6/30/2018. Cholesterol Management in the 21 st Century: Who Do We Treat? How Low Do We Go? DISCLOSURES
|
|
|
- Cuthbert McCarthy
- 5 years ago
- Views:
Transcription
1 holesterol Management in the 21 st entury: Who Do We Treat? How Low Do We Go? Theodore Feldman MD FA FAP Head of ardiology, FIU Wertheim School of Medicine Medical Director, Prevention and ommunity Health Miami ardiac and Vascular Institute Baptist Health South Florida DISLOSURES SPEAKERS BUREAU: SANOFI, REGENERON, BOERINGER INGELHEIM, ELI LILLY, NOVO NORDISK, NOVARTIS, AKEA, PORTOLA, MERK 2013 A/AHA Guideline on the Treatment of Blood holesterol to Reduce Atherosclerotic ardiovascular Risk in Adults Endorsed by the American Association of ardiovascular and Pulmonary Rehabilitation, American Pharmacists Association, American Society for Preventive ardiology, Association of Black ardiologists, Preventive ardiovascular Nurses Association, and WomenHeart: The National oalition for Women with Heart Disease 1
2 itation This slide set is adapted from the 2013 A/AHA Guideline on the Treatment of Blood holesterol to Reduce Atherosclerotic ardiovascular Risk in Adults. E-Published on November 12, 2013, available at: [ 016/j.jacc and NHLBI harge to the Expert Panel Evaluate higher quality randomized controlled trial (RT) evidence for cholesterol-lowering drug therapy to reduce ASVD risk Use ritical Questions (Qs) to create the evidence search from which the guideline is developed holesterol Panel: 3 Qs Risk Assessment Work Group: 2 Qs Lifestyle Management Work Group: 3 Qs RTs and systematic reviews/meta-analyses of RTs independently assessed as fair-to-good quality Develop recommendations based on RT evidence Less expert opinion than in prior guidelines lassification of Recommendations and Levels of Evidence A recommendation with Level of Evidence B or does not imply that the recommendation is weak. Many important clinical questions addressed in the guidelines do not lend themselves to clinical trials. Although randomized trials are unavailable, there may be a very clear clinical consensus that a particular test or therapy is useful or effective. *Data available from clinical trials or registries about the usefulness/ efficacy in different subpopulations, such as sex, age, history of diabetes, history of prior myocardial infarction, history of heart failure, and prior aspirin use. For comparative effectiveness recommendations (lass I and IIa; Level of Evidence A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated. 2
3 4 Statin Benefit Groups linical ASVD* LDL >190 mg/dl, Age >21 years Primary prevention Diabetes: Age years, LDL mg/dl Primary prevention - No Diabetes : 7.5% 10-year ASVD risk, Age years, LDL mg/dl, *Atherosclerotic cardiovascular disease Requires risk discussion between clinician and patient before statin initiation. Statin therapy may be considered if risk decision is uncertain after use of ASVD risk calculator. Guideline Scope Focus on treatment of blood cholesterol to reduce ASVD risk in adults Emphasize adherence to a heart healthy lifestyle as foundation of ASVD risk reduction See Lifestyle Management Guideline Identify individuals most likely to benefit from cholesterol-lowering therapy 4 statin benefit groups Identify safety issues New Perspective on LDL & Non-HDL Goals Lack of RT evidence to support titration of drug therapy to specific LDL and/or non-hdl goals Strong evidence that appropriate intensity of statin therapy should be used to reduce ASVD risk in those most likely to benefit Quantitative comparison of statin benefits with statin risk Nonstatin therapies did not provide ASVD risk reduction benefits or safety profiles comparable to statin therapy 3
4 Why Not ontinue to Treat to Target? Major difficulties: 1. urrent RT data do not indicate what the target should be 2. Unknown magnitude of additional ASVD risk reduction with one target compared to another 3. Unknown rate of additional adverse effects from multidrug therapy used to achieve a specific goal 4. Therefore, unknown net benefit from treat-totarget approach 4 Statin Benefit Groups (Revised Figure) IA IA IB IA IIaB 1 linical Flow (Revised Figure-con t) 4
5 Intensity of Statin Therapy *Individual responses to statin therapy varied in the RTs and should be expected to vary in clinical practice. There might be a biologic basis for a less-than-average response. Evidence from 1 RT only: down-titration if unable to tolerate atorvastatin 80 mg in IDEAL (Pedersen et al). Although simvastatin 80 mg was evaluated in RTs, initiation of simvastatin 80 mg or titration to 80 mg is not recommended by the FDA due to the increased risk of myopathy, including rhabdomyolysis. linical ASVD Initiating Statin therapy *Fasting lipid panel preferred. In a nonfasting individual, a nonfasting non-hdl >220 mg/dl may indicate genetic hypercholesterolemia that requires further evaluation or a secondary etiology. If nonfasting triglycerides are >500 mg/dl, a fasting lipid panel is required. It is reasonable to evaluate the potential for ASVD benefits and for adverse effects, and to consider patient preferences, in initiating or continuing a moderate- or high-intensity statin, in individuals with ASVD >75 years of age. Primary Prevention Global Risk Assessment To estimate 10-year ASVD* risk New Pooled ohort Risk Equations White and black men and women More accurately identifies higher risk individuals for statin therapy Focuses statin therapy on those most likely to benefit You may wish to avoid initiating statin therapy in high-risk groups found not to benefit (higher grades of heart failure and hemodialysis) 5
6 Primary Prevention Statin Therapy Thresholds for initiating statin therapy derived from 3 exclusively primary prevention RTs Before initiating statin therapy, clinicians and patients engage in a discussion of the potential for ASVD risk reduction benefits, potential for adverse effects, drug-drug interactions, and patient preferences alculators don t write Rx, physicians do! Individuals Not in a Statin Benefit Group In those for whom a risk decision is uncertain: These factors may inform clinical decision making: Family history of premature ASVD Elevated lifetime risk of ASVD LDL 160 mg/dl hs-rp 2.0 mg/l oronary artery calcium (A) score 300 Agaston units Ankle brachial index (ABI)<0.9 Their use still requires discussion between clinician and patient Statin Therapy: Monitoring Response- Adherence *Fasting lipid panel preferred. In a nonfasting individual, a nonfasting non-hdl >220 mg/dl may indicate genetic hypercholesterolemia that requires further evaluation or a secondary etiology. If nonfasting triglycerides are >500 mg/dl, a fasting lipid panel is required. In those already on a statin, in whom baseline LDL is unknown, an LDL <100 mg/dl was observed in most individuals receiving high-intensity statin therapy in RTs. See guideline text 6
7 Safety RTs & meta-analyses of RTs used to identify important safety considerations Allow estimation of net benefit from statin therapy o ASVD risk reduction versus adverse effects Expert guidance on management of statin-associated adverse effects, including muscle symptoms Advise use of additional information including pharmacists, manufacturers prescribing information, & drug information centers for complex cases Management of Muscle Symptoms on Statin Therapy It is reasonable to evaluate and treat muscle symptoms including pain, cramping, weakness, or fatigue in statin-treated patients according to the management algorithm To avoid unnecessary discontinuation of statins, obtain a history of prior or current muscle symptoms to establish a baseline before initiating statin therapy 7
8 Management of Muscle Symptoms on Statin Therapy (con t) If unexplained severe muscle symptoms or fatigue develop during statin therapy: Promptly discontinue the statin Address possibility of rhabdomyolysis with: K reatinine urine analysis for myoglobinuria Management of Muscle Symptoms on Statin Therapy (con t) If mild-to-moderate muscle symptoms develop during statin therapy: Discontinue the statin until the symptoms are evaluated Evaluate the patient for other conditions* that might increase the risk for muscle symptoms If after 2 months without statin Rx, muscle symptoms or elevated K levels do not resolve completely, consider other causes of muscle symptoms *Hypothyroidism, reduced renal or hepatic function, rheumatologic disorders such as polymyalgia rheumatica, steroid myopathy, vitamin D deficiency or primary muscle diseases Statin-Treated Individuals Nonstatin Therapy onsiderations Use the maximum tolerated intensity of statin onsider addition of a nonstatin cholesterol-lowering drug(s) If a less-than-anticipated therapeutic response persists Only if ASVD risk-reduction benefits outweigh the potential for adverse effects in higher-risk persons: linical ASVD <75 years of age Baseline LDL 190 mg/dl Diabetes mellitus 40 to 75 years of age Nonstatin cholesterol-lowering drugs shown to reduce ASVD events in RTs are preferred 8
9 Three Principles Do not focus on LDL or non-hdl- cholesterol levels as treatment goals o Lipid panel to monitor adherence For those shown to benefit, use statins inexpensive (5 of 7 generic) medications proven to reduce ASVD risk In primary prevention decisions, use a clinicianpatient discussion to determine: global risk reduction strategy potential for benefit and harms of statin therapy Patient preferences (shared decision making) Future Updates to the Blood holesterol Guideline This is a comprehensive guideline for the evidencebased treatment of blood cholesterol to reduce ASVD risk These guidelines represent a change from previous guidelines that aligns recommendations closely to the evidence For primary prevention, they are patient-centered Guidelines will change in the future as high-quality data will improve future cholesterol treatment guidelines Educational Objectives 1. Summarize results of clinical trials of PSK9 inhibition 2. ompare risks and benefits of available lipid-lowering agents 3. ompare LDL- lowering efficacy of available lipidmodifying agents 4. Identify patients who may benefit from PSK9 inhibition 9
10 IMPROVE-IT: Primary Endpoint ITT ardiovascular death, MI, documented unstable angina requiring rehospitalization, coronary revascularization ( 30 days), or stroke HR I (0.887, 0.988) p=0.016 Simva 34.7% 2742 events NNT= 50 EZ/Simva 32.7% 2572 events 7-year event rates annon P et al. N Engl J Med. 2015;372: PSK9i: Rapid Progress From Discovery to linic PSK9i = PSK9 inhibition. Seidah NG. Proc Natl Acad Sci US. 2003;100(3): Abifadel M. Nat Genet. 2003;34(2): Maxwell KN. Proc Natl Acad Sci US. 2004;101(18): Rashid S. Proc Natl Acad Sci US. 2005;102(15): Lagace TA et al. JI. 2006;116: ohen J. N Engl J Med. 2006;354(12): Zhao Z. Am J Hum Genet. 2006;79(3): Hooper AJ. Atherosderosis. 2007;193(2): han J. Proc Natl Acad Sci US. 2009;106(24): Stein et al. N Engl J Med. 2012;366: European Medicines Agency. Available at: Global Assessment of Plaque Regression with a PSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) Phase 3 study 970 patients with coronary artery disease, taking lipid-lowering therapy, and undergoing coronary catheterization Randomized to receive evolocumab or placebo every 4 weeks for 78 weeks Primary endpoint: hange in percent atheroma volume (assessed by IVUS) from baseline to week
11 GLAGOV: Primary Endpoint Percent Atheroma Volume Nicholls SJ, et al. JAMA. 2016; 316: PSK9 Outcome Trials FOURIER (evolocumab) N = 22,500 Duration 5 years SPIRE-1 (bococizumab)* N = 12,000, LDL- 70 and <100 mg/dl SPIRE-2 (bococizumab)* N = 6,300, LDL- 100 mg/dl ODYSSEY Outcomes (alirocumab) N = 18,000 Duration 5 6 years * Discontinued 1 November Trial Design 27,564 high-risk, stable patients with established V disease (prior MI, prior stroke, or symptomatic PAD) Screening, Lipid Stabilization, and Run-in High or moderate intensity statin therapy (± ezetimibe) Evolocumab S 140 mg Q2W or 420 mg QM LDL- 70 mg/dl or non-hdl- 100 mg/dl RANDOMIZED DOUBLE BLIND S Q2W or QM Sabatine MS, et al. Am Heart J. 2016; 173: Follow-up Q 12 weeks 11
12 Summary of Effects of PSK9i Evolocumab LDL- by 59% First V outcomes in patients on statin Safe and well-tolerated 59% reduction P< Absolute 56 mg/dl HR 0.85 ( ) P< HR 0.80 ( ) P< Evolocumab (median 30 mg/dl, IQR mg/dl) VD, MI, stroke UA, cor revasc VD, MI, stroke Sabatine MS et al. NEJM. 2017; 376: Total Primary Endpoint Events # Events 1st Event HR ( ) Evolocumab Murphy SA et al. Presented at AHA Total Primary Endpoint Events 2714 Total Events RR 0.82 (95%I ) P<0.001 # Events Addition al Events RR 0.74 ( ) st Event HR ( ) Evolocumab Murphy SA et al. Presented at AHA
13 Primary Endpoint Events: Wei, Lin, Weissfeld Model HR (95% I) 1 Event (n=2907) 2 Events (n=1333) 3 Events (n=373) 4+ Events (n=152) HR 0.60 HR 0.76 HR 0.74 HR Evolocumab 1. Better 0 Better Murphy SA et al. Presented at AHA Total Primary Endpoint Events Number of Events Prevented for 1,000 Patients Treated with Evolocumab for 3 Years Events per 1,000 Patients First Event Only Total Events Murphy SA et al. Presented at AHA Giugliano RP et al. Lancet 2017;390:
14 Giugliano RP et al. Lancet 2017;390: Benefit of EvoMab Based on Time from Qualifying MI V Death, MI, or Stroke Qualifying MI <2 yrs ago 24% RRR 10.8% 2.9% HR 0.76 NNT 35 (95% I ) 7.9% P<0.001 Evolocumab Qualifying MI 2 yrs ago 13% RRR HR 0.87 (95% I ) P= % 8.3% 1.0% NNT 101 P interaction= Months after Randomization Sabatine MS et al. Presented Sabatine MS. at AHA Benefit of EvoMab Based on Multivessel Disease V Death, MI, or Stroke Multivessel Disease 30% RRR HR 0.70 (95% I ) P<0.001 Evolocumab 12.6% 3.4% NNT % No Multivessel Disease 11% RRR HR 0.89 (95% I ) P= % 7.6% 1.3% NNT 78 P interaction= Months after Randomization Sabatine MS et al. Presented at AHA Sabatine MS. AHA 14
15 Adjusted for age, sex, race, BMI, diabetes, HTN, smoking, egfr, HF, prior MI, ABG/PI, and Hx stroke or TIA. VD / MI / Stroke 6% 4% 2% Peripheral Artery Disease and Risk in Patients PAD with MI/Stroke N=1036 PAD N=1784 PAD no MI/Stroke N=748 16% 16% MI or Stroke and no PAD N=11996 MI or Stroke and no PAD N= % 14% Adjusted HR 14% 13.0 P= % 12% % 10% ( ) P< % 10.3% P= % 8% 7.6% 7.6% 0% Days from Randomization VD / MI / Stroke 6% 4% 2% 0% Days from Randomization Bonaca MP et al. irculation 2018;137: Major Adverse Limb Events Major Adverse Limb Events All Patients N=27,564 Evolocumab 0.5% 42% RRR 0.45% HR % ( ) P= % 0.27% 0.2% Outcome HR 95% I 0.1% MALE 0.58 ( ) ALI or major amputation 0.52 ( ) ALI 0.55 ( ) Major amputation 0.57 ( ) 0.0% Urgent revascularization 0.69 ( ) Days from Randomization Bonaca MP et al. irculation 2018;137: MAE or MALE In Patients with and without PAD MAE or MALE PAD Evolocumab N=3,642 16% PAD 27% N=3,642 RRR 15.0% 14% PAD PAD HR HR % ARR 4.1% 95% I ( ) NNT 25 12% ( ) ARR P= P= % NNT 2.5y 10% 25 8% 7.8% No No PAD 1.5% 1.5% ARR 6% 6.3% NNT ARR 67 NNT 2.5y No PAD 4% 67 N=23,922 HR % 95% I ( ) P<0.001 p-interaction = % Days from Randomization Bonaca MP et al. irculation 2018;137:
16 MAE or MALE In Patients with PAD and no MI or Stroke Evolocumab PAD (no MI/stroke, N=1505) MAE or MALE 14% 48% RRR 12.8% 12% HR 0.52 ( ) 10% P= % 6.5% 6% 4% 2% 0% Days from Randomization PAD 6.3% ARR NNT 2.5y 16 Bonaca MP et al. irculation 2018;137: Benefit of EvoMab by Risk Strata Bohula EA et al. Presented at AHA ODYSSEY Outcomes ~18,600 men and women aged 40 years with AS 52 weeks before randomization Randomized to receive biweekly alirocumab (75 mg or 150 mg, titrated to achieve LDL- 25 mg/dl and <50 mg/dl) or placebo Primary efficacy measure: time to first occurrence of HD death, nonfatal MI, fatal or nonfatal ischemic stroke, or hospitalization for unstable angina Duration: 64 months (4-month run-in, 60 months randomized treatment; 2 years follow-up) Schwartz GG, et al. Am Heart J. 2014;168:
17 The ODYSSEY OUTOMES Trial: Topline Results Alirocumab in Patients After Acute oronary Syndrome Gregory G. Schwartz, Michael Szarek, Deepak L. Bhatt, Vera Bittner, Rafael Diaz, Jay Edelberg, Shaun G. Goodman, orinne Hanotin, Robert Harrington, J. Wouter Jukema, Guillaume Lecorps, Angèle Moryusef, Robert Pordy, Matthew Roe, Harvey D. White, Andreas Zeiher, Ph. Gabriel Steg On behalf of the ODYSSEY OUTOMES Investigators and ommittees American ollege of ardiology 67th Scientific Sessions March 10, 2018 linicaltrials.gov: NT Residual Risk After Acute oronary Syndrome A.18 Remains high despite evidence based preventive therapies Is related, in part, to levels of low density lipoprotein cholesterol (LDL ) Is reduced when LDL is lowered by Statin therapy, compared with placebo 1 High intensity, compared with moderate intensity statin therapy 2 Ezetimibe, compared with placebo, added to statin 3 1. Schwartz GG, et al. JAMA 2001;285: annon P, et al.nejm 2004;350: annon P, et al.nejm 2015;372: A.18 Alirocumab PSK9 is a validated target for risk reduction in stable atherosclerotic cardiovascular disease 1 3 A fully human monoclonal antibody against PSK9 Produces substantial and sustained reductions in LDL and other atherogenic lipoproteins 2 Has been safe and well tolerated in studies to date 4 PSK9, proprotein convertase subtilisin/kexin type 9 1. Sabatine et al, NEJM 2017;376: Robinson JG et al. NEJM 2015;372: Ridker PM et al. NEJM 2017;376: Robinson JG et al.ja 2017;69:
18 A.18 Study Hypothesis Alirocumab, versus placebo, reduces cardiovascular (V) morbidity and mortality after recent acute coronary syndrome (AS) in patients with elevated levels of atherogenic lipoproteins despite intensive or maximum tolerated statin therapy Schwartz GG, et al. Am Heart J 2014;168: e1. A.18 Main Inclusion riteria Age 40 years AS 1to12monthspriortorandomization Acute myocardialinfarction (MI) orunstable angina High intensity statin therapy* Atorvastatin 40 to 80 mg daily or Rosuvastatin 20 to 40 mg daily or Maximum tolerated dose of one of these agents for 2 weeks Inadequate control of lipids LDL 70 mg/dl (1.8 mmol/l) or Non HDL 100 mg/dl (2.6 mmol/l) or ApolipoproteinB 80 mg/dl *Patients not on statins were authorized to participate if tolerability issues were present and documented Schwartz GG, et al. Am Heart J 2014;168: e1. A.18 Key Exclusion riteria Uncontrolled hypertension NYHA class III or IV heart failure; LVEF <25% if measured History of hemorrhagic stroke Fasting triglycerides >400 mg/dl (4.52 mmol/l) Use of fibrates other than fenofibrate or fenofibric acid Recurrent AS within 2 weeks prior to randomization visit oronary revascularization performed within 2 weeks prior to randomization visit, or planned after randomization Liver transaminases >3 ULN; hepatitis B or infection reatine kinase >3 ULN egfr <30 ml/min/1.73 m 2 Positive pregnancy test egfr, estimated glomerular filtration rate; ULN, upper limit of normal Schwartz GG, et al. Am Heart J 2014;168: e1. 18
19 A.18 Primary Efficacy Outcome Time of first occurrence of: oronary heart disease (HD) death, or Non fatal MI, or Fatal or non fatal ischemic stroke, or Unstable angina requiring hospitalization* All outcomes adjudicated by the linical Events ommittee, under the auspices of the Duke linical Research Institute (DRI). Members were unaware of treatment assignment and lipid levels *Required all of the following: 1. Hospital admission >23 h for MI symptoms, tempo in prior 48 hours and/or 20 min of chest discomfort at rest 2. New EG findings consistent with ischemia or infarction 3. Angiographically significant obstructive coronary disease Schwartz GG, et al. Am Heart J 2014;168: e1. Major Secondary Efficacy Endpoints A.18 Tested in the following hierarchical sequence: HD event: HD death, non fatal MI, unstable angina requiring hospitalization, or ischemia driven coronary revascularization* Major HD event: HD death or non fatal MI V event: V death, non fatal HD event, or non fatal ischemic stroke All cause death, non fatal MI, non fatal ischemic stroke HD death V death All cause death *Revascularization performed because of recurrent AS, new or progressive symptoms of myocardial ischemia or new or progressive abnormalities on functional testing, except revascularization due to restenosis at a prior coronary intervention site. Other Secondary and Safety Endpoints A.18 Other secondary endpoints omponents of the primaryendpointconsidered individually: HD death Non fatal MI Fatalandnon fatalischemic stroke Unstable angina requiring hospitalization Ischemia driven coronary revascularization ongestive heart failurerequiringhospitalization Safety endpoints Adverse events Laboratory assessments 10 19
20 A.18 Treatment Assignment Post ASpatients(1to12months) Run in period of 2 16 weeks on high intensity or maximum tolerated dose of atorvastatin or rosuvastatin At least one lipid entry criterion met Randomization Alirocumab S Q2W S Q2W Patient and investigators remained blinded to treatment and lipid levels for the entire duration of the study Schwartz GG, et al. Am Heart J 2014;168: e1. A.18 A Target Range for LDL- We attempted to maximize the number of patients in the target range and minimize the number below target by blindly titrating alirocumab (75 or 150 mg S Q2W) or blindly switching to placebo. Below target Acceptable range Target range Alirocumab Undesirably high baseline range LDL (mg/dl) Schwartz GG, et al. Am Heart J 2014;168: e1. ODYSSEY OUTOMES: 18,924 patients randomized at 1315 A.18 sites in 57 countries, Nov 2, 2012 Nov 11, 2017 Western Europe entral/eastern Europe anada/usa anada 361 Austria 58 Bosnia Herzegovina 156 Macedonia 132 US 2511 Belgium 197 Bulgaria 333 Poland 926 Denmark 352 roatia 70 Romania 145 Finland 116 zech Republic 381 Russian Federation 1109 France 185 Estonia 216 Serbia 255 Germany 509 Georgia 131 Slovakia 340 Greece 70 Hungary 224 Slovenia 36 Italy 275 Latvia 80 Turkey 78 Netherlands 686 Lithuania 188 Ukraine 639 Norway 97 Portugal 174 Asia Spain 826 Sweden hina Hong Kong 17 Switzerland 88 UK 292 India 521 Japan 204 Korea 94 Malaysia 110 Philippines 116 Singapore 49 Sri Lanka 314 Latin America We thank the patients, Taiwan 93 Argentina 592 Thailand 161 their families, all Brazil 928 Rest of World Australia investigators and hile olombia 354 coordinators involved in Israel 582 Guatemala 25 New Zealand 257 this study, and DRI Mexico 349 South Africa 505 Peru
21 Patient Disposition Randomized 18,924 patients A. 1 8 Alirocumab Follow up*: median 2.8 (Q1 Q ) years 8242 (44%) patients with potential follow up 3 years 1955 patients experienced a primary endpoint 726 patients died Premature treatment discontinuation Blinded switch to placebo (2 consecutive LDL values<15mg/dl) Patients lost to follow up (vital status) 1343 (14.2%) 1496 (15.8%) 730 (7.7%) Not applicable 14 9 *Ascertainment was complete for 99.1% and 99.8% of potential patient years of follow up for the primary endpoint and all cause death, respectively Baseline Demographics haracteristic Alirocumab Age, years, median (Q1 Q3) 58 (52 65) 58 (52 65) Female, n (%) 2390 (25.3) 2372 (25.1) Medical history, n (%) Hypertension 6205 (65.6) 6044 (63.9) Diabetes mellitus 2693 (28.5) 2751 (29.1) urrent tobacco smoker 2282 (24.1) 2278 (24.1) Prior MI 1790 (18.9) 1843 (19.5) A. 1 8 Baseline Index Events haracteristic Alirocumab Time from index AS to randomization, 2.6 ( ) 2.6 ( ) months, median (Q1 Q3) AS type, n (%) NSTEMI 4574 (48.4) 4601 (48.7) STEMI 3301 (35.0) 3235 (34.2) Unstable angina 1568 (16.6) 1614 (17.1) Revascularization for index AS, n (%) 6798 (71.8) 6878 (72.7) A
22 A.18 Baseline Lipid haracteristics haracteristic, mg/dl, median (Q1 Q3) Alirocumab 92.5% of patients qualified on the basis of LDL 70 mg/dl LDL 87 (73 104) 87 (73 104) Non HDL 115 (99 136) 115 (99 137) Apolipoprotein B 79 (69 93) 80 (69 93) HDL 43 (37 50) 42 (36 50) Triglycerides 129 (94 181) 129 (95 183) Lipoprotein(a) 21 (7 59) 22 (7 60) BaselineLipid Lowering Therapy Therapy, n (%) Alirocumab High dose atorvastatin/rosuvastatin 8380 (88.6) 8431 (89.1) Low /moderate dose atorvastatin/rosuvastatin 830 (8.8) 777 (8.2) Other statin 19 (0.2) 27 (0.3) Ezetimibe, with or without statin 269 (2.8) 285 (3.0) No lipid lowering therapy* 87 (0.9) 91 (1.0) A. 1 8 *Patients not on statins were authorized to participate if tolerability issues were present and documented Guideline Recommended Post AS Medications A. 1 8 Medication, n (%) Alirocumab Aspirin 9050 (95.6) 9036 (95.5) P2Y 12 antagonist 8296 (87.7) 8245 (87.1) AE I/ARB 7356 (77.7) 7360 (77.8) Beta blocker 7998 (84.5) 7992 (84.5) AE I,angiotensin converting enzyme inhibitor;arb, angiotensin receptor blocker 22
23 LDL : ITT and On Treatment Analyses Mean LDL- (mg/dl) ITT On treatment* Alirocumab ITT On treatment* Months Since Randomization A. 1 8 *Excludes LDL values after premature treatment discontinuation or blinded switch to placebo All LDL values, including those after premature treatment discontinuation, blinded down titration, or blinded switch to placebo A.18 Mean LDL- (mg/dl) LDL-: On-Treatment Analysis mg/dl 62.7% mg/dl 61.0% Alirocumab Months Since Randomization Excludes LDL values after premature treatment discontinuation or blinded switch to placebo Approximately 75% of months of active treatment were at the 75 mg dose mg/dl 54.7% Primary Efficacy Endpoint: MAE ARR* 1.6% A. 1 8 MAE: HD death, nonfatal MI, ischemic stroke, or unstable angina requiring hospitalization HR 0.85 (95% I 0.78, 0.93) P= *Based on cumulative incidence 31 23
24 Primary Efficacy and omponents Endpoint, n (%) Alirocumab HR (95% I) Log rank P value MAE 903 (9.5) 1052 (11.1) 0.85 (0.78, 0.93) A. 1 8 HD death 205 (2.2) 222 (2.3) 0.92 (0.76, 1.11) 0.38 Non fatal MI 626 (6.6) 722 (7.6) 0.86 (0.77, 0.96) Ischemic stroke 111 (1.2) 152 (1.6) 0.73 (0.57, 0.93) 0.01 Unstable angina 37 (0.4) 60 (0.6) 0.61 (0.41, 0.92) Main Secondary Efficacy Endpoints: Hierarchical Testing A.18 Endpoint, n (%) Alirocumab HR (95% I) Log rank P value HD event 1199 (12.7) 1349 (14.3) 0.88 (0.81, 0.95) Major HD event 793 (8.4) 899 (9.5) 0.88 (0.80, 0.96) V event 1301 (13.7) 1474 (15.6) 0.87 (0.81, 0.94) Death, MI, ischemic 973 (10.3) 1126 (11.9) 0.86 (0.79, 0.93) stroke HD death 205 (2.2) 222 (2.3) 0.92 (0.76, 1.11) 0.38 V death 240 (2.5) 271 (2.9) 0.88 (0.74, 1.05) 0.15 All cause death 334 (3.5) 392 (4.1) 0.85 (0.73, 0.98) 0.026* *Nominal P value 33 All ause Death A. 1 8 ARR 0.6% HR 0.85 (95% I 0.73, 0.98) P=0.026* *Nominal P value Based on cumulative incidence 34 24
25 Other Efficacy Endpoints Endpoint n (%) Alirocumab HR (95% I) Log rank P value A. 1 8 Ischemia driven coronary revascularization Hospitalization for HF 731 (7.7) 828 (8.8) 0.88 (0.79, 0.97) (1.9) 179 (1.9) 0.98 (0.79, 1.20) Post Hoc Analysis: All ause Death by Baseline LDL Subgroups 100 mg/dl HR=0.71 A. 1 8 <80 mg/dl HR 0.89 (95% I 0.69, 1.14) 80 to <100 mg/dl HR 1.03 (95% I 0.78, 1.36) 40 OMPARISON OF FOURIER AND ODYSSEY EVOLUAMAB ALIROUMAB TITLE FOURIER ODYSSEY TYPE OF PTS MI,VS, OR PAD (+/- 3y) 4-52 wks POST AS # PTS 27,564 18,600 HIGH INT. STATIN 69% 89% BASELINE LDL 92 MG/DL 89MG/DL PSK9 DOSING 140MG2W/420MGQ4W 75/150Q2W to LDL 25-50MG/DL LDL- HANGE 56MG/DL 66.4MG/DL DURATION 2.2Y 3Y ENDPOINT 1-VD, MI, VA, UA HOSP., OR REVAS 2-VD, MI, OR VA HD DEATH, MI, ISHEMI VA OR UA HOSP RESULTS 0.81 EP HR 0.85 ( ) 2EP HR(0.73-0,88) 0.85 ( ) 0.88 ( ) 25
26 ombination therapy for lowering LDL- Statins plus PSK9 inhibitor: >75% estimated Statins plus ezetimibe: 60 to 70% reduction with high dose atorva/rosuva Statin plus low dose/alternative day statin: 40 to 50% LDL- reduction Ezetimibe plus bempedoic acid ( experimental therapy in phase 3 trials); 45% LDL- reduction 26
2013 Cholesterol Guidelines. Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc.
 2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines. Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc.
 2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY. Harvey D White on behalf of The STABILITY Investigators
 STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY Harvey D White on behalf of The STABILITY Investigators Lipoprotein- associated Phospholipase A 2 (Lp-PLA 2 ) activity:
STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY Harvey D White on behalf of The STABILITY Investigators Lipoprotein- associated Phospholipase A 2 (Lp-PLA 2 ) activity:
LDL Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial
 LDL Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial Marc P. Bonaca, Patrice Nault, Robert P. Giugliano, Anthony C. Keech, Armando
LDL Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial Marc P. Bonaca, Patrice Nault, Robert P. Giugliano, Anthony C. Keech, Armando
Observations on US CVD Prevention Guidelines. Donald M. Lloyd-Jones, MD ScM FACC FAHA
 Observations on US CVD Prevention Guidelines Donald M. Lloyd-Jones, MD ScM FACC FAHA What are Guidelines? Evidence Base for Guidelines Tricoci, JAMA 2009 Evidence Base for Guidelines Tricoci, JAMA 2009
Observations on US CVD Prevention Guidelines Donald M. Lloyd-Jones, MD ScM FACC FAHA What are Guidelines? Evidence Base for Guidelines Tricoci, JAMA 2009 Evidence Base for Guidelines Tricoci, JAMA 2009
Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice
 Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice Vera Bittner, MD, MSPH Professor of Medicine Section Head, Preventive Cardiology Medical Director, Cardiac Rehabilitation
Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice Vera Bittner, MD, MSPH Professor of Medicine Section Head, Preventive Cardiology Medical Director, Cardiac Rehabilitation
4/24/15. AHA/ACC 2013 Guideline Key Points
 Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
FOURIER STUDY GREYLOCK PRESS: CTS PRODUCT SAMPLE - FOURIER YES. Did the study achieve its main objective?
 FOURIER STUDY Did the study achieve its main objective? 2 15% 1 5% 9.8% YES FOURIER compared Repatha with placebo in patients who were taking a statin and had hardening or narrowing of the arteries and
FOURIER STUDY Did the study achieve its main objective? 2 15% 1 5% 9.8% YES FOURIER compared Repatha with placebo in patients who were taking a statin and had hardening or narrowing of the arteries and
Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB
 Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB on behalf
Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB on behalf
New Cholesterol Guidelines What the LDL are we supposed to do now?!
 New Cholesterol Guidelines What the LDL are we supposed to do now?! Michael D. Shapiro Assistant Professor of Medicine and Radiology Knight Cardiovascular Institute Oregon Health & Science University 2013
New Cholesterol Guidelines What the LDL are we supposed to do now?! Michael D. Shapiro Assistant Professor of Medicine and Radiology Knight Cardiovascular Institute Oregon Health & Science University 2013
FOURIER: Enough Evidence to Justify Widespread Use? Did It fulfill Its Expectations?
 FOURIER: Enough Evidence to Justify Widespread Use? Did It fulfill Its Expectations? CVCT Washington, DC November 3, 2017 Marc S. Sabatine, MD, MPH Chairman, TIMI Study Group Lewis Dexter, MD, Distinguished
FOURIER: Enough Evidence to Justify Widespread Use? Did It fulfill Its Expectations? CVCT Washington, DC November 3, 2017 Marc S. Sabatine, MD, MPH Chairman, TIMI Study Group Lewis Dexter, MD, Distinguished
Making War on Cholesterol with New Weapons: How Low Can We/Should We Go? Shaun Goodman
 Making War on Cholesterol with New Weapons: How Low Can We/Should We Go? Shaun Goodman Disclosures Research grant support, speaker/consulting honoraria: Sanofi and Regeneron Including ODYSSEY Outcomes
Making War on Cholesterol with New Weapons: How Low Can We/Should We Go? Shaun Goodman Disclosures Research grant support, speaker/consulting honoraria: Sanofi and Regeneron Including ODYSSEY Outcomes
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Effect of the PCSK9 Inhibitor Evolocumab on Cardiovascular Outcomes
 Effect of the PCSK9 Inhibitor Evolocumab on Cardiovascular Outcomes MS Sabatine, RP Giugliano, SD Wiviott, FJ Raal, CM Ballantyne, R Somaratne, J Legg, SM Wasserman, R Scott, MJ Koren, and EA Stein for
Effect of the PCSK9 Inhibitor Evolocumab on Cardiovascular Outcomes MS Sabatine, RP Giugliano, SD Wiviott, FJ Raal, CM Ballantyne, R Somaratne, J Legg, SM Wasserman, R Scott, MJ Koren, and EA Stein for
2/10/2016. Perspectives on the 2013 ACC/AHA Cholesterol Guidelines. Disclosures. ATP-III Update 2004
 Perspectives on the 2013 ACC/AHA Cholesterol Guidelines Donald M. Lloyd-Jones, MD ScM Senior Associate Dean Chair and Professor of Preventive Medicine Northwestern Feinberg School of Medicine Disclosures
Perspectives on the 2013 ACC/AHA Cholesterol Guidelines Donald M. Lloyd-Jones, MD ScM Senior Associate Dean Chair and Professor of Preventive Medicine Northwestern Feinberg School of Medicine Disclosures
Managing Dyslipidemia in Disclosures. Learning Objectives 03/05/2018. Speaker Disclosures
 Managing Dyslipidemia in 2018 Glen J. Pearson, BSc, BScPhm, PharmD, FCSHP, FCCS Professor of Medicine (Cardiology) Co-Director, Cardiac Transplant Clinic; Associate Chair, Health Research Ethics Boards;
Managing Dyslipidemia in 2018 Glen J. Pearson, BSc, BScPhm, PharmD, FCSHP, FCCS Professor of Medicine (Cardiology) Co-Director, Cardiac Transplant Clinic; Associate Chair, Health Research Ethics Boards;
Contemporary management of Dyslipidemia
 Contemporary management of Dyslipidemia Todd Anderson Feb 2018 Disclosure Statement Within the past two years: I have not had an affiliation (financial or otherwise) with a commercial organization that
Contemporary management of Dyslipidemia Todd Anderson Feb 2018 Disclosure Statement Within the past two years: I have not had an affiliation (financial or otherwise) with a commercial organization that
Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin
 Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin Marc S. Sabatine, MD, MPH on behalf of the PEGASUS-TIMI 54 Executive
Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin Marc S. Sabatine, MD, MPH on behalf of the PEGASUS-TIMI 54 Executive
The FOURIER Trial. On behalf of the FOURIER Investigators. American Heart Association Scientific Sessions November 13, 2017
 The FOURIER Trial Sabina A. Murphy, Terje R. Pedersen, Zbigniew A. Gaciong, Richard Ceska, Marat V. Ezhov, Derek Connolly, Oleg Kraydashenko, J. Wouter Jukema, Kalman Toth, Matti J. Tikkanen, Kyungah Im,
The FOURIER Trial Sabina A. Murphy, Terje R. Pedersen, Zbigniew A. Gaciong, Richard Ceska, Marat V. Ezhov, Derek Connolly, Oleg Kraydashenko, J. Wouter Jukema, Kalman Toth, Matti J. Tikkanen, Kyungah Im,
EVIDENCE TO DATE EVOLOCUMAB (REPATHA)
 and Clinical Outcomes in Patients with Cardiovascular Disease, March 2017 1 CLINICAL QUESTION In patients with atherosclerotic cardiovascular disease and LDL >1.8mmol/L or non-hdl > 2.6mmol/L, how does
and Clinical Outcomes in Patients with Cardiovascular Disease, March 2017 1 CLINICAL QUESTION In patients with atherosclerotic cardiovascular disease and LDL >1.8mmol/L or non-hdl > 2.6mmol/L, how does
MS Sabatine, RP Giugliano, AC Keech, PS Sever, SA Murphy and TR Pedersen, for the FOURIER Steering Committee & Investigators
 Evolocumab Reduces Cardiovascular Events in Patients with Baseline LDL-C
Evolocumab Reduces Cardiovascular Events in Patients with Baseline LDL-C
Landmesser U et al. Eur Heart J 2017; https://doi.org/ /eurheartj/ehx549
 2017 Update of ESC/EAS Task Force on Practical Clinical Guidance for PCSK9 inhibition in Patients with Atherosclerotic Cardiovascular Disease or in Familial Hypercholesterolaemia Cardiovascular Outcomes
2017 Update of ESC/EAS Task Force on Practical Clinical Guidance for PCSK9 inhibition in Patients with Atherosclerotic Cardiovascular Disease or in Familial Hypercholesterolaemia Cardiovascular Outcomes
Clinical Efficacy and Safety of Achieving Very Low LDL-C Levels With the PCSK9 Inhibitor Evolocumab in the FOURIER Outcomes Trial
 Clinical Efficacy and Safety of Achieving Very Low LDL-C Levels With the PCSK9 Inhibitor Evolocumab in the FOURIER Outcomes Trial RP Giugliano, TR Pedersen, AC Keech, PS Sever, JG Park, and MS Sabatine,
Clinical Efficacy and Safety of Achieving Very Low LDL-C Levels With the PCSK9 Inhibitor Evolocumab in the FOURIER Outcomes Trial RP Giugliano, TR Pedersen, AC Keech, PS Sever, JG Park, and MS Sabatine,
No relevant financial relationships
 MANAGEMENT OF LIPID DISORDERS Balancing Benefits and harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial relationships baron@medicine.ucsf.edu
MANAGEMENT OF LIPID DISORDERS Balancing Benefits and harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial relationships baron@medicine.ucsf.edu
John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam
 Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
New ACC/AHA Guidelines on Lipids: Are PCSK9 Inhibitors Poised for a Breakthrough?
 New ACC/AHA Guidelines on Lipids: Are PCSK9 Inhibitors Poised for a Breakthrough? Sidney C. Smith, Jr. MD, FACC, FAHA Professor of Medicine/Cardiology University of North Carolina at Chapel Hill Immediate
New ACC/AHA Guidelines on Lipids: Are PCSK9 Inhibitors Poised for a Breakthrough? Sidney C. Smith, Jr. MD, FACC, FAHA Professor of Medicine/Cardiology University of North Carolina at Chapel Hill Immediate
2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults
 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation,
2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation,
Novel PCSK9 Outcomes. in Perspective: Lessons from FOURIER & ODYSSEY LDL-C. ASCVD Risk. Suboptimal Statin Therapy
 LDL-C Novel PCSK9 Outcomes Suboptimal Statin Therapy ASCVD Risk in Perspective: Lessons from FOURIER & ODYSSEY Jennifer G. Robinson, MD, MPH Professor, Departments of Epidemiology & Medicine Director,
LDL-C Novel PCSK9 Outcomes Suboptimal Statin Therapy ASCVD Risk in Perspective: Lessons from FOURIER & ODYSSEY Jennifer G. Robinson, MD, MPH Professor, Departments of Epidemiology & Medicine Director,
Does IMPROVE-IT & FOURIER Confirm or Refute the LDL Hypothesis?
 Does IMPROVE-IT & FOURIER Confirm or Refute the LDL Hypothesis? Controversies and Advances in the Treatment of Cardiovascular Disease The Seventeenth in the Series Beverly Hills, November 16, 2017 Sanjay
Does IMPROVE-IT & FOURIER Confirm or Refute the LDL Hypothesis? Controversies and Advances in the Treatment of Cardiovascular Disease The Seventeenth in the Series Beverly Hills, November 16, 2017 Sanjay
Saudi Arabia February Pr Michel KOMAJDA. Université Pierre et Marie Curie Hospital Pitié Salpétrière
 Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
New Strategies for Lowering LDL - Are They Really Worth It?
 New Strategies for Lowering LDL - Are They Really Worth It? Gregg C. Fonarow, MD, FACC, FAHA, FHFSA Eliot Corday Professor of CV Medicine and Science Director, Ahmanson-UCLA Cardiomyopathy Center Co-Director,
New Strategies for Lowering LDL - Are They Really Worth It? Gregg C. Fonarow, MD, FACC, FAHA, FHFSA Eliot Corday Professor of CV Medicine and Science Director, Ahmanson-UCLA Cardiomyopathy Center Co-Director,
What have We Learned in Dyslipidemia Management Since the Publication of the 2013 ACC/AHA Guideline?
 What have We Learned in Dyslipidemia Management Since the Publication of the 2013 ACC/AHA Guideline? Salim S. Virani, MD, PhD, FACC, FAHA Associate Professor, Section of Cardiovascular Research Baylor
What have We Learned in Dyslipidemia Management Since the Publication of the 2013 ACC/AHA Guideline? Salim S. Virani, MD, PhD, FACC, FAHA Associate Professor, Section of Cardiovascular Research Baylor
Major recommendations for statin therapy for ASCVD prevention
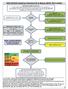 2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
PCSK9 Inhibitors and Modulators
 PCSK9 Inhibitors and Modulators Pam R. Taub MD, FACC Director of Step Family Cardiac Rehabilitation and Wellness Center Associate Professor of Medicine UC San Diego Health System Disclosures Speaker s
PCSK9 Inhibitors and Modulators Pam R. Taub MD, FACC Director of Step Family Cardiac Rehabilitation and Wellness Center Associate Professor of Medicine UC San Diego Health System Disclosures Speaker s
CVD risk assessment using risk scores in primary and secondary prevention
 CVD risk assessment using risk scores in primary and secondary prevention Raul D. Santos MD, PhD Heart Institute-InCor University of Sao Paulo Brazil Disclosure Honoraria for consulting and speaker activities
CVD risk assessment using risk scores in primary and secondary prevention Raul D. Santos MD, PhD Heart Institute-InCor University of Sao Paulo Brazil Disclosure Honoraria for consulting and speaker activities
Characterization of Types and Sizes of Myocardial Infarction Reduced with Evolocumab in FOURIER
 Characterization of Types and Sizes of Myocardial Infarction Reduced with Evolocumab in FOURIER Stephen D Wiviott, Robert P Giugliano, David A Morrow, Gaetano M De Ferrari, Basil S Lewis, Kurt Huber, Julia
Characterization of Types and Sizes of Myocardial Infarction Reduced with Evolocumab in FOURIER Stephen D Wiviott, Robert P Giugliano, David A Morrow, Gaetano M De Ferrari, Basil S Lewis, Kurt Huber, Julia
Lipid Therapy: Statins and Beyond. Ivan Anderson, MD RIHVH Cardiology
 Lipid Therapy: Statins and Beyond Ivan Anderson, MD RIHVH Cardiology Outline The cholesterol hypothesis and lipid metabolism The Guidelines 4 Groups that Benefit from Lipid therapy Initiation and monitoring
Lipid Therapy: Statins and Beyond Ivan Anderson, MD RIHVH Cardiology Outline The cholesterol hypothesis and lipid metabolism The Guidelines 4 Groups that Benefit from Lipid therapy Initiation and monitoring
Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM
 Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM Efficacy by subgroup, and safety when LDL-C
Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM Efficacy by subgroup, and safety when LDL-C
Drug Class Monograph
 Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009
 The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009 Learning Objectives 1. Understand the role of statin therapy in the primary and secondary prevention of stroke 2. Explain
The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009 Learning Objectives 1. Understand the role of statin therapy in the primary and secondary prevention of stroke 2. Explain
Fasting or non fasting?
 Vascular harmony Robert Chilton Professor of Medicine University of Texas Health Science Center Director of Cardiac Catheterization labs Director of clinical proteomics Which is best to measure Lower continues
Vascular harmony Robert Chilton Professor of Medicine University of Texas Health Science Center Director of Cardiac Catheterization labs Director of clinical proteomics Which is best to measure Lower continues
Weigh the benefit of statin treatment: LDL & Beyond
 Weigh the benefit of statin treatment: LDL & Beyond Duk-Woo Park, MD, PhD Heart Institute, University of Ulsan College of Medicine, Asan Medical, Seoul, Korea FOURIER Further cardiovascular OUtcomes Research
Weigh the benefit of statin treatment: LDL & Beyond Duk-Woo Park, MD, PhD Heart Institute, University of Ulsan College of Medicine, Asan Medical, Seoul, Korea FOURIER Further cardiovascular OUtcomes Research
LLL Session - Nutrition support in diabetes and dyslipidemia. Dyslipidemia: targeting the management of cardiovascular risk factors. M.
 ESPEN Congress Leipzig 2013 LLL Session - Nutrition support in diabetes and dyslipidemia Dyslipidemia: targeting the management of cardiovascular risk factors M. Leon Sanz (ES) Dyslipidemia: Targeting
ESPEN Congress Leipzig 2013 LLL Session - Nutrition support in diabetes and dyslipidemia Dyslipidemia: targeting the management of cardiovascular risk factors M. Leon Sanz (ES) Dyslipidemia: Targeting
Treatment of Cardiovascular Risk Factors. Kevin M Hayes D.O. F.A.C.C. First Coast Heart and Vascular Center
 Treatment of Cardiovascular Risk Factors Kevin M Hayes D.O. F.A.C.C. First Coast Heart and Vascular Center Disclosures: None Objectives What do risk factors tell us What to check and when Does treatment
Treatment of Cardiovascular Risk Factors Kevin M Hayes D.O. F.A.C.C. First Coast Heart and Vascular Center Disclosures: None Objectives What do risk factors tell us What to check and when Does treatment
New evidences in heart failure: the GISSI-HF trial. Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy
 New evidences in heart failure: the GISSI-HF trial Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy % Improving survival in chronic HF and LV systolic dysfunction: 1 year all-cause mortality 20
New evidences in heart failure: the GISSI-HF trial Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy % Improving survival in chronic HF and LV systolic dysfunction: 1 year all-cause mortality 20
THE 2013 ACC/AHA GUIDELINES ON THE TREATMENT OF BLOOD CHOLESTEROL
 THE 2013 ACC/AHA GUIDELINES ON THE TREATMENT OF BLOOD CHOLESTEROL Anne Carol Goldberg, MD, FACP, FAHA, FNLA Associate Professor of Medicine Washington University School of Medicine National Lipid Association
THE 2013 ACC/AHA GUIDELINES ON THE TREATMENT OF BLOOD CHOLESTEROL Anne Carol Goldberg, MD, FACP, FAHA, FNLA Associate Professor of Medicine Washington University School of Medicine National Lipid Association
4 th and Goal To Go How Low Should We Go? :
 4 th and Goal To Go How Low Should We Go? : Evaluating New Lipid Lowering Therapies Catherine Bourg Rebitch, PharmD, BCACP Clinical Associate Professor Disclosure The presenter has nothing to disclose
4 th and Goal To Go How Low Should We Go? : Evaluating New Lipid Lowering Therapies Catherine Bourg Rebitch, PharmD, BCACP Clinical Associate Professor Disclosure The presenter has nothing to disclose
Evolving Concepts on Lipid Management from Ezetimibe (IMPROVE IT) to PCSK9 Inhibitors
 Evolving Concepts on Lipid Management from Ezetimibe (IMPROVE IT) to PCSK9 Inhibitors Sidney C. Smith, Jr. MD, FACC, FAHA, FESC Professor of Medicine/Cardiology University of North Carolina at Chapel Hill
Evolving Concepts on Lipid Management from Ezetimibe (IMPROVE IT) to PCSK9 Inhibitors Sidney C. Smith, Jr. MD, FACC, FAHA, FESC Professor of Medicine/Cardiology University of North Carolina at Chapel Hill
What Role do the New PCSK9 Inhibitors Have in Lipid Lowering Treatment?
 What Role do the New PCSK9 Inhibitors Have in Lipid Lowering Treatment? Jennifer G. Robinson, MD, MPH Professor, Departments of Epidemiology & Medicine Director, Prevention Intervention Center University
What Role do the New PCSK9 Inhibitors Have in Lipid Lowering Treatment? Jennifer G. Robinson, MD, MPH Professor, Departments of Epidemiology & Medicine Director, Prevention Intervention Center University
Deep Dive into Contemporary Cholesterol Management. Kim Allan Williams, Sr., MD, FACC Pamela B. Morris, MD, FACC 7 October 2016 Mexico City
 Deep Dive into Contemporary Cholesterol Management Kim Allan Williams, Sr., MD, FACC Pamela B. Morris, MD, FACC 7 October 2016 Mexico City Introduction: Pamela B. Morris, MD, FACC COMING TO CONSENSUS IN
Deep Dive into Contemporary Cholesterol Management Kim Allan Williams, Sr., MD, FACC Pamela B. Morris, MD, FACC 7 October 2016 Mexico City Introduction: Pamela B. Morris, MD, FACC COMING TO CONSENSUS IN
Update on Lipid Management in Cardiovascular Disease: How to Understand and Implement the New ACC/AHA Guidelines
 Update on Lipid Management in Cardiovascular Disease: How to Understand and Implement the New ACC/AHA Guidelines Paul Mahoney, MD Sentara Cardiology Specialists Lipid Management in Cardiovascular Disease
Update on Lipid Management in Cardiovascular Disease: How to Understand and Implement the New ACC/AHA Guidelines Paul Mahoney, MD Sentara Cardiology Specialists Lipid Management in Cardiovascular Disease
Lipids & Hypertension Update
 Lipids & Hypertension Update No financial disclosures Michael W. Cullen, MD, FACC Senior Associate Consultant, Assistant Professor of Medicine Mayo Clinic Department of Cardiovascular Diseases 34 th Annual
Lipids & Hypertension Update No financial disclosures Michael W. Cullen, MD, FACC Senior Associate Consultant, Assistant Professor of Medicine Mayo Clinic Department of Cardiovascular Diseases 34 th Annual
Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes?
 Late Breaking Clinical Trial Session at AHA 2017 Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes? The REAL-CAD Study in 13,054 Patients With Stable Coronary Artery Disease Takeshi
Late Breaking Clinical Trial Session at AHA 2017 Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes? The REAL-CAD Study in 13,054 Patients With Stable Coronary Artery Disease Takeshi
Confusion about guidelines: How should we treat lipids?
 Confusion about guidelines: How should we treat lipids? Anne Carol Goldberg, MD, FACP, FAHA, FNLA Professor of Medicine Washington University School of Medicine American College of Physicians Missouri
Confusion about guidelines: How should we treat lipids? Anne Carol Goldberg, MD, FACP, FAHA, FNLA Professor of Medicine Washington University School of Medicine American College of Physicians Missouri
PCSK9 Agents Drug Class Prior Authorization Protocol
 PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
Alirocumab Treatment Effect Did Not Differ Between Patients With and Without Low HDL-C or High Triglyceride Levels in Phase 3 trials
 Alirocumab Treatment Effect Did Not Differ Between Patients With and Without Low HDL-C or High Triglyceride Levels in Phase 3 trials G. Kees Hovingh, 1 Richard Ceska, 2 Michael Louie, 3 Pascal Minini,
Alirocumab Treatment Effect Did Not Differ Between Patients With and Without Low HDL-C or High Triglyceride Levels in Phase 3 trials G. Kees Hovingh, 1 Richard Ceska, 2 Michael Louie, 3 Pascal Minini,
New Guidelines in Dyslipidemia Management
 The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The TNT Trial Is It Time to Shift Our Goals in Clinical
 The TNT Trial Is It Time to Shift Our Goals in Clinical Angioplasty Summit Luncheon Symposium Korea Assoc Prof David Colquhoun 29 April 2005 University of Queensland, Wesley Hospital, Brisbane, Australia
The TNT Trial Is It Time to Shift Our Goals in Clinical Angioplasty Summit Luncheon Symposium Korea Assoc Prof David Colquhoun 29 April 2005 University of Queensland, Wesley Hospital, Brisbane, Australia
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2
 2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
Dapagliflozin and Outcomes in Patients with Peripheral Artery Disease: Insights from DECLARE-TIMI 58
 Dapagliflozin and Outcomes in Patients with Peripheral Artery Disease: Insights from DECLARE-TIMI 58 Marc P. Bonaca MD MPH for the DECLARE TIMI 58 Investigators American College of Cardiology March 2019
Dapagliflozin and Outcomes in Patients with Peripheral Artery Disease: Insights from DECLARE-TIMI 58 Marc P. Bonaca MD MPH for the DECLARE TIMI 58 Investigators American College of Cardiology March 2019
Introduction. Objective. Critical Questions Addressed
 Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
Disclosures. Overview 9/30/ ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults
 2013 ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults 2014 AAHP Fall Seminar Sherry Myatt, PharmD, BCPS Assistant Director of Pharmacy for
2013 ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults 2014 AAHP Fall Seminar Sherry Myatt, PharmD, BCPS Assistant Director of Pharmacy for
New Horizons in Dyslipidemia Management in Primary Care
 New Horizons in Dyslipidemia Management in Primary Care Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or
New Horizons in Dyslipidemia Management in Primary Care Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or
PCSK9 antibodies: A new therapeutic option for the treatment of hypercholesterolemia
 : 262-267, 2017 Περίληψη Διάλεξης PCSK9 antibodies: A new therapeutic option for the treatment of hypercholesterolemia I. Gouni-Bethold Polyclinic for Endocrinology, Diabetes, and Preventive Medicine University
: 262-267, 2017 Περίληψη Διάλεξης PCSK9 antibodies: A new therapeutic option for the treatment of hypercholesterolemia I. Gouni-Bethold Polyclinic for Endocrinology, Diabetes, and Preventive Medicine University
4/7/ The stats on heart disease. + Deaths & Age-Adjusted Death Rates for
 + Update on Lipid Management Stacey Gardiner, MD Assistant Professor Division of Cardiovascular Medicine Medical College of Wisconsin + The stats on heart disease Over the past 10 years for which statistics
+ Update on Lipid Management Stacey Gardiner, MD Assistant Professor Division of Cardiovascular Medicine Medical College of Wisconsin + The stats on heart disease Over the past 10 years for which statistics
EBBINGHAUS: - A Cognitive Study of Patients Enrolled in the FOURIER Trial
 EBBINGHAUS: - A Cognitive Study of Patients Enrolled in the FOURIER Trial RP Giugliano, F Mach, K Zavitz, AC Keech, TR Pedersen, MS Sabatine, P Sever, C Kurtz, N Honarpour, BR Ott, on behalf of the EBBINGHAUS
EBBINGHAUS: - A Cognitive Study of Patients Enrolled in the FOURIER Trial RP Giugliano, F Mach, K Zavitz, AC Keech, TR Pedersen, MS Sabatine, P Sever, C Kurtz, N Honarpour, BR Ott, on behalf of the EBBINGHAUS
ACC/AHA GUIDELINES ON LIPIDS AND PCSK9 INHIBITORS
 ACC/AHA GUIDELINES ON LIPIDS AND PCSK9 INHIBITORS Ziyad Ghazzal MD, FACC, FSCAI Professor of Medicine Deputy Vice President/Dean Associate Dean for Clinical Affairs American University of Beirut Adjunct
ACC/AHA GUIDELINES ON LIPIDS AND PCSK9 INHIBITORS Ziyad Ghazzal MD, FACC, FSCAI Professor of Medicine Deputy Vice President/Dean Associate Dean for Clinical Affairs American University of Beirut Adjunct
2016 EUROPEAN GUIDELINES ON CVD PREVENTION IN CLINICAL PRACTICE
 2016 EUROPEAN GUIDELINES ON CVD PREVENTION IN CLINICAL PRACTICE Massimo F Piepoli, MD, PhD, FESC, Piacenza, Italy on behalf of the 6 th Joint Task Force 2 3 Guidelines still based upon the principles of
2016 EUROPEAN GUIDELINES ON CVD PREVENTION IN CLINICAL PRACTICE Massimo F Piepoli, MD, PhD, FESC, Piacenza, Italy on behalf of the 6 th Joint Task Force 2 3 Guidelines still based upon the principles of
Workshop. Todd Anderson MD / Jacques Genest MD
 Workshop Todd Anderson MD / Jacques Genest MD Game-Changing Trials 2017 FOURIER Evolocumab n=27,564 HR 0.80 CANTOS Canakinumab n=10,061 HR 0.85 COMPASS Rivaroxaban + ASA n=27,395 HR 0.76 Key Secondary
Workshop Todd Anderson MD / Jacques Genest MD Game-Changing Trials 2017 FOURIER Evolocumab n=27,564 HR 0.80 CANTOS Canakinumab n=10,061 HR 0.85 COMPASS Rivaroxaban + ASA n=27,395 HR 0.76 Key Secondary
Reducing Inflammation to Reduce Cardiovascular Risk: The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS)
 New York City Cardiovascular Symposium December 10, 2017 Reducing Inflammation to Reduce Cardiovascular Risk: The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Paul M Ridker, MD, MPH
New York City Cardiovascular Symposium December 10, 2017 Reducing Inflammation to Reduce Cardiovascular Risk: The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Paul M Ridker, MD, MPH
Dyslipidemia and Combination Therapy: A Framework for Clinical Decision Making
 Dyslipidemia and Combination Therapy: A Framework for Clinical Decision Making Shashank Sinha, MD Pamela B. Morris, MD, FACC 8 October 2016 Mexico City Introduction: Pamela B. Morris, MD, FACC COMING TO
Dyslipidemia and Combination Therapy: A Framework for Clinical Decision Making Shashank Sinha, MD Pamela B. Morris, MD, FACC 8 October 2016 Mexico City Introduction: Pamela B. Morris, MD, FACC COMING TO
ATP IV: Predicting Guideline Updates
 Disclosures ATP IV: Predicting Guideline Updates Daniel M. Riche, Pharm.D., BCPS, CDE Speaker s Bureau Merck Janssen Boehringer-Ingelheim Learning Objectives Describe at least two evidence-based recommendations
Disclosures ATP IV: Predicting Guideline Updates Daniel M. Riche, Pharm.D., BCPS, CDE Speaker s Bureau Merck Janssen Boehringer-Ingelheim Learning Objectives Describe at least two evidence-based recommendations
Lipid Panel Management Refresher Course for the Family Physician
 Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Update on Dyslipidemia and Recent Data on Treating the Statin Intolerant Patient
 Update on Dyslipidemia and Recent Data on Treating the Statin Intolerant Patient Steven E. Nissen MD Chairman, Department of Cardiovascular Medicine Cleveland Clinic Disclosure Consulting: Many pharmaceutical
Update on Dyslipidemia and Recent Data on Treating the Statin Intolerant Patient Steven E. Nissen MD Chairman, Department of Cardiovascular Medicine Cleveland Clinic Disclosure Consulting: Many pharmaceutical
Disclosure. No relevant financial relationships. Placebo-Controlled Statin Trials
 PREVENTING CARDIOVASCULAR DISEASE IN WOMEN: Current Guidelines for Hypertension, Lipids and Aspirin Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
PREVENTING CARDIOVASCULAR DISEASE IN WOMEN: Current Guidelines for Hypertension, Lipids and Aspirin Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease. Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.
 Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
PCSK9 Inhibitors Are They Worth The Money? Michael J. Blaha MD MPH
 PCSK9 Inhibitors Are They Worth The Money? Michael J. Blaha MD MPH Presented by: Michael J. Blaha November 16, 2017 1 Financial Disclosures Grants: Amgen Foundation, Aetna Foundation Advisory Boards: Amgen,
PCSK9 Inhibitors Are They Worth The Money? Michael J. Blaha MD MPH Presented by: Michael J. Blaha November 16, 2017 1 Financial Disclosures Grants: Amgen Foundation, Aetna Foundation Advisory Boards: Amgen,
Lipids: new drugs, new trials, new guidelines
 Lipids: new drugs, new trials, new guidelines Milan Gupta, MD, FRCPC, FCCS State of the Heart Co-Chair Associate Clinical Professor of Medicine, McMaster University Assistant Professor of Medicine, University
Lipids: new drugs, new trials, new guidelines Milan Gupta, MD, FRCPC, FCCS State of the Heart Co-Chair Associate Clinical Professor of Medicine, McMaster University Assistant Professor of Medicine, University
The ODYSSEY OUTCOMES Trial: Topline Results. Alirocumab in Patients After Acute Coronary Syndrome
 The ODYSSEY OUTCOMES Trial: Topline Results Alirocumab in Patients After Acute Coronary Syndrome Gregory G. Schwartz, Michael Szarek, Deepak L. Bhatt, Vera Bittner, Rafael Diaz, Jay Edelberg, Shaun G.
The ODYSSEY OUTCOMES Trial: Topline Results Alirocumab in Patients After Acute Coronary Syndrome Gregory G. Schwartz, Michael Szarek, Deepak L. Bhatt, Vera Bittner, Rafael Diaz, Jay Edelberg, Shaun G.
Disclosure. No relevant financial relationships. Placebo-Controlled Statin Trials
 MANAGEMENT OF HYPERLIPIDEMIA AND CARDIOVASCULAR RISK IN WOMEN: Balancing Benefits and Harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
MANAGEMENT OF HYPERLIPIDEMIA AND CARDIOVASCULAR RISK IN WOMEN: Balancing Benefits and Harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
Disclosures. Choosing a Statin/New Therapies. Case. How else would you do to treat him? LDL-C Reduction with Different Statin Strategies
 Disclosures I have no disclosures relevant to this talk Choosing a Statin/New Therapies Aryan Aiyer, MD Assistant Professor of Medicine University of Pittsburgh School of Medicine UPMC Heart and Vascular
Disclosures I have no disclosures relevant to this talk Choosing a Statin/New Therapies Aryan Aiyer, MD Assistant Professor of Medicine University of Pittsburgh School of Medicine UPMC Heart and Vascular
Lipid Management C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute
 Lipid Management 2018 C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute Disclosures No Financial Disclosures Disclosures I am an Interventional Cardiologist I put STENTS in for
Lipid Management 2018 C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute Disclosures No Financial Disclosures Disclosures I am an Interventional Cardiologist I put STENTS in for
Update on Lipid Guidelines and Intense Treatment of LDL-C with PCSK9 Inhibitors Carl J. Lavie, MD,FACC,FACP,FCCP
 Update on Lipid Guidelines and Intense Treatment of LDL-C with PCSK9 Inhibitors Carl J. Lavie, MD,FACC,FACP,FCCP Professor of Medicine Medical-Director, Preventive Cardiology John Ochsner Heart and Vascular
Update on Lipid Guidelines and Intense Treatment of LDL-C with PCSK9 Inhibitors Carl J. Lavie, MD,FACC,FACP,FCCP Professor of Medicine Medical-Director, Preventive Cardiology John Ochsner Heart and Vascular
Antithrombotic Therapy for Long-Term Secondary Prevention Considerations for Long-Term DAPT
 Antithrombotic Therapy for Long-Term Secondary Prevention Considerations for Long-Term DAPT Marc P. Bonaca, MD, MPH Vascular Section, Cardiovascular Division Investigator TIMI Study Group Brigham and Women
Antithrombotic Therapy for Long-Term Secondary Prevention Considerations for Long-Term DAPT Marc P. Bonaca, MD, MPH Vascular Section, Cardiovascular Division Investigator TIMI Study Group Brigham and Women
THE CRUCIAL PROBLEM OF ASCVD Can New Therapeutic Options Resolve It? THE CRUCIAL PROBLEM OF ASCVD Can New Therapeutic Options Resolve It?
 James A. Underberg, MD, MS, FACPM, FACP, FNYAM, FASPC, FNLA Lipidology and Cardiovascular Disease Prevention Clinical Assistant Professor of Medicine NYU Medical School and NYU Center for CV Prevention
James A. Underberg, MD, MS, FACPM, FACP, FNYAM, FASPC, FNLA Lipidology and Cardiovascular Disease Prevention Clinical Assistant Professor of Medicine NYU Medical School and NYU Center for CV Prevention
Get a Statin or Not? Learning objectives. Presentation overview 4/3/2018. Treatment Strategies in Dyslipidemia Management
 Get a Statin or Not? Treatment Strategies in Dyslipidemia Management Michelle Chu, PharmD, BCACP, CDE Assistant Professor of Clinical Pharmacy, USC School of Pharmacy Sahar Dagher, PharmD Virtual Care
Get a Statin or Not? Treatment Strategies in Dyslipidemia Management Michelle Chu, PharmD, BCACP, CDE Assistant Professor of Clinical Pharmacy, USC School of Pharmacy Sahar Dagher, PharmD Virtual Care
Industry Relationships and Institutional Affiliations
 Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study Christopher
Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study Christopher
Should I use statins?
 I know the trials in heart failure but how do I manage my patient? Should I use statins? Aldo P Maggioni, MD, FESC ANMCO Research Center Firenze, Italy Disclosures Aldo P Maggioni served as a member of
I know the trials in heart failure but how do I manage my patient? Should I use statins? Aldo P Maggioni, MD, FESC ANMCO Research Center Firenze, Italy Disclosures Aldo P Maggioni served as a member of
Disclosures No relationships (not even to an employer) No off-label uses. Cholesterol Lowering Guidelines: What now?
 Disclosures No relationships (not even to an employer) No off-label uses Cholesterol Lowering Guidelines: What now?, FACP 1 2 65-year-old white woman Total cholesterol 175mg/dL HDL 54 mg/dl LDL 96 mg/dl
Disclosures No relationships (not even to an employer) No off-label uses Cholesterol Lowering Guidelines: What now?, FACP 1 2 65-year-old white woman Total cholesterol 175mg/dL HDL 54 mg/dl LDL 96 mg/dl
Decline in CV-Mortality
 Lipids id 2013 What s Changed? Christopher Granger, MD Disclosure Research contracts: AstraZeneca, GSK, Merck, Sanofi- Aventis, BMS, Pfizer, The Medicines Company, Medtronic Foundation, and Boehringer
Lipids id 2013 What s Changed? Christopher Granger, MD Disclosure Research contracts: AstraZeneca, GSK, Merck, Sanofi- Aventis, BMS, Pfizer, The Medicines Company, Medtronic Foundation, and Boehringer
2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD
 2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD How do you interpret my blood test results? What are our targets for these tests? Before the ACC/AHA Lipid Guidelines A1c:
2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD How do you interpret my blood test results? What are our targets for these tests? Before the ACC/AHA Lipid Guidelines A1c:
New Guidelines in Dyslipidemia Management
 The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
Design and Analysis of a Cancer Prevention Trial: Plans and Results. Matthew Somerville 09 November 2009
 Design and Analysis of a Cancer Prevention Trial: Plans and Results Matthew Somerville 09 November 2009 Overview Objective: Review the planned analyses for a large prostate cancer prevention study and
Design and Analysis of a Cancer Prevention Trial: Plans and Results Matthew Somerville 09 November 2009 Overview Objective: Review the planned analyses for a large prostate cancer prevention study and
Pharmacy Drug Class Review
 Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Statins and PCSK9 inhibitors for stroke prevention
 Statins and PCSK9 inhibitors for stroke prevention Haralampos Milionis Professor of Internal Medicine School of Medicine, University of Ioannina Ioannina, Greece Reduction in CV events (%) Every 1 mmol/l
Statins and PCSK9 inhibitors for stroke prevention Haralampos Milionis Professor of Internal Medicine School of Medicine, University of Ioannina Ioannina, Greece Reduction in CV events (%) Every 1 mmol/l
Disclosures. Objectives 2/11/2017
 Role of Non-Statin Therapy in CV Risk Reduction James A. Underberg, MD, MS, FACPM, FACP, FASH, FNLA,FASPC Clinical Assistant Professor of Medicine NYU School of Medicine NYU Langone Center for Cardiovascular
Role of Non-Statin Therapy in CV Risk Reduction James A. Underberg, MD, MS, FACPM, FACP, FASH, FNLA,FASPC Clinical Assistant Professor of Medicine NYU School of Medicine NYU Langone Center for Cardiovascular
David Y. Gaitonde, MD, FACP Endocrinology DDEAMC, Fort Gordon
 David Y. Gaitonde, MD, FACP Endocrinology DDEAMC, Fort Gordon I have no actual or potential conflicts of interest in relation to this program or presentation. Raphael School of Athens, 1509-1511 Apply
David Y. Gaitonde, MD, FACP Endocrinology DDEAMC, Fort Gordon I have no actual or potential conflicts of interest in relation to this program or presentation. Raphael School of Athens, 1509-1511 Apply
Educational Objectives. Disease Trajectories and CVD Risk Reduction. Hypercholesterolemia Support for LDL-C Causality
 Educational Objectives At the conclusion of this activity, participants should be able to: Evaluate the extent of residual CVD risk to which ASCVD patients are exposed, and treat additional CVD risk elements
Educational Objectives At the conclusion of this activity, participants should be able to: Evaluate the extent of residual CVD risk to which ASCVD patients are exposed, and treat additional CVD risk elements
Lipid Management 2013 Statin Benefit Groups
 Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Case Studies The Role of Non-Statin Therapies for LDL-C Lowering in the Management of ASCVD Risk
 Case Studies The Role of Non-Statin Therapies for LDL-C Lowering in the Management of ASCVD Risk Kim K. Birtcher, PharmD, MS, AACC Clinical Professor University of Houston College of Pharmacy Houston,
Case Studies The Role of Non-Statin Therapies for LDL-C Lowering in the Management of ASCVD Risk Kim K. Birtcher, PharmD, MS, AACC Clinical Professor University of Houston College of Pharmacy Houston,
