CHAPTER 1 Section 3.1, page 3 Section 3.1, page 3. CHAPTER 3 Section 1.1, page 1 Section 1.1, page 1
|
|
|
- Ralf Parrish
- 6 years ago
- Views:
Transcription
1
2 CHANGE M JUNE 15, 2016 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 3.1, page 3 Section 3.1, page 3 CHAPTER 3 Section 1.1, page 1 Section 1.1, page 1 CHAPTER 4 Section 9.1, pages 5 and 6 Section 9.1, pages 5 through 7 CHAPTER 7 Section 27.1, pages 1 and 2 Section 27.1, pages 1 and 2 CHAPTER 8 Section 16.1, page 1 Section 16.1, page 1 2
3 CHANGE M JUNE 15, 2016 SUMMARY OF CHANGES CHAPTER 1 1. Section 3.1. This change confirms Intrapulmonary Percussive Ventilation (IPV) may be considered for cost-sharing when the diagnosis is Cystic Fibrosis (CF). EFFECTIVE DATE: 04/30/2009. CHAPTER 3 2. Section 1.1. This change adds as exclusion, unproven, the use of a stellate ganglion block for the treatment of Post-Traumatic Stress Disorder. EFFECTIVE DATE: As stated in the issuance. CHAPTER 4 3. Section 9.1. This change confirms Left Atrial Appendage (LAA) closure for the prevention of embolism in patients with non-valvular atrial fibrillation is covered when performed with an FDA approved device used according to labeled specifications (e.g. Watchman device). EFFECTIVE DATE: 07/02/2015. CHAPTER 7 4. Section 27.1 This change allows Botox injections for the treatment of palmar hyperhidrosis. EFFECTIVE DATE: 01/01/2013. CHAPTER 8 5. Section This change confirms the use of Intrapulmonary Percussive Ventilation for the treatment of Cystic Fibrosis (CF). EFFECTIVE DATE: 04/30/
4
5 TRICARE Policy Manual M, February 1, 2008 Chapter 1, Section 3.1 Rare Diseases 2.13 Effective February 4, 2011, Radiesse Voice laryngoplasty injections may be cost-shared for the treatment of type 1 laryngeal cleft (also described as supraglottic interarytenoid defects that extend no further than the true vocal folds) Effective November 27, 1995, Orthotopic Liver Transplantation (OLT) may be cost-shared for the treatment of Crigler-Najjar Syndrome Type I. OLT may be performed both prior to the onset of neurological symptoms or after the onset of neurological symptoms Effective June 5, 2013, off-label use of intravenous immune globulin for the treatment of Hashimoto s Encephalopathy, may be considered in exceptional circumstances where there is progressive neurologic decline despite appropriate steroid therapy or where steroid therapy is contraindicated Effective April 30, 2009, Intrapulmonary Percussive Ventilation (IPV) may be considered for cost-sharing when the diagnosis is Cystic Fibrosis (CF). See Chapter 8, Section 16.1 for policy regarding IPVs Effective January 4, 2013, allogeneic hematopoietic cell transplant (CPT 2 procedure code 38240) for the treatment of primary plasma cell leukemia Off-label use of Photodynamic Therapy (CPT 2 procedure code 67221) with Visudyne (HCPCS J3396) may be considered for cost-sharing for the treatment of retinal astrocytic hamartoma in Tuberous Sclerosis. The effective date is February 1, Effective June 25, 2014, intracranial angioplasty with stenting (CPT 2 procedure code 61635) of the venous sinuses may be considered for cost-sharing for the treatment of pseudotumor cerebri (also known as idiopathic intracranial hypertension and benign intracranial hypertension) Effective February 1, 2012, OLT (CPT 2 procedure code 47135) for the treatment of Acute Intermittent Porphyria Effective December 1, 2014, Photodynamic Therapy for the treatment of Central Serous Chorioretinopathy. 3.0 EXCLUSIONS 3.1 The off-label use of rituximab for the treatment of pediatric linear Immunoglobulin A (IgA) dermatosis is unproven. 3.2 Proton Beam Therapy (PBT)/radiosurgery/radiotherapy for the treatment of thymoma is unproven. - END - 2 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 3 C-163, June 15, 2016
6
7 TRICARE Policy Manual M, February 1, 2008 Anesthesia Chapter 3 Section 1.1 Anesthesia Issue Date: Authority: 32 CFR 199.4(b)(2)(viii), (c)(2)(vii), (c)(3)(viii), and (g)(40) 1.0 CPT 1 PROCEDURE CODES , 99100, 99116, 99135, POLICY 2.1 Anesthesia services and supplies are covered. 2.2 See Section 1.2 for conscious sedation. 2.3 See the TRICARE Reimbursement Manual (TRM), Chapter 1, Section 9 for information on reimbursement of anesthesia. 3.0 EXCLUSIONS 3.1 Hypnotherapy. 3.2 A separate benefit is not payable for general anesthesia administered by the attending physician (surgeon or obstetrician) or dentist, or by the surgical, obstetrical or dental assistant. This exclusion does not apply to cases involving administration of local or regional anesthesia such as local anesthesia administered by a surgeon in the surgeon s office, by an obstetrician in a delivery room, or by an orthopedic surgeon in an operating room. 3.3 Acupuncture. 3.4 The use of a Stellate Ganglion Block (SGB) (CPT 1 procedure code 64510) for the treatment of Post-Traumatic Stress Disorder (PTSD) is unproven. - END - 1 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 1 C-163, June 15, 2016
8
9 TRICARE Policy Manual M, February 1, 2008 Chapter 4, Section 9.1 Cardiovascular System All procedures are performed in a Centers for Medicare and Medicaid Services (CMS) approved facility that has been determined to be competent in performing the evaluation, procedure, and follow-up necessary to ensure optimal patient outcomes Transcatheter Aortic Valve Replacement (TAVR) for the treatment of severe symptomatic aortic stenosis is proven safe and effective for patients who are not candidates for Surgical Aortic Valve Replacement (SAVR) Percutaneous transluminal mechanical thrombectomy (CPT 4 procedure codes 37184, 37185, 37186) with stent retrievers for the treatment of adults with acute ischemic stroke is proven safe and effective TAVR for the treatment of severe symptomatic aortic stenosis in high-risk operative patients is considered proven safe and effective Intracranial angioplasty with stenting (CPT 4 procedure code 61635) of the venous sinuses may be considered for cost-sharing for the treatment of pseudotumor cerebri (also known as idiopathic intracranial hypertension and benign intracranial hypertension) Cardiography: electrocardiograms, rhythm strips, stress testing; Cardiovascular Monitoring: continuous ambulatory monitors (e.g., Holter monitor, Zio Patch), mobile cardiac telemetry, and event monitors; Implantable and Wearable Cardiac Devices: pacemakers, defibrillators, and loop recorders are covered when approved by the FDA and in accordance with Chapter 8, Section Left Atrial Appendage (LAA) closure for the prevention of embolism in patients with nonvalvular atrial fibrillation is covered when performed with an FDA approved device used according to labeled specifications (e.g., WATCHMAN device). 4.0 EXCLUSIONS 4.1 Thermogram; cephalic (CPT 4 procedure code 93760); peripheral (CPT 4 procedure code 93762) are unproven. 4.2 Percutaneous Myocardial Laser Revascularization (PMR) is unproven. 4.3 Cardiomyoplasty (Cardiac Wrap) for treatment of heart failure is unproven. 4.4 Minimally Invasive CABG surgery to include Minimally Invasive Direct Coronary Artery Bypass (MIDCAB) and Port Access Coronary Artery Bypass (PACAB) are unproven. 4.5 Percutaneous Transluminal Angioplasty (PTA) in the treatment of obstructive lesions of the vertebral and cerebral arteries is unproven. PTA of the carotid artery without stenting is unproven. PTA of the carotid artery with stenting but without embolic protection (CPT 4 procedure code 37216) is unproven. 4.6 Signal-Average Electrocardiography (CPT 4 procedure code 93278) is unproven. 4 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 5 C-163, June 15, 2016
10 TRICARE Policy Manual M, February 1, 2008 Chapter 4, Section 9.1 Cardiovascular System 4.7 Percutaneous transluminal mechanical thrombectomy vein(s) including intraprocedural pharmacological thrombolytic injections and fluroscopic guidance (CPT 5 procedure code 37187) is unproven. 4.8 Percutaneous transluminal mechanical thrombectomy, vein(s) including intraprocedural pharmacological thrombolytic injections and fluroscopic guidance, repeat treatment on subsequent day during course of thrombolytic therapy (CPT 5 procedure code 37188) is unproven. 4.9 LAA closure using the LARIAT Suture Delivery Device is excluded as the LARIAT device fails to meet the off-label device requirements in Chapter 8, Section EFFECTIVE DATES 5.1 March 1, 2001, for gamma and beta intracoronary radiotherapy (brachytherapy). 5.2 January 1, 2002, for TMR. 5.3 October 1, 2003, for VADs as destination therapy. 5.4 December 1, 2003, for endovenous RFA/obliteration. 5.5 January 1, 2005, for ABPM. 5.6 March 17, 2005, for PTA of the carotid artery with stenting in beneficiaries at high risk for CEA. 5.7 March 21, 2006, for percutaneous transluminal mechanical thrombectomy for acute limb ischemia. 5.8 January 1, 2007, for pulmonary vein isolation/ablation. 5.9 January 1, 2009, for endovenous laser ablation/therapy May 1, 2011, for endovenous RFA/obliteration for the treatment of incompetent perforator veins July 27, 2012, for endovenous laser ablation/therapy for the treatment of incompetent perforator veins February 8, 2012, for TAVR for the treatment of severe symptomatic aortic stenosis in patients who are not candidates for SAVR July 27, 2012, for TAVR, for the treatment of severe symptomatic aortic stenosis in high-risk operative patients June 25, 2014, for intracranial angioplasty with stenting of the venous sinuses for the treatment of pseudotumor cerebri. 5 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 6 C-163, June 15, 2016
11 TRICARE Policy Manual M, February 1, 2008 Chapter 4, Section 9.1 Cardiovascular System 5.15 November 30, 2014, for continuous ambulatory Electrocardiogram (ECG) recording greater than 48 hours January 7, 2015, for percutaneous transluminal mechanical thrombectomy with stent retrievers July 2, 2015, for LAA closure for the prevention of embolism in patients with non-valvular atrial fibrillation. - END - 7 C-163, June 15, 2016
12
13 TRICARE Policy Manual M, February 1, 2008 Medicine Chapter 7 Section 27.1 Botulinum Toxin Injections Issue Date: October 12, 1998 Authority: 32 CFR 199.4(c)(2)(iii) and (c)(2)(iv) 1.0 CPT 1 PROCEDURE CODES 46505, , 64640, 64653, HCPCS PROCEDURE CODES J J DESCRIPTION These procedures involve the injection of small amounts of botulinum toxin into selected muscles for the nonsurgical treatment of the conditions relating to spasticity, various dystonias, nerve disorders, and muscular tonicity deviations. 4.0 POLICY 4.1 Botulinum toxin A (AbobotulinumtoxinA/OnabotulinumtoxinA/IncobotulinumtoxinA), Botulinum toxin B (RimabotulinumtoxinB), and any other Federal Drug Administration (FDA) approved botulinum toxin injectable drugs may be considered for cost-sharing for their FDA approved indications, unless otherwise excluded by the program. 4.2 Botox (OnabotulinumtoxinA-chemodenervation-CPT 1 procedure code 46505) may be considered for off-label cost-sharing for the treatment of chronic anal fissure unresponsive to conservative therapeutic measures, effective May 1, Botulinum toxin A injections may be considered for off-label cost-sharing for the treatment of spasticity resulting from Cerebral Palsy (CP), effective November 1, Botox (OnabotulinumtoxinA) and Myobloc (RimabotulinumtoxinB) injections may be considered for off-label cost-sharing for the treatment of sialorrhea associated with Parkinson s disease patients who are refractory to, or unable to tolerate, systemic anticholinergics, effective October 1, Botox (OnabotulinumtoxinA) injections for laryngeal dystonia (adductor spasmodic dysphonia) and oromandibular dystonia (jaw-closing dystonia) may be considered for cost-sharing. 1 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 1 C-93, July 16, 2013
14 TRICARE Policy Manual M, February 1, 2008 Chapter 7, Section 27.1 Botulinum Toxin Injections 4.6 Botox (OnabotulinumtoxinA) injections may be considered for off-label cost-sharing for the treatment of palmar hyperhidrosis that is refractory to topical and pharmacological therapies, effective January 1, Off-label use. Effective July 27, 2012, off-label uses of Botulinum toxin A (AbobotulinumtoxinA/OnabotulinumtoxinA/IncobotulinumtoxinA), Botulinum toxin B (Rimabotulinumtoxin B), and any other FDA approved botulinum toxin injectable drugs may be approved for cost-sharing by the contractor in accordance with Chapter 8, Section 9.1, paragraph EXCLUSIONS 5.1 Botulinum toxin A injections are unproven for the following indications: Lower back pain/lumbago. Episodic migraine, chronic daily headache, cluster headache, cervicogenic headache, and tension-type headache. 5.2 Botox (OnabotulinumtoxinA-chemodenervation-CPT 2 procedure code 64612) for the treatment of muscle spasms secondary to cervical degenerative disc disease and spinal column stenosis is unproven. 5.3 Botulinum toxin A used for cosmetic indications (e.g., frown lines and brow furrows) is excluded from coverage. 6.0 EFFECTIVE DATES 6.1 May 1, 2007, for coverage of chronic anal fissure unresponsive to conservative therapeutic measures (CPT 2 procedure code 46505). 6.2 October 1, 2009, for coverage of sialorrhea associated with Parkinson s disease patients who are refractory to, or unable to tolerate, systemic anticholinergics (CPT 2 procedure code 64653). Effective January 1, 2011, use CPT 2 procedure code November 14, 1990, for coverage of laryngeal or oromandibular dystonia. 6.4 January 1, 2013, for coverage of palmar hyperhidrosis that is refractory to topical and pharmacological therapies. - END - 2 CPT only 2006 American Medical Association (or such other date of publication of CPT). All Rights Reserved. 2 C-163, June 15, 2016
15 TRICARE Policy Manual M, February 1, 2008 Other Services Chapter 8 Section 16.1 Mucus Clearance Devices Issue Date: June 5, 1995 Authority: 32 CFR HCPCS PROCEDURE CODES A7025, A7026, E0480, E E0484, S DESCRIPTION 2.1 Mucus clearance devices are designed to clear mucus secretions from the lungs of patients with mucociliary clearance impairment. 2.2 Some mucus clearance devices resemble a combination of a smoker s pipe and a referee s whistle. It consists of a hardened plastic mouthpiece at one end, a plastic perforated cover at the opposite end, and a valve on the inside created by a high-density stainless steel ball resting in a plastic circular cone. 2.3 Other bronchial drainage systems include an air oscillator and an inflatable vest and uses high-frequency chest wall oscillations, which also clear mucus from the airway wall. This type of system is a mechanical form of Chest Physical Therapy (CPT) used as an alternative to conventional CPT in patients with Cystic Fibrosis (CF). 3.0 POLICY 3.1 Mucus clearance devices may be cost-shared for beneficiaries with mucus producing lung diseases, including, but not limited to CF and Chronic Obstructive Pulmonary Disease (COPD) (which encompasses both chronic bronchitis and emphysema), and for beneficiaries with secretory impairment that requires mucus clearance. 3.2 The mucus clearance device used must be U.S. Food and Drug Administration (FDA) approved. Coverage can only begin effective the date of FDA approval. 3.3 Intrapulmonary Percussive Ventilation (IPV) (Healthcare Common Procedure Coding System (HCPCS) code E0481) may be considered for cost-sharing when the diagnosis is CF. - END - 1 C-163, June 15, 2016
16
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE HEAL TH AFFAIRS EAST CENTRETE HPARKWAY AURORA, CO
 OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE HEAL TH AFFAIRS 16401 EAST CENTRETE HPARKWAY AURORA, CO 8001 1-9066 DEFEN E HEALTH AGENC MB&RS CHANGE 150 6010.57-M DECEMBER 10, 2015 PUBLICATIONS SYSTEM CHANGE
OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE HEAL TH AFFAIRS 16401 EAST CENTRETE HPARKWAY AURORA, CO 8001 1-9066 DEFEN E HEALTH AGENC MB&RS CHANGE 150 6010.57-M DECEMBER 10, 2015 PUBLICATIONS SYSTEM CHANGE
Chapter 1 Section 3.1
 Administration Chapter 1 Section 3.1 Issue Date: May 18, 1994 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(g)(15)(ii) Copyright: CPT only 2006 American Medical Association (or such other date of publication
Administration Chapter 1 Section 3.1 Issue Date: May 18, 1994 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(g)(15)(ii) Copyright: CPT only 2006 American Medical Association (or such other date of publication
CHANGE M MAY 22, CHAPTER 1 Section 3.1, pages 1 through 4 Section 3.1, pages 1 through 5
 CHANGE 182 6010.57-M MAY 22, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 3.1, pages 1 through 4 Section 3.1, pages 1 through 5 2 CHANGE 182 6010.57-M MAY 22, 2017 SUMMARY OF CHANGES CHAPTER 1
CHANGE 182 6010.57-M MAY 22, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 3.1, pages 1 through 4 Section 3.1, pages 1 through 5 2 CHANGE 182 6010.57-M MAY 22, 2017 SUMMARY OF CHANGES CHAPTER 1
HEAl.1TH AFFAIRS EASTCENTRETECH PARKWAY AURORA, CO
 OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE HEAl.1TH AFFAIRS 16401 EASTCENTRETECH PARKWAY AURORA, CO 80011-9066 OEFEN E HEAL TH AGENCY MB&RS CHANGE 156 6010.57-M FEBRUARY 10, 2016 PUBLICATIONS SYSTEM
OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE HEAl.1TH AFFAIRS 16401 EASTCENTRETECH PARKWAY AURORA, CO 80011-9066 OEFEN E HEAL TH AGENCY MB&RS CHANGE 156 6010.57-M FEBRUARY 10, 2016 PUBLICATIONS SYSTEM
Chapter 4 Section 20.1
 Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 61000-61626, 61680-62264, 62268-62284, 62290-63048, 63055-64484, 64505-64595,
Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 61000-61626, 61680-62264, 62268-62284, 62290-63048, 63055-64484, 64505-64595,
Chapter 4 Section 20.1
 Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
Table of Contents. 1.0 Description of the Procedure, Product, or Service Safety and Provider Compliance... 1
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Botox. Botox (onabotulinum toxin A) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.01 Subject: Botox Page: 1 of 8 Last Review Date: September 15, 2017 Botox Description Botox (onabotulinum
Chapter 4 Section 20.1
 Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Pharmacy Prior Authorization AETA BETTER HEALTH MICHIGA Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Circle Yes or No Y N. [If yes, no further questions.]
![Circle Yes or No Y N. [If yes, no further questions.] Circle Yes or No Y N. [If yes, no further questions.]](/thumbs/83/88529512.jpg) 02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
02/18/2016 Prior Authorization AETA BETTER HEALTH PE MEDICAID & AETA BETTER HEALTH KIDS Botulinum Toxins (PA88) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Diagnostic & Therapeutic Cardiac Catheterization Coder 2017
 Diagnostic & Therapeutic Cardiac Catheterization Coder 2017 Including peripheral and cardiovascular services and procedures Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare
Diagnostic & Therapeutic Cardiac Catheterization Coder 2017 Including peripheral and cardiovascular services and procedures Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management
 Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
Neuromuscular Blocking Agents
 Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Peripheral and Cardiology Coder 2018
 Peripheral and Cardiology Coder 2018 Cardiovascular Services and Procedures Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN
Peripheral and Cardiology Coder 2018 Cardiovascular Services and Procedures Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN
 THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
THE CLINICAL USE OF BOTULINUM TOXIN IN THE TREATMENT OF MOVEMENT DISORDERS, SPASTICITY, AND SOFT TISSUE PAIN Spasmodic torticollis (cervical dystonia), blepharospasm, and writer s cramp are specific types
Cardiac Rhythm Management Coder 2018
 Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date:
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Botulinum Toxins (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Covered Critical Illness Conditions Appendix
 Covered Critical Illness Conditions Appendix Effective Date: February 1, 2010 This Appendix contains definitions for those Conditions that are covered under the Manulife Financial Group Critical Illness
Covered Critical Illness Conditions Appendix Effective Date: February 1, 2010 This Appendix contains definitions for those Conditions that are covered under the Manulife Financial Group Critical Illness
2017 PHYSICIAN PROCEDURE CODE CHANGES
 2017 PHYSICIAN PROCEDURE CODE CHANGES Effective for dates of service on or after 1/1/2017, refer to the New Codes listed below for billing. The discontinued codes are not valid for billing dates of service
2017 PHYSICIAN PROCEDURE CODE CHANGES Effective for dates of service on or after 1/1/2017, refer to the New Codes listed below for billing. The discontinued codes are not valid for billing dates of service
Chapter 5 Section 1.1. Diagnostic Radiology (Diagnostic Imaging)
 Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) 1.0 CPT 1 PROCEDURE CODES 70010-72292, 73000-76499, 77071-77084,
Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) 1.0 CPT 1 PROCEDURE CODES 70010-72292, 73000-76499, 77071-77084,
University of Florida Department of Surgery. CardioThoracic Surgery VA Learning Objectives
 University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
Chapter 13 Worksheet Code It
 Class: Date: Chapter 13 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. A cardiac catheterization diverts blood from the heart to the aorta. 2. Selective vascular
Class: Date: Chapter 13 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. A cardiac catheterization diverts blood from the heart to the aorta. 2. Selective vascular
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Corporate Medical Policy
 Corporate Medical Policy Oscillatory Devices for the Treatment of Respiratory Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: oscillatory_devices_for_treatment_of_respiratory_conditions
Corporate Medical Policy Oscillatory Devices for the Treatment of Respiratory Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: oscillatory_devices_for_treatment_of_respiratory_conditions
Chapter 5 Section 1.1
 Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) Copyright: CPT only 2006 American Medical Association (or such
Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) Copyright: CPT only 2006 American Medical Association (or such
Sample page. POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
 2018 Complete Guide for Interventional Radiology An in-depth guide to interventional radiology coding, billing, and reimbursement for facilities and physicians POWER UP YOUR CODING with Optum360, your
2018 Complete Guide for Interventional Radiology An in-depth guide to interventional radiology coding, billing, and reimbursement for facilities and physicians POWER UP YOUR CODING with Optum360, your
A Place For Airway Clearance Therapy In Today s Healthcare Environment
 A Place For Airway Clearance Therapy In Today s Healthcare Environment Michigan Society for Respiratory Care 2015 Fall Conference K. James Ehlen, MD October 6, 2015 Objectives Describe patients who will
A Place For Airway Clearance Therapy In Today s Healthcare Environment Michigan Society for Respiratory Care 2015 Fall Conference K. James Ehlen, MD October 6, 2015 Objectives Describe patients who will
CHAPTER 1 Section 11.1, pages 1 and 2 Section 11.1, pages 1 and 2
 CHANGE 12 6010.60-M NOVEMBER 2, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 11.1, pages 1 and 2 Section 11.1, pages 1 and 2 CHAPTER 4 Section 6.1, pages 1 and 2 Section 6.1, pages 1 and 2 Section
CHANGE 12 6010.60-M NOVEMBER 2, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 11.1, pages 1 and 2 Section 11.1, pages 1 and 2 CHAPTER 4 Section 6.1, pages 1 and 2 Section 6.1, pages 1 and 2 Section
Medicare National Coverage Determinations Manual
 Medicare National Coverage Determinations Manual Chapter 1, Part 1 (Sections 10 80.12) Coverage Determinations Transmittals for Chapter 1, Part 1 Table of Contents (Rev. 182, 05-22-15) Foreword - Purpose
Medicare National Coverage Determinations Manual Chapter 1, Part 1 (Sections 10 80.12) Coverage Determinations Transmittals for Chapter 1, Part 1 Table of Contents (Rev. 182, 05-22-15) Foreword - Purpose
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb)
 Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
Botox (onabotulinumtoxina) Dysport (abobotulinumtoxina) Xeomin (incobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective
CHAPTER 1 Section 12.1, pages 1 and 2 Section 12.1, pages 1 and 2
 CHANGE 192 6010.57-M NOVEMBER 1, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 12.1, pages 1 and 2 Section 12.1, pages 1 and 2 CHAPTER 4 Section 6.1, pages 1 and 2 Section 6.1, pages 1 and 2 Section
CHANGE 192 6010.57-M NOVEMBER 1, 2017 REMOVE PAGE(S) INSERT PAGE(S) CHAPTER 1 Section 12.1, pages 1 and 2 Section 12.1, pages 1 and 2 CHAPTER 4 Section 6.1, pages 1 and 2 Section 6.1, pages 1 and 2 Section
CPT Code Details
 CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
CORRECTIONS DOCUMENT CPT 2009
 CORRECTIONS DOCUMENT CPT 2009 Front Matter Modifiers (See Appendix A for Definitions) 22 Unusual Increased procedural services Correct the description for consistency with the primary listing in Appendix
CORRECTIONS DOCUMENT CPT 2009 Front Matter Modifiers (See Appendix A for Definitions) 22 Unusual Increased procedural services Correct the description for consistency with the primary listing in Appendix
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL REVASCULARIZATION
 MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
FY2015 Proposed Hospital Inpatient Rule Summary
 FY2015 Proposed Hospital Inpatient Rule Summary Cardiac Rhythm Management (CRM) Electrophysiology (EP) Interventional Cardiology (IC) Peripheral Intervention (PI) On April 30, 2014, the Centers for Medicare
FY2015 Proposed Hospital Inpatient Rule Summary Cardiac Rhythm Management (CRM) Electrophysiology (EP) Interventional Cardiology (IC) Peripheral Intervention (PI) On April 30, 2014, the Centers for Medicare
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM
 REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
2017 Cardiology Survival Guide
 2017 Cardiology Survival Guide Chapter 4: Cardiac Catheterization/Percutaneous Coronary Intervention A cardiac catheterization involves a physician inserting a thin plastic tube (catheter) into an artery
2017 Cardiology Survival Guide Chapter 4: Cardiac Catheterization/Percutaneous Coronary Intervention A cardiac catheterization involves a physician inserting a thin plastic tube (catheter) into an artery
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc )
 Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Botulinum toxins: abobotulinumtoxina (Dysport ), incobotulinumtoxina (Xeomin ), onabotulinumtoxina (Botox ), & rimabotulinumtoxinb (Myobloc ) These services may or may not be covered by your HealthPartners
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014
 Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Medication Prior Authorization Form
 (OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
(OnabotulinumtoxinA) Dysport (abobotulinumtoxina) Myobloc (rimabotulinumtoxinb) Xeomin (incobotulinumtoxina) Policy Number: 1042 Policy History Approve Date: 12/11/2015 Revise Dates: 7/7/2016 Next Review:
Thrombectomy, open, arteriovenous fistula without revision, autogenous or nonautogenous dialysis graft (separate procedure)
 Dialysis Vascular Access Coverage, Coding and Reimbursement Overview Hospital Outpatient 2019 Edition All Reimbursement Amounts are Listed at ational Unadjusted Medicare Rates and Do ot Include the 2%
Dialysis Vascular Access Coverage, Coding and Reimbursement Overview Hospital Outpatient 2019 Edition All Reimbursement Amounts are Listed at ational Unadjusted Medicare Rates and Do ot Include the 2%
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
(For items 1-12, each question specifies mark one or mark all that apply.)
 Form 121 - Report of Cardiovascular Outcome Ver. 9.2 COMMENTS -Affix label here- Member ID: - - To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: - Central Case No.:
Form 121 - Report of Cardiovascular Outcome Ver. 9.2 COMMENTS -Affix label here- Member ID: - - To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: - Central Case No.:
Cardiac Valve/Structural Therapies
 Property of Dr. Chad Rammohan Cardiac Valve/Structural Therapies Chad Rammohan, MD FACC Medical Director, El Camino Hospital Cardiac Catheterization Lab Director, Interventional and Structural Cardiology,
Property of Dr. Chad Rammohan Cardiac Valve/Structural Therapies Chad Rammohan, MD FACC Medical Director, El Camino Hospital Cardiac Catheterization Lab Director, Interventional and Structural Cardiology,
MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection
 POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
Summary of Research and Writing Activities In Cardiovascular Disease
 Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
Index. cardiology.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute ischemic stroke TOAST classification of, 270 Acute myocardial infarction (AMI) cardioembolic stroke following, 207 208 noncardioembolic
Index Note: Page numbers of article titles are in boldface type. A Acute ischemic stroke TOAST classification of, 270 Acute myocardial infarction (AMI) cardioembolic stroke following, 207 208 noncardioembolic
Cardiac Rhythm Management Coder 2017
 Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Aetna Health Management HMO Products SouthEast Region (Including Arkansas) Medical and Non-Medical Approvals and Denials from 10/01/2017 to 12/31/2017
 Aetna Health Management HMO Products SouthEast Region (Including Arkansas) Medical and Non-Medical Approvals and Denials from 10/01/2017 to 12/31/2017 Code Inpatient Medical and Non-Medical Approvals and
Aetna Health Management HMO Products SouthEast Region (Including Arkansas) Medical and Non-Medical Approvals and Denials from 10/01/2017 to 12/31/2017 Code Inpatient Medical and Non-Medical Approvals and
Watchman and Structural update..the next frontier. Ari Chanda, MD Cardiology Associates of Fredericksburg
 Watchman and Structural update..the next frontier Ari Chanda, MD Cardiology Associates of Fredericksburg Different Left Atrial Appendage (LAA) morphologies Watchman (the device) Fabric Anchors Device structure
Watchman and Structural update..the next frontier Ari Chanda, MD Cardiology Associates of Fredericksburg Different Left Atrial Appendage (LAA) morphologies Watchman (the device) Fabric Anchors Device structure
Adult Cardiology Clinical Privileges
 Name: Effective from / / to / / Initial privileges (initial appointment) (reappointment) Renewal of privileges All new applicants should meet the following requirements as approved by the governing body,
Name: Effective from / / to / / Initial privileges (initial appointment) (reappointment) Renewal of privileges All new applicants should meet the following requirements as approved by the governing body,
Introducing the COAPT Trial
 physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
BOTOX. Description. Section: Prescription Drugs Effective Date: January 1, 2013 Subsection: CNS Original Policy Date: December 7, 2011
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.12.01 Subject: Botox Page: 1 of 6 Last Review Status/Date: December 6, 2012 BOTOX Description Botox (onabotulinum
b. To facilitate the management decision of a patient with an equivocal stress test.
 National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
Clinical Policy: Holter Monitors Reference Number: CP.MP.113
 Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Student Outline. Improving Transportation Safety: Commercial Driver Medical Examiner Training CHAPTER 1. General FMCSA Information
 Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy
 Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
Prior Authorization Update: Botulinum Toxins
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
2
 1 2 Although the term "cardiomyopathy" could theoretically apply to almost any disease affecting the heart, it is usually reserved for "severe myocardial disease leading to heart failure".cardiomyopathy
1 2 Although the term "cardiomyopathy" could theoretically apply to almost any disease affecting the heart, it is usually reserved for "severe myocardial disease leading to heart failure".cardiomyopathy
2013 Coding Changes. Diagnostic Radiology. Nuclear Medicine
 2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
High Frequency Chest Wall Oscillating Devices (HFCWO) (Airway Clearance Systems)
 High Frequency Chest Wall Oscillating Devices (HFCWO) (Airway Clearance Systems) Date of Origin: 05/2015 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 07/2016 Developed By: Medical
High Frequency Chest Wall Oscillating Devices (HFCWO) (Airway Clearance Systems) Date of Origin: 05/2015 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 07/2016 Developed By: Medical
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System
 Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System The Centers for Medicare and Medicaid Services (CMS) released its proposed rule for calendar year (CY) 2017
Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System The Centers for Medicare and Medicaid Services (CMS) released its proposed rule for calendar year (CY) 2017
Detailed Order Request Checklists for Cardiology
 Next Generation Solutions Detailed Order Request Checklists for Cardiology 8600 West Bryn Mawr Avenue South Tower Suite 800 Chicago, IL 60631 www.aimspecialtyhealth.com Appropriate.Safe.Affordable 2018
Next Generation Solutions Detailed Order Request Checklists for Cardiology 8600 West Bryn Mawr Avenue South Tower Suite 800 Chicago, IL 60631 www.aimspecialtyhealth.com Appropriate.Safe.Affordable 2018
TEXAS - MAC - PART B - TRAILBLAZER RADIOLOGY TABLE OF CONTENTS
 RADIOLOGY TABLE OF CONTENTS CPT to LCD ID CodeMap Mappings... 3 - Automatic Implantable Cardiac Defibrillator (AICD) - 4C-58AB-R1 11 L26529- Cardiac Catheterization - 4C-50AB-R3... 20 L26534- Transthoracic
RADIOLOGY TABLE OF CONTENTS CPT to LCD ID CodeMap Mappings... 3 - Automatic Implantable Cardiac Defibrillator (AICD) - 4C-58AB-R1 11 L26529- Cardiac Catheterization - 4C-50AB-R3... 20 L26534- Transthoracic
Delineation of Privileges Department of Internal Medicine Division of Cardiovascular Medicine
 Delineation of Privileges Department of Internal Medicine Division of Cardiovascular Medicine Name: Please Print or Type LEVEL I CORE PRIVILEGES General Medicine: To qualify for the subspecialty of Cardiovascular
Delineation of Privileges Department of Internal Medicine Division of Cardiovascular Medicine Name: Please Print or Type LEVEL I CORE PRIVILEGES General Medicine: To qualify for the subspecialty of Cardiovascular
Chapter 4 Section 13.2
 Surgery Chapter 4 Section 13.2 Issue Date: November 9, 1982 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(e)(15) 1.0 CPT 1 PROCEDURE CODES 43644, 43770-43774, 43842, 43846, 43848 2.0 HCPCS PROCEDURE CODES
Surgery Chapter 4 Section 13.2 Issue Date: November 9, 1982 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(e)(15) 1.0 CPT 1 PROCEDURE CODES 43644, 43770-43774, 43842, 43846, 43848 2.0 HCPCS PROCEDURE CODES
Mitral Regurgitation
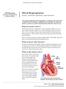 UW MEDICINE PATIENT EDUCATION Mitral Regurgitation Causes, symptoms, diagnosis, and treatment This handout describes mitral regurgitation, a disease of the mitral valve. It explains how this disease is
UW MEDICINE PATIENT EDUCATION Mitral Regurgitation Causes, symptoms, diagnosis, and treatment This handout describes mitral regurgitation, a disease of the mitral valve. It explains how this disease is
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: botulinum_toxin_injection 8/1985 10/2015 10/2016 10/2015 Description of Procedure or Service Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: botulinum_toxin_injection 8/1985 10/2015 10/2016 10/2015 Description of Procedure or Service Description
Automatic External Defibrillators
 Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
MEDICAL POLICY Cardioverter Defibrillators
 POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
Intrathecal Opioid Therapy for Management of Chronic Pain
 Intrathecal Opioid Therapy for Management of Chronic Pain Date of Origin: 01/2000 Last Review Date: 09/27/2017 Effective Date: 09/27/2017 Dates Reviewed: 11/2002, 12/2003, 12/2004, 12/2005, 12/2006, 12/2007,
Intrathecal Opioid Therapy for Management of Chronic Pain Date of Origin: 01/2000 Last Review Date: 09/27/2017 Effective Date: 09/27/2017 Dates Reviewed: 11/2002, 12/2003, 12/2004, 12/2005, 12/2006, 12/2007,
Chapter 4 Section 13.2
 TRICARE Policy Manual 6010.60-M, April 1, 2015 Surgery Chapter 4 Section 13.2 Issue Date: November 9, 1982 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(e)(15) Copyright: CPT only 2006 American Medical Association
TRICARE Policy Manual 6010.60-M, April 1, 2015 Surgery Chapter 4 Section 13.2 Issue Date: November 9, 1982 Authority: 32 CFR 199.2(b) and 32 CFR 199.4(e)(15) Copyright: CPT only 2006 American Medical Association
Angela Clements, CPC, CEMC, COSC Internal Consultant
 Angela Clements, CPC, CEMC, COSC Internal Consultant aclements@ochsner.org angelaclements0@gmail.com Disclaimer The following information was put together based on my experience, research and expertise
Angela Clements, CPC, CEMC, COSC Internal Consultant aclements@ochsner.org angelaclements0@gmail.com Disclaimer The following information was put together based on my experience, research and expertise
Definitions. Peace of mind today and tomorrow. CRITICAL ILLNESS Basic benefit Deluxe benefit. CRITICAL ILLNESS MULTI-PROTECTION (per child)
 Definitions Peace of mind today and tomorrow CRITICAL ILLNESS Basic benefit Deluxe benefit CRITICAL ILLNESS MULTI-PROTECTION (per child) Here are the definitions of the critical and non-critical illnesses
Definitions Peace of mind today and tomorrow CRITICAL ILLNESS Basic benefit Deluxe benefit CRITICAL ILLNESS MULTI-PROTECTION (per child) Here are the definitions of the critical and non-critical illnesses
Title: Automatic External Defibrillators Division: Medical Management Department: Utilization Management
 Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
11/19/2013. Cardiac Rehabilitation Coverage and Documentation Requirements. Phases of Cardiac Rehabilitation. Phase II
 Cardiac Rehabilitation Coverage and Documentation Requirements Phases of Cardiac Rehabilitation Phase I: Acute in-hospital phase of CR Phase II: is the initial outpatient phase of the program Phase III:
Cardiac Rehabilitation Coverage and Documentation Requirements Phases of Cardiac Rehabilitation Phase I: Acute in-hospital phase of CR Phase II: is the initial outpatient phase of the program Phase III:
April 6, 2017 VIA ELECTRONIC MAIL
 April 6, 2017 VIA ELECTRONIC MAIL Patricia Brooks, RHIA Centers for Medicare and Medicaid Services CMM, HAPG, Division of Acute Care Mail Stop C4-08-06 7500 Security Boulevard Baltimore, Maryland 21244-1850
April 6, 2017 VIA ELECTRONIC MAIL Patricia Brooks, RHIA Centers for Medicare and Medicaid Services CMM, HAPG, Division of Acute Care Mail Stop C4-08-06 7500 Security Boulevard Baltimore, Maryland 21244-1850
Recommended Evaluation Data Excerpt from NVIC 04-08
 Recommended Evaluation Data Excerpt from NVIC 04-08 Purpose: This document is an excerpt from the Medical and Physical Evaluations Guidelines for Merchant Mariner Credentials, contained in enclosure 3
Recommended Evaluation Data Excerpt from NVIC 04-08 Purpose: This document is an excerpt from the Medical and Physical Evaluations Guidelines for Merchant Mariner Credentials, contained in enclosure 3
L: Cardiovascular. Saskatchewan Association of Licensed Practical Nurses, Competency Profile for LPNs, 3rd Ed. 107
 L: Cardiovascular Saskatchewan Association of Licensed Practical Nurses, Competency Profile for LPNs, 3rd Ed. 107 Major Competency Area: L Cardiovascular Competency: L-1 Cardiovascular Nursing Date: January
L: Cardiovascular Saskatchewan Association of Licensed Practical Nurses, Competency Profile for LPNs, 3rd Ed. 107 Major Competency Area: L Cardiovascular Competency: L-1 Cardiovascular Nursing Date: January
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablation, radiofrequency, anesthetic considerations for, 479 489 Acute aortic syndrome, thoracic endovascular repair of, 457 462 aortic
Index Note: Page numbers of article titles are in boldface type. A Ablation, radiofrequency, anesthetic considerations for, 479 489 Acute aortic syndrome, thoracic endovascular repair of, 457 462 aortic
Chapter 4 Section Combined Heart-Kidney Transplantation (CHKT)
 Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
Neurotoxins (Botox, Dysport, Myobloc and Xeomin )
 Last Review Date: November 10, 2017 Number: MG.MM.PH.01mC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: November 10, 2017 Number: MG.MM.PH.01mC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
