W Y Zhang, A Li Wan Po, H S Dua, A Azuara-Blanco
|
|
|
- Miranda Powell
- 5 years ago
- Views:
Transcription
1 Br J Ophthalmol 2001;85: Centre for Evidence-Based Pharmacotherapy, School of Life and Health Sciences, Aston University, Aston Triangle, Birmingham B4 7ET, UK W Y Zhang ALiWanPo Division of Ophthalmology, Clinical Laboratory Sciences, Queens Medical Centre, Nottingham NG7 2UH, UK H S Dua The Eye Clinic, Aberdeen Royal Infirmary, Aberdeen AB25 2ZN, UK A Azuara-Blanco Correspondence to: Dr W Y Zhang w.y.zhang@aston.ac.uk Accepted for publication 7 February 2001 Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension W Y Zhang, A Li Wan Po, H S Dua, A Azuara-Blanco Abstract Aim To evaluate the comparative eycacy and tolerance of latanoprost versus timolol through a meta-analysis of randomised controlled trials (RCTs). Methods Systematic retrieval of RCTs of latanoprost versus timolol to allow pooling of results from head to head comparison studies. Quality of trials was assessed based on randomisation, masking, and withdrawal. Sensitivity analyses were used to estimate the evects of quality of study on outcomes. The data sources were Medline, Embase, Scientific Citation Index, Merck Glaucoma, and Pharmacia and Upjohn ophthalmology databases. There were 1256 patients with open angle glaucoma or ocular hypertension reported in 11 trials of latanoprost versus timolol. The main outcome measures were (i) percentage intraocular pressure (IOP) reduction for eycacy; (ii) relative risk, risk diverence, and number needed to harm for side evects such as hyperaemia, conjunctivitis, increased pigmentation, hypotension, and bradycardia expressed as dichotomous outcomes; and (iii) reduction in systemic blood pressure and heart rate as side evects. Results Both 0.005% latanoprost once daily and 0.5% timolol twice daily reduced IOP. The percentage reductions in IOP from baseline (mean (SE)) produced by latanoprost and timolol were 30.2 (2.3) and 26.9 (3.4) at 3 months. The diverence in IOP reduction between the two treatments were 5.0 (95% confidence intervals 2.8, 7.3). However, latanoprost caused iris pigmentation in more patients than timolol (relative risk = 8.01, 95% confidence intervals 1.87, 34.30). The 2 year risk with latanoprost reached 18% (51/ 277). Hyperaemia was also more often observed with latanoprost (relative risk =2.20, 95% confidence intervals 1.33, 3.64). Timolol caused a significant reduction in heart rate of 4 beats/minute (95% confidence interval 2, 6). Conclusion This meta-analysis suggests that latanoprost is more evective than timolol in lowering IOP. However, it often causes iris pigmentation. While current evidence suggests that this pigmentation is benign, careful lifetime evaluation of patients is still justified. (Br J Ophthalmol 2001;85: ) Glaucoma is one of the most frequent causes of blindness in the industrialised world. The economic burden including direct and indirect costs of the disease in the UK was estimated to be 132 million in The mainstay of drug treatment for glaucoma is timolol, a topical β blocker. However, β blockers are contraindicated in patients with cardiovascular or pulmonary disorders. 2 4 Pilocarpine, a cholinergic agonist, is sometimes used but it needs to be administered four times per day and causes miosis, myopia, and occasionally retinal detachment and progressive closure of the anterior chamber angle. 5 6 Given such problems, the search for new evective and safer antiglaucoma agents continues. Among the recently introduced agents, three are widely used as alternatives when β blockers are contraindicated or fail to control intraocular pressure latanoprost (a prostaglandin F 2α analogue), dorzolamide (a topical carbonic anhydrase inhibitor), and brimonidine (a selective α 2 agonist). Latanoprost appears highly promising because of its comparable or better eycacy when compared with timolol. We therefore undertook a comparison of the evects of topical latanoprost and timolol on intraocular pressure (IOP) based on published randomised controlled trials conducted by both pharmaceutical companies and academics. Materials and methods RETRIEVAL OF PUBLISHED STUDIES Reports of randomised controlled trials (RCTs) of latanoprost versus timolol were identified through a systematic search consisting of: (1) an electronic search of Medline, Embase, and Scientific Citation Index; (2) searches of reference lists of original reports and review articles, retrieved through the electronic searches; (3) searches for manufacturers databases including Pharmacia Upjohn ophthalmology database and Merck glaucoma database. The computerised searches covered the period 1966 to end of July The medical subject heading (MeSH) search used in Medline and Embase consisted of three stages, each contained any possible MeSH relevant to the target diseases, drugs, and study methods as shown in Table 1. All MeSHs were exploded. Three stages were then combined to produce citations associated with randomised controlled trials of latanoprost and timolol in the treatment of glaucoma.
2 984 Zhang, Li Wan Po, Dua, et al Table 1 MeSH search strategy Stage 1 Diseases Stage 2 Drugs Stage 3 Study methods 1 exp glaucoma* or exp glaucoma, open angle* 4 exp prostaglandins F, synthetic* 7 exp clinical trials* or exp randomised controlled trials* 2 exp ocular hypertension* 5 exp adrenergic β antagonists* 8 exp double blind method* 3 1 or and 5 9 exp single blind method* 10 exp random allocation* 11 7 or 8 or 9 or 10 Finally, we combined three stages together using: 3 and 6 and 11 exp = explode. *MeSH search including all subject headings under the title Potentially relevant RCTs identified and screened for retrieval (n = 25) RCTs retrieved for more detailed evaluation (n = 24) Potentially appropriate RCTs to be included in the systematic review (n = 19) RCTs included in the systematic review (n = 14) RCTs included in the pooling (n = 11) Figure 1 A keyword search was undertaken in the Scientific Citation Index using the words glaucoma/ocular hypertension, latanoprost/ prostaglandin*, timolol/beta-blocker*/beta blocker*/β-blocker*/β blocker*. We then read the titles and abstracts of retrieved citations to identify possible RCTs. We also wrote to the manufacturers with our final lists to identify other possible RCTs which our searches failed to identify. INCLUSION AND EXCLUSION CRITERIA Only randomised controlled trials directly comparing latanoprost with timolol were included. To facilitate interpretation only studies undertaken in open angle glaucoma (including primary and secondary open angle glaucoma) or ocular hypertension were included. QUALITY ASSESSMENT Quality of studies was assessed based on randomisation, masking, and withdrawal as proposed by Jadad. 7 However, we did not allocate any additional score to an RCT according to whether it described the method of randomisation. In our view, this is a feature of the reporting of the trials and allocation of additional points may be arbitrary. A randomised study was defined as one in which the investigators reported it as being randomised RCT excluded, because of closed angle glaucoma (n = 1) 13 RCTs excluded, because of non-comparison between latanoprost versus timolol (n = 5) RCTs excluded, because of duplication (n = 3), other outcomes (n = 2) RCTs excluded from primary pooling of efficacy data, because of follow up studies after RCTs (n = 3) Flow of the RCTs included in our systematic review. without necessarily defining the randomisation method explicitly since in the past this was not a requirement in the reporting of RCTs. Masking was diverentiated as double blind, single blind and open label. Parallel and crossover designs were also categorised. The percentage of withdrawals was calculated. The impact of all these quality components on our metaanalysis was assessed using sensitivity analysis. DATA EXTRACTION Two of us (WYZ, ALWP) undertook data extraction independently. Any disagreement was resolved by discussion. A customised form was used to record the authors of the study, the year of publication, design of trial (double blind or single blind, parallel or crossover), location of trial, length of study, number of subjects, patient age, sex, type of glaucoma, baseline IOP, and end point IOP. In addition, we recorded the proportion of withdrawals, number of patients reporting local side evects (such as hyperaemia, conjunctivitis, and increased iris pigmentation) and systemic side evects (such as bradycardia, hypotension, and headache). STATISTICAL ANALYSIS We abstracted the mean and standard error of the IOP at baseline and end point from individual studies to calculate the mean IOP reduction (IOPR) and within group standard error (SE IOPR ) using IOPR = IOP baseline IOP endpoint The percentage IOP reduction (IOPR%) and its standard error (SE IOPR% ) was then estimated by IOPR% = IOPR /IOP baseline and SE IOPR% =SE IOPR /IOP baseline. The diverence of the IOP reduction and its standard error between treatment groups was then calculated for each individual study. For estimating the weighted pooled diverence in evect, the method previously described by us was used. 8 The relative risk (RR), risk diverence (RD), and number needed to harm (NNH) were estimated for the adverse evects using intention to treat analysis. Interval estimation of relative risk and risk diverence were as described by Rothman. 9 In the pooling of relative risk and risk diverence, the method described by DerSimonian and Laird 10 was used. The number needed to harm and its 95% confidence intervals (95% CI) were estimated as described by Cook. 11
3 Meta-analysis of RCTs comparing latanoprost with timolol in the treatment of open angle glaucoma or ocular hypertension 985 Table 2 Characteristics of randomised controlled trials comparing latanoprost and timolol End point IOP (mean (SE)) Types of glaucoma Baseline IOP (mean (SE)) POAG OH Others Latanoprost Timolol Latanoprost Timolol Mean age (range, years) Sex (M/F) Withdrawals (%) Timolol (%) Location Length No Trial (ref) Design Latanoprost (%) eve/mor 0.5 bid Scand 6 months (6) 116/ (40 85) (0.5) 24.6 (0.3) 17.0 (0.4) 17.9 (0.3) Alm et al 1995 (27) DB-P, DB-C Aquino et al 1999 (28) DB-P eve 0.5 bid Philippines 3 months 60 / / / / / / Camras et al 1996 (29) DB-P eve 0.5 bid USA 6 months (7) 114/ (30 90) / / Drance et al 1998 (30) DB-C eve 0.5 bid Canada 3 weeks 36 3 (8) 24/ (0.4) 15.3 (0.4) 11.8 (0.3) 12.2 (0.3) 0.5 bid Germany 6 weeks (7) 9/21 62 (40 79) (2.6) 27.2 (1.4) 19.8 (2.5) 22.6 (0.9) /0.005 bid/eve DB-P, DB-C Diestelhorst et al 1997 (31) DB-P eve 0.5 bid Germany 1 month 46 2 (7) 20/26 60 (20 77) (1.2) 24.8 (0.9) 20.3 (0.8) 22.7 (1.1) Diestelhorst et al 1998 (32) DB-P eve 0.5 bid Italy 12 months 36 2 (6) 21/15 46 (35 58) / / Mastropasqua et al 1999 (33) Mishima et al 1996 (34) DB-P mor 0.5 bid Japan 3 months (11) 87/91 57 (22 81) / / / 23.1 (0.2) 23.1 (0.2) 16.8 (0.3) 18.8 (0.3) Nicolela et al 1996 (35) DB-C mor 0.5 bid Canada 1 week 15 0 (0) 7/8 63 (47 80) (1.3) 26.7 (1.3) 19.9 (0.9) 21.4 (0.6) Rulo et al 1994 (36) SB-P bid 0.5 bid Holland 1 week 20 1 (5) 8/12 63 (40 84) (1.8) 24.2 (0.9) / / Watson et al 1996 (37) DB-P eve 0.5 bid UK 6 months (9) 191/ (39 88) (0.3) 26.5 (0.3) 17.1 (0.2) 17.7 (0.2) Total (9) No = number of patients; IOP = intraocular pressure (mm Hg); SE = standard error. DB-P = double blind parallel, SB-P = single blind parallel, DB-C = double blind crossover, DB-P, DB-C = parallel design between latanoprost and timolol, but crossover design between diverent regimens of latanoprost. For example, in Alm s study, 27 patients receiving latanoprost were divided into two groups, one received latanoprost in the morning and placebo in the evening for 3 month and then latanoprost in the evening and placebo in the morning, another started with evening application and then switched after 3 months. In Diestelhorst s report, patients were treated with latanoprost % twice daily or 0.005% once daily for 3 weeks in a crossover design. Ten patients received timolol 0.5% twice daily as control. / = not reported. POAG = primary open angle glaucoma, OH = ocular hypertension, Others = including other types of open angle glaucoma, eg, exfoliation syndrome and pigment dispersion syndrome. Scand = Scandinavia. mor = morning regimen, eve = evening regimen, bid = twice per day. A random evects model was used if trials were heterogeneous on the basis of the Q statistic for heterogeneity 12 and the reason for the heterogeneity could not be identified. In addition, to compare latanoprost and timolol, the study also investigated the evects of the evening regimen, morning regimen, and twice daily regimen of latanoprost in reducing IOP. Results CHARACTERISTICS OF TRIALS Twenty five potential RCTs associated with latanoprost and timolol in the treatment of glaucoma were identified through the literature search Eleven of them met our inclusion criteria The flow of the RCTs included in our analysis is shown in Figure 1. Randomised controlled trials included were undertaken in various countries including the USA, Canada, Japan, the UK, other European nations, and Scandinavia (Table 2). There were eight double blind parallel studies, two double blind crossover studies, and one single blind parallel studies. Five are multicentre RCTs Length of studies varied from 1 week to 1 year. Latanoprost % or 0.006% eye drops were directly compared with timolol 0.5% eye drops in all of the studies. Patients received two identical dropper bottles labelled morning or evening. For patients treated with timolol, both bottles contained timolol, whereas for those treated with latanoprost, one contained the vehicle. A total of 1256 patients were included in the analysis. Withdrawals varied from 0% to 11%. The range of mean ages was from 46 to 67 years. Of the data available on sex, 597 of the patients were male and 593 were female. Of the data available on types of glaucoma, 410 subjects had primary open angle glaucoma (POAG), 465 ocular hypertension (OH), and 137 other types of chronic open angle glaucoma (others). IOP was used as the primary outcome for eycacy in all of the studies included in the metaanalysis. Baseline and end point IOP were summarised in Table 2. EYcacy IOP reduction The percentage reductions in IOP with latanoprost and timolol at various time points are shown in Table 3. Both drugs significantly decreased IOP. Latanoprost showed better IOP lowering evects than timolol with an additional 4 7% reduction. The diverences were all statistically significant except for the result from a single 12 month study (Fig 2). SIDE EFFECTS (1) Short term Latanoprost caused hyperaemia and iris pigmentation in more patients than timolol (Table 4). The risk for hyperaemia was over twice that seen with timolol (RR = 2.20, 95% CI 1.33, 3.65). The number needed to harm was 21 (14, 42) relative to timolol. Treating 21 patients with latanoprost will on average lead to one more patient developing hyperaemia. Moreover, of 478 patients who were treated
4 986 Zhang, Li Wan Po, Dua, et al Table 3 Percentage IOP reduction from baseline with latanoprost and timolol Trials Trial Favours timolol 1 week Diestelhorst et al 1998 n = 46 (32) Nicolela et al 1996 n = 15 (35) Rulo et al 1994 n = 20 (36) 1 month Diestelhorst et al 1998 n = 46 (32) Mishima et al 1996 n = 184 (34) Watson et al 1996 n = 294 (37) 3 months Alm et al 1995 n = 267 (27) Aquino et al 1999 n = 60 (28) Mastropasqua et al 1999 n = 36 (33) Mishima et al 1996 n = 184 (34) Watson et al 1996 n = 294 (37) Percentage IOP reduction (mean (SE)) Latanoprost Timolol DiVerence of the reduction (95% CI) p Value 1 week Diestelhorst et al (6.2) 11.3 (5.4) 8.5 ( 7.6, 24.7) 0.30 Nicolela et al (5.8) 19.8 (5.3) 5.62 ( 9.9, 21.1) 0.48 Rulo et al (2.8) 24.4 (2.9) 6.85 ( 1.0, 14.7) 0.09 Pooled 28.7 (2.3) 21.2 (2.3) 6.9 (0.4, 13.4) 0.04 χ 2 heter month Diestelhorst et al (5.6) 8.5 (2.4) 11.0 ( 0.9, 22.8) 0.07 Mishima et al (1.3) 19.9 (1.1) 5.2 (1.9, 8.4) 0.00 Watson et al (1.5) 34.0 (1.5) 0.4 ( 3.9, 4.6) 0.86 Pooled 27.3 (4.0) 20.9 (6.3) 3.8 (1.2, 6.3) 0.00 χ 2 heter 24.64** 94.22** months Alm et al (2.6) 29.7 (1.7) 4.0 ( 2.1, 10.1) 0.20 Aquino et al (4.0) 31.7 (3.8) 5.41 ( 5.5, 16.3) 0.16 Mastropasqua et al (2.9) 21.7 (2.8) 3.2 ( 4.7, 11.1) 0.42 Mishima et al (1.3) 19.0 (1.1) 7.8 ( 4.4, 11.2) 0.00 Watson et al (1.4) 32.8 (1.5) 1.9 ( 2.2, 6.1) 0.37 Pooled 31.2 (2.3) 26.9 (3.4) 5.0 (2.8, 7.3) 0.00 χ 2 heter 24.01** 65.50** months Alm et al (2.6) 27.2 (1.7) 4.8 ( 1.3, 11.0) 0.12 Camras et al (1.2) 19.4 (1.0) 7.1 (4.0, 10.2) 0.00 Mastropasqua et al (4.3) 20.0 (3.0) 4.5 ( 5.7, 14.8) 0.39 Watson et al (1.4) 33.2 (1.5) 1.5 ( 2.8, 5.6) 0.47 Pooled 29.9 (2.6) 25.0 (3.7) 5.0 (2.8, 7.3) 0.00 χ 2 heter 21.14** 64.61** months Mastropasqua et al (4.6) 19.2 (3.1) 4.9 ( 5.9, 15.8) 0.37 IOP= introcular pressure. Percentage IOP reduction = (baseline IOP end point IOP)/baseline IOP 100%. SE = standard error. χ 2 heter = χ 2 test statistic for heterogeneity. **p<0.001, random evects model were used for pooling. pooled pooled pooled 6 months Alm et al 1995 n = 267 (27) Camras et al 1996 n = 268 (29) Mastropasqua et al 1999 n = 36 (33) Watson et al 1996 n = 294 (37) pooled 12 months Mastropasqua et al 1999 n = 36 (33) Favours latanoprost 6.9% (0.4%, 13.4%), p = 0.04 Q = 0.07 (NS) 3.8% (1.2%, 6.3%), p = Q = 4.57 (NS) 5.0% (2.8%, 7.3%), p = Q = 4.97 (NS) 5.0% (2.8%, 7.3%), p = Q = 4.57 (NS) Difference in IOP reduction Figure 2 DiVerence in percentage IOP reduction from baseline between latanoprost and timolol. Mean diverence and associated 95% confidence interval ( n ). IOP = intraocular pressure; Q = statistic of χ 2 test for heterogeneity; NS = no statistical heterogeneity. Numbers in parentheses are references. with latanoprost, 21 (4.39%) developed iris pigmentation. In contrast, none of the patients treated with timolol showed this evect (0/387). (2) Long term Three studies explored the long term iris darkening evects of latanoprost for up to 2 years after the randomised controlled blinded phases The risks of iris pigmentation are shown in Figure 3. There was an increased incidence of pigmentation with time although in none of the studies did the diverence reach statistical significance at the usual 5% level. The iris pigmentation appears more likely to be with brownish mixed iris colour eyes and this may explain the apparently diverent incidence rates in the diverent countries (Table 5). Four studies compared systemic adverse reactions to timolol versus latanoprost, such as their evects on systolic blood pressure and heart rate. Timolol caused slowing of heart rate after 3 or 6 months of treatment (Table 6), and this returned to the baseline level after switching to latanoprost. 25 EFFECTS OF DIFFERENT REGIMENS OF LATANOPROST The evening regimen was compared with the morning regimen of latanoprost (Table 7). The pooled percentage IOP reductions (mean (SE)) were 33.2 (1.4) and 28.1 (1.1) for the evening regimen and the morning regimen respectively. The pooled diverence was 5.1% (p = 0.006) (Table 7). In addition, the once daily evening regimen of latanoprost was compared with the twice daily regimen (Table 8). Although the evening regimen was marginally better than the twice daily regimen (p=0.08) in one of the trials, pooling with other trials produced numerically
5 Meta-analysis of RCTs comparing latanoprost with timolol in the treatment of open angle glaucoma or ocular hypertension 987 Table 4 Risk of side evects with latanoprost and timolol Side evects No of trials Crude event rate Latanoprost Timolol but not statistically diverent IOP reductions (5.5%, p=0.17) (Table 8). SENSITIVITY ANALYSES Sensitivity analyses were undertaken to evaluate the evect of quality of randomised controlled trials in terms of the study design and withdrawal rate. Trials designed as double blind RR (95% CI) RD (95% CI) NNH (95% CI) Withdrawals due to adverse evects 2 9/277 14/ (0.30, 1.66) 0.02 ( 0.05, 0.16) NA Hyperaemia 6 51/586 20/ (1.33, 3.65)** 0.05 (0.02, 0.07)** 21 (14, 42)** Conjunctivitis 3 7/419 5/ (0.25, 2.53) ( 0.001, 0.02) NA Increased pigmentation 4 21/478 0/ (1.87, 34.30)* 0.03 (0.01, 0.04)** 36 (22, 91)** Hypotension 1 0/149 2/ (0.01, 4.02) 0.01 ( 0.04, 0.01) NA Bradycardia 1 0/87 2/ (0.01, 4.29) 0.02 ( 0.06, 0.02) NA RR = relative risk; RD = risk diverence or attribute risk; NNH = number needed to harm; CI = confidence interval. For trials with event rate of zero, 0.5 was added to each cell of the individual 2 2 table to calculate RR, RD, or NNH. *p<0.05, **p<0.01. % Patients with increased pigmentation % (4/128) 4.9% (12/249) 10.1% (15/149) 18.4% (51/277) 6.6% (12/183) 8.6% (22/255) 0 6 months 12 months 6 months 12 months 6 months 12 months USA UK (Watson et al 1996, 1998) Scandinavia (Camaras et al 1996, 1998) (Alm et al 1995, 2000) Figure 3 Percentage of patients with increased iris pigmentation after use of latanoprost. Error bar shows 95% confidence intervals. Table 5 Iris colour Number of patients with increased pigmentation with long term treatment of latanoprost parallel, double blind crossover, and single blind parallel studies were stratified and the percentage IOP reduction between latanoprost and timolol were compared. There were no statistically significant diverences between the groups. In addition, we divided the studies into two groups according to withdrawal rate (less than 10% and more than 10%). The results showed that the withdrawal rate was not a significant factor (Table 9). Discussion Glaucoma which causes optic nerve damage and visual field loss is the most important cause of irreversible blindness worldwide. About 66.8 millions people have glaucoma, 6.7 million of whom are bilaterally blind. 38 Pharmacological treatments for glaucoma aim to lower IOP and thereby reduce the risk of optic nerve damage. Studies have shown that reduction of IOP prevents development of glaucoma or visual field loss and indeed if the IOP is substantially lowered through treatment, the rate of progression of glaucoma is reduced even among those patients with normal tension glaucoma. 42 Latanoprost is one of the first prostaglandins to be used on a chronic basis in glaucoma patients. Our meta-analysis based on 11 USA, 1 year experience 25 UK, 2 year experience 26 Scandinavia, 2 year experience 24 No Increased pigmentation (%) No Increased pigmentation (%) No Increased pigmentation (%) Blue/grey 21 0 (0) 27 0 (0) 89 0 Blue/grey with slightly brown 36 0 (0) 76 1 (1) 89 1 (1) Blue/grey-brown 34 3 (9) (15) 33 5 (15) Green with slightly brown 4 0 (0) 2 0 (0) 3 0 (0) Green-brown 46 6 (12) (49) (45) Yellow-brown 23 3 (13) 3 2 (67) 2 2 (100) Brown 80 0 (0) 20 2 (10) 7 0 (0) Total (5) (18) (9) Table 6 Trial Mean reduction of systemic blood pressure and heart rate and their 95% confidence intervals after using timolol or latanoprost Timolol Latanoprost Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (beats/min) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (beats/min) Camras et al weeks 2.0 ( 2.3, 6.3) 0.0 ( 2.3, 2.3) 2 (0, 4) 1.0 ( 3.2, 5.2) 0.0 ( 2.6, 2.6) 1 ( 2, 4) 3 months 3.0 ( 1.4, 7.4) 0.0 ( 2.3, 2.3) 4 (2, 6)** 0.0 ( 4.3, 4.3) 0.0 ( 2.4, 2.4) 2 ( 1, 5) 6 months 3.0 ( 1.4, 7.4) 0.0 ( 2.6, 2.6) 4 (2, 6)** 0.0 ( 4.4, 4.4) 0.0 ( 2.6, 2.6) 1 ( 2, 4) Drance et al weeks 5.0 (1.5, 8.5) 2.0 (0.2, 4.2) / 0.6 ( 4.1, 2.9) 0.4 ( 2.4, 1.6) / Nicolela et al week 1.3 ( 12.6, 15.2) 0.2 ( 7.7, 8.1) 0 ( 8, 8) 1.8 ( 11.3, 14.9) 0.3 ( 7.3, 7.9) 5 ( 8, 18) Watson et al months / / 2 ( 1, 5) / / / BP = blood pressure. **p<0.01.
6 988 Zhang, Li Wan Po, Dua, et al Table 7 Percentage IOP reduction from baseline with evening regimen and morning regimen of latanoprost (3 month treatment) Trial Percentage IOP reduction (mean (SE)) Evening Morning DiVerence of IOP reduction p Value Alm et al (1.6) 31.0 (2.0) 4.6 (2.6) 0.08 Alm et al (3.6) / / / Mastropasqua et al (2.9) / / / Mishima et al / 26.8 (1.3) / / Watson et al (1.4) / / / Pooled 33.2 (1.4) 28.1 (1.1) 5.1 (1.8) χ 2 heter 12.00** 3.02 / / IOP = introcular pressure. Percentage IOP reduction = (baseline IOP end point IOP)/baseline IOP 100%. SE = standard error. χ 2 heter = χ 2 test statistic for heterogeneity. **p<0.001, random evects model were used for pooling. Table 8 Percentage IOP reduction from baseline with evening regimen and twice daily (bid) regimen of latanoprost (3 month treatment) Trial Percent IOP reduction (mean (SE)) Evening Twice daily DiVerence of IOP reduction p Value Alm et al (1.7) / / / Alm et al (3.6) 27.7 (3.7) 9.0 (5.2) 0.08 Mastropasqua et al (2.9) / / / Watson et al (1.4) / / / Pooled 33.2 (1.4) 27.7 (3.7) 5.5 (4.0) 0.17 χ 2 heter 12.00** / / / IOP = introcular pressure. Percentage IOP reduction = (baseline IOP end point IOP)/baseline IOP 100%. SE = standard error. χ 2 heter = χ 2 test statistic for heterogeneity. **p<0.001, random evects model were used for pooling. Table 9 Sensitivity analysis to evaluate the evect of quality of randomised controlled trials on IOP reduction between latanoprost and timolol DiVerence in percentage IOP reduction 95% Confidence intervals Design of trial All trials , 7.8 Double blind, parallel , 7.8 Double blind, crossover , 21.1 Single blind, parallel , 14.7 Withdrawals All trials , 7.8 Withdrawals <10% , 7.5 Withdrawals >10% , 11.2 All pooling was undertaken using fixed evect model as no heterogeneity was detected by Q test. published clinical trials shows that 0.005% latanoprost applied topically once daily is superior to 0.5% timolol twice daily in reducing IOP. Latanoprost brings about an additional 5% decrease in IOP (95% CI 3%, 7%), or an average 1.6 mm Hg (p<0.001) further lowering in IOP when compared to timolol. The studies include both company sponsored and non-company sponsored RCTs and were undertaken in various countries including North America (USA and Canada), Asia (Japan and Philippines), and Europe (UK, Germany, Holland, Italy, and Scandinavia). The results are similar to those of a previous meta-analysis of eight company sponsored studies, which demonstrated that latanoprost produced an additional 1.7 mm Hg (p<0.001) reduction in IOP compared with timolol. 43 It has been suggested that the time of IOP measurement is important when comparing latanoprost with timolol. 37 The peak IOP reducing evect of latanoprost is reached 8 12 hours after the drug has been administered, and at this time point timolol is at trough values. 37 Therefore, a single time measurement of IOP in the morning for example, at 9 am, would catch the peak value of latanoprost but not the trough value of timolol when both are administered the previous evening. In order to avoid such bias, we calculated the mean value of IOP measured in the morning, noon, and afternoon for our analysis except for the Japanese trial, which only measured IOP in the morning. In the Japanese trial, although the IOP was measured in the morning at 9 am, latanoprost was comparable with timolol because it was administered in the morning while the second dose of timolol was administered in the evening. The comparison therefore presented trough values for both drugs. Alm et al 27 compared evening and morning administrations of latanoprost and found that the evening application was more evective than the morning application at 3 months but not at 6 months. This may be caused by carryover evect from the crossover design. Konstas et al 44 also failed to establish the superiority of an evening regimen over a morning regimen from his 2 month crossover study. To exclude carryover evect, only the results from the first period before crossover were used to compare the two treatment regimens (Table 7). The results confirm that the once daily evening regimen of 0.005% latanoprost is superior to the once daily morning regimen. Poor compliance is a major problem with most topical agents in the treatment of open angle glaucoma because they usually require at least three times daily administrations. In contrast, latanoprost only needs a once daily administration. Some studies have compared the once daily regimen with the twice daily regimen of this agent and did not find any statistical diverence with the same concentration. 45 Others have reported the superiority of the higher concentration of latanoprost (0.005%) once daily over the lower concentration of latanoprost (0.0015%). Our meta analysis shows no statistically significant diverence in IOP reductions between the two regimens of 0.005% latanoprost twice daily. Thus, once daily application appears adequate for this agent. This is obviously due to the longer action of latanoprost, which may be particularly useful for the elderly. Compliance should be good but comparative data with other treatments are necessary. Unlike β blockers and some other currently used medications such as carbonic anhydrase inhibitors and α 2 agonists, latanoprost acts on outflow rather than formation of aqueous humour Because latanoprost increases uveoscleral outflow, 48 it can theoretically reduce IOP below episcleral venous pressure. This may be advantageous in patients with normal tension glaucoma. In fact, one trial 30 suggested that 0.005% latanoprost produced better lowering of ocular perfusion pressure than 0.5% timolol in normal tension glaucoma. Latanoprost may reduce IOP without reducing systemic blood pressure. In contrast, timolol may reduce both of these. Bradycardia and bronchospasm caused by ophthalmic timolol have been
7 Meta-analysis of RCTs comparing latanoprost with timolol in the treatment of open angle glaucoma or ocular hypertension 989 reported in patients with cardiovascular or pulmonary disorders. Therefore, caution is necessary in the use of timolol in such patients. In contrast, latanoprost does not alter heart rate and blood pressure, and does not avect respiratory function in asthmatic patients. 55 The major adverse reaction to latanoprost is an increase in iris pigmentation. This risk may be as high as 18% within a 2 year period and varies according to the types of iris colour (Table 5). The iris darkening associated with latanoprost may reflect induction of tyrosinase, the rate limiting enzyme in the formation of melanin. 56 Latanoprost does not induce iris melanocyte mitosis. 57 While recent evidence suggests that the problem is purely cosmetic, ongoing surveillance is necessary. UK prices suggest that if we adopt a conservative approach and assume that latanoprost once daily and timolol twice daily are equivalent in evectiveness, latanoprost is three times more expensive than timolol. However, a rigorous economic evaluation considering differences in both eycacy and side evects of these two agents is required. Intercountry diverences in prices will obviously also need to be considered. In summary, latanoprost is superior to timolol for reducing intraocular pressure. Increase in iris pigmentation is still a concern. For patients in whom timolol is contraindicated, latanoprost is in our view a suitable alternative. 1 Coyle D, Drummond M. The economic burden of glaucoma in UK. PharmacoEconomics 1995;7: Diggory P, Cassels-Brown A, Vail A, et al. Avoiding unsuspected respiratory side-evects of topical timolol with cardioselective or sympathomimetic agents. Lancet 1995; 345: Fishman WH, Fuksbrumer MS, Tannenbaum M. Topical ophthalmic beta-adrenergic blockade for the treatment of glaucoma and ocular hypertension. J Clin Pharmacol 1994; 34: McMahon CD, ShaVer RN, Hoskins HD, et al. Adverse evects experienced by patients taken timolol. Am J Ophthalmol 1979;88: CalissendorV B, Maren N, Wetrell K, et al. Timolol versus pilocarpine separately or combined with acetazolamideevects on intraocular pressure. Acta Ophthalmol 1980;58: Zadok D, Geyer O, Zadok J, et al. Combined timolol and pilocarpine alone and timolol alone in the treatment of glaucoma. Am J Ophthalmol 1994;117: Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of randomised clinical trials: is blinding necessary. Control Clin Trials 1996;17: Zhang WY, Li Wan Po A. Analgesic eycacy of paracetamol and of its combination with codeine and caveine in surgical pain a meta-analysis. J Clin Pharm Ther 1996;21: Rothman KJ. Modern epidemiology. Boston: Little, Brown, 1986: DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7: Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment evect. BMJ 1995;320: Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomised clinical trials. Stat Med 1991;10: Aung T, Wong HT, Yip CC, et al. A randomised double-masked study comparing latanoprost with timolol in patients with chronic angle-closure glaucoma. The Association for Reearch in Vision and Ophthalmology (ARVO) meeting. Fort Lauderdale, Florida, USA, May 9 14, Konstas AG, Maltezos A, Gandi S, et al. Comparison of 24-hour intraocular pressure reduction with two dosing regimens of latanoprost and timolol maleate in patients with primary open-angle glaucoma. Am J Ophthalmol 1999;128: Stewart WC, Day DG, Sharp ED, et al. EYcacy and safety of timolol solution once daily vs timolol gel added to latanoprost. Am J Ophthalmol 1999;128: Bucci MG. Intraocular pressure-lowering evects of latanoprost monotherapy versus latanoprost or pilocarpine in comparison with timolol: a randomised, observer-masked multicenter study in patients with open-angle glaucoma. J Glaucoma 1999;8: Racz P, Ruzsonyi MR, Nagy ZT, et al. Around-the-clock intraocular pressure reduction with once-daily application of latanoprost by itself or in combination with timolol. Arch Ophthalmol 1996;114: Alm A, Widengard I, Kjellgren D, et al. Latanoprost administered once daily caused a maintained reduction of intraocular pressure in glaucoma patients treated concomitantly with timolol. Br J Ophthalmol 1995;79: Camras CB, Alm A, Watson P, et al. Latanoprost, a prostaglandin analog, for glaucoma therapy. EYcacy and safety after 1 year of treatment in 198 patients. Ophthalmology 1996;103: Alm A. Comparison phase III clinical trial of latanoprost and timolol in patients with elevated intraocular pressure. Adv Prostaglandin, Theomboxane, & Leukotriene Res 1995; 23: Fristrom B. A 6-month, randomised, double-masked comparison of latanoprost with timolol in patients with open angle glaucoma or ocular hypertension. Acta Ophthalmol Scand 1996;74: Thygesen J, Aaen K, Theodorsen F, et al. Short-term evect of latanoprost and timolol eye drops on tear fluid and the ocular surface in patients with primary open-angle glaucoma and ocular hypertension. Acta Ophthalmol Scand 2000;78: Orzalesi N, Rossetti L, Invernizzi T, et al. A comparison of the evect of timolol, latanoprost, and dorzolaminde on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Association for Research in Vision and Ophthalmology (ARVO) meeting. Fort Lauderdale, Florida, USA, 9 14 May Alm A, Widengard I. Latanoprost: experience of 2-year treatment in Scandinavia. Acta Ophthalmol Scand 2000;78: Camras CB and the US Latanoprost Study Group. Latanoprost treatment for glaucoma: evects of treating for 1 year and of switching from timolol. Am J Ophthalmol 1998;126: Watson PG, Alvarado JA. Latanoprost: two years experience of its use in the United Kingdom. Ophthalmology 1998;105: Alm A, Stjernschantz J, the Scandinavian Latanoprost Study Group. EVects on intraocular pressure and side evects of 0.005% latanoprost applied once daily, evening or morning a comparison with timolol. Ophthalmology 1995; 102: Aquino MV, Lat-Luna M. The evect of latanoprost vs timolol on intraocular pressure in patients with glaucoma and ocular hypertension. Asian J Ophthalmol 1999;1: Camras CB and the US Latanoprost Study Group. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma. A six-month, masked, multicenter trial in the United States. Ophthalmology 1996; 103: Drance SM, Crichton A, Mills RP. Comparison of the evect of latanoprost 0.005% and timolol 0.5% on the calculated ocular perfusion pressure in patients with normal-tension glaucoma. Am J Ophthalmol 1998;125: Diestelhorst M, Roters S, Krieglstein GK. The evect of latanoprost 0.005% once daily versus % twice daily on intraocualr pressure and aqueous humor protein concentration in glaucoma patients. A randomised, doublemasked comparison with timolol 0.5%. Graefes Arch for Clin Exp Ophthalmol 1997;235: Diestelhorst M. Almegard B. Comparison of two fixed combinations of latanoprost and timolol in open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 1998;236: Mastropasqua L, Carpineto P, Ciancaglini A, et al. A 12-month, randomised, double-masked study comparing latanoprost with timolol in pigmentary glaucoma. Ophthalmology 1999;106: Mishima HK, Masuda K, Kitazawa Y, et al. A comparison of latanoprost and timolol in primary open-angle glaucoma and ocular hypertension: a 12-week study. Arch Ophthalmol 1996;114: Nicolela MT, Buckley AR, Walman BE, et al. A comparative study of the evects of timolol and latanoprost on blood flow velocity of the retrobulbar vessels. Am J Ophthalmol 1996;122: Rulo AH, Greve EL, Hoyng PF. Additive evect of latanoprost, a prostaglandin F 2α analogue, and timolol in patients with elevated intraocular pressure. Br J Ophthalmol 1994;78: Watson P, Stjernschantz J, Beck L, et al. A six-month, randomised, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. Ophthalmology 1996;103: Alward WLM. Medical management of glaucoma. N Engl J Med 1998;339: Ontoso IA, Grima FG, Ontoso EA, et al. Does medical treatment of mild intraocular hypertension prevent glaucoma. Eur J Epidemiolol 1997; 13: Kass MA. Timolol treatment prevents or delays glaucomatous visual field loss in individuals with ocular hypertension: a five year randomised double-masked, clinical trial. Trans Am Ophthalmol Soc 1990;87: Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology 1998;105:
8 990 Zhang, Li Wan Po, Dua, et al 42 Collaborative Normal-Tension Glaucoma Study Group. Comparation of glaucomatous between untreated patients with normal-tension glaucoma and patiens with therapeutically reduced intraocular pressure. Am J Ophthalmol 1998;126: Zimmerman T. Latanoprost in glaucoma management. Global Xalatan (latanoprost) Expert Meeting, Hong Kong, 14 December Konstas AG, Maltezos A, Gandi S, et al. Comparison of 24-hour intraocular pressure reduction with two dosing regimens of latanoprost and timolol maleate in patients with primary open-angle glaucoma. Am J Ophthalmol 1999;128: Alm A, Widengard I, Kjellgren D, et al. Latanoprost administered once daily caused a maintained reduction of intraocular pressure in glaucoma patients treated concomitantly with timolol. Br J Ophthalmol 1995;79: Lusky M, Ticho U, Glovinsky J, et al. A comparative study of two dose regemens of latanoprost in patients with elevated intraocular pressure. Ophthalmology 1997;104: Camras CB. Mechanism of the prostaglandin-induced reduction of intraocular pressure in humans. In: Samuelsson B, Paoletti R, Ramwell PW, Vane JR, eds. Adv Prostaglandin Thromboxane Leukot Res, Vol 23. New York: Raven Press 1995: Toris CB, Camras CB, Yablonski ME. EVects of PhXA41, a new prostaglandin F 2α analog on aqueous humor dynamics in human eyes. Ophthalmology 1993;100: Leier CV, Baker ND, Weber PA. Cardiovascular evects of ophthalmic timolol. Ann Intern Med 1986;104: Dinai Y, Sharir M, Naveh NN, et al. Bradycardia induced by interaction between quinidine and ophthalmic timolol. Ann Intern Med 1985;103: Pringle SD, MacEwen CJ. Severe bradycardia due to interaction of timolol eye drops and verapamil. BMJ 1987;294: Avorn J, Glynn RJ, Guiwitz JH. Adverse pulmonary evects of topical beta blockers used in the treatment of glaucoma. J Glaucoma 1993;2: Jones FL Jr, Ekberg NL. Exacerbation of asthma by timolol. N Engl J Med 1979;301: Nelson WL, Fraunfelder FT, Sills JM, et al. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, Am J Ophthalmol 1986; 102: Hedner J, Everts B, Moller CS. Latanoprost and respiratory function in asthmatic patients. Arch Ophthalmol 1999: Lindsey JD, Jones HL, Weinreb RN. Latanoprost increases tyrosinase immunoreactivity in human irirs organ cultures. The Association for Research in Vision and Ophthalmology (ARVO) Meeting. Fort Lauderdale. Florida, USA, 9 14 May Wang N, Lindsey JD, Jones HL, et al. Latanoprost does not induce mitosis in melanocytes within human iris organ cultures. The Association for Research in Vision and Ophthalmology (ARVO) Meeting. Fort Lauderdale, Florida, USA, 9 14 May Br J Ophthalmol: first published as /bjo on 1 August Downloaded from on 24 April 2019 by guest. Protected by copyright.
M Diestelhorst, L-I Larsson,* for The European Latanoprost Fixed Combination Study Group...
 199 SCIENTIFIC REPORT A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension
199 SCIENTIFIC REPORT A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension
Effect of brimonidine on intraocular pressure in normal tension glaucoma: A short term clinical trial
 European Journal of Ophthalmology / Vol. 13 no. 7, 2003 / pp. 611-615 Effect of brimonidine on intraocular pressure in normal tension glaucoma: A short term clinical trial S.A. GANDOLFI, L. CIMINO, P.
European Journal of Ophthalmology / Vol. 13 no. 7, 2003 / pp. 611-615 Effect of brimonidine on intraocular pressure in normal tension glaucoma: A short term clinical trial S.A. GANDOLFI, L. CIMINO, P.
VI.2.2 Summary of treatment benefits
 EU-Risk Management Plan for Bimatoprost V01 aetiology), both OAG and ACG can be secondary conditions. Secondary glaucoma refers to any case in which another disorder (e.g. injury, inflammation, vascular
EU-Risk Management Plan for Bimatoprost V01 aetiology), both OAG and ACG can be secondary conditions. Secondary glaucoma refers to any case in which another disorder (e.g. injury, inflammation, vascular
Efficacy and Safety of Latanoprost Eye Drops for Glaucoma Treatment: A 1-Year Study in Japan
 Efficacy and Safety of Latanoprost Eye Drops for Glaucoma Treatment: A 1-Year Study in Japan Masanobu Suzuki,* Hiromu K. Mishima,* Kanjiro Masuda, Makoto Araie, Yoshiaki Kitazawa and Ikuo Azuma *Department
Efficacy and Safety of Latanoprost Eye Drops for Glaucoma Treatment: A 1-Year Study in Japan Masanobu Suzuki,* Hiromu K. Mishima,* Kanjiro Masuda, Makoto Araie, Yoshiaki Kitazawa and Ikuo Azuma *Department
A Short-term Study of the Additive Effect of Latanoprost 0.005% and Brimonidine 0.2%
 A Short-term Study of the Additive Effect of Latanoprost 0.005% and Brimonidine 0.2% Haydar Erdoğan, İlker Toker, Mustafa Kemal Arıcı, Ahmet Aygen and Ayşen Topalkara Department of Ophthalmology, School
A Short-term Study of the Additive Effect of Latanoprost 0.005% and Brimonidine 0.2% Haydar Erdoğan, İlker Toker, Mustafa Kemal Arıcı, Ahmet Aygen and Ayşen Topalkara Department of Ophthalmology, School
Pooling Data from Similar Randomized Clinical Trials Comparing Latanoprost with Timolol; Medical Results and Statistical Aspects
 Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1235 Pooling Data from Similar Randomized Clinical Trials Comparing Latanoprost with Timolol; Medical Results and Statistical
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1235 Pooling Data from Similar Randomized Clinical Trials Comparing Latanoprost with Timolol; Medical Results and Statistical
Efficacy of latanoprost in management of chronic angle closure glaucoma. Kumar S 1, Malik A 2 Singh M 3, Sood S 4. Abstract
 Original article Efficacy of latanoprost in management of chronic angle closure glaucoma Kumar S 1, Malik A 2 Singh M 3, Sood S 4 1 Associate Professor, 2 Assistant Professor, 4 Professor, Department of
Original article Efficacy of latanoprost in management of chronic angle closure glaucoma Kumar S 1, Malik A 2 Singh M 3, Sood S 4 1 Associate Professor, 2 Assistant Professor, 4 Professor, Department of
Efficacy and Tolerability of Latanoprost 0.005% in Treatment of Primary Open Angle Glaucoma (POAG)
 Original Article Efficacy and Tolerability of Latanoprost.5% in Treatment of Primary Open Angle Glaucoma (POAG) Muhammad Imran Janjua, Saira Bano, Ali Raza Pak J Ophthalmol 217, Vol. 33, No. 3.....................................................................................................
Original Article Efficacy and Tolerability of Latanoprost.5% in Treatment of Primary Open Angle Glaucoma (POAG) Muhammad Imran Janjua, Saira Bano, Ali Raza Pak J Ophthalmol 217, Vol. 33, No. 3.....................................................................................................
Latanoprost 0.005% v/s Timolol Maleate 0.5% Pressure Lowering Effect in Primary Open Angle Glaucoma
 Original Article Latanoprost 0.005% v/s Timolol Maleate 0.5% Pressure Lowering Effect in Primary Open Angle Glaucoma Arshad Ali Lodhi, Khalid Iqbal Talpur, Mahtab Alam Khanzada Pak J Ophthalmol 2008, Vol.
Original Article Latanoprost 0.005% v/s Timolol Maleate 0.5% Pressure Lowering Effect in Primary Open Angle Glaucoma Arshad Ali Lodhi, Khalid Iqbal Talpur, Mahtab Alam Khanzada Pak J Ophthalmol 2008, Vol.
IOP measurements: 8.00 am (trough drug levels) and am (peak drug levels)(2 hours post dose)
 This overview of 2% eye drops was presented by Dr. Ravin N. Das, at Hotel Satya Ashoka, on 19-6-2004 in place of Dr. H. S. Ray due to unforseen circumstances. This dinner meeting was sponsored by Cipla
This overview of 2% eye drops was presented by Dr. Ravin N. Das, at Hotel Satya Ashoka, on 19-6-2004 in place of Dr. H. S. Ray due to unforseen circumstances. This dinner meeting was sponsored by Cipla
Patients with ocular hypertension or
 ... REPORTS... An Economic Analysis of Switching to Latanoprost from a β-blocker or Adding Brimonidine or Latanoprost to a β-blocker in Open-Angle Glaucoma or Ocular Hypertension William C. Stewart, MD;
... REPORTS... An Economic Analysis of Switching to Latanoprost from a β-blocker or Adding Brimonidine or Latanoprost to a β-blocker in Open-Angle Glaucoma or Ocular Hypertension William C. Stewart, MD;
A Comparative Study of Latanoprost (Xalatan) and Isopropyl Unoprostone (Rescula) in Normal and Glaucomatous Monkey Eyes
 A Comparative Study of Latanoprost (Xalatan) and Isopropyl Unoprostone (Rescula) in Normal and Glaucomatous Monkey Eyes Janet B. Serle,* Steven M. Podos,* Yoshiaki Kitazawa, and Rong-Fang Wang* Departments
A Comparative Study of Latanoprost (Xalatan) and Isopropyl Unoprostone (Rescula) in Normal and Glaucomatous Monkey Eyes Janet B. Serle,* Steven M. Podos,* Yoshiaki Kitazawa, and Rong-Fang Wang* Departments
OCULAR PHARMACOLOGY GLAUCOMA. increased intraocular pressure. normally mm Hg. when to Tx no fixed level.
 OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
A. Incorrect! Acetazolamide is a carbonic anhydrase inhibitor given orally or by intravenous injection.
 Pharmacology - Problem Drill 20: Drugs that Treat Glaucoma Question No. 1 of 10 1. is a topical carbonic anhydrase inhibitor. Question #01 (A) Acetazolamide (B) Clonidine (C) Dorzolamide (D) Apraclonidine
Pharmacology - Problem Drill 20: Drugs that Treat Glaucoma Question No. 1 of 10 1. is a topical carbonic anhydrase inhibitor. Question #01 (A) Acetazolamide (B) Clonidine (C) Dorzolamide (D) Apraclonidine
Practical approach to medical management of glaucoma DR. RATHINI LILIAN DAVID
 Practical approach to medical management of glaucoma DR. RATHINI LILIAN DAVID Glaucoma is one of the major causes of visual loss worldwide. The philosophy of glaucoma management is to preserve the visual
Practical approach to medical management of glaucoma DR. RATHINI LILIAN DAVID Glaucoma is one of the major causes of visual loss worldwide. The philosophy of glaucoma management is to preserve the visual
The Systemic Effect Of Topical Timolol On Some Cardiovascular Parameters In Owerri Municipality
 ISPUB.COM The Internet Journal of Third World Medicine Volume 5 Number 1 The Systemic Effect Of Topical Timolol On Some Cardiovascular Parameters In Owerri Municipality G Oze, M Emegwamuo, P Eleanya, H
ISPUB.COM The Internet Journal of Third World Medicine Volume 5 Number 1 The Systemic Effect Of Topical Timolol On Some Cardiovascular Parameters In Owerri Municipality G Oze, M Emegwamuo, P Eleanya, H
Persistency and clinical outcomes associated with latanoprost and beta-blocker monotherapy: Evidence from a European retrospective cohort study
 European Journal of Ophthalmology / Vol. 13 / Suppl. 4, 2003 / pp. S21-S29 Persistency and clinical outcomes associated with latanoprost and beta-blocker monotherapy: Evidence from a European retrospective
European Journal of Ophthalmology / Vol. 13 / Suppl. 4, 2003 / pp. S21-S29 Persistency and clinical outcomes associated with latanoprost and beta-blocker monotherapy: Evidence from a European retrospective
Glaucoma Disease Progression Role of Intra Ocular Pressure. Is Good Enough, Low Enough?
 Glaucoma Disease Progression Role of Intra Ocular Pressure Is Good Enough, Low Enough? Glaucoma Diseases Progression Key Considerations Good number of patients may be diagnosed only after some damage the
Glaucoma Disease Progression Role of Intra Ocular Pressure Is Good Enough, Low Enough? Glaucoma Diseases Progression Key Considerations Good number of patients may be diagnosed only after some damage the
Managing the Patient with POAG
 Managing the Patient with POAG Vision Institute Annual Fall Conference Mitchell W. Dul, OD, MS, FAAO mdul@sunyopt.edu Richard J. Madonna, MA, OD, FAAO rmadonna@sunyopt.edu Ocular Hypertension (OHT) Most
Managing the Patient with POAG Vision Institute Annual Fall Conference Mitchell W. Dul, OD, MS, FAAO mdul@sunyopt.edu Richard J. Madonna, MA, OD, FAAO rmadonna@sunyopt.edu Ocular Hypertension (OHT) Most
Evolution of the Definition of Primary Open-Angle Glaucoma
 OCULAR BLOOD FLOW IN GLAUCOMA MANAGEMENT AHMED HOSSAM ABDALLA PROFESSOR AND HEAD OF OPHTHALMOLOGY DEPARTEMENT ALEXANDRIA UNIVERSITY Evolution of the Definition of Primary Open-Angle Glaucoma Former definition
OCULAR BLOOD FLOW IN GLAUCOMA MANAGEMENT AHMED HOSSAM ABDALLA PROFESSOR AND HEAD OF OPHTHALMOLOGY DEPARTEMENT ALEXANDRIA UNIVERSITY Evolution of the Definition of Primary Open-Angle Glaucoma Former definition
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy
 BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
effect of previous argon laser trabeculoplasty
 Graefe s Arch Clin Exp Ophthalmol (2006) 244: 1073 1076 CLINICAL INVESTIGATION DOI 10.1007/s00417-005-0198-x Esther Arranz-Marquez Miguel A. Teus Effect of previous argon laser trabeculoplasty on the ocular
Graefe s Arch Clin Exp Ophthalmol (2006) 244: 1073 1076 CLINICAL INVESTIGATION DOI 10.1007/s00417-005-0198-x Esther Arranz-Marquez Miguel A. Teus Effect of previous argon laser trabeculoplasty on the ocular
Original Article Intraocular Pressure Lowering Effect Pak Armed Forces Med J 2015; 65(1): 94-8
 Original Article Intraocular Pressure Lowering Effect Pak Armed Forces Med J 2015; 65(1): 94-8 COMPARISON OF INTRAOCULAR PRESSURE LOWERING EFFECT OF LATANOPROST AND TIMOLOL COMBINATION VERSUS LATANOPROST
Original Article Intraocular Pressure Lowering Effect Pak Armed Forces Med J 2015; 65(1): 94-8 COMPARISON OF INTRAOCULAR PRESSURE LOWERING EFFECT OF LATANOPROST AND TIMOLOL COMBINATION VERSUS LATANOPROST
Collaboration in the care of glaucoma patients and glaucoma suspects. Barry Emara MD FRCS(C) Nico Ristorante November 29, 2012
 Collaboration in the care of glaucoma patients and glaucoma suspects Barry Emara MD FRCS(C) Nico Ristorante November 29, 2012 Goals of Collaboration Patient-centred and evidence based approach Timely access
Collaboration in the care of glaucoma patients and glaucoma suspects Barry Emara MD FRCS(C) Nico Ristorante November 29, 2012 Goals of Collaboration Patient-centred and evidence based approach Timely access
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults
 East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
Glaucoma Clinical Update. Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012
 Glaucoma Clinical Update Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Objectives Understand the different categories of glaucoma Recognize the symptoms and signs of open angle and angle-closure
Glaucoma Clinical Update Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Objectives Understand the different categories of glaucoma Recognize the symptoms and signs of open angle and angle-closure
Long Term Care Formulary RS 14. RESTRICTED STATUS Topical Medical Treatment of Glaucoma 1 of 5
 RESTRICTED STATUS Topical Medical Treatment of Glaucoma 1 of 5 PREAMBLE Significance: Glaucoma occurs in 1-2% of white people aged over 40 years, rising to 5% at 70 years and exponentially with advancing
RESTRICTED STATUS Topical Medical Treatment of Glaucoma 1 of 5 PREAMBLE Significance: Glaucoma occurs in 1-2% of white people aged over 40 years, rising to 5% at 70 years and exponentially with advancing
Abbreviated Update: Ophthalmic Glaucoma Agents
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Abbreviated Update: Ophthalmic Glaucoma Agents Month/Year
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Abbreviated Update: Ophthalmic Glaucoma Agents Month/Year
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 28 May 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 May 2008 COSOPT 20 mg/5 mg/ml, eye drops, solution in single dose container Box of 60 0.2 ml single dose containers
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 May 2008 COSOPT 20 mg/5 mg/ml, eye drops, solution in single dose container Box of 60 0.2 ml single dose containers
F Honrubia, 1 J García-Sánchez, 2 V Polo, 1 J M Martínez de la Casa, 2 J Soto 3. Clinical science
 1 Ophthalmology Service, Hospital Miguel Servet, Zaragoza, Spain; 2 Ophthalmology Service, Hospital Clínico, Madrid, Spain; 3 Medical Unit, Pfizer Spain, Madrid, Spain Correspondence to: Dr J Soto, Medical
1 Ophthalmology Service, Hospital Miguel Servet, Zaragoza, Spain; 2 Ophthalmology Service, Hospital Clínico, Madrid, Spain; 3 Medical Unit, Pfizer Spain, Madrid, Spain Correspondence to: Dr J Soto, Medical
53 year old woman attends your practice for routine exam. She has no past medical history or family history of note.
 Case 1 Normal Tension Glaucoma 53 year old woman attends your practice for routine exam. She has no past medical history or family history of note. Table 1. Right Eye Left Eye Visual acuity 6/6 6/6 Ishihara
Case 1 Normal Tension Glaucoma 53 year old woman attends your practice for routine exam. She has no past medical history or family history of note. Table 1. Right Eye Left Eye Visual acuity 6/6 6/6 Ishihara
Class Update with New Drug Evaluation: Glaucoma Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Treatments on the Horizon
 Latanoprostene bunod (Vesneo) Treatments on the Horizon Dominick L Opitz, OD, FAAO Associate Professor Illinois College of Optometry Valeant (B+L) Nitrous oxide-donating prostaglandin F2-alpha analogue
Latanoprostene bunod (Vesneo) Treatments on the Horizon Dominick L Opitz, OD, FAAO Associate Professor Illinois College of Optometry Valeant (B+L) Nitrous oxide-donating prostaglandin F2-alpha analogue
Summary of the risk management plan (RMP) for Izba (travoprost)
 EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
Elements for a Public Summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Epidemiology of the disease Incidence and prevalence Increased pressure in the eye occurs in more than 100 million people, and
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Epidemiology of the disease Incidence and prevalence Increased pressure in the eye occurs in more than 100 million people, and
Meta-analysis of timolol on diurnal and nighttime intraocular pressure and blood pressure
 Eur J Ophthalmol 2010; 20 ( 6) : 1035-1041 Original Article Meta-analysis of timolol on diurnal and nighttime intraocular pressure and blood pressure Princeton Wen-Yuan Lee 1, Aoife Doyle 1, Jeanette A.
Eur J Ophthalmol 2010; 20 ( 6) : 1035-1041 Original Article Meta-analysis of timolol on diurnal and nighttime intraocular pressure and blood pressure Princeton Wen-Yuan Lee 1, Aoife Doyle 1, Jeanette A.
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC)
 Guidelines for the medical treatment of chronic open angle glaucoma and ocular hypertension Summary: DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Diagnosis and management of ocular hypertension (OHT)
Guidelines for the medical treatment of chronic open angle glaucoma and ocular hypertension Summary: DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Diagnosis and management of ocular hypertension (OHT)
Divakar Gupta Glaucoma Fellow, Duke Eye Center 5/14/16
 Divakar Gupta Glaucoma Fellow, Duke Eye Center 5/14/16 Pathophysiology of glaucoma Consider risk factors of glaucoma Understand the side effects of glaucoma medications Diagnostic testing Leading cause
Divakar Gupta Glaucoma Fellow, Duke Eye Center 5/14/16 Pathophysiology of glaucoma Consider risk factors of glaucoma Understand the side effects of glaucoma medications Diagnostic testing Leading cause
Jill Hutzelmann, Susan Owens, Arthur Shedden, Ingrid Adamsons, Enrique Vargas, and the International Clinical Equivalence Study Group
 Br J Ophthalmol 1998;8:149 153 149 Merck Research Laboratories, West Point, PA, USA J Hutzelmann S Owens A Shedden I Adamsons National Institute of Ophthalmology, Lima, Peru E Vargas Correspondence to:
Br J Ophthalmol 1998;8:149 153 149 Merck Research Laboratories, West Point, PA, USA J Hutzelmann S Owens A Shedden I Adamsons National Institute of Ophthalmology, Lima, Peru E Vargas Correspondence to:
Xalatan and Xalatan /betablocker fixed combination : role in glaucoma therapy
 Xalatan and Xalatan /betablocker fixed combination : role in glaucoma therapy Grant McLaren St John Eye Hospital Division of Ophthalmology University of Witwatersrand Johannesburg South Africa 1 Goals
Xalatan and Xalatan /betablocker fixed combination : role in glaucoma therapy Grant McLaren St John Eye Hospital Division of Ophthalmology University of Witwatersrand Johannesburg South Africa 1 Goals
Original Article. Abstract. Introduction. C Olali, G Malietzis, S Ahmed, E Samaila 1, M Gupta
 Original Article Real-life experience study of the safety and efficacy of travoprost 0.004% / timoptol 0.50% fixed combination ophthalmic solution in intraocular pressure control C Olali, G Malietzis,
Original Article Real-life experience study of the safety and efficacy of travoprost 0.004% / timoptol 0.50% fixed combination ophthalmic solution in intraocular pressure control C Olali, G Malietzis,
Glaucoma: target intraocular pressures and current treatments James McAllister FRCS, FRCOphth
 Glaucoma: target intraocular pressures and current treatments James McAllister FRCS, FRCOphth Skyline Imaging Ltd Our Drug review of glaucoma management describes the use of target intraocular pressures
Glaucoma: target intraocular pressures and current treatments James McAllister FRCS, FRCOphth Skyline Imaging Ltd Our Drug review of glaucoma management describes the use of target intraocular pressures
Effect of Latanoprost, Prostaglandin F 2 and Nipradilol on Isolated Bovine Ciliary Muscle
 Effect of Latanoprost, Prostaglandin F 2 and Nipradilol on Isolated Bovine Ciliary Muscle Takeshi Yoshitomi*, Kazutsuna Yamaji*, Hitoshi Ishikawa and Yoshitaka Ohnishi* *Department of Ophthalmology, Wakayama
Effect of Latanoprost, Prostaglandin F 2 and Nipradilol on Isolated Bovine Ciliary Muscle Takeshi Yoshitomi*, Kazutsuna Yamaji*, Hitoshi Ishikawa and Yoshitaka Ohnishi* *Department of Ophthalmology, Wakayama
CLINICAL SCIENCES. Comparison of the Early Effects of Brimonidine and Apraclonidine as Topical Ocular Hypotensive Agents
 Comparison of the Early Effects of Brimonidine and Apraclonidine as Topical Ocular Hypotensive Agents Todd L. Maus, MD; Cherie Nau; Richard F. Brubaker, MD CLINICAL SCIENCES Objective: To compare the mechanism
Comparison of the Early Effects of Brimonidine and Apraclonidine as Topical Ocular Hypotensive Agents Todd L. Maus, MD; Cherie Nau; Richard F. Brubaker, MD CLINICAL SCIENCES Objective: To compare the mechanism
The Uniocular Drug Trial and Second-Eye Response to Glaucoma Medications
 The Uniocular Drug Trial and Second-Eye Response to Glaucoma Medications Tony Realini, MD, 1 Robert D. Fechtner, MD, 2 Sean-Paul Atreides, MD, 3 Stephen Gollance, MD 2 Purpose: To determine if the intraocular
The Uniocular Drug Trial and Second-Eye Response to Glaucoma Medications Tony Realini, MD, 1 Robert D. Fechtner, MD, 2 Sean-Paul Atreides, MD, 3 Stephen Gollance, MD 2 Purpose: To determine if the intraocular
APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS. Each millilitre contains latanoprost 50 µg and benzalkonium chloride 0,02 % m/v as preservative.
 SCHEDULING STATUS: S4 APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS PROPRIETARY NAME (and dosage form): XALATAN Eye Drops COMPOSITION: Each millilitre contains latanoprost 50 µg and benzalkonium chloride
SCHEDULING STATUS: S4 APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS PROPRIETARY NAME (and dosage form): XALATAN Eye Drops COMPOSITION: Each millilitre contains latanoprost 50 µg and benzalkonium chloride
KEY MESSAGES. Details of the evidence supporting these recommendations can be found in the above CPG, available on the following websites:
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study
 Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
Elements for a public summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Glaucoma is an eye disease that can result in damage to the optic nerve and loss of vision (blindness). It is the major cause
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Glaucoma is an eye disease that can result in damage to the optic nerve and loss of vision (blindness). It is the major cause
Cite this article as: BMJ, doi: /bmj e0 (published 1 July 2005)
 Cite this article as: BMJ, doi:10.1136/bmj.38506.594977.e0 (published 1 July 2005) Treatment of ocular and open angle glaucoma: meta-analysis of randomised controlled trials Philip C Maier, Jens Funk,
Cite this article as: BMJ, doi:10.1136/bmj.38506.594977.e0 (published 1 July 2005) Treatment of ocular and open angle glaucoma: meta-analysis of randomised controlled trials Philip C Maier, Jens Funk,
COMPARISON OF IOP LOWERING EFFICACY BRIMONIDINE-TIMOLOL VERSUS DORZOLAMIDE-TIMOLOL FIXED COMBINATION THERAPY
 COMPARISON OF IOP LOWERING EFFICACY BRIMONIDINE-TIMOLOL VERSUS DORZOLAMIDE-TIMOLOL FIXED COMBINATION THERAPY DR. HIDAYATULLA KHAN, M.S. (Ophthalmology) SILVER CRESCENT EYE HOSPITAL Noorkhan Bazar, Hyderabad
COMPARISON OF IOP LOWERING EFFICACY BRIMONIDINE-TIMOLOL VERSUS DORZOLAMIDE-TIMOLOL FIXED COMBINATION THERAPY DR. HIDAYATULLA KHAN, M.S. (Ophthalmology) SILVER CRESCENT EYE HOSPITAL Noorkhan Bazar, Hyderabad
Changes in Trend of Newly Prescribed Anti-Glaucoma Medications in Recent Nine Years in a Japanese Local Community
 The Open Ophthalmology Journal,, 4, 7-7 Open Access Changes in Trend of Newly Prescribed Anti-Glaucoma Medications in Recent Nine Years in a Japanese Local Community Kenji Kashiwagi Department of Community
The Open Ophthalmology Journal,, 4, 7-7 Open Access Changes in Trend of Newly Prescribed Anti-Glaucoma Medications in Recent Nine Years in a Japanese Local Community Kenji Kashiwagi Department of Community
Effect of a Single Drop of Latanoprost on Intraocular Pressure and Blood-Aqueous Barrier Permeability in Patients with Uveitis
 Kobe J. Med. Sci., Vol. 48, No. 6, pp. 153-159, 2002 Effect of a Single Drop of Latanoprost on Intraocular Pressure and Blood-Aqueous Barrier Permeability in Patients with Uveitis YOSHIAKI KIUCHI, KOJI
Kobe J. Med. Sci., Vol. 48, No. 6, pp. 153-159, 2002 Effect of a Single Drop of Latanoprost on Intraocular Pressure and Blood-Aqueous Barrier Permeability in Patients with Uveitis YOSHIAKI KIUCHI, KOJI
Building a Major Ophthalmic Pharmaceutical Company. Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015
 Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Impact of Education on Knowledge Attitude and Practice (KAP) of Glaucoma Patients towards their Disease Management- a Study
 Research Article Impact of Education on Knowledge Attitude and Practice (KAP) of Glaucoma Patients towards their Disease Management- a Study R. Jothi, *Siddhartha Pal, A M. Ismail, R. Senthamarai, C. Rajesh
Research Article Impact of Education on Knowledge Attitude and Practice (KAP) of Glaucoma Patients towards their Disease Management- a Study R. Jothi, *Siddhartha Pal, A M. Ismail, R. Senthamarai, C. Rajesh
Generics are Cheaper and Just as Effective
 Disclosures Generics are Cheaper and Just as Effective Robert L. Stamper, MD UCSF Department of Ophthalmology No current pharmaceutical industry connections Data & Safety Monitor for Cypass - Chair - Transcend
Disclosures Generics are Cheaper and Just as Effective Robert L. Stamper, MD UCSF Department of Ophthalmology No current pharmaceutical industry connections Data & Safety Monitor for Cypass - Chair - Transcend
24-h Efficacy of Glaucoma Treatment Options
 Adv Ther (2016) 33:481 517 DOI 10.1007/s12325-016-0302-0 REVIEW 24-h Efficacy of Glaucoma Treatment Options Anastasios G. P. Konstas. Luciano Quaranta. Banu Bozkurt. Andreas Katsanos. Julian Garcia-Feijoo.
Adv Ther (2016) 33:481 517 DOI 10.1007/s12325-016-0302-0 REVIEW 24-h Efficacy of Glaucoma Treatment Options Anastasios G. P. Konstas. Luciano Quaranta. Banu Bozkurt. Andreas Katsanos. Julian Garcia-Feijoo.
Medical Treatment in Pediatric Glaucoma
 Medical Treatment in Pediatric Glaucoma By Nader Bayoumi, MD Lecturer of Ophthalmology Ophthalmology Department Alexandria University Alexandria, Egypt ESG 2012 Pediatric glaucoma is a surgical disease
Medical Treatment in Pediatric Glaucoma By Nader Bayoumi, MD Lecturer of Ophthalmology Ophthalmology Department Alexandria University Alexandria, Egypt ESG 2012 Pediatric glaucoma is a surgical disease
THE CHRONIC GLAUCOMAS
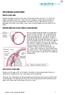 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
Glaucoma therapy may take your breath away
 Age and Ageing 1997; 26: 63-67 REVIEW Glaucoma therapy may take your breath away PAUL DIGGORX WENDY A. FRANKS 1 Department of Elderly Care Medicine, Mayday Hospital, Mayday Road, Croydon CR7 7YE, UK 'Consultant
Age and Ageing 1997; 26: 63-67 REVIEW Glaucoma therapy may take your breath away PAUL DIGGORX WENDY A. FRANKS 1 Department of Elderly Care Medicine, Mayday Hospital, Mayday Road, Croydon CR7 7YE, UK 'Consultant
LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%)
 Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Science & Technologies. UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2
 UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2 1 Clinical Hospital, Shtip 2 University Eye Clinic,Skopje Introduction: Glaucoma is a chronical progressive
UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2 1 Clinical Hospital, Shtip 2 University Eye Clinic,Skopje Introduction: Glaucoma is a chronical progressive
Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review
 REVIEW Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review Andrea Russo Ivano Riva Teodoro Pizzolante Federico Noto Luciano Quaranta Cattedra
REVIEW Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review Andrea Russo Ivano Riva Teodoro Pizzolante Federico Noto Luciano Quaranta Cattedra
Completed Clinical Research Trials Monte Dirks, M.D.
 Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE. Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension 1.1 Short title Glaucoma 2 Background
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension 1.1 Short title Glaucoma 2 Background
Present relevant clinical findings of four landmark glaucoma trials OHTS, EMGT, CNTGS and CIGTS.
 Course title: The Glaucoma Compass Course length: 1 hour +/- 31 slides Corse Description: Even with the technology and available information, glaucoma decision making can still be confusing. How should
Course title: The Glaucoma Compass Course length: 1 hour +/- 31 slides Corse Description: Even with the technology and available information, glaucoma decision making can still be confusing. How should
South East London Area Prescribing Committee Chronic Open Angle Glaucoma and Ocular Hypertension Treatment Pathway
 Proceed to 2 nd line treatment if further reduction in IOP required and there is good response to PGAs or (& no South East London Area Prescribing Committee Chronic Open Angle Glaucoma and Ocular Hypertension
Proceed to 2 nd line treatment if further reduction in IOP required and there is good response to PGAs or (& no South East London Area Prescribing Committee Chronic Open Angle Glaucoma and Ocular Hypertension
ROBERT L. SHIELDS, M.D. GLAUCOMA SURGERY AND TREATMENT
 1 CURRICULUM VITAE ROBERT L. SHIELDS, M.D. GLAUCOMA SURGERY AND TREATMENT University of Colorado Health Eye Center Cherry Creek 2000 South Colorado Boulevard Annex Building, Suite 100 Denver, Colorado
1 CURRICULUM VITAE ROBERT L. SHIELDS, M.D. GLAUCOMA SURGERY AND TREATMENT University of Colorado Health Eye Center Cherry Creek 2000 South Colorado Boulevard Annex Building, Suite 100 Denver, Colorado
Clinical effectiveness and safety of brimonidine (0.2%) versus Dorzolamide (2.0%) in primary open angle
 ISSN: 2231-3354 Received on: 07-10-2011 Revised on: 13:10:2011 Accepted on: 21-10-2011 Clinical effectiveness and safety of brimonidine (0.2%) versus Dorzolamide (2.0%) in primary open angle Sharma Neetu,
ISSN: 2231-3354 Received on: 07-10-2011 Revised on: 13:10:2011 Accepted on: 21-10-2011 Clinical effectiveness and safety of brimonidine (0.2%) versus Dorzolamide (2.0%) in primary open angle Sharma Neetu,
8 USE IN SPECIFIC POPULATIONS Patients with Open-Angle Glaucoma or Ocular Hypertension
 3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Isopto Carpine safely and effectively. See full prescribing information for Isopto Carpine. Isopto
3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Isopto Carpine safely and effectively. See full prescribing information for Isopto Carpine. Isopto
National Institute for Health and Clinical Excellence. Glaucoma. Guideline Consultation Comments Table. 29 September November 2008
 National Institute for Health and Clinical Excellence Glaucoma Guideline Consultation Comments Table 29 September 2008 24 November 2008 Stat us Organisa tion Order no. Version Page no Line no/se ction
National Institute for Health and Clinical Excellence Glaucoma Guideline Consultation Comments Table 29 September 2008 24 November 2008 Stat us Organisa tion Order no. Version Page no Line no/se ction
evaluation of these two combined
 British Journal of Ophthalmology, 1978, 62, 296-31 Atenolol versus adrenaline eye drops and an evaluation of these two combined CALBERT I. PHILLIPS, SHEILA M. GORE,* AND PATRICIA M. GUNN From the Department
British Journal of Ophthalmology, 1978, 62, 296-31 Atenolol versus adrenaline eye drops and an evaluation of these two combined CALBERT I. PHILLIPS, SHEILA M. GORE,* AND PATRICIA M. GUNN From the Department
The retinal renin-angiotensin system: implications for therapy in diabetic retinopathy
 (2002) 16, S42 S46 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh : implications for therapy in diabetic retinopathy AK Sjølie 1 and N Chaturvedi 2 1 Department
(2002) 16, S42 S46 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh : implications for therapy in diabetic retinopathy AK Sjølie 1 and N Chaturvedi 2 1 Department
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people in the United States alone, including 4-10% of the population older than 40 years, have
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people in the United States alone, including 4-10% of the population older than 40 years, have
Glaucoma. Glaucoma. Optic Disc Cupping
 Glaucoma What is Glaucoma? Bruce James A group of diseases in which damage to the optic nerve occurs as a result of intraocualar pressure being above the physiological norm for that eye Stoke Mandeville
Glaucoma What is Glaucoma? Bruce James A group of diseases in which damage to the optic nerve occurs as a result of intraocualar pressure being above the physiological norm for that eye Stoke Mandeville
Abbreviated Class Update: Ophthalmics, Glaucoma agents. Month/Year of Review: August 2012 End date of literature search: June 2012
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
The role of IOP levels in determining the onset and progression
 Effects of Topical Hypotensive Drugs on Circadian IOP, Blood Pressure, and Calculated Diastolic Ocular Perfusion Pressure in Patients with Glaucoma Luciano Quaranta, Federico Gandolfo, Raffaele Turano,
Effects of Topical Hypotensive Drugs on Circadian IOP, Blood Pressure, and Calculated Diastolic Ocular Perfusion Pressure in Patients with Glaucoma Luciano Quaranta, Federico Gandolfo, Raffaele Turano,
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v
![[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v [TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v](/thumbs/94/119684929.jpg) [TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN TRAVOPR-v2-270214 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people
[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN TRAVOPR-v2-270214 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people
L atanoprost, a prostaglandin (PG) F2α related compound, is
 297 CLINICAL SCIENCE Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost K Kashiwagi, S Tsukahara... See end of article for authors affiliations...
297 CLINICAL SCIENCE Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost K Kashiwagi, S Tsukahara... See end of article for authors affiliations...
MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT
 MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT Hussein Hollands, MD, MS (epid) Peter J. Kertes, MD, CM, FRCS(C) 72 The purpose of
MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT Hussein Hollands, MD, MS (epid) Peter J. Kertes, MD, CM, FRCS(C) 72 The purpose of
MODERN DIAGNOSTICS AND TREATMENT OF GLAUCOMA
 Semmelweis University, Ph.D. School, Clinical Sciences, Ophthalmology Head of Program: Ildikó Süveges, M.D., Ph.D., D.Sc. Tutor: Gábor Holló, M.D., Ph.D. MODERN DIAGNOSTICS AND TREATMENT OF GLAUCOMA Ph.D.
Semmelweis University, Ph.D. School, Clinical Sciences, Ophthalmology Head of Program: Ildikó Süveges, M.D., Ph.D., D.Sc. Tutor: Gábor Holló, M.D., Ph.D. MODERN DIAGNOSTICS AND TREATMENT OF GLAUCOMA Ph.D.
BJO Online First, published on September 1, 2009 as /bjo
 BJO Online First, published on September 1, 2009 as 10.1136/bjo.2009.158071 Efficacy and Tolerability of Bimatoprost versus Travoprost in Patients Previously on Latanoprost: a 3-Month, Randomised, Masked-Evaluator,
BJO Online First, published on September 1, 2009 as 10.1136/bjo.2009.158071 Efficacy and Tolerability of Bimatoprost versus Travoprost in Patients Previously on Latanoprost: a 3-Month, Randomised, Masked-Evaluator,
A,kCetazolamide lowers intraocular pressure
 Ocular and systemic effects of acetazolamide in nephrectomized rabbits Zvi Friedman,* Theodore Krupin, and Bernard Becker The effects of acetazolamide on intraocular pressure (IOP) were studied on rabbits
Ocular and systemic effects of acetazolamide in nephrectomized rabbits Zvi Friedman,* Theodore Krupin, and Bernard Becker The effects of acetazolamide on intraocular pressure (IOP) were studied on rabbits
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The term ocular hypertension usually refers to any situation in which the pressure inside the eye, called intraocular pressure,
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The term ocular hypertension usually refers to any situation in which the pressure inside the eye, called intraocular pressure,
Financial Disclosure. Prostaglandin Analogs (PGs) The Glaucoma Grab Bag: Practical Guidelines for Effective Glaucoma Therapy
 The Glaucoma Grab Bag: Practical Guidelines for Effective Glaucoma Therapy Danica J. Marrelli, OD, FAAO University of Houston College of Optometry Financial Disclosure I have received I have received speaking
The Glaucoma Grab Bag: Practical Guidelines for Effective Glaucoma Therapy Danica J. Marrelli, OD, FAAO University of Houston College of Optometry Financial Disclosure I have received I have received speaking
Glaucoma. Glaucoma. Glaucoma. Trevor Arnold, MS, DVM, DACVO
 Glaucoma Trevor Arnold, MS, DVM, DACVO Glaucoma Physiology of Aqueous Humor Produced in the ciliary body Flows out the iridocorneal angle and ciliary cleft High intraocular pressures are caused by a decreased
Glaucoma Trevor Arnold, MS, DVM, DACVO Glaucoma Physiology of Aqueous Humor Produced in the ciliary body Flows out the iridocorneal angle and ciliary cleft High intraocular pressures are caused by a decreased
GLAUCOMA (2006) PHILIPPINE GLAUCOMA SOCIETY
 GLAUCOMA (2006) PHILIPPINE GLAUCOMA SOCIETY CPM 9 TH EDITION Philippine Glaucoma Society (PGS) Correspondence to: Eye Referral Center, T. M. Kalaw Street, Ermita, Manila Telephone: 524-7119/525-9360 Mobile:
GLAUCOMA (2006) PHILIPPINE GLAUCOMA SOCIETY CPM 9 TH EDITION Philippine Glaucoma Society (PGS) Correspondence to: Eye Referral Center, T. M. Kalaw Street, Ermita, Manila Telephone: 524-7119/525-9360 Mobile:
Economic Evaluation of Ophthalmic Solutions for Glaucoma Treatment Including Consideration of Clinical Efficacy
 Regular Article Jpn J Pharm Health Care Sci 40(9) 500 506 (2014) Economic Evaluation of Ophthalmic Solutions for Glaucoma Treatment Including Consideration of Clinical Efficacy Takashi Tomita 1, Hiroaki
Regular Article Jpn J Pharm Health Care Sci 40(9) 500 506 (2014) Economic Evaluation of Ophthalmic Solutions for Glaucoma Treatment Including Consideration of Clinical Efficacy Takashi Tomita 1, Hiroaki
The second most common causes of blindness worldwide. ( after cataract) The commonest cause of irreversible blindness in the world Estimated that 3%
 The second most common causes of blindness worldwide. ( after cataract) The commonest cause of irreversible blindness in the world Estimated that 3% of our population age > 40 have glaucoma In the past:
The second most common causes of blindness worldwide. ( after cataract) The commonest cause of irreversible blindness in the world Estimated that 3% of our population age > 40 have glaucoma In the past:
Saudi Arabia February Pr Michel KOMAJDA. Université Pierre et Marie Curie Hospital Pitié Salpétrière
 Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
Restoring Sensitivity to Timolol After Long-Term Drift in Primary Open-Angle Glaucoma
 Investigative Ophthalmology & Visual Science, Vol., No. 2, February 90 Copyright Association for esearch in Vision and Ophthalmology estoring Sensitivity to Timolol After ong-term Drift in Primary Open-Angle
Investigative Ophthalmology & Visual Science, Vol., No. 2, February 90 Copyright Association for esearch in Vision and Ophthalmology estoring Sensitivity to Timolol After ong-term Drift in Primary Open-Angle
PRACTICAL APPROACH TO MEDICAL MANAGEMENT OF GLAUCOMA
 PRACTICAL APPROACH TO MEDICAL MANAGEMENT OF GLAUCOMA DR. RAVI THOMAS, DR. RAJUL PARIKH, DR. SHEFALI PARIKH IJO MAY 2008 PRESENTER AT JDOS : DR. RAHUL SHUKLA T.N. SHUKLA EYE HOSPITAL TERMINOLOGY POAG: PRIMARY
PRACTICAL APPROACH TO MEDICAL MANAGEMENT OF GLAUCOMA DR. RAVI THOMAS, DR. RAJUL PARIKH, DR. SHEFALI PARIKH IJO MAY 2008 PRESENTER AT JDOS : DR. RAHUL SHUKLA T.N. SHUKLA EYE HOSPITAL TERMINOLOGY POAG: PRIMARY
Regional Institute of Ophthalmology, Indira Gandhi Institute of Medical Sciences, Patna, India 2
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2014;28(5):399-407 http://dx.doi.org/10.3341/kjo.2014.28.5.399 Original Article Comparing the Efficacy of (0.005%), Bimatoprost (0.03%), Travoprost
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2014;28(5):399-407 http://dx.doi.org/10.3341/kjo.2014.28.5.399 Original Article Comparing the Efficacy of (0.005%), Bimatoprost (0.03%), Travoprost
Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.
 Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% 1 Important Information Any discussion of the potential use or
Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% 1 Important Information Any discussion of the potential use or
Retrospective analysis of risk factors for late presentation of chronic glaucoma
 24 Glaxo Department of Ophthalmic Epidemiology, Moorfields Eye Hospital, City Road, London EC1V 2PD S Fraser C Bunce R Wormald Correspondence to: Mr S G Fraser. Accepted for publication 31 July 1998 Retrospective
24 Glaxo Department of Ophthalmic Epidemiology, Moorfields Eye Hospital, City Road, London EC1V 2PD S Fraser C Bunce R Wormald Correspondence to: Mr S G Fraser. Accepted for publication 31 July 1998 Retrospective
9 PM Eye Drops (Latanoprost 0.005%)
 Published on: 10 Jul 2014 9 PM Eye Drops (Latanoprost 0.005%) Composition Each ml contains: Latanoprost... 50 mcg Benzalkonium Chloride, NF... 0.02%w/v (as preservative) aqueous vehicle... q.s. Dosage
Published on: 10 Jul 2014 9 PM Eye Drops (Latanoprost 0.005%) Composition Each ml contains: Latanoprost... 50 mcg Benzalkonium Chloride, NF... 0.02%w/v (as preservative) aqueous vehicle... q.s. Dosage
CHARTING THE NEW COURSE FOR MIGS
 CHARTING THE NEW COURSE FOR MIGS SEE WHAT S ON THE HORIZON CyPass Micro-Stent the next wave in micro-invasive glaucoma surgery. MICRO-INVASIVE GLAUCOMA SURGERY (MIGS) OFFERS A REVOLUTIONARY APPROACH TO
CHARTING THE NEW COURSE FOR MIGS SEE WHAT S ON THE HORIZON CyPass Micro-Stent the next wave in micro-invasive glaucoma surgery. MICRO-INVASIVE GLAUCOMA SURGERY (MIGS) OFFERS A REVOLUTIONARY APPROACH TO
Gonioscopy and 3-Mirror Retinal Evaluation Workshop Edeline Lu, O.D., FAAO Benedicte Gonzalez, O.D., MPH, FAAO Tina Zheng, O.D.
 Gonioscopy and 3-Mirror Retinal Evaluation Workshop Edeline Lu, O.D., FAAO Benedicte Gonzalez, O.D., MPH, FAAO Tina Zheng, O.D., FAAO Please silence all mobile devices and remove items from chairs so others
Gonioscopy and 3-Mirror Retinal Evaluation Workshop Edeline Lu, O.D., FAAO Benedicte Gonzalez, O.D., MPH, FAAO Tina Zheng, O.D., FAAO Please silence all mobile devices and remove items from chairs so others
