1.1. Selective serotonergic vasoconstrictors (triptans) and pain re-activation an update
|
|
|
- Brenda Shields
- 6 years ago
- Views:
Transcription
1 1.1. Selective serotonergic vasoconstrictors (triptans) and pain re-activation an update Introduction Selective serotonergic vasoconstrictors (triptans) are indicated for the acute treatment of with or without aura in adults. The subcutaneous formulation of sumatriptan and the nasal formulation of zolmitriptan are also indicated for acute treatment of cluster headache. Triptans activate the 5HT(1B/1D) receptors and have low affinity for 5-HT(1A), 5-HT(5A) and 5-HT(7) receptors. They exert their effect by stimulating vasoconstriction in the basilar artery and in the blood vessels of the dura mater, this action is presumed to result in the relief of (and cluster headaches) [1]. Among the triptanes, sumatriptan (Imigran ) was granted marketing authorization in the 1991, followed by naratriptan (Naramig ), zolmitriptan (Zomig ), rizatriptan (Maxalt ), almotritptan (Almogran ), eletriptan (Relpax ) and frovatriptan (Fromirex ). The current observation describes the association between triptans and pain re-activation in 19 patients. A previous report regarding this association in 8 patients has been sent to the Medical Evaluation Board in 2002 [2]. Reports Lareb received 19 reports of re-activation of pain associated with the use of triptans, in a period from July 18, 1996 till January 15, The reports are listed in Table 1. Table 1. Reports of pain re-activation associated with the use of triptans Patient, Number, Drug, daily dose Indication for use Concomitant Medication Suspected adverse drug reaction Time to onset, Action with drug Sex, Age, outcome Source *A verapamil pain burning M, temazepam no change *B sumatriptan 100mg psyllium leg pain F, ethinylestradiol/cypro teron withdrawn *C tendon pain 2 hours no change
2 *D 27177, F, 21-30, General Practitioner sumatriptan 50 mg abdominal pain nausea hyperhidrosis 15 minutes, drug withdrawn *E F, sumatriptan 100 mg loratadine fluconazole burning skin 1-2 hours no change *F M, zolmitriptan 1.25 mg joint ache hours withdrawn *G naratriptan 5 mg cramp legs hours F, no change *H flurazepam burning skin M, naproxen no change diazepam salbutamol cimetidine I sumatriptan 50 mg alendronate bone pain 1 hour not changed J F, metropolol atorvastatine pain trauma activated hours discontinued K F, medroxyprogesterone pain trauma activated discontinued Consumer
3 L sumatriptan 50 mg b.i.d. if necessary atenolol 25 mg o.d. cimetidine tibolon postoperative pain periphereal vascular disease 5,5 year not 7 years M M, tamsulosine diclofenac pain trauma activated N F, sumatriptan 50 mg ethinylestradiol/desog estrel leg pain 3 hours withdrawn O F, rizatriptan 10 mg o.d. abdominal pain lower 1 day withdrawn Consumer P pain during injection minutes Q rizatriptan 10 mg zolpidem pain trauma activated 1 hour no change R F, Consumer sumatriptan 50 mg paraesthesia upper limb pain in extremity 1 hour dose reduced not S sumatriptan 75 mg od paracetamol naproxen pain trauma activated 15 minutes withdrawn Consumer
4 * cases were already described in the report in 2002 In the reports below is described in detail to show the relation of symptoms with previous trauma, surgical inventions or inflammatory diseases. Patient A A year-old man regularly uses sumatriptan injections. When he has a superficial wound, it starts to ache smart after use of sumatriptan. At the moment he has many grazes after a fall. Soon after administration of sumatriptan by injection the injuries smarted intensively for several hours. Patient B After intake of sumatriptan, a year-old woman experienced pain in a leg, which was operated for varicosis several years ago. She the same day. The same reaction had occurred in the past after use of sumatriptan. After use of rizatriptan, she experienced no problems. Patient C A year-old woman experienced aggravation of tendinitis related pain, about two hours after injection of sumatriptan). Only the tendons already affected were involved. Patient D A year-old woman experienced, within 15 minutes after oral intake of sumatriptan, abdominal pain, exactly like the pain she felt five years ago during pancreatitis. She within an hour, no lab tests were performed. Patient E: A year-old woman had a partially sun-burned skin on her back. The next day she took sumatriptan and 1-2 hours hereafter she experienced aggravation of pain in the burned parts of the skin, lasting for several hours. Patient F A year-old man had a fissure of his fibula. He used 1.25 mg of zolmitriptan for two days and nine days thereafter. On both occasions he experienced aggravation of the joint-ache. Once the fracture consolidated and the pain
5 decreased, the aggravation of pain after intake of zolmitriptan also diminished. Patient. Patient G A year-old woman experienced severe cramps (during 5 hours) in her recently operated leg after oral intake of 5 mg of naratriptan. A positive re-challenge was seen, up to three times. The patient had no problems after intake of 2.5 mg of naratriptan. Patient. Patient H A year-old man experienced severe burning of the skin, on parts which had previously been exposed too long to the sun. This lasted for several hours. Patient I A year-old woman is known with osteoporosis and rheumatoid arthritis. One hour after intake of sumatriptan for, she experienced stiffness and pain in her bones. Patient. Patient J A year-old female had a knee operation with a bad healing wound in her lower leg. After subcutaneous injection of sumatriptan for, she experienced a violent pain in this wound. The pain lasted for several hours. A positive re-challenge was observed. Patient K A year old woman had a sun burned skin. Soon after subcutaneous injection of sumatriptan she experienced aggravation of pain with a stinging sensation in this sun-burned area, lasting for about two minutes. Re-administration resulted in similar symptoms. Patient L Patient had used sumatriptan for seven years. Two years ago she had a hand operation. Since that moment, she suffers from hand pain. In that year also atenolol had been started, which she had used for two years now. During the last two to three times of sumatriptan use, she noticed painful blue swollen fingers in her operated hand. Outcome is. She will start with zolmitriptan.
6 Patient M A male of years had used sumatriptan injections for nine years. Recently he had a varicose operation of his left leg. After injection of sumatriptan for in his other leg, he experienced pain, stinging and numbness on the varicose operation location. After one hour, the pain decreased. Patient N A year-old woman experienced severe pain in one varicose leg, three hours after intake of sumatriptan for. She had to lie down, because of the leg pain. The leg pain disappeared the following day. Patient O A year-old female with Crohn s disease experienced lower abdominal pain, one day after intake of rizatriptan for. This lasted for two weeks. After a month she took another rizatriptan, resulting in lower abdominal pain in the same location. Years before she had the same experience with the use of ibuprofen. Patient P A female of years experienced pain in her leg, minutes after administration of sumatriptan subcutaneously. In the past, this leg was operated for varicose. Outcome is. Patient Q A year-old woman had severe pain in her formerly broken ankle, one hour after intake of rizatriptan for. The pain lasted for 2 weeks. A year before, she had the same experience of pain activation following rizatriptan intake after a wrist fracture and later also after a wrist operation. Patient R A woman, aged 61-70, had used sumatriptan for seven years. Recently a neuroma of the brachial plexus had been diagnosed. Pressure or touching of the neuroma results in paresthesia, numbness and pain in the arm and hand. One hour after intake of sumatriptan worsening of this paresthesia and pain in this arm occurred. Patient S
7 A year-old woman experienced severe pain at the site of a bone fracture, fifteen minutes after intake of sumatriptan 50 mg, 1.5 tablet, for. Naproxen and paracetamol were insufficient for pain relief, therefore patient was treated with oxycodone. Patient. A week later, a positive re-challenge was observed. Other sources of information SmPC Pain re-activation is not mentioned in the SmPCs of sumatriptan (Imigran ), naratriptan (Naramig ), zolmitriptan (Zomig ), rizatriptan (Maxalt ), almotritptan (Almogran ), eletriptan (Relpax ) and frovatriptan (Fromirex ) [1,3-9]. In the SmPC of naratriptan only pain is mentioned, without further description. In the other SmPCs several sites of pain are included. The most extensive description of pain perception as adverse reaction is to be found in the SmPC of frovatriptan, which includes headache, ear pain, eye pain, pharyngolaryngeal pain, abdominal pain, lip pain, pain in the salivary glands, tooth pain, chest pain, back pain, kidney pain, skeletal pain, arthralgia, pain in extremities and pain in breasts [9]. Literature One publication presents case reports reported to the Pharmacovigilance centres of New Zealand and the Netherlands. Thirteen cases of pain activation and eight cases of aggravation of pain in inflammatory diseases are shown. It is suggested that higher concentrations, as is the case for the subcutaneous formulation, gives a higher rate of pain activation. Pain was mostly severe, but shortlasting, however pain was prolonged in some patients with inflammatory diseases [10]. Furthermore an internet communication from the Belgian Pharmacovigilance Centre can be found. They reported a case of a 19-year-old woman, who experienced in multiple occasions a reactivation of pain in old burns and abrasions shortly after an injection of sumatriptan for [11]. Databases Table 2. Reports of post-traumatic pain, procedural pain and inflammatory pain with triptans in the databases of the Netherlands Pharmacovigilance Centre Lareb, the WHO- and Eudravigilance (EMA) database [12,13]. Database Preferred Terms Number of reports ROR (95% CI) Lareb* Post traumatic pain ( ) Procedural pain 1** WHO Post-traumatic pain ( ) Procedural pain 2** ( ) Inflammatory pain*** Eudravigilance Post traumatic pain ( ) Procedural pain 2**
8 Database Preferred Terms Number of reports ROR (95% CI) Inflammatory pain 1** *13 cases have other preferred terms ** numbers too low to calculate the ROR *** only reports in association with sumatriptan Prescription data Table 3. Number of patients using triptans in the Netherlands between 2009 and 2013 [14]. Drug Sumatriptan 104, , , , ,820 Naratriptan 8,340 8,107 7,849 7,371 6,794 Zolmitriptan 11,677 11,810 11,591 11,163 10,529 Rizatriptan 73,786 78,300 80,616 79,571 76,715 Almotriptan 5,135 5,124 4,916 4,720 4,239 Eletriptan 4,816 5,227 5,635 6,037 6,383 Frovatriptan 8,388 9,723 10,830 11,430 11,345 Total 202, , , , ,790 Mechanism Stimulation of the 5-HT1B receptor leads to vasoconstriction of the cranial arteries. Stimulation of the presynaptic 5-HT1D receptors is primarily on the endings of the primary nociceptive nerve fibers in the peripheral nervous system. This inhibits neurogenic inflammation by inhibiting the release of the neuropeptides CGRP, VIP and substance P. Serotonin is a major component of the inflammatory chemical milieu and contributes to the pain of tissue injury via actions on multiple receptor subtypes. [15]. It is known to be involved with pain sensitizing at inflammatory sites and in pain processing [16]. The binding of triptans with the 5-HT1B and 5-HT1D receptor subtypes may disrupt the physiological balance between serotonin and receptors. This disruption might increase the susceptibility to pain mediated through activation of excitatory receptors such as the vascular 5-HT2B and the neuronal 5-HT7 receptors which have been demonstrated to increase pain [17]. The 5-HT7 receptor occurs in peripheral sensory neurons and it has been demonstrated that sumatriptan displays moderate binding affinity for this receptor [18]. It
9 is suggested that high concentrations of sumatriptan may enhance neurogenic inflammation through activation of the 5-HT7 receptor [10]. Discussion and conclusion Lareb has received 19 reports of pain re-activation in association with triptans. The lower level term pain trauma activated or post procedural pain was only used in a small number (6) of cases. Additional 13 cases were found by word-mining. Initial pain in patients involved inflammations or inflammatory diseases (skin, intestinal tract, bones), injuries (skin, tendons, bones) or pain after surgical procedures. Re-activation of pain following use of triptans developed in almost all cases within hours (minutes to 1 day) after administration, which is consistent with the pharmacological action of these drugs. In nearly all patients, the resolution of pain was within hours (minutes to 1 day) after administration. In one patient with Crohn s disease (O) complaints of abdominal pain lasted for 2 weeks, which is in line with the experienced pain in inflammatory diseases [10]. In eight patients a positive re-challenge was observed. In seven cases pain reactivation occurred after subcutaneous administration of sumatriptan, in all other cases an oral formulation was used. In a single patient (G), a dose relationship was suggested, because no problems were encountered with a lower dose of naritriptan. Surprisingly in one patient (B) no reaction on rizatriptan occurred, although on multiple occasions he had experienced a reaction after administration of oral sumatriptan 100 mg. The association of pain-reactivation in relation to triptans was supported by the WHO database and Eudravigilance database. It should be noted that the lower level terms pain trauma activated (preferred term post-traumatic pain ) and pain inflammation activated (preferred term inflammatory pain ) were added to the WHO adverse reaction terminology (WHOART) only in the early years of this millennium, after the detection of this association by case reports in New Zealand and the Netherlands. No word-mining exercise could be performed in the WHO or Eudravigilance database. Therefore, it is expected that perhaps more cases of pain re-activation might be present in the WHO or Eudravigilance database with different coded terms. The publication of the cases from New Zealand and the Netherlands [10] has not been confirmed by other publications on this association; only an internet communication could be retrieved. Still it is of importance to acknowledge the possible role of triptans in a patient with pain reactivation. Moreover in patients with inflammatory diseases such as rheumatoid arthritis or colitis, it could mimic an exacerbation of the disease. Pain re-activation should be mentioned in the SmPC of triptans References 1. Dutch SmPC Imigran subcutaneous. (version date: , access date: ) 2. Netherlands Pharmacovigilance Centre Lareb. Selective serotonergic vasoconstrictors in suspected association with pain activation. (version date: 2002, access date: ) 3. Dutch SmPC Imigran tablet. (version date: , access date: )
10 4. Dutch SmPC Naramig. (version date: , access date: ) 5. Dutch SmPC Zomig. (version date: , access date: ) 6. Dutch SmPC Maxalt. (version date: , access date: ) 7. Dutch SmPC Almogran. (version date: , access date: ) 8. Dutch SmPC Relpax. (version date: , access date: ) 9. Dutch SmPC Fromirex. (version date: , access date: ) Coulter DM, Passier JL, Clark DW, van Puijenbroek EP. Activation of pain by sumatriptan. Headache 2003;43(9): Sumatriptan en reactivatie van oude letsels. (version date: 2010, access date: ) WHO database Vigimine. (version date: , access date: ) (access restricted). 13. Eudravigilance database. (version date: , access date: ) (access restricted). 14. GIP database - Drug Information System of the Dutch Health Care Insurance Board. (version date: , access date: ) Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3): Durham PL, Russo AF. New insights into the molecular actions of serotonergic anti drugs. Pharmacol.Ther. 2002;94(1-2): Hamel E. The biology of serotonin receptors: focus on pathophysiology and treatment. Can.J Neurol Sci 1999;26 Suppl 3:S2-S6 18. Pierce PA, Xie GX, Peroutka SJ, Levine JD. Dual effect of the serotonin agonist, sumatriptan, on peripheral neurogenic inflammation. Reg Anesth. 1996;21(3): This signal has been raised on July It is possible that in the meantime other information became available. For the latest information, including the official SmPC s, please refer to website of the MEB
ONZETRA XSAIL (sumatriptan) nasal powder
 ONZETRA XSAIL (sumatriptan) nasal powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
ONZETRA XSAIL (sumatriptan) nasal powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
SUMAVEL DOSEPRO (sumatriptan succinate) solution for injection
 SUMAVEL DOSEPRO (sumatriptan succinate) solution for injection Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
SUMAVEL DOSEPRO (sumatriptan succinate) solution for injection Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
1.1. Parenteral medroxyprogesterone and injection site necrosis and -atrophy
 20-4-2012 1.1. Parenteral and injection site necrosis and -atrophy Introduction Medroxyprogesterone is a progestogen which is used as systemic hormonal. Parenteral (Depo-provera, Megestron and Sayana )
20-4-2012 1.1. Parenteral and injection site necrosis and -atrophy Introduction Medroxyprogesterone is a progestogen which is used as systemic hormonal. Parenteral (Depo-provera, Megestron and Sayana )
Zomig. Zomig / Zomig-ZMT (zolmitriptan) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.22 Subject: Zomig Page: 1 of 5 Last Review Date: November 30, 2018 Zomig Description Zomig / Zomig-ZMT
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.22 Subject: Zomig Page: 1 of 5 Last Review Date: November 30, 2018 Zomig Description Zomig / Zomig-ZMT
ADVANCES IN MIGRAINE MANAGEMENT
 ADVANCES IN MIGRAINE MANAGEMENT Joanna Girard Katzman, M.D.MSPH Assistant Professor, Dept. of Neurology Project ECHO, Chronic Pain Program University of New Mexico Outline Migraine throughout the decades
ADVANCES IN MIGRAINE MANAGEMENT Joanna Girard Katzman, M.D.MSPH Assistant Professor, Dept. of Neurology Project ECHO, Chronic Pain Program University of New Mexico Outline Migraine throughout the decades
Medication For Migraine Chart: Table 1: Acute Treatment when the attack begins
 Medication For Migraine Chart: Table 1: Acute Treatment when the attack begins Page a Analgesics (painkillers) Non-steroidal antiinflammatory drugs (NSAIDs) Prescription required Brand Name Formulation
Medication For Migraine Chart: Table 1: Acute Treatment when the attack begins Page a Analgesics (painkillers) Non-steroidal antiinflammatory drugs (NSAIDs) Prescription required Brand Name Formulation
Zomig. Zomig / Zomig-ZMT (zolmitriptan) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.22 Subject: Zomig Page: 1 of 5 Last Review Date: March 16, 2018 Zomig Description Zomig / Zomig-ZMT
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.22 Subject: Zomig Page: 1 of 5 Last Review Date: March 16, 2018 Zomig Description Zomig / Zomig-ZMT
Table 1. Reports of tendinitis in which a HMG-CoA-reductase inhibitor was the suspect drug. Concomitant Medication. naproxen meloxicam
 1.1. HMG-CoA-reductase inhibitors and or Introduction HMG-CoA-reductase inhibitors are widely used for treatment of primary hypercholesterolemia or mixed dyslipidemia and for treatment of homozygote familiar
1.1. HMG-CoA-reductase inhibitors and or Introduction HMG-CoA-reductase inhibitors are widely used for treatment of primary hypercholesterolemia or mixed dyslipidemia and for treatment of homozygote familiar
1.1. Bisphosphonates and stomatitis
 1.1. Bisphosphonates and Introduction Bisphosphonates are used in primary and secondary prevention in the treatment of postmenopausal because of their effect on the bone resorption and are also indicated
1.1. Bisphosphonates and Introduction Bisphosphonates are used in primary and secondary prevention in the treatment of postmenopausal because of their effect on the bone resorption and are also indicated
Overview of reports of adverse drug reactions associated with changes of the package of Thyrax (levothyroxine) from a bottle to a blister
 Overview of reports of adverse reactions associated with changes of the package of from a bottle to a blister Introduction The was granted marketing authorization in the Netherlands on 6 June, 1980. Levothyroxine
Overview of reports of adverse reactions associated with changes of the package of from a bottle to a blister Introduction The was granted marketing authorization in the Netherlands on 6 June, 1980. Levothyroxine
Sumatriptan Tablets, Nasal Spray (Imitrex), Nasal Powder (Onzetra Xsail), sumatriptan and naproxen sodium (Treximet tablets)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 0 Subject: Sumatriptan Page: 1 of 6 Last Review Date: November 30, 2018 Sumatriptan Description Sumatriptan
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 0 Subject: Sumatriptan Page: 1 of 6 Last Review Date: November 30, 2018 Sumatriptan Description Sumatriptan
1.1. Bisphosphonates and depressive reactions
 1.1. Bisphosphonates and depressive reactions Introduction Bisphosphonates are the most commonly prescribed medications for the treatment of [1]. Alendronate (Fosamax ) is indicated for the treatment and
1.1. Bisphosphonates and depressive reactions Introduction Bisphosphonates are the most commonly prescribed medications for the treatment of [1]. Alendronate (Fosamax ) is indicated for the treatment and
Overview of inhaled and nasal corticosteroids and haematoma
 Overview of inhaled and nasal corticosteroids and haematoma Introduction Inhaled glucocorticoids (ICS) are widely used to treat asthma and chronic obstructive pulmonary disease (COPD). Nasal glucocorticoids
Overview of inhaled and nasal corticosteroids and haematoma Introduction Inhaled glucocorticoids (ICS) are widely used to treat asthma and chronic obstructive pulmonary disease (COPD). Nasal glucocorticoids
Maxalt. Maxalt / Maxalt-MLT (rizatriptan) Description. Section: Prescription Drugs Effective Date: April 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Maxalt Page: 1 of 5 Last Review Date: March 18, 2016 Maxalt Description Maxalt / Maxalt-MLT (rizatriptan)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Maxalt Page: 1 of 5 Last Review Date: March 18, 2016 Maxalt Description Maxalt / Maxalt-MLT (rizatriptan)
Disclosures. Triptans for Kids 5/16/13
 5/16/13 Disclosures Triptans for Kids Amy A. Gelfand, MD GelfandA@neuropeds.ucsf.edu Departments of Neurology and Pediatrics UCSF Child Neurology and Headache Center I receive grant funding from: NIH/NINDS
5/16/13 Disclosures Triptans for Kids Amy A. Gelfand, MD GelfandA@neuropeds.ucsf.edu Departments of Neurology and Pediatrics UCSF Child Neurology and Headache Center I receive grant funding from: NIH/NINDS
1.1. Lenalidomide and (aggravation of) psoriasis
 20-4-2012 1.1. Lenalidomide and (aggravation of) psoriasis Introduction Lenalidomide (Revlimid ), was registered on the Dutch market in June 2007 and is indicated for the treatment of multiple myeloma
20-4-2012 1.1. Lenalidomide and (aggravation of) psoriasis Introduction Lenalidomide (Revlimid ), was registered on the Dutch market in June 2007 and is indicated for the treatment of multiple myeloma
Sumatriptan Tablets, Nasal Spray (Imitrex), Nasal Powder (Onzetra Xsail), sumatriptan and naproxen sodium (Treximet tablets)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 05.70.10 Subject: Sumatriptan Page: 1 of 6 Last Review Date: March 16, 2018 Sumatriptan Description Sumatriptan
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 05.70.10 Subject: Sumatriptan Page: 1 of 6 Last Review Date: March 16, 2018 Sumatriptan Description Sumatriptan
Abortive Agents. Available Strengths. Formulary Limits. Tablet: 5mg, 10mg ODT: 5mg, 10 mg 25mg, 50mg, 100mg. 5mg/act, 20mg/act
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Migraine Therapy P&T DATE: 9/12/2017 CLASS: Neurological Disorders REVIEW HISTORY 12/16, 9/15, 2/15, 2/10, 5/07 LOB: MCL
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Migraine Therapy P&T DATE: 9/12/2017 CLASS: Neurological Disorders REVIEW HISTORY 12/16, 9/15, 2/15, 2/10, 5/07 LOB: MCL
MIGRAINE UPDATE. Objectives & Disclosures. Learn techniques used to diagnose headaches. Become familiar with medications used for headache treatment.
 MIGRAINE UPDATE Karen L. Bremer, MD November 16, 2018 Objectives & Disclosures Learn techniques used to diagnose headaches. Become familiar with medications used for headache treatment. Disclosure: I am
MIGRAINE UPDATE Karen L. Bremer, MD November 16, 2018 Objectives & Disclosures Learn techniques used to diagnose headaches. Become familiar with medications used for headache treatment. Disclosure: I am
Migraine. What are the symptoms of a migraine attack?
 Migraine Migraine causes attacks of headaches, often with feeling sick or vomiting. Treatment options include: avoiding possible 'triggers', painkillers, antiinflammatory painkillers, anti-sickness medicines,
Migraine Migraine causes attacks of headaches, often with feeling sick or vomiting. Treatment options include: avoiding possible 'triggers', painkillers, antiinflammatory painkillers, anti-sickness medicines,
1.1. An overview of reports on agomelatine
 1.1. An overview of reports on agomelatine Introduction Agomelatine (Valdoxan ) was registered for the European market on February 19th 2009 and is indicated for treatment of major depressive episodes
1.1. An overview of reports on agomelatine Introduction Agomelatine (Valdoxan ) was registered for the European market on February 19th 2009 and is indicated for treatment of major depressive episodes
Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1].
![Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1]. Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1].](/thumbs/72/66705207.jpg) 1.1. Aripiprazole and aggravated Introduction Aripiprazole is an atypical antipsychotic drug which acts through a combination of partial agonism at dopamine D2 - and serotonin 5-HT1a receptors and antagonism
1.1. Aripiprazole and aggravated Introduction Aripiprazole is an atypical antipsychotic drug which acts through a combination of partial agonism at dopamine D2 - and serotonin 5-HT1a receptors and antagonism
1.1. Angiotensin II Receptor Antagonists and Taste Disorders
 1.1. Angiotensin II Receptor and Taste Disorders Introduction The angiotensin II receptor antagonists (ARBs) and angiotensin II receptor antagonist combination with hydrocholorothiazide available on the
1.1. Angiotensin II Receptor and Taste Disorders Introduction The angiotensin II receptor antagonists (ARBs) and angiotensin II receptor antagonist combination with hydrocholorothiazide available on the
Anti-Migraine Agents
 DRUG POLICY BENEFIT APPLICATION Anti-Migraine Agents Benefit determinations are based on the applicable contract language in effect at the time the services were rendered. Exclusions, limitations or exceptions
DRUG POLICY BENEFIT APPLICATION Anti-Migraine Agents Benefit determinations are based on the applicable contract language in effect at the time the services were rendered. Exclusions, limitations or exceptions
Headache Questionnaire
 Date: All Headache Patients We would appreciate your cooperation in filling out this form. In our evaluation of headache, your history is typically our most valuable tool for diagnosis and subsequent treatment.
Date: All Headache Patients We would appreciate your cooperation in filling out this form. In our evaluation of headache, your history is typically our most valuable tool for diagnosis and subsequent treatment.
HMFP Comprehensive Headache Center Department of Anesthesia, Critical Care and Pain Medicine Beth Israel Deaconess Medical Center Instructor in
 HMFP Comprehensive Headache Center Department of Anesthesia, Critical Care and Pain Medicine Beth Israel Deaconess Medical Center Instructor in Anesthesia and Neurology Harvard Medical School Limited time
HMFP Comprehensive Headache Center Department of Anesthesia, Critical Care and Pain Medicine Beth Israel Deaconess Medical Center Instructor in Anesthesia and Neurology Harvard Medical School Limited time
Migraine Management. Jane Melling Headache nurse Mater Misericordiae Hospital
 Migraine Management Jane Melling Headache nurse Mater Misericordiae Hospital Migraine facts Among the most common disorders of the nervous system 3 rd most prevalent medical disorder on the planet (lancet
Migraine Management Jane Melling Headache nurse Mater Misericordiae Hospital Migraine facts Among the most common disorders of the nervous system 3 rd most prevalent medical disorder on the planet (lancet
Pharmacy Medical Necessity Guidelines: Migraine Medications
 Pharmacy Medical Necessity Guidelines: Effective: September 18, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED)
Pharmacy Medical Necessity Guidelines: Effective: September 18, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED)
1.1. An overview of reports on sitagliptin
 1.1. An overview of reports on Introduction Sitagliptin (Januvia ) was registered for the European marked on March 21 st 27 with the Netherlands as rapporteur. It is indicated as treatment of for patients
1.1. An overview of reports on Introduction Sitagliptin (Januvia ) was registered for the European marked on March 21 st 27 with the Netherlands as rapporteur. It is indicated as treatment of for patients
Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol
 Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol Introduction The European Medicines Agency s Pharmacovigilance Risk Assessment Committee (PRAC)
Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol Introduction The European Medicines Agency s Pharmacovigilance Risk Assessment Committee (PRAC)
1.1. Tamsulosin and depressive reactions
 1.1. and depressive reactions Introduction (Omnic ) is an adrenergic alpha1-receptor (alpha1-ar) blocker. It is registered for the treatment of lower urinary tract symptoms (LUTS) related to benign prostatic
1.1. and depressive reactions Introduction (Omnic ) is an adrenergic alpha1-receptor (alpha1-ar) blocker. It is registered for the treatment of lower urinary tract symptoms (LUTS) related to benign prostatic
Management of headache
 Management of headache TJ Steiner Imperial College London Based on European principles of management of common headache disorders TJ Steiner, K Paemeleire, R Jensen, D Valade, L Savi, MJA Lainez, H-C Diener,
Management of headache TJ Steiner Imperial College London Based on European principles of management of common headache disorders TJ Steiner, K Paemeleire, R Jensen, D Valade, L Savi, MJA Lainez, H-C Diener,
Vestibular Migraine. Information for patients and carers. Department of Neurology and Otolaryngology Aberdeen Royal Infirmary
 Vestibular Migraine Information for patients and carers Department of Neurology and Otolaryngology Aberdeen Royal Infirmary What is vestibular migraine? Migraine is a disabling headache disorder that affects
Vestibular Migraine Information for patients and carers Department of Neurology and Otolaryngology Aberdeen Royal Infirmary What is vestibular migraine? Migraine is a disabling headache disorder that affects
PACKAGE LEAFLET: INFORMATION FOR THE USER. Imigran Nasal Spray 10 mg and 20 mg. Sumatriptan
 PACKAGE LEAFLET: INFORMATION FOR THE USER Imigran Nasal Spray 10 mg and 20 mg Sumatriptan Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER Imigran Nasal Spray 10 mg and 20 mg Sumatriptan Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
Headache A Practical Approach
 Headache A Practical Approach Integrated Pain Symposium December 1, 2017 Alyssa Lettich. MD Neurosciences Institute/Neurosciences Clinical Program Medical Director Headache and Pain Development Teams Disclosures:
Headache A Practical Approach Integrated Pain Symposium December 1, 2017 Alyssa Lettich. MD Neurosciences Institute/Neurosciences Clinical Program Medical Director Headache and Pain Development Teams Disclosures:
Package leaflet: Information for the user. Sumatriptan Amneal 100 mg tablets. sumatriptan
 Package leaflet: Information for the user Sumatriptan Amneal 50 mg tablets Sumatriptan Amneal 100 mg tablets sumatriptan Read all of this leaflet carefully before you start taking this medicine because
Package leaflet: Information for the user Sumatriptan Amneal 50 mg tablets Sumatriptan Amneal 100 mg tablets sumatriptan Read all of this leaflet carefully before you start taking this medicine because
Prevention and Treatment of Migraines CAITLIN BARNES, PHARM.D. CANDIDATE AMBULATORY CARE JOE CAMMILLERI, PHARM.D. NATOHYA MALLORY, PHARM.D.
 Prevention and Treatment of Migraines CAITLIN BARNES, PHARM.D. CANDIDATE AMBULATORY CARE JOE CAMMILLERI, PHARM.D. NATOHYA MALLORY, PHARM.D. Objectives Present patient case Review epidemiology/pathophysiology
Prevention and Treatment of Migraines CAITLIN BARNES, PHARM.D. CANDIDATE AMBULATORY CARE JOE CAMMILLERI, PHARM.D. NATOHYA MALLORY, PHARM.D. Objectives Present patient case Review epidemiology/pathophysiology
2 What you need to know before you take Naramig
 Package Leaflet: Information for the User Naramig 2.5 mg film-coated tablets naratriptan hydrochloride tablets for migraine Read all of this leaflet carefully before you start taking this medicine because
Package Leaflet: Information for the User Naramig 2.5 mg film-coated tablets naratriptan hydrochloride tablets for migraine Read all of this leaflet carefully before you start taking this medicine because
Migraine: Past, Present and Future Edward O Sullivan September 12 th 2015 Dublin 12/09/2015
 1 Migraine: Past, Present and Future Edward O Sullivan September 12 th 2015 Dublin Tour de France 2015: Mark Renshaw Yesterday at the end of the stage 17 I came down with a migraine before the final climb
1 Migraine: Past, Present and Future Edward O Sullivan September 12 th 2015 Dublin Tour de France 2015: Mark Renshaw Yesterday at the end of the stage 17 I came down with a migraine before the final climb
For beclomethasone inhalation, increased sensitivity of the skin for haematomas is already labelled [3;4].
![For beclomethasone inhalation, increased sensitivity of the skin for haematomas is already labelled [3;4]. For beclomethasone inhalation, increased sensitivity of the skin for haematomas is already labelled [3;4].](/thumbs/88/117213243.jpg) Beclomethasone/formoterol and haematoma Introduction Beclomethasone/formoterol (Foster ) is a combination product of a corticosteroid and a long-acting beta2-agonist, indicated for the regular treatment
Beclomethasone/formoterol and haematoma Introduction Beclomethasone/formoterol (Foster ) is a combination product of a corticosteroid and a long-acting beta2-agonist, indicated for the regular treatment
Faculty Disclosure. Karen L. Bremer, MD. Dr. Bremer has listed no financial interest/arrangement that would be considered a conflict of interest.
 Faculty Disclosure Karen L. Bremer, MD Dr. Bremer has listed no financial interest/arrangement that would be considered a conflict of interest. HEADACHE UPDATE Karen L. Bremer, MD November 10, 2017 karen.bremer@creighton.edu
Faculty Disclosure Karen L. Bremer, MD Dr. Bremer has listed no financial interest/arrangement that would be considered a conflict of interest. HEADACHE UPDATE Karen L. Bremer, MD November 10, 2017 karen.bremer@creighton.edu
Triptan Quantity Limit
 *- Florida Healthy Kids Triptan Quantity Limit Override(s) Quantity Limit Approval Duration 1 year Oral Tablets Axert (almotriptan) tablets Relpax (eletriptan) tablets 6 tablets (6.25 mg) 12 tablets (12.5
*- Florida Healthy Kids Triptan Quantity Limit Override(s) Quantity Limit Approval Duration 1 year Oral Tablets Axert (almotriptan) tablets Relpax (eletriptan) tablets 6 tablets (6.25 mg) 12 tablets (12.5
1.1. Sitagliptin and dyspnoea
 1.1. Sitagliptin and Introduction Recently an overview of possible ADRs reported to Lareb in patients using sitagliptin, was presented to the Medicines Evaluation Board (MEB) (Quarterly report 1 st quarter
1.1. Sitagliptin and Introduction Recently an overview of possible ADRs reported to Lareb in patients using sitagliptin, was presented to the Medicines Evaluation Board (MEB) (Quarterly report 1 st quarter
ZOMIG 2.5 mg Tablet ASTRAZENECA
 10-14 ZOMIG 2.5 mg Tablet ASTRAZENECA zolmitriptan TradeMark Presentation Zomig is presented as tablets for oral administration containing 2.5 mg of zolmitriptan. Indication Zomig is indicated for the
10-14 ZOMIG 2.5 mg Tablet ASTRAZENECA zolmitriptan TradeMark Presentation Zomig is presented as tablets for oral administration containing 2.5 mg of zolmitriptan. Indication Zomig is indicated for the
Acute Migraine Treatment in Adults
 PL Detail-Document #301104 This PL Detail-Document gives subscribers additional insight related the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER November 2014 Acute Migraine Treatment
PL Detail-Document #301104 This PL Detail-Document gives subscribers additional insight related the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER November 2014 Acute Migraine Treatment
What is in this leaflet
 Package leaflet: Information for the user 2.5 mg film-coated tablets Naratriptan Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Package leaflet: Information for the user 2.5 mg film-coated tablets Naratriptan Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
M0BCore Safety Profile. Pharmaceutical form(s)/strength: ordispersible tablet SE/H/PSUR/0022/002 Date of FAR:
 M0BCore Safety Profile Active substance: Zolmitriptan Pharmaceutical form(s)/strength: ordispersible tablet P-RMS: SE/H/PSUR/0022/002 Date of FAR: 15.03.2014 4.3 Contraindications Zolmitriptan is contraindicated
M0BCore Safety Profile Active substance: Zolmitriptan Pharmaceutical form(s)/strength: ordispersible tablet P-RMS: SE/H/PSUR/0022/002 Date of FAR: 15.03.2014 4.3 Contraindications Zolmitriptan is contraindicated
Page: 1 of 6. Aimovig (erenumab-aooe) injection, Ajovy (fremanezumab-vfrm) injection, Emgality (galcanezumab-gnim)
 Page: 1 of 6 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Last Review Date: November 30, 2018 Description Aimovig (erenumab-aooe) injection, Ajovy (fremanezumab-vfrm)
Page: 1 of 6 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Last Review Date: November 30, 2018 Description Aimovig (erenumab-aooe) injection, Ajovy (fremanezumab-vfrm)
Drug Therapy Guidelines
 Drug Therapy Guidelines Applicable Medical Benefit Effective: 5/1/18 Pharmacy- Formulary 1 x Next Review: 3/19 Pharmacy- Formulary 2 x Date of Origin: 8/29/06 Triptans: almotriptan, Amerge, Axert, Frova,
Drug Therapy Guidelines Applicable Medical Benefit Effective: 5/1/18 Pharmacy- Formulary 1 x Next Review: 3/19 Pharmacy- Formulary 2 x Date of Origin: 8/29/06 Triptans: almotriptan, Amerge, Axert, Frova,
AUTOIMMUNE DISORDERS IN THE ACUTE SETTING
 AUTOIMMUNE DISORDERS IN THE ACUTE SETTING Diagnosis and Treatment Goals Aimee Borazanci, MD BNI Neuroimmunology Objectives Give an update on the causes for admission, clinical features, and outcomes of
AUTOIMMUNE DISORDERS IN THE ACUTE SETTING Diagnosis and Treatment Goals Aimee Borazanci, MD BNI Neuroimmunology Objectives Give an update on the causes for admission, clinical features, and outcomes of
Sumatriptan Tablets, Nasal Spray (Imitrex), Nasal Powder (Onzetra Xsail), sumatriptan and naproxen sodium (Treximet tablets)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 05.70.10 Subject: Sumatriptan Page: 1 of 5 Last Review Date: December 2, 2016 Sumatriptan Description Sumatriptan
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 05.70.10 Subject: Sumatriptan Page: 1 of 5 Last Review Date: December 2, 2016 Sumatriptan Description Sumatriptan
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
UTILIZATION MANAGEMENT CRITERIA
 SUMATRIPTAN (ALSUMA, IMITREX, SUMAVEL DOSEPRO, ZECUITY ) UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAME: Serotonin 5-HT1 receptor agonists Imitrex (sumatriptan), Alsuma (sumatriptan),
SUMATRIPTAN (ALSUMA, IMITREX, SUMAVEL DOSEPRO, ZECUITY ) UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: BRAND (generic) NAME: Serotonin 5-HT1 receptor agonists Imitrex (sumatriptan), Alsuma (sumatriptan),
Case Presentation. Case Presentation. Case Presentation. Truths about Headaches (2017) Most headaches were muscle-tension headaches
 Agenda Case presentation Migraine Morphology Primary and Premonitory Phase Secondary Headache Aura Headache Primer on Pain Medication Overuse Headache Case Presentation RT is a 25 year old woman with daily
Agenda Case presentation Migraine Morphology Primary and Premonitory Phase Secondary Headache Aura Headache Primer on Pain Medication Overuse Headache Case Presentation RT is a 25 year old woman with daily
ACUTE MIGRAINE: OLD AND NEW DRUGS JOHN ROBROCK MD FORT WILLIAM FAMILY HEALTH TEAM
 ACUTE MIGRAINE: OLD AND NEW DRUGS JOHN ROBROCK MD FORT WILLIAM FAMILY HEALTH TEAM Conflict of Interest Declaration: Nothing to Disclose Presenter: John Robrock, MD Title of Presentation: Acute Migraine:
ACUTE MIGRAINE: OLD AND NEW DRUGS JOHN ROBROCK MD FORT WILLIAM FAMILY HEALTH TEAM Conflict of Interest Declaration: Nothing to Disclose Presenter: John Robrock, MD Title of Presentation: Acute Migraine:
Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab
 Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab Introduction Lareb previously published yearly overviews of reports (most recently in 2017) in consultation
Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab Introduction Lareb previously published yearly overviews of reports (most recently in 2017) in consultation
An overview of reports on lacosamide
 An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
Adult & Pediatric Patients. Stanford Health Care, Division Pain Medicine
 Acute Treatment Strategies in Adult & Pediatric Patients Theresa Mallick Searle, MS, RN BC, ANP BC Disclosures Speakers Bureau: Allergan, Depomed Acute Treatment Strategies in Adult & Pediatric Patients
Acute Treatment Strategies in Adult & Pediatric Patients Theresa Mallick Searle, MS, RN BC, ANP BC Disclosures Speakers Bureau: Allergan, Depomed Acute Treatment Strategies in Adult & Pediatric Patients
Sumatriptan Injection (Imitrex / Alsuma / Sumavel / Zembrace)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.11 Subject: Sumatriptan Injection Page: 1 of 6 Last Review Date: March 16, 2018 Sumatriptan Injection
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.11 Subject: Sumatriptan Injection Page: 1 of 6 Last Review Date: March 16, 2018 Sumatriptan Injection
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Williams CM, Maher CG, Latimer J, et al. Efficacy
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Williams CM, Maher CG, Latimer J, et al. Efficacy
CONSULTATION ADMITTANCE FORM
 CONSULTATION ADMITTANCE FORM Last Name: First Name: Address: City Postal Code: Home Phone: Work Phone: Age: Birth date (dd/mm/yr): Sex: M / F Height Weight Occupation: Alberta Health Care #: PLEASE CHECK
CONSULTATION ADMITTANCE FORM Last Name: First Name: Address: City Postal Code: Home Phone: Work Phone: Age: Birth date (dd/mm/yr): Sex: M / F Height Weight Occupation: Alberta Health Care #: PLEASE CHECK
ACTIVE EDGE CHIROPRACTIC
 ACTIVE EDGE CHIROPRACTIC HEALTH HISTORY QUESTIONNAIRE PERSONAL INFORMATION Name: Female Male Alberta Health Care# Address: City: Province: Postal Code: Telephone: Home: Work: Cell: Email: Occupation: Birth
ACTIVE EDGE CHIROPRACTIC HEALTH HISTORY QUESTIONNAIRE PERSONAL INFORMATION Name: Female Male Alberta Health Care# Address: City: Province: Postal Code: Telephone: Home: Work: Cell: Email: Occupation: Birth
New Zealand Data Sheet
 Arrow - Sumatriptan Sumatriptan Injection 6mg/0.5mL Presentation New Zealand Data Sheet ARROW SUMATRIPTAN Injection 6mg/0.5mL is a clear, colourless to pale yellow solution in a prefilled syringe. Uses
Arrow - Sumatriptan Sumatriptan Injection 6mg/0.5mL Presentation New Zealand Data Sheet ARROW SUMATRIPTAN Injection 6mg/0.5mL is a clear, colourless to pale yellow solution in a prefilled syringe. Uses
Regulatory Status FDA approved indication: Migranal Nasal Spray is indicated for the acute treatment of migraine headaches with or without aura (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: November 30, 2018 Migranal Nasal Spray
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: November 30, 2018 Migranal Nasal Spray
Clinical Learning Days November 10, 2017
 Migraine Clinical Learning Days November 10, 2017 Alyssa Lettich. MD Neurosciences Institute/Neurosciences Clinical Program Medical Director Headache Disclosures: none Learning Objectives: At the conclusion
Migraine Clinical Learning Days November 10, 2017 Alyssa Lettich. MD Neurosciences Institute/Neurosciences Clinical Program Medical Director Headache Disclosures: none Learning Objectives: At the conclusion
MIGRAINE A MYSTERY HEADACHE
 MIGRAINE A MYSTERY HEADACHE The migraine is a chronic neurological disease that is characterized by moderate to severe episodes of headache that is mostly associated with other central nervous system (CNS)
MIGRAINE A MYSTERY HEADACHE The migraine is a chronic neurological disease that is characterized by moderate to severe episodes of headache that is mostly associated with other central nervous system (CNS)
Clinical Policy: Triptans Reference Number: CP.CPA.217 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: Reference Number: CP.CPA.217 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.217 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
A case of a patient with chronic headache. Focus on Migraine. None related to the presentation Grants to conduct clinical trials from: Speaker bureau:
 Chronic Daily Headache Bassel F. Shneker, MD, MBA Associate Professor Vice Chair, OSU Neurology The Ohio State University Wexner Medical Center Financial Disclosures None related to the presentation Grants
Chronic Daily Headache Bassel F. Shneker, MD, MBA Associate Professor Vice Chair, OSU Neurology The Ohio State University Wexner Medical Center Financial Disclosures None related to the presentation Grants
How do we treat migraine? New SIGN Guidelines
 How do we treat migraine? New SIGN Guidelines Managing your migraine Migraine Trust, Edinburgh 2018 Callum Duncan Consultant Neurologist Aberdeen Royal Infirmary Chair SIGN Guideline 155 Premonitory Mood
How do we treat migraine? New SIGN Guidelines Managing your migraine Migraine Trust, Edinburgh 2018 Callum Duncan Consultant Neurologist Aberdeen Royal Infirmary Chair SIGN Guideline 155 Premonitory Mood
Acute Migraine Treatment: What you and your family should know to help you make the best choices with your doctor
 Acute Migraine Treatment: What you and your family should know to help you make the best choices with your doctor TAKE CONTROL OF YOUR MIGRAINES! ABOUT THIS PATIENT GUIDE: Migraine attacks are often debilitating
Acute Migraine Treatment: What you and your family should know to help you make the best choices with your doctor TAKE CONTROL OF YOUR MIGRAINES! ABOUT THIS PATIENT GUIDE: Migraine attacks are often debilitating
NEW ZEALAND DATA SHEET
 Naramig Tablets Naratriptan 2.5mg NEW ZEALAND DATA SHEET Presentation Green film coated, D shaped, biconvex tablets engraved GX CE5 on one face. Each tablet contains 2.5mg of naratriptan as naratriptan
Naramig Tablets Naratriptan 2.5mg NEW ZEALAND DATA SHEET Presentation Green film coated, D shaped, biconvex tablets engraved GX CE5 on one face. Each tablet contains 2.5mg of naratriptan as naratriptan
Home Address. City Postal Code Home Telephone # Business Telephone # Address. Emergency Contact Name, Address, Phone#
 Date Name / / last first middle initial Personal Health # - Male Female Home Address City Postal Code Home Telephone # Business Telephone # Cell # E-Mail Address Best way to contact you: Home # Work #
Date Name / / last first middle initial Personal Health # - Male Female Home Address City Postal Code Home Telephone # Business Telephone # Cell # E-Mail Address Best way to contact you: Home # Work #
ACUTE TREATMENT FOR MIGRAINE. Cristina Tassorelli
 The European Headache School 2012 ACUTE TREATMENT FOR MIGRAINE Cristina Tassorelli Headache Science Centre, IRCCS Neurological Institute C. Mondino Foundation - Pavia University Centre for Adaptive Disorders
The European Headache School 2012 ACUTE TREATMENT FOR MIGRAINE Cristina Tassorelli Headache Science Centre, IRCCS Neurological Institute C. Mondino Foundation - Pavia University Centre for Adaptive Disorders
How varicose veins occur
 Varicose veins are a very common problem, generally appearing as twisting, bulging rope-like cords on the legs, anywhere from groin to ankle. Spider veins are smaller, flatter, red or purple veins closer
Varicose veins are a very common problem, generally appearing as twisting, bulging rope-like cords on the legs, anywhere from groin to ankle. Spider veins are smaller, flatter, red or purple veins closer
A new questionnaire for assessment of adverse events associated with triptans: methods of assessment influence the results. Preliminary results
 J Headache Pain (2004) 5:S112 S116 DOI 10.1007/s10194-004-0123-4 Michele Feleppa Fred D. Sheftell Luciana Ciannella Amedeo D Alessio Giancarlo Apice Nino N. Capobianco Donato M.T. Saracino Walter Di Iorio
J Headache Pain (2004) 5:S112 S116 DOI 10.1007/s10194-004-0123-4 Michele Feleppa Fred D. Sheftell Luciana Ciannella Amedeo D Alessio Giancarlo Apice Nino N. Capobianco Donato M.T. Saracino Walter Di Iorio
Bedside to bench and back Challenging Conventional Wisdom, Migraine
 Bedside to bench and back Challenging Conventional Wisdom, Migraine Lecture handout for Association for Research In Otolaryngology Meeting in Phoenix, Feb 16, 2008 Timothy C. Hain, MD, Chicago IL. Definition
Bedside to bench and back Challenging Conventional Wisdom, Migraine Lecture handout for Association for Research In Otolaryngology Meeting in Phoenix, Feb 16, 2008 Timothy C. Hain, MD, Chicago IL. Definition
Update on Diagnosis and Management of Migraines
 Update on Diagnosis and Management of Migraines Joel J. Heidelbaugh, MD, FAAFP, FACG Clinical Professor Departments of Family Medicine and Urology University of Michigan Learning Objectives To distinguish
Update on Diagnosis and Management of Migraines Joel J. Heidelbaugh, MD, FAAFP, FACG Clinical Professor Departments of Family Medicine and Urology University of Michigan Learning Objectives To distinguish
Lack of effect after substitution of methylphenidate prolonged-release
 Lack of effect after substitution of methylphenidate prolonged-release Introduction Methylphenidate prolonged-release (Concerta, Equasym XL, Medikinet CR, Ritalin LA, Kinecteen ) is indicated for the treatment
Lack of effect after substitution of methylphenidate prolonged-release Introduction Methylphenidate prolonged-release (Concerta, Equasym XL, Medikinet CR, Ritalin LA, Kinecteen ) is indicated for the treatment
Lareb has received 35 reports on all DOACs in association with the occurrence of paraesthesia.
 Direct oral anti-coagulants and paraesthesia Introduction Apixiban (Eliquis ), (Xarelto ), edoxaban (Lixiana ) and (Pradaxa ) belong to the group of direct oral anti-coagulants (DOACs). Apixaban, and edoxaban
Direct oral anti-coagulants and paraesthesia Introduction Apixiban (Eliquis ), (Xarelto ), edoxaban (Lixiana ) and (Pradaxa ) belong to the group of direct oral anti-coagulants (DOACs). Apixaban, and edoxaban
Package leaflet: Information for the user. Zomig 2.5 mg tablets zolmitriptan
 Package leaflet: Information for the user Zomig 2.5 mg tablets zolmitriptan Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep
Package leaflet: Information for the user Zomig 2.5 mg tablets zolmitriptan Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep
It does not take the place of talking to your doctor or pharmacist.
 ZOMIG Zolmitriptan Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about Zomig. It does not contain all the information that is known
ZOMIG Zolmitriptan Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about Zomig. It does not contain all the information that is known
A Review of the Pharmacokinetics, Pharmacodynamics and Effi cacy of Zolmitriptan in the Acute Abortive Treatment of Migraine
 A Review of the Pharmacokinetics, Pharmacodynamics and Effi cacy of Zolmitriptan in the Acute Abortive Treatment of Migraine A.A. Kalanuria 1 and B.L. Peterlin 1,2 REVIEW 1 Drexel University College of
A Review of the Pharmacokinetics, Pharmacodynamics and Effi cacy of Zolmitriptan in the Acute Abortive Treatment of Migraine A.A. Kalanuria 1 and B.L. Peterlin 1,2 REVIEW 1 Drexel University College of
Various Types of Pain Defined
 Various Types of Pain Defined Pain: The International Association for the Study of Pain describes pain as, An unpleasant sensory and emotional experience associated with actual or potential tissue damage,
Various Types of Pain Defined Pain: The International Association for the Study of Pain describes pain as, An unpleasant sensory and emotional experience associated with actual or potential tissue damage,
MEASURE #1: MEDICATION PRESCRIBED FOR ACUTE MIGRAINE ATTACK Headache
 MEASURE #1: MEDICATION PRESCRIBED FOR ACUTE MIGRAINE ATTACK Headache Measure Description Percentage of patients age 12 years and older with a diagnosis of migraine who were prescribed a guideline recommended
MEASURE #1: MEDICATION PRESCRIBED FOR ACUTE MIGRAINE ATTACK Headache Measure Description Percentage of patients age 12 years and older with a diagnosis of migraine who were prescribed a guideline recommended
PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET IMIGRAN MIGRANE RELIEF 50mg film coated tablets Sumatriptan Patient information leaflet In this leaflet: 1. what these tablets do 2. check before you take 3. how to take the
PATIENT INFORMATION LEAFLET IMIGRAN MIGRANE RELIEF 50mg film coated tablets Sumatriptan Patient information leaflet In this leaflet: 1. what these tablets do 2. check before you take 3. how to take the
Carpal Tunnel Release
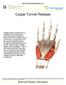 Carpal Tunnel Release Carpal tunnel syndrome is a condition in the hand and wrist caused by excessive pressure on the median nerve in the wrist. Compression of the nerve typically causes numbness and tingling
Carpal Tunnel Release Carpal tunnel syndrome is a condition in the hand and wrist caused by excessive pressure on the median nerve in the wrist. Compression of the nerve typically causes numbness and tingling
Power Line to your Line to your Circuit Line to the station neighborhood house breaker living room. Outlet lamp Lamp with socket, Light bulb
 IMC 606 Neuroscience and Behavior Module Dr. Margaret Paroski Analysis of Sensory Lesions You walk into your living room and turn on the lamp. But no light comes on. What would you do? You would probably
IMC 606 Neuroscience and Behavior Module Dr. Margaret Paroski Analysis of Sensory Lesions You walk into your living room and turn on the lamp. But no light comes on. What would you do? You would probably
Clinical Policy: Triptans Reference Number: CP.HNMC.217 Effective Date: Last Review Date: Line of Business: Medicaid Medi-Cal
 Clinical Policy: Reference Number: CP.HNMC.217 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.HNMC.217 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Name: Date: Sex: Male Female Date of Birth(DD/MM/YY): Address: City: Postal Code: Phone #: (Home) (Work) (Cell) (Other) Address:
 Name: Date: Sex: Male Female Date of Birth(DD/MM/YY): Address: City: Postal Code: Phone #: (Home) (Work) (Cell) (Other) Email Address: Emergency Contact Name and Phone Number: Family Doctor Name and Address:
Name: Date: Sex: Male Female Date of Birth(DD/MM/YY): Address: City: Postal Code: Phone #: (Home) (Work) (Cell) (Other) Email Address: Emergency Contact Name and Phone Number: Family Doctor Name and Address:
Please fill out the following form in as much detail as possible. Please Print. Name. Address. City State Zip. Home Phone Office Phone.
 CASE NO. Please fill out the following form in as much detail as possible. Please Print Date Name Address City State Zip Home Phone Office Phone E-mail Address Age Date of Birth Occupation Sex (M) (F)
CASE NO. Please fill out the following form in as much detail as possible. Please Print Date Name Address City State Zip Home Phone Office Phone E-mail Address Age Date of Birth Occupation Sex (M) (F)
ATORIS 10, 20, 40 mg film-coated tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER ATORIS 10, 20, 40 mg film-coated tablets ATORVASTATIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
PACKAGE LEAFLET: INFORMATION FOR THE USER ATORIS 10, 20, 40 mg film-coated tablets ATORVASTATIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
CHIROPRACTIC NEW PATIENT HEALTH HISTORY
 CHIROPRACTIC NEW PATIENT HEALTH HISTORY Name: Last: First: MI: Address: City: Province: Postal Code: E-mail address: Cell Phone: Home Phone: Age: Birth Date: Alberta Health #: Occupation: Employer: Marital
CHIROPRACTIC NEW PATIENT HEALTH HISTORY Name: Last: First: MI: Address: City: Province: Postal Code: E-mail address: Cell Phone: Home Phone: Age: Birth Date: Alberta Health #: Occupation: Employer: Marital
Page: 1 of 5. Sumatriptan Tablets and Nasal Spray (Imitrex) / sumatriptan and naproxen sodium (Treximet tablets)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 0 Subject: Sumatriptan (Imitrex / Treximet) Page: 1 of 5 Last Review Date: September 12, 2014 Sumatriptan
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 0 Subject: Sumatriptan (Imitrex / Treximet) Page: 1 of 5 Last Review Date: September 12, 2014 Sumatriptan
Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS. Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement
 Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement 2 as 20 Experts published and leaders in their respective field 12 month lead in
Prof Wayne Derman MBChB,BSc (Med)(Hons) PhD, FFIMS Pain Management in the Elite Athlete: The 2017 IOC Consensus Statement 2 as 20 Experts published and leaders in their respective field 12 month lead in
History of Present Condition
 Name: Date: Address: City: Province: Postal Code: Home Phone: Cell Phone: Work Phone: Email: Marital Status: Name Of Family Physician (MD): Age: Occupation: Employer: Extended Health Care Company: Policy
Name: Date: Address: City: Province: Postal Code: Home Phone: Cell Phone: Work Phone: Email: Marital Status: Name Of Family Physician (MD): Age: Occupation: Employer: Extended Health Care Company: Policy
HMG-CoA-reductase inhibitors and nightmares or abnormal dreaming
 HMG-CoA-reductase inhibitors and or abnormal dreaming Introduction HMG-CoA-reductase inhibitors are widely used in primary and secondary prevention of cardiovascular diseases because of their effects on
HMG-CoA-reductase inhibitors and or abnormal dreaming Introduction HMG-CoA-reductase inhibitors are widely used in primary and secondary prevention of cardiovascular diseases because of their effects on
OH, MY ACHING HEAD! I HAVE NO DISCLOSURES OR CONFLICTS OF INTERESTS TO DECLARE MANAGING HEADACHE IN THE OUTPATIENT SETTING SECONDARY HEADACHES
 1 JUSTIN A. OSSMAN, MD CHATTANOOGA FAMILY MEDICINE UPDATE OH, MY ACHING HEAD! MANAGING HEADACHE IN THE OUTPATIENT SETTING 2 I HAVE NO DISCLOSURES OR CONFLICTS OF INTERESTS TO DECLARE OBJECTIVES International
1 JUSTIN A. OSSMAN, MD CHATTANOOGA FAMILY MEDICINE UPDATE OH, MY ACHING HEAD! MANAGING HEADACHE IN THE OUTPATIENT SETTING 2 I HAVE NO DISCLOSURES OR CONFLICTS OF INTERESTS TO DECLARE OBJECTIVES International
Pharmacy Updates Summary
 All of the following changes were reviewed and approved by the SFHP Pharmacy & Therapeutics (P&T) Committee on 4/16/2014 Effective date: 5/15/2014 Therapeutic Classes reviewed: ADHD Ophthalmic antihistamines
All of the following changes were reviewed and approved by the SFHP Pharmacy & Therapeutics (P&T) Committee on 4/16/2014 Effective date: 5/15/2014 Therapeutic Classes reviewed: ADHD Ophthalmic antihistamines
Migranal Nasal Spray. Migranal Nasal Spray (dihydroergotamine) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: June 22, 2017 Migranal Nasal Spray
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.60 Subject: Migranal Nasal Spray Page: 1 of 5 Last Review Date: June 22, 2017 Migranal Nasal Spray
