For beclomethasone inhalation, increased sensitivity of the skin for haematomas is already labelled [3;4].
|
|
|
- Berenice Magdalene McDowell
- 5 years ago
- Views:
Transcription
1 Beclomethasone/formoterol and haematoma Introduction Beclomethasone/formoterol (Foster ) is a combination product of a corticosteroid and a long-acting beta2-agonist, indicated for the regular treatment of asthma where use of a combination product is appropriate or the symptomatic treatment of patients with severe COPD (FEV1 < 50% of predicted normal value). Beclomethasone dipropionate given by inhalation has a glucocorticoid anti-inflammatory action within the lungs, resulting in reduced symptoms and exacerbations of asthma with less adverse effects than when corticosteroids are administered systemically. Formoterol is a selective beta2-adrenergic agonist that produces relaxation of bronchial smooth muscle in patients with reversible airway obstruction. The bronchodilating effect sets in rapidly, within one to three minutes after inhalation, and has a duration of 12 hours after dose administration. Beclomethasone/formoterol was granted market authorisation in the Netherlands in 2007 [1]. Topical and oral glucocorticoids cause thinning of the skin, telangiectasia, and easy bruising. These effects may result from loss of extracellular ground substance within the dermis, due to an inhibitory effect on dermal fibroblasts. There are reports of increased skin bruising and in patients using inhaled corticosteroids. Easy bruising in association with inhaled corticosteroids is more frequent in elderly patients[2]. Elderly patients have more fragile skin and are more sensitive to haematoma. For inhalation, increased sensitivity of the skin for haematomas is already labelled [3;4]. Reports From August 2009 until July 2017 the Netherlands Pharmacovigilance centre Lareb received six reports of haematoma associated with the use of. Furthermore, Lareb received 17 reports of haematoma associated with the use of inhaled alone. These last reports are shown in the appendix. To retrieve these reports from the Lareb database the MedDRA Preferred Terms (PTs) haematoma, ecchymosis,, terms containing haemorrhage, bleeding tendency and contusion were used. Table 1. Reports of associated with haematoma in the Lareb database Patient, Sex, Age Drug Indication for use Concomitant medication Suspected adverse drug reaction Action with drug A pharmacist COPD nifedipine, levothyroxine, tiotropium, theophylline, zopiclon haematoma 10 months (8 hours after administration), dose not, positive rechallenge B , F, General practitioner C , F, 51-60, fluticasonfuroate nasal spray 27,5ug/do, 1 dd 2, Allergic rhinitis inhalation levocetirizine haematoma 5 months, dose not montelukast haematoma 4 weeks, drug D , other health professionnal 100/6ug/do, COPD salbutamol/ipratro pium haematoma, chronic obstructive pulmonary disease exacerbation, drug fatal due to underlying disease
2 Patient, Sex, Age E , F , M, Drug Indication for use Drug use for Concomitant medication Suspected adverse drug reaction ecchymosis haematoma, skin atrophy, haemorrhagic diathesis Action with drug 3, with sequel 2 drug Additional information on the cases is given below: Case A: Past use of the drug also led to a haematoma on the leg. Case B: Large haematomas (10 cm). Suspicion of adverse drug effect of was confirmed by her treating dermatologist. Case D: The reporter mentions that the patient experienced a lot of frequently occurring haematomas. The patient started taking on an unspecified date. In the same period, the patient experienced hematomas on legs, hands and arms. Case E: Haematoma on her lower arms. It took three weeks for the haematomas to resolve and the last two years the haematomas got worse. Case F: Haematomas on her whole body, especially on her arms and legs. Little skin damage results immediately in bleeding. A coagulation test did not show any abnormalities. Other sources of information SmPC The SmPC of does not mention haematoma as adverse drug reaction[1]. However, the SmPC of dipropionate (Qvar ) and the SmPC of dipropionate ERC do mention an increased sensitivity of the skin for haematomas [3;4]. Literature There are several publications that describe the occurrence of haematoma when using inhaled corticosteroids. Malo et al.[5] performed a double-blind crossover study of sixty-nine asthmatic subjects, in which, after a baseline period, they received inhaled or fluticasone (at half the dose of ) for two 4-month periods each. Although the frequency of bruising reported by the questionnaire was not different, there were more bruises on examination for inhaled than for inhaled fluticasone (p=0.04). Tashkin et al [6]. performed a double-blind, randomized, placebo-controlled clinical trial of triamcinolone acetonide versus placebo in participants with mild-to-moderate COPD. Their goal was to define the relationship between skin bruising and inhaled corticosteroid therapy versus placebo in subjects with COPD. Moderate-to-high doses of inhaled corticosteroids resulted in an increased incidence of easy bruising and impairment in skin healing in middle-aged to elderly persons with COPD. A review by Guillot [7] described that thinning of the skin and easy bruising of inhaled corticosteroids are frequent and dose dependent. These adverse effects are probably present in about half of the patients treated with inhaled corticosteroids. The risk of these adverse effects is more important among elderly people and increases with the duration of the treatment and the daily dosage. Gerritsen et al.[8] describe the occurrence of haematoma when using inhaled fluticasone. Databases Table 2. Reports of haematoma associated with the use of in the Lareb, WHO and Eudravigilance database [9;10]. Database Drug ADR Number of reports ROR (95% CI) Lareb haematoma [ ] ecchymosis 1 - WHO* haematoma [ ] ecchymosis 2-1 -
3 Database Drug ADR Number of reports ROR (95% CI) Eudravigilance* haematoma [ ] ecchymosis *WHO and Eudravigilance data consist mainly of Lareb data. Prescription data[11]. Drug ,497 68,360 87, , ,870 Mechanism Glucocorticoids reduce subcutaneous collagen and cause atrophic changes in the skin, resulting in haematoma and paper-thin skinfolds. Purpura has been observed during glucocorticoid treatment and an increased fragility of the capillaries is thought to occur in about 60% of these patients [12]. Numerous physicochemical properties of inhaled corticosteroids including lipophilicity, the rate of dissolution, receptor-binding affinity, and receptor-binding half-life affect systemic availability and potentially systemic adverse effects. Beclomethasone has a high lipophilicity and a long residence time at the glucocorticoid receptor. These factors increase the efficacy of, but also increase the ratio of airway to systemic activity and the potential for systemic adverse effects especially at higher doses [13]. Discussion and conclusion The Netherlands Pharmacovigilance Centre Lareb received six reports of haematoma associated with the use of. These reports concerned five women and one man, with ages varying from 34 to 70 years. The risk of systemic adverse effects of inhaled corticosteroids increases with the duration of treatment which is in accordance with the long latency seen in four of the six reports (five months till three years). In elderly patients, senile can occur, which could be an alternative explanation for the haematomas seen in these patients. Three patients were older than 60 years. The received reports do not describe the degree to which these patients were also receiving intermittent oral glucocorticoids. Furthermore, information about thrombocytes and INR of these patients was not available. The association of and haematoma is supported by a statistically significant disproportionality in the database of Lareb and the WHO. Haematoma have been described for inhaled corticosteroids in literature. For, as well as for other inhaled corticosteroids. For inhaled alone, it is already mentioned in the SmPC. It is important that users of the inhalation are also aware that increased sensitivity of the skin for haematomas can occur. Reference List (1) Dutch SmPC Foster. cbg-meb nl/ib-teksten/h34610 pdf 2016 December 20 [cited 2017 Nov 6]; (2) Saag KG, Furst DE, Barnes PJ. Major side effects of inhaled glucocorticoids. Up to Date 2016 April 1 [cited 2017 Nov 6];Available from: URL: (3) Dutch SmPC Beclometasondipropionaat ERC. cbg-meb nl/ib-teksten/h21940 pdf 2011 May 26 [cited 2017 Nov 6]; (4) Dutch SmPC Qvar. cbg-meb nl/ib-teksten/h21937 pdf 2011 May 26 [cited 2017 Nov 6]; (5) Malo JL, Cartier A, Ghezzo H, Mark S, Brown J, Laviolette M, Boulet LP. Skin bruising, adrenal function and markers of bone metabolism in asthmatics using inhaled and fluticasone. Eur Respir J 1999 May;13(5): (6) Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients: results from Lung Health Study II. Chest 2004 Oct;126(4): (7) Guillot B. Skin reactions to inhaled corticosteroids. Clinical aspects, incidence, avoidance, and management. Am J Clin Dermatol 2000 Mar;1(2): (8) Gerritsen RF, Borgsteede SD, Harmark LVD. Bekend, maar niet altijd herkend. Pharmaceutisch Weekblad 2008;143(36):38-9. (9) Eudravigilance database. eudra org (access restricted) 2016 [cited 2017 Nov 6]; (10) WHO database Vigilyze. who-umc org/#/ 2017 October 28 [cited 2017 Nov 6]; (11) GIPdatabase - Drug Information System of the Dutch Health Care Insurance Board. gipdatabank nl 2016 November 24 [cited 2017 Sep 25]; (12) Corticosteroids - glucocorticoids. In: Aronson JK, editor. Meyler's Side Effects of Drugs. 15 ed p (13) Review of Systemic Adverse Effects Associated with Corticosteroids. micromedexsolutions com 2016 November 16 [cited 2017 Nov 9];
4 Appendix: reports of haematoma associated with the use of inhaled alone ID Sex, age, source Drug, daily dose, F, 71 years and General F, 71 years and M, 61-70, M, 71 years and M, 61-70, F, 71 years and M, 71 years and F, 71 years and F, 51-60, 34711, F, 51-60, General F, 41-50, M, 71 years and F, 71 years and F, 71 years and, salbutamol Bronchitis acute NOS 250ug/do, Beclomethasone inhalation 400ug/do, drug use for Emphysema, 100ug/do, inhalation 100ug, 100ug/do, formoterol inhalation 6ug/do, 50ug/do, Concomitant medication bisacodyl, lormetazepam, oxazepam, sotalol paracetamol, salbutamol, acetylcysteine, acetazolamide, theophylline Suspected adverse drug reactions action with drug, After chronic use, dose not Unknown, dose not salbutamol, perindopril 2 dose not terbutaline, sterculiagom/rhamnus frangula granulate temazepam, salbutamol, acetylcysteine metoprolol, acetylsalicylic acid, kinidine, acetylcysteine, theophylline bruise 10 dose not 12 months, dose not 18 months, dose not terbutaline, salmeterol bruise 10 drug salbutamol Unknown, drug ureum cream, pantoprazole, sumatriptan, estradiol/dydrogesterone fluticasone-propionate, pantoprazole pizotifen, paracetamol/codeine, atenolol ecchymosis Haematoma, skin atrophy After chronic use, drug not Years, dose not 9 dose not 4 dose not salbutamol haematoma Years, dose not not alendronate, calciumdiorotate, losartan Nausea, 1 month, dose not not haematoma Months, dose not not
5 COPD, tiotropium 2,5ug/do M, 5-7, Other health professional F, 71 years and General 100ug/do, 100ug/do, COPD budesonide nasal spray, losartan, omeprazole, domperidone, hydrochlorothiazide, bisacodyl, nifedipine, vaselinecetomacrogol cream haematoma Haematoma, rash 2 weeks,, not 2 days, drug This signal has been raised on January 8, It is possible that in the meantime other information became available. For the latest information, including the official SmPC s, please refer to website of the MEB
Overview of inhaled and nasal corticosteroids and haematoma
 Overview of inhaled and nasal corticosteroids and haematoma Introduction Inhaled glucocorticoids (ICS) are widely used to treat asthma and chronic obstructive pulmonary disease (COPD). Nasal glucocorticoids
Overview of inhaled and nasal corticosteroids and haematoma Introduction Inhaled glucocorticoids (ICS) are widely used to treat asthma and chronic obstructive pulmonary disease (COPD). Nasal glucocorticoids
Inhaled and intranasal fluticasone propionate and haematoma
 Inhaled and intranasal and haematoma Introduction Fluticasone propionate is a locally acting potent corticosteroid and is registered in the Netherlands since November 1990. It is marketed as nasal drops
Inhaled and intranasal and haematoma Introduction Fluticasone propionate is a locally acting potent corticosteroid and is registered in the Netherlands since November 1990. It is marketed as nasal drops
1.1. Angiotensin II Receptor Antagonists and Taste Disorders
 1.1. Angiotensin II Receptor and Taste Disorders Introduction The angiotensin II receptor antagonists (ARBs) and angiotensin II receptor antagonist combination with hydrocholorothiazide available on the
1.1. Angiotensin II Receptor and Taste Disorders Introduction The angiotensin II receptor antagonists (ARBs) and angiotensin II receptor antagonist combination with hydrocholorothiazide available on the
1.1. Bisphosphonates and stomatitis
 1.1. Bisphosphonates and Introduction Bisphosphonates are used in primary and secondary prevention in the treatment of postmenopausal because of their effect on the bone resorption and are also indicated
1.1. Bisphosphonates and Introduction Bisphosphonates are used in primary and secondary prevention in the treatment of postmenopausal because of their effect on the bone resorption and are also indicated
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Table 1. Numbers of reports received by Lareb in the national reporting database for methylphenidate in adults Methylphenidate
 1.1. Overview of reports on methylphenidate in adults Introduction Methylphenidate (Ritalin ) is indicated as part of a comprehensive treatment programme for Attention Deficit Hyperactivity Disorder (ADHD)
1.1. Overview of reports on methylphenidate in adults Introduction Methylphenidate (Ritalin ) is indicated as part of a comprehensive treatment programme for Attention Deficit Hyperactivity Disorder (ADHD)
Fluticasone inhalation and behavioural changes in children
 Fluticasone inhalation and al changes in children Introduction Fluticasone (Flixotide ) is an inhalation corticosteroid available on the Dutch market since 1994. It is registered for the prophylactic treatment
Fluticasone inhalation and al changes in children Introduction Fluticasone (Flixotide ) is an inhalation corticosteroid available on the Dutch market since 1994. It is registered for the prophylactic treatment
1.1. Parenteral medroxyprogesterone and injection site necrosis and -atrophy
 20-4-2012 1.1. Parenteral and injection site necrosis and -atrophy Introduction Medroxyprogesterone is a progestogen which is used as systemic hormonal. Parenteral (Depo-provera, Megestron and Sayana )
20-4-2012 1.1. Parenteral and injection site necrosis and -atrophy Introduction Medroxyprogesterone is a progestogen which is used as systemic hormonal. Parenteral (Depo-provera, Megestron and Sayana )
Medications Affecting The Respiratory System
 Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
1.1. Lenalidomide and (aggravation of) psoriasis
 20-4-2012 1.1. Lenalidomide and (aggravation of) psoriasis Introduction Lenalidomide (Revlimid ), was registered on the Dutch market in June 2007 and is indicated for the treatment of multiple myeloma
20-4-2012 1.1. Lenalidomide and (aggravation of) psoriasis Introduction Lenalidomide (Revlimid ), was registered on the Dutch market in June 2007 and is indicated for the treatment of multiple myeloma
HMG-CoA-reductase inhibitors and nightmares or abnormal dreaming
 HMG-CoA-reductase inhibitors and or abnormal dreaming Introduction HMG-CoA-reductase inhibitors are widely used in primary and secondary prevention of cardiovascular diseases because of their effects on
HMG-CoA-reductase inhibitors and or abnormal dreaming Introduction HMG-CoA-reductase inhibitors are widely used in primary and secondary prevention of cardiovascular diseases because of their effects on
1.1. Sitagliptin and dyspnoea
 1.1. Sitagliptin and Introduction Recently an overview of possible ADRs reported to Lareb in patients using sitagliptin, was presented to the Medicines Evaluation Board (MEB) (Quarterly report 1 st quarter
1.1. Sitagliptin and Introduction Recently an overview of possible ADRs reported to Lareb in patients using sitagliptin, was presented to the Medicines Evaluation Board (MEB) (Quarterly report 1 st quarter
1.1. An overview of reports on sitagliptin
 1.1. An overview of reports on Introduction Sitagliptin (Januvia ) was registered for the European marked on March 21 st 27 with the Netherlands as rapporteur. It is indicated as treatment of for patients
1.1. An overview of reports on Introduction Sitagliptin (Januvia ) was registered for the European marked on March 21 st 27 with the Netherlands as rapporteur. It is indicated as treatment of for patients
Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab
 Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab Introduction Lareb previously published yearly overviews of reports (most recently in 2017) in consultation
Update overview 2018 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab Introduction Lareb previously published yearly overviews of reports (most recently in 2017) in consultation
Overview of reports of adverse drug reactions associated with changes of the package of Thyrax (levothyroxine) from a bottle to a blister
 Overview of reports of adverse reactions associated with changes of the package of from a bottle to a blister Introduction The was granted marketing authorization in the Netherlands on 6 June, 1980. Levothyroxine
Overview of reports of adverse reactions associated with changes of the package of from a bottle to a blister Introduction The was granted marketing authorization in the Netherlands on 6 June, 1980. Levothyroxine
Table 1. Reports of tendinitis in which a HMG-CoA-reductase inhibitor was the suspect drug. Concomitant Medication. naproxen meloxicam
 1.1. HMG-CoA-reductase inhibitors and or Introduction HMG-CoA-reductase inhibitors are widely used for treatment of primary hypercholesterolemia or mixed dyslipidemia and for treatment of homozygote familiar
1.1. HMG-CoA-reductase inhibitors and or Introduction HMG-CoA-reductase inhibitors are widely used for treatment of primary hypercholesterolemia or mixed dyslipidemia and for treatment of homozygote familiar
BUDESONIDE AND FORMOTEROL (SYMBICORT ): Α A REVIEW
 Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Dual-Controller Asthma Therapy: Rationale and Clinical Benefits
 B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
1.1. Tamsulosin and depressive reactions
 1.1. and depressive reactions Introduction (Omnic ) is an adrenergic alpha1-receptor (alpha1-ar) blocker. It is registered for the treatment of lower urinary tract symptoms (LUTS) related to benign prostatic
1.1. and depressive reactions Introduction (Omnic ) is an adrenergic alpha1-receptor (alpha1-ar) blocker. It is registered for the treatment of lower urinary tract symptoms (LUTS) related to benign prostatic
ASTHMA PROTOCOL CELLO
 ASTHMA PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives, non medical and medical therapy and a scheme for inhaler dosage.
ASTHMA PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives, non medical and medical therapy and a scheme for inhaler dosage.
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN VI.1 Summary of risk management plan for RELVAR ELLIPTA (fluticasone furoate/vilanterol) This is a summary of the risk management plan (RMP) for RELVAR ELLIPTA.
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN VI.1 Summary of risk management plan for RELVAR ELLIPTA (fluticasone furoate/vilanterol) This is a summary of the risk management plan (RMP) for RELVAR ELLIPTA.
1.1. Bisphosphonates and depressive reactions
 1.1. Bisphosphonates and depressive reactions Introduction Bisphosphonates are the most commonly prescribed medications for the treatment of [1]. Alendronate (Fosamax ) is indicated for the treatment and
1.1. Bisphosphonates and depressive reactions Introduction Bisphosphonates are the most commonly prescribed medications for the treatment of [1]. Alendronate (Fosamax ) is indicated for the treatment and
Chronic Obstructive Pulmonary Disease
 Page 1 of 5 Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease (COPD) is an 'umbrella' term for people with chronic bronchitis, emphysema, or both. With COPD the airflow to the
Page 1 of 5 Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease (COPD) is an 'umbrella' term for people with chronic bronchitis, emphysema, or both. With COPD the airflow to the
Treatment. Assessing the outcome of interventions Traditionally, the effects of interventions have been assessed by measuring changes in the FEV 1
 58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD)
 Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) Zahava Picado, PharmD PGY1 Pharmacy Practice Resident Central Texas Veterans Healthcare System Temple, TX October
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) Zahava Picado, PharmD PGY1 Pharmacy Practice Resident Central Texas Veterans Healthcare System Temple, TX October
Management of COPD and CHF: drugs that should be preferred or avoided
 Management of COPD and CHF: drugs that should be preferred or avoided Dr John T. Parissis, Heart Failure Unit, Attikon University Hospital, Athens, Greece Disclosures: Research grants by Abbott USA and
Management of COPD and CHF: drugs that should be preferred or avoided Dr John T. Parissis, Heart Failure Unit, Attikon University Hospital, Athens, Greece Disclosures: Research grants by Abbott USA and
Medicine Dr. Kawa Lecture 4 - Treatment of asthma :
 Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
Policy Evaluation: Step Therapy Prior Authorization of Combination Inhaled Corticosteroid / Long-Acting Beta-Agonists
 Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Policy Evaluation: Step Therapy Prior
Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Policy Evaluation: Step Therapy Prior
31 - Respiratory System
 31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol)
 EMA/639304/2014 Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Budesonide/Formoterol Teva, which
EMA/639304/2014 Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Budesonide/Formoterol Teva, which
Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic
 Thorax 1984;39:340-344 Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic asthma RICHARD F WILLEY, RONALD J FERGUSSON, DAVID J GODDEN, GRAHAM K CROMPTON,
Thorax 1984;39:340-344 Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic asthma RICHARD F WILLEY, RONALD J FERGUSSON, DAVID J GODDEN, GRAHAM K CROMPTON,
Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1].
![Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1]. Aripiprazole is available as Abilify (orodispersable) tablets (5, 10, 15, 30 mg), oral solution 1mg/ml and solution for injection 7.5 mg/ml [1].](/thumbs/72/66705207.jpg) 1.1. Aripiprazole and aggravated Introduction Aripiprazole is an atypical antipsychotic drug which acts through a combination of partial agonism at dopamine D2 - and serotonin 5-HT1a receptors and antagonism
1.1. Aripiprazole and aggravated Introduction Aripiprazole is an atypical antipsychotic drug which acts through a combination of partial agonism at dopamine D2 - and serotonin 5-HT1a receptors and antagonism
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia.
 The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia. Rennard, Stephen I; Sin, Donald D; Tashkin, Donald P; Calverley, Peter M; Radner, Finn Published in: Annals
The Relationship among COPD Severity, Inhaled Corticosteroid Use, and the Risk of Pneumonia. Rennard, Stephen I; Sin, Donald D; Tashkin, Donald P; Calverley, Peter M; Radner, Finn Published in: Annals
Drug prescriptions (Pharm) Exposure (36/48 months)
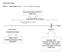 ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
Clinical efficacy of montelukast in anti-inflammatory treatment of asthma and allergic rhinitis
 Clinical efficacy of montelukast in anti-inflammatory treatment of asthma and allergic rhinitis Kim Hyun Hee, MD, PhD. Dept. of Pediatrics The Catholic University of Korea College of Medicine Achieving
Clinical efficacy of montelukast in anti-inflammatory treatment of asthma and allergic rhinitis Kim Hyun Hee, MD, PhD. Dept. of Pediatrics The Catholic University of Korea College of Medicine Achieving
Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol)
 EMA/675937/2014 Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Vylaer Spiromax, which details the measures
EMA/675937/2014 Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Vylaer Spiromax, which details the measures
7.2 Part VI.2 Elements for a Public Summary
 7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
Searching for Targets to Control Asthma
 Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol)
 EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
Choosing an inhaler for COPD made simple. Dr Simon Hart Castle Hill Hospital
 Choosing an inhaler for COPD made simple Dr Simon Hart Castle Hill Hospital 1 Declaration of interests I have received speaker fees, sponsorship to attend conferences, and funding for research from companies
Choosing an inhaler for COPD made simple Dr Simon Hart Castle Hill Hospital 1 Declaration of interests I have received speaker fees, sponsorship to attend conferences, and funding for research from companies
Scottish Medicines Consortium
 Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Drug Class Monograph
 Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
PATHOPHYSIOLOGICAL PROCESS TEMPLATE
 1 PATHOPHYSIOLOGICAL PROCESS TEMPLATE DISEASE: Chronic obstructive pulmonary disease (COPD) DEFINITION: COPD can be defined as a disease in which there is a significant damage to the lungs thus reducing
1 PATHOPHYSIOLOGICAL PROCESS TEMPLATE DISEASE: Chronic obstructive pulmonary disease (COPD) DEFINITION: COPD can be defined as a disease in which there is a significant damage to the lungs thus reducing
HCT Medical Policy. Bronchial Thermoplasty. Policy # HCT113 Current Effective Date: 05/24/2016. Policy Statement. Overview
 HCT Medical Policy Bronchial Thermoplasty Policy # HCT113 Current Effective Date: 05/24/2016 Medical Policies are developed by HealthyCT to assist in administering plan benefits and constitute neither
HCT Medical Policy Bronchial Thermoplasty Policy # HCT113 Current Effective Date: 05/24/2016 Medical Policies are developed by HealthyCT to assist in administering plan benefits and constitute neither
Pharmacological Management of Obstructive Airways in Humans. Introduction to Scientific Research. Submitted: 12/4/08
 Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Other sources of information
 1.1. Fexofenadine and Fexofenadine hydrochloride is a non-sedating H 1 antihistamine. It was registered in the Netherlands in 1997. Fexofenadine is indicated for the relief of symptoms associated with
1.1. Fexofenadine and Fexofenadine hydrochloride is a non-sedating H 1 antihistamine. It was registered in the Netherlands in 1997. Fexofenadine is indicated for the relief of symptoms associated with
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Defining COPD. Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist
 Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
New Therapies for Asthma
 New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
Lareb has received 35 reports on all DOACs in association with the occurrence of paraesthesia.
 Direct oral anti-coagulants and paraesthesia Introduction Apixiban (Eliquis ), (Xarelto ), edoxaban (Lixiana ) and (Pradaxa ) belong to the group of direct oral anti-coagulants (DOACs). Apixaban, and edoxaban
Direct oral anti-coagulants and paraesthesia Introduction Apixiban (Eliquis ), (Xarelto ), edoxaban (Lixiana ) and (Pradaxa ) belong to the group of direct oral anti-coagulants (DOACs). Apixaban, and edoxaban
An overview of reports on lacosamide
 An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
Alberta Childhood Asthma Pathway for Primary Care
 Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Budesonide treatment of moderate and severe asthma in children: A doseresponse
 Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Utilization of LABAs versus SABAs in Albanian Insured Outpatients with COPD during
 Utilization of LABAs versus SABAs in Albanian Insured Outpatients with COPD during 2008-2012 Doi:10.5901/mjss.2013.v4n9p573 Abstract Alida Sina PhD Student, Health Insurance Institute, Tirana, Albania
Utilization of LABAs versus SABAs in Albanian Insured Outpatients with COPD during 2008-2012 Doi:10.5901/mjss.2013.v4n9p573 Abstract Alida Sina PhD Student, Health Insurance Institute, Tirana, Albania
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Patient characteristics Intervention Comparison Length of followup
 ORAL MUCOLYTICS Ref ID: 2511 Bachh AA, Shah NN, Bhargava R et al. Effect oral N- in COPD - A randomised controlled trial. JK Practitioner. 2007; 14(1):12-16. Ref ID: 2511 RCT Single blind; unclear allocation
ORAL MUCOLYTICS Ref ID: 2511 Bachh AA, Shah NN, Bhargava R et al. Effect oral N- in COPD - A randomised controlled trial. JK Practitioner. 2007; 14(1):12-16. Ref ID: 2511 RCT Single blind; unclear allocation
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
International Journal of Medical Science and Education pissn eissn
 COMPARISON OF MONTELUKAST AND KETOTIFEN AS ADD ON THERAPY IN MODERATE AND SEVERE PERSISTENT BRONCHIAL ASTHMA Dr. Gaurav Chhabra 1, Dr. Shubhakaran Sharma 2* Original research article 1,2 Associate professor,
COMPARISON OF MONTELUKAST AND KETOTIFEN AS ADD ON THERAPY IN MODERATE AND SEVERE PERSISTENT BRONCHIAL ASTHMA Dr. Gaurav Chhabra 1, Dr. Shubhakaran Sharma 2* Original research article 1,2 Associate professor,
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo,
 Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo, BRONCHODILATORS: Beta Adrenoreceptor Agonists Actions Adrenoreceptor agonists have many of the same actions as epinephrine/adrenaline,
Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo, BRONCHODILATORS: Beta Adrenoreceptor Agonists Actions Adrenoreceptor agonists have many of the same actions as epinephrine/adrenaline,
What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university
 What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university Management stable COPD Relieve symptoms Improve exercise tolerance Improve health status Prevent
What s new in COPD? Apichart Khanichap MD. Department of Medicine, Faculty of Medicine, Thammasat university Management stable COPD Relieve symptoms Improve exercise tolerance Improve health status Prevent
The FDA Critical Path Initiative
 The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
 CHRONIC OBSTRUCTIVE PULMONARY DISEASE INCIDENCE UP TO 380,000 PEOPLE IN IRELAND HSE FIGURES 110,000 DIAGNOSED AND 200,000 UNDIAGNOSED. AFFECTS MORE MEN THAN WOMEN BUT RATES ARE RISING 1500 DEATHS PER YEAR
CHRONIC OBSTRUCTIVE PULMONARY DISEASE INCIDENCE UP TO 380,000 PEOPLE IN IRELAND HSE FIGURES 110,000 DIAGNOSED AND 200,000 UNDIAGNOSED. AFFECTS MORE MEN THAN WOMEN BUT RATES ARE RISING 1500 DEATHS PER YEAR
This is the publisher s version. This version is defined in the NISO recommended practice RP
 Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 25 May 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Inhaled corticosteroids versus long-acting beta 2 -agonists for chronic obstructive pulmonary disease (Review)
 Inhaled corticosteroids versus long-acting beta 2 -agonists for chronic obstructive pulmonary disease (Review) Spencer S, Evans DJ, Karner C, Cates CJ This is a reprint of a Cochrane review, prepared and
Inhaled corticosteroids versus long-acting beta 2 -agonists for chronic obstructive pulmonary disease (Review) Spencer S, Evans DJ, Karner C, Cates CJ This is a reprint of a Cochrane review, prepared and
Asthma training. Mike Levin Division of Asthma and Allergy Red Cross Hospital
 Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Global Initiative for Asthma (GINA) What s new in GINA 2016?
 Global Initiative for Asthma (GINA) What s new in GINA 2016? GINA Global Strategy for Asthma Management and Prevention GINA: A Brief History Established in 1993 Collaboration between NHLBI and WHO Multiple
Global Initiative for Asthma (GINA) What s new in GINA 2016? GINA Global Strategy for Asthma Management and Prevention GINA: A Brief History Established in 1993 Collaboration between NHLBI and WHO Multiple
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views
 IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
Inflammation in chronic obstructive pulmonary disease : its assessment and the effects of corticosteroids Boorsma, M.
 UvA-DARE (Digital Academic Repository) Inflammation in chronic obstructive pulmonary disease : its assessment and the effects of corticosteroids Boorsma, M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Inflammation in chronic obstructive pulmonary disease : its assessment and the effects of corticosteroids Boorsma, M. Link to publication Citation for published version
Chapter 3: Respiratory System (7 th Edition)
 Chapter 3: Respiratory System (7 th Edition) The Sheffield respiratory guidelines (April 2015) have been removed from the intranet. This is because the COPD section has been superseded by the COPD treatment
Chapter 3: Respiratory System (7 th Edition) The Sheffield respiratory guidelines (April 2015) have been removed from the intranet. This is because the COPD section has been superseded by the COPD treatment
Public Assessment Report Scientific discussion. Orest Easyhaler (budesonide, formoterol fumarate dihydrate) SE/H/1214/02-03/DC
 Public Assessment Report Scientific discussion Orest Easyhaler (budesonide, formoterol fumarate dihydrate) SE/H/1214/02-03/DC This module reflects the scientific discussion for the approval of Orest Easyhaler.
Public Assessment Report Scientific discussion Orest Easyhaler (budesonide, formoterol fumarate dihydrate) SE/H/1214/02-03/DC This module reflects the scientific discussion for the approval of Orest Easyhaler.
Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017
 Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
Aclidinium bromide/formoterol Endpoint category. RR Endpoint. [95% CI 2 ] Study. with event
![Aclidinium bromide/formoterol Endpoint category. RR Endpoint. [95% CI 2 ] Study. with event Aclidinium bromide/formoterol Endpoint category. RR Endpoint. [95% CI 2 ] Study. with event](/thumbs/90/102875145.jpg) Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
International Journal of Medical Research & Health Sciences
 International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
Three s Company - The role of triple therapy in chronic obstructive pulmonary
 Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) October 26 th, 2018 Zahava Picado, PharmD PGY1 Pharmacy Resident Central Texas Veterans Healthcare System Zahava.Picado@va.gov
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) October 26 th, 2018 Zahava Picado, PharmD PGY1 Pharmacy Resident Central Texas Veterans Healthcare System Zahava.Picado@va.gov
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Drug Monograph. Brand Name: Arcapta Neohaler. Generic Name: Indacaterol. Manufacturer¹,²: Novartis
 Drug Monograph Brand Name: Arcapta Neohaler Generic Name: Indacaterol Manufacturer¹,²: Novartis Drug Class¹,²,³, : Beta2-Adrenergic Agonist, Long-Acting Uses: Labeled Uses¹,²,³, : Used for long-term maintenance
Drug Monograph Brand Name: Arcapta Neohaler Generic Name: Indacaterol Manufacturer¹,²: Novartis Drug Class¹,²,³, : Beta2-Adrenergic Agonist, Long-Acting Uses: Labeled Uses¹,²,³, : Used for long-term maintenance
Users of antiasthma drugs in Iceland: a drug utilization study
 Eur Respir J 1997; 10: 1230 1234 DOI: 10.1183/09031936.97.10061230 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Users of antiasthma drugs
Eur Respir J 1997; 10: 1230 1234 DOI: 10.1183/09031936.97.10061230 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Users of antiasthma drugs
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol
 Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol Introduction The European Medicines Agency s Pharmacovigilance Risk Assessment Committee (PRAC)
Overview of reports of thromboembolic adverse drug reactions associated with cyproterone/ethinylestradiol Introduction The European Medicines Agency s Pharmacovigilance Risk Assessment Committee (PRAC)
Combination Beta2-Agonist/Corticosteroid Inhalers Policy Number: Last Review: Origination: Next Review: Policy When Policy Topic is covered:
 Combina ation Beta2-Agonist/Corticosteroid Inhalers Policy Number: 5.01.572 Origination: 06/2014 Last Review: 07/2014 Next Review: 07/2015 Policy BCBSKC will provide coverage for the combination beta2-agonist/corticosteroid
Combina ation Beta2-Agonist/Corticosteroid Inhalers Policy Number: 5.01.572 Origination: 06/2014 Last Review: 07/2014 Next Review: 07/2015 Policy BCBSKC will provide coverage for the combination beta2-agonist/corticosteroid
Tolerance to bronchodilating effects of salmeterol in COPD
 Respiratory Medicine (2003) 97, 1014 1020 Tolerance to bronchodilating effects of salmeterol in COPD J.F. Donohue a, *, S. Menjoge b, S. Kesten b a University of North Carolina School of Medicine, 130
Respiratory Medicine (2003) 97, 1014 1020 Tolerance to bronchodilating effects of salmeterol in COPD J.F. Donohue a, *, S. Menjoge b, S. Kesten b a University of North Carolina School of Medicine, 130
Roflumilast for the management of severe chronic obstructive pulmonary disease
 Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 www.nice.org.uk/ta244 NHS Evidence has accredited the process used by the Centre for Health Technology
Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 www.nice.org.uk/ta244 NHS Evidence has accredited the process used by the Centre for Health Technology
Mario Cazzola, MD, FCCP; Gabriele Di Lorenzo, MD; Felice Di Perna, MD; Francesco Calderaro, MD; Renato Testi, MD; and Stefano Centanni, MD
 Additive Effects of Salmeterol and Fluticasone or Theophylline in COPD* Mario Cazzola, MD, FCCP; Gabriele Di Lorenzo, MD; Felice Di Perna, MD; Francesco Calderaro, MD; Renato Testi, MD; and Stefano Centanni,
Additive Effects of Salmeterol and Fluticasone or Theophylline in COPD* Mario Cazzola, MD, FCCP; Gabriele Di Lorenzo, MD; Felice Di Perna, MD; Francesco Calderaro, MD; Renato Testi, MD; and Stefano Centanni,
500 micrograms/dose Turbohaler dry powder device 500 micrograms/ml injection. 12 micrograms/dose Turbohaler dry powder device
 3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
XOLAIR (omalizumab) Prior Authorization
 MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
Asthma Upate 2018: What s New Since the 2007 Asthma Guidelines of NAEPP?
 10:50-11:50am Asthma Update 2018: What s New Since the 2007 National Asthma Guidelines? SPEAKER Christopher H. Fanta, MD Disclosures The following relationships exist related to this presentation: Christopher
10:50-11:50am Asthma Update 2018: What s New Since the 2007 National Asthma Guidelines? SPEAKER Christopher H. Fanta, MD Disclosures The following relationships exist related to this presentation: Christopher
Roflumilast for the management of severe chronic obstructive pulmonary disease
 Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 guidance.nice.org.uk/ta244 NICE has accredited the process used by the Centre for Health Technology Evaluation
Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 guidance.nice.org.uk/ta244 NICE has accredited the process used by the Centre for Health Technology Evaluation
Sponsor. Generic drug name. Trial indication(s) Protocol number. Protocol title. Clinical trial phase. Study Start/End Dates.
 Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Public Assessment Report Scientific discussion. Salmeterol/Fluticasone Sandoz (salmeterol xinafoate, fluticasone propionate) SE/H/1323/03/DC
 Public Assessment Report Scientific discussion Salmeterol/Fluticasone Sandoz (salmeterol xinafoate, fluticasone propionate) SE/H/1323/03/DC This module reflects the scientific discussion for the approval
Public Assessment Report Scientific discussion Salmeterol/Fluticasone Sandoz (salmeterol xinafoate, fluticasone propionate) SE/H/1323/03/DC This module reflects the scientific discussion for the approval
