PRESCRIBING enewsletter
|
|
|
- Oliver Williamson
- 5 years ago
- Views:
Transcription
1 Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: Dear Gwent Prescriber At its last two meetings (5 th September and 17 th October) ABUHB s Medicines & Therapeutics Committee made the following decisions in relation to the ABUHB Formulary: ABHB FORMULARY UPDATES ABHB s Drug Formulary is at: November 2013 BNF section ACLIDINIUM bromide ( Eklira Genuair ) inhalation powder for COPD ADDED as an option for use in adults with COPD in accordance with AWMSG advice The MTC has followed AWMSG advice and designated aclidinium a Green drug (non specialist initiation appropriate). Full prescribing information on this new LAMA is at: LAMA Options Type of device No of puffs per inhaler Annual cost based on 12 inhalers Glycopyrronium bromide (Seebri Breezhaler) 44mcg OD DPI Aclidinium bromide (Eklira Genuair) 375mcg BD DPI Tiotropium bromide (Spiriva handihaler refill pack) 18mcg OD DPI Tiotropium bromide (Spiriva Respimat) 2.5 mcg 2 puffs OD Prices based on Drug Tariff October 2013 BNF section PERAMPANEL ( Fycompa ) tablets for adjunctive treatment of partial onset seizures ADDED as an option for restricted use where the Wales Patient Access Scheme (WPAS) applies in accordance with AWMSG advice Having now confirmed that the WPAS will apply across Primary and Secondary Care in Wales the MTC has designated perampanel an Amber without Shared Care drug (i.e. specialist initiation only but without the need for Shared Care arrangements for GP repeat prescribing) for the approved indication. Full prescribing information on perampanel at: BNF sections AND DEMECLOCYCLINE Don t use as an antibiotic The MTC agreed to restrict demeclocycline s ABUHB s Formulary inclusion to its licensed indication for the treatment of chronic hyponatraemia associated with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) secondary to malignant disease (where water restriction is ineffective and the patient does not have concomitant cirrhosis) only and that it should not be available in section Antibacterial drugs/tetracyclines. The MTC reached this decision for the following reasons: Page 1 of 8
2 1. The high cost of demeclocycline ( for 28 x 150mg capsules) compared to other oral tetracyclines available on the Formulary ( 1.11 for 8 doxycycline 100mg capsules and 6.22 for 28 lymecycline 408mg capsules), 2. That expert micobiological view was that there was no particular advantage offered by demeclocycline in its antibacterial effect as compared to other tertracyclines available on the Formulary and 3. That only 11 practices had prescribed demeclocycline in the most recent 6 month period of prescribing data (42 items at a total cost of 17,700). Full prescribing information on demeclocycline is at: BNF section INSULIN DEGLUDEC ( Tresiba ) for diabetes mellitus NOT CONSIDERED In the absence of a submission from Novo Nordisk, Tresiba cannot be endorsed by AWMSG for use in accordance with its licensed indication, as a technology appraisal by AWMSG (or NICE) has not been undertaken. The medicine should NOT be prescribed routinely within NHS Wales for this indication. BNF section Linagliptin/metformin ( Jentadueto ) tablets for T2DM ADDED as an option for type 2 diabetes mellitus in accordance with AWMSG advice The MTC has designated Jentadueto an Amber without Shared Care drug (i.e. specialist initiation only but without the need for Shared Care arrangements for GP repeat prescribing). Although the two individual components were designated Green the MTC felt this was the only option considering that since the loss of exclusivity with Takeda s Actos (pioglitazone) the fixed does pioglitazone metformin combination (Competact ) has remained at for 28 days treatment (compared to between 1.64 and 2.40 for 28 days of pioglitazone). Full prescribing information on Jentadueto at: BNF section ULIPRISTAL 5mg ( Esmya ) for uterine fibroids ADDED as an option for the pre operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age in accordance with AWMSG advice The MTC has designated ulipristal 5mg a Red drug (specialist prescribing only) in accordance with the consensus view from ABUHB s Obs & Gynae consultants and in consideration that the duration of treatment with Esmya is limited to 3 months. Cost of Esmya is for 28 tablets. Prescribers should note that ulipristal 30mg (ellaone ) for emergency contraception remains a Green drug. Full prescribing information on Esmya at: BNF section INGENOL MEBUTATE ( Picato ) for actinic keratosis ADDED as an option for non hyperkeratotic, non hypertrophic actinic keratosis in accordance with AWMSG advice In considering AWMSG s view on the appropriate place for prescribing ingenol mebutate gel the MTC felt that: Page 2 of 8
3 As clinical data on the use of more than 1 course of ingenol mebutate gel for 2 or 3 consecutive days, or treatment of more than 1 area of 25cm 2 are not available and As local application site reactions (including erythema, blistering, crusting, erosion, exfoliation, pain and oedema) are all very common ( 1/10) ingenol mebutate gel should be designated Amber without Shared Care (i.e. specialist initiation only but without the need for Shared Care arrangements for GP repeat prescribing). Full prescribing information on Picato gel is at: SPC/ No current BNF listing TESTOSTERONE Skin Patch 300mcg/24hrs ( INTRINSA ) Non Formulary Prescribers should note that this formulation of testosterone (licensed for the treatment of hypoactive sexual desire disorder) apart from being Non Formulary is now also very costly. Intrinsa's UK license was voluntarily withdrawn back in 2012 but it has re appeared and is now significantly more expensive than its original 2007 cost of per pack. The current cost of a pack of 8 patches is 395. This increase has caught the attention of the national press: healthnews/ /drug companies accused of overcharging NHS face fraud inquiry.html See also: No current BNF listing DAPOXETINE ( Priligy ) for premature ejaculation Non Formulary Prescribers should note that the AWMSG have advised that in the absence of a submission from Menarini, dapoxetine ( Priligy ) cannot be endorsed for use within NHS Wales for the treatment of premature ejaculation in men aged 18 to 64 years old. ABHB would not be able to consider a formulary application to appraise dapoxetine locally whilst a Statement Of Advice stands. No BNF section MolluDab (5% potassium hydroxide) for molluscum contagiosum Non Formulary The fact that this new preparation is not classed as a Prescription Only Medicine means that 1. it will not be appraised at a national level by AWMSG 2. it can be bought over the counter from a pharmacy Point 1 above means that any ABUHB Formulary application will require a local appraisal of the evidence by the MTC. In the mean time prescribers should note: The level of evidence required for a medical device to added to Part IXA of the Drug Tariff (which lists MolluDab ) is less than that required for obtaining EU/MHRA marketing authorisation as a licensed medicine. A 2009 Cochrane Review of Interventions for cutaneous molluscum contagiosum ( onlinelibrary.wiley.com/doi/ / cd pub3/abstract) contains this plain language summary: Molluscum contagiosum, in healthy people, is a self limiting, relatively harmless viral skin infection. It affects mainly children and adolescents. It occurs worldwide but is much more frequent in certain geographic areas with warm climates. Molluscum contagiosum usually presents as single or multiple pimples filled with fluid. People may seek treatment for social and aesthetic reasons and because of concerns about spreading the disease to others. Treatment is intended to speed up the healing process. Eleven studies with 495 patients were included in this review. This review found that many common treatments for molluscum, such as physical destruction, have not been adequately evaluated. Several of the treatments that we studied are not part of daily practice. Limitations of several of these studies were: small numbers of patients, the investigators were not blinded, and patients who did not complete the study (which were numerous in some studies) were not included in the analysis. None of the evaluated treatment options were associated with serious adverse effects. Since most lesions will resolve within months, molluscum contagiosum can be left to heal naturally unless better evidence for the superiority of other treatment options emerges. Page 3 of 8
4 Five studies were included in the first 2006 version of the Cochrane Review with 6 more for the 2009 update. Two of the 11 studies assessed topical potassium hydroxide (at 5% and 10%). ABUHB WOUND HEALING PRODUCTS FORMULARY UPDATE ABUHB s Wound Healing Products Formulary is at: BNF section A5.2.4 AQUACEL & AQUACEL EXTRA Convatec has withdrawn the two dressing products Aquacel and Aquacel Ag Both presently on the Wound Healing Products Formulary They have been replaced by Aquacel Extra and Aquacel Ag Extra. These 'Extra' dressings are stronger and more absorbent than Aquacel but due to their extra cost were originally only available in Table 3 of the Formulary and reserved for highly exudating wounds only. The 'Extra' dressings have now been reduced in price to the same as the original dressings. It has been agreed by ABUHB s Wound Healing Group to amend the online version of the Wound Healing Products Formulary 2012 putting the Aquacel Extra dressing into Table 1 replacing the original Aquacel and Aquacel Ag Extra into Table 2 to replace the original Aquacel Ag dressing. Prescribers should note the potential for confusion as printed copies of the Formulary remain unchanged. ABUHB s Wound Healing Products Formulary is at: OTHER PRESCRIBING NEWS Weekly oral METHOTREXATE patients at risk? A review of the most recent quarter s WP10 prescribing data for methotrexate 10mg tablets showed that over 2,000 10mg tablets were dispensed across Gwent between May and July The MTC would like to remind prescribers and dispensers of the NPSA s Safe Practice Checklist in NPSA/2006/13 (at: particularly the points in the box below: All prescribers must avoid the use of as directed in prescribing a specific dose must be applied to each prescription. Bear in mind that patients often understand their dose by the number of tablets they take rather than mg. The required quantity and frequency of dose should be regularly discussed with the patient. Safe Dispensing practice: The strength of tablet supplied to the patient must stay consistent to prevent any confusion about the number of tablets they need to take, and the patient s monitoring document and Patient Medication Record should be checked to confirm the previous supply. It is the view of the MTC that patients receiving both the 2.5mg and 10mg tablets may be particularly at risk of confusion over the number of tablets to take, and the MTC advises that prescribers undertake a review of these patients. Any reasons for variance from the practice of prescribing/supplying solely as 2.5mg tablets should be documented. Note ABUHB acute services follow the policy of only supplying 2.5mg tablets for all weekly oral methotrexate prescriptions. The MTC also considers it good practice for prescribers to specify the day of the week on which the methotrexate should be taken as well as specifying the strength of methotrexate to be supplied. Page 4 of 8
5 BNF ALDOSTERONE ANTAGONISTS in heart failure guidance on monitoring Local guidance on eplerenone (originally issued in Feb 2007 and then revised in Aug 2010 when eplerenone was changed from Red to Amber) has been significantly transformed into more general guidance on the monitoring of aldosterone antagonists (spironolactone and eplerenone) when used to treat left ventricular systolic dysfunction. Prescribers should note that eplerenone is now dually licensed (indication 2 below is new) and that the Amber Traffic Light designation of eplerenone has become less straightforward: 1. in addition to standard therapy including beta blockers, to reduce the risk of cardiovascular mortality and morbidity in stable patients with left ventricular dysfunction (LVEF 40 %) and clinical evidence of heart failure after recent MI (within 3 to 14 days) specialist only initiation i.e. Amber without Shared Care Traffic Light designation. 2. in addition to standard optimal therapy, to reduce the risk of cardiovascular mortality and morbidity in adult patients with NYHA class II (chronic) heart failure and left ventricular systolic dysfunction (LVEF 30%) suitable for non specialist initiation. i.e. Green Traffic Light designation. IN VIEW OF THE CONSIDERABLE DIFFERENCE IN ACQUISITION COSTS BETWEEN THE 2 ALDOSTERONE ANTAGO NISTS, USE OF EPLERENONE FOR THIS SECOND INDICATION SHOULD BE RESERVED FOR PATIENTS INTOLERANT OF SPIRONOLACTONE (E.G. GYNAECOMASTIA). The new guidance is at: ABHBguidelinesFINAL%5BSept2013%5D.pdf Key points to note: PATIENTS SHOULD BE INSTRUCTED TO STOP THEIR ALDOSTERONE ANTAGONIST DURING AN EPISODE OF DIARRHOEA OR WHILE LOOP DIURETIC THERAPY IS INTERRUPTED. CONCURRENT USE OF AN ALDOSTERONE ANTAGONISTS WITH ACE INHIBITORS, ANGIOTENSIN II ANTAGONISTS, HEPA RIN, LOW MOLECULAR WEIGHT HEPARIN OR OTHER DRUGS KNOWN TO CAUSE HYPERKALAEMIA, POTASSIUM SUPPLE MENTS, A DIET RICH IN POTASSIUM (E.G. SPINACH, DRIED APRICOTS AND BANANAS), OR SALT SUBSTITUTES CONTAINING POTASSIUM, (AS WELL AS CONCURRENT USE OF 2 ALDOSTERONE ANTAGONISTS) MAY LEAD TO SEVERE HYPERKALAEMIA. RENAL IMPAIRMENT HIGH RISK OF HYPERKALAEMIA. SEE NOTE ABOVE ABOUT PATIENTS WITH INTER CURRENT ILLNESS AND VOLUME DEPLETION/DEHYDRATION. CONCURRENT USE OF ALDOSTERONE ANTAGONISTS WITH NSAIDS (INCLUDES OVER THE COUNTER PRODUCTS) INCREASES RISK OF NEPHROTOXICITY. BNF Vitamin B Co STRONG & Vitamin B Co time to review? The recent increase in cost of Vitamin B Co (now for 28) has prompted a call for a wider review use of vitamin B in alcoholic liver disease. National guidance supports (NICE CG100 guidance.nice.org.uk/cg100 and CG115) supports the use of prophylactic oral thiamine in harmful or dependent drinkers, but not vitamin B Co or vitamin B Co strong. NICE CG100 recommends that thiamine should be given in doses toward the upper end of the 'BNF' range i.e mg daily in divided doses. Duration of prophylactic oral thiamine treatment? once an individual is alcohol free ABUHB advice is that oral thiamine can be stopped after 3 months. Updated ANTI INFECTIVE GUIDELINES for Primary Care This has recently been updated with new sections on dental infections and diverticulitis and the insertion of hyperlinks to children s doses for a range of common childhood infections The 9 page short version (without all the references) is now available as a Word document on the MTC website at: ABUHB%5BHPA%5DOct2013.doc Page 5 of 8
6 This local guidance is also available as a web based version (more easily viewed on a smart phone or tablet than the full document) at: QR code is: ANTIMICROBIAL RESISTANCE RCGP Toolkit In the UK, 80% of antibiotic prescribing occurs in Primary Care, with over half for respiratory tract infections. With the aim of reducing inappropriate use of antibiotics (and thereby reducing bacterial resistance) the MTC would like to make Primary Care prescribers aware of the following RCGP resources supporting antibiotic prescribing: Training resources Patient information leaflets Self assessment checklist and audit (in English and 5 other languages) Resources for clinicians Antibiotic management guidance External clinical resources All at: BNF Antiepileptic drugs categorised to help prescribers avoid switching risks New guidance on prescribing antiepileptic drugs (AEDs) has been issued by the MHRA to help prescribers and patients understand the potential risks from switching between branded and generic products or between different generic products. The MHRA has now classified all AEDs into 3 categories (using their therapeutic index, solubility and absorption: Category 1 Category 2 Category 3 Advice for doctors Doctors are advised to ensure that their patient is maintained on a specific manufacturer s product Doctors are advised to use their judgement (in consultation with their patient and/or their carer) to determine whether it would be advisable for them to be maintained on a specific manufacturer s product. Doctors are advised that it is usually unnecessary to ensure that their patients are maintained on a specific manufacturer s product. Antiepileptic drugs in category Phenytoin, carbamazepine, phenobarbital, primidone Valproate, lamotrigine, perampanel, retigabine, rufinamide, clobazam, clonazepam, oxcarbazepine, eslicarbazepine, zonisamide, topiramate Levetiracetam, lacosamide, tiagabine, gabapentin, pregabalin, ethosuximide, vigabatrin An information sheet for patients and carers may help in consultations, see: specificinformationandadvice/productspecificinformationandadvice A F/Antiepilepticschangingproducts/index.htm BNF BUCCOLAM (midazolam) two requests Prescribers are reminded that this licensed formulation of oromucosal midazolam is considered suitable for non specialist repeat prescribing (Amber without Shared Care Traffic Light designation) and are requested 1. Not to put Buccolam on repeat prescription but only to issue an acute script when family/carers have run out or Buccolam is about to reach its expiry date. 2. To add 'Please label individual syringes' to prescriptions as individual syringes can be held in different locations for the patient. Page 6 of 8
7 Dispensers should consult with patient carers on whether the labelling of individual pre filled syringes of Buccolam is helpful and print further copies of the dispensing label to stick on to the individual syringes if appropriate. Full prescribing information on Buccolam is at: BNF section 14.4 VARICELLA ZOSTER VACCINE (Zostavax ) A letter from the Chief Medical Officer / Chief Pharmaceutical Officer was issued on 25 th October 2013 see: The letter reminded prescribers that Zostavax, is a live attenuated vaccine and therefore contraindicated in patients on immunosuppressive therapy. This includes patients who have recently received or are currently being treated with biological therapies*. Key prescribing point: As biological therapies are usually administered in a hospital setting, treatment may not be immediately apparent from Primary Care records. * biological therapies include abatacept (Orencia), ustekinumab (Stelara), certolizumab (Cimzia), etanercept (Enbrel), adalimumab (Humira), inflixiamb (Remicade), golimumab (Simponi), rituximab (MabThera), tocilizumab (RoActemra) and anakinra (Kineret). They are commonly indicated for rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn's disease, ulcerative colitis and cancers. immunosuppressive therapies may not be cleared from the blood for several months. Full prescribing information on Zostavax is at: Reporting Adverse Drug Reactions (ADRs) Although the graph below of GP suspected ADR reports (Yellow Cards) in Wales ( ) is a little dated the declining level is a concern, and has led to a suggestion that the number of Yellow Cards submitted per GP practice and per Health Board become a National Prescribing Indicator for Reporting concerns about drug safety (i.e. suspected ADRs) is part of the GMC s ethical professional guidance on prescribing ( uk.org/guidance/ethical_guidance/14323.asp). The MTC would like to highlight extracts from the GMC s guidance below: Page 7 of 8
8 47. You must inform the MHRA about: a. serious suspected adverse reactions to all medicines and all reactions to products marked with a Black Triangle in the BNF and elsewhere using the Yellow Card Scheme. b. adverse incidents involving medical devices, including those caused by human error that put, or have the potential to put, the safety of patients, healthcare professionals or others at risk. These incidents should also be reported to the medical device liaison officer within your organisation. 48. You should provide patients with information about how they can report suspected side effects directly to the MHRA. The MHRA ( collects data on licensed and unlicensed prescription only, pharmacy and over the counter medicines Further guidance on reporting is available from the MHRA: Reporting Adverse Incidents at: bs/documents/publication/con pdf USEFUL LINKS: QR code link to Yellow Card online reporting: Online Yellow Card reporting for suspected ADRs Feedback on any item in this enewsletter is welcome. Suggested agenda items for the MTC are also welcome. Follow Trevor Batt Pharmacist & Professional Secretary to: Aneurin Bevan HB Medicines & Therapeutics Committee Aneurin Bevan Health Board Based at Victoria House, Corporation Rd, Newport NP19 0BH trevor.batt@wales.nhs.uk Tel: (DIRECT LINE) Page 8 of 8
Aneurin Bevan Health Board Medicines & Therapeutics Committee. enewsletter
 Aneurin Bevan Health Board Medicines & Therapeutics Committee August 2013 enewsletter Dear Gwent Prescriber At its last two meetings (23 rd May and 11 th July) ABHB s Medicines & Therapeutics Committee
Aneurin Bevan Health Board Medicines & Therapeutics Committee August 2013 enewsletter Dear Gwent Prescriber At its last two meetings (23 rd May and 11 th July) ABHB s Medicines & Therapeutics Committee
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber At its last
Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber At its last
Shared Care Guideline. The Management of Epilepsies in Children
 THE SOUTH YORKSHIRE & BASSETLAW Shared Care Guideline For The Management of Epilepsies in Children Shared care guideline developed by: Sheffield Children's NHS Foundation Trust; Dr P Baxter Consultant
THE SOUTH YORKSHIRE & BASSETLAW Shared Care Guideline For The Management of Epilepsies in Children Shared care guideline developed by: Sheffield Children's NHS Foundation Trust; Dr P Baxter Consultant
Updated advice for nurses who care for patients with epilepsy
 NICE BULLETIN Updated advice for nurses who care for patients with epilepsy NICE provided the content for this booklet which is independent of any company or product advertised NICE BULLETIN Updated advice
NICE BULLETIN Updated advice for nurses who care for patients with epilepsy NICE provided the content for this booklet which is independent of any company or product advertised NICE BULLETIN Updated advice
Management of Epilepsy in Primary Care and the Community. Carrie Burke, Epilepsy Specialist Nurse
 Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Epilepsy: pharmacological treatment by seizure type. Clinical audit tool. Implementing NICE guidance
 Epilepsy: pharmacological treatment by seizure type Clinical audit tool Implementing NICE guidance 2012 NICE clinical guideline 137 Clinical audit tool: Epilepsy (2012) Page 1 of 25 This clinical audit
Epilepsy: pharmacological treatment by seizure type Clinical audit tool Implementing NICE guidance 2012 NICE clinical guideline 137 Clinical audit tool: Epilepsy (2012) Page 1 of 25 This clinical audit
GWENT FORMULARY DECISIONS
 Dear Gwent Prescriber At its last two meetings (9 th September and 21 st October 2010) the Gwent Partnership Medicines & Therapeutics Committee (GPMTC) made the following decisions in relation to the Aneurin
Dear Gwent Prescriber At its last two meetings (9 th September and 21 st October 2010) the Gwent Partnership Medicines & Therapeutics Committee (GPMTC) made the following decisions in relation to the Aneurin
GENERIC MEDICINES (Non-Innovator Brand) PRESCRIBING POLICY
 GENERIC MEDICINES (Non-Innovator Brand) PRESCRIBING POLICY First issued by/date Issue Version Purpose of Issue/Description of Change Planned Review Date August 2018 1.0 Promoting generic prescribing whenever
GENERIC MEDICINES (Non-Innovator Brand) PRESCRIBING POLICY First issued by/date Issue Version Purpose of Issue/Description of Change Planned Review Date August 2018 1.0 Promoting generic prescribing whenever
Costing statement. Implementing NICE guidance. January NICE clinical guideline 137
 The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care (partial update of NICE clinical guideline 20) Costing statement Implementing NICE guidance
The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care (partial update of NICE clinical guideline 20) Costing statement Implementing NICE guidance
Buccal Midazolam For the treatment of prolonged epileptic seizures, clusters of epileptic seizures and status epilepticus.
 Oxfordshire Clinical Commissioning Group, Oxford University Hospitals NHS Trust and Oxfordshire Health NHS Foundation Trust Shared Care Protocol and Information for GPs Buccal Midazolam For the treatment
Oxfordshire Clinical Commissioning Group, Oxford University Hospitals NHS Trust and Oxfordshire Health NHS Foundation Trust Shared Care Protocol and Information for GPs Buccal Midazolam For the treatment
Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65
 Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Area Drug and Therapeutics Committee Prescribing Supplement No 59 July 2012
 Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
PRESCRIPTION PAD. The Newsletter of the Cumbria Area Prescribing Committee. February 2012 No. 18. Click here to find more. News from the MHRA
 PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 ANEURIN BEVAN UNIVERSITY HEALTH BOARD MEDICINES & THERAPEUTICS COMMITTEE PRESCRIBING enewsletter 28 September 2016 ARCHIVE at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
ANEURIN BEVAN UNIVERSITY HEALTH BOARD MEDICINES & THERAPEUTICS COMMITTEE PRESCRIBING enewsletter 28 September 2016 ARCHIVE at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
THEOPHYLLINE WITH INHALED CORTICOSTEROIDS (TWICS) TRIAL SELF MANAGMENT / ACTION PLANS GENUAIR INHALERS: POTENTIAL SAFETY ISSUE
 I S S U E 4 M A R C H / A R P I L 2 0 1 6 Endorsed December 2014 I N S I D E T H I S I S S U E : Theophylline with Inhaled Corticosteroids (TWICS) Trial Genuair Inhaler: Potential Safety Issue 1 Self Management
I S S U E 4 M A R C H / A R P I L 2 0 1 6 Endorsed December 2014 I N S I D E T H I S I S S U E : Theophylline with Inhaled Corticosteroids (TWICS) Trial Genuair Inhaler: Potential Safety Issue 1 Self Management
Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480
 Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol
 Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
Patient Group Direction for Doxycycline (Tetracycline) Version: 01 Start Date: October 2015 Expiry Date: October 2018
 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
The Epilepsy Prescriber s Guide to Antiepileptic Drugs
 The Epilepsy Prescriber s Guide to Antiepileptic Drugs The Epilepsy Prescriber s Guide to Antiepileptic Drugs Philip N. Patsalos FRCPath, PhD Professor of Clinical Pharmacology and Consultant Clinical
The Epilepsy Prescriber s Guide to Antiepileptic Drugs The Epilepsy Prescriber s Guide to Antiepileptic Drugs Philip N. Patsalos FRCPath, PhD Professor of Clinical Pharmacology and Consultant Clinical
Patient Group Direction for the administration of Levonorgestrel Emergency Contraception for Community Pharmacy
 Patient Group Direction for the administration of Levonorgestrel for Community Pharmacy Author Corporate Lead Chris Toothill (Medicines Management Pharmacist, Governance and Risk, Leeds Community Healthcare
Patient Group Direction for the administration of Levonorgestrel for Community Pharmacy Author Corporate Lead Chris Toothill (Medicines Management Pharmacist, Governance and Risk, Leeds Community Healthcare
Epilepsy 101. Overview of Treatment Kathryn A. O Hara RN. American Epilepsy Society
 Epilepsy 101 Overview of Treatment Kathryn A. O Hara RN American Epilepsy Society Objectives Describe the main treatment options for epilepsy Identify factors essential in the selection of appropriate
Epilepsy 101 Overview of Treatment Kathryn A. O Hara RN American Epilepsy Society Objectives Describe the main treatment options for epilepsy Identify factors essential in the selection of appropriate
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ
 Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ users guide to therapeutic drug monitoring of antiepileptic
Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ users guide to therapeutic drug monitoring of antiepileptic
SHARED CARE AGREEMENT: MELATONIN (CHILDREN)
 NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) where appropriate. DRUG AND INDICATION: Generic drug name: Formulations: MELATONIN 3mg immediate
NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) where appropriate. DRUG AND INDICATION: Generic drug name: Formulations: MELATONIN 3mg immediate
Formulary and Prescribing Guidelines
 Formulary and Prescribing Guidelines SECTION 3: TREATMENT OF BIPOLAR AFFECTIVE DISORDER This section provides information regarding the pharmacological management of Bipolar affective disorder in secondary
Formulary and Prescribing Guidelines SECTION 3: TREATMENT OF BIPOLAR AFFECTIVE DISORDER This section provides information regarding the pharmacological management of Bipolar affective disorder in secondary
Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction
 North Central London Joint Formulary Committee Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction Start date: July 2016 Review date: July 2019 Document
North Central London Joint Formulary Committee Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction Start date: July 2016 Review date: July 2019 Document
NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years
 Title of Project: NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years 1 Reason for the review Respiratory prescribing is long term and can be costly. Appropriate choice and use of inhaled
Title of Project: NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years 1 Reason for the review Respiratory prescribing is long term and can be costly. Appropriate choice and use of inhaled
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD)
 Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
Summary of Lothian Joint Formulary Amendments
 Summary of Lothian Joint Formulary Amendments The purpose of this summary is to detail the main changes to the LJF sections and provide additional information on the reasons for some of the changes. The
Summary of Lothian Joint Formulary Amendments The purpose of this summary is to detail the main changes to the LJF sections and provide additional information on the reasons for some of the changes. The
POLICY FOR THE SAFE USE OF ORAL METHOTREXATE IN SECONDARY CARE. September 2011
 POLICY FOR THE SAFE USE OF ORAL METHOTREXATE IN SECONDARY CARE September 2011 WHSCT Policy for the safe use of oral methotrexate in secondary care Page 1 of 8 Policy Title Policy for the safe use of oral
POLICY FOR THE SAFE USE OF ORAL METHOTREXATE IN SECONDARY CARE September 2011 WHSCT Policy for the safe use of oral methotrexate in secondary care Page 1 of 8 Policy Title Policy for the safe use of oral
Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary
 Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary Biologic Immunomodulators Prior Authorization with Quantity Limit (with a preferred option) OBJECTIVE The intent of the
Biologic Immunomodulators Prior Authorization with Quantity Limit Program Summary Biologic Immunomodulators Prior Authorization with Quantity Limit (with a preferred option) OBJECTIVE The intent of the
Get an Insurance Benefits Review for ORENCIA (abatacept)
 Get an Insurance Benefits Review for ORENCIA (abatacept) If you and your doctor decide that ORENCIA is right for you, you can call 1-800-ORENCIA (1-800-673-6242) to do an Insurance Benefits Review. Our
Get an Insurance Benefits Review for ORENCIA (abatacept) If you and your doctor decide that ORENCIA is right for you, you can call 1-800-ORENCIA (1-800-673-6242) to do an Insurance Benefits Review. Our
PRESCRIBING INCENTIVE SCHEME 2018/19
 PRESCRIBING INCENTIVE SCHEME 2018/19 Summary Harrogate and Rural District CCG is continuing to offer a prescribing incentive scheme to all its member NHS GP practices as encouragement and reward to improve
PRESCRIBING INCENTIVE SCHEME 2018/19 Summary Harrogate and Rural District CCG is continuing to offer a prescribing incentive scheme to all its member NHS GP practices as encouragement and reward to improve
Document Details. Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml
 Title Document Details Patient Group Direction (PGD) Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml Trust Ref No 1445-36348 Local Ref (optional) Main points the document The treatment of
Title Document Details Patient Group Direction (PGD) Ibuprofen 200mg tablets and Ibuprofen oral liquid 100mg in 5ml Trust Ref No 1445-36348 Local Ref (optional) Main points the document The treatment of
Patient Group Direction for LEVONELLE (Version 02) Valid From 1 October September 2019
 Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
North of Tyne Area Prescribing Committee
 DECISION SUMMAY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday 8 th July 2014. Classification of products:
DECISION SUMMAY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday 8 th July 2014. Classification of products:
Xeljanz. Xeljanz, Xeljanz XR (tofacitinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
Lincolnshire Prescribing and Clinical Effectiveness Bulletin
 S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 12 No 10, November 2018 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 12 No 10, November 2018 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
Guidelines for the Prescribing of Sacubitril / Valsartan
 Hull & East Riding Prescribing Committee Guidelines for the Prescribing of Sacubitril / Valsartan 1. BACKGROUND Sacubitril valsartan is an angiotensin receptor neprilysin inhibitor, including both a neprilysin
Hull & East Riding Prescribing Committee Guidelines for the Prescribing of Sacubitril / Valsartan 1. BACKGROUND Sacubitril valsartan is an angiotensin receptor neprilysin inhibitor, including both a neprilysin
Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin)
 14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd
 eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd 08 June 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd 08 June 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
Methotrexate for inflammatory bowel disease: what you need to know
 Methotrexate for inflammatory bowel disease: what you need to know This leaflet aims to answer your questions about taking methotrexate for inflammatory bowel disease (IBD). If you have any questions or
Methotrexate for inflammatory bowel disease: what you need to know This leaflet aims to answer your questions about taking methotrexate for inflammatory bowel disease (IBD). If you have any questions or
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults
 Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
RHEUMATOID ARTHRITIS DRUGS
 Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author s Line Manager:
 For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
Heart Failure (HF) - Primary Care Flow Charts. Pre diagnosis Symptoms or signs suggestive of HF
 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts. Symptoms or signs suggestive of HF. Pre diagnosis. Refer to the Heart Failure Clinic at VHK for
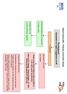 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Prescribing Framework for Methotrexate for Immunosuppression in ADULTS
 Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
The ORENCIA (abatacept) JIA Observational Registry
 Moderate to Severe Juvenile Idiopathic Arthritis (JIA) Injection for Intravenous Use Injection for Subcutaneous Use The ORENCIA (abatacept) JIA Observational Registry Injection for Intravenous Use Injection
Moderate to Severe Juvenile Idiopathic Arthritis (JIA) Injection for Intravenous Use Injection for Subcutaneous Use The ORENCIA (abatacept) JIA Observational Registry Injection for Intravenous Use Injection
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Center for Evidence-based Policy
 P&T Committee Brief Targeted Immune Modulators: Comparative Drug Class Review Alison Little, MD Center for Evidence-based Policy Oregon Health & Science University 3455 SW US Veterans Hospital Road, SN-4N
P&T Committee Brief Targeted Immune Modulators: Comparative Drug Class Review Alison Little, MD Center for Evidence-based Policy Oregon Health & Science University 3455 SW US Veterans Hospital Road, SN-4N
North of England Cancer Network. Policies and Procedures. Standards for the Safe Use of Oral Anticancer Medicines
 \ North of England Cancer Network Policies and Procedures Standards for the Safe Use of Oral Anticancer Medicines NECN Oral Anticancer medicine Policy version 1.6 Page 1 of 17 Issue Date: Feb 2017 Contents
\ North of England Cancer Network Policies and Procedures Standards for the Safe Use of Oral Anticancer Medicines NECN Oral Anticancer medicine Policy version 1.6 Page 1 of 17 Issue Date: Feb 2017 Contents
SHARED CARE GUIDELINE
 SHARED CARE GUIDELINE Title: Lithium Treatment in Adults aged 18-65 years Scope: Pennine Care NHS Foundation Trust NHS Bury NHS Oldham NHS Heywood, Middleton and Rochdale NHS Stockport NHS Tameside & Glossop
SHARED CARE GUIDELINE Title: Lithium Treatment in Adults aged 18-65 years Scope: Pennine Care NHS Foundation Trust NHS Bury NHS Oldham NHS Heywood, Middleton and Rochdale NHS Stockport NHS Tameside & Glossop
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 Aneurin Bevan University Health Board Medicines & Therapeutics Committee July 2014 PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
Aneurin Bevan University Health Board Medicines & Therapeutics Committee July 2014 PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
GWENT FORMULARY DECISIONS
 Dear Gwent Prescriber At its last two meetings (13 th October and 24 th November 2011) ABHB s Medicines & Therapeutics Committee (formerly GPMTC) made the following decisions in relation to the ABHB Joint
Dear Gwent Prescriber At its last two meetings (13 th October and 24 th November 2011) ABHB s Medicines & Therapeutics Committee (formerly GPMTC) made the following decisions in relation to the ABHB Joint
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children
 Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children Background Sleep disturbance in children with neurological or behavioural disorders is common and can be a major
Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children Background Sleep disturbance in children with neurological or behavioural disorders is common and can be a major
Expiry Date: September 2009 Template Version: Page 1 of 7
 GG&C PGD ref 2007/467 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Inclusion criteria: Exclusion criteria:
GG&C PGD ref 2007/467 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Inclusion criteria: Exclusion criteria:
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
Otezla. Otezla (apremilast) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Guidelines for rheumatology patients on biologic therapy. Rheumatology Department Patient Information Leaflet
 Guidelines for rheumatology patients on biologic therapy Rheumatology Department Patient Information Leaflet Introduction In general, people who have biologic therapy for their rheumatic condition (e.g.
Guidelines for rheumatology patients on biologic therapy Rheumatology Department Patient Information Leaflet Introduction In general, people who have biologic therapy for their rheumatic condition (e.g.
pat hways Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16
 pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
Products available Methotrexate tablets 2.5mg ONLY (Methotrexate tablets 10mg are NOT recommended as per NPSA guidance 5 ).
 Methotrexate Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti
Methotrexate Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti
Management pathway: Treatment of epilepsy in adults
 Management pathway: Treatment of epilepsy in adults Key messages Summary of treatment regimens supported by CUHFT/PSHFT consultants to support GP s with prescribing responsibility of antiepileptic drugs.
Management pathway: Treatment of epilepsy in adults Key messages Summary of treatment regimens supported by CUHFT/PSHFT consultants to support GP s with prescribing responsibility of antiepileptic drugs.
Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin)
 9 September 2015 Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) The PHARMAC Board has approved the proposal relating to the funding of the TNF-inhibitor
9 September 2015 Decision relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) The PHARMAC Board has approved the proposal relating to the funding of the TNF-inhibitor
Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014)
 1. Key Points. Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014) 1.1 Aripiprazole long acting injection (LAI) is licensed / indicated
1. Key Points. Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014) 1.1 Aripiprazole long acting injection (LAI) is licensed / indicated
2018 American Academy of Neurology
 Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
EFFECTIVE SHARED CARE AGREEMENT (ESCA)
 WORKING IN PARTNERSHIP WITH EFFECTIVE SHARED CARE AGREEMENT (ESCA) DRUG NAME: ACETYLCHOLINESTERASE-INHIBITORS AND MEMANTINE INDICATION/S COVERED: Dementia in Alzheimers Disease Coastal West Sussex Traffic
WORKING IN PARTNERSHIP WITH EFFECTIVE SHARED CARE AGREEMENT (ESCA) DRUG NAME: ACETYLCHOLINESTERASE-INHIBITORS AND MEMANTINE INDICATION/S COVERED: Dementia in Alzheimers Disease Coastal West Sussex Traffic
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Simponi / Simponi ARIA (golimumab)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Frimley Health Area Prescribing Committee
 Frimley Health Area Prescribing Committee Frimley Health NHS Foundation Trust North East Hampshire and Farnham CCG East Berkshire CCG Surrey Heath CCG Buckinghamshire CCG SHARED CARE Guideline Amber Traffic
Frimley Health Area Prescribing Committee Frimley Health NHS Foundation Trust North East Hampshire and Farnham CCG East Berkshire CCG Surrey Heath CCG Buckinghamshire CCG SHARED CARE Guideline Amber Traffic
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
PATIENT GROUP DIRECTION. Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif )
 PATIENT GROUP DIRECTION Administration of: Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif ) By: Practice Nurses In: General Practice It is the responsibility of the professional working under
PATIENT GROUP DIRECTION Administration of: Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif ) By: Practice Nurses In: General Practice It is the responsibility of the professional working under
PATIENT GROUP DIRECTION PROCEDURE
 PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID
 CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID BACKGROUND Cytokine and cell-adhesion molecule (CAM) antagonists have a major role in the treatment of chronic inflammatory diseases such
CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID BACKGROUND Cytokine and cell-adhesion molecule (CAM) antagonists have a major role in the treatment of chronic inflammatory diseases such
Fencino patch (5) 2. Check the practice has agreed to the protocol and a signed copy is in place.
 Switch protocol: Fentanyl/Durogesic patches to Fencino /Matrifen /Mezolar brand switch Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the
Switch protocol: Fentanyl/Durogesic patches to Fencino /Matrifen /Mezolar brand switch Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the
USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE
 NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
Stelara. Stelara (ustekinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Younger adults with a family history of premature artherosclerotic disease should have their cardiovascular risk factors measured.
 Appendix 2A - Guidance on Management of Hypertension Measurement of blood pressure All adults from 40 years should have blood pressure measured as part of opportunistic cardiovascular risk assessment.
Appendix 2A - Guidance on Management of Hypertension Measurement of blood pressure All adults from 40 years should have blood pressure measured as part of opportunistic cardiovascular risk assessment.
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Respiratory Inhalers. Identification Guide Version 3
 Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
IMPORTANT INFORMATION FOR HEALTHCARE PROFESSIONALS ON SAFETY AND RISK MINIMISATION FOR INFLIXIMAB
 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare Professionals are asked to report any suspected adverse reactions.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare Professionals are asked to report any suspected adverse reactions.
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women.
 ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
Patient Group Directions (PGDs)
 Patient Group Directions (PGDs) Document level: Trustwide (TW) Code: MP2 Issue number: 4 Lead executive Authors details Type of document Target audience Document purpose Medical Director Senior Clinical
Patient Group Directions (PGDs) Document level: Trustwide (TW) Code: MP2 Issue number: 4 Lead executive Authors details Type of document Target audience Document purpose Medical Director Senior Clinical
 North of Tyne Area Prescribing Committee DECISION SUMMARY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday
North of Tyne Area Prescribing Committee DECISION SUMMARY North of Tyne Area Prescribing Committee Summary of decisions made regarding new product requests considered at a meeting of the Committee on Tuesday
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Humira (adalimumab) DRUG.00002
 Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit:
 Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Factsheet. Buccolam (midazolam) 10mg in 2mL oromucosal solution. Management of seizures in adult patients
 North Central London Joint Formulary Committee Factsheet Buccolam (midazolam) 10 mg in 2 ml oromucosal solution Management of seizures in adult patients Start date: May 2017 Review date: May 2020 Document
North Central London Joint Formulary Committee Factsheet Buccolam (midazolam) 10 mg in 2 ml oromucosal solution Management of seizures in adult patients Start date: May 2017 Review date: May 2020 Document
The prescribing newsletter for GPs, nurses and pharmacists NHS Northamptonshire Failure to respond to first choice antibiotics
 Tablet Press EXTRA The prescribing newsletter for GPs, nurses and pharmacists NHS Northamptonshire Failure to respond to first choice antibiotics March 2017 Use of Buccolam (buccal midazolam) for breakthrough
Tablet Press EXTRA The prescribing newsletter for GPs, nurses and pharmacists NHS Northamptonshire Failure to respond to first choice antibiotics March 2017 Use of Buccolam (buccal midazolam) for breakthrough
Prescribing guidelines: Management of COPD in Primary Care
 Prescribing guidelines: Management of COPD in Primary Care Establish diagnosis of COPD in patients 35 years with appropriate symptoms with history, examination and spirometry (FEV1/FVC ratio < 70%) Establish
Prescribing guidelines: Management of COPD in Primary Care Establish diagnosis of COPD in patients 35 years with appropriate symptoms with history, examination and spirometry (FEV1/FVC ratio < 70%) Establish
