2018 PHYSICIAN CODING AND PAYMENT GUIDE
|
|
|
- Conrad Reeves
- 5 years ago
- Views:
Transcription
1 2018 AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Effective January 1, 2018 Indication for Use: The MitraClip System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation.
2 POLICY UDATE Policy Update Medicare Coverage The Centers for Medicare and Medicaid Services (CMS) provide coverage for transcatheter mitral valve repair (TMVr) under Coverage with Evidence Development. 1 Among the coverage criteria specified in this National Coverage Determination (NCD): TMVr must be performed by an interventional cardiologist or a cardiothoracic surgeon. Interventional cardiologist(s) and cardiothoracic surgeon(s) may jointly participate in the intraoperative technical aspects of TMVr as appropriate. All TMVr cases must be enrolled in the national transcatheter valve therapy (TVT) registry. Other institutional and operator requirements apply based on multisociety guidelines. Refer to the NCD Decision Memo and MLN Matters Number MM9002 for additional details and requirements. 1,2 Note that local Medicare Administrative Contractors (MACs) may have additional coverage criteria as published in Local Coverage Determinations or articles. Additional Information Abbott is committed to supporting appropriate patient access to the MitraClip therapy and educating providers on the latest coverage, coding and payment policy. For additional questions, please contact the Reimbursement Hotline: Questions@AskAbbottVascular.com
3 Procedure Codes CPT CODE 3 DESCRIPTOR TMVr PROCEDURE WITH IMPLANT Transcatheter mitral valve repair percutaneous approach including transseptal puncture when performed; initial prosthesis Transcatheter mitral valve repair percutaneous approach including transseptal puncture when performed; additional prosthesis (es) during same session (List separately in addition to code for primary procedure). (Use in conjunction with 33418) CY2018 NATIONAL AVERAGE PAYMENT 4 CY2018 TOTAL FACILITY RVUs 4 $1, $ Angiography, radiological supervision, and interpretation performed to guide TMVr (eg, guiding device placement and documenting completion of the intervention) are included in these codes. Do not report diagnostic right and left heart catheterization procedure codes (93451, 93452, 93453, 93456, 93457, 93458, 93459, 93460, 93461, 93530, 93531, 93532, 93533) with or when done intrinsic to the valve repair procedure. TRANSEOPHAGEAL ECHOCARDIOGRAPPHY (TEE) (for intra-procedural monitoring) 93355* Echocardiography, transesophageal (TEE) for guidance of a transcatheter intracardiac or great vessel(s) structural intervention(s) (eg,tavr, transcathether pulmonary valve replacement, mitral valve repair, paravalvular regurgitation repair, left atrial appendage occlusion/closure, ventricular septal defect closure) (peri-and intra-procedural), real-time image acquisition and documentation, guidance with quantitative measurements, probe manipulation, interpretation, and report, including diagnostic transesophageal echocardiography and, when performed, administration of ultrasound contrast, Doppler, color flow, and 3D $ *Note that is bundled and not separately payable when reported on the same physician claim as the TMVr with MitraClip procedure (33418) or with anesthesia services
4 Coding Modifiers and Additional Requirements Additional coding requirements are necessary for TMVr cases enrolled in the TVT Registry and for cases involving two surgeons/co-surgons. MODIFIER -Q0/-Q /-82 ADDITIONAL REQUIRED INFORMATION NOTES Use for physician claims for cases enrolled in the TVT Registry. Use for physician claims for cases where two surgeons / co-surgeons perform TMVR. Note that in scenarios where co-surgeon participation is medically necessary, the submission of supporting documentation is required. 2 Use for assistant surgeon claims for TMVR. Append modifier to assistant surgeon claims; do not append modifier to primary surgeon claims. Use -80 when TMVR is performed at non-teaching community hospitals without surgery residents. Use -82 for when TMVR is performed at teaching hospitals with surgery residents; -82 indicates qualified surgery resident unavailable. Documentation regarding medical necessity required. NOTES NCT National Clinical Trial Number is required for cases enrolled in the TVT Registry. 2
5 Coding for Co-surgeons TMVr s covered by Medicare when performed by a single operator, or by co-surgeons as clinically appropriate. Per the TMVr NCD (20.33), The heart team s interventional cardiologist or a cardiothoracic surgeon must perform the TMVr. Interventional cardiologist(s) and cardiothoracic surgeon(s) may jointly participate in the intra-operative technical aspects of TMVr as appropriate." 2 The Physician Final Rule 2017 states that the -62 modifier for TMVr has a status indicator of one (1) which signifies that co-surgeons may be paid. Both surgeons use the same CPT code and apply the -62 modifier. Each surgeon submits a separate claim for their professional services. CMS general policy regarding co-surgeons, and medical necessity thereof, apply to TMVr procedures. At this time, there are no TMVrspecific criteria or guidance for co-surgeons, nor do we anticipate that CMS will develop such TMVr-specific direction regarding co-surgeons. Each surgeon s role must be clearly defined in the operative notes. See below table for considerations. Local Medicare Administrative Contractors (MAC) will determine the medical necessity of co-surgeons performing TMVr based on the documentation submitted. MACs would likely expect each co-surgeon to produce their own procedure / operative report detailing their role in the procedure and clinical decision-making, as well as the rationale for each surgeon participating in the procedure. While co-surgeons are typically expected to be from different specialties, co-surgeons from the same specialty may be paid at carrier discretion. CONSIDERATIONS Note which tasks you completed. Note which tasks your co-surgeon completed. Avoid using the term we. EXAMPLE I advanced a wire from the right femoral vein to the superior vena cava for placement of the transseptal sheath and needle. Dr. Smith advanced the mitral valve repair device and delivery system through the guide to the left atrium. Instead of We positioned the clip consider, I advanced the implant into the LV, by advancing the delivery catheter handle as Dr. Smith assisted in positioning the Clip below the valve by maintaining our anterior/posterior position with the guide.
6 Diagnosis Codes Below are the diagnosis codes currently included in the NCD for TMVr. 2 It is the responsibility of the physician to determine the appropriate diagnosis code(s) for each patient. As discussed above, participation in the TVT Registry is a requirement of TMVr coverage. Secondary diagnosis code Zoo.6 should be used to denote clinical trial participation for these TMVr claims. 2 ICD-10-CM DIAGNOSIS CODE CODE DESCRIPTOR I34.0 Nonrheumatic mitral (valve) insufficiency I34.1 Nonrheumatic mitral valve prolapse 6 Zoo.6 Encounter for exam for normal comparison and control in clinical research program Private Payers Private payers use a variety of payment methods for reimbursing inpatient services including case rates, percent of billed charges, DRGs, and device carve outs. Policies vary considerably for co-surgeons. Payers should be consulted in advance of the procedure to verify terms and conditions. Please check with your payer regarding appropriate coding and payment information.
7 PAGE 1 PAGE 2 For Implanting Physician(s): This checklist is provided as a visual summary of the information contained in this coding guide. Please see references at the end of this guide. It is the responsibility of the physician to determine the appropriate diagnosis code (s) for each patient. Codes listed below are for reference only. CODES / MODIFIERS / OTHER WHEN USED? INCLUDED NA Diagnosis Codes CPT Codes CPT Code Modifiers I34.0 / I34.1 Nonrheumatic mitral valve disorders 6 When appropriate Zoo.6 Examination of a participant in a clinical trial All cases Transcatheter mitral valve repair; initial prosthesis All cases Transcatheter mitral valve repair; add l prosthesis(es) -Q0/Q1 Investigational / Routine clinical service provided in a clinical research study that is in an approved clinical research study When two surgeons work together as primary surgeons preforming distinct part(s) of a procedure. Cases where two or more clips are implanted All cases When two surgeons/ co-surgeons perform the procedure. Supporting documentation is required to show medical necessity for co-surgeons NCT Number All cases* *NCT number is required for cases enrolled in the TVT registry. There is a separate NCT number for the ongoing Investigational Device Exemption (IDE) trial using MitraClip therapy called the COAPT trial. For coding and billing instructions for MitraClip procedures that are part of this trial please contact the Reimbursement Hotline.
8 PAGE 1 PAGE 2 For Echocardiographer This checklist is provided as a visual summary of the information contained in this coding guide. Please see references at the end of this guide. It is the responsibility of the physician to determine the appropriate diagnosis code (s) for each patient. Codes listed below are for reference only. CODES / MODIFIERS / OTHER WHEN USED? INCLUDED NA Diagnosis Codes I34.0 / I34.1 nonrheumatic mitral valve disorders 6 When appropriate Zoo.6 Examination of a participant in a clinical trial All cases CPT Codes TEE for intra procedural monitoring All cases CPT Code Modifiers -Q0/Q1 Investigational / Routine clinical service provided in a clinical research study that is in an approved clinical research study. All cases NCT Number All cases* *NCT number is required for cases enrolled in the TVT registry. There is a separate NCT number for the ongoing Investigational Device Exemption (IDE) trial using MitraClip therapy called the COAPT trial. For coding and billing instructions for MitraClip procedures that are part of this trial please contact the Reimbursement Hotline.
9 MITRACLIP CLIP DELIVERY SYSTEMS INDICATIONS FOR USE The MitraClip System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation. CONTRAINDICATIONS The MitraClip System is contraindicated in DMR patients with the following conditions: Patients who cannot tolerate procedural anticoagulation or post procedural antiplatelet regimen Active endocarditis of the mitral valve Rheumatic mitral valve disease Evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus WARNINGS DO NOT use MitraClip NT outside of the labeled indication. Treatment of non-prohibitive risk DMR patients should be conducted in accordance with standard hospital practices for surgical repair and replacement. MitraClip is intended to reduce mitral regurgitation. The MitraClip procedure is recommended to be performed when an experienced heart team has determined that reduction of MR to 2+ is reasonably expected following the MitraClip. If MR reduction to 2+ is not achieved, the benefits of reduced symptoms and hospitalizations, improved quality of life, and reverse LV remodeling expected from MitraClip may not occur. The MitraClip Implant should be implanted with sterile techniques using fluoroscopy and echocardiography (e.g., transesophageal [TEE] and transthoracic [TTE]) in a facility with on-site cardiac surgery and immediate access to a cardiac operating room. Read all instructions carefully. Failure to follow these instructions, warnings and precautions may lead to device damage, user injury or patient injury. Use universal precautions for biohazards and sharps while handling the MitraClip System to avoid user injury. Use of the MitraClip should be restricted to those physicians trained to perform invasive endovascular and transseptal procedures and those trained in the proper use of the system. The Clip Delivery System is provided sterile and designed for single use only. Cleaning, re-sterilization and / or reuse may result in infections, malfunction of the device or other serious injury or death.
10 MITRACLIP CLIP DELIVERY SYSTEMS PRECAUTIONS Patient Selection: - Prohibitive risk is determined by the clinical judgment of a heart team, including a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, due to the presence of one or more of the following documented surgical risk factors: 30-day STS predicted operative mortality risk score of 8% for patients deemed likely to undergo mitral valve replacement or 6% for patients deemed likely to undergo mitral valve repair Porcelain aorta or extensively calcified ascending aorta. Frailty (assessed by in-person cardiac surgeon consultation) Hostile chest Severe liver disease/cirrhosis (MELD Score >12) Severe pulmonary hypertension (systolic pulmonary artery pressure >2/3 systemic pressure Unusual extenuating circumstance, such as right ventricular dysfunction with severe tricuspid regurgitation, chemotherapy for malignancy, major bleeding diathesis, immobility, AIDS, severe dementia, high risk of aspiration, internal mammary artery (IMA) at high risk of injury, etc. Evaluable data regarding safety or effectiveness is not available for prohibitive risk DMR patients with an LVEF < 20% or an LVESD > 60mm. MitraClip should be used only when criteria for clip suitability for DMR have been met. The major clinical benefits of MitraClip are reduction of MR to 2+ resulting in reduced hospitalizations, improved quality of life, reverse LV remodeling and symptomatic relief in patients who have no other therapeutic option. No mortality benefit following MitraClip therapy has been demonstrated. The heart team should include a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease and may also include appropriate physicians to assess the adequacy of heart failure treatment and valvular anatomy. The heart team may determine an in-person surgical consult is needed to complete the assessment of prohibitive risk. The experienced mitral valve surgeon and heart team should take into account the outcome of this surgical consult when making the final determination of patient risk status. For reasonable assurance of device effectiveness, pre-procedural evaluation of the mitral valve and underlying pathologic anatomy and procedural echocardiographic assessment are essential. Note the Use by date specified on the package. Inspect all product prior to use. Do not use if the package is open or damaged, or if product is damaged.
11 MITRACLIP CLIP DELIVERY SYSTEMS SPECIAL PATIENT POPULATIONS Mitral Valve Etiology Safety and effectiveness of the MitraClip device has not been established in patients with MR due to underlying ventricular pathology (functional mitral regurgitation or FMR). Pregnancy The MitraClip device has not been tested in pregnant women. Effects on the developing fetus have not been studied. The risks and reproductive effects are unknown at this time. Gender No safety or effectiveness related gender differences were observed in clinical studies. Ethnicity Insufficient subject numbers prevent ethnicity-related analyses on the clinical safety and effectiveness. Anatomic Considerations For optimal results, the following anatomic patient characteristics should be considered. The safety and effectiveness of the MitraClip outside of these conditions has not been established. Use outside these conditions may interfere with placement of the MitraClip Implant or mitral valve leaflet insertion. - The primary regurgitant jet is non-commissural. If a secondary jet exists, it must be considered clinically insignificant - Mitral valve area 4.0 cm2 - Minimal calcification in the grasping area - No leaflet cleft in the grasping area - Flail width < 15 mm and flail gap < 10 mm Pediatrics Safety and effectiveness of the MitraClip device has not been established in pediatric patients.
12 MITRACLIP CLIP DELIVERY SYSTEMS POTENTIAL COMPLICATIONS AND ADVERSE EVENTS The following ANTICIPATED EVENTS have been identified as possible complications of the MitraClip procedure. Allergic reaction (anesthetic, contrast, Heparin, nickel alloy, latex); Aneurysm or pseudo-aneurysm; Arrhythmias; Atrial fibrillation; Atrial septal defect requiring intervention; Arterio-venous fistula; Bleeding; Cardiac arrest; Cardiac perforation; Cardiac tamponade / Pericardial Effusion; Chordal entanglement / rupture; Coagulopathy; Conversion to standard valve surgery; Death; Deep venous thrombus (DVT); Dislodgement of previously implanted devices; Dizziness; Drug reaction to anti-platelet / anticoagulation agents / contrast media; Dyskinesia; Dyspnea; Edema; Emboli (air, thrombus, MitraClip Implant); Emergency cardiac surgery; Endocarditis; Esophageal irritation; Esophageal perforation or stricture; Failure to deliver MitraClip to the intended site; Failure to retrieve MitraClip System components; Fever or hyperthermia; Gastrointestinal bleeding or infarct; Hematoma; Hemolysis; Hemorrhage requiring transfusion; Hypotension / hypertension; Infection; Injury to mitral valve complicating or preventing later surgical repair; Lymphatic complications; Mesenteric ischemia; MitraClip Implanterosion, migration or malposition; MitraClip Implant thrombosis; MitraClip System component(s) embolization; Mitral stenosis; Mitral valve injury; Multi-system organ failure; Myocardial infarction; Nausea / vomiting; Pain; Peripheral ischemia; Prolonged angina; Prolonged ventilation; Pulmonary congestion; Pulmonary thrombo-embolism; Renal insufficiency or failure; Respiratory failure / atelectasis / insufficiency or failure; Respiratory failure / atelectasis / pneumonia; Septicemia; Shock, Anaphylactic or Cardiogenic; Single leaflet device attachment (SLDA); Skin injury or tissue changes due to exposure to ionizing radiation; Stroke or transient ischemic attack (TIA); Urinary tract infection; Vascular trauma, dissection or occlusion; Vessel spasm; Vessel perforation or laceration; Worsening heart failure; Worsening mitral regurgitation; Wound dehiscence
13 Disclaimer The information provided in this document was obtained from third-party sources and is subject to change without notice as a result of changes in reimbursement laws, regulations, rules, policies, and payment amounts. All content is informational only, general in nature, and does not cover all situations or all payers rules and policies. It is the responsibility of the hospital or physician to determine appropriate coding for a particular patient and/or procedure. Any claim should be coded appropriately and supported with adequate documentation in the medical record. A determination of medical necessity is a prerequisite that Abbott assumes will have been made prior to assigning codes or requesting payments. Any codes provided are examples of codes that specify some procedures or which are otherwise supported by prevailing coding practices. They are not necessarily correct coding for any specific procedure using Abbott products. Hospitals and physicians should consult with appropriate payers, including Medicare Administrative Contractors, for specific information on proper coding, billing, and payment levels for healthcare procedures. Abbott makes no express or implied warranty or guarantee that (i) this list of codes and narratives is complete or error-free in this document, (ii) the use of this information will prevent difference of opinions or disputes with payers, (iii) these codes will be covered or (iv) the provider will receive the reimbursement amounts set forth herein. Reimbursement policies can vary considerably from one region to another and may change over time. The FDA-approved/ cleared labeling for all products may not be consistent with all uses described herein. This document is in no way intended to promote the off-label use of medical devices. This content is not intended to instruct hospitals and/or physicians on how to use medical devices or bill for healthcare procedures. References 1. CMS National Coverage Determination for Transcatheter Mitral Valve Repair CMS MLN Matters MM9002 Transcatheter Mitral Valve Repair (TMVR)-National Coverage Determination (NCD) 3. CPT Copyright 2017 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. 4. Centers for Medicare & Medicaid Services 42 CFR Parts 405, 410, 414, 424, and 425 [CMS-1676-F]. CY2018 relative value units and related information used in determining final Medicare payments. Rates are calculated using a 2018 Conversion Factor of National Correct Coding Initiative Policy Manual for Medicare Services, Chapter II, Revision 1/1/ Per CMS Transmittal 1630, released February 26, Transcatheter Mitral Valve Repair (TMVR)-National Coverage Determination (NCD). CMS Manual System. Pub Medicare Calims Processing. Transmittal Abbott 3200 Lakeside Dr., Santa Clara, CA USA Tel: Caution: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use provided inside the product carton (when available), at eifu.abbottvascular.com or at Manuals.sjm.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events MitraClip is a trademark of the Abbott Group of Companies Abbott. All rights reserved. AP US-Rev P
2019 MITRACLIP CODING AND PAYMENT GUIDE
 CLAIM 2019 MITRACLIP AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Hospital Rates: Effective October 1, 2018 Physician Rates: Effective January 1, 2019 References and Brief Summary 1 CLAIM
CLAIM 2019 MITRACLIP AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Hospital Rates: Effective October 1, 2018 Physician Rates: Effective January 1, 2019 References and Brief Summary 1 CLAIM
2018 MITRACLIP PHYSICIAN CODING AND PAYMENT GUIDE
 2018 MITRACLIP PHYSICIAN CODING AND PAYMENT GUIDE MEDICARE COVERAGE The Centers for Medicare and Medicaid Services (CMS) provide coverage for transcatheter mitral valve repair (TMVr) under Coverage with
2018 MITRACLIP PHYSICIAN CODING AND PAYMENT GUIDE MEDICARE COVERAGE The Centers for Medicare and Medicaid Services (CMS) provide coverage for transcatheter mitral valve repair (TMVr) under Coverage with
FY 2019 HOSPITAL CODING AND PAYMENT GUIDE
 FY 2019 HOSPITAL CODING AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Effective October 1, 2018 Indication for Use: The MitraClip System is indicated for the percutaneous reduction of significant
FY 2019 HOSPITAL CODING AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Effective October 1, 2018 Indication for Use: The MitraClip System is indicated for the percutaneous reduction of significant
FY 2018 PHYSICIAN CODING AND PAYMENT GUIDE
 RMATION FY 2018 AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Effective October 1, 2017 Indication for Use: The MitraClip NT Clip Delivery System is indicated for the percutaneous reduction
RMATION FY 2018 AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Effective October 1, 2017 Indication for Use: The MitraClip NT Clip Delivery System is indicated for the percutaneous reduction
Percutaneous Mitral Valve Repair. Transesophageal Echo Acquisition Guide
 TEE Screening MitraClip Percutaneous Mitral Valve Repair Transesophageal Echo Acquisition Guide Suggested Settings n Each view should be performed with and without color flow Doppler using color compare
TEE Screening MitraClip Percutaneous Mitral Valve Repair Transesophageal Echo Acquisition Guide Suggested Settings n Each view should be performed with and without color flow Doppler using color compare
MitraClip Transcatheter Mitral Valve Repair
 MitraClip Transcatheter Mitral Valve Repair A Breakthrough Technology for Your Select High-Surgical-Risk Patients with Severe DMR * Indications: MitraClip Clip Delivery System: The MitraClip Clip Delivery
MitraClip Transcatheter Mitral Valve Repair A Breakthrough Technology for Your Select High-Surgical-Risk Patients with Severe DMR * Indications: MitraClip Clip Delivery System: The MitraClip Clip Delivery
Percutaneous Mitral Valve Repair. Transthoracic Echo Acquisition Guide
 TTE Screening MitraClip Percutaneous Mitral Valve Repair Transthoracic Echo Acquisition Guide Settings and General Comments n Digital archived images should include three (3) or more cardiac cycles unless
TTE Screening MitraClip Percutaneous Mitral Valve Repair Transthoracic Echo Acquisition Guide Settings and General Comments n Digital archived images should include three (3) or more cardiac cycles unless
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine
 Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine Mitral regurgitation, regurgitant flow between the
Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine Mitral regurgitation, regurgitant flow between the
MitraClip World Wide Commercial Experience
 MitraClip World Wide Commercial Experience Valves Repaired. Lives Improved. Carlos G. Hernandez, MBA Sr. Product Manager, Strategic Planning Abbott Vascular October, 2014 MitraClip Transcatheter Mitral
MitraClip World Wide Commercial Experience Valves Repaired. Lives Improved. Carlos G. Hernandez, MBA Sr. Product Manager, Strategic Planning Abbott Vascular October, 2014 MitraClip Transcatheter Mitral
Percutaneous Mitral Valve Repair
 Percutaneous Mitral Valve Repair Policy Number: Original Effective Date: MM.06.027 08/01/2015 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/01/2017 Section: Surgery Place(s)
Percutaneous Mitral Valve Repair Policy Number: Original Effective Date: MM.06.027 08/01/2015 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/01/2017 Section: Surgery Place(s)
Percutaneous Mitral Valve Repair
 Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Introducing the COAPT Trial
 physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Preparing for Your Transcatheter Mitral Valve Repair Procedure
 WHAT YOU NEED TO KNOW Preparing for Your Transcatheter Mitral Valve Repair Procedure HOW SHOULD YOU PREPARE FOR YOUR PROCEDURE? In the days before your procedure, it is important that you: Take all your
WHAT YOU NEED TO KNOW Preparing for Your Transcatheter Mitral Valve Repair Procedure HOW SHOULD YOU PREPARE FOR YOUR PROCEDURE? In the days before your procedure, it is important that you: Take all your
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY
 SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
8/31/2016. Mitraclip in Matthew Johnson, MD
 Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
WATCHMAN REIMBURSEMENT GUIDE. This comprehensive guide provides an overview of the coding, coverage and payment landscape for the WATCHMAN system.
 WATCHMAN REIMBURSEMENT GUIDE This comprehensive guide provides an overview of the coding, coverage and payment landscape for the WATCHMAN system. For questions regarding WATCHMAN reimbursement, please
WATCHMAN REIMBURSEMENT GUIDE This comprehensive guide provides an overview of the coding, coverage and payment landscape for the WATCHMAN system. For questions regarding WATCHMAN reimbursement, please
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Mitral Valve Disease, When to Intervene
 Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
Update on Percutaneous Therapies for Structural Heart Disease. William Thomas MD Director of Structural Heart Program Tucson Medical Center
 Update on Percutaneous Therapies for Structural Heart Disease William Thomas MD Director of Structural Heart Program Tucson Medical Center NCVH 2014- Tucson Disclosure of Financial Interest Research: Stock
Update on Percutaneous Therapies for Structural Heart Disease William Thomas MD Director of Structural Heart Program Tucson Medical Center NCVH 2014- Tucson Disclosure of Financial Interest Research: Stock
Diagnostic and interventional venous procedures (lower extremity)
 2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
Outcomes of the Initial Experience with Commercial Transcatheter Mitral Valve Repair in the U.S.
 ACC 2015 LBCT Outcomes of the Initial Experience with Commercial Transcatheter Mitral Valve Repair in the U.S. A report from the STS/ACC TVT Registry Paul Sorajja, MD, Saibal Kar, MD, Amanda Stebbins,
ACC 2015 LBCT Outcomes of the Initial Experience with Commercial Transcatheter Mitral Valve Repair in the U.S. A report from the STS/ACC TVT Registry Paul Sorajja, MD, Saibal Kar, MD, Amanda Stebbins,
2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE
 2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE Contents Overview of Central Venous Access s for Hemodialysis 2 Procedures Using Hemodialysis s 2 Physician Reimbursement for Hemodialysis s 3
2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE Contents Overview of Central Venous Access s for Hemodialysis 2 Procedures Using Hemodialysis s 2 Physician Reimbursement for Hemodialysis s 3
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
1. Technology Name: WATCHMAN Left Atrial Appendage Closure Technology. 3. Trade Brand of Technology: WATCHMAN Left Atrial Appendage Closure Technology
 1. Technology Name: REASANZ (serelaxin) Injection 1 mg/ml Solution for I.V. Infusion 2. Manufacturer Name: Novartis Pharmaceuticals Corporation 3. Trade Brand of Technology: The brand name of the new technology
1. Technology Name: REASANZ (serelaxin) Injection 1 mg/ml Solution for I.V. Infusion 2. Manufacturer Name: Novartis Pharmaceuticals Corporation 3. Trade Brand of Technology: The brand name of the new technology
Catheter-based mitral valve repair MitraClip System
 Percutaneous Mitral Valve Repair: Results of the EVEREST II Trial William A. Gray MD Director of Endovascular Services Associate Professor of Clinical Medicine Columbia University Medical Center The Cardiovascular
Percutaneous Mitral Valve Repair: Results of the EVEREST II Trial William A. Gray MD Director of Endovascular Services Associate Professor of Clinical Medicine Columbia University Medical Center The Cardiovascular
Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat
 Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat Innovative Procedures, Devices & State of the Art Care for Arrhythmias, Heart Failure & Structural Heart Disease October 8-10,
Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat Innovative Procedures, Devices & State of the Art Care for Arrhythmias, Heart Failure & Structural Heart Disease October 8-10,
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Centers for Medicare and Medicaid Services. National Coverage of Transcatheter Valve Technologies December 2015
 Centers for Medicare and Medicaid Services National Coverage of Transcatheter Valve Technologies December 2015 National Coverage Determination (NCD) for Transcatheter Aortic Valve Replacement (TAVR) (20.32)
Centers for Medicare and Medicaid Services National Coverage of Transcatheter Valve Technologies December 2015 National Coverage Determination (NCD) for Transcatheter Aortic Valve Replacement (TAVR) (20.32)
Vascular Plug Procedures 2014 CODING AND PAYMENT REFERENCE GUIDE ST. JUDE MEDICAL - CARDIOVASCULAR DIVISION
 Vascular Plug Procedures 2014 CODING AND PAYMENT REFERENCE GUIDE ST. JUDE MEDICAL - CARDIOVASCULAR DIVISION IMPORTANT: St. Jude Medical provides this reference guide for general information purposes only
Vascular Plug Procedures 2014 CODING AND PAYMENT REFERENCE GUIDE ST. JUDE MEDICAL - CARDIOVASCULAR DIVISION IMPORTANT: St. Jude Medical provides this reference guide for general information purposes only
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
ABBOTT CODING GUIDE STRUCTURAL HEART AND VALVES CONGENITAL DEFECTS SURGICAL HEART VALVES AMPLATZER PFO OCCLUDER. Effective January 1, 2019
 ABBOTT CODING GUIDE STRUCTURAL HEART AND VALVES Effective January 1, 2019 STRUCTURAL HEART AND VALVES Effective Janaury 1, 2019 Introduction The Structural Heart and Valves Coding Guide is intended to
ABBOTT CODING GUIDE STRUCTURAL HEART AND VALVES Effective January 1, 2019 STRUCTURAL HEART AND VALVES Effective Janaury 1, 2019 Introduction The Structural Heart and Valves Coding Guide is intended to
Detailed Order Request Checklists for Cardiology
 Next Generation Solutions Detailed Order Request Checklists for Cardiology 8600 West Bryn Mawr Avenue South Tower Suite 800 Chicago, IL 60631 www.aimspecialtyhealth.com Appropriate.Safe.Affordable 2018
Next Generation Solutions Detailed Order Request Checklists for Cardiology 8600 West Bryn Mawr Avenue South Tower Suite 800 Chicago, IL 60631 www.aimspecialtyhealth.com Appropriate.Safe.Affordable 2018
University of Florida Department of Surgery. CardioThoracic Surgery VA Learning Objectives
 University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
Eulogio Garcia MD Hospital Clínico San Carlos Madrid - Spain
 Eulogio Garcia MD Hospital Clínico San Carlos Madrid - Spain Device Landscape 2010 PERCUTANEOUS TECHNIQUES Percutaneous indirect annuloplasty Percutaneous direct annuloplasty Edge to Edge ( E-Valve ) Non
Eulogio Garcia MD Hospital Clínico San Carlos Madrid - Spain Device Landscape 2010 PERCUTANEOUS TECHNIQUES Percutaneous indirect annuloplasty Percutaneous direct annuloplasty Edge to Edge ( E-Valve ) Non
Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System GO TO INDEX COMMONLY BILLED CODES
 2015 Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System COMMONLY BILLED CODES 2015 INDEX For Right Ventricular Outflow Tract Conduit Dysfunction.... 1 ICD-9-CM Diagnosis
2015 Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System COMMONLY BILLED CODES 2015 INDEX For Right Ventricular Outflow Tract Conduit Dysfunction.... 1 ICD-9-CM Diagnosis
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
Clinical Indications for Echocardiography
 Clinical Indications for Echocardiography Echocardiography is widely utilised and potential applications are increasing with advances in technology. The aim of this document is two-fold: 1) To define clinical
Clinical Indications for Echocardiography Echocardiography is widely utilised and potential applications are increasing with advances in technology. The aim of this document is two-fold: 1) To define clinical
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
Organic mitral regurgitation
 The best in heart valve disease Organic mitral regurgitation Ewa Szymczyk Department of Cardiology Medical University of Lodz, Poland I have nothing to declare Organic mitral regurgitation leaflet abnormality
The best in heart valve disease Organic mitral regurgitation Ewa Szymczyk Department of Cardiology Medical University of Lodz, Poland I have nothing to declare Organic mitral regurgitation leaflet abnormality
Adult Echocardiography Examination Content Outline
 Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
2018 Diagnosis Coding Fact Sheet
 The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
TREATMENT OF MITRAL REGURGITATION RAJA NAZIR FACC
 TREATMENT OF MITRAL REGURGITATION RAJA NAZIR FACC NATURAL HISTORY OF MITRAL REGURGITATION Abdallah El Sabbagh et al. JIMG 2018;11:628-643 TREATMENT OPTIONS SURGERY REPAIR REPLACEMENT PERCUTANEOUS INTERVENTIONS
TREATMENT OF MITRAL REGURGITATION RAJA NAZIR FACC NATURAL HISTORY OF MITRAL REGURGITATION Abdallah El Sabbagh et al. JIMG 2018;11:628-643 TREATMENT OPTIONS SURGERY REPAIR REPLACEMENT PERCUTANEOUS INTERVENTIONS
WATCHMAN. For questions regarding WATCHMAN reimbursement, please contact:
 WATCHMAN IMPORTANCE OF DOCUMENTATION & THE IMPACT ON MS- DRG ASSIGNMENT This guide stresses the importance of documentation in capturing the appropriate acuity level for patients considered WATCHMAN candidates.
WATCHMAN IMPORTANCE OF DOCUMENTATION & THE IMPACT ON MS- DRG ASSIGNMENT This guide stresses the importance of documentation in capturing the appropriate acuity level for patients considered WATCHMAN candidates.
Advanced Anesthesia. Presented by: Shelly Cronin, CPC, CPMA, CANPC, CGSC, CGIC. Agenda
 Advanced Anesthesia Presented by: Shelly Cronin, CPC, CPMA, CANPC, CGSC, CGIC 1 Agenda Understanding key terms Review coding concepts & modifiers Documentation standards How to avoid coding pitfalls New
Advanced Anesthesia Presented by: Shelly Cronin, CPC, CPMA, CANPC, CGSC, CGIC 1 Agenda Understanding key terms Review coding concepts & modifiers Documentation standards How to avoid coding pitfalls New
THINK OUTSIDE THE PILLBOX
 THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
CPT Category III Codes
 CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 20 new Category III codes (0543T-0562T) and one revised Category III code (0402T) and guidelines accepted by
CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 20 new Category III codes (0543T-0562T) and one revised Category III code (0402T) and guidelines accepted by
Coronary intravascular ultrasound (IVUS)
 2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
2018 Cerebrovascular Reimbursement Coding Fact Sheet
 The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
Diagnostic and interventional venous procedures (lower extremity)
 Coding and Medicare national payment guide 2018 Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips is gathered
Coding and Medicare national payment guide 2018 Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips is gathered
Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft
 MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
Patient guide: pfm Nit-Occlud PDA coil occlusion system. Catheter occlusion of. Patent Ductus Arteriosus. with the
 Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
THINK OUTSIDE THE PILLBOX
 THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
Watchman and Structural update..the next frontier. Ari Chanda, MD Cardiology Associates of Fredericksburg
 Watchman and Structural update..the next frontier Ari Chanda, MD Cardiology Associates of Fredericksburg Different Left Atrial Appendage (LAA) morphologies Watchman (the device) Fabric Anchors Device structure
Watchman and Structural update..the next frontier Ari Chanda, MD Cardiology Associates of Fredericksburg Different Left Atrial Appendage (LAA) morphologies Watchman (the device) Fabric Anchors Device structure
Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality)
 2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
2015 Procedural Payment Guide
 Contents Introduction Disclaimer (print page 2) Description of Payment Methods (print page 3) Procedural Payment Guide: Cardiac Rhythm Management and Electrophysiology Procedures (print page range: 4-17)
Contents Introduction Disclaimer (print page 2) Description of Payment Methods (print page 3) Procedural Payment Guide: Cardiac Rhythm Management and Electrophysiology Procedures (print page range: 4-17)
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Routine MitraClip. Image Guidance Step by Step
 Routine MitraClip Image Guidance Step by Step Douglas C. Shook, MD, FASE Director, Cardiothoracic Anesthesia Fellowship Director, Cardiac Interventional Anesthesia Department of Anesthesiology BRIGHAM
Routine MitraClip Image Guidance Step by Step Douglas C. Shook, MD, FASE Director, Cardiothoracic Anesthesia Fellowship Director, Cardiac Interventional Anesthesia Department of Anesthesiology BRIGHAM
7. Echocardiography Appropriate Use Criteria (by Indication)
 Criteria for Echocardiography 1133 7. Echocardiography Criteria (by ) Table 1. TTE for General Evaluation of Cardiac Structure and Function Suspected Cardiac Etiology General With TTE 1. Symptoms or conditions
Criteria for Echocardiography 1133 7. Echocardiography Criteria (by ) Table 1. TTE for General Evaluation of Cardiac Structure and Function Suspected Cardiac Etiology General With TTE 1. Symptoms or conditions
Cardiac Valve/Structural Therapies
 Property of Dr. Chad Rammohan Cardiac Valve/Structural Therapies Chad Rammohan, MD FACC Medical Director, El Camino Hospital Cardiac Catheterization Lab Director, Interventional and Structural Cardiology,
Property of Dr. Chad Rammohan Cardiac Valve/Structural Therapies Chad Rammohan, MD FACC Medical Director, El Camino Hospital Cardiac Catheterization Lab Director, Interventional and Structural Cardiology,
University of Wisconsin - Madison Cardiovascular Medicine Fellowship Program UW CICU Rotation Goals and Objectives
 Background: The field of critical care cardiology has evolved considerably over the past 2 decades. Contemporary critical care cardiology is increasingly focused on the management of patients with advanced
Background: The field of critical care cardiology has evolved considerably over the past 2 decades. Contemporary critical care cardiology is increasingly focused on the management of patients with advanced
1. CARDIOLOGY. These listings cannot be correctly interpreted without reference to the Preamble. Anes. $ Level
 1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
Echocardiography as a diagnostic and management tool in medical emergencies
 Echocardiography as a diagnostic and management tool in medical emergencies Frank van der Heusen MD Department of Anesthesia and perioperative Care UCSF Medical Center Objective of this presentation Indications
Echocardiography as a diagnostic and management tool in medical emergencies Frank van der Heusen MD Department of Anesthesia and perioperative Care UCSF Medical Center Objective of this presentation Indications
2018 COMMONLY BILLED CODES
 Melody Transcatheter Pulmonary Valve Ensemble II Transcatheter Valve Delivery System 2018 COMMONLY BILLED CODES INDEX For Right Ventricular Outflow Tract Conduit Dysfunction... 1 Hospital Inpatient Coding
Melody Transcatheter Pulmonary Valve Ensemble II Transcatheter Valve Delivery System 2018 COMMONLY BILLED CODES INDEX For Right Ventricular Outflow Tract Conduit Dysfunction... 1 Hospital Inpatient Coding
Treatment of Inter-MitraClip Regurgitation Due to Posterior Leaflet Cleft by Use of The Amplatzer Vascular Plug II device
 Volume 1, Issue 3 Case Report ISSN: 2572-9292 Treatment of Inter-MitraClip Regurgitation Due to Posterior Leaflet Cleft by Use of The Amplatzer Vascular Plug II device Neha M. Mantri, Gagan D. Singh, Thomas
Volume 1, Issue 3 Case Report ISSN: 2572-9292 Treatment of Inter-MitraClip Regurgitation Due to Posterior Leaflet Cleft by Use of The Amplatzer Vascular Plug II device Neha M. Mantri, Gagan D. Singh, Thomas
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
CPT Code Details
 CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived flow reserve measurement ( vessel or graft) during angiography pharmacologically induced stress; each additional
CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived flow reserve measurement ( vessel or graft) during angiography pharmacologically induced stress; each additional
Common Codes for ICD-10
 Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Transcatheter therapy, venous infusion for thrombolysis, any method, including radiological supervision and interpretation, initial treatment day
 Potential CPT Codes 1 CPT CPT Description Physician Work RVU Total RVU (In-Facility) 2018 National Avg. Medicare Physician Payment (In-Facility) Mechanical Thrombectomy 37187 37188 Percutaneous transluminal
Potential CPT Codes 1 CPT CPT Description Physician Work RVU Total RVU (In-Facility) 2018 National Avg. Medicare Physician Payment (In-Facility) Mechanical Thrombectomy 37187 37188 Percutaneous transluminal
2018 Endovascular Reimbursement Coding Fact Sheet
 The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE. Updated September 2018
 CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
2017 COMMONLY BILLED CODES
 Melody Transcatheter Pulmonary Valve Ensemble II Transcatheter Valve Delivery System 2017 COMMONLY BILLED CODES INDEX For Right Ventricular Outflow Tract Conduit Dysfunction... 1 Hospital Inpatient Coding
Melody Transcatheter Pulmonary Valve Ensemble II Transcatheter Valve Delivery System 2017 COMMONLY BILLED CODES INDEX For Right Ventricular Outflow Tract Conduit Dysfunction... 1 Hospital Inpatient Coding
ASE 2011 Appropriate Use Criteria for Echocardiography
 ASE 2011 Appropriate Use Criteria for Echocardiography Table 1. TTE for General Evaluation of Cardiac Structure and Function 1 2 Suspected Cardiac Etiology General With TTE Symptoms or conditions potentially
ASE 2011 Appropriate Use Criteria for Echocardiography Table 1. TTE for General Evaluation of Cardiac Structure and Function 1 2 Suspected Cardiac Etiology General With TTE Symptoms or conditions potentially
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Mitral Regurgitation
 Mitral Regurgitation Focus on Percutaneous Repair Steven J. Yakubov, MD FACC FSCAI System Chief, Structural Heart Diseaese, OhioHealth John H. McConnell Chair of Advanced Structural Heart Disease Medical
Mitral Regurgitation Focus on Percutaneous Repair Steven J. Yakubov, MD FACC FSCAI System Chief, Structural Heart Diseaese, OhioHealth John H. McConnell Chair of Advanced Structural Heart Disease Medical
ECHOCARDIOGRAPHY. Patient Care. Goals and Objectives PF EF MF LF Aspirational
 Patient Care Be able to: Perform and interpret basic TTE and X cardiac Doppler examinations Perform and interpret a comprehensive X TTE and cardiac Doppler examination Perform and interpret a comprehensive
Patient Care Be able to: Perform and interpret basic TTE and X cardiac Doppler examinations Perform and interpret a comprehensive X TTE and cardiac Doppler examination Perform and interpret a comprehensive
Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2)
 Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2) Definition Vascular surgery is the specialty concerned with the diagnosis and management of congenital and acquired diseases of the
Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2) Definition Vascular surgery is the specialty concerned with the diagnosis and management of congenital and acquired diseases of the
RESPECT Safety Findings
 CO-1 SCAI Town Hall Meeting Monday, October 31, 2016 Washington, DC RESPECT Safety Findings John D. Carroll, M.D., MSCAI Professor of Medicine Cardiology University of Colorado School of Medicine University
CO-1 SCAI Town Hall Meeting Monday, October 31, 2016 Washington, DC RESPECT Safety Findings John D. Carroll, M.D., MSCAI Professor of Medicine Cardiology University of Colorado School of Medicine University
Patient Information. Atrial Septal Defect (ASD) Repair
 Patient Information Atrial Septal Defect (ASD) Repair Table of Contents Overview................................. 4 Symptoms................................ 5 Causes..................................
Patient Information Atrial Septal Defect (ASD) Repair Table of Contents Overview................................. 4 Symptoms................................ 5 Causes..................................
INTRO LEFT VENTRICULAR ASSIST DEVICE (LVAD) ACUTE MCS MECHANICAL CIRCULATORY SUPPORT (MCS)
 ABBOTT CODING GUIDE MECHANICAL CIRCULATORY SUPPORT (MCS) LEFT VENTRICULAR ASSIST DEVICE (LVAD), (HEARTMATE II OR HEARTMATE 3 LVADS) ACUTE MCS (CENTRIMAG OR PEDIMAG PUMPS) Effective January 1, 2019 MECHANICAL
ABBOTT CODING GUIDE MECHANICAL CIRCULATORY SUPPORT (MCS) LEFT VENTRICULAR ASSIST DEVICE (LVAD), (HEARTMATE II OR HEARTMATE 3 LVADS) ACUTE MCS (CENTRIMAG OR PEDIMAG PUMPS) Effective January 1, 2019 MECHANICAL
Ultrasound Reimbursement Information for Anesthesiology 1
 GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
Aortic Stenosis and TAVR TARUN NAGRANI, MD INTERVENTIONAL AND ENDOVASCULAR CARDIOLOGIST, SOMC
 Aortic Stenosis and TAVR TARUN NAGRANI, MD INTERVENTIONAL AND ENDOVASCULAR CARDIOLOGIST, SOMC No Financial Disclosures Aortic Stenosis AS is an insidious disease with a long latency period followed by
Aortic Stenosis and TAVR TARUN NAGRANI, MD INTERVENTIONAL AND ENDOVASCULAR CARDIOLOGIST, SOMC No Financial Disclosures Aortic Stenosis AS is an insidious disease with a long latency period followed by
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Mitral Regurgitation
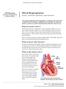 UW MEDICINE PATIENT EDUCATION Mitral Regurgitation Causes, symptoms, diagnosis, and treatment This handout describes mitral regurgitation, a disease of the mitral valve. It explains how this disease is
UW MEDICINE PATIENT EDUCATION Mitral Regurgitation Causes, symptoms, diagnosis, and treatment This handout describes mitral regurgitation, a disease of the mitral valve. It explains how this disease is
Current status: Percutaneous mitral valve therapy
 Current status: Percutaneous mitral valve therapy Ted Feldman, M.D., FSCAI FACC FESC Evanston Hospital ESC Stockholm 2010 Disclosures Research Grants Abbott, Edwards Consultant Abbott, Edwards 2 Percutaneous
Current status: Percutaneous mitral valve therapy Ted Feldman, M.D., FSCAI FACC FESC Evanston Hospital ESC Stockholm 2010 Disclosures Research Grants Abbott, Edwards Consultant Abbott, Edwards 2 Percutaneous
Transcatheter Aortic Valve Implantation Procedure (TAVI)
 Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Intravascular Ultrasound
 Scan for mobile link. Intravascular Ultrasound Intravascular ultrasound (IVUS) uses a transducer or probe to generate sound waves and produce pictures of the coronary arteries. IVUS can show the entire
Scan for mobile link. Intravascular Ultrasound Intravascular ultrasound (IVUS) uses a transducer or probe to generate sound waves and produce pictures of the coronary arteries. IVUS can show the entire
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014
 Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
2019 FAQ EP Coding and Reimbursement Physicians and Facilities
 2019 FAQ EP Coding and Reimbursement Physicians and Facilities RESOURCES TO ASSIST YOU WITH THE REIMBURSEMENT PROCESS Reimbursement and Coding Guide Coding and Reimbursement Frequently Asked Questions
2019 FAQ EP Coding and Reimbursement Physicians and Facilities RESOURCES TO ASSIST YOU WITH THE REIMBURSEMENT PROCESS Reimbursement and Coding Guide Coding and Reimbursement Frequently Asked Questions
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #358: Patient-Centered Surgical Risk Assessment and Communication National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Quality ID #358: Patient-Centered Surgical Risk Assessment and Communication National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Men s Health Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
