R 2 Molecular Formula Molecular Weight* A 0 CH(CH 3 ) 2 H C 87 H 98 N 0 O 28 Cl 2.6 HCl A CH 3 H C 87 H 98 N 0 O 28 Cl 2.6 HCl B 0 CH(CH 3 ) 2 H C 88 H 00 N 0 O 28 Cl 2.6 HCl 86.7 B CH 3 H C 88 H 00 N 0 O 28 Cl 2.6 HCl 86.7 B 2 CH(CH 3 ) 2 CH 3 C 89 H 02 N 0 O 28 Cl 2.6 HCl *Anhydrous free base The B 0 INN chemical name is: 5,3-dichloro-38- de(methoxycarbonyl)-7-demethyl-9-deoxy-56- O-[2-deoxy-2-[(0-methylundecanoyl)amino]-ß-Dglucopyranuronosyl]-38-[[3-(dimethylamino)propyl] carbamoyl]-42-o-a-d-mannopyranosyl-5-nmethyl(ristomycin A aglycone) hydrochloride. DALVANCE is supplied in clear glass vials as a sterile, lyophilized, preservative-free, white to off-white to pale yellow solid. Each vial contains dalbavancin HCl equivalent to 500 mg of anhydrous dalbavancin as the free base, plus lactose monohydrate (29 mg) and mannitol (29 mg) as excipients. Sodium hydroxide or hydrochloric acid may be added to adjust the ph at the time of manufacture. The powder is to be reconstituted and further diluted for IV infusion [see Dosage and Administration (2.3) and How Supplied/Storage and Handling (6)]. 2 CLINICAL PHARMACOLOGY 2. Mechanism of Action Dalbavancin is an antibacterial drug [see Clinical Pharmacology (2.4)]. 2.2 Pharmacodynamics The antibacterial activity of dalbavancin appears to best correlate with the ratio of area under the concentration-time curve to minimal inhibitory concentration (AUC/MIC) for Staphylococcus aureus based on animal models of infection. An exposure-response analysis of a single study in patients with complicated skin and skin structure infections supports the two-dose regimen [see Dosage and Administration (2.) and Clinical Pharmacology (2.3)]. Cardiac Electrophysiology: In a randomized, positive- and placebo-controlled, thorough QT/QTc study, 200 healthy subjects received dalbavancin 000 mg IV, dalbavancin 500 mg IV, oral moxifloxacin 400 mg, or placebo. Neither dalbavancin 000 mg nor dalbavancin 500 mg (supratherapeutic dose) had any clinically relevant adverse effect on cardiac repolarization. 2.3 Pharmacokinetics Dalbavancin pharmacokinetic parameters have been characterized in healthy subjects, patients, and specific populations. Pharmacokinetic parameters following administration of a single intravenous 000 mg dose were as shown in Table 3. The pharmacokinetics of dalbavancin can be described using a three-compartment model. Table 3. Dalbavancin Pharmacokinetic Parameters in Healthy Subjects Parameter Single 000 mg Dose C max (mg/l) 287 (3.9) AUC 0-24 (mg h/l) 385 (2.8) AUC 0-Day7 (mg h/l) 60 (4.) 2 AUC 0-inf (mg h/l) (40.9) 2 Terminal t ½ (h) 346 (6.5) 2,3 CL (L/h) (46.8) 2 All values are presented as mean (% coeffi cient of variation) Data from 50 healthy subjects. 2 Data from 2 healthy subjects. 3 Based upon population pharmacokinetic analyses of data from patients, the effective half-life is approximately 8.5 days (204 hours). In healthy subjects, dalbavancin AUC 0-24h and C max both increased proportionally to dose following single IV dalbavancin doses ranging from 40 mg to 500 mg, indicating linear pharmacokinetics. The mean plasma concentration-time profile for dalbavancin at the recommended two-dose regimen of 000 mg followed one week later by 500 mg is shown in Figure 2. Figure 2. Mean (± standard deviation) dalbavancin plasma concentrations versus time in healthy subjects (n=0) following IV administration over 30 minutes of 000 mg dalbavancin (Day ) and 500 mg dalbavancin (Day 8). Dalbavancin plasma concentration, (mg/l) (+ Standard Deviation) Time, days No apparent accumulation of dalbavancin was observed following multiple IV infusions administered once weekly for up to eight weeks, with 000 mg on Day followed by up to seven weekly 500 mg doses, in healthy adults with normal renal function. Distribution: Dalbavancin is reversibly bound to human plasma proteins, primarily to albumin. The plasma protein binding of dalbavancin is approximately 93% and is not altered as a function of drug concentration, renal impairment, or hepatic impairment. The mean concentrations of dalbavancin achieved in skin blister fluid remain above 30 mg/l up to 7 days (approximately 46 hours) post dose, following 000 mg IV dalbavancin. The mean ratio of the AUC 0-44 hrs in skin blister fluid/auc 0-44 hrs in plasma is 0.60 (range 0.44 to 0.64). Metabolism: In vitro studies using human microsomal enzymes and hepatocytes indicate that dalbavancin is not a substrate, inhibitor, or inducer of CYP450 isoenzymes. A minor metabolite of dalbavancin (hydroxy-dalbavancin) has been observed in the urine of healthy subjects. Quantifiable concentrations of the hydroxydalbavancin metabolite have not been observed in human plasma (lower limit of quantitation = 0.4 µg/ml) [see Drug Interactions (7.2)]. Excretion: Following administration of a single 000 mg dose in healthy subjects, 20% of the dose was excreted in feces through 70 days post dose. An average of 33% of the administered dalbavancin dose was excreted in urine as unchanged dalbavancin and approximately 2% of the administered dose was excreted in urine as the metabolite hydroxy-dalbavancin through 42 days post dose. Specific Populations Renal Impairment: The pharmacokinetics of dalbavancin were evaluated in 28 subjects with varying degrees of renal impairment and in 5 matched control subjects with normal renal function. Following a single dose of 500 mg or 000 mg dalbavancin, the mean plasma clearance (CL T ) was reduced %, 35%, and 47% in subjects with mild (CL CR 50 to 79 ml/min), moderate (CL CR 30 to 49 ml/min), and severe (CL CR less than 30 ml/min), renal impairment, respectively, compared to subjects with normal renal function. The clinical significance of the decrease in mean plasma CL T, and the associated increase in AUC 0- noted in these pharmacokinetic studies of dalbavancin in subjects with severe renal impairment has not been established [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
10 FULL PRESCRIBING INFORMATION DALVANCE (dalbavancin) for injection No dosage adjustment is necessary for patients with CL CR greater than 30 ml/min or patients receiving hemodialysis. The recommended twodose regimen for dalbavancin in patients with severe renal impairment who are not receiving regularly scheduled hemodialysis is 750 mg followed one week later by 375 mg. Dalbavancin pharmacokinetic parameters in subjects with end-stage renal disease receiving regularly scheduled hemodialysis (three times/ week) are similar to those observed in subjects with mild to moderate renal impairment, and less than 6% of an administered dose is removed after three hours of hemodialysis. Therefore, no dosage adjustment is recommended for patients receiving regularly scheduled hemodialysis, and dalbavancin may be administered without regard to the timing of hemodialysis in such patients [see Dosage and Administration (2.) and Overdosage (0)]. Hepatic Impairment: The pharmacokinetics of dalbavancin were evaluated in 7 subjects with mild, moderate, or severe hepatic impairment (Child-Pugh class A, B or C) and compared to those in nine matched healthy subjects with normal hepatic function. The mean AUC hrs was unchanged in subjects with mild hepatic impairment compared to subjects with normal hepatic function; however, the mean AUC hrs decreased 28% and 3% in subjects with moderate and severe hepatic impairment respectively, compared to subjects with normal hepatic function. The clinical significance of the decreased AUC hrs in subjects with moderate and severe hepatic function is unknown. No dosage adjustment is recommended for patients with mild hepatic impairment. Caution should be exercised when prescribing dalbavancin to patients with moderate or severe hepatic impairment as no data are available to determine the appropriate dosing. Gender: Clinically significant gender-related differences in dalbavancin pharmacokinetics have not been observed either in healthy subjects or in patients with infections. No dosage adjustment is recommended based on gender. Geriatric Patients: Clinically significant age-related differences in dalbavancin pharmacokinetics have not been observed in patients with infections. No dosage adjustment is recommended based solely on age. Pediatric Patients: The pharmacokinetics of dalbavancin in pediatric populations <2 years of age have not been established. Drug Interactions Nonclinical studies demonstrated that dalbavancin is not a substrate, inhibitor, or inducer of CYP450 isoenzymes. In a population pharmacokinetic analysis, dalbavancin pharmacokinetics were not affected by co-administration with known CYP450 substrates, inducers or inhibitors, nor by individual medications including acetaminophen, aztreonam, fentanyl, metronidazole, furosemide, proton pump inhibitors (omeprazole, esomeprazole, pantoprazole, lansoprazole), midazolam, and simvastatin. 2.4 Microbiology Mechanism of Action Dalbavancin, a semisynthetic lipoglycopeptide, interferes with cell wall synthesis by binding to the D-alanyl-D-alanine terminus of the stem pentapeptide in nascent cell wall peptidoglycan, thus preventing cross-linking. Dalbavancin is bactericidal in vitro against Staphylococcus aureus and Streptococcus pyogenes at concentrations similar to those sustained throughout treatment in humans treated according to the recommended dosage regimen. Mechanism of Resistance The development of bacterial isolates resistant to dalbavancin has not been observed, either in vitro, in studies using serial passage, or in animal infection experiments. Interaction with Other Antimicrobials When tested in vitro, dalbavancin demonstrated synergistic interactions with oxacillin and did not demonstrate antagonistic or synergistic interactions with any of the following antibacterial agents of various classes: gentamicin, vancomycin, levofloxacin, clindamycin, quinupristin/dalfopristin, linezolid, aztreonam, rifampin or daptomycin. The clinical significance of these in vitro findings is unknown. Dalbavancin has been shown to be active against the following microorganisms, both in vitro and in clinical infections [see Indications and Usage ()]. Gram-positive bacteria Staphylococcus aureus (including methicillinresistant isolates) Streptococcus pyogenes Streptococcus agalactiae Streptococcus anginosus group (including S. anginosus, S. intermedius, S. constellatus) The following in vitro data are available, but their clinical significance is unknown. In addition, at least 90% of organisms in the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the dalbavancin susceptible breakpoint of 0.2 mcg/ml. However, the safety and efficacy of dalbavancin in treating clinical infections due to these bacteria have not been established in adequate well-controlled clinical trials. Gram-positive bacteria Enterococcus faecium (vancomycin-susceptible isolates only) Enterococcus faecalis (vancomycin-susceptible isolates only) Susceptibility Test Methods When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug for treatment. Dilution Techniques Quantitative methods are used to determine minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method.,2 When determining dalbavancin MICs, polysorbate-80 (P-80), should be added at a final concentration of 0.002% to freshly prepared or frozen microtiter trays. The MIC values should be interpreted according to the criteria provided in Table 4. Diffusion Techniques Dalbavancin disks for diffusion susceptibility testing are not available. Disk diffusion is not a reliable method for determining the in vitro activity of dalbavancin. Table 4. Susceptibility Test Interpretive Criteria for Dalbavancin Pathogen MIC (mcg/ml) a Zone Diameter (mm) S I R S I R Staphylococcus aureus (including methicillinresistant isolates) Streptococcus pyogenes, Streptococcus agalactiae, and Streptococcus anginosus group a The current absence of data on resistant isolates precludes defi ning any category other than Susceptible. If isolates yield MIC results other than susceptible, they should be submitted to a reference laboratory for additional testing. A report of Susceptible indicates that the antibacterial agent is likely to inhibit growth of the pathogen if the antibacterial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. Quality Control Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test.,2 Standard dalbavancin powder should provide the following range of MIC values noted in Table 5. Table 5. Acceptable MIC Quality Control Ranges for Dalbavancin Quality Control Strain MIC Range (µg/ml) Staphylococcus aureus ATCC Streptococcus pneumoniae ATCC 4969 a Enterococcus faecalis ATCC ATCC = American Type Culture Collection a This organism may be used for validation of susceptibility test results when testing Streptococcus species other than S. pneumoniae.
11 FULL PRESCRIBING INFORMATION DALVANCE (dalbavancin) for injection 3 NONCLINICAL TOXICOLOGY 3. Carcinogenesis, Mutagenesis, Impairment of Fertility Long-term studies in animals to determine the carcinogenic potential of dalbavancin have not been conducted. Dalbavancin was not genotoxic in a mammalian HGPRT gene mutation assay, an in vitro chromosome aberration assay in Chinese Hamster Ovary cells, or an in vivo mouse micronucleus assay. Impaired fertility in the rat was not observed at a dose of 5 mg/kg/day (.2 times the human dose on an exposure basis). Reductions in male and female fertility and increased embryo resorptions occurred at a dose of 45 mg/kg/day (3.5 times the human dose on an exposure basis), at which signs of parental toxicity were also observed. 3.2 Animal Toxicology and/or Pharmacology Increases in serum levels of liver enzymes (ALT, AST), associated with microscopic findings in the liver were noted in toxicology studies in rats and dogs where dalbavancin was administered daily for 28 to 90 days. Hepatocellular necrosis was observed in dogs dosed at 0 mg/kg/day for longer than 2 months, i.e., at approximately 5 to 7 times the expected human dose on an exposure basis. Histiocytic vacuolation and hepatocyte necrosis were observed in rats dosed daily at 40 and 80 mg/kg/day, respectively, for 4 weeks, (approximately 3 and 6 times the expected human dose on an exposure basis, respectively). In addition, renal toxicity characterized by increases in serum BUN and creatinine and microscopic kidney findings was observed in rats and dogs at doses 5 to 7 times the expected human dose on an exposure basis. The relationship between these findings in the animal toxicology studies after 28 and 90 consecutive days of dosing to the indicated clinical dosing of 2 doses 7 days apart are unclear. 4 CLINICAL STUDIES Acute Bacterial Skin and Skin Structure Infections: Adult patients with ABSSSI were enrolled in two Phase 3, randomized, doubleblind, double-dummy clinical trials of similar design (Trial and Trial 2). The Intent-to-Treat (ITT) population included,32 randomized patients. Patients were treated for two weeks with either a two-dose regimen of intravenous DALVANCE (000 mg followed one week later by 500 mg) or intravenous vancomycin (000 mg or 5 mg/kg every 2 hours, with the option to switch to oral linezolid after 3 days). DALVANCE-treated patients with creatinine clearance of less than 30 ml/min received 750 mg followed one week later by 375 mg. Approximately 5% of patients also received a protocol-specified empiric course of treatment with intravenous aztreonam for coverage of Gram-negative pathogens. The specific infections in these trials included cellulitis (approximately 50% of patients across treatment groups), major abscess (approximately 30%), and wound infection (approximately 20%). The median lesion area at baseline was 34 cm 2. In addition to local signs and symptoms of infection, patients were also required to have at least one systemic sign of disease at baseline, defined as temperature 38 C or higher (approximately 85% of patients), white blood cell count greater than 2,000 cells/mm 3 (approximately 40%), or 0% or more band forms on white blood cell differential (approximately 23%). Across both trials, 59% of patients were from Eastern Europe and 36% of patients were from North America. Approximately 89% of patients were Caucasian and 58% were males. The mean age was 50 years and the mean body mass index was 29. kg/m 2. The primary endpoint of these two ABSSSI trials was the clinical response rate where responders were defined as patients who had no increase from baseline in lesion area 48 to 72 hours after initiation of therapy, and had a temperature consistently at or below 37.6 C upon repeated measurement. Table 6 summarizes overall clinical response rates in these two ABSSSI trials using the pre-specified primary efficacy endpoint in the ITT population. Table 6. Clinical Response Rates in ABSSSI Trials at Hours after Initiation of Therapy DALVANCE Vancomycin/ Linezolid Difference (95% CI) 3 Trial 240/288 (83.3%) 233/285 (8.8%).5% (-4.6, 7.9) Trial 2 285/37 (76.8%) 288/368 (78.3%) -.5% (-7.4, 4.6) There were 7 patients who did not receive treatment and were counted as non-responders: 6 dalbavancin patients (3 in each trial) and one vancomycin/linezolid patient in Trial 2. 2 Patients who died or used non-study antibacterial therapy or had missing measurements were classifi ed as non-responders. 3 The 95% Confi dence Interval (CI) is computed using the Miettinen and Nurminen approach, stratifi ed by baseline fever status. A key secondary endpoint in these two ABSSSI trials evaluated the percentage of ITT patients achieving a 20% or greater reduction in lesion area from baseline at hours after initiation of therapy. Table 7 summarizes the findings for this endpoint in these two ABSSSI trials. Table 7. Patients in ABSSSI Trials with Reduction in Lesion Area of 20% or Greater at Hours after Initiation of Therapy DALVANCE Vancomycin/ Linezolid Difference (95% CI) 3 Trial 259/288 (89.9%) 259/285 (90.9%) -.0% (-5.7, 4.0) Trial 2 325/37 (87.6%) 36/368 (85.9%).7% (-3.2, 6.7) There were 7 patients (as described in Table 6) who did not receive treatment and were counted as non-responders. 2 Patients who died or used non-study antibacterial therapy or had missing measurements were classifi ed as non-responders. 3 The 95% CI is computed using the Miettinen and Nurminen approach, stratifi ed by baseline fever status. Another secondary endpoint in these two ABSSSI trials was the clinical success rate assessed at a follow-up visit occurring between Days 26 to 30. Clinical Success at this visit was defined as having a decrease in lesion size (both length and width measurements), a temperature of 37.6 C or lower, and meeting pre-specified criteria for local signs: purulent discharge and drainage absent or mild and improved from baseline, heat/warmth & fluctuance absent, swelling/ induration & tenderness to palpation absent or mild. Table 8 summarizes clinical success rates at a follow-up visit for the ITT and clinically evaluable population in these two ABSSSI trials. Note that there are insufficient historical data to establish the magnitude of drug effect for antibacterial drugs compared with placebo at the follow-up visits. Therefore, comparisons of DALVANCE to vancomycin/linezolid based on clinical success rates at these visits cannot be utilized to establish non-inferiority. Table 8. Clinical Success Rates in ABSSSI Trials at Follow-Up (Day 26 to 30) Trial Trial 2 DALVANCE Vancomycin/ Linezolid ITT 24/288 (83.7%) 25/285 (88.%) CE 22/226 (93.8%) 220/229 (96.%) ITT 327/37 (88.%) 3/368 (84.5%) CE 283/294 (96.3%) 257/272 (94.5%) Difference (95% CI) 3-4.4% (-0.,.4) -2.3% (-6.6, 2.0) 3.6% (-.3, 8.7).8% (-.8, 5.6) There were 7 patients (as described in Table 6) who did not receive treatment and were counted as failures in the ITT analysis. 2 Patients who died, used non-study antibacterial therapy, or had an unplanned surgical intervention 72 hours after the start of therapy were classifi ed as Clinical Failures. 3 The 95% CI is computed using the Miettinen and Nurminen approach, stratifi ed by baseline fever status. Table 9 shows outcomes in patients with an identified baseline pathogen, using pooled data from Trials and 2 in the microbiological ITT (microitt) population. The outcomes shown in the table are clinical response rates at 48 to 72 hours and clinical success rates at follow-up (Day 26 to 30), as defined above. Pathogen Staphylococcus aureus Methicillinsusceptible Methicillinresistant Streptococcus agalactiae Streptococcus pyogenes Streptococcus anginosus group Table 9. Outcomes by Baseline Pathogen (MicroITT) Early Clinical Response at hours Early Responder 2 DALVANCE 206/257 (80.2) 34/67 (80.2) 72/90 (80.0) 6/2 (50.0) 28/37 (75.7) 8/22 (8.8) Comparator 29/256 (85.5) 63/89 (86.2) 56/67 (83.6) /4 (78.6) 24/36 (66.7) 23/ 25 (92.0) 20% reduction in lesion size DALVANCE 239/257 (93.0) 56/67 (93.4) 83/90 (92.2) 0/2 (83.3) 32/37 (86.5) 2/22 (95.5) Comparator 232/256 (90.6) 73/89 (9.5) 59/67 (88.) 0/4 (7.4) 27/36 (75.0) 25/25 (00.0) Clinical Success at Day 26 to 30 DALVANCE 27/257 (84.4) 42/67 (85.0) 75/90 (83.3) 0/2 (83.3) 33/37 (89.2) 2/22 (95.5) Comparator 229/256 (89.5) 7/89 (90.5) 57/67 (85.) /4 (78.6) 32/36 (88.9) 23/25 (92.0) There were 2 patients in the dalbavancin arm with methicillin-susceptible S. aureus at baseline who did not receive treatment and were counted as non-responders/failures. 2 Early Responders are patients who had no increase from baseline in lesion area 48 to 72 hours after initiation of therapy, and had a temperature consistently at or below 37.6 C upon repeated measurement. 3 All patients in the clinical trials had blood cultures obtained at baseline. A total of 6 ABSSSI patients receiving dalbavancin had positive cultures at baseline for one of the above pathogens. Isolates included eleven S. aureus, three S. agalactiae, one S. pyogenes, and one S anginosus. Eleven of these patients (69%) were clinical responders at hours and clinical successes at Day 26 to 30.
profiles Decision: Dalvance
 Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
Page CONTRAINDICATIONS Hypersensitivity to dalbavancin (4)
 IGLIGTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use DALVANCE safely and effectively. See full prescribing information for DALVANCE. DALVANCE (dalbavancin)
IGLIGTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use DALVANCE safely and effectively. See full prescribing information for DALVANCE. DALVANCE (dalbavancin)
Dalvance (dalbavancin) for injection
 DALVANCE (dalbavancin) for injection Coding & Billing Reference guide For more information: Call the Durata CONNECTS SM program toll-free 1.855.DURATA4 (1.855.387.2824) TM Dalvance (dalbavancin) for injection
DALVANCE (dalbavancin) for injection Coding & Billing Reference guide For more information: Call the Durata CONNECTS SM program toll-free 1.855.DURATA4 (1.855.387.2824) TM Dalvance (dalbavancin) for injection
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
ABSSSI Case Study days (clinical evaluation)
 ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
1 INDICATIONS AND USAGE. 1.1 Limitation of Use FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication
 ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZINPLAVA safely and effectively. See full prescribing information for ZINPLAVA. ZINPLAVA (bezlotoxumab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZINPLAVA safely and effectively. See full prescribing information for ZINPLAVA. ZINPLAVA (bezlotoxumab)
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING. 150 mg*/vial 12
 Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OTIPRIO safely and effectively. See full prescribing information for OTIPRIO. OTIPRIO (ciprofloxacin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OTIPRIO safely and effectively. See full prescribing information for OTIPRIO. OTIPRIO (ciprofloxacin
Staphylococcal enterocolitis: Adult Patients (18 years of age and older): 500 mg to 2 g orally in 3 or 4 divided doses for 7 to 10 days. (2.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FIRVANQ safely and effectively. See full prescribing information for FIRVANQ. FIRVANQ (vancomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FIRVANQ safely and effectively. See full prescribing information for FIRVANQ. FIRVANQ (vancomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use epinastine hydrochloride ophthalmic solution safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use epinastine hydrochloride ophthalmic solution safely and effectively. See full prescribing information
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
VORAXAZE (glucarpidase) For Injection, for intravenous use Initial U.S. Approval: 2012
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VORAXAZE safely and effectively. See full prescribing information for VORAXAZE. VORAXAZE (glucarpidase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VORAXAZE safely and effectively. See full prescribing information for VORAXAZE. VORAXAZE (glucarpidase)
KHAPZORY (levoleucovorin) for injection, for intravenous use Initial U.S. Approval: 1952 (d,l-leucovorin)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
VIBATIV - telavancin hydrochlorideâ injection, powder, lyophilized, for solutionâ Theravance, Inc
 VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
WARNING: FETAL RISK See full prescribing information for complete boxed warning.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
Cefadroxil for Oral Suspension USP 250 mg/5 ml and 500 mg/5 ml
 Cefadroxil for Oral Suspension USP 250 mg/5 ml and 500 mg/5 ml Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil for oral suspension and other antibacterial
Cefadroxil for Oral Suspension USP 250 mg/5 ml and 500 mg/5 ml Rx only To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil for oral suspension and other antibacterial
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
ELSPAR (asparaginase) For injection, intravenous or intramuscular Initial U.S. Approval: 1978
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
ORBACTIV (oritavancin) for injection, for intravenous use Initial U.S. Approval: 2014
 HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use RBACTIV safely and effectively. See full prescribing information for RBACTIV. RBACTIV (oritavancin)
HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use RBACTIV safely and effectively. See full prescribing information for RBACTIV. RBACTIV (oritavancin)
HIGHLIGHTS OF PRESCRIBING INFORMATION CONTRAINDICATIONS. None.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. ONTAK (denileukin diftitox) Injection
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. ONTAK (denileukin diftitox) Injection
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
DALVANCE (DALBAVANCIN) FOR INJECTION REIMBURSEMENT AT-A-GLANCE
 (DALBAVACI) FR IJECTI REIMBURSEMET AT-A-GLACE For more information: Call the CECTS SM program toll-free 1.855.387.2824 Program Coordinators are available 8:00 AM to 8:00 PM Eastern time, Monday through
(DALBAVACI) FR IJECTI REIMBURSEMET AT-A-GLACE For more information: Call the CECTS SM program toll-free 1.855.387.2824 Program Coordinators are available 8:00 AM to 8:00 PM Eastern time, Monday through
DOSAGE FORMS AND STRENGTHS Cream: Each gram contains 10 mg of ozenoxacin (1%) (3).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XEPI TM safely and effectively. See full prescribing information for XEPI TM. XEPI TM (ozenoxacin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XEPI TM safely and effectively. See full prescribing information for XEPI TM. XEPI TM (ozenoxacin)
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aztreonam Pharmaceutical form(s)/strength: 500 mg, 1 g and 2 g powder for solution for injection and infusion NB! 75mg aztreonam for nebulisation to treat infections
M0BCore Safety Profile Active substance: Aztreonam Pharmaceutical form(s)/strength: 500 mg, 1 g and 2 g powder for solution for injection and infusion NB! 75mg aztreonam for nebulisation to treat infections
Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
YONDELIS (trabectedin) DOSING & ADMINISTRATION GUIDE
 YONDELIS (trabectedin) DOSING & ADMINISTRATION GUIDE INDICATION YONDELIS (trabectedin) is indicated for the treatment of patients with unresectable or metastatic liposarcoma or leiomyosarcoma who received
YONDELIS (trabectedin) DOSING & ADMINISTRATION GUIDE INDICATION YONDELIS (trabectedin) is indicated for the treatment of patients with unresectable or metastatic liposarcoma or leiomyosarcoma who received
ALTARGO TM Retapamulin. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w)
 ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Cefprozil For Oral Suspension USP 125 mg/5 ml and 250 mg/5 ml Lupin Limited Mumbai 400 098
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Cefprozil For Oral Suspension USP 125 mg/5 ml and 250 mg/5 ml Lupin Limited Mumbai 400 098
VANCOCIN CAPSULES (vancomycin hydrochloride)
 PRODUCT INFORMATION VANCOCIN CAPSULES (vancomycin hydrochloride) This preparation for the treatment of colitis is for oral use only. Vancocin must be given orally for treatment of staphylococcal enterocolitis
PRODUCT INFORMATION VANCOCIN CAPSULES (vancomycin hydrochloride) This preparation for the treatment of colitis is for oral use only. Vancocin must be given orally for treatment of staphylococcal enterocolitis
400 mg IV (over 5 to 60 minutes) every 12 hours. > 30 to mg IV (over 5 to 60 minutes ) every 12 hours. 15 to 30
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
SIVEXTRO (tedizolid phosphate) for injection, for intravenous use SIVEXTRO (tedizolid phosphate) tablet, for oral use Initial U.S.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIVEXTRO safely and effectively. See full prescribing information for SIVEXTRO. SIVEXTRO (tedizolid
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIVEXTRO safely and effectively. See full prescribing information for SIVEXTRO. SIVEXTRO (tedizolid
Administer as an intravenous infusion over 35 to 60 minutes (2.1, 2.3) Dilution required prior to administration (2.2)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EXONDYS 51 safely and effectively. See full prescribing information for EXONDYS 51. EXONDYS 51 (eteplirsen)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EXONDYS 51 safely and effectively. See full prescribing information for EXONDYS 51. EXONDYS 51 (eteplirsen)
ERAXIS (anidulafungin) for injection, for intravenous use Initial U.S. Approval: 2006
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERAXIS safely and effectively. See full prescribing information for ERAXIS. ERAXIS (anidulafungin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERAXIS safely and effectively. See full prescribing information for ERAXIS. ERAXIS (anidulafungin)
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ETOPOPHOS safely and effectively. See full prescribing information for ETOPOPHOS. ETOPOPHOS (etoposide
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ETOPOPHOS safely and effectively. See full prescribing information for ETOPOPHOS. ETOPOPHOS (etoposide
CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
LUPIN LIMITED SAFETY DATA SHEET
 LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Cefprozil Tablets USP 250 mg and 500 mg Lupin Limited Mandideep 462 046 INDIA Lupin
LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Cefprozil Tablets USP 250 mg and 500 mg Lupin Limited Mandideep 462 046 INDIA Lupin
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
Important Safety Information for Feraheme (ferumoxytol) Injection
 Dear Radiologist: (ferumoxytol) Injection for intravenous (IV) use is an IV iron replacement product indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease
Dear Radiologist: (ferumoxytol) Injection for intravenous (IV) use is an IV iron replacement product indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease
DOSING GUIDE. Indications. Important Safety Information. Enable the immune system. RECOGNIZE. RESPOND.
 DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Daptomycin (CUBICIN) 500 mg Lyophilized Powder for IV Infusion. CUBICIN contains daptomycin, a cyclic lipopeptide antibacterial agent.
 DAPTOMYCIN 0500778/2 CUBICIN TM 500 mg Lyophilized Powder for IV Infusion Antibacterial/Cyclic Lipopeptide Antibiotic 1. Product Description 1.1. Name of Pharmaceutical Product Daptomycin (CUBICIN) 500
DAPTOMYCIN 0500778/2 CUBICIN TM 500 mg Lyophilized Powder for IV Infusion Antibacterial/Cyclic Lipopeptide Antibiotic 1. Product Description 1.1. Name of Pharmaceutical Product Daptomycin (CUBICIN) 500
Page 1 of 9. ERWINAZE (asparaginase Erwinia chrysanthemi) for injection, intramuscular (IM) or intravenous (IV) use Initial U.S.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERWINAZE safely and effectively. See full prescribing information for ERWINAZE. ERWINAZE (asparaginase
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERWINAZE safely and effectively. See full prescribing information for ERWINAZE. ERWINAZE (asparaginase
Core Safety Profile. Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g LV/H/PSUR/0002/001 Date of FAR:
 Core Safety Profile Active substance: Ceftriaxone Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g P-RMS: LV/H/PSUR/0002/001 Date of FAR: 14.10.2009 Section 4.2 (Posology and method
Core Safety Profile Active substance: Ceftriaxone Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g P-RMS: LV/H/PSUR/0002/001 Date of FAR: 14.10.2009 Section 4.2 (Posology and method
12 to 17 years. 7 to 11 years
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
KEVEYIS (dichlorphenamide) tablets, for oral use Initial U.S. Approval: 1958
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
ILUMYA (tildrakizumab-asmn) injection, for subcutaneous use Initial U.S. Approval: 2018
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILUMYA safely and effectively. See full prescribing information for ILUMYA. ILUMYA (tildrakizumab-asmn)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILUMYA safely and effectively. See full prescribing information for ILUMYA. ILUMYA (tildrakizumab-asmn)
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
ACTEMRA IV Dosing & Administration Pocket Guide
 IMPORTANT SAFETY INFORMATION BOXED WARNING Serious Infections Serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial, invasive fungal, viral, and other opportunistic
IMPORTANT SAFETY INFORMATION BOXED WARNING Serious Infections Serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial, invasive fungal, viral, and other opportunistic
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Centers for Medicare & Medicaid Services (CMS) Grants New Technology Add-on Payment (NTAP) to ZEMDRI (plazomicin)
 Centers for Medicare & Medicaid Services (CMS) Grants New Technology Add-on Payment (NTAP) to ZEMDRI (plazomicin) Effective October 1, 2018 CMS has granted an NTAP designation for certain qualifying use
Centers for Medicare & Medicaid Services (CMS) Grants New Technology Add-on Payment (NTAP) to ZEMDRI (plazomicin) Effective October 1, 2018 CMS has granted an NTAP designation for certain qualifying use
PRODUCT INFORMATION 1 ABOUT THIS GUIDE DOSAGE FORM AND STRENGTH STORAGE AND HANDLING
 DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
AROMASIN 25mg (Tablets)
 APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
ADVERSE REACTIONS The most common (>10%) adverse reactions are hypercalcemia, nausea, and diarrhea. (6.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MOZOBIL safely and effectively. See full prescribing information for MOZOBIL. MOZOBIL (plerixafor
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MOZOBIL safely and effectively. See full prescribing information for MOZOBIL. MOZOBIL (plerixafor
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml SI/H/PSUR/0002/002 Date of FAR:
 0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CEFAZOLIN injection safely and effectively. See full prescribing information for CEFAZOLIN injection.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CEFAZOLIN injection safely and effectively. See full prescribing information for CEFAZOLIN injection.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION One sachet contains 5.631g fosfomycin trometamol equivalent to 3.0 g fosfomycin.
 Doctor leaflet 1. NAME OF THE MEDICINAL PRODUCT MONUROL 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One sachet contains 5.631g fosfomycin trometamol equivalent to 3.0 g fosfomycin. Excipients: One sachet
Doctor leaflet 1. NAME OF THE MEDICINAL PRODUCT MONUROL 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One sachet contains 5.631g fosfomycin trometamol equivalent to 3.0 g fosfomycin. Excipients: One sachet
Daptomycin for injection is not indicated for the treatment. Daptomycin for injection is not indicated for the treatment of
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DAPTOMYCIN FOR INJECTION safely and effectively. See full prescribing information for DAPTOMYCIN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DAPTOMYCIN FOR INJECTION safely and effectively. See full prescribing information for DAPTOMYCIN
ERAXIS (anidulafungin) for injection, for intravenous use Initial U.S. Approval: 2006
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERAXIS safely and effectively. See full prescribing information for ERAXIS. ERAXIS (anidulafungin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ERAXIS safely and effectively. See full prescribing information for ERAXIS. ERAXIS (anidulafungin)
CEREZYME Genzyme. 70 mg (52 mg) (18 mg)
 CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CONTRAINDICATIONS None.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PAZEO safely and effectively. See full prescribing information for PAZEO. PAZEO (olopatadine hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PAZEO safely and effectively. See full prescribing information for PAZEO. PAZEO (olopatadine hydrochloride
DOSAGE FORMS AND STRENGTHS mg or 500 mg single dose vials as sterile, pyrogen-free lyophilizates.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZINECARD safely and effectively. See full prescribing information for ZINECARD. ZINECARD (dexrazoxane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZINECARD safely and effectively. See full prescribing information for ZINECARD. ZINECARD (dexrazoxane)
OTIPRIO an antibiotic medicine given to your child at the time of ear tube surgery
 Bring this Conversation Starter with you to your child s next doctor appointment an antibiotic medicine given to your child at the time of ear tube surgery Use this questionnaire to start a conversation
Bring this Conversation Starter with you to your child s next doctor appointment an antibiotic medicine given to your child at the time of ear tube surgery Use this questionnaire to start a conversation
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
DOSING & ADMINISTRATION GUIDE
 DOSING & ADMINISTRATION GUIDE EXONDYS51.com INDICATION 1 EXONDYS 51 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is
DOSING & ADMINISTRATION GUIDE EXONDYS51.com INDICATION 1 EXONDYS 51 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENDARI TM safely and effectively. See full prescribing information for ENDARI. ENDARI (L-glutamine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENDARI TM safely and effectively. See full prescribing information for ENDARI. ENDARI (L-glutamine
Referral Form. Referring physician s phone: Referring physician s fax:
 (ferumoxytol) Injection for intravenous (IV) use Referral Form Referring gphysician s name: Referring physician s phone: Referring physician s fax: Dear Dr or Infusion Center : I am referring my patient
(ferumoxytol) Injection for intravenous (IV) use Referral Form Referring gphysician s name: Referring physician s phone: Referring physician s fax: Dear Dr or Infusion Center : I am referring my patient
CONTRAINDICATIONS Hypersensitivity to any component of this product (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
Feraheme (ferumoxytol) Injection Coding Tool
 AMAG Assist Patient Assistance and Reimbursement Support (ferumoxytol) Injection Coding Tool (ferumoxytol) Injection for intravenous (IV) use is indicated for the treatment of iron deficiency anemia in
AMAG Assist Patient Assistance and Reimbursement Support (ferumoxytol) Injection Coding Tool (ferumoxytol) Injection for intravenous (IV) use is indicated for the treatment of iron deficiency anemia in
New Zealand Data Sheet COLISTIN-LINK
 New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
AEMCOLO (rifamycin) delayed-release tablets, for oral use. Initial U.S. Approval: 2018
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AEMCOLO safely and effectively. See full prescribing information for AEMCOLO. AEMCOLO (rifamycin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AEMCOLO safely and effectively. See full prescribing information for AEMCOLO. AEMCOLO (rifamycin)
PHARMACY DOSING AND ORDERING GUIDE
 PHARMACY DOSING AND ORDERING GUIDE FIRST AND ONLY APPROVED TREATMENT FOR PATIENTS WITH VOD WITH RENAL OR PULMONARY DYSFUNCTION POST HSCT VOD=veno-occlusive disease Indication Defitelio (defibrotide sodium)
PHARMACY DOSING AND ORDERING GUIDE FIRST AND ONLY APPROVED TREATMENT FOR PATIENTS WITH VOD WITH RENAL OR PULMONARY DYSFUNCTION POST HSCT VOD=veno-occlusive disease Indication Defitelio (defibrotide sodium)
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
WARNING: CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA (CDAD) and COLITIS See full prescribing information for complete boxed warning.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CLINDAMYCIN IN 0.9% SODIUM CHLORIDE injection safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CLINDAMYCIN IN 0.9% SODIUM CHLORIDE injection safely and effectively. See full prescribing information
ALTARGO. Retapamulin. Retapamulin 10 mg per gram (1% w/w)
 ALTARGO Retapamulin 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) 2. PHARMACEUTICAL FORM Ointment 3. CLINICAL PARTICULARS 3.1 Indications Retapamulin is indicated for
ALTARGO Retapamulin 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) 2. PHARMACEUTICAL FORM Ointment 3. CLINICAL PARTICULARS 3.1 Indications Retapamulin is indicated for
Wound Infection Patient Case Study 64-year old female with BMI 34 kg/m 2 on warfarin
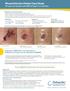 Wound Infection Patient Case Study 64-year old female with BMI 34 kg/m 2 on warfarin Background and Presentation Patient is a 64-year old obese female, BMI 34 kg/m 2. She is a diabetic on insulin and also
Wound Infection Patient Case Study 64-year old female with BMI 34 kg/m 2 on warfarin Background and Presentation Patient is a 64-year old obese female, BMI 34 kg/m 2. She is a diabetic on insulin and also
ADMINISTRATION GUIDE
 DOSING AND ADMINISTRATION GUIDE INDICATION LARTRUVO is indicated, in combination with doxorubicin, for the treatment of adult patients with soft tissue sarcoma (STS) with a histologic subtype for which
DOSING AND ADMINISTRATION GUIDE INDICATION LARTRUVO is indicated, in combination with doxorubicin, for the treatment of adult patients with soft tissue sarcoma (STS) with a histologic subtype for which
PRESCRIBING INFORMATION. Cloxacillin for Injection. Cloxacillin Powder for Solution (as cloxacillin sodium) 500mg powder/vial.
 PRESCRIBING INFORMATION Pr Cloxacillin for Injection Cloxacillin Powder for Solution (as cloxacillin sodium) 500mg powder/vial 1g powder/vial 2g powder/vial 10g powder/vial STERILE Antibiotic SteriMax
PRESCRIBING INFORMATION Pr Cloxacillin for Injection Cloxacillin Powder for Solution (as cloxacillin sodium) 500mg powder/vial 1g powder/vial 2g powder/vial 10g powder/vial STERILE Antibiotic SteriMax
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
CRIFOS 4 GM Injection (Fosfomycin sodium)
 Published on: 30 Sep 2016 CRIFOS 4 GM Injection (Fosfomycin sodium) Composition CRIFOS 4 GM Each vial contains: Fosfomycin Sodium BP equivalent to Fosfomycin..4 g Excipients q.s. Dosage Form Powder for
Published on: 30 Sep 2016 CRIFOS 4 GM Injection (Fosfomycin sodium) Composition CRIFOS 4 GM Each vial contains: Fosfomycin Sodium BP equivalent to Fosfomycin..4 g Excipients q.s. Dosage Form Powder for
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
How to Order OTIPRIO (ciprofloxacin otic suspension) 6%
 How to Order (ciprofloxacin otic suspension) 6% Product: (ciprofloxacin otic suspension) 6% Each carton contains 1 ml of 6% (60 mg/ml, w/v) ciprofloxacin in a 2 ml single-patient use glass vial fitted
How to Order (ciprofloxacin otic suspension) 6% Product: (ciprofloxacin otic suspension) 6% Each carton contains 1 ml of 6% (60 mg/ml, w/v) ciprofloxacin in a 2 ml single-patient use glass vial fitted
ZOMAX IV HIKMA PHARMACEUTICALS
 09-15 ZOMAX IV HIKMA PHARMACEUTICALS azithromycin for injection For IV infusion only DESCRIPTION Zomax (azithromycin for injection) contains the active ingredient azithromycin, an azalide, a subclass of
09-15 ZOMAX IV HIKMA PHARMACEUTICALS azithromycin for injection For IV infusion only DESCRIPTION Zomax (azithromycin for injection) contains the active ingredient azithromycin, an azalide, a subclass of
CONTRAINDICATIONS Known hypersensitivity to daptomycin (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
Dosing and Administration Guide for ARZERRA
 Dosing and Administration Guide for ARZERRA INDICATIONS for ARZERRA (ofatumumab) In combination with chlorambucil, for the treatment of previously untreated patients with chronic lymphocytic leukemia (CLL)
Dosing and Administration Guide for ARZERRA INDICATIONS for ARZERRA (ofatumumab) In combination with chlorambucil, for the treatment of previously untreated patients with chronic lymphocytic leukemia (CLL)
ENTEREG (alvimopan) Capsules Initial U.S. Approval: 2008
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENTEREG safely and effectively. See full prescribing information for ENTEREG. ENTEREG (alvimopan)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENTEREG safely and effectively. See full prescribing information for ENTEREG. ENTEREG (alvimopan)
