Agent(s) FDA Labeled Indications Dosage and Administration. Perinatal/Infantile-onset hypophosphatasia. Juvenile-onset hypophosphatasia
|
|
|
- Gilbert Hart
- 5 years ago
- Views:
Transcription
1 Strensiq (asfotase alfa) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE Agent(s) FDA Labeled Indications Dosage and Administration Strensiq (asfotase alfa) injection Perinatal/Infantile-onset hypophosphatasia Juvenile-onset hypophosphatasia Perinatal/Infantile-onset hypophosphatasia: 2 mg/kg subcutaneously three times per week or 1 mg/kg subcutaneously six times per week. The dose of Strensiq may be increased for lack of efficacy (e.g., no improvement in respiratory status, growth, or radiographic findings) up to 9 mg/kg per week administered as 3 mg/kg subcutaneously three times per week Juvenile-onset hypophosphatasia: 2 mg/kg subcutaneously three times per week or 1 mg/kg six times per week Injection site reactions may limit the tolerability of the six times per week regimen in both Perinatal/Infantile and Juvenile-onset hypophosphatasia CLINICAL RATIONALE Hypophosphatasia (HPP) is a rare genetic disease, characterized by mutations in the tissue non-specific alkaline phosphatase (TNSALP) gene, leading to a diminished activity of the TNSALP enzyme in target tissues and accumulation on TNSALP substrates, including inorganic pyrophosphate, in inhibitor of mineralization. 2 TNSALP controls skeletal and dental mineralization by hydrolyzing inorganic pyrophosphate, an inhibitor of hydroxyapatite crystal growth. Insufficient activity can lead to chest wall instability and respiratory complications in perinatal and infantile forms. Natural substrates of TNSALP that accumulate in hypophosphatasia include inorganic pyrophosphate (PPi), phosphoethanolamine (PEA), and pyridoxal 5 -phosphate (PLP), the principal circulating form of vitamin B6. 4 Perinatal HPP features extreme skeletal disease obvious at birth; survival beyond birth is rare. Infantile HPP develops prior to 6 months of age and has an estimated 50% mortality during KS_PS_Strensiq_PA_ProgSum_AR1018 Page 1 of 6
2 infancy typically due to respiratory complications. Patients develop rickets, failure to thrive, hypotonia, myopathy, and is often complicated by hypercalcemia, nephrocalcinosis, craniosynostosis, and vitamin B6-dependent seizures. Although spontaneous improvement sometimes occurs in infantile hypophosphatasia, substantial bone disease and weakness often persist. Skeletal deterioration typically results in death from respiratory insufficiency. In both forms, hypomineralization leads to thoracic instability, fractures, and deformities, and sometimes even pulmonary hypoplasia in perinatal HPP. 3 Juvenile HPP tends to be less severe than those that appear in infancy. Affected children may have short stature, bowed legs, enlarged wrist and ankle joints (metaphyseal flares that appear as swollen joints ), muscle weakness, and abnormal skull shape. 3 Although the disease spectrum is a continuum, six clinical forms are usually recognized based on age at diagnosis and severity of features: 3 Perinatal (severe) hypophosphatasia characterized by respiratory insufficiency and hypercalcemia Perinatal (benign) hypophosphatasia with prenatal skeletal manifestations that slowly resolve into one of the milder forms Infantile hypophosphatasia with onset between birth and age six months of rickets without elevated serum alkaline phosphatase activity Childhood (juvenile) hypophosphatasia that ranges from low bone mineral density for age with unexplained fractures to rickets, and premature loss of primary teeth with intact roots Adult hypophosphatasia characterized by stress fractures and pseudofractures of the lower extremities in middle age, sometimes associated with early loss of adult dentition Odontohypophosphatasia characterized by premature exfoliation of primary teeth and/or severe dental caries without skeletal manifestations A diagnosis of HPP is based upon identification of characteristic signs and symptoms, a detailed patient history, a thorough clinical evaluation, and a variety of laboratory tests including routine x-ray and biochemical studies. Proper diagnosis of HPP is easy for physicians who are familiar or experienced with this disorder. However, most physicians have little or no knowledge of HPP. Consequently, affected individuals and families may face a frustrating delay in diagnosis. Now, mutation analysis of the ALPL gene is available from commercial laboratories. Clinical Testing and Workup The diagnosis is rarely first suspected from a routine panel of biochemical tests that includes measuring the activity of alkaline phosphatase in blood. Instead signs and symptoms have led to this routine test where the low levels of alkaline phosphatase must be recognized. Individuals with HPP have reduced serum alkaline phosphatase activity for their age, except for the extremely rare individual with pseudohypophosphatasia who has normal activity levels. Identification of deficient alkaline phosphatase activity is consistent with HPP, but not conclusive since other conditions can result in this finding. Additionally, some individuals who are genetic carriers of HPP, but who do not develop any symptoms of the disorder, may also have low blood ALP levels. Importantly, the range of serum ALP activity varies by age. Healthy children normally have higher ALP levels than healthy adults. If the laboratory doing the testing only gives the normal range of ALP activity in adults in its report, a diagnosis of HPP in a child can be missed because the child s ALP activity will mistakenly be believed to be normal. KS_PS_Strensiq_PA_ProgSum_AR1018 Page 2 of 6
3 In the U.S. and elsewhere, a suspected diagnosis of HPP can be further supported by measuring the serum level of vitamin B6. This test is performed by several commercial laboratories. Individuals with HPP have elevated levels of pyridoxal 5 -phosphate (PLP: the active form of vitamin B6) in the blood because PLP is normally broken down by TNSALP. PLP is elevated even in individuals with mild HPP. However, some genetic carriers of HPP who do not develop any symptoms can have an elevated PLP level as well. In the past, blood or urine was tested for increased amounts of phosphoethanolamine (PEA), another chemical normally broken down by TNSALP. However, this finding is not specific to HPP and can occur because of other metabolic bone diseases. Additionally, some individuals with HPP have normal PEA levels. Screening for elevated PLP is preferred over screening for PEA because it is more sensitive, more precise, and less expensive. In the most severe cases of HPP, specifically the perinatal and infantile forms, x-ray studies can reveal diagnostic changes within the bones. However, these changes may not be recognized as being associated with HPP, except by radiologists familiar with the disorder. Molecular genetic testing can support a diagnosis of HPP. Molecular genetic testing can detect mutations in the ALPL gene known to cause the disorder, but it is only available as a diagnostic service at specialized laboratories. 4 Efficacy 1 Strensiq is the first approved therapy for perinatal, infantile and juvenile-onset HPP. Strensiq is a formulation of asfotase alfa, which is a soluble glycoprotein composed of two identical polypeptide chains. Srensiq is a tissue nonspecific alkaline phosphatase produced by recombinant DNA technology in a Chinese hamster ovary cell line. Strensiq was evaluated in 2 studies for perinatal/infantile-onset hypophosphatasia (HPP) identified in the label as study 1 and study 2. Study 1 was a 24-week prospective single-arm trial in 11 patients with severe perinatal/infantile-onset HPP. Study 2 was a prospective open-label study in 59 patients with perinatal/infantile-onset HPP. Survival and invasive ventilation-free survival were compared in both study 1 and study 2 with a historical cohort of untreated patients with similar clinical characteristics. The results of this comparison are shown in Table 1 below: Table 1 KS_PS_Strensiq_PA_ProgSum_AR1018 Page 3 of 6
4 Study 3 (as identified in the prescribing information) was a prospective open-label 24-week trial that included 8 juvenile-onset HPP and 5 perinatal/infantile-onset HPP patients. Growth and skeletal manifestations were compared with a historical cohort of 32 untreated patients with similar clinical characteristics. Strensiq treated patients showed better z-scores for height and weight compared to the historical cohorts. All 8 Strensiq treated patients were rated as responders (defined as a 2 or higher on the Radiographic Global Impression of Change scale by month 54 of treatment for skeletal manifestations. Gait was also assessed using a modified Performance Oriented Mobility Assessment-Gait (MPOMA-G) scale in all 8 patients and using the 6-minute walk test in 7 of the 8 patients in this study. Step length improved by at least 1 point in either foot in 6 out of the 8 patients compared to 1 out of 6 control patients. The proportion of patients who had 6-minute walk test percent predicted values within the normal range for age, sex, and height-matched peers increased from 0 out of 8 patients at baseline to 6 patients by month 48. All 6 of these patients were also able to walk longer distances at this time point compared to baseline. Safety 1 The 80 mg/0.8 ml vial of Strensiq should not be used in pediatric patients weighing less than 40 kg because the systemic exposure of asfotase alfa achieved with the 80 mg/0.8 ml (higher concentration) is lower than that achieved with the other strength vials (lower concentration). A lower exposure may not be adequate for this subgroup of patients. REFERENCES 1. Stensiq prescribing information. Alexion. January Cohen A, Drake MT. UpToDate Epidemiology and etiology of osteomalacia. Last updated August Mornet, E. Hypophophatasia. Orphanet J Rare Dis. 2007;2:40 4. National Organization for Rare Disorders (NORD). Hypophosphatasia. Accessed August KS_PS_Strensiq_PA_ProgSum_AR1018 Page 4 of 6
5 Strensiq (asfotase alfa) Prior Authorization OBJECTIVE The intent of the Strensiq Prior Authorization (PA) Program is to encourage appropriate selection of patients for therapy according to product labeling and/or clinical guidelines and/or clinical studies. Criteria will approve doses that are at or below the maximum FDA labeled dose. TARGET AGENT(S) Strensiq (asfotase alfa) Agent GPI Multisource code Strensiq (asfotase alfa) 18 mg/0.45 ml injection M, N, O, Y 28 mg/0.7 ml injection M, N, O, Y 40 mg/1 ml injection M, N, O, Y 80 mg/0.8 ml injection M, N, O, Y PRIOR AUTHORIZATION CRITERIA FOR APPROVAL Initial Evaluation The requested agent will be approved when ALL of the following are met: 1. The patient has a diagnosis of either perinatal/infantile- OR juvenile-onset hypophosphatasia (HPP) ALL of the following: A. The patient was 18 years of age at onset B. The patient has/had clinical manifestations consistent with hypophospatasia at the age of onset prior to age 18 (e.g. vitamin B6- dependent seizures, skeletal abnormalities: such as rachitic chest deformity leading to respiratory problems or bowed arms/legs, failure to thrive ) C. The patient has/had radiographic imaging to support the diagnosis of hypophospatasia at the age of onset prior to age 18 (e.g. infantile rickets, alveolar bone loss, craniosynostosis) D. Molecular genetic test has been completed confirming mutations in the ALPL gene that encodes the tissue nonspecific isoenzyme of ALP (TNSALP) E. Reduced activity of unfractionated serum alkaline phosphatase (ALP) in the absence of bisphosphonate therapy (i.e. below the normal lab reference range for age and sex) F. ONE of the following: i. Elevated serum concentration of pyridoxal 5'-phosphate (PLP) in the absence of vitamin supplements within one week prior to the test OR ii. Elevated urine concentration of phosphoethanolamine (PEA) OR iii. Elevated urinary inorganic pyrophosphate (PPi) KS_PS_Strensiq_PA_ProgSum_AR1018 Page 5 of 6
6 2. The prescriber is a specialist in the area of the patient s diagnosis (e.g. endocrinologist) or the prescriber has consulted with a specialist in the area of the patient s diagnosis 3. The patient does not have any FDA labeled contraindication(s) to the requested agent 4. The prescriber has provided the patient s weight 5. The requested quantity is within FDA labeled dosing based on the patient s weight Length of Approval: 6 months Renewal Evaluation The requested agent will be approved when ALL the following are met: 1. The patient has been previously approved for the requested agent through the Prime Therapeutics PA process 2. The prescriber is a specialist in the area of the patient s diagnosis (e.g. endocrinologist) or the prescriber has consulted with a specialist in the area of the patient s diagnosis 3. The patient has shown clinical improvement with the requested agent as evidenced by an improvement and/or stabilization (upon subsequent renewals) respiratory status, growth, or radiographic findings from baseline (documentation from the medical record is required to be submitted) 4. The patient does not have any FDA labeled contraindication(s) to the requested agent 5. The prescriber has provided the patient s weight 6. The requested quantity is within FDA labeled dosing based on the patient s weight Length of Approval: 12 months Policy published Policy effective KS_PS_Strensiq_PA_ProgSum_AR1018 Page 6 of 6
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Strensiq (asfotase alfa) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Strensiq (asfotase alfa) Prime Therapeutics will review Prior Authorization
Strensiq (asfotase alfa) Page 1 of 5 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Strensiq (asfotase alfa) Prime Therapeutics will review Prior Authorization
Hypophosphatasia. Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX
 Hypophosphatasia Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX Hypophosphatasia Case Teeth 57 y/o male with a hyperlipidemia and hypogonadism. Noted to have an alkaline phosphatase of
Hypophosphatasia Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX Hypophosphatasia Case Teeth 57 y/o male with a hyperlipidemia and hypogonadism. Noted to have an alkaline phosphatase of
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Strensiq) Reference Number: CP.PHAR.328 Effective Date: 03.01.17 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Strensiq) Reference Number: CP.PHAR.328 Effective Date: 03.01.17 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: Asfotase Alfa (Strensiq) Reference Number: CP.PHAR.328 Effective Date: Last Review Date: 11.17
 Clinical Policy: (Strensiq) Reference Number: CP.PHAR.328 Effective Date: 03.01.17 Last Review Date: 11.17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Strensiq) Reference Number: CP.PHAR.328 Effective Date: 03.01.17 Last Review Date: 11.17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
STRENSIQ (asfotase alfa)
 STRENSIQ (asfotase alfa) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
STRENSIQ (asfotase alfa) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
An Introduction to. Hypophosphatasia. Michael P. Whyte, M.D. Canadian adaptation by Cheryl Rockman-Greenberg, MDCM
 An Introduction to Hypophosphatasia Michael P. Whyte, M.D. Canadian adaptation by Cheryl Rockman-Greenberg, MDCM Soft Bones Canada Inc. was formed in 2013 to provide information and a community to educate,
An Introduction to Hypophosphatasia Michael P. Whyte, M.D. Canadian adaptation by Cheryl Rockman-Greenberg, MDCM Soft Bones Canada Inc. was formed in 2013 to provide information and a community to educate,
A Genetic Overview of Hypophosphatasia
 A Genetic Overview of Hypophosphatasia A Background in Genetics Cells are the basic building blocks of all living organisms. Our body is composed of trillions of cells. Each of these cells contains deoxyribonucleic
A Genetic Overview of Hypophosphatasia A Background in Genetics Cells are the basic building blocks of all living organisms. Our body is composed of trillions of cells. Each of these cells contains deoxyribonucleic
Take the next step with Strensiq.
 Introducing Strensiq : the first and only treatment for people with perinatal/infantile- and juvenile-onset hypophosphatasia (HPP) Take the next step with Strensiq. Strensiq can improve bone health in
Introducing Strensiq : the first and only treatment for people with perinatal/infantile- and juvenile-onset hypophosphatasia (HPP) Take the next step with Strensiq. Strensiq can improve bone health in
Pediatric metabolic bone diseases
 Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Do not use the 80 mg/0.8 ml vial of STRENSIQ in pediatric patients weighing less than 40 kg [see Clinical Pharmacology (12.3)]. **
![Do not use the 80 mg/0.8 ml vial of STRENSIQ in pediatric patients weighing less than 40 kg [see Clinical Pharmacology (12.3)]. ** Do not use the 80 mg/0.8 ml vial of STRENSIQ in pediatric patients weighing less than 40 kg [see Clinical Pharmacology (12.3)]. **](/thumbs/76/73630682.jpg) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STRENSIQ safely and effectively. See full prescribing information for STRENSIQ. STRENSIQ (asfotase
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STRENSIQ safely and effectively. See full prescribing information for STRENSIQ. STRENSIQ (asfotase
Case Report: Perinatal Lethal Form of Hypophosphatasia
 Med. J. Cairo Univ., Vol. 83, No. 2, December: 273-277, 201 5 www.medicaljournalofcairouniversity.net Case Report: Perinatal Lethal Form of Hypophosphatasia NAHLA BARAKAT, M.D.*; WALEED SHABAAN, M.R.C.P.*
Med. J. Cairo Univ., Vol. 83, No. 2, December: 273-277, 201 5 www.medicaljournalofcairouniversity.net Case Report: Perinatal Lethal Form of Hypophosphatasia NAHLA BARAKAT, M.D.*; WALEED SHABAAN, M.R.C.P.*
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
DIAGNOSING X-LINKED HYPOPHOSPHATEMIA (XLH) BIOCHEMICAL TESTING CONSIDERATIONS
 DIAGNOSING X-LINKED HYPOPHOSPHATEMIA (XLH) BIOCHEMICAL TESTING CONSIDERATIONS XLH IS CHARACTERIZED BY CHRONIC HYPOPHOSPHATEMIA XLH is a hereditary, progressive, lifelong disorder. In children and adults,
DIAGNOSING X-LINKED HYPOPHOSPHATEMIA (XLH) BIOCHEMICAL TESTING CONSIDERATIONS XLH IS CHARACTERIZED BY CHRONIC HYPOPHOSPHATEMIA XLH is a hereditary, progressive, lifelong disorder. In children and adults,
Report on the Deliberation Results
 Report on the Deliberation Results June 9, 2015 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] Strensiq Subcutaneous Injection
Report on the Deliberation Results June 9, 2015 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] Strensiq Subcutaneous Injection
Strensiq is supplied as a single-use vial containing 40 or 100 mg/ml of asfotase alfa rch.
 AUSTRALIAN PRODUCT INFORMATION STRENSIQ (ASFOTASE ALFA RCH) SOLUTION FOR INJECTION 1 NAME OF THE MEDICINE Strensiq (asfotase alfa rch) solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
AUSTRALIAN PRODUCT INFORMATION STRENSIQ (ASFOTASE ALFA RCH) SOLUTION FOR INJECTION 1 NAME OF THE MEDICINE Strensiq (asfotase alfa rch) solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Sachin Soni DNB Pediatrics
 Sachin Soni DNB Pediatrics Vitamin D physiology Introduction Etiology Clinical feature Radiology Diagnosis Lab Treatment Source: -Fish, liver and oil, - Human milk (30-40 IU/L) - Exposure to sun light
Sachin Soni DNB Pediatrics Vitamin D physiology Introduction Etiology Clinical feature Radiology Diagnosis Lab Treatment Source: -Fish, liver and oil, - Human milk (30-40 IU/L) - Exposure to sun light
Hypophosphatasia: Rare but there
 Review Article 10.5005/jp-journals-10031-1169 1 Vikas K Jhingala, 2 Pradeep Tangade, 3 Himanshu Punia, 4 Vipul Gupta, 5 Vikas Singh 6 Monika Kalra, 7 Suyash Chaudhary ABSTRACT Hypophosphatasia (HP) is
Review Article 10.5005/jp-journals-10031-1169 1 Vikas K Jhingala, 2 Pradeep Tangade, 3 Himanshu Punia, 4 Vipul Gupta, 5 Vikas Singh 6 Monika Kalra, 7 Suyash Chaudhary ABSTRACT Hypophosphatasia (HP) is
Clinical and Genetic Findings of Turkish Hypophosphatasia Cases
 ORIGINAL ARTICLE DOI: 10.4274/jcrpe.4549 J Clin Res Pediatr Endocrinol Clinical and Genetic Findings of Turkish Hypophosphatasia Cases Halil Sağlam 1, Şahin Erdöl 2, Sevil Dorum 2 1 Uludağ University Faculty
ORIGINAL ARTICLE DOI: 10.4274/jcrpe.4549 J Clin Res Pediatr Endocrinol Clinical and Genetic Findings of Turkish Hypophosphatasia Cases Halil Sağlam 1, Şahin Erdöl 2, Sevil Dorum 2 1 Uludağ University Faculty
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Rickets Simple complement 1. What factor influences on vitamin D absorption at the level of small intestine? A. Normal absorption of lipids B.
 Rickets Simple complement 1. What factor influences on vitamin D absorption at the level of small intestine? A. Normal absorption of lipids B. Increased concentration of proteins in foods C. Decreasing
Rickets Simple complement 1. What factor influences on vitamin D absorption at the level of small intestine? A. Normal absorption of lipids B. Increased concentration of proteins in foods C. Decreasing
Hypophosphatasia (HPP) is a rare inherited disorder. focus Neurosurg Focus 38 (5):E10, 2015
 neurosurgical focus Neurosurg Focus 38 (5):E10, 2015 Enzyme replacement therapy for congenital hypophosphatasia allows for surgical treatment of related complex craniosynostosis: a case series Libby Kosnik-Infinger,
neurosurgical focus Neurosurg Focus 38 (5):E10, 2015 Enzyme replacement therapy for congenital hypophosphatasia allows for surgical treatment of related complex craniosynostosis: a case series Libby Kosnik-Infinger,
X-Linked Hypophosphatemia (XLH) Case Study: A Dynamic Development Model for Rare Disease
 X-Linked Hypophosphatemia (XLH) Case Study: A Dynamic Development Model for Rare Disease Alison Skrinar, PhD Executive Director, Clinical Outcomes Research and Evaluation (CORE) www.ultragenyx.com Dynamic
X-Linked Hypophosphatemia (XLH) Case Study: A Dynamic Development Model for Rare Disease Alison Skrinar, PhD Executive Director, Clinical Outcomes Research and Evaluation (CORE) www.ultragenyx.com Dynamic
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Drug Discovery, Vol. 11, No. 27, February 1, 2016 COMMUNICATION COMMUNICATION ISSN 2278 540X EISSN 2278 5396 Drug Discovery An International Journal FDA Approved Drugs October 2015 Vidhya V Publication
Drug Discovery, Vol. 11, No. 27, February 1, 2016 COMMUNICATION COMMUNICATION ISSN 2278 540X EISSN 2278 5396 Drug Discovery An International Journal FDA Approved Drugs October 2015 Vidhya V Publication
burosumab (Crysvita )
 burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
In fall 2015, our field achieved a milestone in the management
 PERSPECTIVE JBMR Hypophosphatasia: Enzyme Replacement Therapy Brings New Opportunities and New Challenges Michael P Whyte Department of Internal Medicine, Division of Bone and Mineral Diseases, Washington
PERSPECTIVE JBMR Hypophosphatasia: Enzyme Replacement Therapy Brings New Opportunities and New Challenges Michael P Whyte Department of Internal Medicine, Division of Bone and Mineral Diseases, Washington
Clinical Outcome Assessments: Use of Normative Data in a Pediatric Rare Disease
 VALUE IN HEALTH 21 (2018) 508 514 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Clinical Outcome Assessments: Use of Normative Data in a Pediatric Rare Disease
VALUE IN HEALTH 21 (2018) 508 514 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Clinical Outcome Assessments: Use of Normative Data in a Pediatric Rare Disease
4/15/2016 ANALYSIS OF CRANIOFACIAL MORPHOLOGY IN A TNAP KNOCKOUT MOUSE MODEL CRANIOSYNOSTOSIS CURRENT TREAMENT: CRANIAL VAULT REMODELING SURGERY
 CRANIOSYNOSTOSIS Infant with craniosynostosis ANALYSIS OF CRANIOFACIAL MORPHOLOGY IN A TNAP KNOCKOUT MOUSE MODEL Premature fusion of cranial bones 1/2500 live births May involve one or multiple sutures
CRANIOSYNOSTOSIS Infant with craniosynostosis ANALYSIS OF CRANIOFACIAL MORPHOLOGY IN A TNAP KNOCKOUT MOUSE MODEL Premature fusion of cranial bones 1/2500 live births May involve one or multiple sutures
Request for Prior Authorization Growth Hormone (Norditropin
 Request for Prior Authorization Growth Hormone (Norditropin, Nutropin/AQ ) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Growth Hormone require a
Request for Prior Authorization Growth Hormone (Norditropin, Nutropin/AQ ) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Growth Hormone require a
Results Confirm and Extend 40-Week Findings that Treatment with Crysvita is Superior to Conventional Therapy
 Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Updates to the Alberta Drug Benefit List. Effective March 1, 2018
 Updates to the Alberta Drug Benefit List Effective March 1, 2018 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780) 498-8370
Updates to the Alberta Drug Benefit List Effective March 1, 2018 Inquiries should be directed to: Pharmacy Services Alberta Blue Cross 10009 108 Street NW Edmonton AB T5J 3C5 Telephone Number: (780) 498-8370
= Developmental disorders of chondro-osseous tissue
 = Developmental disorders of chondro-osseous tissue Common Orthopedic Problems Dwarfism Short Stature Pathologic Short Stature Normal Variant Short Stature Proportionate Short Stature Midget Endocrine/nutritional
= Developmental disorders of chondro-osseous tissue Common Orthopedic Problems Dwarfism Short Stature Pathologic Short Stature Normal Variant Short Stature Proportionate Short Stature Midget Endocrine/nutritional
Thomas Carpenter Yale University School of Medicine New Haven, Connecticut, USA
 A Randomized, Open-label Phase 2 Study of, a Fully Human Anti-FGF23 Monoclonal Antibody, in 52 Children with X-linked Hypophosphatemia (XLH): 40-Week Results Thomas Carpenter Yale University School of
A Randomized, Open-label Phase 2 Study of, a Fully Human Anti-FGF23 Monoclonal Antibody, in 52 Children with X-linked Hypophosphatemia (XLH): 40-Week Results Thomas Carpenter Yale University School of
X-linked hypophosphatemic rickets across the lifespan
 X-linked hypophosphatemic rickets across the lifespan AACE Midwest Regional Meeting Erik A. Imel, M.D. Associate Professor of Medicine and Pediatrics Indiana Center for Musculoskeletal Health July 28,
X-linked hypophosphatemic rickets across the lifespan AACE Midwest Regional Meeting Erik A. Imel, M.D. Associate Professor of Medicine and Pediatrics Indiana Center for Musculoskeletal Health July 28,
In addition to bone health, emerging science reveals a non-skeletal benefit of vitamin D for several other health outcomes.
 Vitamin D AT A GLANCE Introduction Vitamin D comprises a group of fat-soluble compounds that are essential for maintaining the mineral balance in the body. The vitamin D form synthesized in humans is called
Vitamin D AT A GLANCE Introduction Vitamin D comprises a group of fat-soluble compounds that are essential for maintaining the mineral balance in the body. The vitamin D form synthesized in humans is called
DISEASES WITH ABNORMAL MATRIX
 DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Natpara (parathyroid hormone) Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Natpara (parathyroid hormone) Prime Therapeutics will review Prior Authorization
Natpara (parathyroid hormone) Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Natpara (parathyroid hormone) Prime Therapeutics will review Prior Authorization
Conventional therapy n=32. RGI-C Score (Primary Endpoint) LS Mean* (0.83, 1.45) P< Substantial healing, % patients (RGI-C +2.
 Ultragenyx and Kyowa Kirin Announce Topline Phase 3 Study Results Demonstrating Superiority of Crysvita (burosumab) Treatment to Oral Phosphate and Active Vitamin D in Children with X-Linked Hypophosphatemia
Ultragenyx and Kyowa Kirin Announce Topline Phase 3 Study Results Demonstrating Superiority of Crysvita (burosumab) Treatment to Oral Phosphate and Active Vitamin D in Children with X-Linked Hypophosphatemia
CHAPTER 13 SKELETAL SYSTEM
 CHAPTER 13 SKELETAL SYSTEM Structure and Function Functions of the skeletal system Provides shape and support Protects internal organs Stores minerals and fat Produces blood cells and platelets Assists
CHAPTER 13 SKELETAL SYSTEM Structure and Function Functions of the skeletal system Provides shape and support Protects internal organs Stores minerals and fat Produces blood cells and platelets Assists
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr STRENSIQ (asfotase alfa) Solution for Injection 40 mg/ml & 100 mg/ml Enzyme Replacement Therapy ATC code: A16AB13 STRENSIQ (asfotase alfa),
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr STRENSIQ (asfotase alfa) Solution for Injection 40 mg/ml & 100 mg/ml Enzyme Replacement Therapy ATC code: A16AB13 STRENSIQ (asfotase alfa),
1. Adults; a. Risk factors. b. Who should be tested for vitamin D deficiency? c. Investigations. d. Who do we treat and how do we treat? 2.
 Vitamin D and Bone Health: A Practical Clinical Guideline for Patient Management For Adults and Children Adapted from existing local guidance, National Osteoporosis Society Practical Guides and from Royal
Vitamin D and Bone Health: A Practical Clinical Guideline for Patient Management For Adults and Children Adapted from existing local guidance, National Osteoporosis Society Practical Guides and from Royal
Pediatric Injuries/Fractures. Rena Heathcote
 Pediatric Injuries/Fractures Rena Heathcote INTRODUCTION Incidence Anatomy of the Growing Bone Injury Patterns What can we X-ray PEDIATRIC FRACTURES INCIDENCE What makes children susceptible to fractures?
Pediatric Injuries/Fractures Rena Heathcote INTRODUCTION Incidence Anatomy of the Growing Bone Injury Patterns What can we X-ray PEDIATRIC FRACTURES INCIDENCE What makes children susceptible to fractures?
Skeletal. Ostase (Bone alkaline phosphatase) Analyte Information
 Skeletal Ostase (Bone alkaline phosphatase) Analyte Information 1 2012-04-04 Ostase (Bone alkaline phosphatase - BAP) Introduction Ostase is a trademark of an immunoassay for the quantitative measurement
Skeletal Ostase (Bone alkaline phosphatase) Analyte Information 1 2012-04-04 Ostase (Bone alkaline phosphatase - BAP) Introduction Ostase is a trademark of an immunoassay for the quantitative measurement
The Medical Assessment of Fractures in Suspected Child Maltreatment: Infants and Young Children with Skeletal Injury CPS Podcast
 The Medical Assessment of Fractures in Suspected Child Maltreatment: Infants and Young Children with Skeletal Injury CPS Podcast September 27, 2018 Introduction: Hello everyone, my name is Dominique Piché
The Medical Assessment of Fractures in Suspected Child Maltreatment: Infants and Young Children with Skeletal Injury CPS Podcast September 27, 2018 Introduction: Hello everyone, my name is Dominique Piché
Surgical management for tibial shaft fractures using flexible nailing in adult hypophosphatasia. Jay N. Patel, Priyal V. Bhagat, Li Sun, Juluru Rao
 Patel et al. 1 ACCEPTED CASE REPORT MANUSCRIPT PEER PEER REVIEWED REVIEWED PROVISIONAL OPEN ACCESS PDF Surgical management for tibial shaft fractures using flexible nailing in adult hypophosphatasia Jay
Patel et al. 1 ACCEPTED CASE REPORT MANUSCRIPT PEER PEER REVIEWED REVIEWED PROVISIONAL OPEN ACCESS PDF Surgical management for tibial shaft fractures using flexible nailing in adult hypophosphatasia Jay
Lower Extremity Malalignment: When to Refer and When to Reassure?
 Lower Extremity Malalignment: When to Refer and When to Reassure? Mary Aschenbrener, PA-C Minnesota Academy of Physician Assistants 03/18/16 Cary H. Mielke, MD Chief of Staff Orthopaedic Burn Spinal cord
Lower Extremity Malalignment: When to Refer and When to Reassure? Mary Aschenbrener, PA-C Minnesota Academy of Physician Assistants 03/18/16 Cary H. Mielke, MD Chief of Staff Orthopaedic Burn Spinal cord
X-linked hypophosphatemic rickets across the lifespan
 X-linked hypophosphatemic rickets across the lifespan AACE Midwest Regional Meeting Erik A. Imel, M.D. Associate Professor of Medicine and Pediatrics Indiana Center for Musculoskeletal Health July 28,
X-linked hypophosphatemic rickets across the lifespan AACE Midwest Regional Meeting Erik A. Imel, M.D. Associate Professor of Medicine and Pediatrics Indiana Center for Musculoskeletal Health July 28,
PROTOCOLS. Osteogenesis imperfecta. Principal investigator. Co-investigators. Background
 Osteogenesis imperfecta Principal investigator Leanne M. Ward, MD, FRCPC, paediatric endocrinologist Division of Endocrinology and Metabolism, Children s Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa
Osteogenesis imperfecta Principal investigator Leanne M. Ward, MD, FRCPC, paediatric endocrinologist Division of Endocrinology and Metabolism, Children s Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa
PEDIATRIC SLEEP GUIDELINES Version 1.0; Effective
 MedSolutions, Inc. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Requests for patients with atypical symptoms or clinical presentations
MedSolutions, Inc. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Requests for patients with atypical symptoms or clinical presentations
Case 4 Generalised bone pain
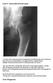 Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
MO Ped Trial FAQs. Page 1
 MO Ped Trial FAQs 1. Is MO, HMO, HME, and MHE all the same disease? Yes. Multiple Osteochondromas (MO) has many names, including Hereditary Multiple Osteochondromas (HMO), Multiple Hereditary (or hereditary
MO Ped Trial FAQs 1. Is MO, HMO, HME, and MHE all the same disease? Yes. Multiple Osteochondromas (MO) has many names, including Hereditary Multiple Osteochondromas (HMO), Multiple Hereditary (or hereditary
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
Overview. Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases. People Centred Positive Compassion Excellence
 Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Hypophosphatasia Recent Advances in Diagnosis and Treatment
 8 The Open Bone Journal, 2009, 1, 8-15 Hypophosphatasia Recent Advances in Diagnosis and Treatment Beck C. 1, Morbach H. 1, Stenzel M. 2, Collmann H. 4, Schneider P. 3 and Girschick H.J. *,1 Open Access
8 The Open Bone Journal, 2009, 1, 8-15 Hypophosphatasia Recent Advances in Diagnosis and Treatment Beck C. 1, Morbach H. 1, Stenzel M. 2, Collmann H. 4, Schneider P. 3 and Girschick H.J. *,1 Open Access
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Mecasermin (Increlex) Reference Number: CP.PHAR.150 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Clinical Policy: Mecasermin (Increlex) Reference Number: CP.PHAR.150 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
The University of Arizona Pediatric Residency Program. Primary Goals for Rotation. Genetics
 The University of Arizona Pediatric Residency Program Primary Goals for Rotation Genetics 1. GOAL: Understand the role of the pediatrician in preventing genetic disease, and in counseling and screening
The University of Arizona Pediatric Residency Program Primary Goals for Rotation Genetics 1. GOAL: Understand the role of the pediatrician in preventing genetic disease, and in counseling and screening
Professor Md. Mahtab Uddin Hassan FCPS(Med) MRCP(UK) FRCP(Edin) Professor of Medicine AKMMCH
 Professor Md. Mahtab Uddin Hassan FCPS(Med) MRCP(UK) FRCP(Edin) Professor of Medicine AKMMCH Introduction The sunshine vitamin is a hot topic. Vitamin D deficiency and insufficiency is becoming a global
Professor Md. Mahtab Uddin Hassan FCPS(Med) MRCP(UK) FRCP(Edin) Professor of Medicine AKMMCH Introduction The sunshine vitamin is a hot topic. Vitamin D deficiency and insufficiency is becoming a global
Inherited Calcium and Magnesium Disorders
 Inherited Calcium and Magnesium Disorders Martin Konrad University Children s Hospital Münster, Germany IPNA / ESPN Master Class, Leuven, Sep 2nd 2015 Outline Hypercalcemia Hypomagnesemia Outline Hypercalcemia
Inherited Calcium and Magnesium Disorders Martin Konrad University Children s Hospital Münster, Germany IPNA / ESPN Master Class, Leuven, Sep 2nd 2015 Outline Hypercalcemia Hypomagnesemia Outline Hypercalcemia
HYPERCALCEMIA. Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences
 HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss. A Publication of The Bone and Cancer Foundation
 Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Package leaflet: Information for the user. Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa
 Package leaflet: Information for the user Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa This medicine is subject to additional monitoring. This will allow quick identification
Package leaflet: Information for the user Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa This medicine is subject to additional monitoring. This will allow quick identification
Genetic examination of diseases affecting bone development. and structure in newborns
 Genetic examination of diseases affecting bone development and structure in newborns Examination of molecular genetic markers in osteopenic preterm infants PhD Thesis Simone Funke, MD University of Pécs
Genetic examination of diseases affecting bone development and structure in newborns Examination of molecular genetic markers in osteopenic preterm infants PhD Thesis Simone Funke, MD University of Pécs
Bone Densitometry. What is a Bone Density Scan (DXA)? What are some common uses of the procedure?
 Scan for mobile link. Bone Densitometry What is a Bone Density Scan (DXA)? Bone density scanning, also called dual-energy x-ray absorptiometry (DXA) or bone densitometry, is an enhanced form of x-ray technology
Scan for mobile link. Bone Densitometry What is a Bone Density Scan (DXA)? Bone density scanning, also called dual-energy x-ray absorptiometry (DXA) or bone densitometry, is an enhanced form of x-ray technology
Klinefelter syndrome ( 47, XXY )
 Sex Chromosome Abnormalities, Sex Chromosome Aneuploidy It has been estimated that, overall, approximately one in 400 infants have some form of sex chromosome aneuploidy. A thorough discussion of sex chromosomes
Sex Chromosome Abnormalities, Sex Chromosome Aneuploidy It has been estimated that, overall, approximately one in 400 infants have some form of sex chromosome aneuploidy. A thorough discussion of sex chromosomes
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme-replacement
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme-replacement
بسم هللا الرحمن الرحيم. Rickets
 بسم هللا الرحمن الرحيم Rickets 1 Rickets IS defined as failure of mineralization of growing bone or osteoid tissue due to vitamin D deficiency. Vitamin D appear as rickets in children and as oteomalacia
بسم هللا الرحمن الرحيم Rickets 1 Rickets IS defined as failure of mineralization of growing bone or osteoid tissue due to vitamin D deficiency. Vitamin D appear as rickets in children and as oteomalacia
Summary ID#Z019 Clinical Study Summary: Study B9R-JE-6001
 CT Registry ID#Z019 Page 1 Summary ID#Z019 Clinical Study Summary: Study B9R-JE-6001 Title of Study: Evaluation of Growth Promoting Effect and Safety of Growth Hormone in Achondroplasia Investigator(s):
CT Registry ID#Z019 Page 1 Summary ID#Z019 Clinical Study Summary: Study B9R-JE-6001 Title of Study: Evaluation of Growth Promoting Effect and Safety of Growth Hormone in Achondroplasia Investigator(s):
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.
![[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4. [If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.](/thumbs/86/94943423.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Enzyme-Replacement Therapy in Life-Threatening Hypophosphatasia
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Enzyme-Replacement Therapy in Life-Threatening Hypophosphatasia Michael P. Whyte, M.D., Cheryl R. Greenberg, M.D., Nada J. Salman,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Enzyme-Replacement Therapy in Life-Threatening Hypophosphatasia Michael P. Whyte, M.D., Cheryl R. Greenberg, M.D., Nada J. Salman,
3. Has bone specific alkaline phosphatase level increased OR does the member have symptoms related to active Paget s?
 Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Metabolic, Endocrine, and Hematologic disease 주선영 가톨릭대학교인천성모병원
 Metabolic, Endocrine, and Hematologic disease 주선영 가톨릭대학교인천성모병원 1 Metabolic disease Ca, P metabolism Vit D metabolism Rickets Nutritional Hypophosphatemic Renal osteodystrophy Hypophosphatasia Hypervitaminosis
Metabolic, Endocrine, and Hematologic disease 주선영 가톨릭대학교인천성모병원 1 Metabolic disease Ca, P metabolism Vit D metabolism Rickets Nutritional Hypophosphatemic Renal osteodystrophy Hypophosphatasia Hypervitaminosis
within the TNSALP gene (11-13 and Henthom, P. S., M. Raducha, V. Fimiani, K. N. Fedde, and M. P. Whyte, manuscript
 Alkaline Phosphatase: Placental and Tissue-nonspecific lsoenzymes Hydrolyze Phosphoethanolamine, Inorganic Pyrophosphate, and Pyridoxal 5'-Phosphate Substrate Accumulation in Carriers of Hypophosphatasia
Alkaline Phosphatase: Placental and Tissue-nonspecific lsoenzymes Hydrolyze Phosphoethanolamine, Inorganic Pyrophosphate, and Pyridoxal 5'-Phosphate Substrate Accumulation in Carriers of Hypophosphatasia
General Approval Criteria for ALL Growth Hormone agents: (ALL criteria must be met)
 Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS)
 https://www.printo.it/pediatric-rheumatology/gb/intro Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS) Version of 2016 1. WHAT IS CAPS 1.1 What is it? Cryopyrin-Associated Periodic
https://www.printo.it/pediatric-rheumatology/gb/intro Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS) Version of 2016 1. WHAT IS CAPS 1.1 What is it? Cryopyrin-Associated Periodic
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Substrate Reduction Therapy Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Substrate Reduction Therapy! Prime Therapeutics will review Prior Authorization
Substrate Reduction Therapy Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Substrate Reduction Therapy! Prime Therapeutics will review Prior Authorization
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FORTEO (teriparatide) INJECTION
 FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS (PFIC)
 The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. PROGRESSIVE FAMILIAL INTRAHEPATIC
The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. PROGRESSIVE FAMILIAL INTRAHEPATIC
PRIMARY HYPERPARATHYROIDISM WITH RICKETS. KRITHIKA.P Dr.L.N.Padmasani Unit 1 Sri Ramachandra Medical College
 PRIMARY HYPERPARATHYROIDISM WITH RICKETS KRITHIKA.P Dr.L.N.Padmasani Unit 1 Sri Ramachandra Medical College Presenting Complaints v 17 year old developmentally normal adolescent boy, first of a twin pregnancy,
PRIMARY HYPERPARATHYROIDISM WITH RICKETS KRITHIKA.P Dr.L.N.Padmasani Unit 1 Sri Ramachandra Medical College Presenting Complaints v 17 year old developmentally normal adolescent boy, first of a twin pregnancy,
Bone density scanning and osteoporosis
 Bone density scanning and osteoporosis What is osteoporosis? Osteoporosis occurs when the struts which make up the mesh-like structure within bones become thin causing them to become fragile and break
Bone density scanning and osteoporosis What is osteoporosis? Osteoporosis occurs when the struts which make up the mesh-like structure within bones become thin causing them to become fragile and break
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Xgeva. Xgeva (denosumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
CUROSURF (poractant alfa) intratracheal suspension Initial U.S. Approval: 1999
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUROSURF safely and effectively. See full prescribing information for CUROSURF. CUROSURF (poractant
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUROSURF safely and effectively. See full prescribing information for CUROSURF. CUROSURF (poractant
BONEFOS 800 mg. Bonefos adalah obat baru yang terdaftar tahun Informasi di bawah ini merupakan informasi update tahun 2008.
 Bonefos adalah obat baru yang terdaftar tahun 2007. Informasi di bawah ini merupakan informasi update tahun 2008. BONEFOS 800 mg Important information, please read carefully! Composition 1 tablet contains
Bonefos adalah obat baru yang terdaftar tahun 2007. Informasi di bawah ini merupakan informasi update tahun 2008. BONEFOS 800 mg Important information, please read carefully! Composition 1 tablet contains
Familial Mediterranean Fever
 www.printo.it/pediatric-rheumatology/gb/intro Familial Mediterranean Fever Version of 2016 1. WHAT IS FMF 1.1 What is it? Familial Mediterranean Fever (FMF) is a genetically transmitted disease. Patients
www.printo.it/pediatric-rheumatology/gb/intro Familial Mediterranean Fever Version of 2016 1. WHAT IS FMF 1.1 What is it? Familial Mediterranean Fever (FMF) is a genetically transmitted disease. Patients
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Cystic Fibrosis Transmembrane Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Prime Therapeutics
Cystic Fibrosis Transmembrane Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Prime Therapeutics
Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) Inhibitors Prior Authorization with Quantity Limit Program Summary
 Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) Inhibitors Prior Authorization with Quantity Limit Program Summary Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) Inhibitors Prior Authorization
Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) Inhibitors Prior Authorization with Quantity Limit Program Summary Proprotein Convertase Subtilisin/Kexin type 9(PCSK9) Inhibitors Prior Authorization
OTL Drug Development. Genetic Disorders of Bone and Mineral Metabolism. Michael P. Whyte, M.D. 9/7/2017
 ASBMR Fellows Program September 7, 2017 Genetic Disorders of Bone and Mineral Metabolism Michael P. Whyte, M.D. Division of Bone and Mineral Diseases, Department of Internal Medicine, Washington University
ASBMR Fellows Program September 7, 2017 Genetic Disorders of Bone and Mineral Metabolism Michael P. Whyte, M.D. Division of Bone and Mineral Diseases, Department of Internal Medicine, Washington University
Adalimumab M Clinical Study Report Final R&D/13/224
 Diagnosis and Main Criteria for Inclusion: A parent or guardian had voluntarily signed and dated an informed consent form, approved by an institutional review board/independent ethics committee, after
Diagnosis and Main Criteria for Inclusion: A parent or guardian had voluntarily signed and dated an informed consent form, approved by an institutional review board/independent ethics committee, after
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
CYSTARAN (cysteamine hydrochloride) ophthalmic solution
 CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
Fibrous Dysplasia in Children. Professor Nick Shaw Birmingham Children s Hospital, UK
 Fibrous Dysplasia in Children Professor Nick Shaw Birmingham Children s Hospital, UK Overview What is Fibrous Dysplasia? Clinical Presentations Endocrine Problems Skeletal Problems Treatment Options Fibrous
Fibrous Dysplasia in Children Professor Nick Shaw Birmingham Children s Hospital, UK Overview What is Fibrous Dysplasia? Clinical Presentations Endocrine Problems Skeletal Problems Treatment Options Fibrous
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Cystic Fibrosis Transmembrane Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Prime Therapeutics
Cystic Fibrosis Transmembrane Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Prime Therapeutics
A case of hereditary hypophosphataemic rickets with hypercalciuria (HHRH)
 Case Reports A case of hereditary hypophosphataemic rickets with hypercalciuria (HHRH) M N Lucas 1, Savithri Dias 2 Sri Lanka Journal of Child Health, 2006; 35:141-3 (Key words: hereditary hypophosphataemic
Case Reports A case of hereditary hypophosphataemic rickets with hypercalciuria (HHRH) M N Lucas 1, Savithri Dias 2 Sri Lanka Journal of Child Health, 2006; 35:141-3 (Key words: hereditary hypophosphataemic
Failure to thrive (faltering growth) Dr. Khalid
 Failure to thrive (faltering growth) Dr. Khalid FTT (faltering growth) The term 'failure to thrive' is a clinical state of growth failure due to any causes, characterized by any one or more of the following
Failure to thrive (faltering growth) Dr. Khalid FTT (faltering growth) The term 'failure to thrive' is a clinical state of growth failure due to any causes, characterized by any one or more of the following
Matrix Vesicles in Osteomalacic Hypophosphatasia Bone Contain Apatite-Like Mineral Crystals
 American Journal of Pathology, Vol. 151, No. 6, December 1997 Copyright X) American Society for Investigative Patbology Short Communication Matrix Vesicles in Osteomalacic Hypophosphatasia Bone Contain
American Journal of Pathology, Vol. 151, No. 6, December 1997 Copyright X) American Society for Investigative Patbology Short Communication Matrix Vesicles in Osteomalacic Hypophosphatasia Bone Contain
