Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies
|
|
|
- Lynn James
- 5 years ago
- Views:
Transcription
1 1188 Br J Ophthalmol 2001;85: ORIGINAL ARTICLES Clinical science Moorfields Eye Hospital, London, UK ACPoon G Geerling J K G Dart G E Fraenkel Institute of Ophthalmology, London, UK G Geerling J K G Dart J T Daniels Correspondence to: Mr John Dart, Moorfields Eye Hospital, 162 City Road, London EC1V 2PD, UK jdart@ucl.ac.uk Accepted for publication 14 February 2001 Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies Alexander C Poon, Gerd Geerling, John K G Dart, Graham E Fraenkel, Julie T Daniels Abstract Background/aims Autologous serum drops have been reported to be beneficial in keratoconjunctivitis sicca (KCS) and persistent epithelial defects (PED). A clinical pilot study was carried out to examine these potential uses and in vitro toxicity testing on corneal epithelial cell cultures was performed to compare the evect of serum drops with unpreserved hypromellose (hydroxypropylmethylcellulose 0.3%). Methods Patients with KCS and PED, unresponsive to conventional treatment were recruited. Patients were examined before treatment, at 1 and 2 weeks after initiation, and then 2 weekly until treatment ceased. Symptoms were assessed at each visit. Clinical examination included Schirmer s test without anaesthesia, rose bengal staining, and fluorescein staining. Epithelial defects were measured with the slit beam. In the laboratory, cultured human corneal epithelial cells were exposed to serum drops and hypromellose, and their viability evaluated with fluorescent viability staining (Calcein AM ethidium homodimer) and an ATP assay. Results Autologous serum was used in 15 eyes of 13 patients with PED and 11 eyes of nine patients with KCS. In two patients serum drops were started after penetrating keratoplasty (PK). The PKs were performed for perforations secondary to PEDs. Of the 15 eyes with PED, nine healed at a mean of 29 days and six failed. The mean duration of PED before the use of serum drops was 48.2 days. Of the 11 eyes with KCS, six had improved subjective scores and fluorescein scores, and five had improved rose bengal scores after the use of serum drops. For the two patients who used serum eyedrops post- PK, there was a stable and intact epithelium at 1 week. Cessation of serum drops during the postoperative period led to deterioration in the subjective and objective scores in both patients. One developed a PED that responded to reinstitution of serum drops. The morphology and ATP levels of cultured epithelial cells exposed to serum were better maintained than those exposed to hypromellose. Conclusion Autologous serum drops are useful for PED and KCS. This evect may be related to a number of active factors in serum including growth factors, fibronectin, vitamin A, and anti-proteases. In vitro toxicity testing demonstrated that serum drops have reduced toxicity compared with unpreserved hypromellose. Currently regulatory restrictions in the UK have prevented the establishment of a prospective randomised controlled trial examining the eycacy of autologous serum drops for the management of this group of ocular surface disorders. (Br J Ophthalmol 2001;85: ) Serum is the fluid component of blood devoid of its cellular components and clotting factors. Various components of serum, such as albumin and immunoglobulin, are used as treatment for medical conditions. In ophthalmology, there have been reports of the use of autologous serum for diseases of the external eye particularly keratoconjunctivitis sicca (KCS) and persistent epithelial defects (PED). 1 5 Tears perform a vital role in maintaining the health of the corneal epithelium. They provide antibacterial factors, prevent desiccation of the cornea, and contain substances like epidermal growth factor (EGF), fibronectin, basic fibroblast growth factor (bfgf), and factors that are thought to maintain epithelial health. 6 In keratoconjunctivitis sicca the corneal epithelium develops punctate staining, filamentary keratitis, and ulceration due to partial or total tear deficiency. Patients with severe keratoconjunctivitis sicca benefit from intensive topical lubricants, punctal occlusion, and occlusive glasses. However, despite maximal therapy many patients continue to have symptoms and signs of keratoconjunctivitis. Serum drops have been found to be beneficial for extremely dry eyes Fox et al found that 15 out of 15 dry eye patients had improved subjective and objective scores after
2 Autologous serum eyedrops for dry eyes and epithelial defects 1189 Table 1 Subjective scores for KCS patients Grade Description 0 No symptoms 1 Symptoms were mild, and they did not make me uncomfortable 2 Symptoms were moderate, they did make me uncomfortable, but they did not interfere with my activities 3 Symptoms were severe, they did make me uncomfortable, and they did not interfere with my activities 4 Symptoms were very severe, they did make me uncomfortable, and they did interfere with my activities the use of serum drops. 1 Serum may be helpful because of the presence of substances that enhance epithelial growth and health. These substances, including growth factors outlined above, are normally found in tears and are much decreased in KCS. Epithelial defects can be caused by a variety of insults to the cornea. Conditions that may lead to non-healing epithelial defects include KCS, exposure keratopathy, neurotrophic cornea, stem cell failure, and post-infectious ulcers. There is evidence suggesting that growth factors and proteins found in the serum may help in the healing of epithelial defects Tsubota et al found that in 16 eyes with PED treated with autologous serum drops, 62.6% were healed within 1 month when their mean duration of PED before serum drops was 7.2 months. 3 At Moorfields Eye Hospital a pilot study was performed to examine the eycacy of autologous serum drops in patients with KCS and PED. A laboratory study was carried out concurrently to evaluate the toxicity of serum drops on human corneal epithelial cell cultures. Materials and methods CLINICAL STUDIES The clinical features of a prospective cohort of patients who were prescribed serum drops are presented. From July 1998 to June 1999, 29 eyes of 24 patients from one of the author s (JKGD) external disease and corneal clinics were started on autologous serum drops. Patients were started on serum drops if they were older than 16 years of age and satisfied one of the following criteria: (1) Dry eye patients with epitheliopathy: (a) symptoms of dry eyes, (b) break up time (BUT) <5 seconds and Schirmer s test without anaesthesia <5 mm at 5 minutes, (c) presence of rose bengal and fluorescein staining of the ocular surface. (2) Patients with epithelial defects of at least 1 week s duration where conventional therapy had failed. Conventional therapy included antibiotic ointment four times a day, eyelid closure procedures such as botulinum induced ptosis and/or tarsorrhaphy, and therapeutic contact lens wear. (3) Patients who had just undergone keratoplasty and who had previously demonstrated failure to epithelialise a graft due to existing ocular surface disease (for example, Sjögren s, Stevens-Johnson syndrome, graft versus host disease, ocular cicatricial pemphigoid, and stem cell deficiency). Patients were not started on serum eye drops if the epithelial defect was classified as a progressive corneal melt caused by an immunological process such as rheumatoid melt or Mooren s ulceration. Patients with active microbial infection, acute herpes simplex or herpes zoster keratitis, drug toxicity, vitamin A deficiency, or recurrent corneal erosion syndrome were also excluded. Patients in whom venesection was not possible (for example, poor venous access), pregnant or lactating females, and those without personal freezing facilities were also excluded. Patients were examined before treatment and then at 1 and 2 weeks after initiation of treatment. Following that, patients were seen every 2 weeks until serum drops were stopped. Patients were asked to grade their dry eye symptoms from 0 to 4 at each assessment. Table 1 gives the description of each score. Other treatments used in the past and concurrently for PED or KCS were recorded. A history of systemic disease was recorded together with systemic medications used. A full ocular history was also obtained. Clinical examination included Schirmer s test without anaesthesia, conjunctival rose bengal staining, and corneal fluorescein staining. Scoring of the rose bengal (0 18) and fluorescein (0 15) staining was based on a scoring system suggested by the National Eye Institute/Industry workshop on clinical trials in dry eyes. 21 Epithelial defects were measured in two dimensions, the longest and its perpendicular dimension. Serum drops were produced either as a 100% or a 50% formulation. The intention was to start all patients on a solution of 50% serum and 50% unpreserved chloramphenicol eye drops (0.5% chloramphenicol with boric acid 1.5%, borax 0.3%, and purified water). However unpreserved chloramphenicol was not available for 1 month and 100% serum was used for patients enrolled during that period. In addition, one patient was found to be allergic to chloramphenicol and was changed to 100% serum. Chloramphenicol was chosen because it is relatively non-toxic and has bacteriostatic properties that would help prevent colonisation of the dropper bottle Sixteen patients were treated with 50% serum and six with 100% (see Tables 2 and 3). Autologous serum drops were produced in the following manner. (1) Venesection was performed at the cubital fossa under aseptic conditions. (2) 25 ml of blood was collected into a sterile container (50 ml of blood if 100% serum was to be produced). (3) Blood was left standing for 2 hours at room temperature to allow clotting to take place. (4) Blood was centrifuged at 4000 rpm for 10 minutes. (5) In a laminar flow cabinet, 3 ml aliquots of serum were removed with disposable pipettes and then deposited into sterile dropper bottles preloaded with 3 ml of chloramphenicol. (For those using 100% serum, 6 ml aliquots of serum were deposited into empty sterile bottles.)
3 1190 Poon, Geerling, Dart, et al Table 2 Summary of the PED patients Patient Eye Age Sex Primary diagnosis Percentage of serum Other Rx tried in past* Other Rx evective at same time* Duration of PED (weeks) Size of PED (mm) Four bottles of autologous serum were dispensed to each patient in containers with coolant bags keeping the serum cold. Patients were instructed to use the drops eight times a day during waking hours in the avected eye. They were asked to keep the current bottle in the home refrigerator at approximately 5 C and to store the extra bottles at 4 C in a freezer. Each bottle was discontinued after 1 week of use. Bottles were cultured only where microbial keratitis was identified on clinical criteria. In all patients, autologous serum drops were started with little change to the current treatment regime. However, lubrication with drops such as hypromellose (hydroxypropylmethylcellulose 0.3%) was decreased so that the frequency of serum and lubricant application was equal to that of lubrication before serum was started. For the PED patients, failure was defined and serum drops were stopped if (1) surgical intervention was necessary, (2) progress was deemed to be too slow after a trial of 1 month, or (3) the PED was enlarging. Success was defined and serum drops were weaned if closure of the epithelial defect occurred. Outcome measures for the KCS group were the subjective scores from the questionnaires and the objective scores from fluorescein and rose bengal staining. Serum drops were stopped if patients experienced worsening of symptoms, or if there was no subjective or objective improvement after 1 month. Weaning of serum, for those patients who had benefited from its use, occurred at 2 months. They were asked to taper it to four times a day until the current bottle was finished. Schirmer s <5 mm Outcome Time of healing for successes (weeks) Time of cessation for failures (weeks) Reason for failure and other relevant diagnoses 1 R 41 M Diabetic neurotrophic keratitis 100 SR, P SC, P No S 1 1 L Diabetic neurotrophic keratitis 100 SR, P SC, P Yes F 2 Enlarging ulcers at 2 weeks 2 R 75 F Neurotrophic HSV 100 B SR No S 4 3 L 66 F Post-HSK 100 SR, P SR, P No F 8 Slow progress 4 L 70 F Post-infectious 50 SK, B No F 10 Slow progress 5 R 84 F OCP 100 P Yes S 3 days 5 L OCP 100 P, SR Yes F 3 Microbial keratitis (coag ve staphylococcus) 6 R 75 F HZO 50 B No S 4 7 L 75 M RA 50 AMT, P, SR P Yes S 8 Stitch abscess (microbe unknown) 8 R 17 F Vernal, post-infectious ulcer in graft 50 SK No S 1 9 L 57 M Neurotrophic HSV and post-pk for perforation 50 LT LT Yes F 2 Slow progress 10 R 51 M OCP 50 AMT,P,SR P Yes S 6 11 R 85 M OCP 50 SR, P P Yes F 2 Increasing discomfort 12 L 81 F Post-infectious, KCS 50 P P Yes S 4 Candida infectious crystalline keratopathy 13 R 86 M Post-infectious, OCP 50 P, SR P 1 7x8 Yes S 4 B = botulinum induced ptosis, P = punctal occlusion, AMT = amniotic membrane transplant, SR = silicone rubber contact lens, SC = scleral contact lens, S = success, F = failure. *All patients were using lubricating ointment unless a therapeutic contact lens was used. Diagnosis made at the same time as when epithelium was found to be healed. Recurrent PED after three attempts at stopping serum. Each time the PED responded to recommencement of serum. There was time limitation to treatment because patient was from overseas and lack of improvement at 2 weeks led to decision to perform a central tarsorrhaphy and abandon serum drops. In the two patients using serum after penetrating keratoplasty, drops were stopped at 1 month. Success was defined as the presence of a stable intact epithelium over the donor cornea at 1 month postoperatively. Failure was the absence of a stable intact epithelium at that time. IN VITRO TOXICITY STUDIES In vitro assays were performed to determine the relative toxicity of a range of tear substitutes on corneal epithelial cells without the complex influence of corneal pathologies. The hypothesis was that diverent tear substitutes would have diverent evects upon corneal epithelial cell survival. Primary corneal epithelial cells were obtained from human corneal donor tissue stored in Optisol at 4 C following trephination of the central corneal button for a corneal graft. Corneal rims were used up to 5 days post mortem. Following incubation at 37 C for 2 hours with 1.2 IU/ml Dispase II (neutral protease; Boehringer Mannheim, Germany) the epithelium was stripped ov with gentle scraping from the limbus to the centre into phosphate buvered saline (PBS). This was centrifuged at 100 g for 5 minutes and the cells grown using routine cell culture methods and keratinocyte serum free medium (K-SFM, Gibco Life Technologies, Paisley, UK). 24 Immunohistochemistry with mouse monoclonal antibodies (ICN-flow, UK) using the alkaline phosphatase method 25 stained the cells strongly positive for human cytokeratin-3 thus confirming that the cells were of corneal epithelial origin. All experiments were performed on cells at passage 2 4; 36 hours after seeding them at a
4 Autologous serum eyedrops for dry eyes and epithelial defects 1191 Figure 1 This is a 74 year old man with KCS secondary to rheumatoid arthritis. History includes left infective corneal melt 3 years ago requiring a tectonic PK. This was followed by recurrent epithelial breakdown unresponsive to intensive lubrication and SRCL wear. The cornea perforated again requiring a repeat PK 3 months before the start of serum drops. The PED recurred and amniotic membrane transplantation was tried but failed to heal it. Serum drops were started subsequently. (A, B) Show a PED with and without fluorescein and cobalt blue illumination before the start of serum. (C) Shows that healing occurred in 2 months (epithelial bullae at site of PED). Serum was stopped after 2 months, and the PED recurred (D). It failed to respond to any therapy apart from the reinstitution of serum. (E) and (F) show healed defect with and without cobalt blue illumination. The PED recurred with further attempts at weaning and healed with reinstitution of serum. At the last review serum was weaned for the fourth time. density of into 96 well culture plates, wells were washed twice with buvered saline and exposed to 200 µl undiluted unpreserved hydroxypropylmethylcellulose 0.3% as produced by the Pharmacy Department of Moorfields Eye Hospital or 50% and 100% serum drops (as used in the clinical study) for 2 hours. For controls wells were incubated with 1% benzalkonium chloride (positive control) or culture medium (negative control). Following this the total ATP of the cultured cells was quantified. Also, ATP was quantified after exposure of the cells to 100% serum of six healthy normal volunteers versus 100% serum of eight patients with at least one systemic immunosuppressive medication for ocular cicatricial pemphigoid (for example, cyclophosphamide or azathioprine). In addition toxicity was visualised by means of a viability stain which demonstrates abnormal cell membrane permeability. In the viability staining the live/dead assay was used (Molecular Probes, Eugene, OR, USA). In this the non-fluorescent Calcein-AM is converted into green fluorescent calcein by intracellular esterase and indicates the presence of active cell metabolism. The second dye, ethidium homodimer, is usually excluded by viable cells. However, it permeates damaged cell membranes and binds to nucleic acids resulting in red fluorescence. Dead or dying cells stain red while viable cells stain green. For this assay the test agents were removed, the cells washed twice with PBS and then incubated with 100 µl of 4 µm ethidium homodimer with 2 µmol Calcein-AM for 30 minutes at room temperature. The cells were then immediately viewed at 485 nm excitation and 530 nm (Calcein)/645 nm (ethidium) emission wave length. In the ATP assay all intracellular ATP was extracted and preserved from each cell culture by adding 50 µl of a somatic cell extraction reagent (DCS, Innovative Diagnostik Systeme, Hamburg/Germany) to each well. Following 20 minutes of incubation at room temperature, 50 µl of luciferin-luciferase was added to each well and the resulting luminescence read immediately (Dynatech, ML 1000). The mean ATP count of the wells was calculated. All experiments were performed in triplicate and repeated twice The percentage of cell growth inhibition (CGI) for each drug and test situation, compared with the cell culture incubated with culture medium, was calculated as follows: 1.0 (Test MI/MO MI) 100 = %CGI where MO = mean counts for no inhibition control cultures (that is, after incubation with culture medium), MI = mean counts for maximum inhibition control cultures (that is, after incubation with benzalkonium chloride 1%) and Test = mean counts for triplicate test situation (that is, serum drops or hypromellose). Wilcoxon sign rank test for non-parametric data was used to determine the significance of diverences. Results CLINICAL STUDIES Autologous serum was used in 15 eyes of 13 patients with PED and 11 eyes of nine patients with KCS. In two patients, serum drops were started after penetrating keratoplasty (PK). The PKs were performed for perforations secondary to PEDs. The female:male ratios were 8:7, 9:1, 1:1 in the PED, KCS, and the post-pk group, respectively. The means and ranges of age were 66 (17 86), 56 (41 79), 41 (40 42) for the PED, KCS, and the post-pk group respectively. PERSISTENT EPITHELIAL DEFECT GROUP In the PED group, before initiation of the serum drops, three eyes had botulinum induced ptosis, seven had tried therapeutic contact lens (TCL) wear, two had received amniotic membrane transplantation, and one had a lateral tarsorrhaphy to encourage epithelial healing. In addition, two had superficial keratectomies to remove necrotic tissue and improve the stromal bed and thus facilitate epithelial growth. Unless contraindicated (for example, with therapeutic contact lens use) all patients were using antibiotic ointment for lubrication. Four eyes did not have a lid closing procedure or a TCL as therapy for PED. This was because, for the patients involved, the contralateral eyes had poor best corrected vision
5 1192 Poon, Geerling, Dart, et al Table 3 Summary of the KCS patients Patient Eye Age Sex Primary Serum Subj 1 condition % Subj RB 1 Fluo 2 month RB 1 1 month Fluo 2 1 month Subj 2 months and contact lens fitting on the ipsilateral eye was not possible due to short fornices in OCP or lens intolerance. In five patients a TCL was used at the same time as serum drops. Nine of the 15 eyes had a Schirmer s test of less than 5 mm and six of the nine were eyes of patients with rheumatoid arthritis or OCP. All of the nine eyes had occluded ipsilateral upper and lower puncta. A summary of the patients is presented in Table 2. Figure 1 shows a patient whose response to serum supports the beneficial action of serum on epithelial defects. Of the 15 eyes with PED, nine healed at a mean of 29 days (range 3 60 days, median 28 days), and six failed. The mean duration of PED before the use of serum drops was 48.2 days. In six eyes 100% serum drops were used and there were three successes and three failures. For nine eyes, 50% serum was used and there were six successes and three failures. One patient with diabetic neurotrophic keratopathy had an enlarged defect after 2 weeks use. Three patients developed an infection during the course of treatment. One had a stitch abscess away from the epithelial defect. An organism was not identified from culture of scrapings and sutures. Another patient developed candida infectious crystalline keratopathy although the persistent epithelial defect had healed. In the third patient a keratitis caused by coagulase negative staphylococcus developed around the site of the epithelial defect. As the epithelium had healed in the first two patients they were classified as successes. A scanty growth of the same organism that caused the keratitis was found on culturing the residual serum of the second and third patients. The serum was macroscopically clear and not grossly contaminated in both cases. Five out of the nine eyes that were successes had epithelial breakdown on cessation of the serum. In two out of the five, reepithelialisation occurred with conventional therapy (without serum). Healing occurred only with reinitiation of serum in another. In the other two eyes, epithelial breakdown coincided with the starting of intensive topical RB 1 2 months Duration of use Side evects Other Rx at same time Other Rx previously a 1 R 52 F Linear * * * 3 weeks Itch, pain and V, H IgA discharge 2 R 79 F OCP b 7 months Allergic to CAP H 3 R 52 F OCP month H 3 L OCP month H 4 R 55 F Sjøgren * * * 1 day Small peripheral sterile infiltrate with overlying defect 5 R 73 F Sjøgren * * * 1 week Swelling and V, A redness 6 R 69 F Sjøgren months 7 R 55 F Sjøgren month H, SC, SR 8 R 45 F SJS b 3 months SR 9 R 41 M SJS weeks A 9 L SJS weeks A a = All patients had puncti occluded and were on 10 minute to 2 hourly hypromellose or saline (without preservative) drops and twice to four times daily ointment. b = continued drops for more than 2 months. 1 = National Eye Institute Rose Bengal scoring system (0 18). 2 = National Eye Institute Fluorescein scoring system (0 15). RB = rose bengal score, Fluo = fluorescein score, Subj = subjective score, Rx = treatment, V = viscotears, A = acetylcysteine, H = hyaluronic acid (Healon), SC = scleral contact lens, SR = silicone rubber contact lens. OCP = ocular cicatricial pemphigoid, SJS = Stevens-Johnson syndrome, CAP = chloramphenicol. *Patients felt worse and stopped serum drops. They were not available for review at time of discomfort while they were still on serum. At time of review a week or more later the findings were the same as the pretreatment parameters. medications that took place at the same time as cessation of serum. One patient started amphotericin (0.15%) hourly day and night for candida keratitis. The other started ofloxacin drops four times a day and dexamethasone 2 hourly by day for treatment of anterior segment inflammation secondary to infective posterior scleritis. The patient with candida keratitis required a therapeutic graft and then a conjunctival flap to stabilise the ocular surface. The other patient had a PED at last review, 2 weeks after the start of the antibiotics. Serum was not recommenced in these two patients. KERATOCONJUNCTIVITIS SICCA GROUP Patients with KCS had symptoms of dry eyes for more than 1 year. All had been treated with intensive topical lubricants consisting of frequent unpreserved drops and ointment. In addition their puncta were all occluded either by the disease process or surgically. One had tried silicone rubber contact lenses for comfort and five had tried 0.1% sodium hyaluronate drops with limited success. Of the 11 eyes with KCS, six had improved subjective scores, five had improved rose bengal scores, and six had improved fluorescein scores after the use of serum drops. Patients with decreased discomfort noted an improvement within 48 hours after serum was started. Table 3 is a summary of the patients and their scores. Figure 2 demonstrates the beneficial evect of serum on one patient. In the KCS group, assuming that those who stopped the drops because of discomfort had increased subjective scores, five patients had worse symptomatic and objective scores while on serum drops. One patient with rheumatoid arthritis developed a marginal infiltrate and an overlying epithelial defect after 1 day of use. Another with Stevens-Johnson syndrome had increased ocular surface fluorescein staining. Two patients complained of increased discomfort and stopped the serum. However, when reviewed in the clinic 1 week after cessation of serum, they essentially showed no
6 Autologous serum eyedrops for dry eyes and epithelial defects 1193 Figure 2 A 52 year old female with OCP, bilateral KCS, and left PK. Bilateral epitheliopathy was secondary to KCS. (A) and (B) show right and left eyes respectively. Serum drops were started and (C) and (D) were taken at 2 week review. Despite the absence of fluorescein in (C) and (D), there are obviously fewer surface irregularities and episcleral injection in the second set of photographs. Both objective and subjective scores had improved. change in their clinical picture. One patient had an allergic reaction to chloramphenicol and was successfully treated with 100% serum. In eight of the KCS eyes 50% serum drops were used and three had improved subjective and/or objective scores. The remaining three KCS eyes had 100% drops and they all had improved subjective and/or objective scores. The patient who was allergic to chloramphenicol was changed from 100% serum to 50% serum diluted with normal saline and she was able to detect the diverence and insisted on being restarted on 100% serum. Patients who were benefiting from serum were weaned ov serum at 2 months to reduce the demands on clinical resources. In two patients the clinical circumstances required serum to be continued for more than 2 months. All other patients reported decreased comfort after cessation and were overed recommencement of serum if symptoms and signs deteriorated significantly. POST-PENETRATING KERATOPLASTY GROUP The two patients who used serum eye drops post-pk had stable and intact epithelium at 1 week. Cessation of serum drops at 1 month postoperatively led to deterioration in the subjective and objective scores in both patients. One patient with graft versus host disease had two previous graft failures from epithelial instability and secondary perforations. Serum drops were started after the third PK. At 1 month postoperatively serum drops were stopped. Although she was on hourly unpreserved hypromellose (0.3%) and Lacrilube ointment four times a day, she developed an epithelial defect that was unchanged at 2 weeks. It healed within 21 days after reinstitution of serum drops. The other patient had Stevens-Johnson syndrome and perforated secondary to a PED, which required a tectonic PK. Serum was commenced immediately postoperatively and was weaned at 1 month. Fluorescein score at 1 month postoperatively, while he was on serum was less than 4 out of a possible 15. At review 1 month after cessation of serum, the score was more than 10. Although there was more corneal epithelial fluorescein staining after cessation of serum, the epithelium was maintained with intensive lubrication. IN VITRO TOXICITY STUDIES The live/dead fluorescent staining following incubation with culture medium (negative control) revealed that the cultured primary human corneal epithelial cells all remained viable. Incubation with 50% serum drops (Fig 3A) preserved the cell membrane integrity much better than with unpreserved hydroxypropylmethylcellulose 0.3% (Fig 3B). The mean reduction in cellular viability as measured with the ATP assay for the various test substances is given in Table 4. Fifty per cent serum reduced cell viability significantly less than unpreserved hypromellose (p = 0.04). The following trends were present: 100% serum seemed to maintain cellular ATP levels better than 50% serum (p = 0.11) and serum of patients on systemic immunosuppressive therapy seemed to be less toxic than serum of healthy normal individuals (p = 0.08) but neither finding was statistically significant.
7 1194 Poon, Geerling, Dart, et al Figure 3 Human corneal epithelial cells after 2 hours incubation with (A) hypromellose 0.3% and (B) 50% serum drops. The green fluorescent calcein demonstrates viable intracellular esterase activity. The red fluorescence results from ethidium-homodimer bound to nucleic acids as a consequence of a pathological increase in cell membrane permeability. Discussion The favourable result associated with the use of autologous serum in patients with dry eyes and persistent epithelial defect has been described by various authors This pilot study supports those claims. Epithelial defects in nine out of 15 eyes healed during treatment with autologous serum. In all 15 eyes previous treatment with antibiotic ointment four times a day had failed. Eleven out of 15 had failed to heal with an eyelid closing procedure (botulinum induced ptosis, lateral tarsorrhaphy), and/or therapeutic contact lens application. The average duration of PED before the use of serum drops was 48 days. Healing occurred at a mean of 29 days and a median of 28 days in the successful cases. Epithelial defects recurred in five eyes after cessation of the serum. Figure 1 shows a patient s eye whose corneal epithelial healing appear to depend on the use of serum drops. In two patients, serum was used after tectonic penetrating keratoplasty performed for perforations secondary to PED. Both patients had poor ocular surfaces and aqueous tear deficiency and had a stable epithelium 1 week postoperatively. Both experienced deterioration when serum was stopped. For the KCS patients, six out of 11 eyes had improved subjective and fluorescein scores and five had improved rose bengal scores. All patients were on maximal conventional therapy for KCS. In five eyes previous topical sodium hyaluronate (0.1%) application had not given satisfactory relief. Four out of these five patients stated that serum gave more relief. Fetal calf serum is extensively used in the laboratory to promote cell growth in culture. Tsubota et al showed that fetal calf serum accelerates the migration of corneal epithelial cells in vitro. 3 Serum also has been used for treating canine corneal ulcers with success. 26 The reason why serum is of benefit to the ocular surface is not entirely clear, but epidermal growth factor (EGF) is present in both tears and serum 4 and has been found to be helpful in healing of traumatic epithelial Table 4 Results of ATP assay: mean percentage of cell viability reduction (SD) following 2 hours of incubation with test agent. 50% serum reduced the viability of cultured cells significantly less than unpreserved hypromellose (p = 0.04). 100% serum maintained cellular ATP levels better than 50% serum (p = 0.11) and serum of patients on systemic immunosuppressive therapy was less toxic than serum of healthy normal individuals (p = 0.08) although these trends did not reach statistical significance % Viability reduction p Value (in comparison with negative control) p Value for other tests Hypromellose 0.3% unpreserved (n = 4 tests) 87 (14) % serum (serum diluted 1:1 v/v with 0.5% chloramphenicol eye 58 (25) (v Hypromellose 0.3%) drops,n=5patients) 100% serum of normal individual (n = 6) 34 (10) (v 50% serum) 100% serum of patients with dry eyes treated with autologous 24 (22) 0.72 serum (n = 4) 100% serum of immunosuppressed patients (n = 8) 26 (7) (v serum of normal individuals)
8 Autologous serum eyedrops for dry eyes and epithelial defects 1195 abrasions EGF may also facilitate epithelialisation because of its anti-apoptotic properties Acidic and basic fibroblast growth factors (afgf, bfgf) were found to speed up healing of epithelial defects in rabbits. 20 Fibronectin, another component of serum, has also been found to have some evect in helping epithelialisation but a randomised control trial showed no significant evect compared to placebo except in eyes with large baseline defects (10 mm 2 or more). 10 Vitamin A is found in much higher concentrations in serum compared to tears and may decrease the progression to squamous metaplasia in KCS. 4 Mucin expression by cultured conjunctival epithelial cells is upregulated by human serum 4 and this may contribute to the beneficial evects of serum in KCS. Serum antiproteases such as α 2 macroglobulin have been thought to inhibit corneal collagenases and be beneficial in conditions such as alkali burns. 29 Neural factors such as substance P are important for corneal epithelial migration. 18 In neurotrophic ulcers, these substances are decreased and may be supplemented by topical serum. Stability data on serum are limited but Tsubota reported levels of EGF, TGF, and vitamin A in 20% serum (diluted with saline) and 100% serum remained stable at 4 C for at least 1 month and at 20 C for at least 3 months. 3 4 In dry eyes pharmaceutical products are used to substitute the deficient tear film. In the majority of patients they are suycient to relieve symptoms and to prevent progressive ocular surface disease. However, in absolute tear deficiency pharmaceutical tear substitutes are often inevective in controlling the symptoms and signs of the disease. Despite their frequent application epithelial defects may occur, persist, or even progress to corneal perforation, as was the case in the patients of the post-pk group in the current study. Our in vitro observations confirm that pharmaceutical tear substitutes, such as unpreserved hydroxypropylmethylcellulose 0.3%, are inappropriate to maintain intracellular ATP levels and cell membrane integrity of cultured human corneal epithelial cells. Both are well established parameters of cellular viability and/or proliferation for in vitro evaluations of toxicity. Certainly the complex physical and molecular interactions of tear film and ocular surface in vivo can not be substituted in vitro. In vivo studies are however much more diycult to standardise. Furthermore, physiological tear substitutes such as serum are usually reserved for the more severe aqueous deficient dry eyes. As the ocular surface epithelia are more susceptible to toxicity in this situation in vitro evaluations may be more appropriate Furthermore, the assay period was 2 hours and hence not long enough for a depletion of nutrients to be responsible for the relative decrease in viability observed with hypromellose. If the cells had been cultured at a similar density in phosphate buvered saline for his time period toxicity would not have been evident. Owing to the precautions taken in defining the culture model (for example, primary human epithelial cells, cultured under fully defined conditions) we believe that the model used here allows extrapolation to the acute toxicity of pharmaceutical and natural tear substitutes on human corneal epithelium in vivo. Pharmaceutical tear substitutes provide lubrication but not nutrition. Previous attempts to construct a physiological tear sub stitute focused on the ionic composition. Compared to the complex composition of serum such substitutes are very limited in their replacement of the nutritional component of endogenous tears. Our in vitro experiments show that serum, despite being diluted with chloramphenicol, is able to maintain the viability of human corneal epithelial cells in culture. This suggests that serum may have a therapeutic role in addition to a pharmaceutical role as a tear substitute. The concentration of serum in drops has varied with diverent studies. Fox used 30% serum diluted with normal saline and all of 15 patients had improved subjective and objective scores. 1 Tsubota suggested using 20% serum diluted with saline and achieved good results. 3 4 In this study we used 50% serum, and because of concern with microbial contamination of the serum bottles we used chloramphenicol as a diluent. Chloramphenicol was chosen because of its relatively low epithelial toxicity. In one patient 100% was much preferred compared to 50% serum diluted with unpreserved normal saline. In the KCS group three out of eight of those using 50% drops compared to three out of three of those using 100% drops had improved scores. This, as well as the finding that 100% serum inhibits cellular growth less than 50% serum diluted with 0.5% chloramphenicol, suggests a higher percentage of serum may give a greater evect. However the frequency of venepuncture and the amount of blood needed is doubled with the use of 100% drops. 55% of our KCS patients had improved scores. This is less than that found by Fox and Tsubota, 1 4 which may relate to diverences in the patient population or to the diverent composition of the dispensed drops. Three patients with PED developed infectious keratitis while on serum drops. Two of them were using 50% serum with chloramphenicol and the other was using 100% serum. All three patients had risk factors that may have contributed to the infection. One patient with rheumatoid arthritis and secondary KCS had a corneal graft with sutures in situ. The next patient had KCS and corneal graft with sutures in situ and had previously had an episode of microbial keratitis related to sutures. The third patient had KCS and a therapeutic contact lens was in use for corneal perforation secondary to persistent epithelial
9 1196 Poon, Geerling, Dart, et al defect. This patient had refused a tectonic PK. Contaminated serum bottles may have introduced bacteria to the compromised ocular surface. The potential for microbial growth in serum and the contamination of serum dropper bottles in clinical use has not been evaluated. Meanwhile we are using chloramphenicol with serum unless contraindicated by hypersensitivity. Chloramphenicol was chosen as it is our drug of choice in patients with PED for prophylaxis against microbial keratitis. No significant complications have been reported with the use of serum drops. There is one case report of immunoglobulin deposition in the cornea of a patient with persistent epithelial defect. 31 We did not encounter this in our series. However, one patient with rheumatoid arthritis did develop peripheral infiltration and ulceration after 1 day of use. This may be related to the use of serum. Rheumatoid factor and other antigens together with autoantibodies present in serum could theoretically cause immune complex deposition and secondary inflammation. Of the patients with KCS who responded to serum, the evect was rapid. Most claimed the evect was almost instantaneous and all said the symptoms were improved by 48 hours. For the PED patients who were successes, a positive response was usually seen by 2 weeks, but the average healing time was approximately 1 month. Serum drops have been shown to have low toxicity in vitro and have demonstrated encouraging results in patients with keratoconjunctivitis sicca and epithelial defects who have failed to respond to currently available treatment. Their use should also be considered in patients requiring penetrating or lamellar keratoplasty in whom problems with epithelial healing are anticipated. As this study was uncontrolled and unmasked, there were inherent problems in interpretation of the results. The true value of autologous serum requires a randomised controlled trial. Regulatory restrictions in the UK, because of the absence of standards regarding the manufacture of biological materials as eye drops, have currently precluded the use of a randomised controlled trial to assess the use of serum drops. Currently the results of uncontrolled studies on the evect of serum drops are favourable and several issues concerning the manufacturing and storage of the drops need resolution. These include quantification of the antimicrobial properties of serum to determine whether a preservative is needed, the minimum concentration required for an acceptable positive evect, optimum storage times and identification of the factors in serum that lead to the evect on epithelial healing. The goal of future studies is to emulate the evect of serum, for the management of this challenging group of disorders, without the need for biological materials. This work wassupported in part by funding from the NHS Executive (locally organised research grant 485), special trustees of Moorfields Eye Hospital, and the Deutsche Forschungsgemeinschaft (DFG Ge 895/4-1; the views expressed in this publication are those of the authors and not necessarily those of the funding bodies. 1 Fox RI, Chan R, Michelson J, et al. Beneficial evect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 1984;29: Tseng SCG, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol 1997;124: Tsubota K, Goto E, Shimmura S, et al. Treatment of persistent epithelial defect by autologous serum application. Ophthalmology 1999;106: Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren s syndrome. Br J Ophthalmol 1999;83: Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol 1996;122: Nishida T. Cornea. In: Krachmer, Mannis, Holland, Pally, eds. Cornea. St Louis: Mosby, 1998;1: Schultz GS, Davis JB, Eiferman RA. Growth factors and corneal epithelium. Cornea 1988;7: Ubels JL, Edelhauser HF, Austin KH. Healing of experimental corneal wounds treated with topically applied retinoids. Am J Ophthalmol 1983;95: Nishida T, Ohasai Y, Awata T, et al. Fibronectin a new therapy for corneal trophic ulcer. Arch Ophthalmol 1983; 101: Gordon JF, Johnson P, Musch DC. Topical fibronectin ophthalmic solution in the treatment of persistent defects of the corneal epithelium. Am J Ophthalmol 1995;119: Cluskey P, Wakefield D, York L. Topical fibronectin therapy in persistent corneal ulceration. Aust NZ J Ophthalmol 1987;15: Phan TM, Foster CS, BoruchoV SA, et al. Topical fibronectin in the treatment of persistent corneal epithelial defects and trophic ulcers. Am J Ophthalmol 1987;104: Eiferman R, Brightwell J, Rowsey J, et al. Acceleration of corneal epithelial resurfacing and keratocyte migration in primate epikeratophakia by biosynthetic human EGF. Invest Ophthalmol Vis Sci 1985;(suppl):26 14 Eiferman R, Schultz GS. Treatment of alkali burns in rabbits with epidermal growth factor. Invest Ophthalmol Vis Sci 1987;(suppl)28: Singh G, Foster CS. Epidermal growth factors in alkaliburned corneal epithelial wound healing. Am J Ophthalmol 1987;103: Reim M, Kehrer T, Lund M. Clinical application of epidermal growth factor in patients with severe eye burns. Ophthalmologica 1988;197: Scardovi C, De Felice GP, Gazzaniga A. Epidermal growth factor in the topical treatment of traumatic corneal ulcers. Ophthalmologica 1993;206: Nishida T, Nakamura M, Ofuji K, et al. Synergistic evects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol 1996;169: Pastor JC, Calonge M. Epidermal growth factor and corneal wound healing: a multicenter study. Cornea 1992;11: Fredj-Reygrobellet D, Plouet J, Delayre T, et al. EVects of afgf and bfgf on wound healing in rabbit corneas. Curr Eye Res 1987;6: Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J 1995;21: Nakamura M, Nishida T, Mishima H, et al. EVects of antimicrobials on corneal epithelial migration. Curr Eye Res 1993;12: Berry M, Gurung A, Easty DL. Toxicity of antibiotics and antifungals on cultured human corneal cells: evect of mixing, exposure and concentrations. Eye 1995;9: Daniels JT, Harris IR, Kearney JN, et al. Calcium: a crucial consideration in serum-free keratinocyte culture. Exp Dermatol 1995;4: Nelson JD, Drake MM, Brewer JT, et al. Evaluation of a physiological tear substitute in patients with keratoconjunctivitis sicca. In: Sullivan DA, ed. Lacrimal gland, tear film, and dry eye syndromes. New York: Plenum Press, 1994: Kirschner SE. Persistent corneal ulcers. What to do when ulcers won t heal. Vet Clin N Am Small Anim Pract 1990;20: Collins MK, Perkins GR, Rodriguez Tarduchy G, et al. Growth factors as survival factors: regulation of apoptosis. Bioessays 1994;16: Rodeck U, Jost M, Kari C, et al. EGF-R dependent regulation of keratinocyte survival. J Cell Sci 1997;110: Berman MB. Collagenase inhibitors: rationale for their use in treating corneal ulceration. Int Ophthalmol Clin 1975;15: Pasternak AS, Miller WM. First-order toxicity assays for eye irritation using cell lines: parameters that avect in vitro evaluation. Fundam Appl Toxicol 1995;25: Wang XM. A new microcellular cytotoxicity test based on calcein AM release. Human Immunol 1993;37:
10 Autologous serum eyedrops for dry eyes and epithelial defects Avisar R, Creter D, Levinsky H, et al. Comparative study of tear substitutes and their immediate evect on the precorneal tear film. Isr J Med Sci 1997;33: Gobbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology 1992;99: Burstein NL. The evects of topical drugs and preservatives on the tears and corneal epithelium in dry eye. Trans Ophthalmol Soc UK 1985;104(Pt 4): What's in the next issue Future content 35 Ubbels JL, Williams KK, Lopez Bernal D, et al. Evaluation of evects of a physiologic artificial tear on the corneal epithelial barrier: lectrical resistance and carboxyfluorescein permeability. In: Sullivan DA, ed. Lacrimal gland, tear film, and dry eye syndromes. New York: Plenum Press, 1994: McDonnell PJ, Schanzlin DJ, Rao NA. Immunoglobulin deposition in the cornea after application of autologous serum. Arch Ophthalmol 1988;106: See which articles have just been accepted for publication and preview the table of contents for the next issue a month before it is published Br J Ophthalmol: first published as /bjo on 1 October Downloaded from on 10 September 2018 by guest. Protected by copyright.
JMSCR Vol 07 Issue 04 Page April 2019
 www.jmscr.igmpublication.org Index Copernicus Value: 79.54 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v7i4.19 Original Research Article Use of Plasma Rich in Growth Factors
www.jmscr.igmpublication.org Index Copernicus Value: 79.54 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v7i4.19 Original Research Article Use of Plasma Rich in Growth Factors
Novel therapies for the treatment of persistent corneal epithelial defects
 Novel therapies for the treatment of persistent corneal epithelial defects Disclosures I have no financial interests in any of the techniques or products discussed. Bennie H. Jeng, M.D. Associate Professor
Novel therapies for the treatment of persistent corneal epithelial defects Disclosures I have no financial interests in any of the techniques or products discussed. Bennie H. Jeng, M.D. Associate Professor
Subject Index. Atopic keratoconjunctivitis (AKC) management 16 overview 15
 Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary
 Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,4 Agent Indication Dosage and Administration Restasis (cyclosporine ophthalmic emulsion)
Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,4 Agent Indication Dosage and Administration Restasis (cyclosporine ophthalmic emulsion)
A tear in the eye is a jewel.
 Long-term use of autologous serum eye drops for the treatment of dry eye disease Munira T. Hussain MS program, CLRA 692 Advisor: Stephen Sonstein A tear in the eye is a jewel. -Arabian proverb BACKGROUND
Long-term use of autologous serum eye drops for the treatment of dry eye disease Munira T. Hussain MS program, CLRA 692 Advisor: Stephen Sonstein A tear in the eye is a jewel. -Arabian proverb BACKGROUND
Trifluridine Ophthalmic Solution, 1% Sterile
 Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
rhngf for neurotrophic keratitis first line
 September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Medical Affairs Policy
 Medical Affairs Policy Service: Corneal Treatments and Specialized Contact Lenses (Corneal remodeling, Corneal transplant, Corneal collagen crosslinking, Intrastromal Rings- INTACS, Keratoconus treatments,
Medical Affairs Policy Service: Corneal Treatments and Specialized Contact Lenses (Corneal remodeling, Corneal transplant, Corneal collagen crosslinking, Intrastromal Rings- INTACS, Keratoconus treatments,
Learning Objectives. Disclosures 2/2/ BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD
 2015 BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD Tueng T. Shen, M.D., Ph.D. Professor of Ophthalmology Adjunct, Bioengineering and Global Health Feb. 13 th, 2015 Learning
2015 BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD Tueng T. Shen, M.D., Ph.D. Professor of Ophthalmology Adjunct, Bioengineering and Global Health Feb. 13 th, 2015 Learning
What are some common conditions that affect the cornea?
 What are some common conditions that affect the cornea? Injuries After minor injuries or scratches, the cornea usually heals on its own. Deeper injuries can cause corneal scarring, resulting in a haze
What are some common conditions that affect the cornea? Injuries After minor injuries or scratches, the cornea usually heals on its own. Deeper injuries can cause corneal scarring, resulting in a haze
Amniotic Membrane Transplantation In Ocular Surface Disorders
 Orginal Article Amniotic Membrane Transplantation In Ocular Surface Disorders Khalid Iqbal Talpur, Faiz Muhammad Halepota, Muhammad Pak J Ophthalmol 2005, Vol. 22 No. 3.................................................................................................
Orginal Article Amniotic Membrane Transplantation In Ocular Surface Disorders Khalid Iqbal Talpur, Faiz Muhammad Halepota, Muhammad Pak J Ophthalmol 2005, Vol. 22 No. 3.................................................................................................
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution)
 VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
THERAPEUTIC CONTACT LENSES
 THERAPEUTIC CONTACT LENSES Prof. Univ. Dr. Adriana Stanila Victor Papilian Faculty of Medicine Emergency Academic Hospital Sibiu Ocular Surface Research Center ROMANIA INTRODUCTION therapeuein greac =
THERAPEUTIC CONTACT LENSES Prof. Univ. Dr. Adriana Stanila Victor Papilian Faculty of Medicine Emergency Academic Hospital Sibiu Ocular Surface Research Center ROMANIA INTRODUCTION therapeuein greac =
Treatment of dry eye by autologous serum application in Sjögren s syndrome
 390 Br J Ophthalmol 1999;83:390 395 ORIGINAL ARTICLES Clinical science Ophthalmology, Tokyo Dental College, Chiba, Japan K Tsubota E Goto H Fujita M Ono H Inoue S Shimmura Ophthalmology, Keio University
390 Br J Ophthalmol 1999;83:390 395 ORIGINAL ARTICLES Clinical science Ophthalmology, Tokyo Dental College, Chiba, Japan K Tsubota E Goto H Fujita M Ono H Inoue S Shimmura Ophthalmology, Keio University
NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY
 NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY Authors: Prof univ. dr. Adriana Stănilă, Dr. Elena Mihai, Dr. Adrian Teodoru, Dr. IonuŃ Costache The Clinical Department of Op
NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY Authors: Prof univ. dr. Adriana Stănilă, Dr. Elena Mihai, Dr. Adrian Teodoru, Dr. IonuŃ Costache The Clinical Department of Op
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
CORNEAL CONDITIONS CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
Ophthalmology Times Case Study Yasmin Mali, MD. Case Study
 Ophthalmology Times Case Study Yasmin Mali, MD Case Study A 57 year old female with presented with ocular irritation and discomfort in both eyes for several months. Patient was previously started on a
Ophthalmology Times Case Study Yasmin Mali, MD Case Study A 57 year old female with presented with ocular irritation and discomfort in both eyes for several months. Patient was previously started on a
Blood Derived Therapies in Refractory Ocular Surface Disease
 Blood Derived Therapies in Refractory Ocular Surface Disease John E. Conto, OD, FAAO, ABCMO Assistant Professor of Ophthalmology and Visual Science Please silence all mobile devices and remove items from
Blood Derived Therapies in Refractory Ocular Surface Disease John E. Conto, OD, FAAO, ABCMO Assistant Professor of Ophthalmology and Visual Science Please silence all mobile devices and remove items from
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye...
 Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
Page 1 RED EYES. conjunctivitis keratitis episcleritis / scleritis. Frank Larkin Moorfields Eye Hospital. acute glaucoma anterior uveitis
 The RED EYE and ALLERGIC EYE DISEASE DIAGNOSIS & MANAGEMENT Frank Larkin Moorfields Eye Hospital RED EYES conjunctivitis keratitis episcleritis / scleritis acute glaucoma anterior uveitis post-op. / trauma
The RED EYE and ALLERGIC EYE DISEASE DIAGNOSIS & MANAGEMENT Frank Larkin Moorfields Eye Hospital RED EYES conjunctivitis keratitis episcleritis / scleritis acute glaucoma anterior uveitis post-op. / trauma
Bringing tears to your eyes: Serum Eyedrops. DR Akila CHANDRASEKAR Consultant in Transfusion Medicine NHS Blood & Transplant
 Bringing tears to your eyes: Serum Eyedrops DR Akila CHANDRASEKAR Consultant in Transfusion Medicine NHS Blood & Transplant Disclaimer This talk will focus on service provision by NHSBT No claims made
Bringing tears to your eyes: Serum Eyedrops DR Akila CHANDRASEKAR Consultant in Transfusion Medicine NHS Blood & Transplant Disclaimer This talk will focus on service provision by NHSBT No claims made
Ocular Surface Management in Corneal Transplantation, a Review
 CLINICAL INVESTIGATIONS Ocular Surface Management in Corneal Transplantation, a Review Kazuo Tsubota* *Departments of Ophthalmology, Tokyo Dental College, Chiba, and Keio University School of Medicine,
CLINICAL INVESTIGATIONS Ocular Surface Management in Corneal Transplantation, a Review Kazuo Tsubota* *Departments of Ophthalmology, Tokyo Dental College, Chiba, and Keio University School of Medicine,
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress MANAGEMENT OF CORNEAL ULCERS IN SMALL ANIMALS Robin G Stanley, BVSc(Hons), FACVSc-Ophthalmology Animal Eye Care
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress MANAGEMENT OF CORNEAL ULCERS IN SMALL ANIMALS Robin G Stanley, BVSc(Hons), FACVSc-Ophthalmology Animal Eye Care
Gas Permeable Scleral Contact Lens. Description
 Subject: Gas Permeable Scleral Contact Lens Page: 1 of 6 Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 3 Gas Permeable
Subject: Gas Permeable Scleral Contact Lens Page: 1 of 6 Last Review Status/Date: December 2013 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 3 Gas Permeable
Dry Eye Assessment and Management Study ELIGIBILITY OCULAR EVALUATION FORM
 Page 1 of 13 BEFORE COMPLETING THE OCULAR EXAMINATION, YOU MUST BE ABLE TO ANSWER YES TO THE FOLLOWING QUESTIONS: Have you done MMP9? (SVonly) The Following are done at Baseline: Have you done Tear Osmolarity?
Page 1 of 13 BEFORE COMPLETING THE OCULAR EXAMINATION, YOU MUST BE ABLE TO ANSWER YES TO THE FOLLOWING QUESTIONS: Have you done MMP9? (SVonly) The Following are done at Baseline: Have you done Tear Osmolarity?
D amage to the ocular surface in Sjögren s syndrome (SS)
 1279 EXTENDED REPORT Albumin as a tear supplement in the treatment of severe dry eye S Shimmura, R Ueno, Y Matsumoto, E Goto, A Higuchi, J Shimazaki, K Tsubota... See end of article for authors affiliations...
1279 EXTENDED REPORT Albumin as a tear supplement in the treatment of severe dry eye S Shimmura, R Ueno, Y Matsumoto, E Goto, A Higuchi, J Shimazaki, K Tsubota... See end of article for authors affiliations...
Strategies for Anterior Segment Disease Management Mile Brujic, OD, FAAO 1409 Kensington Blvd Bowling Green, OH
 Strategies for Anterior Segment Disease Management Mile Brujic, OD, FAAO 1409 Kensington Blvd Bowling Green, OH 43402 brujic@prodigy.net 419-261-9161 Summary As optometry s scope of practice continues
Strategies for Anterior Segment Disease Management Mile Brujic, OD, FAAO 1409 Kensington Blvd Bowling Green, OH 43402 brujic@prodigy.net 419-261-9161 Summary As optometry s scope of practice continues
Dry eye syndrome. Lacrimal gland. Tear duct into nose. 1 of 6
 Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
Advanced Diagnosis and Management of OSD and Tear Dysfunction
 Advanced Diagnosis and Management of OSD and Tear Dysfunction Arthur B. Epstein, OD, FAAO Phoenix, Eye Care & The Dry Eye Center of Arizona Phoenix, AZ, USA Our understanding of the ocular surface has
Advanced Diagnosis and Management of OSD and Tear Dysfunction Arthur B. Epstein, OD, FAAO Phoenix, Eye Care & The Dry Eye Center of Arizona Phoenix, AZ, USA Our understanding of the ocular surface has
Breaking the Cycle. Yijie (Brittany) Lin, MD, MBA, Reena Garg, MD New York Eye and Ear Infirmary of Mount Sinai
 Lin, Garg Ophthalmology Times 1 Breaking the Cycle Yijie (Brittany) Lin, MD, MBA, Reena Garg, MD New York Eye and Ear Infirmary of Mount Sinai Abstract A 32 year-old female with a history of LASIK surgery
Lin, Garg Ophthalmology Times 1 Breaking the Cycle Yijie (Brittany) Lin, MD, MBA, Reena Garg, MD New York Eye and Ear Infirmary of Mount Sinai Abstract A 32 year-old female with a history of LASIK surgery
T a variety of ocular disorders with
 ACTA OPHTHALMOLOGICA SCANDINAVICA 1997 - A comparative study of polyacrylic acid (Viscotears@) liquid gel versus polyvinylalcohol in the treatment of dry eyes J. Brodwall', G. Alme', S. Gedde-Dahl', J.
ACTA OPHTHALMOLOGICA SCANDINAVICA 1997 - A comparative study of polyacrylic acid (Viscotears@) liquid gel versus polyvinylalcohol in the treatment of dry eyes J. Brodwall', G. Alme', S. Gedde-Dahl', J.
Sclerokeratoplasty David S. Chu, M.D. Cases
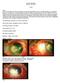 Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Dry Eye Disease Diagnosis and Treatment Pearls from the Trenches (2 hours) Mile Brujic, O.D Kensington Blvd. Bowling Green, OH 43402
 Dry Eye Disease Diagnosis and Treatment Pearls from the Trenches (2 hours) Mile Brujic, O.D. 1409 Kensington Blvd. Bowling Green, OH 43402 Summary As our understanding of dry eye disease has evolved, so
Dry Eye Disease Diagnosis and Treatment Pearls from the Trenches (2 hours) Mile Brujic, O.D. 1409 Kensington Blvd. Bowling Green, OH 43402 Summary As our understanding of dry eye disease has evolved, so
Post-LASIK infections
 Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
This indication includes the temporary relief of burning, irritation, and/or discomfort due to dryness of the eye.
 Page 1 of 5 SCHEDULING STATUS Schedule 0 PROPRIETARY NAME AND DOSAGE FORM REFRESH LIQUIGEL COMPOSITION REFRESH LIQUIGEL lubricant eye drops contain carboxymethylcellulose sodium 10 mg/ml. Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 0 PROPRIETARY NAME AND DOSAGE FORM REFRESH LIQUIGEL COMPOSITION REFRESH LIQUIGEL lubricant eye drops contain carboxymethylcellulose sodium 10 mg/ml. Preservative:
Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns
 1 Cornea and Refractive Surgery Services, Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India 2 Department of Biostatistics, All India Institute
1 Cornea and Refractive Surgery Services, Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India 2 Department of Biostatistics, All India Institute
Corporate Presentation NASDAQ: EYEG
 Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
Dr Jo-Anne Pon. Dr Sean Every. 8:30-9:25 WS #70: Eye Essentials for GPs 9:35-10:30 WS #80: Eye Essentials for GPs (Repeated)
 Dr Sean Every Ophthalmologist Southern Eye Specialists Christchurch Dr Jo-Anne Pon Ophthalmologist Southern Eye Specialists, Christchurch Hospital, Christchurch 8:30-9:25 WS #70: Eye Essentials for GPs
Dr Sean Every Ophthalmologist Southern Eye Specialists Christchurch Dr Jo-Anne Pon Ophthalmologist Southern Eye Specialists, Christchurch Hospital, Christchurch 8:30-9:25 WS #70: Eye Essentials for GPs
Acute Eyes for ED. Enis Kocak. The Alfred Ophthalmology
 Acute Eyes for ED Enis Kocak The Alfred Ophthalmology The problem with eyes Things to cover Ocular anatomy Basic assessment Common presentations Eye first aid and procedures Ophthalmic emergencies What
Acute Eyes for ED Enis Kocak The Alfred Ophthalmology The problem with eyes Things to cover Ocular anatomy Basic assessment Common presentations Eye first aid and procedures Ophthalmic emergencies What
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
Package Leaflet - Information for the User. TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone
 Package Leaflet - Information for the User TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone Read all of this leaflet carefully before you start using this medicine because it
Package Leaflet - Information for the User TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone Read all of this leaflet carefully before you start using this medicine because it
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only
 Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Paracentral Corneal Melting in Four Patients With Chronic Graft-Versus-Host Disease
 Paracentral Corneal Melting in Four Patients With Chronic Graft-Versus-Host Disease Neil Chungfat, MD, MEng 1,2 ; Jocelyn Rowe, MD 1,3 ; Neal P. Barney, MD 1 ; Sarah Nehls, MD 1 The authors have no financial
Paracentral Corneal Melting in Four Patients With Chronic Graft-Versus-Host Disease Neil Chungfat, MD, MEng 1,2 ; Jocelyn Rowe, MD 1,3 ; Neal P. Barney, MD 1 ; Sarah Nehls, MD 1 The authors have no financial
EYE CARE PROTOCOL FOR PATIENTS IN ITU
 EYE CARE PROTOCOL FOR PATIENTS IN ITU Back to contents Developed by SUE LIGHTMAN PROFESSOR OF CLINICAL OPHTHALMOLOGY/CONSULTANT OPHTHALMOLOGIST MOORFIELDS EYE HOSPITAL Amended for UCLU ICU by Caroline
EYE CARE PROTOCOL FOR PATIENTS IN ITU Back to contents Developed by SUE LIGHTMAN PROFESSOR OF CLINICAL OPHTHALMOLOGY/CONSULTANT OPHTHALMOLOGIST MOORFIELDS EYE HOSPITAL Amended for UCLU ICU by Caroline
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
LIMBAL TRANSPLANTATION IN THE MANAGEMENT OF CHRONIC CONTACT-LENS-ASSOCIATED EPITHELIOPATHY
 LIMBAL TRANSPLANTATION IN THE MANAGEMENT OF CHRONIC CONTACT-LENS-ASSOCIATED EPITHELIOPATHY CHRISTOPHER JENKINS, STEPHEN TUFT, CHRISTOPHER LIU and ROGER BUCKLEY London SUMMARY We describe the clinical management
LIMBAL TRANSPLANTATION IN THE MANAGEMENT OF CHRONIC CONTACT-LENS-ASSOCIATED EPITHELIOPATHY CHRISTOPHER JENKINS, STEPHEN TUFT, CHRISTOPHER LIU and ROGER BUCKLEY London SUMMARY We describe the clinical management
Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device.
 Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device. Effective Date: 2/23/2005 Version 1 ProKera is a corneal-epithelial device consisting of an ophthalmic conformer
Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device. Effective Date: 2/23/2005 Version 1 ProKera is a corneal-epithelial device consisting of an ophthalmic conformer
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ALCAINE TM proxymetacaine hydrochloride Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alcaine Eye Drops contain the active ingredient proxymetacaine
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ALCAINE TM proxymetacaine hydrochloride Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alcaine Eye Drops contain the active ingredient proxymetacaine
ICD-10 Coding for Contact Lens Problems. The EyeCodingForum.com
 ICD-10 Coding for Contact Lens Problems The EyeCodingForum.com Jeffrey Restuccio, CPC, CPC-H, MBA Memphis TN (901) 517-1705 jeff@eyecodingforum.com www.eyecodingforum.com EyeCodingForum.com 1 Coding for
ICD-10 Coding for Contact Lens Problems The EyeCodingForum.com Jeffrey Restuccio, CPC, CPC-H, MBA Memphis TN (901) 517-1705 jeff@eyecodingforum.com www.eyecodingforum.com EyeCodingForum.com 1 Coding for
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
History. Examination. Diagnosis/Course
 History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
Mustard Gas Induced Ocular Surface Disorders
 Challenging Case Mustard Gas Induced Ocular Surface Disorders Section Editor: Alireza Baradaran-Rafii, MD Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran Sulfur
Challenging Case Mustard Gas Induced Ocular Surface Disorders Section Editor: Alireza Baradaran-Rafii, MD Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran Sulfur
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Learning Objectives:
 Viral keratitis and antivirals Learning Objectives: Recognise and distinguish different types of viral keratitis HSV HZO Adenovirus Discuss the use of antiviral agents in the treatment of herpetic infections
Viral keratitis and antivirals Learning Objectives: Recognise and distinguish different types of viral keratitis HSV HZO Adenovirus Discuss the use of antiviral agents in the treatment of herpetic infections
Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.]
![Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.]](/thumbs/94/118693790.jpg) Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Description VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment
Viroptic (trifluridine) solution [Monarch Pharmaceuticals, Inc.] Description VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PAINFUL PAINLESS Contact lens user BOV
 Common Causes Allergies Infections Ocular Cornea, uveitis, endophthalmitis Orbital Orbital cellulitis Inflammation Uveitis Scleritis / episcleritis Glaucomas Trauma Foreign bodies Chemical injuries History
Common Causes Allergies Infections Ocular Cornea, uveitis, endophthalmitis Orbital Orbital cellulitis Inflammation Uveitis Scleritis / episcleritis Glaucomas Trauma Foreign bodies Chemical injuries History
Clinical Study Topical 100% Serum Eye Drops for Treating Corneal Epithelial Defect after Ocular Surgery
 BioMed Research International Volume 2013, Article ID 521315, 7 pages http://dx.doi.org/10.1155/2013/521315 Clinical Study Topical 100% Serum Eye Drops for Treating Corneal Epithelial Defect after Ocular
BioMed Research International Volume 2013, Article ID 521315, 7 pages http://dx.doi.org/10.1155/2013/521315 Clinical Study Topical 100% Serum Eye Drops for Treating Corneal Epithelial Defect after Ocular
Cornea & External Disease research at Moorfields
 Recruiting Research Studies Cornea & External Disease research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
Recruiting Research Studies Cornea & External Disease research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
CONTRAINDICATIONS None.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PAZEO safely and effectively. See full prescribing information for PAZEO. PAZEO (olopatadine hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PAZEO safely and effectively. See full prescribing information for PAZEO. PAZEO (olopatadine hydrochloride
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE
 REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
BY N. N. SOODt AND V. J. MARMION- St. Paul's Eye Hospital, Liverpool
 Brit. J. Ophthal. (1964) 48, 609. SUPERFICIAL HERPETIC KERATITIS TREATED WITH 5-IODO-2'-DEOXYURIDINE* BY N. N. SOODt AND V. J. MARMION- St. Paul's Eye Hospital, Liverpool THE results of treating herpetic
Brit. J. Ophthal. (1964) 48, 609. SUPERFICIAL HERPETIC KERATITIS TREATED WITH 5-IODO-2'-DEOXYURIDINE* BY N. N. SOODt AND V. J. MARMION- St. Paul's Eye Hospital, Liverpool THE results of treating herpetic
MAXITROL* Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment, USP 3.5 mg (as neomycin sulfate), 6000 IU/g, 0.
 PRESCRIBING INFORMATION Pr MAXITROL* Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment, USP 3.5 mg (as neomycin sulfate), 6000 IU/g, 0.1% w/w Pr MAXITROL* Neomycin and Polymyxin B
PRESCRIBING INFORMATION Pr MAXITROL* Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment, USP 3.5 mg (as neomycin sulfate), 6000 IU/g, 0.1% w/w Pr MAXITROL* Neomycin and Polymyxin B
Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work?
 See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282816021 Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work? Conference
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282816021 Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work? Conference
PATIENT INFORMATION ON CORNEAL GRAFT
 PATIENT INFORMATION ON CORNEAL GRAFT (TRANSPLANT) SURGERY M ANANDAN What is the cornea? The clear window of the eye approximately 0.5mm thick and 12mm across. It lies in front of the fluid filled anterior
PATIENT INFORMATION ON CORNEAL GRAFT (TRANSPLANT) SURGERY M ANANDAN What is the cornea? The clear window of the eye approximately 0.5mm thick and 12mm across. It lies in front of the fluid filled anterior
MATERIALS AND METHODS
 Clinical Trials Evaluation of Cord Therapy in Acute Ocular Chemical Burns Namrata Sharma, 1 Manik Goel, 1 Thirumurthy Velpandian, 1 Jeewan S. Titiyal, 1 Radhika Tandon, 1 and Rasik B. Vajpayee 1,2,3 From
Clinical Trials Evaluation of Cord Therapy in Acute Ocular Chemical Burns Namrata Sharma, 1 Manik Goel, 1 Thirumurthy Velpandian, 1 Jeewan S. Titiyal, 1 Radhika Tandon, 1 and Rasik B. Vajpayee 1,2,3 From
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML-NEO Liquifilm Ophthalmic Suspension COMPOSITION FML-NEO Liquifilm Ophthalmic Suspension contains per ml: Fluorometholone 1,0
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML-NEO Liquifilm Ophthalmic Suspension COMPOSITION FML-NEO Liquifilm Ophthalmic Suspension contains per ml: Fluorometholone 1,0
Fitting Keratoconus and Other Complicated Corneas
 Fitting Keratoconus and Other Complicated Corneas Christine W Sindt OD FAAO Professor, Clinical Ophthalmology Director, Contact Lens Service University of Iowa Disclosure Consultant: ALCON Vision Care
Fitting Keratoconus and Other Complicated Corneas Christine W Sindt OD FAAO Professor, Clinical Ophthalmology Director, Contact Lens Service University of Iowa Disclosure Consultant: ALCON Vision Care
Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
Persistent epithelial defect (PED) of the cornea is defined as
 CLINICAL SCIENCE Plasma Rich in Growth Factors as a Therapeutic Agent for Persistent Corneal Epithelial Defects Silvia López-Plandolit, MD,* María-Celia Morales, DSci, PhD,* Vanessa Freire, Pre-PhD,* Jaime
CLINICAL SCIENCE Plasma Rich in Growth Factors as a Therapeutic Agent for Persistent Corneal Epithelial Defects Silvia López-Plandolit, MD,* María-Celia Morales, DSci, PhD,* Vanessa Freire, Pre-PhD,* Jaime
Shizuka Koh, M.D. Ph. D.
 CURRICULUM VITAE Page 1 CURRICULUM VITAE Shizuka Koh, M.D. Ph. D. Shizuka Koh, M.D. Ph. D. Department of Ophthalmology University of Rochester 247 Meliora Hall Rochester, NY 14627 Phone : 585-275-8675
CURRICULUM VITAE Page 1 CURRICULUM VITAE Shizuka Koh, M.D. Ph. D. Shizuka Koh, M.D. Ph. D. Department of Ophthalmology University of Rochester 247 Meliora Hall Rochester, NY 14627 Phone : 585-275-8675
Failure of amniotic membrane transplantation in the treatment of acute ocular burns
 Br J Ophthalmol 2001;85:1065 1069 1065 ORIGINAL ARTICLES Clinical science Failure of amniotic membrane transplantation in the treatment of acute ocular burns Annie Joseph, Harminder S Dua, Anthony J King
Br J Ophthalmol 2001;85:1065 1069 1065 ORIGINAL ARTICLES Clinical science Failure of amniotic membrane transplantation in the treatment of acute ocular burns Annie Joseph, Harminder S Dua, Anthony J King
Ocular Surface Reconstruction
 OCULAR SURFACE From Tissue Transplantation to Cell Therapy Abraham Solomon, MD Abstract: The most difficult part in ocular surface reconstruction for total limbal stem cell deficiency is restoring a healthy
OCULAR SURFACE From Tissue Transplantation to Cell Therapy Abraham Solomon, MD Abstract: The most difficult part in ocular surface reconstruction for total limbal stem cell deficiency is restoring a healthy
Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation
 1215 SCIENTIFIC REPORT Management of acute ative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation A Heiligenhaus, H Li, E E Hernandez Galindo, J M Koch, K-P Steuhl,
1215 SCIENTIFIC REPORT Management of acute ative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation A Heiligenhaus, H Li, E E Hernandez Galindo, J M Koch, K-P Steuhl,
A Non-Randomised, Patient Completed Questionnaire Report.
 Vismed Gel (Non-Preserved Sodium Hyaluronate eye drops) for the Treatment of Symptoms of Ocular Dryness and Discomfort in Patients with Sjögren's Syndrome. A Non-Randomised, Patient Completed Questionnaire
Vismed Gel (Non-Preserved Sodium Hyaluronate eye drops) for the Treatment of Symptoms of Ocular Dryness and Discomfort in Patients with Sjögren's Syndrome. A Non-Randomised, Patient Completed Questionnaire
1998 DESCRIPTION Evaluation of Subjective and Objective tests for diagnosing tear-film disorders known to cause ocular irritation.
 DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 18 th Oct 2004 TEST Mixed tests TO Ocular Irritation / Dry Eye REFERENCES DIAGNOSE VERSION of TEST Multiple tests DESCRIPTION Evaluation of Subjective
DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 18 th Oct 2004 TEST Mixed tests TO Ocular Irritation / Dry Eye REFERENCES DIAGNOSE VERSION of TEST Multiple tests DESCRIPTION Evaluation of Subjective
Keratoconjunctivitis sicca in canines diagnostic methods and routine testing
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Keratoconjunctivitis sicca in canines diagnostic methods and routine testing Author : JAMES OLIVER Categories : Vets Date
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Keratoconjunctivitis sicca in canines diagnostic methods and routine testing Author : JAMES OLIVER Categories : Vets Date
ALCAINE Eye Drops contain proxymetacaine hydrochloride. The chemical structure of proxymetacaine hydrochloride is:
 PRODUCT INFORMATION ALCAINE* (proxymetacaine hydrochloride Eye Drops 0.5% NAME OF THE MEDICINE ALCAINE Eye Drops contain proxymetacaine hydrochloride. The chemical structure of proxymetacaine hydrochloride
PRODUCT INFORMATION ALCAINE* (proxymetacaine hydrochloride Eye Drops 0.5% NAME OF THE MEDICINE ALCAINE Eye Drops contain proxymetacaine hydrochloride. The chemical structure of proxymetacaine hydrochloride
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Dry eye syndrome in diabetic children
 European Journal of Ophthalmology / Vol. 17 no. 6, 2007 / pp. 873-878 Dry eye syndrome in diabetic children A. AKINCI 1, E. CETINKAYA 2, Z. AYCAN 2 1 Department of Pediatric Ophthalmology 2 Department
European Journal of Ophthalmology / Vol. 17 no. 6, 2007 / pp. 873-878 Dry eye syndrome in diabetic children A. AKINCI 1, E. CETINKAYA 2, Z. AYCAN 2 1 Department of Pediatric Ophthalmology 2 Department
Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions. April 2018
 Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions This Clinical Practice Guide provides evidence-based information about current best practice in the management
Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions This Clinical Practice Guide provides evidence-based information about current best practice in the management
Package leaflet: Information for the user. <product name> 3 mg/ml eye drops, solution. Ofloxacin
 Package leaflet: Information for the user 3 mg/ml eye drops, solution Ofloxacin Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Package leaflet: Information for the user 3 mg/ml eye drops, solution Ofloxacin Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Postoperative follow up and treatment after refractive surgery
 Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1999, by the Massachusetts Medical Society VOLUME 340 J UNE 3, 1999 NUMBER TREATMENT OF SEVERE OCULAR-SURFACE DISORDERS WITH CORNEAL EPITHELIAL STEM-CELL
The New England Journal of Medicine Copyright, 1999, by the Massachusetts Medical Society VOLUME 340 J UNE 3, 1999 NUMBER TREATMENT OF SEVERE OCULAR-SURFACE DISORDERS WITH CORNEAL EPITHELIAL STEM-CELL
Human Tissues Department, Biotechnology Research Center, Tripoli- Libya P.O.Box: 30313,
 Human Tissues Department, Biotechnology Research Center, Tripoli- Libya P.O.Box: 30313, Abstract Amniotic membranes obtained from placentae of healthy mother donors screened seronegative to HIV, HBV, and
Human Tissues Department, Biotechnology Research Center, Tripoli- Libya P.O.Box: 30313, Abstract Amniotic membranes obtained from placentae of healthy mother donors screened seronegative to HIV, HBV, and
Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
 pissn: 11-8942 eissn: 92-9382 Korean J Ophthalmol 14;28(2):115-121 http://dx.doi.org/.3341/kjo.14.28.2.115 Original Article Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
pissn: 11-8942 eissn: 92-9382 Korean J Ophthalmol 14;28(2):115-121 http://dx.doi.org/.3341/kjo.14.28.2.115 Original Article Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
Corporate Presentation December Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG
 Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management
 JOURNAL OF CASE REPORTS 2014;4(2):419-423 Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management Dian Eka Putri, Lukman Edwar, Made Susiyanti Department of Ophthalmology,
JOURNAL OF CASE REPORTS 2014;4(2):419-423 Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management Dian Eka Putri, Lukman Edwar, Made Susiyanti Department of Ophthalmology,
PRODUCT INFORMATION ALCAINE. Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP. 5 mg/ml. Topical Anesthetic
 PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
Proposed PIL NL/H/0653/001/IB/024/G. MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate
 Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
Application of Umbilical Cord Serum Eyedrops for the Treatment of Neurotrophic Keratitis
 Application of Umbilical Cord Serum Eyedrops for the Treatment of Neurotrophic Keratitis Kyung-Chul Yoon, MD, PhD, 1 In-Cheon You, MD, 1 Seong-Kyu Im, MD, 1 Tae-Sun Jeong, MD, 1 Yeoung-Geol Park, MD, PhD,
Application of Umbilical Cord Serum Eyedrops for the Treatment of Neurotrophic Keratitis Kyung-Chul Yoon, MD, PhD, 1 In-Cheon You, MD, 1 Seong-Kyu Im, MD, 1 Tae-Sun Jeong, MD, 1 Yeoung-Geol Park, MD, PhD,
arthritis "Contact lens" cornea in rheumatoid (opposite). Brit. J. Ophthal. (I970) 54, 410 Peterborough District Hospital
 Brit. J. Ophthal. (I970) 54, 410 "Contact lens" cornea in rheumatoid arthritis A. J. LYNE Peterborough District Hospital It has been noted that patients suffering from long-standing rheumatoid arthritis
Brit. J. Ophthal. (I970) 54, 410 "Contact lens" cornea in rheumatoid arthritis A. J. LYNE Peterborough District Hospital It has been noted that patients suffering from long-standing rheumatoid arthritis
