PRODUCT MONOGRAPH SANTYL OINTMENT. (Collagenase)
|
|
|
- Annabella Allen
- 6 years ago
- Views:
Transcription
1 PRODUCT MONOGRAPH SANTYL OINTMENT (Collagenase) Topical Enzymatic Debriding Agent Smith & Nephew Inc. Date of Authorization: Mississauga, Ontario December 12, 2014 L5N 6H8 Control number:
2 NAME OF DRUG SANTYL OINTMENT (Collagenase) THERAPEUTIC CLASSIFICATION Topical enzymatic debriding agent STRUCTURAL FORMULA AND CHEMISTRY Collagenase is a large protein molecule of biologic origin, being derived from the fermentation of Clostridium histolyticum. The enzyme may be obtained in two forms, one a protein of 105,000 molecular weight, collagenase A and a molecule of 57,000 molecular weight, collagenase B. SANTYL contains a mixture of these two forms in an indeterminable ratio. Both collagenase A and collagenase B require free sulfhydryl groups for formation of enzyme substrate complex. Collagenase also requires enzyme bound zinc for activity. The optimal ph range for enzymatic activity is 7 to 8. Like all enzymes having sulfhydryl groups at the active site, inactivation with heavy metals, such as mercury, silver, cobalt, magnesium, and manganese can occur. The specific substrate for collagenase is collagen. However, minimal amounts of other amidase and esterase activity may be found in the collagenase enzyme preparation. DESCRIPTION Each gram of SANTYL Ointment contains 250 units of collagenase enzyme per gram of white petrolatum U.S.P. The potency assay of collagenase is based on the digestion of undenatured collagen (from bovine Achilles tendon) at ph 7.2 and 37 C for 24 hours. The number of peptides cleaved are measured by reaction with ninhydrin. Peptides released by a trypsin digestion control are subtracted. One net collagenase unit will solubilize ninhydrin reactive material equivalent to 4 micromoles of leucine. ACTION Collagenase digests collagen in the physiologic ph and temperature range. Since collagen accounts for 75% of the dry weight of skin tissue, collagenase is useful in the removal of detritus from the craters of dermal ulcers or skin burns. Rapid debridement contributes toward the formation of granulation tissue and subsequent epithelization of these lesions.
3 INDICATIONS AND CLINICAL USES SANTYL Ointment is a sterile ointment indicated for the debridement of dermal ulcers 3,4,5,6,7,25,26,27,28,30,31,32 or severely burned areas. 8,9,0,12,13,29 CONTRAINDICATIONS Application is contraindicated in patients who have shown local or systemic hypersensitivity to collagenase. WARNINGS Debilitated patients should be closely monitored for systemic bacterial infections because of the theoretical possibility that debriding enzymes may increase the risk of bacteremia. PRECAUTIONS The enzyme's optimal ph range is 7 to 8. 14,15 Significantly lower ph conditions have a definite adverse effect on the enzyme's activity and appropriate precautions should be taken. The enzymatic activity is also adversely affected by detergents and hexachlorophene 11,17 and heavy metal ions 14,16 such as mercury and silver which are used in some antiseptics and by cobalt, magnesium and manganese. When it is suspected such materials have been used, the site should be carefully cleansed by repeated washings with normal saline before SANTYL Ointment is applied. Soaks containing metal ions or acidic solutions such as Burow's (aluminium acetate) solution should be avoided because of the metal ion and low ph. Cleansing materials such as hydrogen peroxide or Dakin's (dilute sodium hypochlorite) solution do not interfere with the activity of the enzyme. The ointment should be confined to the area of the lesion in order to avoid the risk of irritation or maceration of normal skin; however, the enzyme does not damage newly forming granulation tissue. 30,31 A slight erythema has been noted occasionally in the surrounding tissue particularly when the enzyme ointment was not confined to the lesion. This can be readily controlled by more careful application or by protecting the healthy skin with a material such as Lassar's (zinc oxide - 25%) paste. Since the enzyme is a protein, sensitization may develop with prolonged use although this has not been observed to date.
4 ADVERSE REACTIONS Irritation, maceration or erythema have been noted where prolonged contact of normal skin with SANTYL has been allowed, either by application of the ointment to areas of normal skin or by excessive application of ointment to the wound crater with subsequent spread to normal skin when bandages are applied. The reported incidence for this type of reaction was 1.8%. No systemic adverse effects have been reported due to SANTYL. TREATMENT OF OVERDOSAGE SANTYL Ointment can be rendered inert by the application of Burow's Solution U.S.P. (ph ) to the treatment site. If this should be necessary, reapplication should be made only with caution. PHARMACOLOGY Since collagen accounts for 75% of the dry weight of skin tissue, the ability of collagenase to digest insoluble fibrous collagen by peptide bond cleavage under physiological conditions of ph and temperature, makes it particularly effective in the removal of detritus. Collagenase thus contributes towards the formation of granulation tissue and subsequent epithelization 3,4 of dermal ulcers and severely burned areas. Hot water burns, being resistant to enzymatic debridement and approximating the conditions at the periphery of contact burns and other lesions, present a suitable basis for testing the efficacy of the debriding action of collagenase. In a study of these burns on guinea pigs, the average debridement of the collagenase treated animals was 83%, while that of the placebo was 8%. This demonstrates the ability of collagenase to debride a refractory type of burn and to provide a healthy base for the healing process. 18 TOXICOLOGY 1. Investigation of the enzyme collagenase, derived from Clostridium histolyticum, began in 1949 with the work of MacLennan, Mandl and Howes.1 They had selected the strains H4 and from 80 strains of C. histolyticum because of their high enzyme yields and low toxicity. MacLennan et al. established that H4 and strains of C. histolyticum elaborated no or relatively minute amounts of the lethal neurotoxin (toxin). The complete or almost complete absence of toxin was later confirmed by Howes, Mandl and Zaffuto 33. In the same publication, the pharmacological effects of collagenase were also described.
5 It is important to note that the enzyme incorporated into SANTYL (collagenase) Ointment, is derived from a subculture of the H4 strain described in the paper by Howes, Mandl and Zaffuto. As described in the paper, the enzyme displays a low order of toxicity. 2. Acute Systemic Toxicity of Collagenase Powder 19 The acute toxicity of four lots of collagenase powder, administered intravenously to male mice, was determined. The results of these studies are presented below: Collagenase Lot LD 50 (mg/kg) Confidence Limits (mg/kg) LD 1 (mg/kg) Slope Function of Curve 3. Toxicity Study of Collagenase Powder Administered Intravenously to Rabbits As a result of reports that high daily intravenous doses of collagenase produced a drop in platelet counts, three intravenous toxicity studies in rabbits were carried out. The results of these studies are described below: a) Collagenase powder in dosages of 0.25 mg/kg/day and 2.5 mg/kg/day was administered five times a week for three weeks to rabbits. The powder in a dosage of 7.5 mg/kg/day was administered five times a week to rabbits for a total of seven days. Rectal temperatures taken before and one hour after collagenase administration indicated the collagenase solutions, as used, were pyrogenic. The data collected indicated that collagenase powder, when administered daily, 5 days a week intravenously, in doses of 0.25 mg/kg/day for 3 weeks, 2.5 mg/kg/day for 3 weeks and 7.5 mg/kg/day was non-toxic to rabbits. No toxic symptoms were noted in any of the treated animals and all remained in good condition during the course of the study. None showed any evidence of bleeding from body orifices, hyperemia of the eye or hemorrhage in the tissues and viscera examined on the post mortem examination. No gross or histopathology was noted in any of the animals. The dose regimens of 2.5 and 7.5 mg/kg/day produced a decrease in blood platelets. This
6 effect, however, was reversible at the 2.5 mg/kg/day dose level but was not followed in the animals treated with the 7.5 mg/kg/day for reversibility. Alterations in the platelet count were not associated with any toxic manifestations in the animals. 22 b) In a second study, the effect of intravenous administration of collagenase powder at a dose of 0.25 mg/kg/day, 5 times a week for 3 weeks was studied in rabbits. It was concluded from the experimental data, that collagenase powder was non-toxic to rabbits when administered in this manner. No toxic symptoms were noted in any of the treated animals. All remained in good condition during the course of the study. None showed any evidence of bleeding from the body orifices, hyperemia of the eye or hemorrhage in any tissues and viscera examined on post mortem examination. Further, no gross pathology or histopathology was noted in the animals. c) In a third study rabbits were injected with collagenase powder at a concentration of 0.25 mg/kg/body weight, 2.5 mg/kg/body weight and 7.5 mg/kg/body weight. The rabbits were on the intravenous injection schedule for a total of three weeks. All three dose levels caused a pyrogenic reaction to occur in some of the test animals. After the first and second days of injections, increased vascularization was noted until at the twelfth day after the onset, when the condition almost disappeared. No gross pathological conditions were noted throughout the experiment except for the unexplained death of one rabbit eight days after the start of the experiment. It was concluded that in the dosages used, the enzyme was pyrogenic in some instances. The high dose level seemed to cause a temporary loss of weight or a very slow increase in weight during the experiment. It would seem that the consistent I.V. injection of the higher dosage levels resulted in a relative decrease in platelet counts and hematocrit values but that this alteration has a tendency towards self correction after a short rest period of two to five days. Toxicity Studies of the Final Product 1. Subacute Dermal Toxicity The backs of twenty rabbits were closely clipped to compare areas of skin measuring about 7x7 inches. The denuded areas of half of the animals in both groups were scarified.
7 For 15 consecutive working days, the test (250 units collagenase per gram of petrolatum) or control (petrolatum) material was gently inuncted into the backs of the animals at a daily dosage level of 3.5 grams per kilogram of body weight. A usually mild and transient erythema that subsided even before the end of the study was the only sign of local toxicity. There were no remarkable findings with respect to possible toxic effects exerted by the formulation under study based on clinical laboratory findings. No evidence of systemic toxicity was noted based on histopathologic criteria Draize Eye Test (Primary Irritation to Eye Mucosa) It was established that SANTYL Ointment is not irritating when 100 mg is instilled into the eyes of rabbits Treatment of Infected Scalds in Rabbits The influence of collagenase on the incidence of bacteremia associated with infected lesions of the skin was studied. The results obtained support the conclusion that collagenase does not affect the likelihood of infection or predispose the animal to bacteremia Debridement with SANTYL (collagenase) Hot water burns were artificially produced on six guinea pigs. Three animals were treated with SANTYL Ointment 0.3% and three with white petrolatum for three days. There was significant debridement in each group (80% collagenase, 68% white petrolatum). 24 DOSAGE AND ADMINISTRATION SANTYL Ointment should be applied once daily in the following manner: 1. Prior to application, the lesions should be gently cleansed with a gauze pad saturated in normal saline, (buffered to ph ) or hydrogen peroxide to remove any film and digested material. 2. Whenever infection is present, as evidenced by positive cultures, pus, inflammation or odour, it is desirable to use an appropriate topical antibacterial
8 agent. Neomycin-Bacitracin-Polymyxin B (Neosporin) has been found compatible with SANTYL Ointment. This antibiotic should be applied to the lesion in powder form or solution prior to the application of SANTYL Ointment. Should the infection not respond, therapy with SANTYL Ointment should be discontinued until remission of the infection. 3. SANTYL Ointment should be applied (using a wooden tongue depressor or spatula) directly to deep wounds. When dealing with shallow wounds, SANTYL Ointment should be applied to a sterile gauze pad which is then applied to the wound. The wound is covered with sterile gauze pad and secured with clear tape or Kling bandage. 4. Crosshatching thick eschar with a #11 blade is helpful in speeding up debridements. It is also desirable to remove as much loosened detritus as can be done readily with forceps and scissors. 5. All excess ointment should be removed each time dressing is changed. 6. Use of the ointment should be terminated when sufficient debridement of necrotic tissue has occurred and granulation is well underway. DOSAGE FORMS SANTYL Ointment is available as a sterile ointment in a white petrolatum base packaged in 15, and 30 gram tubes.
9 BIBLIOGRAPHY 1. MacLennan, J.D., Mandl, I. and Howes, E.L.: J. Clin. Invest. 32:1317, Mandl, I.: Advances in Enzymology, Interscience Publ. 23:163, Boxer, A.M., Gottesman, N., Bernstein, H. and Mandl, I.: Geriatrics 24:75, German, F.M.: In Collagenase, Gordon & Breach, Publ., Haimovici, H.: In Collagenase, Gordon & Breach, Publ., Hoover, N.W. and Ivins, J.C.: AMA Arch. Surg. 79:701, Mazurek, I.: Med. Welt. 22:150, Zimmermann, W.E.: In Collagenase, Gordon & Breach, Publ., Gruenagel, H.H.: Med. Clin. 58:442, Connell, J.F., Jr., Bowe, J.J., DelGuercio, L. and Rousselott, L.M.: Am. J. Surg. 93:694, Howes, E.L., Mandl, I., Zaffuto, S. and Ackermann, W.: Sur. Gyn. Obst., 109:177, Rehn, J.: Med. Clin. 58:799, Krauss, H., Koslowski, L. and Zimmermann, W.E.: Langenbecks Arch. Klin. Chir. 303:23, Mandl, I., Zipper, H. and Ferguson, L.T.: Arch. Bio. Chem. Biophys., 74:465, Tytell, A.A. and Hewson, K.: Proc. Soc. Exp. Biol. Med., 74:555, Mandl, I., MacLennan, J. and Howes, E.L.: J. Clin. Invest., 32:1323, Howes, E.L.: 20th Congr. Soc. Int. of Chir. Rome, Linder, J.M., M.D., FACS.: Debridement of experimental burns in guinea pigs with collagenase/abc; See Volume II, Exhibit V. 19. Johnson & Johnson: Acute systemic toxicity studies in the mouse and subacute dermal toxicity studies in the rabbit, See Volume II, Exhibit II. 20. NIH Data: Report on collagenase experiment in rabbits. See Volume II, Exhibit I. 21. Rakieten, N., M.L.: Draize Eye Test, primary irritation to eye mucosa made on collagenase/abc Ointment Lot No C-03. See Volume II., Exhibit III. 22. Rakieten, N., M.L.: Toxicity studies on collagenase powder Lot No C-04 Potency 60,000 C Units per gram, following daily intravenous administration to rabbits, See Volume II, Exhibit I. 23. Rakieten, N., M.L.: Treatment with collagenase of infected scalds in rabbits. See Volume II, Exhibit IV.
10 24. Bothwell, J.W.: Debridement with collagenase ointment, Letter to Dr. G. Hildick- Smith, March 2, See Volume II, Exhibit VI. 25. Bardfeld, L.A.: Treatment of dermal ulcers of the lower extremity with collagenase, in Mandl, I.: Collagenase, New York, Gordon & Breach, 1972, pp Barrett, D., Klibanski, A.: Collagenase debridement. Amer. J. Nurs. 73: , May Lee, L.K., Ambrus, J.L.: Collagenase therapy for decubitus ulcers. Geriatrics 30:91-98, May Locke, R., Heifitz, N.: Collagenase as an aid in healing. J. Amer. Podiatry Assoc. 65: , March Mahler, D.L.: The use of collagenase ointment for various clinical purposes. In preparation. 30. Rao, D.B., et al.: Collagenase in the treatment of dermal and decubitus ulcers. J. Amer. Geriat. Soc. 23:22-30, January Varma, A.O., et al.: Debridement of dermal ulcers with collagenase. Surg. Gynecol. Obstet. 136: , February Vetra, H. and Whittaker, D.: Hydrotherapy and topical collagenase for decubitus ulcers. Geriatrics 30:53-58, August Howes, E.L., Mandl, I. and Zaffuto, S.: J. Bact. 79:191, (1960).
CATRIX WOUND DRESSING CARTILAGE POWDER
 CATRIX WOUND DRESSING CARTILAGE POWDER Distributed By: Lescarden Inc. NY, NY (USA) Customer Relations: Toll Free (USA) (888) 581-2076. (212) 687-1050 Internet: www.catrix.com E-mail: info@catrix.com CATRIX
CATRIX WOUND DRESSING CARTILAGE POWDER Distributed By: Lescarden Inc. NY, NY (USA) Customer Relations: Toll Free (USA) (888) 581-2076. (212) 687-1050 Internet: www.catrix.com E-mail: info@catrix.com CATRIX
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
POLYHEAL MICRO - INSTRUCTIONS FOR USE
 POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
Trifluridine Ophthalmic Solution, 1% Sterile
 Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and. hydrocortisone ointment, USP)
 CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution)
 VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
PRESCRIBING INFORMATION. BaciJect. Bacitracin for injection U.S.P. Powder for Solution. 50,000 Units/Vial. Antibiotic
 PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
WOUNDS. Emergency Procedures in PT
 WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types. Summary
 Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP)
 CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
WOUND DRESSING IN DIABETIC FOOT
 Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
POVIDONE-IODINE BETADINE
 POVIDONE-IODINE BETADINE NAME OF PRODUCT BETADINE 10% OINTMENT Read this leaflet carefully before you start using BETADINE ointment. Keep this leaflet. You may need to read it again. If you have any further
POVIDONE-IODINE BETADINE NAME OF PRODUCT BETADINE 10% OINTMENT Read this leaflet carefully before you start using BETADINE ointment. Keep this leaflet. You may need to read it again. If you have any further
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
HYDROCORTISONE OINTMENT USP,
 HYDROCORTISONE- hydrocortisone ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- HYDROCORTISONE OINTMENT USP, 1% Rx only For External Us e Only Not For Ophthalmic Us e DESCRIPTION:
HYDROCORTISONE- hydrocortisone ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- HYDROCORTISONE OINTMENT USP, 1% Rx only For External Us e Only Not For Ophthalmic Us e DESCRIPTION:
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
Skin Integrity and Wound Care
 Skin Integrity and Wound Care By Dr. Amer Hasanien & Dr. Ali Saleh Skin Integrity and Wound Care Skin integrity: the presence of normal Skin & Uninterrupted skin layers by wounds. Factors affecting appearance
Skin Integrity and Wound Care By Dr. Amer Hasanien & Dr. Ali Saleh Skin Integrity and Wound Care Skin integrity: the presence of normal Skin & Uninterrupted skin layers by wounds. Factors affecting appearance
January Adult Burn Injured patients
 Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if Trust wide): Review date (when this version goes out of date): Explicit definition
Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if Trust wide): Review date (when this version goes out of date): Explicit definition
NEOSPORIN G.U. Irrigant Sterile (neomycin sulfate polymyxin B sulfate solution for irrigation)
 NEOSPORIN G.U. Irrigant Sterile (neomycin sulfate polymyxin B sulfate solution for irrigation) NEOSPORIN G.U.SOLUTION NOT FOR INJECTION DESCRIPTION NEOSPORIN G.U. Irrigant is a concentrated sterile antibiotic
NEOSPORIN G.U. Irrigant Sterile (neomycin sulfate polymyxin B sulfate solution for irrigation) NEOSPORIN G.U.SOLUTION NOT FOR INJECTION DESCRIPTION NEOSPORIN G.U. Irrigant is a concentrated sterile antibiotic
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
CASE 1: TYPE-II DIABETIC FOOT ULCER
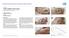 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
WOUND DRESSING Daily Dressing Packets
 AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP)
 NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) NEOSPORIN SOLUTION DESCRIPTION NEOSPORIN Ophthalmic Solution (neomycin and polymyxin B
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) NEOSPORIN SOLUTION DESCRIPTION NEOSPORIN Ophthalmic Solution (neomycin and polymyxin B
Appropriate Dressing Selection For Treating Wounds
 Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:
![The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula: The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:](/thumbs/74/71271167.jpg) Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
Galen ( A.D) Advanced Wound Dressing
 Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
Each gram of the ointment contains 0.25 mg Fluocinolone Acetonide in a base containing White Petrolatum.
 FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory. Neomycin Sulphate, Polymyxin B Sulfate and Bacitracin Zinc Powder
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory NEOSPORIN ANTIBIOTIC POWDER Neomycin Sulphate, Polymyxin B Sulfate and Bacitracin Zinc Powder QUALITATIVE AND QUANTITATIVE
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory NEOSPORIN ANTIBIOTIC POWDER Neomycin Sulphate, Polymyxin B Sulfate and Bacitracin Zinc Powder QUALITATIVE AND QUANTITATIVE
Comparative study of collagenase and papain-urea based preparations in the management of chronic nonhealing limb ulcers
 Comparative study of collagenase and papain-urea based preparations in the management of chronic nonhealing limb ulcers Hosamath Vijaykumar, Sreekar Agumbe Pai*, Vijay Pandey and Prasannakumar Kamble Department
Comparative study of collagenase and papain-urea based preparations in the management of chronic nonhealing limb ulcers Hosamath Vijaykumar, Sreekar Agumbe Pai*, Vijay Pandey and Prasannakumar Kamble Department
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used
 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
Disclosures for Tarik Alam. Wound Bed Preparation. Wound Prognosis. Session Objectives. Debridement 4/26/2015
 Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Summary of Toxicity Studies on Imazapyr
 Summary of Toxicity Studies on Imazapyr Technical Department, Cyanamid (Japan) Ltd. (Received July 15, 1997 ; Accepted August 20, 1997) DESCRIPTIO OF THE TEST COMPOUD Imazapyr is a nonselective herbicide
Summary of Toxicity Studies on Imazapyr Technical Department, Cyanamid (Japan) Ltd. (Received July 15, 1997 ; Accepted August 20, 1997) DESCRIPTIO OF THE TEST COMPOUD Imazapyr is a nonselective herbicide
with' some material which has enough body' to act as a splint,' EVERY surgeon has his own pet method of dressing skin
 A METHOD OF SPLINTING SKIN GRAFTS. BY JOHN STAIGE DAVIS, M.D., OF BALTIMORE, MD., Assistant Surgeon in the Out-patient Department of Johns Hopkins Hospital. EVERY surgeon has his own pet method of dressing
A METHOD OF SPLINTING SKIN GRAFTS. BY JOHN STAIGE DAVIS, M.D., OF BALTIMORE, MD., Assistant Surgeon in the Out-patient Department of Johns Hopkins Hospital. EVERY surgeon has his own pet method of dressing
DOVONEX (calcipotriene) Cream, 0.005% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY. Not for Ophthalmic, Oral or Intravaginal Use.
 DOVONEX (calcipotriene) Cream, 0.005% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY. Not for Ophthalmic, Oral or Intravaginal Use. DESCRIPTION Dovonex (calcipotriene) Cream, 0.005% contains calcipotriene monohydrate,
DOVONEX (calcipotriene) Cream, 0.005% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY. Not for Ophthalmic, Oral or Intravaginal Use. DESCRIPTION Dovonex (calcipotriene) Cream, 0.005% contains calcipotriene monohydrate,
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
Cordran Cream and Cordran Ointment Flurandrenolide, USP
 Cordran Cream and Cordran Ointment Flurandrenolide, USP DESCRIPTION Cordran (flurandrenolide, USP) is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy,
Cordran Cream and Cordran Ointment Flurandrenolide, USP DESCRIPTION Cordran (flurandrenolide, USP) is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy,
Understanding Debridement
 Understanding Debridement Figure 1. Wound Healing Process Wound Blood Clot Blood Blood Vessel Fat Tissue The wound in the skin exposes deep tissue layers to the air. Scab Scab Exudate Granulation Tissue
Understanding Debridement Figure 1. Wound Healing Process Wound Blood Clot Blood Blood Vessel Fat Tissue The wound in the skin exposes deep tissue layers to the air. Scab Scab Exudate Granulation Tissue
INTRODUCTION TO WOUND DRESSINGS
 WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
Facility Name: Name: Date: Tracheostomy Care Evaluation Checklist
 Name: Date: Tracheostomy Care Evaluation Checklist Objective: Learner will be able to demonstrate tracheostomy care with a disposable inner cannula. Review facility protocol for caring for Airway Management:
Name: Date: Tracheostomy Care Evaluation Checklist Objective: Learner will be able to demonstrate tracheostomy care with a disposable inner cannula. Review facility protocol for caring for Airway Management:
DESCRIPTION: Each gram of ointment contains 500 units of Bacitracin in a low melting special base containing White Petrolatum and Mineral Oil.
 BACITRACIN- bacitracin ointment Paddock Laboratories, LLC ---------- Bacitracin Ophthalmic Ointment USP DESCRIPTION: Each gram of ointment contains 500 units of Bacitracin in a low melting special base
BACITRACIN- bacitracin ointment Paddock Laboratories, LLC ---------- Bacitracin Ophthalmic Ointment USP DESCRIPTION: Each gram of ointment contains 500 units of Bacitracin in a low melting special base
Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.]
![Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.]](/thumbs/94/118693790.jpg) Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Description VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment
Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Description VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment
Beyond the Basics ImprovingYour Wound Care Knowledge. Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN
 Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Thermal Dermal Burn Modeling in Rats and Minipigs
 Thermal Dermal Burn Modeling in Rats and Minipigs Comparative Biosciences, Inc. 786 Lucerne Drive Sunnyvale, CA 94085 Telephone: 408.738.9261 www.compbio.com Premier Preclinical Contract Research Organization
Thermal Dermal Burn Modeling in Rats and Minipigs Comparative Biosciences, Inc. 786 Lucerne Drive Sunnyvale, CA 94085 Telephone: 408.738.9261 www.compbio.com Premier Preclinical Contract Research Organization
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/451/98-FINAL June 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS BACITRACIN SUMMARY REPORT (1)
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/451/98-FINAL June 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS BACITRACIN SUMMARY REPORT (1)
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
Coly-Mycin S Otic with Neomycin and Hydrocortisone (colistin sulfate neomycin sulfate thonzonium bromide hydrocortisone acetate otic suspension)
 Coly-Mycin S Otic with Neomycin and Hydrocortisone (colistin sulfate neomycin sulfate thonzonium bromide hydrocortisone acetate otic suspension) DESCRIPTION Coly-Mycin S Otic with Neomycin and Hydrocortisone
Coly-Mycin S Otic with Neomycin and Hydrocortisone (colistin sulfate neomycin sulfate thonzonium bromide hydrocortisone acetate otic suspension) DESCRIPTION Coly-Mycin S Otic with Neomycin and Hydrocortisone
Prontosan. Clean. Easy Wound Healing. Wound Cleansing
 Prontosan Clean. Easy Wound Healing. Wound Cleansing CoE Infection Control Prontosan the unique combination of Betaine & Polihexanide reduces healing time removes and prevents biofilm prevents infections
Prontosan Clean. Easy Wound Healing. Wound Cleansing CoE Infection Control Prontosan the unique combination of Betaine & Polihexanide reduces healing time removes and prevents biofilm prevents infections
Mean percent reduction in ulcer area from baseline at six weeks 62 % SANTYL Ointment + supportive care* + sharp debridement 1 (P<0.
 Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
New Zealand Data Sheet COLISTIN-LINK
 New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
Wound Management. E. Foy White-Chu, MD, CWSP
 Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Collagenase Types 1 through 7
 COLLAGENASE Tissue Dissociation/Cell Isolation PRODUCT HIGHLIGHTS Clostridium histolyticum contains two distinct but related genes for collagenase. The col G gene codes for a 936 amino acid protein designated
COLLAGENASE Tissue Dissociation/Cell Isolation PRODUCT HIGHLIGHTS Clostridium histolyticum contains two distinct but related genes for collagenase. The col G gene codes for a 936 amino acid protein designated
The Ointment is back!
 Now Available for Plaque Psoriasis The Ointment is back! Bioequivalent to Dovonex * Ointment Before prescribing, please see attached full Prescribing Information. The same Delivers the reliability you
Now Available for Plaque Psoriasis The Ointment is back! Bioequivalent to Dovonex * Ointment Before prescribing, please see attached full Prescribing Information. The same Delivers the reliability you
Sodium Bromide, Liquid Sodium Bromide
 MATERIAL SAFETY DATA SHEET SODIUM BROMIDE SOLUTION January 2017 A. GENERAL INFORMATION Trade Name (Common Name or Synonym) - Sodium Bromide, Liquid Sodium Bromide B. COMPOSITION INFORMATION ON INGREDIENTS
MATERIAL SAFETY DATA SHEET SODIUM BROMIDE SOLUTION January 2017 A. GENERAL INFORMATION Trade Name (Common Name or Synonym) - Sodium Bromide, Liquid Sodium Bromide B. COMPOSITION INFORMATION ON INGREDIENTS
DOSAGE AND ADMINISTRATION
 Pr COLISTIMETHATE FOR INJECTION USP Colistimethate for Injection contains the sodium salt of colistimethate which is a polypeptide antibiotic with an approximate molecular weight of 1 750. The empirical
Pr COLISTIMETHATE FOR INJECTION USP Colistimethate for Injection contains the sodium salt of colistimethate which is a polypeptide antibiotic with an approximate molecular weight of 1 750. The empirical
CSL Behring LLC Albuminar -25 US Package Insert Albumin (Human) USP, 25% Revised: 01/2008 Page 1
 Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
1. Identification of the substance/preparation and of the company undertaking
 Q-CHEM nv Leeuwerweg 138 B-3803 Sint-Truiden BELGIUM Tel. +32 (0)11 78 57 17 Fax +32 (0)11 68 15 65 Mobile +32 (0)474 95 13 91 E-mail: q-chem@skynet.be BTW/VAT: BE 865.950.672 BIC: KREDBEBB Bank: KBC 733-0209642-55
Q-CHEM nv Leeuwerweg 138 B-3803 Sint-Truiden BELGIUM Tel. +32 (0)11 78 57 17 Fax +32 (0)11 68 15 65 Mobile +32 (0)474 95 13 91 E-mail: q-chem@skynet.be BTW/VAT: BE 865.950.672 BIC: KREDBEBB Bank: KBC 733-0209642-55
Wound Care Discharge Instructions
 Wound Care Discharge Instructions Wound Care Discharge Instructions DESCRIPTION Caring for your wound is important to promote healing, avoid infection, and minimize scarring. Wounds heal more quickly when
Wound Care Discharge Instructions Wound Care Discharge Instructions DESCRIPTION Caring for your wound is important to promote healing, avoid infection, and minimize scarring. Wounds heal more quickly when
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Test Report No.: GZHG OT Date: Oct 11, 2011 Page 1 of 7 PERSONAL SAFETY CORPORATION 1655 PROGRESS DRIVE, HIAWATHA, LOWA 52233, USA
 Test Report No.: GZHG824985OT Date: Oct, 2 Page of 7 PERSONAL SAFETY CORPORATION 655 PROGRESS DRIVE, HIAWATHA, LOWA 52233, USA The following sample was submitted and identified on behalf of the client
Test Report No.: GZHG824985OT Date: Oct, 2 Page of 7 PERSONAL SAFETY CORPORATION 655 PROGRESS DRIVE, HIAWATHA, LOWA 52233, USA The following sample was submitted and identified on behalf of the client
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Introduction. Follow standard infection control precautions
 Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Total Contact Cast System
 Total Contact Cast System Instructions for Use Products Included in Cutimed Off-Loader Select kit Qty Cutimed Cavity Sterile 1 ea. Cutisorb Cotton Gauze 2" x 2" 4 ea. Delta-Lite Conformable Fiberglass
Total Contact Cast System Instructions for Use Products Included in Cutimed Off-Loader Select kit Qty Cutimed Cavity Sterile 1 ea. Cutisorb Cotton Gauze 2" x 2" 4 ea. Delta-Lite Conformable Fiberglass
Table of Contents. Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings
 Table of Contents Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings Dialysis Port Care Know What Type of Vascular Access You Have. Fistula: An artery in your forearm
Table of Contents Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings Dialysis Port Care Know What Type of Vascular Access You Have. Fistula: An artery in your forearm
NPUAP Mission. Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries. npuap.org
 Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Successful IV Starts Revised February 2014
 Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION
 NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
EXPERIMENTAL THERMAL BURNS I. A study of the immediate and delayed histopathological changes of the skin.
 EXPERIMENTAL THERMAL BURNS I A study of the immediate and delayed histopathological changes of the skin. RJ Brennan, M.D. and B. Rovatti M.D. The purpose of this study was to determine the progressive
EXPERIMENTAL THERMAL BURNS I A study of the immediate and delayed histopathological changes of the skin. RJ Brennan, M.D. and B. Rovatti M.D. The purpose of this study was to determine the progressive
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
DOSAGE FORMS AND STRENGTHS Cream: Each gram contains 10 mg of ozenoxacin (1%) (3).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XEPI TM safely and effectively. See full prescribing information for XEPI TM. XEPI TM (ozenoxacin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XEPI TM safely and effectively. See full prescribing information for XEPI TM. XEPI TM (ozenoxacin)
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Bactisure Wound Lavage. Advancing Biofilm Removal
 Wound Lavage Advancing Biofilm Removal Wound Lavage Wound Lavage is used to remove debris, including microorganisms, from wounds using pulsed (jet) lavage. The clear, colorless, low-odor solution is a
Wound Lavage Advancing Biofilm Removal Wound Lavage Wound Lavage is used to remove debris, including microorganisms, from wounds using pulsed (jet) lavage. The clear, colorless, low-odor solution is a
INDICATIONS AND USAGE SULFAMYLON Cream is a topical agent indicated for adjunctive therapy of patients with second- and third- degree burns.
 SULFAMYLON- mafenide acetate cream Mylan Ins titutional Inc. ---------- DESCRIPTION SULFAMYLON Cream is a soft, white, nonstaining, water-miscible, anti-infective cream for topical administration to burn
SULFAMYLON- mafenide acetate cream Mylan Ins titutional Inc. ---------- DESCRIPTION SULFAMYLON Cream is a soft, white, nonstaining, water-miscible, anti-infective cream for topical administration to burn
1 g of white to slightly yellowish opaque cream contains 0.2 g (20 %) micronised azelaic acid.
 SKINOREN 20% Azelaic Acid Cream Presentation 1 g of white to slightly yellowish opaque cream contains 0.2 g (20 %) micronised azelaic acid. Uses Actions The antimicrobial property of azelaic acid and a
SKINOREN 20% Azelaic Acid Cream Presentation 1 g of white to slightly yellowish opaque cream contains 0.2 g (20 %) micronised azelaic acid. Uses Actions The antimicrobial property of azelaic acid and a
Support. Regranex360 SM is a program that supports patients who are using REGRANEX (becaplermin) Gel, 0.01% for diabetic sores on their legs or feet.
 When caring for your sore, count on Regranex360 SM Support Regranex360 SM is a program that supports patients who are using REGRANEX (becaplermin) Gel, 0.01% for diabetic sores on their legs or feet. Important
When caring for your sore, count on Regranex360 SM Support Regranex360 SM is a program that supports patients who are using REGRANEX (becaplermin) Gel, 0.01% for diabetic sores on their legs or feet. Important
Human experience with a biodegradable polyurethane scaffold I: Short-term implantation
 Human experience with a biodegradable polyurethane scaffold I: Short-term implantation A/Prof. John E Greenwood AM BSc(Hons), MBChB, MD, FRCS(Eng.), FRCS(Plast.), FRACS Director, Burns Unit, Royal Adelaide
Human experience with a biodegradable polyurethane scaffold I: Short-term implantation A/Prof. John E Greenwood AM BSc(Hons), MBChB, MD, FRCS(Eng.), FRCS(Plast.), FRACS Director, Burns Unit, Royal Adelaide
DEBRIDEMENT. Four Methods of Debridement
 Wound Definition Debridement is the removal of devitalized tissue and foreign matter from a wound. These materials support the growth of harmful organisms and may delay wound healing. Although debridement
Wound Definition Debridement is the removal of devitalized tissue and foreign matter from a wound. These materials support the growth of harmful organisms and may delay wound healing. Although debridement
WOUND MANAGEMENT IN THE ELDERLY. Evelyn Cook, RN, CIC Associate Director
 WOUND MANAGEMENT IN THE ELDERLY Evelyn Cook, RN, CIC Associate Director OBJECTIVES Discuss skin changes in elderly Discuss wound care management program Discuss infection prevention implications SKIN CHANGES
WOUND MANAGEMENT IN THE ELDERLY Evelyn Cook, RN, CIC Associate Director OBJECTIVES Discuss skin changes in elderly Discuss wound care management program Discuss infection prevention implications SKIN CHANGES
Consider the possibility of pressure ulcer development
 Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
Basic Dressing Categories
 Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile
 PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
PRESCRIBING INFORMATION Including Patient Medication Information. Colistimethate for Injection USP (colistimethate sodium)
 PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
Thiophanate-methyl -MATERIAL SAFETY DATA SHEET
 Thiophanate-methyl -MATERIAL SAFETY DATA SHEET 1. Chemical Product Identification Product Name: Thiophanate-methyl Molecular Formula: C 12 H 14 N 4 O 4 S 2 Molecular Weight: 342.4 Structural Formula: Chemical
Thiophanate-methyl -MATERIAL SAFETY DATA SHEET 1. Chemical Product Identification Product Name: Thiophanate-methyl Molecular Formula: C 12 H 14 N 4 O 4 S 2 Molecular Weight: 342.4 Structural Formula: Chemical
Topical antimicrobials (antiseptics) Iodine, Silver, Honey
 Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
SDMA Categorisation of Wound Care and Associated Products
 Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Rx only FOR TOPICAL DERMATOLOGIC USE ONLY, NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE
 STZC:R1 ERTACZO TM (SERTACONAZOLE NITRATE) CREAM, 2% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY, NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE DESCRIPTION: ERTACZO TM (sertaconazole nitrate) Cream, 2%,
STZC:R1 ERTACZO TM (SERTACONAZOLE NITRATE) CREAM, 2% Rx only FOR TOPICAL DERMATOLOGIC USE ONLY, NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE DESCRIPTION: ERTACZO TM (sertaconazole nitrate) Cream, 2%,
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology
 The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING. 150 mg*/vial 12
 Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP)
 NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) DESCRIPTION Neosporin Ophthalmic Solution (neomycin and polymyxin B sulfates and gramicidin
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) DESCRIPTION Neosporin Ophthalmic Solution (neomycin and polymyxin B sulfates and gramicidin
Collagenase Assay Kit
 Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen
Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen
