Special Topic Section
|
|
|
- Allen Powell
- 5 years ago
- Views:
Transcription
1 Special Topic Section Cerebrovasc Dis 2004;18:69 74 DOI: / Received: March 8, 2004 Accepted: March 8, 2004 Published online: June 1, 2004 International Carotid Stenting Study: Protocol for a Randomised Clinical Trial Comparing Carotid Stenting with Endarterectomy in Symptomatic Carotid Artery Stenosis Roland L. Featherstone Martin M. Brown Lucy J. Coward on behalf of the ICSS Investigators Institute of Neurology, University College London, London, UK Key Words Carotid stenosis W Stenting W Endarterectomy W Randomised clinical trial W Stroke prevention Abstract Background: Carotid stenting avoids general anaesthesia, cranial nerve injury and the discomforts of surgical treatment of carotid stenosis. A systematic review of the randomised trials showed no overall difference in the major risks of endovascular treatment for carotid stenosis compared with surgery, but the confidence intervals were wide and both methods carried a significant risk of stroke. The use of protection devices appears to improve the safety of endovascular treatment, but there are little randomised data available about long-term outcomes. We have therefore set up an international, multicentre, randomised, controlled, open, prospective clinical trial, namely the International Carotid Stenting Study (ICSS), also known as CAVATAS-2. The objectives of the ICSS are to compare the risks, benefits and cost-effectiveness of a treatment policy of referral for carotid stenting compared with referral for carotid endarterectomy. Methods: Centres are required to have a team with audited expertise in carotid endarterectomy and stenting procedures, including at least one neurologist or stroke physician, a surgeon and an interventionalist. Attendance at a carotid stenting training course is required. Centres with more limited experience can join the trial as probationary centres, but stenting must then be proctored by an experienced interventionalist. Symptomatic patients are included over the age of 40 years with atherosclerotic carotid stenosis, suitable for both stenting and surgery, and are randomised in equal proportions between carotid endarterectomy and stenting. Stents and other devices are chosen for use at the discretion of the interventionalists but must be approved by the devices committee. The protocol recommends that a cerebral protection system should be used whenever the operator thinks one can be safely deployed. The combination of aspirin and clopidogrel is recommended to cover stenting procedures. Standard or eversion endarterectomy is allowed using local or general anaesthesia, shunts or patches. All patients will receive best medical care. Patients will be followed up by neurologists at 30 days after treatment, 6 months after randomisation and then annually up to 5 years after randomisation. The primary outcome measure is the difference in the long-term rate of fatal or disabling stroke in any territory between patients randomised to stenting or surgery. Secondary outcome measures include any stroke, myocardial infarction or death within 30 days of treatment, treatment- ABC Fax karger@karger.ch S. Karger AG, Basel /04/ $21.00/0 Accessible online at: Prof. Martin M. Brown Box 6, The National Hospital for Neurology and Neurosurgery Queen Square London WC1N 3BG (UK) Tel , Fax , m.brown@ion.ucl.ac.uk
2 related cranial nerve palsy or haematoma. Restenosis (170%) on ultrasound follow-up, economic measures and quality of life will also be analysed. The sample size is estimated at 1,500 patients, which will provide 95% confidence intervals of B 3.0 percentage points for the outcome measure of 30-day disabling stroke and death rate and B 3.3 percentage points for the outcome measure of death or stroke during follow-up. The trial office monitors outcome events at individual centres and a rate of events above a given threshold triggers a blinded assessment of the events, submitted to the chairman of the data-monitoring committee. Conclusions: The ICSS protocol incorporates a number of novel features to ensure patient safety, including the concept of probationary centres, proctoring of inexperienced investigators and monitoring of individual centre results on an ongoing basis. The protocol is also designed to mirror routine clinical practice as far as possible, so that the results will be widely applicable and relevant to determining the place of carotid stenting in clinical practice in the future. Introduction Copyright 2004 S. Karger AG, Basel Symptomatic carotid stenosis is usually treated by surgical endarterectomy, which has been convincingly shown in randomised trials to reduce the subsequent rate of recurrent stroke [1, 2]. Endovascular treatment involving the deployment of a stent has become an established alternative treatment for coronary and peripheral vascular disease and is increasingly used to treat carotid stenosis [3]. Compared to carotid endarterectomy, carotid stenting has the advantages of avoiding general anaesthesia, the discomforts of an incision in the neck and the risks of cranial nerve injury and neck haematoma. However, the use of endovascular techniques to treat carotid stenosis in patients with cerebrovascular disease has remained controversial. We previously organised a randomised trial, the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), which compared endovascular treatment with carotid endarterectomy. The results showed no difference in the major risks or benefits of the two types of treatment, but the results were not conclusive and the trial showed that both methods carried a significant risk of causing stroke at the time of treatment [4]. Techniques for endovascular treatment for carotid stenosis have improved since the end of randomisation in CAVATAS in 1997, with the introduction of dedicated carotid artery stents and protection devices. However, the existing data are not sufficient to establish the safety and effectiveness of carotid stenting in comparison with the well-established technique of carotid endarterectomy [5]. In particular, a systematic review of the existing randomised trial data showed no evidence of a difference in major outcomes, but the confidence intervals were wide [6]. The review concluded that current evidence did not support a shift away from recommending carotid endarterectomy as the standard treatment for carotid stenosis. There is therefore a need for further randomised trials of carotid stenting compared with carotid endarterectomy. The need for a trial to follow and build on the experience of CAVATAS led us to establish the International Carotid Stenting Study (ICSS), which is also known as CAVATAS-2. The ICSS is an international, multicentre, randomised, controlled, open, prospective clinical trial comparing carotid endarterectomy with carotid stenting. This article describes the main features of the ICSS trial protocol agreed by the Trial Steering Committees in August 2003 after an initial pilot period and approved by one of the UK Multicentre Research Ethics Committee. The protocol incorporates a number of novel features, including measures to ensure patient safety, the concept of probationary centres, proctoring of inexperienced investigators and monitoring of individual centre results on an ongoing basis. The objectives of the ICSS are to compare the risks, benefits and cost-effectiveness of a treatment policy of referral for carotid stenting compared with referral for carotid endarterectomy. Methods Centre Qualifications Each centre must have a team consisting at least one each of a neurologist or stroke physician (to see patients prior to randomisation and for follow-up), a designated surgeon experienced in endarterectomy and a designated consultant interventionalist with expertise in carotid angiography and the techniques of angioplasty and stenting. Before enrolment, surgeons and interventionalists must provide documentary evidence of their experience, which is assessed by an accreditation committee. The centres are required to confirm that they hold joint vascular meetings at which the investigators discuss the management of individual patients with carotid stenosis. Surgeons are expected to have performed a minimum of 50 carotid operations with an annual rate of at least 10 cases per year. Radiologists are expected to have performed a minimum of 50 stenting procedures, of which at least 10 should be in the carotid artery. Both surgeons and radiologists are expected to show a stroke and death rate within 30 days of treatment, consistent with the centres in the European Carotid Surgery Trial which had an average rate of 7.0% 70 Cerebrovasc Dis 2004;18:69 74 Featherstone/Brown/Coward
3 with a 95% confidence interval of % [1]. In practice, the accreditation committee has required a mortality and morbidity rate of 6% or less. Attendance at training sessions in carotid stenting provided by credentialing centres is required for all radiologists prior to participation. Probationary Centres The trial protocol recognises that many centres may lack the necessary experience of carotid stenting. Centres fulfilling the other requirements for entry but where interventionalists (or surgeons) have insufficient experience of carotid stenting (or endarterectomy) may join the ICSS for a probationary period. Probationary centres randomise patients within the ICSS protocol between surgery and stenting. Stenting (or surgical) procedures carried out on randomised patients by the probationary interventionalists (or surgeons) are required to be proctored by an experienced carotid interventionalist (or surgeon), until the proctor is satisfied with the skills and performance of the procedure. Probationary centres will become fully enrolled in the ICSS once they can satisfy the full entry requirements for stenting (or surgical) experience. The results in patients randomised during the probationary period will be analysed separately and together with the main data. Patient Evaluation Patients will be seen by the study neurologist or stroke physician prior to randomisation to confirm suitability for the study. Mandatory investigation is required for entry into the study to confirm the presence and severity of the ipsilateral stenosis and to assess contralateral carotid disease. Non-invasive investigations are acceptable so long as two independent investigations are performed and are concordant (e.g. ultrasonography and magnetic resonance angiography). Ultrasonography alone is only acceptable if it is standard practice to treat on the basis of ultrasound alone at the individual centre and the centre has been able to provide proof of the reliability of their ultrasonographic imaging through clinical audit. Inclusion and exclusion criteria are listed in table 1. Randomisation Randomisation is made by a telephone call or fax to a computerised service provided by the Oxford Clinical Trials Service Unit. Randomisation will be stratified by centre with minimisation of the main risk factors and balanced between the arms. Patients who need treatment of both carotid arteries will only be randomised for the carotid artery to be treated first. Crossovers The protocol specifies that patients who are randomised to stenting after ultrasound or other non-invasive investigation, in which subsequent angiography, prior to stenting, reveals one or more exclusion criteria (e.g. tortuous anatomy) should be treated by surgery, if appropriate, or medical care only if surgery is not appropriate (e.g. stenosis less than 50%). These patients will continue in the trial and will be analysed on an intention-to-treat basis. Stenting Protocol Stents and other devices are chosen for use at the discretion of the interventionalists but must be CE marked and approved by the devices committee. A list of approved devices is circulated to centres regularly and updated as new technology is approved. The protocol specifies that stenting will be carried out as soon as possible after Table 1. Summary of inclusion and exclusion criteria in the ICSS a Inclusion criteria 1 Symptomatic, extracranial, internal or bifurcation, atheromatous carotid artery stenosis suitable for both stenting and surgery and deemed to require treatment 2 Severity of the stenosis of the randomised artery should be at least 50% (measured by the NASCET method or non-invasive equivalent) 3 Symptoms must have occurred in the 12 months before randomisation; it is recommended that the time between symptoms and randomisation should be less than 6 months, but patients with symptoms occurring between 6 and 12 months may be included if the randomising physician considers treatment indicated 4 The patient must be clinically stable 5 Patients must be willing to have either treatment, be able to provide informed consent and be willing to participate in follow-up 6 Patients must be able to undergo their allocated treatment as soon as possible after randomisation 7 Patients may be any age greater than 40; there is no upper age limit 8 Patients should only be randomised if the investigator is uncertain which of the two treatments is best for that patient at that time b Exclusion criteria 1 Patients refusing either treatment 2 Patients unable or unwilling to give informed consent 3 Patients unwilling or unable to participate in follow-up for whatever reason 4 Patients who have had a major stroke with no useful recovery of function within the territory of the treatable artery 5 Patients with a stenosis that is unsuitable for stenting because of one or more of: Tortuous anatomy proximal or distal to the stenosis Presence of visible thrombus Proximal common carotid artery stenotic disease Pseudo-occlusion ( string sign ) 6 Patients not suitable for surgery due to anatomical factors, e.g. high stenosis or rigid neck 7 Patients in whom it is planned to carry out coronary artery bypass grafting or other major surgery within 1 month of carotid stenting or endarterectomy 8 Carotid stenosis caused by non-atherosclerotic disease, e.g. dissection, fibromuscular disease or neck radiotherapy 9 Previous carotid endarterectomy or stenting in the randomised artery 10 Patients in whom common carotid artery surgery is planned NASCET = North American Symptomatic Endarterectomy Trial. randomisation using percutaneous transluminal interventional techniques from the femoral, brachial or common carotid artery by a designated interventional consultant using an appropriate stent. The protocol recommends that a cerebral protection system should be used whenever the operator thinks one can be safely deployed. Pre- ICSS Protocol Cerebrovasc Dis 2004;18:
4 medication is discretionary, but the combination of aspirin and clopidogrel is recommended as good practice to cover stenting procedures. Intraprocedural heparin is mandatory at a dose determined by the operator; postprocedural heparin may be given according to clinical requirements. It is accepted that virtually all patients will require predilatation of the stenosis by balloon angioplasty prior to stent deployment to minimise the embolic load caused by passage of the endoluminal stent through the stenosis. Details of the procedure, including all periprocedural complications, drug therapy and the types of devices used in the procedure, are reported to the trial central office. Endarterectomy Protocol Endarterectomy is to be done as soon as possible after randomisation using whichever procedures are standard at the individual centre. Local or general anaesthesia, shunts or patches, and standard or eversion endarterectomy may be performed as required by the operating surgeon. Medical Treatment All patients will receive best medical care including antiplatelet therapy or anticoagulation (when appropriate) and control of medical risk factors such as hypertension, smoking and hyperlipidaemia before treatment and throughout the period of follow-up. Follow-Up Patients will be followed up by a neurologist or stroke physician at the participating centres at 30 days after treatment, 6 months after randomisation and then annually after randomisation. At each visit, levels of impairment will be assessed using the modified Rankin scale and any outcome events notified to the central office. Patients are also asked to complete an EQ-5D quality of life assessment questionnaire [7] at baseline and follow-up for return to the central office. Data on length of stay in hospital will be recorded to inform the economic analysis. Doppler ultrasound will be used to assess carotid artery patency at 1 month after treatment and then annually after randomisation. Outcome Event Reporting Outcome events (table 2) will be documented in detail by the investigating centre, censored after receipt at the central office to remove clues as to the treatment received, and then adjudicated by independent neurologists. Deaths of UK patients will be tracked by flagging patients against the UK Registry of Births and Deaths. Disability after stroke and cranial nerve palsy will be assessed 30 days after treatment or onset, using the modified Rankin scale. Duration of symptoms will be recorded and outcome events will be classified as disabling if the Rankin score is 3 or more for more than 30 days after onset. The trial office will monitor outcome events at individual centres. If there are two consecutive deaths or three consecutive major events at a single centre within 30 days of treatment in the same arm of the study, or a cumulative major event or death rate of more than 10% over 20 cases, then a blinded assessment of the relevant outcome events will be performed and submitted to the chairman of the data-monitoring committee. The chairman may then recommend further action, such as suspending randomisation at the centre. Table 2. Definitions of outcome events used in the ICSS Transient ischaemic attack: an acute disturbance of focal neurological function with symptoms lasting less than 24 h attributed to cerebrovascular disease Transient monocular blindness (amaurosis fugax): acute total or partial loss of vision in one eye with recovery within 24 h attributed to vascular disease; this will be included as a variety of transient ischaemic attack Stroke: an acute disturbance of focal neurological function with symptoms lasting more than 24 h resulting from intracranial vascular disturbance; it must be established whether the cause is infarction or haemorrhage (primary intracranial or subarachnoid); visual loss resulting from embolic or haemodynamic retinal ischaemia lasting more than 24 h will be included within the category of stroke Myocardial infarction: two of the following have to be documented: specific cardiac enzymes more than twice the upper limit of normal, a history of chest discomfort for at least half an hour, or the development of specific abnormalities (e.g. Q waves) on a standard 12-lead electrocardiogram Cranial nerve palsy: weakness or sensory impairment in the distribution of one of the cranial nerves attributed to treatment Haematoma: bleeding attributed to the treatment of carotid narrowing requiring new surgery, transfusion or prolonging hospital stay Disabling outcome events: disability after stroke and cranial nerve palsy will be assessed using the modified Rankin scale; the Rankin scale score will be recorded at 1 and 6 months after treatment and then at annual follow-up; outcome events will be classified as disabling if the Rankin scale score is 3 or greater; investigators will be asked to estimate the Rankin scale score at 1 and 3 months after onset of new stroke when they see the patient more than 3 months after onset of stroke Recovered strokes: in patients who make a full recovery from stroke or other outcome events, the duration from onset to full recovery will be recorded in days Data Analysis The primary analysis of the study will determine the difference in the long-term rate of fatal or disabling stroke in any territory of patients with severe symptomatic stenosis after randomisation to a policy of carotid stenting compared with surgery, based on intention to treat. Secondary analyses will determine the differences in the combined outcome of stroke, myocardial infarction or death, and the rate of other outcome events within 30 days of treatment; the rate of symptomatic and asymptomatic restenosis and the rate of ipsilateral stroke and disabling stroke in any territory during follow-up, and the cost-effectiveness of carotid stenting compared to surgery. Sample Size The planned sample size is 1,500. We do not anticipate any large difference in the principal outcome between surgery and stenting. We propose to estimate this difference and present a confidence interval for difference in 30-day death, stroke or myocardial infarc- 72 Cerebrovasc Dis 2004;18:69 74 Featherstone/Brown/Coward
5 tion and for 3-year survival free of disabling stroke or death. For 1,500 patients, the 95% confidence interval will be the observed difference B 3.0 percentage points for the outcome measure of 30-day stroke, myocardial infarction and death rate and B 3.3 percentage points for the outcome measure of death or disabling stroke over 3 years of follow-up. However, the trial will have the power to detect major differences in the risks of the two procedures, for example if stenting proves to be much more risky than surgery or associated with more symptomatic restenosis. The differences detectable with a power of 80% are 4.7 percentage points for 30-day outcome and 5.1 percentage points for survival free of disabling stroke. Similar differences are detectable for secondary outcomes. Meta-Analysis The principal investigators of the three ongoing randomised carotid stenting studies based in Europe have respectively agreed to combine the results of the ICSS, SPACE and EVA-3S in a systematic meta-analysis of their data. To facilitate the meta-analysis, it has been agreed that all three trials will collect identical baseline vascular risk factors, will follow up patients with Doppler ultrasound annually and will collect data on outcome events using similar definitions and measures of disability. Discussion The ICSS is designed to mirror routine clinical practice as far as possible, so that the results will be widely applicable. Thus, there is a considerable degree of flexibility built into the study. In particular, centres are not required to use a single manufacturer s device for stenting or cerebral protection, although all devices used have to carry a CE mark and have prior approval from the devices committee. Likewise, no specific medical regime is laid down to accompany treatment, although the currently recognised best practise is strongly encouraged. Similarly, a range of variations of the endarterectomy procedure are acceptable. This approach strikes a balance between collecting data that reflect the typical clinical experience with these procedures across a variety of countries and centres and producing a protocol defined enough to allow useful meta-analysis, when data from the ICSS are combined with those from other studies. It is particularly relevant in this context that the trial incorporates a range of centres with various degrees of prior experience of carotid stenting, including probationary centres where investigators treat patients within the trial under supervision. Thus, the study should contribute to the power of the evidence base with respect to stenting, whilst not being so proscriptive in design as to be of little relevance to future clinical practice. An important area of potential bias when comparing a relatively new procedure such as stenting with an established one, i.e. carotid endarterectomy, is the effect of the learning curve. It is possible that centres relatively inexperienced in stenting will have initially higher complication rates. The influence of the early part of the learning curve for carotid stenting in the ICSS will be limited by careful training and supervision of individual interventionalists under the proctoring system. The total experience of carotid percutaneous transluminal angioplasty and stenting of individual interventionalists will be recorded prior to entry into the trial. This will allow the average duration of the learning curve to be analysed, taking into account the current experience of the individual interventionalists. This information may have implications for the interpretation of the results of the trial and for the future training and supervision of the procedure. There may also be a learning curve at experienced centres related to changes in technology. New stents and protection devices will be approved by the devices committee during the course of the trial to allow the use of new designs if appropriate. The trial will therefore allow the tracking of new technology. Because randomisation is balanced between centres, any overall change in risks related to the introduction of new technology should be detectable, so long as it is sufficiently large. Concern regarding distal embolisation of debris during carotid artery stenting has led to the introduction and increasing use of cerebral protection devices, which appear to reduce the risk of stroke significantly. For example, the global carotid stenting registry reported a comparison of subgroups of symptomatic patients stented with and without protection, in which events occurred in 2.8 and 5.9% of patients, respectively [3]. A systematic review of carotid stenting case series reported up to June 2002 also determined that the stroke and death rate within 30 days of treatment was significantly lower at 1.8% in patients treated with protection devices compared to 5.5% in patients treated without protection [8]. This has led to the recommendation in the ICSS protocol that a cerebral protection device should be used whenever one can be safely deployed. We accept that there is a school of thought which argues that protection devices are not essential, may cause additional hazards in some patients and increase costs. However, the major concern of the steering committee has been to ensure the overall safety of the procedures. Our investigators, especially surgeons partaking in the trial, have a strong preference in favour of protection devices. Moreover, the use of a protection device matches the stenting procedures more closely with the surgical procedures, in which the surgeon invariably protects the brain from embolism by clamping the distal carotid artery during endarterectomy. We have concluded ICSS Protocol Cerebrovasc Dis 2004;18:
6 that it would not be practical to randomise patients between the use or not of a protection device within the stenting arm because of the large sample size required. The policy of recommending a protection device is supported by the recently reported findings from EVA-3S [9]. Concern has been expressed that the recommendation in the ICSS protocol to use a combination of aspirin and clopidogrel to cover stenting, but not surgery, may disadvantage the surgery arm. However, the ICSS is not designed to be a scientific experiment comparing the use of a stent with the use of a knife, in which every other variable is kept constant. Instead, the ICSS is designed to compare two contrasting clinical management strategies, namely referral to a policy of a surgical team compared with referral to a stenting team. Thus, the two arms will differ in more ways than just the procedure used to treat the stenosis. For example, in many centres, the two arms will have different operators, nursing and ancillary staff and different postoperative care and types of ward. Differences in the antiplatelet regime therefore simply reflect current differences in the way in which patients are managed in the two situations, given that very few surgeons are prepared to use the combination of clopidogrel and aspirin in the immediate perioperative period because of the excess bleeding induced. Good communication with investigators is an essential component of a successful clinical trial. The trial office publishes regular newsletters and holds an annual investigators meeting to review progress, discuss the protocol and encourage feedback. A website with the address has been set up to facilitate communication. Information on progress of the trial and downloadable versions of the trial data collection forms are available on the website. By February 2004, 24 centres from 9 countries in Europe, Australia and Canada were actively enrolling patients into the trial. The trial plans to enroll another 12 centres to achieve its sample size of 1,500 patients by the end of We believe that carotid stenting should be performed as part of a randomised clinical trial at this stage of its development, because this will ensure careful assessment and follow-up of all patients treated in the trial. Supervision from the data-monitoring committee ensures that continuing treatment with the new technique remains ethical. Acknowledgements The ICSS has been funded by grants from the Stroke Association, Sanofi Synthelabo and the European Commission. M.M.B. s Chair in Stroke Medicine at University College London is supported by the Reta Lila Weston Trust for Medical Research. References 1 European Carotid Surgery Trialists Collaborative Group: Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351: North American Symptomatic Carotid Endarterectomy Trial Collaborators: Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339: Wholey MH, Al-Mubarek N, Wholey MH: Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv 2003;60: CAVATAS Investigators: Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): A randomised trial. Lancet 2001;357: Brown MM, Hacke W: Carotid artery stenting: The need for randomised trials. Cerebrovasc Dis 2004;18: Coward LJ, Featherstone RL, Brown MM: Percutaneous transluminal angioplasty and stenting for carotid artery stenosis (Cochrane Review); in: The Cochrane Library, issue 2. Chichester, Wiley, Brazier J, Usherwood T, Harper R, Thomas K: Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol 1998;51: Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB: Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: A systematic review of the literature. Stroke 2003;34: EVA-3S Investigators: Carotid angioplasty and stenting with and without cerebral protection. Clinical alert from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial. Stroke 2004;35:e18 e Cerebrovasc Dis 2004;18:69 74 Featherstone/Brown/Coward
ICSS Safety Results NOT for PUBLICATION. June 2009 ICSS ICSS ICSS ICSS. International Carotid Stenting Study: Main Inclusion Criteria
 Safety Results NOT for The following slides were presented to the Investigators Meeting on 22/05/09 and most of them were also presented at the European Stroke Conference on 27/05/09 They are NOT for in
Safety Results NOT for The following slides were presented to the Investigators Meeting on 22/05/09 and most of them were also presented at the European Stroke Conference on 27/05/09 They are NOT for in
Articles. Funding Medical Research Council, the Stroke Association, Sanofi-Synthélabo, European Union.
 Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial International
Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial International
Percutaneous transluminal angioplasty and stenting for carotid artery stenosis (Review)
 Percutaneous transluminal angioplasty and stenting for carotid artery stenosis (Review) Ederle J, Featherstone RL, Brown MM This is a reprint of a Cochrane review, prepared and maintained by The Cochrane
Percutaneous transluminal angioplasty and stenting for carotid artery stenosis (Review) Ederle J, Featherstone RL, Brown MM This is a reprint of a Cochrane review, prepared and maintained by The Cochrane
Treatment Considerations for Carotid Artery Stenosis. Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery
 Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Carotid Artery Stenting
 Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective)
 Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
New Trials in Progress: ACT 1. Jon Matsumura, MD Cannes, France June 28, 2008
 New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary
 SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO
 Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
CAROTID STENTING A 2009 UPDATE. Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital
 CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
Carotid Artery Stenting
 Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
03/30/2016 DISCLOSURES TO OPERATE OR NOT THAT IS THE QUESTION CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE
 CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE Elizabeth L. Detschelt, M.D. Allegheny Health Network Vascular and Endovascular Symposium April 2, 2016 DISCLOSURES I have no
CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE Elizabeth L. Detschelt, M.D. Allegheny Health Network Vascular and Endovascular Symposium April 2, 2016 DISCLOSURES I have no
Carotid Artery Stenting Versus
 Carotid Artery Stenting Versus Carotid Endarterectomy Seong-Wook Park, MD, PhD, FACC,, Seoul, Korea Stroke & Carotid artery stenosis Stroke & Carotid artery stenosis Cerebrovascular disease is one of the
Carotid Artery Stenting Versus Carotid Endarterectomy Seong-Wook Park, MD, PhD, FACC,, Seoul, Korea Stroke & Carotid artery stenosis Stroke & Carotid artery stenosis Cerebrovascular disease is one of the
BULgarian Carotid Artery Stenting versus Surgery Study (BULCASSS): Randomized single center trial
 BULgarian Carotid Artery Stenting versus Surgery Study (): Randomized single center trial Ivo Petrov, M. Konteva, H. Dimitrov, K. Kichukov Tokuda Hospital Sofia Cardiology Department Background Carotid
BULgarian Carotid Artery Stenting versus Surgery Study (): Randomized single center trial Ivo Petrov, M. Konteva, H. Dimitrov, K. Kichukov Tokuda Hospital Sofia Cardiology Department Background Carotid
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Index. interventional.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
Disclosures. State of the Art Management of Carotid Stenosis. NIH funding for clinical trials Consultant for Scientia Vascular and Medtronic
 State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE?
 UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS
 CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
6. Endovascular aneurysm repair
 Introduction The standard treatment for aortic aneurysm, open repair, involves a large abdominal incision and cross-clamping of the aorta. In recent years, a minimally invasive technique, endovascular
Introduction The standard treatment for aortic aneurysm, open repair, involves a large abdominal incision and cross-clamping of the aorta. In recent years, a minimally invasive technique, endovascular
ORIGINAL CONTRIBUTION
 ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
Guidelines for Ultrasound Surveillance
 Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Debata II: Carotidal stenting v.s. carotidal endatherectomy- surgical side
 Debata II: Carotidal stenting v.s. carotidal endatherectomy- surgical side Academician Mitrev Z, Special hospital for surgery Filip Vtori Skopje - Macedonija Oktomvri, 2008 History Hippocrates, 400 B.C.
Debata II: Carotidal stenting v.s. carotidal endatherectomy- surgical side Academician Mitrev Z, Special hospital for surgery Filip Vtori Skopje - Macedonija Oktomvri, 2008 History Hippocrates, 400 B.C.
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS. 73 year old NS right-handed male applicant for $1 Million life insurance
 MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine
 Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Carotid Artery Stent: Is it ready for prime time?
 2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS. 73 year old NS right-handed male applicant for $1 Million Life Insurance
 MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
GALA GENERAL ANAESTHESIA vs LOCAL ANAESTHESIA FOR CAROTID SURGERY HOSPITAL DISCHARGE OR 7 DAY POST-SURGERY FOLLOW-UP FORM
 GALA GENERAL ANAESTHESIA vs LOCAL ANAESTHESIA FOR CAROTID SURGERY HOSPITAL DISCHARGE OR 7 DAY POST-SURGERY FOLLOW-UP FORM To the surgeon and anesthetist: Please complete questions 1-29 (pages 1, 2 & 3)
GALA GENERAL ANAESTHESIA vs LOCAL ANAESTHESIA FOR CAROTID SURGERY HOSPITAL DISCHARGE OR 7 DAY POST-SURGERY FOLLOW-UP FORM To the surgeon and anesthetist: Please complete questions 1-29 (pages 1, 2 & 3)
Corporate Medical Policy
 Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS
 TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
CLINICAL TIMELINE EVA-3S CREST ICSS SPACE SAPPHIRE
 Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY
 GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY Full Title of Guideline: Author (include email and role): Guideline for Recovery Room Management of Patients after Carotid
GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY Full Title of Guideline: Author (include email and role): Guideline for Recovery Room Management of Patients after Carotid
The most important recommendations from the 2017 ESVS/ESC guideline on the management of carotid artery disease
 The most important recommendations from the 2017 ESVS/ESC guideline on the management of carotid artery disease GJ de Borst Department of Vascular Surgery RECOMMENDATION GRADING CRITERIA What is new
The most important recommendations from the 2017 ESVS/ESC guideline on the management of carotid artery disease GJ de Borst Department of Vascular Surgery RECOMMENDATION GRADING CRITERIA What is new
Articles. Funding Medical Research Council, Stroke Association, Sanofi-Synthélabo, European Union.
 Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial Leo H Bonati, Joanna Dobson, Roland
Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial Leo H Bonati, Joanna Dobson, Roland
Carotid Artery Stenosis
 Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
Carotid Artery Stenting (CAS) Pathophysiology. Technical Considerations. Plaque characteristics: relevant concepts. CAS and CEA
 Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Slide 1. Slide 2 Conflict of Interest Disclosure. Slide 3 Stroke Facts. The Treatment of Intracranial Stenosis. Disclosure
 Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of endovascular stent insertion for intracranial atherosclerotic disease Improving
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of endovascular stent insertion for intracranial atherosclerotic disease Improving
Extracranial to intracranial bypass for intracranial atherosclerosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Extracranial to intracranial bypass for intracranial atherosclerosis In cerebrovascular disease, blood vessels
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Extracranial to intracranial bypass for intracranial atherosclerosis In cerebrovascular disease, blood vessels
Carotid Artery Stenting Today: A Few Updating Remarks
 Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
THE GALA TRIAL PROTOCOL. March 2003
 THE GALA TRIAL General Anaesthesia versus Local Anaesthesia for Carotid Endarterectomy PROTOCOL March 2003 A multicentre randomised trial conducted mainly in Europe to compare primarily the risk of stroke,
THE GALA TRIAL General Anaesthesia versus Local Anaesthesia for Carotid Endarterectomy PROTOCOL March 2003 A multicentre randomised trial conducted mainly in Europe to compare primarily the risk of stroke,
Carotid Endarterectomy for Symptomatic Complete Occlusion of the Internal Carotid Artery
 2011 65 4 239 245 Carotid Endarterectomy for Symptomatic Complete Occlusion of the Internal Carotid Artery a* a b a a a b 240 65 4 2011 241 9 1 60 10 2 62 17 3 67 2 4 64 7 5 69 5 6 71 1 7 55 13 8 73 1
2011 65 4 239 245 Carotid Endarterectomy for Symptomatic Complete Occlusion of the Internal Carotid Artery a* a b a a a b 240 65 4 2011 241 9 1 60 10 2 62 17 3 67 2 4 64 7 5 69 5 6 71 1 7 55 13 8 73 1
Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk
 Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk Amy B. Reed, MD, a Peter Gaccione, MA, b Michael Belkin, MD, b Magruder C. Donaldson, MD, b John A. Mannick, MD,
Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk Amy B. Reed, MD, a Peter Gaccione, MA, b Michael Belkin, MD, b Magruder C. Donaldson, MD, b John A. Mannick, MD,
Carotid artery stenting in the elderly: the time has come
 88 Journal of Geriatric Cardiology June 2007 Vol 4 No 2 Symposium: Review Article Carotid artery stenting in the elderly: the time has come Dipsu Patel, Neil E Strickman St. Luke s Episcopal Hospital/Texas
88 Journal of Geriatric Cardiology June 2007 Vol 4 No 2 Symposium: Review Article Carotid artery stenting in the elderly: the time has come Dipsu Patel, Neil E Strickman St. Luke s Episcopal Hospital/Texas
Update on the only remaining Carotid Multicenter Randomised International Trial in the World:ACST-2
 Update on the only remaining Carotid Multicenter Randomised International Trial in the World:ACST-2 Alison Halliday MD Professor of Vascular Surgery University of Oxford Disclosure Statement of Financial
Update on the only remaining Carotid Multicenter Randomised International Trial in the World:ACST-2 Alison Halliday MD Professor of Vascular Surgery University of Oxford Disclosure Statement of Financial
Surgical Treatment of Carotid Disease
 Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
RCSIsmjoriginal article
 Percutaneous transluminal carotid artery angioplasty and stenting Abstract heading Background: Carotid angioplasty with stenting is becoming a more popular treatment option for severe carotid occlusive
Percutaneous transluminal carotid artery angioplasty and stenting Abstract heading Background: Carotid angioplasty with stenting is becoming a more popular treatment option for severe carotid occlusive
More than strokes occur
 Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
I have the following potential conflicts of interest to report. honorarium: 1. St Jude Medical 2. Biotronik 3. Boston Scientific
 Stenting carotideo nel paziente sintomatico alla luce dei nuovi trials Savona, 11 Aprile 2015 Gioel GabrioSecco, MD, PhD Emodinamica e CardiologiaInterventistica Ospedale SantiAntonio e Biagio e Cesare
Stenting carotideo nel paziente sintomatico alla luce dei nuovi trials Savona, 11 Aprile 2015 Gioel GabrioSecco, MD, PhD Emodinamica e CardiologiaInterventistica Ospedale SantiAntonio e Biagio e Cesare
Michael Horowitz, MD Pittsburgh, PA
 Michael Horowitz, MD Pittsburgh, PA Introduction Cervical Artery Dissection occurs by a rupture within the arterial wall leading to an intra-mural Hematoma. A possible consequence is an acute occlusion
Michael Horowitz, MD Pittsburgh, PA Introduction Cervical Artery Dissection occurs by a rupture within the arterial wall leading to an intra-mural Hematoma. A possible consequence is an acute occlusion
CONSIDER FOR ACST-2 ACST-2 protocol: Version 2.0, July ISRCTN
 Asymptomatic Carotid Surgery Trial-2 ACST 2 A large, simple randomised trial to compare carotid endarterectomy with carotid artery stenting to prevent stroke If a patient needs treatment for asymptomatic
Asymptomatic Carotid Surgery Trial-2 ACST 2 A large, simple randomised trial to compare carotid endarterectomy with carotid artery stenting to prevent stroke If a patient needs treatment for asymptomatic
O ne quarter of strokes occur in the posterior
 586 PAPER Vertebral artery origin angioplasty and primary stenting: safety and restenosis rates in a prospective series G C Cloud, F Crawley, A Clifton, DJHMcCabe, M M Brown, H S Markus... See end of article
586 PAPER Vertebral artery origin angioplasty and primary stenting: safety and restenosis rates in a prospective series G C Cloud, F Crawley, A Clifton, DJHMcCabe, M M Brown, H S Markus... See end of article
Downloaded from:
 Antithrombotic Trialists Collaboration. (inc. Meade, TW), (2002) Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high
Antithrombotic Trialists Collaboration. (inc. Meade, TW), (2002) Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high
Advances in the treatment of posterior cerebral circulation symptomatic disease
 Advances in the treatment of posterior cerebral circulation symptomatic disease Athanasios D. Giannoukas MD, MSc(Lond.), PhD(Lond.), FEBVS Professor of Vascular Surgery Faculty of Medicine, School of Health
Advances in the treatment of posterior cerebral circulation symptomatic disease Athanasios D. Giannoukas MD, MSc(Lond.), PhD(Lond.), FEBVS Professor of Vascular Surgery Faculty of Medicine, School of Health
Antithrombotic therapy in patients with transient ischemic attack / stroke (acute phase <48h)
 Antithrombotic therapy in patients with transient ischemic attack / stroke (acute phase
Antithrombotic therapy in patients with transient ischemic attack / stroke (acute phase
Algorithmic selection of emboli protection device during the procedure of carotid artery stunting
 Algorithmic selection of emboli protection device during the procedure of carotid artery stunting Yasuhiro Kawabata, Tetsuya Tsukahara, Shunichi Fukuda, Tomokazu Aoki, Satoru Kawarazaki Department of Neurosurgery,
Algorithmic selection of emboli protection device during the procedure of carotid artery stunting Yasuhiro Kawabata, Tetsuya Tsukahara, Shunichi Fukuda, Tomokazu Aoki, Satoru Kawarazaki Department of Neurosurgery,
Carotid Artery Disease and Carotid Endartarectomy. Information for Patients
 Carotid Artery Disease and Carotid Endartarectomy Introduction Information for Patients The brain requires a constant supply of blood and oxygen; interruption of that supply for more than just a few minutes
Carotid Artery Disease and Carotid Endartarectomy Introduction Information for Patients The brain requires a constant supply of blood and oxygen; interruption of that supply for more than just a few minutes
Summary of some of the landmark articles:
 Summary of some of the landmark articles: The significance of unruptured intracranial saccular aneurysms: Weibers et al Mayo clinic. 1987 1. 131 patients with 161 aneurysms were followed up at until death,
Summary of some of the landmark articles: The significance of unruptured intracranial saccular aneurysms: Weibers et al Mayo clinic. 1987 1. 131 patients with 161 aneurysms were followed up at until death,
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre
 Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Dr Julia Hopyan Stroke Neurologist Sunnybrook Health Sciences Centre Objectives To learn what s new in stroke care 2010-11 1) Acute stroke management Carotid artery stenting versus surgery for symptomatic
Extracranial Carotid Artery/Stenting
 Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2018 Origination: 4/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2018 Origination: 4/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS
 CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Coutts S, Wein T (Writing Group Chairs) on Behalf of the PREVENTION of STROKE Writing
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Coutts S, Wein T (Writing Group Chairs) on Behalf of the PREVENTION of STROKE Writing
Recommendations for Follow-up After Vascular Surgery Arterial Procedures SVS Practice Guidelines
 Recommendations for Follow-up After Vascular Surgery Arterial Procedures 2018 SVS Practice Guidelines vsweb.org/svsguidelines About the guidelines Published in the July 2018 issue of Journal of Vascular
Recommendations for Follow-up After Vascular Surgery Arterial Procedures 2018 SVS Practice Guidelines vsweb.org/svsguidelines About the guidelines Published in the July 2018 issue of Journal of Vascular
SAMIR R. KAPADIA, MD Department of Cardiovascular Medicine, Cleveland Clinic
 INTERPRETING KEY TRIALS CME CREDIT EDUCATIONAL OBJECTIVE: Readers will discuss the risks and benefits of carotid artery stenting vs endarterectomy with prospective candidates for these procedures OLCAY
INTERPRETING KEY TRIALS CME CREDIT EDUCATIONAL OBJECTIVE: Readers will discuss the risks and benefits of carotid artery stenting vs endarterectomy with prospective candidates for these procedures OLCAY
Assessment of the procedural etiology of stroke resulting from carotid artery stenting
 Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
TITLE: Carotid Artery Stenting versus Carotid Endarterectomy: A Review of the Clinical and Cost-Effectiveness
 TITLE: Carotid Artery Stenting versus Carotid Endarterectomy: A Review of the Clinical and Cost-Effectiveness DATE: 22 September 2009 CONTEXT AND POLICY ISSUES: A stroke is an interruption of blood supply
TITLE: Carotid Artery Stenting versus Carotid Endarterectomy: A Review of the Clinical and Cost-Effectiveness DATE: 22 September 2009 CONTEXT AND POLICY ISSUES: A stroke is an interruption of blood supply
Sample size for clinical trials
 British Standards Institution Study Day Sample size for clinical trials Martin Bland Prof. of Health Statistics University of York http://martinbland.co.uk/ Outcome variables for trials An outcome variable
British Standards Institution Study Day Sample size for clinical trials Martin Bland Prof. of Health Statistics University of York http://martinbland.co.uk/ Outcome variables for trials An outcome variable
Contemporary Management of Carotid Disease What We Know So Far
 Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers
 The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
Amaurosis fugax: some aspects of management
 Journal of Neurology, Neurosurgery, and Psvchiatry 1982;45:1-6 Amaurosis fugax: some aspects of management PJ PARKIN, BE KENDALL, J MARSHALL, WI McDONALD From the National Hospital for Nervous Diseases,
Journal of Neurology, Neurosurgery, and Psvchiatry 1982;45:1-6 Amaurosis fugax: some aspects of management PJ PARKIN, BE KENDALL, J MARSHALL, WI McDONALD From the National Hospital for Nervous Diseases,
The Great Swedish Debate. Håkan Pärsson Department Vascular Surgery Helsingborgs Lasarett, University Lund
 The Great Swedish Debate Håkan Pärsson Department Vascular Surgery Helsingborgs Lasarett, University Lund My Disclosures Trying to bribe the moderators What do my patients expect? Balanced information
The Great Swedish Debate Håkan Pärsson Department Vascular Surgery Helsingborgs Lasarett, University Lund My Disclosures Trying to bribe the moderators What do my patients expect? Balanced information
Introduction. Risk factors of PVD 5/8/2017
 PATHOPHYSIOLOGY AND CLINICAL FEATURES OF PERIPHERAL VASCULAR DISEASE Dr. Muhamad Zabidi Ahmad Radiologist and Section Chief, Radiology, Oncology and Nuclear Medicine Section, Advanced Medical and Dental
PATHOPHYSIOLOGY AND CLINICAL FEATURES OF PERIPHERAL VASCULAR DISEASE Dr. Muhamad Zabidi Ahmad Radiologist and Section Chief, Radiology, Oncology and Nuclear Medicine Section, Advanced Medical and Dental
Appendix A: Summary of evidence from surveillance
 Appendix A: Summary of evidence from surveillance 8-year surveillance (2016) stroke and transient ischaemic attack in over 16s (2008) NICE guideline CG68 Summary of new evidence from surveillance... 1
Appendix A: Summary of evidence from surveillance 8-year surveillance (2016) stroke and transient ischaemic attack in over 16s (2008) NICE guideline CG68 Summary of new evidence from surveillance... 1
Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis
 HOSPITAL CHRONICLES 2008, 3(3): 136 140 ORIGINAL ARTICLE Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis Antonios Polydorou, MD Hemodynamic
HOSPITAL CHRONICLES 2008, 3(3): 136 140 ORIGINAL ARTICLE Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis Antonios Polydorou, MD Hemodynamic
NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015)
 Title of Project: NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015) 1 Reason for the review In the UK, low dose aspirin (75mg) is licensed for the prevention of thrombotic
Title of Project: NHS Dumfries & Galloway Aspirin Discontinuation Audit May 2011 (updated August 2015) 1 Reason for the review In the UK, low dose aspirin (75mg) is licensed for the prevention of thrombotic
Aortic arch pathology. Cerebral ischemia following carotid artery stenosis.
 Important: -Subclavian Steal Syndrome -Cerebral ischemia Aortic arch pathology. Cerebral ischemia following carotid artery stenosis. Mina Aubeed & Alba Hernández Pinilla Aortic arch pathology Common arch
Important: -Subclavian Steal Syndrome -Cerebral ischemia Aortic arch pathology. Cerebral ischemia following carotid artery stenosis. Mina Aubeed & Alba Hernández Pinilla Aortic arch pathology Common arch
Protokollanhang zur SPACE-2-Studie Neurology Quality Standards
 Protokollanhang zur SPACE-2-Studie Neurology Quality Standards 1. General remarks In contrast to SPACE-1, the neurological center participating in the SPACE-2 trial will also be involved in the treatment
Protokollanhang zur SPACE-2-Studie Neurology Quality Standards 1. General remarks In contrast to SPACE-1, the neurological center participating in the SPACE-2 trial will also be involved in the treatment
Flow-diverting stents (in the Treatment of intracranial aneurysms)
 National Hospital for Neurology and Neurosurgery Flow-diverting stents (in the Treatment of intracranial aneurysms) Lysholm Department of Neuroradiology If you would like this document in another language
National Hospital for Neurology and Neurosurgery Flow-diverting stents (in the Treatment of intracranial aneurysms) Lysholm Department of Neuroradiology If you would like this document in another language
Clinical experience amongst surgeons in the Asymptomatic Carotid Surgery Trial-1 (ACST-1)
 Clinical experience amongst surgeons in the Asymptomatic Carotid Surgery Trial-1 (ACST-1) Short Title: Clinical experience in the Asymptomatic Carotid Surgery Trial-1 (ACST-1) Authors: Anne Huibers 1,2,
Clinical experience amongst surgeons in the Asymptomatic Carotid Surgery Trial-1 (ACST-1) Short Title: Clinical experience in the Asymptomatic Carotid Surgery Trial-1 (ACST-1) Authors: Anne Huibers 1,2,
How to Choose Between Carotid Stenting and Carotid Endarterectomy for Stroke Prevention
 How to Choose Between Carotid Stenting and Carotid Endarterectomy for Stroke Prevention Christopher J. White MD, MSCAI Chief of Medical Services, Professor and Chairman of Medicine Ochsner Medical Center
How to Choose Between Carotid Stenting and Carotid Endarterectomy for Stroke Prevention Christopher J. White MD, MSCAI Chief of Medical Services, Professor and Chairman of Medicine Ochsner Medical Center
Stroke prevention by carotid endarterectomy. Citation Hong Kong Practitioner, 1998, v. 20 n. 9, p
 Title Stroke prevention by carotid endarterectomy Author(s) Lau, H; Cheng, SWK Citation Hong Kong Practitioner, 1998, v. 20 n. 9, p. 484-490 Issued Date 1998 URL http://hdl.handle.net/10722/45393 Rights
Title Stroke prevention by carotid endarterectomy Author(s) Lau, H; Cheng, SWK Citation Hong Kong Practitioner, 1998, v. 20 n. 9, p. 484-490 Issued Date 1998 URL http://hdl.handle.net/10722/45393 Rights
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Diagnosis and management of carotid atherosclerosis
 Follow the link from the online version of this article to obtain certified continuing medical education credits bmj.com Cardiology updates from BMJ Group are at bmj.com/specialties/cardiovascular-medicine
Follow the link from the online version of this article to obtain certified continuing medical education credits bmj.com Cardiology updates from BMJ Group are at bmj.com/specialties/cardiovascular-medicine
Carotid. The. Issue. Now approved by the FDA, carotid stenting moves into the spotlight in endovascular care.
 The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
Original Article. Abstract. Introduction. Patients and Methods:
 Original Article Endovascular stenting for carotid artery stenosis: Results of local experience at Aga Khan University Hospital, Karachi Muhammad Azeem Uddin, Tanveer ul Haq, Ishtiaque Ahmed Chishty, Zafar
Original Article Endovascular stenting for carotid artery stenosis: Results of local experience at Aga Khan University Hospital, Karachi Muhammad Azeem Uddin, Tanveer ul Haq, Ishtiaque Ahmed Chishty, Zafar
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Articles. Funding British Heart Foundation; UK National Health Service Management Executive; UK Stroke Association.
 with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised
with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised
AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS
 Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Two major randomised trials concerning the surgical treatment of carotid artery stenosis
 944 * See end of article for authors affiliations c CAROTID Correspondence to: Dr Carlo Di Mario, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK; c.dimario@rbh.nthames.nhs.uk General cardiology
944 * See end of article for authors affiliations c CAROTID Correspondence to: Dr Carlo Di Mario, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK; c.dimario@rbh.nthames.nhs.uk General cardiology
Recanalization of Chronic Carotid Artery Occlusion Objective Improvement Of Cerebral Perfusion
 Recanalization of Chronic Carotid Artery Occlusion Objective Improvement Of Cerebral Perfusion Paul Hsien-Li Kao, MD Assistant Professor National Taiwan University Medical School and Hospital ICA stenting
Recanalization of Chronic Carotid Artery Occlusion Objective Improvement Of Cerebral Perfusion Paul Hsien-Li Kao, MD Assistant Professor National Taiwan University Medical School and Hospital ICA stenting
Post-op Carotid Complications A Nursing Perspective of What to Watch Out for
 Post-op Carotid Complications A Nursing Perspective of What to Watch Out for By Kariss Peterson, ARNP Swedish Medical Center Inpatient Neurology Team 1 Post-op Carotid Management Objectives Review the
Post-op Carotid Complications A Nursing Perspective of What to Watch Out for By Kariss Peterson, ARNP Swedish Medical Center Inpatient Neurology Team 1 Post-op Carotid Management Objectives Review the
Guideline scope Stroke and transient ischaemic attack in over 16s: diagnosis and initial management (update)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Stroke and transient ischaemic attack in over s: diagnosis and initial management (update) 0 0 This will update the NICE on stroke and
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Stroke and transient ischaemic attack in over s: diagnosis and initial management (update) 0 0 This will update the NICE on stroke and
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Fast-track CEA: a 3-year experience
 Fast-track CEA: a 3-year experience Giorgio L. Poletto, MD Milano, Italy 6th ACST-2 Collaborators Meeting, Palau de Congresos, Valencia. 24th and 25th September 2018. Stroke prevention Primary prevention:
Fast-track CEA: a 3-year experience Giorgio L. Poletto, MD Milano, Italy 6th ACST-2 Collaborators Meeting, Palau de Congresos, Valencia. 24th and 25th September 2018. Stroke prevention Primary prevention:
Carotid Stenosis (carotid artery disease)
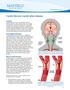 1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
Cerebral Protection for Carotid Artery Stenting: Safety, Efficacy and Limitations
 Review Safety and Efficacy of Catheter-based Carotid Artery Stenting Acta Cardiol Sin 2009;25:177 82 Cerebral Protection for Carotid Artery Stenting: Safety, Efficacy and Limitations Steve Leu, 1 Wen-Neng
Review Safety and Efficacy of Catheter-based Carotid Artery Stenting Acta Cardiol Sin 2009;25:177 82 Cerebral Protection for Carotid Artery Stenting: Safety, Efficacy and Limitations Steve Leu, 1 Wen-Neng
For the ICSS Investigators. 7 th Munich Vascular Conference Munich, 7 December 2017
 Restenosis and its impact on recurrent stroke risks after CAS and CEA for symptomatic carotid stenosis results from the International Carotid Stenting Study Leo H Bonati, John Gregson, Joanna Dobson, Dominick
Restenosis and its impact on recurrent stroke risks after CAS and CEA for symptomatic carotid stenosis results from the International Carotid Stenting Study Leo H Bonati, John Gregson, Joanna Dobson, Dominick
Risk Factors For Stroke, Myocardial Infarction, or Death Following Carotid Endarterectomy: Results From the International Carotid Stenting Study
 Eur J Vasc Endovasc Surg (2015) 50, 688e694 Risk Factors For Stroke, Myocardial Infarction, or Death Following Carotid Endarterectomy: Results From the International Carotid Stenting Study D. Doig a, E.L.
Eur J Vasc Endovasc Surg (2015) 50, 688e694 Risk Factors For Stroke, Myocardial Infarction, or Death Following Carotid Endarterectomy: Results From the International Carotid Stenting Study D. Doig a, E.L.
