Therapeutic outcome of cyclic VAD (vincristine, doxorubicin and dexamethasone) therapy in primary systemic AL amyloidosis patients
|
|
|
- Marlene Daniel
- 5 years ago
- Views:
Transcription
1 Original Therapeutic outcome of cyclic VAD (vincristine, doxorubicin and dexamethasone) therapy in primary systemic AL amyloidosis patients Ko-ichi Tazawa, Masayuki Matsuda, Takuhiro Yoshida, Takahisa Gono, Nagaaki Katoh, Yasuhiro Shimojima, Wataru Ishii, Tomohisa Fushimi, Jun Koyama*, Shu-ichi Ikeda Department of Medicine (Neurology and Rheumatology), Shinshu University School of Medicine, Matsumoto *Department of Medicine (Cardiology), Shinshu University School of Medicine, Matsumoto Correspondence to: Dr. M. Matsuda, Department of Internal Medicine (Neurology and Rheumatology), Shinshu University School of Medicine, Asahi, Matsumoto , Japan. TEL: FAX:
2 Abstract Objective: Intensive chemotherapy targeting plasma cell dyscrasia has been recently employed for the treatment of primary systemic AL amyloidosis. We prospectively studied the clinical usefulness of cyclic VAD (vincristine, doxorubicin and dexamethasone) in patients with primary systemic AL amyloidosis who were ineligible for high-dose melphalan with autologous stem cell support. Patients and methods: Eight patients (mean age, 60.4±8.8 years) were treated with cyclic VAD until the disappearance of M-protein from both serum and urine. Of these, seven showed nephrotic syndrome before the start of VAD irrespective of a decrease in creatinine clearance. Serial follow-up studies after VAD evaluated hematological status and organ function. Results: Four patients (50%) showed a marked decrease in abnormal plasma cells in the bone marrow and normalized κ/λ ratios of serum free light chain in conjunction with disappearance of M-protein after 1 to 3 courses of VAD. There were no serious adverse events, and nephrotic syndrome gradually improved with no hematological relapse in the follow-up period of 3 to 5 years. The remaining 4 patients showed worsening of congestive heart failure and/or systemic edema ascribable to dexamethasone, resulting in cessation of cyclic VAD before disappearance of M-protein. All of these patients died of multiple organ failure or required permanent hemodialysis within 1 year after the start of cyclic VAD. Conclusions: Cyclic VAD is a potent therapeutic option in primary systemic AL amyloidosis, but in patients with renal or cardiac dysfunction careful management for adverse events, especially body fluid retention, is necessary. Keywords: primary systemic AL amyloidosis, cyclic VAD (vincristine, doxorubicin and dexamethasone), plasma cell dyscrasia, nephrotic syndrome 2
3 INTRODUCTION Primary systemic AL amyloidosis is an intractable disorder caused by plasma cell dyscrasia. Amyloid fibrils, formed by degradation of immunoglobulin light chains produced by abnormal plasma cells, gradually accumulate in multiple vital organs, including the heart, kidneys and gastrointestinal (GI) tract (1). To avoid progressive dysfunction of these organs, various chemotherapies targeting plasma cell dyscrasia have been employed for the treatment of primary systemic AL amyloidosis. Among them high-dose melphalan with autologous peripheral blood stem cell transplantation (auto-pbsct) has been widely accepted as the most effective chemotherapy with regard to hematological response in the last decade (2). Nevertheless, this treatment has the disadvantage of high mortality ascribable to subclinical or overt dysfunction in multiple vital organs (3, 4). VAD (vincristine, doxorubicin and dexamethasone) is another intensive chemotherapy for primary systemic AL amyloidosis (5-7). In this study we performed cyclic VAD in Japanese patients with systemic AL amyloidosis according to criteria related to the severity of organ involvement. One half of the patients showed improvement of clinical symptoms, particularly nephrotic syndrome, after complete hematological remission, and we focus upon the clinical benefits and adverse effects of cyclic VAD. PATIENTS AND METHODS Patients Eight patients with primary systemic AL amyloidosis (4 men and 4 women; age range, 45 to 71 years; mean, 60.4±8.8 years), who were selected according to criteria reported by Gono et al (8), were enrolled in this study. None of the patients had received any medical treatment for plasma cell dyscrasia at enrollment. To detect amyloid deposition, alkaline Congo red staining was performed in more than 2 biopsied tissues, and in at least one of these specimens immunohistochemical staining was carried out 3
4 using different antibodies to ALκ, ALλ, amyloid A, transthyretin and β2 microglobulin in order to confirm it as AL type (9). Multiple myeloma was excluded by a serum M-protein level of lower than 3.0 g/dl, plasma cells less than 10% of the total number of nucleated cells in the bone marrow and no osteolytic lesions on X-ray examinations according to the diagnostic criteria proposed by the International Myeloma Working Group (10). The percentage of plasma cells in the bone marrow was examined on smear specimens treated with Wright-Giemsa staining. To identify M-protein in serum and urine, immunofixation was performed in all the patients, and other information was obtained from their medical records. Free light chains (FLCs) in serum were simultaneously measured using a commercially available kit based on a latex-enhanced immunoassay (The Binding Site, Birmingham, UK) on a Behring BN II nephelometric analyzer (Dade Behring, Deerfield, IL, USA). Both κ- and λ-type FLCs were separately determined, and the κ/λ ratio was calculated. Normal values of the κ/λ ratio range from 0.26 to At study entry the extent of amyloid-induced organ involvement was evaluated in each patient according to the clinical criteria proposed by Comenzo et al (11). The Local Ethical Committee approved this study. Treatment The VAD regimen was as follows: vincristine 0.4 mg/day and doxorubicin 9 mg/m 2 /day by continuous intravenous infusion on days 1-4 (total dose: vincristine 1.6 mg, doxorubicin 36 mg/m 2 ), and dexamethasone 40 mg/day by intravenous infusion on days 1-4, 9-12 and (total dose 480 mg). All treatments were repeated every 4 weeks until disappearance of M-protein from both serum and urine on immunofixation, which was defined as complete hematological remission, as long as there were no serious complications. Interferon-α was used at a dose of 3 MIU/week as a maintenance therapy after achieving complete hematological remission. M-protein was investigated on immunofixation at 3- to 6-month intervals after its disappearance. When M-protein reappeared in either serum or urine, readministration of VAD was actively considered. 4
5 Informed consent was obtained from all the patients before starting VAD. Evaluation of therapeutic and toxic effects Therapeutic effects of cyclic VAD were evaluated in terms of amyloidosis-related organ involvement, particularly renal function, and performance status of the patients. Because almost all the patients in this study showed nephrotic syndrome, we employed daily protein excretion in urine as well as total protein and albumin in serum as a therapeutic marker of renal involvement. Serum levels of brain natriuretic peptide (BNP) and echocardiographic examinations assessed cardiac involvement. A standard M-mode scan of the left atrium (LA) and ventricle (LV) was made in order to measure the thickness of the interventricular septum (IVS), fractional shortening of LV (FS), LV dimensions in the end-diastole and LA dimensions. The LV outflow tract velocity was recorded on pulsed Doppler, positioning the sample volume just below the aortic valve in the apical 4-chamber view. Analysis of Doppler flow was performed with computerized planimetry (Color Cineview Plus, TomTee Imaging Systems). Three or more consecutive beats were averaged for each measurement. To quantitatively evaluate diastolic function, we measured the peak velocities of early (E) and late filling (A) waves of transmitral flow (TMF), E/A ratio and deceleration time of E waves. The Southwestern Oncology Group (SWOG) criteria were used for evaluating performance status of the patients (12). Toxic effects were also assessed during and after the chemotherapy. Survival curves were constructed by Kaplan-Meier s method, depending on whether the patients achieved complete hematological remission or not. In these curves starting and end points were set at commencement of therapy and death, respectively. Statistics A log-rank test was employed for detecting a statistically significant difference between survival curves. A p-level less than 0.05 was considered to be statistically significant. Commercially available statistics software was used for data analysis 5
6 (StatView for Macintosh, Abacus Concepts, Berkeley, CA, USA). RESULTS Hematological response Clinical profiles of the patients are shown in Table 1. Four of 8 patients had M-protein in serum, which was identified as IgGλ in 3 and IgAκ in 1 on immunofixation. Despite the absence of M-protein in serum, the remaining 4 patients showed BJPλ in urine. All of the patients demonstrated abnormal FLC κ/λ ratios in serum and clonal expansion of plasma cells in the bone marrow on flow cytometry except for case 6, in whom the FLC κ/λ ratio was within normal limits. The main clinical symptoms of the patients consisted of nephrotic syndrome in 7 patients (cases 1, 2, 4 to 8), amyloid cardiomyopathy in one (case 5) and urinary bladder hemorrhage in one (case 3). Case 5 showed both nephrotic syndrome and cardiomyopathy. Cases 1 to 3 showed complete hematological remission with no serious adverse events after 2 or 3 courses of VAD. Case 4 achieved complete hematological remission after 1 course of VAD, but the second cycle of this chemotherapy could not be performed because of syncopal attacks ascribable to temporary worsening of cardiac and/or autonomic nerve function. Cases 2 to 4 were treated with interferon-α after achieving complete hematological remission, but case 1 refused this maintenance therapy. The administration of interferon-α was stopped in cases 2 and 3 because of adverse effects, such as thrombocytopenia and depression, at 48 and 12 months after commencement, respectively. Cases 1 to 4 showed no reappearance of M-protein in either serum or urine in the serial follow-up studies. The FLC κ/λ ratio was normalized in conjunction with the disappearance of M-protein in these 4 patients, and remained within almost normal levels for 4 years after cyclic VAD therapy (Fig. 1A). The remaining 4 patients (cases 5 to 8) showed serious adverse events ascribable to VAD, such as encephalopathy, glucocorticoid-induced fluid retention and worsening 6
7 of renal and heart failure, and became unable to continue this therapy before the disappearance of M-protein. At cessation of VAD therapy, M-protein was detectable on immunofixation in these 4 patients, but the FLC κ/λ ratio was within normal limits in cases 6 and 8. Survival curves The patients were classified into remission (cases 1 to 4) and non-remission (cases 5 to 8) groups. Kaplan-Meier s survival curves of the 2 patient groups are shown in Fig. 2. The fifty-percent survival period was 7.0±2.0 months for the non-remission group, while it could not be calculated for the remission group because all the patients are still alive. The numbers of patients alive 300 days after commencement of therapy were 4 in the remission group and only 1 in non-remission. Causes of death in the non-remission group were congestive heart failure in case 5 and uremia in cases 6 and 7. Case 8 in the non-remission group is still alive, but hemodialysis had to be started 330 days after commencement of VAD. Therapeutic outcomes Therapeutic outcomes in the remission group are shown in Fig. 1. Cases 1, 2 and 4 showed improvement of nephrotic syndrome with an increase in serum levels of total protein and albumin and a decrease of more than 50% in urinary protein excretion 1 year after VAD. Serum levels of BNP (normal <20 pg/ml) decreased from 84.9 pg/ml and pg/ml to 38.0 pg/ml and pg/ml in cases 2 and 4, respectively, 1 year after VAD. Nevertheless, no obvious change was seen in other cardiac indices, including the thickness of IVS, FS and TMF E/A, even 4 years after VAD. Performance status of the remission group was grade 1 or 2, and did not change during and after VAD. All patients in the non-remission group showed rapid worsening of performance status in parallel with an increase in urinary protein excretion and a decrease in serum albumin (data not shown). 7
8 DISCUSSION VAD is widely used in multiple myeloma as a standard therapeutic regimen targeting abnormal plasma cells in the bone marrow. These cells usually exist also in a form of plasma cell dyscrasia in primary systemic AL amyloidosis, although the number is much lower than in multiple myeloma. Considering that immunoglobulin light chains secreted from abnormal plasma cells turn into amyloid with conformational change, VAD should also be effective also in primary systemic AL amyloidosis with regard both to reduction of the precursor protein and to avoiding progressive dysfunction of vital organs. To increase the possibility of complete hematological remission, VAD can be employed as pretreatment for high-dose melphalan with auto-pbsct in primary systemic AL amyloidosis because of the absence of negative effects on mobilization of hematopoietic stem cells in addition to the rapid reduction of pathogenetic plasma cells (6-8, 13). Several recent reports have demonstrated that some patients with primary systemic AL amyloidosis show good therapeutic outcomes after cyclic administration of VAD alone (5, 14). In the present study cyclic VAD showed good therapeutic outcomes with respect to both hematological response and visceral organ function in half of the patients who were ineligible for high-dose melphalan with auto-pbsct. Interferon-α has been reported to show a good therapeutic effect in primary systemic AL amyloidosis (15), and we tried to use this drug in our patients after VAD. Cases 2 to 4 were treated with interferon-α, but case 1 refused this maintenance therapy. Three patients with nephrotic syndrome showed an increase in serum levels of albumin in parallel with a decrease in daily protein excretion in urine after achieving complete hematological remission. Another notable point in our study is that complete hematological remission persisted for a long period after 1 to 3 courses of VAD irrespective of maintenance therapy with interferon-α. These good therapeutic outcomes may be partly ascribed to the small number of abnormal plasma cells in primary systemic AL amyloidosis. Abnormal plasma cells can be classified into several 8
9 subpopulations according to surface markers, and in multiple myeloma high proportions of the immature subtype have been reported to correlate well with a poor response to treatment (16, 17). Further studies are required in order to clarify whether subtypes of abnormal plasma cells are related to therapeutic outcomes also in primary systemic AL amyloidosis. Doxorubicin and vincristine, which are included in the VAD regimen, can cause cardiac toxicity and neuropathy, respectively. VAD is, therefore, excluded as a therapeutic option in patients with primary systemic AL amyloidosis showing severe cardiac involvement and/or neuropathy. In our study cardiac involvement was present in 4 patients, but of these, 3 were considered to be eligible for VAD on the basis of well-preserved Doppler-based indices on echocardiography. The remaining 1 patient received 1 course of VAD at her request despite the presence of severe cardiac involvement. No patient showed any symptoms ascribable to polyneuropathy before or after cyclic VAD. The most notable point concerning adverse effects of VAD in our study is that corticosteroid-induced fluid retention was much more frequently seen than previously thought. Six patients in our study (75%) showed obvious corticosteroid-induced fluid retention. Among them, 4 had to discontinue cyclic VAD therapy before achieving complete hematological remission because of adverse events, such as intestinal pseudo-obstruction and congestive heart failure ascribable to an excess of body fluid. These 6 patients were considered to have more severe impairment of renal and/or cardiac function before starting VAD with regard to serum BNP, 24 hr creatinine clearance and daily protein excretion compared with the remaining 2 patients. As overt or subclinical visceral organ involvement often exists in patients with primary systemic AL amyloidosis, intensive management of body fluids is necessary during and after cyclic VAD therapy, particularly when marked hypoalbuminemia due to nephrotic syndrome and/or cardiac dysfunction are present. Cyclic VAD is risky in the advanced stage of primary systemic AL amyloidosis, and the enrollment criteria we employed in 9
10 this study may be helpful in selecting patients eligible for this intensive chemotherapy (8). In conclusion, cyclic VAD therapy is effective for approximately 50% of patients with primary systemic AL amyloidosis, although corticosteroid-induced fluid retention frequently occurs as an adverse effect, particularly in those with severe nephrotic syndrome and/or cardiac dysfunction. This therapy should be actively considered as a therapeutic option when high-dose melphalan with auto-pbsct is inappropriate because of advanced age or vital organ involvement. Acknowledgments The authors are grateful to Drs. Y. Hoshii and T. Ishihara, Department of Pathology, Yamaguchi University School of Medicine, for their help with immunohistochemical staining of the biopsy specimens, and also to Dr. T. Yamada, Department of Laboratory Medicine, Jichi University School of Medicine, for determination of the serum free light chain. This work was supported by a grant from the Intractable Disease Division, the Ministry of Health and Welfare, Research Committee for Epochal Diagnosis and Treatment of Amyloidosis in Japan. 10
11 REFERENCES 1 Kyle RA, Gertz MA. Primary systemic amyloidosis. Clinical and laboratory features in 474 cases. Semin Hematol 32: 45-59, Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood 99: , Sanchorawala V, Wright DG, Seldin DC, et al. An overview of the use of high-dose melphalan with autologous stem cell transplantation for the treatment of AL amyloidosis. Bone Marrow Transplant 28: , Gertz MA, Lacy MQ, Dispenzieri A, et al. Stem cell transplantation for management of primary systemic amyloidosis. Am J Med 113: , Wardley AM, Jayson GC, Goldsmith DJA, et al. The treatment of nephrotic syndrome caused by primary (light chain) amyloid with vincristine, doxorubicin and dexamethasone. Br J Cancer 78: , van Gameren II, Hazenberg BPC, Jager PL, et al: AL amyloidosis treated with induction chemotherapy with VAD followed by high dose melphalan and autologous stem cell transplantation. Amyloid 9: , Sezer O, Schmid P, Schweigert M, et al. Rapid reversal of nephritic syndrome due to primary systemic AL amyloidosis after VAD and subsequent high-dose chemotherapy with autologous stem cell support. Bone Marrow Transplant 20: , Gono T, Matsuda M, Shimojima Y, et al. VAD with or without subsequent high-dose melphalan followed by autologous stem cell support in AL amyloidosis: Japanese experience and criteria for patient selection. Amyloid 11: , Hoshii Y, Setoguchi M, Iwata T, et al. Useful polyclonal antibodies against synthetic peptides corresponding to immunoglobulin light chain constant region for immunohistochemical detection of immunoglobulin light chain amyloidosis. Pathol Int 51: ,
12 10 The International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma, and related disorders: a report of the International Myeloma Working Group. Br J Haematol 121: , Comenzo RL, Vosburgh E, Falk RH, et al. Dose-intensive melphalan with blood stem-cell support for the treatment of AL amyloidosis: survival and responses in 25 patients. Blood 91: , Commenzo RL, Vosburgh E, Simms RW, et al. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood 88: , Gono T, Matsuda M, Dohi N, et al. Nephrotic syndrome due to primary AL amyloidosis, successfully treated with VAD and subsequent high-dose melphalan followed by autologous peripheral blood stem cell transplantation. Intern Med 42: 72-77, Matsuda M, Gono T, Katoh N, et al. Nephrotic syndrome due to primary systemic AL amyloidosis, successfully treated with VAD (vincristine, doxorubicin and dexamethasone) alone. Intern Med 47: , Dhodapkar MV, Jagannath S, Vesole D, et al. Treatment of AL-amyloidosis with dexamethazone plus alpha interferon. Leuk Lymphoma 27: , Matsuda M, Gono T, Shimojima Y, Hoshii Y, Ikeda S. Phenotypic analysis of plasma cells in bone marrow using flow cytometry in AL amyloidosis. Amyloid 10: , Kawano MM, Mahmoud MS, Huang N, et al. High proportions of VLA-5 - immature myeloma cells correlate well with poor response to treatment in multiple myeloma. Br J Haematol 91: ,
13 FIGURE LEGENDS Figure 1: The free light chain (FLC) κ/λ ratio in serum was normalized in patients with primary systemic AL amyloidosis who achieved complete hematological remission with cyclic VAD, and remained within the relatively normal range for 4 years (A, broken line: upper and lower limits of FLC). Serum levels of total protein (C) and albumin (D) gradually increased in parallel with a decrease in urinary protein excretion (B) in these patients. Closed triangles: case 1, closed circles: case 2, open squares: case 3, closed squares: case 4. Figure 2: Kaplan-Meier s survival curves of the remission and non-remission groups. Solid line: the remission group (n=4), broken line: the non-remission group (n=4). Censored patients are ticked along the survival curves. 13
14 Table 1. Clinical profiles of the patients and outcomes of cyclic VAD Case Normal values Age/sex 62/F 71/M 67/M 62/F 45/F 54/F 54/M 68/M Organ involvement Kidney Heart Urinary bladder hemorrhage Liver Performance status Laboratory data before VAD M-protein** Serum IgGλ IgGλ - IgAκ IgGλ Urine BJPλ BJPλ BJPλ BJPκ BJPλ BJPλ BJPλ BJPλ FLC κ/λ ratio Before treatment On finishing cyclic VAD Abnormal plasma cells in bone marrow (%)*** Serum brain natriuretic peptide (pg/ml) <20 Serum albumin (g/dl) Blood urea nitrogen (mg/dl) Creatinine (mg/dl) h creatinine clearance (L/day) Daily protein excretion (g/day) VAD cycles performed * 2* 2* 2* Complete hematological remission Maintenance therapy with interferon- α Duration (months) **** Adverse events - IP - Syncope CHF Fluid retention RF IP Fluid retention Fluid retention Encephalopathy Fluid retention Fluid retention *cessation of cyclic VAD before complete hematological remission, **detected by immunofixation, ***detected by flow cytometry, ****still being administered. BJP: Bence Jones protein, FLC: free light chain, IP: intestinal pseudo-obstruction, CHF: congestive heart failure, RF: renal failure
15 10 A (g/day) 5 B 2.5 (g/dl) 7 C (g/dl) 4 D Before treatment 1 2 Figure (years)
16 1 Survival rate (months) Figure 2
Serum Levels of Free Light Chain before and after Chemotherapy in Primary Systemic AL Amyloidosis
 ORIGINAL ARTICLE Serum Levels of Free Light Chain before and after Chemotherapy in Primary Systemic AL Amyloidosis Masayuki MATSUDA, Toshiyuki YAMADA**, Takahisa GONO, Yasuhiro SHIMOJIMA, Wataru ISHII,
ORIGINAL ARTICLE Serum Levels of Free Light Chain before and after Chemotherapy in Primary Systemic AL Amyloidosis Masayuki MATSUDA, Toshiyuki YAMADA**, Takahisa GONO, Yasuhiro SHIMOJIMA, Wataru ISHII,
Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012
 Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012 Support AL Amyloidosis (Light Chain Amyloidosis) Effective Date: 01/01/2013 Document: ARB0413:01 Revision Date: 10/24/2012 Code(s):
Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012 Support AL Amyloidosis (Light Chain Amyloidosis) Effective Date: 01/01/2013 Document: ARB0413:01 Revision Date: 10/24/2012 Code(s):
& 2004 Nature Publishing Group All rights reserved /04 $
 (2004) 33, 381 388 & 2004 Nature Publishing Group All rights reserved 0268-3369/04 $25.00 www.nature.com/bmt Amyloidosis High-dose intravenous melphalan and autologous stem cell transplantation as initial
(2004) 33, 381 388 & 2004 Nature Publishing Group All rights reserved 0268-3369/04 $25.00 www.nature.com/bmt Amyloidosis High-dose intravenous melphalan and autologous stem cell transplantation as initial
The New England Journal of Medicine
 A TRIAL OF THREE REGIMENS FOR PRIMARY AMYLOIDOSIS: COLCHICINE ALONE, MELPHALAN AND PREDNISONE, AND MELPHALAN, PREDNISONE, AND COLCHICINE ROBERT A. KYLE, M.D., MORIE A. GERTZ, M.D., PHILIP R. GREIPP, M.D.,
A TRIAL OF THREE REGIMENS FOR PRIMARY AMYLOIDOSIS: COLCHICINE ALONE, MELPHALAN AND PREDNISONE, AND MELPHALAN, PREDNISONE, AND COLCHICINE ROBERT A. KYLE, M.D., MORIE A. GERTZ, M.D., PHILIP R. GREIPP, M.D.,
Cardiac Involvement is Underdiagnosed in Patients with Biopsy-Proven Systemic AL Amyloidosis
 Original Article 41 Cardiac Involvement is Underdiagnosed in Patients with Biopsy-Proven Systemic AL Amyloidosis Haoyi Zheng 1, Amitabha Mazumder 2, Stuart D. Katz 3 1. Cardiac Imaging, The Heart Center,
Original Article 41 Cardiac Involvement is Underdiagnosed in Patients with Biopsy-Proven Systemic AL Amyloidosis Haoyi Zheng 1, Amitabha Mazumder 2, Stuart D. Katz 3 1. Cardiac Imaging, The Heart Center,
Amyloidosis: What to do and how to diagnose: An Update 2017
 Amyloidosis: What to do and how to diagnose: An Update 2017 Jonathan L. Kaufman, MD Associate Professor Hematology & Oncology Winship Cancer Institute of Emory University Amyloidosis Protein Conformation/Deposition
Amyloidosis: What to do and how to diagnose: An Update 2017 Jonathan L. Kaufman, MD Associate Professor Hematology & Oncology Winship Cancer Institute of Emory University Amyloidosis Protein Conformation/Deposition
Sheena Surindran Grand Rounds 2/15/11
 Sheena Surindran Grand Rounds 2/15/11 Affects 5 12 person per million / year 5 10% associated with myeloma Median survival without treatment is 12 40 months Most commonly affected organs are kidney, heart
Sheena Surindran Grand Rounds 2/15/11 Affects 5 12 person per million / year 5 10% associated with myeloma Median survival without treatment is 12 40 months Most commonly affected organs are kidney, heart
EBMT2008_22_44:EBMT :26 Pagina 424 CHAPTER 27. HSCT for primary amyloidosis in adults. J. Esteve
 EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 424 * CHAPTER 27 HSCT for primary amyloidosis in adults J. Esteve EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 425 CHAPTER 27 Amyloidosis in adults 1. Introduction
EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 424 * CHAPTER 27 HSCT for primary amyloidosis in adults J. Esteve EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 425 CHAPTER 27 Amyloidosis in adults 1. Introduction
Protocol. Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Systemic amyloidosis with cardiac involvement
 034 Cardiology Systemic amyloidosis with cardiac involvement Amyloidosis is a term used to describe a group of disorders consisting of abnormalities in and the accumulation of amyloid protein. This protein
034 Cardiology Systemic amyloidosis with cardiac involvement Amyloidosis is a term used to describe a group of disorders consisting of abnormalities in and the accumulation of amyloid protein. This protein
A.M.W. van Marion. H.M. Lokhorst. N.W.C.J. van de Donk. J.G. van den Tweel. Histopathology 2002, 41 (suppl 2):77-92 (modified)
 chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
A classic case of amyloid cardiomyopathy
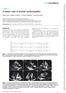 Images in... A classic case of amyloid cardiomyopathy Hayan Jouni, 1 William G Morice, 2 S Vincent Rajkumar, 3 Joerg Herrmann 4 1 Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota, USA
Images in... A classic case of amyloid cardiomyopathy Hayan Jouni, 1 William G Morice, 2 S Vincent Rajkumar, 3 Joerg Herrmann 4 1 Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota, USA
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis. Original Policy Date
 MP 7.03.29 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
MP 7.03.29 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
Instructions for Plasma Cell Disorders (PCD) Post-HCT Data (Form 2116 Revision 3)
 (Form 2116 Revision 3) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the Plasma Cell Disorders (PCD) Post-HCT Data Form. E-mail comments regarding the
(Form 2116 Revision 3) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the Plasma Cell Disorders (PCD) Post-HCT Data Form. E-mail comments regarding the
Mayo Clinic, Rochester, Minnesota; 2 Tufts Medical Center, Boston, Massachusetts; 3
 NEOD001 Demonstrates Organ Biomarker Responses in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction: Final Results From a Phase 1/2 Study Morie A. Gertz, 1 Raymond L. Comenzo, 2 Heather
NEOD001 Demonstrates Organ Biomarker Responses in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction: Final Results From a Phase 1/2 Study Morie A. Gertz, 1 Raymond L. Comenzo, 2 Heather
Plasma Cell Disorders (PCD) Pre-HCT Data
 Plasma Cell Disorders (PCD) Pre-HCT Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Recipient ID: Date of HCT for which this form is being completed: HCT type: (check
Plasma Cell Disorders (PCD) Pre-HCT Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Recipient ID: Date of HCT for which this form is being completed: HCT type: (check
Review Article Autologous Stem Cell Transplant for AL Amyloidosis
 Bone Marrow Research Volume 2012, Article ID 238961, 5 pages doi:10.1155/2012/238961 Review Article Autologous Stem Cell Transplant for AL Amyloidosis Vivek Roy Division of Hematology/Oncology, Mayo Clinic,
Bone Marrow Research Volume 2012, Article ID 238961, 5 pages doi:10.1155/2012/238961 Review Article Autologous Stem Cell Transplant for AL Amyloidosis Vivek Roy Division of Hematology/Oncology, Mayo Clinic,
Citation for published version (APA): Hovenga, S. (2007). Clinical and biological aspects of Multiple Myeloma s.n.
 University of Groningen Clinical and biological aspects of Multiple Myeloma Hovenga, Sjoerd IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen Clinical and biological aspects of Multiple Myeloma Hovenga, Sjoerd IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
2016: Plasma Cell Disorders Pre-HCT Data
 2016: Plasma Cell Disorders Pre-HCT Data Registry Use Only Sequence Number: Date Received: Key Fields CIBMTR Center Number: Date of HCT for which this form is being completed: / / YYYY MM DD HCT type (check
2016: Plasma Cell Disorders Pre-HCT Data Registry Use Only Sequence Number: Date Received: Key Fields CIBMTR Center Number: Date of HCT for which this form is being completed: / / YYYY MM DD HCT type (check
Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes before and after 2006
 (2011) 46, 970 975 & 2011 Macmillan Publishers Limited All rights reserved 0268-3369/11 www.nature.com/bmt ORIGINAL ARTICLE Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes
(2011) 46, 970 975 & 2011 Macmillan Publishers Limited All rights reserved 0268-3369/11 www.nature.com/bmt ORIGINAL ARTICLE Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes
Amyloidosis and Waldenström s Macroglobulinemia
 Amyloidosis and Waldenström s Macroglobulinemia Morie A. Gertz, Giampaolo Merlini, and Steven P. Treon Primary systemic amyloidosis is an immunoglobulin light chain disorder that is 1/5th as common as
Amyloidosis and Waldenström s Macroglobulinemia Morie A. Gertz, Giampaolo Merlini, and Steven P. Treon Primary systemic amyloidosis is an immunoglobulin light chain disorder that is 1/5th as common as
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/26/2013 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/26/2013 Section: Transplants
Generalized Primary Amyloid Lymphadenopathy
 Korean J Hematol Vol. 44, No. 4, December, 2009 Case Report Generalized Primary Amyloid Lymphadenopathy Jin Hyun Park, M.D. 2, Ji Hyun Kwon, M.D. 2, Ji Won Kim, M.D. 2, Hyeon Jin Cho, M.D. 2, Ki Hwan Kim,
Korean J Hematol Vol. 44, No. 4, December, 2009 Case Report Generalized Primary Amyloid Lymphadenopathy Jin Hyun Park, M.D. 2, Ji Hyun Kwon, M.D. 2, Ji Won Kim, M.D. 2, Hyeon Jin Cho, M.D. 2, Ki Hwan Kim,
The Long-term Outcomes after VAD plus SCT Therapy in a Patient with AL Amyloidosis and Severe Factor X Deficiency
 doi: 10.2169/internalmedicine.9263-17 http://internmed.jp CASE REPORT The Long-term Outcomes after VAD plus SCT Therapy in a Patient with AL Amyloidosis and Severe Factor X Deficiency Dosuke Iwadate 1,EikoHasegawa
doi: 10.2169/internalmedicine.9263-17 http://internmed.jp CASE REPORT The Long-term Outcomes after VAD plus SCT Therapy in a Patient with AL Amyloidosis and Severe Factor X Deficiency Dosuke Iwadate 1,EikoHasegawa
Prognostic Significance of Strain Doppler Imaging in Light-Chain Amyloidosis
 JACC: CARDIOVASCULAR IMAGING VOL. 3, NO. 4, 21 21 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION ISSN 1936-878X/1/$36. PUBLISHED BY ELSEVIER INC. DOI:1.116/j.jcmg.29.11.13 ORIGINAL RESEARCH Prognostic
JACC: CARDIOVASCULAR IMAGING VOL. 3, NO. 4, 21 21 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION ISSN 1936-878X/1/$36. PUBLISHED BY ELSEVIER INC. DOI:1.116/j.jcmg.29.11.13 ORIGINAL RESEARCH Prognostic
Serum Cardiac Troponin T in Cardiac Amyloidosis: Serial Observations in Five Patients
 Tohoku J. Exp. Med., 2006, 208, 163-167 ctnt in Cardiac Amyloidosis 163 Serum Cardiac Troponin T in Cardiac Amyloidosis: Serial Observations in Five Patients Case Report TAKAO KATO, YUKIHITO SATO, KAZUYA
Tohoku J. Exp. Med., 2006, 208, 163-167 ctnt in Cardiac Amyloidosis 163 Serum Cardiac Troponin T in Cardiac Amyloidosis: Serial Observations in Five Patients Case Report TAKAO KATO, YUKIHITO SATO, KAZUYA
Amyloidosis. Rajkumar SV, Dispenzieri A, Kyle RA. Mayo Clin Proc 2006;81:
 Advances in AL amyloidosis Yonsei University College of Medicine i Jin Seok Kim Amyloidosis Amyloidosis results from a sequence of changes in protein folding that leads to the deposition of insoluble amyloid
Advances in AL amyloidosis Yonsei University College of Medicine i Jin Seok Kim Amyloidosis Amyloidosis results from a sequence of changes in protein folding that leads to the deposition of insoluble amyloid
Jo Abraham MD Division of Nephrology University of Utah
 Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
AL AMYLOIDOSIS: AN UPDATE. Simrit Parmar, MD COMY, BANGKOK
 AL AMYLOIDOSIS: AN UPDATE Simrit Parmar, MD COMY, BANGKOK Amyloidosis and Amyloid Fibrils Disorder of protein folding Structurally diverse precursors adopt an abnormal common fibrillar conformation New
AL AMYLOIDOSIS: AN UPDATE Simrit Parmar, MD COMY, BANGKOK Amyloidosis and Amyloid Fibrils Disorder of protein folding Structurally diverse precursors adopt an abnormal common fibrillar conformation New
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section: Transplants
Forms Revision: Myeloma Changes
 Sharing knowledge. Sharing hope. Forms Revision: Myeloma Changes J. Brunner, PA-C and A. Dispenzieri, MD February 2013 Disclosures Janet Brunner, PA-C I have no relevant conflicts of interest to disclose.
Sharing knowledge. Sharing hope. Forms Revision: Myeloma Changes J. Brunner, PA-C and A. Dispenzieri, MD February 2013 Disclosures Janet Brunner, PA-C I have no relevant conflicts of interest to disclose.
The impact of dialysis on the survival of patients with immunoglobulin light chain (AL) amyloidosis undergoing autologous stem cell transplantation
 Nephrol Dial Transplant (2016) 31: 1284 1289 doi: 10.1093/ndt/gfv328 Advance Access publication 30 November 2015 Original Article The impact of dialysis on the survival of patients with immunoglobulin
Nephrol Dial Transplant (2016) 31: 1284 1289 doi: 10.1093/ndt/gfv328 Advance Access publication 30 November 2015 Original Article The impact of dialysis on the survival of patients with immunoglobulin
The most common form of systemic amyloidosis in the
 Article High-Dose Melphalan and Autologous Stem-Cell Transplantation in Patients with AL Amyloidosis: An 8-Year Study Martha Skinner, MD; Vaishali Sanchorawala, MD; David C. Seldin, MD, PhD; Laura M. Dember,
Article High-Dose Melphalan and Autologous Stem-Cell Transplantation in Patients with AL Amyloidosis: An 8-Year Study Martha Skinner, MD; Vaishali Sanchorawala, MD; David C. Seldin, MD, PhD; Laura M. Dember,
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
NEOD001 for amyloid light-chain (AL) amyloidosis
 NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
Final Analysis of the Phase 1a/b Study of Fibril-Reactive Monoclonal Antibody 11-1F4 (CAEL-101) in Patients with AL Amyloidosis
 Final Analysis of the Phase 1a/b Study of Fibril-Reactive Monoclonal Antibody 11-1F4 (CAEL-101) in Patients with AL Amyloidosis Camille V. Edwards 1, Divaya Bhutani 2, Markus Mapara 2, Jai Radhakrishnan
Final Analysis of the Phase 1a/b Study of Fibril-Reactive Monoclonal Antibody 11-1F4 (CAEL-101) in Patients with AL Amyloidosis Camille V. Edwards 1, Divaya Bhutani 2, Markus Mapara 2, Jai Radhakrishnan
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: , 2015
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1895-1900, 2015 Clinical characteristics of a group of patients with multiple myeloma who had two different λ light chains by immunofixation electrophoresis: A
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1895-1900, 2015 Clinical characteristics of a group of patients with multiple myeloma who had two different λ light chains by immunofixation electrophoresis: A
Laboratory Examination
 Todd Zimmerman, M.D. 64 year old African American male presents to establish care with PCG. Meds: Norvasc 5 mg daily PMHx: HTN x 20 years, poorly controlled SHx: No tobacco, illicit; rare EtOH ROS: Negative
Todd Zimmerman, M.D. 64 year old African American male presents to establish care with PCG. Meds: Norvasc 5 mg daily PMHx: HTN x 20 years, poorly controlled SHx: No tobacco, illicit; rare EtOH ROS: Negative
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
MYE FORMS REVEALED RESPONSE CODES OBJECTIVES. Stringent Complete Response (scr) Complete Response (CR) I have no conflicts of interest to disclose
 I have no conflicts of interest to disclose MYE FORMS REVEALED Janet Brunner, PA-C CIBMTR MKE New10_1.ppt OBJECTIVES 1) Be able to describe the criteria required for each myeloma response code 2) Be able
I have no conflicts of interest to disclose MYE FORMS REVEALED Janet Brunner, PA-C CIBMTR MKE New10_1.ppt OBJECTIVES 1) Be able to describe the criteria required for each myeloma response code 2) Be able
Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT
 The Kidney in Multiple Myeloma Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT Normal Cell Plasma cells produce antibodies that bind to antigens,
The Kidney in Multiple Myeloma Tarek ElBaz, MD. Prof. Internal Medicine Chief, Division of Renal Medicine Al Azhar University President, ESNT Normal Cell Plasma cells produce antibodies that bind to antigens,
Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan
 doi: 10.2169/internalmedicine.9206-17 http://internmed.jp ORIGINAL ARTICLE Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan Chihiro Shimazaki 1, Hiroyuki Hata 2,
doi: 10.2169/internalmedicine.9206-17 http://internmed.jp ORIGINAL ARTICLE Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan Chihiro Shimazaki 1, Hiroyuki Hata 2,
Clinical features and outcomes of systemic amyloidosis with gastrointestinal involvement: a single-center experience
 ORIGINAL ARTICLE Korean J Intern Med 2015;30:496-505 Clinical features and outcomes of systemic amyloidosis with gastrointestinal involvement: a single-center experience A Young Lim 1, Ji Hyeon Lee 1,
ORIGINAL ARTICLE Korean J Intern Med 2015;30:496-505 Clinical features and outcomes of systemic amyloidosis with gastrointestinal involvement: a single-center experience A Young Lim 1, Ji Hyeon Lee 1,
The Imaging Diagnosis of Less Advanced Cases of Cardiac Amyloidosis: The Relative Apical Sparing Pattern
 CASE REPORT The Imaging Diagnosis of Less Advanced Cases of Cardiac Amyloidosis: The Relative Apical Sparing Pattern Koya Ono 1, Go Ishimaru 2, Miho Hayashi 3,YuanBae 4, Takashi Ito 4, Toshiyuki Izumo
CASE REPORT The Imaging Diagnosis of Less Advanced Cases of Cardiac Amyloidosis: The Relative Apical Sparing Pattern Koya Ono 1, Go Ishimaru 2, Miho Hayashi 3,YuanBae 4, Takashi Ito 4, Toshiyuki Izumo
Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan
 doi: 10.2169/internalmedicine.9206-17 http://internmed.jp ORIGINAL ARTICLE Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan Chihiro Shimazaki 1, Hiroyuki Hata 2,
doi: 10.2169/internalmedicine.9206-17 http://internmed.jp ORIGINAL ARTICLE Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan Chihiro Shimazaki 1, Hiroyuki Hata 2,
Elevation of Plasmin-α2-plasmin Inhibitor Complex Predicts the Diagnosis of Systemic AL Amyloidosis in Patients with Monoclonal Protein
 doi: 10.2169/internalmedicine.8999-17 Intern Med Advance Publication http://internmed.jp ORIGINAL ARTICLE Elevation of Plasmin-α2-plasmin Inhibitor Complex Predicts the Diagnosis of Systemic AL Amyloidosis
doi: 10.2169/internalmedicine.8999-17 Intern Med Advance Publication http://internmed.jp ORIGINAL ARTICLE Elevation of Plasmin-α2-plasmin Inhibitor Complex Predicts the Diagnosis of Systemic AL Amyloidosis
Primary Amyloidosis. Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center
 Primary Amyloidosis Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center Systemic Amyloidosis A group of complex diseases caused by tissue
Primary Amyloidosis Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center Systemic Amyloidosis A group of complex diseases caused by tissue
Amyloidosis Information. A General Overview for Patients
 Amyloidosis Information A General Overview for Patients www.amyloidosis.org Amyloidosis was first discovered 150 years ago by the well know German pathologist, Dr. Rudolf Virchow. Although the disease
Amyloidosis Information A General Overview for Patients www.amyloidosis.org Amyloidosis was first discovered 150 years ago by the well know German pathologist, Dr. Rudolf Virchow. Although the disease
Smoldering Myeloma: Leave them alone!
 Smoldering Myeloma: Leave them alone! David H. Vesole, MD, PhD Co-Director, Myeloma Division Director, Myeloma Research John Theurer Cancer Center Hackensack University Medical Center Prevalence 1960 2002
Smoldering Myeloma: Leave them alone! David H. Vesole, MD, PhD Co-Director, Myeloma Division Director, Myeloma Research John Theurer Cancer Center Hackensack University Medical Center Prevalence 1960 2002
Biology of Blood and Marrow Transplantation 12: (2006) 2006 American Society for Blood and Marrow Transplantation
 Biology of Blood and Marrow Transplantation 12:837-844 (2006) 2006 American Society for Blood and Marrow Transplantation 1083-8791/06/1208-0006$32.00/0 doi:10.1016/j.bbmt.2006.04.006 New Staging Systems
Biology of Blood and Marrow Transplantation 12:837-844 (2006) 2006 American Society for Blood and Marrow Transplantation 1083-8791/06/1208-0006$32.00/0 doi:10.1016/j.bbmt.2006.04.006 New Staging Systems
Amyloidosis. James J. Stark, MD, FACP Medical Director Cancer Program Maryview Medical Center. Professor of Medicine Eastern Virginia Medical
 Amyloidosis James J. Stark, MD, FACP Medical Director Cancer Program Maryview Medical Center Professor of Medicine Eastern Virginia Medical School www.starkoncology.com 68 y.o. man admitted to MMC in March,
Amyloidosis James J. Stark, MD, FACP Medical Director Cancer Program Maryview Medical Center Professor of Medicine Eastern Virginia Medical School www.starkoncology.com 68 y.o. man admitted to MMC in March,
M-Protien, what to do next? Ismail A Sharif MD, FRCPc Internal Medicine Day 22 nd April 2016
 + M-Protien, what to do next? Ismail A Sharif MD, FRCPc Internal Medicine Day 22 nd April 2016 + Disclosures Advisory Boards: AMGEN, Lundbeck, NOVARTIS + Subtypes of Plasma Cell Disorders Increased Plasma
+ M-Protien, what to do next? Ismail A Sharif MD, FRCPc Internal Medicine Day 22 nd April 2016 + Disclosures Advisory Boards: AMGEN, Lundbeck, NOVARTIS + Subtypes of Plasma Cell Disorders Increased Plasma
Bone Marrow Core Biopsy Specimens in AL (Primary) Amyloidosis A Morphologic and Immunohistochemical Study of 100 Cases
 Hematopathology / BONE MARROW BIOPSY IN AL AMYLOIDOSIS Bone Marrow Core Biopsy Specimens in AL (Primary) Amyloidosis A Morphologic and Immunohistochemical Study of 100 Cases Niall Swan, MD, 1,2 Martha
Hematopathology / BONE MARROW BIOPSY IN AL AMYLOIDOSIS Bone Marrow Core Biopsy Specimens in AL (Primary) Amyloidosis A Morphologic and Immunohistochemical Study of 100 Cases Niall Swan, MD, 1,2 Martha
Analysis of Free Serum Light Chains in Patients Suffering from Multiple Myeloma Complicated by Light-Chain Amyloidosis
 ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 4, 531 538 ISSN 1899 5276 Copyright by Wroclaw Medical University Lidia Usnarska-Zubkiewicz 1, A, C F, Jadwiga Hołojda 2, B C, F, Jakub Dębski 3, B, Anna Zubkiewicz-Zarębska
ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 4, 531 538 ISSN 1899 5276 Copyright by Wroclaw Medical University Lidia Usnarska-Zubkiewicz 1, A, C F, Jadwiga Hołojda 2, B C, F, Jakub Dębski 3, B, Anna Zubkiewicz-Zarębska
Review of the recent publications from the French group of myeloma on urine vs serum FLC analysis in MM
 Review of the recent publications from the French group of myeloma on urine vs serum FLC analysis in MM Multiple myeloma Response evaluation Kumar Lancet Oncol 2016; 17: e328 46 Cumulative Proportion Surviving
Review of the recent publications from the French group of myeloma on urine vs serum FLC analysis in MM Multiple myeloma Response evaluation Kumar Lancet Oncol 2016; 17: e328 46 Cumulative Proportion Surviving
Evaluation of Myocardial Changes in Familial Amyloid Polyneuropathy after Liver Transplantation
 ORIGINAL ARTICLE Evaluation of Myocardial Changes in Familial Amyloid Polyneuropathy after Liver Transplantation Sadahisa Okamoto 1, Taro Yamashita 1, Yukio Ando 2, Mitsuharu Ueda 2, Yohei Misumi 1, Konen
ORIGINAL ARTICLE Evaluation of Myocardial Changes in Familial Amyloid Polyneuropathy after Liver Transplantation Sadahisa Okamoto 1, Taro Yamashita 1, Yukio Ando 2, Mitsuharu Ueda 2, Yohei Misumi 1, Konen
Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning
 Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning Challenge Question: Role of Autologous Stem Cell Transplant Which of the following is true about eligibility for high-dose
Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning Challenge Question: Role of Autologous Stem Cell Transplant Which of the following is true about eligibility for high-dose
Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
 Original Article Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment Guangzhong Yang, Wenming Chen, Yin Wu Department
Original Article Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment Guangzhong Yang, Wenming Chen, Yin Wu Department
A faint in the emergency department (due to primary systemic amyloidosis
 104 PRACTICAL NEUROLOGY NEUROLOGICAL RARITIES A faint in the emergency department (due to primary systemic amyloidosis Michele T. M. Hu*, Carolyn M. Gabriel*, Helen J. Lachmann, Rosalind King, Philip N.
104 PRACTICAL NEUROLOGY NEUROLOGICAL RARITIES A faint in the emergency department (due to primary systemic amyloidosis Michele T. M. Hu*, Carolyn M. Gabriel*, Helen J. Lachmann, Rosalind King, Philip N.
Bortezomib (Velcade)
 Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Tracking Disease Status for Multiple Myeloma
 Tracking Disease Status for Multiple Myeloma This appendix is intended to provide additional resources when determining: Best response to line of therapy pre-transplant; Disease Status at the Last Evaluation
Tracking Disease Status for Multiple Myeloma This appendix is intended to provide additional resources when determining: Best response to line of therapy pre-transplant; Disease Status at the Last Evaluation
Update on Treatments for Systemic Amyloidosis
 Update on Treatments for Systemic Amyloidosis Laura M. Dember, M.D. Renal, Electrolyte and Hypertension Division University of Pennsylvania ANZSN Update Course Darwin, Australia September 2, 2017 Disclosure
Update on Treatments for Systemic Amyloidosis Laura M. Dember, M.D. Renal, Electrolyte and Hypertension Division University of Pennsylvania ANZSN Update Course Darwin, Australia September 2, 2017 Disclosure
Management Update: Multiple Myeloma. Presented by Prof. Dr. Khan Abul Kalam Azad Professor of Medicine Dhaka Medical College
 Management Update: Multiple Myeloma Presented by Prof. Dr. Khan Abul Kalam Azad Professor of Medicine Dhaka Medical College Introduction Multiple myeloma - clonal plasma cell neoplasm Monoclonal antibody
Management Update: Multiple Myeloma Presented by Prof. Dr. Khan Abul Kalam Azad Professor of Medicine Dhaka Medical College Introduction Multiple myeloma - clonal plasma cell neoplasm Monoclonal antibody
Cardiac Involvement in Four Patients with Immunoglobulin Light-Chain Systemic Amyloidosis
 Yamanashi Med. J. 14(4), 123 ~ 129, 1999 Case Report Cardiac Involvement in Four Patients with Immunoglobulin Light-Chain Systemic Amyloidosis Isao KOHNO, Sadayoshi KOMORI 1), Kimio YAMAMOTO, Souichi SANO,
Yamanashi Med. J. 14(4), 123 ~ 129, 1999 Case Report Cardiac Involvement in Four Patients with Immunoglobulin Light-Chain Systemic Amyloidosis Isao KOHNO, Sadayoshi KOMORI 1), Kimio YAMAMOTO, Souichi SANO,
Diagnostic Approach to Cardiac Amyloidosis
 Kobe J. Med. Sci., Vol. 60, No. 1, pp. E5-E11, 2014 Diagnostic Approach to Cardiac Amyloidosis HILMAN ZULKIFLI AMIN 1, *, SHUMPEI MORI 2, NAOTO SASAKI 2, and KEN-ICHI HIRATA 2 1 Faculty of Medicine, University
Kobe J. Med. Sci., Vol. 60, No. 1, pp. E5-E11, 2014 Diagnostic Approach to Cardiac Amyloidosis HILMAN ZULKIFLI AMIN 1, *, SHUMPEI MORI 2, NAOTO SASAKI 2, and KEN-ICHI HIRATA 2 1 Faculty of Medicine, University
Diagnostic approach to cardiac amyloidosis: A case report
 Diagnostic approach to cardiac amyloidosis: A case report Georgia Vogiatzi, MD, MSc, PhD 1 st Cardiology Department, Hippokration Hospital, Athens Medical School Disclosures I have no relevant relationships
Diagnostic approach to cardiac amyloidosis: A case report Georgia Vogiatzi, MD, MSc, PhD 1 st Cardiology Department, Hippokration Hospital, Athens Medical School Disclosures I have no relevant relationships
FAQs- Janet s Inbox. Janet Brunner, PA-C Wed. Feb. 26, 2014 TRAINING & DEVELOPMENT 1.
 FAQs- Janet s Inbox Janet Brunner, PA-C Wed. Feb. 26, 2014 TRAINING & DEVELOPMENT 1. Disclosures I have no relevant conflicts of interest to disclose. TRAINING & DEVELOPMENT 2. Objectives 1. Determine
FAQs- Janet s Inbox Janet Brunner, PA-C Wed. Feb. 26, 2014 TRAINING & DEVELOPMENT 1. Disclosures I have no relevant conflicts of interest to disclose. TRAINING & DEVELOPMENT 2. Objectives 1. Determine
Management of Multiple Myeloma
 Management of Multiple Myeloma Damian J. Green, MD Fred Hutchinson Cancer Research Center/ Seattle Cancer Care Alliance New Treatment Options Have Improved OS in MM Kumar SK, et al. Blood. 2008;111:2516-2520.
Management of Multiple Myeloma Damian J. Green, MD Fred Hutchinson Cancer Research Center/ Seattle Cancer Care Alliance New Treatment Options Have Improved OS in MM Kumar SK, et al. Blood. 2008;111:2516-2520.
Therapeutic Effects of Lenalidomide on Hemorrhagic Intestinal Myeloma-associated AL Amyloidosis
 CASE REPORT Therapeutic Effects of Lenalidomide on Hemorrhagic Intestinal Myeloma-associated AL Amyloidosis Seiji Nagano 1,MinakoMori 1,AikoKato 1, Yuichiro Ono 1, Kazunari Aoki 1, Hiroshi Arima 1, Yoko
CASE REPORT Therapeutic Effects of Lenalidomide on Hemorrhagic Intestinal Myeloma-associated AL Amyloidosis Seiji Nagano 1,MinakoMori 1,AikoKato 1, Yuichiro Ono 1, Kazunari Aoki 1, Hiroshi Arima 1, Yoko
Myeloma Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant
 (24) 33, 823 828 & 24 Nature Publishing Group All rights reserved 268-3369/4 $25. www.nature.com/bmt Myeloma Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative
(24) 33, 823 828 & 24 Nature Publishing Group All rights reserved 268-3369/4 $25. www.nature.com/bmt Myeloma Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative
Guidelines on the management of AL amyloidosis
 guideline Guidelines on the management of AL amyloidosis Ashutosh D. Wechalekar, 1 Julian D. Gillmore, 1 Jenny Bird, 2 Jamie Cavenagh, 3 Stephen Hawkins, 4 Majid Kazmi, 5 Helen J. Lachmann, 1 Philip N.
guideline Guidelines on the management of AL amyloidosis Ashutosh D. Wechalekar, 1 Julian D. Gillmore, 1 Jenny Bird, 2 Jamie Cavenagh, 3 Stephen Hawkins, 4 Majid Kazmi, 5 Helen J. Lachmann, 1 Philip N.
Major Diagnostic Criteria
 Multiple Myeloma Elizabeth Ann Coleman, PhD, RNP, AOCN Professor and Chair, Dept. of Nursing Science Cooper Chair in Oncology Nursing, College of Nursing Professor, Dept. of Internal Medicine, College
Multiple Myeloma Elizabeth Ann Coleman, PhD, RNP, AOCN Professor and Chair, Dept. of Nursing Science Cooper Chair in Oncology Nursing, College of Nursing Professor, Dept. of Internal Medicine, College
Successful treatment with infliximab and low-dose methotrexate in a Japanese patient with familial Mediterranean fever
 Case report Successful treatment with infliximab and low-dose methotrexate in a Japanese patient with familial Mediterranean fever Akinori Nakamura Masayuki Matsuda Ko-ichi Tazawa Yasuhiro Shimojima Shu-ichi
Case report Successful treatment with infliximab and low-dose methotrexate in a Japanese patient with familial Mediterranean fever Akinori Nakamura Masayuki Matsuda Ko-ichi Tazawa Yasuhiro Shimojima Shu-ichi
Amyloidosis for Practicing Hematologists
 Amyloidosis for Practicing Hematologists Vishal Kukreti, MD Princess Margaret Cancer Centre October 2, 2015 Disclosures Honoraria Celgene, Janssen Ortho, Amgen, Lundbeck Objectives Case Approach subtyping,
Amyloidosis for Practicing Hematologists Vishal Kukreti, MD Princess Margaret Cancer Centre October 2, 2015 Disclosures Honoraria Celgene, Janssen Ortho, Amgen, Lundbeck Objectives Case Approach subtyping,
Hematopoietic Cell Transplantation for Primary Amyloidosis or Waldenstrom s Macroglobulinemia
 Hematopoietic Cell Transplantation for Primary Amyloidosis or Waldenstrom s Macroglobulinemia Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Hematopoietic Cell Transplantation for Primary Amyloidosis or Waldenstrom s Macroglobulinemia Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
CASE 3 AN UNUSUAL CASE OF NEPHROTIC SYNDROME
 CASE 3 AN UNUSUAL CASE OF NEPHROTIC SYNDROME Dr Seethalekshmy N.V., Dr.Annie Jojo, Dr Hiran K.R., Amrita institute of Medical Sciences, Kochi, Kerala Case history 34 year old gentleman Nephrotic range
CASE 3 AN UNUSUAL CASE OF NEPHROTIC SYNDROME Dr Seethalekshmy N.V., Dr.Annie Jojo, Dr Hiran K.R., Amrita institute of Medical Sciences, Kochi, Kerala Case history 34 year old gentleman Nephrotic range
Systemic Light Chain Amyloidosis
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2016 NCCN.org Continue Version 1.2016, 09/04/15 National Comprehensive Cancer Network, Inc. 2015,
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2016 NCCN.org Continue Version 1.2016, 09/04/15 National Comprehensive Cancer Network, Inc. 2015,
Myeloma Support Group: Now and the Horizon. Brian McClune, DO
 Myeloma Support Group: Now and the Horizon Brian McClune, DO Disclosures Consultant to Celgene Objectives Transplant for myeloma- is there any thing new? High risk disease University protocols New therapies?
Myeloma Support Group: Now and the Horizon Brian McClune, DO Disclosures Consultant to Celgene Objectives Transplant for myeloma- is there any thing new? High risk disease University protocols New therapies?
Cardiac amyloidosis an easy, but usually delayed diagnosis
 M K pag 46 Mædica - a Journal of Clinical Medicine CASE REPORTS Cardiac amyloidosis an easy, but usually delayed diagnosis R. SILISTE, MD a ; I. CALANGEA, MD a ; F. VOINEA, MD a ; I. SAVULESCU-FIEDLER,
M K pag 46 Mædica - a Journal of Clinical Medicine CASE REPORTS Cardiac amyloidosis an easy, but usually delayed diagnosis R. SILISTE, MD a ; I. CALANGEA, MD a ; F. VOINEA, MD a ; I. SAVULESCU-FIEDLER,
= Medical Policy. MP Hematopoietic Cell Transplantation for Primary Amyloidosis
 = Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
= Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
Successful Treatment of Immunoglobulin D Myeloma by Bortezomib and Dexamethasone Therapy
 CASE REPORT Successful Treatment of Immunoglobulin D Myeloma by Bortezomib and Dexamethasone Therapy Naohiro Sekiguchi 1, Naoki Takezako 1, Akihisa Nagata 1, Miyuki Wagatsuma 2, Satoshi Noto 1, Kazuaki
CASE REPORT Successful Treatment of Immunoglobulin D Myeloma by Bortezomib and Dexamethasone Therapy Naohiro Sekiguchi 1, Naoki Takezako 1, Akihisa Nagata 1, Miyuki Wagatsuma 2, Satoshi Noto 1, Kazuaki
Hematopoietic Cell Transplantation for Primary Amyloidosis
 Hematopoietic Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Hematopoietic Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Soluble syndecan-1 at diagnosis and during follow up of multiple myeloma: a single institution study
 VOLUME 45 ㆍ NUMBER 2 ㆍ June 30, 2010 THE KOREAN JOURNAL OF HEMATOLOGY ORIGINAL ARTICLE Soluble syndecan-1 at diagnosis and during follow up of multiple myeloma: a single institution study Ji Myung Kim
VOLUME 45 ㆍ NUMBER 2 ㆍ June 30, 2010 THE KOREAN JOURNAL OF HEMATOLOGY ORIGINAL ARTICLE Soluble syndecan-1 at diagnosis and during follow up of multiple myeloma: a single institution study Ji Myung Kim
Expanding Spectrum of Diseases Associated with Plasma Cell Dyscrasias
 Expanding Spectrum of Diseases Associated with Plasma Cell Dyscrasias Eva Honsova Institute for Clinical and Experimental Medicine Prague, Czech Republic eva.honsova@ikem.cz Plasma cell dyscrasias Plasma
Expanding Spectrum of Diseases Associated with Plasma Cell Dyscrasias Eva Honsova Institute for Clinical and Experimental Medicine Prague, Czech Republic eva.honsova@ikem.cz Plasma cell dyscrasias Plasma
Progress in the Treatment of Cardiac Amyloidosis
 Progress in the Treatment of Cardiac Amyloidosis Jignesh Patel MD PhD FACC FRCP Director, Cardiac Amyloid Program Medical Director, Heart Transplant Program Clinical Professor Cedars-Sinai Heart Institute,
Progress in the Treatment of Cardiac Amyloidosis Jignesh Patel MD PhD FACC FRCP Director, Cardiac Amyloid Program Medical Director, Heart Transplant Program Clinical Professor Cedars-Sinai Heart Institute,
Stabilisation of laryngeal AL amyloidosis with long term curcumin therapy
 University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2015 Stabilisation of laryngeal AL amyloidosis with long term curcumin
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2015 Stabilisation of laryngeal AL amyloidosis with long term curcumin
Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma
 Multidisciplinary Cancer Investigation October 2017, Volume 1, Issue 4 DOI: 10.21859/mci-01041 Original Article Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma Hasan Jalaeikhoo
Multidisciplinary Cancer Investigation October 2017, Volume 1, Issue 4 DOI: 10.21859/mci-01041 Original Article Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma Hasan Jalaeikhoo
CAELUM BIOSCIENCES. Corporate Overview May, 2017
 CAELUM BIOSCIENCES Corporate Overview May, 2017 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning
CAELUM BIOSCIENCES Corporate Overview May, 2017 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning
Clinical history. 73 yo man with chest pain Systemic hypertension and WG Stress EKG N Stress echocardiogram: Cardiac catheterization: no CAD
 CASE 8 Clinical history 73 yo man with chest pain Systemic hypertension and WG Stress EKG N Stress echocardiogram: Concentric hypertrophy Hypokinesis of LV-Inf Cardiac catheterization: no CAD Technique
CASE 8 Clinical history 73 yo man with chest pain Systemic hypertension and WG Stress EKG N Stress echocardiogram: Concentric hypertrophy Hypokinesis of LV-Inf Cardiac catheterization: no CAD Technique
Multiple Myeloma: diagnosis and prognostic factors. N Meuleman May 2015
 Multiple Myeloma: diagnosis and prognostic factors N Meuleman May 2015 Diagnosis Diagnostic assessment of myeloma: what should we know? Is it really a myeloma? Is there a need for treatment? What is the
Multiple Myeloma: diagnosis and prognostic factors N Meuleman May 2015 Diagnosis Diagnostic assessment of myeloma: what should we know? Is it really a myeloma? Is there a need for treatment? What is the
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/155242
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/155242
New and Emerging Technology Briefing. Fibrillex (Eprodisate disodium) for secondary amyloidosis. National Horizon Scanning Centre.
 New and Emerging Technology Briefing National Horizon Scanning Centre Fibrillex (Eprodisate disodium) for secondary amyloidosis January 2006 Horizon Scanning Review Early assessments of new or emerging
New and Emerging Technology Briefing National Horizon Scanning Centre Fibrillex (Eprodisate disodium) for secondary amyloidosis January 2006 Horizon Scanning Review Early assessments of new or emerging
Advances in Peritoneal Dialysis, Vol. 29, 2013
 Advances in Peritoneal Dialysis, Vol. 29, 2013 Takeyuki Hiramatsu, 1 Takahiro Hayasaki, 1 Akinori Hobo, 1 Shinji Furuta, 1 Koki Kabu, 2 Yukio Tonozuka, 2 Yoshiyasu Iida 1 Icodextrin Eliminates Phosphate
Advances in Peritoneal Dialysis, Vol. 29, 2013 Takeyuki Hiramatsu, 1 Takahiro Hayasaki, 1 Akinori Hobo, 1 Shinji Furuta, 1 Koki Kabu, 2 Yukio Tonozuka, 2 Yoshiyasu Iida 1 Icodextrin Eliminates Phosphate
37 Novel Therapies for
 37 Novel Therapies for Multiple Myeloma Abstract: Current standard of management for newly diagnosed multiple myeloma are continuously evolving due to the advent of a number of novel agents with different
37 Novel Therapies for Multiple Myeloma Abstract: Current standard of management for newly diagnosed multiple myeloma are continuously evolving due to the advent of a number of novel agents with different
BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib
 BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib Protocol Code Tumour Group Contact Physician ULKAMLAS Leukemia/BMT Dr. Donna Hogge ELIGIBILITY: Acute myeloid
BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib Protocol Code Tumour Group Contact Physician ULKAMLAS Leukemia/BMT Dr. Donna Hogge ELIGIBILITY: Acute myeloid
Malignant myelomatous pleural effusion-is onset of effusion a new prognostic factor?
 Turk J Hematol 2007; 24:180-184 Turkish Society of Hematology CASE REPORT Malignant myelomatous pleural effusion-is onset of effusion a new prognostic factor? Attili S, Ullas B, Lakshm D, Bapsy P.P, Lakshm
Turk J Hematol 2007; 24:180-184 Turkish Society of Hematology CASE REPORT Malignant myelomatous pleural effusion-is onset of effusion a new prognostic factor? Attili S, Ullas B, Lakshm D, Bapsy P.P, Lakshm
Criteria for Disease Assessment Joan Bladé
 Criteria for Disease Assessment Joan Bladé Unidad de Amiloidosis y Mieloma Servicio de Hematología Hospital Clínic de Barcelona COMy Meeting, París, May 4th, 2018 Response Evaluation EBMT, 1998 - CR and
Criteria for Disease Assessment Joan Bladé Unidad de Amiloidosis y Mieloma Servicio de Hematología Hospital Clínic de Barcelona COMy Meeting, París, May 4th, 2018 Response Evaluation EBMT, 1998 - CR and
If unqualified, Complete remission is considered to be Haematological complete remission
 Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
