Massachusetts General Hospital, Transplant Biology Research Center, Harvard
|
|
|
- Sabina Jacobs
- 5 years ago
- Views:
Transcription
1 Observations of Over 11 Years in Recipients of Combined HLA mismatched Kidney and Bone Marrow Transplantation Without Maintenance Immunosuppression Tatsuo Kawai 1,7, David H. Sachs 2, Ben Sprangers 3, Thomas R. Spitzer 4, Susan L. Saidman 5, Emmanuel Zorn 1, Nina Tolkoff-Rubin 1, Fred Preffer 5, Kerry Crisalli 1, Baoshan Gao 1, Waichi Wong 3, Heather Morris 3, Samuel A. LoCascio 3, Peter Sayer 6, Brittany Shonts 3, Winfred W. Williams, Jr. 1, Rex-Neal Smith 5, Robert B. Colvin 4, Megan Sykes 3 and A. Benedict Cosimi 1 1 Massachusetts General Hospital, Transplant Center, Harvard Medical School 2 Massachusetts General Hospital, Transplant Biology Research Center, Harvard Medical School 3 Columbia University, Columbia Center for Translational Immunology 4 Massachusetts General Hospital, Bone Marrow Transplant Unit, Harvard Medical School 5 Massachusetts General Hospital, Department of Pathology, Harvard Medical School 6 Immune Tolerance Network 7 Address correspondence to Tatsuo Kawai, M.D.,Ph.D., Massachusetts General Hospital, Transplant Center, White 521, 55 Fruit Street, Boston, MA02114 tkawai@partners.org 1
2 Contribution: Tatsuo Kawai, A. Benedict Cosimi, David H. Sachs, Megan Sykes, Thomas Spitzer and Robert B. Colvin performed study design, clinical management, data analyses and manuscript writing. Peter Sayer performed study design. Nina Tolkoff-Rubin, Waichi Wong, Winfred W. Williams, Kerry Crisalli and Rex-Neal Smith participated in clinical management. Susan L. Saidman performed DSA assays. Fred Preffer performed lymphocyte subset assays, Baoshan Gao and Emmanuel Zorn performed serum BAFF measurement. Ben Sprangers, Heather Morris, Brittany Shonts and Samuel LoCascio, with supervision from Megan Sykes, performed in vitro assays and data analyses. 2
3 Abstract: We report here the long-term results of HLA mismatched kidney transplantation without maintenance immunosuppression (IS) following combined kidney and bone marrow transplantation (CKBMT). The first three subjects were treated with nonmyeloablative conditioning and an 8-14 month course of calcineurin inhibitor (NKD03). The next two subjects received, in addition, two doses of pre-transplant rituximab (mod NKD03). The last five recipients received two pre and two posttransplant rituximab doses (ITN036). All 10 subjects developed transient chimerism and in seven of these, IS was successfully discontinued. Four subjects continue to be IS free for periods of years, while three required IS after 6-8 years due to recurrence of original disease or chronic allograft glomerulopathy. Donor-specific antibody (DSA) was detectable in some recipients treated with the NKD03 and mod NKD03 regimens. In contrast, no DSA was detected in ITN036 subjects, who showed more consistent and durable B cell depletion, despite high BAFF levels. In conclusion, long-term stable kidney allograft survival without maintenance IS can be achieved following transient mixed chimerism induction. Adequate B cell depletion during the initial 6 months appears to be an important factor in preventing development of de novo DSA. 3
4 Introduction: Short-term results following organ transplantation have been significantly improved by the use of increasingly efficacious immunosuppressive agents. However, their chronic administration results in significant morbidity, especially from an increased incidence of cardiovascular disease(1), infection(2), malignancies(3), de novo diabetes(4) and other metabolic derangements. Unfortunately, the potent immunomodulatory effects of current therapeutic protocols do not prevent the development of chronic rejection, despite their administration being pushed to toxic levels. Therefore, induction of tolerance, defined as the absence of destructive immune responses to a transplanted tissue without ongoing immunosuppressive therapy, remains the ultimate goal of organ transplantation. Since the seminal work reported by Billingham, Brent and Medawar on neonatal tolerance in 1956, numerous tolerance induction strategies have been defined in rodents. However, only a very limited number of these strategies have been successfully translated to large animals and even fewer to primates. Among the few protocols that have been applied successfully in humans, induction of donor chimerism, either transient or permanent, currently appears to be the most promising strategy to achieve renal allograft tolerance. Initial results of clinical trials for tolerance induction in three centers have so far been reported. Using TLI and DBMT, the Stanford group reported successful induction of stable chimerism and renal allograft tolerance in the majority of HLA-identical kidney transplant recipients(5-7). More recently, Leventhal et al. at Northwestern have 4
5 reported the use of an intensive conditioning regimen and donor hematopoietic stem cells for induction of full donor chimerism and tolerance in HLA-mismatched kidney transplant recipients(8). Although the follow-up of these patients is still relatively brief, persistent donor chimerism without GVHD has been reported, allowing weaning from all maintenance immunosuppression by 1 year in approximately half of the patients. At Massachusetts General Hospital, based on decades-long basic studies in animal models(9-12), we have applied combined kidney and donor bone marrow transplantation (CKBMT) for induction of allograft tolerance in both HLA matched(13-15) and HLA-mismatched(16,17) kidney transplant recipients. In this report, we summarize our experience in all 10 subjects, present assays of their anti-t cell responses, B cell depletion and BAFF levels and evaluate the potential relationship between the conditioning regimens and long-term humoral responses. Our observations emphasize the importance of adequate B cell depletion during the initial 6 months to inhibit de novo DSA. METHODS Study subjects: A total of 10 subjects, age 22-46, 6 males and 4 females, were enrolled into these studies. Their original kidney diseases include Alport s syndrome (n=4), polycystic kidney disease (n=2), membrano-proliferative glomerulonephritis (MPGN) type 1(n=2), reflux uropathy (n=1) and focal glomerulosclerosis (n=1) (Table 1). The first three subjects (#1 - #3) received the NKD03 conditioning 5
6 regimen; the next two subjects (#4 and #5) received the modified NKD03 regimen. The last five subjects(#6 - #10) received the ITN036 protocol detailed in Fig. 1. Conditioning Regimen: The initial conditioning regimen (Fig. 1, NKD03) consisted of cyclophosphamide (60 mg/kg/d) administered i.v. on days 5 and 4 with respect to transplantation; humanized anti-cd2 mab (MEDI 507, MedImmune Inc., Gaithersburg, MD) at a test dose of 0.1 mg/kg on day -2 followed by 0 6 mg/kg/dose on days -1, 0 and +1; cyclosporine A (CyA, Novartis Pharmaceuticals Inc., East Hanover, NJ) (5 mg/kg) i.v. on day 1; and thymic irradiation (700 cgy) on day 1. Hemodialysis was performed before and 14 hours after each dose of cyclophosphamide. On Day 0, kidney transplantation was followed by i.v. infusion of unprocessed donor bone marrow (DBM). Oral CyA (Neoral, Novartis Pharmaceuticals, Inc.) was administered postoperatively at 8 to 12 mg/kg/day with target trough blood levels of ng/ml, then tapered and discontinued over several months. The protocol was modified after treatment of the third subject (see Results), with the addition of Rituximab, 375 mg/m²/dose on days -7 and -2; and prednisone, 2 mg/kg/d starting on the day of transplantation and tapering to withdrawal over the next 10 post-transplant days (Fig. 1,modified NKD03). Since subjects treated with this modified NKD03 regimen still developed donor-specific antibodies (DSA) after discontinuation of immunosuppression, the regimen was further modified (Fig.1) to add two more doses of Rituximab (375 mg/m²/dose) on days 5 and 12, plus a more prolonged course of prednisone until day 20, and 6
7 tacrolimus in place of CyA (ITN036). Tacrolimus was slowly tapered over several months and completely discontinued at 8 months after confirming no rejection by a 6 month protocol biopsy. All treatment regimens were approved by the Massachusetts General Hospital Institutional Review Board (IRB) and developed in collaboration with the Immune Tolerance Network (ITN). Biopsies. Kidney allograft biopsies were taken per protocol at day 0 and at months 6, 12, 24 and 36. Some subjects had later protocol biopsies after 5-8 years of follow-up. Indication biopsies were taken for any episode of graft dysfunction. All biopsies were processed for routine light microscopy, immunofluorescence (including C4d stains) and electron microscopy by techniques previously reported (16, 18). In Vitro Immunologic Assays: Standard MLR and CML assays were performed using the methods detailed previously.(14) LDA to quantify cytotoxic T-lymphocyte precursor frequencies and IL-2-producing Th frequencies were performed as described(19). Detection of donor-specific antibodies: Serially collected pre- and posttransplant sera samples were tested for the presence of HLA antibodies using ELISA kits (LAT Class I & II, One Lambda, Canoga Park, CA). Serum BAFF measurement: Concentration of BAFF in the serum was measured using a Quantikine ELISA kit (R&D systems, Minneapolis, MN) according to the manufacturer s instructions. Serum samples were diluted 1:8 in PBS. 7
8 RESULTS: Induction of transient chimerism: As in the previously reported patients, all additional subjects developed transient multilineage mixed chimerism, which became undetectable by 2-3 weeks post-ckbmt(16, 20). Opmerking [CU1]: Strange page break here 8
9 Acute kidney injury (AKI) days after DBMT: We previously described the cytokine syndrome-like manifestations observed after CKBMT as engraftment syndrome (16, 18). In fact, the symptoms have been temporally associated with the loss of peripheral chimerism as well as of the return of hostderived hematopoietic elements. The most troublesome manifestation of this syndrome, and the only manifestation not alleviated by addition of steroids to the post-transplant treatment regimen, was acute kidney injury (AKI), which was observed after day 10 in all patients, although minimally in Subject #1. The biopsies taken during AKI showed endothelial injury with CD8 + T cell infiltration, as reported previously in detail(18). The peak serum creatinine (Cr.) level ranged from 3 5 to 15 4 mg/dl during days Among the ten subjects who developed AKI, four recovered without additional treatment. Renal allograft function was normalized in two subjects in conjunction with Thymoglobulin and plasma exchange, in one with Thymoglobulin alone and in one with additional steroid therapy. Two kidney allografts failed to recover, one in association with humoral rejection due to preformed anti-donor antibody (Subject #3) and one that developed thrombotic micro-angiopathy associated with high tacrolimus levels (Subject #8). Long-term clinical course of subjects in NKD03 (Subjects #1-5): Initial results, after follow-up periods of up to 2-5 years, in the first five patients, were previously reported(16). As in that report, except for Subject #3, who was retrospectively found to have DSA prior to transplant and suffered early graft failure due to acute antibody-mediated rejection, all renal allografts are functional 9
10 with the longest survival now exceeding 11 years (Subject #1). Four protocol biopsies performed in Subject #1 up to 7 5 years after CKBMT showed no evidence of rejection, including the absence of C4d deposition (Figs. 3A-C). Subject #2 was successfully tapered off of IS by 14 months, after treatment for suspected humoral rejection (transient C4d deposition without DSA) at around day 45. He remained stable until year 7 following immunosuppression withdrawal (Fig. 2). At that point, urinary protein first became detectable. Allograft biopsy revealed no rejection but recurrence of his original disease, MPGN type I (C3 glomerulopathy) (Figs. 3D-F). Mycophenolate mofetil (MMF) monotherapy for recurrent MPGN was initiated after the 8 th year. Although he has been inconsistently compliant, he is currently stable (serum creatinine 2.1 mg/dl) (Fig. 2) with minimal urinary protein ( mg/l). Subject #3 had shown high PRA (50%) but did not show DSA by ELISA and crossmatch was negative before transplantation. However, he lost his renal allograft with severe acute humoral rejection on day 10 (Fig. 2). Retrospective analysis with LUMINEX revealed preformed DSA against HLA class I (FIG. 4A) and his humoral rejection was retrospectively concluded to be due to preformed DSA. This subject subsequently underwent successful second transplantation with conventional IS. Subject #4 received the modified NKD03 regimen (Fig. 1). Protocol biopsies as early as 11 months and up to day 731 stained positive for C4d(16), but did not have features of active rejection. However, his five-year protocol biopsy revealed glomerular basement membrane duplication by light and electron microscopy with capillaritis and continued C4d deposition with minimal interstitial fibrosis (Fig. 10
11 3I). Although his renal function was stable with serum creatinine 1.6 at that time (Fig. 2), MMF was initiated 6 years after IS had been withdrawn. Since proteinuria became detectable after 7 years post-transplant and his kidney function has been slowly deteriorating, a brief course of IVIG and rituximab were administered and MMF has recently been switched to belatacept (Fig. 2) to prevent further progression of chronic rejection. Subject #5 remained stable for 5 years (Fig. 2) with no evidence of rejection or C4d deposition, despite intermittent detection of weak DSA after 3 years (Fig.4). Subsequently, after suffering multiple severe episodes of gout, his renal function deteriorated and C4d deposition transiently became detectable in the biopsy at 6 years. Despite a protocol biopsy at 6.3 years that showed minimal transplant glomerulopathy (cg1) (Figs. 3J, K) with negative C4d staining (Fig. 3L), renal function became unstable with serum Cr. level mg/dl after the 7 th year (Fig. 2) and Belatacept has recently been initiated. Clinical course of subjects in ITN036 (Subjects #6-10): Subject # 6 developed the typically observed AKI after day 10 and recovered without additional treatment. His IS was slowly tapered and completely discontinued at 8 months after CKBMT. He is currently doing well with a serum Cr. level of 1.5 mg/dl for almost 5 years after transplantation, without ongoing IS (Fig. 2). His biopsies have shown no evidence of rejection (Figs.3M and N) but showed de novo C4d deposition in a biopsy taken at 2 years (Fig.3O). Immunosuppressive therapy for Subject #7 was discontinued at 8 months. She is currently 4.7 years Opmerking [CU2]: It seems strange to mention 2 years and not the ensuing 3 years. Did C4d persist? 11
12 after CKBMT with normal kidney function (serum Cr. 0.8 mg/dl) (Fig. 2) with no evidence of rejection or C4d staining in the 2-year protocol biopsy (Figs. 3P-R). Subject # 8 failed to recover from AKI. Biopsy performed on day 22 revealed arterial intimal matrix expansion with infiltrating mononuclear cells and arterial fibrinoid necrosis without C4d deposition in peritubular capillaries. No DSA was detectable. Differential diagnosis included acute cellular rejection type III versus intra-renal thrombotic microangiopathy, possibly due to tacrolimus toxicity. Tacrolimus was discontinued and three doses of anti-thymocyte globulin were administered with MMF. Despite these treatments, her kidney function gradually failed and she was returned to CAPD at 7 months after transplantation. Subject #9 developed a transient AKI after day 10 and recovered without additional treatment. Her immunosuppression was slowly tapered and was discontinued at 8 months after transplantation. She is currently well at almost 4.3 years after CKBMT with normal kidney function (serum Cr. 1 0 mg/dl)(fig. 2). The protocol biopsy at 2 years showed no evidence of rejection (Figs.3S-U). In Subject #10, kidney function gradually returned to normal by 2 months after AKI and a 6- month protocol biopsy did not show rejection. His immunosuppression was discontinued at 8 months. One month later, he developed acute allograft pyelonephritis with moderately elevated serum Cr. (2 2 from 1 6 mg/dl). This was treated with antibiotics and the kidney function recovered to base line. However, 3 weeks after the resolution of his infection, he developed severe acute cellular rejection (Banff 2B)(Fig. 3V) with no C4d deposition (Fig. 3X). Several DSA assays were negative. He was treated with steroid pulses and anti-thymocyte 12
13 globulin, following which his renal function improved but never fully recovered (Fig. 2). A renal biopsy 6 months later showed interstitial and intimal fibrosis without active inflammation (Fig. 3W). His kidney function remained compromised thereafter and he recently received a successful second kidney transplant with conventional IS at 3 years after CKBMT. Anti-donor T cell responses: MLR, CML, CTLp and HTLp assays in NKD03 subjects were previously reported.(16, 21) All NKD03 subjects developed donor specific nonresponsiveness (DSN) by 3 to 9 months in these assays. Serial in vitro immunological assays to test anti-donor T cell responses were also performed in the four ITN036 subjects who discontinued their immunosuppression. In these subjects, DSN or donor-specific hyporesponsiveness (DSH) also developed in MLR by 3 to 18 months, but anti-donor responses were again evident at months in Subjects #7 and #9 (Table 2). High incidence of DSA production in the modified NKD03 subjects: DSA has never been detected in Subjects #1, 6, 7, 8, 9 and 10. DSA was transiently detected only once in Subject #2 with no evidence of rejection. Anti- Met opmaak: Markeren Opmerking [CU3]: Please look at Table 2- the last time point has been removed for Pts 6,7 and 9, making this statement seem untrue. Please replace with the table version I will send with these comments. Met opmaak: Markeren Met opmaak: Markeren Met opmaak: Markeren HLA class II DSA has been persistently positive in Subject #4, and was associated with persistent C4d deposition and glomerulopathy. In Subject #5, weak anti-class II DSA has been detected intermittently after 3 years with transient C4d deposition (Fig. 3). In ITN036 subjects, no DSA has been detectable even in Subject #10 who developed severe acute cellular rejection. 13
14 B cells (CD3-CD19+) were almost completely eliminated from the circulation for 6 months in ITN036 subjects: Recovery of CD3 - CD19 + cells in the two subjects (#1 and #2) after treatment with the original NKD03 regimen was observed by days 50 and 100, respectively. With two doses of pretransplant rituximab (modified NKD03), depletion of CD3 - CD19 + cells was extended to day 150 (Subjects #4 and #5) but complete loss of CD3 - CD19 + cells from the peripheral blood was not observed. In contrast, peripheral blood CD3 - CD19 + cells were less than 1-2/mm 3 for 6 months in all five ITN036 subjects treated with 4 doses of peritransplant rituximab (Fig. 45).(20) High serum BAFF levels in the recipients treated with rituximab: Serum BAFF levels were less than 2 ng/ml at all time points assessed in Subject #1. Subject #2 had high BAFF levels initially until day 100 and humoral rejection was suspected at around day 45, when his BAFF level was highest (Fig. 5B, arrow). BAFF levels waned thereafter to less than 2 ng/ml after day 100 and he successfully discontinued his IS. In contrast, high BAFF levels were detected over 300 days in two subjects treated with the modified NKD03 regimen. Persistently high BAFF was also detectable for more than 300 days in the ITN036 subjects (Fig. 4). 14
15 DISCUSSION We report here that induction of long-term (greater than11 years) stable renal allograft function without maintenance immunosuppression can be achieved after induction of transient lymphohematopoietic chimerism in approximately one-half of recipients of HLA-mismatched CKBMT. Consistent with the fact that chimerism in these recipients was transient, there has been no GVHD observed in our preclinical (10, 11) or clinical studies(16), which is an attractive attribute of this approach for tolerance induction. Apparently, the mechanism of tolerance induction after transient chimerism in nonhuman primates and humans differs from that observed in murine mixed chimerism models, where central deletional pathways have been demonstrated in conjunction with chimerism that persists indefinitely.(12, 22).(9, 23) Nevertheless, our NHP studies have consistently demonstrated the necessity for DBM engraftment, resulting in at least transient measurable donor chimerism, especially in lymphoid lineages, for successful induction of tolerance(10, 11, 24). Our nonhuman primate studies have shown that co-stimulatory blockade, with agents directed against CD40/CD154(11) or B7/CD28 (manuscript in preparation) were important in achieving consistent tolerance. In these clinical trials, we have used an anti-cd2 monoclonal antibody (MEDI507) which provides both T-cell depletion and co-stimulatory blockade via the CD2/LFA-3 interaction in our clinical protocol(16). Our preclinical studies have also emphasized that the kidney allograft itself plays a critical role in the induction and maintenance of tolerance. In our studies in NHP, the same conditioning regimen that achieves 15
16 renal allograft tolerance after induction of transient chimerism, has consistently failed to induce tolerance of isolated heart allografts in monkeys(25). However, heart allograft tolerance is regularly achieved by co-transplanting the kidney from the same donor(26). Moreover, patients who lost chimerism after receiving the same regimen as NKDO3 and BMT for hematologic malignancies, but without a kidney transplant, failed to develop donor-specific hyporesponsiveness (19), in marked contrast to those receiving kidney transplantation with the same BMT regimen (21). We hypothesize that generation of donor-specific regulatory cells in the thymus during the original chimeric state is a process that is maintained bycombined with continueding interactions of recipient T cells with as yet undefined donor cells or antigens in the kidney allograft and is responsible for maintenance of the tolerance (21). One potentially important renal cell is the renal tubular epithelial cell, that has been reported to participate in the induction of allospecific tolerance in rodents and, by in vitro assays in humans (27-29). Studies are in progress in our laboratories to assess the possible role of this cell in the NHP tolerance model upon which these clinical studies are based. Despite the encouraging results of successful long-term IS-free renal allograft survival in our initial clinical trials, obstacles remain before we anticipate more widespread application of this approach. The first obstacle is acute kidney injury (AKI) that has been observed at approximately day 10 in all subjects, although minimally in Subject #1. We have previously reported this complication as engraftment syndrome which has been described previously following both autologous and allogeneic bone marrow transplantation (30). However, 16
17 engraftment syndrome may not adequately describe the phenomenon we have observed, since it was always associated both with recovery of host hematopoietic cells, potentially including homeostatic recovery of memory T cells, and with rapid loss of chimerism. We have noted that the AKI of engraftment syndrome has never been observed in our MHC-mismatched NHP model, in which chimerism is also lost, but at a more gradual pace, usually over 2-3 months(10, 11, 31). Our current interpretation of this observations is that the pretransplant cyclophosphamide-based regimen chosen for the clinical trials(16), in place of low dose total body irradiation (TBI) used in the NHP preparative regimen(10, 11, 31, 32), may be less effective in preventing rapid homeostatic recovery of host memory T cells, which, when rapidly expanding, may have had destructive effector function at the level of donor renal endothelial cells. This hypothesis is consistent with the observation of CD8 T cell activation immediately following conditioning (18) and the appearance of CD8 T cells in peritubular capillary endothelial cells during the period of AKI (18). For this reason, in a current modification of our CKBMT protocol, we are testing the effect of substituting a low dose of TBI for pre-transplant cyclophosphamide (in progress). Also, although the phenomenon of engraftment syndrome has been observed in autologous and allogeneic bone marrow transplantation [ ], the AKI observed in those cases was much milder than that observed in our protocol. One likely reason for this difference is that in the former cases, the recipients had normal kidneys, while the recipients of our CKBMT protocol had also just 17
18 received a kidney transplant, with attendant inflammatory changes which would likely make it more susceptible to any additional injury during the early posttransplant period. Concurrent calcineurin inhibitor toxicity would undoubtedly also contribute to renal dysfunction, and it is likely that the combination of tacrolimus toxicity and engraftment syndrome contributed heavily to the renal failure from TMA observed in Subject # 8. In our modified protocol, we will therefore discontinue treatment with calcineurin inhibitors during the duration of engraftment syndrome, should it occur despite the change from cyclophosphamide to irradiation in the preparative regimen. Another obstacle, observed especially in the modified NKD03 subjects, was the frequent development of either transient or persistent DSA, despite specific loss of anti-donor T cell responses. As depletion of B cells enhances B cell activating factor (BAFF) production which has been found to significantly enhance the differentiation of marginal zone B cells into immunoglobulin secreting cells in a T cell dependent manner(33), we measured peripheral blood B cells (CD19+) and serum BAFF levels after transplantation. In Subject #2, humoral rejection, suspected around day 45 while tapering IS, was associated with high BAFF levels (>8 ng/ml). His BAFF levels subsequently waned to the baseline level by day 100 and IS was successfully withdrawn without DSA development. In the two modified NKD03 subjects, B cells were not completely depleted and recovered within 6 months. In addition, persistently high serum BAFF levels were detected in these patients, which may have contributed to 18
19 enhanced DSA production. In contrast to the results in the modified NKD03 protocol, the intensified anti-b cell treatment included in the ITN036 regimen was associated with almost no evidence of DSA production. In these ITN036 subjects, although BAFF levels were elevated similarly to those observed in the modified NKD03 patients, peripheral blood B cells were essentially nondetectable for more than 6 months and there was no development of DSA. Thus, high BAFF levels may not be a risk factor for DSA production if B cells are profoundly depleted. Although anti-hla antibodies are thought to represent T cell-dependent responses, we found no apparent relationship between DSA production and antidonor T cell responses in these clinical studies. Thus, modified NKD03 subjects developed DSA despite undetectable anti-donor T cell responses and Subjects #7 and #9 in ITN036 have not developed DSA despite persistent anti-donor CTL responses. Our clinical observations therefore suggest that B cell depletion for 6 months by rituximab, as achieved in the ITN036 subjects, may avoid the DSA obstacle to tolerance induction via the mixed chimerism approach. In this regard, we are currently investigating a conditioning regimen that combines B cell depletion and anti-baff antibody in nonhuman primates. In conclusion, our clinical studies have shown that immunosuppressionfree stable renal allograft function can be achieved in HLA-mismatched donorrecipient pairs, with follow up times of greater than 11 years, by induction of transient chimerism through DBMT. We hope that the relatively small modifications of the protocol we have described will avoid the remaining 19
20 obstacles to more widespread application of this technology for clinical tolerance induction. Acknowledgement: This research was performed as a project of the Immune Tolerance Network (N01-AI-15416), an international clinical research consortium and supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation. Mechanistic studies were supported by NIH grant RO1 AI No potential conflict of interest relevant to this article was reported. We thank the Clinical Trials Group of the Immune Tolerance Network (ITN) and Eleanor Ramos, M.D., for their participation in the planning and conduct of these studies; the ITN Drug Withdrawal Committee for their help in determining appropriate conditions and means for withdrawal of immunosuppressive therapy; We thank Drs. James Markmann, Dicken Ko, Martin Hertl, Nahel Elias (transplant surgery), Steve McAfee, Bimalangshu Dey (hematology) and Mses. Sandy Debronkart, N.P. for clinical management. We also appreciate Dr. Stanley Jordan (Cedars-Sinai) and his team for the clinical management of Subject #4. We acknowledge Drs. Joren Madsen and James Markmann (both Massachusetts General Hospital) for critical review of the manuscript. Disclosure: 20
21 The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. This manuscript was also not prepared or funded by any commercial organization. 21
22 Figure Legends Fig. 1: Nonmyeloablative conditioning regimens. The initial conditioning regimen (Fig. 1, NKD03) consisted of cyclophosphamide (60 mg/kg/d) administered i.v. on days 5 and 4 with respect to transplantation; humanized anti-cd2 mab (MEDI 507, MedImmune Inc., Gaithersburg, MD) (0 6 mg/kg/dose) on days -2, -1, 0 and +1; cyclosporine A (CyA, Novartis Pharmaceuticals Inc., East Hanover, NJ) (5 mg/kg) i.v. on day 1; and thymic irradiation (700 cgy) on day 1. Hemodialysis was performed before and 14 hours after each dose of cyclophosphamide. On Day 0, kidney transplantation was followed by i.v. infusion of unprocessed donor bone marrow (DBM). Oral CyA (Neoral, Novartis Pharmaceuticals, Inc.) was administered postoperatively at 8 to 12 mg/kg/day with target trough blood levels of ng/ml, then tapered and discontinued over several months. The protocol was modified after treatment of the third subject (see Results), with the addition of Rituximab, 375 mg/m²/dose on days -7 and -2; and prednisone, 2 mg/kg/d starting on the day of transplantation and tapering to withdrawal over the next 10 post-transplant days (modified NKD03). Since subjects treated with this modified NKD03 still developed donor specific antibodies (DSA) after discontinuation of immunosuppression, the regimen was further modified (Fig.1) to add two more doses of Rituximab (375 mg/m²/dose) on days 5 and 12, plus a more prolonged course of prednisone until day 20, and tacrolimus in place of CyA (ITN036). Tacrolimus was slowly tapered over several months and completely discontinued at 8 months after confirming no rejection by a 6 month 22
23 protocol biopsy. All treatment regimens were approved by the Massachusetts General Hospital Institutional Review Board (IRB) and developed in collaboration with the Immune Tolerance Network (ITN). The initial preparative conditioning regimen (NKD03) consisted of cyclophosphamide (60 mg/kg/d) on days 5 and 4, humanized anti-cd2 mab (0 6 mg/kg/dose) on days -2, -1, 0 and +1; cyclosporine A targeting trough levels of mg/ml beginning on day 1; and thymic irradiation (7Gy) on day 1. Kidney transplantation was followed by i.v. infusion of donor bone marrow (DBM). CyA was tapered and discontinued over 9-14 months. In the modified NKD03 regimen, administration of Rituximab (375 mg/m²/dose) on days -7 and -2 and a short course of post-transplant prednisone were added. In the ITN036 regimen, two more dose of Rituximab (375 mg/m²/dose) on days +5 and +12 and a longer course of prednisone were added and post-transplant cyclosporine was replaced with an 8 months course of tacrolimus, targeting trough levels of 8-12 ng/ml. Fig. 2: Clinical course after CKBMT Blue lines indicate serum Creatinine levels (mg/dl) after CKBMT. Green bars indicate induction immunosuppression (CyA:cyclosporine, Tac:tacrolimus). Orange /blue bars indicate reinstitution of maintenance immunosuppression (MMF: mycophenolate mofetil, actino: actinomycin, Pred: prednisone), CTLA4Ig (belatacept). Fig. 3: Late renal allograft biopsies (all protocol biopsies unless noted). 23
24 A-C (Subject #1 at 7.5 years): Protocol biopsy shows no evidence of rejection by light microscopy (LM) (A). The glomeruli are normal by electron microscopy (EM) (B) and no C4d staining is detectable by immunofluorescence (IF)( C) D, E, F (Subject #2 at 8 years): An indication biopsy for proteinuria shows prominent lobular mesangial expansion with widespread glomerular basement membrane (GBM) duplication (D, arrow). Granular dense deposits in the duplicated GBM are seen by EM (E). Granular and segmental staining for C3 along the GBM and in the mesangium is evident by IF, but no immunoglobulin was detected (F), indicative of recurrent C3 glomerulopathy (originally classified as MPGN, type I).. G,H,I (Subject #4 at 5 years): Widespread GBM duplication (arrow) and glomerulitis is seen by LM (G). EM shows prominent duplication of the GBM without deposits and reactive endothelial cells (H). C4d deposition is detected in peritubular capillaries (I). These findings are indicative of chronic, active antibody mediated rejection, which at this time was subclinical. J,K,L (Subject #5 at 6 years): The biopsy shows minimal glomerulitis by LM (j), slight segmental GBM duplication by LM (J) and EM (K) and no C4d deposition by IF (L) M,N,O (Subject #6 at 2 years): LM shows normal glomeruli with rare foci of interstitial mononuclear inflammation affecting <5% of the cortex (M). EM shows minimal focal GBM duplication and normal endothelium (N). C4d was present in the majority of the peritubular capillaries (O). 24
25 P,Q, R (Subject #7 at 2 years): LM is within normal limits (P). EM reveals a normal GBM and endothelium; foot process effacement is present in a minority of the capillaries (20%) (Q). There is no C4d deposition (R). S,T, U (Subject #9 at 2 years): The kidney biopsy is within normal limits and shows no evidence of rejection by LM (S) with normal glomeruli by EM (T). There is no evidence of recurrent MPGN. No C4d is detected (U). V,W,X: (Subject #10): Indication biopsy at 9.5 months shows acute cellular rejection with endarteritis (V). A protocol biopsy six months later shows complete resolution of the inflammatory process and residual interstitial and intimal fibrosis (W). No C4d deposition is detectable at 9.5 months (X) or at other times. Light microscopy stains: H&E, A, M, V; PAS, D, G, J. P, S. Immunofluorescence stains: C4d, C, F, I, L, O, R, U, X. Fig. 4: Detection of donor specific antibody (DSA) DSAs are detected by ELISA. NKD03: Subject #1: DSA never detected. Subject #2: DSA transiently detected once at 7 years. Subject 3: Although pre-transplant DSA was negative by ELISA, retrospective Luminex showed positive anti-class I DSA (B44) before transplantation. The subject developed acute humoral rejection on day10 with DSA (B44 and DR4). Mod NKD03: Subjects 4: Anti-donor DR17 antibody has been persistently positive since soon after stopping immunosuppression. Subject 5: Weak antidonor DR53 antibody has been intermittently detectable. 25
26 ITN036: In contrast to NKD03 subjects, No DSA has been detectable in any ITN036 subject including Subject #10 who developed severe acute cellular rejection. Fig. 5: A: CD3 - CD19 + cell depletion after CKBMT In two NKD03 subjects, recovery of CD3 - CD19 + cells (B cells) was found by day 50 (Subject #1) and day 120 (Subject #2)(gray lines). With addition of two doses of pre-transplant rituximab (modified NKD03), B cells were partially depleted and recovered by days 150 and 180 (blue lines). With 4 doses of peri-transplant rituximab (ITN036), B cells were nearly completely depleted (less than 1-2/mm 2 ) for 180 days.. B: Serum BAFF Serial serum BAFF levels were measured by ELISA. Serum BAFF levels were less than 2 ng/ml at all time points assessed in Subject #1. Subject #2 had high BAFF levels initially until day 100 and humoral rejection was suspected at around day 45, when his BAFF level was highest (Fig. 5B). BAFF levels waned thereafter to less than 2 ng/ml after day 100 and he successfully discontinued his IS. In contrast, high BAFF levels were detected over 300 days in two subjects treated with the modified NKD03 regimen. Persistently high BAFF was also detectable for more than 300 days in the ITN036 subjects. 26
27 References 1. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2): Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El- Amm JM, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20(4): Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69(16): Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. J Endocrinol Invest. 2000;23(7): Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4): Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N Engl J Med. 2011;365(14): Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, et al. Tolerance and Withdrawal of Immunosuppressive Drugs in Patients Given Kidney and Hematopoietic Cell Transplants. Am J Transplant Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and Tolerance Without GVHD or Engraftment Syndrome in HLA- Mismatched Combined Kidney and Hematopoietic Stem Cell Transplantation. Sci Transl Med. 2012;4(124):124ra Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169(2): Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2): Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9): Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14(4): Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68(4): Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6(9):
28 15. Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, et al. Long-Term Follow-Up of Recipients of Combined Human Leukocyte Antigen- Matched Bone Marrow and Kidney Transplantation for Multiple Myeloma With End-Stage Renal Disease. Transplantation Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4): Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368(19): Farris AB, Taheri D, Kawai T, Fazlollahi L, Wong W, Tolkoff-Rubin N, et al. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am J Transplant. 2011;11(7): Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-cd2 mab, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35(7): LoCascio SA, Morokata T, Chittenden M, Preffer FI, Dombkowski DM, Andreola G, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90(12): Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11(6): Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153(3): Sykes M, Sachs DH. Mixed chimerism. Philos Trans R Soc Lond B Biol Sci. 2001;356(1409): Kimikawa M, Sachs DH, Colvin RB, Bartholomew A, Kawai T, Cosimi AB. Modifications of the conditioning regimen for achieving mixed chimerism and donor-specific tolerance in cynomolgus monkeys. Transplantation. 1997;64(5): Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73(11): Tonsho M MT, Klarin D, Boskovic S, Nadazdin O, Benichou G, Cosimi AB, Smith NR, Colvin RB, Kawai T, Madsen JC., editor Induction of Tolerance to Heart Allografts by Donor Kidney Co-Transplantation in Nonhuman Primates (NHPs).. American Transplant Congress ; Boston. 27. Braun MY, McCormack A, Webb G, Batchelor JR. Evidence for clonal anergy as a mechanism responsible for the maintenance of transplantation tolerance. Eur J Immunol. 1993;23(7):
29 28. Frasca L, Marelli-Berg F, Imami N, Potolicchio I, Carmichael P, Lombardi G, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53(3): Starke A, Lindenmeyer MT, Segerer S, Neusser MA, Rusi B, Schmid DM, et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int.78(1): Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(9): Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68(11): Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming Memory T-Cell Responses for Induction of Delayed Tolerance in Nonhuman Primates. Am J Transplant. 2012;12(2): Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J Immunol. 2007;178(9):
A Tolerance Approach to the Transplantation of Vascularized Tissues
 A Tolerance Approach to the Transplantation of Vascularized Tissues The 9th New Jersey Symposium on Biomaterials Science and Regenerative Medicine October 29-31, 2008 David H. Sachs, M.D. Harvard Medical
A Tolerance Approach to the Transplantation of Vascularized Tissues The 9th New Jersey Symposium on Biomaterials Science and Regenerative Medicine October 29-31, 2008 David H. Sachs, M.D. Harvard Medical
TRANSPLANT IMMUNOLOGY. Shiv Pillai Ragon Institute of MGH, MIT and Harvard
 TRANSPLANT IMMUNOLOGY Shiv Pillai Ragon Institute of MGH, MIT and Harvard Outline MHC / HLA Direct vs indirect allorecognition Alloreactive cells: where do they come from? Rejection and Immunosuppression
TRANSPLANT IMMUNOLOGY Shiv Pillai Ragon Institute of MGH, MIT and Harvard Outline MHC / HLA Direct vs indirect allorecognition Alloreactive cells: where do they come from? Rejection and Immunosuppression
Tolerance Induction in Transplantation
 Tolerance Induction in Transplantation Reza F. Saidi, MD, FACS, FICS Assistant Professor of Surgery Division of Organ Transplantation Department of Surgery University of Massachusetts Medical School Percent
Tolerance Induction in Transplantation Reza F. Saidi, MD, FACS, FICS Assistant Professor of Surgery Division of Organ Transplantation Department of Surgery University of Massachusetts Medical School Percent
HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression
 brief report HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression Tatsuo Kawai, M.D., A. Benedict Cosimi, M.D., Thomas R. Spitzer, M.D., Nina Tolkoff-Rubin, M.D., Manikkam Suthanthiran,
brief report HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression Tatsuo Kawai, M.D., A. Benedict Cosimi, M.D., Thomas R. Spitzer, M.D., Nina Tolkoff-Rubin, M.D., Manikkam Suthanthiran,
Introduction. University School of Medicine, Stanford, CA. Received 19 June 2014, revised 03 October 2014 and accepted for publication 04 October 2014
 American Journal of Transplantation 15; 15: 695 74 Wiley Periodicals Inc. C Copyright 15 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 1.1111/ajt.1391 Chimerism,
American Journal of Transplantation 15; 15: 695 74 Wiley Periodicals Inc. C Copyright 15 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 1.1111/ajt.1391 Chimerism,
HLA and Non-HLA Antibodies in Transplantation and their Management
 HLA and Non-HLA Antibodies in Transplantation and their Management Luca Dello Strologo October 29 th, 2016 Hystory I 1960 donor specific antibodies (DSA): first suggestion for a possible role in deteriorating
HLA and Non-HLA Antibodies in Transplantation and their Management Luca Dello Strologo October 29 th, 2016 Hystory I 1960 donor specific antibodies (DSA): first suggestion for a possible role in deteriorating
Review of Rituximab and renal transplantation. Dr.E Nemati. Professor of Nephrology
 Review of Rituximab and renal transplantation Dr.E Nemati Professor of Nephrology Introductio n Rituximab is a chimeric anti-cd20 monoclonal antibody. The CD20 antigen is a transmembrane nonglycosylated
Review of Rituximab and renal transplantation Dr.E Nemati Professor of Nephrology Introductio n Rituximab is a chimeric anti-cd20 monoclonal antibody. The CD20 antigen is a transmembrane nonglycosylated
Management of Rejection
 Management of Rejection I have no disclosures Disclosures (relevant or otherwise) Deborah B Adey, MD Professor of Medicine University of California, San Francisco Kidney and Pancreas Transplant Center
Management of Rejection I have no disclosures Disclosures (relevant or otherwise) Deborah B Adey, MD Professor of Medicine University of California, San Francisco Kidney and Pancreas Transplant Center
Post-Transplant Monitoring for the Development of Anti-Donor HLA Antibodies
 Post-Transplant Monitoring for the Development of Anti-Donor HLA Antibodies Lorita M Rebellato, Ph.D., D (ABHI) Associate Professor Department of Pathology The Brody School of Medicine at ECU Scientific
Post-Transplant Monitoring for the Development of Anti-Donor HLA Antibodies Lorita M Rebellato, Ph.D., D (ABHI) Associate Professor Department of Pathology The Brody School of Medicine at ECU Scientific
Renal Pathology- Transplantation. Eva Honsova Institute for Clinical and Experimental Medicine Prague, Czech Republic
 Renal Pathology- Transplantation Eva Honsova Institute for Clinical and Experimental Medicine Prague, Czech Republic eva.honsova@ikem.cz Kidney has a limited number of tissue reactions by which the kidney
Renal Pathology- Transplantation Eva Honsova Institute for Clinical and Experimental Medicine Prague, Czech Republic eva.honsova@ikem.cz Kidney has a limited number of tissue reactions by which the kidney
Immunopathology of T cell mediated rejection
 Immunopathology of T cell mediated rejection Ibrahim Batal MD Columbia University College of Physicians & Surgeons New York, NY, USA Overview Pathophysiology and grading of TCMR TCMR is still a significant
Immunopathology of T cell mediated rejection Ibrahim Batal MD Columbia University College of Physicians & Surgeons New York, NY, USA Overview Pathophysiology and grading of TCMR TCMR is still a significant
RENAL EVENING SPECIALTY CONFERENCE
 RENAL EVENING SPECIALTY CONFERENCE Harsharan K. Singh, MD The University of North Carolina at Chapel Hill Disclosure of Relevant Financial Relationships No conflicts of interest to disclose. CLINICAL HISTORY
RENAL EVENING SPECIALTY CONFERENCE Harsharan K. Singh, MD The University of North Carolina at Chapel Hill Disclosure of Relevant Financial Relationships No conflicts of interest to disclose. CLINICAL HISTORY
Transplantation in highly sensitised patients treated with intravenous immunoglobulin and Rituximab
 ORIGINAL ARTICLE Advance Access publication 1 February 2010 Transplantation in highly sensitised patients treated with intravenous immunoglobulin and Rituximab Ana Carina Ferreira 1, Sandra Brum 1, Vasco
ORIGINAL ARTICLE Advance Access publication 1 February 2010 Transplantation in highly sensitised patients treated with intravenous immunoglobulin and Rituximab Ana Carina Ferreira 1, Sandra Brum 1, Vasco
Overview of New Approaches to Immunosuppression in Renal Transplantation
 Overview of New Approaches to Immunosuppression in Renal Transplantation Ron Shapiro, M.D. Professor of Surgery Surgical Director, Kidney/Pancreas Transplant Program Recanati/Miller Transplantation Institute
Overview of New Approaches to Immunosuppression in Renal Transplantation Ron Shapiro, M.D. Professor of Surgery Surgical Director, Kidney/Pancreas Transplant Program Recanati/Miller Transplantation Institute
Statement of Disclosure
 Statement of Disclosure Mark Haas serves as a paid consultant on pathology adjudication committees for two industry-sponsored clinical trials: Shire ViroPharma Treatment of Acute ABMR AstraZeneca Treatment
Statement of Disclosure Mark Haas serves as a paid consultant on pathology adjudication committees for two industry-sponsored clinical trials: Shire ViroPharma Treatment of Acute ABMR AstraZeneca Treatment
Summary of T32 Research Joshua Weiner June 2015
 Summary of T32 Research Joshua Weiner June 2015 Project 1: Regulatory T Cell (Treg) Expansion Xenotransplantation could help make up the difference in the numbers of organs needed for transplantation and
Summary of T32 Research Joshua Weiner June 2015 Project 1: Regulatory T Cell (Treg) Expansion Xenotransplantation could help make up the difference in the numbers of organs needed for transplantation and
Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab
 TRANSPLANTATION Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab Khadijeh Makhdoomi, 1,2 Saeed Abkhiz, 1,2 Farahnaz Noroozinia, 1,3
TRANSPLANTATION Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab Khadijeh Makhdoomi, 1,2 Saeed Abkhiz, 1,2 Farahnaz Noroozinia, 1,3
The Banff Classification for Diagnosis of Renal Allograft Rejection: Updates from the 2017 Banff Conference
 The Banff Classification for Diagnosis of Renal Allograft Rejection: Updates from the 2017 Banff Conference Mark Haas Cedars-Sinai Medical Center Los Angeles, California, USA Statement of Disclosure Mark
The Banff Classification for Diagnosis of Renal Allograft Rejection: Updates from the 2017 Banff Conference Mark Haas Cedars-Sinai Medical Center Los Angeles, California, USA Statement of Disclosure Mark
FIT Board Review Corner March 2016
 FIT Board Review Corner March 2016 Welcome to the FIT Board Review Corner, prepared by Sarah Spriet, DO, and Tammy Peng, MD, senior and junior representatives of ACAAI's Fellows-In-Training (FITs) to the
FIT Board Review Corner March 2016 Welcome to the FIT Board Review Corner, prepared by Sarah Spriet, DO, and Tammy Peng, MD, senior and junior representatives of ACAAI's Fellows-In-Training (FITs) to the
Intruduction PSI MODE OF ACTION AND PHARMACOKINETICS
 Multidisciplinary Insights on Clinical Guidance for the Use of Proliferation Signal Inhibitors in Heart Transplantation Andreas Zuckermann, MD et al. Department of Cardio-Thoracic Surgery, Medical University
Multidisciplinary Insights on Clinical Guidance for the Use of Proliferation Signal Inhibitors in Heart Transplantation Andreas Zuckermann, MD et al. Department of Cardio-Thoracic Surgery, Medical University
Literature Review Transplantation
 Literature Review 2010- Transplantation Alexander Wiseman, M.D. Associate Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas Transplant Programs University of
Literature Review 2010- Transplantation Alexander Wiseman, M.D. Associate Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas Transplant Programs University of
Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function
 ArtIcle Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function Guodong Chen, 1 Jingli Gu, 2 Jiang Qiu, 1 Changxi
ArtIcle Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function Guodong Chen, 1 Jingli Gu, 2 Jiang Qiu, 1 Changxi
Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant
 SDC, Patients and Methods Complement-dependent lymphocytotoxic crossmatch test () Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant donor-specific CXM was
SDC, Patients and Methods Complement-dependent lymphocytotoxic crossmatch test () Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant donor-specific CXM was
Biopsy Features of Kidney Allograft Rejection Banff B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary
 Biopsy Features of Kidney Allograft Rejection Banff 2017 B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary Treatment of allograft dysfunction should rely on the biopsy findings
Biopsy Features of Kidney Allograft Rejection Banff 2017 B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary Treatment of allograft dysfunction should rely on the biopsy findings
Monoclonal Gammopathies and the Kidney. Tibor Nádasdy, MD The Ohio State University, Columbus, OH
 Monoclonal Gammopathies and the Kidney Tibor Nádasdy, MD The Ohio State University, Columbus, OH Monoclonal gammopathy of renal significance (MGRS) Biopsies at OSU (n=475) between 2007 and 2016 AL or AH
Monoclonal Gammopathies and the Kidney Tibor Nádasdy, MD The Ohio State University, Columbus, OH Monoclonal gammopathy of renal significance (MGRS) Biopsies at OSU (n=475) between 2007 and 2016 AL or AH
Posttransplant Human Leukocyte Antigen Antibodies in Stable Kidney Transplant Recipients
 Trends in Transplant. 2014;8:3-9 Gregor Bartel, Georg A. Böhmig: Alloantibodies and Graft Function Posttransplant Human Leukocyte Antigen Antibodies in Stable Kidney Transplant Recipients Gregor Bartel
Trends in Transplant. 2014;8:3-9 Gregor Bartel, Georg A. Böhmig: Alloantibodies and Graft Function Posttransplant Human Leukocyte Antigen Antibodies in Stable Kidney Transplant Recipients Gregor Bartel
Steroid Minimization: Great Idea or Silly Move?
 Steroid Minimization: Great Idea or Silly Move? Disclosures I have financial relationship(s) within the last 12 months relevant to my presentation with: Astellas Grants ** Bristol Myers Squibb Grants,
Steroid Minimization: Great Idea or Silly Move? Disclosures I have financial relationship(s) within the last 12 months relevant to my presentation with: Astellas Grants ** Bristol Myers Squibb Grants,
Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism
 Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism THOMAS R. SPITZER Bone Marrow Transplant Program, Harvard Medical School, Boston, Massachusetts, USA Key Words.
Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism THOMAS R. SPITZER Bone Marrow Transplant Program, Harvard Medical School, Boston, Massachusetts, USA Key Words.
Dr. Yi-chi M. Kong August 8, 2001 Benjamini. Ch. 19, Pgs Page 1 of 10 TRANSPLANTATION
 Benjamini. Ch. 19, Pgs 379-399 Page 1 of 10 TRANSPLANTATION I. KINDS OF GRAFTS II. RELATIONSHIPS BETWEEN DONOR AND RECIPIENT Benjamini. Ch. 19, Pgs 379-399 Page 2 of 10 II.GRAFT REJECTION IS IMMUNOLOGIC
Benjamini. Ch. 19, Pgs 379-399 Page 1 of 10 TRANSPLANTATION I. KINDS OF GRAFTS II. RELATIONSHIPS BETWEEN DONOR AND RECIPIENT Benjamini. Ch. 19, Pgs 379-399 Page 2 of 10 II.GRAFT REJECTION IS IMMUNOLOGIC
Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome
 Pediatr Nephrol (2003) 18:833 837 DOI 10.1007/s00467-003-1175-4 BRIEF REPORT Gina-Marie Barletta William E. Smoyer Timothy E. Bunchman Joseph T. Flynn David B. Kershaw Use of mycophenolate mofetil in steroid-dependent
Pediatr Nephrol (2003) 18:833 837 DOI 10.1007/s00467-003-1175-4 BRIEF REPORT Gina-Marie Barletta William E. Smoyer Timothy E. Bunchman Joseph T. Flynn David B. Kershaw Use of mycophenolate mofetil in steroid-dependent
Transplantation. Immunology Unit College of Medicine King Saud University
 Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
SELECTED ABSTRACTS. All (n) % 3-year GS 88% 82% 86% 85% 88% 80% % 3-year DC-GS 95% 87% 94% 89% 96% 80%
 SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
Acute Renal Endothelial Injury During Marrow Recovery in a Cohort of Combined Kidney and Bone Marrow Allografts
 Wiley Periodicals Inc. C 2011 The Authors Journal compilation C 2011 The American Society of Transplantation and the American Society of Transplant Surgeons Acute Renal Endothelial Injury During Marrow
Wiley Periodicals Inc. C 2011 The Authors Journal compilation C 2011 The American Society of Transplantation and the American Society of Transplant Surgeons Acute Renal Endothelial Injury During Marrow
Strategies for Desensitization
 Strategies for Desensitization Olwyn Johnston MB, MRCPI, MD, MHSc BC Nephrology Day October 8 th 2010 Pre-transplant crossmatch (CMX) with donor lymphocytes has been standard of practice Positive CDC CXM
Strategies for Desensitization Olwyn Johnston MB, MRCPI, MD, MHSc BC Nephrology Day October 8 th 2010 Pre-transplant crossmatch (CMX) with donor lymphocytes has been standard of practice Positive CDC CXM
Tolerance and Chimerism after Renal and Hematopoietic-Cell Transplantation
 T h e n e w e ng l a nd j o u r na l o f m e dic i n e brief report Tolerance and Chimerism after Renal and Hematopoietic-Cell Transplantation John D. Scandling, M.D., Stephan Busque, M.D., Sussan Dejbakhsh-Jones,
T h e n e w e ng l a nd j o u r na l o f m e dic i n e brief report Tolerance and Chimerism after Renal and Hematopoietic-Cell Transplantation John D. Scandling, M.D., Stephan Busque, M.D., Sussan Dejbakhsh-Jones,
NIH Public Access Author Manuscript Am J Transplant. Author manuscript; available in PMC 2012 June 1.
 NIH Public Access Author Manuscript Published in final edited form as: Am J Transplant. 2011 June ; 11(6): 1236 1247. doi:10.1111/j.1600-6143.2011.03566.x. Mechanisms of Donor-Specific Tolerance in Recipients
NIH Public Access Author Manuscript Published in final edited form as: Am J Transplant. 2011 June ; 11(6): 1236 1247. doi:10.1111/j.1600-6143.2011.03566.x. Mechanisms of Donor-Specific Tolerance in Recipients
Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients
 Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism Thomas R. Spitzer. doi: /theoncologist.
 Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism Thomas R. Spitzer The Oncologist 2000, 5:215-223. doi: 10.1634/theoncologist.5-3-215 The online version of this
Nonmyeloablative Allogeneic Stem Cell Transplant Strategies and the Role of Mixed Chimerism Thomas R. Spitzer The Oncologist 2000, 5:215-223. doi: 10.1634/theoncologist.5-3-215 The online version of this
Case Report A Clinical and Pathological Variant of Acute Transplant Glomerulopathy
 Case Report A Clinical and Pathological Variant of Acute Transplant Glomerulopathy Miklos Z. Molnar, 1 G. V. Ramesh Prasad, 2 Darren A. Yuen, 2,3 Serge Jothy, 4 and Jeffrey S. Zaltzman 2,5 1 Division of
Case Report A Clinical and Pathological Variant of Acute Transplant Glomerulopathy Miklos Z. Molnar, 1 G. V. Ramesh Prasad, 2 Darren A. Yuen, 2,3 Serge Jothy, 4 and Jeffrey S. Zaltzman 2,5 1 Division of
Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani, 2 Rajni Agarwal-Hashmi, 3 Melissa Aldinger, 4 Franco Locatelli 1
 Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
BK virus infection in renal transplant recipients: single centre experience. Dr Wong Lok Yan Ivy
 BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
Desensitization in Kidney Transplant. James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver
 Desensitization in Kidney Transplant James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Organ Shortage Currently there are >90,000 patients on the kidney
Desensitization in Kidney Transplant James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Organ Shortage Currently there are >90,000 patients on the kidney
Results of a Phase 1 Trial of Treg Adoptive Cell Transfer (TRACT) in De Novo Living Donor Kidney Transplant Recipients
 Results of a Phase 1 Trial of Treg Adoptive Cell Transfer (TRACT) in De Novo Living Donor Kidney Transplant Recipients A Skaro, A LeFever, J Mathew, L Gallon, J Hie, C Hansen, D Stare, G Johnson, J Leventhal
Results of a Phase 1 Trial of Treg Adoptive Cell Transfer (TRACT) in De Novo Living Donor Kidney Transplant Recipients A Skaro, A LeFever, J Mathew, L Gallon, J Hie, C Hansen, D Stare, G Johnson, J Leventhal
Case 3. ACCME/Disclosure. Laboratory results. Clinical history 4/13/2016
 Case 3 Lynn D. Cornell, M.D. Mayo Clinic, Rochester, MN Cornell.Lynn@mayo.edu USCAP Renal Case Conference March 13, 2016 ACCME/Disclosure Dr. Cornell has nothing to disclose Clinical history 57-year-old
Case 3 Lynn D. Cornell, M.D. Mayo Clinic, Rochester, MN Cornell.Lynn@mayo.edu USCAP Renal Case Conference March 13, 2016 ACCME/Disclosure Dr. Cornell has nothing to disclose Clinical history 57-year-old
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
Kidney Summary. Mark Haas Cedars-Sinai Medical Center Los Angeles, California, USA
 Kidney Summary Mark Haas Cedars-Sinai Medical Center Los Angeles, California, USA Key Issues to Address re: the Classification 1. Incorporation of i-ifta + tubulitis into the TCMR classification - Defining
Kidney Summary Mark Haas Cedars-Sinai Medical Center Los Angeles, California, USA Key Issues to Address re: the Classification 1. Incorporation of i-ifta + tubulitis into the TCMR classification - Defining
This study is currently recruiting participants.
 A Two Part, Phase 1/2, Safety, PK and PD Study of TOL101, an Anti-TCR Monoclonal Antibody for Prophylaxis of Acute Organ Rejection in Patients Receiving Renal Transplantation This study is currently recruiting
A Two Part, Phase 1/2, Safety, PK and PD Study of TOL101, an Anti-TCR Monoclonal Antibody for Prophylaxis of Acute Organ Rejection in Patients Receiving Renal Transplantation This study is currently recruiting
Why Do We Need New Immunosuppressive Agents
 Why Do We Need New Immunosuppressive Agents 1 Reducing acute rejection rates has not transplanted into better long-term graft survival Incidence of early acute rejection episodes by era Relative risk for
Why Do We Need New Immunosuppressive Agents 1 Reducing acute rejection rates has not transplanted into better long-term graft survival Incidence of early acute rejection episodes by era Relative risk for
Chapter 4: Steroid-resistant nephrotic syndrome in children Kidney International Supplements (2012) 2, ; doi: /kisup.2012.
 http://www.kidney-international.org & 2012 KDIGO Chapter 4: Steroid-resistant nephrotic syndrome in children Kidney International Supplements (2012) 2, 172 176; doi:10.1038/kisup.2012.17 INTRODUCTION This
http://www.kidney-international.org & 2012 KDIGO Chapter 4: Steroid-resistant nephrotic syndrome in children Kidney International Supplements (2012) 2, 172 176; doi:10.1038/kisup.2012.17 INTRODUCTION This
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding
RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT. J. H. Helderman,MD,FACP,FAST
 RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT J. H. Helderman,MD,FACP,FAST Vanderbilt University Medical Center Professor of Medicine, Pathology and Immunology Medical Director, Vanderbilt Transplant
RECURRENT AND DE NOVO RENAL DISEASES IN THE ALLOGRAFT J. H. Helderman,MD,FACP,FAST Vanderbilt University Medical Center Professor of Medicine, Pathology and Immunology Medical Director, Vanderbilt Transplant
Emerging Drug List EVEROLIMUS
 Generic (Trade Name): Manufacturer: Everolimus (Certican ) Novartis Pharmaceuticals NO. 57 MAY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence: For use with cyclosporine
Generic (Trade Name): Manufacturer: Everolimus (Certican ) Novartis Pharmaceuticals NO. 57 MAY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence: For use with cyclosporine
NAPRTCS Annual Transplant Report
 North American Pediatric Renal Trials and Collaborative Studies NAPRTCS 2014 Annual Transplant Report This is a privileged communication not for publication. TABLE OF CONTENTS PAGE II TRANSPLANTATION Section
North American Pediatric Renal Trials and Collaborative Studies NAPRTCS 2014 Annual Transplant Report This is a privileged communication not for publication. TABLE OF CONTENTS PAGE II TRANSPLANTATION Section
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Altered regulatory T cell homeostasis in patients with CD4 + lymphopenia following allogeneic hematopoietic stem cell transplantation
 Altered regulatory T cell homeostasis in patients with CD4 + lymphopenia following allogeneic hematopoietic stem cell transplantation Ken-ichi Matsuoka,, Robert J. Soiffer, Jerome Ritz J Clin Invest. 2010;120(5):1479-1493.
Altered regulatory T cell homeostasis in patients with CD4 + lymphopenia following allogeneic hematopoietic stem cell transplantation Ken-ichi Matsuoka,, Robert J. Soiffer, Jerome Ritz J Clin Invest. 2010;120(5):1479-1493.
What are the key challenges we face in kidney transplantation today?
 TRANSPLANTATION RESEARCH R E V I E W Open Access What are the key challenges we face in kidney transplantation today? Jeremy R Chapman* Abstract Transplantation is more predictable than it was 20 to 30
TRANSPLANTATION RESEARCH R E V I E W Open Access What are the key challenges we face in kidney transplantation today? Jeremy R Chapman* Abstract Transplantation is more predictable than it was 20 to 30
10 Center for Infection and Immunity, Mailman School of Public Health, Columbia
 Original Basic ScienceçGeneral Effect of Ex Vivo Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques Raimon Duran-Struuck,
Original Basic ScienceçGeneral Effect of Ex Vivo Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques Raimon Duran-Struuck,
Histopathology: Glomerulonephritis and other renal pathology
 Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Acute Graft-versus-Host Disease (agvhd) Udomsak Bunworasate Chulalongkorn University
 Acute Graft-versus-Host Disease (agvhd) Udomsak Bunworasate Chulalongkorn University Graft-versus-Host Disease (GVHD) Background GVHD is an immunologic reaction of the donor immune cells (Graft) against
Acute Graft-versus-Host Disease (agvhd) Udomsak Bunworasate Chulalongkorn University Graft-versus-Host Disease (GVHD) Background GVHD is an immunologic reaction of the donor immune cells (Graft) against
NAPRTCS Annual Transplant Report
 North American Pediatric Renal Trials and Collaborative Studies NAPRTCS 2010 Annual Transplant Report This is a privileged communication not for publication. TABLE OF CONTENTS PAGE I INTRODUCTION 1 II
North American Pediatric Renal Trials and Collaborative Studies NAPRTCS 2010 Annual Transplant Report This is a privileged communication not for publication. TABLE OF CONTENTS PAGE I INTRODUCTION 1 II
Modulating STAT Signaling to Promote Engraftment of Allogeneic Bone Marrow Transplant
 Modulating STAT Signaling to Promote Engraftment of Allogeneic Bone Marrow Transplant Jacopo Mariotti, M.D. Center for Cancer Research, NCI, NIH Istituto Nazionale Tumori, Milano Verona; May 22, 2009 Non-myeloablative
Modulating STAT Signaling to Promote Engraftment of Allogeneic Bone Marrow Transplant Jacopo Mariotti, M.D. Center for Cancer Research, NCI, NIH Istituto Nazionale Tumori, Milano Verona; May 22, 2009 Non-myeloablative
3/6/2017. Prevention of Complement Activation and Antibody Development: Results from the Duet Trial
 Prevention of Complement Activation and Antibody Development: Results from the Duet Trial Jignesh Patel MD PhD FACC FRCP Medical Director, Heart Transplant Cedars-Sinai Heart Institute Disclosures Name:
Prevention of Complement Activation and Antibody Development: Results from the Duet Trial Jignesh Patel MD PhD FACC FRCP Medical Director, Heart Transplant Cedars-Sinai Heart Institute Disclosures Name:
Case Presentation Turki Al-Hussain, MD
 Case Presentation Turki Al-Hussain, MD Director, Renal Pathology Chapter Saudi Society of Nephrology & Transplantation Consultant Nephropathologist & Urological Pathologist Department of Pathology & Laboratory
Case Presentation Turki Al-Hussain, MD Director, Renal Pathology Chapter Saudi Society of Nephrology & Transplantation Consultant Nephropathologist & Urological Pathologist Department of Pathology & Laboratory
Innovation In Transplantation:
 Innovation In Transplantation: Improving outcomes Thomas C. Pearson Department of Surgery Emory Transplant Center CHOA Symposium October 22, 2016 Disclosures Belatacept preclinical and clinical trial were
Innovation In Transplantation: Improving outcomes Thomas C. Pearson Department of Surgery Emory Transplant Center CHOA Symposium October 22, 2016 Disclosures Belatacept preclinical and clinical trial were
Update on Transplant Glomerulopathy
 Update on Transplant Glomerulopathy Miklos Z Molnar, MD, PhD, FEBTM, FERA, FASN Associate Professor of Medicine Division of Nephrology, Department of Medicine University of Tennessee Health Science Center
Update on Transplant Glomerulopathy Miklos Z Molnar, MD, PhD, FEBTM, FERA, FASN Associate Professor of Medicine Division of Nephrology, Department of Medicine University of Tennessee Health Science Center
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Lionel Rostaing, Nassim Kamar: Alefacept and Transplantation. Alefacept Treatment in the Setting of Transplantation
 Trends in Transplant. 2014;8:35-40 Lionel Rostaing, Nassim Kamar: Alefacept and Transplantation Alefacept Treatment in the Setting of Transplantation Lionel Rostaing and Nassim Kamar Department of Nephrology
Trends in Transplant. 2014;8:35-40 Lionel Rostaing, Nassim Kamar: Alefacept and Transplantation Alefacept Treatment in the Setting of Transplantation Lionel Rostaing and Nassim Kamar Department of Nephrology
Microcirculation Inflammation Associates With Outcome in Renal Transplant Patients With De Novo Donor-Specific Antibodies
 American Journal of Transplantation 2013; 13: 485 492 Wiley Periodicals Inc. Brief Communication C Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons
American Journal of Transplantation 2013; 13: 485 492 Wiley Periodicals Inc. Brief Communication C Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons
Acute Antibody-Mediated Rejection in Renal Transplantation: Current Clinical Management
 Curr Transpl Rep (2014) 1:78 85 DOI 10.1007/s40472-014-0012-y KIDNEY TRANSPLANTATION (ML HENRY, SECTION EDITOR) Acute Antibody-Mediated Rejection in Renal Transplantation: Current Clinical Management Carrie
Curr Transpl Rep (2014) 1:78 85 DOI 10.1007/s40472-014-0012-y KIDNEY TRANSPLANTATION (ML HENRY, SECTION EDITOR) Acute Antibody-Mediated Rejection in Renal Transplantation: Current Clinical Management Carrie
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018
 Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Nephrotic syndrome minimal change disease vs. IgA nephropathy. Hadar Meringer Internal medicine B Sheba
 Nephrotic syndrome minimal change disease vs. IgA nephropathy Hadar Meringer Internal medicine B Sheba The Case 29 year old man diagnosed with nephrotic syndrome 2 weeks ago and complaining now about Lt.flank
Nephrotic syndrome minimal change disease vs. IgA nephropathy Hadar Meringer Internal medicine B Sheba The Case 29 year old man diagnosed with nephrotic syndrome 2 weeks ago and complaining now about Lt.flank
Literature Review: Transplantation July 2010-June 2011
 Literature Review: Transplantation July 2010-June 2011 James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Kidney Transplant Top 10 List: July Kidney
Literature Review: Transplantation July 2010-June 2011 James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Kidney Transplant Top 10 List: July Kidney
Interstitial Inflammation
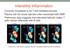 Interstitial Inflammation Currently considered to be T cell-mediated process Plasma cell rich acute rejection often associated with AMR Preliminary data suggests that interstitial follicular helper T cells
Interstitial Inflammation Currently considered to be T cell-mediated process Plasma cell rich acute rejection often associated with AMR Preliminary data suggests that interstitial follicular helper T cells
Pathology of Kidney Allograft Dysfunction. B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary
 Pathology of Kidney Allograft Dysfunction B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary The gold standard for exploration of the cause of an allograft dysfunction is to perform
Pathology of Kidney Allograft Dysfunction B. Ivanyi, MD Department of Pathology, University of Szeged, Szeged, Hungary The gold standard for exploration of the cause of an allograft dysfunction is to perform
DE-MYSTIFYING THE BLACK BOX OF TRANSPLANT IMMUNOLOGY
 2016 DE-MYSTIFYING THE BLACK BOX OF TRANSPLANT IMMUNOLOGY James H Lan, MD, FRCP(C), D(ABHI) Clinical Assistant Professor, University of British Columbia Nephrology & Kidney Transplantation, Vancouver General
2016 DE-MYSTIFYING THE BLACK BOX OF TRANSPLANT IMMUNOLOGY James H Lan, MD, FRCP(C), D(ABHI) Clinical Assistant Professor, University of British Columbia Nephrology & Kidney Transplantation, Vancouver General
HLA Part II: My Patient Has DSA, Now What?
 2017 CST-Astellas Canadian Transplant Fellows Symposium HLA Part II: My Patient Has DSA, Now What? James Lan, MD, FRCPC, D(ABHI) Dr. Lan completed his nephrology training at the University of British Columbia.
2017 CST-Astellas Canadian Transplant Fellows Symposium HLA Part II: My Patient Has DSA, Now What? James Lan, MD, FRCPC, D(ABHI) Dr. Lan completed his nephrology training at the University of British Columbia.
The new Banff vision of the role of HLA antibodies in organ transplantation: Improving diagnostic system and design of clinical trials
 The new Banff vision of the role of HLA antibodies in organ transplantation: Improving diagnostic system and design of clinical trials Carmen Lefaucheur 1 2 Banff 2015: Integration of HLA-Ab for improving
The new Banff vision of the role of HLA antibodies in organ transplantation: Improving diagnostic system and design of clinical trials Carmen Lefaucheur 1 2 Banff 2015: Integration of HLA-Ab for improving
Year 2004 Paper one: Questions supplied by Megan
 QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
Utility of protocol kidney biopsies for de novo donor- specific antibodies
 Received: 6 June 2017 Revised: 24 July 2017 Accepted: 29 July 2017 DOI: 10.1111/ajt.14466 BRIEF COMMUNICATION Utility of protocol kidney biopsies for de novo donor- specific antibodies Sandesh Parajuli
Received: 6 June 2017 Revised: 24 July 2017 Accepted: 29 July 2017 DOI: 10.1111/ajt.14466 BRIEF COMMUNICATION Utility of protocol kidney biopsies for de novo donor- specific antibodies Sandesh Parajuli
Eculizumab chez les receveurs de greffe rénal à haut risque immunologique. Mark D. Stegall Mayo Clinic, Rochester, MN
 Eculizumab chez les receveurs de greffe rénal à haut risque immunologique Mark D. Stegall Mayo Clinic, Rochester, MN Disclosure. Dr Mark Stegall. Institution : Mayo Clinic, Rochester. Research contracts
Eculizumab chez les receveurs de greffe rénal à haut risque immunologique Mark D. Stegall Mayo Clinic, Rochester, MN Disclosure. Dr Mark Stegall. Institution : Mayo Clinic, Rochester. Research contracts
Acute Renal Endothelial Injury During Marrow Recovery in a Cohort of Combined Kidney and Bone Marrow Allografts
 Acute Renal Endothelial Injury During Marrow Recovery in a Cohort of Combined Kidney and Bone Marrow Allografts The Harvard community has made this article openly available. Please share how this access
Acute Renal Endothelial Injury During Marrow Recovery in a Cohort of Combined Kidney and Bone Marrow Allografts The Harvard community has made this article openly available. Please share how this access
Peritubular capillaries C4d deposits in renal allograft biopsies and anti HLA I/II alloantibodies screening Incidence and clinical importance
 ORIGINAL ARTICLE Port J Nephrol Hypert 2008; 22(1): 37-42 Peritubular capillaries C4d deposits in renal allograft biopsies and anti HLA I/II alloantibodies screening Incidence and clinical importance Helena
ORIGINAL ARTICLE Port J Nephrol Hypert 2008; 22(1): 37-42 Peritubular capillaries C4d deposits in renal allograft biopsies and anti HLA I/II alloantibodies screening Incidence and clinical importance Helena
Belatacept: An Update of Ongoing Clinical Trials
 Belatacept: An Update of Ongoing Clinical Trials Michael D. Rizzari, MD University of Wisconsin Madison School of Medicine and Public Health, Madison, Wisconsin Abstract Belatacept is a fusion protein
Belatacept: An Update of Ongoing Clinical Trials Michael D. Rizzari, MD University of Wisconsin Madison School of Medicine and Public Health, Madison, Wisconsin Abstract Belatacept is a fusion protein
Intravenous immunoglobulin in BK virus nephropathy
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2014 Intravenous immunoglobulin in BK virus nephropathy Elizabeth I. Anyaegbu Driscoll Children's Hospital Stanley
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2014 Intravenous immunoglobulin in BK virus nephropathy Elizabeth I. Anyaegbu Driscoll Children's Hospital Stanley
Index. electron microscopy, 81 immunofluorescence microscopy, 80 light microscopy, 80 Amyloidosis clinical setting, 185 etiology/pathogenesis,
 A Acute antibody-mediated rejection (Acute AMR) clinical features, 203 clinicopathologic correlations, 206 pathogenesis, 205 206 204 205 light microscopy, 203 204 Acute cellular rejection (ACR) clinical
A Acute antibody-mediated rejection (Acute AMR) clinical features, 203 clinicopathologic correlations, 206 pathogenesis, 205 206 204 205 light microscopy, 203 204 Acute cellular rejection (ACR) clinical
Interpretation of Renal Transplant Biopsy. Arthur H. Cohen Wake Forest University School of Medicine Winston-Salem, North Carolina USA
 Interpretation of Renal Transplant Biopsy Arthur H. Cohen Wake Forest University School of Medicine Winston-Salem, North Carolina USA Renal Transplant Biopsies Tissue Processing Ideal world process as
Interpretation of Renal Transplant Biopsy Arthur H. Cohen Wake Forest University School of Medicine Winston-Salem, North Carolina USA Renal Transplant Biopsies Tissue Processing Ideal world process as
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
3/6/2017. Treatment of Detected Antibodies. I have financial relationship(s) with: Thoratec/St. Jude/Abbott Consultant CareDx Consultant/Speaker
 Treatment of Detected Antibodies Sean Pinney, MD Director, Advanced Heart Failure & Transplantation Mount Sinai Hospital New York, NY Sean Pinney, MD Associate Professor of Medicine Icahn School of Medicine
Treatment of Detected Antibodies Sean Pinney, MD Director, Advanced Heart Failure & Transplantation Mount Sinai Hospital New York, NY Sean Pinney, MD Associate Professor of Medicine Icahn School of Medicine
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
BK Virus (BKV) Management Guideline: July 2017
 BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
Considering the early proactive switch from a CNI to an mtor-inhibitor (Case: Male, age 34) Josep M. Campistol
 Considering the early proactive switch from a CNI to an mtor-inhibitor (Case: Male, age 34) Josep M. Campistol Patient details Name DOB ESRD Other history Mr. B.I.B. 12 January 1975 (34yo) Membranous GN
Considering the early proactive switch from a CNI to an mtor-inhibitor (Case: Male, age 34) Josep M. Campistol Patient details Name DOB ESRD Other history Mr. B.I.B. 12 January 1975 (34yo) Membranous GN
Chapter 6: Idiopathic focal segmental glomerulosclerosis in adults Kidney International Supplements (2012) 2, ; doi: /kisup.2012.
 http://www.kidney-international.org chapter 6 & 2012 KDIGO Chapter 6: Idiopathic focal segmental glomerulosclerosis in adults Kidney International Supplements (2012) 2, 181 185; doi:10.1038/kisup.2012.19
http://www.kidney-international.org chapter 6 & 2012 KDIGO Chapter 6: Idiopathic focal segmental glomerulosclerosis in adults Kidney International Supplements (2012) 2, 181 185; doi:10.1038/kisup.2012.19
Non-myeloablative allogeneic stem cell transplantation focusing on immunotherapy of life-threatening malignant and non-malignant diseases
 Critical Reviews in Oncology/Hematology 39 (2001) 25 29 www.elsevier.com/locate/critrevonc Non-myeloablative allogeneic stem cell transplantation focusing on immunotherapy of life-threatening malignant
Critical Reviews in Oncology/Hematology 39 (2001) 25 29 www.elsevier.com/locate/critrevonc Non-myeloablative allogeneic stem cell transplantation focusing on immunotherapy of life-threatening malignant
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
Transplantation Immunology
 Transplantation Immunology Mitchell S. Cairo, MD Professor of Pediatrics, Medicine and Pathology Chief, Division, Pediatric Hematology & Blood & Marrow Transplantation Children s Hospital New York Presbyterian
Transplantation Immunology Mitchell S. Cairo, MD Professor of Pediatrics, Medicine and Pathology Chief, Division, Pediatric Hematology & Blood & Marrow Transplantation Children s Hospital New York Presbyterian
Transplantation Immunology
 Transplantation Immunology MHC Restricted Allograft Rejection Mitchell S. Cairo, MD Professor of Pediatrics, Medicine and Pathology Chief, Division, Pediatric Hematology & Blood & Marrow Transplantation
Transplantation Immunology MHC Restricted Allograft Rejection Mitchell S. Cairo, MD Professor of Pediatrics, Medicine and Pathology Chief, Division, Pediatric Hematology & Blood & Marrow Transplantation
ABO INCOMPATILIBITY AND TRANSPLANTATION
 ABO INCOMPATILIBITY AND TRANSPLANTATION Aleksandar Mijovic Consultant Haematologist/Senior Lecturer King s College Hospital/NHS Blood and Transplant London, UK RTC Edu Meeting May 2017 ABO antigens Expressed
ABO INCOMPATILIBITY AND TRANSPLANTATION Aleksandar Mijovic Consultant Haematologist/Senior Lecturer King s College Hospital/NHS Blood and Transplant London, UK RTC Edu Meeting May 2017 ABO antigens Expressed
