ANNEX III AMENDMENTS TO THE SUMMARIES OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLETS
|
|
|
- Morris Murphy
- 6 years ago
- Views:
Transcription
1 ANNEX III AMENDMENTS TO THE SUMMARIES OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLETS 41
2 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE SUMMARY OF PRODUCT CHARACTERISTICS FOR CABERGOLINE CONTAINING MEDICINAL PRODUCTS 4.2 Posology and method of administration The following should be reflected as appropriate: Restriction of the maximum dose to 3 mg/day 4.3 Contraindications: For long-term treatment: Evidence of cardiac valvulopathy as determined by pre-treatment echocardiography. 4.4 Special warnings and precautions for use: Fibrosis and cardiac valvulopathy and possibly related clinical phenomena: Fibrotic and serosal inflammatory disorders such as pleuritis, pleural effusion, pleural fibrosis, pulmonary fibrosis, pericarditis, pericardial effusion, cardiac valvulopathy involving one or more valves (aortic, mitral and tricuspid) or retroperitoneal fibrosis have occurred after prolonged usage of ergot derivatives with agonist activity at the serotonin 5HT 2B receptor, such as cabergoline. In some cases, symptoms or manifestations of cardiac valvulopathy improved after discontinuation of cabergoline. Erythrocyte sedimentation rate (ESR) has been found to be abnormally increased in association with pleural effusion/fibrosis. Chest x-ray examination is recommended in cases of unexplained ESR increases to abnormal values. Valvulopathy has been associated with cumulative doses, therefore, patients should be treated with the lowest effective dose. At each visit, the risk benefit profile of cabergoline treatment for the patient should be reassessed to determine the suitability of continued treatment with cabergoline. Before initiating long-term treatment: All patients must undergo a cardiovascular evaluation, including echocardiogram, to assess the potential presence of asymptomatic valvular disease. It is also appropriate to perform baseline investigations of erythrocyte sedimentation rate or other inflammatory markers, lung function/chest X-ray and renal function prior to initiation of therapy. In patients with valvular regurgitation, it is not known whether cabergoline treatment might worsen the underlying disease. If fibrotic valvular disease is detected, the patient should not be treated with cabergoline (see section 4.3). During long-term treatment: Fibrotic disorders can have an insidious onset and patients should be regularly monitored for possible manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of: Pleuro-pulmonary disease such as dyspnoea, shortness of breath, persistent cough or chest pain. Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb oedema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis. Cardiac failure; cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur. 42
3 Clinical diagnostic monitoring for development of fibrotic disorders, as appropriate, is essential. Following treatment initiation, the first echocardiogram must occur within 3-6 months, thereafter, the frequency of echocardiographic monitoring should be determined by appropriate individual clinical assessment with particular emphasis on the above-mentioned signs and symptoms, but must occur at least every 6 to 12 months. Cabergoline should be discontinued if an echocardiogram reveals new or worsened valvular regurgitation, valvular restriction or valve leaflet thickening (see Section 4.3). The need for other clinical monitoring (e.g. physical examination including, cardiac auscultation, X- ray, CT scan) should be determined on an individual basis. Additional appropriate investigations such as erythrocyte sedimentation rate, and serum creatinine measurements should be performed if necessary to support a diagnosis of a fibrotic disorder. Section 4.4 Special warnings and precautions for use (for cabergoline-containing medicinal products authorised in the indication of anovulation and infertility only) Before administration of cabergoline pregnancy should be excluded. Because clinical experience is still limited and the product has a long half-life, as a precautionary measure it is recommended that once regular ovulatory cycles have been achieved women seeking pregnancy discontinue product name] one month before intended conception. 4.8 Undesirable effects: The following should be included under Cardiac disorders: Very common: cardiac valvulopathy (including regurgitation) and related disorders (pericarditis and pericardial effusion). 43
4 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE PACKAGE LEAFLET FOR CABERGOLINE CONTAINING MEDICINAL PRODUCTS Section 2 Before you take [product name] : Do not take [product name] if you: -will be treated with [product name] for a long period and have or had fibrotic reactions (scar tissue) affecting your heart. Take special care with [product name] [ ].. - If you have or had fibrotic reactions (scar tissue) affecting your heart, lungs or abdomen. In case you are treated with [product name] for a long period, your physician will check before starting treatment whether your heart, lungs and kidneys are in good condition. He/she will also have an echocardiogram (an ultrasound test of the heart) taken before treatment is started and at regular intervals during treatment. If fibrotic reactions occur treatment will have to be discontinued. Section 2 Before you take [product name]. The following should be included under Pregnancy and breast feeding (for cabergoline-containing medicinal products authorised in the indication of anovulation and infertility only): Before you can start using cabergoline you have to exclude that you are pregnant. Additionally you should take care not becoming pregnant for at least one month once you have stopped treatment with cabergoline. Section 4 Possible side effects : Very common side effect (affecting more than one person in ten): heart valve and related disorders e.g. inflammation (pericarditis) or leaking of fluid in the pericardium (pericardial effusion). The early symptoms may be one or more of the following: difficulty breathing, shortness of breath, chest or back pain and swollen legs. If you experience any one of these symptoms you must tell your doctor immediately. 44
5 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE SUMMARY OF PRODUCT CHARACTERISTICS FOR PERGOLIDE CONTAINING MEDICINAL PRODUCTS 4.2 Posology and method of administration The following should be reflected as appropriate: Restriction of the maximum dose to 3 mg/day. 4.3 Contraindication: Evidence of cardiac valvulopathy as determined by pre-treatment echocardiography. 4.4 Special warnings and precautions for use: Fibrosis and cardiac valvulopathy and possibly related clinical phenomena: Fibrotic and serosal inflammatory disorders such as pleuritis, pleural effusion, pleural fibrosis, pulmonary fibrosis, pericarditis, pericardial effusion, cardiac valvulopathy involving one or more valves (aortic, mitral and tricuspid) or retroperitoneal fibrosis have occurred after prolonged usage of ergot derivatives with agonist activity at the serotonin 5HT 2B receptor, such as pergolide. In some cases, symptoms or manifestations of cardiac valvulopathy improved after discontinuation of pergolide. There is evidence that higher dose and/or cumulative exposure are risk factors for development of valvular pathology. However, valvulopathy and fibrotic reactions have been reported during treatment with pergolide at doses less than 0.5mg/day. Before initiating treatment: All patients must undergo a cardiovascular evaluation, including echocardiogram, to assess the potential presence of asymptomatic valvular disease. In patients with valvular regurgitation, it is not known whether pergolide treatment might worsen the underlying disease. If fibrotic valvular disease is detected, the patient should not be treated with pergolide (see section 4.3). It is also appropriate to perform baseline investigations of erythrocyte sedimentation rate or other inflammatory markers, lung function/chest X-ray and renal function prior to initiation of therapy. During treatment: Fibrotic disorders can have an insidious onset and patients should be regularly monitored for possible manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of: Pleuro-pulmonary disease such as dyspnoea, shortness of breath, persistent cough or chest pain. Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb oedema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis. Cardiac failure; cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur. Clinical diagnostic monitoring for development of valvular disease or fibrosis, as appropriate, is essential. Following treatment initiation, the first echocardiogram must occur within 3-6 months, thereafter, the frequency of echocardiographic monitoring should be determined by appropriate individual clinical assessment with particular emphasis on the above-mentioned signs and symptoms, but must occur at least every 6 to 12 months. 45
6 Pergolide should be discontinued if an echocardiogram reveals new or worsened valvular regurgitation, valvular restriction or valve leaflet thickening (see Section 4.3). The need for other clinical monitoring (e.g. physical examination including, cardiac auscultation, X- ray, CT scan) should be determined on an individual basis. Additional appropriate investigations such as erythrocyte sedimentation rate, and serum creatinine measurements should be performed if necessary to support a diagnosis of a fibrotic disorder. 4.8 Undesirable effects: The following should be included under Cardiac disorders: Very common: cardiac valvulopathy (including regurgitation) and related disorders (pericarditis and pericardial effusion). 46
7 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE PACKAGE LEAFLET FOR PERGOLIDE CONTAINING MEDICINAL PRODUCTS Section 2 Before you take [product name] : Do not take [product name] if you: - have or had fibrotic reactions (scar tissue) affecting your heart. Take special care with [product name] If you have or had fibrotic reactions (scar tissue) affecting your heart, lungs or abdomen. Before treatment your physician will check whether your heart, lungs and kidneys are in good condition. He/she will also have an echocardiogram (an ultrasound test of the heart) taken before treatment is started and at regular intervals during treatment. If fibrotic reactions occur treatment will have to be discontinued. Section 3 How to take [product name] Do not take more than 3 <if relevant include colour of the tablet> tablets (3 x 1000 microgram tablets) per day. Section 4 Possible side effects : Very common side effect (affecting more than one person in ten): heart valve and related disorders e.g. inflammation (pericarditis) or leaking of fluid in the pericardium (pericardial effusion). The early symptoms may be one or more of the following: difficulty breathing, shortness of breath, chest or back pain and swollen legs. If you experience any one of these symptoms you must tell your doctor immediately. 47
ANNEX III AMENDMENTS TO THE SUMMARIES OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLETS
 ANNEX III AMENDMENTS TO THE SUMMARIES OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLETS 25 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE SUMMARY OF PRODUCT CHARACTERISTICS FOR BROMOCRIPTINE CONTAINING
ANNEX III AMENDMENTS TO THE SUMMARIES OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLETS 25 AMENDMENTS TO BE INCLUDED IN THE RELEVANT SECTIONS OF THE SUMMARY OF PRODUCT CHARACTERISTICS FOR BROMOCRIPTINE CONTAINING
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Cabergoline Teva Pharma 2 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg cabergoline. Excipient with
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Cabergoline Teva Pharma 2 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg cabergoline. Excipient with
Package leaflet: Information for the user. Cabergoline Teva 2 mg tablets. cabergoline
 Package leaflet: Information for the user Cabergoline Teva 1 mg tablets Cabergoline Teva 2 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the user Cabergoline Teva 1 mg tablets Cabergoline Teva 2 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains
Chemical name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea.
![Chemical name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea. Chemical name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea.](/thumbs/77/75685136.jpg) PRODUCT INFORMATION DOSTINEX NAME OF THE MEDICINE The active ingredient in Dostinex is cabergoline. Chemical name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea.
PRODUCT INFORMATION DOSTINEX NAME OF THE MEDICINE The active ingredient in Dostinex is cabergoline. Chemical name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea.
APO-CABERGOLINE TABLET
 APO-CABERGOLINE TABLET NAME OF THE MEDICINE Cabergoline Chemical Name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea
APO-CABERGOLINE TABLET NAME OF THE MEDICINE Cabergoline Chemical Name: 1-[(6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindole[4,3-fg]- quinoline-9-carbonyl]-1-(3-dimethyl-aminopropyl)-3-ethylurea
Package leaflet: Information for the user. Cabergoline Teva 0.5 mg tablets cabergoline
 Package leaflet: Information for the user Cabergoline Teva 0.5 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Cabergoline Teva 0.5 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
Pre-existing information not covered by the CSP is highlighted in yellow
 Produktinformationen för Cabergoline ratiopharm 2 mg tablett, MTnr 23665, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet inte marknadsförs
Produktinformationen för Cabergoline ratiopharm 2 mg tablett, MTnr 23665, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet inte marknadsförs
Produktinformationen för Cabergoline 1A Farma 3 mg tablett, MTnr 45933, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte
 Produktinformationen för Cabergoline 1A Farma 3 mg tablett, MTnr 45933, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet inte marknadsförs
Produktinformationen för Cabergoline 1A Farma 3 mg tablett, MTnr 45933, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet inte marknadsförs
Package leaflet: Information for the patient. Cabergoline Sandoz 0.5 mg tablets. cabergoline
 Package leaflet: Information for the patient Cabergoline Sandoz 0.5 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Cabergoline Sandoz 0.5 mg tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient. Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains
Atrioventricular Valve Dysplasia
 Atrioventricular Valve Dysplasia How does the heart work? The heart is the organ responsible for pumping blood to and from all tissues of the body. The heart is divided into right and left sides. The job
Atrioventricular Valve Dysplasia How does the heart work? The heart is the organ responsible for pumping blood to and from all tissues of the body. The heart is divided into right and left sides. The job
Package leaflet: Information for the patient. Dostinex 500 microgram Tablets cabergoline
 Package leaflet: Information for the patient Dostinex 500 microgram Tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Dostinex 500 microgram Tablets cabergoline Read all of this leaflet carefully before you start taking this medicine because it contains important information
PRODUCT MONOGRAPH APO-CABERGOLINE. Cabergoline Tablets USP. 0.5 mg DOPAMINE RECEPTOR AGONIST
 PRODUCT MONOGRAPH APO-CABERGOLINE Cabergoline Tablets USP.5 mg DOPAMINE RECEPTOR AGONIST APOTEX INC. DATE OF PREPARATION: 15 Signet Drive June 16, 216 Toronto, Ontario M9L 1T9 Control Number: 128573 APO-CABERGOLINE
PRODUCT MONOGRAPH APO-CABERGOLINE Cabergoline Tablets USP.5 mg DOPAMINE RECEPTOR AGONIST APOTEX INC. DATE OF PREPARATION: 15 Signet Drive June 16, 216 Toronto, Ontario M9L 1T9 Control Number: 128573 APO-CABERGOLINE
Package leaflet: Information for the user Parlodel 5 mg Hard Capsules Bromocriptine mesilate
 Package leaflet: Information for the user Parlodel 5 mg Hard Capsules Bromocriptine mesilate Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Parlodel 5 mg Hard Capsules Bromocriptine mesilate Read all of this leaflet carefully before you start taking this medicine because it contains important information
PRODUCT MONOGRAPH DOSTINEX
 PRODUCT MONOGRAPH PR DOSTINEX (cabergoline) Tablets.5 mg Dopamine Receptor Agonist Pfizer Canada Inc. 17,3 Trans-Canada Highway Kirkland, Quebec H9J 2M5 Revision Date: 23 July 213 Dist. by: Paladin Labs
PRODUCT MONOGRAPH PR DOSTINEX (cabergoline) Tablets.5 mg Dopamine Receptor Agonist Pfizer Canada Inc. 17,3 Trans-Canada Highway Kirkland, Quebec H9J 2M5 Revision Date: 23 July 213 Dist. by: Paladin Labs
Severe Hypertension. Pre-referral considerations: 1. BP of arm and Leg 2. Ambulatory BP 3. Renal causes
 Severe Hypertension *Prior to making a referral, call office or Doc Halo, to speak with a Cardiologist or APP to discuss patient and possible treatment options. Please only contact the patient's cardiologist.
Severe Hypertension *Prior to making a referral, call office or Doc Halo, to speak with a Cardiologist or APP to discuss patient and possible treatment options. Please only contact the patient's cardiologist.
Right-Sided Congestive Heart Failure Basics
 Right-Sided Congestive Heart Failure Basics OVERVIEW Failure of the right side of the heart to pump blood at a sufficient rate to meet the needs of the body or to prevent blood from pooling within the
Right-Sided Congestive Heart Failure Basics OVERVIEW Failure of the right side of the heart to pump blood at a sufficient rate to meet the needs of the body or to prevent blood from pooling within the
Learning About Mitral Regurgitation (MR) Causes Symptoms Treatment Options
 Learning About Mitral Regurgitation (MR) Causes Symptoms Treatment Options How Your Heart Works Your heart pumps blood through your lungs to replenish it with oxygen, and then pumps the oxygen-rich blood
Learning About Mitral Regurgitation (MR) Causes Symptoms Treatment Options How Your Heart Works Your heart pumps blood through your lungs to replenish it with oxygen, and then pumps the oxygen-rich blood
Annex C. (variation to nationally authorised medicinal products)
 Annex C (variation to nationally authorised medicinal products) Annex I Scientific conclusions and grounds for variation to the terms of the marketing authorisations Scientific conclusions Taking into
Annex C (variation to nationally authorised medicinal products) Annex I Scientific conclusions and grounds for variation to the terms of the marketing authorisations Scientific conclusions Taking into
REDERGIN 1.5 mg tablets; 4.5 mg tablets; 1 mg/ml oral solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER REDERGIN 1.5 mg tablets; 4.5 mg tablets; 1 mg/ml oral solution ERGOLOID MESYLATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
PACKAGE LEAFLET: INFORMATION FOR THE USER REDERGIN 1.5 mg tablets; 4.5 mg tablets; 1 mg/ml oral solution ERGOLOID MESYLATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Lung cancer is one of the most common types of cancer in European men and women. There are two main types of lung cancer: small
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Lung cancer is one of the most common types of cancer in European men and women. There are two main types of lung cancer: small
Annex III. Amendments to relevant sections of the product information
 Annex III Amendments to relevant sections of the product information Note: These amendments to the relevant sections of the Summary of Product Characteristics and Package Leaflet are the outcome of the
Annex III Amendments to relevant sections of the product information Note: These amendments to the relevant sections of the Summary of Product Characteristics and Package Leaflet are the outcome of the
DISCLOSURE. Echocardiography in Systemic Diseases: Questions. Relevant Financial Relationship(s) None. Off Label Usage None 5/7/2018
 Echocardiography in Systemic Diseases: Questions Sunil Mankad, MD, FACC, FCCP, FASE Associate Professor of Medicine Mayo Clinic College of Medicine Director, Transesophageal Echocardiography Associate
Echocardiography in Systemic Diseases: Questions Sunil Mankad, MD, FACC, FCCP, FASE Associate Professor of Medicine Mayo Clinic College of Medicine Director, Transesophageal Echocardiography Associate
Manage TB Dr. Dina Nair National Institute for Research in Tuberculosis, Chennai. Lecture 07 Clinical manifestations of TB Session 02
 Manage TB Dr. Dina Nair National Institute for Research in Tuberculosis, Chennai Lecture 07 Clinical manifestations of TB Session 02 Hello, welcome to the second session on Clinical manifestations of tuberculosis.
Manage TB Dr. Dina Nair National Institute for Research in Tuberculosis, Chennai Lecture 07 Clinical manifestations of TB Session 02 Hello, welcome to the second session on Clinical manifestations of tuberculosis.
Annex I. Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s)
 Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for ipratropium/salbutamol,
Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for ipratropium/salbutamol,
Package leaflet: Information for the user Cyklokapron 500 mg Solution for Injection tranexamic acid
 Package leaflet: Information for the user Cyklokapron 500 mg Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important information
Package leaflet: Information for the user Cyklokapron 500 mg Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important information
ECHOCARDIOGRAPHY. Patient Care. Goals and Objectives PF EF MF LF Aspirational
 Patient Care Be able to: Perform and interpret basic TTE and X cardiac Doppler examinations Perform and interpret a comprehensive X TTE and cardiac Doppler examination Perform and interpret a comprehensive
Patient Care Be able to: Perform and interpret basic TTE and X cardiac Doppler examinations Perform and interpret a comprehensive X TTE and cardiac Doppler examination Perform and interpret a comprehensive
ERDHEIM-CHESTER DISEASE LUNG & HEART ISSUES
 ERDHEIM-CHESTER DISEASE LUNG & HEART ISSUES GIULIO CAVALLI, M.D. INTERNAL MEDICINE AND CLINICAL IMMUNOLOGY IRCCS SAN RAFFAELE HOSPITAL VITA-SALUTE SAN RAFFAELE UNIVERSITY MILAN, ITALY cavalli.giulio@hsr.it
ERDHEIM-CHESTER DISEASE LUNG & HEART ISSUES GIULIO CAVALLI, M.D. INTERNAL MEDICINE AND CLINICAL IMMUNOLOGY IRCCS SAN RAFFAELE HOSPITAL VITA-SALUTE SAN RAFFAELE UNIVERSITY MILAN, ITALY cavalli.giulio@hsr.it
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Page 1 of 7
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 7 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prilocard 5mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 7 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prilocard 5mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
Cardiac Mass in a 15-Year-Old Boy
 Cardiac Mass in a 15-Year-Old Boy Echocardiographic Case Report Hortensia Vuçini Department of Cardiology and Cardiac Surgery UHC Mother Theresa Tirana, Albania October 20, 2007 Case Presentation 15 year-old
Cardiac Mass in a 15-Year-Old Boy Echocardiographic Case Report Hortensia Vuçini Department of Cardiology and Cardiac Surgery UHC Mother Theresa Tirana, Albania October 20, 2007 Case Presentation 15 year-old
Transcatheter Aortic Valve Implant (TAVI) Sussex Cardiac Centre
 Transcatheter Aortic Valve Implant (TAVI) Sussex Cardiac Centre Information for patients and carers Contents Valvular heart disease 3 What are the symptoms 3 Transcatheter aortic valve implantation 4 What
Transcatheter Aortic Valve Implant (TAVI) Sussex Cardiac Centre Information for patients and carers Contents Valvular heart disease 3 What are the symptoms 3 Transcatheter aortic valve implantation 4 What
Review of Cardiac Imaging Modalities in the Renal Patient. George Youssef
 Review of Cardiac Imaging Modalities in the Renal Patient George Youssef ECHO Left ventricular hypertrophy (LVH) assessment Diastolic dysfunction Stress ECHO Cardiac CT angiography Echocardiography - positives
Review of Cardiac Imaging Modalities in the Renal Patient George Youssef ECHO Left ventricular hypertrophy (LVH) assessment Diastolic dysfunction Stress ECHO Cardiac CT angiography Echocardiography - positives
Scientific conclusions
 Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Heart Valves: Before and after surgery
 Heart Valves: Before and after surgery Tim Sutton, Consultant Cardiologist Middlemore Hospital, Auckland Auckland Heart Group Indications for intervention in Valvular disease To prevent sudden death and
Heart Valves: Before and after surgery Tim Sutton, Consultant Cardiologist Middlemore Hospital, Auckland Auckland Heart Group Indications for intervention in Valvular disease To prevent sudden death and
Annex III. Amendments to relevant sections of the Product Information
 Annex III Amendments to relevant sections of the Product Information Note: These amendments to the relevant sections of the product information are the outcome of the referral procedure. The product information
Annex III Amendments to relevant sections of the Product Information Note: These amendments to the relevant sections of the product information are the outcome of the referral procedure. The product information
One problem above the diaphragm and one problem below the diaphragm
 One problem above the diaphragm and one problem below the diaphragm Wouter Meersseman, MD, PhD Universitary Hospital Leuven General Internal Medicine Leuven 13 Dec 2013 83-year old lady Past medical history
One problem above the diaphragm and one problem below the diaphragm Wouter Meersseman, MD, PhD Universitary Hospital Leuven General Internal Medicine Leuven 13 Dec 2013 83-year old lady Past medical history
Cardiothoracic and Cardiothoracic Surgery ICD-10-CM 2014: Reference Mapping Card
 2014: Reference Mapping Card 162.3 Malignant neoplasm upper lobe lung 162.5 Malignant neoplasm lower lobe lung 162.9 lung/bronchus 396.2 396.3 Mitral insufficiency, aortic stenosis Mitral aortic valve
2014: Reference Mapping Card 162.3 Malignant neoplasm upper lobe lung 162.5 Malignant neoplasm lower lobe lung 162.9 lung/bronchus 396.2 396.3 Mitral insufficiency, aortic stenosis Mitral aortic valve
Looking Outside the Box: Incidental Extracardiac Finding in Echo
 Looking Outside the Box: Incidental Extracardiac Finding in Echo Dr. Aijaz Shah Head of Division, Adult Echocardiography Laboratory Prince Sultan Cardiac Centre Riyadh Case 1 17 year old boy presented
Looking Outside the Box: Incidental Extracardiac Finding in Echo Dr. Aijaz Shah Head of Division, Adult Echocardiography Laboratory Prince Sultan Cardiac Centre Riyadh Case 1 17 year old boy presented
Summary of the risk management plan (RMP) for Kyprolis (carfilzomib)
 EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
Pericardial Effusion
 Pericardial Effusion How does the heart work? The heart is the organ responsible for pumping blood to and from all tissues of the body. The heart is divided into right and left sides. The job of the right
Pericardial Effusion How does the heart work? The heart is the organ responsible for pumping blood to and from all tissues of the body. The heart is divided into right and left sides. The job of the right
Aortic regurgitation in seropositive juvenile arthritis
 Annals of the Rheumatic Diseases, 1981, 40, 229-234 Aortic regurgitation in seropositive juvenile arthritis A. M. LEAK,' M. W. MILLAR-CRAIG,2 AND BARBARA M. ANSELL1 From the 'Juvenile Rheumatism Unit,
Annals of the Rheumatic Diseases, 1981, 40, 229-234 Aortic regurgitation in seropositive juvenile arthritis A. M. LEAK,' M. W. MILLAR-CRAIG,2 AND BARBARA M. ANSELL1 From the 'Juvenile Rheumatism Unit,
Sodium Chloride 0.9% w/v Intravenous Infusion BP Solution for Infusion Sodium chloride
 Package leaflet: Information for the user Sodium Chloride 0.9% w/v Intravenous Infusion BP Solution for Infusion Sodium chloride Read all of this leaflet carefully before using this medicine because it
Package leaflet: Information for the user Sodium Chloride 0.9% w/v Intravenous Infusion BP Solution for Infusion Sodium chloride Read all of this leaflet carefully before using this medicine because it
The safety concerns defined with the reference product apply to Buprenorphine-Naloxone 2/0.5 mg, 8/2 mg, sublingual tablets.
 These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
Scientific conclusions
 Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 18 Scientific conclusions Overall
Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 18 Scientific conclusions Overall
New Zealand Data Sheet
 New Zealand Data Sheet APO-BROMOCRIPTINE Presentation APO-BROMOCRIPTINE 2.5mg tablets are white, oval, slope-faced & flat-faced tablets with bevelled edges. Scored and engraved APO 2.5 on slope side, plain
New Zealand Data Sheet APO-BROMOCRIPTINE Presentation APO-BROMOCRIPTINE 2.5mg tablets are white, oval, slope-faced & flat-faced tablets with bevelled edges. Scored and engraved APO 2.5 on slope side, plain
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
UCLA-LIVESTRONG LIVESTRONG Survivorship Center of Excellence One in three individuals with receive a cancer diagnosis in their lifetime 10.6 million A
 Cardiovascular Health After Cancer: Common and Overlooked Issues for Post-Cancer Care Barbara Natterson Horowitz, M.D. UCLA Division of Cardiology David Geffen School of Medicine at UCLA UCLA-LIVESTRONG
Cardiovascular Health After Cancer: Common and Overlooked Issues for Post-Cancer Care Barbara Natterson Horowitz, M.D. UCLA Division of Cardiology David Geffen School of Medicine at UCLA UCLA-LIVESTRONG
SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW
 SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW Lung disease can be a serious complication of scleroderma. The two most common types of lung disease in patients with scleroderma are interstitial
SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW Lung disease can be a serious complication of scleroderma. The two most common types of lung disease in patients with scleroderma are interstitial
Saluki heart pathology study
 Heart conditions by MaryDee Sist, DVM Originally published in Baraka Book, Autumn-Winter 2001 For the last decade I have been involved in Saluki heart research. Ouroriginalgoalwastoexaminethe incidence
Heart conditions by MaryDee Sist, DVM Originally published in Baraka Book, Autumn-Winter 2001 For the last decade I have been involved in Saluki heart research. Ouroriginalgoalwastoexaminethe incidence
Annex III. Amendments to the relevant sections of the product information
 Annex III Amendments to the relevant sections of the product information Note: This product information is the outcome of the referral procedure to which this Commission decision relates. The product information
Annex III Amendments to the relevant sections of the product information Note: This product information is the outcome of the referral procedure to which this Commission decision relates. The product information
Clinical Indications for Echocardiography
 Clinical Indications for Echocardiography Echocardiography is widely utilised and potential applications are increasing with advances in technology. The aim of this document is two-fold: 1) To define clinical
Clinical Indications for Echocardiography Echocardiography is widely utilised and potential applications are increasing with advances in technology. The aim of this document is two-fold: 1) To define clinical
Rotation: Echocardiography: Transthoracic Echocardiography (TTE)
 Rotation: Echocardiography: Transthoracic Echocardiography (TTE) Rotation Format and Responsibilities: Fellows rotate in the echocardiography laboratory in each clinical year. Rotations during the first
Rotation: Echocardiography: Transthoracic Echocardiography (TTE) Rotation Format and Responsibilities: Fellows rotate in the echocardiography laboratory in each clinical year. Rotations during the first
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: , VERSION 1.
 RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
PRODUCT INFORMATION. DESERIL Methysergide 1mg (as maleate) Tablets
 NAME OF THE MEDICINE Methysergide PRODUCT INFORMATION DESERIL Methysergide 1mg (as maleate) Tablets H O H CH 2 CH 3 CNH CH 2 OH N H CH 3 Methysergide H 3 C 1-Methyl-d-lysergic acid-(1-hydroxybut-2-yl)amide
NAME OF THE MEDICINE Methysergide PRODUCT INFORMATION DESERIL Methysergide 1mg (as maleate) Tablets H O H CH 2 CH 3 CNH CH 2 OH N H CH 3 Methysergide H 3 C 1-Methyl-d-lysergic acid-(1-hydroxybut-2-yl)amide
Package leaflet: Information for patient
 Package leaflet: Information for patient Apresoline Ampoules 20mg Powder for concentrate for solution for injection or infusion Hydralazine hydrochloride Read all of this leaflet carefully before you start
Package leaflet: Information for patient Apresoline Ampoules 20mg Powder for concentrate for solution for injection or infusion Hydralazine hydrochloride Read all of this leaflet carefully before you start
Case Report. Progression of Left Valve Disease during Use of Dopaminergic Drugs. Abstract. Case Report. Keywords. Discussion
 Progression of Left Valve Disease during Use of Dopaminergic Drugs Lívia Santana Barbosa, Ana Clara Tude Rodrigues, Shirlei Novillo Pereira, Hugo Leonardo Medeiros Vieira, Kamila Fernanda Staszko, José
Progression of Left Valve Disease during Use of Dopaminergic Drugs Lívia Santana Barbosa, Ana Clara Tude Rodrigues, Shirlei Novillo Pereira, Hugo Leonardo Medeiros Vieira, Kamila Fernanda Staszko, José
Degenerative Valve Disease (DVD)
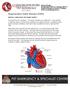 Degenerative Valve Disease (DVD) BRIEFLY, HOW DOES THE HEART WORK? The heart has four chambers. The upper chambers are called atria. One chamber is called an atrium, and the lower chambers are called ventricles.
Degenerative Valve Disease (DVD) BRIEFLY, HOW DOES THE HEART WORK? The heart has four chambers. The upper chambers are called atria. One chamber is called an atrium, and the lower chambers are called ventricles.
Tests Your Pulmonologist Might Order. Center For Cardiac Fitness Pulmonary Rehab Program The Miriam Hospital
 Tests Your Pulmonologist Might Order Center For Cardiac Fitness Pulmonary Rehab Program The Miriam Hospital BASIC ANATOMY OF THE LUNGS Lobes of Lung 3 lobes on the Right lung 2 lobes on the Left Blood
Tests Your Pulmonologist Might Order Center For Cardiac Fitness Pulmonary Rehab Program The Miriam Hospital BASIC ANATOMY OF THE LUNGS Lobes of Lung 3 lobes on the Right lung 2 lobes on the Left Blood
Mitral Valve Disease (MVD)
 Mitral Valve Disease (MVD) What is the mitral valve? The heart contains 4 valves within it. Each valve is present to allow unidirectional flow and to prevent flow backwards. The mitral valve is the valve
Mitral Valve Disease (MVD) What is the mitral valve? The heart contains 4 valves within it. Each valve is present to allow unidirectional flow and to prevent flow backwards. The mitral valve is the valve
srmp Atorvastatin Medical Valley
 srmp Atorvastatin Medical Valley VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology This product is indicated for the lowering of cholesterol blood levels and the prevention of
srmp Atorvastatin Medical Valley VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology This product is indicated for the lowering of cholesterol blood levels and the prevention of
PATIENT MEDICATION INFORMATION. MEGACE OS Megestrol acetate Oral Suspension
 PATIENT MEDICATION INFORMATION MEGACE OS Megestrol acetate Oral Suspension Read this carefully before you start taking MEGACE OS and each time you get a refill. This leaflet is a summary and will not tell
PATIENT MEDICATION INFORMATION MEGACE OS Megestrol acetate Oral Suspension Read this carefully before you start taking MEGACE OS and each time you get a refill. This leaflet is a summary and will not tell
MATRIX VHD FORM. State the name of the patient ( Product Recipient ) for whom you are providing the information contained in this form.
 MATRIX VHD FORM A. Patient Information State the name of the patient ( Product Recipient ) for whom you are providing the information contained in this form. (First Name) (Middle Initial) (Last Name) (Date
MATRIX VHD FORM A. Patient Information State the name of the patient ( Product Recipient ) for whom you are providing the information contained in this form. (First Name) (Middle Initial) (Last Name) (Date
Valvular Heart Disease
 Valvular Heart Disease O.P.Yadava C.E.O. & Chief Cardiac Surgeon National Heart Institute New Delhi 1. What are Valves and what is the Anatomy of the Human Heart? The heart is a marvel of nature. It is
Valvular Heart Disease O.P.Yadava C.E.O. & Chief Cardiac Surgeon National Heart Institute New Delhi 1. What are Valves and what is the Anatomy of the Human Heart? The heart is a marvel of nature. It is
PACKAGE LEAFLET: INFORMATION FOR THE USER. Human Albumin Biotest 20%, solution for infusion Human albumin
 PACKAGE LEAFLET: INFORMATION FOR THE USER Human Albumin Biotest 20%, solution for infusion Human albumin Read this entire leaflet carefully before you start using this medicine, because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Human Albumin Biotest 20%, solution for infusion Human albumin Read this entire leaflet carefully before you start using this medicine, because it contains important
Appendix to Gibson et al. Application of a decision rule and a D-dimer assay in the
 Appendix to Gibson et al. Application of a decision rule and a D-dimer assay in the diagnosis of pulmonary embolism (Thromb Haemost 2010; 103.4) Case 1 You are paged by an emergency room physician, who
Appendix to Gibson et al. Application of a decision rule and a D-dimer assay in the diagnosis of pulmonary embolism (Thromb Haemost 2010; 103.4) Case 1 You are paged by an emergency room physician, who
Abstract Clinical and paraclinical studies on myocardial and endocardial diseases in dog
 Abstract The doctoral thesis entitled Clinical and paraclinical studies on myocardial and endocardial diseases in dog was motivated by the study of the most frequent cardiopathies in dogs, which involves
Abstract The doctoral thesis entitled Clinical and paraclinical studies on myocardial and endocardial diseases in dog was motivated by the study of the most frequent cardiopathies in dogs, which involves
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Transcatheter Aortic Valve Implant (TAVI)
 Transcatheter Aortic Valve Implant (TAVI) Sussex Cardiac Centre Information for Patients and Carers Contents Advice pre and post transcatheter aortic valve implant 2 Valvular heart disease 3 What are the
Transcatheter Aortic Valve Implant (TAVI) Sussex Cardiac Centre Information for Patients and Carers Contents Advice pre and post transcatheter aortic valve implant 2 Valvular heart disease 3 What are the
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus. Rapid Cardiac Echo (RCE)
 Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
MITRAL STENOSIS. Joanne Cusack
 MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center
 Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center Pinpoint & properly assign the appropriate heart failure codes Left- vs. Right-sided Left ventricular failure (LVF) may
Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center Pinpoint & properly assign the appropriate heart failure codes Left- vs. Right-sided Left ventricular failure (LVF) may
PERICARDIAL DIAESE. Kaijun Cui Associated professor Sichuan University
 PERICARDIAL DIAESE Kaijun Cui Associated professor Sichuan University CLASSIFICATION acute pericarditis pericardial effusion cardiac tamponade constrictive pericarditis congenitally absent pericardium
PERICARDIAL DIAESE Kaijun Cui Associated professor Sichuan University CLASSIFICATION acute pericarditis pericardial effusion cardiac tamponade constrictive pericarditis congenitally absent pericardium
Preparing for Your Transcatheter Mitral Valve Repair Procedure
 WHAT YOU NEED TO KNOW Preparing for Your Transcatheter Mitral Valve Repair Procedure HOW SHOULD YOU PREPARE FOR YOUR PROCEDURE? In the days before your procedure, it is important that you: Take all your
WHAT YOU NEED TO KNOW Preparing for Your Transcatheter Mitral Valve Repair Procedure HOW SHOULD YOU PREPARE FOR YOUR PROCEDURE? In the days before your procedure, it is important that you: Take all your
Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Acne vulgaris (or simply acne) is a common human skin disease, characterized by areas of skin with seborrhea (scaly red skin),
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Acne vulgaris (or simply acne) is a common human skin disease, characterized by areas of skin with seborrhea (scaly red skin),
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
Summary of the risk management plan (RMP) for Rezolsta (darunavir / cobicistat)
 EMA/608280/2014 Summary of the risk management plan (RMP) for Rezolsta (darunavir / cobicistat) This is a summary of the risk management plan (RMP) for Rezolsta, which details the measures to be taken
EMA/608280/2014 Summary of the risk management plan (RMP) for Rezolsta (darunavir / cobicistat) This is a summary of the risk management plan (RMP) for Rezolsta, which details the measures to be taken
Summary of risk management plan for Trazimera (trastuzumab)
 Summary of risk management plan for Trazimera (trastuzumab) Summary of risk management plan for PF-05280014 (trastuzumab) 1 This is a summary of the RMP for PF-05280014. The RMP details important risks
Summary of risk management plan for Trazimera (trastuzumab) Summary of risk management plan for PF-05280014 (trastuzumab) 1 This is a summary of the RMP for PF-05280014. The RMP details important risks
Annex I. Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s)
 Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for morphine,
Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for morphine,
THE MEDTRONIC TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) SYSTEM
 THE MEDTRONIC TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) SYSTEM 1 A Guide for Patients With Severe Aortic Stenosis medtronic.com/tavr Table of Contents 2 3 We have created this booklet to help you learn
THE MEDTRONIC TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) SYSTEM 1 A Guide for Patients With Severe Aortic Stenosis medtronic.com/tavr Table of Contents 2 3 We have created this booklet to help you learn
Package leaflet: Information for the user. Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion
 Package leaflet: Information for the user Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion Read all of this leaflet carefully before you start using
Package leaflet: Information for the user Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion Read all of this leaflet carefully before you start using
PART VI Summary of the RMP
 PART VI Summary of the RMP Summary of Risk Management Plan for ORKAMBI This is a summary of the risk management plan (RMP) for ORKAMBI. The RMP details important risks of ORKAMBI, how these risks can be
PART VI Summary of the RMP Summary of Risk Management Plan for ORKAMBI This is a summary of the risk management plan (RMP) for ORKAMBI. The RMP details important risks of ORKAMBI, how these risks can be
An Uncommon Cardiac Etiology of Liver Cirrhosis, Recurrent Ascites, Atrial Fibrillation and Congestive Heart Failure
 Cronicon OPEN ACCESS EC CARDIOLOGY Case Report An Uncommon Cardiac Etiology of Liver Cirrhosis, Recurrent Ascites, Atrial Fibrillation and Congestive Heart Failure Montaser Y Ismail 1 *, Mohammed I Nassar
Cronicon OPEN ACCESS EC CARDIOLOGY Case Report An Uncommon Cardiac Etiology of Liver Cirrhosis, Recurrent Ascites, Atrial Fibrillation and Congestive Heart Failure Montaser Y Ismail 1 *, Mohammed I Nassar
Package leaflet: Information for the user Salofalk 500mg gastro-resistant tablets Mesalazine
 Package leaflet: Information for the user Salofalk 500mg gastro-resistant tablets Mesalazine Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Salofalk 500mg gastro-resistant tablets Mesalazine Read all of this leaflet carefully before you start taking this medicine because it contains important information
Annex III. Amendments to relevant sections of the summary of product characteristics and package leaflets
 Product Information as approved by the CMDh on 19 March 2014, pending endorsement by the European Commission Annex III Amendments to relevant sections of the summary of product characteristics and package
Product Information as approved by the CMDh on 19 March 2014, pending endorsement by the European Commission Annex III Amendments to relevant sections of the summary of product characteristics and package
Glucophage XR is contra-indicated during breast-feeding.
 Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
What s New in Cardiac MRI
 What s New in Cardiac MRI Katie M. Hawthorne, MD Director, Cardiac MRI Main Line Health Philadelphia Cardiovascular Summit November 18, 2017 Cardiac MRI: Disclosure 2 Disclosures No financial disclosures
What s New in Cardiac MRI Katie M. Hawthorne, MD Director, Cardiac MRI Main Line Health Philadelphia Cardiovascular Summit November 18, 2017 Cardiac MRI: Disclosure 2 Disclosures No financial disclosures
Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S)
 Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR for bimatoprost,
Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR for bimatoprost,
Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole)
 EMA/370707/2016 Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma, which details the
EMA/370707/2016 Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma, which details the
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology HIV is a virus that attacks the immune system (the body s natural defences) and weakens it by destroying certain white blood cells
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology HIV is a virus that attacks the immune system (the body s natural defences) and weakens it by destroying certain white blood cells
SAMPLE HLTEN610A. TAFE NSW Training and Education Support Industry Skills Unit, Meadowbank. Practise in the cardiovascular nursing environment
 TAFE NSW Training and Education Support Industry Skills Unit, Meadowbank HLTEN610A Practise in the cardiovascular nursing environment Version 1.0 Flexible Learner Resource Product Code: ISO 9001 HLTEN610A
TAFE NSW Training and Education Support Industry Skills Unit, Meadowbank HLTEN610A Practise in the cardiovascular nursing environment Version 1.0 Flexible Learner Resource Product Code: ISO 9001 HLTEN610A
FAMILIAL MEDITERRANEAN FEVER (FMF) RAKAN TELFAH not MD, not PHD
 FAMILIAL MEDITERRANEAN FEVER (FMF) RAKAN TELFAH not MD, not PHD FMF Is a hereditary autoinflammatory disease caused by mutations in Mediterranean fever gene (MEFV). It is inherited in an autosomal recessive
FAMILIAL MEDITERRANEAN FEVER (FMF) RAKAN TELFAH not MD, not PHD FMF Is a hereditary autoinflammatory disease caused by mutations in Mediterranean fever gene (MEFV). It is inherited in an autosomal recessive
Annex III. Summary of Product Characteristics and Package Leaflet
 Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
Case 2 Dwayne A. Williams CASE 2
 CASE 2 A 40- year- old male with no past medical history presents with bilateral flank pain and dark colored urine for 5 days. During family history taking, he states his father died from kidney failure
CASE 2 A 40- year- old male with no past medical history presents with bilateral flank pain and dark colored urine for 5 days. During family history taking, he states his father died from kidney failure
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
Annex III. Amendments to relevant sections of the Product Information
 Annex III Amendments to relevant sections of the Product Information Note: These amendments to the relevant sections of the Summary of Product Characteristics and package leaflet are the outcome of the
Annex III Amendments to relevant sections of the Product Information Note: These amendments to the relevant sections of the Summary of Product Characteristics and package leaflet are the outcome of the
