1. Introduction Ljubljana, Slovenia 4 Department of Medical Psychology, Hannover Medical School, Hannover, Germany
|
|
|
- Ambrose Stevens
- 5 years ago
- Views:
Transcription
1 Hindawi Publishing Corporation Journal of Diabetes Research Volume 2015, Article ID , 8 pages Clinical Study Reduced Worries of Hypoglycaemia, High Satisfaction, and Increased Perceived Ease of Use after Experiencing Four Nights of MD-Logic Artificial Pancreas at Home (DREAM4) Claudia Ziegler, 1 Alon Liberman, 2 Revital Nimri, 2 Ido Muller, 2 Simona KlemenIiI, 3 Nataša Bratina, 3 Sarah Bläsig, 1 Kerstin Remus, 1 Moshe Phillip, 2 Tadej Battelino, 3 Olga Kordonouri, 1 Thomas Danne, 1 and Karin Lange 4 1 Diabetes Centre for Children and Adolescents, Kinder- und Jugendkrankenhaus AUF DER BULT, Hannover, Germany 2 The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children s Medical Center of Israel, Petah Tikva, Israel 3 Department of Pediatric Endocrinology, Diabetes and Metabolism, University Medical Centre-University Children s Hospital, 1000 Ljubljana, Slovenia 4 Department of Medical Psychology, Hannover Medical School, Hannover, Germany Correspondence should be addressed to Claudia Ziegler; ziegler-claudia@web.de Received 19 January 2015; Accepted 5 March 2015 Academic Editor: Viral Shah Copyright 2015 Claudia Ziegler et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aims. This study assesses the impact of using an AP-system at home on fear of hypoglycaemia. In addition, satisfaction and acceptance of the new technology are evaluated. Methods. In a multicentre, multinational study of 75 patients using the MD- Logic AP during four consecutive nights in home setting 59 of them (aged years, 54% male, HbA1c 7.89 ± 0.69% [62.72 ± 7.51 mmol/mol], diabetes duration 11.6 ± 8.4 yrs) answered standardized questionnaires (HFS, adapted TAM, and AP satisfaction) before and after using the AP. Results. After experiencing the AP in home setting worries of hypoglycaemia were significantly reduced (before 1.04 ± 0.53 versus after 0.90 ± 0.63; P = 0.017). Perceived ease of use as a measure of acceptance with the AP significantly increased after personal experience (before 4.64 ± 0.94 versus after 5.06 ± 1.09; P = 0.002). The overall satisfaction mean score after using the AP was 3.02 ± 0.54 (range 0 4), demonstrating a high level of satisfaction with this technology. Conclusions. The four-night home-based experience of using MD Logic AP was associated with reduced worries of hypoglycaemia, high level of satisfaction, and increased perceived ease of use of the new technology in children, adolescents, and adults. 1. Introduction Current research is focusing on the artificial pancreas (AP) or the so-called closed-loop systems (CLS) to optimize metabolic control in patients with type 1 diabetes mellitus (T1DM). An AP combines continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) with a control algorithm to calculate insulin delivery in response to sensor data. Different artificial pancreas systems from various research groups have shown the superiority of the artificial pancreas compared to standard CSII therapy regarding overall glucose control and risk of nocturnal hypoglycaemia. These results were achieved during controlled conditions of an inpatient environment (review by [1]). Now first studies started to evaluate the AP system at the patient s home. The Diabetes Wireless Artificial Pancreas Consortium (DREAM- Project, [2]) assessed the MD-Logic artificial pancreas system [3, 4] outside hospital settings under real-life conditions. Meanwhile the safety and efficacy of the MD-Logic automated insulin delivery system was demonstrated in hospital setting [1]aswellasindiabetescamps[2] and in home setting [5, 6].Briefly,TheMD-Logicisawirelessfullyautomated closed-loop system based on a fuzzy logic theory algorithm, a learning algorithm, a personalized system setting, and alerts
2 2 Journal of Diabetes Research module. The alerts module includes real-time alarms such as impending hypoglycaemia and long standing hyperglycaemia. The algorithm for alerts integrates information derived from past glucose levels and insulin delivery (time and dose) as well as models of insulin pharmacodynamics. The hypoglycaemia alarms are designed to operate in instances when impending hypoglycaemia cannot be avoided by holding insulin alone [4]. While the metabolic efficacy of the existing AP systems is impressing, its psychological impact remains to be evaluated. The majority of patients accept and use CSII continuously, but there are also reports of some patients who discontinued this technology [7, 8]. CGM use was less effective in adolescents due to the low rate of young people willing to use CGM continuously [9]. Barriers mentioned against CGM use were, for example, technical aspects like alarms and inaccurate readingsand bodyimageconcernstoweartwodevices [10]. Barrierslikefearofhypoglycaemiaandhumanfactorslikethe emotional acceptance of wearing the devices and trusting the accuracy seem to play a leading part for the acceptance and efficacy of these technologies. Until now the acceptance of CLS was rarely assessed. Elleri and colleagues [11] prospectively asked parents of children with T1DM if they would trust an AP-system. The majority (90%) reported secure feelings. A sample of 132 adults with T1DM also indicated positive attitudes towards the new technology [12]. However, these patients and parents had no real-life access to the system. Little is known on the psychological impact of an AP system in the patient s home [13]. Systematic studies on fear of hypoglycaemia and satisfaction with and acceptance of an AP in children, adolescents, and adults with T1DM in the home setting have not yet been evaluated. In this study the impact of using an AP-system during four consecutive nights in a home setting regarding fear of hypoglycaemia is assessed among children, adolescents, and adults in a multicentre study. In addition, satisfaction and acceptance of this new technology are evaluated as main psychological predictors of a potential long-term use of the AP system. 2. Materials and Methods 2.1. Trial Design. This study on the psychological impact of using an AP for four consecutive nights in home setting is part of the DREAM Project (DREAM4) conducted in three multinational centres from Israel, Slovenia, and Germany.Themainstudyfocusedonthefeasibility,safety,and efficacy of the MD-Logic AP. It is a two-arm study, each arm covering four consecutive nights comparing the MD- Logic AP ( closed-loop arm) with sensor-augmented pump therapy ( control arm). Patients were randomly assigned either to Group A (first closed-loop and then control arm) or to Group B (vice versa) with a week washout between the two periods [5, 6]. Before intervention and after experiencing the MD-Logic AP participants answered structured questionnaires on fear of hypoglycaemia, satisfaction with the technology, and acceptance of the MD-Logic AP. The study was conducted in compliance with the protocol, the Declaration of Helsinki, and applicable regulatory and good clinical practice requirements. All patients and parents provided a written informed consent prior to trial initiation Participants and Eligibility Criteria. Overall 45 patients from the Schneider Children s Medical Center in Israel (Petah Tikva), 15 patients from the University Medical Centre- University Children s Hospital in Slovenia (Ljubljana), and 15 patients from the Kinder- und Jugendkrankenhaus AUF DER BULT in Germany (Hanover) were recruited between November 2012 and January Main inclusion criteria were type 1 diabetes (>1 yr since diagnosis), age 10 years and 65 years, CSII therapy for at least three months, experience in using CGM, HbA 1c 7% to <10% (53 86 mmol/mol), patients living with at least one other adult person, and an internet access at patient s home. Main exclusion criteria were concomitant diseases that influence metabolic control, participation in any other study, pregnancy, a history of diabetic ketoacidosis or severe hypoglycaemia within the last month, medications, or other conditions that may influence metabolic control, compromise safety, or prevent subjects from completing the study [5, 6]. For organisational reasons sixty participants were offered to answer psychological questionnaires, 30 from Israel, 15 from Slovenia, and 15 from Germany Psychological Assessment Fear of Hypoglycaemia. Fear of hypoglycaemia was assessed with the Hypoglycaemia Fear Survey (HFS). The HFS is based on cognitive-behavioural theory of anxiety distinguishing emotional and behavioural components. Accordingly the HFS includes a Behaviour Subscale (HFS-B) and a Worry Subscale (HFS-W). The HFS adult-version consists of 10 behaviouroravoidanceitems(items1 10)and17worryor affectitems(items11 27)tobeansweredona5-pointLikert scale. Higher total scores reflect greater fear of hypoglycaemia. Higher scores on the behaviour subscale reflect a greater tendency to avoid hypoglycaemia and/or its negative consequences. Higher scores on the Worry Subscale indicate more worries concerning episodes of hypoglycaemia and its consequences. A study with 158 individuals with type 1 diabetes indicated good internal reliability: Cronbach s alpha for the entire scale was.90, for the Behaviour Subscale.60, and for the Worry Subscale.89. The instrument proved to have a high test-retest stability (after 6 weeks.89,.81, and.85 (P < 0.001)) and a good construct validity as the HFS covaries with elevated HbA 1c and is sensitive to a hypoglycaemia awareness training [14]. TheHFSwasadaptedtobeansweredbychildrenwith T1DM. The final pediatric HFS (C-HFS) questionnaire consists of 10 behaviour or avoidance items (items 1 10) and 15 worry or affect items (items 11 25) to be answered on a 5- point Likert scale. A study with adolescents demonstrated adequate internal consistency for the C-HFS-Total Score and the C-HFS-W Score (.86,.91), with a lower Cronbach s alpha for the C-HFS-B Score (.54) [15]. Green reported similar results [16]. Construct validity was demonstrated by a significant correlation between State-trait Anxiety Inventory for children scores and C-HFS-Total scores and C-HFS-W Scores [15].
3 Journal of Diabetes Research 3 Age (years), mean (SD) Children (10 14 yrs) (n =20) 12.3 (1.17) Adolescents (14 18 yrs) (n =20) 15.6 (0.86) Table 1: Baseline patient characteristics. Adults (>18 yrs) (n =19) (9.96) P value Israel (n =29) (6.43) Slovenia (n =15) (12.00) Germany (n =15) (12.96) P value Male (%) HbA 1c (%), mean (SD) HbA 1c (mmol/mol, IFCC), mean (SD) Diabetes duration (years), mean (SD) CSII duration (years), mean (SD) 7.97 (0.72) (7.90) 7.25 (3.06) 5.68 (3.35) 7.93 (0.76) (8.33) 8.78 (3.31) 6.32 (2.82) 7.78 (0.58) (6.32) (10.82) 8.96 (5.58) (0.71) (7.78) 9.21 (5.42) 5.77 (3.52) 7.89 (0.74) (8.13) (12.29) 7.45 (3.24) 7.63 (0.53) (5.75) (7.85) 8.74 (5.70) Regular sensor use (%) All (n =59) (9.98) 7.89 (0.69) (7.51) (8.44) 6.95 (4.24) Acceptance of the Artificial Pancreas. The acceptance of an artificial pancreas was assessed with the adapted TAM Questionnaire. This instrument developed by van Bon et al. [12] is based on the Technology Acceptance Model (TAM). The questionnaire consists of two items assessing Intention to Use (items 1-2), eight items on Perceived Usefulness and its determinants (items 3 10), three items on Perceived Ease of Use (items 11 13), and one item on Trust (item 14). The items are answered on a 7-point Likert scale. Higher scores indicate a higher degree of acceptance of the AP. In a study with 132 patients with T1DM Cronbach s alpha was.91, reflecting a good internal consistency. Satisfaction with Use of an Artificial Pancreas. The questionnaire was developed and validated specifically for closed-loop studies [17]. The questionnaire consists of 14 items (e.g., item 1: in general to which extent were you satisfied with using the artificial pancreas system? ). Items are answered on a 5- point Likert scale. A higher score indicates a higher degree of satisfaction with the AP. All questionnaires were translated linguistically in patients native language; the validation of the translation was performed by each study centre. Sociodemographic characteristics (age, gender, and family status) and clinical characteristics (HbA 1c, onset of diabetes, start of CSII, and sensor use) were collected from patients files. Metabolic control was assessed by DCA 2000 in all centres Statistical Methods. All analyses were performed with SPSS for Windows version 22. The descriptive statistics are reported as percentages or means and standard deviations (SD). Comparison between pre- and postassessment was performed using paired Fisher-t-test or Wilcoxon signedrank test. Effects of age-group, gender, or regular sensor usewereanalysedbyusinganovaorkruskal-wallish test. Associations between fear of hypoglycaemia, acceptance, satisfaction and HbA 1c, diabetes duration, and pump duration were calculated via Spearman s rho. Cronbach s alpha was performed by analyses of reliability. Varimax rotated factor analyses were applied to assess the structure of the questionnaires. Two-sided P values 0.05 were considered statistically significant. 3. Results 3.1. Study Sample. Overall 59 patients (54% male, age 19.9 ± 9.9 yrs, diabetes duration 11.6 ± 8.4 yrs, HbA 1c 7.89 ± 0.69% [62.7 ± 7.5 mmol/mol]) answered the questionnaires before andafterusingtheapforfourconsecutivenightsathome(29 patients from Israel, 15 from Slovenia, and 15 from Germany). One additional patient withdrew consent. Baseline demographic and diabetes characteristics were similar over centres. CGM use turned out to differ between centres (Table 1). Patients from Germany had previous continuous CGM use less often (3 versus 12) compared to Israel (18 versus 11) or Slovenia (8 versus 7). Overall, significantly fewer adult than younger patients had used the device continuously at baseline (Table 1) Fear of Hypoglycaemia. This questionnaire was completed by 58 participants. Internal Consistency. For the total scale of the adult version Cronbach salphawas.88,suggestingahighlevelofreliability. The Behaviour Subscale had an alpha of.61, and the Worry Subscalehadanalphaof.90,comparabletotheresults published by Cox and colleagues [14]. Cronbach s alpha for the pediatric version was.69, demonstrating an adequate reliability. As reported elsewhere the Behaviour Subscale shows consistently a lower internal consistency [15, 16]. At study entry overall HFS items mean score was 1.33 ± 0.41; for the Behaviour Subscale it was 1.78 ± 0.49 and for the Worry Subscale was 1.04 ± 0.53 (range 0 4). After four nights on the AP, the HFS Worry Score decreased (1.04 ± 0.53 versus 0.90 ± 0.63; P = 0.017). The HFS Total Score and HFS Behaviour Score remained on a low level of anxiety (Figure 1). There were no significant differences among all HFS scales at study entry or at follow-up in relation to the patients demographic or diabetes characteristics (each P>0.1).
4 4 Journal of Diabetes Research Table 2: Acceptance of an artificial pancreas analysis. Pre Post Delta P value Total acceptance All 4.69 (0.87) 4.76 (1.06) 0.07 (0.77) Intention to Use All 4.7 (1.25) 4.76 (1.64) 0.01 (1.44) Perceived Usefulness All 4.66 (0.91) 4.67 (1.07) 0.01 (0.86) Perceived Ease of Use All 4.64 (0.94) 5.06 (1.09) 0.42 (0.95) Children (n = 17) 4.54 (1.04) 4.54 (1.15) 0.00 (0.67) Total acceptance Adolescents (n = 19) 4.69 (0.77) 4.88 (1.18) 0.19 (0.86) Adults (n = 19) 4.81 (0.82) 4.83 (0.86) 0.01 (0.80) Values are expressed as mean (SD). 95% CI: fear of hypoglycemia (range 0 4) 2,00 1,75 1,50 1,25 1,00 0,75 P = Before After P = Before After P = Before After HFS total HFS behavior HFS worry Figure 1: Hypoglycaemia Fear Survey (HFS) before and after 4 nights with the MD-Logic artificial pancreas in home setting (n = 58) Acceptance of the Artificial Pancreas. 55 patients answered all items of the TAM questionnaire. Internal Consistency. For the total scale Cronbach s alpha was.90, reflecting a good internal consistency. The Intention to Use subscale had an alpha of.83 and the Perceived Usefulness subscale revealed a Cronbach s alpha of.87, reflectinganadequatereliability.the PerceivedEaseofUse subscalehadanalphaof.71. Factor Analysis. The principal components analysis revealed the presence of four components with eigenvalues exceeding 1, explaining 43.7%, 9.8%, 8.6%, and 7.2% of the variance. The overall TAM score at study entry was 4.69 ± 0.87; for the Intention to Use subscale it was 4.75 ± 1.25, for the Perceived Usefulness subscale was 4.66 ± 0.91, for the Perceived Ease of Use subscale was 4.64 ± 0.94, and for the Trust item was 4.86 ± 1.24 (range each 0 6). After fournightsonapathomethe PerceivedEaseofUse score increased (4.64 ± 0.94 versus 5.06 ± 1.09; P = 0.002). The other subscales of TAM remained on a high level (Table 2) with no significant association to age, diabetes duration, gender, or metabolic control (each P > 0.1). Patients using CGM continuously reported a higher acceptance of AP on all TAM scales compared to those with no regular use (Table 3). Accordingly there were significant centre-differences on TAM Intention to Use subscale (P = 0.032) and TAM Perceived Usefulness subscale (P = 0.038) with lower scores in the German sample compared to the ones from Slovenia and Israel Satisfaction. The satisfaction questionnaire was completed by 57 patients. Factor Analysis. The principal components analysis revealed the presence of five components with eigenvalues exceeding 1, explaining 34.47%, 11.59%, 9.98%, 8.08%, and 7.47% of variance. Every item has only one high correlation with one factor, ranging from.56 to.87. Five scales can be identified: scale 1 Perceived Usefulness of Alarms (items 8, 9, and 12); scale2 Trust (items2,6,and11),scale3 EaseofUse (items 3,5,and7);scale4 Satisfaction (items1,13,and14),andscale 5 Freedom (items 4, 10). Internal Consistency. For the total scale Cronbach s alpha was.84, reflecting a good internal consistency. Only if item 10 would have been deleted there is an increase in Cronbach s alphato.85.the PerceivedUsefulnessofAlarms subscale showedanalphaof.75, Trust subscalehadanalphaof.73, Ease of Use subscale had an alpha of.72, Satisfaction subscale had an alpha of.77, and Freedom subscale had an alpha of.56 reflecting a lower internal consistency. The overall satisfaction score was 3.02 ± 0.54; for Perceived Usefulness of Alarms subscale it was 2.82 ± 0.77, for Trust subscale was 3.07 ± 0.79, for Ease of Use subscale was 3.26 ± 0.73, for Satisfaction subscale was 3.16 ± 0.77, and for Freedom subscale was 2.66 ± 0.91 (range 0 4). There were significant differences of Ease of Use subscale (P = 0.004) between the age groups with significant lower mean scores in children than in adolescents/adults (Table 4). Significant differences of overall satisfaction mean score, Perceived Usefulness of Alarms subscale, Satisfaction subscale, and Freedom subscale according to regular sensor use with significant higher mean scores in patients using CGM continuously were observed (P = 0.001, P = 0.002, P = 0.005,andP = 0.009). There were no significant centre differences on overall satisfaction scores and all subscales.
5 Journal of Diabetes Research 5 Table 3: Association between acceptance of an artificial pancreas and regular sensor use initial. Regular sensor use Regular sensor use Yes No P value Total acceptance All 5.07 (0.59), (0.93), Intention to Use All 5.20 (1.01), (1.32), Perceived Usefulness All 5.02 (0.67), (0.98), Perceived Ease of Use All 5.03 (0.77), (0.96), Children 5.05 (0.81), (0.90),7 Total acceptance Adolescents 5.09 (0.42), (0.81), 8 Adults 5.07 (0.49), (0.90), 14 Values are expressed as mean (SD), n. Table 4: Satisfaction scores after 4 nights with the MD-Logic artificial pancreas in home setting. Children Adolescents Adults P value Total satisfaction 2.96 (0.52) 3.06 (0.60) 3.04 (0.53) Perceived Usefulness of Alarms 2.84 (0.88) 2.74 (0.80) 2.87 (0.64) Trust 2.95 (0.70) 3.22 (0.80) 3.07 (0.88) Ease of Use 2.85 (0.83) 3.35 (0.60) 3.60 (0.55) Satisfaction 3.15 (0.69) 3.25 (0.91) 3.07 (0.75) Freedom 3.03 (0.66) 2.58 (0.95) 2.37 (1.01) Values are expressed as mean (SD). 4. Discussion Thisanalysisofthepsychologicalimpactofusingtheautomated closed-loop MD-Logic system under real-life conditions in the patients home demonstrated reduced worries of hypoglycaemia with the artificial pancreas. Among children as well as adolescents and adult patients with T1DM alike there was a high level of satisfaction and increased acceptance of controlling nocturnal blood glucose automatically. Hypoglycaemia, especially at night, is a major concern of patients and parents. It can impair well-being and is generally accepted as major obstacle to reach near-normoglycaemia [18, 19]. NewtechnologieslikeCSIIandCGMcanimprove glycemic control but still cannot solve the problem of nocturnal hypoglycaemia sufficiently [20]. Recent studies of our group and others with a night-time closed-loop system demonstrated that the closed-loop system is effective reducing the rate of nocturnal hypoglycaemia and increasing time within range in the home setting [5, 6, 21]. The present results confirm that these positive clinical results translate into positive psychological well-being with the MD-Logic system reducing worries of hypoglycaemia and increasing acceptance and satisfaction with this new technology in all age groups under real-life conditions. It should be noted that fear of hypoglycaemia scores at study entry were already relatively low in all age groups but comparable to those reported in the literature for adults [22, 23], adolescents [24], and children [15]. This low HFS level had to be expected as patients with a particular history of severe hypoglycaemia were not included in the study for safety reasons. Nevertheless, despite the already low baseline level of HFS, a significant reduction in the Worry Subscale after using the AP system was found. This scale is known to reflect the cognitive level of fear of hypoglycaemia. Thus these findings may relate to AP patients experiencing less and even more reliable alarms compared to using sensoraugmented pump therapy (SAP). A significant reduction of hypoglycaemiaandalowerrateofhypoglycaemiaalarms duringtheclosedloopnightswiththemd-logicapversus control nights were demonstrated in the interim findings of themainstudy[5]. This may reinforce patients trust in CLS and reduce their worries concerning episodes of hypoglycaemia. Our current findings are in contrast to another study on short-term use effects of CGM on fear of hypoglycaemia [25]. Without automated closed-loop insulin adjustment no reduction of fear of hypoglycaemia was observed with CGM alone. The authors argued that this finding may have been related to the low CGM accuracy and a high rate of false alarms. Adolescents especially reported frequent alarms as a barrier to using CGM continuously [26]. InourstudytheBehaviourSubscaleofHFSremained unchangedonalowlevel.thiscanbeexplainedduetothe short time of the study. After only four nights it is unlikely that a major behaviour change can be observed. In another study after a two-month blood glucose awareness training, which focused on behavioural aspects, both scales were significantly reduced [27]. Currently 60-day studies with the MD-Logic under home conditions are underway. It will be interesting to analyse if such a longer period will eventually lead to changes in behavioural parameters. Nevertheless, our findings indicate an improvement in well-being in patients with T1DM using the MD-AP with less worries concerning episodes of hypoglycaemia and its consequences. Satisfaction with the CLS has been assessed with a newly developed questionnaire to assess CL-satisfaction. The CL-satisfaction questionnaire demonstrated good internal
6 6 Journal of Diabetes Research reliability. In general, satisfaction with CLS has been relatively highinourstudy,withameanscoreofabout3(ona0-to 4-point scale). CL-satisfaction was related to age, with lower satisfaction regarding Ease of Use of the AP in children than in adolescents and adults. This finding raises the issue that children (10 14 yrs) need the support and positive motivation of their caregivers for managing their diabetes tasks with the CLS. As a practical consequence, the developments of age appropriate education materials and specific curricula for children and their caregivers before starting the AP need to be implemented. Despite the considerable technical prerequisites of using thecls,thebarriersofclsindailylifewereratedverylow, especially in patients with regular CGM use. Potential hassles concerning the interpretation of a lot of data are considered a major barrier to CGM use. Therefore previous regular CGM experience at baseline may have given the patient sufficient knowledge to understand the more complex issues related to the CLS (e.g., sensor information and alarms). The CLS finally allows them to profit from the benefits of realtime CGM without the need for making sense of fluctuating glucose levels. Similar results were seen in a study comparing CGM before starting CSII versus CGM after using CSII. The group with CGM use before the start of CSII eventually turned out to use CGM more frequently [28]. A potential recommendation for future success with the CLS may be implementing a longer CGM experience prior to starting CLS. The level of acceptance of CLS has been assessed with the adapted TAM questionnaire [12]. In general, acceptance of an AP has been relatively high in all age groups even before participants had any experience with the CLS overnight at home,withmeanscoreofabout4(ona0to6scale).after4 nights with an AP participants reported significantly higher Perceived Ease of Use of the AP independently of age. Likewise the other acceptance scales remained on a high level. These findings demonstrate high acceptance before and after CL experience. Similar to the satisfaction results participants with regular sensor use reported significant higher acceptancescoresofanapthanparticipantswithoutregularsensor use. It can be summarized that patient satisfaction with and acceptance of the AP have been relatively high, and patients who used CGM regularly before starting AP reported higher satisfaction with and higher overall acceptance of an AP. Recently a study was published regarding the psychosocial impact of overnight CLS at home for 15 adolescents with T1DM by Cambridge Group [13]. High satisfaction with theclosed-loopsystemandadecreaseofthemeanhfstotal score were reported to be similar to our data. However, inferential statistical analysis and comparison to our data were not possibleduetothesmallsamplesizeofthecambridgegroup. The major strength of this study is that it provides evidence of the psychological effect of a CLS under real life conditions for different age groups. As the patients are asked to wear two devices (sensor and pump) as well as a laptop with the algorithm this high acceptance level of the system by patients is reassuring. Long-term adherence to CLS tasks will be necessary for the efficacy of this new technology. In CSII users with poor adherence to CSII tasks the efficacy of CSII in youth is limited [29].ClearlyCLSmayreducetheburden of several diabetes tasks and could provide a significant benefit to the patients. They will be relieved from giving boluses,adjustingthebasalrateorcalculatinginsulin-tocarb ratios. Nevertheless, the patients involvement in some of the diabetes management tasks will remain when using the CLS. They will still need to treat (rare) hypoglycaemia with carbohydrates, change the insulin catheter and sensor, or check the blood glucose for sensor calibration. Thus, in spiteofthepotentialeaseindiabetesmanagementthrough the CLS, the human factor still needs to be taken into consideration. Thestudycoveredatotalperiodof4nightswiththe CLS. Important short-term effects of the MD-Logic AP on fear of hypoglycaemia and satisfaction with and acceptance of an AP were demonstrated. Several limitations of the present study have to be kept in mind. This study may not be adequately powered as the psychological aspects were not the primary end points. Also, the pediatric participants may not be able to provide all answers correctly. In a next step the psychological effect of an AP during long-term overnight and day-and-night use will be studied. Moreover, this study included only subjects without DKA or recent severe hypoglycaemia, but previous studies have shown that patients in poor metabolic control benefitted to a much greater extent from new technologies like SAP [30]. Thus it will be a future challenge to evaluate if the AP technology could also provide a significant step forward for subgroups with frequent acute diabetes complications. 5. Conclusions In conclusion, this study demonstrated the positive psychological effect of an AP system in patient s home on fear of hypoglycaemia and satisfaction with and acceptance of an AP in children, adolescents, and adults with T1DM. By using the MD-Logic AP for four consecutive nights in home setting worries of hypoglycaemia were reduced in all age groups. In addition high satisfaction with and increasing acceptance of this new technology were reported after using the MD-Logic AP in home setting. This may predict an effective long-term useoftheapsystembythepatientsinthefuture. Abbreviations AP: Artificial pancreas CGM: Continuous glucose monitoring CLS: Closed-loop system CSII: Continuous subcutaneous insulin infusion DREAM: Diabetes Wireless Artificial Pancreas Consortium HFS: Hypoglycaemia Fear Survey SAP: Sensor-augmented pump therapy TAM: Technology Acceptance Model T1DM: Type 1 diabetes mellitus. Disclosure Moshe Phillip is a member of the Advisory Board of AstraZeneca, Sanofi, Animas, Medtronic, and Bayer Health
7 Journal of Diabetes Research 7 Care and Board Member of C.G.M.3 Ltd., Consultant of Bristol-Myers Squibb, D-medical, Ferring Pharmaceuticals, and Andromeda Biotech. The Institute headed by Moshe Phillip received research support from Medtronic, Novo Nordisk, Abbott Diabetes Care, Eli Lilly, Roche, Dexcom, Sanofi, Insulet Corporation, Animas, Andromeda, and Macrogenics. Moshe Phillip has been paid lecture fees by Sanofi, Novo Nordisk, Roche, and Pfizer. He is a Stock/ Shareholder of C.G.M.3 Ltd. and DreaMed Diabetes Ltd. Moshe Phillip reports two patent applications. Tadej Battelino is a board member of Novo Nordisk, Sanofi, Medtronic, and Bayer Health Care and Consultant of Spring. Tadej Battelino s institution received research grant support, with receipt of travel and accommodation expenses in some cases, from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Sandoz, and Diamyd. Tadej Battelino received honoraria for participating on the speaker s bureaux of Eli Lilly, Bayer, Novo Nordisk, Medtronic, Sanofi, and Roche. Tadej Battelino and Nataša Bratina were supported in part by the Slovenian National Research Agency Grant no. P They are shareholder of DreaMed Diabetes Ltd. Thomas Danne received honoraria for consulting, speaking engagements from Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Roche, Abbott, Dexcom, GSK, Cellnovo, Bayer Diabetes Care, and Johnson & Johnson. He is shareholder of DreaMed Diabetes Ltd. Olga Kordonouri received honoraria for scientific lectures from PriMedia, consulting fees and travel reimbursement from Sanofi and Roche Diagnostics. She is shareholder of DreaMed Diabetes Ltd. Karin Lange received honoraria for consulting and scientific lectures from Bayer Diabetes Care, Berlin Chemie, Eli Lilly, Merck, MSD, Novo Nordisk, Roche Diagnostics, and Sanofi. Conflict of Interests Claudia Ziegler, Alon Liberman, Revital Nimri, Ido Muller, Simona Klemenčič, Sarah Bläsig, and Kerstin Remus declared no conflict of interests. There is no other potential conflict of interests relevant to this paper. Authors Contribution This study was an investigator-initiated trial. The study and protocol were designed by the investigators Tadej Battelino, Moshe Phillip, Olga Kordonouri, and Thomas Danne. ClaudiaZieglerandKarinLangecontributedtothestudyconcept and design, researched the data, contributed to the statistical analysis and data interpretation, and wrote and edited the paper. Moshe Phillip and Revital Nimri coordinated the study, codesigned and implemented the glucose controller, and participated in data analysis and interpretation. Alon Liberman, Ido Muller, Simona Klemenčič, Nataša Bratina, Tadej Battelino, Thomas Danne, Olga Kordonouri, Sarah Bläsig, and Kerstin Remus contributed to the study concept and design, supervised the study, researched data, participated in data analysis and interpretation, and reviewed and editedthepaper.thefirst(claudiaziegler)andlastauthors (KarinLange)hadfullaccesstothedataofthestudyandtake responsibility for the accuracy of the analysis and integrity of the work as a whole. All authors reviewed and accepted the final version of the paper. Acknowledgments The authors wish to thank the patients and their families for their participation. This study was supported in part by Sanofi; Medtronic Diabetes supplied software to interface with Paradigm Veo System; and Intel Israel donated laptops for the study. No sponsor had any role in study design, data collection, data analysis, data interpretation, or writing of the paper. References [1] R. Nimri, T. Danne, O. Kordonouri et al., The Glucositter overnight automated closed loop system for type 1 diabetes: a randomized crossover trial, Pediatric Diabetes,vol.14,no.3,pp , [2]M.Phillip,T.Battelino,E.Atlasetal., Nocturnalglucose control with an artificial pancreas at a diabetes camp, The New England Journal of Medicine,vol.368,no.9,pp ,2013. [3]E.Atlas,R.Nimri,S.Miller,E.A.Grunberg,andM.Phillip, MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes, Diabetes Care,vol.33,no.5,pp ,2010. [4] S. Miller, R. Nimri, E. Atlas, E. A. Grunberg, and M. Phillip, Automatic learning algorithm for the MD-logic artificial pancreas system, Diabetes Technology & Therapeutics, vol. 13, no. 10, pp , [5] R. Nimri, I. Muller, E. Atlas et al., Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis, Pediatric Diabetes,vol.15,no.2,pp.91 99,2014. [6] R. Nimri, I. Muller, E. Atlas et al., A multicenter, multinational, single blind, MD-logic overnight type 1 diabetes control in the home setting, In evaluation for publication. [7] S.E.Hofer,B.Heidtmann,K.Raileetal., Discontinuationof insulin pump treatment in children, adolescents, and young adults. A multicenter analysis based on the DPV database in Germany and Austria, Pediatric Diabetes,vol.11,no.2,pp , [8] L. de Vries, Y. Grushka, Y. Lebenthal, S. Shalitin, and M. Phillip, Factors associated with increased risk of insulin pump discontinuation in pediatric patients with type 1 diabetes, Pediatric Diabetes,vol.12,no.5,pp ,2011. [9]TheJuvenileDiabetesResearchFoundationContinuousGlucose Monitoring Study Group, Continuous glucose monitoring and intensive treatment of type 1 diabetes, The New England Journal of Medicine, vol. 359, no. 14, pp , [10] L.Gonder-Frederick,J.Shepard,andN.Peterson, Closed-loop glucose control: psychological and behavioral considerations, Journal of Diabetes Science and Technology, vol.5,no.6,pp , [11] D. Elleri, C. L. Acerini, J. M. Allen et al., Parental attitudes towards overnight closed-loop glucose control in children with type 1 diabetes, Diabetes Technology and Therapeutics, vol.12, no. 1, pp , [12] A.C.vanBon,T.B.Brouwer,G.vonBasum,J.B.L.Hoekstra, and J. H. DeVries, Future acceptance of an artificial pancreas in
8 8 Journal of Diabetes Research adults with type 1 diabetes, Diabetes Technology & Therapeutics, vol. 13, no. 7, pp , [13]K.D.Barnard,T.Wysocki,J.M.Allenetal., Closingloop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents, BMJ Open Diabetes Research and Care, vol. 2, Article ID , [14] D. J. Cox, A. Irvine, L. Gonder-Frederick, G. Nowacek, and J. Butterfield, Fear of hypoglycemia: quantification, validation, and utilization, Diabetes Care,vol.10,no.5,pp ,1987. [15] L.Gonder-Frederick,M.Nyer,J.A.Shepard,K.Vajda,andW. Clarke, Assessing fear of hypoglycemia in children with type 1diabetesandtheirparents, Diabetes Management, vol.1,pp , [16] L. B. Green, T. Wysocki, and B. M. Reineck, Fear of hypoglycemia in children and adolescents with diabetes, Journal of Pediatric Psychology,vol.15,no.5,pp ,1990. [17] R. Nimri, An overnight automated closed-loop, MD-logic system at patients home, Diabetes Technology & Therapeutics, vol. 15, supplement 1, pp. A-1 A-154, [18] K. Barnard, S. Thomas, P. Royle, K. Noyes, and N. Waugh, Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review, BMC Pediatrics, vol. 10, article 50, [19] A. Haugstvedt, T. Wentzel-Larsen, M. Graue, O. Søvik, and B. Rokne, Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study, Diabetic Medicine,vol.27, no.1,pp , [20] M. L. Katz, L. K. Volkening, B. J. Anderson, and L. M. Laffel, Contemporary rates of severe hypoglycaemia in youth with type 1 diabetes: variability by insulin regimen, Diabetic Medicine,vol.29,no.7,pp ,2012. [21] R. Hovorka, D. Elleri, H. Thabit et al., Overnight closed-loop insulindeliveryinyoungpeoplewithtype1diabetes:afreeliving, randomized clinical trial, Diabetes Care, vol.37,no.5, pp , [22] C. Hendrieckx, J. A. Halliday, J. P. Bowden et al., Severe hypoglycaemia and its association with psychological well-being in Australian adults with type 1 diabetes attending specialist tertiary clinics, Diabetes Research and Clinical Practice,vol.103, no. 3, pp , [23] L. A. Gonder-Frederick, K. A. Vajda, K. M. Schmidt et al., Examining the behaviour subscale of the Hypoglycaemia Fear Survey: an international study, Diabetic Medicine, vol. 30, no. 5, pp , [24] L. A. Gonder-frederick, C. D. Fisher, L. M. Ritterband et al., Predictors of fear of hypoglycemia in adolescents with type 1 diabetes and their parents, Pediatric Diabetes, vol.7,no.4,pp , [25] R. J. Davey, K. Stevens, T. W. Jones, and P. A. Fournier, The effect of short-term use of the Guardian RT continuous glucose monitoring system on fear of hypoglycaemia in patients with type 1 diabetes mellitus, Primary Care Diabetes, vol.6,no.1, pp.35 39,2012. [26] M. Tansey, L. Laffel, J. Cheng et al., Satisfaction with continuous glucose monitoring in adults and youths with type1 diabetes, Diabetic Medicine, vol. 28, no. 9, pp , [27] H. Schachinger, K. Hegar, N. Hermanns et al., Randomized controlled clinical trial of blood glucose awareness training (BGAT III) in Switzerland and Germany, Journal of Behavioral Medicine, vol. 28, no. 6, pp , [28] J.Moreno-Fernandez,F.J.Gomez,M.Gazquezetal., Real-time continuous glucose monitoring or continuous subcutaneous insulin infusion, what goes first?: results of a pilot study, Diabetes Technology and Therapeutics,vol.15,no.7,pp , [29] M. O Connell, S. Donath, and F. Cameron, Poor adherence to integral daily tasks limits the efficacy of CSII in youth, Pediatric Diabetes,vol.12,no.6,pp ,2011. [30] J. Hermanides, K. Nørgaard, D. Bruttomesso et al., Sensoraugmented pump therapy lowers HbA 1c in suboptimally controlled Type1 diabetes; a randomized controlled trial, Diabetic Medicine, vol. 28, no. 10, pp , 2011.
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control
The Digital Clinic: Unlock the Power of Diabetes Data Eran Atlas - CEO Copyright DreaMed Diabetes, Ltd. 2017, All Rights Reserved
 The Digital Clinic: Unlock the Power of Diabetes Data Eran Atlas - CEO Basic Copyright Notice & Disclaimer Basic Copyright Notice & Disclaimer 2017 This presentation is copyright protected. All rights
The Digital Clinic: Unlock the Power of Diabetes Data Eran Atlas - CEO Basic Copyright Notice & Disclaimer Basic Copyright Notice & Disclaimer 2017 This presentation is copyright protected. All rights
The artificial pancreas: the next step in connectivity and digital treatment of type 1 diabetes
 The artificial pancreas: the next step in connectivity and digital treatment of type 1 diabetes Roman Hovorka PhD FMedSci University of Cambridge, UK Duality of interest declaration Advisory Panel: Research
The artificial pancreas: the next step in connectivity and digital treatment of type 1 diabetes Roman Hovorka PhD FMedSci University of Cambridge, UK Duality of interest declaration Advisory Panel: Research
What is the role of insulin pumps in the modern day care of patients with Type 1 diabetes?
 What is the role of insulin pumps in the modern day care of patients with Type 1 diabetes? Dr. Fiona Wotherspoon Consultant in Diabetes and Endocrinology Dorset County Hospital Fiona.Wotherspoon@dchft.nhs.uk
What is the role of insulin pumps in the modern day care of patients with Type 1 diabetes? Dr. Fiona Wotherspoon Consultant in Diabetes and Endocrinology Dorset County Hospital Fiona.Wotherspoon@dchft.nhs.uk
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes Individuals: With type 1 diabetes
 Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes. pump. pump
 Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
T he benefits of intensive treatment of
 Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Effect of Continuous Glucose Monitoring on Hypoglycemia in Type 1 Diabetes TADEJ BATTELINO, MD, PHD 1 MOSHE PHILLIP,
Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Effect of Continuous Glucose Monitoring on Hypoglycemia in Type 1 Diabetes TADEJ BATTELINO, MD, PHD 1 MOSHE PHILLIP,
[Frida Svendsen and Jennifer Southern] University of Oxford
![[Frida Svendsen and Jennifer Southern] University of Oxford [Frida Svendsen and Jennifer Southern] University of Oxford](/thumbs/95/124874384.jpg) In adolescents with poorly controlled type 1 diabetes mellitus, could a bionic, bihormonal pancreas provide better blood glucose control than continuous subcutaneous insulin infusion therapy? [Frida Svendsen
In adolescents with poorly controlled type 1 diabetes mellitus, could a bionic, bihormonal pancreas provide better blood glucose control than continuous subcutaneous insulin infusion therapy? [Frida Svendsen
Insulin Pumps and Glucose Sensors in Diabetes Management
 Diabetes Update+ 2014 Congress Whistler, British Columbia Friday March 21, 2014ǀ 8:15 8:45 am Insulin Pumps and Glucose Sensors in Diabetes Management Bruce A Perkins MD MPH Division of Endocrinology Associate
Diabetes Update+ 2014 Congress Whistler, British Columbia Friday March 21, 2014ǀ 8:15 8:45 am Insulin Pumps and Glucose Sensors in Diabetes Management Bruce A Perkins MD MPH Division of Endocrinology Associate
Insulin Pump Therapy in children. Prof. Abdulmoein Al-Agha, FRCPCH(UK)
 Insulin Pump Therapy in children Prof. Abdulmoein Al-Agha, FRCPCH(UK) aagha@kau.edu.sa Highlights Evolution of insulin pump Pumps mimics Pancreas Goals of diabetes care What lowers HbA1c Criteria for selection
Insulin Pump Therapy in children Prof. Abdulmoein Al-Agha, FRCPCH(UK) aagha@kau.edu.sa Highlights Evolution of insulin pump Pumps mimics Pancreas Goals of diabetes care What lowers HbA1c Criteria for selection
Updates in Diabetes Technology
 Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna. Ipoglicemie e Monitoraggio Glicemico
 Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna Ipoglicemie e Monitoraggio Glicemico Management of Hypoglycaemia.if hypoglycemia is a problem, the principles
Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna Ipoglicemie e Monitoraggio Glicemico Management of Hypoglycaemia.if hypoglycemia is a problem, the principles
Development of a New Fear of Hypoglycemia Scale: Preliminary Results
 Development of a New Fear of Hypoglycemia Scale: Preliminary Results Jodi L. Kamps, 1 PHD, Michael C. Roberts, 2 PHD, ABPP, and R. Enrique Varela, 3 PHD 1 Children s Hospital of New Orleans, 2 University
Development of a New Fear of Hypoglycemia Scale: Preliminary Results Jodi L. Kamps, 1 PHD, Michael C. Roberts, 2 PHD, ABPP, and R. Enrique Varela, 3 PHD 1 Children s Hospital of New Orleans, 2 University
How I use the latest technology to investigate and treat a person with Type 1 Diabetes. Dr Pratik Choudhary
 How I use the latest technology to investigate and treat a person with Type 1 Diabetes Dr Pratik Choudhary Disclosures Advisory board and speaker fees from Medtronic, Roche, Abbott and Johnson and Johnson,
How I use the latest technology to investigate and treat a person with Type 1 Diabetes Dr Pratik Choudhary Disclosures Advisory board and speaker fees from Medtronic, Roche, Abbott and Johnson and Johnson,
ARTICLE. Keywords Clinical science. Devices. Diabetes in childhood. Exercise. Hypoglycaemia. Diabetologia DOI /s z
 DOI 10.1007/s00125-017-4395-z ARTICLE Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial Klemen Dovc
DOI 10.1007/s00125-017-4395-z ARTICLE Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial Klemen Dovc
ATTD Session: Needs and solutions in Type 1 diabetes from youth to seniors: towards software prescriptions
 ATTD Session: Needs and solutions in Type 1 diabetes from youth to seniors: towards software prescriptions Moshe Phillip MD The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes National
ATTD Session: Needs and solutions in Type 1 diabetes from youth to seniors: towards software prescriptions Moshe Phillip MD The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes National
Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim
 Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim Division of Endocrinology and Metabolism, Samsung Medical Center, Sungkyunkwan University School of Medicine Conflict of interest
Control of Glycemic Variability for Reducing Hypoglycemia Jae Hyeon Kim Division of Endocrinology and Metabolism, Samsung Medical Center, Sungkyunkwan University School of Medicine Conflict of interest
Artificial Pancreas Device System (APDS)
 Medical Policy Manual Durable Medical Equipment, Policy No. 77 Artificial Pancreas Device System (APDS) Next Review: October 2019 Last Review: October 2018 Effective: November 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Durable Medical Equipment, Policy No. 77 Artificial Pancreas Device System (APDS) Next Review: October 2019 Last Review: October 2018 Effective: November 1, 2018 IMPORTANT REMINDER
DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson
 DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson March 3 rd, 2018 Who is this guy? Accelerating Progress 1 Oh that guy! Accelerating Progress 2 STATE OF T1D CARE
DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson March 3 rd, 2018 Who is this guy? Accelerating Progress 1 Oh that guy! Accelerating Progress 2 STATE OF T1D CARE
The Diamond Study: Continuous Glucose Monitoring In Patients on Mulitple Daily Insulin Injections
 8/5/217 The Diamond Study: Continuous Glucose Monitoring In Patients on Mulitple Daily Insulin Injections Richard M. Bergenstal, MD Executive Director International Diabetes Center at Park Nicollet Minneapolis,
8/5/217 The Diamond Study: Continuous Glucose Monitoring In Patients on Mulitple Daily Insulin Injections Richard M. Bergenstal, MD Executive Director International Diabetes Center at Park Nicollet Minneapolis,
PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS)
 PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS) Pathways for Future Treatment and Management of Diabetes H. Peter Chase, MD Carousel of Hope Symposium Beverly Hilton, Beverly
PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS) Pathways for Future Treatment and Management of Diabetes H. Peter Chase, MD Carousel of Hope Symposium Beverly Hilton, Beverly
Research Article Measures of Adherence and Challenges in Using Glucometer Data in Youth with Type 1 Diabetes: Rethinking the Value of Self-Report
 Hindawi Diabetes Research Volume 2017, Article ID 1075428, 4 pages https://doi.org/10.1155/2017/1075428 Research Article Measures of Adherence and Challenges in Using Glucometer Data in Youth with Type
Hindawi Diabetes Research Volume 2017, Article ID 1075428, 4 pages https://doi.org/10.1155/2017/1075428 Research Article Measures of Adherence and Challenges in Using Glucometer Data in Youth with Type
Original Article. Nicholas B. Argento, MD 1 ; Katherine Nakamura, PhD 2 ABSTRACT
 Original Article Nicholas B. Argento, MD 1 ; Katherine Nakamura, PhD 2 ABSTRACT Objective: Little information is available on personal real-time continuous glucose monitoring (PCGM) in patients 65 years
Original Article Nicholas B. Argento, MD 1 ; Katherine Nakamura, PhD 2 ABSTRACT Objective: Little information is available on personal real-time continuous glucose monitoring (PCGM) in patients 65 years
Adherence to therapy. Kamlesh Khunti University of Leicester, UK. William Polonsky University of California San Diego, USA
 Adherence to therapy Kamlesh Khunti University of Leicester, UK William Polonsky University of California San Diego, USA 1 Dualities of interest Kamlesh Khunti: Honoraria for speaking, advising or research
Adherence to therapy Kamlesh Khunti University of Leicester, UK William Polonsky University of California San Diego, USA 1 Dualities of interest Kamlesh Khunti: Honoraria for speaking, advising or research
Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier?
 Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier? Alan B Schorr DO FAAIM FACE www.sugardoc.com abs@sugardoc.com Disclosures
Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier? Alan B Schorr DO FAAIM FACE www.sugardoc.com abs@sugardoc.com Disclosures
Corporate Medical Policy
 Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Subject Index. Breastfeeding, self-monitoring of blood glucose 56
 Subject Index Animas Vibe 86, 130 Artificial pancreas clinical studies inpatient studies 175 180 outpatient studies outcome assessment 182, 183 technology 180, 181 telemedicine 182 components glucose sensor
Subject Index Animas Vibe 86, 130 Artificial pancreas clinical studies inpatient studies 175 180 outpatient studies outcome assessment 182, 183 technology 180, 181 telemedicine 182 components glucose sensor
NEWS BRIEFING Advances in Technology. moderated by: Irl Hirsch, MD University of Washington Medical Center
 NEWS BRIEFING Advances in Technology moderated by: Irl Hirsch, MD University of Washington Medical Center 1 EMBARGO POLICY All recordings are for personal use only and not for rebroadcast online or in
NEWS BRIEFING Advances in Technology moderated by: Irl Hirsch, MD University of Washington Medical Center 1 EMBARGO POLICY All recordings are for personal use only and not for rebroadcast online or in
DIAGNOSIS OF DIABETES NOW WHAT?
 DIAGNOSIS OF DIABETES NOW WHAT? DISCUSS GOALS FOR DIABETES CARE IDENTIFY COMMON COMPLIANCE- ADHERENCE ISSUES DESCRIBE TECHNOLOGY TO ASSIST AND / OR IMPROVE DIABETES CARE WHAT DO WE WANT OUR PATIENTS TO
DIAGNOSIS OF DIABETES NOW WHAT? DISCUSS GOALS FOR DIABETES CARE IDENTIFY COMMON COMPLIANCE- ADHERENCE ISSUES DESCRIBE TECHNOLOGY TO ASSIST AND / OR IMPROVE DIABETES CARE WHAT DO WE WANT OUR PATIENTS TO
Artificial Pancreas Device Systems
 Artificial Pancreas Device Systems Policy Number: 1.01.30 Last Review: 1/2019 Origination: 1/2017 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for
Artificial Pancreas Device Systems Policy Number: 1.01.30 Last Review: 1/2019 Origination: 1/2017 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for
The next five years in diabetes technology: closed-loop systems
 The next five years in diabetes technology: closed-loop systems J. H. (Hans) DeVries Academic Medical Center at the University of Amsterd, the Netherlands Keystone, CO, 19 July 2013 Disclosure AP@home,
The next five years in diabetes technology: closed-loop systems J. H. (Hans) DeVries Academic Medical Center at the University of Amsterd, the Netherlands Keystone, CO, 19 July 2013 Disclosure AP@home,
Medical Policy. MP Artificial Pancreas Device Systems
 Medical Policy MP 1.01.30 BCBSA Ref. Policy: 1.01.30 Last Review: 01/30/2018 Effective Date: 04/01/2018 Section: Durable Medical Equipment Related Policies 1.01.20 Continuous or Intermittent Monitoring
Medical Policy MP 1.01.30 BCBSA Ref. Policy: 1.01.30 Last Review: 01/30/2018 Effective Date: 04/01/2018 Section: Durable Medical Equipment Related Policies 1.01.20 Continuous or Intermittent Monitoring
hypoglycaemia unawareness keystone 18 July 2014
 hypoglycaemia unawareness keystone 18 July 2014 Hypoglycaemia unawareness: ( Impaired awareness of hypoglycaemia ) Philip Home Newcastle University Philip Home Duality of interest Manufacturers of glucose-lowering
hypoglycaemia unawareness keystone 18 July 2014 Hypoglycaemia unawareness: ( Impaired awareness of hypoglycaemia ) Philip Home Newcastle University Philip Home Duality of interest Manufacturers of glucose-lowering
THE INFLUENCE OF PSYCHOSOCIAL AND TREATMENT-RELATED VARIABLES ON ADHERENCE AND METABOLIC CONTROL IN ADOLESCENTS WITH TYPE 1 DIABETES MELLITUS
 THE INFLUENCE OF PSYCHOSOCIAL AND TREATMENT-RELATED VARIABLES ON ADHERENCE AND METABOLIC CONTROL IN ADOLESCENTS WITH TYPE 1 DIABETES MELLITUS Lene J. Kristensen, Niels H. Birkebaek, Anne H. Mose, Morten
THE INFLUENCE OF PSYCHOSOCIAL AND TREATMENT-RELATED VARIABLES ON ADHERENCE AND METABOLIC CONTROL IN ADOLESCENTS WITH TYPE 1 DIABETES MELLITUS Lene J. Kristensen, Niels H. Birkebaek, Anne H. Mose, Morten
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
C o n n e c t e d I n s u l i n D e l i v e r y S y s t e m
 Technology Innovation Alert C o n n e c t e d I n s u l i n D e l i v e r y S y s t e m 6 July 12 July, 2018 1 4 0 0 Start ups Companies Universities Individuals Interesting Innovation Compliance with
Technology Innovation Alert C o n n e c t e d I n s u l i n D e l i v e r y S y s t e m 6 July 12 July, 2018 1 4 0 0 Start ups Companies Universities Individuals Interesting Innovation Compliance with
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond)
 Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
James J. Chamberlain, MD 1, Dana Dopita, RN, MSN, CDE 1, Emily Gilgen, RD, CD, CDE 1, and Annie Neuman, MPA, PA-C 1.
 604633DSTXXX10.1177/1932296815604633Journal of Diabetes Science and TechnologyChamberlain et al research-article2015 Original Article Impact of Frequent and Persistent Use of Continuous Glucose Monitoring
604633DSTXXX10.1177/1932296815604633Journal of Diabetes Science and TechnologyChamberlain et al research-article2015 Original Article Impact of Frequent and Persistent Use of Continuous Glucose Monitoring
Future Direction of Artificial Pancreas. Roy W. Beck, MD, PhD. JAEB Center for Health Research Tampa, Florida
 Future Direction of Artificial Pancreas Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer,
Future Direction of Artificial Pancreas Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer,
Artificial Pancreas Goes Outpatient: A New Diabetes Ecosystem
 Journal of Diabetes Science and Technology Volume 7, Issue 6, November 2013 Diabetes Technology Society EDITORIAL Artificial Pancreas Goes Outpatient: A New Diabetes Ecosystem Eric, M.D., Ph.D., 1,2,3
Journal of Diabetes Science and Technology Volume 7, Issue 6, November 2013 Diabetes Technology Society EDITORIAL Artificial Pancreas Goes Outpatient: A New Diabetes Ecosystem Eric, M.D., Ph.D., 1,2,3
No disclosures 8/4/2017. Cari Berget RN, MPH, CDE Clinical Research Nurse, Diabetes Educator. Disclosure to Participants
 Cari Berget RN, MPH, CDE Clinical Research Nurse, Diabetes Educator Barbara Davis Center for Diabetes Aurora, CO Disclosure to Participants No disclosures FEAR OF HYPOGLYCEMIA AND IMPLICATIONS FOR CLINICAL
Cari Berget RN, MPH, CDE Clinical Research Nurse, Diabetes Educator Barbara Davis Center for Diabetes Aurora, CO Disclosure to Participants No disclosures FEAR OF HYPOGLYCEMIA AND IMPLICATIONS FOR CLINICAL
Cowen Investor Conference March confidently live life with ease
 Cowen Investor Conference March 2018 confidently live life with ease Forward-Looking Statements This presentation contains certain forward-looking statements that involve risks and uncertainties that could
Cowen Investor Conference March 2018 confidently live life with ease Forward-Looking Statements This presentation contains certain forward-looking statements that involve risks and uncertainties that could
Faculty Disclosure. No, nothing to disclose Yes, please specify: Novo-Nordisk. Roche. Abbott. Funded Research. Ownership/ Equity Position
 Diabeloop Closed-Loop does better than sensor-augmented-pump on blood glucose during 3 days with intensive physical eercises: a randomized crossover trial. Sylvia Franc 1, MD, Sophie Borot, MD, PhD, Pierre-Yves
Diabeloop Closed-Loop does better than sensor-augmented-pump on blood glucose during 3 days with intensive physical eercises: a randomized crossover trial. Sylvia Franc 1, MD, Sophie Borot, MD, PhD, Pierre-Yves
UvA-DARE (Digital Academic Repository) The artificial pancreas Kropff, J. Link to publication
 UvA-DARE (Digital Academic Repository) The artificial pancreas Kropff, J. Link to publication Citation for published version (APA): Kropff, J. (2017). The artificial pancreas: From logic to life General
UvA-DARE (Digital Academic Repository) The artificial pancreas Kropff, J. Link to publication Citation for published version (APA): Kropff, J. (2017). The artificial pancreas: From logic to life General
The Realities of Technology in Type 1 Diabetes
 The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
Continuous Glucose Monitoring
 Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
CAROLINAS CHAPTER/AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS Annual Meeting HILTON HEAD ISLAND FRIDAY PRESENTATION
 CAROLINAS CHAPTER/AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS 2016 Annual Meeting HILTON HEAD ISLAND FRIDAY PRESENTATION September 9-11, 2016 ~ Sonesta Resort ~ Hilton Head Island, SC This continuing
CAROLINAS CHAPTER/AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS 2016 Annual Meeting HILTON HEAD ISLAND FRIDAY PRESENTATION September 9-11, 2016 ~ Sonesta Resort ~ Hilton Head Island, SC This continuing
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Continuous or Intermittent Monitoring
Designed with your patients lives in mind
 The Accu-Chek Insight diabetes therapy system Designed with your patients lives in mind With pre-filled insulin cartridge Designed for easy patient training The Accu-Chek Insight diabetes therapy system
The Accu-Chek Insight diabetes therapy system Designed with your patients lives in mind With pre-filled insulin cartridge Designed for easy patient training The Accu-Chek Insight diabetes therapy system
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (<18 years of age)
 Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
What is a CGM? (Continuous Glucose Monitor) The Bionic Pancreas Is Coming
 The Bionic Pancreas Is Coming Montana Diabetes Professional Conference October 23, 2014 H. Peter Chase, MD Professor of Pediatrics University of Colorado Barbara Davis Center Stanford: Bruce Buckingham,
The Bionic Pancreas Is Coming Montana Diabetes Professional Conference October 23, 2014 H. Peter Chase, MD Professor of Pediatrics University of Colorado Barbara Davis Center Stanford: Bruce Buckingham,
Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES
 Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES Many parents whose child is diagnosed with Type 1 diabetes wonder: Why is this happening to my child?
Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES Many parents whose child is diagnosed with Type 1 diabetes wonder: Why is this happening to my child?
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus
 Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Patrick J. Sullivan, President & CEO March 7, 2016 Raymond James 37 th Annual Institutional Conference
 Patrick J. Sullivan, President & CEO March 7, 2016 Raymond James 37 th Annual Institutional Conference Forward Looking Statement This presentation may contain forward-looking statements concerning Insulet's
Patrick J. Sullivan, President & CEO March 7, 2016 Raymond James 37 th Annual Institutional Conference Forward Looking Statement This presentation may contain forward-looking statements concerning Insulet's
Presented by Dr. Bruce Perkins, MD MPH Dr. Michael Riddell, PhD
 Type 1 Diabetes and Exercise: Optimizing the Medtronic MiniMed Veo Insulin Pump and Continuous Glucose Monitoring (CGM) for Better Glucose Control 1,2 for Healthcare Professionals Presented by Dr. Bruce
Type 1 Diabetes and Exercise: Optimizing the Medtronic MiniMed Veo Insulin Pump and Continuous Glucose Monitoring (CGM) for Better Glucose Control 1,2 for Healthcare Professionals Presented by Dr. Bruce
15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016
 15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016 Sponsored by: Abbott Diabetes Care and Lilly Diabetes The REPOSE Trial (Relative Effectiveness of Pumps over Structured Education) Background
15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016 Sponsored by: Abbott Diabetes Care and Lilly Diabetes The REPOSE Trial (Relative Effectiveness of Pumps over Structured Education) Background
These comments are an attempt to summarise the discussions at the manuscript meeting. They are not an exact transcript.
 1-Aug-2016 Dear Prof. Heller Manuscript ID BMJ.2016.034070 entitled "A cluster randomised trial comparing insulin pump therapy to multiple injections during flexible intensive insulin therapy for type
1-Aug-2016 Dear Prof. Heller Manuscript ID BMJ.2016.034070 entitled "A cluster randomised trial comparing insulin pump therapy to multiple injections during flexible intensive insulin therapy for type
CGM and Closing The Loop
 CGM and Closing The Loop Dualities Research: Helmsely Charitable Trust, ADA, JDRF, NIDDK Consulting: Abbott Diabetes Care, Roche, Intarcia, Valeritas, Adocia, Big Foot Like With Pumps, We ve Come A Long
CGM and Closing The Loop Dualities Research: Helmsely Charitable Trust, ADA, JDRF, NIDDK Consulting: Abbott Diabetes Care, Roche, Intarcia, Valeritas, Adocia, Big Foot Like With Pumps, We ve Come A Long
Diabetes and Technology. Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE
 Diabetes and Technology Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE Disclosure Speaker s Bureau: Janssan Pharmaceuticals Current Technology V-Go by Valeritas Continuous Sensors (personal
Diabetes and Technology Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE Disclosure Speaker s Bureau: Janssan Pharmaceuticals Current Technology V-Go by Valeritas Continuous Sensors (personal
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness
 Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
When Will CGM Replace SMBG? Roy W. Beck, MD, PhD. JAEB Center for Health Research Tampa, Florida
 When Will CGM Replace SMBG? Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer, the JAEB Center
When Will CGM Replace SMBG? Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer, the JAEB Center
JDRF Perspective on Closed Loop
 JDRF Perspective on Closed Loop Aaron J. Kowalski, Ph.D. Assistant Vice President Treatment Therapies Juvenile Diabetes Research Foundation International 1 Presenter Disclosure Aaron Kowalski Disclosed
JDRF Perspective on Closed Loop Aaron J. Kowalski, Ph.D. Assistant Vice President Treatment Therapies Juvenile Diabetes Research Foundation International 1 Presenter Disclosure Aaron Kowalski Disclosed
Initiation of insulin pump therapy in children at diagnosis of type 1 diabetes resulted in improved long-term glycemic control
 Pediatric Diabetes 2016 doi: 10.1111/pedi.12357 All rights reserved 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd. Pediatric Diabetes Original Article Initiation of insulin pump therapy
Pediatric Diabetes 2016 doi: 10.1111/pedi.12357 All rights reserved 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd. Pediatric Diabetes Original Article Initiation of insulin pump therapy
Comparing the use of SMBG vs. CGM data to Optimize Glucose Control in T2DM
 Comparing the use of SMBG vs. CGM data to Optimize Glucose Control in T2DM For the first time using CGM to assess glucose control achieved in both groups Richard M. Bergenstal, MD International Diabetes
Comparing the use of SMBG vs. CGM data to Optimize Glucose Control in T2DM For the first time using CGM to assess glucose control achieved in both groups Richard M. Bergenstal, MD International Diabetes
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid
 Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2018 Origination: 11/2001 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2018 Origination: 11/2001 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City
Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid
 Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid Policy #: 038 Latest Review Date: December 2016 Category: DME Policy Grade: B Background/Definitions: As a general
Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid Policy #: 038 Latest Review Date: December 2016 Category: DME Policy Grade: B Background/Definitions: As a general
8/5/2017. Disclosure to Participants. What is PCORI? Outline. To Test or Not To Test. What is PCORI? THE MONITOR TRIAL
 THE MONITOR TRIAL Effect of Glucose Monitoring on Patient and Provider Outcomes in Non-insulin Treated Diabetes Laura Young, MD, PhD Assistant Professor of Medicine University of North Carolina To Test
THE MONITOR TRIAL Effect of Glucose Monitoring on Patient and Provider Outcomes in Non-insulin Treated Diabetes Laura Young, MD, PhD Assistant Professor of Medicine University of North Carolina To Test
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Artificial Pancreas Device Systems Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Artificial Pancreas Device Systems Professional Institutional Original
Artificial Pancreas Device Systems Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Artificial Pancreas Device Systems Professional Institutional Original
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
Subheadings adapted to the terminology in the respective databases were included in the searches.
 Table 1. Literature searches related to the literature review (Chapter 2) * Search terms ** Diabetes Mellitus, Type 1 OR type 1 diabetes + parent OR parenting + hypoglycemia + fear Diabetes Mellitus, Type
Table 1. Literature searches related to the literature review (Chapter 2) * Search terms ** Diabetes Mellitus, Type 1 OR type 1 diabetes + parent OR parenting + hypoglycemia + fear Diabetes Mellitus, Type
CONTINUOUS OR INTERMITTENT GLUCOSE MONITORING IN INTERSTITIAL FLUID
 FLUID Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
FLUID Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Commissioning Policy Individual Funding Request
 Commissioning Policy Individual Funding Request Continuous Glucose Monitors Prior Approval Policy Date Adopted: 13 October 2017 Version: 1718.2 Document Control Title of document Continuous Glucose Monitors
Commissioning Policy Individual Funding Request Continuous Glucose Monitors Prior Approval Policy Date Adopted: 13 October 2017 Version: 1718.2 Document Control Title of document Continuous Glucose Monitors
Continuous Glucose Monitoring Devices Pharmacy Policy
 Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
The Hypoglycaemia-Hyperglycaemia Minimizer System in the Management of Type 1 Diabetes. Brian L Levy, Thomas W McCann, Jr and Daniel A Finan
 Diabetes The Hypoglycaemia-Hyperglycaemia Minimizer System in the Management of Type 1 Diabetes Brian L Levy, Thomas W McCann, Jr and Daniel A Finan Animas Corporation, Wayne, USA DOI: http://doi.org/10.17925/ee.2016.12.01.18
Diabetes The Hypoglycaemia-Hyperglycaemia Minimizer System in the Management of Type 1 Diabetes Brian L Levy, Thomas W McCann, Jr and Daniel A Finan Animas Corporation, Wayne, USA DOI: http://doi.org/10.17925/ee.2016.12.01.18
SCIENTIFIC PROGRAM. PARALLEL INDUSTRY WORKSHOP Industry session not included in main event PARALLEL INDUSTRY WORKSHOP
 1 SCIENTIFIC PROGRAM Wednesday, 15 February, 2017 Blue Amphitheater Maillot 252 PARALLEL INDUSTRY WORKSHOP PARALLEL INDUSTRY WORKSHOP PARALLEL INDUSTRY WORKSHOP 14:30-16:00 Industry session not included
1 SCIENTIFIC PROGRAM Wednesday, 15 February, 2017 Blue Amphitheater Maillot 252 PARALLEL INDUSTRY WORKSHOP PARALLEL INDUSTRY WORKSHOP PARALLEL INDUSTRY WORKSHOP 14:30-16:00 Industry session not included
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING SYSTEMS/ EXTERNAL INSULIN PUMP THERAPY FOR DIABETES EFFECTIVE DATE: 08/17/17
 MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 11 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 11 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
More Than 1 Year of Hybrid Closed Loop in Pediatrics. Gregory P. Forlenza, MD Assistant Professor Barbara Davis Center
 More Than 1 Year of Hybrid Closed Loop in Pediatrics Gregory P. Forlenza, MD Assistant Professor Barbara Davis Center Disclosure Dr. Forlenza has served as a consultant for Abbott Diabetes Care and conducts
More Than 1 Year of Hybrid Closed Loop in Pediatrics Gregory P. Forlenza, MD Assistant Professor Barbara Davis Center Disclosure Dr. Forlenza has served as a consultant for Abbott Diabetes Care and conducts
Bringing closed-loop home: recent advances in closed-loop insulin delivery
 REVIEW C URRENT OPINION Bringing closed-loop home: recent advances in closed-loop insulin delivery Hood Thabit and Roman Hovorka Purpose of review To highlight the recent advances in closed-loop research,
REVIEW C URRENT OPINION Bringing closed-loop home: recent advances in closed-loop insulin delivery Hood Thabit and Roman Hovorka Purpose of review To highlight the recent advances in closed-loop research,
Diabetes Management: Current High Tech Innovations
 Diabetes Management: Current High Tech Innovations How Far We ve Come in the Last 40 Years William V. Tamborlane, MD Department of Pediatrics Yale School of Medicine Disclosures I am a consultant for:
Diabetes Management: Current High Tech Innovations How Far We ve Come in the Last 40 Years William V. Tamborlane, MD Department of Pediatrics Yale School of Medicine Disclosures I am a consultant for:
TABLE OF CONTENTS. HLC102A - Continuous Glucose Monitoring (CGM): Technologies and Global Markets
 TABLE OF CONTENTS CHAPTER 1 INTRODUCTION 1 STUDY GOALS AND OBJECTIVES 1 REASONS FOR DOING THIS STUDY 1 CONTRIBUTIONS TO THE STUDY AND INTENDED AUDIENCE 1 SCOPE AND FORMAT 1 METHODOLOGY AND INFORMATION
TABLE OF CONTENTS CHAPTER 1 INTRODUCTION 1 STUDY GOALS AND OBJECTIVES 1 REASONS FOR DOING THIS STUDY 1 CONTRIBUTIONS TO THE STUDY AND INTENDED AUDIENCE 1 SCOPE AND FORMAT 1 METHODOLOGY AND INFORMATION
Artificial Pancreas, shortly close to home? BC Pediatric Diabetes Day, Feb 26, 2016
 Artificial Pancreas, shortly close to home? BC Pediatric Diabetes Day, Feb 26, 2016 Rémi Rabasa- Lhoret, M.D. (Endocrinology), Ph.D. CSPQ Director Pla:orm for Research in Obesity, Metabolism and Diabetes
Artificial Pancreas, shortly close to home? BC Pediatric Diabetes Day, Feb 26, 2016 Rémi Rabasa- Lhoret, M.D. (Endocrinology), Ph.D. CSPQ Director Pla:orm for Research in Obesity, Metabolism and Diabetes
T he ultimate goal of diabetes technology
 D I A B E T E S Current Application of Continuous Glucose Monitoring in the Treatment of Diabetes Pros and cons JEROEN HERMANIDES, MD 1 MOSHE PHILLIP, MD, PHD 2 J. HANS DEVRIES, MD, PHD 1 T he ultimate
D I A B E T E S Current Application of Continuous Glucose Monitoring in the Treatment of Diabetes Pros and cons JEROEN HERMANIDES, MD 1 MOSHE PHILLIP, MD, PHD 2 J. HANS DEVRIES, MD, PHD 1 T he ultimate
INSULIN INITIATION AND INTENSIFICATION WITH A FOCUS ON HYPOGLYCEMIA REDUCTION
 INSULIN INITIATION AND INTENSIFICATION WITH A FOCUS ON HYPOGLYCEMIA REDUCTION Jaiwant Rangi, MD, FACE Nov 10 th 2018 DISCLOSURES Speaker Novo Nordisk Sanofi-Aventis Boheringer Ingleheim Merck Abbvie Abbott
INSULIN INITIATION AND INTENSIFICATION WITH A FOCUS ON HYPOGLYCEMIA REDUCTION Jaiwant Rangi, MD, FACE Nov 10 th 2018 DISCLOSURES Speaker Novo Nordisk Sanofi-Aventis Boheringer Ingleheim Merck Abbvie Abbott
Lessons From The Type 1 Diabetes Exchange
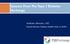 Lessons From The Type 1 Diabetes Exchange Andrew Ahmann, MD Harold Schnitzer Diabetes Health Center at OHSU Presenter Disclosure Information In compliance with the accrediting board policies, the American
Lessons From The Type 1 Diabetes Exchange Andrew Ahmann, MD Harold Schnitzer Diabetes Health Center at OHSU Presenter Disclosure Information In compliance with the accrediting board policies, the American
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid
 Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2014 Origination: 11/2001 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City
Continuous or Intermittent Glucose Monitoring in Interstitial Fluid Policy Number: 1.01.20 Last Review: 9/2014 Origination: 11/2001 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City
Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial
 Diabetologia (2009) 52:1250 1257 DOI 10.1007/s00125-009-1365-0 ARTICLE Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial M. A. O Connell
Diabetologia (2009) 52:1250 1257 DOI 10.1007/s00125-009-1365-0 ARTICLE Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial M. A. O Connell
Hypoglycemia a barrier to normoglycemia Are long acting analogues and pumps the answer to the barrier??
 Hypoglycemia a barrier to normoglycemia Are long acting analogues and pumps the answer to the barrier?? Moshe Phillip Institute of Endocrinology and Diabetes National Center of Childhood Diabetes Schneider
Hypoglycemia a barrier to normoglycemia Are long acting analogues and pumps the answer to the barrier?? Moshe Phillip Institute of Endocrinology and Diabetes National Center of Childhood Diabetes Schneider
Fear of hypoglycemia, parenting stress, and metabolic control for children with type 1 diabetes and their parents
 The final publication is available at Springer via http://dx.doi.org/10.1007/s10880-017-9489-8 Fear of hypoglycemia, parenting stress, and metabolic control for children with type 1 diabetes and their
The final publication is available at Springer via http://dx.doi.org/10.1007/s10880-017-9489-8 Fear of hypoglycemia, parenting stress, and metabolic control for children with type 1 diabetes and their
Effective Health Care Program
 Comparative Effectiveness Review Number 57 Effective Health Care Program Methods for Insulin Delivery and Glucose Monitoring: Comparative Effectiveness Executive Summary Background Diabetes mellitus is
Comparative Effectiveness Review Number 57 Effective Health Care Program Methods for Insulin Delivery and Glucose Monitoring: Comparative Effectiveness Executive Summary Background Diabetes mellitus is
NDSS Helpline
 Continuous GLUCOSE MONITORING A guide to using CGM for children and young people with type 1 diabetes NDSS Helpline 1300 136 588 ndss.com.au The National Diabetes Services Scheme is an initiative of the
Continuous GLUCOSE MONITORING A guide to using CGM for children and young people with type 1 diabetes NDSS Helpline 1300 136 588 ndss.com.au The National Diabetes Services Scheme is an initiative of the
Diabetes Care 34: , with an increased risk of SH. In a
 Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Factors Predictive of Severe Hypoglycemia in Type 1 Diabetes Analysis from the Juvenile Diabetes Research Foundation
Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Factors Predictive of Severe Hypoglycemia in Type 1 Diabetes Analysis from the Juvenile Diabetes Research Foundation
RESEARCH. open access
 open access Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised
open access Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised
QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3?
 500 1 QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3? WHAT ADDITIONAL DATA ARE NEEDED? AACE/ACE CGM Consensus Conference:
500 1 QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3? WHAT ADDITIONAL DATA ARE NEEDED? AACE/ACE CGM Consensus Conference:
National Best Practice Guides: Inpatients on Continuous Subcutaneous Insulin Infusion (CSII)
 Gold Sponsors: Silver Sponsors: National Best Practice Guides: Inpatients on Continuous Subcutaneous Insulin Infusion (CSII) #abcdipn17 @uk_ipn Bronze Sponsors: The sponsoring pharmaceutical companies
Gold Sponsors: Silver Sponsors: National Best Practice Guides: Inpatients on Continuous Subcutaneous Insulin Infusion (CSII) #abcdipn17 @uk_ipn Bronze Sponsors: The sponsoring pharmaceutical companies
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Diabetes Management with Continuous Glucose Monitoring & Multiple Daily Injections. Aaron Michels MD
 Diabetes Management with Continuous Glucose Monitoring & Multiple Daily Injections Aaron Michels MD Outline SMBG & CGM by age group JDRF CGM Trial Sensor Augmented Insulin Pump Therapy for A1c Reduction
Diabetes Management with Continuous Glucose Monitoring & Multiple Daily Injections Aaron Michels MD Outline SMBG & CGM by age group JDRF CGM Trial Sensor Augmented Insulin Pump Therapy for A1c Reduction
Today s Goals 10/6/2017. New Frontiers in Diabetes Technology. Disclosures
 New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
Type 1 diabetes (T1D) is one of the most common chronic
 DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Supplement 1, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2015.1512 ORIGINAL ARTICLE Diabetes Technology and Therapy in the Pediatric Age Group Shlomit
DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Supplement 1, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2015.1512 ORIGINAL ARTICLE Diabetes Technology and Therapy in the Pediatric Age Group Shlomit
