NEW ANTIMICROBIALS. Sarah McClain, PharmD, BCPS Infectious Diseases Pharmacy Resident Carilion Clinic Roanoke Memorial Hospital April 17, 2015
|
|
|
- Randolph Harvey
- 5 years ago
- Views:
Transcription
1 NEW ANTIMICROBIALS Sarah McClain, PharmD, BCPS Infectious Diseases Pharmacy Resident Carilion Clinic Roanoke Memorial Hospital April 17, 2015
2 DISCLOSURES I have no disclosures or conflicts of interest.
3 OBJECTIVES Pharmacists For each new antimicrobial: Classify the agent based on pharmaceutical class and mechanism of action. Describe the microbiological spectrum of activity and indications for use. Review dosing adjustments for patients with hepatic and renal impairment. Identify common adverse effects and major drug interactions. Evaluate key clinical trials. Pharmacy Technicians For each new antimicrobial: Identify the brand and generic names and pharmaceutical class. Review indications for use and common adverse effects. Describe storage and preparation requirements.
4 OVERVIEW Antifungals Isavuconazonium (Cresemba ) Gram Negative Antibacterials Ceftazidime/ avibactam (Avycaz ) Ceftolozane/ tazobactam (Zerbaxa ) Gram Positive Antibacterials Tedizolid (Sivextro ) Dalbavancin (Dalvance ) Oritavancin (Orbactiv )
5 LEGISLATION Food and Drug Administration Safety and Innovation Act Generating Antibiotic Incentives Now (GAIN) Act (Title VIII) Passed by Congress in July 2012 Qualified Infectious Disease Product (QIDP) designation More lenient clinical trial requirements Abbreviated New Drug Application Expedited FDA review (priority/fast track) 5 years of additional market exclusivity content/uploads/2013/10/fda.jpg
6 ISAVUCONAZONIUM SULFATE
7 ISAVUCONAZONIUM SULFATE (CRESEMBA ) Class: Triazole Antifungal FDA Approval Date: March 6, 2015 FDA Approved Indications: Invasive Aspergillosis Invasive Mucormycosis Orphan Drug Designation for Invasive Candidiasis (11/2014) Cresemba Prescribing Information. Astellas, Accessed March
8 ISAVUCONAZONIUM SULFATE (CRESEMBA ) Prodrug of isavuconazole Microbiological Spectrum: Broad! Yeasts (Candida spp., Cryptococcus neoformans) Molds (Aspergillus spp., Fusarium spp., Mucorales) Dimorphic Fungi Mechanism of Action Target: Fungal cell membrane Blocks conversion of lanosterol to ergosterol by inhibiting lanosterol 14 alpha demethylase Cresemba Prescribing Information. Astellas, Accessed March 2015.
9 ISAVUCONAZONIUM SULFATE (CRESEMBA ) Pharmacokinetics Absorption 98% bioavailability, IV=PO Distribution Extensive >99% protein bound Metabolism Hydrolysis to active drug by esterases in bloodstream CYP3A4, CYP3A5 Elimination Hepatic Pharmacodynamics No association between plasma AUC or isavuconazole concentration and efficacy QTc interval Shortens ( msec) Dose dependent Cresemba Prescribing Information. Astellas, Accessed March 2015.
10 ISAVUCONAZONIUM SULFATE (CRESEMBA ): DOSING & ADMINISTRATION Loading Dose: 372 mg isavuconazonium sulfate Q8H x 6 doses Maintenance Dose: 372 mg isavuconazonium sulfate once daily Intravenous Vial: 372 mg isavuconazonium sulfate (200 mg isavuconazole) Stored in the fridge 250 ml NS or D5W Administration: 1 hour In line filter required micron pore size Oral Capsules: 186 mg isavuconazonium sulfate (100 mg isavuconazole) Blister packs Administer with or without food Cresemba Prescribing Information. Astellas, Accessed March 2015.
11 ISAVUCONAZONIUM SULFATE (CRESEMBA ): WARNINGS & PRECAUTIONS Hepatic Adverse Drug Reactions LFT elevations (generally reversible) Hepatitis, cholestasis, hepatic failure reported Severe underlying medical conditions Infusion Related Reactions Hypotension, dyspnea, chills, dizziness, paresthesias reported Hypersensitivity Reactions Anaphylaxis and Stevens Johnson syndrome with other azoles Embryo Fetal Toxicity Increased perinatal mortality in animal models Drug Interactions Drug Particulates Cresemba Prescribing Information. Astellas, Accessed March 2015.
12 ISAVUCONAZONIUM SULFATE (CRESEMBA): ADVERSE EFFECTS Common Nausea (26%) Vomiting (25%) Diarrhea (22%) Headache (17%) Elevated LFTs (16%) Hypokalemia (14%) Dyspnea & cough (12%) Peripheral edema (11%) Back pain (10%) Serious Occurred in 55% of patients in trials (223/403) 14% permanently discontinued treatment (56/403) Altered mental status Acute renal failure Elevated bilirubin Convulsion/epilepsy Dyspnea Cresemba Prescribing Information. Astellas, Accessed March 2015.
13 ISAVUCONAZONIUM SULFATE (CRESEMBA): DRUG INTERACTIONS Contraindications Ketoconazole 5x increased exposure Rifampin 97% decreased exposure Lopinavir/ritonavir 96% increased isavuconazole exposure Possible decreased exposure to lopinavir/ritonavir and loss of antiretroviral efficacy Serious Immunosuppressants Cyclosporine, Sirolimus, Tacrolimus, Mycophenolate Mofetil Atorvastatin Digoxin Midazolam Buproprion Cresemba Prescribing Information. Astellas, Accessed March 2015.
14 ISAVUCONAZONIUM SULFATE (CRESEMBA): SPECIAL POPULATIONS Pregnancy Category C Limited geriatric data Not for lactating mothers No renal dose adjustment No pediatric data Not studied in severe hepatic impairment Cresemba Prescribing Information. Astellas, Accessed March 2015.
15 ISAVUCONAZONIUM SULFATE (CRESEMBA ): CLINICAL TRIALS Invasive Aspergillosis Design: Randomized, double blind, non inferiority, active control Isavuconazonium sulfate versus voriconazole Primary treatment of invasive fungal disease caused by Aspergillus spp. Stratified by history of allogeneic bone marrow transplant, uncontrolled malignancy and geography Patient Population Mean age: 51 years (17 87) 78% Caucasian 60% male 95% with pulmonary disease At least one species of Aspergillus identified in 30% of patients A. fumigatus and A. flavus most common Cresemba Prescribing Information. Astellas, Accessed March 2015.
16 ISAVUCONAZONIUM SULFATE (CRESEMBA ): CLINICAL TRIALS Invasive Aspergillosis Results Maximum treatment duration = 84 days Mean treatment duration = 47 days for both groups 8 9 days of IV administration before changing to PO All Cause Mortality at Day % isavuconazonium sulfate vs 20.2% voriconazole (95% CI 8.0% to 5.9%) Overall Response Success at End of Treatment 35% isavuconazonium sulfate vs 38.9% voriconazole (95% CI 16.3% to 8.4%) Non inferior to voriconazole for the treatment of invasive aspergillosis Cresemba Prescribing Information. Astellas, Accessed March 2015.
17 ISAVUCONAZONIUM SULFATE (CRESEMBA ): CLINICAL TRIALS Invasive Mucormycosis Design: Open label, non comparative Safety and efficacy evaluation for isavuconazonium sulfate in patients with invasive mucormycosis Patient Population (n=37) Mean age: 49 years (22 79) 68% Caucasian 81% male Risk factors for mucormycosis: 60% hematologic malignancy, 35% HSCT, 27% neutropenic, 27% on corticosteroids, 49% on T cell immunosuppressant, 11% diabetes Rhizopus oryzae most common pathogen 59% pulmonary, 43% sinus, 19% ocular, 16% CNS, 14% bone Cresemba Prescribing Information. Astellas, Accessed March 2015.
18 ISAVUCONAZONIUM SULFATE (CRESEMBA ): CLINICAL TRIALS Invasive Mucormycosis Results Median duration of treatment 102 days for patients classified as primary 33 days for patients classified as refractory 85 days for patients classified as intolerant All Cause Mortality at Day 42 38% (14/37) Overall Response Success at End of Treatment 31% (11/35) Cresemba Prescribing Information. Astellas, Accessed March 2015.
19 ASSESSMENT Which of the following is false? A. Isavuconazonium sulfate is a prodrug of isavuconazole, a difluorinated triazole antifungal B. Isavuconazonium sulfate may be used as salvage therapy for refractory MRSA bacteremia C. Isavuconazonium sulfate requires a loading dose that is given over 48 hours D. Isavuconazonium sulfate causes drug drug interactions primarily through CYP3A4 inhibition E. Isavuconazonium sulfate must be administered with an in line filter due to the potential for precipitate formation
20 ASSESSMENT Which of the following is false? A. Isavuconazonium sulfate is a prodrug of isavuconazole, a difluorinated triazole antifungal B. Isavuconazonium sulfate may be used as salvage therapy for refractory MRSA bacteremia C. Isavuconazonium sulfate requires a loading dose that is given over 48 hours D. Isavuconazonium sulfate causes drug drug interactions primarily through CYP3A4 inhibition E. Isavuconazonium sulfate must be administered with an in line filter due to the potential for precipitate formation Answer B (slides 6 18)
21 CEFTAZIDIME/ AVIBACTAM
22 CEFTAZIDIME/AVIBACTAM (AVYCAZ ) Class: 3 rd Generation Cephalosporin + Novel Beta Lactamase Inhibitor FDA Approval Date: February 25, 2015 FDA Approved Indications: Complicated Intra Abdominal Infections (ciai) Used in combination with metronidazole Complicated Urinary Tract Infections (cuti) Including pyelonephritis content/uploads/2015/03/avycaz.jpg Avycaz Prescribing Information. Actavis, Accessed March 2015.
23 CEFTAZIDIME/AVIBACTAM (AVYCAZ ) Microbiological Spectrum: Gram negatives only Pseudomonas aeruginosa Most ESBLs Some carbapenemases KPCs, OXAs, no NDMs Mechanism of Action Target: Bacterial cell wall synthesis Binds penicillin binding proteins (PBPs) Bactericidal Avycaz Prescribing Information. Actavis, Accessed March 2015.
24 CEFTAZIDIME/AVIBACTAM (AVYCAZ ) Pharmacokinetics Absorption IV only Distribution <10% protein bound Metabolism Minimal 80 90% ceftazidime eliminated unchanged No avibactam metabolism Elimination Renal Pharmacodynamics Time dependent killing Time > MIC No effect on QTc interval Mandell, Douglas, Bennett PPID 7 th Ed. Avycaz Prescribing Information. Actavis, Accessed March 2015.
25 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): DOSING & ADMINISTRATION 2.5 g (2 g ceftazidime g avibactam) IV every 8 hours Duration of Treatment: ciai 5 14 days, cuti 7 14 days Renal Dose Adjustment Required Estimated CrCl Dose ml/min 1.25g (1g/0.25g) IV q8h ml/min 0.94g (0.75g/0.19g) IV q12h 6 15 ml/min 0.94g (0.75g/0.19g) IV q24h 5 ml/min 0.94g (0.75g/0.19g) IV q48h Administration Preparation Compatible with NS, D5W, LR Dilute in ml Stable hours Give over 2 hours Compatibility with other drugs not established Avycaz Prescribing Information. Actavis, Accessed March 2015.
26 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): WARNINGS & PRECAUTIONS Decreased Efficacy in Patients with Baseline CrCl ml/min Monitor creatinine clearance at least daily in patients with changing renal function Adjust dose accordingly Hypersensitivity Reactions Anaphylaxis and serious skin reactions possible Clostridium difficile Associated Diarrhea Central Nervous System Reactions Seizures and other neurologic events possible Adjust dose accordingly in patients with renal impairment Development of Drug Resistant Bacteria Avycaz Prescribing Information. Actavis, Accessed March 2015.
27 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): ADVERSE EFFECTS Common Vomiting (14%) Nausea (10%) Constipation (10%) Anxiety (10%) Positive Direct Coombs Test (7.3%) Without evidence of hemolytic anemia < 5% Rash Hypokalemia Elevated LFTs Prolonged PT Blood dyscrasias Eosinophilia Thrombocytopenia Avycaz Prescribing Information. Actavis, Accessed March 2015.
28 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): DRUG INTERACTIONS Probenecid Drug Drug Decreases elimination of avibactam by 56 70% OAT inhibition Co administration not recommended Drug Lab False positive glucose in the urine Enzymatic glucose oxidase reactions recommended Avycaz Prescribing Information. Actavis, Accessed March 2015.
29 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): SPECIAL POPULATIONS Pregnancy Category B Ceftazidime excreted in milk No pediatric data Limited geriatric data Renal dose adjustment required Avycaz Prescribing Information. Actavis, Accessed March 2015.
30 CEFTAZIDIME/AVIBACTAM (AVYCAZ ): CLINICAL TRIALS Design Ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra abdominal infections (ciai) 69.3% male 55.4% Caucasian Ceftazidime/avibactam versus imipenem/cilastatin for complicated urinary tract infections (cuti) 75% female 58.8% Caucasian Results Similar overall rates of efficacy, adverse events, and mortality Increased mortality in ciai studies for subgroup of patients with baseline CrCl ml/min 2.5% (8/31) on ceftazidime/avibactam + metronidazole vs 8.6% (3/35) on meropenem Under dosing Delayed surgical intervention Baseline pathogens unlikely to respond to study drug Avycaz Prescribing Information. Actavis, Accessed March 2015.
31 CEFTOLOZANE/ TAZOBACTAM
32 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ) Class: Novel Cephalosporin + Beta Lactamase Inhibitor FDA Approval Date: December 19, 2014 FDA Approved Indications: Complicated Intra Abdominal Infections (ciai) Used in combination with metronidazole Complicated Urinary Tract Infections (cuti) Including pyelonephritis images.forbes.com/davidkroll/files/2014/12/zerbaxa_box_angle_w_vial.jpg Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
33 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ) Microbiological Spectrum: Broad Gram negative coverage Pseudomonas aeruginosa Acinetobacter baumannii Most ESBLs No carbapenemases Exception: Some strains of Pseudomonas Gram positives: Streptococci Mechanism of Action Target: Bacterial cell wall synthesis Binds penicillin binding proteins (PBPs) Bactericidal Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
34 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ) Pharmacokinetics Absorption IV only Distribution Ceftolozane 16 21% protein bound Tazobactam 30% protein bound Metabolism Ceftolozane eliminated unchanged Tazobactam is hydrolyzed to inactive metabolite (M1) Elimination Renal Pharmacodynamics Time dependent killing Time > MIC No effect on cardiac electrophysiology Mandell, Douglas, Bennett PPID 7 th Ed. Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
35 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): DOSING & ADMINISTRATION 1.5 g (1 g ceftolozane g tazobactam) IV every 8 hours Duration of Treatment: ciai 4 14 days, cuti 7 days Renal Dose Adjustment Required Estimated CrCl Dose ml/min 750 mg IV q8h ml/min 375 mg IV q8h ESRD or Hemodialysis 750 mg IV x 1, then 150mg IV q8h Administration Preparation Compatible with NS or D5W Dilute in 100 ml Stable 24 hours Give over 1hour Compatibility with other drugs not established Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
36 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): WARNINGS & PRECAUTIONS Decreased Efficacy in Patients with Baseline CrCl ml/min Monitor creatinine clearance at least daily in patients with changing renal function Adjust dose accordingly Hypersensitivity Reactions Anaphylaxis possible Clostridium difficile Associated Diarrhea Development of Drug Resistant Bacteria Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
37 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): ADVERSE EFFECTS Common Nausea (7.9%) Diarrhea (6.2%) Headache (5.8%) Pyrexia (5.6%) Serious 2% permanently discontinued treatment (20/1032) 1.9% in comparator arms Renal impairment led to discontinuation in 0.5% (5/1015) None in comparator arms Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
38 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): SPECIAL POPULATIONS Pregnancy Category B No lactation data No pediatric data Limited geriatric data Renal dose adjustment required No drug interactions! Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
39 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): CLINICAL TRIALS Complicated Intra Abdominal Infections: ASPECT ciai Design: Randomized, double blind, multi center phase III trial Ceftolozane/tazobactam + metronidazole versus meropenem x 4 14 days Appendicitis, cholecystitis, diverticulitis, gastric/duodenal/intestinal perforations, intra abdominal abscess, peritonitis Patient Population (n=979) Mean age: 52 years 57.8% male 34.2% with diffuse peritonitis Results Ceftolozane/tazobactam non inferior to meropenem 83% vs 87.3% Similar rates of adverse reactions Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March Solomkin et al. CID 2015; epub.
40 CEFTOLOZANE/TAZOBACTAM (ZERBAXA ): CLINICAL TRIALS Complicated Urinary Tract Infections: ASPECT cuti Design: Randomized, double blind, multi center phase III trial Ceftolozane/tazobactam versus levofloxacin x 7 days Primary endpoint: Complete resolution or marked improvement and microbiologic eradication at test of cure visit 7±2 days after last dose Patient Population (n=1068) Mean age: 50.5 years 74% female 7.8% with bacteremia 82% with pyelonephritis Results Ceftolozane/tazobactam and levofloxacin response rates were similar when adjusted for baseline levofloxacin resistance 82.6% vs 79.7% Similar rates of adverse reactions Zerbaxa Prescribing Information. Cubust Pharmaceuticals, Accessed March Solomkin et al. CID 2015; epub.
41 ASSESSMENT Which of the following is a key difference between ceftazidime/avibactam and ceftolozane/tazobactam? A. Renal dose adjustment is required for ceftazidime/avibactam but not ceftolozane/tazobactam B. Ceftazidime/avibactam exhibits time dependent bactericidal effects whereas ceftolozane/tazobactam is concentrationdependent and bacteriostatic C. Ceftolozane/tazobactam has been extensively studied for pyelonephritis in pregnancy D. Only ceftazidime/avibactam covers Pseudomonas aeruginosa E. Ceftazidime/avibactam is active against some carbapenemaseproducing organisms
42 ASSESSMENT Which of the following is a key difference between ceftazidime/avibactam and ceftolozane/tazobactam? A. Renal dose adjustment is required for ceftazidime/avibactam but not ceftolozane/tazobactam B. Ceftazidime/avibactam exhibits time dependent bactericidal effects whereas ceftolozane/tazobactam is concentrationdependent and bacteriostatic C. Ceftolozane/tazobactam has been extensively studied for pyelonephritis in pregnancy D. Only ceftazidime/avibactam covers Pseudomonas aeruginosa E. Ceftazidime/avibactam is active against some carbapenemaseproducing organisms Answer E (slide 24)
43 TEDIZOLID PHOSPHATE
44 TEDIZOLID (SIVEXTRO ) Tedizolid phosphate is the prodrug of tedizolid Class: 2 nd Generation Oxazolidinone FDA Approval Date: June 20, 2014 FDA Approved Indications: Acute Bacterial Skin and Skin Structure Infections (ABSSSI) Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March
45 TEDIZOLID (SIVEXTRO ) Microbiological Spectrum: Gram positives Staphylococci MRSA Streptococci Enterococci VRE No Gram negatives, obligate anaerobes or atypicals Mechanism of Action Target: Bacterial protein synthesis Binds 50s subunit of bacterial ribosome Bacteriostatic Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
46 TEDIZOLID (SIVEXTRO ) Pharmacokinetics Absorption F=91%, IV=PO C max reached 3 hours after oral dose, 1 hour after IV Distribution 70 90% protein bound Metabolism Converted to tedizolid by phosphatases No other metabolism Elimination Hepatic 82% fecal, 18% urine Pharmacodynamics AUC/MIC best correlates with activity No effect on cardiac repolarization Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March Mandell, Douglas, Bennett PPID 7 th Ed.
47 TEDIZOLID (SIVEXTRO ): DOSING & ADMINISTRATION 200 mg IV/PO every 24 hours Duration of Treatment: 6 days Preparation Reconstitute with 4 ml SWFI Dilute in 250 ml NS Shaking may cause foaming Only compatible with NS Stable for 24 hours Administration Give IV over 1 hour Compatibility with other drugs not established Oral may be administered with or without food Missed doses Take ASAP, anytime up to 8 hours prior to next dose Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
48 TEDIZOLID (SIVEXTRO ): WARNINGS & PRECAUTIONS Patients with Neutropenia Safety and efficacy not adequately evaluated Reduced activity in animal models Clostridium difficile Associated Diarrhea Development of Drug Resistant Bacteria Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
49 TEDIZOLID (SIVEXTRO ): ADVERSE EFFECTS Common Nausea (8%) Headache (6%) Diarrhea (4%) Vomiting (3%) Dizziness (2%) Median time to onset 5 days Less myelosuppression? Less optic and peripheral neuropathy? Serious Occurred in 1.8% of patients in trials (12/662) 0.5% permanently discontinued treatment (3/662) Less than comparator (linezolid, 0.9%) Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
50 TEDIZOLID (SIVEXTRO ): DRUG INTERACTIONS No clinically significant Weak MAOI interactions Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
51 TEDIZOLID (SIVEXTRO ): SPECIAL POPULATIONS Pregnancy Category C No human lactation data No pediatric data Insufficient geriatric data No dose adjustments Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
52 TEDIZOLID (SIVEXTRO ): CLINICAL TRIALS ESTABLISH 1 & 2 Design: Randomized, double blind, multicenter, noninferiority ABSSSI Tedizolid 200 mg PO daily x 6 days versus linezolid 600 mg PO twice daily x 10 days ESTABLISH 2: Minimum 1 day of IV therapy before PO Primary Endpoint Early clinical response ESTABLISH 1: No increase from baseline lesion at hours after first dose and oral temperature 99.7 F ESTABLISH 2: 20% decrease in lesion area at hours after first dose JAMA 2013; 309(6): Lancet Infect Dis Aug;14(8): Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
53 TEDIZOLID (SIVEXTRO ): CLINICAL TRIALS ESTABLISH 1 Patient Population (n=667) Median age: 43 years (18 86) 84% Caucasian 61% Male Results Tedizolid non inferior to linezolid Early clinical response: 79.5% vs 79.4% Similar rates of adverse events ESTABLISH 2 Patient Population (n=666) Median age: 46 years (17 86) 86% Caucasian 68% Male Results Tedizolid non inferior to linezolid Early clinical response: 85% vs 83% Similar rates of adverse events JAMA 2013; 309(6): Lancet Infect Dis Aug;14(8): Sivextro Prescribing Information. Cubust Pharmaceuticals, Accessed March 2015.
54 ORITAVANCIN
55 ORITAVANCIN (ORBACTIV ) Class: 2 nd Generation Lipoglycopeptide FDA Approval Date: August 6, 2014 FDA Approved Indications: Acute Bacterial Skin and Skin Structure Infections (ABSSSI) Orbactiv Prescribing Information. The Medicines Company, Accessed March
56 ORITAVANCIN (ORBACTIV ) Microbiological Spectrum: Gram positives Staphylococci MRSA Streptococci Enterococci Mechanism of Action Target: Bacterial cell wall synthesis Inhibits peptidoglycan synthesis Disrupts bacterial membrane integrity Bactericidal Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
57 ORITAVANCIN (ORBACTIV ) Pharmacokinetics Absorption IV only Distribution 85% protein bound Metabolism Not metabolized Elimination Slowly excreted in feces and urine Terminal half life = 245 hrs Pharmacodynamics AUC/MIC No effect on QTc interval Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
58 ORITAVANCIN (ORBACTIV ): DOSING & ADMINISTRATION Dose: 1200 mg IV x 1 Preparation 400 mg vials Reconstitute with 40 ml SWFI Dilute in 1000 ml D5W Incompatible with NS Stable 6 hours (RT) or 12 hours (refrigerated) Administration Give over 3 hours Compatibility with other drugs not established Do not co administer Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
59 ORITAVANCIN (ORBACTIV ): WARNINGS & PRECAUTIONS Potential Risk of Bleeding with Concomitant Warfarin Use Warfarin exposure may be increased Coagulation Test Interference Artificial prolongation of PT INR for up to 24 hours and aptt for up to 48 hours Non phospholipid dependent test (chromogenic anti Xa) may be used if unavoidable Hypersensitivity Infusion Reactions Clostridium difficile Associated Diarrhea Osteomyelitis Development of Drug Resistant Bacteria Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
60 ORITAVANCIN (ORBACTIV ): ADVERSE EFFECTS Common Headache (7.1%) Nausea (9.9%) Vomiting (4.6%) Limb & subcutaneous abscesses (3.8%) Diarrhea (3.7%) Serious Discontinued due to adverse reactions in 3.7% (36/976) Cellulitis (4/976) Ostemyelitis (3/976) Delayed hypersensitivity reactions? Not reported Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
61 ORITAVANCIN (ORBACTIV ): DRUG INTERACTIONS Weak inhibitor: CYP 2C9, 2C19 Weak inducer: CYP 3A4, 2D6 Unfractionated Heparin Contraindicated Warfarin Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
62 ORITAVANCIN (ORBACTIV ): SPECIAL POPULATIONS Pregnancy Category C No human lactation data No pediatric data Insufficient geriatric data Caution in renal impairment Not studied in severe hepatic impairment Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
63 ORITAVANCIN (ORBACTIV ): CLINICAL TRIALS SOLO 1 & 2 Design: Randomized, double blind, non inferiority, phase III trial Oritavancin 1200 mg IV x 1 or vancomycin 1000mg or 15 mg/kg IV q12h x 7 10 days Adults with ABSSSI (cellulitis, abscess, wound infection) Excluded if received antibiotics in 14 days prior to randomization Primary Endpoint Cessation of the spread of erythema, temperature 99.7 F, and lack of use of rescue antibiotics hours into treatment Secondary Endpoints Clinical cure 7 14 days after treatment (investigator determined) Lesion size reduction 20% into treatment NEJM 2014; 370(23): CID 2015; 60(2): Orbactiv Prescribing Information. The Medicines Company, Accessed March 2015.
64 ORITAVANCIN (ORBACTIV ): CLINICAL TRIALS SOLO 1 Patient Population (n=954) Mean age: 46 years 63% male 58% Caucasian Results Primary: 82.3% vs 78.9% Clinical cure: 79.6% vs 80% Lesion size: 86.9% vs 82.9% SOLO 2 Patient Population (n=1005) Mean age: 45 years 67% male 71% Caucasian Results Primary: 80.1% vs 82.9% Clinical cure: 82.7% vs 80.5% Lesion size: 85.9% vs 85.3% NEJM 2014; 370(23): CID 2015; 60(2): Orbactiv Prescribing Information. The Medicines Company, Accessed March Oritavancin non inferior to vancomycin.
65 DALBAVANCIN
66 DALBAVANCIN (DALVANCE ) Class: 2 nd Generation Lipoglycopeptide FDA Approval Date: May 23, 2014 FDA Approved Indications: Acute Bacterial Skin and Skin Structure Infections (ABSSSI) Dalvance Prescribing Information. Durata Therapeutics, Accessed March
67 DALBAVANCIN (DALVANCE ) Microbiological Spectrum: Gram positives Staphylococci MRSA Streptococci Enterococci Mechanism of Action Target: Bacterial cell wall synthesis Inhibits peptidoglycan synthesis Disrupts bacterial membrane integrity Bactericidal Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
68 DALBAVANCIN (DALVANCE ) Pharmacokinetics Absorption IV only Distribution 93% protein bound Metabolism Not a CYP substrate Minimal Hydroxy dalbavancin Elimination Renal and fecal Pharmacodynamics AUC/MIC No effect on cardiac electrophysiology Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
69 DALBAVANCIN (DALVANCE ): DOSING & ADMINISTRATION Initial Dose: 1000 mg IV x 1 Follow Up Dose (7 days later): 500 mg IV x 1 CrCl < 30 ml/min: 750 mg IV x 1, then 375 mg IV x 1 Preparation 500 mg vials Reconstitute with 25 ml SWFI Dilute with D5W Final concentration: 1 5 mg/ml Stable 48 hours Administration Give over 30 minutes Compatibility with other drugs not established Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
70 DALBAVANCIN (DALVANCE ): WARNINGS & PRECAUTIONS Hypersensitivity Reactions Infusion Reactions Clostridium difficile Associated Diarrhea Hepatic Effects Development of Drug Resistant Bacteria Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
71 DALBAVANCIN (DALVANCE ): ADVERSE EFFECTS Common Nausea (5.5%) Headache (4.7%) Diarrhea (4.4%) Rash (2.7%) Pruritus (2.1%) Median duration: 4 days Serious Occurred in 6.1% (109/1778) 6.5% in comparator arm Led to treatment discontinuation in 3% (53/1778) ALT elevations > 3x ULN more common 0.8% vs 0.2% All reversible Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
72 DALBAVANCIN (DALVANCE ): SPECIAL POPULATIONS Pregnancy Category C No lactation data No pediatric data Limited geriatric data Renal dose adjustment, studied in HD No data for moderate to severe hepatic impairment No drug drug or drug lab interactions! Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
73 DALBAVANCIN (DALVANCE ): CLINICAL TRIALS DISCOVER 1 & 2 Design: Randomized, double blind, double dummy, multi center, non inferiority phase III trials Dalbavancin versus vancomycin for at least 3 days Option to switch to oral linezolid to complete days of treatment Adults with ABSSSI (cellulitis, abscesses, wound infections) Excluded if used antibiotics within the previous 14 days Primary Endpoint Early clinical response: Cessation of spread of infection related erythema and the absence of fever at hours Secondary Endpoints Clinical status at end of therapy Investigator s assessment of outcome at end of therapy NEJM 2014; 370: Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
74 DALBAVANCIN (DALVANCE ): CLINICAL TRIALS DISCOVER 1 & 2 Patient Population (n=1312) Mean age: 48.9 years (18 85) 90% Caucasian 60% male No significant differences between groups Results Early clinical response: 79.7% dalbavancin vs 79.8% Secondary outcomes Clinical status: 90.7% dalbavancin vs 92.1% Assessment of outcome: 96% dalbavancin vs 96.7% Less adverse events and shorter duration in dalbavancin group Dalbavancin non inferior to vancomycin followed by linezolid. NEJM 2014; 370: Dalvance Prescribing Information. Durata Therapeutics, Accessed March 2015.
75 ASSESSMENT The use of unfractionated heparin is contraindicated during the 48 hours following the administration of which of the following antimicrobials: A. Oritavancin B. Ceftolozane/tazobactam C. Dalbavancin D. Tedizolid E. Ceftazidime/avibactam
76 ASSESSMENT The use of unfractionated heparin is contraindicated during the 48 hours following the administration of which of the following antimicrobials: A. Oritavancin B. Ceftolozane/tazobactam C. Dalbavancin D. Tedizolid E. Ceftazidime/avibactam Answer A (slide 62)
77 FYI ONLY: NEW ANTIVIRALS Influenza Peramivir (Rapivab) 12/2014 HIV Dolutegravir/ abacavir/ lamivudine (Triumeq) 8/2014 Darunavir/ cobicistat (Prezcobix) 1/2015 Atazanavir/ cobicistat (Evotaz) 1/2015 Hepatitis C Ledipasvir/ sofosbuvir (Harvoni) 10/2014 Paritaprevir/ ritonavir/ ombitasvir, dasabuvir (Viekira Pak) 12/2014
78 FYI ONLY: NEW TOPICAL AGENTS Topical Antifungals Efinaconazole (Jublia) solution 6/2014 Luliconozole (Luzu) cream 11/2013 Topical Antibacterials Finafloxacin Otic Suspension (Xtoro) 12/2014
79 ANTIMICROBIAL PIPELINE Drug Class Phase Meropenem + RPX709 Ceftaroline + avibactam Imipenem + relebactam Plazomicin Carbapenem + BLI Cephalosporin + BLI Carbapenem + BLI Aminoglycoside Enterobacteriaceae Pseudomonas spp. Acinetobacter spp. ESBL KPC NDM WT MDR NDM WT MDR 3 Y Y N Y Y N Y Y 2 Y Y N N N N N N 2 Y Y N Y? N? N 2 Y Y? N N N N N Eravacycline Fluorocycline 2 Y Y? N N N?? Brilacidin Peptide defense protein mimetic 2 Y????? N N BLI = Beta Lactamase Inhibitor, NDM = New Delhi Metallo Beta Lactamase, WT = Wild Type, KPC = Klebsiella pneumoniae Carbapenemase, MDR = Multi Drug Resistant Boucher H et al. Clin Infect Dis 2013;56(12):
80 NEW ANTIMICROBIALS Sarah McClain, PharmD, BCPS Infectious Diseases Pharmacy Resident Carilion Clinic Roanoke Memorial Hospital April 17, 2015
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
CE Prn. Pharmacy Continuing Education from WF Professional Associates ABOUT WFPA LESSONS TOPICS ORDER CONTACT PHARMACY EXAM REVIEWS
 CE Prn Pharmacy Continuing Education from WF Professional Associates ABOUT WFPA LESSONS TOPICS ORDER CONTACT PHARMACY EXAM REVIEWS New Antimicrobials March 2016 Drug resistant infections are becoming more
CE Prn Pharmacy Continuing Education from WF Professional Associates ABOUT WFPA LESSONS TOPICS ORDER CONTACT PHARMACY EXAM REVIEWS New Antimicrobials March 2016 Drug resistant infections are becoming more
New Antibiotics. Will Roland, MD
 New Antibiotics Will Roland, MD FDA Approvals 2010 - ceftaroline 2011 - fidaxomicin, telaprevir, boceprevir, spinosad 2012 bedaquiline, raxibacumab, Stribild, 2013 sofosbuvir, simeprevir, dolutegravir
New Antibiotics Will Roland, MD FDA Approvals 2010 - ceftaroline 2011 - fidaxomicin, telaprevir, boceprevir, spinosad 2012 bedaquiline, raxibacumab, Stribild, 2013 sofosbuvir, simeprevir, dolutegravir
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Xerava (eravacycline) NEW PRODUCT SLIDESHOW
 Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Infectious Disease in the Critically Ill Patient
 Infectious Disease in the Critically Ill Patient Heather L. Evans, MD MS FACS Director of Surgical Infectious Disease Harborview Medical Center Asst. Professor UW Department of Surgery New Antibiotics:
Infectious Disease in the Critically Ill Patient Heather L. Evans, MD MS FACS Director of Surgical Infectious Disease Harborview Medical Center Asst. Professor UW Department of Surgery New Antibiotics:
NEW DRUGS PART II 7/7/ ANNUAL MEETING ANTIBIOTICS AND ANTIFUNGALS DISCLOSURE BACTERIA OBJECTIVES ANTIBIOTIC RESISTANCE
 ANTIBIOTICS AND ANTIFUNGALS NEW DRUGS PART II JACQUELINE KING, PHARM.D. PHARMACY OPERATIONS SUPERVISOR UFHEALTH CANCER CENTER JACQUELINE.KING@ORLANDOHEALTH.COM TARA MCNULTY, CPHT, RPHT PROJECT MANAGER
ANTIBIOTICS AND ANTIFUNGALS NEW DRUGS PART II JACQUELINE KING, PHARM.D. PHARMACY OPERATIONS SUPERVISOR UFHEALTH CANCER CENTER JACQUELINE.KING@ORLANDOHEALTH.COM TARA MCNULTY, CPHT, RPHT PROJECT MANAGER
December 3, 2015 Severe Sepsis and Septic Shock Antibiotic Guide
 Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
Severe Sepsis and Septic Shock Antibiotic Guide Surviving Sepsis: The choice of empirical antimicrobial therapy depends on complex issues related to the patient s history, including drug intolerances,
Antibiotics to treat multi-drug-resistant bacterial infections
 Antibiotics to treat multi-drug-resistant bacterial infections July 2013 JUNE 2013 Copyright 2013 Tetraphase Pharmaceuticals, Inc. 1 Forward Looking Statements and Other Important Cautions Any statements
Antibiotics to treat multi-drug-resistant bacterial infections July 2013 JUNE 2013 Copyright 2013 Tetraphase Pharmaceuticals, Inc. 1 Forward Looking Statements and Other Important Cautions Any statements
AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use Initial U.S. Approval: 2015
 HIGHLIGHTS OF PRESCRIBING INFORMATION HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AVYCAZ safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AVYCAZ safely and effectively. See full prescribing information
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications
 Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
XERAVATM (eravacycline): A Novel Fluorocycline Antibacterial
 XERAVATM (eravacycline): A Novel Fluorocycline Antibacterial Matteo Bassetti, MD, PhD Infectious Diseases Division University of Udine and Santa Maria Misericordia University Hospital Udine, Italy Disclosures
XERAVATM (eravacycline): A Novel Fluorocycline Antibacterial Matteo Bassetti, MD, PhD Infectious Diseases Division University of Udine and Santa Maria Misericordia University Hospital Udine, Italy Disclosures
New Antimicrobials & Agents in the Pipeline
 New Antimicrobials & Agents in the Pipeline Keenan Ryan, PharmD PGY2 Pharmacy Resident in Infec=ous Diseases University of New Mexico College of Pharmacy October 2015 Learning Objectives By the end of
New Antimicrobials & Agents in the Pipeline Keenan Ryan, PharmD PGY2 Pharmacy Resident in Infec=ous Diseases University of New Mexico College of Pharmacy October 2015 Learning Objectives By the end of
AVYCAZ (ceftazidime and avibactam) Dosing and Administration
 AVYCAZ (ceftazidime and avibactam) Dosing and Administration DOSAGE OF AVYCAZ BY INDICATION IN PATIENTS WITH NORMAL RENAL FUNCTION (CREATININE CLEARANCE GREATER THAN 50 ml/min) 1 DOSAGE ADJUSTMENTS IN
AVYCAZ (ceftazidime and avibactam) Dosing and Administration DOSAGE OF AVYCAZ BY INDICATION IN PATIENTS WITH NORMAL RENAL FUNCTION (CREATININE CLEARANCE GREATER THAN 50 ml/min) 1 DOSAGE ADJUSTMENTS IN
Jill Michaud, Pharm.D., BCPS. I have no actual or potential conflicts of interest related to this presentation
 Jill Michaud, Pharm.D., BCPS I have no actual or potential conflicts of interest related to this presentation Focus New drugs you might use in the Emergency department New drugs patients might be on Indications
Jill Michaud, Pharm.D., BCPS I have no actual or potential conflicts of interest related to this presentation Focus New drugs you might use in the Emergency department New drugs patients might be on Indications
Hepatitis C Treatment and the Role of the Pharmacist
 Infectious Diseases Odds and Ends: Focus on Developments in Hepatology and Gram-Negative Infections Lindsey Childs-Kean, PharmD, MPH, BCPS Clinical Assistant Professor University of Florida College of
Infectious Diseases Odds and Ends: Focus on Developments in Hepatology and Gram-Negative Infections Lindsey Childs-Kean, PharmD, MPH, BCPS Clinical Assistant Professor University of Florida College of
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
New Antimicrobials & Agents in the Pipeline
 Learning Objectives New Antimicrobials & Agents in the Pipeline Keenan Ryan, PharmD PGY2 Pharmacy Resident in Infec=ous Diseases University of New Mexico College of Pharmacy October 2015 By the end of
Learning Objectives New Antimicrobials & Agents in the Pipeline Keenan Ryan, PharmD PGY2 Pharmacy Resident in Infec=ous Diseases University of New Mexico College of Pharmacy October 2015 By the end of
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aztreonam Pharmaceutical form(s)/strength: 500 mg, 1 g and 2 g powder for solution for injection and infusion NB! 75mg aztreonam for nebulisation to treat infections
M0BCore Safety Profile Active substance: Aztreonam Pharmaceutical form(s)/strength: 500 mg, 1 g and 2 g powder for solution for injection and infusion NB! 75mg aztreonam for nebulisation to treat infections
Dosing & administration guide
 For first-line treatment of invasive aspergillosis and invasive mucormycosis in adults Dosing & administration guide INDICATIONS AND USAGE CRESEMBA (isavuconazonium sulfate) is an azole antifungal indicated
For first-line treatment of invasive aspergillosis and invasive mucormycosis in adults Dosing & administration guide INDICATIONS AND USAGE CRESEMBA (isavuconazonium sulfate) is an azole antifungal indicated
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CRESEMBA safely and effectively. See full prescribing information for CRESEMBA. CRESEMBA (isavuconazonium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CRESEMBA safely and effectively. See full prescribing information for CRESEMBA. CRESEMBA (isavuconazonium
New Antimicrobials: Now and In the Future
 New Antimicrobials: Now and In the Future David C. Hooper, M.D. Division of Infectious Diseases Infection Control Unit Massachusetts General Hospital Harvard Medical School Conflicts of Interest Melinta
New Antimicrobials: Now and In the Future David C. Hooper, M.D. Division of Infectious Diseases Infection Control Unit Massachusetts General Hospital Harvard Medical School Conflicts of Interest Melinta
Summary of the risk management plan (RMP) for Zerbaxa (ceftolozane / tazobactam)
 EMA/513109/2015 Summary of the risk management plan (RMP) for Zerbaxa (ceftolozane / tazobactam) This is a summary of the risk management plan (RMP) for Zerbaxa, which details the measures to be taken
EMA/513109/2015 Summary of the risk management plan (RMP) for Zerbaxa (ceftolozane / tazobactam) This is a summary of the risk management plan (RMP) for Zerbaxa, which details the measures to be taken
! Macrolide antibacterial. Fidaxomicin (Dificid ) package labeling. Optimer Pharmaceuticals, Inc. May 2011.
 Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study
 Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
VIII Updating Course of Antimicrobials and Infectious Diseases 2018
 The Author 2018. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/).
The Author 2018. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/).
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
400 mg IV (over 5 to 60 minutes) every 12 hours. > 30 to mg IV (over 5 to 60 minutes ) every 12 hours. 15 to 30
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
SWISS SUMMARY OF THE RISK MANAGEMENT PLAN (RMP) FOR ZERBAXA. (Ceftolozane/Tazobactam)
 PAGE 1 SWISS SUMMARY OF THE RISK MANAGEMENT PLAN (RMP) FOR ZERBAXA (Ceftolozane/Tazobactam) Active substance(s): Ceftolozane/Tazobactam Product(s) concerned: ZERBAXA Market Authorisation Holder: MSD Merck
PAGE 1 SWISS SUMMARY OF THE RISK MANAGEMENT PLAN (RMP) FOR ZERBAXA (Ceftolozane/Tazobactam) Active substance(s): Ceftolozane/Tazobactam Product(s) concerned: ZERBAXA Market Authorisation Holder: MSD Merck
New Drug Update Biography. Objectives 2/18/2015. Wesley Lindsey, Pharm.D.
 New Drug Update 2015 Wesley Lindsey, Pharm.D. Associate Clinical Professor Auburn University: Harrison School of Pharmacy Biography Undergraduate: University of North Carolina at Asheville Pharmacy: Campbell
New Drug Update 2015 Wesley Lindsey, Pharm.D. Associate Clinical Professor Auburn University: Harrison School of Pharmacy Biography Undergraduate: University of North Carolina at Asheville Pharmacy: Campbell
Itraconazole DIGICON. Composition: MOLECULAR INTRODUCTION
 Itraconazole DIGICON Composition: Itraconazole 100 mg MOLECULAR INTRODUCTION Itraconazole is a triazole medicine used to treat fungal infections. It is effective against a broad spectrum of fungi including:
Itraconazole DIGICON Composition: Itraconazole 100 mg MOLECULAR INTRODUCTION Itraconazole is a triazole medicine used to treat fungal infections. It is effective against a broad spectrum of fungi including:
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Voriconazole Effective Date... 3/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 4004 Table of Contents Coverage Policy... 1 General Background...
Cigna Drug and Biologic Coverage Policy Subject Voriconazole Effective Date... 3/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 4004 Table of Contents Coverage Policy... 1 General Background...
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
NDA Briefing Document Anti-Infective Drugs Advisory Committee 05 December 2014
 CEFTAZIDIME-AVIBACTAM FOR INJECTION for Treatment of Complicated Intra-abdominal Infection (used in combination with metronidazole), Complicated Urinary Tract Infection including Acute Pyelonephritis,
CEFTAZIDIME-AVIBACTAM FOR INJECTION for Treatment of Complicated Intra-abdominal Infection (used in combination with metronidazole), Complicated Urinary Tract Infection including Acute Pyelonephritis,
Fungi are eukaryotic With rigid cell walls composed largely of chitin rather than peptidoglycan (a characteristic component of most bacterial cell
 Antifungal Drugs Fungal infections (Mycoses) Often chronic in nature. Mycotic infections may be superficial and involve only the skin (cutaneous mycoses extending into the epidermis) Others may penetrate
Antifungal Drugs Fungal infections (Mycoses) Often chronic in nature. Mycotic infections may be superficial and involve only the skin (cutaneous mycoses extending into the epidermis) Others may penetrate
Summary of the risk management plan (RMP) for Cresemba (isavuconazole)
 EMA/576866/2015 Summary of the risk management plan (RMP) for Cresemba (isavuconazole) This is a summary of the risk management plan (RMP) for Cresemba, which details the measures to be taken in order
EMA/576866/2015 Summary of the risk management plan (RMP) for Cresemba (isavuconazole) This is a summary of the risk management plan (RMP) for Cresemba, which details the measures to be taken in order
PRUFLOX Tablets (Prulifloxacin)
 Published on: 10 Jul 2014 PRUFLOX Tablets (Prulifloxacin) Composition Each Film coated tablet contains: Prulifloxacin... 600 mg Colours: Red Oxide of Iron & Titanium Dioxide IP Dosage Form Tablet Pharmacology
Published on: 10 Jul 2014 PRUFLOX Tablets (Prulifloxacin) Composition Each Film coated tablet contains: Prulifloxacin... 600 mg Colours: Red Oxide of Iron & Titanium Dioxide IP Dosage Form Tablet Pharmacology
ABSSSI Case Study days (clinical evaluation)
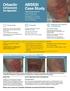 ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
Frequency of Occurrence and Antimicrobial Susceptibility of Bacteria from ICU Patients with Pneumonia
 Frequency of Occurrence and Antimicrobial Susceptibility of Bacteria from ICU Patients with Pneumonia Helio S. Sader, M.D.* Mariana Castanheira, Ph.D. Rodrigo E. Mendes, Ph.D. Robert K. Flamm, Ph.D. JMI
Frequency of Occurrence and Antimicrobial Susceptibility of Bacteria from ICU Patients with Pneumonia Helio S. Sader, M.D.* Mariana Castanheira, Ph.D. Rodrigo E. Mendes, Ph.D. Robert K. Flamm, Ph.D. JMI
Acute Cholangitis. Kelsey Knotts PharmD Candidate Class of 2016
 Acute Cholangitis Kelsey Knotts PharmD Candidate Class of 2016 Learning Objectives 1. Describe the mechanism of the development of acute cholangitis 2. Identify common causative organisms in acute cholangitis
Acute Cholangitis Kelsey Knotts PharmD Candidate Class of 2016 Learning Objectives 1. Describe the mechanism of the development of acute cholangitis 2. Identify common causative organisms in acute cholangitis
Innovations in Antimicrobials Something Old, Something New
 FSHP Disclosure Innovations in Antimicrobials Something Old, Something New Harrison Bachmeier, PharmD, BCPS Pharmacist Specialist-Infectious Diseases LeeHealth Fort Myers & Cape Coral, FL I do not have
FSHP Disclosure Innovations in Antimicrobials Something Old, Something New Harrison Bachmeier, PharmD, BCPS Pharmacist Specialist-Infectious Diseases LeeHealth Fort Myers & Cape Coral, FL I do not have
Development of C sporins. Beta-lactam antibiotics - Cephalosporins. Second generation C sporins. Targets - PBP s
 Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
Nightmare Bacteria. Disclosures. Technician Objectives. Pharmacist Objectives. Carbapenem Resistance in Carbapenem Resistance in 2017
 Nightmare Bacteria How to Deal with the Reality of Carbapenem-resistant Organisms Disclosures I have no conflicts of interest relative to the content of this presentation Matthew L. Brown, Pharm.D., BCPS
Nightmare Bacteria How to Deal with the Reality of Carbapenem-resistant Organisms Disclosures I have no conflicts of interest relative to the content of this presentation Matthew L. Brown, Pharm.D., BCPS
WARNING, CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS,
 Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
WARNING: RISK OF SERIOUS INFECTIONS
 RA PROGRESSION INTERRUPTED 1 DOSAGE AND ADMINISTRATION GUIDE No structural damage progression was observed at week 52 in 55.6% and in 47.8% of patients receiving KEVZARA 200 mg + MTX or 150 mg + MTX, compared
RA PROGRESSION INTERRUPTED 1 DOSAGE AND ADMINISTRATION GUIDE No structural damage progression was observed at week 52 in 55.6% and in 47.8% of patients receiving KEVZARA 200 mg + MTX or 150 mg + MTX, compared
Core Safety Profile. Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g LV/H/PSUR/0002/001 Date of FAR:
 Core Safety Profile Active substance: Ceftriaxone Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g P-RMS: LV/H/PSUR/0002/001 Date of FAR: 14.10.2009 Section 4.2 (Posology and method
Core Safety Profile Active substance: Ceftriaxone Pharmaceutical form(s)/strength: Powder for solution for injection, 1 g P-RMS: LV/H/PSUR/0002/001 Date of FAR: 14.10.2009 Section 4.2 (Posology and method
Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml SI/H/PSUR/0002/002 Date of FAR:
 0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
Macrolides & Ketolides. Objectives. Protein Synthesis
 Macrolides & Ketolides Elizabeth D. Hermsen, Pharm.D. Infectious Diseases Research Fellow University of Minnesota College of Pharmacy bjectives Participant should be able to explain macrolide/ketolide
Macrolides & Ketolides Elizabeth D. Hermsen, Pharm.D. Infectious Diseases Research Fellow University of Minnesota College of Pharmacy bjectives Participant should be able to explain macrolide/ketolide
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication
 ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
TP Larry Tsai, MD Chief Medical Officer Tetraphase Pharmaceuticals
 TP-676 Larry Tsai, MD Chief Medical Officer Tetraphase Pharmaceuticals Tetraphase Pharmaceuticals Overview Tetraphase is developing novel antibiotics for serious and life-threatening Gram-negative MDR
TP-676 Larry Tsai, MD Chief Medical Officer Tetraphase Pharmaceuticals Tetraphase Pharmaceuticals Overview Tetraphase is developing novel antibiotics for serious and life-threatening Gram-negative MDR
POMALYST (pomalidomide) for Previously Treated Multiple Myeloma
 POMALYST (pomalidomide) for Previously Treated What is POMALYST? POMALYST (pomalidomide) capsule is an oral immunomodulatory therapy (a thalidomide analogue) indicated for patients with multiple myeloma
POMALYST (pomalidomide) for Previously Treated What is POMALYST? POMALYST (pomalidomide) capsule is an oral immunomodulatory therapy (a thalidomide analogue) indicated for patients with multiple myeloma
The sensitivity of fungal microorganisms to fluconazole is as follows:
 FLUCAN Composition Each capsule contains Fluconazole 50, 100 or 150 mg Capsules Action Fluconazole is a triazole anti-fungal, which in sensitive fungi, inhibits cytochrome P450-dependent enzymes resulting
FLUCAN Composition Each capsule contains Fluconazole 50, 100 or 150 mg Capsules Action Fluconazole is a triazole anti-fungal, which in sensitive fungi, inhibits cytochrome P450-dependent enzymes resulting
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ZERBAXA. Ceftolozane and Tazobactam powder for injection
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ZERBAXA Ceftolozane and Tazobactam powder for injection 1.5 gram (g) per vial Containing ceftolozane 1 g (as ceftolozane sulfate) and tazobactam
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ZERBAXA Ceftolozane and Tazobactam powder for injection 1.5 gram (g) per vial Containing ceftolozane 1 g (as ceftolozane sulfate) and tazobactam
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory ZODERM E CREAM. Oxiconazole Nitrate Cream
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory ZODERM E CREAM Oxiconazole Nitrate Cream QUALITATIVE AND QUANTITATIVE COMPOSITION ZODERM E CREAM contains: Oxiconazole
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory ZODERM E CREAM Oxiconazole Nitrate Cream QUALITATIVE AND QUANTITATIVE COMPOSITION ZODERM E CREAM contains: Oxiconazole
Treatment of febrile neutropenia in patients with neoplasia
 Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Treatment of febrile neutropenia in patients with neoplasia George Samonis MD, PhD Medical Oncologist Infectious Diseases Specialist Professor of Medicine The University of Crete, Heraklion,, Crete, Greece
Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
PRODUCT CIRCULAR CASPOFUNGIN. Antifungal
 PRODUCT CIRCULAR CASPOFUNGIN CANCIDAS Powder for I.V. Injection Antifungal THERAPEUTIC CLASS CASPOFUNGIN (CANCIDAS ) is a sterile, lyophilized product for intravenous infusion that contains a semi-synthetic
PRODUCT CIRCULAR CASPOFUNGIN CANCIDAS Powder for I.V. Injection Antifungal THERAPEUTIC CLASS CASPOFUNGIN (CANCIDAS ) is a sterile, lyophilized product for intravenous infusion that contains a semi-synthetic
OTE. Peramivir: A Novel Intravenous Neuraminidase Inhibitor for the Treatment of Influenza. Vol. 30, Issue 9 June 2015.
 HAR Vol. 30, Issue 9 June 2015 M A N OTE Established 1985 Peramivir: A Novel Intravenous Neuraminidase Inhibitor for the Treatment of Influenza Vincent Solomon, PharmD Peramivir: A Novel Intravenous Neuraminidase
HAR Vol. 30, Issue 9 June 2015 M A N OTE Established 1985 Peramivir: A Novel Intravenous Neuraminidase Inhibitor for the Treatment of Influenza Vincent Solomon, PharmD Peramivir: A Novel Intravenous Neuraminidase
Ninlaro. (ixazomib) New Product Slideshow
 Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
DOSING GUIDE. Indications. Important Safety Information. Enable the immune system. RECOGNIZE. RESPOND.
 DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
PRODUCT INFORMATION TARGOCID
 NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
Vancomycin Orion , Version 2 Public Summary of the Risk Management Plan
 Vancomycin Orion 18.1.2016, Version 2 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Vancomycin Orion 18.1.2016, Version 2 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
LUNCH AND LEARN. April 10, 2015
 LUNCH AND LEARN New Drugs 2014 Part 1 April 10, 2015 Featured Speaker: Mary Lynn Moody, RPh Director, Business Development Drug Information Group Clinical Associate Professor University of Illinois at
LUNCH AND LEARN New Drugs 2014 Part 1 April 10, 2015 Featured Speaker: Mary Lynn Moody, RPh Director, Business Development Drug Information Group Clinical Associate Professor University of Illinois at
CANCIDAS (caspofungin acetate) for injection, for intravenous use Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CANCIDAS safely and effectively. See full prescribing information for CANCIDAS. CANCIDAS (caspofungin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CANCIDAS safely and effectively. See full prescribing information for CANCIDAS. CANCIDAS (caspofungin
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
PRODUCT INFORMATION 1 ABOUT THIS GUIDE DOSAGE FORM AND STRENGTH STORAGE AND HANDLING
 DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
Neutropenic Sepsis Guideline
 Neutropenic Sepsis Guideline Neutropenic Sepsis Guideline - definitions Suspected or proven infection in a neutropenic patient is a MEDICAL EMERGENCY and is an indication for immediate assessment and prompt
Neutropenic Sepsis Guideline Neutropenic Sepsis Guideline - definitions Suspected or proven infection in a neutropenic patient is a MEDICAL EMERGENCY and is an indication for immediate assessment and prompt
Vancomycin Orion , Version 1.0. Public Summary of the Risk Management Plan
 Vancomycin Orion 18.6.2015, Version 1.0 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Vancomycin Orion 18.6.2015, Version 1.0 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Overcoming the PosESBLities of Enterobacteriaceae Resistance
 Overcoming the PosESBLities of Enterobacteriaceae Resistance Review of current treatment options Jamie Reed, PharmD Pharmacy Grand Rounds August 28, 2018 Rochester, MN 2018 MFMER slide-1 Disclosure No
Overcoming the PosESBLities of Enterobacteriaceae Resistance Review of current treatment options Jamie Reed, PharmD Pharmacy Grand Rounds August 28, 2018 Rochester, MN 2018 MFMER slide-1 Disclosure No
Package leaflet: Information for the user. Piperacillin/Tazobactam Actavis 4 g / 0.5 g powder for solution for infusion. piperacillin / tazobactam
 Package leaflet: Information for the user Piperacillin/Tazobactam Actavis 2 g / 0.25 g powder for solution for infusion Piperacillin/Tazobactam Actavis 4 g / 0.5 g powder for solution for infusion piperacillin
Package leaflet: Information for the user Piperacillin/Tazobactam Actavis 2 g / 0.25 g powder for solution for infusion Piperacillin/Tazobactam Actavis 4 g / 0.5 g powder for solution for infusion piperacillin
Cubicin A Guide to Dosing
 Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES
 DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
The CLSI Approach to Setting Breakpoints
 The CLSI Approach to Setting Breakpoints Jean B. Patel, PhD, D(ABMM) Deputy Director, Office of Antimicrobial Resistance Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic
The CLSI Approach to Setting Breakpoints Jean B. Patel, PhD, D(ABMM) Deputy Director, Office of Antimicrobial Resistance Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic
Other β-lactam. A. Carbapenems:
 A. Carbapenems: Other β-lactam Carbapenems are synthetic β-lactam antibiotics Differ in structure from the penicillins in that the sulfur atom of the thiazolidine ring. Imipenem, meropenem, doripenem,
A. Carbapenems: Other β-lactam Carbapenems are synthetic β-lactam antibiotics Differ in structure from the penicillins in that the sulfur atom of the thiazolidine ring. Imipenem, meropenem, doripenem,
9/7/2018. Faculty. Overcoming Challenges in the Management of Invasive Fungal Infections. Learning Objectives. Faculty Disclosure
 Faculty Overcoming Challenges in the Management of Invasive Fungal James S. Lewis II, PharmD, FIDSA ID Clinical Pharmacy Coordinator Oregon Health and Science University Departments of Pharmacy and Infectious
Faculty Overcoming Challenges in the Management of Invasive Fungal James S. Lewis II, PharmD, FIDSA ID Clinical Pharmacy Coordinator Oregon Health and Science University Departments of Pharmacy and Infectious
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Isavuconazonium sulfate (Cresemba, Astellas Pharma US Inc.) June 2017 Non-Formulary
 Isavuconazonium sulfate (Cresemba, Astellas Pharma US Inc.) June 2017 Non-Formulary Criteria for Formulary Consideration of Isavuconazonium Sulfate Efficacy Isavuconazonium is approved by the FDA for treatment
Isavuconazonium sulfate (Cresemba, Astellas Pharma US Inc.) June 2017 Non-Formulary Criteria for Formulary Consideration of Isavuconazonium Sulfate Efficacy Isavuconazonium is approved by the FDA for treatment
APX001 A novel broad spectrum antifungal agent in development for the treatment of invasive fungal infections
 APX001 A novel broad spectrum antifungal agent in development for the treatment of invasive fungal infections TIMM, Belgrade, Serbia October, 2017 New antifungal drugs in the pipeline S15 Dr. Michael Hodges
APX001 A novel broad spectrum antifungal agent in development for the treatment of invasive fungal infections TIMM, Belgrade, Serbia October, 2017 New antifungal drugs in the pipeline S15 Dr. Michael Hodges
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 1Q18 January February
 BRAND NAME Prevymis TM GENERIC NAME Letermovir MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL November 9, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of new
BRAND NAME Prevymis TM GENERIC NAME Letermovir MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL November 9, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of new
Abiraterone Acetate is an antiandrogen used in the treatment of Castration-Resistant Prostate Cancer(CRPC).
 For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin)
 SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
National Center for Emerging and Zoonotic Infectious Diseases The Biggest Antibiotic Resistance Threats
 National Center for Emerging and Zoonotic Infectious Diseases The Biggest Antibiotic Resistance Threats Jean B. Patel, PhD, D(ABMM) Science Lead, Antibiotic Resistance and Coordination Unit Centers for
National Center for Emerging and Zoonotic Infectious Diseases The Biggest Antibiotic Resistance Threats Jean B. Patel, PhD, D(ABMM) Science Lead, Antibiotic Resistance and Coordination Unit Centers for
Achaogen Launches ZEMDRI (plazomicin), a Once-Daily Aminoglycoside for use in complicated Urinary Tract Infections (cuti)
 Achaogen Launches ZEMDRI (plazomicin), a Once-Daily Aminoglycoside for use in complicated Urinary Tract Infections (cuti) -- ZEMDRI now available for ordering in the U.S. -- -- ZEMDRI demonstrated in vitro
Achaogen Launches ZEMDRI (plazomicin), a Once-Daily Aminoglycoside for use in complicated Urinary Tract Infections (cuti) -- ZEMDRI now available for ordering in the U.S. -- -- ZEMDRI demonstrated in vitro
Drafting a Coverage Authorization Request Letter
 Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
VABOMERE (meropenem and vaborbactam) for injection, for intravenous use Initial U.S. Approval: 2017
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VABOMERE safely and effectively. See full prescribing information for VABOMERE. VABOMERE (meropenem
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VABOMERE safely and effectively. See full prescribing information for VABOMERE. VABOMERE (meropenem
Drug Class Monograph
 Drug Class Monograph Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drugs: alogliptin, alogliptin/metformin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin),
Drug Class Monograph Class: Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drugs: alogliptin, alogliptin/metformin, Januvia (sitagliptin), Janumet (sitagliptin/metformin), Janumet XR (sitagliptin/metformin),
14 41 minutes Vd 218 t ½ 22 to 70 Clearance hours Protein Binding 71%, active metabolite 42% Bioavailability 3%
 Brand Name: Yupelri Generic Name: revefenacin Manufacturer: Mylan Drug Class: Anticholinergic Uses: Labeled Uses: inhalation solution for maintenance treatment of COPD 1 Unlabeled Uses: no off-label indications
Brand Name: Yupelri Generic Name: revefenacin Manufacturer: Mylan Drug Class: Anticholinergic Uses: Labeled Uses: inhalation solution for maintenance treatment of COPD 1 Unlabeled Uses: no off-label indications
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Giving the Proper Dose: How Can The Clinical and Laboratory Standards Institute(CLSI)Help?
 Giving the Proper Dose: How Can The Clinical and Laboratory Standards Institute(CLSI)Help? Pranita D. Tamma, M.D., M.H.S. Director, Pediatric Antimicrobial Stewardship Johns Hopkins University School of
Giving the Proper Dose: How Can The Clinical and Laboratory Standards Institute(CLSI)Help? Pranita D. Tamma, M.D., M.H.S. Director, Pediatric Antimicrobial Stewardship Johns Hopkins University School of
New insights in antibiotic and antifungal therapy in the compromised host
 New insights in antibiotic and antifungal therapy in the compromised host Claudio Viscoli University of Genova Ospedale Policlinico San Martino, Genova Potential conflicts of interest (last 5 years) Received
New insights in antibiotic and antifungal therapy in the compromised host Claudio Viscoli University of Genova Ospedale Policlinico San Martino, Genova Potential conflicts of interest (last 5 years) Received
DOSAGE AND ADMINISTRATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NOXAFIL safely and effectively. See full prescribing information for NOXAFIL. NOXAFIL (posaconazole)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NOXAFIL safely and effectively. See full prescribing information for NOXAFIL. NOXAFIL (posaconazole)
The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses.
 1. NAME OF THE MEDICINAL PRODUCT Avopenin 800 mg film-coated tablets Avopenin 1 g film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Phenoxymethylpenicillin potassium 800 mg or 1 g For a full
1. NAME OF THE MEDICINAL PRODUCT Avopenin 800 mg film-coated tablets Avopenin 1 g film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Phenoxymethylpenicillin potassium 800 mg or 1 g For a full
PEDIATRIC PHARMACOTHERAPY
 PEDIATRIC PHARMACOTHERAPY Volume 23 Number 6 June 2017 C Ceftaroline: An Alternative Broad Spectrum Antibiotic for Pediatric Infections Marcia L. Buck, PharmD, FCCP, FPPAG, BCPPS eftaroline was approved
PEDIATRIC PHARMACOTHERAPY Volume 23 Number 6 June 2017 C Ceftaroline: An Alternative Broad Spectrum Antibiotic for Pediatric Infections Marcia L. Buck, PharmD, FCCP, FPPAG, BCPPS eftaroline was approved
New Medicines Committee Briefing. July Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection
 New Medicines Committee Briefing July 2014 Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection (unlicensed indication) Fosfomycin trometamol to be reviewed for use within:
New Medicines Committee Briefing July 2014 Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection (unlicensed indication) Fosfomycin trometamol to be reviewed for use within:
